This cohort study examines whether intrauterine exposure to selective serotonin reuptake inhibitors and prenatal or postnatal depressive symptoms are associated with brain morphologic trajectory in offspring.
Key Points
Question
Is intrauterine exposure to selective serotonin reuptake inhibitors (SSRIs) and prenatal or postnatal depressive symptoms associated with brain morphologic trajectory in offspring?
Findings
In this cohort study of 3198 mother-infant dyads, compared with no maternal SSRI exposure or depressive symptoms, prenatal SSRI use was associated with less cerebral gray matter in children that persisted from 7 to 15 years of age and greater increases in volumes of the amygdala and fusiform gyrus in children that did not persist until early adolescence.
Meaning
Maternal SSRI use during pregnancy may be associated with altered brain development in offspring.
Abstract
Importance
Clinical decision-making on antidepressant treatment during pregnancy, particularly selective serotonin reuptake inhibitors (SSRIs), is challenging, as both prenatal SSRI exposure and maternal depressive symptoms may be associated with negative outcomes in offspring.
Objective
To investigate the association between intrauterine SSRI exposure and maternal depressive symptoms and structural brain development in offspring from mid-childhood to early puberty.
Design, Setting, and Participants
This prospective, population-based cohort study was embedded in the Generation R Study in Rotterdam, the Netherlands. All pregnant individuals with an expected delivery date between April 1, 2002, and January 31, 2006, were invited to participate. Data were analyzed from February 1 to September 30, 2022.
Exposure
Maternal-reported SSRI use verified by pharmacy records. In mid-pregnancy and 2 and 6 months after delivery, participants reported depressive symptoms using the Brief Symptom Inventory and were divided into 5 groups: SSRI use during pregnancy (n = 41; 80 scans), SSRI use only before pregnancy (n = 77; 126 scans), prenatal depressive symptoms without prenatal SSRI use (n = 257; 477 scans), postnatal depressive symptoms only (n = 74; 128 scans), and nonexposed control individuals (n = 2749; 4813 scans).
Main Outcomes and Measures
The main outcome was brain morphometry in offspring, including global and cortical brain volumes, measured at 3 magnetic resonance imaging assessments from 7 to 15 years of age.
Results
The study included 3198 mother-child dyads. A total of 3198 mothers (100%) identified as women; mean (SD) age at intake was 31.1 (4.7) years. Children (1670 [52.2%] female) underwent brain imaging assessment from 7 to 15 years of age with 5624 total scans. Most brain gray matter volumes showed an inverted U–shaped trajectory. Compared with nonexposed controls, children prenatally exposed to SSRIs had less cerebral gray matter (β [SE], −20 212.2 [7285.6] mm3; P = .006), particularly within the corticolimbic circuit, which persisted up to 15 years of age. Children exposed to SSRIs prenatally showed a steeper increase in volumes of the amygdala (age interaction: β [SE], 43.3 [13.4] mm3; P = .006) and fusiform gyrus (age interaction: β [SE], 168.3 [51.4] mm3; P = .003) from 7 to 15 years of age. These volumetric differences in the amygdala and fusiform observed in childhood did not persist until early adolescence. Prenatal depression was associated with a smaller volume in the rostral anterior cingulate gyrus (β [SE], −166.3 [65.1] mm3; P = .006), and postnatal depression was associated with a reduced fusiform gyrus (β [SE], −480.5 [189.2] mm3; P = .002). No association of SSRI use before pregnancy with brain outcomes was observed.
Conclusions and Relevance
The results of this cohort study suggest that prenatal SSRI exposure may be associated with altered developmental trajectories of brain regions involved in emotional regulation in offspring. Further research on the functional implications of these findings is needed.
Introduction
During pregnancy, approximately 7% to 20% of women experience depressive symptoms and anxiety.1 In the US, about 10% of pregnant individuals receive antidepressant treatment, mostly selective serotonin reuptake inhibitors (SSRIs).1 In northern Europe, the prevalence of prenatal SSRI use is lower and approximately 1% to 2%.2 SSRIs are often used throughout pregnancy as maintenance treatment to prevent relapse of psychiatric symptoms.3 Maternal depression and prenatal exposure to SSRIs have been associated with negative outcomes in offspring, including adverse neurodevelopment.4,5,6,7,8 Currently, prescribing SSRIs to pregnant individuals is generally considered safe. However, methodologic limitations complicate understanding of potential direct consequences of prenatal SSRI exposure for offspring. Confounding by indication is 1 such limitation, which arises from the possibility that pregnant individuals who use SSRIs may have characteristics or conditions, including more severe depressive symptoms, that may be independently associated with adverse outcomes in offspring.8,9 Correspondingly, separating the potential negative consequences of prenatal SSRI exposure from maternal depressive symptoms and controlling for confounding are challenging. We used a hierarchical approach based on animal and human imaging studies.5,6,7,8,9,10 In the primary analyses, we examined the associations between prenatal exposure to SSRIs or maternal depressive symptoms and global volumes, including total gray and white matter, and volumes of the hippocampus and amygdala. In secondary analyses, we examined frontolimbic structures and somatosensory and higher-order visual areas.
SSRIs inhibit the reuptake of serotonin, a crucial neurotransmitter broadly distributed across brain areas that influences cognition, attention, and emotional regulation.5,10 Animal studies have shown that serotonin regulates neuronal proliferation, differentiation, migration, and synaptogenesis.10,11 Furthermore, a prenatal SSRI exposure model using serotonin transporter knockout mice demonstrated somatosensory cortex and corticolimbic circuits alterations.12 In humans, prenatal SSRI exposure has been associated with an increased risk of neurodevelopmental, affective, and anxiety disorders emerging from early childhood to puberty.8,13,14,15,16 Knowledge on prenatal SSRI treatment and brain morphometry in humans is growing. Previously, our research group showed that prenatal SSRI exposure was associated with reduced fetal head growth assessed by ultrasonography.17 To our knowledge, only 3 structural magnetic resonance imaging (MRI) studies have examined prenatal SSRI exposure and brain volume in humans, of which 2 were retrospective studies in neonates.18,19,20 SSRI-exposed newborns had larger amygdala and superior frontal cortices.19 Also, prenatal SSRI exposure was associated with lower white matter diffusivity in infants.18 However, these 2 studies had small samples (14-16 infants).18,19 The third study showed that prenatal SSRI exposure (n = 235) was associated with a larger surface area in the left superior parietal cortex and a thicker left lateral occipital cortex in children aged 9 to 10 years.20 However, the study was based on retrospective maternal reports of SSRI use and depressive symptoms during pregnancy, which could be prone to recall bias. Importantly, exposure to maternal depressive symptoms (without SSRI use) during pregnancy was also associated with differences in cortical thickness and volumes in the limbic, frontal, and temporal lobes in neonates and young children. A systematic review of neuroimaging studies also indicated changes in white matter microstructure and functional connectivity in limbic and mesocortical networks.21
The prospective association of prenatal SSRI exposure with structural brain developmental trajectories is still largely unknown.22 This prospective, population-based cohort study investigated the association of prenatal SSRI exposure and prenatal and postnatal maternal depressive symptoms with offspring brain morphometry in children aged 7 to 15 years. In an effort to address confounding by indication, we triangulated results from different contrasting groups: SSRI use only before pregnancy, prenatal depressive symptoms, and postnatal depressive symptoms only.
