Abstract
Aims
Premature ventricular beats (PVBs) in athletes are often benign, but sometimes they may be a sign of an underlying disease. We evaluated the prevalence, burden, and morphology of PVBs in healthy voluntary athletes and controls with the main purpose of defining if certain PVB patterns are ‘common’ and ‘training related’ and, as such, are more likely benign.
Methods and results
We studied 433 healthy competitive athletes [median age 27 (18–43) years, 74% males] and 261 age- and sex-matched sedentary subjects who volunteered to undergo 12-lead 24 h ambulatory electrocardiogram (ECG) monitoring (24H ECG), with a training session in athletes. Ventricular arrhythmias (VAs) were evaluated in terms of their number, complexity [i.e. couplet, triplet, or non-sustained ventricular tachycardia (NSVT)], exercise inducibility, and morphology. Eighty-six percent of athletes and controls exhibited a total of ≤10 PVBs/24 h, and >90% did not show any couplets, triplets, or runs of NSVT > 3 beats. An higher number of PVBs correlated with increasing age (P < 0.01) but not with sex and level of training. The most frequent morphologies among the 36 athletes with >50 PVBs were the infundibular (44%) and fascicular (22%) ones. In a comparison between athletes and sedentary individuals, and male and female athletes, no statistically significant differences were found in PVBs morphologies.
Conclusion
The prevalence and complexity of VAs at 24H ECG did not differ between athletes and sedentary controls and were not related to the type and amount of sport or sex. Age was the only variable associated with an increased PVB burden. Thus, no PVB pattern in the athlete can be considered ‘common’ or ‘training related’.
Keywords: Pre-participation screening, Premature ventricular beats, Sports cardiology, Ventricular arrhythmias, Sudden cardiac death
Graphical Abstract
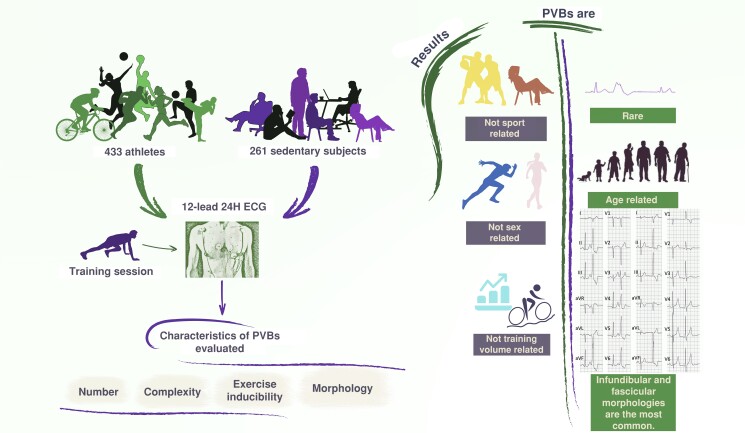
Graphical Abstract.
The study enrolled 433 athletes and 261 sedentary subjects who underwent 12-lead 24H ECG. Premature ventricular beats were found to be rare in athletes and sedentary subjects, with infundibular and fascicular morphologies being the most common; their incidence and morphologies were not related to the type and amount of sport, nor to sex, and tended to increase with age. 12-lead 24H ECG, 12-lead 24 h electrocardiogram monitoring; PVBs, premature ventricular beats.
What’s new?
Premature ventricular beats (PVBs) are rare in athletes and sedentary healthy subjects who are supposed to be healthy.
The PVBs burden and morphologies are not related to the type and amount of sport, nor to sex, and tend to increase with age.
There are no PVBs patterns that can be considered ‘common’ or ‘training related’, although certain characteristics (frequent/complex PVBs with morphologies other than infundibular or fascicular) may be associated with a higher disease probability and thus deserve to be investigated more in depth.
Introduction
Premature ventricular beats (PVBs) are not an infrequent finding in the general population of athletes during pre-participation screening (PPS).1–3 Although they are often benign, sometimes they may represent a sign of an underlying heart disease at risk of sudden cardiac death (SCD).4 The clinical significance and characteristics to be considered for risk stratification of PVBs have been much debated in the literature, and many aspects are still not fully clarified.
An initial hypothesis was that a certain number of PVBs could be considered an expression of the athlete’s heart due to exercise-induced heart remodelling; only when the number of PVBs was particularly high did the probability of an underlying disease increased.5 More recent studies suggested that the prevalence of ventricular arrhythmias (VAs) in young competitive athletes is low, independent of the type and intensity of sports activity, and similar to sedentary subjects.6 Importantly, it has also been demonstrated that some PVB patterns are associated with an increased probability of an underlying disease at risk of SCD.7–9
According to this new evidence, a modern approach to the interpretation and risk stratification of PVBs in the athlete has been proposed, taking into consideration parameters other than just the number, such as morphology, complexity, and relation to exercise.10,11 Similar to the classification of resting electrocardiogram (ECG) changes, PVB characteristics were divided into two groups: ‘common’ and ‘uncommon’,12 but the definition was based on the risk rather than on prevalence and relation to training on healthy athletes. In other words, although some PVBs characteristics are associated with a lower probability of an underlying disease, it remains to be demonstrated whether they can be considered common/training-related features of the athlete’s heart.
To overcome this limitation, this study was designed to compare the prevalence, burden, morphology, and relation to training of PVBs in a court of healthy athletes and a control group of healthy sedentary subjects who volunteered to undergo 12-lead 24 h ambulatory ECG monitoring (24H ECG).
