Abstract
Checkpoint markers and immune checkpoint inhibitors have been increasingly identified and developed as potential immunotherapeutic targets in various human cancers. Despite valuable efforts to discover novel immune checkpoints and their ligands, the precise roles of their therapeutic functions, as well as the broad identification of their counterpart receptors, remain to be addressed. In this context, it has been suggested that various putative checkpoint receptors can be induced upon activation. In the tumor microenvironment, T cells, as crucial immune response against malignant diseases as well as other immune central effector cells, such as natural killer cells, are regulated via co-stimulatory or co-inhibitory signals from immune or tumor cells. Studies have shown that exposure of T cells to tumor antigens upregulates the expression of inhibitory checkpoint receptors, leading to T-cell dysfunction or exhaustion. Although targeting immune checkpoint regulators has shown relative clinical efficacy in some tumor types, most trials in the field of cancer immunotherapies have revealed unsatisfactory results due to de novo or adaptive resistance in cancer patients. To overcome these obstacles, combinational therapies with newly discovered inhibitory molecules or combined blockage of several checkpoints provide a rationale for further research. Moreover, precise identification of their receptors counterparts at crucial checkpoints is likely to promise effective therapies. In this review, we examine the prospects for the application of newly emerging checkpoints, such as T-cell immunoglobulin and mucin domain 3, lymphocyte activation gene-3, T-cell immunoreceptor with Ig and ITIM domains (TIGIT), V-domain Ig suppressor of T-cell activation (VISTA), new B7 family proteins, and B- and T-cell lymphocyte attenuator, in association with immunotherapy of malignancies. In addition, their clinical and biological significance is discussed, including their expression in various human cancers, along with their roles in T-cell-mediated immune responses.
Keywords: cancers, checkpoints inhibitors and immunotherapy, immune checkpoints
Introduction
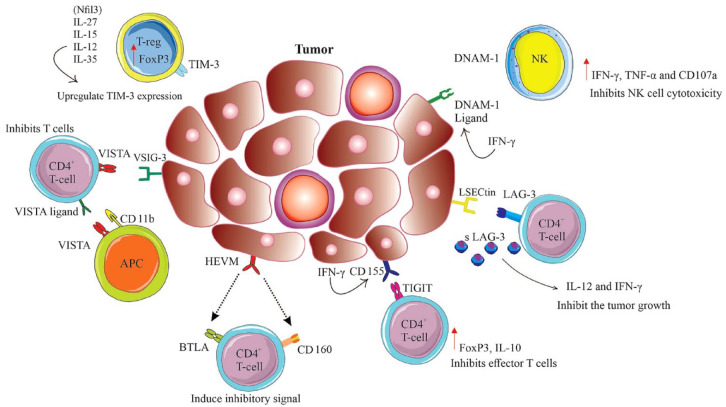
Tumor-associated antigens or immune checkpoint molecules play a pivotal role in the immune regulation of malignancies whose biological significance is crucial in cancer diagnosis, prognosis, and treatment. The checkpoints that are placed either on the surface of various immune cells including T lymphocytes or on tumor cells act like a switching protein through the induction of various signals to control the over-activation of T cells. In cancers, T-cell dysfunctionality may be due to persistent antigen exposure that is associated with overexpression of multiple inhibitory receptors that ultimately reduce T-cell proliferation capacity or functionality. Blockade of checkpoint proteins with immune checkpoint inhibitors prevents the ‘off’ signal from being sent while the blocked condition accumulates and increases T cells in the blood circulation that further amplifies their movement into the tumor to kill cancer cells. In addition, immune checkpoint therapies may consider a novel treatment strategy for infectious diseases and autoimmune disorders. Interestingly, under normal circumstances, inhibitory checkpoint proteins prevent autoimmune damage by suppressing immune responses. In malignancies, the cancerous cells hire mechanisms by which they can protect themselves from being attacked by immune cells through stimulating immune checkpoint targets. 1 To rescue the recognition of the immune response or to maintain homeostasis of the immune system while reducing undesirable immune responses, tumor cells continuously modify their expressions at their molecular level, while in this context, the types of cancers and their various stages significantly influence the compositions of the tumor microenvironment (TME). A great body of research has emphasized that in the TME, immune checkpoint molecules play an important role in the progression and outcome of tumors, and intensive research has eventually led to the development of various cancer immunotherapeutic reagents including monoclonal antibodies. Further to receptors and ligands of immune checkpoints on the cell membrane, various soluble immune checkpoints have been also discovered.2,3 The soluble immune checkpoints that circulate in the serum of patients are produced either under alternative splicing of mRNA or under cleavage of membrane-bound proteins. These circulating isoforms also play a crucial role in cancer immunotherapy by the interaction between soluble receptors or soluble ligands with full-length ligands or full-length receptors, respectively. Despite the aforementioned therapeutic competence of the immune checkpoints, these circulating biomarkers may additionally be useful for the determination of the prognosis of various cancers or/and screening of patients as a first line of diagnosis prior to intensive and invasive cancer diagnostic approaches.4–6 Among various types of surface and soluble receptors or ligands, cytotoxic tumor lymphocyte antigen 4 (CTLA-4) and programmed cell death-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) are the most studied molecules for which blocking antibodies are developed and currently ipilimumab and pembrolizumab have been examined in various clinical trials, respectively.7–9 In this review, we discuss the role of recently targeted checkpoint molecules, including T-cell immunoglobulin and mucin domain 3 (TIM-3), lymphocyte activation gene-3 (LAG-3), T-cell immunoreceptor with Ig and ITIM domains (TIGIT), V-domain Ig suppressor of T-cell activation (VISTA), new B7 family proteins, and B- and T-cell lymphocyte attenuator (BTLA) in the diagnosis, prognosis, and treatments of various cancers.
Classical checkpoints regulators PD-1 and CTLA-4 and their recent clinical application
Adaptive and innate immune systems both interact independently or complementary via the expression of a wide family of inhibitory receptors. Of interest, malignant cells can escape or exhaust the immune system through alteration of the dynamic balance between stimulatory and inhibitory signaling molecules. In this context, T cells, B cells, and natural killer (NK) cells exhibit coinhibitory molecules belonging to the B7-CD28 group of proteins such as CTLA-4 and PD-1 receptors that can adjust the production of antigen-specific T cells while negatively regulating immune tolerance. 10 The PD-L1 that is expressed on antigen-presenting cells (APCs) or cancer cells serves as a ligand for the PD-1 receptor, binds to PD-1 on immune cells, and suppresses immune responses through either suppression of T-cell activation or escaping of cancer cells from immune surveillance. 11 In clinical studies, benefits of monotherapy either with PD-1 or CTLA-4 blockers were associated with low response rates while few patients were found to have a response to therapy. 12 A number of studies revealed that the majority of metastatic melanoma patients could not respond properly to ipilimumab, nivolumab, and pembrolizumab as evaluated by objective response rate.13,14 On the other hand, it has been confirmed that a combination of CTLA-4 and PD-1 blockade can improve the response rates along with the survival rates in multiple cancer types including melanoma, renal cell carcinoma (RCC), colorectal cancer, small-cell lung cancer, mesothelioma, and sarcoma.15–20 Although combining CTLA-4 and PD-1 blockers has been shown to improve treatment outcomes in multiple cancer types, it has been associated with the induction of adverse events in affected patients. In this regard, besides further research to evaluate the combination of other CTLA-4 blockers (tremelimumab) and other PD-1/PD-L1 blockers (cemiplimab, durvalumab, avelumab, and atezolizumab) and their associated dose titration or administration sequence, the search for efficacious combination therapies with newly discovered inhibitory molecules continues.
T-cell immunoglobulin and mucin domain 3
TIM-3, also known as hepatitis A virus cellular receptor 2, which is encoded by HAVCR, comprises TIM-1, TIM-3, and TIM-4 in humans and Tim-1 through Tim-8 in mice. 21 Among various tumor-infiltrating cells in the TME, Tim-3 as the cell terminal dysfunction biomarker is mostly expressed at higher levels by cluster of differentiation 8 (CD8+) tumor-infiltrating lymphocytes (TILs) and cluster of differentiation 4 (CD4+) regulatory T cells (Tregs) and until now, four specific ligands including galectin-9, phosphatidylserine (PtdSer), high mobility group protein B1 (HMGB1), and carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM-1) have been introduced for this immune checkpoint. 22 Further support for the role of TIM-3 is provided by the analysis of genetic polymorphisms. A recent study indicates that TIM-3 and lectin, galactoside-binding soluble 9 (LGALS9) polymorphisms are associated with clear cell renal cell carcinoma (ccRCC) risk and overall survival (OS). 23 Generally, and as mentioned earlier, the dysfunctionality of T cells in malignancies is due to loss of cytotoxicity or deficiency in the production of pro-inflammatory cytokines along with increased expression of various checkpoint proteins. Hence, it has been speculated that antagonizing transcription factor T-cell factor 1 (TCF-1) with Tim-3 can reduce the stemness of CD8+ T cells, the cells that are crucial mediators of tumor clearance. TCF-1 probably maintains stemness and restrains effector differentiation in CD8+ T cells; however, the contribution of Tim-3 to terminal dysfunction of CD8+ T cells is not clearly understood. Elevated expression levels of Tim-3 have also been reported on CD4+ Tregs in human and murine tumors, which were further associated with Foxp3 expression. Interestingly, 60% of fox3+ TILs were found to be TIM-3+, a condition that highlights the crucial role of Tim-3+ Tregs in advanced tumor stages. 24 Furthermore, TIM-3 expression in non-T cells, including macrophages, dendritic cells (DC), and NK cells, also has been reported in several studies; however, the regulation of Tim-3 expression in these cells remains to be uncovered.25–27 Besides the influence of various transcriptional factors, diverse signaling pathways additionally influence the regulation of Tim-3 expression on T cells. For instance, both nuclear factor interleukin 3 regulated (Nfil3) and interleukin (IL)-27, IL-15, IL-12, and IL-35 as novel members of the IL-12 cytokine family upregulate TIM-3 expression in humans and animal models of various malignancies.28–31 TIM-3+, PD-1+, and CD8+ TILs have shown inhibition of the production of the cytokines such as tumor necrosis factor-α (TNF-α), interferon-gamma (IFN-γ), and IL-2. 32 Interestingly, treatment with TIM-3 blockade antibody significantly increased IFN-γ and IL-22/IL-17 levels while it decreased IL-10, that is correlated with enhanced Th1- and Th17-mediated immune response and decreased Treg immune response, respectively. 33 As a prognostic marker in cancer, Tim-3 expression has shown an association with negative outcomes and poor OS in several solid tumors while it can be a valuable therapeutic target. 34 In a study conducted by Pu et al., 35 high expression of TIM-3 was significantly associated with an increased risk of mortality among patients with primary osteosarcoma. In fresh tumor tissue samples of these patients, overexpression of TIM-3 further was reported. Importantly, a positive correlation of TIM-3 expression with lymph node metastasis, tumor grade, and PD-1 expression should not be neglected. 34 In hematological cancers, a recent study with broad immunogen analysis that investigated immune cell co-stimulation/inhibition and cancer antigen expression patterns in association with cancer subtypes or genomics deserves particular attention. 36 In this context, the influence of cancer cell molecular phenotype on immune cell infiltration and function has been described elsewhere. 36 Recently, it has been revealed that in patients with acute myeloid leukemia (AML), there is a correlation between the levels of TIM-3 expression and NK cells cytotoxicity. In this context, the elevated TIM-3+ cell expression within the circulating NK cells has shown positive prognostic value in patients with M1+2 grade but not M4+5. This study indicates that NK cell circulation along with TIM-3 expression levels serves as positive prognostic biomarkers in effective immunity against AML. 37 Consistent with this study, in patients with newly diagnosed AML, the high TIM-3 expression from peripheral blood (PB) samples was also associated with decreased OS rates suggesting that TIM-3 might be considered as a weak prognosis biomarker in patients with AML. 38 In patients with chronic lymphocytic leukemia (CLL), an increased Tim-3 expression pattern on NK cells was observed in comparison to healthy controls. 39 Consistently, low hemoglobin level, high absolute lymphocyte count, and high serum C-reactive protein level have been reported as a poor prognostic profile. Recently, an in vitro investigation on CD8+ T cells isolated from PB of patients with early-stage CLL who were treated with anti-PD-1 and anti-TIM-3 blocking antibodies showed no improvement in CD8+ T cell proliferation. 40 This observation indicates that pretreatment of CD8+ T cells with blocking antibodies in CLL patients does not influence the restoration of CD8+ T-cell functionality, at least in the early clinical stages. These findings provide a rationale for further in vitro and in vivo studies to investigate the efficacy of checkpoint inhibitors for CLL patients. From an epigenetic regulation point of view, the regulation of TIM-3 via DNA methylation of the encoding genes and also its crucial ligand galectin 9 (LGALS9) in association with molecular and immune correlates in malignant melanoma and patients’ survival deserves attention. It has been revealed that DNA methylation has a pivotal role in various biological activities of T cells including T-cell differentiation or exhaustion and tumorigenesis. Holderried et al. 41 have found that TIM-3 and LGALS9 mRNA expression and methylation levels correlated significantly with tumor immune cell infiltration. Although the expression of TIM-3 is associated with T-cell exhaustion, they reported a significantly better OS in association with high TIM-3 and LGALS9 mRNA expression. For both TIM-3 and LGALS9, significant positive correlations between mRNA expression and gene body methylation were reported while inverse correlations between mRNA expression with promoter methylation were identified. Since TIM-3/LGALS9 methylation levels were evaluated in isolated immune cells, melanocyte, and melanoma cell lines, it would be of interest to examine methylation analysis of isolated, exhausted T cells, to understand whether hypomethylation of 5′-C-phosphate-G-3′ (CpG) sites located in the TIM-3 promoter area might serve as a surrogate biomarker for T-cell exhaustion. Finally, it has been reported that TIM-3 expression in melanoma cells is associated with non-responsiveness to PD-1 immune checkpoint blockade (ICB). Importantly, the upregulation of TIM-3 has been observed after PD-1-targeted ICB. Therefore, the analysis of TIM-3 methylation could be a promising predictive approach to select the subgroup of melanoma patients who would benefit from TIM-3-targeted ICB. In addition, the increased co-expression of TIM-3 along with PD-1, CTLA-4, LAG-3, and TIGIT on TILs in early breast cancers (BCs) has indicated these immune checkpoints might synergistically inhibit the response to the tumor. 42 Hence, from a therapeutic point of view, the combined inhibition of these immune checkpoints may synergistically enhance the T-cell response to various tumor antigens. In this context, the in vitro study of combined PD-L1 and TIM-3 blockade indicates enhanced expansion of fit human CD8+ antigen-specific T cells for adoptive immunotherapy. 43 Recently, various clinical trials include TIM-3 as a novel target in cancer immunotherapy. Ongoing clinical trials with Tim-3-specific monoclonal antibodies or antagonist agents are presented in Table 1.
Table 1.
