Abstract

Methane is a powerful greenhouse gas that is produced in large quantities in marine sediments. Microbially mediated oxidation of methane in sediments, when in balance with methane production, prevents the release of methane to the overlying water. Here, we present a gene-based reactive transport model that includes both microbial and geochemical dynamics and use it to investigate whether the rate of growth of methane oxidizers in sediments impacts the efficiency of the microbial methane filter. We focus on iron- and methane-rich coastal sediments and, with the model, show that at our site, up to 10% of all methane removed is oxidized by iron and manganese oxides, with the remainder accounted for by oxygen and sulfate. We demonstrate that the slow growth rate of anaerobic methane-oxidizing microbes limits their ability to respond to transient perturbations, resulting in periodic benthic release of methane. Eutrophication and deoxygenation decrease the efficiency of the microbial methane filter further, thereby enhancing the role of coastal environments as a source of methane to the atmosphere.
Keywords: microbial methane oxidation, gene-centric reactive transport modeling, greenhouse gas, sediment biogeochemistry, cell-specific methane oxidation rates, microbial growth rates
Short abstract
With a novel gene-based reactive transport model, we show that the slow growth of anaerobic methane oxidizers limits methane oxidation during transient perturbations, thereby allowing the release of methane to the overlying water.
Introduction
Methane (CH4) is an important greenhouse gas and its atmospheric concentration has more than doubled since the start of the industrial revolution.1 Methanogenesis accounts for the final step in the degradation of organic matter in marine sediments and accounts for a substantial fraction of naturally produced CH4.2 Methane emissions from the seafloor are limited, however, because most CH4 is converted to CO2 via microbially mediated anaerobic and aerobic CH4 oxidation.3 Enhanced eutrophication (i.e., enhanced nutrient input and organic matter loading) and deoxygenation can alter the balance between CH4 production and its oxidation, potentially resulting in high benthic CH4 release.4,5 Coastal zones are especially vulnerable to such environmental perturbations because of their relatively shallow sulfate–methane transition zone (SMTZ).6 It is therefore critical to better understand and quantify the effects of perturbations on marine CH4 dynamics and the efficiency of the microbial CH4 filter to constrain future CH4 release from marine coastal systems.
Sedimentary CH4 is predominantly oxidized by microbes
using oxygen (O2) and sulfate ( ) as electron acceptors.3 However, recent discoveries show that alternative
anaerobic
pathways such as CH4 oxidation coupled to Fe and Mn oxide
reduction can also play a role.7−9 The quantitative role of metal-dependent
anaerobic oxidation of CH4 is largely unknown. Nitrate
and nitrite can also be used as electron acceptors to oxidize CH4,10 but because of their relatively
low concentrations in marine sediments, they are expected to play
a limited role.11 Microbial oxidation rates
of CH4 coupled to different electron acceptors are often
estimated via geochemical modeling or incubations with radiotracers.12−14 However, quantification of the in situ cell-specific rates and doubling
times that ultimately control the ability of microorganisms to adapt
to changing environmental conditions remains a challenge. This specifically
holds for slow growing microbes, such as anaerobic methanotrophic
archaea (ANME).15,16 As a consequence, the role of
microbes in the sedimentary CH4 filter and their response
to anthropogenic perturbations are not well understood.
) as electron acceptors.3 However, recent discoveries show that alternative
anaerobic
pathways such as CH4 oxidation coupled to Fe and Mn oxide
reduction can also play a role.7−9 The quantitative role of metal-dependent
anaerobic oxidation of CH4 is largely unknown. Nitrate
and nitrite can also be used as electron acceptors to oxidize CH4,10 but because of their relatively
low concentrations in marine sediments, they are expected to play
a limited role.11 Microbial oxidation rates
of CH4 coupled to different electron acceptors are often
estimated via geochemical modeling or incubations with radiotracers.12−14 However, quantification of the in situ cell-specific rates and doubling
times that ultimately control the ability of microorganisms to adapt
to changing environmental conditions remains a challenge. This specifically
holds for slow growing microbes, such as anaerobic methanotrophic
archaea (ANME).15,16 As a consequence, the role of
microbes in the sedimentary CH4 filter and their response
to anthropogenic perturbations are not well understood.
Recently,
reactive transport models (RTMs) that include microbial
dynamics were developed to describe nitrogen dynamics in the water
column of oxygen minimum zones.17,18 In these models, functional
gene abundances were used as a proxy for the cell abundances that
are associated with a given redox pathway. These studies show that
the use of the functional gene approach in RTMs increases their predictive
power. Here, we present a gene-based RTM for sediments, in which we
use the same principles17,18 to investigate the
controls on the microbial CH4 filter. We applied the model
to sediments from a brackish coastal site in the Bothnian Sea that
is rich in CH4 and Fe oxides and where both geochemical
and microbial data suggest a high potential for anaerobic CH4 oxidation coupled to 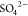 and to Fe and Mn oxides.19,20 The RTM is calibrated with porewater and solid phase depth profiles
and depth-dependent oxidation and reduction rates of key geochemical
processes. With the RTM, we show that O2 and
and to Fe and Mn oxides.19,20 The RTM is calibrated with porewater and solid phase depth profiles
and depth-dependent oxidation and reduction rates of key geochemical
processes. With the RTM, we show that O2 and  are the key electron acceptors
for CH4 oxidation. Metal oxides can also play an appreciable
role,
accounting for up to 10% of the total CH4 oxidized. The
relatively slow growth rate of ANMEs in comparison to other microbes
prevents their rapid adjustment to quickly changing environmental
conditions. In dynamic systems with large temporal changes in porewater
O2 and
are the key electron acceptors
for CH4 oxidation. Metal oxides can also play an appreciable
role,
accounting for up to 10% of the total CH4 oxidized. The
relatively slow growth rate of ANMEs in comparison to other microbes
prevents their rapid adjustment to quickly changing environmental
conditions. In dynamic systems with large temporal changes in porewater
O2 and 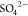 concentrations, such as coastal
zones,
this leads to periods of high benthic CH4 release. With
a sensitivity analysis, we investigate the response of the microbial
communities to changes in environmental parameters such as bottom
water O2 and organic matter and metal oxide deposition.
We show that continued coastal eutrophication and deoxygenation will
decrease the efficiency of the microbial CH4 filter. Ultimately,
this will enhance the importance of the oxidation of CH4 in the water column, which is the last barrier before CH4 is released to the atmosphere.
concentrations, such as coastal
zones,
this leads to periods of high benthic CH4 release. With
a sensitivity analysis, we investigate the response of the microbial
communities to changes in environmental parameters such as bottom
water O2 and organic matter and metal oxide deposition.
We show that continued coastal eutrophication and deoxygenation will
decrease the efficiency of the microbial CH4 filter. Ultimately,
this will enhance the importance of the oxidation of CH4 in the water column, which is the last barrier before CH4 is released to the atmosphere.
Materials and Methods
Study Area and Sampling
The Öre Estuary is located
at the Swedish coast in the Bothnian Sea (Figure 1A). The estuary is oligotrophic and has a
surface area of approximately 70 km2, a mean depth of 10
m, and a bottom water salinity of ca. 6. This study focuses on site
NB8 that is located in the deepest part of the estuary (Figure 1B). The site is characterized
by oxygenated bottom waters, bioirrigation to a depth of ca. 10 cm,
and high rates of organic matter deposition (Figure SA.1).19 Biogeochemical processes
in the Öre Estuary are strongly impacted by pulses of high
Fe, Mn, and organic carbon input from the Öre River that occur
every ca. 20 years and are thought to be coupled to hydrological changes
on land.19,21 Microbial community analysis by 16S rRNA
gene amplicons at our site revealed a high relative archaeal abundance
of up to 90% ANMEs 2a,b.20 The ANMEs 2a,b
become abundant in the SMTZ and follow the Fe content below the SMTZ.
This indicates the potential of ANMEs 2a,b to couple CH4 oxidation to both 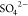 and Fe oxide reduction.
and Fe oxide reduction.
Figure 1.
(A) Location of the Öre Estuary in the Bothnian Sea. (B) Location of sampling site NB8 in the Öre Estuary. Figure drawn using Ocean Data View.22
Sediment was collected during a field campaign
with R/V
Botnica in June 2019 using a Gemini gravity corer (8 cm inner
diameter). In total, 11 cores were collected. Core 1 was used for
porewater and solid phase analyses; core 2 was used for CH4 sampling; core 3 was used for O2 micro-profiling; cores
4–5 were used for the determination of Fe and Mn reduction
and 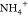 production rates; cores 6–7 were
used to determine sulfate reduction rates (SRR); cores 8–9
were used to determine CH4 production rates; core 10 was
used to determine the sediment porosity; and core 11 was used to determine
sedimentary bioirrigation rates in the sediment.
production rates; cores 6–7 were
used to determine sulfate reduction rates (SRR); cores 8–9
were used to determine CH4 production rates; core 10 was
used to determine the sediment porosity; and core 11 was used to determine
sedimentary bioirrigation rates in the sediment.
