Key Points
Question
What is the risk of developing inflammatory bowel disease in children and adults with atopic dermatitis (AD)?
Finding
This population-based cohort study of 409 431 children and 625 083 adults with AD found a statistically significant increased risk of incident, or new-onset, inflammatory bowel disease of 44% in children and 34% in adults compared with patients without AD, with this risk increasing with worsening AD severity.
Meaning
The risk of new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease.
This cohort study examines the association between atopic dermatitis and inflammatory bowel disease in children and adults.
Abstract
Importance
Data on the association between atopic dermatitis (AD) and inflammatory bowel disease (IBD) are inconsistent. Few studies have examined the association of AD or AD severity with risk of ulcerative colitis (UC) and Crohn disease (CD) separately.
Objectives
To examine the risk of new-onset IBD, UC, and CD in children and adults with AD.
Design, Setting, and Participants
This population-based cohort study assessed patients with AD matched with up to 5 controls on age, practice, and index date. Treatment exposure was used as a proxy for AD severity. Data were retrieved from The Health Improvement Network, a UK electronic medical record database, for January 1, 1994, to February 28, 2015. Data analysis was performed from January 8, 2020, to June 30, 2023.
Main Outcomes and Measures
Outcomes of interest were incident IBD, UC, and CD. Logistic regression was used to examine the risk for each outcome in children and adults with AD compared with controls.
Results
A total of 1 809 029 pediatric controls were matched to 409 431 children with AD (93.2% mild, 5.5% moderate, and 1.3% severe). The pediatric cohort ranged in median age from 4 to 5 years (overall range, 1-10 years), was predominantly male (936 750 [51.8%] controls, 196 996 [51.6%] with mild AD, 11 379 [50.7%] with moderate AD, and 2985 [56.1%] with severe AD), and with similar socioeconomic status. A total of 2 678 888 adult controls were matched to 625 083 adults with AD (65.7% mild, 31.4% moderate, and 2.9% severe). The adult cohort ranged in median age from 45 to 50 years (overall range, 30-68 years) and was predominantly female (1 445 589 [54.0%] controls, 256 071 [62.3%] with mild AD, 109 404 [55.8%] with moderate AD, and 10 736 [59.3%] with severe AD). In fully adjusted models, children with AD had a 44% increased risk of IBD (hazard ratio [HR], 1.44; 95% CI, 1.31-1.58) and a 74% increased risk of CD (HR, 1.74; 95% CI, 1.54-1.97), which increased with worsening AD; however, they did not have increased risk of UC (HR, 1.09; 95% CI, 0.94-1.27) except for those with severe AD (HR, 1.65; 95% CI, 1.02-2.67). Adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk of IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk of CB, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk of UC, with risk increasing with worsening AD.
Conclusion and Relevance
In this cohort study, children and adults with AD had an increased risk of IBD, with risk varying by age, AD severity, and IBD subtype. These findings provide new insights into the association between AD and IBD. Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms.
Introduction
Atopic dermatitis (AD) has been associated with an increasing number of comorbid diseases, including inflammatory bowel disease (IBD).1,2,3 However, studies on the association between AD and IBD, including ulcerative colitis (UC) and Crohn disease (CD), have shown mixed results.4,5,6,7,8,9 Although an increased prevalence of IBD has been observed with AD, others have found an association among adults only and not children.10 Studies examining the incidence of UC and CD in individuals with AD have shown incongruent findings.11,12,13,14 The cross-sectional nature of some studies limits our ability to measure the true incidence rates of IBD and understand the direction of this association.7,8,9,10,14 In addition, the influence of AD severity or confounders, such as smoking, has not always been addressed. We also argue that the risk of UC and CD should be examined separately because these are distinct entities.15 We present the results of our analysis of the association between AD and IBD, including UC and CD, in children and adults using a large population-based cohort from the UK.
Methods
Study Design
This cohort study used data from The Health Improvement Network (THIN), an electronic health records database of patients registered with UK general practices in which general practitioners serve as the primary point of contact for care, which is thought to be representative of the general UK population. Variables are captured using Read codes, a comprehensive numerical system that records diagnosis and tests.16 Diagnostic codes for AD, IBD, CD, and UC have been validated in THIN.17,18,19,20,21 Data on race or ethnicity were not available because THIN does not systematically collect data on ethnicity. All data were collected from January 1, 1994, to February 28, 2015. This study was granted exempt status with the requirement of informed consent waived by the institutional review board of the University of Pennsylvania and approved by the Scientific Review Committee of CSD Medical Research in the UK because data were collected by a third party and deidentified before being made available. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Definition of Study Population
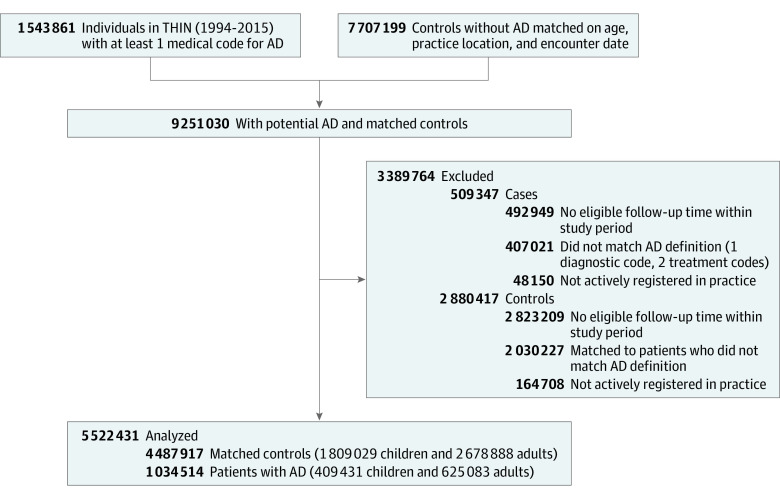
Patients with AD were matched with up to 5 controls without AD on age (±3 years), practice location, and having an encounter within 6 months of the AD index date, ensuring that follow-up occurred during similar periods and by similar practitioners (Figure 1). The presence of at least 1 of 5 common diagnostic codes for AD and 2 AD-related treatment codes were used to classify patients as having AD, an algorithm validated in the literature with a positive predictive value of 86% for physician-confirmed AD.18 The AD index date was defined as the last of diagnosis date, practice registration, or Vision date (ie, date of implementation of Vision software, version 3 in THIN providing quality assurance). Patients were stratified into pediatric (aged <18 years) or adult (aged ≥18 years) cohorts based on age at baseline.
