Abstract
The spike (S) glycoprotein of the SARS-CoV-2 virus binds to the host cell receptor and promotes the virus’s entry into the target host cell. This interaction is primed by host cell proteases like furin and TMPRSS2, which act at the S1/S2 and S2´ cleavage sites, respectively. Both cleavage sites have serine or proline residues flanking either the single or polybasic region and were found to be conserved in coronaviruses. Unravelling the effects of these conserved residues on the virus entry and infectivity might facilitate the development of novel therapeutics. Here, we have investigated the role of the conserved serine and proline residues in the SARS-CoV-2 spike mediated entry, fusogenicity, and viral infectivity by using the HIV-1/spike-based pseudovirus system. A conserved serine residue mutation to alanine (S2´S-A) at the S2´ cleavage site resulted in the complete loss of spike cleavage. Exogenous treatment with trypsin or overexpression of TMPRSS2 protease could not rescue the loss of spike cleavage and biological activity. The S2´S-A mutant showed no significant responses against E-64d, TMPRSS2 or other relevant inhibitors. Taken together, serine at the S2´ site in the spike protein was indispensable for spike protein cleavage and virus infectivity. Thus, novel interventions targeting the conserved serine at the S2´ cleavage site should be explored to reduce severe disease caused by SARS-CoV-2-and novel emerging variants.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03749-y.
Keywords: SARS-CoV-2, Conserved serine, Spike cleavage site, Cell entry and viral infectivity
Introduction
The recently emerged severe acute respiratory syndrome 2 coronavirus (SARS-CoV-2) is an enveloped RNA virus that belongs to the family Coronaviridae and the lineage betacoronavirus (Sarbeco virus) (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses 2020). The SARS-CoV-2 virus shares genetic similarities with two other vital Coronaviruses that cause severe disease in humans, the severe acute respiratory syndrome (SARS) and the Middle East Respiratory Syndrome (MERS), which originated in 2003 and 2012, respectively (Maldonado et al. 2021). These enveloped viruses contain a structural glycoprotein, spike (S), which is the major viral surface protein responsible for the host cells’ attachment through binding to the ACE2 (angiotensin-converting enzyme 2) receptor and then facilitating a process of fusion and entry to the host cell for the release of the viral genome (Li 2016; Hatmal et al. 2020; Jackson et al. 2022).
The S glycoprotein belongs to the class I fusion proteins and shares standard features with other enveloped RNA viruses such as HIV-1 enveloped glycoprotein gp160 (Wu Zhang and Leng Yap 2004), Influenza HA protein (Amitai 2021), Respiratory Syncytial Virus (RSV) F protein (Walls et al. 2017) and other viruses of the Paramyxoviridae family, including Hendra and Nipah viruses (Weissenhorn et al. 1999; Rey and Lok 2018). The S glycoprotein is present on the virus surface as a homotrimer protein, consisting of three identical monomeric proteins, thus forming a complex trimeric structure on the surface (Bosch et al. 2003; Huang et al. 2020). The S glycoprotein of SARS-CoV-2 consists of approximately 1273 amino acid (aa), with a molecular size of 180–200 kDa, and mainly consists of an N-terminal extracellular domain, a transmembrane domain that helps in virus anchoring to the host cell, and a short C -terminal domain (Huang et al. 2020). The extracellular N terminal domain is glycosylated during synthesis in the host cell cytoplasm, which helps proper protein folding, transport, and host immune evasion (Walls et al. 2019; Shajahan et al. 2020; Sanda et al. 2021).
The S glycoprotein is synthesized as an uncleaved inactive precursor (Belouzard et al. 2012; Huang et al. 2020) and to initiate the infection, the spike must be cleaved by the host cell proteases, forming S1 and S2 subunits: The S1 subunit mainly contains the receptor binding domain (RBD) which initiates the virus attachment to the host cell receptor, ACE2 and the S2 subunit mainly mediates the virus fusion to the host cell membrane (Hoffmann et al. 2020b; Chen et al. 2021). The S glycoprotein contains two cleavage sites: the S1/S2 site, which is proteolytically processed by furin-like proteases and S2´ site, which is mainly primed by the serine protease 2 (TMPRSS2, Transmembrane Serine Protease 2) (Bestle et al. 2020; Essalmani et al. 2022). The cleavage of the S glycoprotein largely determines the virus entry, infectivity, and pathogenicity (Hoffmann et al. 2020a; Peacock et al. 2021).
The presence of the multi-basic amino acids in the SARS-CoV-2 S glycoprotein cleavage sites ease in efficient proteolytic processing of the S glycoprotein (Hoffmann et al. 2020a; Peacock et al. 2021). However, it is observed that SARS-CoV-2 and other Coronaviruses harbor proline and serine residues flanking the polybasic S1/S2 furin cleavage site and serine residue at the S2´ cleavage site. The role of these conserved residues in SARS-CoV-2 virus infectivity and pathogenicity is unknown. In this study, we sought to assess the effect of these conserved proline and serine residues at the S glycoprotein cleavage sites by introducing an alanine mutation and generating mutant pseudoviruses using the HIV-1/spike glycoprotein pseudovirus system. Our study concludes that the conserved serine residue at the S2´ site, which might interact with the serine protease, TMPRSS2, is critical for the S glycoprotein cleavage. Thus, serine mutation to alanine has a deleterious effect on virus entry and hence could decrease the virus fusogenicity and infectivity. Hence, interventions targeting the conserved serine residue at the S2´ site could be beneficial for reducing disease severity.
Materials and methods
Plasmids and cell lines
The Wuhan-Hu-1 SARS-CoV-2 full-length spike (GenBank: MN908947.3, Surface glycoprotein) was codon optimized and cloned into pcDNA3.1 vector (Thermo Fisher Scientific, USA). HIV-1 pNL4-3.Luc.R-E- plasmid was a kind gift from Dr. Kalpana Luthra, AIIMS, India. The Vero E6 and BHK-21 cell lines were kind gifts from Dr. Sudhanshu Vrati, RCB, India. All the cell lines: HEK293T, HEK293T-hACE2, Vero E6 and BHK-21 cells were maintained in complete Dulbecco’s Modified Eagle Media (DMEM) (Gibco) containing 10% fetal bovine serum (FBS, Gibco), 1% glutamine (Gibco) and 1% penicillin/streptomycin (Gibco) and routinely tested for mycoplasma-free status.
Pseudovirus production, infectivity titer and virus entry assay
Pseudoviruses were prepared as described previously (Adedeji et al. 2013). Titration of the pseudoviruses was performed in HEK293T cells overexpressing hACE2. Briefly, HEK293T-hACE2 (40,000/well) or HEK293T-hACE2-TMPRSS2 (40,000/well) were seeded in 100ul of complete DMEM. Later, 100 µl of pseudovirus supernatant was added to the wells and incubated for 48 h. After the incubation, britelite plus reporter substrate (PerkinElmer) was used to lyse the cells. The luciferase luminescence was measured by a Perkin Elmer luminometer. Luminescence from different groups were compared in relative light units (RLU) directly. Increased or decreased RLU were compared in terms of percentages to compare the drugs’ effect. RLU from the wells, where drug was not added, was used as a control to determine drugs’ effect.
HEK293T cells (40,000/well) were seeded in a 96-well plate and post 24 h, cells were co-transfected with plasmids encoding SARS-CoV-2 spike glycoprotein and pLN4 backbone expressing luciferase. Chemical inhibitors like E-64d, 20 µM, Sigma-Aldrich, E8640; camostat mesylate, 100 µM, Sigma-Aldrich, SML0057; NH4Cl, 20 mM, Sigma-Aldrich, 254,134, Batimastat, 20µM, Sigma-Aldrich, SML0041 and Cytochalasin D, 1µM, Sigma-Aldrich, C8273 were added post 24 h of transfection. After two hours of compounds’ addition, hACE2 and hACE2-TMPRSS2 over expressing HE293T cells were overlaid on spike transfected HEK293T cells. TPCK-Trypsin (2 mg/ml) treatment was given post 22 h of transfection for 1 h with serum free media, later, the media was changed to complete DMEM containing 10% FBS. After 16 h, britelite substrate was used to lyse the cells and luminescence was measured by PerkinElmer luminometer in RLU units. Luminescence from different groups were compared in relative light units (RLU) directly. Increased or decreased RLU were compared in terms of percentages to show the drugs’ effect. RLU from the wells, where drug was not added, was used as a control to determine drugs’ effect.
Pseudovirus stability
Pseudoviruses were generated as described in the previous section. PSV titration was performed in HEK293T-hACE2 cells as explained earlier. PSV worth 1 million RLU (in 100 µl volume) was taken from each mutant sample and incubated with HEK293T-hACE2 (40,000/well in 100 µl volume) at different time points (24 h, 3 day and 7 day) post pseudovirus harvesting. Incubation was done for 48 h and britelite plus reporter substrate (PerkinElmer) was used to lyse the cells. The luciferase luminescence was measured by a Perkin Elmer luminometer. Luminescence from different groups were compared in relative light units (RLU) directly. Increased or decreased RLU were compared in terms of percentages to compare the PSV stability.