We hypothesized that children prenatally exposed to SSRIs would have altered trajectories of brain morphologic growth, particularly in the corticolimbic and somatosensory circuits, compared with nonexposed control individuals. We also expected volumetric differences, albeit to a lesser extent, in children exposed to prenatal and postnatal maternal depressive symptoms.
Methods
Setting and Participants
The current study was embedded within the Generation R Study, a large, population-based cohort in Rotterdam, the Netherlands, that spans from fetal life until adolescence.23,24 Recruitment details have been described elsewhere.23,24 Briefly, pregnant individuals with a delivery date between April 1, 2002, and January 31, 2006, who were living in Rotterdam were invited to participate. Mothers who participated during pregnancy were eligible (eFigure 1 in the Supplement). We excluded mothers without data on SSRI use or information on prenatal depressive symptoms. Children of participating mothers underwent at least 1 neuroimaging assessment. Children with unusable MRI data (mostly poor image quality, but also incidental findings) were excluded. The medical ethics committee of the Erasmus Medical Centre, Rotterdam, approved the study. Participants provided written informed consent for each phase of the study (fetal, preschool, childhood, and adolescence). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Prenatal SSRI Use and Maternal Depressive Symptoms
To optimize ascertainment of maternal SSRI use in pregnancy, information was collected using maternal-reported questionnaires and prescription records from pharmacies. During the first trimester, pregnant individuals were asked whether they had used medications in the previous 6 months (during pregnancy, only before pregnancy, or stopped when they knew they were pregnant). In the second and third trimester, we asked which medication was used in the preceding 3 months. From these questionnaires, we assessed SSRI exposure and timing (before or during pregnancy). To validate filled SSRI prescriptions, we used pharmacy data to verify SSRI use. The agreement between self-reports and prescription records was high: Yule Y was 0.94.17,25
Information on maternal psychopathology was obtained during pregnancy (20 weeks’ gestation) and postnatally (2 and 6 months) with the Brief Symptom Inventory, a validated self-report questionnaire containing 53 items. The 6-item depression scale of the Brief Symptom Inventory was used (Cronbach α at 3 assessments ranged from 0.82 to 0.88). According to Dutch normative data, mothers with a score higher than 0.75 had clinically relevant depressive symptoms25 (eMethods in Supplement 1).
Based on the aforementioned information, pregnant individuals were classified into 5 groups. First, the reference (control) group included individuals not using SSRIs who had low scores for depressive symptoms during pregnancy. Second, the prenatal SSRI exposure group included individuals with and without depressive symptoms who used SSRIs during pregnancy. Third, the group with SSRI use before pregnancy included individuals with and without depressive symptoms who used SSRIs before pregnancy. Fourth, the group with prenatal depression included individuals not using SSRIs before and during pregnancy who had clinically relevant depressive symptoms during pregnancy. Fifth, the group with postnatal depression only included individuals not using an SSRI before or during pregnancy who had clinically relevant depressive symptoms postnatally only.
Image Acquisition and Neuroimaging Processing
Children underwent 3 neuroimaging waves: T1, T2, and T3. Age at each time point is shown in eFigure 2 in Supplement 1. Magnetic resonance imaging scans were obtained at T1 using a Discovery MR750 3.0T MRI system (GE HealthCare) and at T2 and T3 using a dedicated Discovery MR750w 3.0T MRI system (GE HealthCare). Technical details are provided in the eMethods in Supplement 1.
Cortical reconstruction and volumetric segmentation were performed with FreeSurfer, version 6.0 (Athinoula A. Martinos Center for Biomedical Imaging).26 FreeSurfer morphometry has shown good test-retest reliability across scanners and field strengths.27 Briefly, nonbrain tissue was removed, voxel intensities were adjusted for B1 inhomogeneity, whole-brain tissue segmentation was done, and a surface-based model of the cortex was reconstructed. Cortical labeling was performed using the Desikan-Killiany Atlas (eMethods in Supplement 1).
Covariates
Based on the literature,18,19,20,22 the following potential covariates were selected: maternal age at intake, national origin (Dutch, non-Dutch [including European, Western American, and Western Asian], and non-Dutch, non-Western [including African, Cape Verdean, Dutch Antillean, Indonesian, Moroccan, non-Western American, non-Western Asian, Oceanian, Surinamese, and Turkish]), educational level, marital status, substance use (tobacco, cannabis, and alcohol), benzodiazepine use during pregnancy, and household income and child sex and age at the assessment. National origin was determined based on the country of birth of the mother and her parents using a questionnaire with categories corresponding to those used by the Dutch Central Bureau of Statistics. Because many of the national origins have a small number of participants in the Generation R Study, we operationalized these data following prior work in the Generation R Study as a 3-category variable. Details are provided in the eMethods in Supplement 1.
Statistical Analysis
Data were analyzed from February 1 to September 30, 2022. The association of prenatal SSRI exposure and depressive symptoms with brain morphometry from ages 7 to 15 years was examined with linear mixed-effects models. We used a hierarchical approach. To minimize multiple testing, we conducted follow-up analyses only if associations with the primary outcomes (total gray and white matter and hippocampus and amygdala) were observed. Cortical regions of interest, including cortical volumes of frontal, cingulate, somatosensory (postcentral gyrus), and higher-order visual areas (fusiform gyrus, parahippocampal gyrus), were included in the second tier to investigate how frontolimbic systems could contribute to the observed global associations.10,19,22,28,29 Exposure and confounder variables were included as fixed effects with a random intercept to allow for the repeated observations of participants. Main associations and interaction terms of the exposure and child age with the brain outcomes were included.
The fully adjusted model was corrected for child sex and age at neuroimaging assessment and maternal age at intake, national origin, marital status, educational level, substance use (tobacco, cannabis, and alcohol), benzodiazepine use during pregnancy, and household income. Models with subcortical and cortical volumes included intracranial volume to account for head size. To account for scanner differences, a random-effect term was added in the model. We additionally explored interaction by child sex. Directed acyclic graphs and model equations are shown in the eMethods and eFigure 3 in Supplement 1.