Figure 1. Flow Diagram of Study Participant Selection.
AD indicates atopic dermatitis; THIN, The Health Improvement Network.
Definition of AD Severity
The severity of AD is not captured in THIN; therefore, treatment exposure was used as a proxy. Patients with AD were classified as having mild AD by default. Patients were then classified as having moderate AD at the first of (1) second potent topical corticosteroid prescription within 1 year or (2) first topical calcineurin inhibitor prescription (reserved for moderate AD in the UK). Patients were classified as having severe AD at (1) first systemic immunosuppressant prescription, (2) any prescription for phototherapy, or (3) dermatologic referral because most patients with AD are treated by their general practitioner. Once defined as having moderate AD, they remained as such until they developed severe AD, and once defined as having severe AD, they remained as such until the end of follow-up. This approach has been used in similar studies and is considered an acceptable method when using data from large medical record databases.18,22,23,24,25,26,27,28
Follow-Up Time
Follow-up began at the latest of first AD diagnosis (or diagnosis date for controls), registration within the practice, or Vision date. End of follow-up occurred at the earliest of outcome diagnosis, patient transfer out of practice, withdrawal of practice from THIN, death, or end of study.
Outcome of Interest
The primary study outcomes were (1) incident IBD, (2) UC, and (3) CD. Outcomes were identified using codes selected a priori. Patients were classified as having incident IBD if they received a code after the index date and on or before the end of the study period. Patients with a history of IBD, CD, or UC at cohort entry were excluded.
Covariates of Interest Defined at Cohort Entry
Covariates of interest included age, sex, Townsend index (measure of deprivation), history of asthma or allergic rhinitis, use of systemic steroids, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), smoking, and alcohol use (ie, drinking) in adults because these variables are not accurately recorded for children. Use of systemic corticosteroids was defined as having any prescription in THIN during the time of the study and not as a cumulative dose. For smoking and drinking, codes that captured a patient’s status as current, former, or never were used. Body mass index was treated as a categorical variable. Missing categories for smoking, drinking, and BMI were created to capture missing data and included in the models.
Statistical Analysis
Data analysis was performed from January 8, 2020, to June 30, 2023. Descriptive statistics were used to summarize cohort characteristics. Dichotomous and continuous variables were tested using χ2 and 2-tailed, unpaired t tests or Mann-Whitney tests. Cumulative incidence rates (IRs) are reported per 1000 person-years. Cox proportional hazard regression models were used to examine unadjusted hazard ratios (HRs) with age and severity treated as time-updated covariates. Models were adjusted for confounders using a purposeful selection approach.29 Covariates with a biological plausible association with AD or IBD and/or a 2-sided P < .05 in univariate models were added to the full model and removed one at a time or maintained if P < .10 and if there was a change in the point estimate by more than 10% to 15%. Sensitivity analyses were conducted to address effect modification by comorbid asthma and/or allergic rhinitis and ascertainment bias by restricting analyses to patients with at least 5 years of follow-up and seen at least once yearly by their general practitioner during the study period. In addition, we performed sensitivity analysis excluding patients who received medications that could have been given for IBD and restricted to patients who were defined as having severe AD at least 1 year before developing the outcome of interest. Last, we examined the effect of unmeasured confounding by determining the E-value for the observed association (ie, HR) and the limit of the CI closest to the null. This method for addressing unmeasured confounding has been previously described in the literature.30 All analysis were conducted in Stata, version 17.0 (StataCorp LLC).
Results
Pediatric Cohort
A total of 1 809 029 pediatric controls were matched to 409 431 children with AD (381 678 [93.2%] mild, 22 433 [5.5%] moderate; and 5320 [1.3%] severe). The median age in controls was 4 (IQR, 2-9) years and in mild, moderate, and severe AD was 4 years (IQR, 1-8) years, 9 (IQR, 4-14) years, and 5 (IQR, 1-10) years, respectively. The pediatric cohort was predominantly male (936 750 [51.8%] controls, 196 996 [51.6%] with mild AD, 11 379 [50.7%] with moderate AD, and 2985 [56.1%] with severe AD). Similar socioeconomic status was observed across the cohort. Median follow-up time was 5 (IQR, 2-9) years in controls and 5 to 7 (IQR, 2-13) years across AD groups. The prevalence of allergic rhinitis, asthma, and use of systemic corticosteroids was higher in patients with AD compared with controls, and, overall, those with severe AD had a higher prevalence of history of IBD. These patients were excluded from further analysis (Table 1).
