Summary
Background
Cardiovascular disease and dementia often coexist at advanced stages. Yet, longitudinal studies examining the interplay between atherosclerosis and its risk factors on brain health in midlife are scarce. We aimed to characterise the longitudinal associations between cerebral glucose metabolism, subclinical atherosclerosis, and cardiovascular risk factors in middle-aged asymptomatic individuals.
Methods
The Progression of Early Subclinical Atherosclerosis (PESA) study is a Spanish longitudinal observational cohort study of 4184 asymptomatic individuals aged 40–54 years (NCT01410318). Participants with subclinical atherosclerosis underwent longitudinal cerebral [18F]fluorodeoxyglucose ([18F]FDG)-PET, and annual percentage change in [18F]FDG uptake was assessed (primary outcome). Cardiovascular risk was quantified with SCORE2 and subclinical atherosclerosis with three-dimensional vascular ultrasound (exposures). Multivariate regression and linear mixed effects models were used to assess associations between outcomes and exposures. Additionally, blood-based biomarkers of neuropathology were quantified and mediation analyses were performed. Secondary analyses were corrected for multiple comparisons using the false discovery rate (FDR) approach.
Findings
This longitudinal study included a PESA subcohort of 370 participants (median age at baseline 49·8 years [IQR 46·1–52·2]; 309 [84%] men, 61 [16%] women; median follow-up 4·7 years [IQR 4·2–5·2]). Baseline scans took place between March 6, 2013, and Jan 21, 2015, and follow-up scans between Nov 24, 2017, and Aug 7, 2019. Persistent high risk of cardiovascular disease was associated with an accelerated decline of cortical [18F]FDG uptake compared with low risk (β=–0·008 [95% CI –0·013 to –0·002]; pFDR=0·040), with plasma neurofilament light chain, a marker of neurodegeneration, mediating this association by 20% (β=0·198 [0·008 to 0·740]; pFDR=0·050). Moreover, progression of subclinical carotid atherosclerosis was associated with an additional decline in [18F]FDG uptake in Alzheimer's disease brain regions, not explained by cardiovascular risk (β=–0·269 [95% CI –0·509 to –0·027]; p=0·029).
Interpretation
Middle-aged asymptomatic individuals with persistent high risk of cardiovascular disease and subclinical carotid atherosclerosis already present brain metabolic decline, suggesting that maintenance of cardiovascular health during midlife could contribute to reductions in neurodegenerative disease burden later in life.
Funding
Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III, Santander Bank, Pro-CNIC Foundation, BrightFocus Foundation, BBVA Foundation, “la Caixa” Foundation.
Introduction
Atherosclerosis—the gradual build-up of cholesterol, cellular waste products, inflammatory cells, fibrin, and calcium into atheroma plaques in the arterial wall—is the main underlying cause of cardiovascular disease.1 However, clinical manifestation of atherosclerosis does not occur until decades later when there is narrowing of the arteries or disruption of the atheroma.1 Therefore, early identification of subclinical atherosclerosis is increasingly recommended by cardiovascular disease primary prevention strategies.2 The Progression of Early Subclinical Atherosclerosis (PESA) study is an ongoing longitudinal prospective cohort study of over 4000 asymptomatic middle-aged (40–54 years) individuals exhaustively evaluated for cardiovascular risk and thoroughly imaged for multiterritorial subclinical atherosclerosis.3, 4 Over 60% of these asymptomatic individuals presented subclinical atherosclerosis at the baseline visit, which include around 40% of those classified as having low long-term cardiovascular risk.5
Epidemiological studies suggest that about one-third of all cases of Alzheimer's disease, the most common form of dementia,6 could be attributable to potentially modifiable cardiovascular risk factors, such as midlife hypertension, obesity, physical inactivity, smoking, and diabetes.7 Moreover, the presence of intracranial or extracranial atherosclerosis is linked to dementia at advanced stages.8 Even in cognitively unimpaired individuals, midlife carotid atherosclerosis has been associated with worse cognitive performance, smaller brain volumes, and reduced cerebral blood flow.9, 10 Additionally, we recently showed that cardiovascular risk and subclinical carotid atherosclerosis in asymptomatic middle-aged PESA individuals were linked to brain hypometabolism.11 Brain glucose metabolism, as measured by [18F]fluorodeoxyglucose ([18F]FDG)-PET, has long been regarded as a proxy of brain health.12 Even though it is commonly used as a marker of neurodegeneration,6 [18F]FDG-PET is relatively unspecific because it is strongly coupled to cerebral perfusion and sensitive to cerebrovascular injury13 and neuroinflammation.14 Regional patterns of cerebral hypometabolism convey some degree of specificity that is useful to differentiate among dementias.12 For instance, in our previous cross-sectional study, reduced glucose consumption was detected in parietotemporal regions, which are typically affected in Alzheimer's disease.11 Nevertheless, additional biomarkers can be used in combination with cerebral [18F]FDG-PET to gain insights into the specific pathophysiological mechanisms at work. In this regard, recently developed blood-based brain biomarkers are a promising tool for routine clinical diagnosis of different neurological conditions15 and include, among others, neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) as markers of neurodegeneration and reactive astrogliosis, respectively, and plasma biomarkers of Alzheimer's pathology (ie, amyloid β and phosphorylated tau 181, 217, or 231).15 A precise understanding of the potential mechanisms underlying decreased cerebral glucose consumption is relevant because, although reduced cerebral blood flow can be reverted, neurodegeneration itself is generally irreversible.
Research in context.
Evidence before this study
Previous work on cross-sectional and longitudinal [18F]fluorodeoxyglucose ([18F]FDG)-PET studies in midlife with a focus on cardiovascular risk factors and atherosclerosis was thoroughly reviewed before undertaking this study. We searched PubMed from inception to March 16, 2023, with the terms “cerebral FDG-PET” or “cerebral glucose metabolism” in combination with “subclinical atherosclerosis”, “midlife”, and “longitudinal”. No filters related to dates or languages were applied. Only two studies came up but were discarded because no [18F]FDG-PET analyses were done. When the terms “subclinical”, “midlife”, or “longitudinal” were removed from the search, the number of retrieved studies increased to six, eight, and ten, respectively. However, those studies were either cross-sectional or analysed participants older than 72 years. Additional searches were done with the term “cardiovascular risk factors” instead of “subclinical atherosclerosis”. In this case, 19 studies were retrieved, but mostly referred to brain MRI findings in middle-age to late-age adults (aged 30–89 years). When the terms “midlife” or “longitudinal” were removed from the search, the number of studies increased to 31 and 363, respectively. None of the studies found used plasma biomarkers to assess neuropathology related to decreased cerebral glucose metabolism in midlife.
Added value of this study
To our knowledge, our study presents the first longitudinal evidence of the association of subclinical atherosclerosis progression and its associated cardiovascular risk factors with glucose hypometabolism in brain regions typically involved in Alzheimer's disease, in midlife, decades before its typical age of onset. This work included longitudinal evaluation of blood-based biomarkers, providing valuable data on potential neuropathological changes happening in midlife. We present precise quantification of subclinical atherosclerosis by three-dimensional vascular ultrasound, and the study is further strengthened by its longitudinal design, the age of the individuals assessed, and the thorough characterisation of subclinical atherosclerosis.
Implications of all the available evidence
Our study builds upon existing evidence characterising the effect of the cardiovascular profile on brain health, but goes one step further by pointing to a prolonged exposure to modifiable cardiovascular risk factors during midlife as a cause for neurodegeneration that might affect the brain's ability to cope with future pathology. Also, in light of our results, screening for carotid disease holds a strong potential to identify people vulnerable to brain alterations and future cognitive impairment. This work might have important implications for clinical practice because it supports the implementation of primary cardiovascular prevention strategies early in life as a valuable approach for healthy cerebral longevity.
