Abstract
Background:
Humans have been consuming medicinal plants (as herbs/ spices) to combat illness for centuries while ascribing beneficial effects predominantly to the plant/phytochemical constituents, without recognizing the power of obligatory resident microorganism’ communities (MOCs) (live/dead bacteria, fungus, yeast, molds etc.) which remain after industrial microbial reduction methods. Very little is known about the taxonomic identity of residual antigenic microbial associated molecular patterns (MAMPs) debris in our botanical over the counter (OTC) products, which if present would be recognized as foreign (non-self) antigenic matter by host pattern recognition receptors (PRRs) provoking a host immune response; this the basis of vaccine adjuvants. As of today, only few research groups have removed the herbal MAMP biomass from herbs, all suggesting that immune activation may not be from the plant but rather its microbial biomass; a hypothesis we corroborate.
Purpose:
The purpose of this work was to conduct a high through put screening (HTPS) of over 2500 natural plants, OTC botanical supplements and phytochemicals to elucidate those with pro-inflammatory; toll like receptor 4 (TLR4) activating properties in macrophages.
Study Design:
The HTPS was conducted on RAW 264.7 cells vs. lipopolysaccharide (LPS) E. coli 0111:B4, testing iNOS / nitric oxide production as a perimeter endpoint. The data show not a single drug/chemical/ phytochemical and approximately 98 % of botanicals to be immune idle (not effective) with only 65 pro-inflammatory (hits) in a potency range of LPS. Method validation studies eliminated the possibility of false artifact or contamination, and results were cross verified through multiple vendors/ manufacturers/lot numbers by botanical species. Lead botanicals were evaluated for plant concentration of LPS, 1,3:1,6-β-glucan, 1,3:1,4-β-D-glucan and α-glucans; where the former paralleled strength in vitro. LPS was then removed from plants using high-capacity endotoxin poly lysine columns, where bioactivity of LPS null “plant” extracts were lost. The stability of E.Coli 0111:B4 in an acid stomach mimetic model was confirmed. Last, we conducted a reverse culture on aerobic plate counts (APCs) from select hits, with subsequent isolation of gram-negative bacteria (MacConkey agar). Cultures were 1) heat destroyed (retested/ confirming bioactivity) and 2) subject to taxonomical identification by genetic sequencing 18S, ITS1, 5.8 s, ITS2 28S, and 16S.
Conclusion:
The data show significant gram negative MAMP biomass dominance in A) roots (e.g. echinacea, yucca, burdock, stinging nettle, sarsaparilla, hydrangea, poke, madder, calamus, rhaponticum, pleurisy, aconite etc.) and B) oceanic plants / algae’s (e.g. bladderwrack, chlorella, spirulina, kelp, and “OTC Seamoss-blends“ (irish moss, bladderwrack, burdock root etc), as well as other random herbs (eg. corn silk, cleavers, watercress, cardamom seed, tribulus, duckweed, puffball, hordeum and pollen). The results show a dominance of gram negative microbes (e.g. Klebsilla aerogenes, Pantoae agglomerans, Cronobacter sakazakii), fungus (Glomeracaea, Ascomycota, Irpex lacteus, Aureobasidium pullulans, Fibroporia albicans, Chlorociboria clavula, Aspergillus_sp JUC-2), with black walnut hull, echinacea and burdock root also containing gram positive microbial strains (Fontibacillus, Paenibacillus, Enterococcus gallinarum, Bromate-reducing bacterium B6 and various strains of Clostridium).
Conclusion:
This work brings attention to the existence of a functional immune bioactive herbal microbiome, independent from the plant. There is need to further this avenue of research, which should be carried out with consideration as to both positive or negative consequences arising from daily consumption of botanicals highly laden with bioactive MAMPS.
Keywords: Herbs, Edible microbiome, Medicinal microbiome, Bugs as drugs, Immune boosting
1. Introduction
Humans have been orally consuming medicinal plants for centuries to treat a myriad of human illnesses, many of which happen to be derived from the richest source of microbes found in the natural world; ocean and soil. While the plant microbiome has been extensively studied in relation to its growth, health, maturation, influence on environmental ecology (Berihu et al., 2023; Cook et al., 2023; Yin et al., 2023; Zhou et al., 2022) and food safety (Kazi et al., 2018; Wassermann et al., 2022), very little is known about the characterization or concentration of bioactive remnants of microbial debris in edible foods, including pathogenic after being intentionally destroyed. Specifically, there is limited knowledge about foods which would as a design by mother nature, contain high concentration of microbial/pathogen associated molecular patterns (MAMPs/PAMPS) with known capacity to modulate the immune system, this the fundamental basis for the development of vaccines, adjuvants and immunotherapies (Dong et al., 2014; Dubey et al., 2014; Nelson, 2022; Roeder et al., 2004; Rossi and Mastroeni, 2022; Zelechowska et al., 2022). Immune provocation is based on the ability to distinguish self from non-self, accomplished by host pattern recognition receptors (PRRs) that recognize foreign MAMPS (non-self) and include among others – the lipid A subregion of bacterial lipopolysaccharide (LPS) from gram negative rods. Concurrently, the human gut-immune axis, which plays a pivotal role in human immune resilience, houses cognate PRRs that are acted upon by these same MAMPS. However, despite advances in metagenomics, and the unveiling of taxonomy of live MOCS in raw foods (e.g. lettuce, bananas, olives, figs, sorghum etc. (Abdullaeva et al., 2021; Abera et al., 2022; Agarussi et al., 2022; Frohling et al., 2018; Higgins et al., 2018; Hong et al., 2023; Nakayasu et al., 2022; Soto-Giron et al., 2021)), research into the edible post processed heat destroyed microbiome and its effect on human immune activation is sparse.
Botanical microorganism community (MOC) biomass is a function of nature, which predicates its collective phenotype. Edible MAMPS in herbs, would not only mechanistically activate the Toll-like receptors including TLR4 such as lipoproteins, byglycans, acylated lipopeptides, lipoteichoic acid, peptidoglycans but also other TLRs (e.g. imidazoquinolines (TLR7) modulin, RNA from bacteria (TLR8) the synthetic dsRNA molecule polyinosinic-polycytidylic acid (poly-IC), glycolipids, fibrinogen/fibronectin, heat shock proteins, uric acid, flagellin (TLR5), ssRNA of microbial origin and unmethylated CpG rich DNA (TLR9) or for (TRL3) poly C derivatives). (Ehrchen et al., 2009; Galluzzi et al., 2012; Gulyas et al., 2022; Wolska et al., 2009a).
The diversity of plant microbiomes are a design of nature that would by logic be a function of; 1) the plants inherent bactericidal/ fungicidal properties (e.g. garlic (bulb), oregano, clove, thyme, green tea (leaves)) 2) the physical location of the plant/ part - from roots below soil (rhizome rich micro-biome)/ to leaves being above the soil (in direct exposure to sanitizing UV sunlight / pressurized rain wash off) 3) mitigating MOC changes during maturation to harvest 4) variation in circumscribing water systems, (storm,farm, industrial, oceanic) 5) wide array of MOC secondary metabolites produced 6) the adhesive nature of the plant enabling MOC concentration (e.g. sea alginates) 7) moisture and humidity (tropical to drought) 8) and post-harvest storage handling to modern advanced microbial reduction techniques employed.
Botanical food processing in civilized societies for over the counter (OTC) products assure the elimination of food borne pathogens (e.g. Escherichia coli, Staphylococcus aureus, Salmonella enterica ssp typhimurium, Pseudomonas aeruginosa, Bacillus subtilis, Candida albicans and Aspergillus niger). This requires use of broad based destructive processes ranging from dry/wet heat, fumigants (ethylene oxide/ propylene oxide) under vacuum or pressure, ultra-violet radiation, chlorine dioxide, ozone, CO2, or sophisticated oxidation techniques. (Jefri et al., 2022; Ma et al., 2022; Shi et al., 2021; Sholtes et al., 2016). Point of sale (POS) products sold as capsules, tablets or powders, therefore, contain not only the plant’s inherent content but also its defunct epiphytic microbiome, comprised of lack of viable pathogens, its dead microbiome and all other residual non-pathogenic live bacteria which remain as aerobic plate counts (APCs), reported as yeasts, molds and fungus in accordance to limits set by local pharmacopeia authorities, the world health organization (WHO) or Food Chemicals Codex monographs. (Becker et al., 2019; Jefri et al., 2022; Jovanovic et al., 2022; Ma et al., 2022; Shi et al., 2021; Sholtes et al., 2016).
While people have consumed plant microbiomes for thousands of years, only a small number of research teams - including our own – have suggested that the immune boosting (direct activation of innate or acquired immunity) effects of “specific herbs” may have little to do with the plant, but rather are inflicted by ingesting a decomposing bioactive microbiome. Pioneering research teams who’s work we confirm have shown MOC gram negative TLR4 activating MAMP biomass present in black walnut, echinacea root, ginseng root, alfalfa seeds (Pugh et al., 2008), astragalus root (Denzler et al., 2010; Koehler et al., 2020), angelica root; (Montenegro et al., 2015), and wheat (Kohchi et al., 2006; Mizuno and Soma, 1992; Nishizawa et al., 1992; Taniguchi et al., 2009). Meanwhile TLR4 MAMP agonists are being used in vaccines such as Cervarix® (human papillomavirus vaccine) and Shringrix® (herpes zoster) (Luchner et al., 2021) and there is greater acceptance of the idea that eating dead microbes can affect the immune system, with emergence of the field of para-probiotics (Vallejo-Cordoba et al., 2020). Similarly, the concept is well exemplified by the body of work on edible fungal beta-1,3/1,6-D-glucan derived from mushrooms or Saccharomyces cerevisiae (yeast), as well as spirulina (a gram negative rod) which can trigger anti-cancer immunity and provide greater response to infection through the gut-immune-systemic plexus (Al-Batshan et al., 2001; Albeituni et al., 2016; Geller et al., 2019; Li et al., 2010; Liu et al., 2015; Nelson et al., 2017; Ohno et al., 1988; Vetvicka, 2011; Vetvicka and Vetvickova, 2020; Xiang et al., 2012; Xin et al., 2022).
Mechanistically, once ingested, MAMPS activate immune cells (such as macrophages) and epithelial cells in gut-associated lymphoid tissues (GALT)/Peyer’s patches as well as subcomponent lamina propria via mechanisms involving TLRs, C-type lectin receptors, and NOD-like receptors (Crunkhorn, 2022; Patin et al., 2019). Once activated, MAMP/ PRRs transmit corresponding antigenic information via M cells to antigen presenting cells (APCs) which then transition toward mesenteric lymph (Mo et al., 2022) and systemic lymph via dendritic cells (Debnath et al., 2022; Iqbal et al., 2014; Paradia et al., 2022; Stander et al., 2022) triggering T cell clonal expansion. (Gallichan and Rosenthal, 1996; Smith et al., 2002; Woolverton et al., 1992). TLRs in the gut immune plexus have been identified on cell surfaces (TLR1, 2, 4, 5, 6) or / in (endosomal TLR3, 7, 8, 9) (Oblak and Jerala, 2011; Wolska et al., 2009b).
The data in this work, while suggesting a new area of plant medicine, apart from the plant itself, does not negate the incredible value of the plants phytochemical constituents having been reported extensively in the literature as having powerful “anti’ medicinal drug like properties” (anti-inflammatory, anti-pain (analgesic), anti-pyretic, anti-malarial, anti-bacterial, anti-protozoal, anti-parasitic, anti-oxidant, anti-viral etc.) (Aziman et al., 2014; Chen, 2022; Janthamala et al., 2021; Klein et al., 2013; Lu et al., 2019; Mazzio et al., 2016; Raposo et al., 2021; Romualdo et al., 2020; Sharifzadeh et al., 2016; Siddiqui et al., 2022; Silva et al., 2013; Zhang et al., 2021a; Zhang et al., 2021c; Zheng et al., 2018) In fact, these chemical attributes are the rationale behind extensive historical botanical / spice use in food preservation, (Chahal et al., 2021; Choi et al., 2017; El-Azzouny et al., 2018; Hosseinzadeh et al., 2014; Kim et al., 2020; Nabavi et al., 2015; Rodriguez-Garcia et al., 2016; Syahidah et al., 2017) and treatment of human infections (by lethal destruction to microbes) (Fahey et al., 2015; Howshigan et al., 2015; Tong et al., 2011; Zaidi et al., 2015). As of today, most all anti-cancer studies involving plants are focusing and reporting on phytochemicals acting on specific biotargets, as in blocking cell cycle, apoptosis, inducing / reducing transcription factors, oncogenes/ tumor suppressor proteins, receptors, cytokines and inflammatory signaling. (Al-Khayri et al., 2022; Shen et al., 2005). Contrarily and logistically, herbs that support systemic tumor immune boosting are cross parallel to those that include MAMPS, which are frequently heat/pH stable and may work synergistically with inherent plant compounds.
Here, in this work, we originally set out to conduct a routine high throughput screening of > 2000 botanicals of OTC nutraceuticals (sold through basic retail outlets worldwide) with intent to elucidate if any; could invoke macrophage polarization toward an M1 anti-cancer fighter phenotype (pro-inflammatory, anti-tumorigenic) which mechanistically could reverse an acquiescent M2 (anti-inflammatory,pro-tumorigenic) phenotype (Muller et al., 2017; Orange et al., 2016) beneficial to reawaken T-cell mediated adaptive host immune response to recognize self-malignant cells. (Chauhan and Kanwar, 2021; Evans et al., 2021; Galluzzi et al., 2012; Ghochikyan et al., 2014; Wang et al., 2023). The findings in our work, however, took a sharp turn after observing an unexplainable blatant inflammatory property beholden to specific herbs, on par with LPS. Here we establish a connection with previous research (sparse) suggesting that the plants MAMP laden microbiome and not the plant itself, appears to be directly aligned with activation of TLR4 signaling.
Methods & Materials.
Hanks Balanced Salt Solution, (4-(2-hydroxyethyl)-1-piper-azineethanesulfonic acid) (HEPES), ethanol, sulfanilamide, 96 well plates, chemicals, general reagents, and supplies, were all purchased from either Sigma-Aldrich, (St Louis, MO, U.S.A.) or V.W.R. (Radnor, PA, U.S.A.). Natural products were purchased from Frontier Natural Products Co-op (Norway, IA, U.S.A.), Monterey Bay Spice Company (Watsonville, CA, U.S.A.), Mountain Rose Herbs (Eugene, OR, U.S.A.), Mayway Traditional Chinese Herbs (Oakland, CA, U.S.A.), Kalyx Natural Marketplace (Camden, NY, U.S.A.), Futureceuticals (Momence, IL, U.S. A.), Organic fruit vegetable market: New Leaf (Tallahassee, FL, U.S.A.), Florida Food Products Inc. (Eustis, FL, U.S.A.), Patel Brothers Indian Grocery (Tampa, FL, U.S.A.), Starwest Botanicals (Sacramento, CA, U.S. A.), Monteray, Bay Spice Co. (Watsonville, CA, U.S.A.), Banyan Botanicals (Williams, OR, U.S.A.), Bulk Supplements (Henderson, NV), Swanson Health Products (Fargo, ND, U.S.A.), Raven Moon Emporium (Rock Hill, SC, U.S.A.), Pure Organic (Solana Beach, CA, U.S.A.), NOW Foods, Supplements (Bloomingdale, IL, U.S.A.), American Standard Supplements (Ontario, CA), Carlyle Nutritional’s (Melville, NY, U.S.A.), Horbaach Health (Melville, NY, U.S.A.), Solaray (Salt Lake City, UT, U.S. A.), Terra Dolce Mycological Natural Products (Eugene, OR, U.S.A.), Daiwa Health Development (Gardena CA, U.S.A.), Earthborn Elements (Lindon, UT, U.S.A.), Natures Way (Green Bay, WI, U.S.A.), Nusapure (Los Angeles, CA, U.S.A.), T.N. Vitamins (Farmingdale, NY, U.S.A.), Maui Herbs (Kihei, Hawaii, U.S.A.), Vadik Herbs (Concord, California, U.S.A.), Arizona Nutritional Supplements (Chandler AZ, U.S.A.), Tame the Spirit Herbs (Mount Vernon,KY, U.S.A.), Terra Vita/ Terra Health (Sheridan, WY, U.S.A.), Western Botanicals (Spanish Fork, UT, U.S.A.), Toogood Botanic Co. (Richmond Hill, ON, Canada) and Mauwe / Maui Herbs (Maui, Hawaii, U.S.A.).
1.1. Cell culture
RAW 264.7 macrophages cells were purchased from American Type Culture Collection (ATCC) (Manassas, VA, U.S.A.). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing high glucose [4500 mg/L] supplemented with 7 % fetal bovine serum (FBS), 4 mM L-glutamine, and penicillin/streptomycin (100 U/ 0.1 mg/mL). Culture conditions were maintained at 37 °C in 5 % CO2 /atmosphere, and every 2–3 days, the media was replaced for sub-culture. For experiments, plating media consisted of DMEM (minus phenol red) [glucose 4500 mg/L], 5 % FBS, and penicillin/streptomycin (100 U/0.1 mg/mL). A stock solution of LPS from E. coli 0111: B4 was prepared in HBSS at 1 mg/mL and stored at –20 °C.
1.2. Library sample preparation
All natural chemicals, phytochemicals, and reference drugs were subject to stock preparation either being dissolved in dimethyl sulfoxide (DMSO) or ethanol [5–20 mg/mL], where all crude herbs in FAMUs botanical library were prepared in absolute ethanol [50 mg/mL] after being diced, macerated, and powdered, etc. then stored at –20 °C in the dark. All plants were cataloged by manufacturer, botanical and common names and given an internal I.D. number. All dilutions were prepared in sterile HBSS + 5 mM HEPES, adjusted to a pH of 7.4, ensuring solvent concentration of DMSO or absolute ethanol stayed at<0.5 % at working concentration.
1.3. Cell viability
Cell viability was assessed as a routine background measure, not a study endpoint, using resazurin [7-Hydroxy-3H-phenoxazin-3-one 10-oxide] (Alamar Blue) indicator dye. A working solution of resazurin was prepared in sterile HBSS minus phenol red (0.5 mg/mL), then added (15 % v/v) to each sample. Samples were returned to the incubator for 2–4 h, and the reduction of the dye by viable cells (to resorufin, a fluorescent compound) was quantitatively analyzed using a Synergy HTX multi-mode reader Bio-Tek Inc. (Winooski, VT, U.S.A.) with settings at [550 nm / 580 nm], [excitation/emission].
1.4. Hit Determination: Nitrite /indirect iNOS expression
Quantification of nitrite (NO2 − ) was determined using the Greiss reagent. The Greiss reagent was prepared by mixing an equal volume of 1.0 % sulfanilamide in 10 % phosphoric acid and 0.1 % N-(1-naphthyl)-ethylenediamine in deionized water, which was added directly to the cell supernatant post-treatment (experimental media consisting of DMEM - phenol red) and incubated at room temperature for 5 min. Controls and blanks were run simultaneously and subtracted from the final value to eliminate interference. Samples were analyzed at 540 nm on a Synergy H.T.X. multi-mode reader: Bio-Tek (Winooski, VT, USA).
