Abstract
Aims
Sudden cardiac death (SCD) is challenging to predict. Electrocardiogram (ECG)-derived heart rate-corrected QT-interval (QTc) is used for SCD-risk assessment. QTc is preferably determined manually, but vendor-provided automatic results from ECG recorders are convenient. Agreement between manual and automatic assessments is unclear for populations with aberrant QTc. We aimed to systematically assess pairwise agreement of automatic and manual QT-intervals and QTc.
Methods and results
A multi-centre cohort enriching aberrant QTc comprised ECGs of healthy controls and long-QT syndrome (LQTS) patients. Manual QT-intervals and QTc were determined by the tangent and threshold methods and compared to automatically generated, vendor-provided values. We assessed agreement globally by intra-class correlation coefficients and pairwise by Bland–Altman analyses and 95% limits of agreement (LoA). Further, manual results were compared to a novel automatic QT-interval algorithm. ECGs of 1263 participants (720 LQTS patients; 543 controls) were available [median age 34 (inter-quartile range 35) years, 55% women]. Comparing cohort means, automatic and manual QT-intervals and QTc were similar. However, pairwise Bland–Altman-based agreement was highly discrepant. For QT-interval, LoAs spanned 95 (tangent) and 92 ms (threshold), respectively. For QTc, the spread was 108 and 105 ms, respectively. LQTS patients exhibited more pronounced differences. For automatic QTc results from 440–540 ms (tangent) and 430–530 ms (threshold), misassessment risk was highest. Novel automatic QT-interval algorithms may narrow this range.
Conclusion
Pairwise vendor-provided automatic and manual QT-interval and QTc results can be highly discrepant. Novel automatic algorithms may improve agreement. Within the above ranges, automatic QT-interval and QTc results require manual confirmation, particularly if T-wave morphology is challenging.
Keywords: QT-interval, QTc, Electrocardiogram, Misassessment, Long QT syndrome, Sudden cardiac death
Graphical Abstract
Graphical abstract.
What’s new?
One of the largest and diverse cohorts enriched for aberrant QT-intervals to assess pairwise agreement between vendor-provided automatic and manual QT-interval and heart-rate corrected QT (QTc).
Automatic and manual QT-intervals and QTc values are highly discrepant.
A wide range of automatic QT-interval and QTc results is prone to misinterpretation.
We recommend a range for reviewing automatic QT-interval and QTc results manually, particularly when the T-wave morphology is challenging.
Introduction
Sudden cardiac death (SCD) results in the loss of life-years in often young and previously healthy individuals,1–3 and most SCD events occur in the general population.4–7 A prolonged ventricular repolarization, reflected by a prolonged QT-interval and heart rate (HR)-corrected QT (QTc) on the electrocardiogram (ECG), predisposes to SCD.8–13 QT-interval and QTc prolongation are observed in the context of structural heart disease, cardiac ischaemia, and congestive heart failure but may also be induced by several drugs both for cardiac and non-cardiac morbidities.14
Since ECGs are non-invasive and easily accessible, the QT-interval and QTc are widely used across medical specialties to guide risk adjudication and decision-making.15–17 The QT-interval is also an important aspect for diagnosing the long-QT syndrome (LQTS), a cardiac arrhythmia disorder associated with malignant arrhythmias and SCD.16–19 Despite the importance of QT-interval and QTc prolongation for SCD risk, no commonly accepted standard exists for their assessment.16,17 QT-interval experts advocate manual and serial measurements by the tangent or threshold methods.20 Although measurements by both experts and non-experts are reliable, many physicians, including cardiologists, appear to misadjudicate a normal vs. abnormal QT-interval.21,22 In fact, for convenience, many healthcare professionals routinely use QT-interval and QTc values that are automatically provided by ECG recorders. The agreement between automatic QT-interval and QTc results and those assessed manually is not sufficiently clear.
Here, we systematically quantified the agreement between vendor-provided automatic and manual QT-interval and QTc assessments. QT-interval and QTc assessments are particularly challenging in case of complex T-wave morphologies as often present in LQTS patients.23 To evaluate a cohort enriched for aberrant QTc values and T-wave morphologies, we therefore investigated a large cohort of both controls and LQTS patients with various degrees of LQTS expressivity and hypothesized that a discrepancy between automatic and manual QT-interval and QTc findings was more pronounced among the latter. Secondarily, we accessed measurement agreement using a recently reported, academia-developed QT algorithm.24
Methods
Study cohort
Patients with confirmed pathogenic mutations in the LQTS Type-1 (KCNQ1), Type-2 (KCNH2), and Type-3 (SCN5A) genes were included as cases and healthy genotype-negative family members as controls. Participants were recruited at two large academic cardiology departments, the Amsterdam UMC, in Amsterdam, the Netherlands, and the University Hospital of the Ludwig-Maximilians-Universität Munich, Germany, as described previously.25
Data collection and management
We used the first available 12 lead resting ECG (index-ECG) of all participants. When the index-ECG was unavailable, a subsequent ECG was used. We excluded ECGs with missing automatic QT-interval and QTc values or in case of ventricular pacing, arrhythmias hindering QT-interval and QTc analysis (e.g. atrial fibrillation), higher degree conduction disorders, complete bundle branch blocks, or poor ECG quality (see Supplementary material online, Table S1). For our secondary analysis involving the novel automatic algorithm for automatic QT-interval and QTc assessment, we additionally excluded individuals lacking digital raw signal ECG (see Supplementary material online, Figure S1). The AMC Review Board approved the study waiving individual informed consent as only retrospective routine care data were used. All ECGs were pseudonymized. Enrolment ended in December 2016.
Manual ECG measurements
Specific methods of how ECGs were manually measured have been reported before.25 The leads preferred for measurement were lead II, followed by lead V5, followed by any lead if lead II or lead V5 was unsuitable. On all ECGs, three pre-selected, consecutive P-QRS-T complexes including their respective preceding RR-intervals were measured independently by three readers.25 Subsequently, measurements were averaged across complexes and readers. Of note, both the inter- and intra-reader validity of our standard manual QT-interval assessment were systematically examined before.25 More specifically, across manual measurement methods, the inter-reader intra-class correlation coefficient (ICC) was >0.96 with a small systematic error of maximally 15 ms. Similarly, the intra-reader validity was 0.99 with a systematic error of 4 ms. Measurements included manual HR (HRManual), manual PR interval (PRManual), manual QRS duration (QRSManual), and the QT-interval determined by both the tangent (QTTangent) and threshold methods (QTThreshold). For the tangent method, the end of the T-wave was defined as the point where the tangent on the steepest point of the terminal limb of the T-wave intersects with the isoelectric baseline. For the threshold method, the end of the T-wave was defined as the intersection of the terminal limb of the T-wave with the isoelectric baseline (see Supplementary material online, Figure S2).25 Specific instructions on how to deal with complicated T-wave morphology including U waves have been described in the previous publication and are additionally visualized (see Supplementary material online, Figure S2).25 Considering the clinically most widespread use and its shown applicability also at higher HRs,26,27 QT-intervals were corrected for HR using Bazett’s formula (QTcTangent; QTcThreshold). Alternative QTc correction formulas (Fridericia; Framingham; Hodges; Rautaharju) were calculated for comprehensiveness.