Cores for CH4 and  reduction rates were sampled directly
after
core recovery using a core liner with pre-drilled holes with a 2.5
cm depth spacing. For CH4, samples of 10 mL were taken
with cutoff syringes from each hole and immediately transferred to
a 65 mL glass bottle filled with saturated salt solution. The bottles
were stoppered, capped, and stored upside down until analysis. For
reduction rates were sampled directly
after
core recovery using a core liner with pre-drilled holes with a 2.5
cm depth spacing. For CH4, samples of 10 mL were taken
with cutoff syringes from each hole and immediately transferred to
a 65 mL glass bottle filled with saturated salt solution. The bottles
were stoppered, capped, and stored upside down until analysis. For 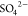 reduction rates, samples of 5
mL were taken
with cutoff syringes from each hole and were closed directly with
parafilm.5
reduction rates, samples of 5
mL were taken
with cutoff syringes from each hole and were closed directly with
parafilm.5
All other cores were brought
back to shore for further processing.
From the core for porewater and solid phase analysis, two bottom water
samples were taken, and subsequently the core was transferred into
intervals of 1–4 cm under a nitrogen atmosphere at bottom water
temperature. Each sediment sample was sliced into a 50 mL centrifuge
tube. The 50 mL centrifuge tubes were centrifuged at 4000 rpm for
20 min to extract porewater. Cores for Fe and Mn oxide reduction and 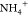 production rates were sliced under an anoxic
atmosphere in 7 different intervals (0–0.5, 0.5–3.5,
3.5–6.5, 17–20, 30–33, 45–48, and 57–60
cm) into plastic beakers, except for the top sample that was sampled
in a 50 mL centrifuge tube. Cores for CH4 production rates
were sliced under an anoxic atmosphere in 6 different intervals (0–4,
9–12, 21–24, 33–36, 49–52, and 69–72)
into geochemical bags. The core to determine the sediment water content
was sliced into intervals of 1–2 cm into pre-weighted 50 mL
greiner tubes.
production rates were sliced under an anoxic
atmosphere in 7 different intervals (0–0.5, 0.5–3.5,
3.5–6.5, 17–20, 30–33, 45–48, and 57–60
cm) into plastic beakers, except for the top sample that was sampled
in a 50 mL centrifuge tube. Cores for CH4 production rates
were sliced under an anoxic atmosphere in 6 different intervals (0–4,
9–12, 21–24, 33–36, 49–52, and 69–72)
into geochemical bags. The core to determine the sediment water content
was sliced into intervals of 1–2 cm into pre-weighted 50 mL
greiner tubes.
Porewater
High-resolution depth
profiles of dissolved
O2 were obtained in a separate sediment core directly after
retrieval using microelectrodes (50 μm resolution) and a two-dimensional
micromanipulator. Calibration was performed with a 2-point calibration
with 100% oxygen-saturated and nitrogen-purged artificial seawater
using the CAL300 calibration chamber (Unisense). Bottom and porewater
samples were filtered through 0.45 μm pore size filters and
subsampled under a nitrogen atmosphere. Subsamples were taken for
analysis of 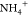 ,
, 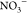 ,
,  , hydrogen sulfide (where H2S
represents the sum of H2S, HS–, and S2–), dissolved Fe, and dissolved Mn. Subsamples for
, hydrogen sulfide (where H2S
represents the sum of H2S, HS–, and S2–), dissolved Fe, and dissolved Mn. Subsamples for 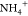 and
and  were stored frozen at −20 °C.
All other subsamples were stored at 4 °C until analysis.
were stored frozen at −20 °C.
All other subsamples were stored at 4 °C until analysis.
Samples for 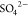 were analyzed with ion chromatography
(detection
limit of <75 μmol L–1; average analytical
uncertainty based on duplicate and triplicate is 1%). For H2S, 0.5 mL of porewater was immediately transferred into a 4 mL glass
vial containing 2 mL of a 2% zinc acetate solution to trap the H2S as ZnS. Sulfide was determined spectrophotometrically by
the complexion of the ZnS precipitate in an acidified solution of
phenylenediamine and ferric chloride.23 Subsamples taken for dissolved Fe and Mn were acidified with 10
μL 30% suprapur HCl per mL of sample and were analyzed by inductively
coupled plasma-optical emission spectrometry (ICP-OES; PerkinElmer
Avio 500). Porewater
were analyzed with ion chromatography
(detection
limit of <75 μmol L–1; average analytical
uncertainty based on duplicate and triplicate is 1%). For H2S, 0.5 mL of porewater was immediately transferred into a 4 mL glass
vial containing 2 mL of a 2% zinc acetate solution to trap the H2S as ZnS. Sulfide was determined spectrophotometrically by
the complexion of the ZnS precipitate in an acidified solution of
phenylenediamine and ferric chloride.23 Subsamples taken for dissolved Fe and Mn were acidified with 10
μL 30% suprapur HCl per mL of sample and were analyzed by inductively
coupled plasma-optical emission spectrometry (ICP-OES; PerkinElmer
Avio 500). Porewater  and
and  concentrations were determined colorimetrically
using the indophenol-blue method24 and
with a Gallery Automated Chemistry Analyzer type,25 respectively. For
concentrations were determined colorimetrically
using the indophenol-blue method24 and
with a Gallery Automated Chemistry Analyzer type,25 respectively. For 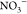 , the standard deviation of duplicate samples
was below 2%.
, the standard deviation of duplicate samples
was below 2%.
Samples for CH4 were prepared for measurement by injecting 10 mL of nitrogen headspace into the bottle. Subsequently, the CH4 concentrations in the headspace were determined by injection of a subsample (50–200 μL) into a Thermo Finnigan Trace GC gas chromatograph (flame ionization detector), after which CH4 concentrations were corrected for sediment porosity.
Solid Phase
Sediment samples that were analyzed for porosity were dried in an oven at 60 °C, and the porosity was determined from the weight loss. Sediment that was sliced under an anoxic atmosphere was freeze-dried. The freeze-dried sediments were ground and homogenized inside an argon-filled glovebox and subsequently separated into a fraction that was stored under oxic conditions (the oxic fraction) and a fraction that was stored under a nitrogen atmosphere (the anoxic fraction). The speciation of solid phase Fe and Mn was determined on the anoxic subsamples to avoid oxidation artifacts.26 A subsample of circa 300 mg from the oxic fraction was decalcified with 2 wash steps of 1 M HCl27 and subsequently dried, powdered, and analyzed for carbon using an elemental analyzer (Fisons Instruments NA 1500 NCS). Organic C content was determined after correction for the weight loss following decalcification.
Sedimentary Fe and Mn speciation was determined on ca. 50 mg from the anoxic fraction using a 5-step sequential extraction procedure (Table SA.1) based on.28−30 After extraction, all solutions were filtered through 0.45 μm pore size filters prior to analysis. Total Fe and Mn in the extraction solutions were determined via ICP-OES. Both Fe(II) and total Fe were measured in the 1 M HCl solution, and Fe(III) was calculated by subtracting the Fe(II) pool from total Fe. The average analytical uncertainty for Fe and Mn is <2%. The sedimentation rate at site NB8 was determined on 210Pb data from a sediment core that was sampled in August 2015 and was found to be 2.75 cm yr–1 (Figure SA.2).
Geochemical Rates
Fe and Mn reduction and  production rates were determined in incubations
with a duration of 2 days.31,32 SRRs were determined
on two separate sediment cores.5,33 The bioirrigation rate
was determined in a 2 day incubation of a sediment core in which the
inert tracer bromide was added to the overlying water.34 Methanogenesis was determined via bottle incubations.35 See Section SA.1 for
a more detailed description of the methods for the rate determinations.
production rates were determined in incubations
with a duration of 2 days.31,32 SRRs were determined
on two separate sediment cores.5,33 The bioirrigation rate
was determined in a 2 day incubation of a sediment core in which the
inert tracer bromide was added to the overlying water.34 Methanogenesis was determined via bottle incubations.35 See Section SA.1 for
a more detailed description of the methods for the rate determinations.