2. Gram negative cell wall
2.0.1. Limulus Amebocyte Lysate (LAL) Testing
Concentrations of gram-negative microbial cell wall in herbs were quantified using the Pierce™ LAL chromogenic endotoxin quantitation kit (Thermofisher scientific, Waltham MA USA), which is based on a modified Limulus Amebocyte Lysate (LAL) reaction with LPS [Catalog number: A39552]. A major modification was needed due to the extreme high sensitivity and rapid signal saturation of this assay. In brief, reagent bottle 1 (LAL solution) and reagent bottle 2 (the chromogenic substrate solution) were diluted by 50 % using endotoxin-free water. Botanical samples (10 μg/mL) were prepared in endotoxin-free water, to which (half the recommended volume) 25 μl of LAL reagent bottle was added, and LPS standards (1 ng/mL to 87 ng/mL) were run on the same plate. After 3.5 min (at room temperature, no heat was applied), 50 μl of chromogen (half the recommended volume) was added, and the plates were read immediately at 1 plate read/ minute until the standard curve of LPS and samples were within the quantitative linear range for each herb. After the final read, stopping reaction was added. Samples were quantitatively analyzed using a Synergy HTX multi-mode reader Bio-Tek Inc. (Winooski, VT, U.S.A.) with settings at 405 nm. Note: we found cross-reactivity of this assay with β-glucans; therefore, we also conducted the glucan assays to ascertain false positives if any.
2.0.2. LPS -Biglycan ELISA
LPS presence was confirmed using the BioAssay™ ELISA for (Lip-oglycan, Endotoxin) manufactured by US Biologicals product number # 026552 and carried out according to the product manufacturer. Samples were read at 450 nm after adding the stopping reaction on an Bio-Tek Inc. (Winooski, VT, U.S.A.).
2.1. β-Glucan assay kit (Yeast and Mushroom)
Quantification of 1,3:1,6-β-glucan and α-glucan (yeast and mushroom) was determined using a kit purchased by Neogen/ Megazyme (Lansing, MI, U.S.A.) and carried out according to the manufacturer’s instructions (Assay procedure K-YBGL 02/21). Briefly, herbal ethanol extracts were solubilized /hydrolyzed in ice-cold sulfuric acid for 2 h, where glucan fragments were re hydrolyzed to glucose using a purified exo-1,3-β glucanase and β-glucosidase solution, followed by hydrolysis of α-Glucans hydrolyzed to D-glucose then measured with amyloglucosidase and invertase using the kits GOPOD reagent. Using a standard curve and blanks, samples were quantitatively analyzed using a Synergy HTX multi-mode reader Bio-Tek Inc. (Winooski, VT, U.S.A.) with settings at 510 nm.
2.2. β-Glucan assay kit (Mixed Linkage)
Quantification of 1,3:1,4-β-D-glucan was determined using a kit purchased by Neogen/ Megazyme (Lansing, MI, U.S.A.) and carried out according to the manufacturer’s instructions (Assay procedure A - K-BGLU 02/21) according to AACC Method 32–23.01/AOAC Method 995.16. Briefly, the following buffers were prepared to carry out the assay: sodium phosphate buffer [20 mM, pH 6.5], sodium acetate buffer (50 mM, pH 4.0), and sodium acetate buffer (200 mM, pH 4.0) Briefly, herbal extracts were diluted in sodium phosphate buffer, placed in boiling water for 60 s, and incubated at 100 °C for 2 min / 50 °C for 5 min prior to the addition of linchinase and further incubation at 1 h – 50 °C. After the addition of sodium acetate buffer, samples were equilibrated to room temp and centrifuged. β -glucosidase was added in 50 mM sodium acetate and then incubated at 50 °C for 10 min prior to the addition of the GOPOD reagent and subsequent quantification at 510 nm against a standard curve after subtracting blanks.
2.3. Endotoxin removal
Gram-negative cell wall was removed from herbal extracts using Pierces™ high-capacity endotoxin removal spin columns, 0.5 mL/ Catalog number: 88,274 (Thermofisher Scientific, Waltham MA, U.S.A.), and experiments were carried out in accordance with the manufacturer’s protocols. Columns were regenerated 4x for reuse and then discarded. Pre and post-column eluents were evaluated for macrophage activation against the standard curve of LPS (E. coli (0111:B4)) up to 5 μg/mL by measuring nitric oxide in RAW 264.7 macrophages at equal concentration set at 170 μg/mL.
2.4. Immunohistochemistry
Herbs were prepared (50 mg /mL EtOH) and centrifuged at 2000 x. Ethanol was removed, and the sample was reconstituted in methanol. Samples were vortexed, centrifuged, and the supernatant discarded, where residue was allowed to dry by evaporation overnight. Plant residue was reconstituted in PBS with 1 % casein blocking buffer for 20 min prior to the addition of 1:500 primary antibody (mouse anti - LPS antibody (Abcam, Cambridge, MA, U.S.A.) in microcentrifuge vials, then placed on a vertical rotator for 2 h at room temperature. After discarding the supernatant, the residue was washed 3X in PBS, centrifuging the residue and discarding the supernatant between washes. The secondary antibody secondary antibody 1:1000 (goat-anti-mouse Alexa 488) prepared in blocking buffer was added to tubes, and samples were placed on a rotator for 1 h at room temperature. After discard, the residue was washed 3x in PBS, centrifuging the residue and discarding supernatant between washes, with the last wash remaining mixed with the residue. Samples were then transferred to a 96-well plate and imaged using a Cytation 5 (Biotek) (Winooski, VT, U.S.A.).
2.5. Capsule to culture studies
Eight pro-inflammatory OTS/POS purchased in 2022 were subject to a starter culture. Samples were unpacked in a sterile working area/hood, and a starter culture of 50 mg/mL was grown at 34 °C for 1 week in RPMI media, no phenol red, with L-glutamine supplemented and 10 % FBS and without pen/strep. After one week, 40 μls of culture supernatant (absent residue) were transferred to 15 mL of media (same) and regrown for another two weeks at 34 °C. At this point, the cultures were highly concentrated and subject to microbiology studies and biological studies. For biological studies, cultures were boiled for 10 min and diluted in PBS with pen/strep and tested in a standard plating medium also containing pen/strep and examined for potency of macrophage activation.
2.6. Microbiology studies
Microbial cultures were subject to 10-fold serial dilutions in buffered peptone water. 2 mL aliquots taken from each dilution tube were transferred to 18 mL of nutrient broth (NB) and incubated at 37 °C for 24 h while shaking at 120 rpm. After 24 h, 10-fold serial dilutions were prepared from the NB medium and 100 μl of the dilutions were plated in duplicate on N.B. agar and MacConkey agar, a selective medium for Gram-negative bacteria and enteric bacteria and differentiated them based on lactose fermentation. The plates were incubated at 37 °C for 24 h (N.B. agar) or 48 h (McConkey agar). The colonies appearing on the plates were then counted, and the counts were recorded. The DNA of the isolates was extracted using DNeasy PowerLyzer ®, PowerSoil® Kit QIAGEN according to manufacturer instructions for further sequencing.
2.7. Sequencing/species identification
Sequencing for both global colonies and gram-negative isolation was carried out using ribosomal 18S, ITS1, 5.8 s, ITS2 28S, and 16S rDNA sequence (1,542 bp) coding regions for species identification and community analysis by L.C. Sciences (Houston, Texas U.S.A.). In brief, the amplified library used for sequencing on the NovaSeq platform pairedend reads (2 × 250 bp). Were ITS2 primer:F (5′-GTGARTCATCGAATCTTTG-3′) R (5′-TCCTCCGCTTATTGATATGC-3′); ITS1 primer:F (5′-GAACCWGCGGARGGATCA-3′) R (5′-GCTGCGTTCTTCATCGATGC-3′). For 16S rDNA.The primers (341F/805R) designed to target the V3 and V4 regions of 16S rDNA generate an amplicon about 465 bp in size. The amplified library is sequenced on a NovaSeq platform with 250 bp pairedend reads mode (2 × 250 bp). Workflow involved DNA extraction, PCR amplification, Product purification, library preparation, and high through put sequencing. Bioinformatics analysis was carried out by raw data files in FASTQ format subject to reads merged by overlapping sequences, data quality control, and chimera filtering, resulting in high-quality clean data. DADA2 (Divisive Amplicon Denoising Algorithm) was used for dereplication (equivalent to 100 % similarity clustering), and generation of representative sequences at single-base resolution, greatly improving the data accuracy and taxonomy resolution. The core function of DADA2 is denoising, leading to the construction of Operational Taxonomic Units-like table, namely ASVs (Amplicon Sequence Variants) table. The resultant characteristic representative ASV sequences are key for downstream analyses, including diversity, taxonomy, and differential analyses. Taxonomy: required use of SILVA (Release 138, https://www.arb-silva.de/documentation/release-138/), NT-16S database, RDA and Unite database for taxonomy, confidence level set to be > 0.7. We use for taxonomy, confidence level set to be > 0.7. Based on the ASV annotation results, the abundance, and differential results at different levels, ie., Domain, Phylum, Class, Order, Family, Genus, and Species, can be determined. Data is reported as Species.
2.8. Stomach simulation
A stomach model simulation was developed to test the ability of hydrochloric acid (HCl) to inactivate the biological activity of LPS. HCl was prepared from 0.007 N to 2 N in sterile water containing 5 mg/ml phenol red, along with a blank. pH was acquired for each acid solution on day 1, 2 and 3 to ensure stability. On day 3, after equilibration with ambient air, neutralization strategies were pilot tested on 500 ul of each HCL concentration with drop by drop addition of 0.05, 0.5 and 2 N NaOH grossly demarcated first by visual targeting using phenol red indicator dye. Volumes of added NaOH were recorded, and end pH was checked using an elite pH spear. All solutions were neutral.
After the above neutralization methods were validated - each concentration of HCl was placed in a 15 mL conical tube filled to 8 mL. LPS was added to all HCl serial dilutions at 5ug/ml. After adding LPS/ the LPS/HCl solutions were neutralized at Time 0 (immediately) 1, 2 and 4 h using the technique above and stored at 4°C. All samples were then tested for macrophage activation in vitro as previous described.
3. Data analysis
General statistical analysis was performed using Graph Pad Prism (version 3.0; Graph Pad Software Inc. San Diego, CA, U.S.A.) with the significance of the difference between the groups assessed using a one-way ANOVA then, followed by Tukey post hoc means comparison test or a Student’s t-test. IC50s were determined by regression analysis using ATT Bioquest analytic tools.
4. Results
4.1. HTPS setup
The pro-inflammatory effects of an in-house natural product and botanical library (2532 compounds) were determined by measuring production (endpoint) in RAW 264.7 macrophages [1 × 10] cells /mL] in 96 well plates using (LPS E.Coli 0111:B4) as a positive control, and cell solvent blank as a negative control. The mechanism by which LPS acts on PRRs / TLR4 is well known where it can trigger endocytosis, initiating recruitment of myeloid differentiation primary response gene 88 (MyD88) to the cytosolic domain. (Kolanowski et al., 2014; Sakai et al., 2017) (Fig. 1). Subsequently, mitogen activated protein kinase signaling elicits translocation of nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) is directed into the nucleus where it upregulates a host of genes involved with leukocyte recruitment (CCL2,CCL6,CCL12, CXCL10, CXCL11,CXCL12,CXCL13), a pro-inflammatory response (IFN-γ, TNF –α, IL-6 or Type I interferons IFN- α and IFN-β), and iNOS induction and production of . (Luchner et al., 2021).
Fig. 1.

Hit Description; Rapid screening of RAW 264.7 macrophages by LPS E. Coli 0111:B4 by quantifying the production of nitric oxide (NO). LPS activates the pattern recognition receptor TLR4, invoking recruitment of myeloid differentiation primary response gene 88 (MyD88) to its cytosolic domain and adaptor protein TIRAP (T.I.R. adaptor protein), initiating translocation of nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) into the nucleus from the cytoplasm where induction of genes (Sakai et al., 2017) encoding chemokines involved in leukocyte recruitment (CCL2, CCL6, CCL12, CXCL10, CXCL11, CXCL12, CXCL13), inflammatory cytokines (TNF –α, IL-6), iNOS, thereby making the easily quantifiable release of nitric oxide (NO) by colorimetric detection using the Griess Reagent a target for high throughput screening.
LPS/ TLR4 agonists are not only used in modern vaccines as adjuvants, to link innate to adaptive immune response (Luchner et al., 2021) but also trigger anti-tumor signaling in cells bearing its receptor with the most studied being tumor associated macrophages (TAM) M1 anti-cancer fighter phenotype (pro-inflammatory, anti-tumorigenic) which reverses the acquiescent M2 (anti-inflammatory pro-tumorigenic and stimulated by IL-4, IL-13, IL-10 phenotype) (Muller et al., 2017; Orange et al., 2016). Administration of LPS, in diverse tumor models leads to activation of M1 TAMS and a host systemic reawakening of the T-cell mediated adaptive host immune response to recognize self-malignant cells (Chauhan and Kanwar, 2021; Evans et al., 2021; Galluzzi et al., 2012; Ghochikyan et al., 2014; Wang et al., 2023).
For rapid screening, is easily detected as an indicator of macrophage activation, using a visible chromogen (Griess Reagent) suitable for high throughput screenings.
The HTPS libraries were cataloged from dry material intake, ascribed an ID, with data records kept on vendor, lot number, botanical identity and cultivation region, with most all plant based botanical products and OTC products accompanied by a certificate of analysis. All stock solutions were prepared for drugs/ phytochemicals in either ethanol or DMSO, and all herbs were macerated, powdered and placed in sterilizing ethanol at 50 mg/ml. All compounds were prepared in stock plates containing endotoxin free HBSS, and tested in vitro, under sterile conditions. All compounds were tested in macrophages cultured in media with pen-strep (note: lethal to gram positive, and gram-negative bacteria). A Tier 1 screening; 2532 compounds (all herbs tested at 317 μg /mL) (Fig. 2) either rendered an absence of response, a pro-inflammatory response or a toxic response, the latter tested in subsequently lower concentrated Tiers. All hits and toxic compounds were rescreened through a series of lower concentrations starting at Tier 2 (158 μg/mL), Tier 3 (79 μg/mL) and lower, for macrophage activation.
Fig. 2.

Study Design: A high throughput screening library comprised of 2532 compounds [366 drugs, 193 phytochemicals, and 1973 OTC /POS botanical ethanol extracts] were evaluated for NO production in macrophages vs. LPS E. coli 0111: B4 positive controls (1 μg/mL). Hits and toxic compounds from Tier 1 (T1) tested at 317 μg/mL were tested at subsequent lower concentrations (T2 and T3), and toxic compounds at lower concentrations if needed. 65/2532 botanicals were Q.C. validated as pro-inflammatory, none of which were phytochemicals or drugs, all absent of interfering artifact or contamination, being predominantly repeatable across vendors (Table 1). These 65 were then subject to a dose–response potency test (Pro-inflammatory 50 concentration PI50) (conc. range: 0.72–371 μg /mL) established from an LPS standard curve (1 ng/ mL-1000 ng/mL) run simultaneously with all samples, having the LPS (Max) value at 500 nm set at 100 % LPS control. All 65 herbal extracts were then tested for the presence of gram-negative cell wall and β-glucans. (Table 1).
The standard screening layout in 96 well plates contained four positive/ four negative controls in Column 1 with samples as shown in Fig. 3. The data showed a lack of macrophage activation by all drugs and phytochemicals, with hits only in botanicals - comparably potent to LPS capacity to elicit production. All hits were subject to validation testing to eliminate plausible artifacts, including 1) reagent control reactions (matrix blanks with the Griess Reagent) 2) consistency across various vendors and lot numbers (product specific) and 3) strength of bio-active response by pro-inflammatory 50 (PI50) concentrations vs. 100 % LPS control and 4) quantification of gram-negative microbial LPS and various β-glucans (Table 1). LPS standard curves for dose - time response were required for every follow-up study in macrophages to maintain quantification by regression matched batch controls, with measurements made on the linear portion of standard curves, relative to cell density.
Fig. 3.
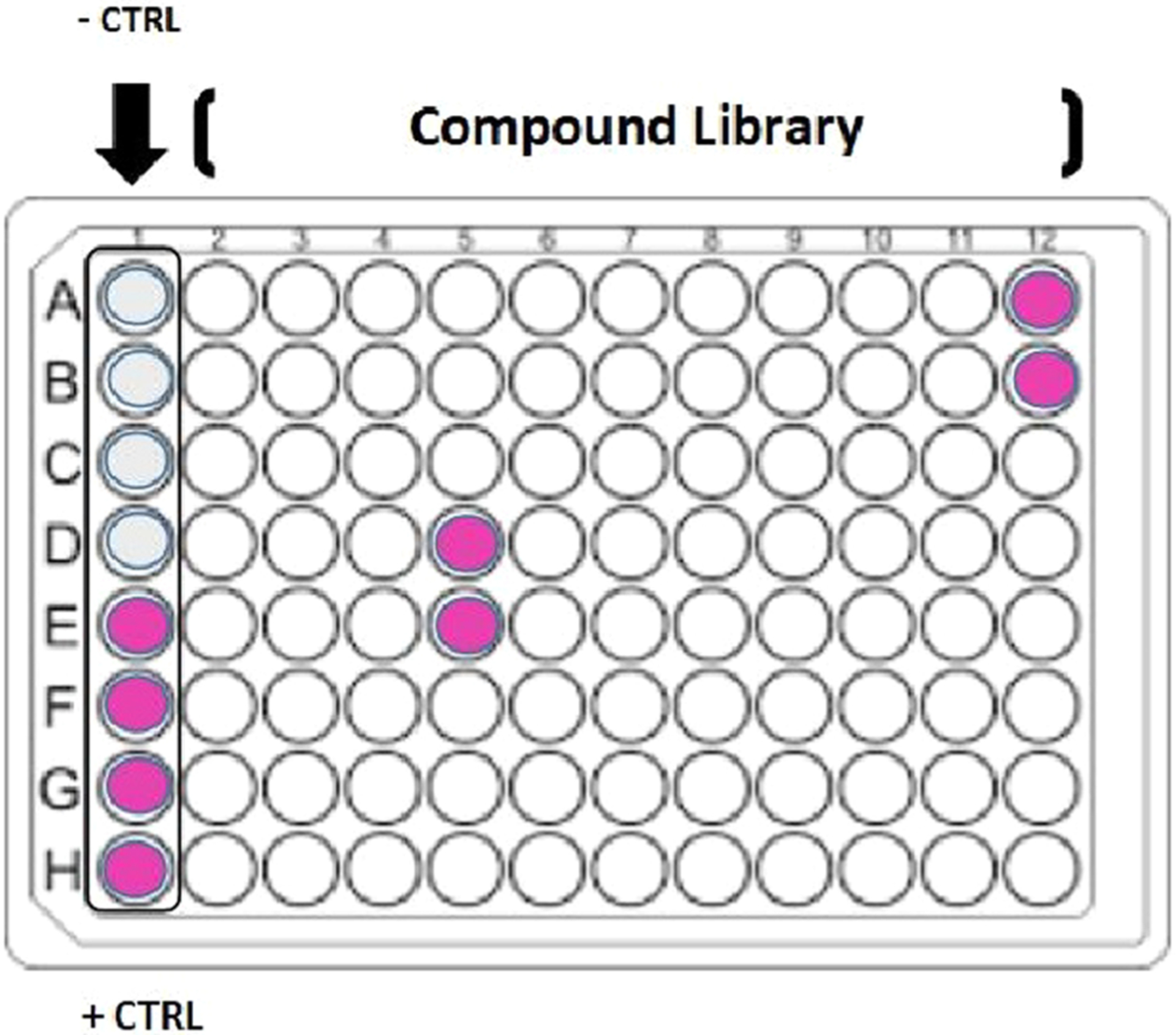
HTS plate setup. Screenings were conducted in 96 well plates, with positive and negative controls in column 1 (A-H).