Current ongoing clinical trials for Tim-3-specific monoclonal antibodies or antagonist agents.
| Name of the compound | Mechanism of action | Study phase | Trial ID | Targeted population | Status |
|---|---|---|---|---|---|
| MBG453 + spartalizumab and stereotactic radiosurgery | Tim-3 inhibitor | Phase I | ClinicalTrials.gov NCT03961971 |
In patients with recurrent glioblastoma multiforme | Active, not recruiting |
| INCMGA00012 + INCAGN02385 and INCAGN02390 | Anti-PD-1, anti-LAG-3, anti-TIM-3 | Phases I and II | ClinicalTrials.gov NCT04370704 |
In patients with melanoma | Recruiting |
| TSR-042 | PD-1 inhibitor dostarlimab | Phase II | ClinicalTrials.gov NCT04139902 |
To test the effects of anti-PI-1 inhibitor (TSR-042) or anti-PD-1/anti-TIM-3 combination (TSR-042/TSR-022) in patients with operable melanoma | Recruiting |
| TSR-042 + TSR-022 | PD-1 inhibitor dostarlimab + Tim-3 inhibitor | ||||
| RO7121661 | PD-1/TIM-3 bispecific antibody | Phase I | ClinicalTrials.gov NCT03708328 |
In participants with advanced and/or metastatic solid tumors | Active, not recruiting |
| TSR-022 + TSR-042 | Anti-TIM-3 antibody, anti-PD-1 antibody | Phase II | ClinicalTrials.gov NCT03680508 |
In treating patients with locally advanced or metastatic liver cancer | Recruiting |
| Sym021 (monotherapy) | Anti-PD-1 | Phase I | ClinicalTrials.gov NCT03311412 |
In patients with locally advanced/unresectable or metastatic solid tumor malignancies or lymphomas that are refractory to available therapy or for which no standard therapy is available | Completed |
| Sym021 + Sym022 | Anti-PD-1 + anti-LAG-3 | ||||
| Sym021 + Sym023 | Anti-PD-1 + anti-TIM-3 | ||||
| Sym021 + Sym022 + Sym023 | Anti-PD-1 + anti-LAG-3 + anti-TIM-3 | ||||
| TSR-022 (conducted in two parts: part 1 consisting of dose escalation and part 2 dose expansion) | Anti-TIM-3 antibody | Phase I | ClinicalTrials.gov NCT02817633 |
In patients with advanced solid tumors (AMBER) | Recruiting |
| RO7121661 (compared with nivolumab) | PD1-TIM3 BsAb | Phase II | ClinicalTrials.gov NCT04785820 |
In patients with advanced or metastatic ESCC refractory or intolerant to fluoropyrimidine- or taxane- and platinum-based regimen | Recruiting |
| RO7247669 (compared with nivolumab) | PD1-LAG3 BsAb | ||||
| BGB-A425 + tislelizumab | Humanized IgG1-variant monoclonal antibody against TIM-3 + humanized IgG4-variant monoclonal antibody against PD-1 | Phases I and II | ClinicalTrials.gov NCT03744468 |
In locally advanced or metastatic solid tumors for phase I, dose escalation and phase II safety lead-in, HNSCC, NSCLC, and RCC participants for phase II | Recruiting |
| BGB-A425 + tislelizumab | |||||
| MBG453 (sabatolimab) | Anti-TIM-3 antibody | Phases I and II | ClinicalTrials.gov NCT04623216 |
In participants with AML/secondary AML who are in complete remission with positive measurable residual disease post-allogeneic hematopoietic stem cell transplantation (MRD+ post-aHSCT) | Recruiting |
| MBG453 (sabatolimab) + azacitidine | Anti-TIM-3 antibody + chemical analog of cytidine | ||||
| MBG453 (sabatolimab) + azacitidine | Anti-TIM-3 antibody + chemical analog of cytidine | Phase III | ClinicalTrials.gov NCT04266301 |
In adult subjects with intermediate, high, or very high-risk myelodysplastic syndrome (MDS) as per IPSS-R, or chronic myelomonocytic leukemia-2 (CMML-2) who have an indication for treatment with azacitidine in the first-line setting and are not eligible for intensive chemotherapy or HSCT according to medical judgment by the investigator | Active, not recruiting |
| HDM201 + MBG453 | Potential antineoplastic activity + anti-TIM-3 antibody Or |
Phase I | ClinicalTrials.gov NCT03940352 |
In subjects with AML or high-risk MDS | Active, not recruiting |
| For all subjects, TP53wt status must be characterized by, at a minimum, no mutations noted in exons 5, 6, 7, and 8 |
aHSCT, autologous hematopoietic stem cell transplantation; ESCC, esophageal squamous cell carcinoma; HNSCC, squamous cell carcinoma of the head and neck; HSCT, autologous hematopoietic stem cell transplantation; LAG-3, lymphocyte activation gene-3; RCC, renal cell carcinoma; TIM-3, T-cell immunoglobulin and mucin domain 3.
Lymphocyte activation gene-3
LAG-3 is a transmembrane molecule closely related to CD4, which has been reported to be expressed in multiple immune cells, including T cells, NK cells, natural killer T (NKT) cells, Treg, activated B cells, natural plasma cells, and plasmacytoid dendritic cells (pDCs).44–48 Due to the structural similarity between LAG-3 and CD4, it has been proposed that major histocompatibility complex class II (MHC-II) molecules are ligands for LAG-3 with approximately 100 times greater binding affinity than CD4. 49 Alternative ligands include galectin-3, a 31-kDa lectin that has been shown to modulate T-cell responses, and liver sinusoidal endothelial cell lectin (LSECtin), which is expressed in liver or human melanoma tissues with its growth-enhancing activity through inhibition of antitumor T-cell-dependent responses.50–52 In melanoma cells, IFN-γ production was inhibited by the interaction between LAG-3 and LSECtin through mediating antigen-specific effector T cells. 52 Since LAG-3 influences CD8+ T-cell function as well as CD4+ T cells, the two alternative ligands mentioned above may serve to explain the intrinsic role of LAG-3 on CD8+ T cells in the TME. Furthermore, in a mouse model of Parkinson’s disease, LAG-3 was reported to bind with α-synuclein that produces fibrils in the central nervous system and this finding further indicates a role of LAG-3 immunologically relevant ligands apart from its potential in TME. 53 In tolerogenic conditions like chronic inflammation or tumor environment, LAG-3 co-expression with other immune checkpoints including PD-1, TIGIT, TIM-3, 2B4, and CD160 on immune and cancer cells can lead to functional exhaustion along with a reduction in proliferation and depletion of cytokine secretion. 54 Notably, the inhibitory effects of LAG-3 on CD8+ T cells are different from those of CTLA-4 or PD-1. In this context, the biochemical study of the PD-1 signaling pathway has shown an increased dephosphorylation of the CD28 receptor upon PD-1 ligation with PD-L1 in comparison with the T-cell receptor (TCR). 55 Equivalently, CTLA-4 inhibits T cell activities via competitive binding with CD80 and CD86 co-stimulatory ligands for CD28. As an alternative mechanism, CTLA-4 may delete CD80/CD86 from APCs. By contrast, LAG-3 may decrease T-cell activation through a TCR reducing pathway. The different mechanism of action between LAG-3 and PD-1 or CTLA-4 highlights the importance of synergistic enhancement of its combined antitumor blockade with PD-1. 56 In Table 2, clinical trials involving combined LAG-3 with PD-1, CTLA-4 checkpoints blockade, or with bispecific agents targeting LAG-3 are presented. In addition, several bispecific agents targeting LAG-3 deserve attention. FS118 is a dual antagonist bispecific agent targeting both LAG-3 and PD-L1 that enhances T-cell activation with enhanced antitumor activity. 57 A phase I first-in-human study of FS118, in patients with advanced cancer and PD-L1 resistance, was well tolerated with no dose-limiting toxicity (DLTs). This result highlights the need for further investigation to determine its clinical benefit in patients who have become refractory to anti-PD-(L)1 therapy. 58 In preclinical studies, FS118 could clear MC38 tumor cells while it induces the shedding of LAG-3. The reduced surface expression of LAG-3 and elevated soluble sLAG-3 (sLAG-3) in the serum indicate its potential importance for combined immunotherapy. MGD013 is another bispecific dual affinity retargeting antibodies (DART) molecule that binds PD-1 and LAG-3, and phase I clinical trials in various malignancies have been undertaken. Elevated LAG-3 mRNA expression has also been reported in the red pulp of the spleen, cerebellum, and thymic medulla. 59 Indeed, altered LAG-3 expression together with its cleavage from the surface of immune or tumor cells governs optimal T-cell activity. Notably, through this cleavage, sLAG-3 is released into circulation, although its biological function has not been clearly understood.60,61 A poor expression of sLAG-3 in PB was positively associated with IL-12 and IFN-γ expression in patients with gastric cancer (GC) and increased sLAG-3 expression has shown better prognosis in these patients. 62 Importantly, the in vivo experiments with GC-bearing mice revealed that elevated levels of sLAG-3 may inhibit tumor growth, along with promotion in the secretion of CD8+ T cells, IL-12, and IFN-γ. Hence, prolonged OS along with increased survival rate was correlated with increased levels of sLAG-3 in GC-bearing mice. In a study of human BC expressing estrogen or progesterone receptors, a high level of sLAG-3 was also associated with better OS. 63 In patients with early BC, following treatment with neoadjuvant chemotherapy significantly increased levels of soluble co-inhibitory checkpoints including LAG-3, PD-L1, and TIM-3 have been reported that may indicate the recovery of immune homeostasis in treated patients. 64 Inconsistently, in CLL and ccRCC patients, sLAG-3 was found lightly associated with poor survival, while in the CLL group, the sLAG-3 could enhance leukemic cell activation along with anti-apoptotic properties.65,66 A recent study has shown a correlation between elevated levels of sLAG-3 and advanced tumor stage in ccRCC patients. 66 Elevated sLAG-3 expression has also been reported in Treg cells in the PB of patients with advanced melanoma and colorectal cancer. 67 This contradiction could be explained by the differential regulatory role of sLAG-3 in governing interaction between LAG-3 and MHC-II or by the individual immune response at different cancer sites.68,69 Last but not least, a negative correlation between sLAG-3 expression and CD8A (cluster of differentiation 8a; T-cell marker) in ccRRCC patients may indicate that elevated sLAG-3 may influence T-cell suppression at the tumor site, which may lead to cancer development in these patients. In addition, in virally mediated tumors, overexpression of the LAG-3 for Epstein–Barr virus (EBV) in GC and human papillomavirus (HPV) in cervical cancer and head and neck squamous cell (HNSC) cancer deserves attention. Significant overexpression of LAG-3 has been reported in EBV+ tumors in gastric cancer (stomach adenocarcinoma), and HPV+ tumors in cervical [cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC)] and HNSC cancer. 70 Interestingly, while the expression of PD-1 and CTLA-4 immune checkpoints were also significantly increased in EBV+ GC and HPV+ cervical cancer, neither the ligands of PD-1 nor the ligands of CTLA-4 were upregulated in HPV+ HNSC cancer. 70 Hence, regarding the overexpression of LAG-3 in HPV+ HNSC cancers, LAG-3 blockade alone or in combination with other immune checkpoint pathways blockade including PD-1 might be of particular benefit. However, the results from a recent phase I/II clinical trial of the LAG-3 inhibitor ieramilimab (LAG525) either with anti-PD-1 spartalizumab (PDR001) or alone in patients with advanced malignancies deserve particular attention. 71 Although ieramilimab was well tolerated as monotherapy and in combination with spartalizumab, a moderate response was seen with combined treatment in patients. Hence, with respect to LAG-3 biology, there are still serious debates including whether ligands other than the MHC-II can be actively involved in the course of immunotherapy. Clinical trials that have been conducted so far on LAG-3 are mainly in combination with the PD-(L)1 axis, hence, whether LAG-3 combination with other inhibitory molecules may show promising results or not should be discovered with further experiments. Certainly, data collection from completed and ongoing clinical trials would be extremely helpful to test the hypothesis. Table 2 summarizes the characteristics of anti-LAG-3-specific monoclonal antibodies or antagonists currently used in clinical trials.
Table 2.
Current ongoing clinical trials for LAG-3-specific monoclonal antibodies or antagonist agents.
| Name of the compound | Mechanism of action | Study phase | Trial ID | Targeted population | Status |
|---|---|---|---|---|---|
| INCMGA00012 + INCAGN02385 + INCAGN02390 | Anti-PD-1 + anti-LAG-3 + anti-TIM-3 | Phase I/II | ClinicalTrials.gov NCT04370704 |
The study will determine recommended phase II dose (RP2D) for all study drugs, based on the safety and tolerability of the following combinations: INCAGN02385 + INCAGN02390 and INCAGN02385 + INCAGN02390 + INCMGA00012 | Recruiting |
| REGN3767 | Anti-LAG-3 | Phase I | ClinicalTrials.gov NCT03005782 |
The primary objectives in the dose-escalation phase are to evaluate the safety and PK to determine the selected dose level(s) for expansion of REGN3767 as monotherapy and in combination with cemiplimab in patients with advanced malignancies, including lymphoma | Active, not recruiting |
| REGN3767 + REGN2810 | Anti-LAG-3 + anti-PD-1 | ||||
| Eftilagimod alpha (IMP321) + pembrolizumab | Soluble LAG-3 fusion protein | Phase II | ClinicalTrials.gov NCT03625323 |
Metastatic NSCLC, recurrent PD-X refractory NSCLC, recurrent, or metastatic HNSCC (TACTI-002) | Active, not recruiting |
| Anti PD-1 mAb | |||||
| RO7247669 | Anti-PD-1/anti-LAG-3 bispecific antibody | Phase I | ClinicalTrials.gov NCT04140500 |
Participants with solid tumors, metastatic melanoma, NSCLC, esophageal squamous cell carcinoma | Recruiting |
| EMB-02 | Anti-PD-1/LAG-3 bispecific antibody | Phase I/II | ClinicalTrials.gov NCT04618393 |
The primary purpose of this study is to identify the RP2Ds and schedule assessed to be safe for EMB-02 and to characterize the safety and tolerability of EMB-02 at the RP2Ds | Recruiting |
| FS118 | Anti-PD-L1/anti-LAG-3 bispecific antibody | Phase I/II | ClinicalTrials.gov NCT03440437 |
This is a phase I/II, multicenter, open-label, multiple-dose, first-in-human study, designed to systematically assess safety and tolerability, to identify the maximum tolerated dose and/or RP2D for FS118 in participants with advanced tumors and to determine the efficacy of FS118 in participants with SCCHN | Recruiting |
| Nivolumab + relatlimab | Anti-PD-1 + anti-LAG-3 | Phase II | ClinicalTrials.gov NCT03623854 |
This phase II trial studies how well nivolumab and relatlimab work in treating participants with chordoma that has spread to other places in the body | Recruiting |
| MGD013 | Anti-PD-1, anti-LAG-3 bispecific DART protein | Phase I | ClinicalTrials.gov NCT03219268 |
In patients with advanced solid tumors, hematologic neoplasms, GC, ovarian cancer, gastroesophageal cancer, HER2-positive breast cancer, HER2-positive GC, diffuse large B cell lymphoma (DLBCL) | Active, not recruiting |
| MGD013 + margetuximab | Anti-PD-1, anti-LAG-3 bispecific DART protein + anti-HER2 (human epidermal growth factor receptor 2) mAb | ||||
| BMS-986016 | Anti-LAG-3 monoclonal antibody | Phase I | ClinicalTrials.gov NCT02966548 |
This study will be used to determine the safety and tolerability of BMS-986016 administered alone and in combination with nivolumab in subjects with advanced solid tumors | Active, not recruiting |
| BMS-986016 + nivolumab | Anti-LAG-3 monoclonal antibody + anti-PD-1 mAb | ||||
| Relatlimab + nivolumab + BMS-986205 | Anti-LAG-3 + anti-PD-1 + reduce kynurenine production | Phase I/II | ClinicalTrials.gov NCT03459222 |
The purpose of this study is to demonstrate the safety and preliminary activity with triple combinations of relatlimab in combination with nivolumab and BMS-986205, or in combination with nivolumab and ipilimumab in immunotherapy-naïve and pretreated populations across select advanced tumor types | Recruiting |
| Relatlimab + nivolumab + ipilimumab | Anti-LAG-3 + anti-PD-1 + anti-CTLA-4 | ||||
| Nivolumab | Anti-PD-1 | Phase II | ClinicalTrials.gov NCT04080804 |
The aim of this study is to potentiate adaptive immunity to enhance the antitumor activity of anti-PD-1 antibody by the addition of anti-CTLA-4 antibody or anti-LAG-3 antibody (relatlimab) given in subjects with resectable locally advanced HNSCC prior to surgical resection | Recruiting |
| Nivolumab + relatlimab | Anti-PD-1 + anti-LAG-3 | ||||
| Nivolumab + ipilimumab | Anti-PD-1 + anti-CTLA-4 | ||||
| Relatlimab | Anti-LAG-3 | Phase II | ClinicalTrials.gov NCT03743766 |
The main goal of this study is to evaluate the antitumor activity of relatlimab and nivolumab in combination in subjects with unresectable or metastatic melanoma who have not received prior treatment with immunotherapy | Recruiting |
| Nivolumab | Anti-PD-1 | ||||
| Relatlimab + nivolumab | Anti-LAG-3 + anti-PD-1 | ||||
| Enoblituzumab + retifanlimab | Anti-B7-H3 antibody + anti-PD-1 antibody | Phase II | ClinicalTrials.gov NCT04634825 |
This is a phase II study of enoblituzumab combined with either retifanlimab or tebotelimab administered as first-line treatment to patients with recurrent or metastatic SCCHN | Terminated |
| Enoblituzumab + tebotelimab | Anti-B7-H3 antibody + PD-1 and LAG-3 bispecific DART molecule | ||||
| Nivolumab + relatlimab | Anti-PD-1 + anti-LAG-3 | Phase II | ClinicalTrials.gov NCT04326257 |
In patients with recurrent and/or metastatic HNSCC (R/M HNSCC) who have failed prior immunotherapy with anti-PD-1 or PD-L1 mAb therapy | Recruiting |
| Nivolumab + ipilimumab | Anti-PD-1 + anti-CTLA-4 | ||||
| Margetuximab + INCMGA00012 | Fc-modified anti-HER2 monoclonal antibody + chemo | Phase II/III | ClinicalTrials.gov NCT04082364 |
In patients with HER2-positive GC or gastroesophageal junction cancer | Active, not recruiting |
| Margetuximab + INCMGA00012 + chemo | Fc-modified anti-HER2 monoclonal antibody + anti-PD-1 + chemo | ||||
| Margetuximab + MGD013 + chemo | Fc-modified anti-HER2 monoclonal antibody + anti-PD-1, anti-LAG-3 dual checkpoint inhibitor DART molecule + chemo | ||||
| Margetuximab + chemo (XELOX or mFOLFOX-6) | Fc-modified anti-HER2 monoclonal antibody + chemo | ||||
| Trastuzumab + chemo (XELOX or mFOLFOX-6) | Anti-HER2 monoclonal antibody + chemo | ||||
| LAG525 + spartalizumab (in unselected patients) | Anti-LAG-3 + anti-PD-1 | Phase II | ClinicalTrials.gov NCT03484923 |
To evaluate the efficacy of novel spartalizumab (PDR001) combinations in previously treated unresectable or metastatic melanoma | Active, not recruiting |
| Capmatinib + spartalizumab | c-Met tyrosine kinase(c-Met) inhibitor + anti-PD-1 | ||||
| Canakinumab + spartalizumab | Anti-interleukin-1 beta + anti-PD-1 | ||||
| Ribociclib + spartalizumab | Cyclin-dependent kinase inhibitor + anti-PD-1 | ||||
| LAG525 + spartalizumab (in LAG-3 positive patients) | Anti-LAG-3 + anti-PD-1 | ||||
| Nivolumab + relatlimab | Anti-PD-1 + anti-LAG-3 | Phase II | ClinicalTrials.gov NCT03642067 |
To evaluate the safety and clinical activity of nivolumab and relatlimab in patients with metastatic or locally advanced microsatellite stable colorectal cancer | Recruiting |
| Nivolumab | Anti-PD-1 | Phase I | ClinicalTrials.gov NCT04658147 |
To determine the safety and tolerability of neoadjuvant/adjuvant nivolumab or nivolumab plus relatlimab in patients with hepatocellular carcinoma | Recruiting |
| Nivolumab + relatlimab | Anti-PD-1 + anti-LAG-3 | ||||
| Nivolumab + relatlimab 480 mg/160 mg (co-administered) or 480 mg/960 mg (sequential administration) | Anti-PD-1 + anti-LAG-3 | Phase II | ClinicalTrials.gov NCT03607890 |
In patients with microsatellite instability high solid tumors refractory to prior PD-(L)1 therapy | Recruiting |
| XmAb®22841 | Anti CTLA-4/LAG-3 bispecific antibody | Phase I | ClinicalTrials.gov NCT03849469 |
In subjects with select advanced solid tumors | Active, not recruiting |
| XmAb®22841 + pembrolizumab | Anti-CTLA-4/LAG-3 bispecific antibody + anti-PD-1 |
CTLA-4, cytotoxic tumor lymphocyte antigen 4; Fc, fragment crystallizable; GC, gastric cancer; HNSCC, squamous cell carcinoma of the head and neck; LAG-3, lymphocyte activation gene-3; mAb, monoclonal antibody; NSCLC, non-small cell lung cancer; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; PK, pharmacokinetics; SCCHN, squamous cell carcinoma of the head and neck.
