Abstract
Chaperone proteins have crucial roles to play in all animal species and are involved in mediating both the folding of newly synthesized peptides into their mature conformation, the refolding of misfolded proteins, and the trafficking of proteins between subcellular compartments. These highly conserved proteins have particularly important roles to play in dealing with disruptions of the proteome as a result of environmental stress since abiotic factors, including temperature, pressure, oxygen, water availability, and pollutants can readily disrupt the conformation and/or function of all types of proteins, e.g., enzymes, transporters, and structural proteins. The current review provides an update on recent advances in understanding the roles and responses of chaperones in aiding animals to deal with environmental stress, offering new information on chaperone action in supporting survival strategies including torpor, hibernation, anaerobiosis, estivation, and cold/freeze tolerance among both vertebrate and invertebrate species.
Keywords: Heat shock proteins, Freeze tolerance, Hibernation, Estivation, Anaerobiosis
Introduction
All living organisms on Earth are subjected to changing environmental conditions, both predictable (e.g., daily, seasonal, tide cycle) and unpredictable, and they must respond/adapt appropriately in order to survive. Adaptative responses to environmental stress can occur at multiple levels including behavioral (e.g., move to new locations), physiological (e.g., entry into dormant/hypometabolic states), and molecular (e.g., altered gene/protein expression, up/downregulation of enzymes, synthesis of specific metabolite or protein protectants). For most organisms, environmental stress conditions must be met “head-on” since opportunities to elude stressful conditions (e.g., changes in temperature, oxygen, salinity, water availability, exposure to chemicals/toxins) are often poor or unattainable.
Multiple strategies of adaptation to environmental stress have evolved and are supported by metabolic responses at whole animal, cellular and biochemical levels (Hochachka and Somero 2002; Storey 2004). A key factor in adaptation to environmental stress is protection, renewal, or modification of the cellular proteome to support the functional machinery of cells. Protection of proteins/enzymes under stress conditions is one key responsibility of chaperone proteins. Initially named heat shock proteins (HSPs), it is now known that upregulation of chaperone proteins is a conserved response to multiple environmental stress conditions including heat, cold, water loss, oxygen restriction, heavy metals, and chemical pollutants, as well as internal disruptions (infection, inflammation, exercise, etc.) or virtually any stress that disrupts the normal conformation or function of cell proteins.
The discovery of chaperone proteins arose from the pioneering work, 60 years ago, of an Italian scientist, Ferruccio Ritossa who identified a heat shock response by fruit flies, Drosophila melanogaster (Ritossa 1962). New chromosomal puffs appeared when flies were placed at an elevated temperature indicating that enhanced gene expression was occurring at these loci. This led to the subsequent discovery of a group of proteins encoded by these genes that were named heat shock proteins (Tissières et al. 1974), as summarized by De Maio et al. (2012). Since then, enormous numbers of research papers have focused on HSPs and other groups of chaperones, identifying many constitutive and inducible chaperones and their involvement in diverse cellular processes. It is now known that chaperone proteins act in several capacities to (a) mediate the correct folding of nascent peptide chains into their functional conformations, (b) limit stress-induced protein misfolding or unfolding, (c) promote the refolding of partially unfolded proteins back into their native conformations, (d) prevent, limit, or break up protein aggregates, and (e) aid in intracellular protein trafficking and assembly (for reviews: Gething and Sambrook 1992; Feder and Hofmann 1999; De Maio 1999; Winter and Jakob 2004; Żwirowski et al. 2017). A general model for HSP expression and action was also developed. Using HSP70 as the example, this included (a) stress-mediated upregulation of a trimeric transcription factor named the heat shock factor (HSF), (b) rapid transcription of hsp70 transcripts, (c) elevated HSP70 protein synthesis to deal with unfolded or malfolded polypeptides, and (d) binding of excess/unused HSP70 to HSF to trigger downregulation once stress conditions are resolved (De Maio 1999). Other inducible HSPs are similarly regulated. HSP110, HSP90, HSP70, HSP60, and HSP40 are probably the best known and most studied chaperone proteins, along with the glucose-regulated proteins (e.g., GRP78, GRP94), but the chaperone family has proven to be huge and often suffers from inconsistent naming. An important paper by Kampinga et al. (2009) brought consistency to naming conventions, although older names still persist in many new publications (including in this review).
Chaperones are not enzymes, but many of them exhibit ATP binding and hydrolysis that aids chaperone action in mediating the translocation of target proteins across membranes, folding or refolding of proteins into their functional conformation, and/or disaggregation of damaged proteins. Indeed, the term “molecular machine” has been promoted as the most appropriate way to describe chaperone action (Saibil 2013). ATP-dependent chaperones include some of the most commonly studied stress-responsive chaperones such as HSP60 and HSP70 and HSP90. HSPs can also be organelle-specific. For example, HSP60, also known as chaperonin, acts inside mitochondria where it is involved in the correct folding of incoming nuclear-encoded peptides into their functional conformations within this organelle. Similarly, GRP78 is localized in the endoplasmic reticulum (ER) where it is a core regulator of protein assembly and mediator of the UPR to suppress protein processing when the ER is overloaded.
The common concept of how chaperones work is that they assist in the correct folding or refolding of peptides into their functional conformations, acting on both nascent peptides and peptides/proteins that become unfolded, as well as breaking up nonfunctional protein aggregates. For example, small HSPs (s-HSPs) can bind to and tag protein aggregates, often to sequester these proteins until needed. These aggregates can be broken up by the action of HSP70/HSP100 allowing individual proteins to move into a refolding pathway that provides quick release of sequestered proteins as needed (Żwirowski et al. 2017; Reinle et al. 2022). Another recent concept about HSP action challenges the idea that chaperones are needed to guide the folding of nascent proteins. Several studies have shown that most newly synthesized polypeptides can fold spontaneously into their functional conformation without the aid of chaperones. This led Saibil (2013) to propose that chaperones act to stabilize the unfolded state of nascent proteins during the transfer from their site of synthesis (typically the endoplasmic reticulum) to their subcellular destination, a journey that often involves traversing an organelle membrane. Upon arrival in the proper place, the chaperone releases the polypeptide that then folds spontaneously into its functional conformation. This different point of view has led to the idea that, instead of just acting to refold stress-damaged unfolded proteins or break up protein aggregates, chaperones are also up-regulated under stress conditions in order to quickly mediate transportation of new polypeptides to their site of action. There, the peptides are released, fold into their proper conformation, and either replace stress-damaged proteins or enhance the levels of specific proteins to deal with the stress condition. Upregulation of chaperone proteins would still be seen as a response to cell stress but with slightly different functional consequences than have been the assumption for many years. This concept is well worth considering since organismal responses to stress almost always entail upregulation of selected proteins/enzymes even though cell metabolic processes are, in general, often strongly suppressed under stress conditions.
Chaperone proteins and environmental stress
Changes in the expression of chaperones, in particular, inducible HSP70, are widely used biomarkers of environmental stress used to evaluate both effects of naturally changing environmental conditions and the consequences of human-driven pollution (e.g., Nadeau et al. 2001; Moreira-de-Sousa et al. 2018). In particular, much research on the consequences of environmental stress (e.g., temperature change, hypoxia/anoxia, dehydration, pollution) among vertebrate species has focused on two main indices: inducible HSP70 and cortisol levels. These markers have been utilized in huge numbers of studies, particularly in ecophysiology research. For example, they are widely used markers of stress due to environmental change among fish species (e.g., Iwama et al. 1998; Beemelmanns et al. 2021; Han et al. 2022).
