Summary
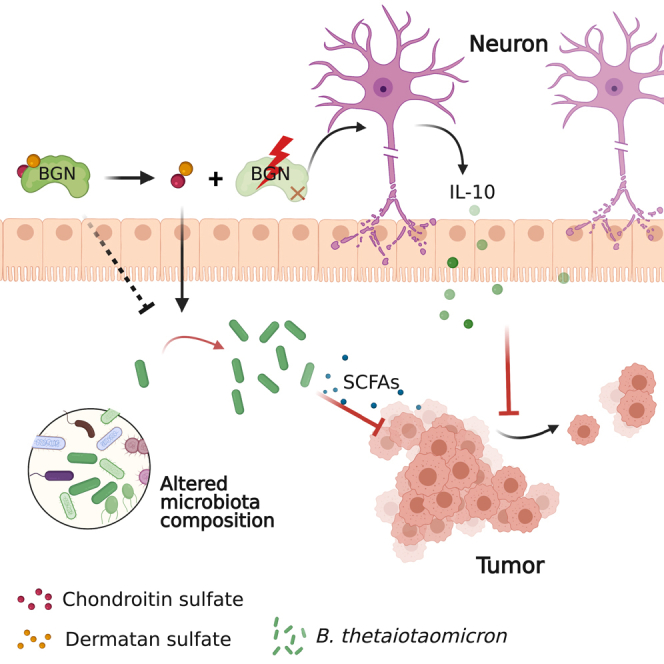
Biglycan (BGN) is a proteoglycan with branch chains and highly expressed in enteric neurons in the tumor tissue of colorectal cancer (CRC), which is negatively associated with survival rates in patients with CRC. However, how the proteoglycan promotes the progress of CRC through interacting with bacteria and regulating the immune response of enteric neurons remains largely unknown. In the present study, we found that biglycan deficiency changed tumor distribution in a colitis-associated colon cancer model. Furthermore, we revealed that BGN deficiency inhibits tumor growth in an allograft tumor model and the migration of cancer cell by upregulating interleukin-10 expression in enteric neurons. Significantly, we demonstrated that biglycan deficiency enriched the abundance of Bacteroides thetaiotaomicron through competing with it for chondroitin sulfate to inhibit CRC progress. Our work provided new insights into the interaction between host proteoglycan and gut microbiota as well as the role of enteric neurons in the tumor microenvironment.
Subject areas: Health sciences, Microbiome, Cancer
Graphical abstract
Highlights
-
•
BGN deficiency impaired gastrointestinal motility and tumor distribution
-
•
BGN regulated the production of IL-10 from enteric neurons to modulate tumor growth
-
•
BGN might compete with Bacteroides thetaiotaomicron for chondroitin sulfate
-
•
BGN partially regulates the abundance of B. thetaiotaomicron to inhibit tumorigenesis
Health sciences; Microbiome; Cancer
Introduction
Colorectal cancer (CRC) is one of the most common malignant cancers, ranking third in incidence and second in mortality among cancers worldwide.1 Increasing evidence shows that a combination of inheritable and environmental factors contribute to the initiation and development of CRC.2 Tumor microenvironment consisting of immune cells, gut microbiota, fibroblasts, epithelial cells, and neural cells have been reported to contribute to the development of CRC.3,4,5 The precise molecular mechanism of CRC remains largely unclear, although inflammation is regarded as a critical risk factor.4 Recently, emerging studies have shown that the enteric nervous system (ENS) is involved in colon inflammation and the development of CRC.6,7,8,9 For example, Jarret et al. revealed that enteric neuron-derived interleukin 18 (IL-18) signaling regulated intestinal immunity and affected the mucosal barrier and invasive bacterial killing.10 Notably, patients with CRC accompanied by perineural invasion suffered from a lower survival rate.11 Consistent with this clinical study, Duchalais et al. demonstrated that colon tumor cell adhered to and migrated along enteric neurons.12 Sadighparvar et al. reported that parasympathetic denervation suppressed the colon carcinogenesis, characterized by the reduction of tumor incidence, tumor volume and weight, as well as cell proliferation.13 Gut microbiota has been shown to modulate adult ENS and nitrergic neurons through TLR2-induced neurogenesis in mice.14 Aktar et al. reported that commensal bacteria, Bacteroides thetaiotaomicron colonization regulated colonic ENS innervation and neurogenic function in germ-free mice.15 The studies implicated that ENS might interact with gut microbiota, involving in neural pathological development of CRC.
Biglycan (BGN), an extracellular matrix protein, belongs to the small leucine-rich repeat proteoglycan family and is expressed in various tissues. BGN is characterized by a protein core with branch chains including chondroitin sulfate (CS) and/or dermatan sulfate (DS).16 The expression of BGN in tumor tissue is relatively higher than that in adjacent tissues and negatively correlated with patient prognosis.17 It has been reported that BGN promotes cancer cells migration in CRC.17,18 Moreover, the role of BGN in nervous system have been investigated. For example, BGN protected neuronal cells from nitric oxide-induced cell apoptosis by inhibiting AMPK-mTOR mediated autophagy and intracellular ROS level.19 The utilization of proteoglycans is crucial for the colonization and proliferation of gut bacteria as well as the health of the host.20 However, how BGN, as a proteoglycan, promotes the progress of CRC by maintaining homeostasis of the gut microecosystem and regulating the function of enteric neurons remained largely unknown. Here, we report a previously unrecognized role of BGN in enteric neurons in promoting tumorigenesis by suppressing IL-10 expression and regulating abundance of B. thetaiotaomicron.
