Summary
Background
The effect of childhood pneumococcal conjugate vaccine implementation in Malawi is threatened by absence of herd effect. There is persistent vaccine-type pneumococcal carriage in both vaccinated children and the wider community. We aimed to use a human infection study to measure 13-valent pneumococcal conjugate vaccine (PCV13) efficacy against pneumococcal carriage.
Methods
We did a double-blind, parallel-arm, randomised controlled trial investigating the efficacy of PCV13 or placebo against experimental pneumococcal carriage of Streptococcus pneumoniae serotype 6B (strain BHN418) among healthy adults (aged 18–40 years) from Blantyre, Malawi. We randomly assigned participants (1:1) to receive PCV13 or placebo. PCV13 and placebo doses were prepared by an unmasked pharmacist to maintain research team and participant masking with identification only by a randomisation identification number and barcode. 4 weeks after receiving either PCV13 or placebo, participants were challenged with 20 000 colony forming units (CFUs) per naris, 80 000 CFUs per naris, or 160 000 CFUs per naris by intranasal inoculation. The primary endpoint was experimental pneumococcal carriage, established by culture of nasal wash at 2, 7, and 14 days. Vaccine efficacy was estimated per protocol by means of a log-binomial model adjusting for inoculation dose. The trial is registered with the Pan African Clinical Trials Registry, PACTR202008503507113, and is now closed.
Findings
Recruitment commenced on April 27, 2021 and the final visit was completed on Sept 12, 2022. 204 participants completed the study protocol (98 PCV13, 106 placebo). There were lower carriage rates in the vaccine group at all three inoculation doses (0 of 21 vs two [11%] of 19 at 20 000 CFUs per naris; six [18%] of 33 vs 12 [29%] of 41 at 80 000 CFUs per naris, and four [9%] of 44 vs 16 [35%] of 46 at 160 000 CFUs per naris). The overall carriage rate was lower in the vaccine group compared with the placebo group (ten [10%] of 98 vs 30 [28%] of 106; Fisher's p value=0·0013) and the vaccine efficacy against carriage was estimated at 62·4% (95% CI 27·7–80·4). There were no severe adverse events related to vaccination or inoculation of pneumococci.
Interpretation
This is, to our knowledge, the first human challenge study to test the efficacy of a pneumococcal vaccine against pneumococcal carriage in Africa, which can now be used to establish vaccine-induced correlates of protection and compare alternative strategies to prevent pneumococcal carriage. This powerful tool could lead to new means to enhance reduction in pneumococcal carriage after vaccination.
Funding
Wellcome Trust.
Introduction
Pneumococcal conjugate vaccine has greatly reduced invasive pneumococcal disease worldwide, both by direct vaccine protection and by reduced carriage and transmission, leading to indirect protection (ie, the herd effect) for unvaccinated populations.1 In Malawi, the 13-valent pneumococcal conjugate vaccine (PCV13) was introduced in 2011 and has resulted in substantial reductions in invasive pneumococcal disease in vaccinated children.2 Unlike in the UK, however, there have been consistent observations of continued high rates of vaccine-type pneumococcal carriage among vaccinated children, and unvaccinated children and adults living with HIV in Malawi.3 This continued vaccine-type carriage, and the observed reduced herd effect in protection against disease,4 are a cause for concern.5
Research in context.
Evidence before this study
We did an individual participant data meta-analysis (published May 4, 2023) to assess the safety of a pneumococcal controlled human infection model, searching for papers published between Jan 1, 2011, and June 30, 2022, with no language restrictions. By updating the search to April 1, 2023, we did not identify additional studies. We searched PubMed with the search terms “pneumococc*” OR “Streptococcus pneumoniae” AND “human infection model” OR “human infection stud*” OR “experimental human infection” OR “controlled human infection”. We found only one study designed to evaluate the effect of 13-valent pneumococcal conjugate vaccine (PCV13) vaccination on experimental pneumococcal colonisation. This double-blind, randomised controlled trial, done in Liverpool, UK, established that the risk ratio of experimental pneumococcal colonisation following PCV13 vaccination compared with control vaccine (hepatitis A) was 0·22 (95% CI 0·09–0·52; p<0·001). Streptococcus pneumoniae serotype 6B was used as the challenge agent, at a single dose of 80 000 colony forming units (CFUs) per 100 μL and natural pneumococcal carriage was observed in 6 of 100 participants in this study. Our group has previously shown feasibility of pneumococcal controlled human infection in Malawi with carriage rates of 44% at a dose of 80 000 CFUs per 100 μL (4 of 9 unvaccinated participants, 95% CI 13·7–78·8).
Added value of this study
We found that PCV13 was protective against experimentally induced pneumococcal carriage (serotype 6B) in healthy Malawian participants but to a lesser extent than observed in participants recruited to a similar UK-based trial. Episodes of natural pneumococcal carriage were high (42%) in our trial compared with published UK studies. We observed a greater PCV13 protective effect in participants without natural carriage (69·1%) compared with participants with natural carriage (51·7%) at the point of experimental pneumococcal inoculation. This is, we believe, the first vaccine trial that makes use of a bacterial human infection study in Africa. Our study provides important experimental data to inform epidemiological observations of high residual carriage of vaccine-serotype S pneumoniae after roll-out of PCV13 within the expanded programme on immunisation in sub-Saharan Africa.
Implications of all the available evidence
PCV13 vaccination was protective against experimental colonisation with pneumococcal serotype 6B in healthy adult volunteers from Malawi. An effective pneumococcal vaccine should interrupt population transmission through prevention of natural colonisation in vaccinated individuals. We have observed differences in protection compared with UK participants; this finding requires further evaluation by means of longitudinal immunological samples obtained from participants. Future research should pool data from the UK and Malawi populations to investigate correlates of protection. Our studies using pneumococcal controlled human infection could suggest means to improve schedules for current vaccines and to test prospective pneumococcal vaccines relevant to populations that need them most.