To assess the popularity of HSPs as markers of environmental stress, we did a quick search on PubMed for “chaperone response to environmental stress” in October 2022. This search resulted in nearly 5000 hits and included papers illustrating HSP/chaperone involvement in organism responses to heat, cold, anoxia/hypoxia, dehydration/drought, salinity, altitude, chemical pollution, pH, heavy metals, electromagnetic radiation, seasonal changes, and multiple diseases among animal, plant, and bacteria species. Furthermore, a broader search for “heat shock proteins” produced over 76,000 hits. These results are clear indicators of the universality of chaperone action as a fundamental life process and a conserved indicator of the environmental stress response.
This review discusses some of the roles played by chaperone proteins in animal defense against environmental stress, relying mainly on some examples from our own laboratory and/or with collaborators that have documented significant upregulation of chaperones in response to diverse environmental stress conditions across a range of animal systems (for earlier reviews, see Storey and Storey 2011; Zhang and Storey 2018). Focusing mainly on terrestrial species, we discuss the responses of chaperones (HSPs and/or GRPs) to a variety of environmental stress conditions including responses to heat, cold, freezing or dehydration stresses in frogs (Storey and Storey 2019; Wu et al. 2020; Malik et al. 2022), anoxia/hypoxia exposure in turtles (Krivoruchko and Storey 2010), torpor or hibernation in mammals (Mamady and Storey 2006; Wu et al. 2015; Luu et al. 2018a, b), cold hardening in insects (Zhang et al. 2011, 2018), and estivation in land snails (Otala lactea) and sea cucumbers (Storey and Storey 2011; Wang et al. 2018, 2021).
Although not a topic of the current review, it should not be forgotten that chaperone protein responses to environmental stress are also tailored at the gene level. Genetic variation both within and among species can be an important element in the survival and adaptation of species and populations to changing environmental conditions. For example, detailed studies of six live-bearing Poeciliid fish species showed substantial variation in HSP genes/proteins both within and between populations. Each species expressed 5–7 HSP70 isoforms but whereas constitutive HSP70 was identical between species, the heat-inducible isoforms showed different patterns of expression among the six species. This genetic variation provides a mechanism that will allow a population to recover from and adapt to environmental change, although undoubtedly to the detriment of numerous individuals (White et al. 1994; Norris et al. 1995).
It is cold outside
The subzero temperatures of winter are a major challenge for ectothermic animals. Freezing means death for the majority of species, and so, adaptive strategies are needed to survive. Many ectotherms avoid freezing by using thermally sheltered environments, including underwater (e.g., various frogs and turtles, insects that have aquatic larvae), underground (e.g., digging below the frost line or moving to underground caverns, or simply using the thermal protection of the snowpack with a freeze avoidance (supercooling) strategy (Merriam et al. 1983; Costanzo et al. 2008; Milsom and Jackson 2011). Other species overwinter in life stages that are relatively easy to protect, such as eggs or pupae, and have developed specific cryoprotectants and pack their extra and intracellular fluids with these as autumn temperatures get colder. Cryoprotectants include antifreeze proteins as well as the production of high concentrations of sugars (e.g., glucose, trehalose) or polyhydric alcohols (e.g., glycerol, sorbitol) that lower the freezing point of body fluids by colligative action and also help to stabilize the proteome (Storey and Storey 2013, 2017). The most remarkable strategy for winter survival is freeze tolerance, an ability to endure the freezing of body water in extracellular spaces while implementing adaptive strategies that prevent freezing of intracellular water (Storey and Storey 1988; 2017; Costanzo et al. 2008). Freeze tolerance has some mechanisms in common with freeze avoidance, e.g., synthesis of low molecular weight cryoprotectants that protect intracellular spaces. However, instead of antifreeze proteins, ice nucleating proteins are used to seed and regulate ice formation in extracellular fluid spaces. As a consequence, significant amounts of water are drawn out of cells to join growing extracellular ice masses and cells shrink in size until a build-up of intracellular osmolytes and cryoprotectants brings intra and extracellular osmolality into equilibrium. Recent research has shown that both strategies for winter survival also involve upregulation of chaperone proteins as an aid to proteome protection.
Chaperones and cold-hardy insects
Two well-studied insect models of cold hardiness are the larvae of goldenrod gall formers that spend the winter inside galls on the tall woody stems of goldenrod plants where they are typically fully exposed to weather conditions above the snowpack. Caterpillars of the moth, Epiblema scudderiana, avoid freezing by synthesizing extremely high concentrations of glycerol to maintain body fluidity, whereas larvae of the fly, Eurosta solidaginis, tolerate freezing of extracellular body fluids and use a combination of glycerol and sorbitol as intracellular cryoprotectants (Storey and Storey 2017). Freeze-avoiding insects also typically produce specific antifreeze proteins to provide additional resistance against freezing, whereas freeze-tolerant species synthesize ice-nucleating proteins that trigger and guide ice formation in extracellular spaces (Duman 2001).
Studies from our lab show that both strategies of subzero survival include upregulation of chaperones for proteome protection over the winter. Figure 1 illustrates changes in four chaperone proteins (Hsp70, Hsp60, Hsp40, and Hsp110) over the autumn, winter, and spring seasons for the goldenrod gall formers. Chaperone proteins are significantly elevated between October and March, peaking at about 1.7-fold higher than September values in December–January for freeze-tolerant E. solidaginis and about 2.5–threefold higher than September values for freeze-avoiding E. scudderiana (Zhang et al. 2011, 2018). The greater elevation of HSPs in E. scudderiana caterpillars likely reflects the unfrozen (supercooled) status of their body fluids, whereas about 65% of the body fluids in E. solidaginis larvae are frozen in extracellular ice masses. This means that the intracellular environment of E. solidaginis is highly concentrated with both native proteins and cryoprotectants and, therefore, requires lesser amounts of chaperones for proteome protection. Interestingly, freeze-tolerant gall fly larvae showed a strong midwinter suppression of Hsp60 to less than half of the September or April values (Fig. 1B). HSP60 is the mitochondrial chaperone, and since freezing effectively cuts off the oxygen supply to cells, this suggests that the larvae do away with a significant percentage of these organelles over the winter as one aspect of their winter diapause state. Indeed, this pattern parallels other metabolic changes in overwintering E. solidaginis larvae that include (a) strong suppression of Na+ K+-ATPase activity from November to February that is followed by a huge increase in this enzyme in April (McMullen and Storey 2008), (b) winter accumulation of anaerobic end products, lactate and alanine (Storey and Storey 1985), and (c) a major reduction in mitochondrial cytochrome c oxidase activity in winter larvae to about one-third of autumn values (indicative of reduced mitochondria numbers) (Storey and Storey 2008). This is strong evidence that the winter diapause in E. solidaginis is largely dependent on anaerobic metabolism while also up-regulating chaperones for the protection of the larval proteome.
Fig. 1.