Construction and Calibration of the Gene-Based RTM
The model
that we applied to our site describes the mass balance
of 9 dissolved and 8 particulate species and is a modified version
of a standard multicomponent RTM based on the principles outlined
by.36 Here, we extended this model to include
the dynamics of key microbial groups that facilitate CH4 oxidation. We included 4 different groups of microbes that correspond
to a particular metabolism:18,37 (1) aerobic CH4 oxidation; (2)  driven anaerobic oxidation of
CH4 (
driven anaerobic oxidation of
CH4 (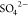 –AOM); (3) Fe oxide driven
anaerobic
oxidation of CH4 (Fe–AOM); and (4) Mn oxide driven
anaerobic oxidation of CH4 (Mn–AOM). In the model,
substrate-dependent microbial growth is described using Michaelis–Menten
kinetics with an optional inhibition factor,17,37 including the thermodynamic potential factor FT;17 that accounts for the Gibbs
free energy available to drive the metabolism. The equation that describes
modeled microbial growth in cell yr–1 cm–3 is defined as
–AOM); (3) Fe oxide driven
anaerobic
oxidation of CH4 (Fe–AOM); and (4) Mn oxide driven
anaerobic oxidation of CH4 (Mn–AOM). In the model,
substrate-dependent microbial growth is described using Michaelis–Menten
kinetics with an optional inhibition factor,17,37 including the thermodynamic potential factor FT;17 that accounts for the Gibbs
free energy available to drive the metabolism. The equation that describes
modeled microbial growth in cell yr–1 cm–3 is defined as
| 1 |
where −qr is the death rate (yr–1), Γr is the microbial abundance (cells cm–3), c is the average dry cell mass (gram cell–1), Zr is the biomass production coefficient (gram mol–1), and Hr is the cell-specific reaction rate (mol yr–1 cell–1). The rate of the processes in mol yr–1 cm–3 is defined as
| 2 |
where Cm is the concentration of the reactant. The model is calibrated with porewater and solid phase depth profiles and depth-dependent production and removal rates of key geochemical processes in the sediment. Model details and settings are given in Section SA.2.
Results and Discussion
Methane Dynamics in Coastal Sediments
At our coastal
site, high rates of organic matter decomposition are evident from
the limited penetration of O2 and nitrate (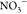 ) in the sediment (i.e., 0.7 and 4 cm, respectively)
and high concentrations of porewater ammonium (
) in the sediment (i.e., 0.7 and 4 cm, respectively)
and high concentrations of porewater ammonium (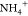 ; up to 3 mmol L–1; Figure 2A). Both the low
salinity and active
; up to 3 mmol L–1; Figure 2A). Both the low
salinity and active 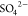 reduction contribute to a shallow
SMTZ
at ca. 20 cm depth, below which CH4 concentrations increase
up to ca. 6 mmol L–1. Despite high SRRs, little
sulfide accumulates in the porewater because of the abundant presence
of Fe oxides Figure 2.19 In the methanic zone, the dissolution
of Fe and Mn oxides leads to high concentrations of dissolved Fe and
Mn (up to 2.8 and 0.6 mmol L–1, respectively). This
is attributed to Fe and Mn oxide-mediated oxidation of CH4 at depth.19
reduction contribute to a shallow
SMTZ
at ca. 20 cm depth, below which CH4 concentrations increase
up to ca. 6 mmol L–1. Despite high SRRs, little
sulfide accumulates in the porewater because of the abundant presence
of Fe oxides Figure 2.19 In the methanic zone, the dissolution
of Fe and Mn oxides leads to high concentrations of dissolved Fe and
Mn (up to 2.8 and 0.6 mmol L–1, respectively). This
is attributed to Fe and Mn oxide-mediated oxidation of CH4 at depth.19
Figure 2.
(A) Porewater depth profiles
of O2, 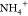 ,
,  ,
,  , H2S, dissolved Fe,
and dissolved
Mn; (B) solid phase depth profiles of total organic carbon, Fe oxides,
and Mn oxides. Due to strong variations in the incorporation of Mn
in the structure of vivianite,38,39 this mineral is not
included in the RTM. (C) Production rates of
, H2S, dissolved Fe,
and dissolved
Mn; (B) solid phase depth profiles of total organic carbon, Fe oxides,
and Mn oxides. Due to strong variations in the incorporation of Mn
in the structure of vivianite,38,39 this mineral is not
included in the RTM. (C) Production rates of 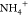 and CH4 and reduction rates
of
and CH4 and reduction rates
of 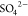 , Fe oxides (FeOx), and
Mn oxides (MnOx).
Colored diamonds are measured concentrations or rates, and the black
lines are modeled concentrations or rates from the RTM.
, Fe oxides (FeOx), and
Mn oxides (MnOx).
Colored diamonds are measured concentrations or rates, and the black
lines are modeled concentrations or rates from the RTM.
We applied our RTM to key porewater and solid-phase
depth profiles
and to measured rates of CH4 and  production and reduction rates of
production and reduction rates of  , Fe oxide, and Mn oxide at our
site (Figure 2). Based
on previous
work,19 we implemented a transient scenario
in which a period of increased organic matter, Fe and Mn oxide deposition
occurred every 20 years (Figure SA.3 and Section SA.2.3). Modeled porewater and solid-phase
depth profiles adequately capture the trends in the measured profiles
(Figure 2). The same
holds for the modeled rates of
, Fe oxide, and Mn oxide at our
site (Figure 2). Based
on previous
work,19 we implemented a transient scenario
in which a period of increased organic matter, Fe and Mn oxide deposition
occurred every 20 years (Figure SA.3 and Section SA.2.3). Modeled porewater and solid-phase
depth profiles adequately capture the trends in the measured profiles
(Figure 2). The same
holds for the modeled rates of  production and Fe and Mn oxide reduction.
The modeled SRR above the SMTZ is similar to the measured rates. However,
below the SMTZ, the depth profiles deviate, likely because of sample
handling issues that also impact potential rates of methane production,
as discussed in Section SA.3. The variations
in organic matter deposition strongly impact temporal CH4 dynamics at our site (Figure 3A,B). After periods of enhanced organic matter deposition,
methanogenesis becomes the key pathway for organic matter degradation,
and porewater CH4 concentrations strongly increase. During
these periods, microbial CH4 oxidation cannot keep up with
the sudden increase in methanogenesis, which leads to periodic benthic
CH4 release of up to 12 μmol m–2 d–1 (Figure 3A). This indicates that, especially in dynamic environments,
such as coastal zones, benthic CH4 release may occur periodically
because microbial abundances need to adjust to the change in CH4 supply and electron acceptor availability.
production and Fe and Mn oxide reduction.
The modeled SRR above the SMTZ is similar to the measured rates. However,
below the SMTZ, the depth profiles deviate, likely because of sample
handling issues that also impact potential rates of methane production,
as discussed in Section SA.3. The variations
in organic matter deposition strongly impact temporal CH4 dynamics at our site (Figure 3A,B). After periods of enhanced organic matter deposition,
methanogenesis becomes the key pathway for organic matter degradation,
and porewater CH4 concentrations strongly increase. During
these periods, microbial CH4 oxidation cannot keep up with
the sudden increase in methanogenesis, which leads to periodic benthic
CH4 release of up to 12 μmol m–2 d–1 (Figure 3A). This indicates that, especially in dynamic environments,
such as coastal zones, benthic CH4 release may occur periodically
because microbial abundances need to adjust to the change in CH4 supply and electron acceptor availability.
Figure 3.
(A) Modeled transient organic matter deposition and benthic CH4 release; (B) heatmap of porewater CH4 dynamics from 1969–2019; (C,D) relative contribution of the various pathways of organic matter degradation and CH4 oxidation in the sediment during enhanced OM deposition and in the last year of the model run (2019), respectively.
The various pathways of CH4 oxidation
are highly dependent
on temporal changes in organic matter deposition. In periods of enhanced
organic matter deposition, O2 is the main electron acceptor
for CH4 oxidation, while in the final year of our model
run (i.e., 2019), 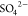 is responsible for ca. 75% of
CH4 oxidation (Figure 3D). This is in accordance with the current understanding
that the
oxidation of CH4 in marine systems is predominantly coupled
to O2 and
is responsible for ca. 75% of
CH4 oxidation (Figure 3D). This is in accordance with the current understanding
that the
oxidation of CH4 in marine systems is predominantly coupled
to O2 and  .2,3 Recent geochemical
and
microbiological evidence, however, shows that Fe and Mn oxides can
also mediate CH4 oxidation,7,8,40 but the quantitative importance of Fe- and Mn–AOM
is largely unknown. Measured and modeled rates in North Sea and Bothnian
Sea sediments suggest that Fe–AOM accounted for ca. 2–3%
of the total anaerobic oxidation of CH4.8,41 In
our model for metal oxide-rich sediment, Fe and Mn oxide-mediated
CH4 oxidation is responsible for ca. 10% of the total CH4 oxidation in the year of sampling. This suggests that in
Fe and Mn oxide-rich sediments, Fe- and Mn–AOM are able to
account for an appreciable fraction of the oxidation of sedimentary
CH4. This is likely especially important in sediments close
to river mouths where the Fe and Mn oxide input is high,42 and the depth of the SMTZ is located relatively
close to the sediment-water interface because of a low salinity.