Results
BGN deficiency alleviated DSS-induced mouse colitis and altered the tumor distribution in a colitis-associated colon cancer mouse model
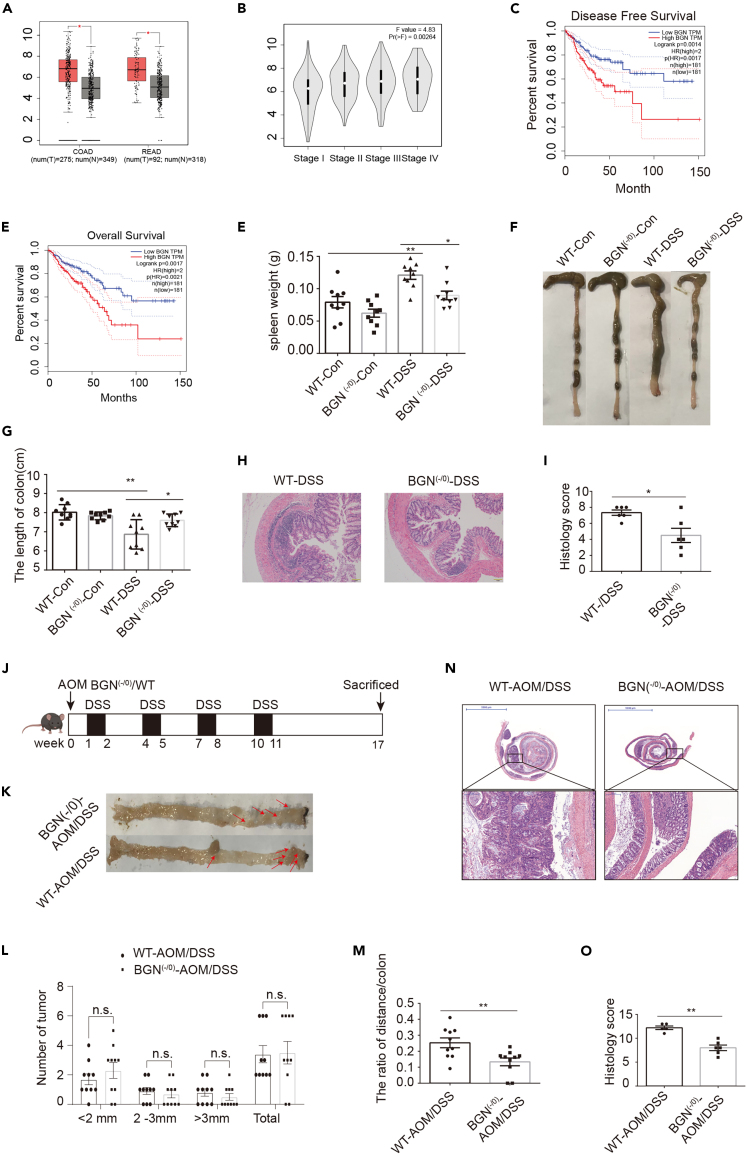
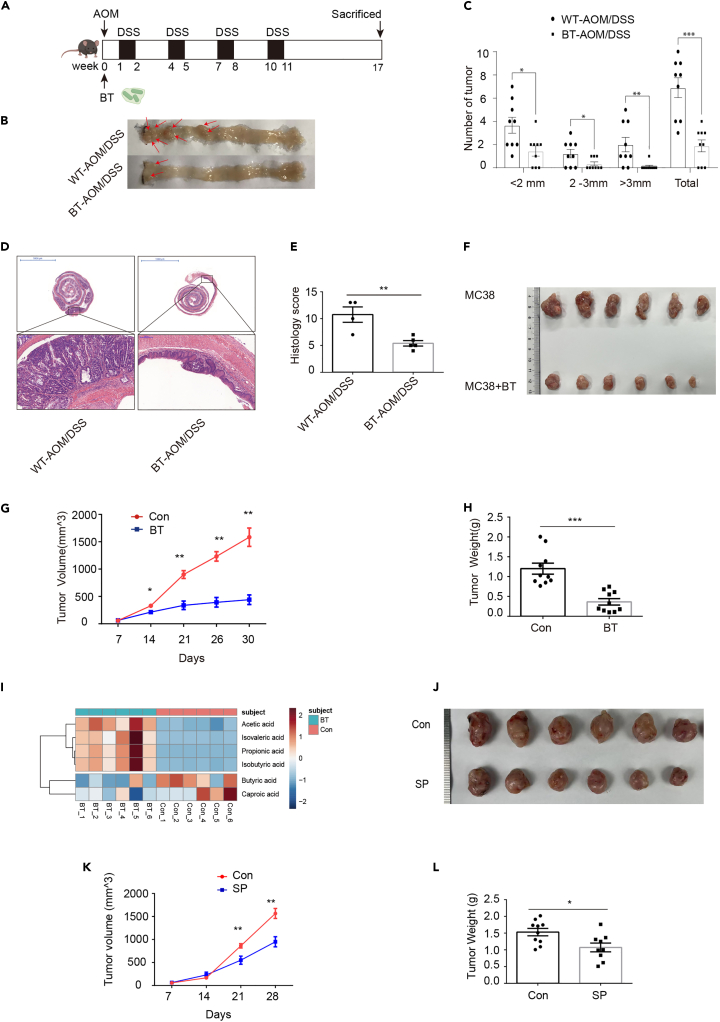
Based on the analysis through the Gene Expression Profiling Interactive Analysis (GEPIA) website from the The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) CRC dataset, BGN expression was higher in tumor tissue compared with adjacent normal tissue and was significantly associated with CRC progression (Figures 1A and 1B). Moreover, the higher expression of BGN was associated with poor disease-free survival and overall survival (Figures 1C and 1D). These results demonstrated that BGN is a risk factor for CRC. To investigate the role of BGN in colitis and CRC, we established a BGN (-/0) mouse model by using CRISPR/Cas9 technology. A total of 122 nucleotides were deleted in exon 2 to generate a frameshift mutant. No DNA was detected in the deletion location (Figure S1). To evaluate the effects of BGN on colitis, BGN (-/0) mice and littermates WT were orally administrated with 2.5% DSS for 7 days. Compared with the WT DSS group, mice in the BGN (-/0) DSS group suffered from the mitigation of colitis. As shown in Figures 1E–1I, the BGN (-/0) DSS group had a lighter spleen weight, longer colon length, and less severe histologic damage. These results showed that BGN deficiency alleviated DSS-induced mouse colitis.
Figure 1.
BGN deficiency alleviates DSS-induced colitis and altered the tumor distribution in colitis-associated colon cancer mice model
(A) BGN was higher expression in CRC tissue than adjacent normal tissue, as analyzed by GEPIA website.
(B) The expression of BGN elevated in CRC tissue along with the stages of CRC, as analyzed by GEPIA website.
(C) High level of BGN reduced the disease-free curves of CRC patients, as analyzed by GEPIA website.
(D) High level of BGN reduced the overall survival curves of CRC patients, as analyzed by GEPIA website.
(E) The spleen weight was higher in DSS-treated mice than that in control mice. BGN deficiency reduced spleen weight in DSS-treated mice (n = 9 mice/group).
(F and G) Representative image of colon in mice colitis. DSS treatment reduced the length of colon and BGN deficiency increased the length of colon in DSS-treated mice (n = 9 mice/group).
(H and I) Representative image of colon sections, stained with H&E (Scale: 1 mm). BGN deficiency reduced the colon pathological scores in mice colitis (n = 6 mice/group).