Vaccine efficacy is often reduced in low-resource settings compared with high-income countries. There are many reasons why this might be the case, including vaccine acceptability and uptake, and health systems issues such as cold chain integrity, high force of infection (high carriage rates in the community), pathogen diversity, and host immunity. In the case of the observed reduced herd effect following PCV13 implementation in infants in Malawi, it has been suggested that force of infection might explain the residual vaccine-type carriage in vaccinated children.3 There is high force of infection of both vaccine-type and non-vaccine type pneumococci in children and in susceptible populations such as people living with HIV or people living in crowded environments.6 It is possible, however, that host immunity developing in environments such as Malawi (these observations are true of many sub-Saharan African countries) results in a different mucosal defence to that observed in UK adults.7 In particular, the experience of seasonal viral infections, early life infections, and nutrition are very different between the two countries.8, 9, 10 Malawi has little seasonal variation of respiratory viruses, which are endemic at low levels all year round.8 Early life infections with bacterial10 and parasitic disease11 remain common and nutrition is variable with seasonal hunger.12 In this context, it is important to understand why vaccine-type pneumococcal carriage persists after vaccination, and to make every effort to optimise the efficacy of this important, expensive vaccine.
We previously developed a controlled human infection model (CHIM) of experimental human pneumococcal carriage (EHPC) in Liverpool, UK,13 to study mucosal immune determinants of pneumococcal carriage and transmission. The EHPC CHIM is safe, reproducible, and acceptable to volunteers in the UK and we used it to show PCV13 vaccine efficacy in prevention of experimental pneumococcal carriage in British university students.14 We then transferred the EHPC CHIM to Malawi, a low-resource high-transmission setting, and have previously shown feasibility and acceptability of this CHIM method for the first time in Africa.15, 16
In this study, we aimed to use the EHPC CHIM to establish the vaccine efficacy of PCV13 against experimental pneumococcal carriage in young healthy adults in Malawi.
Methods
Study design and participants
This was a double-blind, parallel-arm, randomised controlled trial investigating the efficacy of PCV13 or placebo (1:1) against experimental pneumococcal carriage of Streptococcus pneumoniae serotype 6B (strain BHN418; hereafter referred to as S pneumoniae 6B). The study was done according to our open access a priori published trial protocol (https://wellcomeopenresearch.org/articles/6-240/v2)17 with minor approved modifications reported here (see Statistical analysis section) and according to our prespecified statistical analysis plan.
The trial was approved in Malawi by the National Health Sciences Research Committee (16/07/2519) and Pharmacy Medicines and Regulatory Authority (PMRA/CTRC/III/10062020121) and in the UK by the Liverpool School of Tropical Medicine (20-021). Written informed consent was obtained for all participants.
Healthy adult participants were recruited from the community, workplaces, and colleges in Blantyre, Malawi. Study procedures took place in the Ward 3A research clinic, Queen Elizabeth Central Hospital (Blantyre, Malawi). Recruitment commenced on April 27, 2021 and the final visit was completed on Sept 12, 2022. A detailed description of screening, eligibility and recruitment procedures is described elsewhere.17 Briefly, prospective adult participants (aged 18–40 years) were screened by means of a combination of medical history, physical examination, and clinical laboratory tests to confirm eligibility before randomisation. Participants were excluded if at increased risk of invasive pneumococcal disease (eg, pregnancy or HIV infection), or they were close social contacts of individuals at increased risk (eg, breastfeeding an infant), or showed natural carriage of S pneumoniae 6B (precluding elucidation of primary outcome). Participants with natural carriage of pneumococcal serotypes other than 6B were not excluded. In an amendment from our published protocol, participants who tested positive for SARS-CoV-2 (all testing done by PCR) after inoculation were not automatically excluded from the trial but followed up to planned study exit. These participants were not included in the per-protocol analysis.
Randomisation and masking
Per protocol,17 we powered the study to detect a 40% reduction in experimental colonisation from a baseline of 60% (control) to 36% (PCV13) in participants allocated to the 80 000 colony forming units (CFUs) per naris pneumococcal inoculation group (n=70 per group). Another 40 participants had been planned to be randomly assigned at 20 000 CFUs per naris and 20 participants at 160 000 CFUs per naris for an exploratory analysis of the dose (inoculation)–response (experimental carriage) curve. However, on the basis of lower than expected observed carriage rates in masked data (27% observed carriage vs 48% expected carriage across both study groups), the sample size was changed to 40 participants at 20 000 CFUs per naris dose, 80 at 80 000 CFUs per naris dose, and 80 at 160 000 CFUs per naris dose. The primary analysis was changed from the original plans (but a priori before data analysis commenced and prespecified in the statistical analysis plan) to a log-binomial model adjusting for inoculation dose, thereby allowing data from all inoculation doses to be used for the primary analysis.