Western blot
Expi293F cells were transiently transfected for soluble hACE2, RBD and spike proteins using Expi293fectin (Invitrogen) following the manufacturer’s protocol. 25ug of above-mentioned proteins were immunized in BALB/c mice for polyclonal sera. Anti-hACE2/RBD/anti-spike polyclonal sera was used for western blot, flow cytometry and immunofluorescence.
HEK293T cells (1 million/well) were transfected for the spike cleavage site mutants in a 6-well plate. The next day, transfected cells were lysed with RIPA lysis buffer and sonicated for 2 min. Cell lysates were separated on a 10% SDS-PAGE and transferred to a PVDF membrane. Anti-RBD mouse polyclonal sera (1:1000) was used to probe the spike and HRP-conjugated anti-mouse goat antibody (1:2000) was used. GAPDH (1;1000, Invitrogen, AM4300) was used as a loading control. Western blot data was quantified in Image J software. Pixels of the GAPDH, loading control were calculated, and normalized for the sample loading ratio. Later, sample loading ratio was used to normalized each sample. Pixels of each sample were then compared and plotted using GraphPad prism 8 software.
FACS-based surface expression and soluble hACE2 binding assay
FACS-based cell surface expression of spike cleavage site mutants was carried out as described previously (Samal et al. 2018; Khatri et al. 2023). A soluble hACE2-spike binding assay was performed where HEK293T cells were transfected with spike plasmids, and 24 h post transfection, cells were washed twice with FACS buffer (5% FBS in 1 × PBS) and then incubated with 10 μg/ml soluble hACE2 for 1 h on ice. After incubation, cells were washed and incubated with polyclonal mouse anti-human ACE2 antibody (1:500) (R&D Systems, USA) for 1 h, followed by incubation with PE-conjugated goat anti-mouse secondary antibody (1:1000) (Jackson ImmunoResearch, USA). After three washes, the cells were acquired with BD FACS Diva and the data were analyzed with FlowJo software (version 10.0.6, Tree Star Inc.).
Fusion and syncytial formation assay
For fusion and syncytial formation assay, BHK-21 cells were transfected with 0.5 µg of spike- and/or ACE2- and/or TMPRSS2-expressing plasmids using HD FuGENE transfecting reagent (Promega, E2311). Inhibitors (E-64d, 20 µM; camostat mesylate, 100 µM; NH4Cl, 20 mM, Batimastat, 20µM, and Cytochalasin D, 1µM) were added 2 h post-transfection. TPCK-Trypsin (2 mg/ml) was added 22 h post-transfection for 1 h with serum free media, later, the media was changed to DMEM containing 10% FBS. At 24 h post-transfection, cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton in PBS. Then, blocking was done for nonspecific binding with 3% goat serum in PBS for 1 h. Anti-spike polyclonal sera (1:500) was used as the primary antibody overnight at 4°C. Wells were washed thrice with PBS and incubated with Alexafluor488-labelled rabbit anti-mouse IgG (1:1000). Nuclei were counterstained with DAPI (D9542, Sigma-Aldrich, United States) and the imaging was performed with fluorescence microscope (IX-71, Olympus). Statistical analysis of the GFP + area was done using Image J software. GFP + area of the test samples was compared with control sample, where both the samples were first normalized by reducing the same area from the mock sample.
Confocal microscopy
BHK-21 cells (30,000 cells/well) were seeded on a 12mm glass coverslip. Next day, cells were transfected with spike and/or ACE2 and/or TMPRSS2 expressing plasmids. 24-h post-transfection, cells were fixed with 4% paraformaldehyde (Sigma), permeabilized with 0.1% Triton in PBS for 20 min, and blocked with 5% FBS (Fetal Bovine Serum, Gibco) in PBS, blocking buffer, for an hour on room temperature. Anti-spike polyclonal sera (1:500) was used as the primary antibody overnight at 4 °C. Cells were washed thrice with PBS and incubated with Alexafluor488-labelled rabbit anti-mouse IgG (1:1000). Cells were washed thrice for 5 min each with PBS. Nuclei were counterstained with DAPI (D9542, Sigma-Aldrich, United States). Cells were then washed again in PBS three times and mounted with 70% glycerol. Cells were imaged using by an Olympus FluoView FV3000 Lazer scanning confocal microscope using 60 × objective lens. The images were processed by Fiji Image J software.
Molecular dynamics simulation
System Preparation: The crystal structures of Wuhan-1 spike protein without/with ACE2 (Angiotensin-converting enzyme 2) protein were retrieved from the RCSB protein data bank (PDB ID: 7CWL, 6M0J, respectively). For the structural complex preparation, the full crystal structure of Wuhan-spike protein’s RBD domain of 7CWL was aligned with RBD domain of 6M0J crystal structure using Maestro's protein structure alignment tool. To prepare the protein structures of different systems with ACE2 (Wuhan-1 spike wild type, Wuhan-1 spike S686A mutant (mut1), and Wuhan-1 spike S816A mutant (mut2) Maestro's Protein Preparation Wizard module with OPLS4 force field, protein’s missing regions were filled by prime module, and preliminary minimization done with same OPLS4 force field. The systems were prepared with the protonation states of the residues at neutral pH as predicted by the PROPKA module in Protein Preparation Wizard (Kumari et al. 2021, 2023; Mittal et al. 2021).
Molecular dynamics simulation: All simulations were done by the Desmond v6.1 module from the Schrodinger suite [https://doi.org/10.1109/sc.2006.54]. Systems were minimized with the OPLS4 force field and then solvated with a predefined TIP3P water solvent model (Suri et al. 2022). For each of the three complexes, the size and shape of the repeating unit buffered at 10.0 Å distances were determined by placing them all in orthorhombic periodic boundary conditions. Using a steepest-descent integrator for 2000 steps, each complex underwent energy minimization for 100 ps. The Nose-Hover chain thermostat and the Martyna-Tobias-Klein barostat were assigned for NPT ensemble simulations. A time step of 0.002 ps was used with the RESPA integrator. The cut-off radius for short-range Coulombic interactions was 9.0 Å. The M SHAKE algorithm of DESMOND was used to constrain bonds to hydrogen. The simulation was run for a total of 50 ns for each system, and the coordinates were saved at intervals of 10 ps. Scripts written in Schrodinger and VMD were used to post-process the MD simulation trajectory data (Panwar et al. 2022).
Statistical analysis
The data were analyzed on GraphPad Prism version 8 or 9 (GraphPad Software Inc.). Significant differences were calculated by comparing either Wuhan-1 with other variants using one-way ANOVA or inhibitor treated samples with untreated samples using two-way ANOVA (analysis of variance) with multiple comparisons test. p values below 0.05 were considered statistically significant and presented as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Results and discussion
Spike expression and cleavability of cleavage sites mutants
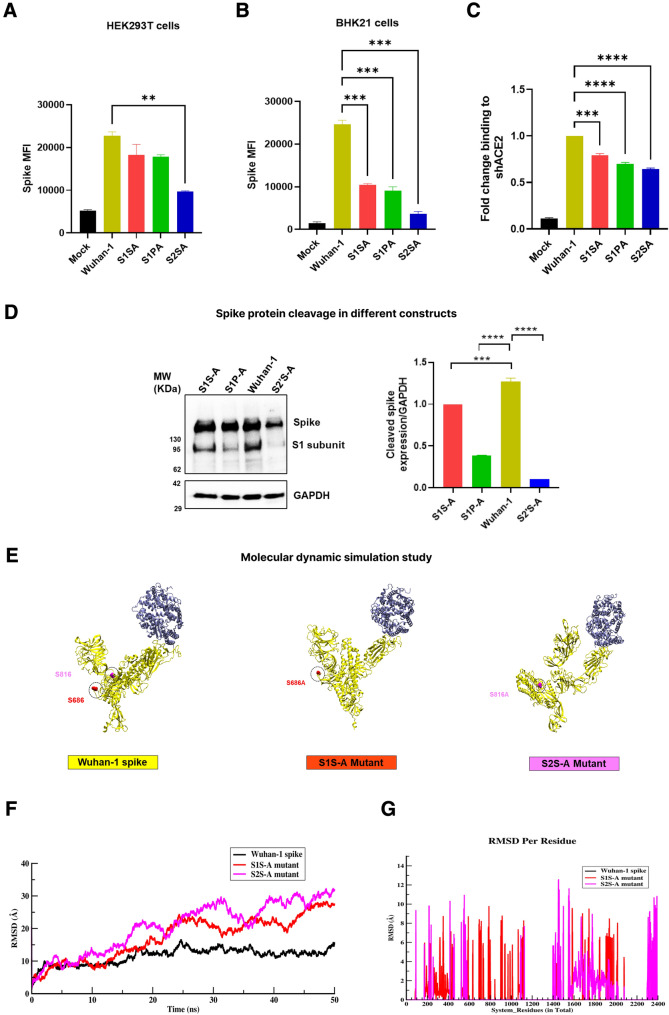
Site-directed mutagenesis was performed on the full-length SARS-CoV-2 Wuhan-1 spike protein (GenBank: MN908947.3) to generate S1S-A, S1P-A, and S2´S-A cleavage site mutants (Fig. 1A, B). Two of these mutations are made around the S1/S2 cleavage site, where proline and serine at 680 and 686 aa positions in SARS-CoV-2 spike protein were mutated to alanine and termed S1P-A and S1S-A mutants, respectively. For the S2´S-A mutant, serine at the 816 aa position was mutated to alanine at the S2´ cleavage site (Fig. 1B). We have tried to alter proline at 812 aa at the S2´ cleavage site to alanine, but we have not succeeded in generating the mutation. Cell surface expression of the spike cleavage site mutants was assessed against Wuhan-1 spike protein using flow cytometry in HEK293T and BHK-21 cells. Spike surface expression was observed higher in S1S-A and S1P-A mutants than in S2´S-A in both cell lines (Fig. 2A, B). In comparison to Wuhan-1 spike, the S2´S-A mutant showed a significant reduction (> twofold) in the spike expression in the HEK293T cells (Fig. 2A). Though HEK293T cells expressed S1S-A and S1P-A mutants relatively like Wuhan-1, BHK-21 cells showed a marked reduction in the expression of both the mutants compared to Wuhan-1 spike (Fig. 2B). Notably, HEK cells have higher protein expression as compared to BHK-21 cells (Thomas and Smart 2005). Though, Wuhan-1 is expressed equally in both cell lines, the mutants could not express well in BHK-21 as compared to HEK cells; we observed the same trend of protein expression in both cell lines showing different percentage values.