Longitudinal studies provide a unique understanding of disease progression,16 yet studies of the effect of midlife atherosclerosis and cardiovascular risk factors on brain metabolic decline are scarce. In this study, we assessed whether longitudinal changes of subclinical atherosclerosis and cardiovascular risk factors in middle-aged asymptomatic PESA participants are linked to declines in brain glucose metabolism as measured by [18F]FDG-PET, and the extent to which these links are mediated by blood-based biomarkers of neuropathology. In view of our previous work,11 we hypothesised that steeper longitudinal reductions of cerebral glucose metabolism in Alzheimer's disease regions will be linked to high risk of cardiovascular disease, sustained hypertension, and increments in subclinical carotid atherosclerosis, even after accounting for cardiovascular risk factors.
Methods
Study design and participants
The PESA study (NCT01410318) is an observational, prospective cohort study that recruited 4184 asymptomatic White individuals aged 40–54 years in 2010 in Madrid, Spain, who have been exhaustively screened for subclinical atherosclerosis and cardiovascular risk factors every 3 years.3 Exclusion criteria at baseline included cardiovascular disease, cancer, or any disease expected to shorten lifespan or influence protocol adherence. Expanded information regarding the study design3 can be found in appendix 2 (p 2) and at the PESA study website. The PESA study was approved by the ethics committee of the Instituto de Salud Carlos III (Madrid, Spain). All participants provided written informed consent.
A PESA subcohort of individuals with subclinical atherosclerosis at baseline (ie, in the highest tertile of plaque thickness or with a coronary artery calcification score ≥1)11, 17 underwent [18F]FDG-PET scans at the baseline and the 6-year follow-up visits.
[18F]FDG-PET image acquisition and processing
The acquisition protocol has been previously described.11, 17 Briefly, upper-body craniocaudal PET scans were acquired in five bed positions (3 min per bed position) on a hybrid PET–MRI Philips Ingenuity system (Philips Healthcare, Cleveland, OH, USA) at 120 min after radiotracer intravenous injection (mean target dose 294 MBq (SD 10) of [18F]FDG). Data were reconstructed and processed as detailed in appendix 2 (p 2). Briefly, scans were pairwise-realigned and normalised to the Montreal Neurological Institute standard space. Cerebral [18F]FDG uptake was normalised to the pons, rendering parametric standard uptake value ratio (SUVR) maps. Follow-up and baseline SUVR maps were subtracted, normalised by baseline SUVR, and divided by follow-up time, yielding outcome images of SUVR annual percentage change. All processing steps were carried out with SPM12. Volume-weighted mean SUVR annual percentage change was calculated in the whole cortex and in a meta-region of interest (ROI) including the cingulate, angular, and temporal gyri that define the hypometabolic signature of Alzheimer's disease.18
Determination of cardiovascular risk and atherosclerosis
Cardiovascular risk factors were recorded from medical interviews, body measurements, and blood samples, as previously described.3, 5, 11 Cardiovascular risk was assessed with the 10-year risk scale SCORE2, which uses the following variables: age, sex, smoking status, blood pressure, and total and HDL cholesterol.19 Annual change was calculated by subtracting follow-up and baseline SCORE2 and dividing the result by follow-up time. Analyses stratifying individuals into SCORE2 risk groups were also done. Because Spain is a region of low cardiovascular disease risk according to SCORE2,19 we used the following thresholds for cardiovascular risk stratification to confer a more restricted classification: low risk (SCORE2 <2·5%), moderate risk (SCORE2 2·5–5%), and high risk (SCORE2 >5%). Groups were defined on the basis of the classification at each visit, and participants were classified into stable, decreased, and increased risk groups.
Arterial hypertension was defined as systolic blood pressure of 140 mm Hg or higher, diastolic blood pressure of 90 mm Hg or higher, or use of antihypertensive medication. Hypertension progression was defined as being stable normotensive or hypertensive, or as a change to normotensive or hypertensive status. SCORE2 and hypertension progression groups with small sample size (n≤10) were excluded from analyses.
A three-dimensional vascular ultrasound with standardised 6 cm acquisition was used to quantify atheroma plaque volume in the carotid and femoral arteries20 (ie, sum of plaque volumes in right and left arteries of each territory). Annual change in atheroma volume was calculated by subtracting follow-up and baseline volumes and dividing the result by follow-up time.
Plasma biomarkers
Blood samples were collected in fasting conditions (minimum of 6 h) at baseline and follow-up PESA visits and plasma was extracted and stored at –80°C. Plasma aliquots were processed for blood-based biomarker investigation at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden. The concentrations of NfL, GFAP, and amyloid β 42 and 40 in plasma were quantified with the Simoa Neurology 4-Plex E Advantage kit, and the concentration of phosphorylated tau 181 was quantified with the p-Tau181 V2 Advantage kit (both from Quanterix Corporation, Billerica, MA, USA). Measurements were performed in one round of analysis by board-certified laboratory technicians who were masked to clinical data, and with a single batch of reagents. Intra-assay coefficients of variation, monitored via internal quality control samples, were below 10%. Samples below the assays’ lower detection limit were discarded. The amyloid β 42/40 ratio was calculated as a more accurate estimation of the amyloid accumulation process, compared with peptides alone. Due to the left-skewed data, outliers were removed based on the IQR rule.
Outcomes
The primary outcome of this study was [18F]FDG-PET SUVR annual percentage change as a measure of the longitudinal evolution of brain glucose metabolism. Additional outcomes included cross-sectional baseline and follow-up [18F]FDG-PET SUVR and plasma biomarkers. The outcomes were assessed individually in relation to the progression of cardiovascular risk, hypertension, and subclinical atheroma plaque volume (exposures).
Statistical analysis
Descriptive summaries of study demographics and measurements were calculated. The study's primary hypothesis is that a greater decline in SUVR would be associated with higher cardiovascular risk, persistent hypertension over time, and increased carotid plaque volume when adjusting for cardiovascular risk factors in Alzheimer's disease brain regions (primary endpoint). All remaining analyses are exploratory secondary endpoints.
All statistical models were adjusted for age, sex, education, and APOE genotype (divided into four groups: ε2/ε3; ε3/ε4 or ε4/ε4; ε3/ε3; and ε2/ε4), and, in the case of SUVR models, by plasma glucose concentrations at the time of scans as well. Linear regression models were used to individually test the association of SUVR annual change with SCORE2 annual change and carotid or femoral plaque volume annual change. Plaque volume data were not normally distributed and were consequently log-transformed (log+1). Two additional models of plaque burden were computed, including as additional covariates the carotid or femoral plaque volume at baseline (plaque model) or annual change and baseline SCORE2 estimates (SCORE2 model). SUVR annual change across hypertension progression groups was compared with ANOVA.
Linear mixed effects models were used to study change in SUVR over time of SCORE2 risk and hypertension progression groups, including an interaction term between follow-up time and the variable of interest and with repeated measurements as random effect. A post-hoc analysis was done to further study the individual contribution of each modifiable cardiovascular risk factor that is part of SCORE2 on longitudinal cerebral glucose metabolism (appendix 2 p 3). These statistical analyses were also conducted with blood-based biomarkers as dependent variables, additionally adjusting for BMI and creatinine.21 Mediation analyses were done with plasma biomarkers as mediators of SUVR annual changes, partial mediation being achieved when both total and indirect effects were statistically significant (p<0·05).
All models were computed either at the ROI level (in the whole cortex and in the meta-ROI of Alzheimer's disease) with R version 4.2.0 and at the voxelwise level with SPM12. In ROI analyses, statistical significance was set at p<0·05 and exploratory secondary endpoints were corrected for multiple comparisons with the false discovery rate (FDR) method.22 In voxelwise analyses, brain regions showing significant effects (p<0·05) were identified by means of the threshold-free cluster-enhancement method, as in the cross-sectional study,11 and FDR correction. All cases with missing data were excluded from the respective analyses.