Expression of four heat shock proteins across the winter season in larvae of cold hardy goldenrod gall insects. A The freeze-avoiding caterpillar of the gall moth Epiblema scudderiana inside its elliptical-shaped gall; B The freeze-tolerant larva of the gall fly Eurosta solidaginis inside its ball gall. Galls were collected in September and held in cloth bags hung on a fence outdoors over the winter with sampling in the middle of each month. Data are from Zhang et al. (2011, 2018). Photographs by JM Storey
Chaperones and freeze-tolerant frogs
Upregulation of chaperone proteins is also a significant adaptation for winter survival by freeze-tolerant wood frogs, Rana sylvatica. Both HSPs (HSP110, HSC70, HSP60, HSP40, and HSP10) and glucose-regulated proteins (GRP78, GRP94) showed tissue-specific upregulation in response to whole-body freezing (24 h at − 2.5 °C), most strongly apparent in the liver, skeletal muscle, and heart (Storey and Storey 2019). Selected chaperones also responded robustly when frogs were exposed to two of the unavoidable consequences of freezing: anoxia due to the cessation of breathing and heartbeat and dehydration due to water exit into extracellular ice masses. Frogs exposed to anoxia alone (24 h under a nitrogen gas atmosphere at 5 °C) showed particularly strong responses by HSP110 in the liver, HSP60 in the liver, muscle, and heart, and HSP10 in the liver and brain (Storey and Storey 2019). It is not surprising that HSP60 and HSP10 responded to anoxia since these are mitochondrial chaperones and are likely needed to help stabilize/conserve these organelles when oxygen is depleted. GRP94 was also elevated under anoxia in most frog tissues but a 2–threefold upregulation of GRP78 occurred only in the heart and skin. In general, chaperone responses to dehydration (40% of total body water lost) were more muted with prominent upregulation of HSP60 in the liver, skin, and kidney, a fourfold increase in kidney HSP40, and twofold increases in HSP10 in the brain and heart. GRP78 also responded robustly to dehydration in the heart and skin, and GRP 94 rose by ~ twofold in muscle. Overall, both stress-specific and tissue-specific responses by HSPs and GRPs were most pronounced in response to freezing, undoubtedly due to the multi-stress nature of freezing that includes both cell/tissue anoxia and dehydration as well as requiring all cells to rely on endogenous carbohydrate fuels and anaerobic glycolysis for ATP production (Storey and Storey 2019).
Freezing had a particularly strong effect on HSP60, the mitochondrial chaperone, with significant increases of 1.5–2.3-fold in the liver, skeletal muscle, brain, skin, and kidney that remained elevated after thawing in wood frog liver, brain, and muscle. Levels of Hsp10, the partner of HSP60, also rose significantly by 1.5–2.0-fold in all tissues during freezing, except brain. This contrasts with the situation for freeze-tolerant insects, but unlike E. solidaginis larvae, wood frogs do not show characteristics of a diapause state, and they are immediately active and move to breeding ponds as soon as the spring melt occurs. HSP60 also responded robustly to anoxia exposure in five of the six tissues assessed (except the kidney), and HSP10 rose in four tissues. These data suggest that the freeze-induced limitation of oxygen delivery to tissues was a main driver behind the upregulation of these mitochondrial chaperones in wood frogs. Two tissues closely associated with water economy in frogs, skin and kidney, also showed upregulated HSP60 in response to dehydration (40% body water lost) (Storey and Storey 2019). These responses by HSP60 may indicate the importance of stabilizing mitochondrial proteins to support an easy resumption of oxygen-based mitochondrial ATP production once breathing and blood circulation are restarted.
Wood frogs are not alone in showing a chaperone response to cold and freezing. Analysis of the liver transcriptome of Cope’s gray treefrog (Dryophytes chrysoscelis), another well-researched freeze-tolerant frog, revealed upregulation of genes encoding HSP70, HSP90, and HSP105 as well as GRP78 in response to cold or freezing (Do Amaral et al. 2020). This supports the need for chaperone proteins as an integral part of vertebrate freezing survival.
Too hot and no escape
Chaperone responses to estivation and dehydration stress
As opposed to cold and frozen, many animals are challenged by the opposite problem—hot and dry. Mechanisms must be available to deal with seasonal high temperatures and/or low water availability, often due to the failure of water sources during the summer dry season. Both physiological and molecular adaptations, including modulation of chaperone proteins, again contribute to survival. One key strategy is estivation, a seasonal dormancy, during which animals retreat into sheltered locations, cease eating and drinking, and strongly suppress metabolic rate to survive using only internal fuel and water reserves. Among terrestrial vertebrate species, estivation necessitates mechanisms to conserve body water. For example, the “water-holding” frogs of Australia and southern Africa bury themselves underground, use their large full bladders to support body hydration for many months (sometimes years), and shed layers of skin to form a cocoon that limits evaporative water loss (Flanigan et al. 1993). Other anurans enter estivation underground with lower body water reserves such as North American spadefoot toads (Scaphiopus couchii) (Lee and Mercer 1967) or African clawed frogs (Xenopus laevis). Instead, they resist body water loss with a colligative strategy of incorporating waste ammonia into urea biosynthesis and retaining high concentrations of urea (normally a secreted waste product of protein catabolism) or other compatible solutes in their tissues (McClanahan, 1967; Luu et al. 2021).
Chaperone proteins play a significant role in anuran resistance to dehydration stress. For example, HSP27 was elevated in two tissues, the kidney and lung, of X. laevis under dehydrating conditions (Luu et al. 2018a, b), potentially adding extra proteome protection to these tissues that are major venues for water loss from the frog body. Interestingly, HSP27 is also known to play a role in inhibiting apoptosis (Concannon et al. 2003; Bruey et al. 2000) and thus may contribute to minimizing cell death during long-term dormancy. HSP70 levels rose significantly in X. laevis liver, lung, and kidney under dehydration stress, HSC70 increased in the liver and kidney, and HSP40 rose significantly in the liver and skeletal muscle (Luu et al. 2018a, b). By contrast, HSP90 levels fell in muscle, kidney, and lung, and HSP60 also decreased in the kidney and lung under drying conditions. HSP90 is known to be highly expressed in unstressed cells (Heikkila 2010) and binds to HSF-1 to inhibit/modulate hsp gene transcription (Zou et al. 1998). Hence, a multi-tissue suppression of HSP90 could support dehydration-mediated activation of HSF-1 to enhance or sustain chaperone production in response to this stress.
Dehydration stress on African clawed frogs (28% of total body water lost) also triggered the UPR that is characterized by upregulation of resident chaperones of the endoplasmic reticulum (Malik et al. 2022). Commonly known as glucose-regulated proteins, three of these proteins (GRP58, GRP75, and GRP94) were significantly upregulated X. laevis tissues. GRP58 was elevated by 1.6–1.8-fold in the lung and skin, GRP75 rose ~ sevenfold in the lung, and GRP94 increased by ~ ninefold in the skin, as compared with control values. Notably, the tissues affected (lung and skin) are the most vulnerable to water loss from the body and probably the first tissues to require enhanced chaperone action to help stabilize the proteome.
Many other animals also estivate in response to hot and dry conditions. Several fish species enter estivation when their aquatic environment dries out. Best studied are African lungfish of the Protopterus genus that dig into the wet mud of their shrinking water source, secrete a slime coat that hardens into a cocoon, and accumulate urea as an osmoprotectant while they estivate for months or even years (Lajus and Alekseev 2019), a strategy not unlike that of X. laevis. A variety of terrestrial snails also estivate, utilizing chaperone proteins to enhance proteome protection during the peak hot months. For example, the European milk snail, Otala lactea, showed 18- and 11-fold increases in HSP10 in hepatopancreas and foot muscle, respectively, after 14 days estivation as well as ~ twofold increases in HSP60, HSP90, and HSP110 in hepatopancreas (Ramnanan et al. 2009). This occurred despite a strong reduction of protein synthesis capacity (by ~ 80%) as measured in vitro when extracts of foot muscle and hepatopancreas from dormant snails were compared with active snails. The activity of energy-expensive processes such as the 20S proteosome and Na+K+ATPase were also suppressed during estivation (Ramnanan and Storey 2006; Ramnanan et al. 2009). These changes are indicative of significant metabolic rate depression coupled with increased proteome protection during estivation.