Fig. 1.
Schematic presentation of conserved residues and cleavage site mutations in spike. A Conserved residues are shown in spike protein of different coronaviruses around the S1/S2 furin and S2´ cleavage sites. Cleavage site amino acids (arginine, R) are in red bold font and the conserved residues (serine, S and proline, P) are in blue bold font. RBD, Receptor binding domain; FP, fusion peptide; N, N-terminal region and C, C-terminal region. Coronaviruses: SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SARS-CoV, severe acute respiratory syndrome coronavirus-1; MERS CoV, Middle East respiratory syndrome coronavirus; HCoV, human coronavirus; and IBV, infectious bronchitis virus. B Cleavage site amino acid residues in SARS-CoV-2 spike. Conserved serine and proline residues are shown in bold black font. Mutations in the conserved serine and proline residues to alanine: S1S-A, S1/S2 site serine to alanine mutation; S1P-A, S1/S2 site proline to alanine mutation and S2´S-A, S2´ cleavage site serine to alanine mutant) are shown in blue bold font. SP signal peptide, NTD N-terminal domain, RBD receptor binding domain, FP fusion peptide, HR1 heptad repeat 1, HR2 heptad repeat 2, TM transmembrane domain and CP cytoplasmic tail
Fig. 2.
Spike expression, ACE2 binding and cleavage of cleavage site mutants. A, B Cell surface expression of spike protein variants in HEK293T and BHK-21 cell lines as measured by flow cytometry. BHK-21 and HEK293T cells were transfected with spike cleavage site mutants for 24 h. Post incubation, spike expressing cells were stained with polyclonal mice anti-spike sera and later, PE-conjugated anti-mouse goat secondary antibody. Samples were analyzed with BD FACS Diva flow cytometer. Statistical significance was determined using one-way Anova in GraphPad prism keeping Wuhan-1 as the control. The experiment was repeated three times and the error bars show mean values with SEM. C Soluble-hACE2 protein binding with spike cleavage site mutants. Spike protein was expressed on HEK293T cell surface and after 24 h of transfection, cells were incubated with 10ug of soluble-hACE2 for an hour, stained with anti-hACE2 mouse polyclonal sera and later with PE-conjugated anti-mouse goat secondary antibody. Samples were acquired with BD FACS Diva flow cytometer. Statistical significance was determined using one-way Anova in GraphPad prism keeping Wuhan-1 as the control. The experiment was repeated three times and the error bars show mean values with SEM. D Western blot analysis of spike expression in HEK293T cells at 24 h post transfection. Cell lysates were prepared using RIPA lysis buffer and run on a 10% SDS-PAGE. After transferring the gel to a PVDF membrane, it was blocked with 5% milk and stained with anti-RBD mouse polyclonal sera (1:1000 dilution) and later with HRP-conjugated goat anti-mouse antibody (1:2000). GAPDH was used as a loading control. Experiment was repeated three times and the error bar show mean values with SEM. For the statistical significance, one-way ANOVA test was performed with multiple comparisons where, Wuhan-1 was used as a control to compare with cleavage site mutants. (p < 0.05), where (p < 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 were considered significant and p > 0.05 was considered nonsignificant (ns). E–G Dynamical measurement of structures over time. E Conformational changes in the protein-complex of spike protein with hACE2. Figure shows hACE2 protein complex with Wuhan-1, S1S-A, and S2´SA, respectively. F RMSD analysis of Wuhan-1/mutant spike with hACE2 protein complex system over the course of MD (molecular dynamics) trajectory simulation. G Changes in RMSD at residual level for S1S-A and S2´S-A mutants in comparison with Wuhan-1 spike
We further assessed the binding of the mutants to soluble hACE2. As shown in Fig. 2C, spike cleavage site mutants’ binding to shACE2 (soluble human ACE2) was reduced compared to the Wuhan-1 spike protein. However, a marked decrease in binding to the hACE2 receptor was found in the S2´S-A mutant. Next, pseudoviruses were generated with these mutants according to the protocol described in the material and methods section. To analyze the cleavage of spike protein incorporated in pseudoviruses, we performed a western blot analysis with the spike mutants’ transfected HEK293T cell lysates at 24 h post-transfection. S1S-A and S1P-A showed a similar level of spike cleavage but were significantly lower than Wuhan-1. The cleavage of the S protein in the S2´S-A mutant was almost negligible, and a significant difference was observed with all three spike mutant constructs (Fig. 2D). A molecular dynamic simulation study was done to explore the conformational changes in the Wuhan-1 and mutants’ spike protein structures (Fig. 2E–G). We determined the stability of all three complexes of spike and ACE2 wild –vs.-mutant proteins via backbone atoms' RMSD values. We observed, both local and global changes in the spike proteins containing the serine mutations at the S1/S2 and S2´ sites, respectively (Fig. 2E). Furthermore, we observed that over the course of simulation, Wuhan-1 spike gained stability after the initial 2 ns and the rest of the two mutant systems: S686A and S816A at the S1/S2 and S2´ sites, respectively, showed their flexible nature throughout the simulation (Fig. 2F). To understand how mutant molecular structures, differ from the wild-type Wuhan-1 spike protein, we computed the per residue RMSD (root mean square deviation), which revealed significant changes at the residue level in mutant structures (Fig. 2G). Overall, the mutant proteins are considerably more flexible than the Wuhan-1 spike. The results indicate that the mutation of the conserved serine residue (816 aa) in the SARS-CoV-2 spike protein plays an essential role in the expression and cleavage of the spike protein. There was a significant reduction in the expression of the S2´S-A mutant compared to the Wuhan-1 or other cleavage site mutants, which could explain why this mutant was not efficiently cleaved in the presence of furin in HEK293T cells.