Automatic ECG measurements
From the vendor-provided automatic output of the ECG recorders, we obtained HR (HRAutomatic), PR-interval (PRAutomatic), QRS duration (QRSAutomatic), QT-interval (QTAutomatic), and QTc (QTcAutomatic). For ECGs recorded with GE Healthcare ECG recorders (General Electric Company, MUSE software, Chicago, IL, USA) applying the company’s proprietary 12SL algorithm;28 QTcAutomatic was determined by Bazett’s formula. The formula remained undisclosed for other manufacturers.
Novel automatic QT-interval algorithm
A novel, automatic QT-interval algorithm was developed and validated previously by our group.29 In short, a QRS-T-complex was defined after excluding measurements from leads that deviated more than 2 standard deviations from the median. On this QRS-T-complex, the QT-interval (QTNovel) was determined using the tangent method, which was HR corrected using Bazett’s formula (QTcNovel).
ECG-based diagnosis of LQTS
Importantly, diagnosing LQTS requires a comprehensive clinical and genetic evaluation. Clinical practice guidelines recommend applying the so called Schwartz score to support a LQTS diagnosis.16,17,30 However, the same clinical practice guidelines suggest to at least consider a significant QTc prolongation indicative of a LQTS diagnosis if, in the absence of QTc-prolonging drugs or other correctable factors, a QTc ≥480 ms is determined on repeated ECGs.16,17 Correct determination of QT-interval and QTc is thus critically important. If QTcAutomatic values deviate markedly from the standard of manual QTc assessment, such a clinically relevant QTc cut-off may be misassessed.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics 27 (IBM, Armonk, NY, USA). Categorical data are presented as frequencies (percentages, %) and compared using χ2 or Fisher’s exact tests as appropriate. Continuous data are presented as mean ± standard deviation (SD) compared by Student’s t-tests or as median and inter-quartile range compared by the Wilcoxon test. Two-sided P-values <0.05 were considered statistically significant.
We assessed agreement between automatic and manual ECG measurements. For global agreement, we calculated the intra-class corelation coefficient (ICC) and 95% confidence intervals (CIs) for multiple measurements based on a two-way consistency model according to Cicchetti and Sparrow.31 For pairwise evaluations, we quantified and visualized Bland–Altman limits of agreement (LoA).32 Analyses are presented for the overall cohort and separately for controls and LQTS patients.
Using manual ECG measurements as the standard, we calculated the absolute difference of QT-interval and QTc values between (i) QT-interval/QTcAutomatic and QT-interval/QTcTangent; (ii) QT-interval/QTcAutomatic and QT-interval/QTcThreshold; and (iii) QT-interval/QTcNovel and QT-interval/QTcTangent. To focus on clinically relevant discrepancies between automatic and manual measurements, we considered the previously described intra- and inter-observer variability of manual measurements25 and determined the percentage of individuals with an absolute pairwise measurement difference ≥ ±15 ms.
For the ECG-based diagnosis of LQTS, based on our Bland–Altman LoAs, we defined and visualized QTcAutomatic ranges at risk for possible misassessment. For both the tangent and threshold methods, from the guideline-suggested cut-off of QTc 480 ms, we deducted the lower LoA and added the upper LoA observed in our overall cohort.
We also examined cut-off-based discrepancies between automatic and manual QTc assessments from 380 to 540 ms. We determined the frequency of QTcAutomatic and QTcNovel ECGs exceeding these cut-offs, whereas the manual QTcTangent and QTcThreshold assessments remained below the cut-off value. Conversely, we determined the frequency of QTcAutomatic and QTcNovel ECGs undercutting the cut-offs, whereas the manual QTcTangent and QTcThreshold assessments remained above the cut-off value.
Results
Cohort characteristics
Of 1577 individuals overall, 1263 were eligible for the comparison between automatic and manual ECG measurements after exclusions (see Supplementary material online, Figure S1). The median age was 34 (interquartile range 35) years, and 55% were females (Table 1). The majority (90.3%) of ECGs was recorded with GE-Healthcare devices applying Bazett’s correction formula. The remaining ECGs were recorded with devices from Welch Allyn (1.3%), AMEDTEC (1.2%), or other manufacturers (7.3%) (see Supplementary material online, Table S2). LQTS patients tended to be younger than controls and had longer mean QT-intervals and QTc both applying the tangent and threshold methods (Table 1).
Table 1.
Cohort characteristics
| Overall cohort | Controls | LQTS | P-value | |
|---|---|---|---|---|
| n = 1263 | n = 543 | n = 720 | ||
| Females, n (%) | 692 (55) | 283 (52) | 409 (57) | 0.10 |
| Age at ECG (years) | 34 (35) | 42 (34) | 27 (34) | <0.001 |
| HRManual (bpm) | 74 ± 20 | 74 ± 19 | 75 ± 22 | 0.19 |
| HRAutomatic(bpm) | 74 ± 20 | 73 ± 19 | 74 ± 22 | 0.32 |
| PRManual (ms) | 146 ± 26 | 148 ± 24 | 144 ± 28 | 0.027 |
| PRAutomatic (ms) | 145 ± 25 | 145 ± 22 | 145 ± 27 | 0.82 |
| QRSManual (ms) | 82 ± 14 | 85 ± 13 | 80 ± 14 | <0.001 |
| QRSAutomatic (ms) | 88 ± 14 | 90 ± 13 | 86 ± 14 | <0.001 |
| QTTangent (ms) | 392 ± 56 | 366 ± 36 | 411 ± 60 | <0.001 |
| QTcTangent (ms) | 427 ± 43 | 399 ± 26 | 448 ± 40 | <0.001 |
| QTThreshold (ms) | 402 ± 56 | 379 ± 38 | 419 ± 60 | <0.001 |
| QTcThreshold (ms) | 438 ± 41 | 412 ± 25 | 458 ± 39 | <0.001 |
| QTAutomatic (ms) | 405 ± 56 | 383 ± 40 | 422 ± 61 | <0.001 |
| QTcAutomatic (ms) | 438 ± 41 | 413 ± 21 | 456 ± 42 | <0.001 |
| Novel automatic analyses | n = 996 | n = 470 | n = 526 | |
| HRNovel (bpm) | 74 ± 20 | 74 ± 19 | 75 ± 22 | 0.51 |
| QTNovel (ms) | 390 ± 51 | 367 ± 33 | 411 ± 55 | <0.001 |
| QTcNovel (ms) | 425 ± 41 | 400 ± 28 | 447 ± 40 | <0.001 |
Formal comparison for controls vs. LQTS patients. Results for QT-interval/QTcNovel analyses restricted to individuals with available digital ECG data.
ECG, electrocardiogram; HR, heart rate; LQTS, long QT syndrome; HRManual, manual HR; HRAutomatic, automatic HR; PRManual, manual PR-interval; PRAutomatic, automatic PR-interval; QRSManual, manual QRS; QRSAutomatic, automatic QRS; QTAutomatic, automatic QT-interval; QTcAutomatic, automatic corrected QT-interval; QTThreshold, threshold QT-interval; QTcThreshold, threshold corrected QT-interval; QTTangent, tangent QT-interval; QTcTangent, tangent corrected QT-interval.