T-cell immunoreceptor with Ig and ITIM domains (TIGIT)
TIGIT, which belongs to the poliovirus receptor (PVR)precursor family, has one extracellular immunoglobulin (Ig) variable domain and a short intracellular domain, while a type I extracellular transmembrane domain shares sequence homology with DNAX accessory molecule-1 (DNAM-1), CD96, CD155, CD111, CD112, CD113, and poliovirus receptor-related 4 (PVRL4). 72 The short intracellular domain includes one immunoreceptor tyrosine-based inhibitory motif (ITIM) and one immunoglobulin tyrosine tail-like motif where both the extracellular and the intracellular domains share 58% sequence homology between humans and mice while in contrast, the ITIM has an identical sequence in mice and humans.72,73 The low expression of TIGIT has been reported on naïve cells including CD4+ T cells, CD8+ T cells, and Tregs along with NK cells and its expression is upregulated to the highest levels in those activated cells that are influenced by various oncogenic expression factors.72,74 Three ligands have been identified for TIGIT including CD112 (also known as nectin-2), CD113, and CD155 (also known as PVR) while the last one is the main ligand in both humans and mice. 75 Notably, most human malignancies have shown overexpression of CD155 and CD112 on various immune cells and in hematopoietic or non-hematopoietic tissue, respectively.76–78 Upregulation of CD155 and CD112 has been found to be caused by IFN-γ in tumor cells. 79 Consistently, it has been also proven that cytokines are produced by DCs through interaction between TIGIT and CD155. 72 TIGIT inhibits effector T cells and NK cells via either cell-extrinsic or cell-intrinsic manner and through interaction with CD155 or DNAM-1 ligand. 80 In addition, the direct inhibitory signals delivered by TIGIT via interaction with PVR and PVRL2 that inhibits human NK cell cytotoxicity are significant. 74 In this context, the mechanisms of action of TIGIT have been recently reviewed elsewhere. 81 Despite the in-depth discovery of the mechanism of action of TIGID, it is still unclear whether all these pathways are active in every TIGIT expression cell or whether each individual immune cell acquires a specific mechanism of action. As we indicated above, on TILs, TIGIT expression increases and its upregulation has been reported in various cancers, including BC, melanoma, GC, chronic myeloid leukemia, colorectal cancer, nasopharyngeal carcinoma, and non-small-cell lung cancer (NSCLC).82–89 For the mentioned malignancies, CD8+ T cells have shown significantly increased levels of TIGIT expression; however, elevated TIGIT levels have been also reported on tumor-infiltrating Treg and NK cells. There are several disappointing clinical outcomes and the expression of TIGIT includes the development of metastases, poor survival, and disease severity along with relapse post-transplantation. Consistent with these findings, there is a study that indicates TIGIT deficiency produced potent antitumor immunity with protection contra B16 experimental lung metastasis in mice. 90 On the other hand, in myelodysplastic syndrome (MDS), which is characterized by abnormal quality and quantity of blood cells including PB cytopenia and dysfunctional bone marrow hematopoiesis, high expression of TIGIT was reported in PB NK and T cells, which was involved in disease progression along with immune escape of MDS. 91 Furthermore, the increased expression of TIGIT in NK, CD8+, and CD4+ restricts the function of NK and T cells through decreasing cytokines expression including IFN-γ, TNF-α, and CD107a. 91 Decreased expression levels of aforementioned cytokines were also observed in higher-risk MDS patients compared to lower-risk patients. Interestingly, TIGIT+ T and NK cells show significantly increased proliferation compared to TIGIT− T and NK cells. The observed results revealed that TIGIT can be considered as a negative immune checkpoint in MDS, with inhibitory effects on cytokines secretion and proliferation ultimately leading to inhibition of antitumor immune response in MDS patients. Consistent with this conclusion, Han et al. 92 recently reported new pathways of antitumor response by TIGIT blockade through analysis of fragment crystallizable gamma receptor (FcγR) interaction and myeloid cell activation. 92 The authors found that the anti-TIGIT therapeutic effect rather is achieved by possible reverse activating signals via FcγRs on myeloid cells with induction of cytokines and chemokines expression, than depletion of TME Treg or any other immune cell expressing TIGIT. The production and control of various cytokines through activating FcγRs have been also previously reviewed in human pathogen defense and autoimmunity. 93 In a recent study on patients with oral squamous cell carcinoma (OSCC), a dysfunctional phenotype including low secretion of IL-2, TNF-α, and IFN-γ was reported for TIGIT highly expressed CD4+ and CD8+ T cells from PB mononuclear cells and TILs. 94 Notably, inhibitory functions such as high expression of Foxp3 and elevated levels of IL-10 were also measured for TIGIT+ CD4+ T cells in these patients. Last but not least, in this study, the in vitro proliferation and cytokine production of CD4+ and CD8+ T cells improved with TIGIT blockade. Connected to these findings, the Fc-dependent antitumor capabilities of anti-TIGIT antibodies, which are independent of Treg depletion, deserve attention in malignant diseases. Hence, a novel design of a new class of engineered Fc-antibodies may enhance the therapeutic abilities of anti-TIGIT antibodies improving their antitumor immune response either alone or in combination with other ICB. In this context, the simultaneous blockade of three different checkpoint receptors including PD-1, LAG-3, and TIGIT along with NBTXR3-enhanced localized radiation combinatorial therapy in an anti-PD1-resistant lung cancer model in mice, has shown improved therapeutic efficacy. 95 Consistently, in preclinical studies of the ICB-resistant colorectal tumor model MC38 expressing human carcinoembryonic antigen, the combined treatment strategy of three independent pathways TIGIT:CD155, PD-1/PD-L1, and transforming growth factor beta (TGF-β) has shown significant antitumor efficacy. 96 In addition, the combination of atezolizumab (anti-PD-L1) and tiragolumab (anti-TIGIT) treatment in an ex vivo assay for microsatellite stable colorectal tumor cells could restore CD4 and CD8 TILs functionality in this type of cancers which are also resistant to anti-PD-1/PD-L1 therapy. 97 Finally, the recent study of dose escalation for etigilimab (anti-TIGIT antibody) alone or in combination with nivolumab (anti-PD-1 antibody) in patients with locally advanced or metastatic solid tumors showed an acceptable safety profile that warrants further research in clinical trials. 98 Besides, recently developed bispecific PD-L1/TIGIT antibodies have shown promising results in preclinical studies including increased OS in transgenic mice. 99 The bispecific PD-L1/TIGIT antibodies have also shown improved human IL-2 secretion by primary human T cells. Consistently, by applying CD122-directed IL-2 complexes in mice treated with radiation and anti-PD-1, the circulating stem-like CD8+ T cells were increased. 100 Notably, a recent study has also indicated that low-affinity IL-2 (laIL-2) to PD-1+ T cells could induce better tumor control with lower toxicity. 101 It should be mentioned that in this study, the laIL-2 could neither activate peripheral CD8+ nor Treg cells due to low binding features to IL-2Rα and IL-2Rβ, while it conversely was able to activate CD8+ T cells in the tumor particularly when coupled with anti-PD-1. Until now, there are two bispecific antibodies targeting PD-L1/TIGIT including HLX301 and PM1022, from Henlius Inc. and Biotheus Inc., respectively, which have been under study. Ongoing clinical trials for TIGIT-specific monoclonal antibodies or antagonists are presented in Table 3.
Table 3.
Current ongoing clinical trials for TIGIT-specific monoclonal antibodies or antagonist agents.
| Name of the compound | Mechanism of action | Study phase | Trial ID | Targeted population | Status |
|---|---|---|---|---|---|
| Elotuzumab (active comparator) + pomalidomide + dexamethasone | Anti-SLAMF7 + angiogenesis and myeloma cell growth inhibitor + binds to GCR | Phase I/II | ClinicalTrials.gov NCT04150965 |
Patients with relapsed refractory multiple myeloma who have relapsed after treatment with prior therapies | Recruiting |
| Relatlimab | Anti-LAG-3 | ||||
| Relatlimab + pomalidomide + dexamethasone | Anti-LAG-3 + angiogenesis and myeloma cell growth inhibitor + binds to GCR | ||||
| BMS-986207 | Anti-TIGIT | ||||
| BMS-986207 + pomalidomide + dexamethasone | Anti-TIGIT + angiogenesis and myeloma cell growth inhibitor + binds to GCR | ||||
| Tislelizumab + ociperlimab Tislelizumab |
Anti-PD-1 + anti-TIGIT Anti-PD-1 |
Phase II | ClinicalTrials.gov NCT04693234 |
Participants with previously treated recurrent or metastatic cervical cancer | Active, not recruiting |
| IBI939 (dose escalation) | Anti-TIGIT | Phase I | ClinicalTrials.gov NCT04353830 |
Subjects with advanced malignancies | Active, not recruiting |
| IBI939 + sintilimab (dose-escalation stage) | Anti-TIGIT + anti-PD-1 | ||||
| IBI939 + sintilimab (expansion stage) | Anti-TIGIT + anti-PD-1 | ||||
| Tislelizumab + ociperlimab | Anti-PD-1 + anti-TIGIT | Phase II | ClinicalTrials.gov NCT04732494 |
Participants with PD-L1 tumor area positivity ⩾10% unresectable, locally advanced, recurrent, or metastatic esophageal squamous cell carcinoma | Recruiting |
| Tislelizumab + placebo | Anti-PD-1 | ||||
| BGB-A1217 (ociperlimab) + tislelizumab | Anti-TIGIT + anti-PD-1 | Phase I | ClinicalTrials.gov NCT04047862 |
Patients with locally advanced and metastatic solid tumors | Recruiting |
| AB154 (domvanalimab) + AB122 (zimberelimab) |
Anti-TIGIT + anti-PD-1 | Early phase I | ClinicalTrials.gov NCT04656535 |
Patients with first or second recurrence of glioblastoma | Recruiting |
| COM902 | A TIGIT inhibitor | Phase I | ClinicalTrials.gov NCT04354246 |
Subjects with advanced malignancies | Recruiting |
| COM701 + BMS-986207 + nivolumab | PVRIG inhibitor + anti-TIGIT + anti-PD-1 | Phase I/II | ClinicalTrials.gov NCT04570839 |
Patients with advanced solid tumors | Recruiting |
| Tislelizumab + ociperlimab | Anti-PD-1 + anti-TIGIT | Phase III | ClinicalTrials.gov NCT04746924 |
The purpose of the study is to compare PFS between arm A (ociperlimab in combination with tislelizumab) and arm B (pembrolizumab in combination with placebo) as assessed by investigators according to response evaluation criteria in solid tumors version 1.1 (RECIST v1.1) and to compare overall survival between arm A and arm B | Recruiting |
| Pembrolizumab + Placebo | Anti-PD-1 | ||||
| Tislelizumab + placebo | Anti-PD-1 | ||||
| Tiragolumab + atezolizumab | Anti-TIGIT + anti-PD-L1 | Phase III | ClinicalTrials.gov NCT04543617 |
Participants with unresectable esophageal squamous cell carcinoma (or those who are unable or unwilling to undergo surgery) and whose cancers have not progressed following definitive concurrent chemoradiotherapy | Recruiting |
| Tiragolumab (placebo) + atezolizumab | Placebo + anti-PD-L1 | ||||
| M6223 | Anti-TIGIT | Phase I | ClinicalTrials.gov NCT04457778 |
Participants with metastatic or locally advanced solid unresectable tumors | Active, not recruiting |
| M6223 + bintrafusp alfa | Anti-TIGIT + anti-PD-L1/TGF-β | ||||
| Tiragolumab + atezolizumab | Anti-TIGIT + anti-PD-L1 | Phase III | ClinicalTrials.gov NCT04294810 |
Participants with previously untreated locally advanced, unresectable or metastatic PD-L1-selected NSCLC, with no EGFR mutation or anaplastic lymphoma kinase translocation | Recruiting |
| Placebo + atezolizumab | Placebo + anti-PD-L1 | ||||
| Pembrolizumab + vibostolimab | Anti-PD-1 + anti-TIGIT | Phase I/II | ClinicalTrials.gov NCT04305054 |
Participants with advanced melanoma and to identify the investigational agent(s) that, when used in combination, are superior to the current treatment options/pembrolizumab monotherapy | Recruiting |
| Pembrolizumab | Anti-PD-1 | ||||
| Pembrolizumab/quavonlimab (coformulation) | Anti-PD-1/anti-CTLA-4 | ||||
| Pembrolizumab/quanvonlimab (coformulation) + lenvatinib | Anti-PD-1/anti-CTLA-4 + multiple kinase inhibitor | ||||
| Pembrolizumab + quavonlimab + vibostolimab Pembrolizumab + quavonlimab + lenvatinib |
Anti-PD-1 + anti-CTLA-4 + anti-TIGIT Anti-PD-1 + anti-CTLA-4 + multiple kinase inhibitor |
Phase I/II | ClinicalTrials.gov NCT04305041 |
Participants with PD-1 refractory melanoma to identify the investigational agent(s) that, when used in combination, are superior to the current treatment options/historical control available | Recruiting |
| Etigilimab + nivolumab | Anti-TIGIT + anti-PD-1 | Phase I/II | ClinicalTrials.gov NCT04761198 |
Subjects with locally advanced or metastatic solid tumors | Recruiting |
| Pembrolizumab + vibostolimab Pembrolizumab + V937 Pembrolizumab |
Anti-PD-1 + anti-TIGIT Anti-PD-1 + binds to intracellular adhesion molecule 1 Anti-PD-1 |
Phase I/II | ClinicalTrials.gov NCT04303169 |
Participants with stage III melanoma who are candidates for neoadjuvant therapy to identify the investigational agent(s) that, when used in combination, are superior to the current treatment options/historical control available | Recruiting |
| Sasanlimab + encorafenib + binimetinib Sasanlimab + axitinib + SEA-TGT |
Anti-PD-1 + BRAF inhibitor + MEK inhibitor Anti-PD-1 + VEGFR1–3, c-Kit, PDGFR inhibitor + anti-TIGIT |
Phase I/II | ClinicalTrials.gov NCT04585815 |
Patients with NSCLC | Active, not recruiting |
| CRC01 + fludarabine + cyclophosphamide | Anti-CD19 CAR-T (PD-1 knockdown, TIGIT knockdown) + DNA synthesis inhibitor + cell apoptosis (DNA crosslinks both between and within DNA strands at guanine N-7 positions) | Phase I/II | ClinicalTrials.gov NCT04836507 |
Adult patients with relapsed or refractory large B-cell lymphoma | Recruiting |
| GPC3 and/or TGF-β targeting CAR-T cells | CD4+ T cells are genetically engineered to express TGFβ-CAR and secret IL-7/CCL19 and/or SCFVs against PD-1/CTLA-4/TIGIT, CD8+ T cells are constructed to express GPC3-DAP10-CAR with knockdown of PD-1/HPK1 | Phase I | ClinicalTrials.gov NCT03198546 |
Human hepatocellular carcinoma patients with GPC3 expression | Recruiting |
CAR-T, chimeric antigen receptor T; EGFR, epidermal growth factor receptor; LAG-3, lymphocyte activation gene-3; NSCLC, non-small-cell lung cancer; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1; PFS, progression-free survival; TGF-β, transforming growth factor beta; TIGIT, T-cell immunoreceptor with Ig and ITIM domains.