(J) Schematic timeline for experimental design, created with Biorender.com.
(K) Representative images of colonic tumors from AOM/DSS-treated BGN (-/0) and WT mice (n = 10 mice/group).
(L) Tumor size and number of macroscopic polyps in AOM/DSS-treated BGN (-/0) and WT mice (n = 10 mice/group).
(M) BGN deficiency changed the ratio of the distance from tumor to distal colon (DC) to the whole length of colon (n = 10 mice/group).
(N and O) Representative image of Swiss-rolled colon sections, stained with H&E (scale bar = 200 μm, 5 mm). BGN deficiency reduced the colon pathological scores in mice CAC (n = 5–6 mice/group).
Data are representative or cumulative results of at least two independent experiments (E, G–H, K, and L). Data are presented as mean ± S.E.M. ∗p < 0.05, ∗∗p < 0.01 and n.s. indicates not significant (p > 0.05).
To further determine the role of BGN in CRC, we established CAC mouse model induced by AOM/DSS treatment. Seventeen weeks after the initiation of carcinogenesis, the WT and BGN (-/0) mice were sacrificed (Figure 1J). Compared with WT mice, the number of tumor (diameter <2 mm, >3 mm and total) was slightly but not significantly decreased in the BGN (-/0) mice (Figures 1K and 1L). Interestingly, the distribution of tumors significantly differed between the WT and BGN (-/0) mice. In WT mice, the tumors were mainly located in the middle colon and the distal colon. In contrast, the tumors in the BGN (-/0) mice were mainly located in the distal colon (Figure 1M). As shown in Figures 1N and 1O, H&E staining and the pathological score indicated that low-grade adenocarcinomas and dysplasia were significantly decreased in BGN (-/0) mice. Taken together, the results suggested that BGN play a crucial role in tumor localization, not tumor initiation and growth.
BGN deficiency inhibited cancer cell migration through enteric neurons
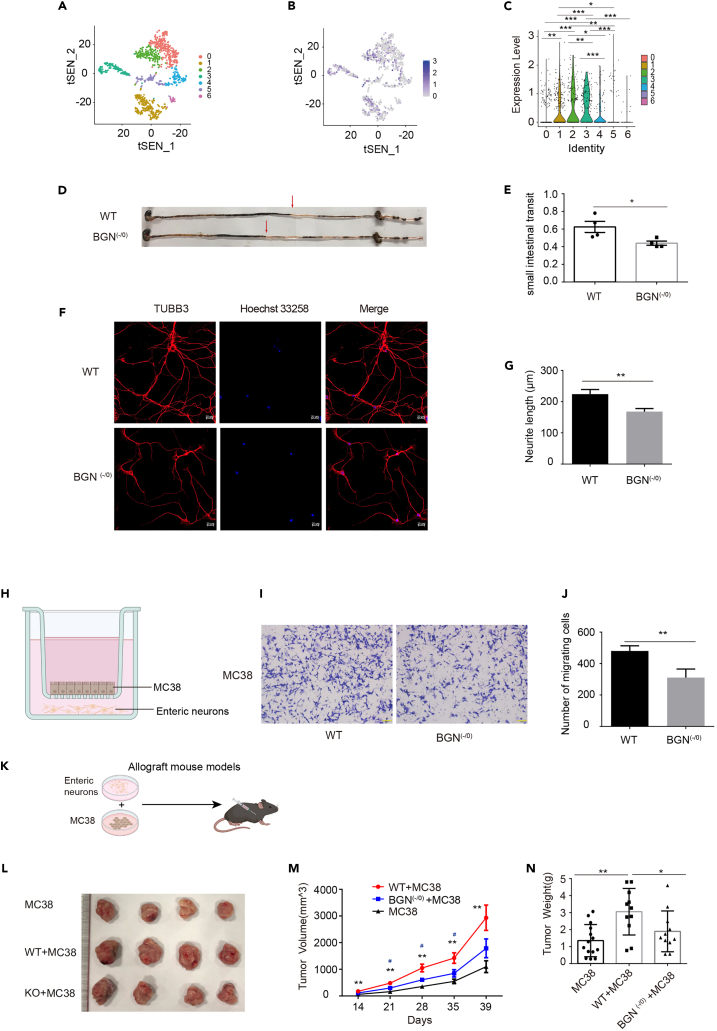
Next, we proposed that the expression of BGN in enteric neurons has a function to affect cancer cells migration. We firstly checked BGN expression profile in ENS using single RNA-seq database. As shown in Figure 2A, the dataset clustered to seven enteric neuron subsets, including 0–6 subsets referred to Chat+Sst+Calcb-putative interneurons, Nos1+ putative inhibitory motor neurons, Calb2+ putative secretomotor/vasodilator neurons, putative secretomotor/vasodilator neurons, Chat+Tac1+ putative excitatory motor neurons, putative excitatory motor neurons, and putative inhibitory motor neurons. BGN was expressed in the above seven enteric neuron subsets, and the higher level of BGN expression was observed in subsets 1, 2 and 3 (Figures 2B and 2C). By using the intestinal transit rate as a parameter in the WT and BGN (-/0) mice to assess gastrointestinal motility, we found that the ink propulsion distance was shorter in the BGN (-/0) mice than in the WT mice after gavage for 20 min (Figures 2D and 2E), which suggested that BGN might regulate gastrointestinal motility through modulating the function of ENS. To further investigate whether BGN deficiency influenced CAC through enteric neurons, we firstly isolated and cultured enteric neurons. The neurite from the BGN (-/0) mice was found to be shorter than that in the WT mice (Figures 2F and 2G). Furthermore, we established a transwell co-culture model of cancer cell and enteric neurons from the WT or BGN (-/0) mice (Figure 2H). As shown in Figure 2J, the ability of cancer cell migration was attenuated in the co-culture model with enteric neurons derived from the BGN (-/0) mice compared with that of the co-culture model with WT enteric neurons. To further determine whether BGN deficiency inhibited tumor development through enteric neurons, we established allograft mouse models by subcutaneously inoculating the combination of MC38 and enteric neurons derived from the WT mice (MC38+ WT) or the BGN (-/0) mice (MC38+ BGN (-/0)) (Figure 2K). We found that the volume, growth, and weight of tumors were significantly reduced in the MC38+ BGN (-/0) group compared with that in the MC38+ WT group. Moreover, the volume, growth, and weight of tumors were significantly increased in the MC38+WT group compared with that in the MC38 group alone (Figures 2L–2N). These results showed that BGN deficiency attenuated tumor growth promoted by enteric neurons in allograft mouse models.
