Abstract
We previously reported on the efficacy of the enzyme-linked immunoglobulin M capture immune complex (IC) biotinylated antigen assay (EMIBA) for the seroconfirmation of early Lyme disease and active infection with Borrelia burgdorferi. In earlier work we identified non-cross-reacting epitopes of a number of B. burgdorferi proteins, including flagellin. We now report on an improvement in the performance of EMIBA with the addition of a biotinylated form of a synthetic non-cross-reacting immunodominant flagellin peptide to the biotinylated B. burgdorferi B31 sonicate antigen source with the avidin-biotinylated peroxidase complex detection system used in our recently developed indirect IgM-capture immune complex-based assay (EMIBA). As in our previous studies, the enzyme-linked immunosorbent assay (ELISA) reactivities of antibodies liberated from circulating ICs (by EMIBA) were compared with those of antibodies in unprocessed serum (antibodies found free in the serum, thus as an IgM-capture ELISA, but not EMIBA, because the antibodies were not liberated from ICs), the sample usually used in standard ELISAs and Western blot assays. The addition of the flagellin epitope enhanced the ELISA signal obtained with untreated sera from many Lyme disease patients but not from healthy controls. In tests with both free antibodies and ICs, with or without the addition of the flagellin epitope to the sonicate, we found the most advantageous combination was IC as the source of antibodies and sonicate plus the flagellin epitope as the antigen. In a blinded study of sera obtained from patients with early and later-phase Lyme disease, EMIBA with the enhanced antigenic preparation compared favorably with other serologic assays, especially for the confirmation of early disease.
Lyme disease (LD) is a multisystem inflammatory disorder (49) due to infection with the spirochete Borrelia burgdorferi sensu lato, which is transmitted by ticks in the Ixodes ricinus complex (5, 50). Soon after the tick bite the pathognomonic skin lesion erythema migrans (EM) occurs in 50 to 75% of patients (49) (and perhaps in as many as 90% of patients [20]), and this is often accompanied by nonspecific constitutional symptoms similar to those of a viral syndrome. If EM is present, the diagnosis of LD can be made without serologic confirmation. However, in the absence of EM (or if the lesion is not identified properly), the symptoms of early LD are nonspecific and objective signs of disease may appear only later in the infection. Thus, in the absence of EM the early and prompt diagnosis of early LD may be difficult to make on purely clinical grounds. Due to the great public concern over LD, the number of blood tests performed exceeds the number of confirmed cases of LD by a factor of 100 (4). False-positive enzyme-linked immunosorbent assay (ELISA) results are common, often resulting in an incorrect diagnosis. It may take 6 to 8 weeks for seroconversion to occur, so serologic confirmation of new infection may be delayed (49). The current recommendation is to confirm all positive or equivocal ELISA results by immunoblotting (8a) due to the high frequency of false-positive ELISA results (i.e., a positive ELISA result for a patient without LD). In our experience misinterpretation of the results of Western blot analysis by practioners contributes to the overdiagnosis of LD (45a).
Since early antibiotic treatment of LD is more effective in curing the infection and preventing progression to later disease, our challenge was to devise a serologic assay capable of detecting antibodies at the earliest time after infection while severely limiting false-positive responses. We have focused on detecting immunoglobulin M (IgM) antibodies, which occur during the initial humoral immune response, and in order to optimize the assay, we decided to incorporate the best of a number of different assays. The IgM capture format was selected in order to eliminate potential false-positive results from rheumatoid factor (9, 29, 52) and competition that might otherwise occur in a direct ELISA due to the relatively greater serum IgG concentration (9, 52). Since previous studies have shown that circulating specific anti-B. burgdorferi IgM antibodies are frequently sequestered within antigen-antibody immune complexes (ICs) (42–44), especially at an early stage of infection, we chose to compare two sources of serum antibodies: IgM free in serum versus IgM bound in ICs. In order to detect low levels of IgM we had previously chosen biotinylated antigen, similar to Hansen et al. (24), and an enzyme-avidin complex (8) to amplify the signal while preserving a low background (7). This combination of techniques, termed the enzyme-linked IgM capture IC biotinylated antigen assay (EMIBA), was used to test a bank of serum samples obtained from the Lyme Disease Center at the Robert Wood Johnson Medical School. It was found to have better sensitivity (98%; 95% confidence interval [CI], 90 to 100%) than IgM ELISA (66%; 95% CI, 53 to 77%) or IgG ELISA (58%; 95% CI, 45 to 70%) and IgM immunoblotting (58%; 95% CI, 45 to 70%) or IgG immunoblotting ELISA (44%; 95% CI, 32 to 57%) and better specificity (96%; 95% CI, 87 to 99%) than IgM ELISA (76%; 95% CI, 64 to 86%) or IgG ELISA (87%; 95% CI, 76 to 93%) and IgM immunoblotting (84%; 95% CI, 72 to 91%) or IgG immunoblotting (93%; 95% CI, 83 to 97%) (7).
Many ELISAs use a crude sonicate of B. burgdorferi to interact with the antibodies in a patient’s serum. One cause for false-positive ELISA results is that serum antibodies to proteins found on organisms other than B. burgdorferi may cross-react with B. burgdorferi antigens (11, 32). Our laboratory has previously identified linear epitopes of B. burgdorferi proteins uniquely recognized by sera from patients with LD (53), including one immunodominant flagellar peptide (19, 41, 53, 54). The present study demonstrates the signal-enhancing property of this flagellin epitope antigen in EMIBA and provides support for our goal of replacing crude sonicates or whole (even recombinant) proteins with a mixture of defined peptide epitopes as antigens in a serodiagnostic assay.
MATERIALS AND METHODS
Growth and preparation of B. burgdorferi sonicate.
High-passage B. burgdorferi B31 (22) from liquid nitrogen storage was grown at 32°C in BSK-H medium made and filtered in our laboratory or purchased from Sigma (St. Louis, Mo.) (3, 39) and supplemented with 6% normal rabbit serum, grown in T flasks (Corning, Corning, N.Y.), and harvested after 5 days (late logarithmic phase). Typically, 250 ml (usual spirochetal cell count of approximately 7 × 107 cells/ml) was harvested by centrifugation at 9,000 × g for 15 min. The cell pellet was washed three times with cold phosphate-buffered saline (PBS; pH 7.2). The final pellet was either stored at −70°C for later use or resuspended in 2 ml of PBS and sonicated (medium setting; Braun-Sonic 2000) for four pulses of 30 s each with 1 min between pulses. The protein content of the sonicate was about 3 mg/ml, as determined by the binchoninic acid (BCA) protein assay (Pierce, Rockford, Ill.).