After confirmation of eligibility, participants were randomly assigned (1:1) to receive intramuscular PCV13 (Prevenar-13 containing serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F; Pfizer, New York, NY, USA) or 0·9% normal saline (control). Research staff (clinical and laboratory) and participants were masked to vaccine allocation. The trial pharmacist (designated Qualified Person, JN) was unmasked to allocation and prepared intervention and control vaccines (identical in appearance and labelled A or B) on the day of randomisation. Three randomisation lists were generated by an independent statistician by means of code written by the trial statistician (MYRH) for each pneumococcal inoculation dose (20 000, 80 000, and 160 000 CFUs per naris) and prospectively uploaded to electronic case report forms with robust allocation procedures done per published protocol.17 The decision to test these doses was informed by previous work in pneumococcal CHIM development.13, 15 Randomisation to PCV13 or saline vaccination did not influence inoculation dose administered, with balanced allocation within each dosage group.17 The randomisation method used an allocation ratio of 1:1 between study groups and was a block randomisation procedure, which made use of variable block sizes from four to eight participants. The randomisation was implemented by means of the blockrand library for the R environment for statistical computation.18
Procedures
28 days following vaccination, participants underwent nasal inoculation with S pneumoniae 6B to establish experimental human pneumococcal carriage with a method that we have used safely in Liverpool for more than 10 years.19 Briefly, experimental inocula of S pneumoniae 6B were prepared at the Liverpool School of Tropical Medicine, UK, following established standard operating procedures, including purity checks. Inocula were shipped to Malawi at −80°C and viability, bacterial count, and purity were confirmed from selected samples before human intranasal instillation. On each experimental day, a sample was thawed, centrifuged, and washed before resuspension in 0·9% normal saline at the prespecified concentration. The prepared inoculum was taken immediately (less than 5 min transit) to the clinical area where 100 μL was instilled into each nostril of each participant. Serial dilutions of the inocula were plated (pre-inoculation and post-inoculation) onto blood agar for dose confirmation. Owing to lower than expected total carriage rates observed in the 80 000 CFUs per naris group, a protocol modification was made (approved by the data, safety, and monitoring committee and research ethics committee) to escalate to 160 000 CFUs per naris after 81 participants had been inoculated at the 80 000 CFUs per naris dose. The first 42 participants were inoculated with 20 000 CFUs per 100 μL, the next 81 participants with 80 000 CFUs per 100 μL and the final 98 with 160 000 CFUs per 100 μL.
Nasal wash samples were collected immediately before and 28 days after vaccination, and after inoculation on days 2, 7, and 14. Sample collection and processing has been previously described.15, 19 Briefly, 5 mL of 0·9% saline was instilled into each naris, retained for 10 s and expelled in to a petri dish; this was repeated twice (10 mL per naris, 20 mL total). The recovered sample was immediately transferred to the laboratory on ice. On receipt in the laboratory, the sample was centrifuged at 3400 g for 10 min. Following centrifugation, 1 mL samples of the supernatant were stored at −80°C in ten pre-labelled tubes. The nasal wash bacterial pellet was resuspended in 100 μL of skim milk, tryptone, glucose, and glycerol with 20 μL plated onto Columbia sheep Blood Agar (Oxoid, Basingstoke, UK) containing 5 μg/mL gentamicin for pneumococcal isolation. Eight serial 1:10 dilutions were made from 20 μL of the suspension by means of the Miles and Misra method to establish the number of CFUs. All plates were incubated at 370°C with 5% CO2 for 18–24 h and inspected by two team members (ie, two of either TC, SS, BG, FT, or CM) to identify pneumococcal colonies by colony morphology. Colonies were then subcultured for optochin sensitivity, bile solubility, and serotyping. Pneumococcal serotypes were confirmed by latex agglutination by means of Immulex Pneumotest reagents (Statens Serum Institute, Copenhagen, Denmark). 800 μL skim milk, tryptone, glucose, and glycerol was added to the remaining nasal wash pellet and divided into three vials (300 μL into the first two tubes and the remainder into the third vial) and stored at −80°C for later molecular detection of pneumococci.
Genomic bacterial DNA was isolated from nasal wash pellets stored in skim milk, tryptone, glucose, and glycerol by means of the Agowa Mag mini-DNA extraction kit (LGC Genomics, Berlin, Germany) as per manufacturer's instruction by means of one of the three samples described previously. The extracted DNA was stored at −20°C for use in lytA/cpsA multiplex PCR. Sequences of the primers and probes used to do PCR were: lytA forward primer 5ʹ-ACGCAATCTAGCAGATGAAGCA-3ʹ; lytA reverse primer 5ʹ-TCGTGCGTTTTAATTCCAGCT-3ʹ; lytA probe 5ʹ-TGCCGAAAACGCTTGATACAGGGAG-3ʹʹ, and cpsA forward primer 5ʹ- AAGTTTGCACTAGAGTATGGGAAGGT-3ʹ; cpsA reverse primer 5ʹ-ACATTATGTCCATGTCTTCGATACAAG-3ʹ; and cpsA probe 5ʹ-TGTTCTGCCCTGAGCAACTGG-3ʹ. The reaction mixture of 20 μl contained 1 μL of each of the primers, 0·4 μL of each of the probes, 2·7 μL of PCR grade water, 10 μL of qScript XLT 1-Step RT-qPCR ToughMix (Quantabio, Cummings Centre, Beverly, MA, USA), and 2·5 μL of sample in a set of duplicate wells. The quantitative PCR (qPCR) reaction was run on a QuantStudio 7 Flex machine (Applied Biosystems, Lincoln Centre Drive, Foster City, CA, USA) with the following programme: 95°C for 10 min; followed by 40 cycles of 95°C for 15 s and then 60°C for 1 min.
Outcomes
The primary endpoint was nasal carriage of S pneumoniae 6B established by microbiological culture of nasal wash at any time after inoculation (sampling timepoints days 2, 7, and 14 post-inoculation; participants expected to attend all visits). Nasal wash samples were recorded as positive if the lytA/cpsA PCR was positive at less than 40 cycles in duplicate samples. Adverse effects, and the effect of natural carriage at recruitment were included as secondary outcomes and are reported here.
Statistical analysis
Data were collected electronically, with laboratory results pushed onto the database once they were available. Data collection tablets included range and consistency checks. Statistical analyses were done according to an a priori statistical analysis plan by means of R version 4.0.2 (R Development Core Team, Vienna, Austria) and GraphPad Prism version 9.0.0 (Graph-Pad Software, San Diego, CA, USA). Non-normally distributed quantitative measurements are summarised by the median (IQR). Exact binomial CIs are reported for estimated proportions. For the primary analysis, the binary response of experimental carriage was compared between study groups by means of a log-binomial model adjusting for inoculation dose group.
Our revised power calculation assumed the overall carriage proportions to be 5% (on the basis of observed, masked data at the time of protocol amendment) in the 20 000 CFUs per naris inoculation dose group, 27% in the 80 000 CFUs per naris dose group, and 48% in the 160 000 CFUs per naris dose group. We planned to detect a relative reduction of 60% in the percentage of PCV13 randomly assigned participants becoming experimental carriers compared with the saline randomly assigned individuals. With a total sample size of 200 individuals we calculated (using simulation) that we had 74% power to detect this difference. The code for the revised power calculation via simulation is available on GitHub.