Figure 2.
BGN deficiency inhibited cancer cell migration and MC38 cell allograft growth through enteric neurons
(A) The mouse enteric neurons were clustered to 7 clusters by tSNE.
(B) Visualization of BGN expression in mouse enteric neurons clusters by tSNE.
(C) BGN was expressed in mouse enteric neurons clusters.
(D and E) Representative image of ink propulsion distance in BGN (-/0) and WT mice and BGN deficiency reduced intestinal propulsion rate (n = 4 mice/group).
(F and G) Representative images of mouse enteric neurons of TUBB3 staining, by Immunofluorescence. BGN deficiency reduced the length of neurite (n = 3 mice/group).
(H) The model of transwell co-culture of cancer cell and enteric neurons (Created with Biorender.com).
(I and J) Representative image presenting transwell co-culture of MC-38 cells and the enteric neurons from BGN (-/0) and WT mice. The number of MC-38 cells migration was decreased in co-culture with BGN (-/0) mice enteric neurons (n = 3 biological replicates/group).
(K) Allograft mouse model (Created with Biorender.com).
(L) Representative image of tumors.
(M) BGN (-/0) mice enteric neurons reduced the tumor size (n = 11–14 mice/group).
(N) BGN (-/0) mice enteric neurons reduced the tumor weight (n = 11–14 mice/group).
Data are representative or cumulative results of at least two independent experiments (D–G, J and L–M). Data are presented as mean ± S.E.M. ∗ indicates WT + MC38 group VS. MC38 group, # indicates WT + MC38 group VS. BGN (-/0) +MC38 group, ∗p < 0.05, ∗∗p < 0.01. ∗∗∗p < 0.001.
BGN deficiency enhanced IL-10 expression in mouse enteric neurons induced by LPS
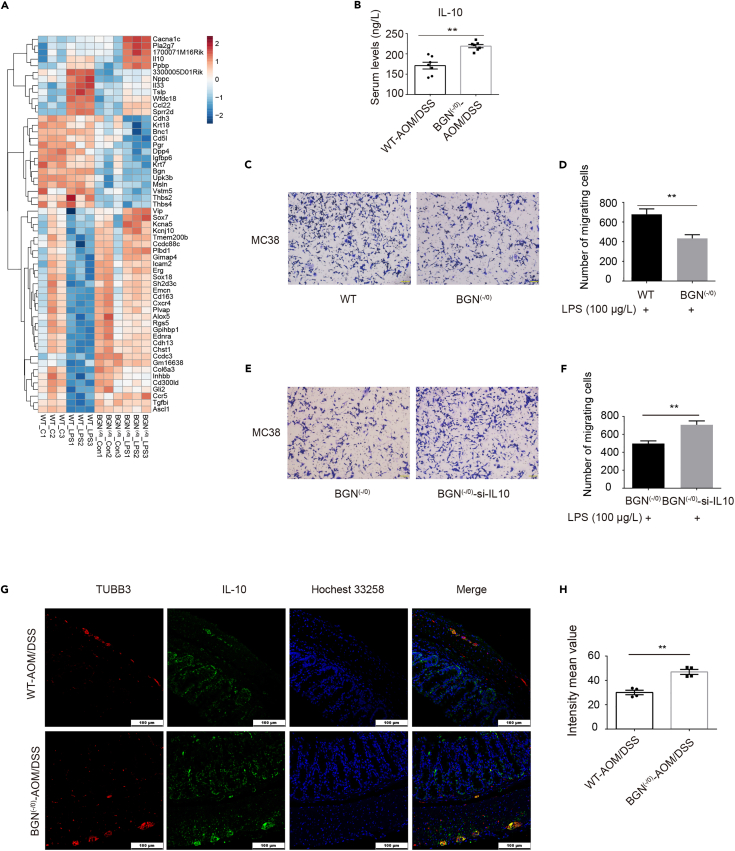
To explore the role of BGN in neurons during colitis-associated tumorigenesis, we firstly extracted enteric neurons derived from WT and BGN (-/0) mice, and simulated the intestinal inflammatory state of gut dysbiosis through lipopolysaccharide (LPS) treatment. We then performed RNA sequencing for these four groups, including WT-Con, WT-LPS, BGN (-/0)_Con and BGN (-/0)-LPS. In total, 673 differentially expressed genes (DEGs) between WT_LPS and WT_Con, 552 DEGs between BGN (-/0)_LPS and BGN (-/0)_Con, and 57 DEGs between BGN (-/0)_LPS and WT_LPS were observed between any two groups. As shown in Figure 3A, 36 upregulated DEGs and 21 downregulated DEGs were detected in the BGN (-/0)_LPS versus WT_LPS (Figure 3A). Venn diagram indicated the distribution of DEGs in the three comparisons. IL-10 was one of the five DEGs upregulated among the three comparisons (Figure S2A). In addition, the serum cytokines in both groups indicated that the levels of GM-CSF, TNF-α, IL-1β, IL-6, and IFN-γ were reduced, whereas the IL-10 level was upregulated in the BGN (-/0) mice compared with the WT mice (Figures 3B and S3A-S3F). We also found that the correlation between IL-10 and BGN was tissue specific and the correlation coefficient was highest in the neurons (brain cortex, R = 0.75, Figure S2E), lower in COAD tumor (R = 0.48, Figure S2F), and irrelevant in COAD normal tissue (R = 0.12, Figure S2G). The analysis further suggested that IL-10 played a role in BGN regulating CRC progression via enteric neurons. Gene ontology (GO) analysis showed that most of the upregulated genes were enriched in chemotaxis, angiogenesis, and inflammatory response (Figure S2C). The downregulated genes were enriched in 16 pathways, including formation of the cornified envelope, prostaglandin Synthesis, antimicrobial humoral response, cellular response to tumor necrosis factor, and regulation of the glycoprotein metabolic process (Figure S2D). Based on this information, we established a transwell co-culture model of cancer cell and WT or BGN (-/0) mice enteric neurons with LPS treatment to simulate colitis-induced tumorigenesis in vitro. Using the co-culture model under inducible inflammation conditions, we found that the ability of cancer cell migration was still attenuated in the co-culture model with enteric neurons derived from BGN (-/0) mice (Figures 3C and 3D) compared with the co-culture model from WT mice. The results were similar to those in the co-culture model without LPS treatment (Figure 2J).
Figure 3.