Automatic vs. manual QT-interval and QTc assessments
In our overall cohort, mean QTTangent, QTThreshold, and QTcTangent were shorter than mean QTAutomatic and QTcAutomatic (P < 0.001 for all), respectively. Mean QTcThreshold was similar to QTcAutomatic (P = 0.56) (Table 1). By overall agreement analysis, QT-intervals determined by the tangent and threshold methods showed excellent agreement with QTAutomatic (ICC 0.94, 95% CI 0.87–0.96 and ICC 0.95, 95% CI 0.95–0.96, respectively; Table 2). Overall agreement with QTcAutomatic was weaker, both for QTc values derived by the tangent (ICC 0.86, 95% CI 0.80–0.90) and the threshold methods (ICC 0.88, 95% CI 0.87–0.89; Table 2). Overall agreement analyses for QTc correction formulas other than Bazett’s formula are reported in Supplementary material online, Table S3.
Table 2.
Pairwise ECG measurement agreement analysis
| Overall cohort | Controls | LQTS | ||||
|---|---|---|---|---|---|---|
| Automatic vs. manual | Novel vs. manual | Automatic vs. manual | Novel vs. manual | Automatic vs. manual | Novel vs. manual | |
| n = 1263 | n = 996 | n = 543 | n = 470 | n = 720 | n = 526 | |
| Mean diff. (lLoA to uLoA) | Mean diff. (lLoA to uLoA) | Mean diff. (lLoA to uLoA) | Mean diff. (lLoA to uLoA) | Mean diff. (lLoA to uLoA) | Mean diff. (lLoA to uLoA) | |
| ICC (95% CI) | ICC (95% CI) | ICC (95% CI) | ICC (95% CI) | ICC (95% CI) | ICC (95% CI) | |
| HR (bpm) | 0 (−7 to 7) | 0 (−10 to 9) | 0 (−7 to 7) | 0 (−6 to 5) | −1 (−8 to 7) | 0 (−12 to 12) |
| 0.99 (0.99–0.99) | 0.99 (0.98–0.99) | 0.99 (0.99–0.99) | 0.99 (0.99–1.0) | 0.99 (0.99–0.99) | 0.98 (0.98–0.98) | |
| PR (ms) | −1 (−25 to 23) | NA | −3 (−22 to 16) | NA | 0 (−27 to 27) | NA |
| 0.94 (0.93–0.95) | 0.95 (0.93–0.96) | 0.93 (0.92–0.94) | ||||
| QRS (ms) | 6 (−14 to 25) | NA | 5 (−15 to 25) | NA | 6 (−13 to 25) | NA |
| 0.81 (0.65–0.88) | 0.77 (0.614–0.85) | 0.83 (0.65–0.90) | ||||
| QT-interval (ms) | ||||||
| Tangent | 13 (−34 to 61) | 0 (−31 to 32) | 17 (−14 to 48) | 1 (−22 to 24) | 11 (−45 to 67) | −1 (−38 to 37) |
| 0.94 (0.87–0.96) | 0.98 (0.97–0.98) | 0.91 (0.40–0.97) | 0.97 (0.97–0.98) | 0.93 (0.90–0.95) | 0.97 (0.97–0.98) | |
| Threshold | 3 (−43 to 49) | NA | 4 (−27 to 36) | NA | 2 (−52 to 57) | NA |
| 0.95 (0.95–0.96) | 0.95 (0.94–0.96) | 0.95 (0.94–0.95) | ||||
| QTc (ms) | ||||||
| Tangent (ms) | 11 (−43 to 65) | 0 (−36 to 36) | 15 (−24 to 53) | 2 (−29 to 32) | 8 (−55 to 70) | −1 (−41 to 39) |
| 0.86 (0.80–0.90) | 0.95 (0.94–0.95) | 0.72 (0.34–0.85) | 0.91 (0.90 0.93) | 0.82 (0.78–0.85) | 0.92 (0.91–0.94) | |
| Threshold (ms) | 0 (−53 to 52) | NA | 1 (−37 to 39) | NA | −2 (−62 to 59) | NA |
| 0.88 (0.87–0.89) | 0.79 (0.75–0.82) | 0.83 (0.80–0.85) | ||||
In each table cell, upper line indicates mean difference and lower and upper limits of agreement based on Bland–Altman analyses [mean diff. (lLoA-uLoA)]; lower line indicates ICC and 95% CI.
HR, heart rate; LQTS, long QT syndrome; LoA, limits of agreement; HR, heart rate; ICC, inter-class correlation coefficient; CI, confidence interval; NA, not available.