Table 1.
65 Lead hits presented by Class (“Plant Category”) Common/ Chinese name and Botanical name (“Description”) cross verified by multiple vendors, lot numbers and of various year of issue (“Multiple Vendors”), creating a hit ratio. Pro-inflammatory 50 (PI50)s were determined from a dose response curve (over 10 concentrations.72–371 ug/mL) of the group representative sample (“Rep ID”) by assessing NO production vs. LPS % Control, n = 3 (“Macrophage Activation”). The lower the value the more potent the botanical, and this value is the most relevant. Gram negative cell wall concentrations (Herbal micro-biome) (tested by LAL) are provided as Endotoxin Units (E.U.) where 1 E.U. = 0.5 ng/ml, and reported as E.U./mg and standard capsules sold OTC. Values were then extrapolated to μg/500 mg, for depicting how many μg of pure LPS would be consumed in a 500 mg OTC sold capsule. Beta glucans in herbal extracts were determined and reported as ng/mg (1–3,1–4) lineage and g/100 g for 1–3,1–6 lineage (yeast mushroom), n = 2.
| BOTANICAL IDENTITY | VENDOR HIT RATIO | BIOLOGICAL | HERB micro-biome | HERB micro-biome | HERB micro-biome | HERB micro-biome | HERB micro-biome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rep ID | Class | Botanical Name/Pharmaceut ical Latin | #Hits | #Vendors | Hit Ratio | Pro-inflammatory (PI50(μg/ml)) | E.U./mg | E.U./500 mg | up LPS per 500 mg capsule | β-glucan 1–3,1–4 | β-glucan1–3,1–6 (g/100g) | ||
| AB192 | Root | Bai Tou Weng: English Pulsatil la Root | Pulsatillae Radix | 3 | 3 | 100% | 26.3 | 1,288 | 643,925 | 322 | ND | ND | ND |
| AB197 | Grass/Straw | Ban Bain Lian: English Chinese Lobelia | Lobelia chinensis herb | 2 | 2 | 100% | 105.3 | 195 | 97,636 | 49 | ND | ND | ND |
| AB266 | Root | Black Cohosh Root | Cimicifuga racemosa | 5 | 6 | 83% | 109.7 | 271 | 135,624 | 68 | ND | 1.3 | 8.4 |
| AB413 | Hull/Bark/Pods | Black Haw Bark | Viburnum prunifolium | 5 | 5 | 100% | 118.0 | 219 | 109,290 | 55 | ND | ND | ND |
| AB309 | Hull/Bark/Pods | Black Walnut Hull | Juglans nigra | 5 | 5 | 100% | 6.7 | 5,000 | 2,499,961 | 1,250 | ND | 0.0 | 8.8 |
| AB289 | Sea Algae/Weed | Bladde rwrack | Fucus vesiculosus | 6 | 6 | 100% | 12.6 | 6,374 | 3,186,900 | 1,593 | ND | ND | ND |
| AB55 | Leaf/Stem | Borage | Borago officinalis | 2 | 2 | 100% | 51.7 | 509 | 254,638 | 127 | ND | ND | ND |
| AB311 | Root | Burdoc kRoot | Arctium lappa | 9 | 9 | 100% | 9.6 | 10,748 | 5,373,844 | 2,687 | ND | 0.9 | 8.0 |
| AB380 | Root | Butchers Broom Root | Ruscus aculeatus | 8 | 8 | 100% | 14.4 | 3,162 | 1,580,995 | 790 | ND | ND | ND |
| AB337 | Root | Calamus Root | Acorus calamus | 6 | 8 | 75% | 23.6 | 391 | 195,685 | 98 | 7.59 | 0.7 | 10.5 |
| AB221 | Root | Cao Wu (Zhi): English Wild Aconit e Root | Aconitum kusnezoffii root prepared | 2 | 2 | 100% | 17.8 | 172 | 86,121 | 43 | ND | ND | ND |
| AB317 | Fruit/Seed | Cardomumseed | Elettariacardamomum | 4 | 4 | 100% | 31.9 | 509 | 254,638 | 127 | ND | 0.6 | 6.0 |
| AB465 | Mushroom | Chanterelies | Cantharelluscibarius | 2 | 2 | 100% | 45.4 | 638 | 318,889 | 159 | ND | 0.0 | 6.7 |
| AB272 | Grass/Straw | Chickweed | Stellariamedia | 3 | 3 | 100% | 46.0 | 242 | 121,074 | 61 | ND | ND | ND |
| AB450 | Sea Algae/Weed | Chlorella | Chlorellavulgaris | 4 | 4 | 100% | 13.4 | 19,811 | 9,905,722 | 4,953 | ND | 0.0 | 8.9 |
| AB267 | Grass/Straw | Cleavers | Galiumaparine | 6 | 6 | 100% | 11.6 | 37,601 | 18,800,621 | 9,400 | 4.8 | 0.8 | 5.3 |
| AB263 | Grass/Straw | Comsilk | Zea mays | 5 | 5 | 100% | 31.3 | 195 | 97,636 | 49 | ND | 0.9 | 5.0 |
| AB281 | Root | Dandelion Root | Taraxacumofficinale | 6 | 8 | 75% | 124.5 | 257 | 128,429 | 64 | ND | 0.8 | 5.2 |
| ab468 | Grass/Straw | Dog Grass Root | Triticumrepens | 1 | 1 | 100% | 44.7 | 195 | 97,636 | 49 | ND | ND | ND |
| AB156 | Sea Algae/Weed | Dulse Powder | Palmariapalmata | 3 | 3 | 100% | 235.5 | 172 | 86,121 | 43 | ND | ND | ND |
| AB304 | Root | Echinacea Root | Echinaceapurpurea | 6 | 6 | 100% | 27.4 | 855 | 427,353 | 214 | ND | 0.8 | 7.2 |
| AB236 | Root | Feng Fang: English Saposhnikoviae Root | Radix Saposhnikoviae | 3 | 3 | 100% | 112.2 | 3,162 | 1,580,995 | 790 | ND | 0.0 | 6.0 |
| AB183 | Grass/Straw | Fu Ping/Duckweed | Spirodelapolyrrhizaherb | 2 | 2 | 100% | 31.2 | 5,197 | 2,598,318 | 1,299 | ND | 0.7 | 2.3 |
| AB260 | Leaf/Stem | Homy Goat Weed | Epimediumsagittatum | 3 | 4 | 75% | 45.4 | 1,089 | 544,460 | 272 | ND | 0.5 | 0.8 |
| AB291 | Root | Hydrangea Root | Hydrangeaarborescens | 5 | 5 | 100% | 9.9 | 855 | 427,353 | 214 | ND | 0.8 | 6.1 |
| AB421 | Sea Algae/Weed | Irish Moss | Chondruscrispus | 3 | 4 | 75% | 5.4 | 7,508 | 3,754,187 | 1,877 | ND | ND | ND |
| AB386 | Root | Kava Kava Root | Pipermethysticum | 7 | 7 | 100% | 33.5 | 1,824 | 911,925 | 456 | ND | 0.0 | 10.3 |
| AB387 | Sea Algae/Weed | Kelp Powder | Ascophyllu m Nodosum | 3 | 5 | 60% | 4.4 | 53,423 | 26,711,363 | 13,356 | ND | ND | ND |
| AB268 | Fruit/Seed | Kola Nut | Colaacuminata | 4 | 4 | 100% | 49.5 | 339 | 169,409 | 85 | ND | 0.5 | 17.6 |
| AB188 | Root | Lou Lu/Rhaponticum Root | Rhaponticum Uniflorum Root | 2 | 2 | 100% | 14.8 | 509 | 254,638 | 127 | ND | ND | ND |
| AB216 | Herb | Ma Bian Cao / Verban a | Herba Verbenae | 2 | 2 | 100% | 43.1 | 242 | 121,074 | 61 | ND | 0.5 | 2.8 |
| AB189 | Fruit/Seed | Ma Bo/ Puffbal 1 | Calvatia Gigantia Sporophore | 2 | 2 | 100% | 23.8 | 3,959 | 1,979,413 | 990 | ND | ND | ND |
| AB445 | Grass/Straw | Mai Ya Fructus Hordei Germin atus | Hordeum vulgare fruit | 2 | 2 | 100% | 30.1 | 132 | 65,795 | 33 | ND | 0.4 | 2.9 |
| AB431 | Fruit/Seed | Milk Thistle | Silybummarianum | 4 | 6 | 67% | 33.7 | 638 | 318,889 | 159 | ND | 0.6 | 4.9 |
| AB469 | Bark | Muira Puama Bark Powder | Crotonechioides | 3 | 3 | 100% | 86.8 | 81 | 40,448 | 20 | ND | 0.6 | 3.8 |
| AB408 | Root | Nettle Root | Urtica dioica | 5 | 5 | 100% | 25.2 | 638 | 318,889 | 159 | ND | ND | ND |
| EM497 | Rice Bran | Orzya Sativa (RiceRoot) Nuo Dao Gen | Orzya Sativa (Rice) | 2 | 2 | 100% | 22.4 | 1,089 | 544,460 | 272 | ND | ND | ND |
| AB10 | Rice Bran | Orzya Sativa BRM4 | Orzya Sativa (Rice) | 5 | 5 | 100% | 334.4 | 132 | 65,795 | 33 | 11.2 | 0.5 | 47.6 |
| AB316 | Hull/Bark/Pods | Paprika | Capsicumannuum | 7 | 8 | 88% | 123.4 | 150 | 74,752 | 37 | ND | ND | ND |
| AB366 | Leaf/Stem | Patchouli | Pogostemoncablin | 5 | 5 | 100% | 29.1 | 242 | 121,074 | 61 | ND | ND | ND |
| AB4 | Leaf’ Stem | Plantain Leaf | Plantago Major | 4 | 4 | 100% | 76.1 | 105 | 52,509 | 26 | ND | 0.8 | 2.4 |
| AB116 | Root | Pleuris y Root | Asclepiastuberosa | 2 | 2 | 100% | 18.4 | 741 | 370,323 | 185 | 3.74 | 0.0 | 3.8 |
| AB312 | Root | Poke Root | Phytolaccaamericana | 3 | 3 | 100% | 6.2 | 9,701 | 4,850,647 | 2,425 | ND | 0.8 | 3.9 |
| AB213 | Pollen | Pu Huang/Pollen | Pollen Typhae | 2 | 2 | 100% | 9.0 | 16,848 | 8,423,818 | 4,212 | ND | 0.0 | 3.2 |
| AB214 | Root | Qian Cao/Madder Root | Radix Rubiae | 2 | 2 | 100% | 14.0 | 314 | 157,154 | 79 | ND | ND | ND |
| AB388 | Root | Saspari 11a | Smilaxmedica | 4 | 4 | 100% | 5.7 | 2,746 | 1,372,898 | 686 | 2.64 | 0.5 | 5.8 |
| AB78 | Sea Algae/Weed | Seamoss | Irish Moss (Chondruscrispus), Bladderwrack (Fucusvesiculosus), Burdock Root (Arcitumlappa) | 1 | 1 | 100% | 16.6 | 6,904 | 3,452,111 | 1,726 | 9.69 | 0.0 | 11.1 |
| AB237 | Fruit/Seed | She Chuang Zi | Cnidiummonnierifruit | 2 | 2 | 100% | 31.3 | 266 | 133,215 | 67 | ND | ND | ND |
| AB180 | Root | Sheng Jiang Pi | Zingiber Officinale Rhizome-Peel | 2 | 2 | 100% | 9.1 | 290 | 145,011 | 73 | ND | ND | ND |
| AB354 | Root | Soapw ort root | Saponasiaofficinalis | 2 | 2 | 100% | 48.9 | 1,824 | 911,925 | 456 | ND | 0.0 | 2.5 |
| AB258 | Microbe | Spirulina | Arthrospiraplatensis | 10 | 10 | 100% | 17.9 | 1,089 | 544,460 | 272 | ND | ND | ND |
| AB245 | Root | Stinging Nettle | Urticadioica | 4 | 4 | 100% | 27.1 | 6,295 | 3,147,325 | 1,574 | ND | 0.0 | 2.9 |
| AB399 | Root | Suma Root | Pfaffiapaniculata | 4 | 4 | 100% | 25.9 | 150 | 74,752 | 37 | ND | ND | ND |
| AB294 | Fruit/Seed | Tribulu s Fruit | Tribulusterrestris | 3 | 3 | 100% | 17.9 | 2,746 | 1,372,898 | 686 | ND | ND | ND |
| AB124 | Mushroom | Turkeytailmushroom | Trametesversicolor | 3 | 3 | 100% | 33.0 | 290 | 145,011 | 73 | ND | 0.0 | 4.4 |
| AB271 | herb | Watercress | Nasturtiumofficinale | 4 | 4 | 100% | 18.5 | 509 | 254,638 | 127 | ND | ND | ND |
| AB334 | Herb | Wormwood Herb | Artemesia Absinthium | 4 | 4 | 100% | 38.2 | 257 | 128,429 | 64 | ND | ND | ND |
| AB184 | Spike | XiaKu Cao/Heal All | Prunellavulgarisspike | 3 | 3 | 100% | 24.7 | 195 | 97,636 | 49 | ND | ND | ND |
| AB262 | Hull/Bark/Pods | Yohimbe Bark | Pausinystaliajohimbe | 4 | 5 | 80% | 141.5 | 290 | 145,011 | 73 | ND | 0.0 | 9.9 |
| AB466 | Root | Yucca Root | Yuccaschidigera | 5 | 5 | 100% | 41.5 | 257 | 128,429 | 64 | ND | 0.0 | 16.9 |
| AB205 | Bulb | Zhe Bei Mu | Fritillariathunbergiibulb | 2 | 2 | 100% | 116.8 | 127 | 63,543 | 32 | ND | 0.0 | 10.5 |
| AB446 | Mushroom | Zhu Ling | Polyporusumbellatusfungus | 2 | 2 | 100% | 53.3 | 314 | 157,154 | 79 | ND | 0.0 | 9.9 |
| AB193 | Flower | Zi Hua Di Ding | Violayedoensisherb | 2 | 2 | 100% | 75.1 | 391 | 195,685 | 98 | ND | ND | ND |
| AB207 | Sea Algae/Weed | Zi Wan | Aster Tataricus root and Rhizome | 2 | 2 | 100% | 35.5 | 143 | 71,582 | 36 | ND | ND | ND |
Table 1 provides basic information on the botanical identity of “hits” by common name, botanical name, the number of vendors tested for the same botanical species, ratio of hits/ vendor tested ( % hit ratio), (immune potency of the herb) as (PI50) and concentrations of gram-negative cell wall (endotoxin unit (EU) or (ug) and β-glucans by lineage (g/100 g) w/w.
4.2. Column removal of gram-negative cell wall
LPS was removed from plants using high-capacity endotoxin removal columns. Method validation studies were first performed to ensure efficacy of colum ns to remove LPS E.Coli 0111:B4 up to 5ug/mL (Fig. 4). Bioactivity of post vs pre column eluents for all samples are provided in Table 2. Endotoxin removal was effective in reversing macrophage activation for all herbs-except for black walnut hull and marine plants. After testing eluants for LPS, it was believed that marine plants either had a greater affinity for MAMPs than the polylysine stationary column packing, or its presence exceeded capacity. This will require further investigation as marine kelps and seaweeds contain adhesive alginates, “seaweed gums,” a main ingredient in microbiological growth agar. The ability of alginates to seed bacterial colonies could result in a natural high-capacity microbial reservoir inherent to oceanic plants. None the less LPS was visualized by centrifuge immunohistochemistry in kelp and bladderwrack residue (Fig. 5A) and confirmed by ELISA (Fig. 5B).
Fig. 4.

Endotoxin Removal Method validation: A method validation was run prior to removing LPS from the 65 herbs. The data represents Pre (100 %) vs Post (% of Pre) by macrophage activation response, measured by iNOS / NO2 production at equal concentrations. LPS (0.62 to 5ug/mL) was tested where the data is expressed as the Mean ± S.E.M., n = 2 column separations. Significant differences for pre vs. post macrophage activation was determined using a t-test *P < 0.01.
Table 2.
Evaluation of post -column eluents on TRL4 macrophage activation. The data represent Pre (100 %) vs. Post (% of Pre) for macrophage activation at 170 μg/mL for all herbs. The data are expressed as the Mean ± S.D. n = 2 columns/ endotoxin removal. All effluents were tested using the LAL (limulus amebocyte lysate) testing, # denotes positive presence of LPS in post column effluent.