.2,3 Recent geochemical
and
microbiological evidence, however, shows that Fe and Mn oxides can
also mediate CH4 oxidation,7,8,40 but the quantitative importance of Fe- and Mn–AOM
is largely unknown. Measured and modeled rates in North Sea and Bothnian
Sea sediments suggest that Fe–AOM accounted for ca. 2–3%
of the total anaerobic oxidation of CH4.8,41 In
our model for metal oxide-rich sediment, Fe and Mn oxide-mediated
CH4 oxidation is responsible for ca. 10% of the total CH4 oxidation in the year of sampling. This suggests that in
Fe and Mn oxide-rich sediments, Fe- and Mn–AOM are able to
account for an appreciable fraction of the oxidation of sedimentary
CH4. This is likely especially important in sediments close
to river mouths where the Fe and Mn oxide input is high,42 and the depth of the SMTZ is located relatively
close to the sediment-water interface because of a low salinity.
Abundance and Growth of Methanotrophs
In our RTM results, three distinct zones of cell abundance in the sediment can be distinguished based on the modeled presence of microbes involved in CH4 oxidation (Figure 4). Aerobic CH4 oxidizers are most abundant in the upper 10 cm of the sediment, while anaerobic CH4 oxidizer abundances are low. Below 10 cm depth in the SMTZ, cell abundances of ANMEs strongly increase. Cell abundances of ANMEs in CH4-rich sediments along continental margins can vary over orders of magnitude and depend on the SMTZ depth. For example, at a site with an extremely shallow SMTZ offshore Oregon (ca. 3 cm), an ANME abundance of 0.7 * 1010 cells cm–3 is reported.43 This contrasts with observations for a North Sea site with a deeper SMTZ (ca. 70 cm), where an abundance of ca. 4 * 106 cells cm–3 was found.41 At our site, the ANMEs are almost absent above the SMTZ and become abundant in the SMTZ, where cell abundances reach ca. 1.25 * 108 cells cm–3 at 17 cm depth (Figure 4). Below the SMTZ, the highest cell abundances (i.e., 2.2 * 108 cells cm–3) are found at the depth where Fe oxides and Mn oxides are present. This is in accordance with the observed 16S rRNA data for our site, where ANMEs 2a,b are absent above the SMTZ but are abundant in the SMTZ and in zones where Fe oxides are present.20
Figure 4.
Top row: depth profiles of the cell abundance of microbes associated
with CH4 oxidation coupled to reduction of O2, 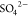 , Fe oxides, and Mn oxides,
cell-specific
rate for each pathway (fmol cell–1 d–1), and microbial community growth (d–1). The maximum
in situ doubling time in the sediment indicates the doubling time
in the last timestep of the model run. Fastest doubling times possible
for O2 cells,
, Fe oxides, and Mn oxides,
cell-specific
rate for each pathway (fmol cell–1 d–1), and microbial community growth (d–1). The maximum
in situ doubling time in the sediment indicates the doubling time
in the last timestep of the model run. Fastest doubling times possible
for O2 cells, 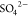 –ANME, FeOX–ANME,
and Mn–ANME
are <1, 124, 203, and 163 days, respectively (Table SA.7). Bottom row: absolute rates of CH4 oxidation
(nmol cm–3 d–1) with depth profiles
of O2,
–ANME, FeOX–ANME,
and Mn–ANME
are <1, 124, 203, and 163 days, respectively (Table SA.7). Bottom row: absolute rates of CH4 oxidation
(nmol cm–3 d–1) with depth profiles
of O2, 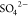 , Fe oxides, and Mn oxides and
CH4 concentration (green). Maximum concentration of CH4 is
ca. 12 mmol L–1. Plots show model data of the last
timestep in the RTM (i.e., for 2019).
, Fe oxides, and Mn oxides and
CH4 concentration (green). Maximum concentration of CH4 is
ca. 12 mmol L–1. Plots show model data of the last
timestep in the RTM (i.e., for 2019).
The doubling time of microbes predominantly determines
how fast
microbes can adjust to varying environmental conditions in the sediment.
Estimated growth rates of aerobic CH4 oxidizing bacteria
are in the order of 12 h to days44 and
are therefore expected to adapt quickly to variations in the availability
of O2. The doubling time of ANMEs is not well known but
has been estimated to be in the order of months.3,16,44 In our model scenario, the maximum doubling
times of 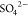 –ANME, FeOx–ANME,
and MnOx–ANME
are 124, 203, and 163 days, respectively (Table SA.7). The in situ doubling times in the last time step of
the model are especially low for FeOx–ANME and MnOx–ANME
(240 and 370 days, respectively), which indicates that their growth
is limited by the presence of electron acceptors at this timepoint.
The growth of
–ANME, FeOx–ANME,
and MnOx–ANME
are 124, 203, and 163 days, respectively (Table SA.7). The in situ doubling times in the last time step of
the model are especially low for FeOx–ANME and MnOx–ANME
(240 and 370 days, respectively), which indicates that their growth
is limited by the presence of electron acceptors at this timepoint.
The growth of 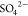 –ANME is relatively fast
(i.e., 130
days). This indicates that ANMEs that couple metal oxide reduction
to CH4 oxidation grow at a slower rate than
–ANME is relatively fast
(i.e., 130
days). This indicates that ANMEs that couple metal oxide reduction
to CH4 oxidation grow at a slower rate than 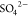 –ANMEs and therefore only
become
important deeper in the sediment when the microbial community has
had sufficient time to grow and accumulate enough biomass. To investigate
the impact of the maximum growth and death rate on microbial abundances
and geochemical depth profiles, we carried out a sensitivity analysis
where we multiplied the growth and death rate by a discrete factor
(0.5; 0.75; 0.9; 1.1; 1.25; and 1.5; Figures SA.4 and SA.5) and assessed the changes in the profiles. We find
that the model is very sensitive to changes in the growth rate and
less so for the death rate, as further discussed in Section SA.4.
–ANMEs and therefore only
become
important deeper in the sediment when the microbial community has
had sufficient time to grow and accumulate enough biomass. To investigate
the impact of the maximum growth and death rate on microbial abundances
and geochemical depth profiles, we carried out a sensitivity analysis
where we multiplied the growth and death rate by a discrete factor
(0.5; 0.75; 0.9; 1.1; 1.25; and 1.5; Figures SA.4 and SA.5) and assessed the changes in the profiles. We find
that the model is very sensitive to changes in the growth rate and
less so for the death rate, as further discussed in Section SA.4.
In sediments where geochemical processes
are in a steady state, 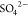 is quantitatively the most important
sink
for CH4.6 However, in highly
transient environments, such as coastal zones, the slow adaptation
of ANMEs to transient geochemical processes can alter the role of
CH4 oxidation by
is quantitatively the most important
sink
for CH4.6 However, in highly
transient environments, such as coastal zones, the slow adaptation
of ANMEs to transient geochemical processes can alter the role of
CH4 oxidation by 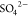 . This has previously been explored
in a
model study for continental margin sediments subject to increased
upward advective flow of CH4. In the corresponding model
scenario, it took >60 years for ANMEs to achieve equilibrium with
the new porewater concentrations.4 Sulfate-reducing
bacteria (SRB) can grow much faster doubling time of <1 day;45 than ANMEs and will typically outcompete ANME-associated
SRB. Therefore, heterotrophic SRB will adapt faster to transient situations
and are expected to play a key role in determining variations in SMTZ
depth. This can have major implications for the role of
. This has previously been explored
in a
model study for continental margin sediments subject to increased
upward advective flow of CH4. In the corresponding model
scenario, it took >60 years for ANMEs to achieve equilibrium with
the new porewater concentrations.4 Sulfate-reducing
bacteria (SRB) can grow much faster doubling time of <1 day;45 than ANMEs and will typically outcompete ANME-associated
SRB. Therefore, heterotrophic SRB will adapt faster to transient situations
and are expected to play a key role in determining variations in SMTZ
depth. This can have major implications for the role of 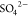 as an electron acceptor in CH4 oxidation and the efficiency of the sedimentary CH4 filter,
as we will show in the example discussed below.
as an electron acceptor in CH4 oxidation and the efficiency of the sedimentary CH4 filter,
as we will show in the example discussed below.
During periods
of enhanced organic matter deposition in our model
scenario, the SMTZ moved upward from ca. 24 to 17 cm (Figure 5A). This upward shift of the
SMTZ is coupled to enhanced heterotrophic 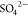 reduction. After this shift of
the SMTZ,
reduction. After this shift of
the SMTZ, 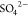 -ANMEs that are present at the
former SMTZ
depth (i.e., 24 cm) no longer have access to
-ANMEs that are present at the
former SMTZ
depth (i.e., 24 cm) no longer have access to 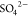 (Figure 5). Therefore, nearly all
(Figure 5). Therefore, nearly all  reduction becomes coupled to organic
matter
degradation, and
reduction becomes coupled to organic
matter
degradation, and 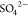 –AOM becomes a negligible
process.