Post hoc group comparisons were performed in cortical regions associated with both prenatal SSRI and depressive symptoms exposure. For nonresponse analyses, we performed a t test or Wilcoxon rank sum test for continuous variables and χ2 tests for categorical variables. Since participants included in the study population and those lost to follow-up differed in multiple characteristics (eTable 3 in Supplement 1), we used inverse probability of attrition weighting to account for potential selection bias (eMethods and eTable 5 in Supplement 1). To assess potential influential observations, we performed an additional analysis to detect influential data in mixed-effects models30 (eMethods, eResults, eFigure 5, and eTable 6 in Supplement 1).
Multiple comparison correction was applied using a false discovery rate (Benjamini-Hochberg procedure).31 Statistical significance was considered at 2-sided P < .05. Missing covariate data were imputed using multivariate imputation by chained equations with 25 imputations; variables and outcomes were not imputed. All analyses were conducted using R, version 4.1.2 (R Project for Statistical Computing).
Results
Descriptive Information
Among 8756 eligible mothers, we excluded 756 without data on SSRI use, 1783 without information on prenatal depressive symptoms, and 2572 because children did not have MRI scans at T1, T2, or T3, leaving 3645 mother-child pairs (eFigure 1 in Supplement 1). After excluding 447 children with unusable MRI data, the final sample included 3198 mother-child pairs with 5624 scans. eTable 1 in Supplement 1 outlines the number of images in each neuroimaging wave.
The mean (SD) age of mothers at intake was 31.1 (4.7) years, and 3198 (100%) identified as women. Of the children, 1670 were female (52.2%) and 1528 were male (47.8%) and underwent brain imaging assessment from 7 to 15 years of age with 5624 total scans. A total of 41 participants were in the group with SSRI use during pregnancy (80 scans), 77 in the group with SSRI use only before pregnancy (126 scans), 257 in the group with prenatal depressive symptoms without prenatal SSRI use (477 scans), 74 in the group with postnatal depressive symptoms only (128 scans), and 2749 in the group of nonexposed control individuals (4813 scans). Compared with nonexposed controls, participants who used SSRIs during pregnancy had lower household income, consumed less alcohol, and were more likely to smoke tobacco and use benzodiazepines (Table 1). Participants who reported clinically relevant prenatal depressive symptoms were younger, had fewer years of education, and were more likely to be non-Dutch. Women with postnatal depression only were younger, had fewer years of education, and had lower income.
Table 1. Demographic Characteristics of the Study Populationa.
| Characteristic | Participant groupsb | ||||
|---|---|---|---|---|---|
| Reference (n = 2749) | Prenatal SSRI exposure (n = 41) | SSRI use before pregnancy (n = 77) | Prenatal depression exposure (n = 257) | Postnatal depression exposure only (n = 74) | |
| Maternal characteristics | |||||
| Age at intake, mean (SD), y | 31.2 (4.5) | 31.9 (5.3) | 31.0 (5.2) | 28.4 (5.7)c | 29.8 (5.5) |
| National origin | |||||
| Dutch | 1763 (64.1) | 27 (65.9) | 51 (66.2) | 72 (28.0)c | 31 (41.9)c |
| Non-Dutchd | 241 (8.8) | <10 | <10 | 18 (7.0) | <10 |
| Non-Dutch, non-Westerne | 745 (27.1) | 10 (24.4) | 23 (29.9) | 167 (65.0) | 39 (52.7) |
| Marital status, with partner | 2504 (91.1) | 34 (82.9) | 70 (90.9) | 170 (66.1)c | 60 (81.1)c |
| Educational level | |||||
| Primary or lower | 136 (4.9) | <10 | <10 | 46 (17.2) | <10 |
| Secondary | 1085 (39.5) | 17 (41.5) | 40 (51.9) | 155 (60.3) | 35 (47.3) |
| Higher | 1528 (55.6) | 19 (46.3) | 35 (45.5) | 56 (21.8) | 32 (43.2) |
| Monthly household income, €f | |||||
| <1200 | 319 (11.6) | 10 (24.4)c | 14 (18.2) | 120 (46.7)c | 19 (25.7)c |
| 1200-2000 | 373 (13.6) | <10 | 11 (14.3) | 54 (21.0) | 14 (18.9) |
| >2000 | 2057 (74.8) | 24 (58.5) | 52 (67.5) | 83 (32.3) | 41 (55.4) |
| Tobacco use | |||||
| Never during pregnancy | 2174 (79.1) | 24 (58.5)c | 157 (61.1)c | 157 (61.1)c | 56 (75.7) |
| Until pregnancy was known | 234 (8.5) | <10 | 13 (5.1) | 13 (5.1) | <10 |
| Continued during pregnancy | 341 (12.4) | 12 (29.3) | 87 (33.9) | 87 (33.9) | <10 |
| Cannabis use | |||||
| Never during pregnancy | 2601 (94.6) | 35 (85.4)c | 72 (93.5) | 229 (89.1)c | 70 (94.6) |
| Before pregnancy | 88 (3.2) | <10 | <10 | <10 | <10 |
| During pregnancy | 60 (2.2) | <10 | <10 | 23 (8.9) | <10 |
| Alcohol use | |||||
| Never during pregnancy | 1013 (36.8) | 17 (41.5) | 32 (41.6) | 124 (48.2)c | 33 (44.6) |
| Until pregnancy was known | 391 (14.2) | 11 (26.8) | <10 | 28 (10.9) | 12 (16.2) |
| Throughout pregnancy, occasionally | 1045 (38.0) | <10 | 29 (37.7) | 94 (36.6) | 20 (27.0) |
| Throughout pregnancy, frequently | 300 (10.9) | <10 | <10 | 11 (4.3) | <10 |
| Used benzodiazepine in pregnancy | 42 (1.5) | 11 (26.8)c | 15 (19.5)c | <10 | <10 |
| Pregnancy complications | |||||
| Preeclampsia | 55 (2.0) | 0 | <10 | <10 | <10 |
| Gestational hypertension | 115 (4.2) | <10 | <10 | <10 | <10 |
| GSI at 20 wk gestation, mean (SD)g | 0.18 (0.20) | 0.78 (0.79)c | 0.31 (0.27)c | 1.06 (0.50)c | 0.42 (0.28)c |
| Depressive symptom score, mean (SD)g | |||||
| At 20 wk gestation | 0.1 (0.2) | 0.82 (0.9)c | 0.17 (0.2)c | 1.42 (0.6)c | 0.3 (0.2)c |
| Offspring aged 2 mo (n = 2216) | 0.09 (0.2) | 0.57 (0.7)c | 0.6 (0.8)c | 0.85 (0.8)c | 1.4 (0.6)c |
| Offspring aged 6 mo (n = 1973) | 0.14 (0.3) | 0.71 (0.8)c | 0.63 (0.8)c | 0.85 (0.9)c | 0.73 (0.7)c |
| Child characteristics | |||||
| Sex | |||||
| Female | 1447 (52.6) | 21 (51.2) | 43 (55.8) | 126 (49.0) | 33 (44.6) |
| Male | 1302 (47.4) | 20 (48.8) | 34 (44.2) | 131 (51.0) | 41 (55.4) |
| Gestational age at birth, mean (SD), wk | 39.9 (1.7) | 38.8 (3.3)c | 40.1 (1.2) | 39.7 (2.1) | 39.8 (1.7) |
| Preterm birth (<37 wk) | 139 (5.1) | <10 | 0 | 23 (8.9) | <10 |
| Low birth weight (<2500 g) | 128 (4.7) | <10 | <10 | 15 (5.8) | <10 |
| Low 5-min Apgar score (<7) | 25 (0.9) | <10 | <10 | <10 | <10 |
| Age at MRI scan, mean (SD), y | |||||
| T1 (n = 830) | 7.03 (0.8) | 6.97 (0.7)c | 6.99 (0.7) | 7.04 (0.7) | 6.97 (0.7) |
| T2 (n = 2458) | 10.3 (0.8) | 10.3 (0.7) | 10.35 (0.8) | 10.47 (0.8) | 10.22 (0.7) |
| T3 (n = 2216) | 13.8 (0.6) | 13.8 (0.5) | 13.81 (0.6) | 13.78 (0.6) | 13.83 (0.7) |
Abbreviations: GSI, Global Severity Index; MRI, magnetic resonance imaging; SSRI, selective serotonin reuptake inhibitor.