Flagellin peptide synthesis and conjugation to albumin.
Synthesis and purification (53) of the non-cross-reacting flagellin epitope sequence VQEGVQQEGAQQP (positions F211 to F223, where F denotes the amino acid residue in flagellin) and conjugation of the peptide to albumin have been described previously (54). For conjugation purposes, the epitope was synthesized with an additional cysteine residue at the carboxy terminus, followed by two beta-alanine residues as a spacer and the epitope sequence. The heterobifunctional reagent m-maleimidobenzoyl-N-hydroxysuccinimide ester (Pierce) was used to cross-link the cysteine thiol group in the peptide to the side chain amino group in the lysine residues of bovine serum albumin (BSA). Molar coupling ratios of between 13 and 22 (peptide:protein) were achieved, as determined by amino acid analysis (54).
Biotinylation of antigens.
Different long-arm biotin hydroxysuccinimide esters, including biotinamidocaproate N-hydroxysuccinimide (NHS) ester (Sigma) and NHS–long-chain (LC) biotin II (Pierce), were equally effective. One milliliter of a 3-mg/ml protein sonicate or epitope-BSA solution was adjusted to pH 9 by adding 0.1 volume of 0.5 M carbonate-bicarbonate buffer (pH 9) immediately before biotinylation. To 1 ml of the pH-adjusted protein sonicate, 7 μl of a 50-mg/ml biotin solution in diethylformamide was added in a glass tube (16 by 100mm), covered, and slowly rotated for 1 to 2 h at room temperature, pipetting up and down every 15 min. The reaction was stopped by adding 0.1 volume of 1 M Tris-HCl (pH 7.5). At this point, the protease inhibitors phenylmethylsulfonyl fluoride (0.5 mM), pepstatin A (0.7 μg/ml), and leupeptin (1 μg/ml) were added to the reacted sonicate. Excess biotinylating reagent was removed from both reactions by dialysis against three 2-liter changes of PBS containing 0.02% sodium azide in the cold with a Slide-A-Lyzer (Pierce) device with a 10,000-Da cutoff. The resulting biotinylated product from the sonicate was assayed for protein by the BCA assay (Pierce). Yields were typically at least 75%. Each biotinylated reagent was diluted in buffer to a final concentration of 6 to 12 μg/ml. The biotinylated product from the sonicate retained full activity in the ELISA for at least 6 months when it was stored at 4°C (7).
Patient study population.
The sera used were well-defined samples from Marshfield Laboratories (36), the University of Minnesota and the Centers for Disease Control and Prevention (CDC) (27a), and the Robert Wood Johnson Lyme Disease Center clinic. Sera with the prefix MC (Marshfield Clinic) were obtained from patients with culture-proven EM and were collected on the same day that lesions were biopsied for culture. The skin biopsy procedure and culture methods have been described previously (34, 36). Samples designated 1 (EM and arthralgias), 2 (Lyme carditis), 3 (EM), 4 (Lyme arthritis), 5 (EM), and 6 (viral syndrome following tick bite) were from patients evaluated at the Lyme Disease Center at the Robert Wood Johnson Medical School and were adjudged to have LD by one of the authors (L.H.S.). All were IgM ELISA positive and fulfilled CDC IgM immunoblotting criteria (16) on evaluation with the MarDx (Carlsbad, Calif.) isotype-specific ELISA and immunoblotting kits (following the manufacturer’s instructions) at the Lyme Disease Diagnostic Laboratory of the University Diagnostic Laboratories of Robert Wood Johnson Medical School. Samples 2, 4, and 5 were also IgG ELISA and IgG immunoblotting positive by the CDC criteria, and sample 3 was IgG ELISA positive but immunoblotting negative (IgG reactivity with 58-, 45-, 41-, and 39-kDa bands). The patient from whom sample 7 was obtained was not thought to have evidence of active Lyme disease solely on the basis of a clinical evaluation by one of the authors (L.H.S.) and was IgG and IgM negative by isotype-specific ELISA and immunoblotting.
Sera designated MC were from patients with EM either single or multiple lesions seen at the Marshfield Clinic. Sera designated MCC were negative control samples obtained at the Marshfield Clinic from individuals without a history of current or past infection with B. burgdorferi. Other negative control samples were obtained at the Lyme Disease Center from patients in whom LD was not suspected on the basis of clinical and laboratory evaluation by one of the authors (L.H.S.). Serum samples 807 (EM), 930 (EM), 948 (negative control), 949 (negative control), and 952 (Lyme arthritis) were obtained from CDC as part of a panel of samples provided and used in previous studies (7); the histories of the patients from whom these samples were obtained were unblinded only after completion of the studies described here. Samples 948 and 949 were negative by isotype-specific ELISA and immunoblotting. All the other samples from CDC used in this study were IgM ELISA and immunoblotting positive; serum sample 807 was also IgG ELISA and immunoblotting positive, and sample 930 fulfilled the IgG immunoblotting criteria, although it was negative by ELISA.
Serum collection.
Blood was drawn, clotted at room temperature, and centrifuged in a Sorvall RC5C, HS-4, rotor at 769 × g for 10 min. Clear serum was drawn off with a pipette. After analysis the serum was stored at −70°C until it was needed for further testing. Freezing-thawing slightly decreased the reactivities of some samples, but it did not reduce the results for any positive samples into the negative range (7).
IC purification.
The polyethylene glycol (PEG) method for IC precipitation was used (7). Briefly, 100 μl of serum was precipitated with an equal volume of a 7% PEG (average molecular weight, 8,000; Sigma) solution in 0.1 M sodium borate–0.075 M sodium chloride buffer (pH 8.4) in the cold. The microcentrifuge tube was vortexed, kept at 4°C for 2 to 16 h, and then centrifuged at 8,320 × g for 15 min in the cold. The supernatant was carefully removed with a Pasteur pipette, and the pellet was resuspended and washed twice with 200 μl of 3.5% PEG in 0.1 M sodium borate–0.075 M sodium chloride buffer (pH 8.4). The pellet was resuspended in its original volume with 0.1 M sodium borate buffer (pH 10.2) and was kept at 4°C until it was used.