The log-binomial model provides a direct estimation of the relative risk (RR) associated with the different predictor variables and, as such, has been recommended for use in randomised clinical trials.20 Specifically, the estimate of βPCV13, the model coefficient for the binary indicator variable for the study group, will estimate the average (for the study population) difference between study groups in the logarithm of the probability of experimental carriage (as defined by the primary study endpoint). This means that exp(βPCV13), the exponentiated model coefficient for the study group, will directly estimate the RR of experimental carriage associated with PCV13 vaccination. If this is less than 1, it means that the vaccine is protective and the vaccine efficacy can then be estimated as vaccine efficacy=1− exp(βPCV13). Log-binomial models can have difficulties with convergence, but we have used the logbin R package which mitigates these issues through the use of more stable expectation–maximisation-type algorithms.21
Categorical variables were compared by means of Fisher's exact test. A p value of less than 0·05 was considered significant; all p values are two tailed. The full statistical analysis plan, providing details of the planned analyses with all R code, is available from GitHub. The trial is registered with the Pan African Clinical Trials Registry, PACTR202008503507113.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Participant recruitment was continuous between April 27, 2021, and Aug 9, 2022, with a brief pause (June 8, 2021–Sept 13, 2021) owing to a national COVID-19 infection wave in Malawi requiring staff to assist with hospital emergency cover.22, 23 The screening, vaccination, and exclusion of participants in the study are shown in figure 1. Participants with SARS-CoV-2 infection were unable to attend the research clinic for scheduled visits and this resulted in missing data in seven participants following inoculation (another seven participants also tested positive for SARS-CoV-2 but did complete all study visits; in total, 14 participants infected with SARS-CoV-2 were removed from analysis). Groups randomly assigned to receive either PCV13 or placebo were broadly similar in terms of age, BMI, and smoking history, and the majority were men; participant characteristics are presented in table 1.
Figure 1.
Trial profile
Some participants were excluded after a delay of >10 weeks between vaccination and inoculation per protocol.17 Delays of >10 weeks occurred primarily due to a national wave of COVID-19 infection in Malawi leading to a pause of study activities between June and September, 2021. CFU=colony-forming unit. PCV13=13-valent pneumococcal conjugate vaccine.
Table 1.
Participant characteristics by randomisation group
| Placebo | PCV13 | Overall | ||
|---|---|---|---|---|
| Study characteristics | ||||
| Participants | 114 (52%) | 107 (48%) | 221 (100%) | |
| SARS-CoV-2 infection | 6 (5%) | 8 (7%) | 14 (6%) | |
| Completed follow-up visits and included in final analysis* | 106 (93%) | 98 (92%) | 204 (92%) | |
| Sex | ||||
| Male | 72 (68%) | 75 (77%) | 147 (72%) | |
| Female | 34 (32%) | 23 (23%) | 57 (28%) | |
| Age, years | 24·6 (22·8–28·5) | 25·5 (23·1–28·0) | 25·3 (22·9–28·5) | |
| BMI, kg/m2 | 22·0 (20·3–23·7) | 21·5 (20·2–24·5) | 21·8 (20·2–23·9) | |
| Smoking status | ||||
| Never smoker | 104 (98%) | 95 (97%) | 199 (98%) | |
| Past smoker | 2 (2%) | 2 (2%) | 4 (2%) | |
| Current smoker | 0 | 1 (1%) | 1 (<1%) | |
Data are n (%) or median (IQR). PCV13=13-valent pneumococcal conjugate vaccine.
The totals from this row are used as the column-wide denominators to calculate percentages in rows further down in the table.
All inoculation doses were within the target range (doubling or halving of target dose). The mean inoculation doses at each target dose were 19 056 CFUs per 100 μL (SD 2808 CFUs per 100 μL) for the 20 000 CFUs per 100 μL target; 75 185 CFUs per 100 μL (13 333 per 100 μL) for the 80 000 CFUs per 100 μL target; and 155 493 per 100 μL (32 738 per 100 μL) for 160 000 CFUs per 100 μL target (appendix p 4).
There were no vaccination or inoculation related serious adverse events, but one serious adverse event was recorded in a participant with SARS-CoV-2 infection who required medical attention. Adverse events were mild and unrelated to pneumococcal vaccination, inoculation, or carriage. All adverse events are reported in the appendix (pp 1–2)—nasal washing resulted in nasal stuffiness in 18 (8%) of 221 participants and there were a wide variety of mild, non-specific symptoms reported in direct questioning in the weeks following inoculation.
Experimentally induced carriage of S pneumoniae 6B was established by culture of nasal wash in two (5%) of 40 volunteers inoculated at 20 000 CFUs per naris, 18 (24%) of 74 inoculated at 80 000 CFUs per naris, and 20 (22%) of 90 inoculated at 160 000 CFUs per naris. Data for the percentage of volunteers found to have S pneumoniae 6B carriage by culture in nasal wash at each timepoint are shown in table 2.
Table 2.