Cell-to-cell fusion and infectivity of S2´S-A mutant significantly reduced
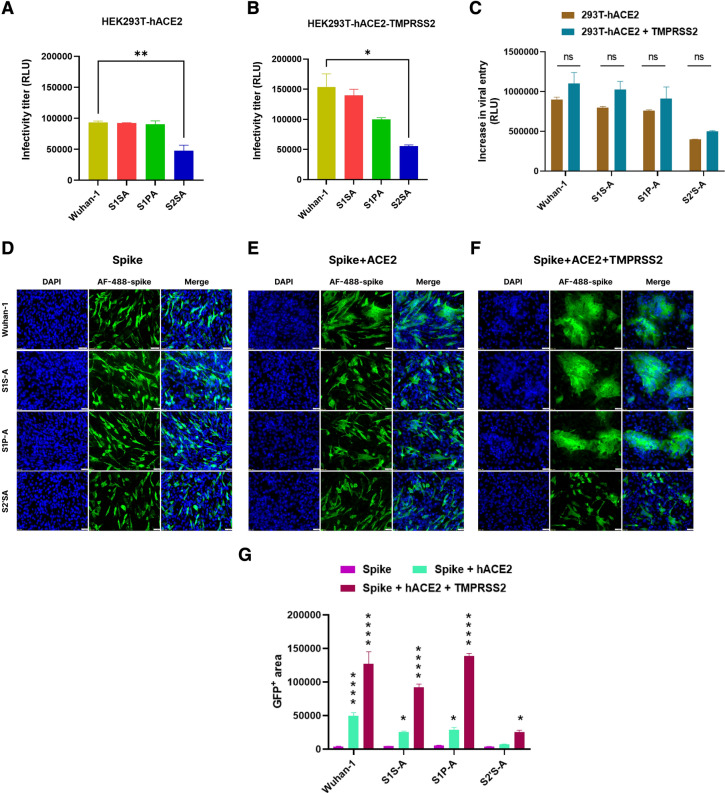
Further, we evaluated the effect of cleavage site mutants on cell-to-cell fusion, viral entry, and infectivity. After generating pseudoviruses for Wuhan-1, S1S-A, S1P-A and S2´S-A spike mutants, their infectivity was measured by a luciferase assay using HEK293T cells overexpressing hACE2 and both hACE2-TMPRSS2 (Fig. 3A, B). Wuhan-1, S1S-A and S1P-A pseudoviruses showed a similar level of viral infectivity in HEK293T-hACE2 cells, whereas about a twofold reduction was seen in the infectivity of the S2´S-A mutant compared to the other three pseudoviruses. Additionally, except for the S2´S-A mutant, the infectivity of all three pseudoviruses was enhanced in HEK293T overexpressing both hACE2 and TMPRSS2 cells; the infectivity titer was increased by 57%, 47%, and 14.28% in Wuhan-1, S1S-A and S1P-A, respectively, while only a 11.65% increment in the infectivity was observed with the S2´S-A mutant (Fig. 3A, B). The data suggest that serine protease TMPRSS2 is less effective in the S2´S-A mutant thus affecting virus entry compared to other cleavage site mutants (Fig. 3C). Pseudovirus stability was also checked at day 3 and day 7 post virus harvesting (Fig. S1). Difference in the infectivity titer of all the pseudoviruses was non-significant at different time points. Overall, titer reduced to 50–60% and 25–35% by day 3 and 7, respectively. BHK-21 cells were transfected with the spike mutants as mentioned earlier, and cellular expression was imaged using mouse anti-spike polyclonal sera and later, anti-mouse Alexafluor488 (Fig. 3D). We investigated hACE2 and TMPRSS2 mediated fusion and syncytium formation in BHK-21 cells expressing the spike in the Wuhan-1 and cleavage site mutants (Fig. 3E–G). The highest fusion was observed with the Wuhan-1 spike in HEK293T-hACE2 cells as compared to the S1S-A, S1P-A, and S2´S-A mutants (Fig. 3E). The S1S-A and S1P-A spike mutants showed about twofold less syncytium formation in the presence of hACE2 as compared to the Wuhan-1 spike protein. Interestingly, the S2´S-A mutant failed to form any syncytium as shown by the GFP positive area graphs (Fig. 3G). In the presence of TMPRSS2 expression in HEK293T- hACE2 cells, there was a significant increase in fusion area in the cases of Wuhan-1, S1S-A and S1P-A mutants; however, in the S2´S-A mutant, no significant change was seen in syncytium formation (Fig. 3F). A confocal microscopy-based similar experiment was also performed to support this data with higher magnification and better resolution (Fig. S2). Next, we evaluated the SARS-CoV-2 spike and hACE2-mediated fusion in the presence of exogenous trypsin. Kim Yeeun et al. have shown that trypsin could enhance virus replication in cell lines (Kim et al. 2022). In the presence of exogenous trypsin (2 ug/ml), only Wuhan-1 showed a significant increase in viral cell entry but no other mutants (Fig. S3A). BHK-21 cells were transfected with spike and hACE2 encoding plasmids and treated with exogenous trypsin (2 ug/ml) for half an hour, later cells were fixed, stained for spike protein, and imaged (Fig. S3B). Wuhan-1 spike showed a significant increment in the fusion and syncytium formation, which is almost twofold compared to the cells where trypsin was not added. S1S-A and S1P-A mutants showed minimal increases in the fusion, and the S2´S-A mutant did not show any change after adding exogenous trypsin. The S2´S-A mutant is incapable of causing cell-to-cell fusion and syncytium in the absence of trypsin and the addition of exogenous trypsin did not help in increasing the fusion, indicating that phenotypically the S2´S-A spike mutant has been changed, which led to the loss of the property of forming cell-to-cell fusion and syncytium formation.
Fig. 3.
Effect of ACE2 and TMPRSS2 on the infectivity, viral entry and fusogenicity of cleavage site mutants. A, B Infectivity titer of pseudovirus variants in HEK293T-hACE2 and HEK293T-hACE2-TMPRSS2 cells, respectively. Spike cleavage site mutants based pseudoviruses were prepared in HEK293T cells. Then, HEK293T-hACE2 and HEK293T-hACE2-TMPRSS2 cells were infected with pseudoviruses for 48 h. Cells were lysed and luciferase titer was measured in a luminometer. Statistical significance was determined using one-way Anova keeping Wuhan-1 as the control. The experiment was repeated three times and the error bars show mean values with SEM. C Quantitative fusion assay of spike variants with HEK293T-hACE2 or HEK293T-hACE2-TMPRSS2 as measured by RLU. The experiment was repeated three times in duplicates. Statistical significance was determined using two-way ANOVA, with multiple comparisons using hACE2 expression as control. The error bars show mean values with SEM. D–F Immunofluorescence assay for spike (D), spike-hACE2 (E) and spike-hACE2-TMPRSS2 (F) mediated cell–cell fusion in BHK-21 cells at 24 h post transfection, respectively. After transfecting the cells with respective plasmids, cells were fixed post 24-h and stained with anti-spike mouse polyclonal sera (1:500) and AlexaFluor-488-labeled anti-mouse antibody (green) (1:1000). Experiment was repeated three times. The images were taken with an Olympus fluorescence microscope. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue); scale bar: 50 μm and magnification 20x. G Immunofluorescence images were quantified by selecting five random GFP + areas in Image J software. Graph was prepared and analyzed in GraphPad prism 9. For the statistical significance two-way ANOVA test was performed with multiple comparisons where, spike expression was used as a control to compare with spike + hACE2 or spike + hACE2 + TMPRSS2 expression. The error bars show mean values with SEM. (p < 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 were considered significant and p > 0.05 was considered nonsignificant (ns)
Effect of E-64d and NH4Cl on cleavage sites mutants
SARS-CoV-2 spike protein interaction with the host cell receptor, hACE2, mediates internalization of the virus through endosomal vesicles, where the Cathepsin L proteases cleave the spike protein, which in turn aids in viral infectivity and pathogenesis (Jackson et al. 2022) Here, we evaluated the role of Cathepsin L protease inhibitor (E-64d) in the cleavage site mutants in cell-to-cell fusion, pseudovirus infection and entry (Fig. 4). Wuhan-1 and S1S-A mutants showed 57.5% and 42.6% reductions in the pseudovirus infectivity in the presence of E-64d (20 µM), whereas no significant reduction in viral infectivity was shown in the case of S1P-A and S2´S-A mutants (Fig. 4A). Interestingly, in the presence of E-64d inhibitor, the viral entry inhibition assay showed a marked reduction in the relative luciferase units (RLU) in Wuhan-1 and cleavage site spike mutants (Fig. 4B); the highest reduction was observed in Wuhan-1 and S1S-A mutant, followed by the S2´S-A and S1P-A mutants. This difference in the results of Fig. 4A, B, might be due to the type of assays performed. In Fig. 4A, for measuring the virus infectivity, pseudoviruses (in prefusion conformation) were incubated with E-64d and then added to the HEK293T-ACE2 cells. Both mutants showed no significant difference suggesting that the mutations might have changed the cleavage site conformation in prefusion state as compared to Wuhan-1, as shown in the molecular docking experiment (Fig. 2E). Thus, in Fig. 4A, the drug might not be able to interact properly with the mutants’ spike protein in its prefusion conformation, hence no significant difference was observed. In the second experiment, to measure the percentage of virus entry inhibition, the HEK293T cells were co-transfected with hACE2 receptor and spike plasmids, followed by the addition of the E-64d drug. The presence of the receptor before adding of the drug might be allowing post conformation changes in the spike protein. The effect that was shown in the presence of drug might be due to the post conformation changes in both Wuhan-1 and mutant’s spike protein, where even after the cleavage site mutations, the site was accessible to E64d and hence the protease inhibitor could act properly.
Fig. 4.