M-capture ELISA.
The M-capture ELISA was performed as described previously (7). Briefly, Immulon 4 microtiter plates (Dynatek, Chantilly, Va.) were coated with 100 μl of 10 μg of affinity-purified anti-human IgM (mu-chain specific) antibody (Rockland, Gilbertsville, Pa.; Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) per ml at 100 μl/well in 0.04 M carbonate-bicarbonate buffer (pH 9.6), slowly rotated for 2 h at room temperature, and kept at 4°C overnight. The plates were washed three times in a plate washer (ELP35; Biotek, Winooski, Vt.) with 10 mM PBS (the NaCl concentration was 0.15 M) containing 0.1% BSA (Sigma) (PBS-B), each well was blocked with 300 μl of PBS-B containing 5% nonfat dry milk (Bio-Rad) with 0.02% sodium azide for 1 to 2 h at 37°C, and the plates were washed twice with PBS-B. Serum samples (100 μl) were diluted in blocking buffer (containing azide) (1:10 for IC and 1:100 for free antibody), and added in duplicate. The plate was slowly rotated overnight at room temperature to optimize IgM capture.
EMIBA was developed so the same positive controls in each run gave an optical density (OD) of approximately 1.0, negative controls gave an OD of less than 0.1, and bare well controls gave an OD of 0.05 or less when read at dual wavelengths (450 and 630 nm; signal at 450 nm, with the background at 630 nm subtracted) on an ELISA plate reader (Bio-Tek). The net OD at 450 nm (OD450) was calculated as follows: net OD450 = (OD450 of sample − OD630 of sample) − (OD450 of blank well − OD630 of blank well). The optimal amounts of antigenic preparations were determined for each batch made (4 to 12 μg/ml), and each batch was standardized with the preceding preparation by using the same positive and negative serum panels. The optimal dilution for dissociated ICs was 1/10, and that for serum was 1/100; there was no increase in reactivity when sera were run at a dilution of 1/10.
The reagent (100 μl), diluted in blocking buffer without sodium azide, was added to each well, and the plate was rotated at 300 rpm on a Titer Plate Shaker (Lab-Line, Melrose Park, Ill.) at room temperature for 1 h. During this time, the avidin-biotinylated peroxidase complex (ABC; Vector Laboratories, Burlingame, Calif.) was formed by adding 1 drop (50 μl) of reagent A (avidin DH) and 1 drop of reagent B (biotinylated peroxidase) to 5 ml of PBS-B (the NaCl concentration was increased to 0.5 M) containing 0.1% Tween 20 (PBS-BT), and the complex was vortexed and kept at room temperature for at least 30 min before use. After complex formation, 7 ml of PBS-BT was added to the ABC reagent as described above. The plate was washed four times with PBS-B, and 100 μl of the diluted ABC reagent was added to each well. The plate was placed on an orbital shaker and shaken at 300 to 400 rpm for 30 min at room temperature. The plate was washed four times with PBS-B (on the Bio-Tek plate washer) followed by two more manual washes with PBS-T (no BSA) for 5 min each time with a multichannel pipette (Brinkmann Transferpette) on a rotator. During the last wash, the two-component 3,3′,5,5′-tetramethylbenzidine substrate solution (Kirkegaard & Perry Laboratories, Inc.) was prepared at room temperature. Substrate (100 μl/well) was added with a repeater pipette (Eppendorf Plus/8), the plate was rotated for 10 min, and the reaction was stopped by adding 1 M phosphoric acid (100 μl/well). The plate was then rotated for 2 more min to homogenize the yellow color. The wells in which a reaction occurred were then read on an ELISA plate reader (Biotek) set for dual wavelengths (450 and 630 nm). Blank and antigen control wells always had readings of less than 0.05 OD units. Sera from 10 subjects in New Jersey who did not have a clinical diagnosis of LD and who were seronegative by both Western blotting and a commercial ELISA (MarDx) were used as negative control samples on each experimental plate; the number of negative controls was in keeping with previous studies (42). The index value was calculated as follows: index value = (mean net OD450 of sample)/(mean net OD450 plus 3 standard deviations for 10 negative controls). An index value above 1.0 was considered positive, whereas an index value of 0.8 to 0.99 (approximately 2 to 3 standard deviations above the mean) was considered equivocal and an index value below 0.8 was considered negative. Each sample was tested in duplicate, with the precision typically being within a 3% average deviation.
Immunoserologic methods.
The procedures for IgM indirect fluorescent-antibody assay (IFA) and IgM immunoblotting have been described previously (35). The polyvalent enzyme immunoassay (EIA) and a recombinant p39-based IgM EIA (dot blot) were supplied in kit form (General Biometrics, San Diego, Calif.) and were used according to the manufacturer’s instructions.
RESULTS
Influence of the addition of the non-cross-reacting flagellin epitope on serologic reactivity with the standard antigen preparation, the borrelial sonicate.
We previously reported on EMIBA, which uses an IgM-capture format with IgM derived from purified, disrupted ICs with a biotinylated sonicate of B. burgdorferi as the antigen and ABC as the detection system (7). The addition of a biotinylated, albumin-conjugated immunodominant flagellin epitope to the standard antigen preparation was studied. Initial studies used serum rather than IC-derived antibodies.
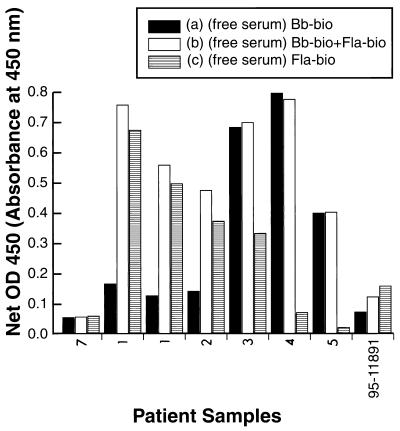
Sample 7, representative of more than 50 samples from subjects without LD from among our non-LD control samples and similar negative control samples from other laboratories, produced a low OD, i.e., below the negative cutoff, in our assay with the sonicate alone, the flagellin epitope alone, or the combination of the flagellin epitope and the sonicate (Fig. 1).
FIG. 1.