Methods
The study protocol was approved by the local Ethics Committee, and all subjects (or parents/guardians if subjects were <18 years old) gave written informed consent. Participants were not paid. Parts of these data were reported in previous publications.6,13
Athletes
According to Italian law, all athletes engaged in competitive sports activity must undergo pre-participation cardiovascular evaluation including family and personal history, physical examination, resting 12-lead ECG, and limited exercise testing focused on the evaluation of VAs. In addition, athletes >35 years old underwent incremental maximal exercise testing to rule out ischaemic heart disease. Further examinations were provided for athletes showing abnormalities at first-line investigations. Athletes who were considered eligible for competitive sports activity according to PPS and who fulfilled the inclusion criteria were offered participation in the study until the pre-specified sample size was reached.
Inclusion criteria were the following: (i) age > 15 years old; (ii) engagement in all competitive sports, with the exception of skill disciplines according to the 2020 ESC Guidelines on Sports Cardiology and Exercise;14 (iii) ≥6 h of exercise per week for at least 1 year; and (iv) no structural cardiovascular disease, excluding mild valvular disease, mild aortic dilation, and patent foramen ovale. In addition to PPS, athletes with >10 isolated PVB or ≥1 repetitive VA [couplets or non-sustained ventricular tachycardia (NSVT)] on the 24H ECG also underwent echocardiography to rule out an underlying heart disease. Those with echocardiographic abnormalities or those with normal echocardiography but with frequent (>500/day) or distinctively exercise-induced VA (excluding ‘fascicular’ or ‘infundibular’ PVBs that carry a low disease probability) were referred for contrast-enhanced cardiac magnetic resonance (CMR). The following information was collected for each participant: type of sport, classified as endurance, power, or mixed according to the 2020 ESC Guidelines on Sports Cardiology and Exercise14 (in case of multiple disciplines, the one accounting for the majority of training hours was considered); hours of training per week; cumulative years of practice; and family history of premature SCD (<50 years old in females and <40 years old in males), coronary artery disease, or cardiomyopathy in first-degree relatives.
Sedentary individuals
During the same period, a group of sedentary subjects was recruited as controls by public advertisements on local press, social networks, and notice boards in the hospital. The sedentary controls had a negative history of heart disease (including arrhythmias) or hypertension, were engaged in ≤2 h per week of leisure-time physical exercise, and were matched to athletes for sex and age class (16–20 years old, 21–30 years old, 31–40 years old, and >40 years old) with a ≈3:2 ratio. Except for sports activity, inclusion criteria were the same as for athletes. Similar to athletes, an underlying structural heart disease was excluded with echocardiography and CMR in selected cases.
Ambulatory electrocardiogram monitoring
A 12-lead 24H ECG (H12, Mortara Instruments Inc.) was performed on all study participants. Athletes were asked to perform a training session of at least 30–60 min during the ambulatory ECG recording. Sedentary subjects were instructed to perform their habitual daily life activities. Recordings were reviewed by two cardiologists (A.Z., G.M.): in particular, every single ectopic beat, pause, or artefact and all families of normal beats were confirmed manually. Recordings with >2 h of artefacts or missing signals were considered inadequate and repeated.
The PVB morphology was classified as left bundle branch block (LBBB)–like if the ectopic QRS complex was predominantly negative in lead V1 and as right bundle branch block (RBBB)–like if the ectopic QRS complex was predominantly positive or isodiphasic in lead V1. Premature ventricular beats with a LBBB/inferior axis (positive QRS complex in aVF and negative QRS complex in V1 and aVL) configuration were considered of ventricular outflow tract origin; PVBs with a QRS duration of ≤130 ms resembling a typical RBBB/left or right axis deviation were considered of fascicular origin.10,11,15 Premature ventricular beats with ≥2 morphologies that accounted for ≥10% of all PVBs were classified as polymorphic.
Statistical analysis
Continuous and categorical variables were expressed as median (with 25th–75th percentiles) and number (%), respectively. Categorical variables were compared using the χ2 or Fisher exact test, as appropriate. Continuous data were compared using the Mann–Whitney U test because normality could not be assumed for any variable. A P-value of <0.05 was considered statistically significant. Univariate analysis was conducted to identify potential significant factors influencing the number of PVBs, while multivariate analysis was performed to assess the most important factors. Data were analysed with SPSS, version 29 (IBM). Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Padova (Padova, Italy).
Results
Comparison between athletes and sedentary controls
During the study period, 1604 competitive athletes who fulfilled the enrolment criteria underwent PPS. Of those athletes, 125 did not meet the enrolment criteria because they were engaged in sports with low cardiovascular demand (n = 90), had cognitive/physical disabilities (n = 19), or were considered not eligible for competitive sports activity because of a cardiovascular disease (n = 16). Of the remaining 1479 athletes, 436 agreed to participate in the study, but three were excluded for demonstration of a non-ischaemic left ventricular (LV) scar on CMR, which was performed for exercise-induced PVBs on the 24H ECG.6 The final population was composed of 433 athletes (74% males, median age 27 years). The athletic group practised a variety of sports including cycling (n = 75, 18%), soccer (n = 74, 17%), running (n = 64, 15%), volleyball (n = 43, 10%), triathlon (n = 33, 8%), rugby (n = 18, 5%), basketball (n = 16, 4%), martial arts (n = 11, 2%), swimming (n = 7, 1%), tennis (n = 11, 2%), rowing (n = 8, 1%), and others (n = 73, 17%). The family history was positive for SCD in 18 athletes (4%), for myocardial infarction in 101 athletes (23%), and for cardiomyopathy in 5 athletes (1%).