In other work, comparative studies of a desiccation-resistant, desert snail species Sphincterochila zonata and a close relative from the Mediterranean region (S. cariosa) that is desiccation-sensitive revealed interesting strategies. Upregulation of HSPs in S. zonata relied mainly on small HSPs (Hsp25 and Hsp30), whereas S. cariosa showed a strong induction of Hsp72, Hsp74, and Hsp90 (Mizrahi et al. 2010). The authors proposed that the desert species (S. zonata) is consistently prepared for heat/desiccation stress (i.e., a preparative defense) that can be supplemented by upregulation of small HSPs as needed, whereas S. cariosa mounts a major adaptive response involving multiple HSP family members when stressed intermittently. A study of another terrestrial snail, Theba pisana, also showed comparable responses along a climatic gradient with desiccation resistance and thermotolerance being greater for populations from warmer environments and constitutive Hsp72, as well as Hsp74 and Hsp90, being highest in warmer locales (Mizrahi et al. 2015).
Hot and wet: estivation in the sea
It is well known that different species living in the marine intertidal zone experience different lengths of aerial emersion and severity of thermal and desiccation stress at low tide (Dong et al. 2008). Not unexpected, high intertidal species maintain higher constitutive levels of HSP70 (a preparative defense) against high temperatures, whereas low intertidal residents rely more on a rapid induction of HSP70 when challenged by temperatures above their normal experience (Dong et al. 2008). Shallow water species can also be heat stressed when high summer temperatures elevate water temperature to unusual levels. To deal with summer heating of inshore shallows, selected species turn to estivation. The sea cucumber, Apostichopus japonicus, is one such species. It is a commercially important food that is raised in aquaculture in Asia. In recent years, much research has gone into understanding the biology and biochemistry of A. japonicus and shown that the animals cease eating and enter estivation during the hot summer months when sea surface temperature can rise to ~ 25 °C as compared with ~ 15 °C in the months when animals are active and feeding (Wang et al. 2018, 2021). This environmental stress frequently leads to cell/tissue death triggered by the apoptosis-inducing factor mitochondrial 1 (AIFM1), thereby compromising the aquaculture harvest. AIFM1 has a normal function as a resident of the intermembrane space of mitochondria, but when released under stress conditions, it translocates to the nucleus where it activates the caspase-independent pathway of apoptosis (Daugas et al. 2000; Sevrioukova 2011). However, various species have evolved pro-survival mechanisms to block/inhibit caspase-dependent or independent apoptosis under stress conditions that could be lethal (Lanneau et al. 2008).
Studies of A. japonicus found that HSP70 binding to AIFM1 inhibits its transfer to the nucleus, thereby blocking the transcription of apoptogenic genes. Recent work by colleagues associated with our lab found an inverse relationship between AIFM1 and HSP70 in A. japonicus intestine (Wang et al. 2018). Both transcript and protein levels of AIFM1 were reduced during estivation by about one-half, whereas HSP70 transcript and protein levels increased strongly (~ threefold and 1.6-fold, respectively), as compared with non-aestivating animals. Furthermore, elevated temperature (25 °C) played a key role. Transcript and protein levels of AIFM1 did not change significantly when sea cucumbers were tested at 15°, but exposure to 25 °C strongly elevated hsp70 transcripts by ~ 3.5-fold and HSP70 protein by ~ twofold over 15 °C values. This provided a mechanism to strengthen the inhibition of AIFM1 by HSP70 and suppress its transfer to the nucleus at 25 °C. Furthermore, co-immunoprecipitation of sea cucumber HSP70 and AIFMI showed a strong binding interaction between these two proteins at 25 °C but not at 15 °C. Another study used an in vitro approach to assess changes in the ultrastructure of sea cucumber intestinal cells in response to high temperature shock (4 h at 25 °C versus 15 °C controls) (Wang et al. 2021). Heat stress led to the appearance of multiple autophagosomes and an expanded rough endoplasmic reticulum but also greatly enhanced the expression of two key ER-resident chaperones, ERP29 (endoplasmic reticulum resident protein 29-like) and protein disulfide-isomerase A6-like (PDIA6). The latter protein aids both the folding of polypeptide chains and catalyzes the formation of disulfide bonds to stabilize protein structure. Furthermore, a new study of A. japonicus coelomocytes revealed differential expression of three HSP90 family members and two cofactors, CDC37 and AHSA1, the latter being known to work with HSP90a to respond to heat stress caused by a switch from 15 to 30 °C (Wang et al. 2021). AHSA1 also stimulates the ATPase activity of HSP90 (Shao et al. 2016). Seven members of the HSP40 family were also upregulated by this high temperature, and a new regulatory player in the A. japonicus heat stress response (alternative splicing of genes) was also identified. Overall, these two studies of sea cucumbers show that both enhanced protection by chaperones and suppressed apoptosis contribute to the survival of summer heat stress.
Out of breath: chaperones and anoxia tolerance
Life without oxygen is impossible for mammals and birds but not always so for most species on Earth. Many species tolerate short or long periods of oxygen deprivation (causing either hypoxia or anoxia) that are generally linked to their lifestyles. For example, intertidal invertebrates are well-prepared for daily interruptions of oxygen supply and temperature change at low tide. Indeed, Han et al. (2017) reported the presence of 19 genes coding for heat shock proteins as well as upregulation of genes for HIF-1 and alanopine dehydrogenase (a functional equivalent of lactate dehydrogenase) in the intertidal limpet Cellana toreuma in response to heat stress at low tide. Various freshwater species are also susceptible to oxygen depletion, and the phenomenon of “winter kill” in ice-locked ponds is quite common both for resident species and for those that move into an aquatic environment to escape subzero temperatures on land (e.g., various frog and turtle species). Some of these species become facultative anaerobes in cold water, switching to oxygen-independent metabolism by relying on glycogen reserves, generating ATP via anaerobic glycolysis, and producing end products that can be excreted across gills (e.g., ethanol) or stored into bone or shell (e.g., lactate, alanine, succinate). For example, the western painted turtle, Chrysemys picta bellii, shows the greatest anoxia tolerance of any vertebrate, being able to endure 24 h anoxia at 26 °C and as much as 6 months without breathing oxygen at 3 °C (Herbert and Jackson 1985). Turtles compensate for oxygen deprivation by using anaerobic metabolism, catabolizing large reserves of glycogen from the liver and sequestering the lactate product into their shell and bones until air breathing is restored and lactate can be retrieved and oxidized (Davis and Jackson 2007). Many gill-breathing fish are also compromised when oxygen is too low, but some, like goldfish (Carassius auratus) and other cyprinids, use a modified version of glycolysis that results in ethanol as the end product. Ethanol is then excreted across the gills, allowing ATP generation to continue without a buildup of waste product (Fagernes et al. 2017).