Table 1. Baseline Characteristics of Pediatric and Adult Cohorts With and Without Atopic Dermatitisa.
| Characteristic | Pediatric cohort (aged <18 y) | Adult cohort (aged ≥18 y) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Atopic dermatitis severity group | Control | Atopic dermatitis severity group | ||||||
| Mild | Moderate | Severe | Mild | Moderate | Severe | ||||
| Total | 1 809 029 | 381 678 (93.2) | 22 433 (5.5) | 5320 (1.3) | 2 678 888 | 410 867 (65.7) | 196 101 (31.4) | 18 115 (2.9) | |
| Age, median (IQR), y | 4 (2-9) | 4 (1-8) | 9 (4-14) | 5 (1-10) | 47 (32-64) | 45 (30-63) | 50 (34-68) | 47 (32-63) | |
| Sex | |||||||||
| Female | 872 279 (48.2) | 184 682 (48.4) | 11 054 (49.3) | 2335 (43.9) | 1 445 589 (54.0) | 256 071 (62.3) | 109 404 (55.8) | 10 736 (59.3) | |
| Male | 936 750 (51.8) | 196 996 (51.6) | 11 379 (50.7) | 2985 (56.1) | 1 233 299 (46.0) | 154 796 (37.7) | 86 697 (44.2) | 7379 (40.7) | |
| Townsend deprivation index | |||||||||
| 1 (Lowest) | 424 409 (24.7) | 89 820 (24.9) | 4768 (22.6) | 1251 (25.0) | 677 724 (26.4) | 102 924 (26.2) | 46 708 (25.0) | 4685 (27.3) | |
| 2 (Low) | 340 677 (19.8) | 71 979 (20.0) | 4106 (19.4) | 1069 (21.4) | 564 890 (22.0) | 84 924 (21.6) | 40 579 (21.7) | 3821 (22.2) | |
| 3 (Moderate) | 355 559 (20.7) | 75 261 (20.9) | 4551 (21.5) | 1033 (20.6) | 534 554 (20.8) | 81 331 (20.7) | 39 255 (21.0) | 3566 (20.8) | |
| 4 (High) | 339 336 (19.8) | 70 649 (19.6) | 4316 (20.4) | 900 (18.0) | 468 773 (18.2) | 73 004 (18.6) | 35 452 (19.0) | 3038 (17.7) | |
| 5 (Highest) | 257 540 (15.0) | 53 113 (14.7) | 3407 (16.1) | 750 (15.0) | 322 027 (12.5) | 50 711 (12.9) | 24 936 (13.3) | 2079 (12.1) | |
| Person time, median (IQR), y | 4.99 (2.02-9.41) | 5.22 (2.12-9.74) | 6.02 (2.57-10.18) | 6.89 (2.68-12.62) | 4.96 (2.09-9.18) | 4.94 (2.05-9.24) | 5.20 (2.20-9.44) | 5.41 (2.14-10.44) | |
| Allergic rhinitis | 75 050 (4.2) | 23 935 (6.3) | 2870 (12.8) | 521 (9.8) | 266 083 (9.9) | 66 023 (16.1) | 29 926 (15.3) | 3062 (16.9) | |
| Asthma | 169 679 (9.4) | 49 782 (13.0) | 6094 (27.2) | 1222 (23.0) | 346 024 (12.9) | 80 267 (19.5) | 42 608 (21.7) | 4584 (25.3) | |
| History of systemic corticosteroid use | 88 904 (4.9) | 23 433 (6.1) | 2671 (11.9) | 737 (13.8) | 396 497 (14.8) | 73 019 (17.8) | 44 533 (22.7) | 8008 (44.2) | |
| History of IBD | 411 (0.02) | 65 (0.02) | 10 (0.04) | 79 (1.5) | 21204 (0.8) | 3397 (0.8) | 1852 (0.9) | 1730 (9.6) | |
| History of CD | 204 (0.01) | 31 (0.01) | 9 (0.04) | 54 (1.0) | 8261 (0.3) | 1262 (0.3) | 629 (0.3) | 949 (5.2) | |
| History of UC | 159 (0.01) | 28 (0.01) | 1 (0.00) | 26 (0.5) | 12 733 (0.5) | 2054 (0.5) | 1185 (0.6) | 903 (5.0) | |
| BMI | |||||||||
| Underweight (<18) | NA | NA | NA | NA | 72 655 (2.7) | 11 504 (2.8) | 4150 (2.1) | 525 (2.9) | |
| Normal (18.5-24.9) | NA | NA | NA | NA | 911 449 (34.0) | 152 480 (37.1) | 66 015 (33.7) | 6972 (38.5) | |
| Overweight (25-29.9) | NA | NA | NA | NA | 707 292 (26.4) | 109 693 (26.7) | 56 021 (28.6) | 4799 (26.5) | |
| Obese (30-34.9) | NA | NA | NA | NA | 285 567 (10.7) | 44 998 (11.0) | 24 088 (12.3) | 1900 (10.4) | |
| Severely obese (35-39.9) | NA | NA | NA | NA | 94 373 (3.5) | 15 720 (3.8) | 8486 (4.3) | 653 (3.6) | |
| Morbidly obese (>40) | NA | NA | NA | NA | 44 721 (1.7) | 8341 (2.0) | 4525 (2.3) | 343 (1.9) | |
| Smoking status | |||||||||
| Never | NA | NA | NA | NA | 1 293 811 (48.3) | 206 577 (50.3) | 89 588 (45.7) | 8653 (47.8) | |
| Current | NA | NA | NA | NA | 576 463 (21.5) | 84 855 (20.6) | 44 195 (22.5) | 3914 (21.6) | |
| Former | NA | NA | NA | NA | 548 828 (20.5) | 92 290 (22.5) | 48 636 (24.8) | 4182 (23.1) | |
| Drinking status | |||||||||
| Never | NA | NA | NA | NA | 300 614 (11.2) | 51 208 (12.5) | 24 278 (12.4) | 2338 (12.9) | |
| Current | NA | NA | NA | NA | 1 655 958 (61.8) | 262 008 (63.8) | 125 921 (64.2) | 11 525 (63.6) | |
| Former | NA | NA | NA | NA | 114 596 (4.3) | 19 708 (4.8) | 10 187 (5.2) | 965 (5.3) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CD, Crohn disease; IBD, inflammatory bowel disease; NA, not applicable; UC, ulcerative colitis.
Data are presented as number (percentage) of patients or controls unless otherwise indicated.