Participants with confirmed Streptococcus pneumoniae serotype 6B (strain BHN418) nasal carriage by microbiological culture from nasal wash
|
Placebo |
PCV13 |
|||
|---|---|---|---|---|
| S pneumoniae serotype 6B carriers | Proportion (95% CI) | S pneumoniae serotype 6B carriers | Proportion (95% CI) | |
| Day 2, CFU per naris | ||||
| 20 000 | 2/19 | 10·5% (1·3–33·1) | 0/21 | 0·0% (0·0–16·1) |
| 80 000 | 11/41 | 26·8% (14·2–42·9) | 4/33 | 12·1% (3·4–28·2) |
| 160 000 | 9/46 | 19·6% (9·4–33·9) | 4/44 | 9·1% (2·5–21·7) |
| Day 7, CFU per naris | ||||
| 20 000 | 1/19 | 5·3% (0·1–26.0) | 0/21 | 0·0% (0·0–16·1) |
| 80 000 | 8/41 | 19·5% (8·8–34·9) | 3/33 | 9·1% (1·9–24·3) |
| 160 000 | 9/46 | 19·6% (9·4–33·9) | 3/44 | 6·8% (1·4–18·7) |
| Day 14, CFU per naris | ||||
| 20 000 | 1/19 | 5·3% (0·1–26·0) | 0/21 | 0·0% (0·0–16·1) |
| 80 000 | 8/41 | 19·5% (8·8–34·9) | 1/33 | 3·0% (0·1–15·8) |
| 160 000 | 10/46 | 21·7% (10·9–36·4) | 3/44 | 6·8% (1·4–18·7) |
| All visits,*CFU per naris | ||||
| 20 000 | 2/19 | 10·5% (1·3–33·1) | 0/21 | 0·0% (0·0–16·1) |
| 80 000 | 12/41 | 29·3% (16·1–45·5) | 6/33 | 18·2% (7·0–35·5) |
| 160 000 | 16/46 | 34·8% (21·4–50·2) | 4/44 | 9·1% (2·5–21·7) |
Data are n/N, proportion (%), or 95% CI. Nasal wash samples were taken 2 days (visit E), 7 days (visit F) and 14 days (visit G) after bacterial inoculation. Differential carriage rates are categorised by inoculation dose and by randomisation allocation (intramuscular saline vs PCV13 28 days before bacterial inoculation). CFU=colony forming unit. PCV13=13-valent pneumococcal conjugate vaccine.
This row aggregates carriage data for individual participants across all three visits.
The experimental carriage rate at each dose was lower in unvaccinated participants than expected. We modelled the dose–response curve (appendix p 5) and showed a plateau function that suggested that further increasing the inoculation dose would not reach the anticipated 60% carriage rate.
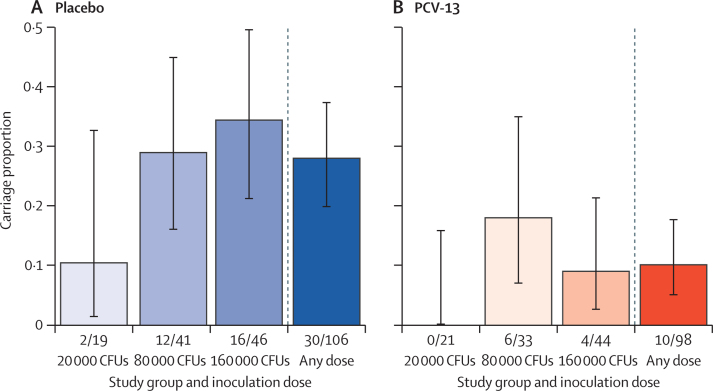
The rate of experimental S pneumoniae 6B carriage is compared by vaccine and placebo groups in figure 2. At every dose, there were fewer participants with experimental S pneumoniae 6B carriage in the vaccine group compared with placebo (figure 2). At 20 000 CFUs per naris, none of the vaccine group and two of the placebo group had S pneumoniae 6B experimental carriage. At 80 000 CFUs per naris there were six carriers in the vaccine group and 12 in the placebo group whereas in the 160 000 CFUs per naris, there were four carriers in the vaccine group and 16 in the placebo group. The log-binomial model adjusting for inoculation dose group showed a 62·4% protection of vaccine compared with placebo (carriage RR 0·38, 95% CI 0·20–0·72, p=0·0033). Our data do not show evidence for a difference in S pneumoniae 6B carriage risk between the 80 000 CFUs per naris and 160 000 CFUs per naris dose, although the log-binomial model did identify a significant reduction in carriage risk for the 20 000 CFUs per naris dose compared with the 160 000 CFUs per naris dose (RR 0·231, 95% CI 0·06–0·92, p=0·038; table 3).
Figure 2.
Streptococcus pneumoniae serotype 6B (BHN418) carriage by study group
Participants randomly assigned to intramuscular 0·9% saline vs PCV13 and inoculation dose. The figure represents carriage proportions by inoculation dose, comparing saline group (A) with PCV13 (B). Number of carriers of total participants are indicated below each bar. Log-binomial regression p value for PCV13 vaccination coefficient, p=0·0033. Fisher's exact test (overall carriage), p=0·0013. Fisher's exact test p value: at doses 20 000 CFU per naris, p=0·22, 80 000 CFUs per naris, p=0·29, and 160 000 CFUs per naris, p=0·005. CFU=colony forming unit. PCV13=13-valent pneumococcal conjugate vaccine.
Table 3.
Summary of the log-binomial regression model fit
| Estimate* | SE | Z statistic | p value | |
|---|---|---|---|---|
| Intercept | 0·327 | 0·201 | −5·573 | <0·0001 |
| PCV13 | 0·376 | 0·333 | −2·936 | 0·0033 |
| 20 000 CFUs per naris | 0·231 | 0·706 | −2·077 | 0·038 |
| 80 000 CFUs per naris | 1·000 | 0·276 | 0·000 | 1·000 |
The baseline carriage risk for a saline-randomised participant inoculated at the CFU 160 000 dose is 33% (95% CI 22·0–48·4). There is a significant effect of PCV13 vaccine on the probability of serotype 6B carriage (risk ratio 0·38, 95% CI 0·20–0·72, p=0·0033). CFU=colony forming unit. PCV13=13-valent pneumococcal conjugate vaccine.
Shows the baseline risk on the Intercept row and the relative risk associated with each of the exposures on the subsequent rows.
As expected from community prevalence data,3 there were high rates of non-experimental pneumococcal carriage in the study groups. A comparison of natural carriage rates (non-S pneumoniae 6B) by vaccine group at each timepoint (pre-vaccination, post-vaccination, day 2, day 7, and day 14 post-inoculation) is shown in figure 3. Data on vaccine-type and non-vaccine-type natural carriage are shown in the appendix (p 6). Natural carriage rates of approximately 20% were found at each timepoint in both PCV13 vaccinated and placebo groups, with 85 (42%) of 204 participants having natural carriage from the point of enrolment to study exit.