| Common Name: Chinese/English | Botanical Name/ Pharmaceutical Latin | Post/Pre % Macrophage Activation | Result |
|---|---|---|---|
| Bai Tou Weng: English Pulsatilla Root | Pulsatillae Radix | 1.6 % ± 0.1 % | No Activity |
| Ban Bain Lian: English Chinese Lobelia | Lobelia chinensis herb | 0.2 % ± 0.1 % | No Activity |
| Black Cohosh Root | Cimicifuga racemosa | 0.5 % ± 0.1 % | No Activity |
| Black Haw Bark | Viburnum prunifolium | 0.2 % ± 0.1 % | No Activity |
| Black Walnut Hull | Juglans nigra | 83.4 % ± 4.2 % | Activity # |
| Bladderwrack | Fucus vesiculosus | 95.3 % ± 2.3 % | Activity # |
| Borage | Borago officinalis | 2.5 % ± 0.5 % | No Activity |
| Burdock Root | Arctium lappa | 0.9 % ± 0.5 % | No Activity |
| Butchers Broom Root | Ruscus aculeatus | 2.4 % ± 0.5 % | No Activity |
| Calamus Root | Acorus calamus | 2.3 % ± 0.5 % | No Activity |
| Cao Wu (Zhi): English Wild Aconite Root | Aconitum kusnezoffii root prepared | 0.3 % ± 0.5 % | No Activity |
| Cardomum seed | Elettaria cardamomum | 2.3 % ± 0.8 % | No Activity |
| Chanterelles | Cantharellus cibarius | 2.0 % ± 1.0 % | No Activity |
| Chickweed | Stellaria media | 5.9 % ± 1.0 % | No Activity |
| Chlorella | Chlorella vulgaris | 61.0 % ± 4.5 % | Moderate Activity # |
| Cleavers | Galium aparine | 2.1 % ± 0.8 % | No Activity |
| Cornsilk | Zea mays | 2.2 % ± 0.9 % | No Activity |
| Dandelion Root | Taraxacum officinale | 2.7 % ± 0.9 % | No Activity |
| Dog Grass Root | Triticum repens | 3.1 % ± 0.9 % | No Activity |
| Dulse Powder | Palmaria palmata | 3.1 % ± 1.0 % | No Activity |
| Echinacea Root | Echinacea purpurea | 1.9 % ± 0.8 % | No Activity |
| Feng Fang: English Saposhnikoviae Root | Radix Saposhnikoviae | 2.4 % ± 1.0 % | No Activity |
| Fu Ping/Duckweed | Spirodela polyrrhiza herb | 1.3 % ± 0.9 % | No Activity |
| Horny Goat Weed | Epimedium sagittatum | 4.1 % ± 0.9 % | No Activity |
| Hydrangea Root | Hydrangea arborescens | 1.2 % ± 0.8 % | No Activity |
| Irish Moss | Chondrus crispus | 2.9 % ± 1.0 % | No Activity |
| Kava Kava Root | Piper methysticum | 1.0 % ± 1.1 % | No Activity |
| Kelp Powder | Ascophyllum Nodosum | 98.4 % ± 3.2 % | Activity # |
| Kola Nut | Cola acuminata | 2.4 % ± 1.0 % | No Activity |
| Lou Lu/Rhaponticum Root | Rhaponticum Uniflorum Root | 17.9 % ± 0.8 % | Weak Activity |
| Ma Bian Cao / Verbana | Herba Verbenae | 6.8 % ± 0.6 % | No Activity |
| Ma Bo/ Puffball | Calvatia Gigantia Sporophore | 18.4 % ± 0.3 % | Weak Activity |
| Mai Ya Fructus Hordei Germinatus | Hordeum vulgare fruit | 2.4 % ± 0.7 % | No Activity |
| Milk Thistle | Silybum marianum | 2.2 % ± 0.7 % | No Activity |
| Muira Puama Bark Powder | Croton echioides | 1.3 % ± 0.7 % | No Activity |
| Nettle Root | Urtica dioica | 3.0 % ± 0.6 % | No Activity |
| Orzya Sativa (Rice Root) Nuo Dao Gen | Orzya Sativa (Rice) | 1.8 % ± 0.6 % | No Activity |
| Orzya Sativa BRM4 | Orzya Sativa (Rice) | 0.7 % ± 0.8 % | No Activity |
| Paprika | Capsicum annuum | 1.6 % ± 0.8 % | No Activity |
| Patchouli | Pogostemon cablin | 2.2 % ± 0.7 % | No Activity |
| Plantain Leaf | Plantago Major | 2.9 % ± 0.8 % | No Activity |
| Pleurisy Root | Asclepias tuberosa | 0.0 % ± 0.8 % | No Activity |
| Poke Root | Phytolacca americana | 0.0 % ± 0.9 % | No Activity |
| Pu Huang /Pollen | Pollen Typhae | 97.3 % ± 0.4 % | Activity # |
| Qian Cao/Madder Root | Radix Rubiae | 0.4 % ± 0.9 % | No Activity |
| Sarsaparilla Root | Smilax medica | 2.4 % ± 1.0 % | No Activity |
| Seamoss | Combination: Irish Moss (Chondrus crispus), Bladderwrack (Fucus vesiculosus), Burdock Root (Arcitum lappa) | 93.7 % ± 2.7 % | Activity # |
| She Chuang Zi | Cnidium monnieri fruit | 3.5 % ± 1.2 % | No Activity |
| Sheng Jiang Pi | Zingiber Officinale Rhizome-Peel | 4.8 % ± 0.9 % | No Activity |
| Soapwort root | Saponasia officinalis | 0.0 % ± 1.1 % | No Activity |
| Spirulina | Arthrospira platensis | 22.8 % ± 1.2 % | Weak Activity # |
| Stinging Nettle | Urtica dioica | 6.5 % ± 0.9 % | No Activity |
| Suma Root | Pfaffia paniculata | 0.5 % ± 1.2 % | No Activity |
| Tribulus Fruit | Tribulus terrestris | 0.3 % ± 0.7 % | No Activity |
| Turkey tail mushroom | Trametes versicolor | 2.6 % ± 0.9 % | No Activity |
| Watercress | Nasturtium officinale | 0.9 % ± 1.0 % | No Activity |
| Wormwood Herb | Artemesia Absinthium | 1.4 % ± 1.0 % | No Activity |
| Xia Ku Cao / Heal All | Prunella vulgaris spike | 1.3 % ± 0.9 % | No Activity |
| Yohimbe Bark | Pausinystalia johimbe | 1.3 % ± 2.9 % | No Activity |
| Yucca Root | Yucca schidigera | 1.6 % ± 1.2 % | No Activity |
| Zhe Bei Mu | Fritillaria thunbergii bulb | 2.3 % ± 1.2 % | No Activity |
| Zhu Ling | Polyporus umbellatus fungus | 4.6 % ± 1.5 % | No Activity |
| Zi Hua Di Ding | Viola yedoensis herb | 1.9 % ± 1.0 % | No Activity |
| Zi Wan | Aster Tataricus root | 1.5 % ± 1.1 % | No Activity |
Fig. 5A.

Centrifuge immunohistochemistry of gram-negative cell wall in kelp (Ascophyllum Nodosum) and bladderwrack (Fucus vesiculousus). The images represent auto fluorescence + cross-reactivity with the secondary antibody (goat anti-mouse Alexa 488) (A) kelp (C) bladderwrack (negative controls), with the addition of primary (mouse anti-LPS) (B) kelp (D) bladderwrack. Images were acquired using a digital imaging system, merging red, green, and blue (RGB) images.
Fig. 5B.

Capture antibody competitive ELISA of LPS in OTC Kelp prepared in sterile endotoxin free PBS. The data represent LPS concentration as reversed O. D. 450 nm and are expressed as the Mean +- S. E. M, n = 3. * Significance from the control using a one-way ANOVA p < 0.05.
4.3. Gram negative LPS pH stability – stomach model simulation
One of the main questions to the concept of this work, would be do edible MAMPS withstand stomach digest or acidity. A basic stomach model was created by examining a wide range of H + acid concentrations above and below the known concentration of HCl released from parietal cells in the stomach (0.16 N, pH 1–2) - range from 0.007 N to 2 N HCL and for 0–4 h (Fig. 6). LPS 5ug/ml was placed in varying acid solutions for 0–4 h, neutralized with NaOH, and retested in RAW 264.7 macrophages for bioactivity vs LPS control. We found no negative effects of LPS upon incubation with HCl up to 2 N, and<0.5 pH with an exposure time of 4 h.
Fig. 6.

LPS stability – using a stomach acidity model. LPS was incubated for 0 to 4 h in varying concentrations of hydrochloric acid, also evaluated for pH (left panel). After neutralization, solutions were tested for biological activity (right panel) on RAW 264.7 macrophages for nitric oxide production vs. LPS control. The data represents macrophage activation (% LPS control) for varying acidity and exposure time and is presented as the Mean ± S.E.M., n = 3. There was no significant loss of biological activity well beyond stomach acidity, with no effect for 4 h of incubation in 2 N HCL at < 0.5 pH.
4.4. Gram positive vs. Gram negative TLR4 activation
Comparable macrophage activity was assessed in response to gram-positive (B. Subtilis), gram-negative (E. Coli) (Fig. 7) and lipoteichoic acid. The latter was 134X more potent than peptidoglycan (PDG) and LPS was 1569X stronger than PDG.
Fig. 7.

Relative potency of gram-negative vs. gram-positive cell wall to activate RAW 264.7 macrophages. The data is expressed as the Mean ± S.E.M., n = 4 with significant differences from peptidoglycan (PDG) (gram-positive cell wall from B. Subtilis) for both Lipoteichoic Acid (gram-positive cell wall from B. Subtilis) and LPS (gram-negative cell wall from E.Coli) determined using a t-test *P < 0.01.
4.5. Capsule to culture - residual live reverse culture
All herbal botanical products pass a 3rd party testing (chain of custody) (Fig. 8) documented by a certificate of analysis for aerobic plate counts, (by the most stringent recommendation), not to exceed 104 colony forming units (CFU)/ gram, total yeasts and molds at < 103 CFU/ gram, total coliforms < 102 CFU/g with the absence of E. Coli / 10 g. Reverse culture of a few immune stimulating botanicals sold at OTC starting at [50 mg/mL herb] was carried out at 34 °C for one week, then diluted and sub-cultured for two weeks at 34 °C, then boiled and retested on macrophages where potency was magnified. Cultures were grown on standard agar and subsequently colony isolation using MacConkey agar for gram-negative presence. DNA was isolated and further subject to 16S amplicon and metagenomics shotgun sequencing to survey bacterial communities (Table 3).
Fig. 8.

Capsule to Culture Schematic: Prior to FAMU OTC/POS botanical library cataloging, manufacturers are required to test each lot/ batch using recommendations for finished products by a regulating authority, including the world health organization (WHO), NSF/ANSI standards, various pharmacopeia’s (European (E. P.) or United States (U.S.P.). A certificate of analysis is issued with information on passing criteria for residual live aerobic plate counts, coliform, yeast, molds, and absence of viable pathogens or toxic mold products. For capsule studies (1), upon receipt of the OTC/POS product, a library I.D. is assigned designating manufacturer, lot number, and botanical identity. (2) We chose 8 inflammatory herbs and tested these by reverse culture in sterile media (no / pen strep) supplemented with F.B.S. at 34◦ C. After three weeks of growth and subculture, samples were transferred to agar (3) to observe colony growth (4) and gram-negative presence (5). DNA from cultures were isolated, and sequencing to identify micro-organism communities (Table 3).
Table 3.
Taxonomical identity of reverse cultured OTC/ POS herbal products. Sequencing for both global colonies and gram negative isolation was carried out using ribosomal 18S, ITS1, 5.8 s, ITS2 28S and 16S rDNA sequence (1,542 bp) coding regions for species identification and community analysis. The data is listed by class, taxonomy, herb tested and abundance.
| Type | Description | Reverse Culture | Value* | Gram- | Gram-Value** |
|---|---|---|---|---|---|
| Gram-negative | s__Klebsiella_aerogenes | Stinging Nettle Root | 58.83 | POS | 54.91 |
| Fungus | s__uncultured_Glomeraceae | Stinging Nettle Root | 34.51 | NA | 14.01 |
| Gram-negative | s | Stinging Nettle Root | 29.12 | POS | 28.37 |
| Fungus | s__uncultured_Ascomycota | Stinging Nettle Root | 25.33 | NA | 2.75 |
| Fungus | s_Cryptococcus_sp._F19–3–1 | Stinging Nettle Root | 12.00 | NA | 0.00 |
| Fungus | s__Dothideomycetes_sp._LS-2013 g | Stinging Nettle Root | 9.82 | NA | 0.00 |
| NA | s__unclassified | Stinging Nettle Root | 5.20 | NA | 0.30 |
| Plant | s__Quercus_suber | Stinging Nettle Root | 4.16 | NA | 0.00 |
| Fungus | s__Aureobasidium_pullulans | Stinging Nettle Root | 3.73 | NA | 0.00 |
| Fungus | s__Naganishia_albida | Stinging Nettle Root | 2.56 | NA | 0.00 |
| Gram-negative | s__Gammaproteobacteria_unclassified | Stinging Nettle Root | 2.09 | POS | 0.71 |
| Gram-negative | s__uncultured_Enterobacter_sp. | Stinging Nettle Root | 1.30 | POS | 0.00 |
| Gram-Positive | s__Clostridium_sensu_stricto_18_unclassified | Stinging Nettle Root | 1.28 | NA | 0.02 |
| Fungus | s__Fissuroma_neoaggregatum | Stinging Nettle Root | 0.75 | NA | 0.00 |
| algal eukaryotes | s__Mallomonas_striata | Stinging Nettle Root | 0.74 | NA | 1.29 |
| Gram-negative | s__Enterobacter_sp. | Stinging Nettle Root | 0.70 | POS | 0.44 |
| Gram-negative | s__Enterobacter_hormaechei | Stinging Nettle Root | 0.60 | POS | 0.40 |
| Fungus | s__Sterigmatomyces_halophilus | Stinging Nettle Root | 0.57 | NA | 0.00 |
| Fungus | s__Malassezia_globosa | Stinging Nettle Root | 0.55 | NA | 0.00 |
| Gram-negative | s__Enterobacter_sp._GIST-NKst8 | Stinging Nettle Root | 0.53 | POS | 0.32 |
| Gram-negative | s__Atlantibacter_hermannii | Stinging Nettle Root | 0.46 | POS | 0.00 |
| Gram-negative | s__Enterobacter_sp._MZ_1 | Stinging Nettle Root | 0.37 | POS | 0.00 |
| Gram-negative | s__Pantoea_septica | Stinging Nettle Root | 0.23 | POS | 0.00 |
| Gram-negative | s__uncultured_Klebsiella_sp. | Stinging Nettle Root | 0.18 | POS | 0.00 |
| Gram-Positive | s__Enterobacteriaceae_unclassified | Stinging Nettle Root | 0.16 | POS | 0.00 |
| Gram-negative | s__Paenibacillus_sp._RAG-53 | Stinging Nettle Root | 0.16 | NA | 0.00 |
| Gram-negative | s__Cronobacter_sakazakii | Yucca Root | 84.02 | POS | 76.41 |
| Fungus | s__Irpex_lacteus | Yucca Root | 21.09 | NA | 0.01 |
| NA | s__unclassified | Yucca Root | 17.99 | NA | 33.70 |
| Fungus | s__Chlorociboria_clavula | Yucca Root | 16.22 | NA | 0.00 |
| Fungus | s__uncultured_Ascomycota | Yucca Root | 14.58 | NA | 0.00 |
| Fungus | s__uncultured_Glomeraceae | Yucca Root | 8.76 | NA | 0.01 |
| Fungus | s_Cryptococcus_sp._F19–3–1 | Yucca Root | 8.61 | NA | 0.00 |
| Fungus | s__Taphrina_pruni | Yucca Root | 6.70 | NA | 0.00 |
| Yeast | s__Meyerozyma_guilliermondii | Yucca Root | 6.17 | NA | 0.00 |
| Fungus | s__uncultured_Chytridiomycota | Yucca Root | 5.88 | NA | 0.00 |
| Fungus | s__Fusarium_verticillioides | Yucca Root | 5.32 | NA | 0.00 |
| Gram-negative | s__Cronobacter_malonaticus | Yucca Root | 2.66 | POS | 2.50 |
| Plant | s__Quercus_suber | Yucca Root | 2.61 | NA | 0.00 |
| Gram-negative | s__Pantoea_agglomerans | Corn Silk | 37.72 | POS | 46.64 |
| Fungus | s__Aspergillus_sp._JUC-2 | Corn Silk | 37.41 | NA | 0.00 |
| Spiromonas | s__Colpodella_angusta | Corn Silk | 23.43 | NA | 0.00 |
| Gram-negative | s__Klebsiella_aerogenes | Corn Silk | 20.10 | POS | 23.60 |
| NA | s__unclassified | Corn Silk | 19.19 | NA | 0.46 |
| Gram-negative | s__Enterobacter_cloacae | Corn Silk | 10.06 | POS | 12.74 |
| Gram-negative | s__Enterobacter_sp._VJ-6 | Corn Silk | 9.89 | POS | 12.63 |
| Gram-negative | s__Escherichia_sp._CPD32 | Corn Silk | 3.70 | POS | 0.03 |
| Gram-negative | s__uncultured_Enterobacter_sp. | Corn Silk | 2.53 | POS | 0.00 |
| Fungus | s__Penicillium_decumbens | Corn Silk | 1.99 | NA | 0.00 |
| Gram-negative | s__Enterobacter_sp._MZ_1 | Corn Silk | 1.76 | POS | 0.00 |
| Fungus | s__Malassezia_restricta | Corn Silk | 1.49 | NA | 0.00 |
| Gram-negative | s__uncultured_Klebsiella_sp. | Corn Silk | 1.38 | POS | 1.53 |
| Gram-negative | s__Enterobacter_sp. | Corn Silk | 1.16 | POS | 0.38 |
| Gram-negative | s__Atlantibacter_hermannii | Corn Silk | 1.03 | POS | 0.00 |
| Gram-negative | s__Gammaproteobacteria_unclassified | Corn Silk | 0.97 | POS | 0.42 |
| Gram-negative | s__Pantoea_septica | Corn Silk | 0.65 | POS | 0.00 |
| Gram-Positive | s__Bacillus_sp._IDA4921 | Corn Silk | 0.58 | NA | 0.00 |
| Gram-negative | s__Enterobacter_ludwigii | Corn Silk | 0.49 | POS | 0.61 |
| Gram-negative | s__Kosakonia_cowanii | Corn Silk | 0.44 | POS | 0.00 |
| Gram-negative | s__Enterobacter_sp._enrichment_culture_clone_HSL39 | Corn Silk | 0.39 | POS | 0.44 |
| Gram-negative | s__Enterobacter_sp._UIWRF0036 | Corn Silk | 0.35 | POS | 0.00 |
| Gram-negative | s__Enterobacteriaceae_unclassified | Corn Silk | 0.35 | POS | 0.00 |
| Gram-Positive | s__Lactobacillus_crispatus | Corn Silk | 0.22 | NA | 0.00 |
| Gram-Positive | s__Enterobacter_asburiae | Corn Silk | 0.21 | NA | 0.31 |
| Type | Description | Reverse culture | Value* | Gram- | |
| Gram-Positive | s__Fontibacillus_unclassified | Black Walnut Hull | 44.24 | NA | |
| Gram-Positive | s__Paenibacillus_unclassified | Black Walnut Hull | 37.04 | NA | |
| Fungus | s__Aureobasidium_pullulans | Black Walnut Hull | 27.17 | NA | |
| Fungus | s__Aspergillus_sp._JUC-2 | Black Walnut Hull | 21.90 | NA | |
| Fungus | s__Malassezia_sympodialis | Black Walnut Hull | 13.27 | NA | |
| NA | s__unclassified | Black Walnut Hull | 11.73 | NA | |
| Fungus | s__Irpex_lacteus | Black Walnut Hull | 10.12 | NA | |
| Gram-Positive | s__Bacillus_sp._IDA4921 | Black Walnut Hull | 4.94 | NA | |
| Fungus | s__Cladosporium_sphaerospermum | Black Walnut Hull | 4.31 | NA | |
| Gram-negative | s__Anaerospora_unclassified | Black Walnut Hull | 3.38 | POS | |
| Gram-Positive | s__Bacillus_thermoamylovorans | Black Walnut Hull | 2.98 | NA | |
| Gram-Positive | s__Geobacillus_thermoleovorans | Black Walnut Hull | 1.31 | NA | |
| Gram-Positive | s__uncultured_Paenibacillus_sp. | Black Walnut Hull | 1.00 | NA | |
| Gram-Positive | s__uncultured_Bacillus_sp. | Black Walnut Hull | 0.81 | NA | |
| Gram-Positive | s__Paenibacillus_yonginensis | Black Walnut Hull | 0.57 | NA | |
| Gram-Positive | s__Fontibacillus_phaseoli | Black Walnut Hull | 0.36 | NA | |
| Gram-Positive | s__Enterococcus_gallinarum | Burdock_Root | 73.12 | NA | |
| Fungus | s__uncultured_Glomeraceae | Burdock_Root | 46.41 | NA | |
| Fungus | s__Fibroporia_albicans | Burdock_Root | 21.61 | NA | |
| Yeast | s__Rhodotorula_sp._CH4 | Burdock_Root | 17.85 | NA | |
| Mite | s__Demodex_folliculorum | Burdock_Root | 9.66 | NA | |
| Gram-Positive | s__Bacillus_sp._IDA4921 | Burdock_Root | 4.06 | NA | |
| Gram-Positive | s__Enterococcus_faecium | Burdock_Root | 3.51 | NA | |
| Algal eukaryotes | s__Mallomonas_striata | Burdock_Root | 1.25 | NA | |
| Gram-Positive | s__bromate-reducing_bacterium_B6 | Burdock_Root | 1.15 | NA | |
| Gram-negative | s__Bilophila_unclassified | Burdock_Root | 0.95 | POS | |
| Gram-Positive | s__Pediococcus_acidilactici | Burdock_Root | 0.74 | NA | |
| Gram-Positive | s__Bacillus_proteolyticus | Burdock_Root | 0.36 | NA | |
| Gram-Positive | s__uncultured_Enterococcus_sp. | Burdock_Root | 0.20 | NA | |
| Gram-Positive | s__Enterococcus_sp._MA3 | Burdock_Root | 0.17 | NA | |
| NA | s__unclassified | Echinacea Root | 37.34 | NA | |
| Fungus | s__uncultured_Glomeraceae | Echinacea Root | 36.53 | NA | |
| Gram-Positive | s__bromate-reducing_bacterium_B6 | Echinacea Root | 18.76 | NA | |