The control group initially included 262 subjects, but one was excluded because frequent and polymorphic PVBs on 24H ECG requested echocardiography that showed mitral valve prolapse with moderate regurgitation. Hence, the final study cohort included 261 sedentary controls. Detailed information about the two groups is shown in Table 1. Athletes presented significantly lower mean and minimum heart rates (HRs) and a significantly higher maximal HR if compared with controls at 24H ECG.
Table 1.
Comparison between athletes and sedentary controls
| Variable | Athletes | Sedentary controls | |
|---|---|---|---|
| n = 433 | n = 261 | ||
| Male | 322 (74%) | 184 (70%) | P = 0.2 |
| Age | 27 (18–43) | 31 (22–46) | P = 0.06 |
| Positive family historya | 124 (28%) | 65 (25%) | P = 0.2 |
| Training time, hours/week | 8 (6–10) | 1 (1–2) | P < 0.01* |
| Sports practice, years | 9 (5–15) | 8 (2–19) | P < 0.01* |
| Mean HR, bpm | 71 (65–75) | 74 (68–81) | P < 0.01* |
| Minimum HR, bpm | 40 (36–43) | 45 (41–49) | P < 0.01* |
| Maximum HR, bpm | 169 (152–180) | 139 (125–153) | P < 0.01* |
Data are given as number (percentage) or median (25th–75th percentiles).
bpm, beats per minute; HR, heart rate.
For premature (<40 years old in men and <50 years old in women) sudden death, inherited cardiomyopathies, or coronary artery disease.
P < 0.05.
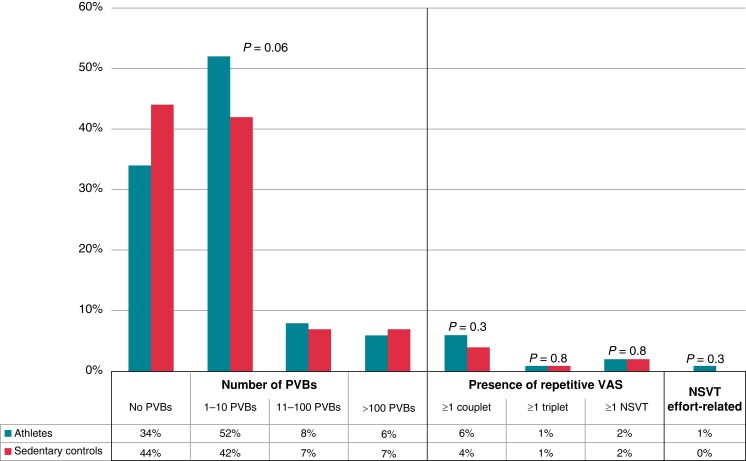
The prevalence and complexity of PVBs recorded by ambulatory ECG monitoring did not differ between athletes and sedentary subjects. In detail, 86% of athletes and 86% of controls exhibited a total of ≤10 PVBs/24 h and 91% of athletes and 93% of controls did not show any couplets, triplets, or runs of NSVT > 3 beats (Figure 1).
Figure 1.
Grading of VAs in 24 h ambulatory ECG monitoring in athletes and age- and sex-matched sedentary controls. NSVT, non-sustained ventricular tachycardia; PVBs, premature ventricular beats; VAs, ventricular arrhythmias.
Determinants of premature ventricular beats in athletes
Sex
Male athletes (n = 322) were significantly older than female athletes (n = 111). However, the two groups did not significantly differ in positive family history, type of sport, training time per week, training time per year, and years of sports practice. The 24H ECG showed significant differences in the mean and minimum HRs, which were lower in male athletes, but not in the maximum HR. Table 2 shows detailed information about the differences between male and female athletes.
Table 2.
Comparison between male and female athletes
| Variable | Male athletes | Female athletes | |
|---|---|---|---|
| n = 322 | n = 111 | ||
| Age | 29 (19–43) | 23 (17–35) | P < 0.008* |
| Positive family historya | 91 (28%) | 33 (30%) | P = 0.9 |
| Training time, hours/week | 8 (6–9) | 7 (6–11) | P = 0.5 |
| Training time, hours/year | 350 (312–450.75) | 350 (308–504) | P = 0.4 |
| Sports practice, years | 10 (5–17) | 7 (5–12) | P = 0.07 |
| Mean HR, bpm | 70 (65–74) | 73 (69–77) | P < 0.001* |
| Minimum HR, bpm | 40 (36–42.25) | 42 (39–46) | P < 0.001* |
| Maximum HR, bpm | 169 (152–179) | 171 (154–184) | P = 0.1 |
Data are given as number (percentage) or median (25th–75th percentiles).
bpm, beats per minute; HR, heart rate.
For premature (<40 years old in men and <50 years old in women) sudden death, inherited cardiomyopathies, or coronary artery disease.
P < 0.05.