This illustrates that ANMEs, because of their slow growth rate, cannot
adjust quickly to a change in the availability of substrate. In our
model scenario, aerobic CH4 oxidation then becomes the
key pathway of CH4 oxidation (Figure 3D).
–AOM becomes a negligible
process.
This illustrates that ANMEs, because of their slow growth rate, cannot
adjust quickly to a change in the availability of substrate. In our
model scenario, aerobic CH4 oxidation then becomes the
key pathway of CH4 oxidation (Figure 3D).
Figure 5.
(A) Integrated rates of methanogenesis (black),
total  reduction (green), and
reduction (green), and 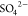 –AOM (gray) as calculated
by the
RTM. The blue dashed line indicates the depth of the SMZT, calculated
at the first depth where the
–AOM (gray) as calculated
by the
RTM. The blue dashed line indicates the depth of the SMZT, calculated
at the first depth where the  concentration is below 0.1 mmol
L–1 within the RTM. (B,C) show depth profiles of
concentration is below 0.1 mmol
L–1 within the RTM. (B,C) show depth profiles of 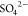 and CH4 (mmol L–1), the abundance of
and CH4 (mmol L–1), the abundance of  –ANME, and the rate of
–ANME, and the rate of  reduction coupled to oxidation
of organic
matter and CH4 for the years 1997 and 2001, respectively.
reduction coupled to oxidation
of organic
matter and CH4 for the years 1997 and 2001, respectively.
Increased organic matter input can lead to an upward
shift of the
SMTZ as a result of increased rates of  reduction and/or methanogenesis.11,46,47 Our model results suggest that
it is unlikely that ANMEs facilitate a rapid upward shift of the SMTZ
through
reduction and/or methanogenesis.11,46,47 Our model results suggest that
it is unlikely that ANMEs facilitate a rapid upward shift of the SMTZ
through 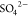 –AOM because their slow
growth rate
hinders a quick adjustment of their biomass to varying CH4 and
–AOM because their slow
growth rate
hinders a quick adjustment of their biomass to varying CH4 and 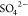 concentrations. Therefore, a sudden
upward
shift of the SMTZ is likely regulated by enhanced heterotrophic/organoclastic
concentrations. Therefore, a sudden
upward
shift of the SMTZ is likely regulated by enhanced heterotrophic/organoclastic  -reduction. This would lead to
the depletion
of
-reduction. This would lead to
the depletion
of 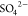 in the zone where ANMEs
are present and
therefore a limited contribution of
in the zone where ANMEs
are present and
therefore a limited contribution of 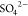 –AOM until ANMEs have had
enough
time to readjust to the new environmental conditions.
–AOM until ANMEs have had
enough
time to readjust to the new environmental conditions.
Rates of CH4 Oxidation
Cell-specific rates of microbes (fmol cell–1 d–1) determine how much substrate microbes can use per time unit. For slow-growing microbes with low energy yields such as ANMEs,15,16 these cell-specific rates are largely unknown.3,44 Cell-specific rates can be determined during long-term or pure-culture incubation experiments. However, rates from laboratory experiments are typically orders of magnitude higher than in situ rates7,13,48 because of changes in, for example, substrate availability and sediment handling. Hence, they should be considered as potential rates.2 Quantification of the role of microbes in CH4 oxidation requires insight into their in situ cell-specific rates in order to couple cell abundances to absolute rates in the sediment. Our model allows us to quantify such in situ cell-specific rates for various microbes and show how these vary with sediment depth.
In our model, cell-specific
rates depend strongly on substrate availability and follow Michaelis–Menten
kinetics (Figure SA.6). The highest cell-specific
rates are observed for aerobic methanotrophs in the zone where oxygen
is pumped into the sediment via bioirrigation at 2 cm depth. Here,
neither O2 nor CH4 is limiting. Cell-specific
rates of  –ANME are highest around
the SMTZ
and reach a value of ca. 1.5 fmol cell–1 d–1, which falls within the range of rates suggested in the literature
of 0.2–10 fmol cell–1 d–1.11,49 Cell-specific rates of FeOx- and MnOx–ANME
are highest in the zones where the respective metal oxides are present.
Cell-specific rates are, however, 1 or 2 orders of magnitude lower
compared to those of aerobic methanotrophs and
–ANME are highest around
the SMTZ
and reach a value of ca. 1.5 fmol cell–1 d–1, which falls within the range of rates suggested in the literature
of 0.2–10 fmol cell–1 d–1.11,49 Cell-specific rates of FeOx- and MnOx–ANME
are highest in the zones where the respective metal oxides are present.
Cell-specific rates are, however, 1 or 2 orders of magnitude lower
compared to those of aerobic methanotrophs and 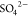 –ANME, despite the fact
that Fe and
Mn oxides are more energetically favorable electron acceptors compared
to
–ANME, despite the fact
that Fe and
Mn oxides are more energetically favorable electron acceptors compared
to  . This is likely the case
because
. This is likely the case
because 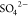 is a solute and therefore more
easily available
to microbes than solids such as Fe and Mn oxides.7 This additionally leads to a slower growth rate for FeOx-
and MnOx–ANMEs.
is a solute and therefore more
easily available
to microbes than solids such as Fe and Mn oxides.7 This additionally leads to a slower growth rate for FeOx-
and MnOx–ANMEs.
The absolute rate of CH4 oxidation
depends on both the
cell-specific rate and the microbial abundance. For aerobic methanotrophs,
the rate is highest in the bioirrigation zone (up to 10 nmol cm–3 d–1) and is near zero in the zone
where O2 penetrates because of CH4 limitation
(Figure 4). This shows
that enhanced oxygenation of the sediment due to bioirrigation can
be an efficient barrier for upward diffusing CH4 and act
as an important control on benthic CH4 emissions. Rates
of 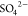 –AOM are strongly
enhanced in a shallow
zone of the SMTZ with rates up to 60 nmol cm–3 d–1 and are low above and below the SMTZ because of substrate
limitation. Absolute rates of Fe- and Mn–AOM are only high
below the SMTZ (up to 1 and 0.3 nmol cm–3 d–1, respectively; Figure 4). Rates for Fe–AOM are in the same range as
found for sediments that were incubated with ferrihydrite 1–5
nmol cm–3 d–1.41 In situ rates of Mn–AOM are largely unknown. However,
in incubation studies, very high rates were observed, i.e., ca. 40
nmol cm–3 d–1;7,40 when
compared to those in our model. This might be because of the strongly
enhanced Mn oxide concentrations in the incubations compared to the
lower contents (<10 μmol g–1 Mn oxide)
in our sediments. Despite the abundant presence of Fe and Mn oxides
above the SMTZ, absolute rates are low because of the low abundance
of the responsible microbes.
–AOM are strongly
enhanced in a shallow
zone of the SMTZ with rates up to 60 nmol cm–3 d–1 and are low above and below the SMTZ because of substrate
limitation. Absolute rates of Fe- and Mn–AOM are only high
below the SMTZ (up to 1 and 0.3 nmol cm–3 d–1, respectively; Figure 4). Rates for Fe–AOM are in the same range as
found for sediments that were incubated with ferrihydrite 1–5
nmol cm–3 d–1.41 In situ rates of Mn–AOM are largely unknown. However,
in incubation studies, very high rates were observed, i.e., ca. 40
nmol cm–3 d–1;7,40 when
compared to those in our model. This might be because of the strongly
enhanced Mn oxide concentrations in the incubations compared to the
lower contents (<10 μmol g–1 Mn oxide)
in our sediments. Despite the abundant presence of Fe and Mn oxides
above the SMTZ, absolute rates are low because of the low abundance
of the responsible microbes.
Environmental Constraints on CH4 Oxidation
Eutrophication and deoxygenation are impacting many coastal ecosystems and have the potential to greatly alter CH4 dynamics in sediments.50−52 Enhanced eutrophication can stimulate methanogenesis, while deoxygenation can lead to less efficient CH4 oxidation in the sediment. The efficiency of CH4 oxidation is also very sensitive to other environmental perturbations, such as sea level rise and changes in precipitation, which can alter bottom water salinity53,54 and variations in riverine fluxes of metals, which can alter the metal oxide deposition.55 To investigate the effect of these perturbations on the efficiency of the sedimentary CH4 filter and the microbial dynamics, we carried out a sensitivity analysis where we changed the (1) bottom water salinity; (2) bottom water O2; (3) organic matter input; and (4) Fe and Mn oxide input.