Pairwise agreement analysis
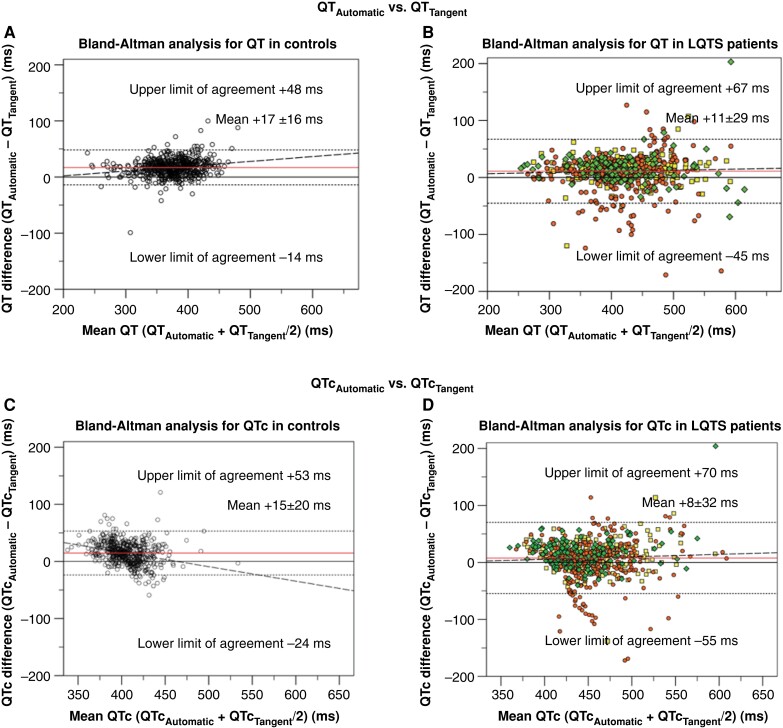
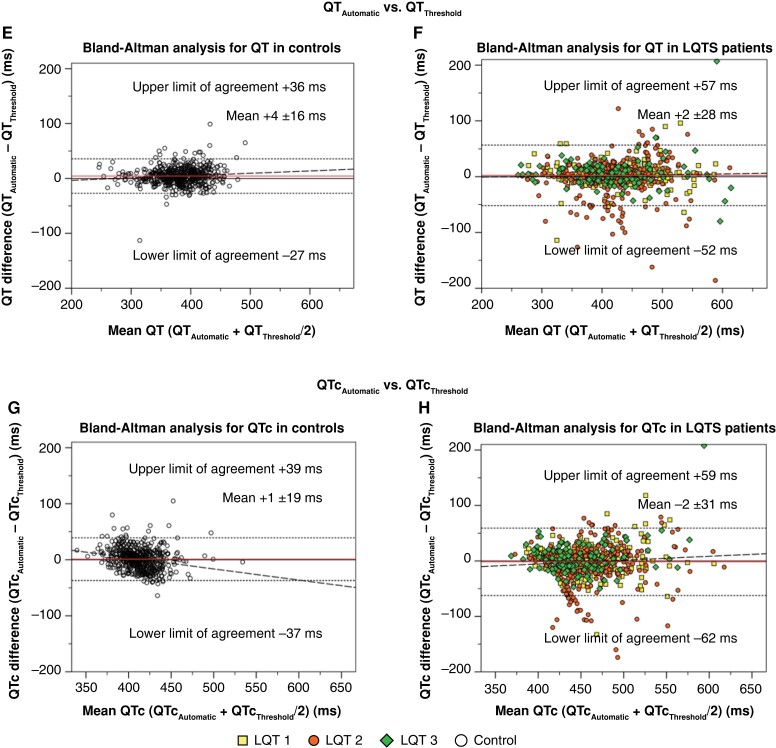
Pairwise agreement by Bland–Altman analyses revealed discrepancies between vendor-provided automatic and manual measurements. For QTAutomatic compared to QTTangent, the LoAs ranged from −34 to 61 ms (spread 95 ms). For QTAutomatic compared to QTThreshold, the spread was marginally narrower (92 ms; −43 to 49 ms). For all comparisons, LQTS patients exhibited wider 95% LoAs than controls (Figure 1; Table 2). For QTc in the overall cohort, QTcAutomatic and QTcTangent LoAs ranged from −43 to 65 ms (spread 108 ms), whereas QTcAutomatic and QTcThreshold LoAs rangend from −53 to 52 ms (spread 105 ms). LQTS patients showed a larger variation in QTc than controls (Figure 1; Table 2). The frequencies of individuals with absolute pairwise measurement differences ≤15 ms are presented in Supplementary material online, Table S4. Pairwise agreement with different QTc correction formulas is presented in Supplementary material online, Table S3.
Figure 1.
Bland–Altman analysis for pairwise automatic vs. manual QT-interval and heart-rate corrected QT (QTc) agreement. All panels display Bland–Altman analyses. Horizontal (red) lines indicate the mean difference between assessment methods. Horizontal dotted lines indicate the upper and lower limits of agreement. Dashed lines indicate the regression line of pairwise differences. (A) Pairwise agreement between automatic QT-interval (QTAutomatic) and tangent QT-interval (QTTangent) among controls. (B) Pairwise agreement between QTAutomatic and QTTangent among LQTS patients. (C) Pairwise agreement between automatic corrected QT-interval (QTcAutomatic) and tangent corrected QT-interval (QTcTangent) among controls. (D) Pairwise agreement between QTcAutomatic and QTcTangent among long QT syndrome (LQTS) patients. (E) Pairwise agreement between QTAutomatic and threshold method QT-interval (QTThreshold) among controls. (F) Pairwise agreement between QTAutomatic and QTThreshold among LQTS patients. (G) Pairwise agreement between QTcAutomatic and threshold method corrected QT-interval (QTcThreshold) among controls. (H) Pairwise agreement between QTcAutomatic and QTcThreshold among LQTS patients.
Cut-off–based discrepancy analysis
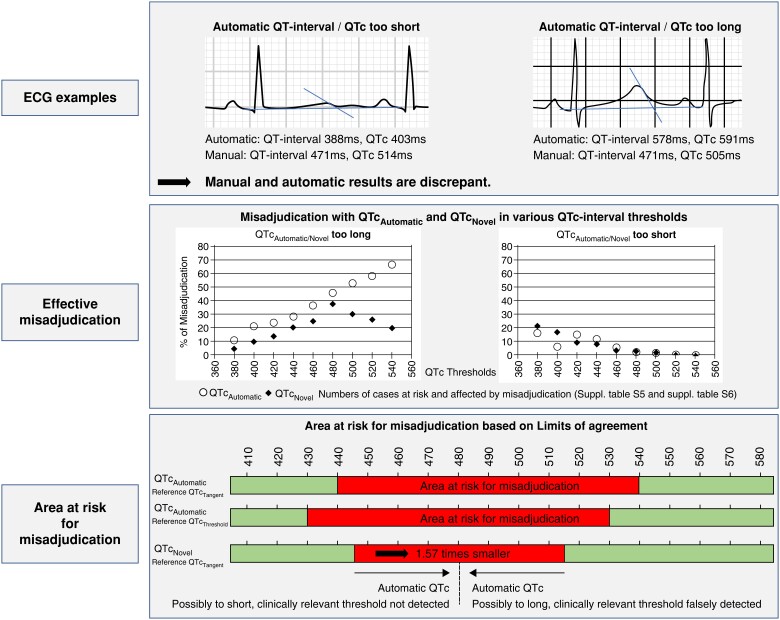
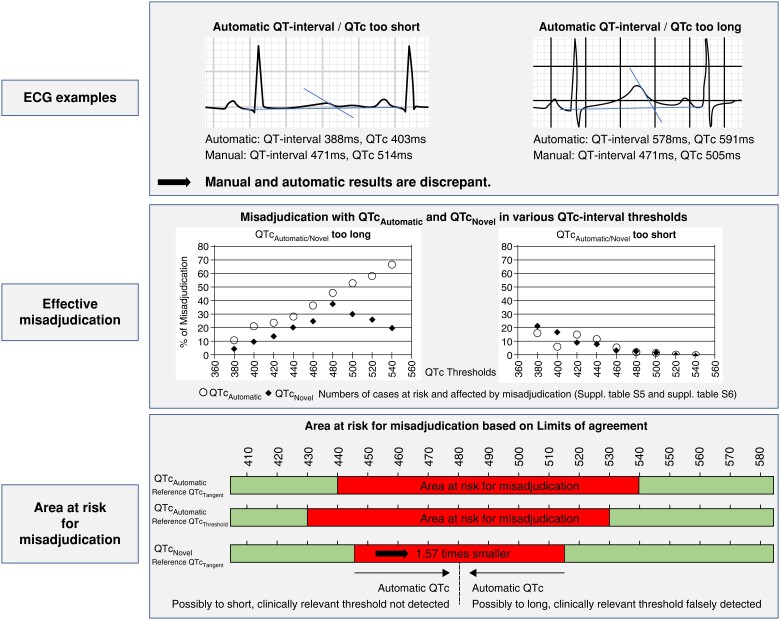
By cut-off–based discrepancy analysis, we identified ECGs where QTcAutomatic either exceeded or undercut the respective cut-off compared to the manual results. For 480 ms as the clinically most relevant QTc cut-off, 45.8% (82/179) of QTcAutomatic results exceeded QTcTangent findings. Of these, 90.2% (74/82) had a pairwise difference of ≥ ±15 ms. QTcAutomatic results less frequently [2.8% (30/1084)] undercut the 480 ms cut-off value, but 90.0% (27/30) exceeded ±15 ms (see Supplementary material online, Table S5; Figure 2).