A post-hoc power analysis was performed to determine the statistical power attainable with our sample size (appendix 2 p 3).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
This study included a final subcohort of 370 PESA participants with two sets of valid [18F]FDG-PET brain images for the longitudinal analysis (figure 1). Baseline scans occurred from March 6, 2013, to Jan 21, 2015, and follow-up scans from Nov 24, 2017, to Aug 7, 2019. Median age at the baseline PET scan was 49·8 years (IQR 46·1–52·2) and the median follow-up time was 4·7 years (IQR 4·2–5·2). 61 (16%) participants were women and 309 (84%) were men; 73 (20%) participants were APOE ε4 carriers (table). On average, SUVR decreased by 0·61% (95% CI 0·51 to 0·70) per year in the whole cortex and by 0·77% (0·68 to 0·87) per year in the hypometabolic Alzheimer's disease signature.
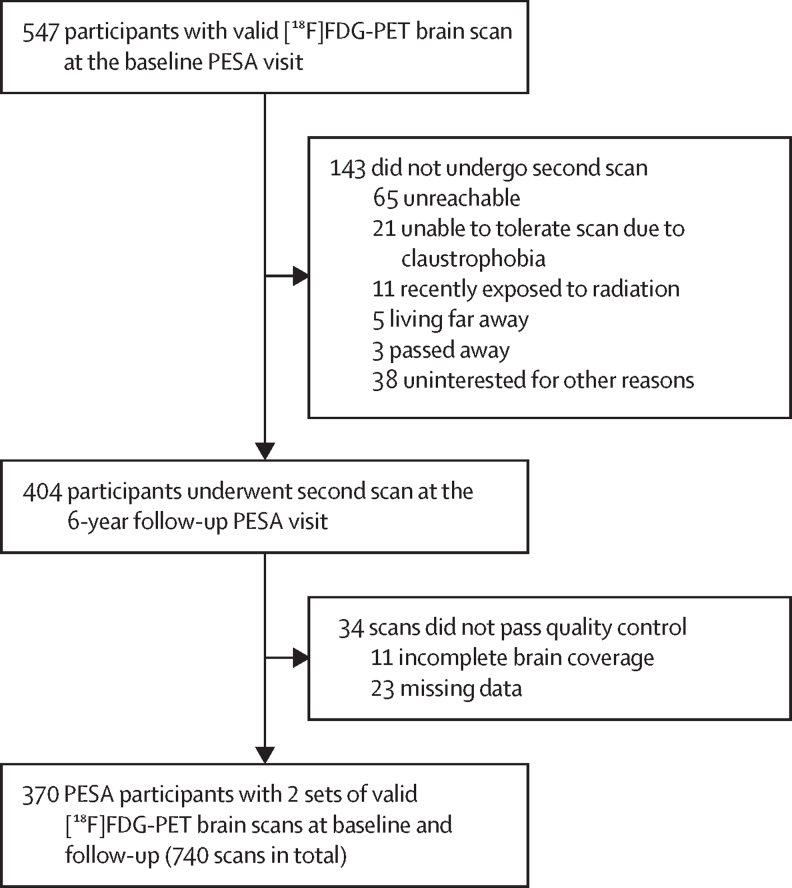
Figure 1.
Study flow
PESA participants with documented subclinical atherosclerosis at baseline underwent two [18F]FDG-PET scans over a 4·7-year period. Of the 547 participants with a valid brain [18F]FDG-PET scan at baseline,11 404 underwent a second scan and, of those, 370 participants were included in the longitudinal analysis. [18F]FDG=[18F]fluorodeoxyglucose.
Table.
Demographics of the studied PESA subcohort
| Study population (n=370, except where otherwise indicated) | ||
|---|---|---|
| Age, years | ||
| At baseline | 49·8 (46·1–52·2) | |
| At follow-up | 54·3 (50·6–57·0) | |
| Follow-up time, years | 4·7 (4·2–5·2) | |
| Sex | ||
| Women | 61 (16%) | |
| Men | 309 (84%) | |
| Race | ||
| White | 370 (100%) | |
| APOE genotype | ||
| ɛ2/ɛ3 | 28 (8%) | |
| ɛ3/ɛ3 | 269 (72%) | |
| ɛ3/ɛ4 | 65 (18%) | |
| ɛ4/ɛ4 | 3 (1%) | |
| ɛ2/ɛ4 | 5 (1%) | |
| SCORE2 risk score, % (n=325) | ||
| At baseline | 3·1% (2·4–4·1) | |
| At follow-up | 4·0% (2·8–5·0) | |
| SCORE2 risk status change between baseline and follow-up (n=325) | ||
| Stable low risk | 50 (15%) | |
| Stable moderate risk | 127 (39%) | |
| Stable high risk | 30 (9%) | |
| Change from low to moderate risk | 47 (14%) | |
| Change from moderate to high risk | 51 (16%) | |
| Systolic blood pressure, mm Hg (n=368) | ||
| At baseline | 120·0 (113·0–129·0) | |
| At follow-up | 120·0 (112·0–132·0) | |
| Diastolic blood pressure, mm Hg (n=368) | ||
| At baseline | 75·0 (70·0–82·0) | |
| At follow-up | 75·0 (69·0–81·0) | |
| Participants with hypertension (n=369) | ||
| At baseline | 73 (20%) | |
| At follow-up | 109 (30%) | |
| Hypertension status change between baseline and follow-up (n=369) | ||
| Stable normotensive | 251 (69%) | |
| Change to hypertensive | 45 (12%) | |
| Stable hypertensive | 64 (17%) | |
| Total cholesterol, mg/dL (n=369) | ||
| At baseline | 208·3 (184·7–228·5) | |
| At follow-up | 203·4 (176·9–227) | |
| HDL cholesterol, mg/dL (n=369) | ||
| At baseline | 43·5 (37·3–50·7) | |
| At follow-up | 48·9 (41·9–57·0) | |
| Smokers (n=362) | ||
| At baseline | 87 (24%) | |
| At follow-up | 70 (19%) | |
| BMI, kg/m2 (n=368) | ||
| At baseline | 27·2 (24·9–29·4) | |
| At follow-up | 26·9 (25·1–29·4) | |
| Serum creatinine, mg/dL (n=369) | ||
| At baseline | 0·8 (0·8–0·9) | |
| At follow-up | 0·9 (0·8–0·9) | |
| Carotid plaque volume, mm3 (n=365) | ||
| At baseline | 3·4 (0–36·1) | |
| At follow-up | 14·8 (0–58·4) | |
| Femoral plaque volume, mm3 (n=344) | ||
| At baseline | 42·2 (0–137·2) | |
| At follow-up | 70·0 (0–186·6) | |
| NfL, pg/mL (n=355) | ||
| At baseline | 8·1 (6·6–10·1) | |
| At follow-up | 9·6 (7·6–12·1) | |
| GFAP, pg/mL (n=359) | ||
| At baseline | 61·2 (47·6–78·6) | |
| At follow-up | 72·2 (55·6–92·4) | |
| Amyloid β 42/40 ratio (n=363) | ||
| At baseline | 0·07 (0·06–0·07) | |
| At follow-up | 0·07 (0·06–0·07) | |
| Phosphorylated tau 181, pg/mL (n=361) | ||
| At baseline | 1·3 (1·0–1·5) | |
| At follow-up | 1·4 (1·1–1·7) | |
Data are median (IQR) or n (%). SCORE2 and hypertension groups were defined on the basis of classification of each individual at baseline and follow-up. SCORE2 and hypertension progression groups with small sample size (n≤10) were excluded from analysis. NfL=neurofilament light chain. GFAP=glial fibrillary acidic protein.