BGN deficiency enhanced IL-10 expression in mouse enteric neurons induced by LPS
(A) Heatmap showing the top 31 DEGS in BGN (-/0) and WT control enteric neurons and LPS-treated BGN (-/0) and WT mouse enteric neurons.
(B) BGN deficiency reduced the level of IL-10 in AOM/DSS-treated mice, as detected by ELISA assay (n = 7 mice/group).
(C and D) Representative image presenting transwell co-culture of MC38 cells and the enteric neurons from BGN (-/0) and WT mice with LPS (100 μg/L). The number of MC38 cells migration was decreased in co-culture with BGN (-/0) mice enteric neurons with LPS (100 μg/L) treatment (n = 3 biological replicates/group).
(E and F) Representative image presenting transwell co-culture of MC38 cells and the enteric neurons from BGN (-/0) and IL-10 knockdown of BGN (-/0) enteric neurons with LPS (100 μg/L). The number of MC38 cells migration was increased in co-culture with IL-10 knockdown of BGN (-/0) mice enteric neurons (n = 3 biological replicates/group).
(G and H) BGN deficiency upregulated the IL-10 expression of enteric neurons in CAC mice (n = 4 mice/group).
Data are representative or cumulative results of at least two independent experiments (B–H). Data are presented as mean ± S.E.M. ∗p < 0.05 and ∗∗p < 0.01.
To further investigate whether BGN regulates IL-10 to inhibit cancer cell migration, we knockdown IL-10 expression in enteric neurons isolated from WT and BGN (-/0) (Figure S2H). The transwell co-culture model showed increased cancer cell migration when the expression of IL-10 in enteric neurons was knockdown (Figures 3E and 3F), moreover, the group with co-culture of knockdown of IL-10 in enteric neurons isolated from WT mice with MC38 cells showed increased cancer cell migration compared with control group (Figures S4A–S4C). Immunofluorescence analysis showed TUBB3 as neuron marker and IL-10 co-localized at enteric neurons in tumor tissue of the CAC mouse model (Figure 3G). The level of IL-10 expression was higher than that in WT mice in CAC model (Figures 3G and 3H). Taken together, the above results implied that BGN inhibited cancer cell migration, partially by up-regulating IL-10 expression of enteric neurons.
BGN modulated gut microbiota composition in AOM/DSS mice
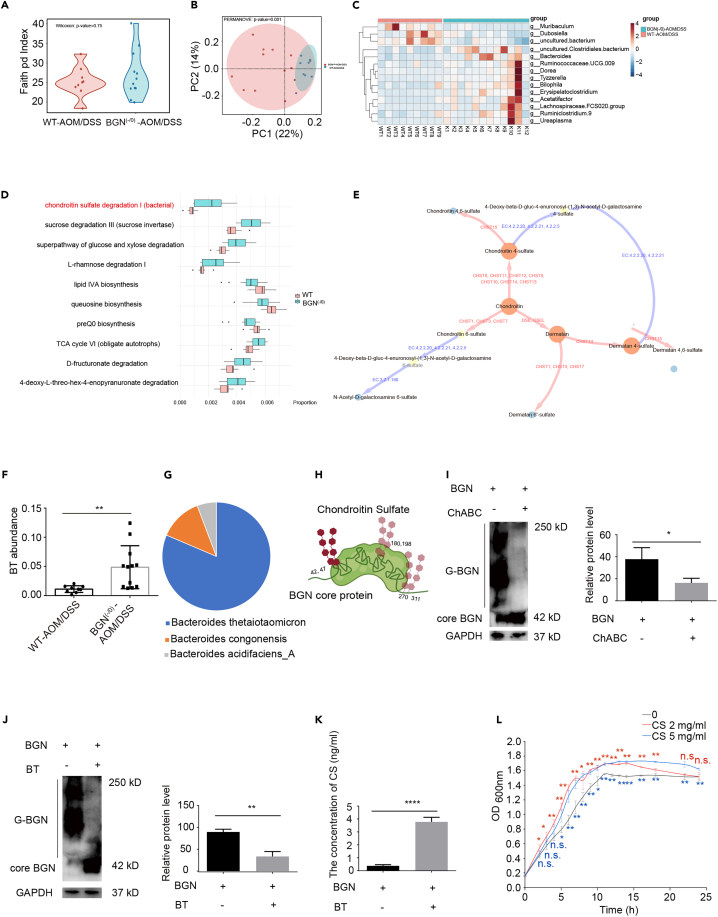
Increased evidences showed genetic factors interacted with the composition of gut microbiota to modulate CRC progress.21,22 To evaluate the effects of BGN on gut microbiota in AOM/DSS mice, fecal samples were subjected to high-throughput sequencing of 16S rRNA gene. The Faith’s PD (Faith’s Phylogenetic Diversity) index was utilized to estimate α-diversity. No significant difference in α-diversity between the two groups was observed (Figure 4A). Beta-diversity based on PcoA analysis using the Bray–Curtis distance showed a significant difference between the WT CAC mice and the BGN (-/0) CAC mice (Figure 4B). A significant difference at the genus level between two groups (p < 0.05) by clustering analysis was observed. As shown in Figure 4C, Muribaculum was enriched in the WT CAC mice, whereas the Clostridiales bacterium, Bacteroides, Ruminococcaceae.UCG.009, Dorea, Tyzzerella, Bilophila, Erysipelatoclostridium, Acetatifactor, Lachnospiraceae.FCS020.group, Ruminiclostridium.9 and Ureaplasma were enriched in the BGN (-/0) CAC mice (Figure 4C).
Figure 4.
BGN modulated gut microbiota composition of AOM/DSS mice
(A) α-diversity (Faith pd Index) analysis in AOM/DSS-treated BGN (-/0) and WT mice (n = 9–12 mice/group).
(B) BGN deficiency changed the β-diversity based on PcoA at the Bray-Curtis distance in AOM/DSS-treated BGN (-/0) and WT mice (n = 9–12 mice/group).
(C) BGN deficiency changed the gut microbiota composition. Heatmap showing the differential genus in the fecal from AOM/DSS-treated BGN (-/0) and WT mice (n = 9–12 mice/group).
(D) The functional abundances were different in AOM/DSS-treated BGN (-/0) and WT mice, analyzed by PICRUSt2 (n = 9–12 mice/group).
(E) The chondroitin sulfate metabolic network. The red edge indicates the corresponding reaction were conducted by human-encoded enzymes, and the blue ones were done by bacteria-encoded enzymes.