Effect of E-64d and NH4Cl on the infectivity, viral entry and fusogenicity of cleavage site mutants. A Inhibition in infectivity titer of pseudovirus variants in HEK293T-hACE2 in the presence of E-64d. After harvesting, the pseudoviruses were incubated with 20 µM of E-64d for 2 h at 37 ºC. Later, HEK293T-hACE2 cells were added to pseudovirus and drug mixture. Post 48 h of cell addition, luciferase readings were taken as RLU. Statistical significance was determined using two-way ANOVA, with multiple comparisons using spike + hACE2 (untreated) as control. The error bars show mean values with SEM. The experiment was repeated three times. B Inhibition in viral entry of pseudovirus variants in HEK293T-hACE2 in the presence of E-64d. 20 µM of cysteine protease inhibitor, E-64d, was added to the entry assay cells for 2 h at 37ºC. Later, HEK293T-hACE2 cells were added to the spike expressing cells and drug compound. Post 48 h of cell addition, luciferase readings were taken as RLU. Statistical significance was determined using two-way ANOVA, with multiple comparisons using spike + hACE2 (untreated) as control. The error bars show mean values with SEM. The experiment was repeated three times. C–E Immunofluorescence assay for fusion inhibition in spike variants of SARS-CoV-2 in BHK-21 cells in the presence of E-64d at 24 h post transfection. The experiment was repeated three times. Statistical significance was determined using two-way ANOVA, with multiple comparisons using hACE2 expression without E-64d (D) as control. The error bars show mean values with SEM. F. Immunofluorescence assay for fusion inhibition in spike variants of SARS-CoV-2 in BHK-21 cells in the presence of NH4Cl (20 mM) at 24 h post transfection. After transfecting the cells with respective plasmids, cells were treated with compound post 2 h of transfection. Later, cells were fixed post 24-h and stained with anti-spike mouse polyclonal sera (1:500) and AlexaFluor-488-labeled anti-mouse antibody (green) (1:1000). Experiment was repeated three times. The images were taken with an Olympus fluorescence microscope. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue); scale bar: 50 μm and magnification 20x. G. Inhibition in infectivity titer of pseudovirus variants in HEK293T-hACE2 in the presence of NH4Cl at 48 h of infection. H Inhibition in viral entry of pseudovirus variants in HEK293T-hACE2 in the presence of NH4Cl. Experiment was repeated three times. For the statistical significance, two-way ANOVA test was performed with multiple comparisons where untreated samples were compared with NH4Cl treated samples. The error bars show mean values with SEM. I Immunofluorescence images for NH4Cl treatment were quantified by selecting five random GFP + areas in Image J software. Graph was prepared and analyzed in GraphPad prism 9. For the statistical significance two-way ANOVA test was performed with multiple comparisons. p < 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 were considered significant and p > 0.05 was considered nonsignificant (ns)
Next, we assessed cell-to-cell fusion inhibition in the presence of E-64d (Fig. 4C–E). Furthermore, a reduction in the cell-to-cell fusion was observed in Wuhan-1, S1S-A, and S1P-A mutants. Consistent with the infectivity data, Wuhan-1 and S1S-A showed significant inhibition in the cell-to-cell fusion and syncytium formation by 65% and 33%, respectively, compared to the control cells where the compound was not added (Fig. 4D). We further sought to test the effect of ammonium chloride (NH4Cl) on the cleavage site mutants. Ammonium chloride helps endosomal acidification, which modulates cathepsin L-mediated proteolytic activity (Ou et al. 2021). The cleavage site mutants did not show any change in fusion in the presence of NH4Cl (Fig. 4F, I). In the presence of 20 mM NH4Cl, a reduction in the viral entry was observed in all the cleavage site mutants, whereas only S1P-A mutant had a moderate reduction in the virus infectivity titer (Fig. 4G, H).
Effect of TMPRSS2 protease inhibitor on cleavage sites mutants pseudoviruses infectivity and fusion
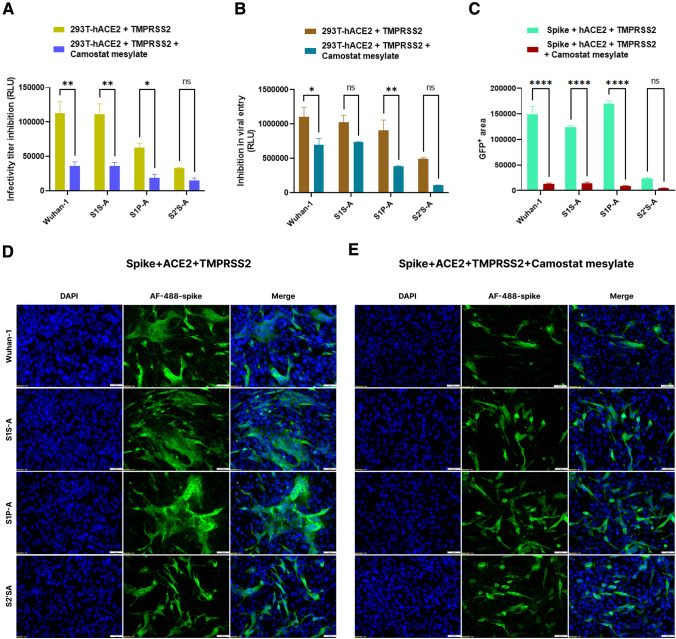
In the presence of TMPRSS2 serine protease inhibitor, camostat mesylate (100µM), the pseudovirus infectivity was significantly reduced in Wuhan-1, S1S-A, and S1P-A mutants by 68.5%, 67% and 69%, respectively, in hACE2 and TMPRSS2 overexpressing HEK293T cells (Fig. 5A). The S2´S-A mutant showed less than a 50% reduction in pseudovirus infectivity under the same conditions. Entry inhibition was observed in Wuhan-1 and S1P-A mutant, which showed a marked reduction in cell entry of 63% and 42%, respectively, but S1S-A and S2´S-A showed no significant differences (Fig. 5B). Further, spike and hACE2-mediated syncytium formation was evaluated in the presence of TMPRSS2 serine protease and its inhibitor, camostat mesylate, in BHK-21 cells (Fig. 5C–E). A graph comparing the GFP positive area in all the mutants showed a significant reduction in cell-to-cell fusion in Wuhan-1, S1S-A, and S1P-A but not in S2´S-A (Fig. 5C). The addition of this inhibitor completely abrogated the fusion and syncytium formation in Wuhan-1, S1S-A, and S1P-A mutants as compared to the control wells shown in Fig. 5D. Camostat mesylate was tested with the control conditions like spike and spike + TMPRSS2 as well shown in Fig. S4. The data corroborated our earlier results, the S2´S-A mutant failed to be activated by TMPRSS2 protease.
Fig. 5.
Effect of camostat mesylate on the infectivity, viral entry and fusogenicity of cleavage site mutants. A Inhibition in infectivity titer of pseudovirus variants in HEK293T-hACE2-TMPRSS2 cells in the presence of camostat mesylate (100 µM) at 48 h of infection. Experiment was repeated three times. The error bars show mean values with SEM. For the statistical significance, two-way ANOVA test was performed with multiple comparisons where untreated samples were compared with camostat mesylate treated samples. B Inhibition in cell entry of pseudovirus variants in HEK293T-hACE2-TMPRSS2 cells in the presence of camostat mesylate. Camostat mesylate (100 µM) was added to the entry assay cells for 2 h at 37 ºC. Later, HEK293T-hACE2 cells were added to the spike expressing cells and drug compound. Post 48 h of cell addition, luciferase readings were taken as RLU. The error bars show mean values with SEM. Experiment was repeated three times. For the statistical significance, two-way ANOVA test was performed with multiple comparisons where untreated samples were compared with camostat mesylate treated samples. C–E. Immunofluorescence assay for fusion inhibition mediated by spike, hACE2 and TMPRSS2 in BHK-21 cells in the presence of camostat mesylate at 24 h post transfection. After transfecting the cells with respective plasmids, cells were treated with compound post 2 h of transfection. Later, cells were fixed post 24-h and stained with anti-spike mouse polyclonal sera (1:500) and AlexaFluor-488-labeled anti-mouse antibody (green) (1:1000). Experiment was repeated three times. The images were taken with an Olympus fluorescence microscope. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue); scale bar: 50 μm and magnification 20x. The error bars show mean values with SEM. For the statistical significance, two-way ANOVA test was performed with multiple comparisons where untreated samples were compared with camostat mesylate treated samples. p < 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 were considered significant and p > 0.05 was considered nonsignificant (ns)
Effect of Batimastat and cytochalasin D on cleavage sites mutants
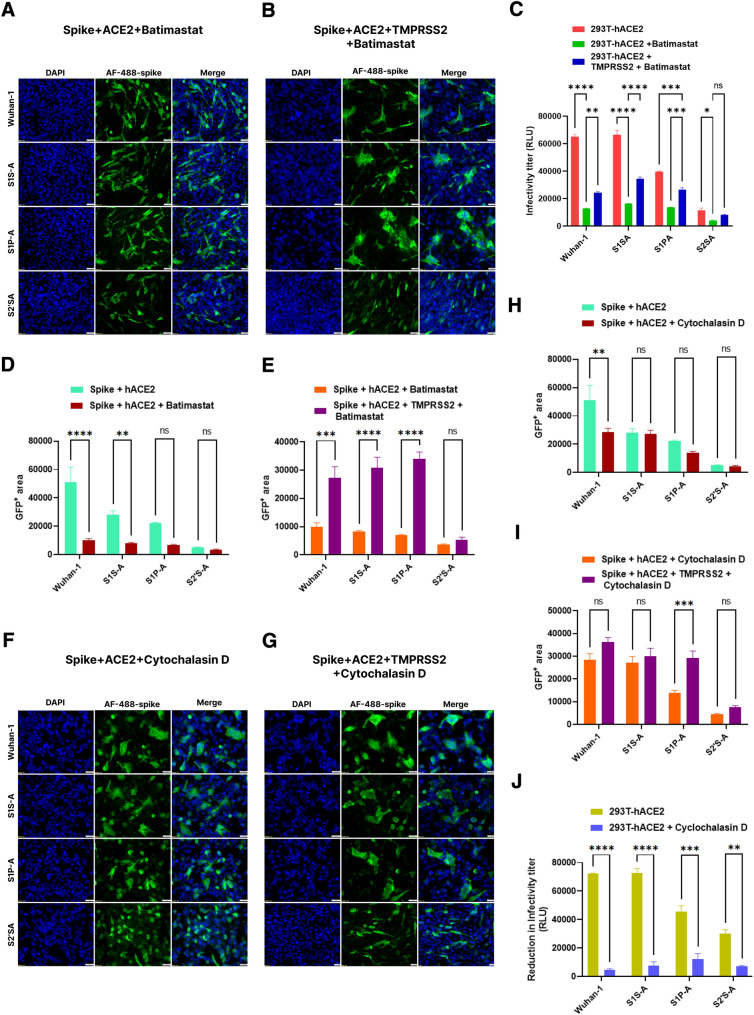