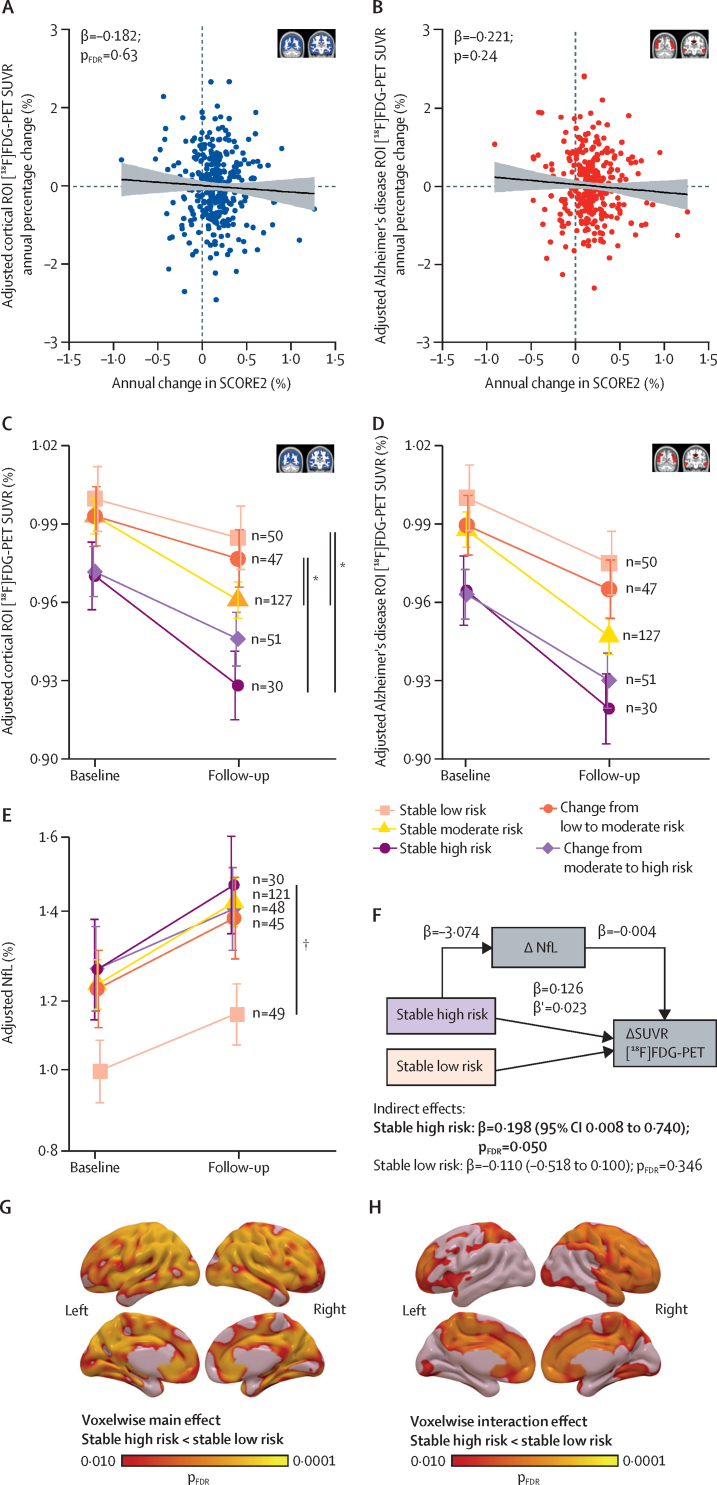
To test whether the progression of cardiovascular risk (measured by SCORE2) was associated with brain glucose metabolism decline, we used linear regression and linear mixed effects models. The decrease observed in [18F]FDG uptake change was not linearly associated with the annual change in SCORE2 in the cortex (β=–0·182 [95% CI –0·585 to 0·221]; pFDR=0·63; figure 2A) nor in the Alzheimer's disease signature (β=–0·221 [–0·586 to 0·144]; p=0·24; figure 2B). When grouping the participants according to SCORE2 categories to stratify them by low, moderate, or high cardiovascular disease risk at each visit (table), individuals at stable low risk over the 4·7-year period had a smaller decline in cortical [18F]FDG uptake (1·48% [95% CI 0·42 to 2·5]) compared with those at sustained high risk, who had the greatest decline over time among the five groups (4·34% [2·83 to 5·86]). A significant difference in cortical SUVR decline between the stable low and high risk groups was observed (group × time interaction; β=–0·008 [95% CI –0·013 to –0·002]; pFDR=0·040; figure 2C; appendix 2 p 4). Cortical SUVR decline was 3·12% (95% CI 2·41 to 3·83) in individuals who remained at moderate risk over time, 1·44% (–0·27 to 2·60) in those who increased from low to moderate risk, and 2·51% (1·22 to 3·80) in those who increased from moderate to high risk. Significant interactions were also found between the stable low-risk and the stable moderate-risk groups, and between individuals whose risk increased from low to moderate and those in stable moderate-risk and stable high-risk groups (figure 2C; appendix 2 p 4). Similar trajectories were found in the Alzheimer's disease meta-ROI, but these did not reach significance (figure 2D; appendix 2 p 4).
Figure 2.
Association between persistent high cardiovascular risk over time and decline in brain glucose metabolism, and its mediation by NfL
(A, B) Scatter plots showing the association between annual change in SCORE2 and the percentage annual change in [18F]FDG-PET SUVR in the cortex (A) and in the Alzheimer's disease meta-ROI (B) with a linear regression model. The line shows the regression fitting and the shading shows 95% CI. (C, D) Mean plots showing the trajectory of [18F]FDG-PET SUVR over time in each SCORE2 progression group in the cortex (C) and in the Alzheimer's disease meta-ROI (D). (E) Mean plot showing the trajectory of plasma NfL over time in each of the SCORE2 progression groups. SUVR and NfL in mean plots were normalised to that of the stable low-risk group at baseline. Group and slope effects were tested with a linear mixed effects model and bars show the 95% CI. (F) Schematic of the mediation analysis output showing that brain metabolism decline was 19·8% (indirect effect) mediated by NfL in the stable high-risk group. β corresponds to the regression coefficients of each model in the direction of the arrow and describe average direct effects. β′ corresponds to the average causal mediation effects. The β coefficients of the indirect effects correspond to the proportion of the SUVR variance mediated by the mediator, NfL. Bold font refers to statistically significant results. (G, H) Statistical voxelwise maps showing the spatial distribution of the main effect (G) and the interaction effect between time and SCORE2 progression groups on [18F]FDG uptake (H), including stable high and stable low. Coloured bars represent the magnitude of the voxel significance corrected for multiple comparisons (pFDR<0·05). All models were adjusted for age, sex, education, and APOE genotype; in the case of SUVR models, by basal glucose concentrations as well; and in the case of NfL models, by BMI and creatinine as well. [18F]FDG=[18F]fluorodeoxyglucose. FDR=false discovery rate. NfL=neurofilament light chain. ROI=region of interest. SUVR=standard uptake value ratio. *pFDR<0·05 (interaction effect). †pFDR<0·05 (main effect).
Next, we longitudinally measured plasma concentrations of NfL, GFAP, and phosphorylated tau 181, as well as the amyloid β 42/40 ratio, to identify potential mechanisms behind the observed cortical [18F]FDG-PET differences among SCORE2 groups. Plasma NfL increased in all groups over time (stable low risk: 16·34% [95% CI 9·70 to 22·99]; stable moderate risk: 20·42% [15·70 to 25·14]; stable high risk: 20·98% [11·80 to 30·15]; change from low to moderate risk: 15·48% [8·60 to 22·36]; change from moderate to high risk: 17·67% [9·53 to 25·82] NfL increase). Stable low-risk individuals presented significantly lower concentrations compared with the stable high-risk group (main effect; β=–3·074 [95% CI –0·428 to –5·743]; pFDR=0·050; figure 2E). Mediation analyses showed a significant indirect (mediating) effect of NfL on the difference in brain metabolism decline between the stable high-risk and the stable low-risk groups (β=0·198 [95% CI 0·008 to 0·740]; pFDR=0·050; figure 2F). No other significant mediation effects were found with NfL or other blood-based biomarkers (appendix 2 p 8). Of note, voxelwise analysis comparing stable low-risk versus stable high-risk groups showed that the main effect was predominant in parietotemporal regions (figure 2G; appendix 2 pp 5–6), whereas the interaction effect occurred in brain areas including the frontal, orbital, and anterior cingulate gyri, and the precuneus (figure 2H; appendix 2 pp 5–6).