Figure 2.
Upper section depicts electrocardiogram (ECG) examples where automatic QT-analysis results in too short (left) or too long (right) measurements compared to manual assessment. Middle section visualizes percentage of automatic corrected QT-interval (QTcAutomatic) and QTcNovel results that are too long (left) or too short (right) compared to manual assessment. Percentage of misassessed ECGs is provided for select corrected QT (QTc) cut-offs from 380 to 540 ms. Lower section illustrates ranges (red) at risk for clinically relevant mis-assessment of QTcAutomatic relative to tangent corrected QT-interval (QTcTangent) (upper bar), QTcAutomatic relative to threshold corrected QT-interval (QTcThreshold) (middle bar), or QTcNovel (lower bar). Findings within red zones should be reviewed manually.
Agreement analysis based on the automatic measurements from the novel algorithm
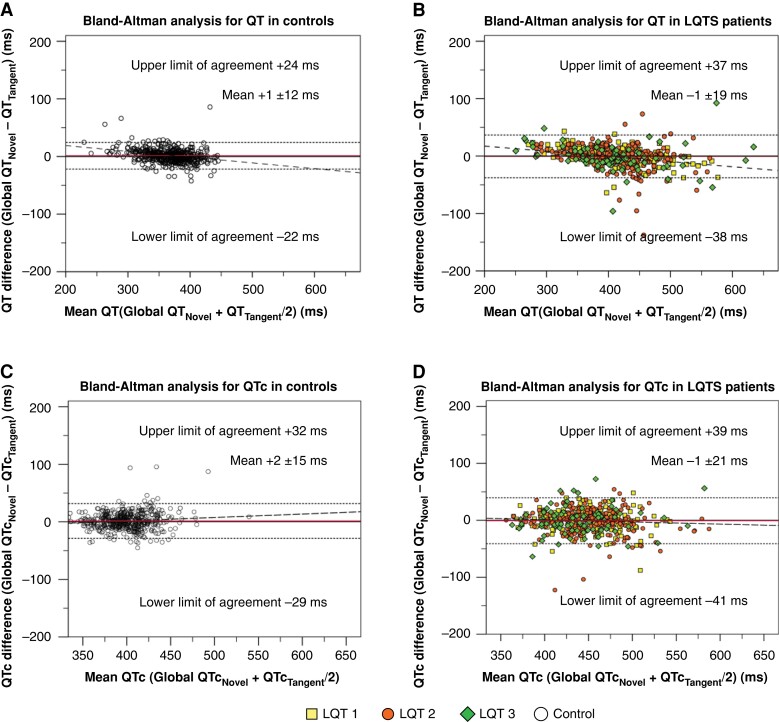
As a secondary analysis, we compared the automatic analysis using a novel algorithm with the manual QT-interval and QTc assessments. Mean QT-interval and QTcNovel values were similar (P = 0.54 and P = 0.59, respectively). Also, overall agreement was very high. Bland–Altman pairwise agreement LoAs ranged from −31 to 32 ms (spread 63 ms) for QT-intervals and from −36 to 36 ms (spread 72 ms) for QTc (Figure 3; Table 2). LoAs were narrower for controls than for LQTS patients. Absolute pairwise measurement differences for QT-interval/QTcNovel are presented in Supplementary material online, Table S4. Cut-off–based discrepancy results for QTcNovel are shown in Supplementary material online, Table S6; Figure 2.
Figure 3.
Bland–Altman analysis for pairwise automatic vs. manual QT-interval and corrected QT (QTc) agreement using a novel automatic algorithm. All panels display Bland–Altman analyses. Horizontal (red) lines indicate the mean difference between assessment methods. Horizontal dotted lines indicate the upper and lower limits of agreement. Dashed lines indicate the regression line of pairwise differences. (A) Pairwise agreement between QTNovel and tangent QT-interval (QTTangent) among controls. (B) Pairwise agreement between QTNovel and QTTangent among long QT syndrome (LQTS) patients. (C) Pairwise agreement between QTcNovel and tangent corrected QT-interval (QTcTangent) among controls. (D) Pairwise agreement between QTcNovel and QTcTangent among LQTS patients.
Area at risk for mis-adjudication
To visualize ranges prone to automatic vs. manual QTc misassessment in clinical application, we present Figure 2. For QTcTangent, from the practice guideline recommended 480 ms QTc cut-off, we deducted the lower (480 ms−43 ms = 437 ms) and added the upper LoA (480 ms + 65 ms = 545 ms) in the overall cohort, resulting in a clinically intuitive QTcAutomatic range from approximately 440-540 ms at risk for mis-assessment. Within this range, 4.8% of QTcAutomatic ECGs were ≤480 ms, whereas the pairwise QTcTangent was >480 ms. Conversely, 53.3% of QTcAutomatic results exceeded 480 ms, whereas the pairwise QTcTangent result was ≤480 ms. QTcThreshold resulted in a similar, clinically intuitive range of 430–530 ms. Using QTcNovel, the range prone to mis-assessment was 1.57 times narrower (445–515 ms) (Figure 2; see Supplementary material online, Table S7).
Automatic vs. manual HR, PR, and QRS measurements
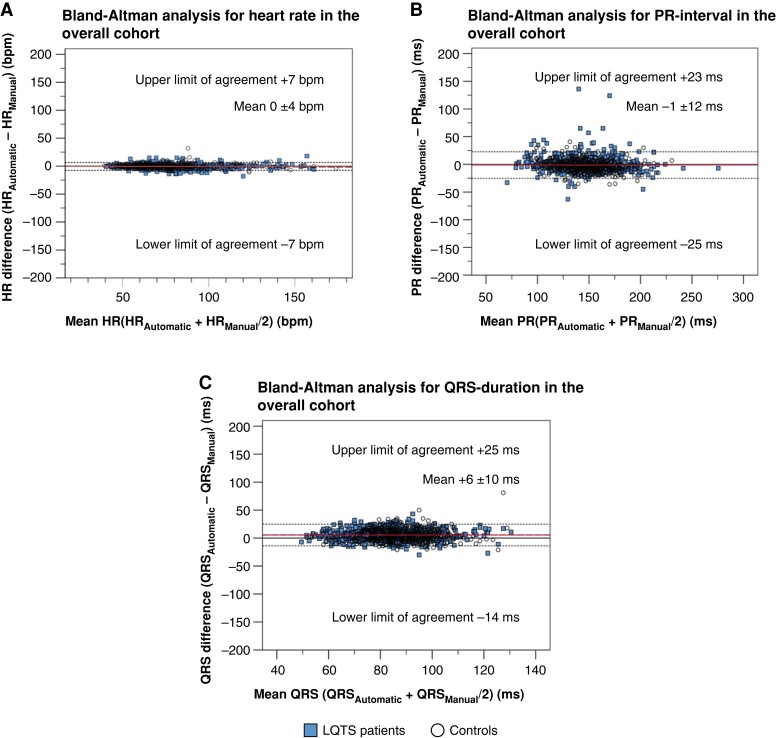
We also analysed HR, PR, and QRS. The mean HR, PR-interval, and QRS-duration were similar between automatic and manual measurements (Table 1), and the overall agreement analysis revealed a good to excellent agreement (ICC >0.80; Table 2). Bland–Altman analyses indicated only a minimal pairwise variation for HR (LoAs from −8 to 7 ms) and small pairwise variations for PR-interval (LoAs from −25 to 23 ms) and for QRS-duration (LoAs from −14 to 25 ms) (Figure 4A–C).