Batimastat is a metalloprotease inhibitor that inhibits cell-to-cell fusion (Hörnich et al. 2021). Hence, we further evaluated the effect of batimastat on the fusion and infectivity of the cleavage site mutants. We expressed the spike, hACE2, and/or TMPRSS2 in BHK-21 cells for a fusion assay in the presence and absence of batimastat (20µM) (Fig. 6A, D). In the presence of batimastat, where spike and hACE2 were co-expressed, Wuhan-1 spike and S1S-A mutant fusion were significantly inhibited. The TMPRSS2 expression along with spike and hACE2 markedly increased the fusion of Wuhan-1, S1S-A, and S1P-A spike but was not shown in the S2´S-A mutant (Fig. 6B, E). Additionally, marked inhibition was observed in the infectivity titer of all the cleavage site spike mutants in HEK293T-hACE2 cells in the presence of batimastat (Fig. 6C). The infectivity titer of Wuhan-1, S1S-A, S1P-A, and S2´S-A mutants was reduced by 80%, 76%, 67.5% and 66.1%, respectively. Consistent with the cell-to-cell fusion data, a significant increase was observed in the infectivity titer of Wuhan-1, S1S-A, and S1P-A mutants when TMPRSS2 was co-expressed in HEKF293T-hACE2 cells. Next, we evaluated the role of Cytochalasin D in cell-to-cell fusion and infectivity of the cleavage site spike mutants. Cytochalasin D is a cytoskeletal drug that could modulate endocytosis-mediated fusion by cytokinesis (Yeung et al. 2021). A significant decrease in cell-to-cell fusion was observed in the Wuhan-1 spike expressed in HEK293T-hACE2 cells but not in other cleavage site constructs in the presence of Cytochalasin D (1µM) (Fig. 6F, H). However, in the presence of TMPRSS2 expression and Cytochalasin D, we did not observe any marked changes in the fusion (Fig. 6G, I). Interestingly, the pseudovirus infectivity titer was significantly reduced in the presence of Cytochalasin D in all the constructs (Fig. 6J). The highest reduction was observed in Wuhan-1 and S1S-A mutant, and S2´S-A showed the minimal virus infectivity reduction.
Fig. 6.
Effect of batimastat and cytochalasin D on the infectivity, viral entry and fusogenicity of cleavage site mutants. A, B Immunofluorescence assay for fusion inhibition in the spike variants of SARS-CoV-2 in BHK-21 cells in the presence of batimastat (20 µM) at 24 h post transfection. Batimastat was added to the spike-hACE2 and spike-hACE2-TMPRSS2 expressing cells. After transfecting the cells with respective plasmids, cells were treated with compound post 2 h of transfection. Later, cells were fixed post 24-h and stained with anti-spike mouse polyclonal sera (1:500) and AlexaFluor 488-labeled anti-mouse antibody (green) (1:1000). Experiment was repeated three times. The images were taken with an Olympus fluorescence microscope. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue); scale bar: 50 μm and magnification 20x. C Inhibition in infectivity titer of pseudovirus variants in HEK293T-hACE2 and HEK293T-hACE2-TMPRSS2 cells in the presence of batimastat at 48 h post infection. The error bars show mean values with SEM. Experiment was repeated three times. For the statistical significance, two-way ANOVA test was performed with multiple comparisons. D, E Immunofluorescence images for batimastat treatment were quantified by selecting five random GFP + areas in Image J software. Graph was prepared and analyzed in GraphPad prism 9. The error bars show mean values with SEM. For the statistical significance, two-way ANOVA test was performed with multiple comparisons where untreated samples (Fig. 4D) were compared with batimastat treated samples in the presence or absence of TMPRSS2. F, G Immunofluorescence assay for fusion inhibition in spike variants of SARS-CoV-2 in BHK-21 cells in the presence of cytochalasin D (1 µM) at 24 h post transfection. Cytochalasin D was added to the spike-hACE2 and spike-hACE2-TMPRSS2 expressing cells. After transfecting the cells with respective plasmids, cells were treated with compound post 2 h of transfection. Later, cells were fixed post 24-h and stained with anti-spike mouse polyclonal sera (1:500) and AlexaFluor-488-labeled anti-mouse antibody (green) (1:1000). Experiment was repeated three times. The images were taken with an Olympus fluorescence microscope. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue); scale bar: 50 μm and magnification 20x. H, I Inhibition in infectivity titer of pseudovirus variants in HEK293T-hACE2 and HEK293T-hACE2-TMPRSS2 cells in the presence of cytochalasin D at 48 h post infection. The error bars show mean values with SEM. Experiment was repeated three times. For the statistical significance, two-way ANOVA test was performed with multiple comparisons. I Immunofluorescence images for cytochalasin D treatment were quantified by selecting five random GFP + areas in Image J software. Graph was prepared and analyzed in GraphPad prism 9. The error bars show mean values with SEM. For the statistical significance, two-way ANOVA test was performed with multiple comparisons where untreated samples (Fig. 4D) were compared with cytochalasin D treated samples in the presence or absence of TMPRSS2. J. Inhibition in infectivity titer of pseudovirus variants in HEK293T-hACE2 cells in the presence of cytochalasin D at 48 h post infection. Experiment was repeated three times. For the statistical significance, two-way ANOVA test was performed with multiple comparisons where untreated samples were compared with cytochalasin D treated samples. p < 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 were considered significant and p > 0.05 was considered nonsignificant (ns)
Conclusion
Fusion inhibitors are an important class of anti-viral drugs that prevent virus entry, thus blocking virus replication. It is important to understand the virus biology and the fusion event for the development of promising fusion inhibitors. The cleavage site in the S protein facilitates virus fusion to the host cell, which is an important step in virus entry and is the major determinant of viral infectivity and pathogenesis. Reports have shown that mutations in the cleavage site sequence, mainly in the polybasic arginine residues, significantly modulate viral entry, thus imparting major effects on virus infectivity and cell-to-cell spread (Wrobel et al. 2020; Vankadari 2020; Chu et al. 2021). Furin is one of the cellular endoproteases that mainly cleaves the paired basic residues and is abundantly present in the respiratory tract. Unlike SARS-CoV-1, the cleavage site of the SARS-CoV-2 has evolved and contains the multi-basic arginine residue “RRAR”, which might result in the efficient cleavage of the S glycoprotein, thus leading to enhanced infectivity and pathogenesis (Mykytyn et al. 2021). Interestingly, flanking the S1/S2 cleavage site sequence, conserved proline and serine residues are present and in the S2´ region, the serine residue is conserved in all the coronaviruses (Fig. 1A). However, the role of these residues in virus fusion and infectivity is not well understood. In our study, substitution of these residues with alanine resulted in reduced expression of spike protein and the binding to soluble-hACE2 (Fig. 2A–C). The efficient spike expression on the virus surface and proper binding to hACE2 host receptor facilitate virus entry, and subsequently determines the virus infectivity. With the emergence of SARS-CoV-2 VoC, it was observed that the mutation in the spike protein modulated the virus entry due to differential spike expression and binding to hACE2 receptor (Alaofi and Shahid 2021; Ghimire et al. 2022). Additionally, alanine substitution at the position of serine in the S2´S-A mutant resulted in severe loss of cleavability into the S2 fragment (Fig. 2D). The results strongly suggest that the host cell proteases need serine residue at the S2´ site for catalytic activity. Additionally, molecular dynamics simulation studies of ancestral Wuhan-1 and the serine mutants’ at S1/S2 and S2´ site show that compared to the Wuhan-1, the mutants are less stable and more flexible, which might be resulting in the impaired interaction of host proteases with the virus cleavage site. M. Pooja et.al have shown by the bioinformatics and molecular simulation studies that TMPRSS2 (serine protease) form the catalytic triad consisting of Serine-441, Histidine-296 and Aspartic acid-345 in the TMPRSS2 which is the active binding site of TMPRSS2, and this interacts with S1 domain (Pooja et al. 2021). In another study by M. Hussain et.al (bioRxiv, 22 Apr 2020 https://doi.org/10.1101/2020.04.21.052639), have shown by the molecular docking simulation studies, that the TMPRSS2 region key functional residues form intermolecular hydrogen bonds with the spike C-terminal cleavage site (serine 816). We suggest, mutating the serine to alanine at S2´ site, induces a conformational change which might be disabling the proper interaction of TMPRSS2 active catalytic site with S2´ site, thus impairing the cleavability. Further structural analysis might shed more information on the events occurring at the RBD S2´ cleavage site and TMPRSS2 interaction sites.