| Gram-Positive | s__Clostridium_sensu_stricto_7_unclassified | Echinacea Root | 12.50 | NA | |
| Gram-Positive | s__Lachnotalea_unclassified | Echinacea Root | 7.78 | NA | |
| Gram-Positive | s__Lysinibacillus_unclassified | Echinacea Root | 7.59 | NA | |
| Gram-Positive | s__Clostridium_sensu_stricto_18_unclassified | Echinacea Root | 7.34 | NA | |
| Fungus | s__Bjerkandera_sp._CPCC_480726 | Echinacea Root | 7.33 | NA | |
| Gram-Positive | s__Clostridium_sensu_stricto_3_unclassified | Echinacea Root | 7.03 | NA | |
| Fungus | s__Malassezia_sympodialis | Echinacea Root | 6.80 | NA | |
| Gram-Positive | s__Clostridium_sp._K7 | Echinacea Root | 3.84 | NA | |
| Fungus | s__Aspergillus_sp._JUC-2 | Echinacea Root | 3.83 | NA | |
| Plant | s__Quercus_suber | Echinacea Root | 3.66 | NA | |
| Gram-Positive | s_Eubacterium]_fissicatena_group_unclassified | Echinacea Root | 2.93 | NA | |
| Gram-Positive | s__Clostridium_sensu_stricto_1_unclassified | Echinacea Root | 2.73 | NA | |
| Fungus | s__Chlorociboria_clavula | Echinacea Root | 2.54 | NA | |
| Gram-Positive | s__Clostridiaceae_unclassified | Echinacea Root | 2.14 | NA | |
| Gram-Positive | s__Clostridium_sp._BTY5 | Echinacea Root | 1.82 | NA | |
| Gram-Positive | s__uncultured_Bacillus_sp. | Echinacea Root | 1.42 | NA | |
| Probiotic Bacteria | s__Lachnospiraceae_unclassified | Echinacea Root | 1.26 | NA | |
| Probiotic Bacteria | s__Caproiciproducens_unclassified | Echinacea Root | 1.26 | NA | |
| Gram-Positive | s__Clostridium_sp._Iso-A2 | Echinacea Root | 0.99 | NA | |
| Probiotic Bacteria | s__Faecalicatena_contorta | Echinacea Root | 0.89 | NA | |
| Gram-Positive | s__Clostridium]_amygdalinum | Echinacea Root | 0.61 | NA | |
| Gram-Positive | s__Clostridium_sensu_stricto_10_unclassified | Echinacea Root | 0.51 | NA | |
| Gram-Positive | s__Clostridium]_xylanolyticum | Echinacea Root | 0.48 | NA | |
| Gram-Positive | s__Bacillus_cereus | Echinacea Root | 0.38 | NA | |
| Gram-Positive | s__Paenibacillus_unclassified | Kava Kava Root | 50.23 | NA | |
| Fungus | s__uncultured_Glomeraceae | Kava Kava Root | 49.20 | NA | |
| Fungus | s__Fibroporia_albicans | Kava Kava Root | 23.00 | NA | |
| Gram-Positive | s__Bacillus_thermoamylovorans | Kava Kava Root | 16.59 | NA | |
| Fungus | s__Malassezia_restricta | Kava Kava Root | 16.23 | NA | |
| Gram-Positive | s__Fontibacillus_unclassified | Kava Kava Root | 10.56 | NA | |
| Fungus | s__Auricularia_polytricha | Kava Kava Root | 8.07 | NA | |
| Fungus | s__Chlorociboria_clavula | Kava Kava Root | 6.59 | NA | |
| Gram-Positive | s__uncultured_Bacillus_sp. | Kava Kava Root | 4.35 | NA | |
| Gram-Positive | s__Geobacillus_thermoleovorans | Kava Kava Root | 2.42 | NA | |
| single cell eukaryotes | s__Colpoda_steinii | Kava Kava Root | 2.40 | NA | |
| Yeast | s__Meyerozyma_guilliermondii | Kava Kava Root | 2.39 | NA | |
| Gram-Positive | s__bromate-reducing_bacterium_B6 | Kava Kava Root | 2.37 | NA | |
| Gram-Positive | s__Bacillus_sp._IDA4921 | Kava Kava Root | 0.96 | NA | |
| Gram-Positive | s__Bacillus_sp._SAB | Kava Kava Root | 0.88 | NA | |
| plant | s__Ginkgo_biloba | Kava Kava Root | 0.86 | NA | |
| Gram-Positive | s__Paenibacillus_sp. | Kava Kava Root | 0.80 | NA | |
| Gram-Positive | s__Enterococcus_faecium | Kava Kava Root | 0.22 | NA | |
| Gram-Positive | s__Ligilactobacillus_unclassified | Kava Kava Root | 0.21 | NA | |
| Gram-Positive | s__Clostridium_sp._KOPRI80182 | Seamoss | 51.95 | NA | |
| NA | s__unclassified | Seamoss | 33.95 | NA | |
| Fungus | s__uncultured_Glomeraceae | Seamoss | 33.80 | NA | |
| Gram-Positive | s__Clostridium_sensu_stricto_1_unclassified | Seamoss | 19.46 | NA | |
| Fungus | s__Aspergillus_sp._JUC-2 | Seamoss | 11.57 | NA | |
| Gram-Positive | s__bromate-reducing_bacterium_B6 | Seamoss | 7.65 | NA | |
| Fungus | s__Bjerkandera_sp._CPCC_480726 | Seamoss | 6.22 | NA | |
| Fungus | s__Cryptococcus_sp._F19–3–1 | Seamoss | 4.54 | NA | |
| Gram-Positive | s__Bacillus_thermoamylovorans | Seamoss | 4.36 | NA | |
| Gram-Positive | s__Clostridium_sensu_stricto_18_unclassified | Seamoss | 3.53 | NA | |
| Fungus | s__Ceriporiopsis_sp._s12–2 | Seamoss | 3.40 | NA | |
| Gram-Positive | s__Clostridium_sensu_stricto_12_unclassified | Seamoss | 2.47 | NA | |
| Gram-Positive | s__uncultured_Bacillus_sp. | Seamoss | 2.00 | NA | |
| Gram-Positive | s__Clostridium_sp._JC242 | Seamoss | 0.97 | NA | |
| Gram-Positive | s__Bacillus_sp._CanS-96 | Seamoss | 0.73 | NA | |
| Gram-Positive | s__Bacillus_cereus | Seamoss | 0.66 | NA | |
| Gram-Positive | s__Bacillus_sp._A17 | Seamoss | 0.65 | NA | |
| Gram-Positive | s__Clostridium]_amygdalinum | Seamoss | 0.21 | NA | |
Table 3 provides a list of microbial communities by reverse culture, which alone invoked macrophage activation, after boiling (Fig. 10). The data here are very rudimentary where we spot-tested herbs such as stinging nettle, corn silk, and yucca root which tested positive for gram-negative cell wall (Fig. 9), where TLR4 macrophage activation to produce [below 12 ng /mL] occurred greatest in LPS laden herbs in addition to black walnut hull (Fig. 10). The most dominant microorganisms in capsule-to-culture reverse culture were as follows:
Fig. 10.

Bioactivity of heat inactivated reverse cultured plant microbiomes. The data are presented as macrophage activation % LPS E.Coli 0111:B4, represented by the Mean ± S.E.M., n-4. Significant differences from the control were determined by a one-way ANOVA, with post hoc Tukey test, *P < 0.05.
Fig. 9.

Representative macroscopic images of agar plates incubated at 37 ◦C for 24 h in nutrient broth agar (NB agar) or 48 h (McConkey agar) isolated from yucca root, stinging nettle, and cornsilk. A) Microbial aerobic growth on NB agar (whole or left half) and B) Gram-negative bacteria growth on MacConkey agar if present (right half) are shown.
Stinging Nettle root [Gram negative/fungus] (Klebseilla aerogenes, Pantoea agglomerans, Gomeracea, Ascomycota, Cryptococcus sp., Dothiedeomycetes sp.),
Yucca root [Gram negatives/fungus] (Cronobacter sakazakii, Irpex lacteus, Chlorociboria clavula, Gomeracea, Ascomycota, Crytptococcus sp. Taphrina pruni).
Corn Silk [Gram negative/fungus/spiromonas] (Pantoea agglomerans, Klebseilla aerogenes, Enterobacter cloacae, Enterobacter sp. Aspergillus_sp, and colpodella angusta),
Black Walnut Hull [Gram Positive/ Fungus] (Fontibacillus, Paenibacillus, Auerobasidium pullulans, Aspeprgillus sp, Malassezia sympodialis, Irpex lacteus).
Echinacea Root [Gram Positive/ Fungus] (Gomeraceae, bromate-reducing bacterium B6, Clostridium sensu stricto 7,18 and 3_unclassified) Lachnotalea, Lysinibacillus).
Kava Kava Root [Gram Positive/ Fungus] (Paenibacillus, Glomeraceae, Fibroporia albicans, Bacillus thermoamylovorans, Malassezia restricta, Fontibacillus, Auricularia polytricha, Chlorociboria clavula).
Seamoss [Gram Positive/ Fungus ] (Clostridium_sp KOPRI80182, Gomeraceae, Clostridium sensu stricto 1, Aspergillus sp. JUC-2, bromate reducing bacterium B6,Bjerkandera sp. CPCC 480726).
Future studies will have to be conducted on many fronts to further investigate the nature of the therapeutic immune modulating plant microbiome.
5. Discussion
Regarding plant-based medicinal research, as seemingly correct, the plant itself and its constituent phytochemicals have received most of the attention over the past century. Whereas, the plant microbiome has received most attention in the study of agriculture in pursuit of understanding MOC roles in ecological balance of soil, plant, water, and air systems (Diedhiou et al., 2016; Shahid and Khan, 2022), with meager research as to impact on human health. Food based MOC debris is by no means ubiquitous in concentration or taxonomy, and it is ever present in all processed foods, beverages, and even bottled drinking water (tap, bottled, mineral, and natural water), to exception of that treated by reverse osmosis. (Tischner et al., 2021; Vasudevan et al., 2021; Zhou et al., 2021). Given that our entire diet from the natural world involves ingestion of MOCS (Irshad et al., 2022; Jiang et al., 2021; Soto-Giron et al., 2021) some which are intentionally added (generally recognized as safe (GRAS)) for fermentation (Chen et al., 2019; Tang et al., 2021; Voidarou et al., 2020; Wang et al., 2017; Yan et al., 2022) there is a pressing need to assess, these bioactive entities in herbs and spices (in particular) for impact on human immune function (F et al., 2022; Neuzil-Bunesova et al., 2022; Ssepuuya et al., 2019).
In this work, we focus on botanicals and related OTC supplements used by consumers world wide which are certified to be devoid of live food borne pathogens (Finger et al., 2021; Karam et al., 2021; Levy et al., 2015; Willis et al., 2015) and contain threshold limits for live aerobic bacteria counts (APC) (Feroz et al., 2016; Garbowska et al., 2015), yeasts, and molds. However, this only describes industry compliance and according to reports on actual shelf products (herbs/spices) pulled, 92.1 % contain live, unidentified bacteria, and 5.3 % have counts that are beyond acceptable APC levels (>100,000 CFU/g), the majority of which are unidentified fungi, mold, or yeasts (Dlugaszewska et al., 2019). Due to the antibacterial properties of the plant/parts, such as those found in thyme, there is great disparitiy between low CFUs/g (eg. 2,100 CFU/g) or oregano, 1,500 CFU/g, to extremely high counts found in paprika (100,000 CFU/g) and pepper (820,000 CFU/g) (Schwab et al., 1982). Again our results confirmed these findings showing extremely high MAMP loads found in paprika for 7 / 8 manufacturers examined globally, and low to no bioactive MAMP content in thyme, oregano or other antibacterial leaf based products.
Our findings, also align with research reporting high levels of gram-negative LPS found in wheat (Mizuno and Soma, 1992; Nishizawa et al., 1992), black walnut, echinacea root, ginseng root, alfalfa seed (Pugh et al., 2008), astragalus root (Denzler et al., 2010; Koehler et al., 2020), the immune boosting herb Juzen-taiho-to (Montenegro et al., 2015) and kelp. (Inagawa et al., 1992a) LPS in wheat flour (LPSw) from Pantoea agglomerans reportedly bestows ameliorative effects on diverse health ailments such as diabetes (Iguchi et al., 1992), ulcer (Inagawa et al., 1992b) and hyperlipidemia (Kobayashi et al., 2018; Mizobuchi et al., 2021; Okutomi et al., 1992).
While some suggest LPS to be therapeutic, the term LPS as endotoxin has a negative connotation. In fact, “endotoxin” is a single derogatory term used to describe cell wall from all gram negative species, despite concentration, or its association with deadly sepsis, health benefits when consumed as spirulina LPS (gram negative rod) or as a therapeutic immune booster vaccine adjuvant. (Luchner et al., 2021) In 1856, Peter Ludvig Panum first referred to LPS as “putrid poison”, which was later termed endotoxin by Richard Friedrich Johannes Pfeiffer in association with Cholera, followed by its chemical extraction from Salmonella typhimurium in 1933 (Kolmos, 2006; Ogawa et al., 2007) to elucidation of its structure. LPS contains 1) a terminal O-antigen hydrophilic polysaccharides (serotype strain) 2) a leading lipid A region (anchor) linked to 3) a core region by 3-deoxy-d-manno-2-octulosonic acid (Kdo) with L- glycero-D-manno-heptose (l,d-Hep) and hexoses and hexosamines (Kong et al., 2004; Mazgaeen and Gurung, 2020). In 1952, Westphal and Lüderitz developed a hot phenol method for LPS purification / extraction from gram negative bacteria and in 1954, identified lipid A as the immune active endotoxic substance, (Osborn, 2019) later classified as a PAMP capable of activating human TLR4 PPR receptors. (Gauthier et al., 2022; Mazgaeen and Gurung, 2020) The ability of LPS to interact with PRRs is directly related to its structure and can differ in its endotoxic potential based on the fatty acid composition of the lipid A structure and /or the O-antigens (lacking) rough vs. rich in O-antigen (smooth). See Review: (Mazgaeen and Gurung, 2020). Meanwhile, humans have been ingesting LPS (e.g. spirulina (Arthrospira platensis) a gram negative rod ) (Besednova et al., 1979) for over five centuries to boost human immune resilience.
5.1. Bacterial LPS and immune response
The use of bacterial MAMPS (including LPS) as an immune therapy to treat human cancers has an extensive history, dating back to the late 1800s, where cancer patients experienced curative remission after contracting acute febrile infection with Streptococcus pyogenes (Carlson et al., 2020; Okuyama et al., 2017). There after, cancers were treated with an “attenuated” mixture of Streptococcus pyogenes (gram positive peptidoglycan) with Serratia marcescens (gram negative LPS) evoking curative remission in approximately 50 % of mesodermal embryonic origin cancers (sarcoma, lymphoma, leukemia, kidney, ovarian etc (Hobohm, 2001; Hoption Cann et al., 2003; McCarthy, 2006). Today, immune-suppressive medicines are main line drugs often used to treat cancer, making infection a leading cause of death of cancer patients. Meanwhile the scientific community is attempting to renovate rediscovery in anti-tumor immunotherapies.
Nevertheless, the study of LPS and its role in cancer yields contradiction within the literature. However, data is consistent when aligned to use of models, end points, and route of administration. On the one hand, studies using LPS in immunocompetent, immune-system-intact animals show inoculation regimes are quite effective in reducing tumor burden (ovarian, liver, glioma etc.) (Ignacio et al., 2019; Okuyama et al., 2017; Won et al., 2003), coinciding with significant changes in boosting of immune function (Chicoine et al., 2001; Chicoine et al., 2007). LPS has also been shown to lessen the side effects of antibiotics and chemotherapies ((e.g., LPS or gram-negative microbes (e. g., Alistipes shahii) (Soma and Inagawa, 2015) and oral administration e. g. (Pantoea agglomerans) establishes complete recovery and remission in 62 % of patients (Morishima and Inagawa, 2016). Likewise , clinical trials using synthetic LPS Lipid A subunits show tumor reduction (such as ONO-4007, DT 5461), not withstanding tolerance which becomes a limiting therapeutic issue (Matsumoto et al., 1998; Matsushita et al., 2003). Alternatively, research establashing LPS as pro-tumor largely employimmune deficient models such as nude mice, cancer cells without an immune co-culture, or by general expression profile that shows TLR4 to be abundant in malignant tumor tissue relative to normal.
5.2. Marine plants and gram negative LPS
The oceans are teeming with plants that have been invaded by microbial communities that share common living quarters, being essential for plant growth (Ramirez-Puebla et al., 2022), preservation of aquatic ecosystems, natural recycling, plant immunity (Weinberger, 2007) and with greater accrual in aged plants (Lemay et al., 2021). The data in this study are in direct alignment with existing marine biology studies such as that on Chlorella (Chlorella vulgaris), Spirulina, Dulse (Palmaria palmate), Irish Moss (Chondrus crispus), Seamoss (combination of Irish Moss and Bladderwrack (Fucus vesiculosus) all showing a high presence of gram negative microbes (and thus LPS). (Miranda et al., 2022; Weigel and Pfister, 2021). Other reports show high gram negative presence in the sea kelps (P. scouleri Laminaria setchellii, N. luetkeana, Chondrus crispus, Laminaria ochroleuca, Palmaria palmate etc.) Granulosicoccus antarcticus, hellea balneolensis (Miranda et al., 2022; Weigel and Pfister, 2021) which reside amongst hundreds of bacterial strains rich in Bacillaceae and Enterobacteriaceae.(Del Olmo et al., 2018; Picon et al., 2021) The same is true for coastal ocean sea grasses, with gram-negative biofilms, dominated by Granulosicoccus sp. (Gammaproteobacteria) bacteroidetes and Alphaproteobacteria and coincide with efficacy of brown seaweeds such as bladderwrack (Fucus vesiculosus) which also bestow tumor suppressive effects (Gupta et al., 2020), on ovarian (Bae et al., 2020) estrogen-dependent cancers (Zhang et al., 2016) head and neck (Blaszczak et al., 2018), thyroid (Shen et al., 2017) and pancreatic cancers (Geisen et al., 2015). These studies all show a pattern by which medicinal use of sea plants can evoke stimulation of natural killer (NK) cells (Zhang et al., 2021b) or greater peripheral blood mononuclear cell counts (Park et al., 2021). These heavily laden microbial reservoirs have been consumed for centuries, with a plethora of recent research showing the same to instill anti-tumor immunity (Ishiguro et al., 2020; Skjanes et al., 2021) in various cancers including colon, breast (Yuan and Walsh, 2006), hormone-related (Skibola et al., 2005) and thyroid (Rosen et al., 2014). For instance, chlorella attenuates tumor growth in animal models (Kubatka et al., 2015) which coincides with increased natural killer cell (NK) activity, macrophage activation and proliferative lymphocytic capacity (Kim et al., 2022). Similar, spirulina, a gram-negative microbe renders LPS that increases phagocytosis and cytokine-mediated inducible nitric oxide production, while enhancing immunity against cancer and infection (Al-Batshan et al., 2001). These investigations are generally in line with reports that certain sea kelps and seaweeds will reduce the risk of developing malignancies of the biliary tract, the prostate, or the breast (Nelson et al., 2017), prostate (Ohno et al., 1988) or breast cancers, reports which represent the foundation of traditional Japanese cultural dietary practices.