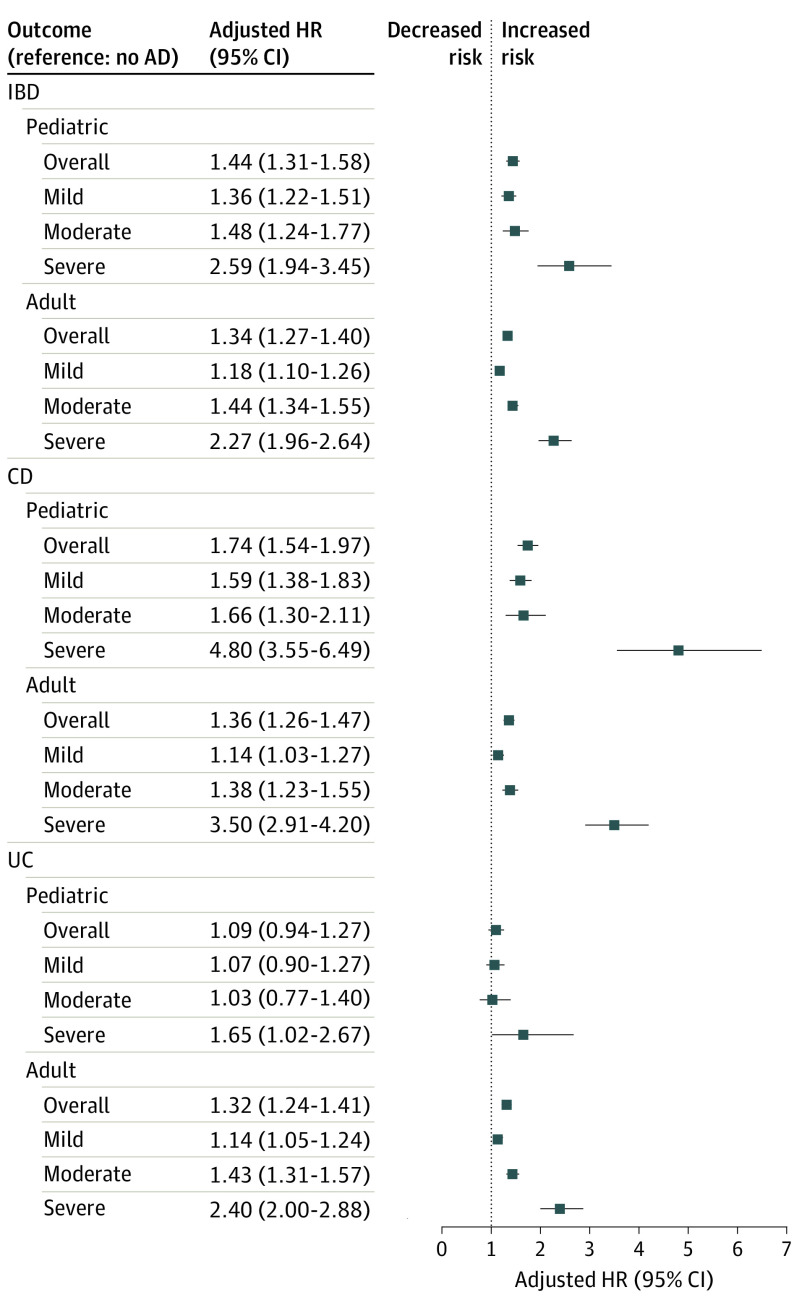
The IRs for IBD and CD were higher across AD groups (range, 0.18-0.95 for IBD and 0.10-0.91 for CD) compared with controls (range, 0.15-0.16 for IBD and 0.07-0.08 for CD). The IRs for UC were similar between controls (range, 0.07-0.08) and patients with mild AD (range, 0.06-0.09) but higher in patients with moderate (range, 0.13-0.23) and severe (range, 0.16-0.41) AD. Compared with controls, AD was associated with a 44% increased risk of IBD (HR, 1.44; 95% CI, 1.31-1.58) and a 74% increased risk of CD (HR, 1.74; 95% CI, 1.54-1.97) but not an increased risk of UC (HR, 1.09; 95% CI, 0.94-1.27). Patients with mild AD had a 36% (HR, 1.36; 95% CI, 1.22-1.51) increased risk, those with moderate AD had a 48% (HR, 1.48; 95% CI, 1.24-1.77) increased risk, and those with severe AD had a 2.6-fold increased risk (HR, 2.59; 95% CI, 1.94-3.45) of IBD. An increased risk of CD was observed across AD cohorts, with risk increasing with AD severity (mild: HR, 1.59; 95% CI, 1.38-1.83; moderate: HR, 1.66; 95% CI, 1.30-2.11; and severe: HR, 4.80; 95% CI, 3.55-6.49). An increased risk of UC was observed only in patients with severe AD (HR, 1.65; 95% CI, 1.02-2.67) (Figure 2). The IBD risk was robust to multiple sensitivity analyses (eTable 1 in Supplement 1). The CD risk remained strong in sensitivity analysis except for patients with moderate AD for whom a change in the direction of the point estimate was observed when excluding patients with asthma and/or allergic rhinitis and restricting analysis to those seen at least yearly during follow-up; however, the CIs remained overlapping.
Figure 2. Adjusted Hazard Ratios (HRs) for Inflammatory Bowel Disease (IBD), Crohn Disease (CD), and Ulcerative Colitis (UC) Stratified by Age and Atopic Dermatitis (AD) Severity.