(F) BGN deficiency improved the relative abundance B. thetaiotaomicron (BT) in AOM/DSS-treated BGN (-/0) mice (n = 9–12 mice/group).
(G) B. thetaiotaomicron was the primary taxon in CS degradation I pathway regulation (n = 9–12 mice/group).
(H) The Schematic of BGN glycosylation site (Created with Biorender.com).
(I) The glycosylation of BGN with CS can be digested by ChABC, detected by Western blotting and quantitative analysis. G-BGN means the glycosylated BGN (n = 3 biological replicates/group).
(J) B. thetaiotaomicron reduced the glycosylation level of BGN, detected by Western blotting and quantitative analysis. G-BGN means the glycosylated BGN (n = 3 biological replicates/group).
(K) B. thetaiotaomicron increased the level of dissociative CS, detected by ELISA (n = 5/group).
(L) The growth curve for B. thetaiotaomicron showed CS promoted B. thetaiotaomicron growth. The blue ∗ indicates 5 mg/mL CS group VS. control group and the red ∗ indicates 2 mg/mL CS group VS. control group.
Data are representative or cumulative results of at least two independent experiments (A–D, F, and I–L). Data are presented as mean ± S.E.M. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 and n.s. indicates not significant (p > 0.05).
Next, PICRUSt2 analysis was applied to dissect the differential microbial function in BGN (-/0) CAC mice compared with that of WT mice. As shown in Figure 4D, 41 pathways were enriched in the AOM/DSS-treated BGN (-/0) mice. Among them, the chondroitin sulfate degradation I pathway was enriched in the BGN (-/0) mice (Figure 4D). Following the KEGG database,23 we built a CS metabolic network (Figure 4E). Impressively, most CS synthesis pathways (from chondroitin to CS) were catalyzed by host-encoded enzymes, such as the CHST family proteins, DSE, and DSEL in Homo sapiens. However, CS degradation was performed by microbiome-encoded enzymes with EC (Enzyme commission) number 3.2.1.180, 4.2.2.20, 4.2.2.21, and 4.2.2.5. To figure out the corresponding taxa for CS degradation I pathway, we annotated over 40000 bacteria genomes from the GTDB (Release 202).24 Another clue is that Bacteroides was the most increased genus in the AOM/DSS-treated BGN (-/0) mice (Figure 4C). We screened taxa from the Bacteroides genus which encode CS degradation related enzymes (3.2.1.180, 4.2.2.20, 4.2.2.21, 4.2.2.5) (Table S2). We then compared the sequenced data with the 16S rRNA of the screened taxa and found that B. thetaiotaomicron, a potential probiotic,25,26 is involved in CS degradation I pathway regulation (Figure 4G), and the abundance of B. thetaiotaomicron was significantly enriched in the BGN (-/0) CAC group (Figure 4F). As shown in the summarized Figure 4H, CS was reported to attach to amino acids 42, 47, 180, 198, 270, and 311 in human BGN, which formed O-linked and N-linked glycosaminoglycans.27
Given that the abundance of B. thetaiotaomicron was significantly enriched in the BGN (-/0) CAC group, we proposed that BGN may compete with B. thetaiotaomicron for CS. To test the hypothesis, we firstly used chondroitinase ABC (ChABC) to confirm the glycosylation of BGN with CS (Figure 4I). We therefore deduced that B. thetaiotaomicron may encode the conserved chondroitinase enzyme. Consistent with our prediction, bioinformatics analysis showed that B. thetaiotaomicron encode a functional conserved chondroitinase enzyme containing a similar motif pattern to five ChABC sequences from Proteus vulgaris, Bacteroides faecis, and B. thetaiotaomicron (Figure S5). Accordingly, B. thetaiotaomicron was found to reduce the glycosylation of BGN (Figure 4J), and the supernatants after B. thetaiotaomicron treatment showed increased levels of CS compared with the control group (Figure 4K). In line with the above western blot results, the growth curve analysis showed that CS could promote the growth of B. thetaiotaomicron (Figure 4L). Overall, the results imply that BGN deficiency in mice may enrich the abundance of B. thetaiotaomicron to inhibit tumorigenesis.
B. thetaiotaomicron and sodium propionate inhibited tumorigenesis in AOM/DSS-induced mouse CAC and MC38 allograft tumor, respectively
To further determine the role of B. thetaiotaomicron in CAC, we constructed AOM/DSS-induced mouse CAC with/without B. thetaiotaomicron treatment. Seventeen weeks after the initiation of carcinogenesis, WT and B. thetaiotaomicron administration mice were sacrificed (Figure 5A). Compared with WT mice, the number of tumor (>3 mm and total) was significantly reduced, and the number of tumor (<2 mm and 2mm–3 mm) was slightly but not significantly decreased in CAC mice with B. thetaiotaomicron administration (Figures 5B and 5C). As shown in Figures 5D and 5E, H&E staining indicated that low-grade adenocarcinomas and dysplasia were significantly reduced, and B. thetaiotaomicron administration group showed decreased pathological score. Consistent with CAC model, MC38 allograft tumor model showed B. thetaiotaomicron administration inhibited the growth of tumor and reduced significantly tumor weight compared with control group (Figures 5F–5H). Short chain fatty acids (SCFAs) were produced from gut microbiota fermented non-digestible carbohydrate, which was reported to inhibit colon cancer development.28 We collected the supernatant of culture of B. thetaiotaomicron and control medium for detecting the SCFAs using GC/MS. The results showed that acetic acid, isovaleric acid, propionic acid, isobutyric acid, butyric acid and caproic acid were detected in two groups (Figures 5I and S6). Among of them, acetic acid and propionic acid were the most enriched in the supernatant of B. thetaiotaomicron culture. The PcoA analysis showed the level of SCFAs were clearly distinctive from each other in the two groups (Figure S6A). Furthermore, we used MC38 allograft tumor model to confirm sodium propionate significantly inhibited the growth and weight of tumor (Figures 5J–5L). These results implicated that enriched B. thetaiotaomicron in BGN (-/0) mice play an important role of inhibiting CRC development through producing SCFAs.
Figure 5.
B. thetaiotaomicron and sodium propionate inhibited tumorgenesis in AOM/DSS-induced mouse CAC and MC38 allograft tumor, respectively
(A) Schematic timeline for experimental design (Created with Biorender.com).
(B) Representative images of colonic tumors from AOM/DSS-treated WT and B. thetaiotaomicron administration mice (n = 9mice/group).
(C) B. thetaiotaomicron administration reduced the number of tumor size (n = 9 mice/group).