Roots
The results reported in this work are also in alignment with agricultural studies pertaining to the root bed in plants showing heavy presence of certain microbes “seed to rhizo-micro-biomes”(Neemisha et al., 2022; Orozco-Mosqueda et al., 2022) which dominate plant growth (Chang et al., 2022; Zhang et al., 2020), rigor, fitness, and crop yield (Abdullaeva et al., 2021) Koprivova and Kopriva, 2022; Li et al., 2022). Microbial communities residing in roots are diverse, some including non – pathogenic B. cereus group, and spp of Enterobacter, Pseudomonas, Cronobacter, with those close to water having the greatest diversity (Sun et al., 2019). In this study, we demonstrate that the roots with the highest MAMP content were: Tou Weng: English Pulsatilla Root (Pulsatillae Radix), Black Cohosh Root (Cimicifuga racemose), Burdock Root (Arctium lappa), Butchers Broom Root (Ruscus aculeatus), Calamus Root (Acorus calamus), Cao Wu (Zhi): English Wild Aconite Root (Aconitum kusnezoffii), Dandelion Root (Taraxacum officinale), Echinacea Root (Echinacea purpurea), Feng Fang Root (Radix Saposhnikoviae) Hydrangea Root (Hydrangea arborescens), Kava Kava Root (Piper methysticum), Lou Lu/Rhaponticum Root (Rhaponticum Uniflorum), Nettle Root (Urtica dioica), Pleurisy Root (Asclepias tuberosa), Poke Root (Phytolacca Americana), Qian Cao/Madder Root (Radix Rubiae), Sasparilla (Smilax medica), Sheng Jiang Pi (Zingiber Officinale), Soapwort root (Saponasia officinalis) and Suma Root (Pfaffia paniculata).
The study of plant lignans and phytochemicals, which are thought to be the cause of these effects, has dominated research on the therapeutic benefits of roots up until this point. While effects of these products on “ immune boosting’ have been reported extensively (e.g Burdock root (Chan et al., 2011; He et al., 2018; Suzuki et al., 2002; Wang and Yang, 1993), Essiac (1998) Kava Kava Root (Minh et al., 2022; Pont-Fernandez et al., 2022), Butchers broom (Masullo et al., 2016; Rodrigues et al., 2021) Dandelion Root (Ren et al., 2022; Talapphet et al., 2021) and Pleurisy Root (Warashina and Noro, 2010), the plants microbiome has not been investigated in the way that would be most appropriate. Further study into relevance of the microbiome of plant will likely unveil it to be a most significant influencer of immune system response.
6. Important considerations
6.1. Microbiome presence in botanical medicinal research; false artifact
When conducting experiments in vitro on botanical bioactive products, a plant’s anti-inflammatory phytochemicals could be masked as false negatives by an overwhelming presence of MAMPs/LPS. Furthermore, the majority of research carried out on plant extracts do not account for the potential interfering variable comprising the plants inactive microbiome, where MAMPS could reside in consirable concentrations, having amphipathic properties, some heat- and pH-stable. These substances are rarely, validated, tested for, or removed during studies investigating physiological effects of plant medicines. This is not the case for phytochemicals, which are often manufactured for cell culture in endotoxin free form. In other words, it would be necessary to completely remove the plant’s microbiome in order to demonstrate that the plant is operating on a biological target of its own merit.
6.2. Chronic inflammation
A second important issue involves possible consequences of routine daily ingestion/ exposure to MAMP laden products. . Long-term use of MAMP-containing goods may cause chronic inflammation, which may fatigue the immune system, upregulate checkpoints, and increase the body’s ability to start carcinogen-mediated carcinogenesis. (Di et al., 2021; Li et al., 2015; Liu et al., 2021). Chronic inflammation, on the other hand, is the root cause of a wide range of human diseases, including auto-immune disorders, osteoporosis, and degenerative joint disease (Mai et al., 2013). These diseases can be significantly reduced by consuming anti-inflammatory plant phytochemicals on a daily basis, which are of great value (Chen et al., 2018; Coutinho-Wolino et al., 2022). Because of this, a lot more research will be needed to understand the long-term health effects of ingesting oral MAMP products, including beta-glucans or probiotics and over an extended period of time.
7. Conclusion
There exists a lack of festidious research efforts on the immune-modulating edible plant based microbiome. As a result, this work serves as a pilot study to offer initial insights and provide direction for future research projects that aim to investigate the relevance of the herbal microbiome as a separate entity of functional foods, which influences innate and acquired immunity as a means of “immune boosting”. Further exploration is required in this area some including the following goals; 1) A larger investigation involving recording thousands of products,and comparisons as to plant’s MOC taxonomy according to its place of cultivated origin 2) To establish a rapid valid methodology to identify structural motifes in MAMP antigens to identify pre-existing live taxonomic species associated with those molecules 3) To ascertain the biological effects of main classes of individual re-cultured bioactive strains that have been inactivated and how these act on human micro-biome signaling in the gut and, at what concentrations and 4) To continue forward tracing and identification of bioactive microbial communities during all stages of plant growth from field to harvesting, collections, storage prior to food processing, and point of sale with regard to immune activation.
Additionally, research should be concentrated on figuring out how the major classes of individual re-cultured bioactive strains, both inactivated and activated, affect the human gut microbiome in terms of signaling, concentration, and their use to treat various pathological conditions. The ultimate objective is to determine whether these bioactive strains have the potential to be used as ingestible immunemodulating antigenic-based supplements or medications that can affect long-term acquired immunity in humans. Without the necessity for the plant to serve as an inert reservoir, it could be conceivable to nurture and develop these microorganisms in a bioreactor, kill them, and use antigenic-based supplements or medications to modify human innate and acquired immunity.
Acknowledgements
This research was supported by the National Institute of Minority Health and Health Disparities of the National Institutes of Health through Grant Number U54 MD 007582.
Abbreviations:
- APC
antigen presenting cells
- ASV
amplicon sequence variants
- CCL
(C-C motif) ligand
- CFU
colony forming units
- CXCL
chemokine (C-X-C motif) ligand
- DADA2
divisive amplicon denoising algorithm
- DMEM
dulbecco’s modified eagle medium
- DMSO
dimethyl Sulfoxide
- GALT
gut-associated lymphoid tissue
- HBSS
hank’s balance salt solution
- HCl
hydrochloric acid
- HEPES
N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid
- HTS
high throughput screening
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LAL
limulus amebocyte lysate
- LPS
lipopolysaccharide
- MAMPs
microbial associated molecular pattern
- MyD88
myeloid differentiation primary response gene 88
- NaOh
sodium hydroxide
- NK
natural killer
- NO2–
nitric oxide
- OTC
over the counter
- PAMPs
pathogen associated molecular patterns
- PBS
phosphate buffered saline
- POLYIC
polyinosinic-polycytidylic acid
- POS
point of sale
- PRR
pathogen/pattern recognition receptors
- TAM
tumor associated macrophage
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- WHO
World Health Organization
Footnotes
CRediT authorship contribution statement
E. Mazzio: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. A. Barnes: Data curation, Formal analysis, Investigation, Methodology. R. Badisa: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. G. Fierros- Romero: Data curation, Formal analysis, Methodology, Resources. H. Williams: Data curation, Formal analysis, Methodology, Resources. S. Council: Visualization, Writing – original draft, Writing – review & editing. K.F.A. Soliman: .
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Statement.
(1) This material is the authors’ own original work, which has not been previously published elsewhere.
(2) The paper is not currently being considered for publication elsewhere.
(3) The paper reflects the authors’ own research and analysis in a truthful and complete manner.
(4) The paper properly credits the meaningful contributions of co-authors and co-researchers.
The results are appropriately placed in the context of prior and existing research.
This research did not involve live animals or humans.
Data availability
No data was used for the research described in the article.
References
- Abdullaeva Y, Ambika Manirajan B, Honermeier B, Schnell S, & Cardinale M (2021). Domestication affects the composition, diversity, and co-occurrence of the cereal seed microbiota. Journal of Advanced Research, 31, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abera S, Shimels M, Tessema T, Raaijmakers JM, Dini-Andreote F, 2022. Back to the roots: defining the core microbiome of Sorghum bicolor in agricultural field soils from the centre of origin. FEMS Microbiology Ecology, 98. [DOI] [PubMed] [Google Scholar]
- Agarussi MCN, Pereira OG, Pimentel FE, Azevedo CF, da Silva VP, FF ES, 2022. Microbiome of rehydrated corn and sorghum grain silages treated with microbial inoculants in different fermentation periods. Scientific Reports 12, 16864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Batshan HA, Al-Mufarrej SI, Al-Homaidan AA, & Qureshi MA (2001). Enhancement of chicken macrophage phagocytic function and nitrite production by dietary Spirulina platensis. Immunopharmacology and Immunotoxicology, 23, 281–289. [DOI] [PubMed] [Google Scholar]
- Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, & Al-Mssallem MQ (2022). Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeituni SH, Ding C, Liu M, Hu X, Luo F, Kloecker G, … Yan J (2016). Yeast-Derived Particulate beta-Glucan Treatment Subverts the Suppression of Myeloid-Derived Suppressor Cells (MDSC) by Inducing Polymorphonuclear MDSC Apoptosis and Monocytic MDSC Differentiation to APC in Cancer. Journal of Immunology, 196, 2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziman N, Abdullah N, Noor ZM, Kamarudin WS, & Zulkifli KS (2014). Phytochemical profiles and antimicrobial activity of aromatic Malaysian herb extracts against food-borne pathogenic and food spoilage microorganisms. Journal of Food Science, 79, M583–M592. [DOI] [PubMed] [Google Scholar]
- Bae H, Lee JY, Yang C, Song G, & Lim W (2020). Fucoidan Derived from Fucus vesiculosus Inhibits the Development of Human Ovarian Cancer via the Disturbance of Calcium Homeostasis, Endoplasmic Reticulum Stress, and Angiogenesis. Marine Drugs, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Stoll D, Schulz P, Kulling S, & Huch M (2019). Microbial Contamination of Organically and Conventionally Produced Fresh Vegetable Salads and Herbs from Retail Markets in Southwest Germany. Foodborne Pathogens and Disease, 16, 269–275. [DOI] [PubMed] [Google Scholar]
- Berihu M, Somera TS, Malik A, Medina S, Piombo E, Tal O, … Freilich S (2023). A framework for the targeted recruitment of crop-beneficial soil taxa based on network analysis of metagenomics data. Microbiome, 11, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besednova NN, Smolina TP, Mikheiskaia LV, & Ovodova RG (1979). Immunostimulating activity of the lipopolysaccharides of blue-green algae. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii, 75–79. [PubMed] [Google Scholar]
- Blaszczak W, Lach MS, Barczak W, & Suchorska WM (2018). Fucoidan Exerts Anticancer Effects Against Head and Neck Squamous Cell Carcinoma In Vitro. Molecules, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RD, Flickinger JC Jr., Snook AE, 2020. Talkin’ Toxins: From Coley’s to Modern Cancer Immunotherapy. Toxins (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal R, Nanda A, Akkol EK, Sobarzo-Sanchez E, Arya A, Kaushik D, Dutt R, Bhardwaj R, Rahman MH, Mittal V, 2021. Ageratum conyzoides L. and Its Secondary Metabolites in the Management of Different Fungal Pathogens. Molecules 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YS, Cheng LN, Wu JH, Chan E, Kwan YW, Lee SM, … Chan SW (2011). A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology, 19, 245–254. [DOI] [PubMed] [Google Scholar]
- Chang J, van Veen JA, Tian C, & Kuramae EE (2022). A review on the impact of domestication of the rhizosphere of grain crops and a perspective on the potential role of the rhizosphere microbial community for sustainable rice crop production. The Science of the Total Environment, 842, Article 156706. [DOI] [PubMed] [Google Scholar]
- Chauhan V, & Kanwar SS (2021). Lipopeptide(s) associated with human microbiome as potent cancer drug. Seminars in Cancer Biology, 70, 128–133. [DOI] [PubMed] [Google Scholar]
- Chen CY, Kao CL, & Liu CM (2018). The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. International Journal of Molecular Sciences, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Su JY, Yang CY, 2022. Ultrasound-Assisted Aqueous Extraction of Chlorogenic Acid and Cynarin with the Impact of Inulin from Burdock (Arctium lappa L.) Roots. Antioxidants (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Liu XW, Huang JL, Baloch S, Xu X, & Pei XF (2019). Microbial diversity and chemical analysis of Shuidouchi, traditional Chinese fermented soybean. Food Research International, 116, 1289–1297. [DOI] [PubMed] [Google Scholar]
- Chicoine E.K. MR Won MC Zahner Intratumoral injection of lipopolysaccharide causes regression of subcutaneously implanted mouse glioblastoma multiforme Neurosurgery 48, 607–614 2001. discussion 614–605. [DOI] [PubMed] [Google Scholar]
- Chicoine MR, Zahner M, Won EK, Kalra RR, Kitamura T, Perry A, & Higashikubo R (2007). The in vivo antitumoral effects of lipopolysaccharide against glioblastoma multiforme are mediated in part by Toll-like receptor 4. Neurosurgery, 60, 372–380. discussion 381. [DOI] [PubMed] [Google Scholar]
- Choi HA, Cheong DE, Lim HD, Kim WH, Ham MH, Oh MH, … Kim GJ (2017). Antimicrobial and Anti-Biofilm Activities of the Methanol Extracts of Medicinal Plants against Dental Pathogens Streptococcus mutans and Candida albicans. Journal of Microbiology and Biotechnology, 27, 1242–1248. [DOI] [PubMed] [Google Scholar]
- Cook C, Magan N, Robinson-Boyer L, & Xu X (2023). Effect on microbial communities in apple orchard soil when exposed short-term to climate change abiotic factors and different orchard management practices. Journal of Applied Microbiology. [DOI] [PubMed] [Google Scholar]
- Coutinho-Wolino KS, Almeida PP, Mafra D, & Stockler-Pinto MB (2022). Bioactive compounds modulating Toll-like 4 receptor (TLR4)-mediated inflammation: Pathways involved and future perspectives. Nutrition Research, 107, 96–116. [DOI] [PubMed] [Google Scholar]
- Crunkhorn S (2022). A bacteria-derived oral tumour vaccine. Nature Reviews. Drug Discovery, 21, 494. [DOI] [PubMed] [Google Scholar]
- Debnath N, Thakur M, Negi Khushboo, N. P, Gautam V, Kumar Yadav A, Kumar D, 2022. Insight of oral vaccines as an alternative approach to health and disease management: An innovative intuition and challenges. Biotechnol Bioeng 119, 327–346. [DOI] [PubMed] [Google Scholar]
- Del Olmo A, Picon A, & Nunez M (2018). The microbiota of eight species of dehydrated edible seaweeds from North West Spain. Food Microbiology, 70, 224–231. [DOI] [PubMed] [Google Scholar]
- Denzler KL, Waters R, Jacobs BL, Rochon Y, & Langland JO (2010). Regulation of inflammatory gene expression in PBMCs by immunostimulatory botanicals. PLoS One1, 5, e12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di ME, Kahkonen B, Liu CH, & Di YP (2021). Lung carcinomas induced by NNK and LPS. Methods in Cell Biology, 163, 175–185. [DOI] [PubMed] [Google Scholar]
- Diedhiou AG, Mbaye FK, Mbodj D, Faye MN, Pignoly S, Ndoye I, … Champion A (2016). Field Trials Reveal Ecotype-Specific Responses to Mycorrhizal Inoculation in Rice. PLoS One1, 11, e0167014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugaszewska J, Ratajczak M, Kaminska D, & Gajecka M (2019). Are dietary supplements containing plant-derived ingredients safe microbiologically? Saudi Pharm J, 27, 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Dai H, Hu X, Xiong SD, & Gao XM (2014). The (1->6)-beta-glucan moiety represents a cross-reactive epitope of infection-induced malignancy surveillance. Journal of Immunology, 192, 1302–1312. [DOI] [PubMed] [Google Scholar]
- Dubey LK, Moeller JB, Schlosser A, Sorensen GL, & Holmskov U (2014). Induction of innate immunity by Aspergillus fumigatus cell wall polysaccharides is enhanced by the composite presentation of chitin and beta-glucan. Immunobiology, 219, 179–188. [DOI] [PubMed] [Google Scholar]
- Ehrchen JM, Sunderkotter C, Foell D, Vogl T, & Roth J (2009). The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. Journal of Leukocyte Biology, 86, 557–566. [DOI] [PubMed] [Google Scholar]
- El-Azzouny MM, El-Demerdash AS, Seadawy HG, & Abou-Khadra SH (2018). Antimicrobial Effect of Garlic (Allium sativum) and Thyme (Zataria multiflora Boiss) Extracts on Some Food Borne Pathogens and Their Effect on Virulence Gene Expression. Cellular and Molecular Biology (Noisy-le-Grand, France), 64, 79–86. [PubMed] [Google Scholar]
- Evans L, Milward K, Attanoos R, Clayton A, Errington R, Tabi Z, 2021. Macrophage Plasticity and Function in the Lung Tumour Microenvironment Revealed in 3D Heterotypic Spheroid and Explant Models. Biomedicines 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- F IJ, De Smet J, Crauwels S, Lievens B, Van Campenhout L, 2022. Meta-analysis of larvae of the black soldier fly (Hermetia illucens) microbiota based on 16S rRNA gene amplicon sequencing. FEMS Microbiology Ecology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Stephenson KK, & Wallace AJ (2015). Dietary amelioration of Helicobacter infection. Nutrition Research, 35, 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feroz F, Shimizu H, Nishioka T, Mori M, & Sakagami Y (2016). Bacterial and Fungal Counts of Dried and Semi-Dried Foods Collected from Dhaka, Bangladesh, and Their Reduction Methods. Biocontrol Science, 21, 243–251. [DOI] [PubMed] [Google Scholar]
- Finger J, Maffei DF, Dias M, Mendes MA, & Pinto UM (2021). Microbiological quality and safety of minimally processed parsley (Petroselinum crispum) sold in food markets, southeastern Brazil. Journal of Applied Microbiology, 131, 272–280. [DOI] [PubMed] [Google Scholar]