Adult Cohort
A total of 2 678 888 controls were matched to 625 083 adult patients with AD (410 867 [65.7%] mild, 196 101 [31.4%] moderate, and 18 115 [2.9%] severe). Median age was 47 (IQR, 32-64) years in controls and in mild, moderate, and severe AD groups was 45 (IQR, 30-63) years, 50 (IQR, 34-68) years, and 47 (IQR, 32-63) years, respectively. The median cohort follow-up time was 5 (IQR, 2-10) years. The adult cohort was predominantly female (1 445 589 [54.0%] controls, 256 071 [62.3%] with mild AD, 109 404 [55.8%] with moderate AD, and 10 736 [59.3%] with severe AD). Socioeconomic status was similar across all cohorts. Patients with AD had a higher prevalence of allergic rhinitis, asthma, and use of systemic corticosteroids compared with controls. The proportion of current and former smokers and/or drinkers was higher in patients with moderate and severe AD. A higher prevalence of IBD, CD, and UC was seen in patients with severe AD. These patients were excluded from further analysis (Table 1).
The IRs for IBD were higher in patients with AD compared with controls (range, 0.39-0.41), with higher rates among those with more severe disease (mild: range, 0.47-0.53; moderate: range, 0.58-0.66; and severe: range, 0.94-1.25). For CD and UC separately, higher IRs were seen across AD groups compared with controls (Table 2). Atopic disease was associated with a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk of IBD, 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk of CD, and 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk of UC compared with controls. When stratified by AD severity, mild AD was associated with an 18% (HR, 1.18; 95% CI, 1.10-1.26), moderate AD with a 44% (HR, 1.44; 95% CI, 1.34-1.55), and severe AD with a 127% (HR, 2.27; 95% CI, 1.96-2.64) increased risk of IBD. Mild AD was associated with a 14% (HR, 1.14; 95% CI, 1.03-1.27), moderate AD with a 38% (HR, 1.38; 95% CI, 1.23-1.55), and severe AD with a 250% (HR, 3.50; 95% CI, 2.91-4.20) increased risk of CD. Mild AD was associated with a 14% (HR, 1.14; 95% CI, 1.05-1.24), moderate AD with a 43% (HR, 1.43; 95% CI, 1.31-1.57), and severe AD with a 140% (HR, 2.40; 95% CI, 2.00-2.88) increased risk of UC (Figure 2). Risk of IBD remained robust in sensitivity analyses (eTable 1 in Supplement 1). Results of the effect of unmeasured confounding on the outcomes of interest are summarized in eTable 2 in Supplement 1.
Table 2. Incidence Rates (95% CIs) of Inflammatory Bowel Disease, Crohn Disease, and Ulcerative Colitis (per 1000 Person-Years) in Pediatric and Adult Cohorts With and Without Atopic Dermatitis.
| Disease | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pediatric cohort (aged <18 y) | Adult cohort (aged ≥18 y) | |||||||
| Control | Atopic dermatitis disease severity | Control | Atopic dermatitis disease severity | |||||
| Mild | Moderate | Severe | Mild | Moderate | Severe | |||
| Inflammatory bowel disease | 0.16 (0.15-0.16) | 0.20 (0.18-0.22) | 0.48 (0.41-0.57) | 0.71 (0.54-0.95) | 0.40 (0.39-0.41) | 0.50 (0.47-0.53) | 0.62 (0.58-0.66) | 1.08 (0.94-1.25) |
| Crohn disease | 0.08 (0.07-0.08) | 0.12 (0.10-0.13) | 0.27 (0.22-0.34) | 0.68 (0.51-0.91) | 0.16 (0.15-0.16) | 0.19 (0.18-0.21) | 0.23 (0.21-0.26) | 0.71 (0.59-0.84) |
| Ulcerative colitis | 0.07 (0.07-0.08) | 0.07 (0.06-0.09) | 0.17 (0.13-0.23) | 0.26 (0.16-0.41) | 0.25 (0.24-0.25) | 0.29 (0.27-0.32) | 0.37 (0.34-0.41) | 0.72 (0.60-0.86) |
Discussion
In this cohort study, AD was associated with an increased risk of IBD ranging from 31% to 58% in children and 27% to 40% in adults. When analyzed separately, we did not observe an increased risk of UC in children with AD except among those with severe AD (65% increased risk). In contrast, children with AD had a 54% to 97% increased risk of CD, with children with severe AD having an almost 5-fold increased risk. For CD specifically, a previous cross-sectional study11 found a 2-fold increased risk among adolescents with AD, and a separate claims-based cohort study12 in the US found an increased risk among children with AD. However, the former study is limited in its ability to examine a temporal relationship because of its cross-sectional design, whereas the latter study did not examine the impact of AD severity and diagnostic codes for AD have not been previously validated in this database. As for UC risk, a previous cohort study11 in a US population–based database did not find an association with AD, whereas a separate cohort study31 from Germany found a 20% increased risk of CD but not of UC in those younger than 40 years after adjusting for age, sex, socioeconomic status, access to health care, and health care utilization; however, these results were not stratified further by age or severity.