V-domain Ig suppressor of T-cell activation
VISTA is a type I Ig membrane protein with 55–65 kDa molecular weight which is also known as differentiation of embryonic stem cells 1, platelet receptor Gi24 precursor, B7-H5, SISP1, death domain 1α (DD1α) and programmed death protein-1 homolog (PD-1H) that belongs to the B7-family while shares 22% homology with PD-L1 but has large structural differences with CD276, CD80, and CD86 among the B7 family. 102 VISTA expression on hematopoietic and myeloid cells has been addressed together with its high expression on mature APCs, which are analyzed for their high CD11b. The extrinsic transformation inhibitory signals induced to T cells by VISTA, when expressed on APCs, further address its action as a ligand on myeloid cells, particularly, APCs. 103 In addition, V-Set and immunoglobulin domain-containing 3 (VSIG-3) is also known as immunoglobulin superfamily member 11 (IGSF11) or brain-specific testis-specific immunoglobulin superfamily (BT-IgSF), and has been identified recently as a ligand of the B7 family member VISTA/PD-1H, and interestingly its inhibitory effect on human T-cell functions through a novel VSIG-3/VISTA pathway has been unveiled. 104 It should be noted that, despite the structural similarity of the VISTA extracellular domain to PD-L1, there is no association of VISTA with the CD28-B7 family. Therefore, the pathways through which VISTA and PD-1 checkpoints govern their inhibitory functions are independent. 105 Finally, but not least, the coinhibitory receptor P-selectin glycoprotein ligand-1 (PSGL-1) has recently been shown to interact with multiple histidine residues in the extracellular domain of VISTA particularly at the acidic pH, a condition that mostly found in TMEs. At acidic pH conditions, the R chain of histidine looses protons and the subsequent post-translational modification facilitates binding to the ligand. 106 Importantly, VISTA-mediated immune suppression is inhibited in vivo by antibodies that block the associated interaction in acidic environments. 106 Remarkably, the insignificant VISTA expression on CD8+, CD4+, Treg, and TILs also deserves attention. 105 On CD4+, VISTA act like a co-inhibitory receptor and its action inhibits T-cell activation, propagation, and cytokines production via anti-CD3 activation. 107 Consistently, VISTA−/− CD4+ T cells have shown strong antigen-specific proliferation along with cytokine production as compared with naïve T cells and further VISTA-neutralizing monoclonal antibodies attenuate VSIG-3-induced T-cell inhibition via decreasing the binding of VSIG-3 and VISTA.104,108 In hematological malignancies, the potential roles of VISTA in immunotherapy also deserve attention. For example, in a study performed by Pagliuca et al., 109 the elevated VISTA expression can influence a patient’s response to chemotherapy by impeding the immune response and facilitating leukemia relapse. In this study, the elevated VISTA expression in myeloid neoplasia versus lymphoid subsets was seen by analyzing of human leukemia and lymphoma cell lines. In addition, the VISTA overexpression in AML cells of nucleophosmin 1 (NPM1) mutant was confirmed in both leukemic and CD3+ cells in short first remission versus long first remission cases according to the length of first remission. By observing these results, authors conclude that the VISTA high expression on AML cells might indicate an early reaction to immune activation-mediated tension for controlling tumor progression. Furthermore, this study highlighted the VISTA as a potential target for the treatment of patients with AML either in the prevention of disease recurrence or in patients with treatment refractory disease. In a murine brain glioma model, the VISTA-deficient animal highly resisted tumor induction, and in this model, depletion of CD4+ T cells enhanced tumor formation. 110 Consistently, elevated VISTA expression is also reported in glioma tissues in Gtex when compared with normal tissues. 111 With worthiness, in a recent study conducted by Zhang et al., 112 based on VISTA and CD8+ TILs in patients with hepatocellular carcinoma (HCC), they classified TMEs into four immune subtypes as follows: VISTA+/CD8+, VISTA+/CD8−, VISTA−/CD8+, and VISTA−/CD8+. 112 In this study, patients with VISTA-positive expression in tumor cells have shown prolonged OS as compared with those with VISTA-negative expression, and the tissue dual positive for VISTA and CD8+ TILs that were measured by various tissue microarray analysis was associated with favorable TME and better OS. In patients with high-grade serous ovarian cancer, VISTA expression on immune and endothelial cells was associated with pathologic type, while conversely its expression on tumor cells was associated with prolonged OS. 113 Furthermore, the association between VISTA expression in tumor cells and a favorable prognostic significance has recently been reported in patients with pancreatic cancer. 114 Consistent with these findings, in prostate cancer patients who were treated with anti-CTLA-4 (ipilimumab), the increased expression of PD-L1 and VISTA inhibitory molecules in independent subsets of macrophages deserves attention. 115 In OSCC, VISTA expression in tumor cells and lymphocytes is associated with IL-33 levels. 116 The IL-33 biology in various cancers has been described elsewhere. 117 In fact until recently and in patients with cancer, VISTA has mostly been described as a negative checkpoint regulator that suppresses T-cell activation with subsequently poor prognosis. Hence, the novel discovery of VISTA protein expression in tumor cells of patients with HCC or patients with high-grade serous ovarian cancer has underscored the importance of its newly found expression on tumor cells that results in a favorable prognosis. This finding may be of great interest to investigate the potentially precise role of VISTA expression in tumor cells and to design a rational combination for cancer immunotherapy. Consistently, a recent study indicates that VISTA expression was promoted in tumor cells following the chemotherapy through the HIF-2α transcription factor. 118 In the same study, the VISTA-blocking antibody 13F3 has shown therapeutic enhancement to carboplatin therapy. Importantly, in a recent study of patients with RCC along with venous tumor thrombus, the increased VISTA expression on immune cells was associated with T-cell exhaustion TOX marker expression and a worse prognosis of the disease. 119 These results further indicate a VISTA inhibitor may potentially have synergistic effects when combined with chemotherapy. Given the significant therapeutic potential of VISTA targeting approaches, some VISTA-specific antagonist agents along with monoclonal antibodies with their potential suppressing VISTA activity that have recently moved into the clinical trials are presented in Table 4.
Table 4.
Current ongoing clinical trials for VISTA-specific monoclonal antibodies or antagonist agents.
| Name of the compound | Mechanism of action | Study phase | Trial ID | Targeted population | Status |
|---|---|---|---|---|---|
| CI-8993 | Human immunoglobulin (Ig) G1κ monoclonal antibody (mAb) against the VISTA ligand | Phase I | ClinicalTrials.gov NCT04475523 |
Patients with relapsed/refractory solid tumors | Recruiting |
| JNJ-61610588 | Human IgG1 kappa anti-VISTA monoclonal antibody | Phase I | ClinicalTrials.gov NCT02671955 |
Participants with advanced cancer | Terminated |
| CA-170 | Small molecule (PD-L1/PD-L2), and (VISTA) antagonist | Phase I | ClinicalTrials.gov NCT02812875 |
Adult patients with advanced solid tumors or lymphomas who have progressed or are nonresponsive to available therapies and for which no standard therapy exists | Completed |
PD-L1, programmed cell death-ligand 1; VISTA, V-domain Ig suppressor of T-cell activation.
New B7 family checkpoints molecules
The newly discovered representatives of the B7 family comprise five members which are B7-H3 (also known as CD276), B7-H4 [(also known as B7S1, B7x, or V-set domain-containing T-cell activation inhibitor 1 (Vtcn 1)], B7-H5 (also known as VISTA, platelet receptor Gi24 precursor or PD-1H), B7-H6 [NK cell cytotoxicity receptor 3 ligand 1 (NCR3LG1)], and B7-H7 [human endogenous retrovirus-H long terminal repeat-associating protein 2 (HHLA2)].120,121 The important features of B7-H5, known as VISTA, and its role in the regulation and suppression of immune reactions in cancer are shown in the previous section. Thus, in this section, apart from B7-H5, we discuss the novel features of other members of the type I membrane B7 family. In mice and humans, there is a sequence similarity between B7-H3 and the extracellular domain of PD-L1; however, in humans, there is also an alternative isoform with a tandem repeat of immunoglobulin variable (IgV) and immunoglobulin constant (IgC) domains (VCVC) that is the most frequently expressed isoform. 120 At the RNA level, the B7-H3 is generally expressed in both lymphoid and nonlymphoid cells while at the protein level, its expression is mainly found in T cells, B cells, monocytes, activated DC, and NK cells. Furthermore, its aberrant expression has been reported in various malignancies such as ovary, colorectum, liver, breast, prostate, brain, RCC, and NSCLC.120,122 The putative receptor which has been identified for B7-H3 is located on activated T cells; however, monocytes and macrophages that are influenced by various disease conditions are also recognized. 123 The B7-H3 co-stimulatory or co-inhibitory action on CD4+ and CD8+ via TCR signaling that enhances IFN-γ production to produce cellular immunity, or through the nuclear factor of activated T cells, nuclear factor kappa B (NFκB), and activator protein 1 factor that influences TCR regulation to inhibit the associated gene transcription has been reported, respectively.124,125 Since the precise B7-H3 receptors have not been identified, this controversial functionality of B7-H3 might be due to other possible binding ligands that need to be discovered. Last, but also important, the potential role of soluble B7-H3 (sB7-H3) released from cells into serum and its blockage of matrix metalloproteinase inhibitor, which consequently accumulates B7-H3 on the cell surface, should be mentioned. In this context, the high circulating serum B7-H3 levels have been associated with various malignancies in patients. 126 The association between the B7-H3 expression and the poor outcome in various human cancers further emphasized the feasibility of this immune checkpoint in the prediction and prognosis of various human cancers. For instance, B7-H3 in human BC is a direct target of microRNA (miR)-29c. 127 Nygren et al. 127 have found that the high expression of miR-29c is associated with a significantly reduced risk of death from BC. 127 Downregulation of miR-29c may play roles in BC progression through deregulating B7-H3 expression in BC. Interestingly, nearly 50 miRNAs that downregulate B7-H3 at protein levels have been identified, and among them, 13 miRNAs including miR-214, miR-363, miR-326, miR-940, miR-29c, miR-665, miR-34b, miR-708, miR-601, miR-124a, miR-380-5p, miR-885-3p, and miR-593 target B7-H3 via binding to its three prime untranslated region (3′-UTR). 127 Consistently, multiple adverse clinical symptoms have been found in association with elevated B7-H3 expression in either tumor cell or diffuse tumor vasculature in patients with ccRCC. 128 In particular, in higher grade and stage RCC, lower miR-187 expression levels are observed. 129 Downregulation of miR-187 may be relatively involved in RCC progression through its interfering influence on B7-H3 expression. In osteosarcoma cells, the expression level of B7-H3 is a direct target of miR-124, and overexpression of this tumor suppressor miRNA, inhibits cell proliferation through targeting B7-H3 in OS tumor tissue in vitro. 130 Interestingly, in recent years, miRNA profiling and sequencing revealed that its expression is dysregulated in cancer mainly via amplification or deletion of miRNA genes or transcriptional control changes. 131 Since miRNAs might act as either oncogenes or tumor suppressors in different conditions, further studies of their alterations and associated signatures can be a useful approach for tumor classification, diagnosis, prognosis, and even immunotherapeutic treatments. Among five major hematologic malignancies, the B7-H3 expression was reported to be highest in patients with AML and lowest in patients with acute lymphoblastic leukemia. 132 Importantly, analyzing B7-H3 expression via the TCGAseq and GSE10358 datasets showed that B7-H3 is associated with negative prognostic value in AML patients. Notably, positive correlation between B7-H3 expression and four genes belonging to the tumor necrosis factor family including TNFRSF4 (OX40), TNFSF9 (CD137L), TNFSF14 (LIGHT), and TNFRSF18 (GITR) was identified in patients with AML. In addition, in these patients, the VISTA (B7-H5) and CD70 genes also showed a positive correlation with B7H3 expression. Consistent with this study, other studies confirm blocking CD70 in patients with AML has effective therapeutic results. 133 Among various strategies in cancer immunotherapy, recently chimeric antigen receptor T (CAR-T) cell therapy in prostate cancer tissues and cells also deserves attention. 134 The high expression of B7-H3 on the surface of PC3, DU145, and LNCaP cells and prostate cancer tissues was efficiently inhibited in vitro and in vivo by B7-H3 CAR-T cells in an antigen-dependent manner. Furthermore, in vitro application of B7-H3 CAR-T cells into tumor cells produced high levels of IFN-γ and TNF-α that indicates B7-H3 can be a potential target for specific CAR-T cells therapy in prostate cancer. There are already studies that explain the precise role of those cytokines in the treatment of cancers. 135 Consistently, in NSCLC treatment with dihydroartemisinin (DHA), the B7H3 expression was also actively involved with the positive effect of the antitumor agent. 136 In this study, DHA treatment largely inhibited the B7-H3 overexpression while it expanded the infiltration of CD8+ T Lymphocytes in the xenografts. This result further highlights the potential effect of B7-H3 blockade in cancer immunotherapy. Another type I membrane B7 family member is the B7-H4 that contains one IgV and one IgC domains with 87% amino acid similarity between humans and mice. 120 Recent studies indicated the broad spectrum of patients’ tumors that have been associated with aberrant B7-H4 expression across a wide variety of cancers, including colorectal, craniopharyngioma, RCC, BC, prostate cancer, pancreatic cancer, esophageal squamous cell carcinoma (ESCC), and cervical cancer.137–144 Furthermore, serum sB7-H4 may be a valuable prognostic marker in patients in this wide spectrum of cancers. In patients with non-metastatic ccRCC, the high levels of sB7-H4 along with elevated PB neutrophil count were associated with either poor progression-free survival (PFS) or OS. 145 Recently, Emaldi and Nunes-Xavier 146 have found increased B7-H4 gene expression in Caki-1 and 786-O renal cancer cells that were treated either with tyrosine kinase inhibitors (axitinib, cabozantinib, and lenvatinib) or mTOR inhibitors (everolimus and temsirolimus). 