At higher salinity, methanogenesis is
suppressed because of enhanced organoclastic  reduction and
reduction and 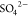 -AOM (Figure 6A). Our sensitivity analysis suggests that
CH4 oxidation is efficient over the full salinity range
because of efficient
CH4 oxidation by O2 and partly by metal oxides
at lower salinity. However, a small benthic CH4 flux of
4 μmol m–2 d–1 is observed
at a salinity of 0. When the bottom water O2 is increased,
methanogenesis slightly decreases and the role of Fe- and Mn–AOM
increases (Figure 6B). At lower bottom water O2, the importance of
-AOM (Figure 6A). Our sensitivity analysis suggests that
CH4 oxidation is efficient over the full salinity range
because of efficient
CH4 oxidation by O2 and partly by metal oxides
at lower salinity. However, a small benthic CH4 flux of
4 μmol m–2 d–1 is observed
at a salinity of 0. When the bottom water O2 is increased,
methanogenesis slightly decreases and the role of Fe- and Mn–AOM
increases (Figure 6B). At lower bottom water O2, the importance of 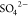 –AOM increases. However,
when bottom
water O2 becomes lower than 50 μmol L–1 oxidation of CH4 is not efficient enough, and enhanced
benthic CH4 release is observed. This flux is potentially
even higher when both the salinity and O2 would decrease.
–AOM increases. However,
when bottom
water O2 becomes lower than 50 μmol L–1 oxidation of CH4 is not efficient enough, and enhanced
benthic CH4 release is observed. This flux is potentially
even higher when both the salinity and O2 would decrease.
Figure 6.

Sensitivity
analysis of CH4 removal via benthic release
(CH4 flux), and depth integrated oxidation rates coupled
to reduction of O2, Mn oxides (MnOx), Fe oxide (FeOx),
and 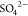 (mmol m–2 d–1) for (A) salinity; (B) bottom water O2; (C) the organic
matter input factor; and (D) Fe and Mn oxide input factor compared
to the baseline scenario. (E–H) Show the corresponding relative
abundance in microbial communities for the same sensitivity analysis
as A–D. The results of the baseline scenario are indicated
by the vertical dashed line. The benthic flux and integrated rates
are averages for the last 50 years of the transient scenario (Figure SA.3). Porewater profiles for the last
time steps of the sensitivity analysis are shown in Figure SA.7.
(mmol m–2 d–1) for (A) salinity; (B) bottom water O2; (C) the organic
matter input factor; and (D) Fe and Mn oxide input factor compared
to the baseline scenario. (E–H) Show the corresponding relative
abundance in microbial communities for the same sensitivity analysis
as A–D. The results of the baseline scenario are indicated
by the vertical dashed line. The benthic flux and integrated rates
are averages for the last 50 years of the transient scenario (Figure SA.3). Porewater profiles for the last
time steps of the sensitivity analysis are shown in Figure SA.7.
Upon enhanced organic
matter input, methanogenesis
strongly increases,
and the oxidation of CH4 coupled to O2 and 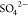 is enhanced (Figure 6C). However, when the deposition
of organic
matter increases >5%, the efficiency of CH4 oxidation
declines
and benthic CH4 release increases. When the deposition
of Fe and Mn oxides increases, methanogenesis slightly decreases,
and the role of Fe- and Mn–AOM increases (Figure 6D). However, O2 and
is enhanced (Figure 6C). However, when the deposition
of organic
matter increases >5%, the efficiency of CH4 oxidation
declines
and benthic CH4 release increases. When the deposition
of Fe and Mn oxides increases, methanogenesis slightly decreases,
and the role of Fe- and Mn–AOM increases (Figure 6D). However, O2 and 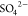 remain the major electron acceptors
for
CH4 oxidation.
remain the major electron acceptors
for
CH4 oxidation.
The microbial composition of the aerobic
and anaerobic CH4 oxidizing microbes in the model strongly
varies (Figure 6E–H).
We show that the
microbial composition does not necessarily reflect the importance
of a related microbially driven process. For example, FeOx- and MnOx–ANME
can account for a high biomass; however, because of their relatively
low cell-specific rates compared to 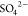 –AOM and especially aerobic
CH4 oxidation, the relative importance of Fe- and Mn–AOM
remains limited. This highlights the importance of combining the microbial
abundances as a proxy for a certain process with the cell-specific
rates of the microbes.
–AOM and especially aerobic
CH4 oxidation, the relative importance of Fe- and Mn–AOM
remains limited. This highlights the importance of combining the microbial
abundances as a proxy for a certain process with the cell-specific
rates of the microbes.
Our modeling results suggest that the
anaerobic oxidation of CH4 coupled to Fe and Mn oxide reduction
is promoted by the following
factors: (1) a low bottom water salinity, since 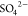 –AOM is low and sulfide
production
is limited, resulting in a higher availability of Fe and Mn oxides;
(2) high bottom water O2, since enhanced recycling of Fe
and Mn increases the sedimentary Fe and Mn oxide content and there
is little escape of dissolved Fe and Mn from the sediment; (3) intermediate
rates of organic matter deposition, since at low organic matter deposition
Fe- and Mn–AOM is limited by CH4 production, while
at high organic matter input the availability of Fe and Mn oxides
decreases because of enhanced sulfide production and subsequent Fe
and Mn oxide dissolution and FeSx precipitation;
(4) a high input of Fe and Mn oxides since this directly promotes
Fe- and Mn–-AOM.
–AOM is low and sulfide
production
is limited, resulting in a higher availability of Fe and Mn oxides;
(2) high bottom water O2, since enhanced recycling of Fe
and Mn increases the sedimentary Fe and Mn oxide content and there
is little escape of dissolved Fe and Mn from the sediment; (3) intermediate
rates of organic matter deposition, since at low organic matter deposition
Fe- and Mn–AOM is limited by CH4 production, while
at high organic matter input the availability of Fe and Mn oxides
decreases because of enhanced sulfide production and subsequent Fe
and Mn oxide dissolution and FeSx precipitation;
(4) a high input of Fe and Mn oxides since this directly promotes
Fe- and Mn–-AOM.
Perspectives
Microbial dynamics strongly determine geochemical processes such as CH4 oxidation in the sediment. Gene-centric modeling, as applied here, is an effective tool to determine the characteristics of slow-growing microbes such as anaerobic CH4 oxidizers and the impact of their activity on the efficiency of the microbial CH4 filter. The incorporation of microbial dynamics in biogeochemical models, as done here for sediments, allows us to investigate key characteristics of microbial communities. Importantly, the inclusion of microbial dynamics in RTMs is especially relevant when assessing the effects of environmental perturbations in systems where slow-growing microbes, such as ANMEs but also anammox bacteria, are involved in critical removal processes. Further improvement of the predictive power of these types of biogeochemical models can be achieved through: (1) a better quantification of microbial abundances either through qPCR to determine the amount of genes or through single-cell methods (such as CARD-FISH, nanoSIMS, or flow cytometry) or very high throughput sequencing/transcriptomics to determine the amount of active cells in the sediment; (2) a better quantification of the half rate constants and maximum cell-specific rates to further constrain the substrate dependent reaction rates (i.e., Michaelis Menten kinetics); (3) the determination of maximum growth rates of microorganisms through incubation studies; (4) the determination of death rates and the key factors that control the death rate of microorganisms; (5) the evaluation of possible inhibition factors on the growth and efficiency of CH4 oxidizing microbes, for example, sulfide inhibition, possibly by incubation studies.
Acknowledgments
We thank the captain and crew and for their assistance during sampling aboard R/V Botnica. We thank Henrik Larsson and Johan Wikner from Umeå Science Centre for support during fieldwork and labwork at the Umeå Marina Forskningscentrum. We thank Coen Mulder, John Visser, Thom Claessen, Arnold van Dijk, Santiago Gonzalez, Martijn Hermans, and Lilia Orozco Ramirez for their analytical assistance. This work was funded by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) NESSC Gravitation Grant 02001001 [CPS, MSMJ], SIAM Gravitation grant 024.002.001 [PDM, MSMJ], and ERC Marix grant 854088 [CPS, MSMJ]. PDM acknowledges support from NWO-Veni grant 212.040.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c02023.
Detailed description of rate measurements and model code. Figures: results of bromide tracer incubations at station NB8; measured and modeled depth profiles of 210Pb and porosity at station NB8; modeled transient fluxes of organic matter, Fe oxides, and Mn oxides at the sediment–water interface; model sensitivity analysis; modeled Michaelis−Menten kinetics of methane oxidation rates; porewater profiles for the sensitivity analysis; Tables: sequential extraction procedure for Fe and Mn; reaction pathways and stoichiometries implemented in the model; environmental parameters; boundary conditions of solids and solutes at the sediment−water interface; reaction equations; and potential CH4 production rates determined for site NB8 (PDF)
Porewater, solids phase and rate depth profiles (ZIP)
Author Present Address
§ P.D.M: Microbial Ecology Cluster, GELIFES, University of Groningen, Broerstraat 5, 9712 CP Groningen, The Netherlands
Author Contributions
WL and CS designed the research and wrote the paper with comments provided by NvH, PDM, AW, and MJ. WL, NvH, and PDM performed the sampling and analyses. WL wrote the model code and performed model simulations. WL, NvH, PDM, AW, MJ, and CS interpreted the data. All authors contributed to the article and approved the submitted version.
