Abstract
Clinical Trials Registration ClinicalTrials.gov Identifier: NCT04518410.
Keywords: ACTIV-2, clinical trial, COVID-19, monoclonal antibodies
In April of 2020, the public-private partnership, Accelerating COVID-19 Therapeutics and Vaccine (ACTIV), a cross National Institutes of Health (NIH) initiative, was created to jumpstart the evaluation of new therapeutics and vaccines for coronavirus disease 2019 (COVID-19) in randomized clinical trials [1]. The process through which the ACTIV trials were developed and the rationale for the use of a master protocol for this purpose has been previously described [2]. The ACTIV-2 trial was initiated to address the need to evaluate monoclonal antibodies and other novel therapies for ambulatory patients with COVID-19, and the AIDS Clinical Trials Group (ACTG) was selected by the NIH and the ACTIV Therapeutics Working Group to lead the protocol development and study conduct [2]. The goal was to develop a platform trial that could rapidly evaluate compounds that were prioritized for study by the ACTIV agent prioritization group [3]. The clinical trial was sponsored by the NIH and designed and led by a team of investigators in the ACTG with funding to the ACTG UM1 awards. The time from concept submission for ACTIV-2 to the first participant enrolled was 2.5 months. The study team worked in collaboration with pharmaceutical companies who were developing the products; however, all aspects of the trial were under the primary sponsorship of the NIH. A clinical research organization (CRO), PPD, was contracted by the NIH to support the ACTG in the implementation of the trial. This supplement includes papers that describe selected key findings and study design and analysis challenges. In this overview, we provide a description of the ACTIV-2 trial and highlight key operational challenges.
STUDY DESIGN
ACTIV-2 (NCT04518410) is a randomized, controlled platform trial designed to evaluate investigational agents in both phase 2 and phase 3 for the treatment of nonhospitalized persons with COVID-19. The rationale for the use of a platform trial in this setting was to allow for the evaluation of multiple therapies while sharing control groups, thus requiring fewer participants than in independently conducted randomized controlled trials. The design also allowed for the continuous introduction of new agents as they became available, with rapid movement of promising agents from phase 2 to phase 3 evaluation. The trial also included standardized quantitative measurement of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral ribonucleic acid (RNA) from samples collected from nasopharyngeal, plasma, and anterior nasal (self-collected) samples at scheduled timepoints to permit the exploration of virologic endpoints as possible future primary surrogate endpoints in COVID-19 trials by assessing the association between changes in viral shedding and clinical outcomes.
The primary objectives for ACTIV-2 were to assess safety, antiviral activity, and clinical endpoints. The coprimary objectives of the study for phase 2 and phase 3 are outlined in Table 1. The full description of secondary and exploratory objectives for the ACTIV-2 protocol are detailed in the full protocol included in the Supplementary Materials. These endpoints remained unchanged throughout the conduct of the trial.
Table 1.
Co Primary Objectives of the ACTIV-2 Study
| Phase | Coprimary Objective |
|---|---|
| 2 | To determine efficacy of the investigational agent to reduce the duration of COVID-19 symptoms through study day 28. |
| 2 | To determine the efficacy of the investigational agent to increase the proportion of participants with NP SARS-CoV-2 RNA below the lower LLoQa at study days 3, 7, and 14 |
| 3 | To determine whether the investigational agent will prevent the composite endpoint of hospitalization due to any cause or death due to any cause through study day 28. Hospitalization is defined as ≥24 hours of acute care, in a hospital or similar acute care facility, including emergency rooms or temporary facilities instituted to address medical needs of those with severe COVID-19 |
| 2 and 3 | To evaluate the safety of the investigational agent. |
Abbreviations: COVID-19, coronavirus disease 2019; LLoQ, lower limit of quantification; NP, nasopharyngeal; RNA, ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Changed from below the limit of detection to the LLoQ in version 2.
The phase 2 portion of the trial focused on evaluating safety and the potential effect of an investigational agent on COVID-19-associated symptoms and viral shedding over 28 days, whereas phase 3 included a larger sample size to also allow for an evaluation of hospitalization and death, because the key outcome for regulatory review of new therapeutics in nonhospitalized individuals at the start of the pandemic was progression to severe disease. To address regulatory requirements for long-term safety assessments for monoclonal antibodies having a prolonged half-life, all participants were followed for 72 weeks. This extended follow up permitted the inclusion of self-reported measures of post-COVID conditions or Long COVID and health-related quality of life. These assessments included the same 13 symptoms present in the acute viral illness dairy completed during the first 28 days of the study plus 14 additional Long COVID symptoms selected based on available literature at the time.