Figure 4.
Bland–Altman analysis for pairwise automatic vs. manual heart rate, PR-interval, and QRS-duration agreement. All panels display Bland–Altman analyses. Horizontal (red) lines indicate the mean difference between assessment methods. Horizontal dotted lines indicate the upper and lower limits of agreement. Dashed lines indicate the regression line of pairwise differences. (A) Pairwise agreement for heart rate (HR) between automatic (HRAutomatic) and manual (HRManual). (B) Pairwise agreement for PR-interval between automatic (PRAutomatic) and manual (PRManual). (C) Pairwise agreement for QRS duration between automatic (QRSAutomatic) and manual (QRSManual).
Discussion
QT-interval and QTc are established markers of ventricular arrhythmia risk in clinical practice, but accurate determination is critical. Besides the standard of manual QT-interval assessment, for convenience, physicians frequently use automatic, vendor-provided measurements. In our cohort, enriched for aberrant QT-interval and QTc values, we systematically investigated the agreement of automatic and manual QT-interval and QTc assessments. We demonstrate that their pairwise agreement is highly discrepant, especially in the presence of aberrant QTc values and T-wave morphologies. The extent of discrepancy limits using automatic QT-interval/QTc values in clinical practice. Hence, we clearly suggest manual review of automatic QTc results, but most importantly for automatic QTc results between 440 and 540 ms. Optimized automatic algorithms to determine QT-interval/QTc may improve pairwise agreement. Also, manufacturers may facilitate recapitulating their automatic QT-interval and QTc measurements by visualizing their measurement landmarks.
Automatic vs. manual measurements
The mean difference between automatic and manual measurement results considers the average of the entire cohort rather than the pairwise difference of individuals. Considering such cohort means, agreement between vendor-provided automatic and manual QT-interval assessment appears good. Yet importantly, by pairwise Bland–Altman analyses, we found a great variability between QT-interval and QTc assessments. The range covering 95% of the pairwise QT-interval measurements spans a wide 95 ms for QTTangent and 92 ms for QTThreshold. For QTcTangent (108 ms) and for QTcThreshold (105 ms), this spread is even wider. All pairwise comparisons thus exceed a degree of variation that is clinically acceptable.
Pairwise agreement between automatic and manual QT-interval assessments has been investigated before. A modestly sized study by Savelieva et al.33 analysed 54 patients with hypertrophic cardiomyopathy and 70 controls. ECGs were interpreted by a single reader averaging ECG complexes in an individually chosen lead. A relevant pairwise disagreement was identified with a 95% LoA spread of 70 ms in controls and of 55 ms in in cardiomyopathy patients. The smaller LoA spread in hypertrophic cardiomyopathy patients might be due to the less complex T-wave morphologies in these patients. In contrast, Hnatkova et al.34 examined a large cohort of healthy individuals. Here, the 95% LoA spread was 10–30 ms, and 94–99% of ECGs exhibited absolute measurement differences ≤15 ms. These findings appear contradictory to our results. However, the authors studied ECGs recorded under highly standardized experimental conditions to compare versions of the same analysis algorithm.34 Instead, we interpreted ECGs obtained in clinical care and thus submit that our study setting better reflects ECG analysis in everyday practice. Also, a ‘Thorough QT study’ reported pairwise Bland–Altman results,35 yet in few healthy individuals without any overt ECG pathologies and limited to an automatic QTc of ≤450 ms. Even in this very homogenous cohort, the LoAs were wide (±32.3 ms). Also, the variability across different vendor provided QTc algorithms has been provided before. In a report by Kligfield et al.,36 the pairwise differences of means of the QT interval were markedly smaller than in our study comparing automatic vs. manual ECG analyses.36 Yet, also in the analysis by Kligfield et al.,36 LQTS patients exhibited larger QT variability compared to normal individuals.
Clinical relevance
Regarding the clinical relevance of our findings, several aspects are important. First, we compared manual QT-interval and QTc results to vendor-provided automatic ones. Such automatic results are frequently used in clinical practice. We counter these easily accessible automatic results with two standardized manual methods, the tangent and threshold methods.20 We further apply the most commonly used Bazett QTc formula in our main analyses. For comprehensiveness, we also report our findings for other QT-interval correction formulas including the one by Fridericia. Importantly, we describe highly discrepant pairwise comparison findings for both QT-interval assessment methods. We thus believe that our discrepant findings are not solely explained by nuances of differing methodology.
Second, interpreting automatic QT-interval and QTc results without manual review may lead to incorrect and possibly consequential conclusions, whenever the length of QT-interval and QTc is of importance. An example is the QT-interval and QTc in the context of drug therapy given the knowledge that numerous drugs interfere with cardiac repolarization.14 Such drugs include antiarrhythmics like amiodarone, antibiotics like moxifloxacin, antidepressants like citalopram, and antipsychotics like quetiapine.14 We cannot comment on the performance of automatic QT-interval and QTc assessments on serial ECG recordings. Yet, a single incorrect QT-interval and QTc could lead to erroneous decisions in drug prescription. Similarly, an incorrect QT-interval and QTc may incorrectly raise the suspicion of LQTS.37 Diagnosing LQTS requires a comprehensive assessment.38 Nevertheless, clinical practice guidelines suggest to at least consider a LQTS diagnosis when QTc is repeatedly ≥480 ms.16,17 Incorrect QTc assessment may hence result in misinterpreting such a clinically relevant cut-off. Considering the wide LoA range of discrepant QT-interval and QTc findings, we submit that both over- and underestimating QT-interval and QTc are serious challenges in clinical practice. We visualize this issue in Figure 2 and suggest a range where automatic QT-interval and QTc results warrant manual review.
Third, our cohort combines LQTS patients and controls. The variability between automatic and manual QT-interval and QTc assessments was larger among the former. An explanation may be challenging T-wave morphologies, including biphasic, notched, and low-amplitude T-waves, which are commonly present in LQTS patients.39–43 Importantly, T-wave morphology is not typically assessed by automated algorithms, while T-wave morphology is an important denominator of repolarization pathology.30 We thus strongly encourage a manual review of automatic QT-interval and QTc assessments in known or suspected LQTS patients or when a challenging T-wave morphology is present.