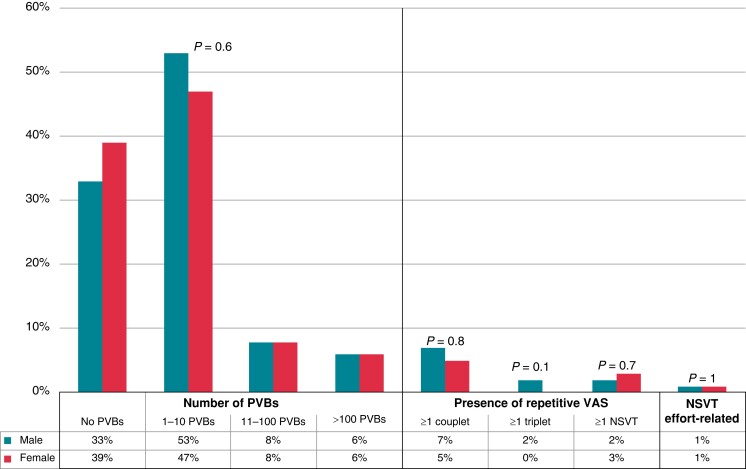
As shown in Figure 2, no differences in the number and complexity of PVBs were detected between the two groups. Particularly, the percentage of male and female athletes with no PVBs, isolated PVBs, couplets, triplets, and NSVT was comparable.
Figure 2.
Grading of VAs on 24 h ambulatory ECG monitoring in male and female athletes. NSVT, non-sustained ventricular tachycardia; PVBs, premature ventricular beats; VAs, ventricular arrhythmias.
Level of training
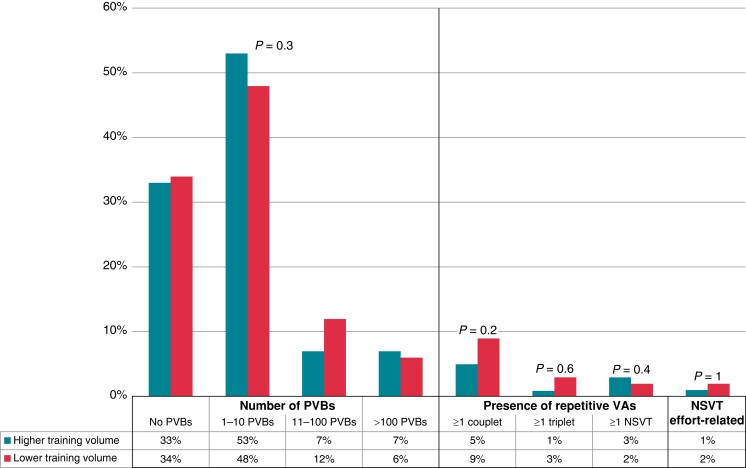
The athletes who practised endurance or mixed sports were divided into two groups according to the hours of training per week: higher training volume [n = 274, median age 25 (25th–75th percentiles, 17–42 years), 75% males], with a training time of ≥7 h/week, and lower training volume [n = 127, median age 33 (25th–75th percentiles, 22–43 years), 74% males], with a training time of <7 h/week. The two groups showed no differences in age (P = 0.1), sex (P = 0.8), positive family history (P = 0.9), and mean (P = 0.7) and maximum (P = 0.9) HRs recorded at the 24H ECG. The minimum HR was significantly lower in the higher training volume group [40 (25th–75th percentiles, 36–43 bpm)] if compared with the lower training volume one [42 (25th–75th percentiles, 36–45 bpm); P = 0.04]. Also, in this case, no differences between these groups were demonstrated in the number and complexity of PVBs recorded at the 24H ECG. A detailed representation is shown in Figure 3.
Figure 3.
Grading of VAs in 24 h ambulatory ECG monitoring in higher training volume and lower training volume groups. NSVT, non-sustained ventricular tachycardia; PVBs, premature ventricular beats; VAs, ventricular arrhythmias.
Age
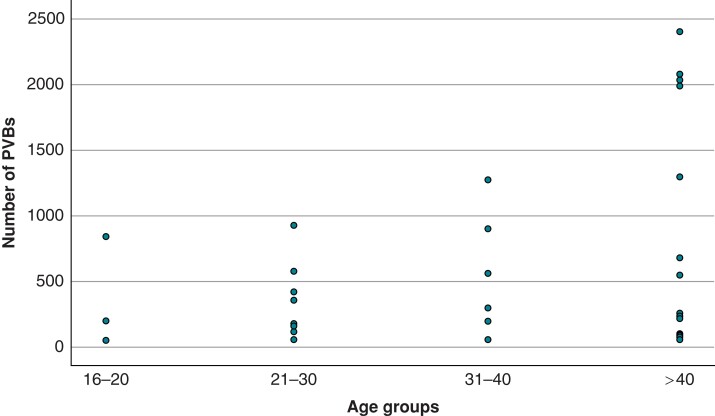
An analysis of variance was performed to study the significance of age, sex, type of sport, hours of training per week, hours of training per year, and years of training on the number of PVBs. In the univariate analysis, the only variable significantly associated with the number of PVBs was age. The correlation between the number of PVBs and age was confirmed also after correction with the other variables (F = 10.530, P < 0.01). Figure 4 shows the distribution of the number of PVBs according to the age group.
Figure 4.
Number of PVBs in athletes divided into four age groups (16–20 years old, 21–30 years old, 31–40 years old, and >40 years old). PVBs, premature ventricular beats.
Morphology of premature ventricular beats
Athletes
We compared the distribution of PVB morphologies on the 24H ECG between the group of athletes with 1–50 PVBs (n = 250) and the group with >50 PVBs (n = 36). The two groups significantly differed for age, with older age in the group with the higher number of PVBs: the median age of athletes with lower PVBs number was 30 years (25th–75th percentiles, 20–43 years) while that of athletes with higher PVB number was 37 years (25th–75th percentiles, 28–46.75 years); P = 0.01. The two groups did not differ in sex (P = 0.5), positive family history (P = 0.1), training time (P = 0.3), years of sports practice (P = 0.1), and mean (P = 0.2), minimum (P = 0.9), and maximum (P = 0.6) HRs recorded at the 24H ECG. Moreover, the two groups did not differ in the type of sport (P = 0.3). As reported in Table 3, the only statistically significant difference between the two groups was about the number of athletes showing monomorphic infundibular PVBs, which was higher in the group of athletes with the greatest number of arrhythmias (29% vs. 44%, P = 0.04).