Studies examining the risk of UC and CD in adults with AD have shown incongruent results. For example, a claims-based US cohort study found no association between AD and UC or CD.11 A separate cohort study in a Danish population also did not find an association between AD and UC or CD.13 This is in contrast to our findings in which AD was associated with a 24% to 41% increased risk of UC and a 26% to 47% increased risk of CD. A Korean cohort study in adults with AD demonstrated a 50% increased risk of UC and a 2-fold increased risk of CD, which aligns with our findings.32 Results from cross-sectional studies have also shown an increased risk of UC and CD, including a nationwide Danish registry study13 that observed an increased risk of UC and CD, and a cross-sectional study14 in Japan that found an increased risk of UC but not CD. Finally, our results are similar to those of a prior systematic review of 3 case-control studies and 1 cohort study that included 143 434 study participants and found pooled relative risks of 1.44 for IBD, 1.38 for CD, and 1.48 for UC.33
The association between AD and IBD may be explained by shared genetic and environmental factors, immune cell activation, and alterations in skin and gut microbiota. Interestingly, although UC is primarily a TH2-driven disease, CD is believed to be a TH1-driven disease. TH17 immunity has also been implicated in CD and UC and some phases of AD and plays a role in chronic tissue inflammation.34,35 Evidence also suggests that interleukin (IL) 25, otherwise known as IL-17E, may have a shared role in the pathogenesis of AD and IBD.36 Interleukin 25 promotes the production of other TH2 cytokines, including IL-4, IL-5, and IL-13.37 Known as a barrier cytokine, it is secreted by gut and skin epithelial cells in response to stimuli.36,38 In addition, IL-25 has anti-inflammatory activity by inhibiting TH17 and TH1 responses, which could result in an increased TH2 response, yet IL-25 is not elevated in CD gut samples.38,39 Although the exact functions of IL-25 remain to be fully elucidated, this potentially shared pathophysiologic mechanism could explain the association between AD and IBD. It is unclear why we saw a stronger association between AD and CD and not UC in our pediatric cohort. Crohn disease is known to be more prevalent in children than UC.40 Diagnosing pediatric IBD is challenging because of the ambiguity of its clinical presentation and lack of specific diagnostic tests.40,41 Generally, UC affects the colon and spreads from the rectum upward. However, rectal sparing has been observed in children with disease isolated to the colon, potentially leading to misdiagnosis.40,42,43 In addition, UC is more prevalent in younger children and can be undistinguishable from indeterminate colitis, a third subtype of IBD, with features of CD and UC and rates as high as 33% in children younger than 2 years.42 A US-based registry of 250 children with IBD found that 33.7% of those with an initial diagnosis of indeterminate colitis were reclassified as having CD after a median follow-up of 1.9 years.44 Others have suggested that genetic factors play a bigger role in children vs adults because known environmental risk factors for IBD (eg, smoking and medication exposure) are not as prevalent in children.45 Mutations in the proinflammatory gene NOD2 (OMIM 605956) have been suggested as a shared genetic risk factor.46 NOD2 was the first gene identified as a risk factor for ileal CD, and individuals who are heterozygous and homozygous for NOD2 variants have a 2- to 4-fold and a 20- to 40-fold increased risk, respectively, of developing CD.46,47,48 However, not all NOD2 carriers develop disease, and although testing for NOD2 gene mutations can identify 25% of patients with CD, it cannot differentiate between UC and CD of the colon.42,46 Aberrant expression of NOD2-mediated innate immunity has been observed in patients with AD.49,50
Studies that examine the association between AD and IBD are important because they shed light on common pathophysiologic mechanisms and because, with the advent of targeted therapeutic approaches, they could influence treatment selection. For example, IL-12/IL-23 inhibitors for psoriasis are also used in the management of IBD.51 However, biologics that block IL-17 may result in new-onset IBD or IBD worsening in patients with psoriasis at higher risk of IBD. There are currently no approved biologics for AD and IBD. Although 2 Janus kinase inhibitors (abrocitinib and upadacitinib) are approved for use in AD,52,53 tofacitinib is approved for UC but not AD.54
Strengths and Limitations
Our study’s strengths include a large cohort size, the use of validated AD algorithms, and the ability to examine the temporal association between AD and IBD and AD severity. Study limitations include using treatments as a proxy for severity, which makes it difficult to separate the effects of treatment exposure from those of AD severity. Although our algorithm for defining AD based on treatment could have also biased our results because medications for AD can be used to treat patients with IBD, sensitivity analysis excluding such patients showed similar results.22,23,27,55 Severity of AD was treated as a time-updated variable, allowing for escalation of severity but not deescalation, meaning a case defined as severe would not have been reclassified as moderate even if AD improved. In this case, we would expect our results to be biased toward the null and not show stronger associations. This strategy has been used in other studies because objective measures are not routinely captured in clinical practice. Outcome misclassification could also have occurred. However, the IR for IBD in controls was similar to those previously published in other UK studies.51,56 Surveillance bias could have affected our findings because severe AD may be followed up more closely; however, a sensitivity analysis limited to patients seen at least 1 time per year yielded similar findings. Residual confounding can still exist because of unmeasured confounders. However, the strength of the association between an unknown confounder and the exposure (ie, AD) and outcome of interest (IBD, UC, or CD) would need to be between 2- and 9-fold to be able to explain away the observed associations.
Conclusions
This cohort study found an increased risk of IBD and CD in adults and children with AD and an increased risk of UC among all adults with AD and children with severe AD. The finding that this risk increases with worsening severity of AD suggests a possible causal association. Future studies in more diverse populations are needed to help substantiate the validity of our findings.