- Frohling A, Rademacher A, Rumpold B, Klocke M, & Schluter O (2018). Screening of microbial communities associated with endive lettuce during postharvest processing on industrial scale. Heliyon, 4, e00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallichan WS, & Rosenthal KL (1996). Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. The Journal of Experimental Medicine, 184, 1879–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, … Kroemer G (2012). Trial Watch: Experimental Toll-like receptor agonists for cancer therapy. Oncoimmunology, 1, 699–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbowska M, Berthold-Pluta A, & Stasiak-Rozanska L (2015). Microbiological quality of selected spices and herbs including the presence of Cronobacter spp. Food Microbiology, 49, 1–5. [DOI] [PubMed] [Google Scholar]
- Gauthier AE, Rotjan RD, & Kagan JC (2022). Lipopolysaccharide detection by the innate immune system may be an uncommon defence strategy used in nature. Open Biology, 12, Article 220146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisen U, Zenthoefer M, Peipp M, Kerber J, Plenge J, Manago A, … Kalthoff H (2015). Molecular Mechanisms by Which a Fucus vesiculosus Extract Mediates Cell Cycle Inhibition and Cell Death in Pancreatic Cancer Cells. Marine Drugs, 13, 4470–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A, Shrestha R, & Yan J (2019). Yeast-Derived beta-Glucan in Cancer: Novel Uses of a Traditional Therapeutic. International Journal of Molecular Sciences, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A, Pichugin A, Bagaev A, Davtyan A, Hovakimyan A, Tukhvatulin A, … Ataullakhanov RI (2014). Targeting TLR-4 with a novel pharmaceutical grade plant derived agonist, Immunomax(R), as a therapeutic strategy for metastatic breast cancer. Journal of Translational Medicine, 12, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas D, Kovacs G, Jankovics I, Meszaros L, Lorincz M, & Denes B (2022). Effects of the combination of a monoclonal agonistic mouse anti-OX40 antibody and toll-like receptor agonists: Unmethylated CpG and LPS on an MB49 bladder cancer cell line in a mouse model. PLoS One1, 17, e0270802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D, Silva M, Radziun K, Martinez DC, Hill CJ, Marshall J, … Reilly GC (2020). Fucoidan Inhibition of Osteosarcoma Cells Is Species and Molecular Weight Dependent. Marine Drugs, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Fan Q, Cai T, Huang W, Xie X, Wen Y, & Shi Z (2018). Molecular mechanisms of the action of Arctigenin in cancer. Biomedicine & Pharmacotherapy, 108, 403–407. [DOI] [PubMed] [Google Scholar]
- Higgins D, Pal C, Sulaiman IM, Jia C, Zerwekh T, Dowd SE, & Banerjee P (2018). Application of high-throughput pyrosequencing in the analysis of microbiota of food commodities procured from small and large retail outlets in a U.S. metropolitan area - A pilot study. Food Research International, 105, 29–40. [DOI] [PubMed] [Google Scholar]
- Hobohm U (2001). Fever and cancer in perspective. Cancer Immunology, Immunotherapy, 50, 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Yuan X, Yang J, Yang Y, Jv H, Li R, … Ruan Y (2023). Selection of rhizosphere communities of diverse rotation crops reveals unique core microbiome associated with reduced banana Fusarium wilt disease. The New Phytologist. [DOI] [PubMed] [Google Scholar]
- Hoption Cann SA, van Netten JP, & van Netten C (2003). Dr William Coley and tumour regression: A place in history or in the future. Postgraduate Medical Journal, 79, 672–680. [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H, Alaw Qotbi AA, Seidavi A, Norris D, & Brown D (2014). Effects of different levels of coriander (Coriandrum sativum) seed powder and extract on serum biochemical parameters, microbiota, and immunity in broiler chicks. ScientificWorldJournal, 2014, Article 628979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howshigan J, Perera K, Samita S, & Rajapakse PS (2015). The effects of an Ayurvedic medicinal toothpaste on clinical, microbiological and oral hygiene parameters in patients with chronic gingivitis: A double-blind, randomised, placebo-controlled, parallel allocation clinical trial. The Ceylon Medical Journal, 60, 126–132. [DOI] [PubMed] [Google Scholar]
- Ignacio RMC, Lee ES, & Son DS (2019). Potential Roles of Innate Immune Chemokine and Cytokine Network on Lipopolysaccharide-Based Therapeutic Approach in Ovarian Cancer. Immune Netw, 19, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi M, Inagawa H, Nishizawa T, Okutomi T, Morikawa A, Soma GI, & Mizuno D (1992). Homeostasis as regulated by activated macrophage. V. Suppression of diabetes mellitus in non-obese diabetic mice by LPSw (a lipopolysaccharide from wheat flour). Chemical and Pharmaceutical Bulletin (Tokyo), 40, 1004–1006. [DOI] [PubMed] [Google Scholar]
- Inagawa H, Nishizawa T, Tsukioka D, Suda T, Chiba Y, Okutomi T, … Mizuno D (1992). Homeostasis as regulated by activated macrophage. II. LPS of plant origin other than wheat flour and their concomitant bacteria. Chemical and Pharmaceutical Bulletin (Tokyo), 40, 994–997. [DOI] [PubMed] [Google Scholar]
- Inagawa H, Saitoh F, Iguchi M, Nishizawa T, Okutomi T, Morikawa A, … Mizuno D (1992). Homeostasis as regulated by activated macrophage. III. Protective effect of LPSw (lipopolysaccharide (LPS) of wheat flour) on gastric ulcer in mice as compared with those of other LPS from various sources. Chemical and Pharmaceutical Bulletin (Tokyo), 40, 998–1000. [DOI] [PubMed] [Google Scholar]
- Iqbal S, Zebeli Q, Mansmann DA, Dunn SM, & Ametaj BN (2014). Oral administration of LPS and lipoteichoic acid prepartum modulated reactants of innate and humoral immunity in periparturient dairy cows. Innate Immunity, 20, 390–400. [DOI] [PubMed] [Google Scholar]
- Irshad MK, Noman A, Wang Y, Yin Y, Chen C, & Shang J (2022). Goethite modified biochar simultaneously mitigates the arsenic and cadmium accumulation in paddy rice (Oryza sativa) L. Environmental Research, 206, Article 112238. [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Robben N, Burghart R, Cote P, Greenway S, Thakkar R, Upreti D, Nakashima A, Suzuki K, Comer J, Tamura M, 2020. Cell Wall Membrane Fraction of Chlorella sorokiniana Enhances Host Antitumor Immunity and Inhibits Colon Carcinoma Growth in Mice. Integr Cancer Ther 19, 1534735419900555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janthamala S, Jusakul A, Kongpetch S, Kimawaha P, Klanrit P, Loilome W, … Techasen A (2021). Arctigenin inhibits cholangiocarcinoma progression by regulating cell migration and cell viability via the N-cadherin and apoptosis pathway. Naunyn-Schmiedeberg’s Archives of Pharmacology, 394, 2049–2059. [DOI] [PubMed] [Google Scholar]
- Jefri U, Khan A, Lim YC, Lee KS, Liew KB, Kassab YW, … Kalusalingam A (2022). A systematic review on chlorine dioxide as a disinfectant. Journal of Medicine and Life, 15, 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wang Z, Li X, Liu S, Song F, & Liu F (2021). Relationship between endophytic microbial diversity and grain quality in wheat exposed to multi-generational CO2 elevation. The Science of the Total Environment, 776, Article 146029. [DOI] [PubMed] [Google Scholar]
- Jovanovic J, Tretiak S, Begyn K, Rajkovic A, 2022. Detection of Enterotoxigenic Psychrotrophic Presumptive Bacillus cereus and Cereulide Producers in Food Products and Ingredients. Toxins (Basel) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam L, Salloum T, El Hage R, Hassan H, & Hassan HF (2021). How can packaging, source and food safety management system affect the microbiological quality of spices and dried herbs? The case of a developing country. International Journal of Food Microbiology, 353, Article 109295. [DOI] [PubMed] [Google Scholar]
- Kazi M, Parlapani FF, Boziaris IS, Vellios EK, & Lykas C (2018). Effect of ozone on the microbiological status of five dried aromatic plants. Journal of the Science of Food and Agriculture, 98, 1369–1373. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim DH, Jo S, Cho MJ, Cho YR, Lee YJ, & Byun S (2022). Immunomodulatory functional foods and their molecular mechanisms. Experimental & Molecular Medicine, 54, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SE, Memon A, Kim BY, Jeon H, Lee WK, & Kang SC (2020). Gastroprotective effect of phytoncide extract from Pinus koraiensis pinecone in Helicobacter pylori infection. Scientific Reports, 10, 9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Ruben C, & Upmann M (2013). Antimicrobial activity of essential oil components against potential food spoilage microorganisms. Current Microbiology, 67, 200–208. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Inagawa H, Kohchi C, Kazumura K, Tsuchiya H, Miwa T, … Soma GI (2018). Oral administration of Pantoea agglomerans-derived lipopolysaccharide prevents development of atherosclerosis in high-fat diet-fed apoE-deficient mice via ameliorating hyperlipidemia, pro-inflammatory mediators and oxidative responses. PLoS One1, 13, e0195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler H, Puchalski K, Ruiz G, Jacobs B, & Langland J (2020). The Role of Endophytic/Epiphytic Bacterial Constituents in the Immunostimulatory Activity of the Botanical, Astragalus membranaceus. The Yale Journal of Biology and Medicine, 93, 239–250. [PMC free article] [PubMed] [Google Scholar]
- Kohchi C, Inagawa H, Nishizawa T, Yamaguchi T, Nagai S, & Soma G (2006). Applications of lipopolysaccharide derived from Pantoea agglomerans (IP-PA1) for health care based on macrophage network theory. Journal of Bioscience and Bioengineering, 102, 485–496. [DOI] [PubMed] [Google Scholar]
- Kolanowski ST, Dieker MC, Lissenberg-Thunnissen SN, van Schijndel GM, van Ham SM, & ten Brinke A (2014). TLR4-mediated pro-inflammatory dendritic cell differentiation in humans requires the combined action of MyD88 and TRIF. Innate Immunity, 20, 423–430. [DOI] [PubMed] [Google Scholar]
- Kolmos HJ (2006). Panum’s studies on “putrid poison” 1856. An early description of endotoxin. Danish Medical Bulletin, 53, 450–452. [PubMed] [Google Scholar]
- Kong QK, Guo HJ, Zhao G, Guo X, Cheng JS, & Wang L (2004). Sequence of Escherichia coli O141 O-antigen gene cluster and analyses of its evolutionary history. Yi Chuan Xue Bao, 31, 1448–1454. [PubMed] [Google Scholar]
- Koprivova A, & Kopriva S (2022). Plant secondary metabolites altering root microbiome composition and function. Current Opinion in Plant Biology, 67, Article 102227. [DOI] [PubMed] [Google Scholar]
- Kubatka P, Kapinova A, Kruzliak P, Kello M, Vybohova D, Kajo K, … Dobrota D (2015). Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition, 31, 560–569. [DOI] [PubMed] [Google Scholar]
- Lemay MA, Davis KM, Martone PT, & Parfrey LW (2021). Kelp-associated Microbiota are Structured by Host Anatomy(1). Journal of Phycology, 57, 1119–1130. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Beck NK, Kossik AL, Patti T, Meschke JS, Calicchia M, & Hellberg RS (2015). Microbial safety and quality of fresh herbs from Los Angeles, Orange County and Seattle farmers’ markets. Journal of the Science of Food and Agriculture, 95, 2641–2645. [DOI] [PubMed] [Google Scholar]
- Li B, Cai Y, Qi C, Hansen R, Ding C, Mitchell TC, & Yan J (2010). Orally administered particulate beta-glucan modulates tumor-capturing dendritic cells and improves antitumor T-cell responses in cancer. Clinical Cancer Research, 16, 5153–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Liu J, Saleem M, Li G, Luan L, Wu M, & Li Z (2022). Reduced chemodiversity suppresses rhizosphere microbiome functioning in the mono-cropped agroecosystems. Microbiome, 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Xu X, Jiang M, Bi Y, Xu J, & Han M (2015). Lipopolysaccharide induces inflammation and facilitates lung metastasis in a breast cancer model via the prostaglandin E2-EP2 pathway. Molecular Medicine Reports, 11, 4454–4462. [DOI] [PubMed] [Google Scholar]
- Liu CH, Chen Z, Chen K, Liao FT, Chung CE, Liu X, … Di YP (2021). Lipopolysaccharide-Mediated Chronic Inflammation Promotes Tobacco Carcinogen-Induced Lung Cancer and Determines the Efficacy of Immunotherapy. Cancer Research, 81, 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Luo F, Ding C, Albeituni S, Hu X, Ma Y, … Yan J (2015). Dectin-1 Activation by a Natural Product beta-Glucan Converts Immunosuppressive Macrophages into an M1-like Phenotype. Journal of Immunology, 195, 5055–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Chang L, Zhou H, Liu X, Li Y, Mi T, & Tong D (2019). Arctigenin Attenuates Tumor Metastasis Through Inhibiting Epithelial-Mesenchymal Transition i”n Hepatocellular Carcinoma via Suppressing GSK3beta-Dependent Wnt/beta-Catenin Signaling Pathway In Vivo and In Vitro. Frontiers in Pharmacology, 10, 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchner M, Reinke S, & Milicic A (2021). TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Yang H, Guan L, Liu X, & Zhang T (2022). Risks of antibiotic resistance genes and antimicrobial resistance under chlorination disinfection with public health concerns. Environment International, 158, Article 106978. [DOI] [PubMed] [Google Scholar]
- Mai CW, Kang YB, & Pichika MR (2013). Should a Toll-like receptor 4 (TLR-4) agonist or antagonist be designed to treat cancer? TLR-4: Its expression and effects in the ten most common cancers. Onco Targets Ther, 6, 1573–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masullo M, Pizza C, & Piacente S (2016). Ruscus Genus: A Rich Source of Bioactive Steroidal Saponins. Planta Medica, 82, 1513–1524. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Aze Y, Akimoto A, & Fujita T (1998). ONO-4007, an antitumor lipid A analog, induces tumor necrosis factor-alpha production by human monocytes only under primed state: Different effects of ONO-4007 and lipopolysaccharide on cytokine production. The Journal of Pharmacology and Experimental Therapeutics, 284, 189–195. [PubMed] [Google Scholar]
- Matsushita K, Kuramitsu Y, Ohiro Y, Kobayashi I, Watanabe T, Kobayashi M, & Hosokawa M (2003). ONO-4007, a synthetic lipid A analog, induces Th1-type immune response in tumor eradication and restores nitric oxide production by peritoneal macrophages. International Journal of Oncology, 23, 489–493. [PubMed] [Google Scholar]
- Mazgaeen L, & Gurung P (2020). Recent Advances in Lipopolysaccharide Recognition Systems. International Journal of Molecular Sciences, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzio EA, Li N, Bauer D, Mendonca P, Taka E, Darb M, … Soliman KF (2016). Natural product HTP screening for antibacterial (E.coli 0157: H7) and anti-inflammatory agents in (LPS from E. coli O111: B4) activated macrophages and microglial cells; focus on sepsis. BMC Complementary and Alternative Medicine, 16, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy EF (2006). The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. The Iowa Orthopaedic Journal, 26, 154–158. [PMC free article] [PubMed] [Google Scholar]
- Minh TN, Van TM, Khanh TD, & Xuan TD (2022). Isolation and Identification of Constituents Exhibiting Antioxidant, Antibacterial, and Antihyperuricemia Activities in Piper methysticum Root. Foods, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K, Weigel BL, Fogarty EC, Veseli IA, Giblin AE, Eren AM, Pfister CA, 2022. The Diversity and Functional Capacity of Microbes Associated with Coastal Macrophytes. mSystems, e0059222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi H, Yamamoto K, Yamashita M, Nakata Y, Inagawa H, Kohchi C, & Soma GI (2021). Prevention of Diabetes-Associated Cognitive Dysfunction Through Oral Administration of Lipopolysaccharide Derived From Pantoea agglomerans. Frontiers in Immunology, 12, Article 650176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno D, & Soma G (1992). Oral or percutaneous administration of lipopolysaccharide of small molecular size may cure various intractable diseases: A new version of Coley’s toxin. Molecular Biotherapy, 4, 166–169. [PubMed] [Google Scholar]
- Mo S, Ru H, Huang M, Cheng L, Mo X, & Yan L (2022). Oral-Intestinal Microbiota in Colorectal Cancer: Inflammation and Immunosuppression. Journal of Inflammation Research, 15, 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro D, Kalpana K, Chrissian C, Sharma A, Takaoka A, Iacovidou M, … Kawamura A (2015). Uncovering potential ‘herbal probiotics’ in Juzen-taiho-to through the study of associated bacterial populations. Bioorganic & Medicinal Chemistry Letters, 25, 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima A, & Inagawa H (2016). Clinical Effects of Orally Administered Lipopolysaccharide Derived from Pantoea agglomerans on Malignant Tumors. Anticancer Research, 36, 3747–3751. [PubMed] [Google Scholar]
- Muller E, Christopoulos PF, Halder S, Lunde A, Beraki K, Speth M, … Corthay A (2017). Toll-Like Receptor Ligands and Interferon-gamma Synergize for Induction of Antitumor M1 Macrophages. Frontiers in Immunology, 8, 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi SF, Di Lorenzo A, Izadi M, Sobarzo-Sanchez E, Daglia M, & Nabavi SM (2015). Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients, 7, 7729–7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayasu M, Takamatsu K, Yazaki K, & Sugiyama A (2022). Plant specialized metabolites in the rhizosphere of tomatoes: Secretion and effects on microorganisms. Bioscience, Biotechnology, and Biochemistry, 87, 13–20. [DOI] [PubMed] [Google Scholar]
- Neemisha Kumar, A., Sharma P, Kaur A, Sharma S, Jain R, 2022. Harnessing rhizobacteria to fulfil inter-linked nutrient dependency on soil and alleviate stresses in plants. Journal of Applied Microbiology. [DOI] [PubMed] [Google Scholar]
- Nelson HS (2022). The return of the mixed respiratory bacterial vaccine. Allergy and Asthma Proceedings, 43, 501–508. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Gao YT, Nogueira LM, Shen MC, Wang B, Rashid A, … Koshiol J (2017). Diet and biliary tract cancer risk in Shanghai. China. PLoS One, 12, e0173935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil-Bunesova V, Ramirez Garcia A, Modrackova N, Makovska M, Sabolova M, Sproer C, … Schwab C (2022). Feed Insects as a Reservoir of Granadaene-Producing Lactococci. Frontiers in Microbiology, 13, Article 848490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa T, Inagawa H, Oshima H, Okutomi T, Tsukioka D, Iguchi M, … Mizuno D (1992). Homeostasis as regulated by activated macrophage. I. Lipopolysaccharide (LPS) from wheat flour: Isolation, purification and some biological activities. Chemical and Pharmaceutical Bulletin (Tokyo), 40, 479–483. [DOI] [PubMed] [Google Scholar]