We encourage manual review of automatic QT-interval and QTc assessments. However, the advantages of confidently using automatic assessments in clinical routine are evident. Yet, this disposal of physicians’ responsibility of manual assessment for the gain of convenience bears a risk of accepting incorrect automatic results. Aiming to minimize such negative effects, we have hence tested a recently developed, novel ECG analysis algorithm.29 Applying this algorithm to our cohort, the discrepancy between automatic and manual QT-interval and QTc assessments was markedly smaller, narrowing the range recommended for manual review 1.57-fold compared with vendor-provided automatic measurements (Figure 2). Pending independent validation, we envision future improvements in automatic QT-interval measurements including the application of artificial intelligence will further narrow and eventually close the range for manual QTc reassessment. In the meantime, clinicians may additionally be supported by visualizations where commercial ECG recorders defined their measurements. We thus encourage manufacturers to routinely display such measurement landmarks.
Limitations
Several aspects require consideration. Most ECGs were recorded using the GE 12SL algorithm limiting transferability to other manufacturers. Importantly, QT-interval measurement methods and QTc correction formulas were not documented for all automatic ECGs. To reduce bias, we have assessed several QT-interval correction formulas, while focusing on the clinically most relevant Bazett formula. Our cohort composition of LQTS patients and their genotype-negative relatives may not fully reflect the general population. However, most ECGs in clinical practice are not recorded in healthy general population individuals but recording is triggered by clinical need. Such need may arise from symptoms or from known pathologies. In both instances, pathologic ECG findings are prevalent. Hence, we intentionally chose our study composition to include a sufficient spectrum of abnormal QT-intervals. Moreover, with this selection we were able—as opposed to other studies with general population or healthy control ECGs—to establish cases vs. controls. Importantly, a yet unpublished comparison of our control ECGs with the population-based Maastricht Study indicated very good comparability. We thus consider our cohort composition a clear strength as it is enriched for challenging T-wave morphologies encountered in clinical practice but not routinely encountered in the general population. Despite our large cohort size and the systematic assessment by three trained readers, independent validation may further increase their relevance.
Conclusion
In conclusion, pairwise agreement of vendor-provided automatic vs. manual assessments of QT-interval and of QTc is highly discrepant, independent of the QT-interval measurement method. Interestingly, we observed no large discrepancies for HR, PR-interval, and QRS-duration, indicating that QT-interval and QTc are more vulnerable to mis-assessment. Our data support that novel, transparent, automatic QT-interval algorithms may reduce discrepancies. Hence, manual review of vendor-provided automatic QT-interval and QTc assessments is critical and clearly recommended. As this may not always be feasible, a manual review should at least be considered for QTc between 440 and 540 ms, especially when T-wave morphologies are challenging.
Supplementary Material
Contributor Information
Benjamin Neumann, Department of Medicine I, LMU University Hospital, LMU Munich, Munich, Germany; German Centre for Cardiovascular Research (DZHK), partner site: Munich Heart Alliance, Munich, Germany.
A Suzanne Vink, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Heart Center, Amsterdam, The Netherlands; Department of Pediatric Cardiology, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Ben J M Hermans, Department of Biomedical Engineering, Maastricht University, Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands.
Krystien V V Lieve, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Heart Center, Amsterdam, The Netherlands.
Didem Cömert, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Heart Center, Amsterdam, The Netherlands.
Britt-Maria Beckmann, Department of Medicine I, LMU University Hospital, LMU Munich, Munich, Germany; Department of Legal Medicine, Goethe Univeristy, University Hospital Frankfurt, Frankfurt am Main, Germany.
Sally-Ann B Clur, Department of Pediatric Cardiology, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Nico A Blom, Department of Pediatric Cardiology, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Department of Pediatric Cardiology, Leiden University Medical Center, Leiden, The Netherlands.
Tammo Delhaas, Department of Biomedical Engineering, Maastricht University, Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Maastricht, The Netherlands.
Arthur A M Wilde, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Heart Center, Amsterdam, The Netherlands; Department of Pediatric Cardiology, Leiden University Medical Center, Leiden, The Netherlands; Princess Al-Jawhara Al-Brahim Center of Excellence in Research of Hereditary Disorders, Jeddah, Kingdom of Saudi Arabia; European Reference Network for Rare, Low Prevalence and Complex Diseases of the Heart (ERN GUARD-Heart), Amsterdam University Medical Center, Amsterdam, The Netherlands.
Stefan Kääb, Department of Medicine I, LMU University Hospital, LMU Munich, Munich, Germany; German Centre for Cardiovascular Research (DZHK), partner site: Munich Heart Alliance, Munich, Germany.
Pieter G Postema, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Heart Center, Amsterdam, The Netherlands.
Moritz F Sinner, Department of Medicine I, LMU University Hospital, LMU Munich, Munich, Germany; German Centre for Cardiovascular Research (DZHK), partner site: Munich Heart Alliance, Munich, Germany.
Authors’ contribution
The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication were the sole responsibility of the authors.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was funded by the German Centre for Cardiovascular Research (DZHK) by a grant to S.K. (81Z0600206) and by the Dutch Heart Foundation by a grant to P.G.P. (2021T061).
Data availability
Data supporting the findings of this study are available upon reasonable request. Access to the data is subject to approval from the corresponding author and compliance with privacy regulations. Interested researchers can contact the corresponding author at msinner@med.lmu.de to request access to the de-identified dataset and supplementary materials.
References
- 1. Bagnall RD, Weintraub RG, Ingles J, Duflou J, Yeates L, Lam Let al. A prospective study of sudden cardiac death among children and young adults. N Engl J Med 2016;374:2441–52. [DOI] [PubMed] [Google Scholar]
- 2. Eckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo-Rojas LMet al. Sudden death in young adults: an autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol 2011;58:1254–61. [DOI] [PubMed] [Google Scholar]
- 3. Priori SG, Aliot E, Blomstrom-Lundqvist C, Bossaert L, Breithardt G, Brugada Pet al. Task force on sudden cardiac death of the European society of cardiology. Eur Heart J 2001;22:1374–450. [DOI] [PubMed] [Google Scholar]
- 4. Myerburg RJ, Goldberger JJ. Sudden cardiac arrest risk assessment: population science and the individual risk mandate. JAMA Cardiol 2017;2:689–94. [DOI] [PubMed] [Google Scholar]
- 5. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm 2018;15:e190–252. [DOI] [PubMed] [Google Scholar]
- 6. Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM Jret al. Risk stratification for sudden cardiac death: a plan for the future. Circulation 2014;129:516–26. [DOI] [PubMed] [Google Scholar]
- 7. Junttila MJ, Castellanos A, Huikuri HV, Myerburg RJ. Risk markers of sudden cardiac death in standard 12-lead electrocardiograms. Ann Med 2012;44:717–32. [DOI] [PubMed] [Google Scholar]
- 8. Straus SM, Bleumink GS, Dieleman JP, van der Lei J, t Jong GW, Kingma JHet al. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med 2004;164:1293–7. [DOI] [PubMed] [Google Scholar]
- 9. Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa Jet al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 2006;47:362–7. [DOI] [PubMed] [Google Scholar]
- 10. Niemeijer MN, van den Berg ME, Deckers JW, Franco OH, Hofman A, Kors JAet al. Consistency of heart rate-QTc prolongation consistency and sudden cardiac death: the Rotterdam study. Heart Rhythm 2015;12:2078–85. [DOI] [PubMed] [Google Scholar]
- 11. Schwartz PJ, Wolf S. QT Interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation 1978;57:1074–7. [DOI] [PubMed] [Google Scholar]
- 12. D'Ascenzi F, Anselmi F, Graziano F, Berti B, Franchini A, Bacci Eet al. Normal and abnormal QT interval duration and its changes in preadolescents and adolescents practicing sport. Europace 2019;21:1566–74. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki H, Horie M, Ozawa J, Sumitomo N, Ohno S, Hoshino Ket al. Novel electrocardiographic criteria for short QT syndrome in children and adolescents. Europace 2021;23:2029–38. [DOI] [PubMed] [Google Scholar]
- 14.CredibleMeds [(last accessed 31 July 2023).]. https://www.crediblemeds.org/ . QTDrugs Lists 2015.