146 In this study, knocking down the expression of B7-H4 by small interfering RNA (siRNA) reduced renal cancer cell viability while increasing sensitivity to drug treatment. These findings highlight the therapeutic potential of B7-H4 in immune checkpoint-targeted therapy. Although the activated T cells and myeloid-derived suppressor cells (MDSCs) express the B7-H4 putative receptor, its counter receptor has not been identified yet. The contact between B7-H4 and its associated receptors inhibits TCR-mediated T-cell proliferation and induces IL-2 production which negatively regulate T-cell responses. Consistently, in a study conducted by Xu et al., 147 the B7-H4 expression at both mRNA and protein levels was measured to be upregulated by IL-2, IFN-α, and IFN-γ in a ccRCC cell line which was obtained from patients with RCC. 147 In this study, the low efficacy of IL-2, IFN-α, and IFN-γ in metastatic RCC could be due to the pathway in which the B7-H4 escapes from the immune response. Finally, it should be considered that immunohistochemical analysis of cervical cancer tissues has also revealed a negative correlation between B7-H4 expression and IL-2 cervical cancer patients. 148 Since this research proposes that the putative B7-H4 receptor could be induced upon activation, the discovery of more specific receptors, even at highly differentiated levels, could improve our understanding of B7-H4 antitumor immunity. It should be taken into account that the B7-H4 mRNA is extensively spread in peripheral tissues but it has low or absent expression at protein level in normal tissues. Hence, its high protein expression in tumor tissues exposes the B7-H4 as a crucial target for immunotherapy. Consistently, the preclinical study with B7-H4-specific CAR-T cells or its antibody-mediated blockade has revealed promising therapeutic results in vitro and in vivo.149,150 Inconsistent with the findings mentioned above, the positive prognostic role of sB7-H4 in patients with NSCLC treated with pembrolizumab should not be neglected. 151 For example, patients with elevated levels of sB7-H4 (>63.9 pg/mL) have shown longer OS and PFS. In this context, unveiling the precise interaction of sB7-H4 with the cell protein that consequently enhances T-cell-mediated immune reaction deserves particular attention. Among the B7 family members identified until now, B7-H6 and B7-H7 are the most recently distinguished proteins. B7-H6 activates NK cells through NKp30 has two Ig domains including IgV and IgC with homologous sequences as well as the other B7 family members. 152 In contrast with the B7-H4, the B7-H6 mRNA is not broadly found in normal tissues while its high expression has been reported in various subsets of human HCC and chronic myeloid leukemia.152,153 Similarly, the expression of human B7-H6 protein is not seen in healthy tissues while its expression is elevated on various human tumors such as cervical cancer, ESCC, BC, small-cell lung cancer, and posterior lipopolysaccharide (LPS) stimulation of glioma cells.154–158 In this context, the negative prognostic value of B7-H6 in human cancers deserves also particular attention. In patients with pancreatic cancer, the B7-H6 expression on tissues has been associated with tumor progression and metastasis while notably the soluble form of it has shown the same profile in these patients. 159 Shorter OS was seen also at high expressed levels of both the B7-H6 cell surface and sB7-H6. In this study, the in vitro knocking out of B7-H6-induced NK cells increased cytokine production and increased the PC tumor cells’ interaction with NK-mediated cytotoxicity. Apart from these findings, the B7-H6 may show opposing functions based on its opposite effects on NK cells. In this context, some reports indicate that the B7-H6-expressing tumor cells can be eliminated by the immune system through cytotoxicity or secretion of various cytokines. Several approaches applied to tumor therapies including radiotherapy, chemotherapy, and immune mediator therapy using cytokines such as TNF-α, have been shown to upregulate B7-H6 expression in tumor cells and enhance their sensitivity to NK cell cytolysis. 160 Although tumor-induced B7-H6 cells stimulate innate immunity, the mechanism by which these cells escape from the immune system should not be overlooked. For example, the B7-H6 induces anti-apoptosis through signal transducer and activator of transcription 3 (STAT3) pathway activation and promotes tumor proliferation. 161 In addition, to speed up tumorigenesis, B7-H6 secretes TNF-α, IFN-γ, and B7-H6-specific bispecific T-cell engager (BiTE) triggers T. 161 In this context, the specific interaction of the B7-H6 on the surface of transformed cells with NKp30 that produces IFN-γ also deserves attention. 162 Notably, low IFN-γ levels in various cancers including H22 hepatoma and B16 melanoma induced PD-L1, PD-L2, CTLA-4, and facilitated tumor immune escape. 163 In human oral squamous carcinoma, PD-L1 surface expression is increased by IFN-γ through the protein kinase D isoform 2 (PKD2) signal pathway while inhibition of PKD2 activity prevents PD-L1 expression along with antitumor effect enhancement of tumor antigen-specific T cell. 164 It should be taken into account that both type IFN I and II regulate a multigenic PD-L1-dependent and PD-L1-independent resistance to ICB and their precise molecular mechanisms have been explained elsewhere. 165 Several factors that influence tumor cells can also regulate B7-H6 expression. For example, its surface expression can be upregulated by endoplasmic reticulum (ER) stress. Protein kinase R-like ER kinase (PERK) that phosphorylates eukaryotic initiation factor-2α (eIF2α) plays a pivotal role in B7H6 induction via ER stress. 166 Obiedat et al. 166 have revealed that nelfinavir and lopinavir enhance eIF2α phosphorylation and subsequently provoke B7-H6 expression, a condition in which the enhanced B7-H6 expression improved melanoma targets for CAR-T cells directed against this immune checkpoint. 166 Furthermore, the class I histone deacetylase inhibitors (HDACi) or siRNA-mediated knockdown of the class I histone deacetylases (HDAC) 2 or 3, downregulates the B7-H6 expression either at transcription or at translation levels, respectively. 162 Such a downregulation at the translation level reduces the NKp30-dependent effector functions of NK cells. 162 Regarding these findings and as a potential cancer treatment, combined immunotherapy with HDACi should be further investigated. Finally, regarding the therapeutic potential of bispecific antibodies targeting B7H6, recent results obtained from a novel B7-H6-targeted IgG-like T cell-engaging antibody in gastrointestinal tumors deserve attention. This study revealed that B7-H6/CD3 IgG-like T-cell engager (ITE) induced redirection of T cells into B7H6 expressing tumor cells yielded various results including proliferation of T cells along with B7-H6-dependent lysis of tumor cells. In addition, in in vitro coculture assays and in vivo colorectal cancer models, cytokine secretion and infiltration of T cells into tumor tissues were observed. 167 These results highlight the importance of additional clinical investigations in B7-H6 targeted therapy. Until now, the regulation of B7-H6 expression in tumor cells is not adequately explored. However, a broad understanding of the actual mechanisms that govern B7-H6 expression is also pivotal for the evaluation of this immune checkpoint as a crucial target in tumor therapies. Last but not least, in the B7 family, the B7-H7 which is known as HHLA2 with its associated receptor CD28H [transmembrane and immunoglobulin domain-containing protein 2 (TMIGD2) or immunoglobulin containing and proline-rich receptor-1 (IGPR1)] is only found in humans. 168 Despite B7-H7 expression in various epithelia of human organs, its expression has also been reported on human monocytes and macrophages. Although there is no expression of B7-H7 on resting T or B cells, IFN-γ or other inflammatory signals like LPS or polyinosinic:polycytidylic acid (poly I:C) upregulate B7-H7 expression on mature monocytes and dendritic cells.121,169 Furthermore, a high expression of CD28H has been reported on naïve T cells, pDCs, and NK cells. 170 Regarding the B7-H7 functionality as a co-stimulatory molecule, the B7-H7 interaction with CD28H on the NK cells activates them via selective synergy with receptors NKp46 and 2B4 that are located on the surface of NK cells. This interaction further induced pro-inflammatory cytokine secretion along with degranulation and lysis of B7-H7+ tumor cells. 171 The high expression of B7-H7 on tumor cells intensifies NK cell functions either via natural or antibody-dependent cellular cytotoxicity. 171 Hence, the antitumor activity of NK cells through the interaction between B7-H7 and its associated receptors CD28H as a potent activator of NK cells should not be neglected. Importantly, the inhibitory function of the B7-H7 on T-cell activation and proliferation via TCR and CD28 signaling pathways also deserves attention. 121 The simultaneously combined TCR and CD28 stimulation increased B7-H7 co-inhibitory action on T cells as much as PD-L1 co-inhibitory activity. 172 Accordingly, there is also a study indicating that B7-H7 prevents the proliferation of CD4+ and CD8+ T cells in the presence of TCR signaling. 121 Interestingly, the blockade of B7-H7 boosted T-cell activity and proliferation, which further emphasized the role of its blockage as a new therapeutic approach. The elevated expression level of B7-H7 in breast, lung, ccRCC, colorectal carcinoma, intrahepatic cholangiocarcinoma, and malignant glioma that is associated with poor prognosis or metastatic disease in patients has drawn the attention of the clinical significance of the B7-H7 expression in human cancer therapy.173–176 In in vivo experiments of human gallbladder cancer, the HHLA2 overexpression promoted tumor progression while its knockdown reduced the sizes of the GBC tumors. 177 Conversely, the HHLA2 ablation inhibited both TGF-β1- and long noncoding RNA H19-induced GBC progression in vitro. However, in a recent study on human ccRCC that evaluated the HHLA2 prognostic value, a positive correlation between increased HHLA2 and survival rates is reported. 178 In this context, neither costimulatory nor co-inhibitory roles of the B7-H7 and CD28H pathways in T-cell activation or for other immune cells have been fully understood. Therefore, the identification of unknown receptors on activated T cells or other immune cells that could interact with tumor-expressed B7-H7 cells should be further investigated. Finally, given the above findings, targeting the interaction between tumor-expressed B7-H7 and endothelial-expressed CD28H, which may enhance angiogenesis in TME, could be a novel therapeutic approach in antitumor immunity. Interestingly, in a recent study, the either stimulatory or inhibitory role of HHLA2 expression in colorectal cancer has been also addressed. 179 Currently ongoing clinical trials for the new B7 family checkpoint molecule-specific antagonists are presented in Table 5.
Table 5.
Current ongoing clinical trials for the new B7 family checkpoint molecule-specific antagonist agents.
| Name of the compound | Mechanism of action | Study phase | Trial ID | Targeted population | Status |
|---|---|---|---|---|---|
| SCRI-CARB7H3(s); B7H3-specific chimeric antigen receptor T (CAR-T) cell | Autologous CD4+ and CD8+ T cells lentivirally transduced to express a B7H3-specific CAR and EGFRt | Phase I | ClinicalTrials.gov NCT04185038 |
Patients with diffuse intrinsic pontine glioma/diffuse midline glioma and recurrent or refractory pediatric central nervous system tumors | Recruiting |
| Temozolomide | Interact with DNA and repair processes | Phases I and II | ClinicalTrials.gov NCT04077866 |
Patients with recurrent glioblastoma Patients with refractory glioblastoma |
Recruiting |
| Temozolomide + B7-H3 CAR-T cells | Interact with DNA and repair processes + a retroviral vector encoding a CAR targeting B7-H3 | ||||
| Second-generation 4-1BBζ B7H3-EGFRt-DHFR | Autologous CD4+ and CD8+ T-cells lentivirally transduced to express a second-generation 4-1BBζ B7H3-EGFRt-DHFR | Phase I | ClinicalTrials.gov NCT04483778 |
Patients with recurrent/refractory solid tumors in children and young adults | Recruiting |
| Second-generation 4-1BBζ B7H3-EGFRt-DHFR (selected) and a second-generation 4-1BBζ CD19-Her2tG | Autologous CD4+ and CD8+ T cells lentivirally transduced to express a second-generation 4-1BBζ B7H3-EGFRt-DHFR (selected) and a second-generation 4-1BBζ CD19-Her2tG | ||||
| CAR-T cell therapy | CAR-T cells targeting HER2, mesothelin, PSCA, MUC1, Lewis-Y, GPC3, AXL, EGFR, Claudin18.2, or B7-H3 | Phase I | ClinicalTrials.gov NCT03198052 |
Immunotherapy of patients with lung cancer | Recruiting |
| 4SCAR-276 | Targeting CD276 (B7-H3)-positive solid tumors | Phase I/II | ClinicalTrials.gov NCT04432649 |
Patients with refractory and/or recurrent solid tumor | Recruiting |
| B7-H3 CAR-T + temozolomide | Targeting B7-H3 antigen + interact with DNA and repair processes | Phase I | ClinicalTrials.gov NCT04385173 |
Patients with recurrent and refractory glioblastoma | Recruiting |
| CAR.B7-H3 + fludarabine + cyclophosphamide | Targeting the B7-H3 antigen + DNA synthesis inhibitor + cell apoptosis (DNA crosslinks both between and within DNA strands at guanine N-7 positions) | Phase I | ClinicalTrials.gov NCT04670068 |
Patients with recurrent epithelial ovarian cancer | Recruiting |
| MGC018 | Anti-B7-H3 antibody drug conjugate | Phase I/II | ClinicalTrials.gov NCT03729596 |
Patients with advanced solid tumors | Active, not recruiting |
| MGC018 + MGA012 | Anti-B7-H3 antibody–drug conjugate + anti-PD-1 antibody | ||||
| Enoblituzumab + retifanlimab | Anti-B7-H3 antibody + anti-PD-1 antibody | Phase II | ClinicalTrials.gov NCT04634825 |
Patients with recurrent or metastatic squamous cell carcinoma of the head and neck | Terminated |
| Enoblituzumab + tebotelimab | Anti-B7-H3 antibody + PD-1 and LAG-3 bispecific DART molecule | ||||
| 4SCAR-T | Targeting GD2, PSMA, and CD276 | Phase I/II | ClinicalTrials.gov NCT04637503 |
Patients with relapsed and refractory neuroblastoma | Recruiting |
| Engineered TILs/CAR-TILs | Targeting HER2, mesothelin, PSCA, MUC1, Lewis-Y, GPC3, AXL, EGFR, Claudin18.2/6, ROR1, GD1, or B7-H3 | Phase 1 | ClinicalTrials.gov NCT04842812 |
Patients with advanced solid tumors | Recruiting |
| BI 765049 | Limited information is currently available | Phase I | ClinicalTrials.gov NCT04752215 |
Adults with advanced solid tumors whose previous cancer treatment was not successful | Recruiting |
| BI 765049 + BI 754091 | Limited information is currently available + anti-PD-1 |
EGFR, epidermal growth factor receptor; LAG-3, lymphocyte activation gene-3; PD-1, programmed cell death-1; SCCHN, squamous cell carcinoma of the head and neck; TIL, tumor-infiltrating lymphocyte.