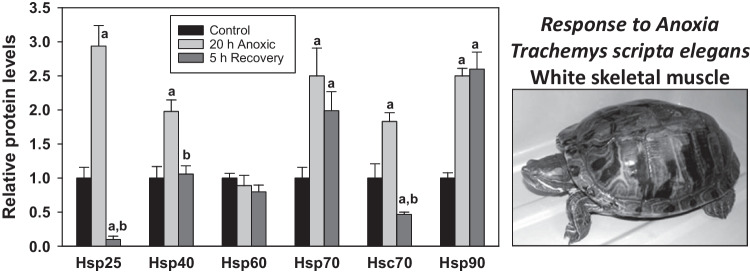
Long-term survival of turtles under water is also supported by upregulation of stress-responsive chaperones. For example, after acclimation at 4 °C, submergence of adult red-eared slider turtles (Trachemys scripta elegans) for 20 h triggered significant 1.5–threefold upregulation of heat shock proteins (Hsp25, Hsp40, Hsp70, Hsc70, Hsp90) in neck retractor white muscle, and this was linked to a fivefold increase in the active form of the heat shock transcription factor (HSF-1) in muscle as well as increased migration of active HSF-1 into the nucleus (Krivoruchko and Storey 2010, 2013, 2015). Kidney and liver also showed elevated amounts of most HSPs after 20 h submergence, whereas HSPs in the heart were unaffected. Most tissues showed a full reversal of HSP content after 5 h of aerobic recovery (Fig. 2). Although enhanced synthesis of chaperone proteins under anoxic conditions is ATP-expensive and would need the support of anaerobic metabolism, an enhanced capacity for chaperone-mediated protein refolding (as opposed to a much larger ATP expenditure for de novo protein synthesis) may be the crucial decision necessary for sustaining long-term viability in a hypometabolic anoxic state. Scott et al. (2003) also reported interesting tissue-specific responses by Hsp73 (constitutive) and Hsp72 (inducible) in the brain, liver, heart, and skeletal muscle of painted turtles (Chrysemys picta) following a 24 h forced dive and a 1 h recovery. Levels of constitutive Hsp73 were unchanged in three tissues but fell strongly in the liver under anoxia and further still after aerobic recovery. Oppositely, the 24 h dive triggered strong increases in inducible Hsp72 in the brain, heart, and skeletal muscle (but not in the liver). After 1 h aerobic recovery, Hsp72 levels remained high in the heart, increased further in the brain and skeletal muscle, and were also strongly elevated in the liver. Elevated expression of Hsp70 and/or Hsc70 was also reported in turtle tissues in response to anoxia (Prentice et al. 2004), and Ramaglia and Buck (2004) documented time-dependent increases in Hsp72, Hsp73, and Hsp90 in tissues of western painted turtles (C. p. belli) over prolonged forced dives of 24–30 h. This latter study provided a very clear picture that distinguishes the roles of Hsp72 (inducible) and Hsp73 (constitutive) confirming that Hsp72 is the crucial stress-responsive protein, whereas Hsp73 plays mainly a “house-keeping” role and can be unchanged or suppressed under stress conditions.
Fig. 2.
Effects of 20 h anoxic submergence and 5 h aerobic recovery on relative levels of heat shock proteins in white skeletal muscle of an anoxia-tolerant turtle, the red-eared Trachemys scripta elegans. Protein levels were standardized to control values, and data are shown as mean ± SEM, n = 4 independent trials for different animals. “a” Significantly different from corresponding control (P < 0.05); “b” significantly different from the corresponding anoxic value, P < 0.05. Data from Krivoruchko and Storey (2010). Photograph by JM Storey
Fast asleep—chaperones in torpor and hibernation
Mammals are typically considered to be homeotherms that sustain a constant body temperature of about 37 °C. However, while true of many large mammals, a wide variety of small mammals (and even some big ones such as bears) allow body temperature to fall to varying extents during sleep, daily torpor, or hibernation (Ruf and Geiser 2015). For many small mammals, entry into torpor is a life-saving response that allows them to “make it through the night” by regulated suppression of metabolic rate to conserve body fuel (glycogen, triglycerides) as much as possible. Indeed, many animals utilize regulated metabolic rate depression as a survival strategy under a variety of names including torpor, hibernation, estivation, diapause, dormancy, anaerobiosis, and anhydrobiosis. For many small mammals, torpor bouts are daily occurrences that allow animals to minimize the consumption of endogenous fuel reserves while resting/sleeping, generally aided by allowing a drop in body temperature (Tb) towards ambient. In various species, the use of daily torpor can also expand seasonally into multiday hibernation particularly under challenging cold environments. Differences in body mass, season, and geography often determine the use and duration of daily torpor versus seasonal hibernation.
Daytime excursions into torpor by the tiny nocturnal South American marsupial, monito del monte (Dromiciops gliroides), provide an excellent example of a species that uses both shallow daily torpor in warm seasons and multi-day hibernation during the winter (Bozinovic et al. 2004). Indeed, heterothermy is the norm for these animals (Nespolo et al. 2021). Tissue-specific responses by HSPs were evaluated between aroused and torpid states to help understand their role in daily survival (Luu et al. 2018a, b). Relative levels of HSP27 and HSP60 (the mitochondrial chaperone) increased significantly in the liver by 2.00- and 2.76-fold, respectively, and HSP60 rose in the heart by 1.73 ± 0.13-fold during torpor. By contrast, there was no change HSPs in the brain during torpor. Interestingly, levels of several stress-sensitive cell cycle regulators (ATR, p-Chk1, p-Chk2, and p21) also rose significantly in these tissues during torpor suggesting that the energy-expensive cell cycle is suppressed to conserve energy while animals are in torpor. Elevation of HSPs during torpor is likely an aid to stabilizing key proteins and pathways during torpor, promoting a quiescent state that can be rapidly reactivated as animals rewarm and return to an active state.
At the opposite end of mammalian phylogeny, but very similar to D. gliroides, is a tiny primate, the gray mouse lemur (Microcebus murinus). This native of Madagascar also uses daily torpor to minimize metabolic costs during its inactive hours by allowing metabolic rate to drop to about one-quarter of the value for aroused animals (Schmid 2000). Furthermore, during the dry season when food is scarce, these animals also transition into bouts of multiday torpor to aid their survival. An analysis of HSP responses to overnight torpor (body temperature fell by no more than 7 °C) among mouse lemurs from a captive research colony showed significant increases in HSP70 and HSP90 in brown adipose tissue and HSP60 in the liver. Again, these changes could help to stabilize the proteome and promote metabolic suppression at a reduced body temperature. Relative expression of mRNA transcripts that code for chaperone proteins also increased during torpor including hspb1 in the liver, hsp90b1 and dnajb1 in the heart, and hspb1 and dnajb1 in brown adipose tissue (Biggar et al. 2015), strongly implicating an upregulation of the respective proteins as an important strategy for stabilizing the torpor state. Indeed, protein levels of HSP70 and HSP90α were elevated by small but significant increases of 1.3- and 1.5-fold, respectively, in brown adipose tissue during torpor as compared with controls and HSP60 in the liver of torpid animals increased by 1.15-fold over that of controls (P < 0.05) (Wu et al. 2015).
Many other small mammals living in seasonally cold environments hibernate during the winter months and undergo multiple day/week bouts of torpor, typically allowing body temperature to fall to near ambient and fueled by either large body fat reserves or stored food caches. Most hibernators go through multiple cycles of torpor/arousal, allowing body temperature to fall to near to ambient (sometimes close to 0 °C) for long periods of time and yet, at intervals, rewarm to ~ 37 °C for short periods of time. Depending on the species, animals may fully awaken and eat cached food or simply re-enter a euthermic sleep state that gradually transitions into the next bout of cold torpor. Chaperone proteins can make key contributions to protecting and stabilizing the proteome not only to support the torpid state but also to stabilize the proteome over these major thermal transitions to/from active and torpor states. For example, several studies have documented changes in HSPs during hibernation of bats. Although most HSPs were unchanged, a seasonal 1.7-fold enhancement of Hsp70 was reported in the muscle of the bat, Murina leucogaster, during winter hibernation (and sustained during arousal) (Lee et al. 2008). Elevation of both total and phosphorylated Hsp27 protein was also reported for heart and skeletal muscle of torpid little brown bats (Myotis lucifugus) (Eddy et al. 2005). Hsp27 is an ATP-independent chaperone that has a wide range of roles (summarized in Storey and Storey 2011). Given the need for high rates of shivering thermogenesis by skeletal muscle to rewarm the bat body during arousal, enhanced levels of HSP70 could be valuable in stabilizing the muscle proteome during long periods of cold torpor so that the proteins/pathways needed for thermogenesis can be rapidly activated when interbout arousal is triggered (Lee et al. 2008). However, interestingly, a study of the heat shock transcription factor 1 (HSF1) and HSP70 expression in hibernating chipmunks found the highest levels of both in nonhibernating summer-active animals, strong suppression during hibernation, and strong upregulation again during interbout arousal (Tsukamoto et al. 2019).