Pooled imputed data are shown (except for maternal depressive symptom scores), and data are reported as number (percentage) of participants unless otherwise indicated. Data reported as less than 10 participants indicate a small number and the potential for self-identification.
Reference was no SSRI use and low score on depression symptoms during pregnancy; prenatal SSRI exposure, children exposed to SSRIs during pregnancy; SSRI use before pregnancy, low score on depression symptoms during pregnancy and maternal SSRI use before pregnancy; prenatal depression exposure, children exposed to clinically relevant depressive symptoms during the pregnancy; and postnatal depression exposure only, no SSRI use and children exposed to clinically relevant depressive symptoms only in the postnatal period.
P < .05 vs reference, derived from analysis of variance and Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables.
Included European, Western American, and Western Asian.
Included African, Cape Verdean, Dutch Antillean, Indonesian, Moroccan, non-Western American, non-Western Asian, Oceanian, Surinamese, and Turkish.
In which €1 is equal to US $1.12 in 2023.
Scores range from 0 to 4, with higher scores indicating more clinically relevant psychological symptoms.
Global and Subcortical Brain Volumes
Overall, the development of global and cortical brain structures from midchildhood to early puberty was nonlinear and best modeled by adding a quadratic age term (eTable 2 in Supplement 1). Only the hippocampal and postcentral gyrus volume trajectories were best modeled linearly.
Table 2 shows the associations of prenatal SSRI use with the repeated measures of global and subcortical brain volume. Figure 1 depicts the estimated trajectories of different brain volumes for each exposure group separately. The development of the cerebral gray matter volume followed an inverted U shape, while white matter increased from ages 7 to 15 years. As shown in Figure 1A, compared with the nonexposed condition, prenatal SSRI exposure was associated with lower cerebral gray matter volume (β [SE], −20 212.2 [7285.6] mm3; P = .006), particularly within the corticolimbic circuit, that persisted up to 15 years of age. Likewise, exposure to SSRIs during pregnancy was associated with lower cerebral white matter (β [SE], −16 336.7 [4167.2] mm3; P < .001) and amygdala (β [SE], −159.1 [31.3] mm3; P < .001) volume from 9 to 12 years of age (Table 2), but these volume differences attenuated with age. The age interaction (β [SE]) for cerebral white matter was 1548.8 [432.2] mm3 (P = .004) and for amygdala was 43.3 (13.4) mm3 (P = .006) (Figure 1B and C and Table 2). Exposure to an SSRI was not associated with volume and growth trajectory of the hippocampus (Figure 1D and Table 2).
Table 2. Association of Prenatal SSRI Use and Prenatal and Postnatal Maternal Depressive Symptoms With Global and Subcortical Brain Volumesa.
| Cerebral gray matter volume | Cerebral white matter volume | Amygdala volume | Hippocampus volume | |||||
|---|---|---|---|---|---|---|---|---|
| β, mean (SE), mm3 | P value | β, mean (SE), mm3 | P value | β, mean (SE), mm3 | P value | β, mean (SE), mm3 | P value | |
| Prenatal SSRI exposure | ||||||||
| Group | −20 212.2 (7285.6) | .006b | −16 336.7 (4167.2) | <.001b | −159.1 (31.3) | <.001b | −209.2 (197.9) | .06 |
| Group × age | 651.7 (1787.5) | .71 | 1548.8 (432.2) | .004 | 43.3 (13.4) | .006b | 11.7 (14.4) | .35 |
| Combined exposure effectc | 6.3 | .03 | 13.6 | <.001b | 15.3 | <.001b | 4.1 | .12 |
| SSRI use before pregnancy | ||||||||
| Group | 4123.5 (7760.8) | .82 | 4035.3 (5249.9) | .34 | −18.5 (79.2) | .82 | 15.4 (129.2) | .82 |
| Group × age | 655.8 (1315.8) | .61 | 1373.4 (752.8) | .06 | 4.3 (12.6) | .70 | −5.1 (15.5) | .74 |
| Combined exposure effectc | 2.3 | .31 | 2.6 | .38 | 0.1 | .96 | 1.1 | .58 |
| Prenatal depression exposure | ||||||||
| Group | −708.5 (4655.5) | .41 | −3819.4 (4025.1) | .59 | −41.9 (47.8) | .11 | 41.1 (163.4) | .58 |
| Group × age | 338.7 (756.4) | .71 | 1495.2 (357.1) | <.001b | 5.2 (6.2) | .52 | −1.6 (7.3) | .82 |
| Combined exposure effectc | 3.9 | .14 | 22.5 | <.001b | 3.2 | .19 | 0.8 | .65 |
| Postnatal depression exposure only | ||||||||
| Group | −3505.0 (7565.4) | .65 | −4772.5 (5453.4) | .17 | −35.4 (50.3) | .56 | −10.0 (124.2) | .84 |
| Group × age | 126.3 (656.4) | .56 | 763.7 (673.5) | .25 | 6.5 (7.2) | .62 | 3.9 (13.8) | .77 |
| Combined exposure effectc | 1.3 | .42 | 3.6 | .20 | 0.2 | .87 | 1.5 | .45 |
Abbreviation: SSRI, selective serotonin reuptake inhibitor.