The authors declare no competing financial interest.
Notes
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supporting Information.
Supplementary Material
References
- Saunois M.; Stavert A. R.; Poulter B.; Bousquet P.; Canadell J. G.; Jackson R. B.; Raymond P. A.; Dlugokencky E. J.; Houweling S.; Patra P. K.; et al. The global methane budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. 10.5194/essd-12-1561-2020. [DOI] [Google Scholar]
- Reeburgh W. Oceanic methane biogeochemistry. Chem. Rev. 2007, 38, 486–513. 10.1002/chin.200720267. [DOI] [PubMed] [Google Scholar]
- Knittel K.; Boetius A. Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 2009, 63, 311–334. 10.1146/annurev.micro.61.080706.093130. [DOI] [PubMed] [Google Scholar]
- Dale A. W.; Van Cappellen P.; Aguilera D. R.; Regnier P. Methane efflux from marine sediments in passive and active margins: Estimations from bioenergetic reaction–transport simulations. Earth Planet. Sci. Lett. 2008, 265, 329–344. 10.1016/j.epsl.2007.09.026. [DOI] [Google Scholar]
- Egger M.; Lenstra W.; Jong D.; Meysman F. J.; Sapart C. J.; Van Der Veen C.; Röckmann T.; Gonzalez S.; Slomp C. P. Rapid sediment accumulation results in high methane effluxes from coastal sediments. PLoS One 2016, 11, e0161609 10.1371/journal.pone.0161609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M.; Riedinger N.; Mogollón J. M.; Jørgensen B. B. Global diffusive fluxes of methane in marine sediments. Nat. Geosci. 2018, 11, 421–425. 10.1038/s41561-018-0122-8. [DOI] [Google Scholar]
- Beal E. J.; House C. H.; Orphan V. J. Manganese- and iron-dependent marine methane oxidation. Science 2009, 325, 184–187. 10.1126/science.1169984. [DOI] [PubMed] [Google Scholar]
- Egger M.; Rasigraf O.; Sapart C. J.; Jilbert T.; Jetten M. S.; Röckmann T.; Van Der Veen C.; Bânda N.; Kartal B.; Ettwig K. F.; Slomp C. P. Iron-mediated anaerobic oxidation of methane in brackish coastal sediments. Environ. Sci. Technol. 2015, 49, 277–283. 10.1021/es503663z. [DOI] [PubMed] [Google Scholar]
- Cai C.; Leu A. O.; Xie G. J.; Guo J.; Feng Y.; Zhao J. X.; Tyson G. W.; Yuan Z.; Hu S. A methanotrophic archaeon couples anaerobic oxidation of methane to Fe(III) reduction. ISME J. 2018, 12, 1929–1939. 10.1038/s41396-018-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghoebarsing A. A.; Pol A.; Van de Pas-Schoonen K. T.; Smolders A. J. P.; Ettwig K. F.; Rijpstra W. I. C.; Schouten S.; Damsté J. S. S.; Op den Camp H. J. M.; Jetten M. S. M.; et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 2006, 440, 918–921. 10.1038/nature04617. [DOI] [PubMed] [Google Scholar]
- Jørgensen B. B. Sulfur Biogeochemical Cycle of Marine Sediments. Geochem. Perspect. 2021, 10, 145–307. 10.7185/geochempersp.10.2. [DOI] [Google Scholar]
- Iversen N.; Jorgensen B. B. Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark) 1. Limnol. Oceanogr. 1985, 30, 944–955. 10.4319/lo.1985.30.5.0944. [DOI] [Google Scholar]
- Treude T.; Boetius A.; Knittel K.; Wallmann K.; Jørgensen B. B. Anaerobic oxidation of methane above gas hydrates at Hydrate Ridge, NE Pacific Ocean. Mar. Ecol.: Prog. Ser. 2003, 264, 1–14. 10.3354/meps264001. [DOI] [Google Scholar]
- Rooze J.; Egger M.; Tsandev I.; Slomp C. P. Iron-dependent anaerobic oxidation of methane in coastal surface sediments: Potential controls and impact. Limnol. Oceanogr. 2016, 61, S267–S282. 10.1002/lno.10275. [DOI] [Google Scholar]
- Dale A. W.; Regnier P.; Van Cappellen P. Bioenergetic controls on anaerobic oxidation of methane (AOM) in coastal marine sediments: a theoretical analysis. Am. J. Sci. 2006, 306, 246–294. 10.2475/ajs.306.4.246. [DOI] [Google Scholar]
- Nauhaus K.; Albrecht M.; Elvert M.; Boetius A.; Widdel F. In vitro cell growth of marine archaeal-bacterial consortia during anaerobic oxidation of methane with sulfate. Environ. Microbiol. 2007, 9, 187–196. 10.1111/j.1462-2920.2006.01127.x. [DOI] [PubMed] [Google Scholar]
- Reed D. C.; Algar C. K.; Huber J. A.; Dick G. J. Gene-centric approach to integrating environmental genomics and biogeochemical models. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 1879–1884. 10.1073/pnas.1313713111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca S.; Hawley A. K.; Katsev S.; Torres-Beltran M.; Bhatia M. P.; Kheirandish S.; Michiels C. C.; Capelle D.; Lavik G.; Doebeli M.; Crowe S. A.; Hallam S. J. Integrating biogeochemistry with multiomic sequence information in a model oxygen minimum zone. Proc. Natl. Acad. Sci. U.S.A. 2016, 113, E5925–E5933. 10.1073/pnas.1602897113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra W. K.; Egger M.; van Helmond N. A. G. M.; Kritzberg E.; Conley D. J.; Slomp C. P. Large variations in iron input to an oligotrophic Baltic Sea estuary: impact on sedimentary phosphorus burial. Biogeosciences 2018, 15, 6979–6996. 10.5194/bg-15-6979-2018. [DOI] [Google Scholar]
- Rasigraf O.; Helmond N. A. G. M.; Frank J.; Lenstra W. K.; Egger M.; Slomp C. P.; Jetten M. S. Microbial community composition and functional potential in Bothnian Sea sediments is linked to Fe and S dynamics and the quality of organic matter. Limnol. Oceanogr. 2020, 65, S113–S133. 10.1002/lno.11371. [DOI] [Google Scholar]
- Sarkkola S.; Nieminen M.; Koivusalo H.; Lauren A.; Kortelainen P.; Mattsson T.; Palviainen M.; Piirainen S.; Starr M.; Finer L. Iron concentrations are increasing in surface waters from forested headwater catchments in eastern Finland. Sci. Total Environ. 2013, 463–464, 683–689. 10.1016/j.scitotenv.2013.06.072. [DOI] [PubMed] [Google Scholar]
- Schlitzer R.“Ocean Data View. 2012”, 2015. Available: http://odv.awi.de.