Another group of chaperone proteins, the glucose-regulated proteins also support hibernation. The first analysis of GRP involvement in hibernation focused on GRP75, a mitochondrial member of the HSP70 family, and showed that the protein was prominently upregulated in multiple tissues of ground squirrels (Spermophilus tridecemlineatus), as compared with active euthermic squirrels (Carey et al. 1999). A subsequent study examined another family member, GRP78, that resides in the endoplasmic reticulum. GRP78 is a key regulator of the folding and assembly of newly synthesized peptides (Mamady and Storey 2006) and the main mediator of the UPR (Schröder and Kaufman 2005). During hibernation, GRP78 protein was prominently elevated in brown adipose and brain of S. tridecemlineatus (~ twofold) mediated by pronounced upregulation of the grp78 gene (by 3–fourfold) under the action of the ATF4 transcription factor (Mamady and Storey 2006). The UPR is a quality control mechanism that can detect and halt protein handling and posttranslational modification in the endoplasmic reticulum when the processing of proteins is backed up or when misfolded proteins accumulate. Given that entry in torpor/hibernation typically involves a huge downward decrease in body temperature, and that the conformation and/or activity of proteins/enzymes are strongly affected by temperature change, it is not surprising that major adjustments to protein processing are required when hibernating species transition into or out of torpor.
Conclusion
Humans struggle mightily to bend the environment to our will and as a result waste vast amounts natural resources on heating/cooling our homes, watering our lawns/crops, polluting the air with exhaust from our cars and factories, and digging unique resources out of the ground. With minor exceptions, such as the busy beaver, animals must find a different way to adapt and endure in the environment into which they are born. Over time, animals have developed/evolved strategies (behavioral, physiological, biochemical) to modulate or re-imagine their basic metabolism to sustain life and adapt to the particular stress conditions of their environment. Chaperone proteins are key to survival and adaptation and are ubiquitous in the animal kingdom. The present review has surveyed some recent advances in understanding chaperone action as part of the range of adaptive strategies that support animal survival under challenging environmental conditions. However, as always with scientific research, there is still much more to be discovered!
Acknowledgements
We thank the many graduate students and colleagues that have contributed to the publications cited in this review and the Natural Sciences and Engineering Research Council of Canada for career-long funding support. We are forever grateful to have landed as graduate students in the laboratory of Peter W. Hochachka at the University of British Columbia and be introduced to the amazing world of Biochemical Adaptation.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Balinsky JB, Coe CGL, van der Schans GS. Amino acid metabolism and urea synthesis in naturally aestivating Xenopus laevis. Comp Biochem Physiol. 1967;22:59–68. doi: 10.1016/0010-406x(67)90166-1. [DOI] [PubMed] [Google Scholar]
- Beemelmanns A, Zanuzzo FS, Xue X, Sandrelli RM, Rise ML, Gamperl AK. The transcriptomic responses of Atlantic salmon (Salmo salar) to high temperature stress alone, and in combination with moderate hypoxia. BMC Genomics. 2021;22:261. doi: 10.1186/s12864-021-07464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar KK, Wu CW, Tessier SN, Zhang J, Pifferi F, Perret M, Storey KB. Modulation of gene expression in key survival pathways during daily torpor in the gray mouse lemur, Microcebus murinus. Genom Proteom Bioinform. 2015;13:111–118. doi: 10.1016/j.gpb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovic F, Ruiz G, Rosenmann J. Energetics and torpor of a South American “living fossil”, the microbiotheriid Dromiciops gliroides. J Comp Physiol B. 2004;174:293–297. doi: 10.1007/s00360-004-0414-8. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2(9):645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Carey HV, Sills NS, Gorham DA (1999) Stress proteins in mammalian hibernation. Am Zool 39(6):825–835
- Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8:61–70. doi: 10.1023/a:1021601103096. [DOI] [PubMed] [Google Scholar]
- Costanzo JP, Lee RE, Jr, Ultsch GR. Physiological ecology of overwintering in hatchling turtles. J Exp Zool A. 2008;309:297–379. doi: 10.1002/jez.460. [DOI] [PubMed] [Google Scholar]
- Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/S0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- Davis EC, Jackson DC. Lactate uptake by skeletal bone in anoxic turtles, Trachemys scripta. Comp Biochem Physiol A. 2007;146:299–304. doi: 10.1016/j.cbpa.2006.10.034. [DOI] [PubMed] [Google Scholar]
- De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;1:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- De Maio A, Santoro MG, Tanguay RM, Hightower LE. Ferruccio Ritossa’s scientific legacy 50 years after his discovery of the heat shock response: a new view of biology, a new society, and a new journal. Cell Stress Chaperon. 2012;17:139–143. doi: 10.1007/s12192-012-0320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Amaral MCF, Frisbie J, Crum RJ, Goldstein DL, Krane CM. Hepatic transcriptome of the freeze-tolerant Cope’s gray treefrog, Dryophytes chrysoscelis: responses to cold acclimation and freezing. BMC Genom. 2020;21:226. doi: 10.1186/s12864-020-6602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Miller LP, Sanders JG, Somero GN. Heat-shock protein 70 (Hsp70) expression in four limpets of the genus Lottia: interspecific variation in constitutive and inducible synthesis correlates with in situ exposure to heat stress. Biol Bull. 2008;215:173–181. doi: 10.2307/25470698. [DOI] [PubMed] [Google Scholar]
- Duman JG. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu Rev Physiol. 2001;63:327–357. doi: 10.1146/annurev.physiol.63.1.327. [DOI] [PubMed] [Google Scholar]
- Eddy SF, McNally JD, Storey KB. Up-regulation of a thioredoxin peroxidase-like protein, proliferation-associated gene, in hibernating bats. Arch Biochem Biophys. 2005;435:103–111. doi: 10.1016/j.abb.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Fagernes CE, Stensløkken KO, Røhr ÅK, Berenbrink M, Ellefsen S, Nilsson GE. (2017) Extreme anoxia tolerance in crucian carp and goldfish through neofunctionalization of duplicated genes creating a new ethanol-producing pyruvate decarboxylase pathway. Sci Rep. 2017;7:7884. doi: 10.1038/s41598-017-07385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Ann Rev Physiol. 1999;6:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Flanigan JE, Withers PC, Fuery CJ, Guppy M. Metabolic depression and Na+/K+ gradients in the aestivating Australian goldfields frog, Neobatrachus wilsmorei. J Comp Physiol B. 1993;163:587–593. doi: 10.1007/BF00302118. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nat. 1992;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Han G, Zhang S, Dong Y. Anaerobic metabolism and thermal tolerance: the importance of opine pathways on survival of a gastropod after cardiac dysfunction. Integr Zool. 2017;12:361–370. doi: 10.1111/1749-4877.12229. [DOI] [PubMed] [Google Scholar]