In our study we could not generate the proline (812 aa) at the S2´ cleavage site mutants, which might have provided additional information on how the conserved proline in the S2´ site is affecting the virus biological functions. The incapability of the S2´S-A mutant to cleave the spike protein efficiently and low expression on the cell surface resulted in less infectivity of this mutant in both HEK293T-hACE2 and HEK293T-hACE2-TMPRSS2 overexpressed cells (Fig. 3A, B). One of the characteristic features of the SARS-CoV-2 spike protein is the formation of syncytium by cell-to-cell fusion, in which infected cells expressing the spike proteins fused to form multi-nucleated cells, a phenomenon also called virological synapses (Lin et al. 2021; Zheng et al. 2021). The fusogenic property facilitates the spread of the virus from cell to cell, and hence determines severe viral pathogenesis and transmission (Arora et al. 2021; Zeng et al. 2022). In our study, we further observed that fusion and syncytial formation are increased when the ancestral Wuhan-1 spike protein is expressed in the presence of ACE2 and TMPRSS2 (Figs. 3D–G and S1), and alanine substitution in S1/S2 region cleavage mutants S1S-A and S1P-A did not have a major impact on cell-to-cell fusion. Also, the fusogenicity was found to be less as compared to the Wuhan-1 spike protein. However, corroborated with our earlier findings, in the S2´S-A mutant, there was a complete loss of fusion and syncytial formation both in spike expressed with hACE2 or spike expressed with hACE2 and TMPRSS2 together. The coronaviruses’ spike protein is also activated by trypsin, an exogenous protease that could facilitate viral entry through the non-endosomal route (Matsuyama et al. 2005; Belouzard et al. 2009; Kim et al. 2022). Replacing conserved proline and serine residues in the SARS-CoV-2 spike protein cleavage site resulted in the ineffectiveness of trypsin in activating cleavage site mutants (Fig. S3). These results further explain the loss of infectivity by cleavage site mutants. Interestingly, the cathepsin inhibitor, E64d was found to be acting better on the cleavage site mutants when the hACE2 and spike was co-expressed before adding the E64d, where as in the virus infectivity assay significant difference was not shown in S1P-A and S1S-A mutants (Fig. 4A). The results suggest that the serine mutations might have pronounced effect in the spike conformation, which is further modulating the pre- and post-fusion conformation. Though, a more pronounced effect was seen in S1P-A and S1S-A mutants in the presence of the TMPRSS2 inhibitor, camostat mesylate (Fig. 5). The S2´S-A cleavage site mutant did not respond to the TMPRSS2 protease inhibitor, which was suggestive of the fact that loss of serine in the S2´ cleavage site inhibits priming by TMPRSS2 protease. Further assessment of the cleavage site mutants’ response to the metalloprotease inhibitor batimastat showed a significant reduction in viral infectivity in S1S-A and S1P-A mutants but not in S2´S-A mutant (Fig. 6).
Altogether, these findings demonstrate the importance of the conserved amino acids: proline and serine, in modulating SARS-CoV-2 spike cleavage site functionality, which in addition is regulating the virus entry and fusion activities. Singh R. et. al have identified potential natural bioactive compounds by bioinformatic and molecular docking analysis as promising fusion inhibitors (Singh et al. 2021). Understanding the fusion process and role of the cleavage site will further facilitate the identification and development of novel cleavage site directed fusion inhibitors. Vankadari N et.al have shown the structural complex of TMPRSS2 with the spike glycoprotein, which might facilitate discovery of potential therapeutic interventions targeting the S2´ site (Vankadari et al. 2022). Currently, serine protease inhibitors such as Camostast and Nafmostast have shown effectively blocking TMPRSS2, thus are effective against the SARS-CoV-2. More precise targeting of the TMPRSS2 key residue and S1 domain serine 816 interacting site might offer attractive perspective in the development of novel antiviral drugs. V. Sethu et. al have shown potential targets against TMPRSS2 and furin cleavage site by virtual screening of many phytochemicals (Vardhan and Sahoo 2022). Both S1/S2 and S2´ cleavage sites of spike protein have distinctive roles in the cleavage process and our study demonstrate novel inhibitors targeting S2´ site might have a better impact in restricting virus entry and infectivity. The presence of polybasic arginine residues in the SARS-CoV-2 cleavage site is adventitious to the virus as it is activated by furin-like proteases which are abundantly present in the respiratory tract and other organs. With the emergence of novel variants of concern (VoC), the conserved proline was naturally replaced by histidine, as shown in the Alpha variant cleavage site, HRRAR ~ S (Lubinski et al. 2022a), and in more virulent VoC, Delta virus, proline was replaced by arginine (RRRAR ~ S) (Mlcochova et al. 2021; Lubinski et al. 2022b). Compared to the Alpha virus, the replacement of proline with arginine at the spike cleavage site in the Delta virus resulted in high virulence and pathogenesis of the virus. Recently, the circulating viruses are Omicron VoC, which harbors the same cleavage site as Alpha VoC, in which proline is replaced by histidine (P681H) (Lubinski et al. 2022c). Omicron VoC viruses are less virulent than Delta viruses. Our study showed similar results, replacing proline with neutral alanine amino acid could decrease virus infectivity and fusogenicity. Notably, the conserved serine in S1/S2 and S2´ sites were not mutated, and has remained conserved till now in the SARS-CoV-2 circulating strains. In our study, despite the presence of the S1/S2 polybasic sequence, a single mutation of the serine at the S2´ site completely abrogated the cleavage and fusion properties and thus resulted in diminished infectivity, which strongly suggests the importance of serine in the S2´ region for virus biological functions. It might be possible that the S2´ site is accessible to other proteases apart from TMPRSS2 due to the presence of two basic residues, lysine (K) and arginine (R); although more experimental evidence is needed to prove the hypothesis. We speculate that novel interventions targeting the conserved serine at the S2´ cleavage site will serve as potential candidates to reduce SARS-CoV-2-induced disease severity and as a checkpoint for novel emerging variants.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank Prof Pramod Kumar Garg, Executive Director, THSTI for full support and valuable input and guidance. We thank Prof. S Pöhlmann, Infection Biology Unit, Göttingen, Germany for providing ACE2-Fc plasmids as a kind gift. We thank Dr. B Graham (VRC/NIAID/NIH) for providing us with the spike construct (SARS-2-CoV S 2P). The following reagent was obtained through BEI Resources, NIAID, NIH: Human Embryonic Kidney Cells (HEK293T) Expressing Human Angiotensin-Converting Enzyme 2, HEK293T-hACE2 Cell Line, NR-52511.The TMPRSS2 plasmid was a gift from Roger Reeves (Addgene plasmid # 53887).
Author contributions
SS and RK conceived the project. RK, BL, GK, VM and A.G conducted most of the experiments. DS and SA performed the molecular dynamic simulation study. SS and RK analyzed the data. SS wrote the original manuscript; RK and BL edited the manuscript.
Funding
This work was supported by the Department of Biotechnology (DBT), Govt. of India and THSTI core funding.