Influence of synthetic flagellin epitope (unprocessed serum). Serum samples from patients were assayed for LD (Lyme specific IgM antibodies) by M-capture ELISA. Either the biotinylated whole-cell sonicate (Bb-bio) alone (a), the biotinylated whole-cell sonicate (Bb-bio) plus biotinylated flagellin synthetic epitope-BSA conjugate (Fla-bio) (b), or the biotinylated flagellin synthetic epitope-BSA conjugate (Fla-bio) alone (c) was used as the antigen source. Results are expressed as net mean OD450, as defined in Materials and Methods. The sets of bars represent data for samples from patients 7, 1 (22 May 1997), 1 (23 May 1997), 2 to 5 and 95-11891 from left to right, respectively.
Samples 1 and 2, serum samples from two patients with LD, showed moderate responses in tests with the sonicate alone; the OD in the same assay with either the flagellin epitope alone or the combination of the flagellin epitope and the sonicate was much higher. This phenomenon was reproducible among assays done on different days, as shown with two assays with sample 1 done on 22 and 23 May 1997 (Fig. 1).
Serum sample 3, from a patient with LD, gave a strong signal with the flagellin epitope antigen or the sonicate alone, but there was no appreciable enhancement in response with the combination of sonicate and the flagellin epitope compared to that with the sonicate alone. Serum samples 4 and 5, from patients with LD, were strongly positive when the sonicate was used, but there was little reactivity when the flagellin epitope alone was used and no enhancement in reactivity when the combination of sonicate and flagellin epitope was used compared with the reactivity obtained with the sonicate alone. Serum sample 95-11891, from a patient with LD, was negative in the standard assay with only the sonicate, but it was positive with the flagellin epitope alone or the combination of the flagellin epitope with the sonicate (Fig. 1). Thus, for sera from some but not all patients with LD, the addition of the flagellin epitope enhanced the OD reading. For one sample described here, the addition of the flagellin epitope increased the OD sufficiently to place a serum sample from a patient with LD within the seropositive range. Seroconfirmation of LD in such a patient was possible only with the addition of the flagellin epitope. In no cases did the addition of the flagellin epitope place a serum sample from a subject without LD into the seropositive range.
Comparison of IC-derived IgM with serum as source of antibodies with and without the addition of the flagellin epitope.
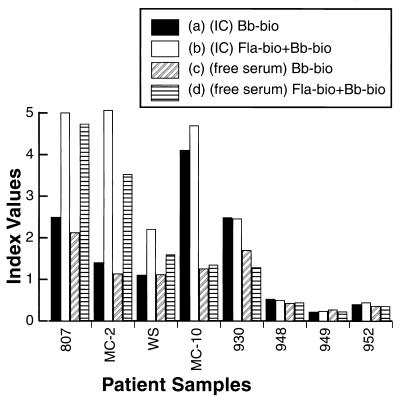
Our previous work demonstrated that IC is superior to free serum antibodies in EMIBA (7). Thus, we compared the use of IC-derived antibodies with the use of free serum antibodies in assays with the standard borrelial sonicate or with the sonicate and the flagellin epitope. An example of the results obtained in such a comparison study is presented in Fig. 2; each assay included at least 10 control serum samples from subjects without LD (data not shown in Fig. 2); these samples were used to calculate a normalization index value. The results obtained with the first four samples from patients with LD, samples 807, MC-2, WS, and MC-10 (confirmed to be positive by three different laboratories), indicate that the addition of the synthetic flagellin epitope enhanced the results obtained with both free antibody and IC-derived antibody for sample MC-10. The absolute enhancement of the free antibody result was much less than that seen in the assay with IC as the source of antibodies. For sample 930 no enhancement of the result obtained with free antibody or IC-derived antibody was found. For control samples from subjects without LD (samples 948, 949 and 952), all four assay methods gave negative results, with no enhancement achieved by the addition of the flagellin epitope.
FIG. 2.
Influence of synthetic epitope flagellin antigen on free and IC-derived L antibodies to B. burgdorferi. Serum samples from patients containing IC-derived antibody (precipitated with PEG and resuspended in PEG) assayed with biotinylated whole-cell sonicate (Bb-bio) alone (a), serum samples containing IC-derived antibody assayed with biotinylated whole-cell sonicate (Bb-bio) plus biotinylated flagellin synthetic epitope-BSA conjugate (Fla-bio) (b), serum samples containing free (untreated) antibody assayed with biotinylated whole-cell sonicate (Bb-bio) alone (c), and serum samples containing free (untreated) antibody assayed with biotinylated whole-cell sonicate (Bb-bio) plus biotinylated flagellin synthetic epitope-BSA conjugate (Fla-bio) (d) as the antigen source were used to detect LD-specific IgM antibodies in M-capture ELISA. Results are expressed as an index value, as defined in Materials and Methods.
The results presented in Fig. 1 and 2 illustrate a variety of serologic results presumably due to different LD antibody repertoires, but in general, sera from LD patients (with or without flagellin epitope enhancement) had a higher OD result when IC was the source of antibodies, consistent with our previous results (7). Neither the use of IC-derived antibodies nor the addition of the synthetic flagellin epitope created false-positive results for sera from subjects without LD.
Blinded study of serum samples with flagellin epitope-enhanced borrelial sonicate as the antigen in EMIBA.
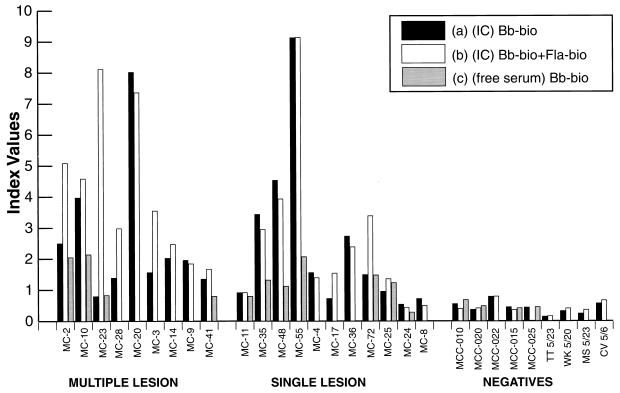
ICs were prepared from the sera in the Marshfield Clinic collection of well-characterized samples from patients from whose EM lesions B. burgdorferi had been cultured (36). Unprocessed serum (free antibodies) and ICs were tested by EMIBA with either the sonicate or a combination of the sonicate and flagellin epitope antigen (Fig. 3). Many of these samples had previously produced inconsistent results in different LD-related immunoassays (36), although the clinical diagnosis had been confirmed by culture of the spirochete from biopsy specimens of EM lesions (Table 1). The samples were analyzed in a blinded fashion, but the results are presented in Fig. 3 in three groups: for patients with (i) multiple EM lesions (disseminated or secondary lesions) or (ii) single lesions and (iii) for negative control subjects.