Figure 3.
Natural Streptococcus pneumoniae nasal carriage by study visit and study group
Participants randomly assigned to intramuscular 0·9% saline vs PCV13. The figure shows natural carriage proportions per study group at the different study visits. The right-most pair of bars show overall carriage, defined as carriage at any study visit. No S pneumoniae serotype 6B (BHN418) natural carriage episodes were observed before experimental challenge (day 0). PCV13=13-valent pneumococcal conjugate vaccine.
The primary analysis of vaccine effect on experimental pneumococcal (S pneumoniae 6B) carriage was repeated for the group with no natural carriage pre-inoculation and separately for the group with natural pneumococcal carriage at the pre-inoculation visit. These analyses showed a greater protective efficacy of vaccine in the group without natural carriage (69·1%) compared with the group with established natural carriage at the time of inoculation (51·7%; table 4).
Table 4.
Tabulated counts and percentages of experimental Streptococcus pneumoniae serotype 6B (BHN418) carriers stratified by natural carriage and randomisation group
| S pneumoniae 6B carriers | Proportion of S pneumoniae 6B carriers (95% CI) | Vaccine efficacy | |
|---|---|---|---|
| No natural carriage | |||
| Placebo | 21/81 | 25·9% (16·8–36·9) | NA |
| PCV13 | 6/75 | 8·0% (3·0–16·6) | 69·1% |
| Natural carriage | |||
| Placebo | 9/25 | 36·0% (18·0–57·5) | NA |
| PCV13 | 4/23 | 17·4% (5·0–38·8) | 51·7% |
Natural carriage is defined here as being a carrier of a non-6B strain at the pre-inoculation visit. PCV13=13-valent pneumococcal conjugate vaccine. NA=not applicable.
Discussion
We have shown in a double-blind, randomised controlled trial, which made use of a human infection study that PCV13 has a protective effect against experimental pneumococcal carriage in healthy young Malawian adults. This is an important result because of the disappointingly high rates of vaccine-type pneumococcal carriage in our context, following national vaccine roll-out. The study offers both reassurance in showing a vaccine effect, and an explanation for the observed persistent carriage in showing a reduced vaccine effect associated with natural carriage at the time of experimental inoculation. It could be that high rates of natural carriage lead to reduced vaccine effectiveness. This is also a landmark study in that it is, to our knowledge, the first vaccine trial that makes use of a bacterial human infection study in Africa.
There are similarities and differences between our study and the experimental human carriage randomised controlled trial of PCV13 published in Liverpool.14 The clear protective effect of vaccine against carriage is seen in both studies, but is less in Malawi (62%, 95% CI 28–80) than in Liverpool (78%, 48–91). The CIs of these estimates overlap, but the lower value in Malawi is consistent with the observed persistent vaccine-type carriage (18%) in vaccinated children3 compared with no persistent vaccine-type carriage in the UK.24 The human infection studies were both carried out in healthy young adults and resulted in no vaccine or inoculation related adverse events.
The first major difference in the human infection studies in Malawi and Liverpool was that more Malawian participants were male compared with the Liverpool study (72% male in Malawi vs 40% in Liverpool, UK).14 The difference in sex between the two studies is of interest as it reflects enthusiasm for human infection studies and participation in research in general in both settings. It has been shown before that male sex is associated with lower experimental carriage rates, but a mechanism has not been proposed as that study, like this one, excluded women caring for young children.25
The second major difference between the UK and Malawi was the difference in natural carriage. In Malawi, more participants had natural pneumococcal carriage during the study than in Liverpool. 42% of young Malawian adults had an episode of natural carriage during the 2 months of the study compared with only 6% in Liverpool14—and those with natural carriage at the point of inoculation had a reduced experimental carriage rate compared with those without pneumococcal carriage at that moment. This result is very likely to be relevant to children who have high rates of carriage (>80%) in our context. We do not have an explanation for the reduced vaccine effect in participants with pre-existing natural carriage but there might be an immunological mechanism preventing optimal antigen presentation of vaccine in the presence of an ongoing carriage. Regulatory T-cell responses are known to be crucial in persistent carriage.26
In the context of the experimental model, these observations suggest that the ecological niche into which the experimental inoculum is pipetted is a determinant of success or otherwise in establishing experimental carriage. There are other data to support this hypothesis. We have described nasopharyngeal microbiomes in which high diversity was associated with experimental carriage, and lower diversity with less carriage.27 More recently, experimental data from a murine model indicate a hierarchy of success dependent on pneumococcal serotype—the importance of this concept can only be tested by further work in our model with additional serotypes.28 It might be that pneumococcal colonisation alters the human nasopharyngeal microbiome to make acquisition of further pneumococcal serotypes more efficient. Further, we have described an association between asymptomatic viral colonisation and the efficiency of experimental colonisation.29, 30 Studies to establish the viral colonisation of the Malawian participants in this study are in progress. If natural carriage determines experimental carriage, then this factor, and the effect of sex, must be included in vaccine evaluation studies in order not to underestimate the protective efficacy of vaccine.
A limitation of our study is the low rate of experimental carriage that was achieved. We increased the inoculation dose to twice that used in Liverpool. Further, we modelled the dose–response function (appendix p 5) and showed that a plateau effect was evident. This curve suggests that little further carriage would have been achieved by the use of higher inoculation doses. In fact, murine data31 suggest that very high doses might result in inflammation and resulting lower rates of experimental carriage.