(D and E) Representative images of Swiss-rolled colon sections, stained with H&E (scale bar = 200 μm, 5 mm). B. thetaiotaomicron administration reduced the pathological scores in CAC mice (n = 4–5 mice/group).
(F) Representative images of MC38 allograft tumors in PBS and B. thetaiotaomicron administration group.
(G and H) B. thetaiotaomicron administration reduced the growth and weight of MC38 allograft tumor (n = 10 mice/group).
(I) The heatmap showed that supernatant of B. thetaiotaomicron medium was enriched in acetic acid, isovaleric acid, propionic acid and isobutyric acid not caproic acid and butyric acid (n = 6/group).
(J) Representative images of MC38 allograft tumors in PBS and Sodium propionate injection group (n = 9–10 mice/group).
(K and L) Sodium propionate reduced the growth and weight of MC38 allograft tumors (n = 9–10 mice/group).
Data are representative or cumulative results of at least two independent experiments (B–H and J–L). Data are presented as mean ± S.E.M. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001.
Discussion
In the present study, we demonstrated that BGN deficiency significantly alleviated colitis and inhibited tumor growth and distribution by inhibiting IL-10 expression in enteric neurons. Specifically, we indicated that BGN regulates the abundance of B. thetaiotaomicron by competing for CS. Furthermore, we determined that enriched B. thetaiotaomicron in BGN (-/0) mice inhibited tumor growth by producing SCFAs, such as propionate. Our results provided new insights into the interaction of host proteoglycan and gut microbiota as well as the role of enteric neurons in the tumor microenvironment.
A few studies have reported that ENS is a vital component of the intestinal tract involved in colorectal carcinogenesis, but the underlying mechanism remains largely unknown. A multifunctional cytokine, IL-10 plays a role in both inflammation and immunity by acting as an inflammatory and immunosuppressive factor.29,30 Recently, Kou et al. reported IL-10 play a significant role in cancer development through modulating tumor microenvironment.31 However, the understanding of IL-10 outside immune cells in tumorigenesis remains unknown. Jarret et al. reported that the production of another cytokine, IL-18, derived from enteric neurons regulated an antimicrobial program by modulating goblet cell antimicrobial peptide release, furthermore they indicated that ENS is an important enteric immune mediator in bacterial defense.10 In the present study, we observed knockdown of IL-10 in enteric neurons promoted cancer cell migration, which further suggested that the interplay of gut microbiota and immune cytokines from ENS plays a crucial role in colon cancer development.
Increasing evidences showed that the host gene-regulated homeostasis of gut microbiota affects tumor growth and distribution. For example, Man et al. found that AIM2−/− mice were more susceptible to colorectal tumorigenesis, and the susceptibility was increased by gut microbiota disturbance. Interestingly, the number and incidence of colon tumors are strikingly reduced in AIM2-deficient mice by co-housing with WT mice.32 In the present study, we found that the BGN (-/0) mice regulated gut microbiota composition and only affected tumor distribution not tumor number, suggesting a complex interplay of host genetics factor and gut microbiota. AOM/DSS CAC model is dependent on the induction of inflammation. Given BGN affecting innate inflammatory response,33 it is possible that BGN deficiency might lead to tumor outgrowth in suboptimal inflammatory conditions. On the other hand, BGN is expressed in other cell types, such as, epithelial cells and immune cells, which suggests BGN expression in other types of cells, especially immune cells play a different role in tumorigenesis. Given MC38 allograft mice built by co-cultured enteric neurons from BGN (-/0) and MC38 cells showed decreased tumor size compared with that of WT mice, conditional BGN knockout mice in enteric neurons should be established to further clarify the role of BGN in in tumorigenesis.
Proteoglycans and glycosaminoglycans(GAGs) are the main components of the extracellular matrix that play critical roles in multiple cancers, including colon cancer.34 BGN, as a proteoglycan, is composed of a core protein with branch chains, including CS and/or DS, which are positively associated with colon cancer.35 Ndeh et al. demonstrated that the versatile core genetic locus of B. thetaiotaomicron regulated GAGs metabolism and found various enzymes that bind and utilize CS/DS in the polysaccharide utilization loci (PULCS/DS/HS) of B. thetaiotaomicron.36 Ndeh et al. also showed that CS is a priority source of carbon for the growth of B. thetaiotaomicron.37,38 Our data indicated that B. thetaiotaomicron treatment reduced BGN glycosylation, which suggests that BGN deficiency may reduce the utilization of CS binding BGN core protein, thus enhancing CS intaking by B. thetaiotaomicron. With regard to the role of B. thetaiotaomicron in cancer cell growth, Ryu et al. recently reported that B. thetaiotaomicron supernatant significantly inhibited HCT116 cell growth.39 Tian et al. reported that SCFAs mix including sodium propionate can prevent development of tumor and attenuated the colonic inflammation in the CAC model.40 Consistently, our results showed that B. thetaiotaomicron derived propionate, also inhibited the growth of MC38 allograft tumors. The results further suggested B. thetaiotaomicron might be a potential probiotic in the preventing CRC progresses.
In summary, our work provided new insights into the role of enteric neurons in the tumor microenvironment of CRC and the interaction between proteoglycan and microbiota through competing for CS. Our study also suggested that B. thetaiotaomicron has the potential to be a microbiological probiotic for CRC treatment strategies.