The ACTIV-2 trial evaluated 7 investigational agents in phase 2, with 3 agents advancing to phase 3. The agents included 4 monoclonal antibody preparations (bamlanivimab at 2 doses by intravenous [IV] administration, amubarvimab plus romlusevimab IV administration, tixagevimab plus cilgavimab by IV and by intramuscular administration, and BMS-986414 [C135-LS]/BMS-986413 [C144-LS] by subcutaneous administration), an inhaled beta interferon, oral camostat (a host serine protease inhibitor), and SAB-185, an intravenously administered novel fully-human immunoglobulin (Ig)G polyclonal Ig studied at 2 doses. Primary results from several of these components of the trial have been described or are in press [4–8]. The agents studied in ACTIV-2, accrual by agent subprotocol, and protocol versions in which they were introduced are outlined in Table 2.
Table 2.
Investigational Agents Evaluated in ACTIV-2
| Investigational Agent |
Subprotocol | Phase | Control Arm | Route of Administration | Risk Group for Severe COVID-19 Progression | Protocol Version Introducing Agent | Total Accrual |
|---|---|---|---|---|---|---|---|
| Bamlanivimab | Bam 7000 mga | II | Placebo | IV | H + L | 1 | 94 |
| Bam 700 mg | II | Placebo | IV | H + L | 1 (LOA 1) | 225 | |
| Bam 700 (phase III) | III | None | IV | H + L | 1 (LOA 3) | 1059 | |
| Amubarvimab/ romlusevimab |
A/R (phase II)b | II | Placebo | IV | H | 2 | 222 |
| A/R (phase III)b | III | Placebo | IV | H | 2 | 624 | |
| SNG001 | SNG001 | II | Placebo | Inhaled (14 days of dosing) |
Initially H + Lc | 3 | 163 |
| Camostat | Camostat | II | Placebo | Oral (7 days of dosing) |
Initially H + Lc | 3 | 167 |
| Tixagevimab/ cilgavimab |
T/C IM | II | Placebo | IM injection | Initially H + Lc | 3 | 164 |
| T/C IVa | II | Placebo | IV | Initially Hc | 3 | 94 | |
| SAB-185 | SAB high dose | II | Placebo | IV | Initially Hc | 4 | 156 |
| SAB low dose (phase II) | II | Placebo | IV | Initially Hc | 4 | 149 | |
| SAB low dose (phase III)d | III | Active comparator | IV | H | 7 | 733 | |
| BMS-986414+ BMS-986413 |
BMS | II | Placebo | SC injection | Initially H + Lc | 5 | 173 |
Abbreviations: A/R, amubarvimab/romlusevimab; BMS, Bristol Myers Squibb; H, higher risk; COVID-19, coronavirus disease 2019; IM, intramuscular injection; IV, intravenous infusion; L, lower risk; LOA, Letter of Amendment; SC, subcutaneous injection; T/C, tixagevimab/cilgavimab.
Subprotocols for which phase II enrollment was terminated early for administrative reasons.
Amubarvimab/Romlusevimab was the only agent with placebo-controlled evaluation in both phase II and phase III.
Initially, agents administered intravenously were restricted to higher risk individuals; however, Protocol Version 6.0 changed inclusion criteria to restrict phase II enrollment to only those at lower risk for progression to severe COVID-19, regardless of the route of administration.
Phase III noninferiority evaluation of SAB-185 low dose stopped early due to futility.