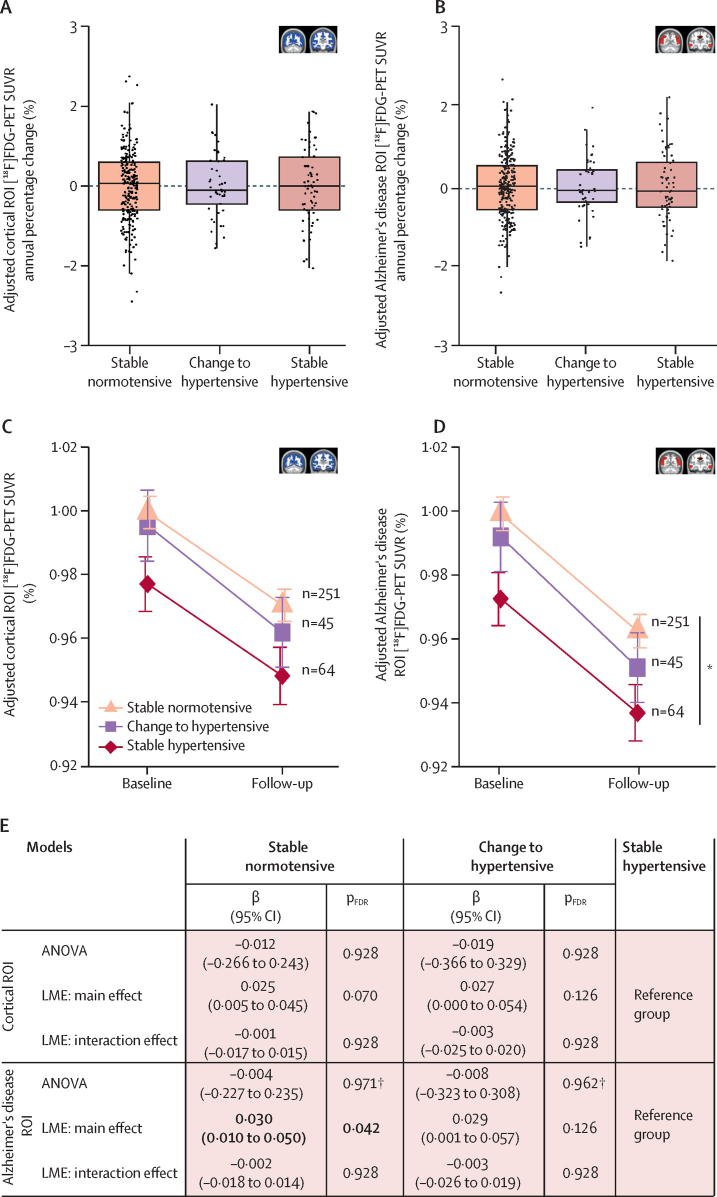
Next, we aimed to evaluate which cardiovascular risk factor had the strongest association with the cerebral metabolic decline observed over time. We first analysed the longitudinal effect of the presence of hypertension on brain metabolism, because hypertension was the cardiovascular risk factor with the strongest association with brain hypometabolism at baseline.11 No significant differences in SUVR annual percentage change (figure 3A, B), or in the interaction effect of group × time on [18F]FDG uptake (figure 3C–E) were found either in the cortex or in Alzheimer's disease-associated brain regions across hypertension progression groups. However, individuals who remained hypertensive showed significantly lower [18F]FDG uptake in the Alzheimer's disease signature than those who remained normotensive (main effect; β=0·030 [95% CI 0·010 to 0·050]; pFDR=0·042; figure 3D, E). In this case, no associations were found between any of the plasma biomarkers measured and hypertension progression (data not shown).
Figure 3.
Association between continuous presence of hypertension and decline in brain glucose metabolism
(A, B) Boxplots showing the association between the progression of hypertension and the annual percentage change in [18F]FDG-PET SUVR in the cortex (A) and in the Alzheimer's disease meta-ROI (B). Group differences were tested with ANOVA. The horizontal line within the boxes shows the median and the upper and lower edges of the boxes show the 75th and 25th percentiles. (C, D) Mean plots showing the trajectory of [18F]FDG-PET SUVR over time in each hypertension progression group in the cortex (C) and in the Alzheimer's disease meta-ROI (D). SUVR was normalised to that of the stable normotensive group at baseline. Group and slope effects were tested with a linear mixed effects model and bars show the 95% CI. The stable hypertensive, change to hypertensive, and stable normotensive groups had a mean cortical SUVR decrease of 2·85% (95% CI 1·79–3·90), 3·28% (2·08–4·48), and 2·78% (2·25–3·31), respectively. (E) Summary table with regression coefficients (β) and pFDR values derived from linear regression and LME models testing the effect of hypertension progression on brain glucose metabolism progression in the cortex and in the Alzheimer's disease meta-ROI. Bold font refers to statistically significant results. All models were adjusted for age, sex, education, APOE genotype, and basal glucose concentrations. [18F]FDG=[18F]fluorodeoxyglucose. FDR=false discovery rate. LME=linear mixed effects. ROI=region of interest. SUVR=standard uptake value ratio. *pFDR<0·05 (main effect). †This p value was not FDR-corrected (only secondary analyses were FDR-corrected).
Linear mixed effects models were used to study the individual effect of each of the modifiable SCORE2 components in individuals who remained at persistent high or low risk at both visits. Because the four SCORE2 components were dichotomised, β coefficients are comparable and represent the magnitude of the weight on [18F]FDG uptake (appendix 2 p 7). Although not significant, the highest β coefficient corresponded to HDL cholesterol, both as main and interaction effects, suggesting this was the cardiovascular risk factor with the largest weight on reduced [18F]FDG brain uptake and on decline of brain metabolism over time.
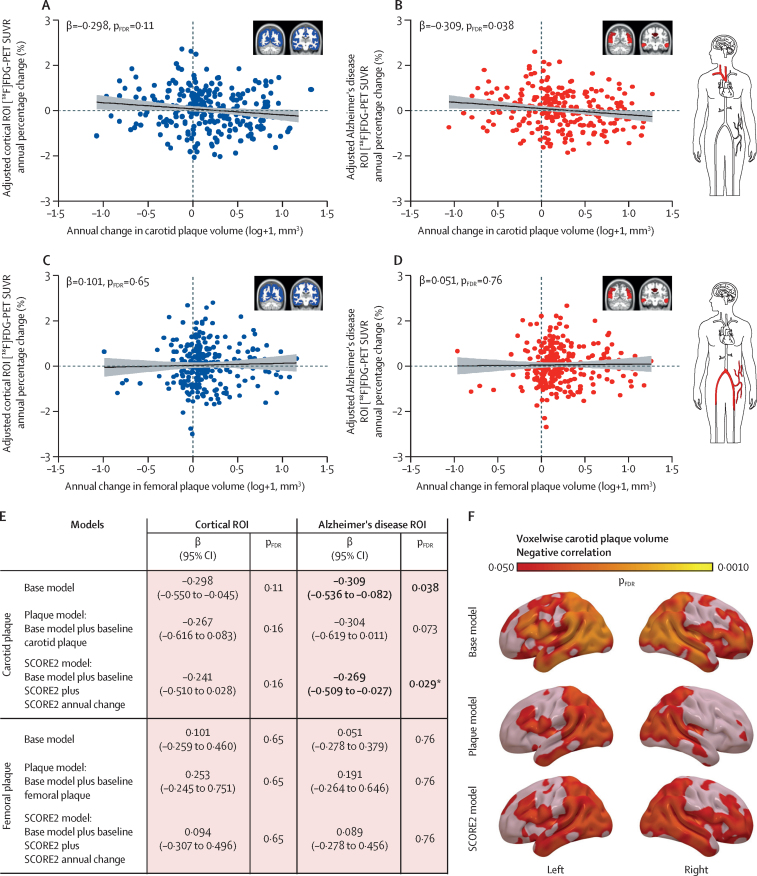
Lastly, we studied the association between longitudinal brain metabolism and subclinical atherosclerosis trajectories. Although no significant association was found between annual change in subclinical carotid atherosclerosis and annual change in SUVR in the cortical ROI (figure 4A), a significant negative association was detected in the Alzheimer's disease hypometabolic signature (β=–0·309 [95% CI –0·536 to –0·082]; pFDR=0·038; figure 4B). As occurred in the cross-sectional study,11 no significant associations were observed with subclinical femoral atherosclerosis (figure 4C–E). Voxelwise analysis showed that the brain regions where progression of carotid atherosclerosis was significantly linked to glucose metabolism decline included the temporal gyrus, hippocampus, occipital lobe, precuneus, and angular gyrus (base model; figure 4F; appendix 2 pp 5–6). When adjusted for baseline carotid plaque volume, this association presented a reduced effect size in the Alzheimer's disease ROI (plaque model; β=–0·304 [95% CI –0·619 to 0·011]; pFDR=0·073; figure 4E), but remained significant in most brain regions identified in the voxelwise analyses (plaque model; figure 4F; appendix 2 pp 5–6). Even after adjustment for annual change in SCORE2 and baseline SCORE2, the association remained significant in Alzheimer's disease regions, indicating that this link was not fully explained by the presence or progression of cardiovascular risk factors (SCORE2 model; β=–0·269 [95% CI –0·509 to –0·027]; p=0·029; figure 4E, F; appendix 2 pp 5–6). When participants without plaque burden at both visits were included in these analyses, the results remained similar (appendix 2 p 9). No associations were noted between plasma biomarkers and annual change in carotid or femoral plaque burden (data not shown).