eTable 1. Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis Stratified by Disease Severity: Primary and Sensitivity Analyses
eTable 2. E-Value Computations for Both Relative Risk and Confidence Interval Closest to the Null
Data Sharing Statement
References
- 1.Huang AH, Roh YS, Sutaria N, et al. Real-world comorbidities of atopic dermatitis in the pediatric ambulatory population in the United States. J Am Acad Dermatol. 2021;85(4):893-900. doi: 10.1016/j.jaad.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 2.Paller A, Jaworski JC, Simpson EL, et al. Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19(6):821-838. doi: 10.1007/s40257-018-0383-4 [DOI] [PubMed] [Google Scholar]
- 3.Roh YS, Huang AH, Sutaria N, et al. Real-world comorbidities of atopic dermatitis in the US adult ambulatory population. J Am Acad Dermatol. 2022;86(4):835-845. doi: 10.1016/j.jaad.2021.11.014 [DOI] [PubMed] [Google Scholar]
- 4.Weng YC, Juan CK, Ho HJ, Chang YL, Wu CY, Chen YJ. Atopic dermatitis does not increase the risk of inflammatory bowel disease: a nationwide cohort study. J Dermatol. 2021;48(2):168-174. doi: 10.1111/1346-8138.15661 [DOI] [PubMed] [Google Scholar]
- 5.Wu LC, Hwang CY, Chung PI, et al. Autoimmune disease comorbidities in patients with atopic dermatitis: a nationwide case-control study in Taiwan. Pediatr Allergy Immunol. 2014;25(6):586-592. doi: 10.1111/pai.12274 [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Lee JH, Koh SJ, Park H. Bidirectional relationship between atopic dermatitis and inflammatory bowel disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;83(5):1385-1394. doi: 10.1016/j.jaad.2020.05.130 [DOI] [PubMed] [Google Scholar]
- 7.Kim M, Choi KH, Hwang SW, Lee YB, Park HJ, Bae JM. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76(1):40-48. doi: 10.1016/j.jaad.2016.08.022 [DOI] [PubMed] [Google Scholar]
- 8.Cohen R, Robinson D Jr, Paramore C, Fraeman K, Renahan K, Bala M. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001-2002. Inflamm Bowel Dis. 2008;14(6):738-743. doi: 10.1002/ibd.20406 [DOI] [PubMed] [Google Scholar]
- 9.Andersen YM, Egeberg A, Gislason GH, Skov L, Thyssen JP. Autoimmune diseases in adults with atopic dermatitis. J Am Acad Dermatol. 2017;76(2):274-280.e1. doi: 10.1016/j.jaad.2016.08.047 [DOI] [PubMed] [Google Scholar]
- 10.Narla S, Silverberg JI. Association between atopic dermatitis and autoimmune disorders in US adults and children: a cross-sectional study. J Am Acad Dermatol. 2019;80(2):382-389. doi: 10.1016/j.jaad.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 11.Schneeweiss MC, Kirchgesner J, Wyss R, et al. Occurrence of inflammatory bowel disease in patients with chronic inflammatory skin diseases: a cohort study. Br J Dermatol. 2022;187(5):692-703. doi: 10.1111/bjd.21704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghersin I, Khateeb N, Katz LH, Daher S, Shamir R, Assa A. Comorbidities in adolescents with inflammatory bowel disease: findings from a population-based cohort study. Pediatr Res. 2020;87(7):1256-1262. doi: 10.1038/s41390-019-0702-3 [DOI] [PubMed] [Google Scholar]
- 13.Egeberg A, Wienholtz N, Gislason GH, Skov L, Thyssen JP. New-onset inflammatory bowel disease in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2017;31(8):e363-e365. doi: 10.1111/jdv.14157 [DOI] [PubMed] [Google Scholar]
- 14.Niwa Y, Sumi H, Akamatsu H. An association between ulcerative colitis and atopic dermatitis, diseases of impaired superficial barriers. J Invest Dermatol. 2004;123(5):999-1000. doi: 10.1111/j.0022-202X.2004.23462.x [DOI] [PubMed] [Google Scholar]
- 15.Agrawal M, Spencer EA, Colombel JF, Ungaro RC. Approach to the management of recently diagnosed inflammatory bowel disease patients: a user’s guide for adult and pediatric gastroenterologists. Gastroenterology. 2021;161(1):47-65. doi: 10.1053/j.gastro.2021.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. doi: 10.1136/bmj.300.6732.1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meer E, Thrastardottir T, Wang X, et al. Risk factors for diagnosis of psoriatic arthritis, psoriasis, rheumatoid arthritis, and ankylosing spondylitis: a set of parallel case-control studies. J Rheumatol. 2022;49(1):53-59. doi: 10.3899/jrheum.210006 [DOI] [PubMed] [Google Scholar]
- 18.Abuabara K, Magyari AM, Hoffstad O, et al. Development and validation of an algorithm to accurately identify atopic eczema patients in primary care electronic health records from the UK. J Invest Dermatol. 2017;137(8):1655-1662. doi: 10.1016/j.jid.2017.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogdie A, Alehashemi S, Love TJ, et al. Validity of psoriatic arthritis and capture of disease modifying antirheumatic drugs in the health improvement network. Pharmacoepidemiol Drug Saf. 2014;23(9):918-922. doi: 10.1002/pds.3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393-401. doi: 10.1002/pds.1335 [DOI] [PubMed] [Google Scholar]
- 21.Lewis JD, Brensinger C, Bilker WB, Strom BL. Validity and completeness of the General Practice Research Database for studies of inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2002;11(3):211-218. doi: 10.1002/pds.698 [DOI] [PubMed] [Google Scholar]
- 22.Wan J, Shin DB, Syed MN, Abuabara K, Lemeshow AR, Gelfand JM. Risk of herpesvirus, serious and opportunistic infections in atopic dermatitis: a population-based cohort study. Br J Dermatol. 2022;186(4):664-672. doi: 10.1111/bjd.20887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverwood RJ, Forbes HJ, Abuabara K, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ. 2018;361:k1786. doi: 10.1136/bmj.k1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverwood RJ, Mansfield KE, Mulick A, et al. Atopic eczema in adulthood and mortality: UK population-based cohort study, 1998-2016. J Allergy Clin Immunol. 2021;147(5):1753-1763. doi: 10.1016/j.jaci.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ascott A, Mansfield KE, Schonmann Y, et al. Atopic eczema and obesity: a population-based study. Br J Dermatol. 2021;184(5):871-879. doi: 10.1111/bjd.19597 [DOI] [PubMed] [Google Scholar]