Table 3.
Comparison of the prevalent morphology of ventricular arrhythmias between athletes with 1–50 PVBs and athletes with >50 PVBs
| Morphology | Athletes with 1–50 PVBs | Athletes with >50 PVBs | |
|---|---|---|---|
| n = 250 | n = 36 | ||
| Infundibular | 70 (29%) | 16 (44%) | P = 0.04* |
| Fascicular (RBBB/QRS ≤ 130 ms) | 69 (28%) | 8 (22%) | P = 0.6 |
| LBBB/superior axis | 23 (8%) | 1 (3%) | P = 0.1 |
| RBBB/QRS > 130 | 50 (20%) | 5 (14%) | P = 0.2 |
| Polymorphic | 38 (15%) | 6 (17%) | P = 0.8 |
Data are given as number (percentage).
LBBB, left bundle branch block; PVBs, premature ventricular beats; RBBB, right bundle branch block.
P < 0.05.
Athletes vs. sedentary subjects
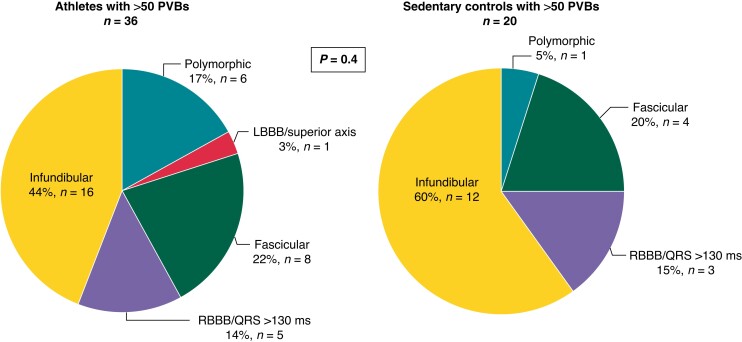
A comparison between athletes with >50 isolated PVBs and sedentary controls [n = 20, median age 41 (25th–75th percentiles, 34.25–49.5 years), 75% males] with >50 isolated PVBs was done. The two groups did not differ in age and sex. No statistically significant differences were found in the distribution of PVB morphologies between the two groups (P = 0.4) (Figure 5). In athletes, we did not notice an increased distribution of PVBs after the training session, which was recorded during 24H ECG in the athletes’ diary and identified with increasing HRs. Infundibular and fascicular resulted in being the most frequent morphologies in both groups.
Figure 5.
Prevalent morphologies of ventricular arrhythmias among 36 athletes and 20 sedentary controls with >50 isolated PVBs. LBBB, left bundle branch block; PVBs, premature ventricular beats; RBBB, right bundle branch block.
Female athletes vs. male athletes
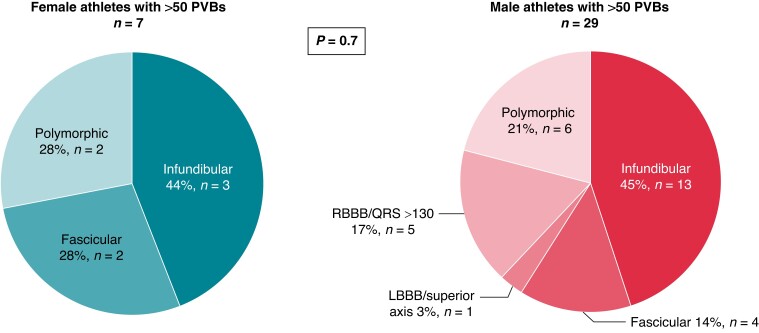
Finally, a comparison between female athletes [n = 7, median age 45 (25th–75th percentiles, 33–50 years)] with >50 isolated PVBs and male athletes [n = 36, median age 33 (25th–75th percentiles, 26–46 years)] with >50 isolated PVBs was done. The two groups did not differ in age (P = 0.3), positive family history (P = 0.1), type of sport (P = 0.2), training time per week (P = 0.1) and per year (P = 0.4), and years of sports practice (P = 0.6). No significant differences were found in the distribution of PVB morphologies between the two groups, with predominance of the infundibular morphology in both (Figure 6).
Figure 6.
Prevalent morphologies of ventricular arrhythmias among female and male athletes with >50 isolated PVBs. LBBB, left bundle branch block; PVBs, premature ventricular beats; RBBB, right bundle branch block.
Discussion
In this study, we compared the prevalence, burden, and morphology of PVBs recorded with 24H ECG between a group of healthy athletes and a control group of healthy sedentary subjects. The presence of an underlying heart disease was ruled out with a rigorous protocol that included CMR for those with frequent or exercise-induced PVBs. The main results are as follows: (i) the mean number of PVBs was low in healthy athletes and did not differ from sedentary subjects; (ii) sex, type of sport, and training level did not affect the burden of PVBs in the athletic population, which instead turned out to be influenced by age; (iii) among athletes with more frequent PVBs, (>50/24 h), infundibular and fascicular morphologies were the most represented, accounting for two-thirds of cases; and (iv) male and female athletes presented similar PVB patterns and morphologies.