RESULTS
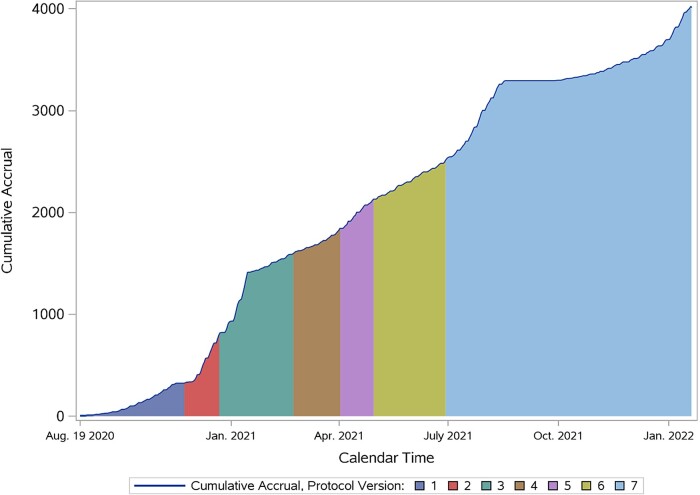
The ACTIV-2 trial enrolled 4043 unique participants at 173 sites in 7 countries (United States, Brazil, South Africa, Mexico, Argentina, Guatemala, and the Philippines) between August 2020 and January 2022. Clinical data available for 4023 participants included in primary analyses of ACTIV-2 are summarized in Table 3. Data from 20 participants were removed from the database due to research misconduct at one site. The timeline for enrollment by protocol version is shown in Figure 1. The participating sites (Supplemental Table 1) included those actively participating in the clinical trial networks supported by the NIH Division of AIDS as well as community-based clinical research sites contracted by the CRO in the United States and globally. Approximately two thirds of the study enrollment was accomplished by the top 30 enrolling sites, reflecting many of the challenges faced by participating sites that delayed their opening, discussed below. The median age of the randomized population was 47 (quartiles: 36, 58) years and 53% identified as female sex at birth. Participants of Hispanic ethnicity comprised 44% of the overall group, reflecting successful engagement with the study's Community Advisory Board, outreach efforts, and site density in areas with large Hispanic populations. The racial composition included 83% White, 10% Black, 5% Asian, and 1% American Indian or Alaska Native. The key baseline demographic and clinical features of the study population are described in Table 3. The protocol enrolled through multiple waves of SARS-CoV-2 variants including alpha, delta, and early omicron variants. Over time, the proportion of participants who had received 1 or more COVID-19 vaccines increased and the protocol was modified to accommodate inclusion of these participants and stratify their risk for progression to hospitalization/death. Across the study population overall, 11% of participants reported having received 1 or more COVID-19 vaccinations before study entry.
Table 3.
Baseline Characteristics: Demographics (All Randomized Participants)
| Characteristic | Statistics/Categories | Total (N = 4023) |
|---|---|---|
| Age (years) | Mean (SD) | 47.3 (14.9) |
| Median (Q1, Q3) | 47 (36, 58) | |
| <55 | 2723 (68%) | |
| 55–<65 | 802 (20%) | |
| ≥65 | 498 (12%) | |
| Sex | Female | 2138 (53%) |
| Male | 1885 (47%) | |
| Gender identity | Cisgender | 4004 (>99%) |
| Transgender spectrum | 16 (<1%) | |
| Not reported | 3 (<1%) | |
| Race | American Indian Or Alaska Native | 34 (1%) |
| Asian | 186 (5%) | |
| Black Or African American | 393 (10%) | |
| Multiple | 34 (1%) | |
| Native Hawaiian Or Other Pacific Islander | 8 (0%) | |
| Other | 101 (3%) | |
| White | 3258 (81%) | |
| Missing | 9 | |
| Ethnicity | Hispanic Or Latino | 1773 (44%) |
| Not Hispanic Or Latino | 2245 (56%) | |
| Missing | 5 | |
| Pregnancy status at study entry | N/A (Male) | 1885 (47%) |
| Not of reproductive potential | 1104 (28%) | |
| Not pregnant | 1011 (25%) | |
| Pregnant | 1 (0%) | |
| Missing | 22 | |
| Country | Argentina | 102 (3%) |
| Brazil | 33 (1%) | |
| Guatemala | 5 (0%) | |
| Mexico | 51 (1%) | |
| Philippines | 3 (0%) | |
| South Africa | 150 (4%) | |
| United States | 3679 (91%) |
Abbreviations: N/A, not applicable: P10, P90, 10th, 90th percentile; Q1, Q3, 25th, 75th percentile; SD, standard deviation.
Figure 1.
ACTIV-2 accrual timeline.
Challenges
A major initial operational challenge that delayed sites opening and enrolling was to identify clinical locations that were available, could safely screen and enroll participants at in-person visits, and were equipped to administer outpatient infusions. This required the availability of trained staff and physical spaces that could comply with infection prevention measures to allow for visits lasting over 2 hours at times. The study supplied personal protective equipment so as not to place a drain on local clinical supplies, which had to be sourced and distributed. Sites were able to use novel prefabricated structures, a large number of which were procured and distributed by the study; however, this also required permits and set up that required additional time. Many of the prefabricated units have remained in place throughout the pandemic and continue to be used for delivery of COVID-19 therapeutics.