FIG. 3.
M-capture ELISA used to detect LD-specific IgM antibodies in patients with multiple lesions, patients with single lesions, and negative control patients. Serum samples from patients containing IC-derived antibody (precipitated with PEG and resuspended in PEG) assayed with biotinylated whole-cell sonicate (Bb-bio) alone (a), serum samples containing IC assayed with biotinylated whole-cell sonicate (Bb-bio) plus biotinylated flagellin synthetic epitope-BSA conjugate (Fla-bio) (b), and free (untreated) serum samples containing free (untreated) antibody assayed with biotinylated whole-cell sonicate (Bb-bio) alone (c) as the antigen source were tested. Results are expressed as an index value, as defined in Materials and Methods.
TABLE 1.
Comparison of M-capture ELISA results with other Lyme test results of other assays for LD for patient samples from patients with primary and secondary LDa
| Type of LD and patient | Culture for B. burgdorferi | M-capture ELISA result with the following:
|
Result of IFA for IgM | Result of EIA (recombinanti dot blot) for IgM | Result of immunoblotting for IgM | Result of polyvalent EIA | ||
|---|---|---|---|---|---|---|---|---|
| Sonicate + flagellin (IC) | Sonicate (IC-derived antibody) | Sonicate (free antibody) | ||||||
| Secondary | ||||||||
| MC-2 | POS | POS | POS | POS | POS | POS | NEG | POS |
| MC-10 | POS | POS | POS | POS | POS | POS | NEG | POS |
| MC-23 | POS | POS | NEG | NEG | POS | POS | POS | POS |
| MC-28 | POS | POS | POS | ND | POS | POS | POS | POS |
| MC-20 | POS | POS | POS | ND | POS | POS | POS | POS |
| MC-3 | POS | POS | POS | ND | POS | NEG | NEG | NEG |
| MC-14 | POS | POS | POS | ND | POS | POS | POS | POS |
| MC-9 | POS | POS | POS | ND | POS | POS | NEG | POS |
| MC-41 | POS | POS | POS | NEG | POS | EQUIV | NEG | POS |
| Primary | ||||||||
| MC-11 | POS | EQUIV | EQUIV | NEG | POS | NEG | NEG | NEG |
| MC-35 | POS | POS | POS | POS | NEG | EQUIV | POS | NEG |
| MC-48 | POS | POS | POS | POS | POS | NEG | NEG | NEG |
| MC-55 | POS | POS | POS | POS | POS | EQUIV | POS | NEG |
| MC-4 | POS | POS | POS | ND | NEG | NEG | NEG | NEG |
| MC-17 | POS | POS | NEG | ND | POS | NEG | NEG | POS |
| MC-36 | POS | POS | POS | ND | NEG | POS | POS | POS |
| MC-72 | POS | POS | POS | ND | EQUIV | NEG | POS | POS |
| MC-25 | POS | POS | EQUIV | POS | POS | NEG | NEG | NEG |
| MC-24 | POS | NEG | NEG | NEG | POS | NEG | NEG | NEG |
| MC-8 | POS | NEG | NEG | ND | NEG | NEG | NEG | NEG |
Although results are presented for the M-capture ELISA done in three ways, the direct comparison to other tests for LD should be done with the serum sample containing IC-derived antibody and assayed with the biotinylated whole-cell sonicate (Bb-bio) plus the biotinylated flagellin synthetic epitope-BSA carrier (Fla-bio) as the antigen source. The other Lyme tests for LD are described in Materials and Methods. POS, positive; NEG, negative; EQUIV, equivocal; ND, not determined.
All ICs from patients with multiple lesions gave an index above 1.2 (above the cutoff value of 1.0) when they were tested with the combined sonicate and flagellin epitope (Figure 3). For seven of these nine samples, the flagellin epitope enhanced the reactivity to a level greater than that achieved with the sonicate alone. With the exception of sample MC-23, all ICs from this group of samples from patients with multiple lesions tested in the positive range with the sonicate only. Sample MC-23 gave negative results in repeated assays without the addition of the flagellin peptide epitope. Both sample MC-23 and sample MC-41 would have had false-negative results if they had been tested with serum (free antibody) as the source of antibody with the standard antigen preparation (the sonicate) rather than with IC-derived IgM and the enhanced antigenic preparation. Sample MC-41 had previously tested negative by an IgM immunoblotting assay and equivocal by an IgM dot blot assay (Table 1).
Among the group of samples from patients with single lesions, 8 of 11 samples (all but samples MC-11, MC-24, and MC-8) had values above the cutoff index value of 1 when IC-derived IgM was tested with the combined antigen preparation (Fig. 3). Testing for IC-derived IgM with only the sonicate would have given false-negative results for two of the positive samples (samples MC-17 and MC-25). In the IgM immunofluorescence assays done previously, the result for sample MC-4 was negative, while the results for samples MC-35 and MC-17 were contradictory (Table 1).
Of the three negative samples in this group, sample MC-11 gave an equivocal result, whereas samples MC-24 and MC-8 were negative when they were tested for either free antibody or IC-derived IgM. Sample MC-8 was negative by all other IgM immunoassays done previously, whereas samples MC-11 and MC-24 gave contradictory results (Table 1).
All nine control serum samples from subjects without LD were negative in tests with free antibody or IC with either sonicate or a combination of sonicate and the flagellin epitope antigen; no enhancement due to the addition of the flagellin epitope was noted (Fig. 3).
In summary, all samples from subjects without LD tested negative by EMIBA, whether for IC-derived IgM or free IgM, with or without the addition of the flagellin epitope. For patients with multiple EM lesions, all samples tested positive in tests with IC when the sonicate and flagellin epitope antigen were used, even though other IgM assays were nonconfirmatory for the diagnosis of LD (Table 1). For the samples from patients with single EM lesions, 8 of 11 were positive by our format with IC-derived IgM and the combination of antigens. By comparison, the previously reported immunofluorescence assay for IgM scored only 6 of 11 samples as positive, while the other assays were less likely to predict the clinical status of the patient (Table 1).