Prevalence and determinants of ventricular arrhythmias
In the literature, there are conflicting outcomes about the role of exercise in determining VAs.16 Palatini et al.17 compared 24H ECG monitoring results of 40 endurance athletes and 40 healthy sedentary subjects and showed a higher prevalence of ventricular ectopy in athletes. Biffi et al.5,18 studied 355 athletes who underwent 24H ECG monitoring for palpitations and/or PVBs recorded on the basal ECG. In most of the athletes with <2000 PVBs/day and no NSVT, an underlying structural heart disease could not be demonstrated, VAs decreased with detraining, and the follow-up was event-free. Hence, the theory is that a certain number of PVBs could be considered an expression of the ‘athlete’s heart’. On the other hand, other studies demonstrated that the burden of VAs in athletes is low and not different from that of sedentary subjects.19–22 Also, our group demonstrated that the prevalence of VAs at 24H ECG did not differ between middle-aged athletes23 and young competitive athletes6 when compared with age-matched sedentary controls and was unrelated to the amount and duration of exercise. Moreover, Delise et al.24 proved the inability of detraining to reduce arrhythmias in athletes, concluding that VAs cannot be considered an expression of the athlete’s heart.
In the present study, about 90% of athletes had <10 PVB/24 h and >90% did not show any couplets, triplets, or runs of NSVT > 3 beats, with the same finding in athletes and sedentary subjects, so the conclusion is that PVBs are rare in healthy athletes such as in sedentary individuals. Moreover, we stratified athletes for the type of sport and training time per week (≥7 h/week vs. <7 h/week): there were no significant differences in the number and complexity of VAs between the subgroups. Therefore, our study supports the idea that there are no links between sports activity and an increased VA burden. We can conclude that PVBs in athletes are neither common nor training related.
Instead, we found a significant correlation between the number of PVBs and age. Indeed, the group with a higher number of PVBs (PVBs > 50/24 h) resulted in being significantly older when compared with the group with 1–50 PVBs in 24 h. Furthermore, age acts as an independent risk factor for the number of PVBs in an analysis of variance including age, sex, type of sport, hours of training per week, hours of training per year, and years of training. These results are in line with previous studies demonstrating that the arrhythmic burden increases with age in the general population.1,2 It may be hypothesized that the gradual development of cardiac fibrosis and the progressive dysregulation of the myocyte calcium homeostasis are the mechanisms involved in the increased electrical instability with ageing.25,26
Morphology of premature ventricular beats
In our population, the majority of athletes with PVBs > 50/24 h showed LBBB/inferior axis (infundibular) or fascicular morphologies, which are considered benign and are usually due to automatic foci in a structurally normal heart.27 This finding is in keeping with previous studies: Verdile et al.28 demonstrated at exercise testing that in athletes with a structurally normal heart, the most frequent morphologies were the infundibular and fascicular ones. Parisi et al.29 showed that these morphologies were characterized by a very low risk of events during follow-up in athletes who carried on with training. Also, Di Florio et al.12 found that PVBs originating from the outflow tract or fascicles of the left bundle were relatively common in young competitive athletes referred for cardiology evaluation, and the probability of having a structural heart disease was 9% in those athletes with these morphologies compared with 38% of athletes with other morphologies (P < 0.001). It was demonstrated that even if the number of these PVBs is high, the prognosis is good.30,31
Of note, only 24 of 322 (7%) healthy athletes showed relatively frequent infundibular or fascicular PVBs on a 24H ECG, and we did not find any differences in the distribution of PVB morphologies according to the type of sport and training volume. For this reason, although we confirm that infundibular and fascicular PVBs are the relatively most common morphologies observed in healthy athletes, they cannot be considered ‘common’ in absolute or ‘training related’.
Clinical implications
Traditionally, the burden of PVBs is considered to correlate with disease probability; for this reason, current international recommendations for the interpretation of the athlete’s ECG suggest to consider as normal the presence of one PVB in a 10 s resting ECG.32 In our study, we disproved this perspective by showing that in healthy athletes, PVBs are neither common nor training related and cannot be considered a feature of the athlete’s heart.
It is now well established that the number of PVBs has no prognostic value, but other properties of PVBs help risk stratification.11,33,34 Actually, recent evidence suggests that morphology, complexity, and relation to exercise are more accurate features than the number in differentiating between probable benign and potentially malignant PVBs.10 In particular, infundibular and fascicular PVBs have low disease probability, especially in case of a negative personal/family history and normal baseline ECG; conversely, other morphologies may be more frequently associated with pathological substrates at risk of SCD.10,35 In our study population, we confirmed that infundibular and fascicular PVBs were the relatively more common PVB patterns, while only 3% (12/433) showed >50 PVBs with morphologies other than infundibular or fascicular. Given the low number, further diagnostic investigation seems to be justified in these athletes. Of our athletes potentially eligible for the study, three were excluded because of an isolated non-ischaemic scar on CMR, which was performed due to the recording of PVBs with RBBB/wide QRS or polymorphic VAs on the study 24H ECG. This finding supports the importance of evaluating the morphology (rather than the number) of PVBs in athletes for an appropriate clinical work-up.