Linear mixed-effect models were used to test the associations. The fully adjusted model corrected for child sex and age at the neuroimaging assessment and maternal age at intake, national origin, marital status, educational level, substance use (tobacco, cannabis, and alcohol), benzodiazepine use during pregnancy, and monthly household income. Intracranial volume was additionally adjusted for subcortical volumes.
Indicates a significant association after a false discovery rate correction for multiple testing.
From likelihood ratio tests.
Figure 1. Developmental Trajectories of Global and Subcortical Structural Brain Volumes Across the Follow-Up Period.
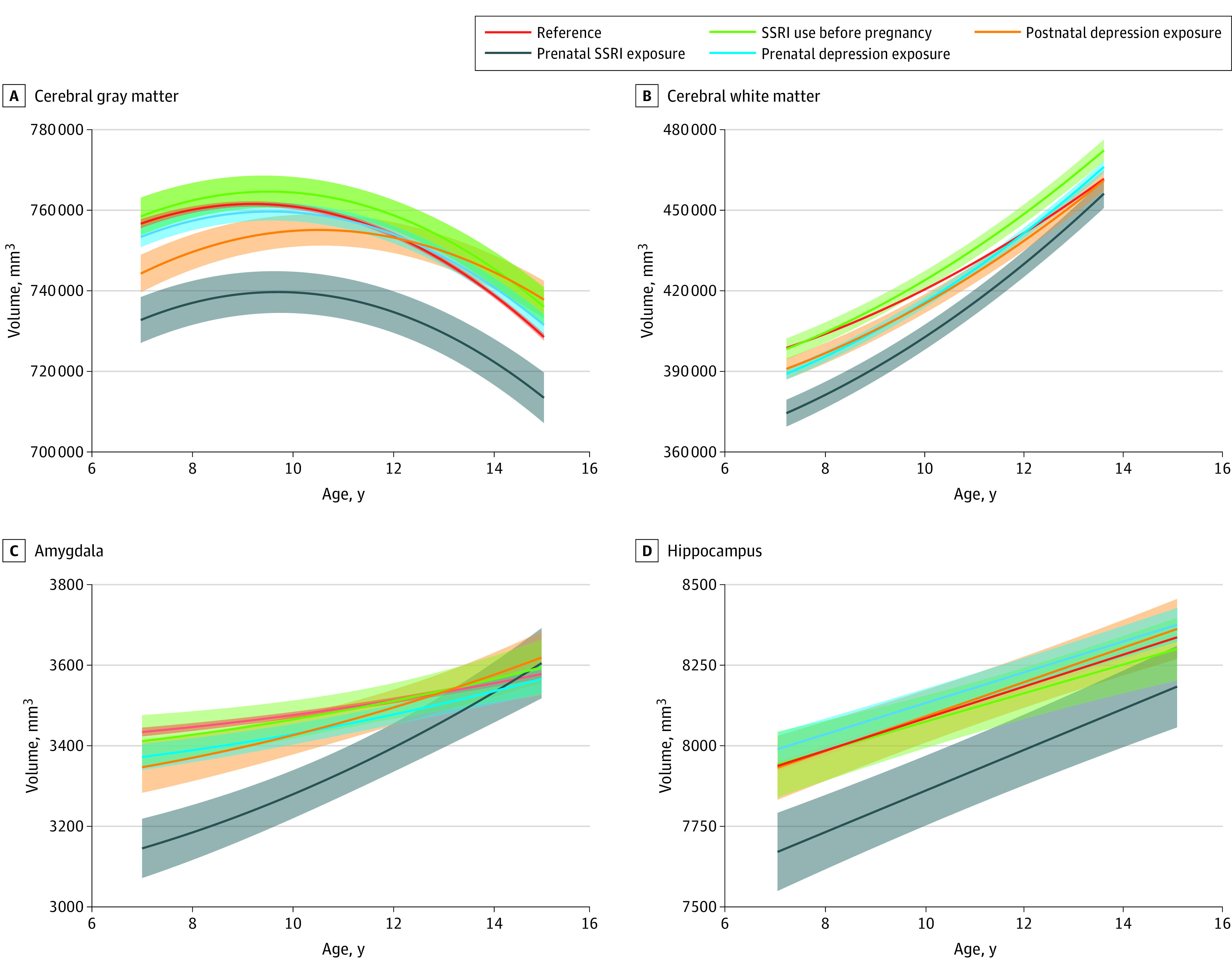
Estimated mean trajectories are shown for global (A and B) and subcortical (C and D) brain volumes for each exposure group, including the reference (n = 2749; 4813 scans), prenatal selective serotonin reuptake inhibitor (SSRI) exposure (n = 41; 80 scans), SSRI use before pregnancy (n = 77; 126 scans), prenatal depression exposure (n = 257; 477 scans), and postnatal depression exposure (n = 74; 128 scans). Adjustment was made for child sex and age at the neuroimaging assessment; maternal age at intake, national origin, marital status, educational level, substance use (tobacco, cannabis, and alcohol), benzodiazepine use during pregnancy, and monthly household income; and child intracranial volume for subcortical volumes. Shaded areas indicate 95% CIs.
No associations of postnatal exposure to depressive symptoms or SSRI use before pregnancy with primary brain outcomes were observed. Prenatal exposure to depressive symptoms was associated with a steeper increase in cerebral white matter volume (age interaction: β [SE], 1495.2 [357.1] mm3; P < .001) (Figure 1B and Table 2). No interaction by child sex in any of the models was observed.
Cortical Brain Volumes
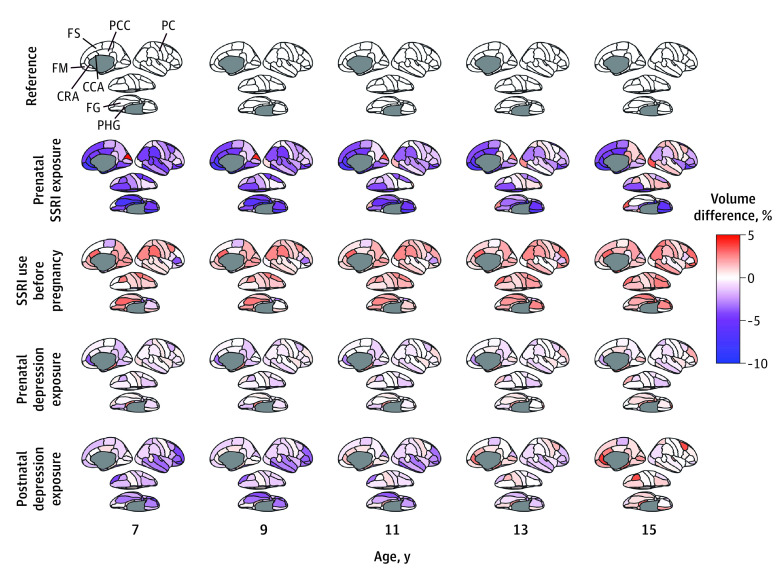
In follow-up analyses, we further explored the developmental trajectories of specific cortical brain regions as defined by the Desikan-Killiany parcellation. Cortical maps from ages 7 to 15 years were plotted and represent estimated volume differences in each exposure group compared with the reference (Figure 2). Prenatal SSRI exposure was consistently associated with lower volume, ranging from 5% to 10% in the frontal, cingulate, and temporal cortex across ages.