- Han B, Meng Y, Tian H, Li C, Li Y, Gongbao C, Fan W, Ma R. Effects of acute hypoxic stress on physiological and hepatic metabolic responses of triploid rainbow trout (Oncorhynchus mykiss) Front Physiol. 2022;13:921709. doi: 10.3389/fphys.2022.921709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila JJ. Heat shock protein gene expression and function in amphibian model systems. Comp Biochem Physiol A Mol Integr Physiol. 2010;156:19–33. doi: 10.1016/j.cbpa.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Herbert CV, Jackson DC. Temperature effects on the responses to prolonged sub- mergence in the turtle Chrysemys picta bellii. I. Blood acid-base and ionic changes during and following anoxic submergence. Physiol Zool. 1985;58:655–669. doi: 10.1086/physzool.58.6.30156070. [DOI] [Google Scholar]
- Hochachka PW, Somero GN. Biochemical adaptation: mechanism and process in physiological evolution. Oxford: Oxford University Press; 2002. [Google Scholar]
- Iwama GK, Thomas PT, Forsyth RB, Mathilakath MV. Heat shock protein expression in fish. Rev Fish Biol Fisheries. 1998;8:35–56. doi: 10.1023/A:1008812500650. [DOI] [Google Scholar]
- Jackson DC, Ultsch GR. Physiology of hibernation under the ice by turtles and frogs. J Exp Zool A Ecol Genet Physiol. 2010;313(6):311–327. doi: 10.1002/jez.603. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperon. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoruchko A, Storey KB. Regulation of the heat shock response under anoxia in the turtle, Trachemys scripta elegans. J Comp Physiol B. 2010;180:403–414. doi: 10.1007/s00360-009-0414-9. [DOI] [PubMed] [Google Scholar]
- Krivoruchko A, Storey KB. Activation of the unfolded protein response during anoxia exposure in the turtle Trachemys scripta elegans. Mol Cell Biochem. 2013;374:91–103. doi: 10.1007/s11010-012-1508-3. [DOI] [PubMed] [Google Scholar]
- Krivoruchko A, Storey KB. Turtle anoxia tolerance: biochemistry and gene regulation. Biochim Biophys Acta. 2015;1850:1188–1196. doi: 10.1016/j.bbagen.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Lajus DL, Alekseev VR (2019) Fish: diapause, dormancy, aestivation, and delay in gonad development. In: Alekseev V, Pinel-Alloul B (eds) Dormancy in aquatic organisms. theory, human use and modeling. Monograph Biolog 92. Springer, Cham. 10.1007/978-3-030-21213-1_4
- Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Mercer EH. Cocoon surrounding desert-dwelling frogs. Sci. 1967;157(3784):87–88. doi: 10.1126/science.157.3784.87. [DOI] [PubMed] [Google Scholar]
- Lee K, Park JY, Yoo W, Gwag T, Lee JW, Byun MW, Choi I. Overcoming muscle atrophy in a hibernating mammal despite prolonged disuse in dormancy: proteomic and molecular assessment. J Cell Biochem. 2008;104:642–456. doi: 10.1002/jcb.21653. [DOI] [PubMed] [Google Scholar]
- Luu BE, Wijenayake S, Malik AI, Storey KB. The regulation of heat shock proteins in response to dehydration in Xenopus laevis. Cell Stress Chaperon. 2018;23:45–53. doi: 10.1007/s12192-017-0822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu BE, Wijenayake S, Zhang J, Tessier SN, Quintero-Galvis JF, Gaitán-Espitia JD, Nespolo RF, Storey KB. Strategies of biochemical adaptation for hibernation in a South American marsupial, Dromiciops gliroides: 2. Control of the Akt pathway and protein translation machinery. Comp Biochem Physiol B. 2018;224:19–25. doi: 10.1016/j.cbpb.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Luu BE, Hawkins LJ, Storey KB. Insights from a vertebrate model organism on the molecular mechanisms of whole-body dehydration tolerance. Mol Cell Biochem. 2021;476:2381–2392. doi: 10.1007/s11010-021-04072-x. [DOI] [PubMed] [Google Scholar]
- Malik AI, Storey JM, Storey KB (2022) Regulation of the unfolded protein response during dehydration stress in African clawed frogs, Xenopus laevis. Cell Stress Chaperon, in press. 10.1007/s12192-022-01275-z [DOI] [PMC free article] [PubMed]
- Mamady H, Storey KB. Up-regulation of the endoplasmic reticulum molecular chaperone GRP78 during hibernation in thirteen-lined ground squirrels. Mol Cell Biochem. 2006;292:89–98. doi: 10.1007/s11010-006-9221-8. [DOI] [PubMed] [Google Scholar]
- McClanahan L. Adaptations of the spadefoot toad Scaphiopus couchii, to desert environments. Comp Biochem Physiol. 1967;20:73–99. doi: 10.1016/0010-406X(67)90726-8. [DOI] [Google Scholar]
- McMullen DC, Storey KB. Suppression of Na+K+-ATPase activity by reversible phosphorylation over the winter in a freeze-tolerant insect. J Insect Physiol. 2008;54:1023–1027. doi: 10.1016/j.jinsphys.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Merriam G, Wegner J, Coldwell D. Invertebrate activity under snow in deciduous woods. Holarctic Ecol. 1983;6:89094. doi: 10.1111/j.1600-0587.1983.tb01069.x. [DOI] [Google Scholar]
- Milsom WK, Jackson DC. Hibernation and gas exchange. Compr Physiol. 2011;1(1):397–420. doi: 10.1002/cphy.c090018. [DOI] [PubMed] [Google Scholar]
- Mizrahi T, Heller J, Goldenberg S, Arad Z. Heat shock proteins and resistance to desiccation in congeneric land snails. Cell Stress Chaperones. 2010;15(4):351–363. doi: 10.1007/s12192-009-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi T, Goldenberg S, Heller J, Arad Z. Natural variation in resistance to desiccation and heat shock protein expression in the land snail Theba pisana along a climatic gradient. Physiol Biochem Zool. 2015;88(1):66–80. doi: 10.1086/679485. [DOI] [PubMed] [Google Scholar]
- Moreira-de-Sousa C, de Souza RB, Fontanetti CS. (2018) HSP70 as a biomarker: an excellent tool in environmental contamination analysis - a review. Water Air Soil Pollut. 2018;229:264. doi: 10.1007/s11270-018-3920-0. [DOI] [Google Scholar]
- Nadeau D, Corneau S, Plante I, Morrow G, Tanguay RM. Evaluation for Hsp70 as a biomarker of effect of pollutants on the earthworm Lumbricus terrestris. Cell Stress Chaperon. 2001;6:153–163. doi: 10.1379/1466-1268(2001)006<0153:efhaab>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nespolo RF, Mejías C, Espinoza A, Quintero-Galvis J, Rezende EL, Fontúrbel FE, Bozinovic F. Heterothermy as the norm, homeothermy as the exception: variable torpor patterns in the South American marsupial Monito del Monte (Dromiciops gliroides) Front Physiol. 2021;12:682394. doi: 10.3389/fphys.2021.682394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CE, di Iorio PJ, Schultz RJ, Hightower LE. Variation in heat shock proteins within tropical and desert species of poeciliid fishes. Mol Biol Evol. 1995;12(6):1048–1062. doi: 10.1093/oxfordjournals.molbev.a040280. [DOI] [PubMed] [Google Scholar]
- Prentice HM, Milton SL, Scheurle D, Lutz PL. The upregulation of cognate and inducible heat shock proteins in the anoxic turtle brain. J Cereb Blood Flow Metab. 2004;24:826–828. doi: 10.1097/01.WCB.0000126565.27130.79. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, Buck LT. Time-dependent expression of heat shock proteins 70 and 90 in tissues of the western painted turtles (Chrysemys picta belli) J Exp Biol. 2004;207:3775–3784. doi: 10.1242/jeb.01211. [DOI] [PubMed] [Google Scholar]