- 15. Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo Met al. Risk stratification in the long-QT syndrome. N Engl J Med 2003;348:1866–74. [DOI] [PubMed] [Google Scholar]
- 16. Priori SG, Blomstrom-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm Jet al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC)Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
- 17. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul Cet al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–63. [DOI] [PubMed] [Google Scholar]
- 18. Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol 2008;51:2291–300. [DOI] [PubMed] [Google Scholar]
- 19. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol 2018;72:e91–e220. [DOI] [PubMed] [Google Scholar]
- 20. Goldenberg I, Moss AJ, Zareba W. QT Interval: how to measure it and what is “normal”. J Cardiovasc Electrophysiol 2006;17:333–6. [DOI] [PubMed] [Google Scholar]
- 21. Viskin S, Rosovski U, Sands AJ, Chen E, Kistler PM, Kalman JMet al. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm 2005;2:569–74. [DOI] [PubMed] [Google Scholar]
- 22. Postema PG, De Jong JS, Van der Bilt IA, Wilde AA. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm 2008;5:1015–8. [DOI] [PubMed] [Google Scholar]
- 23. McLaughlin NB, Campbell R, Murray A. Comparison of automatic QT measurement techniques in the normal 12 lead electrocardiogram. Heart 1995;74:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hermans BJ, Vink AS, Bennis FC, Filippini LH, Meijborg VM, Wilde AAet al. The development and validation of an easy to use automatic QT-interval algorithm. PLoS One 2017;12:e0184352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arja S V, Neumann B, Lieve Krystien VV, Sinner Moritz F, Hofman N, el Kadi Set al. Determination and interpretation of the QT interval. Circulation 2018;138:2345–58. [DOI] [PubMed] [Google Scholar]
- 26. Stramba-Badiale M, Karnad DR, Goulene KM, Panicker GK, Dagradi F, Spazzolini Cet al. For neonatal ECG screening there is no reason to relinquish old Bazett's correction. Eur Heart J 2018;39:2888–95. [DOI] [PubMed] [Google Scholar]
- 27. Pærregaard MM, Hvidemose SO, Pihl C, Sillesen AS, Parvin SB, Pietersen Aet al. Defining the normal QT interval in newborns: the natural history and reference values for the first 4 weeks of life. Europace 2021;23:278–86. [DOI] [PubMed] [Google Scholar]
- 28. Company GE. Marquette 12SL ECG Analysis Program, Revision B. https://www.gehealthcare.co.uk/-/jssmedia/global/uk/diagnostic-cardiology/2018/06/18/marquette-12sl-algorithm.pdf (last accessed 31 July 2023).
- 29. Hermans BJM, Stoks J, Bennis FC, Vink AS, Garde A, Wilde AAM, et al. Support vector machine-based assessment of the T-wave morphology improves long QT syndrome diagnosis. Europace. 2018;20:iii113–ii9. [DOI] [PubMed] [Google Scholar]
- 30. Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J 2013;34:3109–16. [DOI] [PubMed] [Google Scholar]
- 31. Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 1981;86:127–37. [PubMed] [Google Scholar]
- 32. Martin Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;327:307–10. Originally published as volume 1, issue 8476. [PubMed] [Google Scholar]
- 33. Savelieva I, Yi G, Guo X-h, Hnatkova K, Malik M. Agreement and reproducibility of automatic versus manual measurement of QT interval and QT dispersion. Am J Cardiol 1998;81:471–7. [DOI] [PubMed] [Google Scholar]
- 34. Hnatkova K, Gang Y, Batchvarov VN, Malik M. Precision of QT interval measurement by advanced electrocardiographic equipment. Pacing Clin Electrophysiol 2006;29:1277–84. [DOI] [PubMed] [Google Scholar]
- 35. Darpo B, Agin M, Kazierad DJ, Layton G, Muirhead G, Gray Pet al. Man versus machine: is there an optimal method for QT measurements in thorough QT studies? J Clin Pharmacol 2006;46:598–612. [DOI] [PubMed] [Google Scholar]
- 36. Kligfield P, Badilini F, Denjoy I, Babaeizadeh S, Clark E, De Bie Jet al. Comparison of automated interval measurements by widely used algorithms in digital electrocardiographs. Am Heart J 2018;200:1–10. [DOI] [PubMed] [Google Scholar]
- 37. Dagradi F, Spazzolini C, Castelletti S, Pedrazzini M, Kotta MC, Crotti Let al. Exercise training-induced repolarization abnormalities masquerading as congenital long QT syndrome. Circulation 2020;142:2405–15. [DOI] [PubMed] [Google Scholar]
- 38. Wilde AA, Semsarian C, Márquez MF, Shamloo AS, Ackerman MJ, Ashley EAet al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Europace 2022;24:1307–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiang CE, Roden DM. The long QT syndromes: genetic basis and clinical implications. J Am Coll Cardiol 2000;36:1–12. [DOI] [PubMed] [Google Scholar]
- 40. Lupoglazoff J, Denjoy I, Berthet M, Neyroud N, Demay L, Richard Pet al. Notched T waves on Holter recordings enhance detection of patients with LQT2 (HERG) mutations. Circulation 2001;103:1095–101. [DOI] [PubMed] [Google Scholar]
- 41. Moss AJ, Zareba W, Benhorin J, Locati EH, Hall WJ, Robinson JLet al. ECG T-wave patterns in genetically distinct forms of the hereditary long QT syndrome. Circulation 1995;92:2929–34. [DOI] [PubMed] [Google Scholar]
- 42. Lehmann MH, Suzuki F, Fromm BS, Frankovich D, Elko P, Steinman RTet al. T wave “humps” as a potential electrocardiographic marker of the long QT syndrome. J Am Coll Cardiol 1994;24:746–54. [DOI] [PubMed] [Google Scholar]
- 43. Murray A, McLaughlin NB, Bourke JP, Doig JC, Furniss SS, Campbell RW. Errors in manual measurement of QT intervals. Br Heart J 1994;71:386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available upon reasonable request. Access to the data is subject to approval from the corresponding author and compliance with privacy regulations. Interested researchers can contact the corresponding author at msinner@med.lmu.de to request access to the de-identified dataset and supplementary materials.