Figure 2. Cortical Maps Showing Estimated Volume Differences in Cortical Regions Between Each Exposure Group and Reference Across the Follow-Up Period.
Color maps represent the directions and estimated volume differences. Red indicates increased volume, and blue indicates decreased volume compared with the reference group (white). Adjustment was made for child sex and age at the neuroimaging assessment; maternal age at intake, national origin, marital status, educational level, substance use (tobacco, cannabis, and alcohol), benzodiazepine use during pregnancy, and monthly household income; and child intracranial volume. Since the models were built using bilateral averages of each cortical region, lateral, medial, and inferior views of the right hemisphere are shown purely for visualization purposes. CCA indicates cingulate, caudal anterior; CRA, cingulate, rostral anterior; FG, fusiform gyrus; FM, frontal medial orbital; FS, frontal, superior; PC, postcentral gyrus; PCC, posterior cingulate; PHG, parahippocampal gyrus; SSRI, selective serotonin reuptake inhibitor.
eFigure 4 and eTable 4 in Supplement 1 show the persistent association between prenatal SSRI exposure and less cortical volumes across the 10-year follow-up period, including in the superior frontal cortex, medial orbitofrontal cortex, parahippocampal gyrus, rostral anterior cingulate cortex, and posterior cingulate. In the fusiform gyrus, the estimated difference was age dependent: the difference between the prenatal SSRI exposure group and the reference group decreased from 7.4% at age 7 years to 1.2% at age 15 years (Figure 2 and eFigure 4 in Supplement 1). The catch-up growth in the fusiform gyrus was explained by the steeper volume increase in prenatally exposed children (age interaction: β [SE], 168.3 [51.4] mm3; P = .003) (eFigure 4 and eTable 4 in Supplement 1). Catch-up growth of the fusiform gyrus was also observed in children postnatally exposed to maternal depressive symptoms (age interaction: β [SE], 140.3 [51.6] mm3; P = .003) (eFigure 4 and eTable 4 in Supplement 1). However, prenatal SSRI exposure was associated with lower volume in the fusiform gyrus than was exposure to postnatal depressive symptoms (β [SE], −621.4 [164.2] mm3; P = .007).
No associations of SSRI use before pregnancy with cortical volumes were observed. Exposure to prenatal depressive symptoms was only associated with less volume reduction in the rostral anterior cingulate cortex. Prenatal depression was associated with smaller volume in the rostral anterior cingulate gyrus (β [SE], −166.3 [65.1] mm3; P = .006), and postnatal depression was associated with reduced volume in the fusiform gyrus (β [SE], −480.5 [189.2] mm3; P = .002) (eTable 4 in Supplement 1). No interaction by child sex was identified in any of the models.
Discussion
In this prospective, population-based cohort study of mother-child pairs, we observed that prenatal SSRI exposure was associated with reduced global gray and white matter volume in children from 7 to 15 years of age. However, children exposed to SSRIs during gestation showed catch-up growth of the white matter, the amygdala, and the fusiform gyrus during early adolescence. Comparing these findings with those from the contrasting exposure groups suggests a specific association of prenatal SSRI exposure with brain morphometry. Children exposed to prenatal depressive symptoms had smaller rostral anterior cingulate gyri, and those exposed to postnatal depressive symptoms had smaller fusiform gyrus only. SSRI use before conception was not associated with any brain differences.
Several potential mechanisms may underly the association of exposure to prenatal SSRIs and depressive symptoms with brain development. Previous animal studies have demonstrated that high levels of serotonin during the perinatal period may impact the somatosensory and corticolimbic network.4,10,32 Perinatal SSRI exposure could contribute to altered serotonin receptor distribution,10 transcription factors expression,33 or neurotrophic factor level fluctuations.34 In humans, biomarker studies have shown reduced levels of cord trophic factor, such as S100 calcium-binding protein B, in SSRI-exposed neonates.35 Maternal SSRI use may also be associated with epigenetic dysregulation of genes crucial in embryonic brain development (insulinlike growth factor 2, glial cell–derived neurotrophic factor).36 Alterations of corticolimbic structures in the offspring in the present study were in line with these studies.4,10,32,33,34,35,36
While maternal depressive symptoms have been associated with a larger amygdala volume,37,38 we did not find this in the present study; we found a specific association of prenatal SSRI exposure with amygdala and fusiform gyrus maturation. Taken together, our longitudinal results suggest an increased volume growth of the amygdala and fusiform gyrus, a neural circuitry that has been associated with affective disorders in individuals prenatally exposed to SSRIs.
A recent systematic review by Rommel et al8 reported that prenatal exposure to antidepressants was associated with multiple physical, neurodevelopmental, and psychiatric outcomes in offspring. However, the authors suggested that these associations were mostly related to underlying maternal psychopathology rather than a direct association with the medication. The same research team reported similar associations in children whose fathers used antidepressants during pregnancy, indicating another association with parental psychopathology rather than with direct in utero antidepressant exposure.16 To address potential confounding, we included several comparison groups. Despite our efforts to control for confounding, there were still disparities in group characteristics. Notably, women using SSRIs prenatally had higher depression scores and benzodiazepine use compared with the reference group, suggesting a more severe or comorbid depressive phenotype.
Strengths and Limitations
Strengths of the study were the use of a population-based sample, a longitudinal design with the combined use of self-reports and pharmacy records, repeated assessments of pediatric neuroimaging, and multiple sociodemographic confounders. However, several limitations must be discussed. First, we were unable to investigate trimester-specific outcomes of SSRI use and assess associations with specific SSRI types due to the low prevalence of SSRI use. While we used a validated 6-item, self-reported depressive symptom scale, more detailed psychiatric evaluations might further increase the validity of assessments. Our main results were based on a small subsample (n = 41; 80 scans) with prenatal SSRI exposure; thus, findings should be interpreted cautiously. Second, while child brain structure has been associated with a variety of cognitive and sensorimotor functions in the general population,39 the functional implications of the reported morphologic variations have yet to be explored. Future research on the long-term behavioral and psychological outcomes associated with these brain trajectories is needed. Third, even though we adjusted for multiple factors, residual confounding cannot be ruled out (eg, genetic factors, nutrition, stress, and other medical problems during and before pregnancy). Fourth, while sex differences in prenatal programming are commonly observed,40 our study did not find any sex differences. This may be attributed to the limited sample size of SSRI-exposed offspring. Fifth, confounding by indication is difficult to rule out in an observational study even when triangulating different contrasting groups and adjusting for multiple confounders. Thus, these results should be cautiously interpreted.