The primary purpose of this study was to establish, by means of a human infection study, whether PCV13 was protective against carriage in Malawian adults. This result was clear and showed that vaccination does protect against carriage. This result has been shown to be similar in both Liverpool, UK and Blantyre, Malawi, suggesting that the result is generalisable, albeit with consideration of sex and natural carriage incidence. PCV13 boosts circulating anticapsular IgG,32 but also affects humoral and cellular responses in other compartments. The human infection study method allows sampling of serum and nasal fluid for humoral defenses, and nasal mucosa and blood for cellular defense.29 These studies might suggest means by which the vaccine efficacy of PCV13 to prevent vaccine type carriage might be improved.
This online publication has been corrected. The corrected version first appeared at thelancet.com/microbe on October 4, 2023
Data sharing
An anonymised study dataset can be shared within Malawi in line with local data sharing policy. Requests for data sharing outside Malawi can be presented to the Data Research Support Unit, Malawi–Liverpool–Wellcome Research Programme (https://www.mlw.mw/departments/statistical-support-unit/). A full dataset combining Liverpool and Malawi data is in preparation and analyses from this dataset are planned.
Declaration of interests
DF declares grant funding from Pfizer to her institution for separate projects and consulting fees from Pfizer, MSD, and Sanofi. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We also gratefully acknowledge the support of the Clinical Research Support Unit at Malawi–Liverpool–Wellcome Trust Clinical Research Programme, particularly the input of Markus Gmeiner in establishing human infection studies in Malawi. We thank our willing participants for volunteering, and our Community Engagement team for advice. We thank the Grace Bandawe Conference Centre for participant accommodation and clinical colleagues at Mwai Wathu Private Hospital for being available for emergency cover if ever needed. The work was funded by the Wellcome Trust (grant number 211433 awarded to SBG). We are very grateful for the willing support of our data, safety, and monitoring committee (DSMC; Robert Read, Johnstone Kumwenda, Neil French, Mavuto Mukaka). The DSMC have been attentive to detail and very responsive to our requests for advice.
MARVELS consortium members
Mark Alderson, Sandra Antoine, Christopher R Bailey, Debbie Bogaert, Jeremy Brown, Sarah Burr, Marien, L De Jonge, Klara Doherty, David Goldblatt, Gabriela Gomes, Joel Gondwe, Kate Gooding, Jonathon Grigg, Tina Harawa, Rob Heyderman, Jason Hinds, Angela Hyder-Wright, Simon Jochems, Blessings Kapumba, Vella Kaudzu, Robert Kneller, Richard Malley, Jane Mallewa, Lucinda Manda-Taylor, Edward Mangani, Mphatso Mayuni, Henry Mwandumba, Percy Mwenechanya, Mike Parker, Andrew Pollard, Modesta Reuben, and Jeffrey Weiser.
Contributors
All authors had full access to the data and accept responsibility for publication. MYRH and SBG have accessed and verified all the data reported in the study. All authors have read and reviewed the final manuscript. MARVELS consortium members not listed as authors have advised and collaborated on relevant work packages not the topic of this manuscript. DD, BM, MYRH, JR, JN, DF, KJ, and SBG conceptualised the study, AM, EK, and MYRH curated the data, MYRH and EK did the formal analysis, SBG, KJ, DF, and JR acquired funding, DD, BM, TC, and AEC were responsible for the investigation, EN, TC, VN, CN, SS, BG, GT, MC, KJ, RK, LM, FT, AH, NT, TK-N, JN,CM, LC, NPKB, JR, and JN were responsible for the methodology, NT and JN did the project administration, SG and KJ were responsible for the resources, MYRH was responsible for the software, SBG, BM, DD, TC, GC, and KJ were responsible for the supervision, SBG, MYRH, and TC were responsible for the validation, MYRH and SG were responsible for the visualisation, BM, DD, and SBG wrote the original draft), and SG and all authors reviewed and edited the manuscript. A reflexivity statement for this study is provided in the appendix, pp 7–9.
Supplementary Material
References
- 1.Syeed MS, Ghule P, Le L, et al. Pneumococcal vaccination in children: a systematic review and meta-analysis of cost-effectiveness studies. Value Health. 2023;26:598–611. doi: 10.1016/j.jval.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Zeev N, Swarthout TD, Everett DB, et al. Impact and effectiveness of 13-valent pneumococcal conjugate vaccine on population incidence of vaccine and non-vaccine serotype invasive pneumococcal disease in Blantyre, Malawi, 2006–18: prospective observational time-series and case-control studies. Lancet Glob Health. 2021;9:e989–e998. doi: 10.1016/S2214-109X(21)00165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swarthout TD, Fronterre C, Lourenço J, et al. High residual carriage of vaccine-serotype Streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi. Nat Commun. 2020;11 doi: 10.1038/s41467-020-15786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenraads M, Swarthout TD, Bar-Zeev N, et al. Changing incidence of invasive pneumococcal disease in infants less than 90 days of age before and after introduction of the 13-valent pneumococcal conjugate vaccine in Blantyre, Malawi: a 14-year hospital based surveillance study. Pediatr Infect Dis J. 2022;41:764–768. doi: 10.1097/INF.0000000000003606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahl B, O'Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flasche S, Lipsitch M, Ojal J, Pinsent A. Estimating the contribution of different age strata to vaccine serotype pneumococcal transmission in the pre vaccine era: a modelling study. BMC Med. 2020;18:129. doi: 10.1186/s12916-020-01601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swarthout TD, Henrion MYR, Thindwa D, et al. Waning of antibody levels induced by a 13-valent pneumococcal conjugate vaccine, using a 3 + 0 schedule, within the first year of life among children younger than 5 years in Blantyre, Malawi: an observational, population-level, serosurveillance study. Lancet Infect Dis. 2022;22:1737–1747. doi: 10.1016/S1473-3099(22)00438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho A, Mallewa J, Peterson I, et al. Epidemiology of severe acute respiratory illness and risk factors for influenza infection and clinical severity among adults in Malawi, 2011-2013. Am J Trop Med Hyg. 2018;99:772–779. doi: 10.4269/ajtmh.17-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett A, Pollock L, Bar-Zeev N, et al. Community transmission of rotavirus infection in a vaccinated population in Blantyre, Malawi: a prospective household cohort study. Lancet Infect Dis. 2021;21:731–740. doi: 10.1016/S1473-3099(20)30597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel PD, Patel P, Liang Y, et al. Safety and efficacy of a typhoid conjugate vaccine in Malawian children. N Engl J Med. 2021;385:1104–1115. doi: 10.1056/NEJMoa2035916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witek-McManus S, Simwanza J, Chisambi AB, et al. Epidemiology of soil-transmitted helminths following sustained implementation of routine preventive chemotherapy: demographics and baseline results of a cluster randomised trial in southern Malawi. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0009292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson CL, Reynolds T, Merfeld JD, Biscaye P. Relating seasonal hunger and prevention and coping strategies: a panel analysis of Malawian farm households. J Dev Stud. 2017;54:1737–1755. doi: 10.1080/00220388.2017.1371296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira DM, Neill DR, Bangert M, et al. Controlled human infection and rechallenge with Streptococcus pneumoniae reveals the protective efficacy of carriage in healthy adults. Am J Respir Crit Care Med. 2013;187:855–864. doi: 10.1164/rccm.201212-2277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins AM, Wright AD, Mitsi E, et al. First human challenge testing of a pneumococcal vaccine. Double-blind randomized controlled trial. Am J Respir Crit Care Med. 2015;192:853–858. doi: 10.1164/rccm.201503-0542OC. [DOI] [PubMed] [Google Scholar]
- 15.Morton B, Burr S, Chikaonda T, et al. A feasibility study of controlled human infection with Streptococcus pneumoniae in Malawi. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton B, Burr S, Jambo K, et al. A pneumococcal controlled human infection model in Malawi: transfer of an established pneumococcal carriage model from Liverpool, UK to Blantyre, Malawi—a feasibility study. Wellcome Open Res. 2020;5:25. doi: 10.12688/wellcomeopenres.15689.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morton B, Jambo K, Chikaonda T, et al. The influence of pneumococcal conjugate vaccine-13 on nasal colonisation in a controlled human infection model of pneumococcal carriage in Malawi: a double-blinded randomised controlled trial protocol. Wellcome Open Res. 2022;6:240. doi: 10.12688/wellcomeopenres.17172.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snow G. Package ‘blockrand’: randomization for block random clinical trials. R package version 1.5. https://cran.r-project.org/web/packages/blockrand/blockrand.pdf
- 19.Gritzfeld JF, Wright AD, Collins AM, et al. Experimental human pneumococcal carriage. J Vis Exp. 2013;72 doi: 10.3791/50115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rombach I, Knight R, Peckham N, Stokes JR, Cook JA. Current practice in analysing and reporting binary outcome data—a review of randomised controlled trial reports. BMC Med. 2020;18:147. doi: 10.1186/s12916-020-01598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marschner IC, Gillett AC. Relative risk regression: reliable and flexible methods for log-binomial models. Biostatistics. 2012;13:179–192. doi: 10.1093/biostatistics/kxr030. [DOI] [PubMed] [Google Scholar]
- 22.Morton B, Banda NP, Nsomba E, et al. Establishment of a high-dependency unit in Malawi. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morton B, Barnes KG, Anscombe C, et al. Distinct clinical and immunological profiles of patients with evidence of SARS-CoV-2 infection in sub-Saharan Africa. Nat Commun. 2021;12 doi: 10.1038/s41467-021-23267-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi YH, Andrews N, Miller E. Estimated impact of revising the 13-valent pneumococcal conjugate vaccine schedule from 2 + 1 to 1 + 1 in England and Wales: a modelling study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheliotis KS, Jewell CP, Solórzano C, et al. Influence of sex, season and environmental air quality on experimental human pneumococcal carriage acquisition: a retrospective cohort analysis. ERJ Open Res. 2022;8 doi: 10.1183/23120541.00586-2021. 00586-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pido-Lopez J, Kwok WW, Mitchell TJ, Heyderman RS, Williams NA. Acquisition of pneumococci specific effector and regulatory Cd4+ T cells localising within human upper respiratory-tract mucosal lymphoid tissue. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cremers AJ, Zomer AL, Gritzfeld JF, et al. The adult nasopharyngeal microbiome as a determinant of pneumococcal acquisition. Microbiome. 2014;2:44. doi: 10.1186/2049-2618-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abruzzo AR, Aggarwal SD, Sharp ME, Bee GCW, Weiser JN. Serotype-dependent effects on the dynamics of pneumococcal colonization and implications for transmission. MBio. 2022;13 doi: 10.1128/mbio.00158-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jochems SP, Marcon F, Carniel BF, et al. Inflammation induced by influenza virus impairs human innate immune control of pneumococcus. Nat Immunol. 2018;19:1299–1308. doi: 10.1038/s41590-018-0231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rylance J, de Steenhuijsen Piters WAA, Mina MJ, et al. Two randomized trials of the effect of live attenuated influenza vaccine on pneumococcal colonization. Am J Respir Crit Care Med. 2019;199:1160–1163. doi: 10.1164/rccm.201811-2081LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neill DR, Coward WR, Gritzfeld JF, et al. Density and duration of pneumococcal carriage is maintained by transforming growth factor β1 and T regulatory cells. Am J Respir Crit Care Med. 2014;189:1250–1259. doi: 10.1164/rccm.201401-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsi E, McLenaghan D, Wolf AS, et al. Thirteen-valent pneumococcal conjugate vaccine-induced immunoglobulin G (IgG) responses in serum associated with serotype-specific IgG in the lung. J Infect Dis. 2022;225:1626–1631. doi: 10.1093/infdis/jiab331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
An anonymised study dataset can be shared within Malawi in line with local data sharing policy. Requests for data sharing outside Malawi can be presented to the Data Research Support Unit, Malawi–Liverpool–Wellcome Research Programme (https://www.mlw.mw/departments/statistical-support-unit/). A full dataset combining Liverpool and Malawi data is in preparation and analyses from this dataset are planned.