Study limitations
The main limitation of this study is that we enrolled a cohort of volunteers rather than a consecutive series of athletes who underwent PPS. Even though the baseline characteristics of those who did and did not agree to participate were similar, we cannot exclude a selection bias. Also, sedentary individuals may not be representative of the general sedentary population because they were recruited on a voluntary basis. A systematic imaging study of all the athletes with normal PPS and no VAs on 24H ECG was not performed. Moreover, follow-up data on the entire study cohort are lacking, and therefore, we cannot rule out the possibility that they may have developed a disease later in their life. Furthermore, the study mostly included Italian White athletes (Caucasian), and so the results may not be generalized to athletes of other ethnicities. Because of logistical reasons, recruitment of a larger group of athletes than sedentary individuals may have influenced the matching efficiency.
Conclusions
In conclusion, in this study, we found that PVBs are rare in athletes and sedentary subjects who are supposed to be healthy; their burden and morphologies are not related to the type and amount of sport, nor to sex, and tend to increase with age. For these reasons, there are no PVB patterns that can be considered ‘common’ or ‘training related’, although certain characteristics may be associated with a higher disease probability and thus deserve to be investigated more in depth. In particular, frequent/complex PVBs with morphologies other than infundibular or fascicular are rare in healthy athletes, and a careful clinical work-up is indicated before concluding on their benign nature. Our results need to be confirmed by further studies, especially including top/elite athletes who often exercise more than amateur/non-elite athletes who made up our study population.
Contributor Information
Francesca Graziano, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Via Giustiniani 2, 35128, Padova, Italy; Department of Sports Medicine, Semmelweis University, Budapest, Hungary.
Giulio Mastella, Klinikum Rechts der Isar–Innere Medizin I, Technische Universität München, Munchen, Germany.
Bela Merkely, Department of Sports Medicine, Semmelweis University, Budapest, Hungary; Heart and Vascular Center, Semmelweis University, Budapest, Hungary.
Hajnalka Vago, Department of Sports Medicine, Semmelweis University, Budapest, Hungary; Heart and Vascular Center, Semmelweis University, Budapest, Hungary.
Domenico Corrado, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Via Giustiniani 2, 35128, Padova, Italy.
Alessandro Zorzi, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Via Giustiniani 2, 35128, Padova, Italy.
Authors’ contributions
F.G. and A.Z. contributed to the design of the work and the analysis and interpretation of data and drafted the manuscript. G.M. and A.Z. contributed to acquisition and analysis. B.M., H.V., and D.C. contributed to the interpretation of data and critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
The study was funded by the research grant budget for integrated departmental research 2016 (University of Padova, Padova, Italy) and by the Target Projects RF-2013-02356762 grant of the Ministero della Salute, Italy.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
- 1. Hingorani P, Karnad DR, Rohekar P, Kerkar V, Lokhandwala YY, Kothari S. Arrhythmias seen in baseline 24-hour Holter ECG recordings in healthy normal volunteers during phase 1 clinical trials. J Clin Pharmacol 2016;56:885–93. [DOI] [PubMed] [Google Scholar]
- 2. von Rotz M, Aeschbacher S, Bossard M, Schoen T, Blum S, Schneider Set al. Risk factors for premature ventricular contractions in young and healthy adults. Heart 2017;103:702–7. [DOI] [PubMed] [Google Scholar]
- 3. Steriotis AK, Nava A, Rigato I, Mazzotti E, Daliento L, Thiene Get al. Noninvasive cardiac screening in young athletes with ventricular arrhythmias. Am J Cardiol 2013;111:557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bohm P, Meyer T, Narayanan K, Schindler M, Weizman O, Beganton Fet al. Sports-related sudden cardiac arrest in young adults. Europace 2023;25:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biffi A, Maron BJ, Verdile L, Fernando F, Spataro A, Marcello Get al. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol 2004;44:1053–8. [DOI] [PubMed] [Google Scholar]
- 6. Zorzi A, De Lazzari M, Mastella G, Niero A, Trovato D, Cipriani Aet al. Ventricular arrhythmias in young competitive athletes: prevalence, determinants, and underlying substrate. J Am Heart Assoc 2018;7:e009171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calò L, Panattoni G, Tatangelo M, Brunetti G, Graziano F, Monzo Let al. Electrocardiographic characteristics of right-bundle-branch-block premature ventricular complexes predicting absence of left ventricular scar in athletes with apparently structural normal heart. Europace 2023;25:euad217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heidbuchel H, Arbelo E, D’Ascenzi F, Borjesson M, Boveda S, Castelletti Set al. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part 2: ventricular arrhythmias, channelopathies, and implantable defibrillators. Europace 2021;23:147–8. [DOI] [PubMed] [Google Scholar]
- 9. Lee J, Adeola O, Garan H, Stevenson WG, Yarmohammadi H. Electrocardiographic recognition of benign and malignant right ventricular arrhythmias. Europace 2021;23:1338–49. [DOI] [PubMed] [Google Scholar]