- Cline J. D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 1969, 14, 454–458. 10.4319/lo.1969.14.3.0454. [DOI] [Google Scholar]
- Solorzano L. Determination Of Ammonia In Natural Waters By The Phenolhypochlorite Method 1 1 This research was fully supported by U.S. Atomic Energy Commission Contract No. ATS (11-1) GEN 10, P.A. 20. Limnol. Oceanogr. 1969, 14, 799–801. 10.4319/lo.1969.14.5.0799. [DOI] [Google Scholar]
- Doane T. A.; Horwáth W. R. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 2003, 36, 2713–2722. 10.1081/al-120024647. [DOI] [Google Scholar]
- Kraal P.; Slomp C. P.; Forster A.; Kuypers M. M. M.; Sluijs A. Pyrite oxidation during sample storage determines phosphorus fractionation in carbonate-poor anoxic sediments. Geochim. Cosmochim. Acta 2009, 73, 3277–3290. 10.1016/j.gca.2009.02.026. [DOI] [Google Scholar]
- Van Santvoort P. J. M.; De Lange G. J.; Thomson J.; Colley S.; Meysman F. J. R.; Slomp C. P. Oxidation and origin of organic matter in surficial Eastern Mediterranean hemipelagic sediments. Aquat. Geochem. 2002, 8, 153–175. 10.1023/a:1024271706896. [DOI] [Google Scholar]
- Claff S. R.; Sullivan L. A.; Burton E. D.; Bush R. T. A sequential extraction procedure for acid sulfate soils: Partitioning of iron. Geoderma 2010, 155, 224–230. 10.1016/j.geoderma.2009.12.002. [DOI] [Google Scholar]
- Raiswell R.; Vu H. P.; Brinza L.; Benning L. G. The determination of labile Fe in ferrihydrite by ascorbic acid extraction: Methodology, dissolution kinetics and loss of solubility with age and de-watering. Chem. Geol. 2010, 278, 70–79. 10.1016/j.chemgeo.2010.09.002. [DOI] [Google Scholar]
- Lenstra W. K.; Klomp R.; Molema F.; Behrends T.; Slomp C. P. A sequential extraction procedure for particulate manganese and its application to coastal marine sediments. Chem. Geol. 2021, 584, 120538. 10.1016/j.chemgeo.2021.120538. [DOI] [Google Scholar]
- Canfield D. E.; Thamdrup B.; Hansen J. W. The anaerobic degradation of organic matter in Danish coastal sediments: Iron reduction, manganese reduction, and sulfate reduction. Geochim. Cosmochim. Acta 1993, 57, 3867–3883. 10.1016/0016-7037(93)90340-3. [DOI] [PubMed] [Google Scholar]
- Thamdrup B.; Fossing H.; Jørgensen B. B. Manganese, iron and sulfur cycling in a coastal marine sediment, Aarhus bay, Denmark. Geochim. Cosmochim. Acta 1994, 58, 5115–5129. 10.1016/0016-7037(94)90298-4. [DOI] [Google Scholar]
- Fossing H.; Jørgensen B. B. Measurement of bacterial sulfate reduction in sediments: Evaluation of a single-step chromium reduction method. Biogeochemistry 1989, 8, 205–222. 10.1007/bf00002889. [DOI] [Google Scholar]
- Martin W. R.; Banta G. T. The measurement of sediment irrigation rates: A comparison of the Br tracer and 222-Rn/ 226-Ra disequilibrium techniques. J. Mar. Res. 1992, 50, 125–154. 10.1357/002224092784797737. [DOI] [Google Scholar]
- Martins P. D.; de Monlevad J. P. R. C.; Lenstra W. K.; Wallenius A. J.; Medrano M. J. E.; Hermans M.; Slomp C. P.; Welte C. U.; Jetten M. S. M.; van Helmond N. A. G. M.. “Sulfide toxicity as key control on anaerobic oxidation of methane in eutrophic coastal sediments,” 10/2/2022. BioRxiv. https://www.biorxiv.org/content/10.1101/2022.02.10.479873v1.abstract. [DOI] [PMC free article] [PubMed]
- Wang Y. F.; Van Cappellen P. A multicomponent reactive transport model of early diagenesis: Application to redox cycling in coastal marine sediments. Geochem. Cosmochim. Acta 1996, 60, 2993–3014. 10.1016/0016-7037(96)00140-8. [DOI] [Google Scholar]
- Thullner M.; Van Cappellen P.; Regnier P. Modeling the impact of microbial activity on redox dynamics in porous media. Geochim. Cosmochim. Acta 2005, 69, 5005–5019. 10.1016/j.gca.2005.04.026. [DOI] [Google Scholar]
- Rothe M.; Kleeberg A.; Hupfer M. The Occurrence, Identification and Environmental Relevance of Vivianite in Waterlogged Soils and Aquatic Sediments. Earth Sci. Rev. 2016, 158, 51. 10.1016/j.earscirev.2016.04.008. [DOI] [Google Scholar]
- Kubeneck L. J.; Lenstra W. K.; Malkin S. Y.; Conley D. J.; Slomp C. P. Phosphorus burial in vivianite-type minerals in methane-rich coastal sediments. Mar. Chem. 2021, 231, 103948. 10.1016/j.marchem.2021.103948. [DOI] [Google Scholar]
- Leu A. O.; Cai C.; McIlroy S. J.; Southam G.; Orphan V. J.; Yuan Z.; Hu S.; Tyson G. W. Anaerobic methane oxidation coupled to manganese reduction by members of the Methanoperedenaceae. ISME J. 2020, 14, 1030–1041. 10.1038/s41396-020-0590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromokeye D. A.; Kulkarni A. C.; Elvert M.; Wegener G.; Henkel S.; Coffinet S.; Eickhorst T.; Oni O. E.; Richter-Heitmann T.; Schnakenberg A.; et al. Rates and microbial players of iron-driven anaerobic oxidation of methane in methanic marine sediments. Front. Microbiol. 2020, 10, 1–19. 10.3389/fmicb.2019.03041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton S. W.; Raiswell R. The low-temperature geochemical cycle of iron: From continental fluxes to marine sediment deposition. Am. J. Sci. 2002, 302, 774–805. 10.2475/ajs.302.9.774. [DOI] [Google Scholar]
- Boetius A.; Ravenschlag K.; Schubert C. J.; Rickert D.; Widdel F.; Gieseke A.; Amann R.; Jørgensen B. B.; Witte U.; Pfannkuche O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. 10.1038/35036572. [DOI] [PubMed] [Google Scholar]
- ‘t Zandt M. H.; De Jong A. E.; Slomp C. P.; Jetten M. S. The hunt for the most-wanted chemolithoautotrophic spookmicrobes. FEMS Microbiol. Ecol. 2018, 94, fiy064. 10.1093/femsec/fiy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F. Microbiology and ecology of sulfate-and sulfur-reducing bacteria. Biol. Anaerobic Microorg. 1988, 469–585. [Google Scholar]
- Slomp C. P.; Mort H. P.; Jilbert T.; Reed D. C.; Gustafsson B. G.; Wolthers M. Coupled dynamics of iron and phosphorus in sediments of an oligotrophic coastal basin and the impact of anaerobic oxidation of methane. PLoS One 2013, 8, e62386 10.1371/journal.pone.0062386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flury S.; Røy H.; Dale A. W.; Fossing H.; Tóth Z.; Spiess V.; Jensen J. B.; Jørgensen B. B. Controls on subsurface methane fluxes and shallow gas formation in Baltic Sea sediment (Aarhus Bay, Denmark). Geochim. Cosmochim. Acta 2016, 188, 297–309. 10.1016/j.gca.2016.05.037. [DOI] [Google Scholar]
- Jørgensen B. B.; Marshall I. P. G. Slow microbial life in the seabed. Ann. Rev. Mar. Sci 2016, 8, 311–332. 10.1146/annurev-marine-010814-015535. [DOI] [PubMed] [Google Scholar]
- Knittel K.; Lösekann T.; Boetius A.; Kort R.; Amann R. Diversity and distribution of methanotrophic archaea at cold seeps. Appl. Environ. Microbiol. 2005, 71, 467–479. 10.1128/aem.71.1.467-479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R. J.; Rosenberg R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926–929. 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- Breitburg D.; Levin L. A.; Oschlies A.; Grégoire M.; Chavez F. P.; Conley D. J.; Garçon V.; Gilbert D.; Gutiérrez D.; Isensee K.; Jacinto G. S.; Limburg K. E.; Montes I.; Naqvi S. W.; Pitcher G. C.; Rabalais N. N.; Roman M. R.; Rose K. A.; Seibel B. A.; Telszewski M.; Yasuhara M.; Zhang J. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240 10.1126/science.aam7240. [DOI] [PubMed] [Google Scholar]
- Wallenius A. J.; Martins P. D.; Slomp C. P.; Jetten M. S. Anthropogenic and Environmental Constraints on the Microbial Methane Cycle in Coastal Sediments. Front. Microbiol. 2021, 12, 631621. 10.3389/fmicb.2021.631621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavia D.; Field J. C.; Boesch D. F.; Buddemeier R. W.; Burkett V.; Cayan D. R.; Fogarty M.; Harwell M. A.; Howarth R. W.; Mason C.; Reed D. J.; Royer T. C.; Sallenger A. H.; Titus J. G. Climate change impacts on U.S. coastal and marine ecosystems. Estuaries 2002, 25, 149–164. 10.1007/bf02691304. [DOI] [Google Scholar]
- Harley C. D. G.; Hughes A. R.; Hultgren K. M.; Miner B. G.; Sorte C. J. B.; Thornber C. S.; Rodriguez L. F.; Tomanek L.; Williams S. L. The impacts of climate change in coastal marine systems. Ecol. Lett. 2006, 9, 228–241. 10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- Björnerås C.; Weyhenmeyer G. A.; Evans C. D.; Gessner M. O.; Grossart H. P.; Kangur K.; Kokorite I.; Kortelainen P.; Laudon H.; Lehtoranta J.; Lottig N.; Monteith D. T.; Nõges P.; Nõges T.; Oulehle F.; Riise G.; Rusak J. A.; Räike A.; Sire J.; Sterling S.; Kritzberg E. S. Widespread Increases in Iron Concentration in European and North American Freshwaters. Global Biogeochem. Cycles 2017, 31, 1488–1500. 10.1002/2017gb005749. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