Conclusions
This cohort study found that exposure to maternal SSRI use during pregnancy was associated with offspring brain morphometry development from ages 7 to 15 years. We reported smaller volumes of the corticolimbic circuit and catch-up growth of the white matter, amygdala, and fusiform gyrus. This study may increase understanding of the association between in utero SSRI exposure and brain growth. Well-designed replication studies in diverse settings are needed before evidence-based recommendations can be derived, as the prevalence of SSRI use during pregnancy varies across populations.
eMethods. Neuroimaging, Maternal Depressive Symptoms, Psychopathology and SSRI Use, Pregnancy Complications, Covariates, Statistical Analyses
eResults. Nonresponse and Sensitivity Analyses
eFigure 1. Flow Diagram of Study Population
eFigure 2. Showing the Age of Each Participant at Each Study Time Point
eFigure 3. Direct Acyclic Graph Showing the Hypothesized Relationship Between Maternal SSRI Use During Pregnancy and Brain Outcomes
eFigure 4. Developmental Trajectories of Cortical Brain Structural Volumes
eFigure 5. Difference in Parameter Estimates (DFBETAS) for Each Data Point and All Exposure Groups in the Analyses of Primary Outcomes
eTable 1. Number of Images of the Participants in Each Group at the Three Assessments
eTable 2. Comparison of Linear and Nonlinear (Quadratic) Models Using the Likelihood Ratio Test
eTable 3. Nonresponse Analysis
eTable 4. Association of Prenatal SSRI Use and Maternal Depressive Symptoms With Cortical Brain Volumes
eTable 5. Comparison of Results From the Unweighted Model With Inverse Probability of Attrition Weighting (IPAW) Models for the Associations of Prenatal SSRI and Maternal Depressive Symptoms Exposure and Brain Morphology
eTable 6. Impact of Potentially Influential Observations on Primary Outcomes
eReferences
Data Sharing Statement
References
- 1.Vigod SN, Wilson CA, Howard LM. Depression in pregnancy. BMJ. 2016;352:i1547. doi: 10.1136/bmj.i1547 [DOI] [PubMed] [Google Scholar]
- 2.Ververs T, Kaasenbrood H, Visser G, Schobben F, de Jong-van den Berg L, Egberts T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62(10):863-870. doi: 10.1007/s00228-006-0177-0 [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Molenaar N, Agerbo E, et al. Antidepressant discontinuation before or during pregnancy and risk of psychiatric emergency in Denmark: a population-based propensity score-matched cohort study. PLoS Med. 2022;19(1):e1003895. doi: 10.1371/journal.pmed.1003895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gemmel M, Bögi E, Ragan C, et al. Perinatal selective serotonin reuptake inhibitor medication (SSRI) effects on social behaviors, neurodevelopment and the epigenome. Neurosci Biobehav Rev. 2018;85:102-116. doi: 10.1016/j.neubiorev.2017.04.023 [DOI] [PubMed] [Google Scholar]
- 5.Hutchison SM, Mâsse LC, Pawluski JL, Oberlander TF. Perinatal selective serotonin reuptake inhibitor (SSRI) and other antidepressant exposure effects on anxiety and depressive behaviors in offspring: a review of findings in humans and rodent models. Reprod Toxicol. 2021;99:80-95. doi: 10.1016/j.reprotox.2020.11.013 [DOI] [PubMed] [Google Scholar]
- 6.Zou R, Tiemeier H, van der Ende J, et al. Exposure to maternal depressive symptoms in fetal life or childhood and offspring brain development: a population-based imaging study. Am J Psychiatry. 2019;176(9):702-710. doi: 10.1176/appi.ajp.2019.18080970 [DOI] [PubMed] [Google Scholar]
- 7.Suri R, Lin AS, Cohen LS, Altshuler LL. Acute and long-term behavioral outcome of infants and children exposed in utero to either maternal depression or antidepressants: a review of the literature. J Clin Psychiatry. 2014;75(10):e1142-e1152. doi: 10.4088/JCP.13r08926 [DOI] [PubMed] [Google Scholar]
- 8.Rommel AS, Bergink V, Liu X, Munk-Olsen T, Molenaar NM. Long-term effects of intrauterine exposure to antidepressants on physical, neurodevelopmental, and psychiatric outcomes: a systematic review. J Clin Psychiatry. 2020;81(3):19r12965. doi: 10.4088/JCP.19r12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores JM, Avila-Quintero VJ, Bloch MH. Selective serotonin reuptake inhibitor use during pregnancy-associated with but not causative of autism in offspring. JAMA Psychiatry. 2019;76(12):1225-1227. doi: 10.1001/jamapsychiatry.2019.2193 [DOI] [PubMed] [Google Scholar]
- 10.Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci. 2010;31(2):60-65. doi: 10.1016/j.tips.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Grieb ZA, Ragan CM. The effects of perinatal SSRI exposure on anxious behavior and neurobiology in rodent and human offspring. Eur Neuropsychopharmacol. 2019;29(11):1169-1184. doi: 10.1016/j.euroneuro.2019.07.239 [DOI] [PubMed] [Google Scholar]
- 12.Wellman CL, Izquierdo A, Garrett JE, et al. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27(3):684-691. doi: 10.1523/JNEUROSCI.4595-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Marroun H, White T, Verhulst FC, Tiemeier H. Maternal use of antidepressant or anxiolytic medication during pregnancy and childhood neurodevelopmental outcomes: a systematic review. Eur Child Adolesc Psychiatry. 2014;23(10):973-992. doi: 10.1007/s00787-014-0558-3 [DOI] [PubMed] [Google Scholar]
- 14.Lupattelli A, Wood M, Ystrom E, Skurtveit S, Handal M, Nordeng H. Effect of time-dependent selective serotonin reuptake inhibitor antidepressants during pregnancy on behavioral, emotional, and social development in preschool-aged children. J Am Acad Child Adolesc Psychiatry. 2018;57(3):200-208. doi: 10.1016/j.jaac.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malm H, Brown AS, Gissler M, et al. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry. 2016;55(5):359-366. doi: 10.1016/j.jaac.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rommel AS, Momen NC, Molenaar NM, Liu X, Munk-Olsen T, Bergink V. Long-term prenatal effects of antidepressant use on the risk of affective disorders in the offspring: a register-based cohort study. Neuropsychopharmacology. 2021;46(8):1518-1525. doi: 10.1038/s41386-021-01005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Marroun H, Jaddoe VWV, Hudziak JJ, et al. Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Arch Gen Psychiatry. 2012;69(7):706-714. doi: 10.1001/archgenpsychiatry.2011.2333 [DOI] [PubMed] [Google Scholar]