Figure 4.
Association between progression of subclinical carotid plaque volume and decline in brain glucose metabolism
(A–D) Scatter plots showing the association of annual percentage change in [18F]FDG-PET SUVR in the cortex (A, C) and in the Alzheimer's disease meta-ROI (B, D) with annual change in carotid plaque volume (n=261) and with annual change in femoral plaque volume (n=261), respectively. The line shows the regression fittings and the shading shows the 95% CI. (E) Summary table with regression coefficients (β) and pFDR values derived from linear regression models testing the effect of carotid and femoral plaque volume progression on brain glucose metabolism progression in both ROIs. Bold font refers to statistically significant results. (F) Statistical voxelwise maps showing the brain regions where there is a negative correlation between annual in carotid plaque volume and annual percentage change in [18F]FDG-PET SUVR for the different models. Coloured bars show the magnitude of the voxel significance corrected for multiple comparisons (pFDR<0·05). Plots and statistics represent part of the dataset, excluding those participants without atherosclerotic burden. All models were adjusted for age, sex, education, APOE genotype and basal glucose levels (base model). [18F]FDG=[18F]fluorodeoxyglucose. FDR=false discovery rate. ROI=region of interest. SUVR=standard uptake value ratio. *This p value was not FDR-corrected (only secondary analyses were FDR-corrected).
Discussion
Our results shows that asymptomatic middle-aged individuals at high risk of cardiovascular disease over a 5-year period have a steeper decline in cerebral glucose metabolism compared with individuals at low risk. In those at sustained high risk, 20% of the observed decline can be attributed to neurodegeneration and therefore might be irreversible. We also report, for the first time to our knowledge, that the progression of subclinical carotid atherosclerosis during midlife is associated with a greater decline in metabolism in Alzheimer's brain regions, additionally to that attributed to the presence and progression of classical cardiovascular risk factors.
These data confirm and expand on the results from a previous study on a small middle-aged cohort,23 and on our cross-sectional findings in the same PESA cohort in which higher cardiovascular risk was associated with lower cerebral glucose metabolism.11 They are also in line with reports from older longitudinal cohorts of cognitively intact individuals,24 but go one step further by showing that the decline in cerebral glucose consumption starts earlier, during midlife. We found that middle-aged individuals at sustained moderate and high cardiovascular risk over 5 years had a greater decline in cerebral glucose consumption than those who remained at low risk. This association was not found when analysing the continuous SCORE2 changes, suggesting a threshold effect only captured when comparing opposite cardiovascular risk groups. We observed a 1·5%, 3·1%, and 4·3% global decline in cerebral metabolism in the low-risk, moderate-risk, and high-risk groups, respectively, indicating that middle-aged individuals at persistent high cardiovascular risk have an average drop in brain metabolism that is almost three times higher than that of individuals at low risk. This 4·3% drop over 4·7 years of follow-up means that middle-aged individuals at persistent high cardiovascular risk have a decline in cerebral metabolism of 0·9% per year. This is particularly relevant when compared with the reductions in global cortical glucose metabolism of 1·2% and 2·6% per year reported for patients with mild cognitive impairment and Alzheimer's disease, respectively.25 Hence, the observed reductions in cerebral glucose metabolism might affect the brain's ability to cope with neurodegenerative or cerebrovascular disease later in life.
We also observed a 16%, 20%, and 21% increase in NfL plasma concentrations in the low-risk, moderate-risk, and high-risk groups, respectively, suggesting that middle-aged individuals at persistent high risk of cardiovascular disease over 5 years have an average increase in NfL concentrations, a marker of axonal injury, 1·3 times higher than individuals at low risk. Moreover, these high concentrations of NfL in plasma over 5 years partially (20%) mediated the steeper decline in cerebral glucose metabolism detected in individuals who remained at high cardiovascular risk. Plasma NfL is a well established biomarker of neurodegeneration15 and has been reported to increase in older participants at risk of cardiovascular disease.26 We did not observe differences between cardiovascular risk groups in amyloid β, tau, or astrocytic blood biomarkers, nor did they mediate associations with [18F]FDG uptake changes. Taken together, our results suggest that the observed decline in glucose metabolism in middle-aged asymptomatic individuals at sustained high risk of cardiovascular disease has a neurodegenerative component that is independent of Alzheimer's pathology. These findings are consistent with reductions in brain volume observed in middle-aged individuals with high Cardiovascular Risk Factors, Aging and Dementia scores,27 and are highly relevant because neurodegeneration is generally irreversible. Voxelwise analyses show that individuals with stable high risk of cardiovascular disease had lower cerebral glucose metabolism in Alzheimer's disease-vulnerable regions and greater metabolism declines in lateral and medial frontal areas, compared with the low-risk group. Hypometabolism in the frontal lobe is characteristic of cerebrovascular disease,28 although it is not infrequently found in patients with mild cognitive impairment and Alzheimer's disease,29 suggesting that the hypometabolism detected here might have a cerebrovascular component. In addition, we could not detect significant changes in cerebral glucose consumption in participants whose risk of cardiovascular disease increase from low to moderate over the 5 years of follow-up in this study, suggesting that a longer period with a sustained higher cardiovascular risk profile might be needed to produce the brain changes observed in the groups with persistently moderate and high risk.
Multiple midlife cardiovascular risk factors, such as insulin resistance or high serum cholesterol concentrations, have been independently linked to brain [18F]FDG uptake changes,30, 31, 32 but high blood pressure stands out with an important role in reduced brain metabolism, starting in midlife,11, 33 and with downstream sequelae in cognition.34 In concordance with such reports and with our previous cross-sectional findings,11 we herein found that participants who remained hypertensive had lower brain glucose metabolism. However, all progression groups presented similar rates of decline, indicating that the continuous presence of hypertension alone does not explain the steeper decline in brain glucose metabolism observed in individuals with high risk of cardiovascular disease. A synergistic effect of different cardiovascular risk factors contributing to poor general cardiovascular health, rather than an individual risk factor, could have a broader negative impact on brain health.