As the pandemic unfolded and new treatments emerged, the study adapted to address the changing therapeutic landscape. Initially, high-risk participants (based on age and comorbidities) were randomized to placebo-controlled evaluations, but when the first emergency use authorization (EUA) for monoclonal antibodies was approved in the United States, the study adapted to focus on lower risk participants for phase 2 evaluations. In addition, the study design had to adjust to the evolution of SARS-CoV-2 variants and their susceptibility to monoclonal antibodies. For example, for the phase 3 evaluation of the SAB compound, the study was adapted to compare the SAB agent to the combination of casirivimab/imdevimab (ie, an active control), which had EUA authorization at the time for treatment of nonhospitalized adults with COVID-19. However, the omicron variant emerged during enrollment of this evaluation, and with the reduced activity of casirivimab/imdevimab against Omicron and low hospitalization/death event rates during this stage of the pandemic, this phase 3 evaluation was terminated early for operational futility.
Outreach, community engagement, and facilitation of participant enrollment were major activities within the trial. The study developed an active outreach campaign through a collaboration with a dynamic community advisory board comprising a diverse group of individuals personally impacted by COVID-19 and with expertise in community engagement. The group helped to shape the messages of the outreach efforts, which included a website and call center that provided warm handoffs between potential study participants and clinical research sites. Advertising for the trial included a media campaign, “Rise Above COVID”, that directed people to the ACTIV-2 website (RiseAboveCOVID.org) where they could learn more about COVID-19 and clinical trials, enabling them to take action to be tested and consider joining the study if appropriate.
A key operational issue for ACTIV-2 was the need to generate a new protocol version with each additional new agent. To reduce this burden, several agents were introduced in the same protocol version when possible. This led to operational challenges at sites, described further below, when multiple study agents were available concurrently. In addition, the requirement for new versions of the protocol led to regulatory delays at many sites, despite the use of a single institutional review board for sites in the United States. The regulatory complexity of introducing multiple agents was most notable for sites outside the United States.
Sites were required to manage eligibility, consenting, randomization, administration, and supply of investigational agents and placebos with different eligibility requirements and routes of administration. Although an effort was made to minimize differences in eligibility criteria between agents, eligibility requirements necessarily differed with respect to the route of administration, drug-drug interactions, and hepatic and renal function. Because participants were not allowed by the study design to select specific agents for randomization, these criteria needed to be assessed and tracked for all potential participants for all available agents, to identify the correct set of agents for which they were eligible.
Sites were also required to have the capacity to administer products with different routes of administration, risk profiles, and requirements for drug preparation, such as need for a biosafety cabinet, infusion space, nursing personnel for administration, and adequate expertise to manage potential adverse events, such as infusion reactions. It was also vital to ensure adequate supply of all investigational product on site at the time of enrollment for each participant, to maximize the benefit of sharing control groups in evaluating agents in parallel. In addition, on an operational level, the possibility that a participant might be randomized to an agent that required infusion was a significant practical constraint in terms of clinic capacity to undertake multiple simultaneous infusions, compared with dispensing an oral self-administered regimen to participants. As the number of available agents at a given site increased, the length of the informed consent form and consenting process resulted in longer study visits, which may have deterred potentially interested participants.
Strategies applied to decrease site and participant burden included the following: remote consenting, home-based assessments and specimen collection, electronic questionnaires, and transportation services for people with COVID-19 organized by the study. Despite many challenges, the ACTIV-2 team was able to implement several novel assessments, including a COVID-19-specific symptom diary validated in parallel with the conduct of the trial and daily anterior nasal swab sampling that led to important observations on the emergence of monoclonal antibody resistance [4], rapid conversion to negative SARS-CoV-2 nasal culture with monoclonal antibody therapy versus placebo [5], superior effectiveness of 2 versus 1 active monoclonal antibodies in the context of changing variants [6], and the frequency of viral and symptom rebound in the absence of treatment and rarity of their co-occurrence [7].
SUMMARY AND OVERVIEW OF THE SUPPLEMENT
The ACTIV-2 trial represents an unprecedented public-private partnership to rapidly evaluate 8 novel potential therapeutics globally. To date, the study has contributed to regulatory approvals for 2 agents and helped rapidly determine the lack of activity of other therapeutics. Critically important lessons learned during the trial will inform ongoing efforts in COVID-19 clinical trials and planning for future pandemics. Instruments (symptom diaries, questionnaires) developed during the trial continued to be used in the evaluation of newer agents by pharma- and NIH-supported trials. Additional findings from the trial described in this supplement include a more detailed discussion of key features of the ACTIV-2 design and the use of pooled placebo control groups, a manuscript describing the relationship between nasal and plasma viral RNA measures and risk of hospitalization, findings describing the predictors of and frequency of postacute sequelae of COVID-19 after monoclonal antibody therapy, an evaluation of time to event COVID-19 symptom outcome measures, best practices for analysis of quantitative SARS-CoV-2 RNA outcome measures in randomized trials, and analyses of viral kinetics by immune compromise status and variant. It is hoped that the many lessons learned during the conduct of the ACTIV-2 trial will inform future efforts in pandemic response.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Judith S Currier, Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles, Los Angeles, California, USA.