DISCUSSION
Our goal was to design an immunoassay useful for confirming the clinical diagnosis of LD at the earliest possible time; consequently, we developed EMIBA, an indirect IgM-capture IgM assay. The advantages of this format have been described previously (7). By measuring 1 dilution (with a saturating anti-IgM concentration coating the plates), the M-capture assay gives a good approximation of the antibody load for the specific IgM antibody for the disease, accurately correlating the antibody load with the clinical condition (45). The use of biotinylated antigen (24) with an ABC detection system increases sensitivity and obviates the use (and production) of anti-B. burgdorferi secondary labeled antibodies (16) or F(ab)2 fragments (6, 12). We found that by using IC-derived IgM we could detect antibodies in the sera of patients with LD who were negative by standard assays (Table 1) while maintaining negative results with control samples (7). These findings are confirmed in the current studies: EMIBA with IC-derived IgM can detect levels of antibodies well within the positive range for many samples determined to be false negative or equivocal by standard assays (e.g., Fig. 3, samples MC-41 and MC-48) or increase the value of the result for positive samples (e.g., Fig. 3, samples MC-35 and MC-55) without a parallel increase in the OD for control samples, thereby giving a more sensitive assay.
Enhancement of the standard antigenic preparation, a borrelial sonicate, with the addition of an albumin-conjugated flagellin peptide epitope in some cases boosted the OD result without falsely increasing the signal derived from true-negative samples. This enhancement might be clinically important, as with serum samples MC-23 and MC-17 (Fig. 3), in which it corrected an otherwise false-negative result.
The flagellin peptide epitope used here was chosen on the basis of previous studies demonstrating that it was not bound by sera from patients without LD (14, 15). It did not decrease the specificity of the EMIBA, as demonstrated by the absence of enhancement of the values for true-negative samples. The current studies confirm that sera from subjects without LD do not bind to this epitope which is of great importance since flagellin contains many epitopes that are cross-reactive and flagellin-based assays can yield false-positive results in seroconfirmatory tests for LD (1, 31, 51). Although purified flagellin has been useful for the seroconfirmation of Lyme neuroborreliosis (25), attempts to improve the sensitivity of ELISA with flagellin-enriched preparations or purified flagellin antigens have given variable results (10), and false-positive results have been obtained in indirect ELISAs (21); ELISA with recombinant flagellin is also prone to cross-reactivity (33). The flagellin-enriched antigen was as sensitive as the sonicate in one study (21) and more sensitive than the whole sonicate in another (10). Some studies found purified flagellin to be superior (26, 27) and to increase the sensitivity of detection of IgG in patients with EM or neuroborreliosis (although there was no such improvement in patients with acrodermatitis chronica atrophicans [28]); of relevance to our design of an assay for early LD, there was no significant difference in IgM titers for any of the patient groups, nor was the sensitivity of detection of IgM (or IgG) in cerebrospinal fluid from patients with neuroborreliosis affected (28). Potentially even greater specificity and sensitivity may be achieved by replacing the sonicate (or recombinant proteins) entirely with a mixture of peptides serving as the antigen source (54), e.g., synthetic peptide epitopes of p39 (30, 46, 47), p23 (OspC) (38), and perhaps p25 (2), in addition to our p41 (flagellin) epitope, for a better seroconfirmatory test for early LD.
Some studies have shown the superiority of immunoblotting over ELISA for the seroconfirmation of LD (2, 16, 17, 40); of note, Aguero-Rosenfeld et al. (2) used a polyvalent rather than IgM-specific secondary antibody which may contribute to the performance of immunoblotting. Immunoblotting is still not standardized (48, 51), and discussion of the appropriate criteria for a standard immunoblot assay continues, with this discussion being fueled by recent work with receptor operating characteristics analysis for early LD (48). Immunoblotting is labor- and cost-intensive and relatively cumbersome (compared to ELISA), and interpretation of the results is subjective (16, 17, 37, 55).
Table 1 demonstrates that this enhancement of EMIBA yields results that correlate better with the clinical condition (patients with EM and culture positive culture result) than other serologic assays, including Western blotting, or a recombinant p39 dot blot (7, 35). In comparison to other seroconfirmatory assays, the improved EMIBA more accurately demonstrated true positivity for a group of patients with primary symptoms (single lesions), which represent early disease (Fig. 3) (Table 1). Current serologic assays are incapable of confirming the diagnosis of LD for a large proportion of patients with early disease, suggesting to us that a new seroconfirmatory assay rather than a modification of current criteria may be the most appropriate next step in test design and interpretation. EMIBA may be suitable for seroconfirmation of LD in patients with somewhat later stages of LD, as evidenced by the results for patients with multiple lesions (Table 1). Indeed, persistence of IgM seroreactivity (1, 13, 24) has been found in patients with LD, and IgM can be found for more than 1 year in patients who were apparently successfully treated for early LD (18, 23). Thus, measurement of whole unprocessed serum (free antibody in our terminology) for anti-B. burgdorferi IgM reactivity by the standard serologic assays with which we compared that method with EMIBA can produce positive results; these positive results have no known clinical significance, i.e., they do not suggest active infection (18, 23), although misinterpretation of serologic reactivity, especially IgM seroreactivity, which is synonymous with active disease, by referring clinicians is common in our experience (45a, 45b).
EMIBA measures ICs rather than free antibody, as described previously. It substantially reduces the rate of false-positive results, it better correlates with the patient’s clinical status than standard ELISA or immunoblotting, and it is capable of seroconfirmation of LD before ELISA or immunoblotting (7). With the addition of the flagellin epitope, we think the principle of using preselected epitopes rather than crude sonicate or recombinant proteins as an antigenic source was satisfactorily demonstrated. Current studies in our laboratory are expanding on the current work, and we are using other epitopes in the development of better serologic assays (14, 15, 53) and other relevant control groups, including sera from patients other infectious diseases (syphilis, Epstein-Barr virus infection, and other borrelia infections) and noninfectious diseases (lupus and rheumatoid arthritis). The goal of these studies is to develop a standardized antigenic preparation for serologic assays whose results are corroborated in comparison with the results for these other diseases used as controls.
ACKNOWLEDGMENTS
We thank Russell Johnson of the University of Minnesota for sending well-characterized blinded samples for evaluation and William Schrier and Barbara Johnson of CDC for serum samples.