We also observed that changes in subclinical carotid atherosclerosis over 5 years were associated with a longitudinal decline in cerebral glucose metabolism in parietotemporal brain regions of asymptomatic individuals, matching the Alzheimer's disease hypometabolic signature, decades before the typical age of onset. This effect was not mediated by plasma biomarkers of neurodegeneration, neuroinflammation, or Alzheimer's disease, and, thus, cannot be interpreted as a preclinical manifestation of neurodegeneration or Alzheimer's pathology. However, irrespective of its cause, a decline in cerebral glucose metabolism in cognitively unimpaired middle-aged individuals is a strong predictor of subsequent cognitive decline.24 Hence, lower cerebral glucose metabolism in Alzheimer's disease-sensitive brain areas could decrease the brain's resilience (ie, its ability to cope with Alzheimer's pathology later in life).35
The association between subclinical carotid atherosclerosis and changes in cerebral glucose metabolism remained significant in Alzheimer's disease-vulnerable regions after correcting for cardiovascular risk, suggesting that the effect of carotid atherosclerosis on cerebral glucose consumption is additive to that of cardiovascular risk factors. At very early stages of atherosclerosis and in low-risk populations, risk equations are not always able to accurately grasp cardiovascular risk, making the quantification of subclinical atherosclerosis through imaging crucial. Here, carotid atherosclerosis seems to explain some effects in brain glucose metabolism that traditional cardiovascular risk factors cannot. Indeed, this association was only found with carotid plaque burden, not with femoral plaque burden, further strengthening the specificity of the effects of subclinical carotid atherosclerosis on the brain. Moreover, voxelwise analyses showed that associations in parietotemporal regions, particularly in the hypometabolic Alzheimer's disease signature, remained significant even after correction for baseline plaque burden, suggesting that the decline in glucose metabolism was mainly driven by changes in plaque volume. Although PESA participants do not present flow-limiting plaques, our results indicate that subclinical carotid atherosclerosis might reflect a specific vulnerability of the carotid cerebral territory, and could be a surrogate for certain cerebral alterations, such as intracranial atherosclerosis or cerebral small vessel disease, which could directly affect cerebral circulation and metabolism.36 Hence, carotid plaque volume and its associated impact on cerebral metabolism might be modifiable, to some extent, through lifestyle interventions. Notably, multidomain interventions could improve or maintain cognitive functioning in at-risk older people.37
Our study has several limitations. First, the lack of longitudinal cognitive testing precludes us from assessing the extent to which the observed declines in cerebral glucose metabolism have any effect on cognition. However, PESA participants are asymptomatic, middle-aged, active workers, and hence we do not expect this population to have major cognitive deficits. Another limitation is that we cannot fully establish the cause of the observed changes in [18F]FDG uptake beyond those results partially attributable to neurodegeneration; other physiological changes (ie, cerebral hypoperfusion) could explain the observed effects. Also, the relatively short period of follow-up time (4·7 years) and the low number of women included in this subcohort might limit the generalisability of the conclusions. The PESA study has been extended to run until 2029, and to include additional brain assessments, such as brain MRI and cognitive testing,4 which will allow some of these limitations to be addressed in the future. Extrapolation of these findings to the general population should be done cautiously because the PESA subcohort included in this analysis was enriched to have a higher prevalence of atherosclerosis. Nevertheless, this enrichment might have allowed us to attain enough statistical power to detect these effects, which are anticipated to be relatively small in an asymptomatic middle-aged population.
Strengths of this study include the large sample of longitudinal [18F]FDG-PET scans in middle-aged asymptomatic individuals, which allowed us to capture early changes in cerebral glucose metabolism, and the thorough characterisation of atherosclerosis in the PESA cohort, using cutting-edge imaging technology to measure longitudinal changes of atherosclerosis during subclinical phases.20 To the best of our knowledge, such measurements are not available in other longitudinal [18F]FDG-PET studies in middle-aged asymptomatic individuals, and hence our findings cannot be replicated in an independent cohort at the moment. The evaluation of longitudinal blood-based biomarkers of neuropathology, which allowed further insights to be gained on the nature and significance of the brain [18F]FDG uptake changes, is another key strength of our study. Further studies are necessary to investigate whether midlife subclinical carotid atherosclerosis and associated cardiovascular risk factors are causally associated with later cognitive decline and with the development of dementia.
Data sharing
Variables used in this study will be made available to others upon publication and reasonable request by email to the corresponding author with a research proposal that will require further review and approval by the PESA Scientific Committee.
Declaration of interests
CT-P received travel grants from The Company of Biologists and International Society of Cerebral Blood Flow and Metabolism. JS-G is a Philips employee. MS has served as a consultant and at advisory boards for Roche Diagnostics International and NovoNordisk; and is a cofounder of ScanDx Sweden. MS-C has served as a consultant and at advisory boards for Roche Diagnostics International; given lectures in symposia sponsored by Roche Diagnostics and Roche Farma; and was granted with a project funded by Roche Diagnostics International. HZ has served at scientific advisory boards or as a consultant for AbbVie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche; and has served as chair of the Alzheimer's Association Global Biomarkers Standardization Consortium. KB has served at scientific advisory boards or as a consultant for Acumen, ALZpath, BioArctic, Biogen, Eisai, Lilly, Julius Clinical, Novartis, Ono Pharma, Roche Diagnostics, and Siemens Healthineers; and has produced or participated in educational programmes for Biogen, Eisai, and Roche Diagnostics. HZ and KB are cofounders of Brain Biomarker Solutions in Gothenburg, which is a part of the GU Ventures Incubator Program (unrelated to the submitted work). JDG reports research support from GE Healthcare, Roche Diagnostics, and Hoffmann-La Roche; has given lectures in symposia sponsored by General Electric, Philips, Life Molecular Imaging, and Biogen; has served on scientific advisory boards or as a consultant for Prothena and Roche Diagnostics; and is the inventor, founder, and co-owner of BetaScreen. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the PESA participants and the imaging, administrative, and medical PESA teams. The PESA study is equally co-funded by the Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) and Santander Bank (Madrid, Spain) and also receives funding from the Instituto de Salud Carlos III (ISCIII), Madrid, Spain (PI15/02019), the European Regional Development Fund (ERDF—A Way to Build Europe), and the European Social Fund (ESF—Investing in Your Future). CNIC is a Severo Ochoa Center of Excellence (CEX2020-001041-S) and is supported by the ISCIII, the Spanish Ministry for Science and Innovation, and the Pro-CNIC Foundation. CT-P was supported by a “la Caixa” Foundation fellowship (ID 100010434, LCF/BQ/DI19/11730052). MC-C was supported by a Miguel Servet type II research contract (ISCIII, CPII21/00007) and the Fondo de Investigación Sanitaria (ISCIII, PI20/00819). We acknowledge the Sephardic Foundation on Aging and other donors of the Alzheimer's Disease Research (grant number A2022034S), a programme of the BrightFocus Foundation, for support of this research. This work was also partially produced with the support of a 2021 Leonardo Grant for Researchers and Cultural Creators from the BBVA Foundation awarded to MC-C (the Foundation takes no responsibility for the opinions, statements, and contents of this project, which are entirely the responsibility of its authors). BI was supported by the European Research Council (ERC-2018-CoG 819775-MATRIX). MS is supported by the Knut and Alice Wallenberg Foundation (Wallenberg Centre for Molecular and Translational Medicine; KAW2014.0363), the Swedish Research Council (2017-02869, 2021-02678, 2021-06545), the Swedish state under the agreement between the Swedish Government and the County Councils, the ALF-agreement (ALFGBG-813971, ALFGBG-965326), the Swedish Brain Foundation (FO2021-0311), and the Swedish Alzheimer Foundation (AF-740191). MS-C receives funding from the European Research Council (grant agreement number 948677), project “PI19/00155”, funded by ISCIII and co-funded by the EU, and a fellowship from “la Caixa” Foundation (ID 100010434) and from the EU's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement number 847648 (LCF/BQ/PR21/11840004). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018), the EU's Horizon Europe research and innovation programme under grant agreement number 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation, USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C, #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the EU's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement number 860197 (MIRIADE), the EU Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), and the UK Dementia Research Institute at UCL (UKDRI-1003). KB is supported by the Swedish Research Council (#2017-00915, #2022-00732), the Swedish state under the agreement between the Swedish Government and the County Councils, the ALF-agreement (#ALFGBG-715986, #ALFGBG-965240), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721, #AF-968270), Hjärnfonden, Sweden (#FO2017-0243, #ALZ2022-0006), the Alzheimer's Association 2021 Zenith Award (ZEN-21-848495), and the Alzheimer's Association 2022–2025 grant (SG-23-1038904 QC).