B-cell and T-cell lymphocyte attenuator
B- and T-cell lymphocyte attenuator (BTLA) also termed CD272 is an extracellular Ig domain-containing glycoprotein that belongs to B7/CD28 superfamily, whereas its associated ligands HVEM (herpesvirus entry mediator) which is also termed as TNFRSR14 belongs to TNF/TNFR superfamily. 180 Interestingly, the TNF superfamily that interacts with HVEM also includes two ligands such as lymphotoxin alpha (LTalpha) and LIGHT (TNFSF14). 181 The HVEM, as a complex cosignaling molecule, further interacts with the Ig superfamily BTLA and CD160. Based on the HVEM ligand engagement, the HVEM has both stimulatory and inhibitory functions. In this context, following its interaction with TNF members on T and B cells, the HVEM induces stimulatory signals, whereas its interaction with BTLA or CD160 conversely induces an inhibitory signal on T cells. 182 There is a study that indicates the interaction of LIGHT with the HVEM in lymphoid malignancy stimulates a significant elevation in the expression of IL-8 and the upregulation of apoptotic genes. 183 By contrast, the interaction of BTLA with HVEM recruits the protein tyrosine phosphatases Src-homology phosphatase type-1 (SHP-1) and Src-homology phosphatase type-2 (SHP-2) and suppresses TCR activation. 184 Notable, recruitment of SHPs to its cytoplasmic domain blocks also TCR signal transduction after ligation to CTLA-4 or PD-1. 184 Last but not least, ligation of BTLA with HVEM inhibits the secretion of cytokines including IL-2, IFN-γ, IL-4, and IL-10 and downregulates the proper immune response.185,186 Consistently, in a murine TC-1 cervical cancer model, blocking BTLA-HVEM interactions with psBTLA [eukaryotic expression plasmid that expressed the extracellular domain of murine BTLA (soluble form of BTLA)] significantly enhanced antitumor immunity of heat shock protein 70 (HSP70) vaccine by increasing the expression of Th1 cytokines, IL-2, and IFN-γ in the TME. 187 Despite the BTLA expression on T cells and B cells, its expression has been seen further on other critical regulators of pro- and antitumor immunity like NK cells and APCs.188,189 During the past decade, various studies on tumor cells and their microenvironment have revealed the irregular expression of both BTLA and HVEM in effector lymphocytes against tumor cells. For example, the tumor cells immunohistochemistry analysis of patients with stage I–III NSCLC has shown high BTLA expression which was further associated with a positively high level of PD-L1 expression, lymphatic invasion, a shorter relapse-free survival, and poor prognosis. 190 Similarly, the BTLA expression was significantly upregulated on leukemic cells and NK cells from patients with CLL, and further soluble BTLA was found to be increased in the sera of these patients and this was associated with shorter survival time. 191 Consistent with this, in patients with CLL, increased expression of BTLA on the surface of CD4+ and CD8+ T lymphocytes was correlated with poor outcomes and T-cell-mediated immune exhaustion. 192 Notably, blocking BTLA combined with bispecific anti-CD3/anti-CD19 antibody enhanced anti-leukemic responses through CD8+ T cells. In this context, the BTLA/HVEM binding disruption further stimulated IFN-γ+ CD8+ T lymphocytes secretion. The anti- or pro-tumorigenic activities of IFN-γ and its associated receptors (IFN-γ receptor 1/2) have been defined elsewhere. 193 Likewise, in gastric biopsy and PB samples that were taken from patients with GC, an increased BTLA mRNA and protein were identified in advanced stages. Interestingly, in these patients, the HVEM was higher only at the protein level. 194 These recent findings highlight that the BTLA/HVEM/sHVEM (soluble herpes virus entry mediator) inhibitory pathways may serve as potential therapeutic strategies in patients with GC. In an animal model study of epithelial ovarian carcinoma, the combined chemotherapy along with anti-BTLA antibody significantly decreased peritoneal tumor volume and prolonged survival along with increased activated CD4+ and C8+ T lymphocytes in affected mice. 195 In this study, the BTLA expression was identified predominantly on B lymphocytes, particularly on CD19hi B cells, and the elevated expression might be due to the increased secretion of anti-inflammatory cytokines including IL-6 and IL-10 through phosphorylation of STAT3 and serine/threonine kinase (AKT) in B lymphocytes. Notably, the influence of increased anti-inflammatory cytokines like IL-6, IL-10, and TGF-β on tumor progression in ovarian cancer has been reported elsewhere. 196 The synergistic therapeutic value of anti-PD-1 and anti-BTLA against murine glioblastoma also has been reported. 197 A combined anti-PD-1 and anti-BTLA therapy for tumor-bearing mice demonstrated increased OS (up to 60%) compared either with anti-PD-1 (20%) or anti-BTLA (0%) individually (p = 0.003). Furthermore, in this preclinical study, an increased expression of CD4+ IFN-γ (p < 0.0001) and CD8+ IFN-γ (p = 0.0365) was seen along with decreased levels of CD4+ FoxP3+ Treg in the brain (p = 0.0136) on this combined therapy. Another recent study of patients with cutaneous melanoma [skin cutaneous melanoma (SKCM)] has shown that BTLA expression levels increased in metastatic melanoma compared to normal skin tissues and primary melanoma. In this study, increased BTLA expression correlates with improved prognosis in SKCM and was positively associated with enhanced immune cell responses. 198 Notably, the accurate BTLA anticipation in the outcome of melanoma patients that were treated with melanoma-associated antigen 3 (MAGE-A3) blocker or first-line anti-PD-1 further highlights the pivotal role of BTLA as a predictive biomarker in melanoma. Although this study did not support an inhibitory role of BTLA for immune escape and cytotoxic activity of effector lymphocytes, its expression as a single gene and independent good prognostic factor should not be overlooked and deserves to be systematically investigated in vivo and in vitro in other metastatic cancers. In this context, there is a recent study that revealed the tumor cell-intrinsic BTLA receptor could prevent tumor cell proliferation through the protein kinase (ERK1/2, extracellular signal-regulated protein kinase) signaling pathway. 199 Importantly, the interaction of tumor cell-intrinsic BTLA and HVEM prevented extracellular regulation of protein kinase (ERK1/2) and consequently resulted in the inhibition of tumor cell proliferation. This study further highlighted that tumor cell-intrinsic BTLA/HVEM inhibition can be a potential target in cancer immunotherapy. Table 6 summarizes the characteristics of anti-BTLA-specific monoclonal antibodies currently under clinical trials.
Table 6.
Current ongoing clinical trials for BTLA-specific monoclonal antibodies or antagonist agents.
| Name of the compound | Mechanism of action | Study phase | Trial ID | Targeted population | Status |
|---|---|---|---|---|---|
| JS004 | Recombinant humanized IgG4κ monoclonal antibody specific to BTLA | Phase I | ClinicalTrials.gov NCT04278859 |
Patients with advanced solid tumor | Unknown |
| Phase I | ClinicalTrials.gov NCT04477772-in China |
Patients with recurrent/refractory malignant lymphoma | Recruiting | ||
| Phase I | ClinicalTrials.gov NCT04773951-in China |
Patients with advanced solid tumors | Recruiting | ||
| TAB004 + toripalimab | Monoclonal antibody specific to BTLA as monotherapy and in combination with an anti-PD-1 monoclonal antibody | Phase I | ClinicalTrials.gov NCT04137900 |
Patients with advanced unresectable solid tumor + metastatic solid tumor | Recruiting |
BTLA, B- and T-cell lymphocyte attenuator.
Conclusions and future perspectives
In recent years, cancer immunotherapy and particularly immune checkpoints inhibitors (ICIs) including blocking CTLA-4/CD28 or PD-L1/PD-1 axis are being investigated in various preclinical experiments or clinical studies of human malignancies at various stages of disease. Despite some of their therapeutic success, a large proportion of patients indicates inadequate response to ICI due to the adaptive, primary or acquired resistance against immunotherapy. In TME, the PD-L1 overexpression in tumor cells is considered one of the crucial mechanisms involved in tumor immune escape and several factors, including cellular or intracellular oncogenic signals, transcriptional factors, and posttranscriptional modifiers, could modulate its over expression. The soluble PD-L1 (sPD-L1) can also effectively predict metastasis and poor prognosis in various cancers through recruiting the suppressive immune cells into the affected sites. Hence, to increase the effectiveness of the ICI, combined CTLA-4 and PD-1 blockers like ipilimumab and nivolumab have been examined. Furthermore, the anti-PD-1/PD-L1 antibodies (pembrolizumab and nivolumab/atezolizumab and durvalumab) as the most broadly prescribed anticancer therapies have been also investigated in various clinical trials. However, both approaches indicate some degree of limiting immune-related adverse effects along with induction of autoimmunity in treated patients. Importantly, inhibition of some B7:CD28 pathways, such as PD-1 inhibitor, has been found to induce the expression of other immune checkpoints that consequently weakens the influence of the ICI. Based on these observations, there is a need to discover novel targets or ligands that enhance ICI for various checkpoints, along with the feasibility of their combinational therapy in each individual cancer. In this context, those immune checkpoints that were discussed in this review and summarized in Figure 1, such as Tim-3, LAG-3, TIGIT, VISTA, new B7 family proteins, and BTLA are currently investigating to unveil either their potential costimulatory or coinhibitory characteristics as biomarkers in the course of immunotherapy and for the advancement of targeted agents. Furthermore, the complete picture of the checkpoint biology on the surface of various immune cells rather than T cells that are also infiltrating into TME, including macrophages, B cells, neutrophils, DCs, NK cells, cancer-associated fibroblasts, and MDSCs, may open new horizons for the next generation of immunotherapies. Undoubtedly, the discovery of the precise molecular pathways regulated by each of them and their explicit roles in immune homeostasis, along with the identification of their novel ligands could support the innovative approaches to immunotherapy. Finally, due to the plasticity of tumors and to enhance the curative effect of ICIs, additional factors including TME composition, tumor mutation burden, and microsatellite instability, together with the individual properties of the patient’s immune response, should be taken into account for the development of future sequential therapeutic strategies ICIs.
Figure 1.
A summarized overview of mechanisms of action of various immune checkpoints and immune cell components. Signals induced by each immune checkpoint and the interaction via immune mediators are schematically depicted.
Acknowledgments
The authors thank the CinnaGen Medical Biotechnology Research Center.
Footnotes
ORCID iD: Ali N. Kamali  https://orcid.org/0000-0002-6225-9726
https://orcid.org/0000-0002-6225-9726
Contributor Information
Ali N. Kamali, CinnaGen Medical Biotechnology Research Center, Alborz University of Medical Sciences, Simin Dasht Industrial Area, Karaj, Iran; CinnaGen Research and Production Co., Alborz 3165933155, Iran.
José M. Bautista, Department of Biochemistry and Molecular Biology, Faculty of Veterinary Sciences, Complutense University of Madrid, Madrid, Spain Research Institute Hospital 12 de Octubre, Madrid, Spain.
Michael Eisenhut, Department of Pediatrics, Luton and Dunstable University Hospital NHS Foundation Trust, Luton, UK.
Haleh Hamedifar, CinnaGen Medical Biotechnology Research Center, Alborz University of Medical Sciences, Karaj, Iran; CinnaGen Research and Production Co., Alborz, Iran.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Ali N. Kamali: Conceptualization; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
José M. Bautista: Supervision; Validation; Writing – review & editing.
Michael Eisenhut: Validation; Writing – review & editing.
Haleh Hamedifar: Conceptualization; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: All the data supporting the findings of this research are available in the form of tables and image within the article.