- 26.Mansfield KE, Schmidt SAJ, Darvalics B, et al. Association between atopic eczema and cancer in England and Denmark. JAMA Dermatol. 2020;156(10):1086-1097. doi: 10.1001/jamadermatol.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe KE, Mansfield KE, Delmestri A, et al. Atopic eczema and fracture risk in adults: a population-based cohort study. J Allergy Clin Immunol. 2020;145(2):563-571.e8. doi: 10.1016/j.jaci.2019.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schonmann Y, Mansfield KE, Hayes JF, et al. Atopic eczema in adulthood and risk of depression and anxiety: a population-based cohort study. J Allergy Clin Immunol Pract. 2020;8(1):248-257.e16. doi: 10.1016/j.jaip.2019.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med. 2016;4(6):111. doi: 10.21037/atm.2016.02.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 31.Schmitt J, Schwarz K, Baurecht H, et al. Atopic dermatitis is associated with an increased risk for rheumatoid arthritis and inflammatory bowel disease, and a decreased risk for type 1 diabetes. J Allergy Clin Immunol. 2016;137(1):130-136. doi: 10.1016/j.jaci.2015.06.029 [DOI] [PubMed] [Google Scholar]
- 32.Soh H, Lee HJ, Han K, et al. Atopic diseases are associated with development of inflammatory bowel diseases in Korea: a nationwide population-based study. Clin Gastroenterol Hepatol. 2021;19(10):2072-2081.e6. doi: 10.1016/j.cgh.2020.07.049 [DOI] [PubMed] [Google Scholar]
- 33.Shi X, Chen Q, Wang F. The bidirectional association between inflammatory bowel disease and atopic dermatitis: a systematic review and meta-analysis. Dermatology. 2020;236(6):546-553. doi: 10.1159/000505290 [DOI] [PubMed] [Google Scholar]
- 34.Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283-297. doi: 10.1007/s00281-019-00733-8 [DOI] [PubMed] [Google Scholar]
- 35.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123(3):326-338. doi: 10.1111/j.1365-2567.2007.02719.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borowczyk J, Shutova M, Brembilla NC, Boehncke WH. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148(1):40-52. doi: 10.1016/j.jaci.2020.12.628 [DOI] [PubMed] [Google Scholar]
- 37.Deng C, Peng N, Tang Y, et al. Roles of IL-25 in type 2 inflammation and autoimmune pathogenesis. Front Immunol. 2021;12:691559. doi: 10.3389/fimmu.2021.691559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinschek MA, Owyang AM, Joyce-Shaikh B, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204(1):161-170. doi: 10.1084/jem.20061738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caruso R, Sarra M, Stolfi C, et al. Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology. 2009;136(7):2270-2279. doi: 10.1053/j.gastro.2009.02.049 [DOI] [PubMed] [Google Scholar]
- 40.Yu YR, Rodriguez JR. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26(6):349-355. doi: 10.1053/j.sempedsurg.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 41.Griffiths AM. Specificities of inflammatory bowel disease in childhood. Best Pract Res Clin Gastroenterol. 2004;18(3):509-523. doi: 10.1016/j.bpg.2004.01.002 [DOI] [PubMed] [Google Scholar]
- 42.Bousvaros A, Antonioli DA, Colletti RB, et al. ; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Colitis Foundation of America . Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44(5):653-674. doi: 10.1097/MPG.0b013e31805563f3 [DOI] [PubMed] [Google Scholar]
- 43.Rajwal SR, Puntis JW, McClean P, et al. Endoscopic rectal sparing in children with untreated ulcerative colitis. J Pediatr Gastroenterol Nutr. 2004;38(1):66-69. doi: 10.1097/00005176-200401000-00015 [DOI] [PubMed] [Google Scholar]
- 44.Carvalho RS, Abadom V, Dilworth HP, Thompson R, Oliva-Hemker M, Cuffari C. Indeterminate colitis: a significant subgroup of pediatric IBD. Inflamm Bowel Dis. 2006;12(4):258-262. doi: 10.1097/01.MIB.0000215093.62245.b9 [DOI] [PubMed] [Google Scholar]
- 45.Cuffari C. Diagnostic considerations in pediatric inflammatory bowel disease management. Gastroenterol Hepatol (N Y). 2009;5(11):775-783. [PMC free article] [PubMed] [Google Scholar]
- 46.Negroni A, Pierdomenico M, Cucchiara S, Stronati L. NOD2 and inflammation: current insights. J Inflamm Res. 2018;11:49-60. doi: 10.2147/JIR.S137606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599-603. doi: 10.1038/35079107 [DOI] [PubMed] [Google Scholar]
- 48.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411(6837):603-606. doi: 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- 49.Wong CK, Chu IM, Hon KL, Tsang MS, Lam CW. Aberrant expression of bacterial pattern recognition receptor NOD2 of basophils and microbicidal peptides in atopic dermatitis. Molecules. 2016;21(4):471. doi: 10.3390/molecules21040471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiao D, Wong CK, Qiu HN, et al. NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell Mol Immunol. 2016;13(4):535-550. doi: 10.1038/cmi.2015.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasvol TJ, Horsfall L, Bloom S, et al. Incidence and prevalence of inflammatory bowel disease in UK primary care: a population-based cohort study. BMJ Open. 2020;10(7):e036584. doi: 10.1136/bmjopen-2019-036584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.AbbVie. US FDA approves RINVOQ® (upadacitinib) to treat adults and children 12 years and older with refractory, moderate to severe atopic dermatitis. January 14, 2022. Accessed March 3, 2023. https://news.abbvie.com/news/press-releases/us-fda-approves-rinvoq-upadacitinib-to-treat-adults-and-children-12-years-and-older-with-refractory-moderate-to-severe-atopic-dermatitis.htm
- 53.Pfizer. US FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis. January 14, 2022. Accessed March 3, 2023. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- 54.US Food and Drug Administration . FDA approves new treatment for moderately to severely active ulcerative colitis. May 30, 2018. Accessed March 3, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-moderately-severely-active-ulcerative-colitis
- 55.Ascott A, Mulick A, Yu AM, et al. Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol. 2019;143(5):1821-1829. doi: 10.1016/j.jaci.2018.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King D, Reulen RC, Thomas T, et al. Changing patterns in the epidemiology and outcomes of inflammatory bowel disease in the United Kingdom: 2000-2018. Aliment Pharmacol Ther. 2020;51(10):922-934. doi: 10.1111/apt.15701 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis Stratified by Disease Severity: Primary and Sensitivity Analyses
eTable 2. E-Value Computations for Both Relative Risk and Confidence Interval Closest to the Null
Data Sharing Statement