Data availability
The data supporting the findings of this study are available from the corresponding author upon request.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Adedeji AO, Severson W, Jonsson C, et al. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J Virol. 2013;87:8017–8028. doi: 10.1128/JVI.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaofi AL, Shahid M. Mutations of SARS-CoV-2 RBD may alter its molecular structure to improve its infection efficiency. Biomolecules. 2021 doi: 10.3390/biom11091273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai A. Viral surface geometry shapes influenza and coronavirus spike evolution through antibody pressure. PLoS Comput Biol. 2021;17:e1009664. doi: 10.1371/journal.pcbi.1009664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P, Sidarovich A, Krüger N, et al. B.1.617.2 enters and fuses lung cells with increased efficiency and evades antibodies induced by infection and vaccination. Cell Rep. 2021;37:109825. doi: 10.1016/j.celrep.2021.109825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestle D, Heindl MR, Limburg H, et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci Alliance. 2020 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch BJ, van der Zee R, de Haan CAM, Rottier PJM. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–8811. doi: 10.1128/jvi.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Du R, Galvan Achi JM, et al. SARS-CoV-2 cell entry and targeted antiviral development. Acta Pharm Sin B. 2021;11:3879–3888. doi: 10.1016/j.apsb.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Hu B, Huang X, et al. Host and viral determinants for efficient SARS-CoV-2 infection of the human lung. Nat Commun. 2021;12:134. doi: 10.1038/s41467-020-20457-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essalmani R, Jain J, Susan-Resiga D, et al. Distinctive roles of furin and TMPRSS2 in SARS-CoV-2 infectivity. J Virol. 2022;96:e0012822. doi: 10.1128/jvi.00128-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghimire D, Han Y, Lu M. Structural plasticity and immune evasion of SARS-CoV-2 spike variants. Viruses. 2022 doi: 10.3390/v14061255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatmal MM, Alshaer W, Al-Hatamleh MAI, et al. Comprehensive structural and molecular comparison of spike proteins of SARS-CoV-2, SARS-CoV and MERS-CoV, and their interactions with ACE2. Cells. 2020 doi: 10.3390/cells9122638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78:779–784.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörnich BF, Großkopf AK, Schlagowski S, et al. SARS-CoV-2 and SARS-CoV spike-mediated cell-cell fusion differ in their requirements for receptor expression and proteolytic activation. J Virol. 2021 doi: 10.1128/JVI.00002-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yang C, Xu X-F, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri R, Siddqui G, Sadhu S, et al. Intrinsic D614G and P681R/H mutations in SARS-CoV-2 VoCs Alpha, Delta, Omicron and viruses with D614G plus key signature mutations in spike protein alters fusogenicity and infectivity. Med Microbiol Immunol. 2023;212:103–122. doi: 10.1007/s00430-022-00760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Jang G, Lee D, et al. Trypsin enhances SARS-CoV-2 infection by facilitating viral entry. Arch Virol. 2022;167:441–458. doi: 10.1007/s00705-021-05343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Mittal L, Srivastava M, et al. Conformational characterization of the co-activator binding site revealed the mechanism to achieve the bioactive state of FXR. Front Mol Biosci. 2021;8:658312. doi: 10.3389/fmolb.2021.658312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Mittal L, Srivastava M, et al. Deciphering the structural determinants critical in attaining the FXR partial agonism. J Phys Chem B. 2023;127:465–485. doi: 10.1021/acs.jpcb.2c06325. [DOI] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Li Q, Wang Y, Shi Y. Syncytia formation during SARS-CoV-2 lung infection: a disastrous unity to eliminate lymphocytes. Cell Death Differ. 2021;28:2019–2021. doi: 10.1038/s41418-021-00795-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski B, Fernandes MHv, Frazier L, et al. Functional evaluation of the P681H mutation on the proteolytic activation of the SARS-CoV-2 variant B.1.1.7 (Alpha) spike. iScience. 2022;25:103589. doi: 10.1016/j.isci.2021.103589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski B, Frazier LE, Phan MVT, et al. Spike protein cleavage-activation mediated by the SARS-CoV-2 P681R mutation: a case-study from its first appearance in variant of interest (VOI) A.23.1 identified in Uganda. bioRxiv. 2022 doi: 10.1101/2021.06.30.450632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinski B, Jaimes JA, Whittaker GR. Intrinsic furin-mediated cleavability of the spike S1/S2 site from SARS-CoV-2 variant B.1.1.529 (Omicron) bioRxiv. 2022 doi: 10.1101/2022.04.20.488969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado LL, Bertelli AM, Kamenetzky L. Molecular features similarities between SARS-CoV-2, SARS, MERS and key human genes could favour the viral infections and trigger collateral effects. Sci Rep. 2021;11:4108. doi: 10.1038/s41598-021-83595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Ujike M, Morikawa S, et al. Protease-mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc Natl Acad Sci U S A. 2005;102:12543–12547. doi: 10.1073/pnas.0503203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal L, Tonk RK, Awasthi A, Asthana S. Targeting cryptic-orthosteric site of PD-L1 for inhibitor identification using structure-guided approach. Arch Biochem Biophys. 2021;713:109059. doi: 10.1016/j.abb.2021.109059. [DOI] [PubMed] [Google Scholar]
- Mlcochova P, Kemp SA, Dhar MS, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn AZ, Breugem TI, Riesebosch S, et al. SARS-CoV-2 entry into human airway organoids is serine protease-mediated and facilitated by the multibasic cleavage site. Elife. 2021 doi: 10.7554/eLife.64508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou T, Mou H, Zhang L, et al. Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. PLoS Pathog. 2021;17:e1009212. doi: 10.1371/journal.ppat.1009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar S, Kumari A, Kumar H, et al. Structure-based virtual screening, molecular dynamics simulation and in vitro evaluation to identify inhibitors against NAMPT. J Biomol Struct Dyn. 2022;40:10332–10344. doi: 10.1080/07391102.2021.1943526. [DOI] [PubMed] [Google Scholar]
- Peacock TP, Goldhill DH, Zhou J, et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat Microbiol. 2021;6:899–909. doi: 10.1038/s41564-021-00908-w. [DOI] [PubMed] [Google Scholar]
- Pooja M, Reddy GJ, Hema K, et al. Unravelling high-affinity binding compounds towards transmembrane protease serine 2 enzyme in treating SARS-CoV-2 infection using molecular modelling and docking studies. Eur J Pharmacol. 2021;890:173688. doi: 10.1016/j.ejphar.2020.173688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey FA, Lok S-M. Common features of enveloped viruses and implications for immunogen design for next-generation vaccines. Cell. 2018;172:1319–1334. doi: 10.1016/j.cell.2018.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samal S, Das S, Boliar S, et al. Cell surface ectodomain integrity of a subset of functional HIV-1 envelopes is dependent on a conserved hydrophilic domain containing region in their C-terminal tail. Retrovirology. 2018;15:50. doi: 10.1186/s12977-018-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanda M, Morrison L, Goldman R. N- and O-glycosylation of the SARS-CoV-2 spike protein. Anal Chem. 2021;93:2003–2009. doi: 10.1021/acs.analchem.0c03173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajahan A, Supekar NT, Gleinich AS, Azadi P. Deducing the N- and O-glycosylation profile of the spike protein of novel coronavirus SARS-CoV-2. Glycobiology. 2020;30:981–988. doi: 10.1093/glycob/cwaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Bhardwaj VK, Sharma J, et al. Identification of potential plant bioactive as SARS-CoV-2 Spike protein and human ACE2 fusion inhibitors. Comput Biol Med. 2021;136:104631. doi: 10.1016/j.compbiomed.2021.104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Awasthi A, Asthana S. Crystallographic landscape provides molecular insights into the modes of action of diverse ROR-γt modulators. Drug Discov Today. 2022;27:652–663. doi: 10.1016/j.drudis.2021.11.022. [DOI] [PubMed] [Google Scholar]
- Thomas P, Smart TG. HEK293 cell line: a vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Vankadari N. Structure of furin protease binding to SARS-CoV-2 spike glycoprotein and implications for potential targets and virulence. J Phys Chem Lett. 2020;11:6655–6663. doi: 10.1021/acs.jpclett.0c01698. [DOI] [PubMed] [Google Scholar]
- Vankadari N, Ketavarapu V, Mitnala S, et al. Structure of human TMPRSS2 in complex with SARS-CoV-2 spike glycoprotein and implications for potential therapeutics. J Phys Chem Lett. 2022;13:5324–5333. doi: 10.1021/acs.jpclett.2c00967. [DOI] [PubMed] [Google Scholar]
- Vardhan S, Sahoo SK. Virtual screening by targeting proteolytic sites of furin and TMPRSS2 to propose potential compounds obstructing the entry of SARS-CoV-2 virus into human host cells. J Tradit Complement Med. 2022;12:6–15. doi: 10.1016/j.jtcme.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls AC, Tortorici MA, Snijder J, et al. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc Natl Acad Sci. 2017;114:11157–11162. doi: 10.1073/pnas.1708727114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls AC, Xiong X, Park Y-J, et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176:1026–1039.e15. doi: 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W, Dessen A, Calder LJ, et al. Structural basis for membrane fusion by enveloped viruses. Mol Membr Biol. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]
- Wrobel AG, Benton DJ, Xu P, et al. SARS-CoV-2 and bat RaTG13 spike glycoprotein structures inform on virus evolution and furin-cleavage effects. Nat Struct Mol Biol. 2020;27:763–767. doi: 10.1038/s41594-020-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Zhang X, Leng Yap Y. Structural similarity between HIV-1 gp41 and SARS-CoV S2 proteins suggests an analogous membrane fusion mechanism. THEOCHEM. 2004;677:73–76. doi: 10.1016/j.theochem.2004.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung ML, Teng JLL, Jia L, et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell. 2021;184:2212–2228.e12. doi: 10.1016/j.cell.2021.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Evans JP, King T, et al. SARS-CoV-2 spreads through cell-to-cell transmission. Proc Natl Acad Sci U S A. 2022 doi: 10.1073/pnas.2111400119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Zhou L-L, Su Y, Sun Q. Cell fusion in the pathogenesis of COVID-19. Mil Med Res. 2021;8:68. doi: 10.1186/s40779-021-00348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.