References
- 1. Pardoll D. Cancer and the immune system: basic concepts and targets for intervention. Semin Oncol 2015; 42: 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castello A, Rossi S, Toschi L, et al. Soluble PD-L1 in NSCLC patients treated with checkpoint inhibitors and its correlation with metabolic parameters. Cancers (Basel) 2020; 12: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong MP, Enomoto M, Thuy LTT, et al. Clinical significance of circulating soluble immune checkpoint proteins in sorafenib-treated patients with advanced hepatocellular carcinoma. Sci Rep 2020; 10: 3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inomata M, Kado T, Okazawa S, et al. Peripheral PD1-positive CD4 T-lymphocyte count can predict progression-free survival in patients with non-small cell lung cancer receiving immune checkpoint inhibitor. Anticancer Res 2019; 39: 6887–6893. [DOI] [PubMed] [Google Scholar]
- 5. Ishikawa M, Nakayama K, Nakamura K, et al. High PD-1 expression level is associated with an unfavorable prognosis in patients with cervical adenocarcinoma. Arch Gynecol Obstet 2020; 302: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mathé EA, Patterson AD, Haznadar M, et al. Noninvasive urinary metabolomic profiling identifies diagnostic and prognostic markers in lung cancer. Cancer Res 2014; 74: 3259–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ariyan CE, Brady MS, Siegelbaum RH, et al. Robust antitumor responses result from local chemotherapy and CTLA-4 blockade. Cancer Immunol Res 2018; 6: 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dorta-Estremera S, Hegde VL, Slay RB, et al. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV(+) oral cancer. J Immunother Cancer 2019; 7: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou J, Mahoney KM, Giobbie-Hurder A, et al. Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res 2017; 5: 480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zou W, Chen L. Inhibitory b7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008; 8: 467–477. [DOI] [PubMed] [Google Scholar]
- 11. Yi M, Niu M, Xu L, et al. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol 2021; 14: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol 2018; 29: 71–83. [DOI] [PubMed] [Google Scholar]
- 13. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16: 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 15. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (checkmate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016; 17: 883–895. [DOI] [PubMed] [Google Scholar]
- 16. Cella D, Grünwald V, Escudier B, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol 2019; 20: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D’Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol 2018; 19: 416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016; 17: 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 2018; 36: 773–779. [DOI] [PubMed] [Google Scholar]
- 20. Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol 2019; 20: 239–253. [DOI] [PubMed] [Google Scholar]
- 21. Freeman GJ, Casasnovas JM, Umetsu DT, et al. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev 2010; 235: 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Du W, Yang M, Turner A, et al. TIM-3 as a target for cancer immunotherapy and mechanisms of action. Int J Mol Sci 2017; 18: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrzejczak A, Tupikowski K, Tomkiewicz A, et al. The variations’ in genes encoding TIM-3 and its ligand, galectin-9, influence on ccRCC risk and prognosis. Int J Mol Sci 2023; 24: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao X, Zhu Y, Li G, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One 2012; 7: e30676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dao TN, Utturkar S, Atallah Lanman N, et al. TIM-3 expression is downregulated on human NK cells in response to cancer targets in synergy with activation. Cancers 2020; 12: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Mingo Pulido Á, Gardner A, Hiebler S, et al. TIM-3 regulates CD103(+) dendritic cell function and response to chemotherapy in breast cancer. Cancer Cell 2018; 33: 60–74.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ocaña-Guzman R, Torre-Bouscoulet L, Sada-Ovalle I. TIM-3 regulates distinct functions in macrophages. Front Immunol 2016; 7: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hakim MS, Jariah ROA, Spaan M, et al. Interleukin 15 upregulates the expression of PD-1 and TIM-3 on CD4(+) and CD8(+) T cells. Am J Clin Exp Immunol 2020; 9: 10–21. [PMC free article] [PubMed] [Google Scholar]
- 29. Turnis ME, Sawant DV, Szymczak-Workman AL, et al. Interleukin-35 limits anti-tumor immunity. Immunity 2016; 44: 316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang ZZ, Grote DM, Ziesmer SC, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Investig 2012; 122: 1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu C, Sakuishi K, Xiao S, et al. An IL-27/NFIL3 signalling axis drives tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun 2015; 6: 6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu J, Zhang S, Hu Y, et al. Targeting PD-1 and Tim-3 Pathways to reverse CD8 T-Cell exhaustion and enhance ex vivo T-Cell responses to autologous dendritic/tumor vaccines. J Immunother 2016; 39: 171–180. [DOI] [PubMed] [Google Scholar]
- 33. Li Z, Wang Y, Zheng K, et al. Tim-3 blockade enhances the clearance of Chlamydia psittaci in the lung by promoting a cell-mediated immune response. Int Immunopharmacol 2023; 116: 109780. [DOI] [PubMed] [Google Scholar]
- 34. Qin S, Dong B, Yi M, et al. Prognostic values of TIM-3 expression in patients with solid tumors: a meta-analysis and database evaluation. Front Oncol 2020; 10: 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pu F, Chen F, Zhang Z, et al. TIM-3 expression and its association with overall survival in primary osteosarcoma. Oncol Lett 2019; 18: 5294–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dufva O, Pölönen P, Brück O, et al. Immunogenomic landscape of hematological malignancies. Cancer Cell 2020; 38: 424–428. [DOI] [PubMed] [Google Scholar]
- 37. Rakova J, Truxova I, Holicek P, et al. TIM-3 levels correlate with enhanced NK cell cytotoxicity and improved clinical outcome in AML patients. OncoImmunology 2021; 10: 1889822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kamal AM, Nabih NA, Elleboudy NS, et al. Expression of immune check point gene TIM-3 in patients newly diagnosed with acute myeloid leukemia: significance and impact on outcome. Oncol Lett 2021; 21: 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hadadi L, Hafezi M, Amirzargar AA, et al. Dysregulated expression of Tim-3 and NKp30 receptors on NK cells of patients with chronic lymphocytic leukemia. Oncol Res Treat 2019; 42: 202–208. [DOI] [PubMed] [Google Scholar]
- 40. Rezazadeh H, Astaneh M, Tehrani M, et al. Blockade of PD-1 and TIM-3 immune checkpoints fails to restore the function of exhausted CD8(+) T cells in early clinical stages of chronic lymphocytic leukemia. Immunol Res 2020; 68: 269–279. [DOI] [PubMed] [Google Scholar]
- 41. Holderried TAW, de Vos L, Bawden EG, et al. Molecular and immune correlates of TIM-3 (HAVCR2) and galectin 9 (LGALS9) mRNA expression and DNA methylation in melanoma. Clin Epigenetics 2019; 11: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mollavelioglu B, Cetin Aktas E, Cabioglu N, et al. High co-expression of immune checkpoint receptors PD-1, CTLA-4, LAG-3, TIM-3, and TIGIT on tumor-infiltrating lymphocytes in early-stage breast cancer. World J Surg Oncol 2022; 20: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lak S, Janelle V, Djedid A, et al. Combined PD-L1 and TIM3 blockade improves expansion of fit human CD8(+) antigen-specific T cells for adoptive immunotherapy. Mol Ther Methods Clin Dev 2022; 27: 230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity 2004; 21: 503–513. [DOI] [PubMed] [Google Scholar]
- 45. Kisielow M, Kisielow J, Capoferri-Sollami G, et al. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol 2005; 35: 2081–2088. [DOI] [PubMed] [Google Scholar]
- 46. Lino AC, Dang VD, Lampropoulou V, et al. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity 2018; 49: 120–133.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Triebel F, Jitsukawa S, Baixeras E, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 1990; 171: 1393–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Workman CJ, Wang Y, El Kasmi KC, et al. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol 2009; 182: 1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huard B, Prigent P, Tournier M, et al. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol 1995; 25: 2718–2721. [DOI] [PubMed] [Google Scholar]
- 50. Baixeras E, Huard B, Miossec C, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med 1992; 176: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu W, Tang L, Zhang G, et al. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J Biol Chem 2004; 279: 18748–18758. [DOI] [PubMed] [Google Scholar]
- 52. Xu F, Liu J, Liu D, et al. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res 2014; 74: 3418–3428. [DOI] [PubMed] [Google Scholar]
- 53. Mao X, Ou MT, Karuppagounder SS, et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016; 353: 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016; 44: 989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017; 355: 1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abi-Aad SJ, Zouein J, Chartouni A, et al. Simultaneous inhibition of PD-1 and LAG-3: the future of immunotherapy? Immunotherapy 2023; 15: 611–618. [DOI] [PubMed] [Google Scholar]
- 57. Kraman M, Faroudi M, Allen NL, et al. FS118, a bispecific antibody targeting LAG-3 and PD-L1, enhances T-Cell activation resulting in potent antitumor activity. Clin Cancer Res 2020; 26: 3333–3344. [DOI] [PubMed] [Google Scholar]
- 58. Yap TA, LoRusso PM, Wong DJ, et al. A phase 1 first-in-human study of FS118, a tetravalent bispecific antibody targeting LAG-3 and PD-L1 in patients with advanced cancer and PD-L1 resistance. Clin Cancer Res 2023; 29: 888–898. [DOI] [PubMed] [Google Scholar]
- 59. Workman CJ, Rice DS, Dugger KJ, et al. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3). Eur J Immunol 2002; 32: 2255–2263. [DOI] [PubMed] [Google Scholar]
- 60. Clayton KL, Douglas-Vail MB, Nur-ur Rahman AK, et al. Soluble T cell immunoglobulin mucin domain 3 is shed from CD8+ T cells by the sheddase ADAM10, is increased in plasma during untreated HIV infection, and correlates with HIV disease progression. J Virol 2015; 89: 3723–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li N, Wang Y, Forbes K, et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J 2007; 26: 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li N, Jilisihan B, Wang W, et al. Soluble LAG3 acts as a potential prognostic marker of gastric cancer and its positive correlation with CD8+T cell frequency and secretion of IL-12 and INF-γ in peripheral blood. Cancer Biomark 2018; 23: 341–351. [DOI] [PubMed] [Google Scholar]
- 63. Triebel F, Hacene K, Pichon MF. A soluble lymphocyte activation gene-3 (sLAG-3) protein as a prognostic factor in human breast cancer expressing estrogen or progesterone receptors. Cancer Lett 2006; 235: 147–153. [DOI] [PubMed] [Google Scholar]
- 64. Rapoport BL, Steel HC, Benn CA, et al. Dysregulation of systemic soluble immune checkpoints in early breast cancer is attenuated following administration of neoadjuvant chemotherapy and is associated with recovery of CD27, CD28, CD40, CD80, ICOS and GITR and substantially increased levels of PD-L1, LAG-3 and TIM-3. Front Oncol 2023; 13: 1097309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shapiro M, Herishanu Y, Katz BZ, et al. Lymphocyte activation gene 3: a novel therapeutic target in chronic lymphocytic leukemia. Haematologica 2017; 102: 874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang Q, Zhang J, Tu H, et al. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J Immunother Cancer 2019; 7: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Camisaschi C, Casati C, Rini F, et al. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J Immunol 2010; 184: 6545–6551. [DOI] [PubMed] [Google Scholar]
- 68. Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12: 298–306. [DOI] [PubMed] [Google Scholar]
- 69. Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3 – potential mechanisms of action. Nat Rev Immunol 2015; 15: 45–56. [DOI] [PubMed] [Google Scholar]
- 70. Panda A, Rosenfeld JA, Singer EA, et al. Genomic and immunologic correlates of LAG-3 expression in cancer. Oncoimmunology 2020; 9: 1756116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schöffski P, Tan DSW, Martín M, et al. Phase I/II study of the LAG-3 inhibitor ieramilimab (LAG525) ± anti-PD-1 spartalizumab (PDR001) in patients with advanced malignancies. J Immunother Cancer 2022; 10: e003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yu X, Harden K, Gonzalez LC, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009; 10: 48–57. [DOI] [PubMed] [Google Scholar]
- 73. Stanietsky N, Rovis TL, Glasner A, et al. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol 2013; 43: 2138–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stanietsky N, Simic H, Arapovic J, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A 2009; 106: 17858–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Levin SD, Taft DW, Brandt CS, et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol 2011; 41: 902–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang HT, Zhang Z, Fan M, et al. Expression of CD112 in colon carcinoma tissues and cell lines and their clinical significance. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2010; 26: 477–479. [PubMed] [Google Scholar]
- 77. Yong H, Cheng R, Li X, et al. CD155 expression and its prognostic value in postoperative patients with breast cancer. Biomed Pharmacother 2019; 115: 108884. [DOI] [PubMed] [Google Scholar]
- 78. Zhuo B, Li Y, Gu F, et al. Overexpression of CD155 relates to metastasis and invasion in osteosarcoma. Oncol Lett 2018; 15: 7312–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Escalante NK, von Rossum A, Lee M, et al. CD155 on human vascular endothelial cells attenuates the acquisition of effector functions in CD8 T cells. Arterioscler Thromb Vasc Biol 2011; 31: 1177–1184. [DOI] [PubMed] [Google Scholar]
- 80. Liu L, You X, Han S, et al. CD155/TIGIT, a novel immune checkpoint in human cancers (Review). Oncol Rep 2021; 45: 835–845. [DOI] [PubMed] [Google Scholar]
- 81. Harjunpää H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol 2020; 200: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chauvin JM, Pagliano O, Fourcade J, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Investig 2015; 125: 2046–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fathi M, Pustokhina I, Kuznetsov SV, et al. T-cell immunoglobulin and ITIM domain, as a potential immune checkpoint target for immunotherapy of colorectal cancer. IUBMB Life 2021; 73: 726–738. [DOI] [PubMed] [Google Scholar]
- 84. Hu F, Wang W, Fang C, et al. TIGIT presents earlier expression dynamic than PD-1 in activated CD8(+) T cells and is upregulated in non-small cell lung cancer patients. Exp Cell Res 2020; 396: 112260. [DOI] [PubMed] [Google Scholar]
- 85. Xie X, Feng Y, Fan P, et al. Increased co-expression of TIM-3 with TIGIT or 2B4 on CD8+ T cells is associated with poor prognosis in locally advanced nasopharyngeal carcinoma. Biomol Biomed 2023; 23: 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xu D, Zhao E, Zhu C, et al. TIGIT and PD-1 may serve as potential prognostic biomarkers for gastric cancer. Immunobiology 2020; 225: 151915. [DOI] [PubMed] [Google Scholar]
- 87. Yao D, Xu L, Liu L, et al. Increased expression of TIGIT/CD57 in peripheral blood/bone marrow NK cells in patients with chronic myeloid leukemia. Biomed Res Int 2020; 2020: 9531549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang L, Wang J, Wei F, et al. Profiling the dynamic expression of checkpoint molecules on cytokine-induced killer cells from non-small cell lung cancer patients. Oncotarget 2016; 7: 43604–43615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang Q, Gao C, Shao J, et al. TIGIT-related transcriptome profile and its association with tumor immune microenvironment in breast cancer. Biosci Rep 2021; 41: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang Q, Bi J, Zheng X, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol 2018; 19: 723–732. [DOI] [PubMed] [Google Scholar]
- 91. Meng F, Li L, Lu F, et al. Overexpression of TIGIT in NK and T cells contributes to tumor immune escape in myelodysplastic syndromes. Front Oncol 2020; 10: 1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Han JH, Cai MM, Grein J, et al. Effective anti-tumor response by TIGIT blockade associated with FcγR engagement and myeloid cell activation. Front Immunol 2020; 11: 573405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Vogelpoel LT, Baeten DL, de Jong EC, et al. Control of cytokine production by human Fc gamma receptors: implications for pathogen defense and autoimmunity. Front Immunol 2015; 6: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu X, Li Q, Zhou Y, et al. Dysfunctional role of elevated TIGIT expression on T cells in oral squamous cell carcinoma patients. Oral Dis 2021; 27: 1667–1677. [DOI] [PubMed] [Google Scholar]
- 95. Hu Y, Paris S, Bertolet G, et al. Combining a nanoparticle-mediated immunoradiotherapy with dual blockade of LAG3 and TIGIT improves the treatment efficacy in anti-PD1 resistant lung cancer. J Nanobiotechnol 2022; 20: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Franks SE, Fabian KP, Santiago-Sánchez G, et al. Immune targeting of three independent suppressive pathways (TIGIT, PD-L1, TGFβ) provides significant antitumor efficacy in immune checkpoint resistant models. Oncoimmunology 2022; 11: 2124666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Thibaudin M, Limagne E, Hampe L, et al. Targeting PD-L1 and TIGIT could restore intratumoral CD8 T cell function in human colorectal cancer. Cancer Immunol Immunother 2022; 71: 2549–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mettu NB, Ulahannan SV, Bendell JC, et al. A phase 1a/b open-label, dose-escalation study of etigilimab alone or in combination with nivolumab in patients with locally advanced or metastatic solid tumors. Clin Cancer Res 2022; 28: 882–892. [DOI] [PubMed] [Google Scholar]
- 99. Mu S, Liang Z, Wang Y, et al. PD-L1/TIGIT bispecific antibody showed survival advantage in animal model. Clin Transl Med 2022; 12: e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Onyshchenko K, Luo R, Guffart E, et al. Expansion of circulating stem-like CD8(+) T cells by adding CD122-directed IL-2 complexes to radiation and anti-PD1 therapies in mice. Nat Commun 2023; 14: 2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ren Z, Zhang A, Sun Z, et al. Selective delivery of low-affinity IL-2 to PD-1+ T cells rejuvenates antitumor immunity with reduced toxicity. J Clin Investig 2022; 132: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mehta N, Maddineni S, Mathews II, et al. Structure and functional binding epitope of V-domain Ig suppressor of T cell activation. Cell Rep 2019; 28: 2509–2516.e5. [DOI] [PubMed] [Google Scholar]