Carlee Moser, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Joseph J Eron, Department of Medicine, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, North Carolina, USA.
Kara W Chew, Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles, Los Angeles, California, USA.
Davey M Smith, Department of Medicine, University of California, San Diego, La Jolla, California, USA.
Arzhang Cyrus Javan, Division of AIDS, National Institutes of Health, Rockville, Maryland, USA.
David Alain Wohl, Department of Medicine, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, North Carolina, USA.
Eric S Daar, Lundquist Institute at Harbor-UCLA Medical Center, Torrance, California, USA.
Michael D Hughes, Center for Biostatistics in AIDS Research, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
for the ACTIV-2/A5401 Study Team:
Lara Hosey, Jhoanna Roa, Nilam Patel, Frontier Science, Bill Erhardt, Lorraine Waring, Diane Hessinger, and Stacey Adams
Notes
Acknowledgments. We thank the study participants, site staff, site investigators, and the entire ACTIV-2/A5401 study team; the AIDS Clinical Trials Group, including Lara Hosey, Jhoanna Roa, and Nilam Patel; the UW Virology Specialty Laboratory; the ACTG Laboratory Center; Frontier Science; the Harvard Center for Biostatistics in AIDS Research (CBAR) and ACTG Statistical and Data Analysis Center (SDAC); the ACTIV-2 Community Advisory Board; the National Institute of Allergy and Infectious Diseases (NIAID)/Division of AIDS (DAIDS); Bill Erhardt, Lorraine Waring, and Diane Hessinger; the Foundation for the National Institutes of Health and the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership, including Stacey Adams; and the PPD clinical research business of Thermo Fisher Scientific.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers UM1AI068636, UM1AI068634, and UM1AI106701.
Supplement sponsorship. This article appears as part of the supplement “Findings From the ACTIV-2/AIDS Clinical Trials Group A5401 Adaptive Platform Trial of Investigational Therapies for Mild-to-Moderate COVID-19,” sponsored by the National Institutes of Health through a grant to the University of California, Los Angeles.
References
- 1. Collins FS, Stoffels P. Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): an unprecedented partnership for unprecedented times. JAMA 2020; 323:2455–7. [DOI] [PubMed] [Google Scholar]
- 2. LaVange L, Adam SJ, Currier JS, et al. Accelerating COVID-19 therapeutic interventions and vaccines (ACTIV): designing master protocols for evaluation of candidate COVID-19 therapeutics. Ann Intern Med 2021; 174:1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grobler JA, Anderson AS, Fernandes P, et al. Accelerated preclinical paths to support rapid development of COVID-19 therapeutics. Cell Host Microbe 2020; 28:638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choudhary MC, Chew KW, Deo R, et al. Emergence of SARS-CoV-2 escape mutations during Bamlanivimab therapy in a phase II randomized clinical trial. Nat Microbiol 2022; 7:1906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boucau J, Chew KW, Choudhary MC, et al. Monoclonal antibody treatment drives rapid culture conversion in SARS-CoV-2 infection. Cell Rep Med 2022; 3:100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Harrison LJ, Chew KW, et al. Nasal and Plasma Severe Acute Respiratory Syndrome Coronavirus 2 RNA levels are associated with timing of symptom resolution in the ACTIV-2 trial of nonhospitalized adults with coronavirus disease 2019. Clin Infect Dis. 2023; 76:734–37. doi: 10.1093/cid/ciac818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith DM, Li JZ, Moser C, et al. Recurrence of symptoms following a 2-day symptom free period in patients with COVID-19. JAMA Netw Open 2022; 5:e2238867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evering TH, Chew KW, Giganti MJ, et al. ACTIV-2/A5401 Study Team. Safety and efficacy of combination SARS-CoV-2 neutralizing monoclonal antibodies amubarvimab plus romlusevimab in nonhospitalized patients with COVID-19. Ann Intern Med. 2023;176:658–66. doi: 10.7326/M22-3428. Epub 2023 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.