- Oblak A, & Jerala R (2011). Toll-like receptor 4 activation in cancer progression and therapy. Clinical & Developmental Immunology, 2011, Article 609579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Asai Y, Makimura Y, & Tamai R (2007). Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A. Frontiers in Bioscience: a Journal and Virtual Library, 12, 3795–3812. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Yoshida O, Oishi K, Okada K, Yamabe H, & Schroeder FH (1988). Dietary beta-carotene and cancer of the prostate: A case-control study in Kyoto, Japan. Cancer Research, 48, 1331–1336. [PubMed] [Google Scholar]
- Okutomi T, Nishizawa T, Inagawa H, Takano T, Morikawa A, Soma G, & Mizuno D (1992). Homeostasis as regulated by activated macrophage. VII. Suppression of serum cholesterol level by LPSw (a lipopolysaccharide from wheat flour) in WHHL (Watanabe heritable hyperlipidemic) rabbit. Chemical and Pharmaceutical Bulletin (Tokyo), 40, 1268–1270. [DOI] [PubMed] [Google Scholar]
- Okuyama H, Tominaga A, Fukuoka S, Taguchi T, Kusumoto Y, & Ono S (2017). Spirulina lipopolysaccharides inhibit tumor growth in a Toll-like receptor 4-dependent manner by altering the cytokine milieu from interleukin-17/interleukin-23 to interferon-gamma. Oncology Reports, 37, 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange M, Reuter U, & Hobohm U (2016). Coley’s Lessons Remembered: Augmenting Mistletoe Therapy. Integrative Cancer Therapies, 15, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Mosqueda MDC, Fadiji AE, Babalola OO, Glick BR, & Santoyo G (2022). Rhizobiome engineering: Unveiling complex rhizosphere interactions to enhance plant growth and health. Microbiological Research, 263, Article 127137. [DOI] [PubMed] [Google Scholar]
- Osborn MJ (2019). The Way It Was. Annual Review of Microbiology, 73, 1–15. [DOI] [PubMed] [Google Scholar]
- Paradia PK, Bhavale R, Agnihotri T, & Jain A (2022). A Review On Edible Vaccines And Biopharmaceutical Products From Plants. Current Pharmaceutical Biotechnology. [DOI] [PubMed] [Google Scholar]
- Park AY, Nafia I, Stringer DN, Karpiniec SS, Fitton JH, 2021. Fucoidan Independently Enhances Activity in Human Immune Cells and Has a Cytostatic Effect on Prostate Cancer Cells in the Presence of Nivolumab. Mar Drugs 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patin EC, Thompson A, & Orr SJ (2019). Pattern recognition receptors in fungal immunity. Seminars in Cell & Developmental Biology, 89, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picon A, Del Olmo A, & Nunez M (2021). Bacterial diversity in six species of fresh edible seaweeds submitted to high pressure processing and long-term refrigerated storage. Food Microbiology, 94, Article 103646. [DOI] [PubMed] [Google Scholar]
- Pont-Fernandez S, Kheyfets M, Rogers JM, Smith KE, & Epstein DH (2022). Kava (Piper methysticum) in the United States: The quiet rise of a substance with often subtle effects. The American Journal of Drug and Alcohol Abuse, 1–12. [DOI] [PubMed] [Google Scholar]
- Pugh ND, Tamta H, Balachandran P, Wu X, Howell J, Dayan FE, & Pasco DS (2008). The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and lipopolysaccharides. International Immunopharmacology, 8, 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Puebla ST, Weigel BL, Jack L, Schlundt C, Pfister CA, & Mark Welch JL (2022). Spatial organization of the kelp microbiome at micron scales. Microbiome, 10, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Saraiva A, Ramos F, Carrascosa C, Raheem D, Barbara R, & Silva H (2021). The Role of Food Supplementation in Microcirculation-A Comprehensive Review. Biology (Basel), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F, Zhang Y, Qin Y, Shang J, Wang Y, Wei P, … Zhao T (2022). Taraxasterol prompted the anti-tumor effect in mice burden hepatocellular carcinoma by regulating T lymphocytes. Cell Death Discov, 8, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues JPB, Fernandes A, Dias MI, Pereira C, Pires T, R CC, Carvalho AM, Ferreira I, Barros L, 2021. Phenolic Compounds and Bioactive Properties of Ruscus aculeatus L. (Asparagaceae): The Pharmacological Potential of an Underexploited Subshrub. Molecules 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Garcia I, Silva-Espinoza BA, Ortega-Ramirez LA, Leyva JM, Siddiqui MW, Cruz-Valenzuela MR, … Ayala-Zavala JF (2016). Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Critical Reviews in Food Science and Nutrition, 56, 1717–1727. [DOI] [PubMed] [Google Scholar]
- Roeder A, Kirschning CJ, Rupec RA, Schaller M, Weindl G, & Korting HC (2004). Toll-like receptors as key mediators in innate antifungal immunity. Medical Mycology, 42, 485–498. [DOI] [PubMed] [Google Scholar]
- Romualdo GR, Silva EDA, Da Silva TC, Aloia TPA, Nogueira MS, De Castro IA, … Cogliati B (2020). Burdock (Arctium lappa L.) root attenuates preneoplastic lesion development in a diet and thioacetamide-induced model of steatohepatitis-associated hepatocarcinogenesis. Environmental Toxicology, 35, 518–527. [DOI] [PubMed] [Google Scholar]
- Rosen JE, Gardiner P, Saper RB, Pearce EN, Hammer K, Gupta-Lawrence RL, & Lee SL (2014). Kelp use in patients with thyroid cancer. Endocrine, 46, 123–130. [DOI] [PubMed] [Google Scholar]
- Rossi O, & Mastroeni P (2022). Editorial: Pattern recognition receptors at the crosstalk between innate and adaptive immune systems and implications for vaccine development. Frontiers in Cellular and Infection Microbiology, 12, 1088029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai J, Cammarota E, Wright JA, Cicuta P, Gottschalk RA, Li N, … Bryant CE (2017). Lipopolysaccharide-induced NF-kappaB nuclear translocation is primarily dependent on MyD88, but TNFalpha expression requires TRIF and MyD88. Scientific Reports, 7, 1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab AH, Harpestad AD, Swartzentruber A, Lanier JM, Wentz BA, Duran AP, … Read RB Jr. (1982). Microbiological quality of some spices and herbs in retail markets. Applied and Environmental Microbiology, 44, 627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid M, & Khan MS (2022). Tolerance of pesticides and antibiotics among beneficial soil microbes recovered from contaminated rhizosphere of edible crops. Current Research in Microbial Sciences, 3, Article 100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifzadeh A, Javan AJ, Shokri H, Abbaszadeh S, & Keykhosravy K (2016). Evaluation of antioxidant and antifungal properties of the traditional plants against foodborne fungal pathogens. Journal of Medical Mycology, 26, e11–e17. [DOI] [PubMed] [Google Scholar]
- Shen G, Jeong WS, Hu R, & Kong AN (2005). Regulation of Nrf2, NF-kappaB, and AP-1 signaling pathways by chemopreventive agents. Antioxidants & Redox Signaling, 7, 1648–1663. [DOI] [PubMed] [Google Scholar]
- Shen HY, Li LZ, Xue KC, Hu DD, & Gao YJ (2017). Antitumor activity of fucoidan in anaplastic thyroid cancer via apoptosis and anti-angiogenesis. Molecular Medicine Reports, 15, 2620–2624. [DOI] [PubMed] [Google Scholar]
- Shi YG, Zhu CM, Li DH, Shi ZY, Gu Q, Chen YW, … Chen JS (2021). New Horizons in Microbiological Food Safety: Ultraefficient Photodynamic Inactivation Based on a Gallic Acid Derivative and UV-A Light and Its Application with Electrospun Cyclodextrin Nanofibers. Journal of Agricultural and Food Chemistry, 69, 14961–14974. [DOI] [PubMed] [Google Scholar]
- Sholtes KA, Lowe K, Walters GW, Sobsey MD, Linden KG, & Casanova LM (2016). Comparison of ultraviolet light-emitting diodes and low-pressure mercury-arc lamps for disinfection of water. Environmental Technology, 37, 2183–2188. [DOI] [PubMed] [Google Scholar]
- Siddiqui AJ, Jahan S, Singh R, Saxena J, Ashraf SA, Khan A, … Adnan M (2022). Plants in Anticancer Drug Discovery: From Molecular Mechanism to Chemoprevention. Biomed Research International, 2022, 5425485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva N, Alves S, Goncalves A, Amaral JS, & Poeta P (2013). Antimicrobial activity of essential oils from Mediterranean aromatic plants against several foodborne and spoilage bacteria. Food Science and Technology International, 19, 503–510. [DOI] [PubMed] [Google Scholar]
- Skibola CF, Curry JD, VandeVoort C, Conley A, & Smith MT (2005). Brown kelp modulates endocrine hormones in female sprague-dawley rats and in human luteinized granulosa cells. The Journal of Nutrition, 135, 296–300. [DOI] [PubMed] [Google Scholar]
- Skjanes K, Aesoy R, Herfindal L, & Skomedal H (2021). Bioactive peptides from microalgae: Focus on anti-cancer and immunomodulating activity. Physiologia Plantarum, 173, 612–623. [DOI] [PubMed] [Google Scholar]
- Smith KM, Davidson JM, & Garside P (2002). T-cell activation occurs simultaneously in local and peripheral lymphoid tissue following oral administration of a range of doses of immunogenic or tolerogenic antigen although tolerized T cells display a defect in cell division. Immunology, 106, 144–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma G, & Inagawa H (2015). Methods to Prevent or Treat Refractory Diseases by Focusing on Intestinal Microbes Using LPS and Macrophages. Anticancer Research, 35, 4393–4396. [PubMed] [Google Scholar]
- Soto-Giron MJ, Kim JN, Schott E, Tahmin C, Ishoey T, Mincer TJ, … Toledo G (2021). The Edible Plant Microbiome represents a diverse genetic reservoir with functional potential in the human host. Scientific Reports, 11, 24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssepuuya G, Wynants E, Verreth C, Crauwels S, Lievens B, Claes J, … Van Campenhout L (2019). Microbial characterisation of the edible grasshopper Ruspolia differens in raw condition after wild-harvesting in Uganda. Food Microbiology, 77, 106–117. [DOI] [PubMed] [Google Scholar]
- Stander J, Mbewana S, & Meyers AE (2022). Plant-Derived Human Vaccines: Recent Developments. BioDrugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Tian W, Zhang F, Chen Y, Ren XM, Pang FH, … Chen ZJ (2019). Composition and Predictive Functional Analysis of Rhizosphere Bacterial Communities in Riparian Buffer Strips in the Danjiangkou Reservoir, China. Huan Jing Ke Xue, 40, 421–429. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Umezawa T, & Shimada M (2002). Stereochemical diversity in lignan biosynthesis of Arctium lappa L. Bioscience, Biotechnology, and Biochemistry, 66, 1262–1269. [DOI] [PubMed] [Google Scholar]
- Syahidah A, Saad CR, Hassan MD, Rukayadi Y, Norazian MH, & Kamarudin MS (2017). Phytochemical Analysis, Identification and Quantification of Antibacterial Active Compounds in Betel Leaves, Piper betle Methanolic Extract. Pakistan Journal of Biological Sciences, 20, 70–81. [DOI] [PubMed] [Google Scholar]
- Talapphet N, Palanisamy S, Li C, Ma N, Prabhu NM, & You S (2021). Polysaccharide extracted from Taraxacum platycarpum root exerts immunomodulatory activity via MAPK and NF-kappaB pathways in RAW264.7 cells. Journal of Ethnopharmacology, 281, Article 114519. [DOI] [PubMed] [Google Scholar]
- Tang J, Liu Y, Lin B, Zhu H, Jiang W, Yang Q, & Chen S (2021). Effects of ultra-long fermentation time on the microbial community and flavor components of light-flavor Xiaoqu Baijiu based on fermentation tanks. World Journal of Microbiology and Biotechnology, 38, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Yoshioka N, Nishizawa T, Inagawa H, Kohchi C, & Soma G (2009). Utility and safety of LPS-based fermented flour extract as a macrophage activator. Anticancer Research, 29, 859–864. [PubMed] [Google Scholar]
- Tischner Z, Sebok R, Kredics L, Allaga H, Vargha M, Sebestyen A, … Magyar D (2021). Mycological Investigation of Bottled Water Dispensers in Healthcare Facilities. Pathogens, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Jing Y, Zhao D, Zhang L, & Zeng S (2011). Fluoroquinolone-resistant uncomplicated urinary tract infections, Chinese herbal medicine may provide help. African Journal of Traditional, Complementary, and Alternative Medicines, 8, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo-Cordoba B, Castro-Lopez C, Garcia HS, Gonzalez-Cordova AF, & Hernandez-Mendoza A (2020). Postbiotics and paraprobiotics: A review of current evidence and emerging trends. Advances in Food and Nutrition Research, 94, 1–34. [DOI] [PubMed] [Google Scholar]
- Vasudevan U, Gantayat RR, Chidambaram S, Prasanna MV, Venkatramanan S, Devaraj N, … Ganesh N (2021). Microbial contamination and its associations with major ions in shallow groundwater along coastal Tamil Nadu. Environmental Geochemistry and Health, 43, 1069–1088. [DOI] [PubMed] [Google Scholar]
- Vetvicka V (2011). Glucan-immunostimulant, adjuvant, potential drug. World J Clin Oncol, 2, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetvicka V, & Vetvickova J (2020). Anti-infectious and Anti-tumor Activities of beta-glucans. Anticancer Research, 40, 3139–3145. [DOI] [PubMed] [Google Scholar]
- Voidarou C, Antoniadou M, Rozos G, Tzora A, Skoufos I, Varzakas T, Lagiou A, Bezirtzoglou E, 2020. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, & Yang JS (1993). Studies on the chemical constituents of Arctium lappa L. Yao Xue Xue Bao, 28, 911–917. [PubMed] [Google Scholar]
- Wang K, Xiong J, Lu Y, Wang L, & Tian T (2023). SENP1-KLF4 signalling regulates LPS-induced macrophage M1 polarization. The FEBS Journal, 290, 209–224. [DOI] [PubMed] [Google Scholar]
- Wang X, Du H, & Xu Y (2017). Source tracking of prokaryotic communities in fermented grain of Chinese strong-flavor liquor. International Journal of Food Microbiology, 244, 27–35. [DOI] [PubMed] [Google Scholar]
- Warashina T, & Noro T (2010). 8,12;8,20-Diepoxy-8,14-secopregnane glycosides from the aerial parts of Asclepias tuberosa. Chemical and Pharmaceutical Bulletin (Tokyo), 58, 172–179. [DOI] [PubMed] [Google Scholar]
- Wassermann B, Abdelfattah A, Cernava T, Wicaksono W, & Berg G (2022). Microbiome-based biotechnology for reducing food loss post harvest. Current Opinion in Biotechnology, 78, Article 102808. [DOI] [PubMed] [Google Scholar]
- Weigel BL, & Pfister CA (2021). Oxygen metabolism shapes microbial settlement on photosynthetic kelp blades compared to artificial kelp substrates. Environmental Microbiology Reports, 13, 176–184. [DOI] [PubMed] [Google Scholar]
- Weinberger F (2007). Pathogen-induced defense and innate immunity in macroalgae. The Biological Bulletin, 213, 290–302. [DOI] [PubMed] [Google Scholar]
- Willis C, Sadler-Reeves L, Elviss N, Aird H, Fox A, Kaye M, … McLauchlin J (2015). An assessment of the microbiological safety of fresh whole-leaf herbs from retail premises in the United Kingdom with a focus on Salmonella spp. Journal of Applied Microbiology, 119, 827–833. [DOI] [PubMed] [Google Scholar]
- Wolska A, Lech-Maranda E, & Robak T (2009). Toll-like receptors and their role in carcinogenesis and anti-tumor treatment. Cellular & Molecular Biology Letters, 14, 248–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolska A, Lech-Maranda E, & Robak T (2009). Toll-like receptors and their role in hematologic malignancies. Current Molecular Medicine, 9, 324–335. [DOI] [PubMed] [Google Scholar]
- Won EK, Zahner MC, Grant EA, Gore P, & Chicoine MR (2003). Analysis of the antitumoral mechanisms of lipopolysaccharide against glioblastoma multiforme. Anti-Cancer Drugs, 14, 457–466. [DOI] [PubMed] [Google Scholar]
- Woolverton CJ, Holt LC, Mitchell D, & Sartor RB (1992). Identification and characterization of rat intestinal lamina propria cells: Consequences of microbial colonization. Veterinary Immunology and Immunopathology, 34, 127–138. [DOI] [PubMed] [Google Scholar]
- Xiang D, Sharma VR, Freter CE, & Yan J (2012). Anti-tumor monoclonal antibodies in conjunction with beta-glucans: A novel anti-cancer immunotherapy. Current Medicinal Chemistry, 19, 4298–4305. [DOI] [PubMed] [Google Scholar]
- Xin Y, Ji H, Cho E, Roh KB, You J, Park D, & Jung E (2022). Immune-enhancing effect of water-soluble beta-glucan derived from enzymatic hydrolysis of yeast glucan. Biochemistry and Biophysics Reports, 30, Article 101256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Chen H, Sun L, Zhang W, Lu X, Li Z, … Ren Q (2022). The changes of microbial diversity and flavor compounds during the fermentation of millet Huangjiu, a traditional Chinese beverage. PLoS One1, 17, e0262353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Wang YF, Cui HL, Zhou R, Li L, Duan GL, & Zhu YG (2023). Distinctive Structure and Assembly of Phyllosphere Microbial Communities between Wild and Cultivated Rice. Microbiology Spectrum, e0437122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YV, & Walsh NA (2006). Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food and Chemical Toxicology, 44, 1144–1150. [DOI] [PubMed] [Google Scholar]
- Zaidi SF, Muhammad JS, Usmanghani K, & Sugiyama T (2015). Review: Pharmacological ins and outs of medicinal plants against Helicobacter pylori: A review. Pakistan Journal of Pharmaceutical Sciences, 28, 1171–1176. [PubMed] [Google Scholar]
- Zelechowska P, Brzezinska-Blaszczyk E, Agier J, & Kozlowska E (2022). Different effectiveness of fungal pathogen-associated molecular patterns (PAMPs) in activating rat peritoneal mast cells. Immunology Letters, 248, 7–15. [DOI] [PubMed] [Google Scholar]
- Zhang J, Riby JE, Conde L, Grizzle WE, Cui X, & Skibola CF (2016). A Fucus vesiculosus extract inhibits estrogen receptor activation and induces cell death in female cancer cell lines. BMC Complementary and Alternative Medicine, 16, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang YW, Zhu YZ, & Gu XL (2021). Discovery of quality control ingredients in burdock root by combining anti-tumor effects and UHPLC-QqQ-MS/ MS. Biomedical Chromatography, 35, e5187. [DOI] [PubMed] [Google Scholar]
- Zhang P, Cui Z, Guo M, & Xi R (2020). Characteristics of the soil microbial community in the forestland of Camellia oleifera. PeerJ, 8, e9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, An EK, Park HB, Hwang J, Dhananjay Y, Kim SJ, … Jin JO (2021). Ecklonia cava fucoidan has potential to stimulate natural killer cells in vivo. International Journal of Biological Macromolecules, 185, 111–121. [DOI] [PubMed] [Google Scholar]
- Zhang X, Herrera-Balandrano DD, Huang W, Chai Z, Beta T, Wang J, … Li Y (2021). Comparison of Nutritional and Nutraceutical Properties of Burdock Roots Cultivated in Fengxian and Peixian of China. Foods, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Wang X, Liu P, Li M, Dong H, Qiao X, 2018. Semi-Preparative Separation of 10 Caffeoylquinic Acid Derivatives Using High Speed Counter-Current Chromatogaphy Combined with Semi-Preparative HPLC from the Roots of Burdock (Arctium lappa L.). Molecules 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Liu W, Chen Z, Yang D, Qiu Z, Feng H, … Shen Z (2021). The effect of different drinking water in culture medium on feces microbiota diversity. Journal of Water and Health, 19, 267–277. [DOI] [PubMed] [Google Scholar]
- Zhou X, Liu L, Zhao J, Zhang J, Cai Z, & Huang X (2022). High carbon resource diversity enhances the certainty of successful plant pathogen and disease control. The New Phytologist. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