- 103. Xu W, Hiếu T, Malarkannan S, et al. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell Mol Immunol 2018; 15: 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang J, Wu G, Manick B, et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology 2019; 156: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lines JL, Sempere LF, Broughton T, et al. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res 2014; 2: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Johnston RJ, Su LJ, Pinckney J, et al. VISTA is an acidic pH-selective ligand for PSGL-1. Our Nat 2019; 574: 565–570. [DOI] [PubMed] [Google Scholar]
- 107. Le Mercier I, Chen W, Lines JL, et al. VISTA regulates the development of protective antitumor immunity. Cancer Res 2014; 74: 1933–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wang L, et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc Natl Acad Sci U S A 2014; 111: 14846–14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pagliuca S, Gurnari C, Zhang K, et al. Comprehensive transcriptomic analysis of VISTA in acute myeloid leukemia: insights into its prognostic value. Int J Mol Sci 2022; 23: 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Flies DB, Han X, Higuchi T, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4+ T cell-mediated immunity. J Clin Investig 2014; 124: 1966–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang LC, Wang YL, He B, et al. Expression and clinical significance of VISTA, B7-H3, and PD-L1 in glioma. Clin Immunol 2022; 245: 109178. [DOI] [PubMed] [Google Scholar]
- 112. Zhang M, Pang HJ, Zhao W, et al. VISTA expression associated with CD8 confers a favorable immune microenvironment and better overall survival in hepatocellular carcinoma. BMC Cancer 2018; 18: 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zong L, Zhou Y, Zhang M, et al. VISTA expression is associated with a favorable prognosis in patients with high-grade serous ovarian cancer. Cancer Immunol Immunother 2020; 69: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hou Z, Pan Y, Fei Q, et al. Prognostic significance and therapeutic potential of the immune checkpoint VISTA in pancreatic cancer. J Cancer Res Clin Oncol 2021; 147: 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gao J, Ward JF, Pettaway CA, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 2017; 23: 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Starzyńska A, Sobocki BK, Sakowicz-Burkiewicz M, et al. VISTA H-score is significantly associated with a 5-year DFS rate in oral squamous cell carcinoma. J Clin Med 2023; 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yeoh WJ, Vu VP, Krebs P. IL-33 biology in cancer: an update and future perspectives. Cytokine 2022; 157: 155961. [DOI] [PubMed] [Google Scholar]
- 118. Li N, Yang S, Ren Y, et al. Chemotherapy induces immune checkpoint VISTA expression in tumor cells via HIF-2α. Biochem Pharmacol 2023; 210: 115492. [DOI] [PubMed] [Google Scholar]
- 119. Zapała Ł, Kunc M, Sharma S, et al. Immune checkpoint receptor VISTA on immune cells is associated with expression of T-cell exhaustion marker TOX and worse prognosis in renal cell carcinoma with venous tumor thrombus. J Cancer Res Clin Oncol 2023; 149: 4131–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ni L, Dong C. New B7 family checkpoints in human cancers. Mol Cancer Ther 2017; 16: 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhao R, Chinai JM, Buhl S, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci U S A 2013; 110: 9879–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Castellanos JR, Purvis IJ, Labak CM, et al. B7-H3 role in the immune landscape of cancer. Am J Clin Exp Immunol 2017; 6: 66–75. [PMC free article] [PubMed] [Google Scholar]
- 123. Mao Y, Chen L, Wang F, et al. Cancer cell-expressed B7-H3 regulates the differentiation of tumor-associated macrophages in human colorectal carcinoma. Oncol Lett 2017; 14: 6177–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat Immunol 2001; 2: 269–274. [DOI] [PubMed] [Google Scholar]
- 125. Prasad DV, Nguyen T, Li Z, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol 2004; 173: 2500–2506. [DOI] [PubMed] [Google Scholar]
- 126. Azuma T, Sato Y, Ohno T, et al. Serum soluble B7-H3 is a prognostic marker for patients with non-muscle-invasive bladder cancer. PLoS One 2020; 15: e0243379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nygren MK, Tekle C, Ingebrigtsen VA, et al. Identifying microRNAs regulating B7-H3 in breast cancer: the clinical impact of microRNA-29c. Br J Cancer 2014; 110: 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zhang X, Ji J, Zhang G, et al. Expression and significance of B7-H3 and Tie-2 in the tumor vasculature of clear cell renal carcinoma. Onco Targets Ther 2017; 10: 5417–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhao J, Lei T, Xu C, et al. MicroRNA-187, down-regulated in clear cell renal cell carcinoma and associated with lower survival, inhibits cell growth and migration though targeting B7-H3. Biochem Biophys Res Commun 2013; 438: 439–444. [DOI] [PubMed] [Google Scholar]
- 130. Wang L, Kang FB, Sun N, et al. The tumor suppressor miR-124 inhibits cell proliferation and invasion by targeting B7-H3 in osteosarcoma. Tumour Biol 2016; 37: 14939–14947. [DOI] [PubMed] [Google Scholar]
- 131. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther 2016; 1: 15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhang LY, Jin Y, Xia PH, et al. Integrated analysis reveals distinct molecular, clinical, and immunological features of B7-H3 in acute myeloid leukemia. Cancer Med 2021; 10: 7831–7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Riether C, Pabst T, Höpner S, et al. Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat Med 2020; 26: 1459–1467. [DOI] [PubMed] [Google Scholar]
- 134. Li S, Zhang M, Wang M, et al. B7-H3 specific CAR-T cells exhibit potent activity against prostate cancer. Cell Death Discov 2023; 9: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the treatment of cancer. J Interferon Cytokine Res 2019; 39: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hu BQ, Huang JF, Niu K, et al. B7-H3 but not PD-L1 is involved in the antitumor effects of dihydroartemisinin in non-small cell lung cancer. Eur J Pharmacol 2023; 950: 175746. [DOI] [PubMed] [Google Scholar]
- 137. Feng Y, Yang Z, Zhang C, et al. B7-H4 induces epithelial-mesenchymal transition and promotes colorectal cancer stemness. Pathol Res Pract 2021; 218: 153323. [DOI] [PubMed] [Google Scholar]
- 138. Han S, Wang Y, Shi X, et al. Negative roles of B7-H3 and B7-H4 in the microenvironment of cervical cancer. Exp Cell Res 2018; 371: 222–230. [DOI] [PubMed] [Google Scholar]
- 139. Kim N, Park M, Kweon SS, et al. B7-H3 and B7-H4 expression in breast cancer and their association with clinicopathological variables and T cell infiltration. Pathobiology 2020; 87: 179–192. [DOI] [PubMed] [Google Scholar]
- 140. Li A, Zhang N, Zhao Z, et al. Overexpression of B7-H4 promotes renal cell carcinoma progression by recruiting tumor-associated neutrophils via upregulation of CXCL8. Oncol Lett 2020; 20: 1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li H, Piao L, Liu S, et al. B7-H4 is a potential prognostic biomarker of prostate cancer. Exp Mol Pathol 2020; 114: 104406. [DOI] [PubMed] [Google Scholar]
- 142. Piao L, Yang Z, Jin J, et al. B7H4 is associated with stemness and cancer progression in esophageal squamous cell carcinoma. Hum Pathol 2018; 80: 152–162. [DOI] [PubMed] [Google Scholar]
- 143. Qi ZJ, Yu D, Chen CH, et al. The prognostic value of B7H1 and B7H4 expression in pancreatic cancer: a meta-analysis. Int J Biol Markers 2019; 34: 373–380. [DOI] [PubMed] [Google Scholar]
- 144. Wang Y, Deng J, Wang L, et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and VISTA in craniopharyngioma. J Immunother Cancer 2020; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Azuma T, Sato Y, Ohno T, et al. Serum soluble B7-H4 is a prognostic marker for patients with non-metastatic clear cell renal cell carcinoma. PLoS One 2018; 13: e0199719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Emaldi M, Nunes-Xavier CE. B7-H4 immune checkpoint protein affects viability and targeted therapy of renal cancer cells. Cells 2022; 11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Xu Y, Zhu S, Song M, et al. B7-H4 expression and its role in interleukin-2/interferon treatment of clear cell renal cell carcinoma. Oncol Lett 2014; 7: 1474–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Huang C, Zhou L, Chang X, et al. B7-H3, B7-H4, Foxp3 and IL-2 expression in cervical cancer: associations with patient outcome and clinical significance. Oncol Rep 2016; 35: 2183–2190. [DOI] [PubMed] [Google Scholar]
- 149. Iizuka A, Nonomura C, Ashizawa T, et al. A T-cell-engaging B7-H4/CD3-bispecific Fab-scFv antibody targets human breast cancer. Clin Cancer Res 2019; 25: 2925–2934. [DOI] [PubMed] [Google Scholar]
- 150. Smith JB, Lanitis E, Dangaj D, et al. Tumor regression and delayed onset toxicity following B7-H4 CAR T cell therapy. Mol Ther 2016; 24: 1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Genova C, Tasso R, Rosa A, et al. Prognostic role of soluble and extracellular vesicle-associated PD-L1, B7-H3 and B7-H4 in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Cells 2023; 12: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chen L, Feng J, Xu B, et al. Correction to: Expression of B7-H6 expression in human hepatocellular carcinoma and its clinical significance. Cancer Cell Int 2018; 18: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cao Y, Huo L, Zhou L, et al. Expression of B7-H6 in chronic myeloid leukemia and its clinical significance. Int J Clin Exp Pathol 2019; 12: 568–575. [PMC free article] [PubMed] [Google Scholar]
- 154. Che F, Xie X, Wang L, et al. B7-H6 expression is induced by lipopolysaccharide and facilitates cancer invasion and metastasis in human gliomas. Int Immunopharmacol 2018; 59: 318–327. [DOI] [PubMed] [Google Scholar]
- 155. Gutierrez-Silerio GY, Franco-Topete RA, Haramati J, et al. Positive staining of the immunoligand B7-H6 in abnormal/transformed keratinocytes consistently accompanies the progression of cervical cancer. BMC Immunol 2020; 21: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Sun J, Tao H, Li X, et al. Clinical significance of novel costimulatory molecule B7-H6 in human breast cancer. Oncol Lett 2017; 14: 2405–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhang X, Xie W, Wang Z, et al. Expression of a novel immune checkpoint B7-H6 ligand in human small cell lung cancer. Ann Transl Med 2020; 8: 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhou H, Dong J, Guo L, et al. The prognostic value of B7-H6 in esophageal squamous cell carcinoma. Sci Rep 2019; 9: 18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhu Z, Teng KY, Zhou J, et al. B7H6 serves as a negative prognostic marker and an immune modulator in human pancreatic cancer. Front Oncol 2022; 12: 814312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Cao G, Wang J, Zheng X, et al. Tumor therapeutics work as stress inducers to enhance tumor sensitivity to natural killer (NK) cell cytolysis by up-regulating NKp30 ligand B7-H6. J Biol Chem 2015; 290: 29964–29973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hu Y, Zeng T, Xiao Z, et al. Immunological role and underlying mechanisms of B7-H6 in tumorigenesis. Clin Chim Acta 2020; 502: 191–198. [DOI] [PubMed] [Google Scholar]
- 162. Fiegler N, Textor S, Arnold A, et al. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood 2013; 122: 684–693. [DOI] [PubMed] [Google Scholar]
- 163. He YF, Wang XH, Zhang GM, et al. Sustained low-level expression of interferon-gamma promotes tumor development: potential insights in tumor prevention and tumor immunotherapy. Cancer Immunol Immunother 2005; 54: 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chen J, Feng Y, Lu L, et al. Interferon-γ-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology 2012; 217: 385–393. [DOI] [PubMed] [Google Scholar]
- 165. Benci JL, Xu B, Qiu Y, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016; 167: 1540–1554.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Obiedat A, Charpak-Amikam Y, Tai-Schmiedel J, et al. The integrated stress response promotes B7H6 expression. J Mol Med 2020; 98: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhang W, Auguste A, Liao X, et al. A novel B7-H6-targeted IgG-like T cell-engaging antibody for the treatment of gastrointestinal tumors. Clin Cancer Res 2022; 28: 5190–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zhu Y, Yao S, Iliopoulou BP, et al. B7-H5 costimulates human T cells via CD28H. Nat Commun 2013; 4: 2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Janakiram M, Chinai JM, Fineberg S, et al. Expression, clinical significance, and receptor identification of the newest B7 family member HHLA2 protein. Clin Cancer Res 2015; 21: 2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Crespo J, Vatan L, Maj T, et al. Phenotype and tissue distribution of CD28H(+) immune cell subsets. Oncoimmunology 2017; 6: e1362529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zhuang X, Long EO. CD28 homolog is a strong activator of natural killer cells for lysis of B7H7(+) tumor cells. Cancer Immunol Res 2019; 7: 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Rieder SA, Wang J, White N, et al. B7-H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell Mol Immunol 2021; 18: 1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Jing CY, Fu YP, Yi Y, et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. J Immunother Cancer 2019; 7: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Qi Y, Deng G, Xu P, et al. HHLA2 is a novel prognostic predictor and potential therapeutic target in malignant glioma. Oncol Rep 2019; 42: 2309–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zhou QH, Li KW, Chen X, et al. HHLA2 and PD-L1 co-expression predicts poor prognosis in patients with clear cell renal cell carcinoma. J Immunother Cancer 2020; 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhu Z, Dong W. Overexpression of HHLA2, a member of the B7 family, is associated with worse survival in human colorectal carcinoma. Onco Targets Ther 2018; 11: 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zhang Y, Li H, Lv C, et al. HHLA2 promotes tumor progression by long non-coding RNA H19 in human gallbladder cancer. Int J Oncol 2022; 61: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zhang Z, Liu J, Zhang C, et al. Over-expression and prognostic significance of HHLA2, a new immune checkpoint molecule, in human clear cell renal cell carcinoma. Front Cell Dev Biol 2020; 8: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kula A, Dawidowicz M, Mielcarska S, et al. Overexpression and role of HHLA2, a novel immune checkpoint, in colorectal cancer. Int J Mol Sci 2023; 24: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 2003; 4: 670–679. [DOI] [PubMed] [Google Scholar]
- 181. Mauri DN, Ebner R, Montgomery RI, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity 1998; 8: 21–30. [DOI] [PubMed] [Google Scholar]
- 182. Cheung TC. Modulation of T cell proliferation through the LIGHT-HVEM-BTLA cosignaling pathway. Recent Pat DNA Gene Seq 2009; 3: 177–182. [DOI] [PubMed] [Google Scholar]
- 183. Pasero C, Barbarat B, Just-Landi S, et al. A role for HVEM, but not lymphotoxin-β receptor, in LIGHT-induced tumor cell death and chemokine production. Eur J Immunol 2009; 39: 2502–2514. [DOI] [PubMed] [Google Scholar]
- 184. Murphy KM, Nelson CA, Sedý JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol 2006; 6: 671–681. [DOI] [PubMed] [Google Scholar]
- 185. Chemnitz JM, Lanfranco AR, Braunstein I, et al. B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J Immunol 2006; 176: 6603–6614. [DOI] [PubMed] [Google Scholar]
- 186. Otsuki N, Kamimura Y, Hashiguchi M, et al. Expression and function of the B and T lymphocyte attenuator (BTLA/CD272) on human T cells. Biochem Biophys Res Commun 2006; 344: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 187. Han L, Wang W, Fang Y, et al. Soluble B and T lymphocyte attenuator possesses antitumor effects and facilitates heat shock protein 70 vaccine-triggered antitumor immunity against a murine TC-1 cervical cancer model in vivo. J Immunol 2009; 183: 7842–7850. [DOI] [PubMed] [Google Scholar]
- 188. Sekar D, Govene L, del Río ML, et al. Downregulation of BTLA on NKT cells promotes tumor immune control in a mouse model of mammary carcinoma. Int J Mol Sci 2018; 19: 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wang WD, Gao YC, Lu YB, et al. BTLA-expressing CD11c antigen presenting cells in patients with active tuberculosis exhibit low capacity to stimulate T cell proliferation. Cell Immunol 2017; 311: 28–35. [DOI] [PubMed] [Google Scholar]
- 190. Li X, Xu Z, Cui G, et al. BTLA expression in stage I-III non-small-cell lung cancer and its correlation with PD-1/PD-L1 and clinical outcomes. Onco Targets Ther 2020; 13: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, et al. BTLA/HVEM axis induces NK cell immunosuppression and poor outcome in chronic lymphocytic leukemia. Cancers 2021; 13: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Sordo-Bahamonde C, Lorenzo-Herrero S, Martínez-Pérez A, et al. BTLA dysregulation correlates with poor outcome and diminished T cell-mediated antitumor responses in chronic lymphocytic leukemia. Cancer Immunol Immunother 2023; 72: 2529–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ding H, Wang G, Yu Z, et al. Role of interferon-gamma (IFN-γ) and IFN-γ receptor 1/2 (IFNγR1/2) in regulation of immunity, infection, and cancer development: IFN-γ-dependent or independent pathway. Biomed Pharmacother 2022; 155: 113683. [DOI] [PubMed] [Google Scholar]
- 194. Azarafza M, Tehrani M, Valadan R, et al. Role of BTLA/HVEM network in development of gastric cancer. Hum Immunol 2022; 83: 637–644. [DOI] [PubMed] [Google Scholar]
- 195. Chen YL, Lin HW, Chien CL, et al. BTLA blockade enhances cancer therapy by inhibiting IL-6/IL-10-induced CD19(high) B lymphocytes. J Immunother Cancer 2019; 7: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Chen YL, Chang MC, Chen CA, et al. Depletion of regulatory T lymphocytes reverses the imbalance between pro- and anti-tumor immunities via enhancing antigen-specific T cell immune responses. PLoS One 2012; 7: e47190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Choi J, Medikonda R, Saleh L, et al. Combination checkpoint therapy with anti-PD-1 and anti-BTLA results in a synergistic therapeutic effect against murine glioblastoma. OncoImmunology 2021; 10: 1956142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Dong X, Song J, Chen B, et al. Exploration of the prognostic and immunotherapeutic value of B and T lymphocyte attenuator in skin cutaneous melanoma. Front Oncol 2020; 10: 592811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Cheng TY, Liu YJ, Yan H, et al. Tumor cell-intrinsic BTLA receptor inhibits the proliferation of tumor cells via ERK1/2. Cells 2022; 11: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]