Acknowledgments
Contributors
VF, MAM, BI, JDG, and MC-C conceptualised the study. VF, BI, JDG, and MC-C acquired funding. JS-G designed the acquisition methods. CT-P, VF, IG-L, AG-A, AF-O, JDG, and MC-C performed the investigation. CT-P did the formal analysis and produced the figures. VF, JDG, and MC-C supervised the study. MC-C oversaw project administration. CP-H, MS, MS-C, JS-G, HZ, and KB provided infrastructural and technical resources. CT-P, BO, AM-A, IG-L, and AG-A curated the data. JS-G, BO, and JDG validated the data. CT-P, JDG, and MC-C accessed and verified all the underlying data. CT-P, JDG, and MC-C wrote the original draft. All authors had full access to all the data in the study, read and approved the final version of the manuscript, and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From subclinical atherosclerosis to plaque progression and acute coronary events: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1608–1617. doi: 10.1016/j.jacc.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Ortiz A, Jiménez-Borreguero LJ, Peñalvo JL, et al. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: rationale and design. Am Heart J. 2013;166:990–998. doi: 10.1016/j.ahj.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B, Fernández-Ortiz A, Fernández-Friera L, García-Lunar I, Andrés V, Fuster V. Progression of Early Subclinical Atherosclerosis (PESA) study: JACC Focus Seminar 7/8. J Am Coll Cardiol. 2021;78:156–179. doi: 10.1016/j.jacc.2021.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Friera L, Peñalvo JL, Fernández-Ortiz A, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation. 2015;131:2104–2113. doi: 10.1161/CIRCULATIONAHA.114.014310. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 8.Cortes-Canteli M, Iadecola C. Alzheimer's disease and vascular aging: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:942–951. doi: 10.1016/j.jacc.2019.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cermakova P, Ding J, Meirelles O, et al. Carotid intima-media thickness and markers of brain health in a biracial middle-aged cohort: CARDIA brain MRI sub-study. J Gerontol A Biol Sci Med Sci. 2020;75:380–386. doi: 10.1093/gerona/glz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Norby FL, Alonso A, et al. Association of carotid intima-media thickness with brain MRI markers in the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) J Stroke Cerebrovasc Dis. 2022;31 doi: 10.1016/j.jstrokecerebrovasdis.2022.106388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes-Canteli M, Gispert JD, Salvadó G, et al. Subclinical atherosclerosis and brain metabolism in middle-aged individuals: the PESA study. J Am Coll Cardiol. 2021;77:888–898. doi: 10.1016/j.jacc.2020.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Chételat G, Arbizu J, Barthel H, et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer's disease and other dementias. Lancet Neurol. 2020;19:951–962. doi: 10.1016/S1474-4422(20)30314-8. [DOI] [PubMed] [Google Scholar]
- 13.Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pelligrino D. Cerebral blood flow response to functional activation. J Cereb Blood Flow Metab. 2010;30:2–14. doi: 10.1038/jcbfm.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvadó G, Milà-Alomà M, Shekari M, et al. Reactive astrogliosis is associated with higher cerebral glucose consumption in the early Alzheimer's continuum. Eur J Nucl Med Mol Imaging. 2022;49:4567–4579. doi: 10.1007/s00259-022-05897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zetterberg H, Blennow K. Moving fluid biomarkers for Alzheimer's disease from research tools to routine clinical diagnostics. Mol Neurodegener. 2021;16:10. doi: 10.1186/s13024-021-00430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ou Y-N, Xu W, Li J-Q, et al. FDG-PET as an independent biomarker for Alzheimer's biological diagnosis: a longitudinal study. Alzheimers Res Ther. 2019;11:57. doi: 10.1186/s13195-019-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-Friera L, Fuster V, López-Melgar B, et al. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J Am Coll Cardiol. 2019;73:1371–1382. doi: 10.1016/j.jacc.2018.12.075. [DOI] [PubMed] [Google Scholar]
- 18.Sala A, Caprioglio C, Santangelo R, et al. Brain metabolic signatures across the Alzheimer's disease spectrum. Eur J Nucl Med Mol Imaging. 2020;47:256–269. doi: 10.1007/s00259-019-04559-2. [DOI] [PubMed] [Google Scholar]
- 19.SCORE2 working group and ESC Cardiovascular risk collaboration SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42:2439–2454. doi: 10.1093/eurheartj/ehab309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Melgar B, Fernández-Friera L, Oliva B, et al. Subclinical atherosclerosis burden by 3D ultrasound in mid-life: the PESA study. J Am Coll Cardiol. 2017;70:301–313. doi: 10.1016/j.jacc.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 21.Binette AP, Janelidze S, Cullen N, Mattsson-Carlgren N, Hansson O. Creatinine and body mass index influence on plasma amyloid ratio, p-tau217 and neurofilament light. Alzheimers Dement. 2021;17 [Google Scholar]
- 22.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- 23.Borja AJ, Hancin EC, Zhang V, et al. Global brain glucose uptake on 18F-FDG-PET/CT is influenced by chronic cardiovascular risk. Nucl Med Commun. 2021;42:444–450. doi: 10.1097/MNM.0000000000001349. [DOI] [PubMed] [Google Scholar]
- 24.Yu G-X, Zhang T, Hou X-H, et al. Associations of vascular risk with cognition, brain glucose metabolism, and clinical progression in cognitively intact elders. J Alzheimers Dis. 2021;80:321–330. doi: 10.3233/JAD-201117. [DOI] [PubMed] [Google Scholar]
- 25.Ossenkoppele R, Tolboom N, Foster-Dingley JC, et al. Longitudinal imaging of Alzheimer pathology using [11C]PIB, [18F]FDDNP and [18F]FDG PET. Eur J Nucl Med Mol Imaging. 2012;39:990–1000. doi: 10.1007/s00259-012-2102-3. [DOI] [PubMed] [Google Scholar]
- 26.Aparicio H, Himali J, Himali D, et al. Association of plasma NfL levels with risk of cardiovascular disease in the Framingham Heart Study (S33.005) Neurology. 2022;98 [Google Scholar]
- 27.Liu X, Dounavi M-E, Ritchie K, et al. Higher midlife CAIDE score is associated with increased brain atrophy in a cohort of cognitively healthy middle-aged individuals. J Neurol. 2021;268:1962–1971. doi: 10.1007/s00415-020-10383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pascual B, Prieto E, Arbizu J, Marti-Climent J, Olier J, Masdeu JC. Brain glucose metabolism in vascular white matter disease with dementia: differentiation from Alzheimer disease. Stroke. 2010;41:2889–2893. doi: 10.1161/STROKEAHA.110.591552. [DOI] [PubMed] [Google Scholar]
- 29.Shivamurthy VKN, Tahari AK, Marcus C, Subramaniam RM. Brain FDG PET and the diagnosis of dementia. AJR Am J Roentgenol. 2015;204:W76–W85. doi: 10.2214/AJR.13.12363. [DOI] [PubMed] [Google Scholar]
- 30.Willette AA, Bendlin BB, Starks EJ, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiman EM, Chen K, Langbaum JBS, et al. Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer's disease and normal aging. Neuroimage. 2010;49:169–176. doi: 10.1016/j.neuroimage.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pak K, Malén T, Santavirta S, et al. Brain glucose metabolism and aging: a 5-year longitudinal study in a large positron emission tomography cohort. Diabetes Care. 2023;46:e64–e66. doi: 10.2337/dc22-1872. [DOI] [PubMed] [Google Scholar]
- 33.Langbaum JBS, Chen K, Launer LJ, et al. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol Aging. 2012;33:827.e11–827.e19. doi: 10.1016/j.neurobiolaging.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 35.Arenaza-Urquijo EM, Przybelski SA, Lesnick TL, et al. The metabolic brain signature of cognitive resilience in the 80+: beyond Alzheimer pathologies. Brain. 2019;142:1134–1147. doi: 10.1093/brain/awz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Li D, Yuan C, et al. Association of severity between carotid and intracranial artery atherosclerosis. Ann Clin Transl Neurol. 2018;5:843–849. doi: 10.1002/acn3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Variables used in this study will be made available to others upon publication and reasonable request by email to the corresponding author with a research proposal that will require further review and approval by the PESA Scientific Committee.