REFERENCES
- 1.Aguero-Rosenfeld M, Nowakowski J, Bittker S, Cooper D, Nadelman R B, Wormser G P. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol. 1996;34:1–9. doi: 10.1128/jcm.34.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguero-Rosenfeld M, Nowakowski J, McKenna D F, Carbonaro C A, Wormser G P. Serodiagnosis in early Lyme disease. J Clin Microbiol. 1993;31:3090–3095. doi: 10.1128/jcm.31.12.3090-3095.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G, Fish D. The biological and social phenomena of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 5.Benach J, Bosler E M, Hanrahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 6.Berardi V, Weeks K E, Steere A C. Serodiagnosis of early Lyme disease: analysis of IgM and IgG antibody responses by using an antibody-capture enzyme immunoassay. J Infect Dis. 1988;158:754–760. doi: 10.1093/infdis/158.4.754. [DOI] [PubMed] [Google Scholar]
- 7.Brunner M, Stein S, Sigal L H. Enzyme-linked, IgM capture, immune complex, biotinylated antigen assay (EMIBA): a new serologic assay for early Lyme disease. Arthritis Rheum. 1997;40:S142. [Google Scholar]
- 8.Brunner M, Moriera-Filho C, Wachtel S S. On the secretion of H-Y antigen. Cell. 1984;37:615–619. doi: 10.1016/0092-8674(84)90392-1. [DOI] [PubMed] [Google Scholar]
- 8a.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morbid Mortal Weekly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 9.Cerny E H, Farshy C E, Hunter E F, Larsen S A. Rheumatoid factor in syphilis. J Clin Microbiol. 1985;22:89–94. doi: 10.1128/jcm.22.1.89-94.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman J, Benach J. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987;155:756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- 11.Coleman J L, Benach J L. Characterization of antigenic determinants of Borrelia burgdorferi shared by other bacteria. J Infect Dis. 1992;165:658–666. doi: 10.1093/infdis/165.4.658. [DOI] [PubMed] [Google Scholar]
- 12.Coyle P, Deng Z, Schutzer S E, Belman A L, Benach J, Krupp L B, Luft B. Detection of Borrelia burgdorferi antigens in cerebrospinal fluid. Neurology. 1993;43:1093–1097. doi: 10.1212/wnl.43.6.1093. [DOI] [PubMed] [Google Scholar]
- 13.Craft J E, Fischer D K, Shimamoto G T, Steere A C. Antigens of Borrelia burgdorferi recognized during Lyme disease. J Clin Invest. 1986;78:934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Z, Lackland H, Stein S, Li Q, Radziewicz R, Williams S, Sigal L. Molecular mimicry in Lyme disease: monoclonal antibody H9724 to Borrelia burgdorferi flagellin specifically detects chaperonin-HSP60. Biochim Biophys Acta. 1993;1181:97–100. doi: 10.1016/0925-4439(93)90096-j. [DOI] [PubMed] [Google Scholar]
- 15.Dai Z Z. Definition of the epitope on the 41-kDa flagellin of Borrelia burgdorferi for a monoclonal antibody H9724 and identification of a H9724-reactive protein from calf adrenal gland. Ph.D thesis. Piscatawy, N.J: Rutgers University; 1993. [Google Scholar]
- 16.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 17.Engstrom S, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feder H M, Gerber M A, Luger S W, Ryan S W. Persistence of serum antibodies to Borrelia burgdorferi in patients treated for Lyme disease. Clin Infect Dis. 1992;15:788–793. doi: 10.1093/clind/15.5.788. [DOI] [PubMed] [Google Scholar]
- 19.Fikrig E, Huguenel E D, Berland R, Rahn D W, Hardin J A, Flavell R A. Serologic diagnosis of Lyme disease using recombinant outer surface proteins A and B and flagellin. J Infect Dis. 1992;165:1127–1132. doi: 10.1093/infdis/165.6.1127. [DOI] [PubMed] [Google Scholar]
- 20.Gerber M A, Shapiro E D, Burke G S, Parcells V J, Bell G L. Lyme disease in children in southeastern Connecticut. N Engl J Med. 1996;335:1270–1274. doi: 10.1056/NEJM199610243351703. [DOI] [PubMed] [Google Scholar]
- 21.Grodzicki R, Steere A C. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J Infect Dis. 1988;157:790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- 22.Guner E. Retention of B. burgdorferi pathogenicity and infectivity after multiple passages in a co-culture system. Experientia. 1994;50:54–59. doi: 10.1007/BF01992050. [DOI] [PubMed] [Google Scholar]
- 23.Hammers-Berggren S, Lebech A M, Karlsson M, Svenungsson B, Hansen K, Stiernstedt G. Serological follow-up after treatment of patients with erythema migrans and neuroborreliosis. J Clin Microbiol. 1994;32:1519–1532. doi: 10.1128/jcm.32.6.1519-1525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen K, Pii K, Lebech A-M. Improved immunoglobulin M serodiagnosis in Lyme borreliosis by using a mu-capture enzyme-linked immunosorbent assay with biotinylated Borrelia burgdorferi flagella. J Clin Microbiol. 1991;29:166–173. doi: 10.1128/jcm.29.1.166-173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen K, Lebech A M. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi specific immunoglobulin G, A, and M. Ann Neurol. 1991;30:197–205. doi: 10.1002/ana.410300212. [DOI] [PubMed] [Google Scholar]
- 26.Hansen K, Hindersson P, Pedersen N S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988;26:338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen K, Asbrink E. Serodiagnosis of erythema migrans and acrodermatitis chronica atrophicans by the Borrelia burgdorferi flagellum enzyme-linked immunosorbent assay. J Clin Microbiol. 1989;27:545–551. doi: 10.1128/jcm.27.3.545-551.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Johnson B J B, Robbins K E, Bailey R E, Cao B-L, Sviat S L, Craven R B, Mayer L W, Dennis D T. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis. 1996;174:346–353. doi: 10.1093/infdis/174.2.346. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson M, Stiernstedt G, Granstrom M, Asbrink E, Wretlind B. Comparison of flagellum and sonicate antigens for serological diagnosis of Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1990;9:169–177. doi: 10.1007/BF01963833. [DOI] [PubMed] [Google Scholar]
- 29.Lovece S, Stern R, Kagen L J. Effects of rheumatoid factor, antinuclear antibodies and plasma reagin on the serologic assay for Lyme disease. J Rheumatol. 1991;18:1813–1818. [PubMed] [Google Scholar]