- 10. Corrado D, Drezner JA, D’Ascenzi F, Zorzi A. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. Br J Sports Med 2020;54:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brunetti G, Cipriani A, Perazzolo Marra M, De Lazzari M, Bauce B, Calore Cet al. Role of cardiac magnetic resonance imaging in the evaluation of athletes with premature ventricular beats. J Clin Med 2022;11:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Florio A, Fusi C, Anselmi F, Cavigli L, Focardi M, Cameli Met al. Clinical management of young competitive athletes with premature ventricular beats: a prospective cohort study. Int J Cardiol 2021;330:59–64. [DOI] [PubMed] [Google Scholar]
- 13. Cipriani A, Vio R, Mastella G, Ciarmatori N, Del Monte A, Trovato Det al. Burden of premature atrial beats in middle-aged endurance athletes with and without lone atrial fibrillation versus sedentary controls. Eur J Prev Cardiol 2020;27:1555–63. [DOI] [PubMed] [Google Scholar]
- 14. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli Set al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
- 15. Heidbüchel H, Corrado D, Biffi A, Hoffmann E, Panhuyzen-Goedkoop N, Hoogsteen Jet al. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part II: ventricular arrhythmias, channelopathies and implantable defibrillators. Eur J Cardiovasc Prev Rehabil 2006;13:676–86. [DOI] [PubMed] [Google Scholar]
- 16. Graziano F, Juhasz V, Brunetti G, Cipriani A, Szabo L, Merkely Bet al. May strenuous endurance sports activity damage the cardiovascular system of healthy athletes? A narrative review. J Cardiovasc Dev Dis 2022;9:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palatini P, Maraglino G, Sperti G, Calzavara A, Libardoni M, Pessina ACet al. Prevalence and possible mechanisms of ventricular arrhythmias in athletes. Am Heart J 1985;110:560–7. [DOI] [PubMed] [Google Scholar]
- 18. Biffi A, Pelliccia A, Verdile L, Fernando F, Spataro A, Caselli Set al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol 2002;40:446–52. [DOI] [PubMed] [Google Scholar]
- 19. Viitasalo MT, Kala R, Eisalo A. Ambulatory electrocardiographic recording in endurance athletes. Br Heart J 1982;47:213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viitasalo MT, Kala R, Eisalo A. Ambulatory electrocardiographic findings in young athletes between 14 and 16 years of age. Eur Heart J 1984;5:2–6. [DOI] [PubMed] [Google Scholar]
- 21. Talan DA, Bauernfeind RA, Ashley WW, Kanakis C, Rosen KM. Twenty-four hour continuous ECG recordings in long-distance runners. Chest 1982;82:19–24. [DOI] [PubMed] [Google Scholar]
- 22. Bjørnstad H, Storstein L, Meen HD, Hals O. Ambulatory electrocardiographic findings in top athletes, athletic students and control subjects. Cardiology 1994;84:42–50. [DOI] [PubMed] [Google Scholar]
- 23. Zorzi A, Mastella G, Cipriani A, Berton G, Del Monte A, Gusella Bet al. Burden of ventricular arrhythmias at 12-lead 24-hour ambulatory ECG monitoring in middle-aged endurance athletes versus sedentary controls. Eur J Prev Cardiol 2018;25:2003–11. [DOI] [PubMed] [Google Scholar]
- 24. Delise P, Lanari E, Sitta N, Centa M, Allocca G, Biffi A. Influence of training on the number and complexity of frequent VPBs in healthy athletes. J Cardiovasc Med (Hagerstown) 2011;12:157–61. [DOI] [PubMed] [Google Scholar]
- 25. Hatch F, Lancaster MK, Jones SA. Aging is a primary risk factor for cardiac arrhythmias: disruption of intracellular Ca2+ regulation as a key suspect. Expert Rev Cardiovasc Ther 2011;9:1059–67. [DOI] [PubMed] [Google Scholar]
- 26. Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience 2017;39:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenbaum MB. Classification of ventricular extrasystoles according to form. J Electrocardiol 1969;2:289–97. [DOI] [PubMed] [Google Scholar]
- 28. Verdile L, Maron BJ, Pelliccia A, Spataro A, Santini M, Biffi A. Clinical significance of exercise-induced ventricular tachyarrhythmias in trained athletes without cardiovascular abnormalities. Heart Rhythm 2015;12:78–85. [DOI] [PubMed] [Google Scholar]
- 29. Parisi A, Tranchita E, Minganti C, Sperandii F, Guerra E, Calò Let al. Young athletes with ventricular premature beats: continuing or not intense training and competition? Scand J Med Sci Sports 2018;28:541–8. [DOI] [PubMed] [Google Scholar]
- 30. Porcedda G, Brambilla A, Favilli S, Spaziani G, Mascia G, Giaccardi M. Frequent ventricular premature beats in children and adolescents: natural history and relationship with sport activity in a long-term follow-up. Pediatr Cardiol 2020;41:123–8. [DOI] [PubMed] [Google Scholar]
- 31. Beaufort-Krol GCM, Dijkstra SSP, Bink-Boelkens MTE. Natural history of ventricular premature contractions in children with a structurally normal heart: does origin matter? Europace 2008;10:998–1003. [DOI] [PubMed] [Google Scholar]
- 32. Drezner JA, Sharma S, Baggish A, Papadakis M, Wilson MG, Prutkin JMet al. International criteria for electrocardiographic interpretation in athletes: consensus statement. Br J Sports Med 2017;51:704–31. [DOI] [PubMed] [Google Scholar]
- 33. Zorzi A, Vessella T, De Lazzari M, Cipriani A, Menegon V, Sarto Get al. Screening young athletes for diseases at risk of sudden cardiac death: role of stress testing for ventricular arrhythmias. Eur J Prev Cardiol 2020;27:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cipriani A, Zorzi A, Sarto P, Donini M, Rigato I, Bariani Ret al. Predictive value of exercise testing in athletes with ventricular ectopy evaluated by cardiac magnetic resonance. Heart Rhythm 2019;16:239–48. [DOI] [PubMed] [Google Scholar]
- 35. Sabbag A, Essayagh B, Barrera JDR, Basso C, Berni A, Cosyns Bet al. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed by the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace 2022;24:1981–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.