- Ramnanan CJ, Storey KB. Suppression of Na+K+-ATPase activity during estivation in the land snail Otala lactea. J Exp Biol. 2006;209:677–688. doi: 10.1242/jeb.02052. [DOI] [PubMed] [Google Scholar]
- Ramnanan CJ, Allan ME, Groom AG, Storey KB. Regulation of global protein translation and protein degradation in aerobic dormancy. Mol Cell Biochem. 2009;323:9–20. doi: 10.1007/s11010-008-9959-2. [DOI] [PubMed] [Google Scholar]
- Reinle K, Mogk A, Bukau B. The diverse functions of small heat shock proteins in the proteostasis network. J Mol Biol. 2022;434:167157. doi: 10.1016/j.jmb.2021.167157. [DOI] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol Rev Camb Philos Soc. 2015;90:891–926. doi: 10.1111/brv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid J. Daily torpor in the gray mouse lemur (Microcebus murinus) in Madagascar: energetic consequences and biological significance. Oecologia. 2000;123:175–183. doi: 10.1007/s004420051003. [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Scott MA, Locke M, Buck LT. Tissue-specific expression of inducible and constitutive Hsp70 isoforms in the western painted turtle. J Exp Biol. 2003;206:303–311. doi: 10.1242/jeb.00107. [DOI] [PubMed] [Google Scholar]
- Sevrioukova IF. Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid Redox Signal. 2011;14:2545–2579. doi: 10.1089/ars.2010.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Wang L, Zhong C, Qi R, Li Y (2016) AHSA1 regulates proliferation, apoptosis, migration, and invasion of osteosarcoma. Biomed Pharmacother 77:45–51. 10.1016/j.biopha.2015.11.008 [DOI] [PubMed]
- Sottile ML, Nadin SB. Heat shock proteins and DNA repair mechanisms: an updated overview. Cell Stress Chaperon. 2018;23:303–315. doi: 10.1007/s12192-017-0843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecyk JA, Couturier CS, Fagernes CE, Ellefsen S, Nilsson GE. Quantification of heat shock protein mRNA expression in warm and cold anoxic turtles (Trachemys scripta) using an external RNA control for normalization. Comp Biochem Physiol D. 2012;7:59–72. doi: 10.1016/j.cbd.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Storey KB. Functional metabolism: regulation and adaptation. Hoboken, NJ: Wiley-Liss; 2004. [Google Scholar]
- Storey JM, Storey KB. Freezing and cellular metabolism in the gall fly larva, Eurosta solidaginis. J Comp Physiol B. 1985;155:333–337. doi: 10.1007/BF00687475. [DOI] [Google Scholar]
- Storey KB, Storey JM. Freeze tolerance in animals. Physiol Rev. 1988;68(1):27–84. doi: 10.1152/physrev.1988.68.1.27. [DOI] [PubMed] [Google Scholar]
- Storey JM, Storey KB. Insects in winter: cold case files. In: McKechnie AE, editor. Hypometabolism in animals: hibernation, torpor and cryobiology (Lovegrove BG. Pietermaritzburg: University of KwaZulu; 2008. pp. 83–92. [Google Scholar]
- Storey KB, Storey JM. Heat shock proteins and hypometabolism: adaptive strategy for proteome preservation. Res Rep Biol. 2011;2:57–68. doi: 10.2147/RRB.S13351. [DOI] [Google Scholar]
- Storey KB, Storey JM (2013) Molecular biology of freeze tolerance in animals. Compr Physiol 3(3):1283–1308. 10.1002/cphy.c130007 [DOI] [PubMed]
- Storey KB, Storey JM. Molecular physiology of freeze tolerance in vertebrates. Physiol Rev. 2017;97:623–665. doi: 10.1152/physrev.00016.2016. [DOI] [PubMed] [Google Scholar]
- Storey JM, Storey KB. In defense of proteins: chaperones respond to freezing, anoxia or dehydration stress in tissues of freeze tolerant wood frogs. J Exp Zool. 2019;331:392–402. doi: 10.1002/jez.2306. [DOI] [PubMed] [Google Scholar]
- Storey KB, Storey JM, Brooks SPJ, Churchill TA, Brooks RJ. Hatchling turtles survive freezing during winter hibernation. Proc Natl Acad USA. 1988;85:8350–8354. doi: 10.1073/pnas.85.21.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissières A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Tsukamoto D, Hasegawa T, Hirose SI, Sakurai Y, Ito M, Takamatsu N (2019) Circadian transcription factor HSF1 regulates differential HSP70 gene transcription during the arousal-torpor cycle in mammalian hibernation. Sci Rep 9(1):832. 10.1038/s41598-018-37022-7 [DOI] [PMC free article] [PubMed]
- Wang S, Li X, Chen M, Storey KB, Wang T. A potential anti-apoptotic regulation: the interaction of heat shock protein 70 and apoptosis-inducing factor mitochondrial 1 during heat stress and aestivation in sea cucumber. J Exp Zool A. 2018;329:103–111. doi: 10.1002/jez.2180. [DOI] [PubMed] [Google Scholar]
- Wang S, Zheng Y, Chen M, Storey KB. Ultrastructural variation and key ER chaperones response induced by heat stress in intestinal cells of sea cucumber Apostichopus japonicus. J Oceanol Limnol. 2021;39:317–328. doi: 10.1007/s00343-020-9265-8. [DOI] [Google Scholar]
- White CN, Hightower LE, Schultz RJ. Variation in heat-shock proteins among species of desert fishes (Poeciliidae, Poeciliopsis) Mol Biol Evol. 1994;11(1):106–119. doi: 10.1093/oxfordjournals.molbev.a040085. [DOI] [PubMed] [Google Scholar]
- Winter J, Jakob U. Beyond transcription – new mechanisms for the regulation of molecular chaperones. Crit Rev Biochem Mol Biol. 2004;39:297–317. doi: 10.1080/10409230490900658. [DOI] [PubMed] [Google Scholar]
- Wu C-W, Biggar KK, Tessier SN, Zhang J, Pifferi F, Perret M, Storey KB. Induction of antioxidant and heat shock protein responses during torpor in the mouse lemur, Microcebus murinus. Genom Proteom Bioinform. 2015;13:119–126. doi: 10.1016/j.gpb.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-W, Tessier SN, Storey KB. Stress-induced antioxidant defense and protein chaperone response in the freeze-tolerant wood frog Rana sylvatica. Cell Stress Chaperon. 2018;23:1205–1217. doi: 10.1007/s12192-018-0926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-W, Tessier SN, Storey KB. Dehydration stress alters the mitogen-activated-protein kinase signaling and chaperone stress response in Xenopus laevis. Comp Biochem Physiol B. 2020;246–247:110461. doi: 10.1016/j.cbpb.2020.110461. [DOI] [PubMed] [Google Scholar]
- Zhang G, Storey JM, Storey KB. Chaperone proteins and winter survival by a freeze tolerant insect. J Insect Physiol. 2011;57:1115–1122. doi: 10.1016/j.jinsphys.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Zhang G, Storey JM, Storey KB. Elevated chaperone proteins are a feature of winter freeze avoidance by larvae of the goldenrod gall moth, Epiblema scudderiana. J Insect Physiol. 2018;106:106–113. doi: 10.1016/j.jinsphys.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Storey KB (2018) Life in suspended animation: role of chaperone proteins in vertebrate and invertebrate stress adaptation. In: Regulation of heat shock protein responses (Asea AAA, Kaur P, eds.) Springer International, Dordrecht. Chapter 5:95-137.
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/S0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- Żwirowski S, Kłosowska A, Obuchowski I, Nillegoda NB, Piróg A, Ziętkiewicz S, Bukau B, Mogk A, Liberek K. Hsp70 displaces small heat shock proteins from aggregates to initiate protein refolding. EMBO J. 2017;36:783–796. doi: 10.15252/embj.201593378. [DOI] [PMC free article] [PubMed] [Google Scholar]