- 30.Ma B, Christen B, Leung D, Vigo-Pelfrey C. Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J Clin Microbiol. 1992;30:370–376. doi: 10.1128/jcm.30.2.370-376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnarelli L, Anderson J G F, Johnson R C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987;156:183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- 32.Magnarelli L, Miller J N, Anderson J F, Riviere G R. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990;28:1276–1279. doi: 10.1128/jcm.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magnarelli L, Fikrig E, Padula S J, Anderson J F, Flavell R A. Use of recombinant antigens of Borrelia burgdorferi in serologic tests for diagnosis of Lyme borreliosis. J Clin Microbiol. 1996;34:237–240. doi: 10.1128/jcm.34.2.237-240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melski J, Reed K D, Mitchell P D, Barth D G D. Primary and secondary erythema migrans in central Wisconsin. Arch Dermatol. 1993;129:709–716. [PubMed] [Google Scholar]
- 35.Mitchell P, Reed K D, Aspeslet T L, Vandermause M F, Melski J W. Comparison of four immunoserologic assays for detection of antibodies to Borrelia burgdorferi in patients with culture-positive erythema migrans. J Clin Microbiol. 1994;32:1958–1962. doi: 10.1128/jcm.32.8.1958-1962.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell P, Reed K D, Vandermause M F, Melski J W. Isolation of Borrelia burgdorferi from skin biopsy specimens of patients with erythema migrans. Am J Clin Pathol. 1993;99:104–107. doi: 10.1093/ajcp/99.1.104. [DOI] [PubMed] [Google Scholar]
- 37.Pachner A R, Ricalton N S. Western blotting in evaluating Lyme seropositivity and the utility of a gel densitometric approach. Neurology. 1992;42:2185–2192. doi: 10.1212/wnl.42.11.2185. [DOI] [PubMed] [Google Scholar]
- 38.Padula S J, Dias R, Sampieri A, Craven R B, Ryan R W. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J Clin Microbiol. 1994;32:1733–1738. doi: 10.1128/jcm.32.7.1733-1738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollack R, Telford III S R, Spielman A. Standardization of medium for culturing Lyme disease spirochetes. J Clin Microbiol. 1993;31:1251–1255. doi: 10.1128/jcm.31.5.1251-1255.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitz J L, Powell C S, Folds J D. Comparison of seven commercial kits for detection of antibodies to Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1993;12:419–424. doi: 10.1007/BF01967435. [DOI] [PubMed] [Google Scholar]
- 41.Schneider T, Lange R, Ronspeck W, Weigelt W, Kolmel H W. Prognostic B-cell epitopes on the flagellar protein of Borrelia burgdorferi. Infect Immun. 1992;60:316–319. doi: 10.1128/iai.60.1.316-319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schutzer S, Coyle P K, Dunn J J, Luft B J, Brunner M. Early and specific antibody response to ospA in Lyme disease. J Clin Invest. 1994;94:454–457. doi: 10.1172/JCI117346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schutzer S, Coyle P K, Belman A L, Golightly M G, Drulle J. Sequestration of antibody to Borrelia burgdorferi in immune complexes in seronegative Lyme disease. Lancet. 1990;335:312–315. doi: 10.1016/0140-6736(90)90606-6. [DOI] [PubMed] [Google Scholar]
- 44.Schutzer S E, Coyle P K, Brunner M. Identification of specific Borrelia burgdorferi components in circulating antigen-antibody complexes. In: Schutzer S E, editor. Lyme disease: molecular and immunologic approaches. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 135–148. [Google Scholar]
- 45.Siegel J P, Remington J S. Comparison of methods for quantitating antigen-specific immunoglobulin M antibody with a reverse enzyme-linked immunosorbent assay. J Clin Microbiol. 1983;18:63–70. doi: 10.1128/jcm.18.1.63-70.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Sigal L H. Persisting complaints of Lyme disease: a conceptual review. Am J Med. 1994;96:365–374. doi: 10.1016/0002-9343(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 45b.Sigal L H. Special article: pitfalls in the diagnosis and management of Lyme disease. Arthritis Rheum. 1998;41:195–204. doi: 10.1002/1529-0131(199802)41:2<195::AID-ART3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 46.Simpson W J, Cieplak W, Schrumpf M E, Barbour A G, Schwan T G. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol Let. 1994;119:381–388. doi: 10.1111/j.1574-6968.1994.tb06917.x. [DOI] [PubMed] [Google Scholar]
- 47.Simpson W J, Schrumpf M E, Schwan T G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990;28:1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sivak S L, Aguero-Rosenfeld M E, Nowakowski J, Nadelman R B, Wormser G P. Accuracy of IgM immunoblotting to confirm the clinical diagnosis of early Lyme disease. Arch Intern Med. 1996;156:2105–2109. [PubMed] [Google Scholar]
- 49.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 50.Steere A C, Grodzicki R L, Kornblatt A N, Craft J E, Barbour A G, Burgdorfer W, Schmid G P, Johnson J, Malawista S E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 51.Tierno P M, Cadet-Legros J. Methods comparison for diagnosis of Lyme disease. Lab Med. 1996;27:542–546. [Google Scholar]
- 52.Vejtorp M. The interference of IgM rheumatoid factor in enzyme-linked immunosorbent assays of rubella IgM and IgG antibodies. J Virol Methods. 1980;1:1–9. [Google Scholar]
- 53.Yu Z, Carter J M, Sigal L H, Stein S. Multi-well ELISA based on independent peptide-antigens for antibody capture: application to Lyme disease serodiagnosis. J Immunol Methods. 1996;198:23–25. doi: 10.1016/0022-1759(96)00140-8. [DOI] [PubMed] [Google Scholar]
- 54.Yu Z, Carter J M, Lackland H, Sigal L H, Stein S. Presentation of peptide antigens as albumin conjugates for use in detection of serum antibodies by ELISA. Bioconj Chem. 1996;7:338–342. doi: 10.1021/bc960018s. [DOI] [PubMed] [Google Scholar]
- 55.Zoller L, Burkard S, Schafer H. Validity of Western immunoblot band patterns in the serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1991;29:174–182. doi: 10.1128/jcm.29.1.174-182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]