- 18.Jha SC, Meltzer-Brody S, Steiner RJ, et al. Antenatal depression, treatment with selective serotonin reuptake inhibitors, and neonatal brain structure: a propensity-matched cohort study. Psychiatry Res Neuroimaging. 2016;253:43-53. doi: 10.1016/j.pscychresns.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lugo-Candelas C, Cha J, Hong S, et al. Associations between brain structure and connectivity in infants and exposure to selective serotonin reuptake inhibitors during pregnancy. JAMA Pediatr. 2018;172(6):525-533. doi: 10.1001/jamapediatrics.2017.5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreau AL, Voss M, Hansen I, et al. Prenatal selective serotonin reuptake inhibitor exposure, depression and brain morphology in middle childhood: results from the ABCD Study. Biol Psychiatry Glob Open Sci. 2022;3(2)243-254. doi: 10.1101/2021.09.01.21262980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cattarinussi G, Aarabi MH, Sanjari Moghaddam H, et al. Effect of parental depressive symptoms on offspring’s brain structure and function: a systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2021;131:451-465. doi: 10.1016/j.neubiorev.2021.09.046 [DOI] [PubMed] [Google Scholar]
- 22.Rotem-Kohavi N, Williams LJ, Oberlander TF. Advanced neuroimaging: a window into the neural correlates of fetal programming related to prenatal exposure to maternal depression and SSRIs. Semin Perinatol. 2020;44(3):151223. doi: 10.1016/j.semperi.2020.151223 [DOI] [PubMed] [Google Scholar]
- 23.Kooijman MN, Kruithof CJ, van Duijn CM, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243-1264. doi: 10.1007/s10654-016-0224-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White T, Muetzel RL, El Marroun H, et al. Paediatric population neuroimaging and the Generation R Study: the second wave. Eur J Epidemiol. 2018;33(1):99-125. doi: 10.1007/s10654-017-0319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Marroun H, White TJH, van der Knaap NJF, et al. Prenatal exposure to selective serotonin reuptake inhibitors and social responsiveness symptoms of autism: population-based study of young children. Br J Psychiatry. 2014;205(2):95-102. doi: 10.1192/bjp.bp.113.127746 [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11-22. doi: 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- 27.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402-1418. doi: 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Knaap N, Wiedermann D, Schubert D, Hoehn M, Homberg JR. Perinatal SSRI exposure affects brain functional activity associated with whisker stimulation in adolescent and adult rats. Sci Rep. 2021;11(1):1680. doi: 10.1038/s41598-021-81327-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baudat M, de Kort AR, van den Hove DLA, Joosten EA. Early-life exposure to selective serotonin reuptake inhibitors: long-term effects on pain and affective comorbidities. Eur J Neurosci. 2022;55(1):295-317. doi: 10.1111/ejn.15544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieuwenhuis R, te Grotenhuis M, Pelzer B. influence.ME: tools for detecting influential data in mixed effects models. R J. 2012;4(2):38-47. doi: 10.32614/RJ-2012-011 [DOI] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 32.Simpson KL, Weaver KJ, de Villers-Sidani E, et al. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci U S A. 2011;108(45):18465-18470. doi: 10.1073/pnas.1109353108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brivio P, Homberg JR, Riva MA, Calabrese F. Alterations of glutamatergic markers in the prefrontal cortex of serotonin transporter knockout rats: a developmental timeline. Cell Mol Neurobiol. 2019;39(5):715-720. doi: 10.1007/s10571-019-00673-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabrese F, Guidotti G, Middelman A, Racagni G, Homberg J, Riva MA. Lack of serotonin transporter alters BDNF expression in the rat brain during early postnatal development. Mol Neurobiol. 2013;48(1):244-256. doi: 10.1007/s12035-013-8449-z [DOI] [PubMed] [Google Scholar]
- 35.Pawluski JL, Galea LAM, Brain U, Papsdorf M, Oberlander TF. Neonatal S100B protein levels after prenatal exposure to selective serotonin reuptake inhibitors. Pediatrics. 2009;124(4):e662-e670. doi: 10.1542/peds.2009-0442 [DOI] [PubMed] [Google Scholar]
- 36.Cardenas A, Faleschini S, Cortes Hidalgo A, et al. Prenatal maternal antidepressants, anxiety, and depression and offspring DNA methylation: epigenome-wide associations at birth and persistence into early childhood. Clin Epigenetics. 2019;11(1):56. doi: 10.1186/s13148-019-0653-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lupien SJ, Parent S, Evans AC, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A. 2011;108(34):14324-14329. doi: 10.1073/pnas.1105371108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen DJ, Poh JS, Ni SN, et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry. 2017;7(4):e1103-e1103. doi: 10.1038/tp.2017.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baum GL, Cui Z, Roalf DR, et al. Development of structure-function coupling in human brain networks during youth. Proc Natl Acad Sci U S A. 2020;117(1):771-778. doi: 10.1073/pnas.1912034117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hicks LM, Swales DA, Garcia SE, Driver C, Davis EP. Does prenatal maternal distress contribute to sex differences in child psychopathology? Curr Psychiatry Rep. 2019;21(2):7. doi: 10.1007/s11920-019-0992-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Neuroimaging, Maternal Depressive Symptoms, Psychopathology and SSRI Use, Pregnancy Complications, Covariates, Statistical Analyses
eResults. Nonresponse and Sensitivity Analyses
eFigure 1. Flow Diagram of Study Population
eFigure 2. Showing the Age of Each Participant at Each Study Time Point
eFigure 3. Direct Acyclic Graph Showing the Hypothesized Relationship Between Maternal SSRI Use During Pregnancy and Brain Outcomes
eFigure 4. Developmental Trajectories of Cortical Brain Structural Volumes
eFigure 5. Difference in Parameter Estimates (DFBETAS) for Each Data Point and All Exposure Groups in the Analyses of Primary Outcomes
eTable 1. Number of Images of the Participants in Each Group at the Three Assessments
eTable 2. Comparison of Linear and Nonlinear (Quadratic) Models Using the Likelihood Ratio Test
eTable 3. Nonresponse Analysis
eTable 4. Association of Prenatal SSRI Use and Maternal Depressive Symptoms With Cortical Brain Volumes
eTable 5. Comparison of Results From the Unweighted Model With Inverse Probability of Attrition Weighting (IPAW) Models for the Associations of Prenatal SSRI and Maternal Depressive Symptoms Exposure and Brain Morphology
eTable 6. Impact of Potentially Influential Observations on Primary Outcomes
eReferences
Data Sharing Statement