Limitations of the study
Our findings shed new light on the interaction between host proteoglycans and gut microbiota, however, we did not detect the change of CS modification of BGN after B. thetaiotaomicron treatment due to technical limitations. Therefore, the detail CS competition mechanism between BGN and B. thetaiotaomicron needs to be further explored. Also, we observed significant differences in tumor distribution between BGN (-/0) and WT mice in CAC models, however, there was no significant difference of tumor number between two groups. Given the expression of BGN in other cell types, such as epithelial cells and immune cells, it is likely that BGN in other cell types contribute to tumorigenesis of CAC model. Hence, to further understand how BGN expression in enteric neurons affects tumor metastasis, conditional knockout mice in enteric neuron are required.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-beta III Tubulin antibody [2G10] - Neuronal Marker ab78078 | abcam | Cat#ab78078 RRID: AB_2256751 |
| Anti-IL10 Antibody | abcam | Cat#ab189392 RRID:AB_2847946 |
| goat anti-mouse IgG-HRP: sc-2005 | Santa Cruz Biotechnology | Cat#sc-2005 RRID: AB_631736 |
| Goat Anti-Rat IgG H&L (Alexa Fluor® 488) | abcam | Cat#ab150165 RRID:AB_2650997 |
| Alexa Fluor® 647 AffiniPure Rabbit Anti-Mouse IgG (H + L) | Jackson ImmunoResearch Inc. | Code number:315-605-045 RRID: AB_2340244 |
| Bacterial and virus strains | ||
| Bacteroides.thetaiotaomicron VPI 5482 | Gao’s laboratory | ATCC 29148 |
| Chemicals, peptides, and recombinant proteins | ||
| Dextran sulfate sodium salt | MP Biomedicals | Cat#160110 |
| Azoxymethane | Sigma Aldrich | Cat# A5486 |
| Collagenase Type IV | Sigma Aldrich | Cat# C4-28 |
| Protease Inhibitor Cocktail (EDTA-Free) | MedChemExpress | Cat# HY-K0011 |
| Fetal Bovine Serum | ExCell Bio | Cat# FSP500 |
| DMEM high glucose | Thermo Fisher Scientific | Cat# C11965500BT |
| Penicillin-Streptomycin Solution | Thermo Fisher Scientific | Cat#15140122 |
| Trizol Reagent | Thermo Fisher Scientific | Cat#15140122 |
| Neurobasal™ Plus | Thermo Fisher Scientific | Cat#A3582901 |
| Recombinant Murine GDNF | PEPROTECH | Cat#450-44 |
| B-27™ Supplement (50X), serum free | Thermo Fisher Scientific | Cat#17504044 |
| Hoechst 33258 | Thermo Fisher Scientific | Cat#H1398 |
| chondroitinase ABC | Sigma Aldrich | Cat#C2905 |
| sodium propionate | MedChemExpress | Cat#137-4-6 |
| Critical commercial assays | ||
| ELISA Kit for Chondroitin Sulfate (CS) | CLOUD-CLONE-CORP.WUHAN | Cat#CEA723Ge |
| One Step Mouse Genotyping Kit | Vazyme | Cat#PD101-01 |
| ChamQ Universal SYBR qPCR Master Mix | Vazyme | Cat# Q711 |
| HiScript® III RT SuperMix for qPCR (+gDNA wiper) |
Vazyme | Cat#R323 |
| Deposited data | ||
| Mouse fecal 16S rRNA data | This paper | PRJNA803604 https://www.ncbi.nlm.nih.gov/bioproject |
| RNA-Seq sequencing data | This paper | PRJNA803604 https://www.ncbi.nlm.nih.gov/bioproject |
| Experimental models: Cell lines | ||
| HCT116 | ATCC | CCL-247 |
| MC38 | Provided by Prof. Chuanping Si (Institue of Immunology and Molecular Medicine, Jining Medical College) | RRID:CVCL_B288 |
| Experimental models: Organisms/strains | ||
| Mouse: B6: C57BL/6J | Animal core facility of Nanjing medical university | N/A |
| Oligonucleotides | ||
| Primers for qPCR: see Table S1 | This paper | N/A |
| Recombinant DNA | ||
| (BGN)TK-PCDH-copGFP-T2A-Puro | This paper | N/A |
| Software and algorithms | ||
| GraphPad Prim 6.0 | GraphPad Software | https://www.graphpad.com/ |
| QIIME 2 | Bolyen, et al., 201941 | https://docs.qiime2.org/2020.8/ |
| R | R Core Team | https://www.r-project.org/ |
| Fastqc | Babraham Bioinformatics | https://github.com/DaehwanKimLab/hisat2 |
| PICRUSt 2 | Gavin M. Douglas, et al., 202042 | https://github.com/picrust/picrust2 |
| STAMP 2.1.3 | Donovan H Parks, et al.43 | http://kiwi.cs.dal.ca/Software/STAMP |
| usearch v11.0.667_i86linux32 | Robert C. Edgar, et al.44 | http://www.drive5.com/usearch |
| vsearch v2.17.1_linux_x86_64 | Torbjørn Rognes, et al.45 | https://github.com/torognes/vsearch |
| GTDB | Donovan H Parks, et al.,202024 | https://gtdb.ecogenomic.org |
| eggNOG 5.0 | Jaime Huerta-Cepas, et al.46 | http://eggnog.embl.de |
| MEME Suite | Timothy L Bailey, et al.47 | http://meme-suite.org |
| Pfam | Jaina Mistry, et al.48 | http://pfam.xfam.org/ |
| MEGA software | Sudhir Kumar, et al.49 | www.megasoftware.net |
| Clustal X2 | M A Larkin, et al.50 |
http://www.ebi.ac.uk/tools/clustalw2; ftp://ftp.ebi.ac.uk/pub/software/clustalw2/ |
| All original code | This paper | https://github.com/Xingyinliu-Lab/BGN-project-related-codes |
| Mendeley data | This paper | https://doi.org/10.17632/4bk37hy3r3.1 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Xingyin Liu (xingyinliu@njmu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Mouse strain
BGN (-/0) mice on the C57BL/6 background were established by Animal Core Facility of Nanjing Medical University (Nanjing, China) by CRISPR/Cas9 method51 and BGN (-/0) mice with 122 bp deletion in exon 2 were verified by reverse transcription polymerase chain reaction (RT-PCR). All mice were raised at room temperature (23°C–26°C) with free access to food and water with a 12 h light/dark cycle in a barrier facility of Animal Core Facility of Nanjing Medical University. Mice were bred in Male BGN (-/0) mice and littermate wild-type (WT) mice (8–10 weeks) were used all animal experiments. All of the experimental operations were approved by the Committee on the Ethics of Animal Experiments of Nanjing Medical University (Ethics No. 1811064-3).
Cancer cell culture
MC38 cells were from provided by Prof. Chuanping Si (Institute of Immunology and Molecular Medicine, Jining Medical College). The cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) with 10% fetal bovine serum (FBS, ExCell, Uruguay) and 1% Penicillin-Streptomycin (Gibco, USA).
Method details
Isolation and culture of enteric neurons
Isolation and culture of enteric neurons from the colon of three BGN (-/0) mice or littermate WT mice were performed according to previously reported.52,53 BD Matrigel matrix (BD Biosciences, USA) was diluted with DMEM-F12 (1:100), which was added into 12-well plates with cell slide for 30–60 min in 37°C cell incubator before the enteric neurons were seeded. The complete medium of enteric neurons culture used in this study was composed of Neurobasal Plus medium (Gibco, USA), Murine Glial cell line-Derived Neurotrophic Factor (Murine GDNF, PeproTech, USA), NeuroCult SM1 Neuronal Supplement/B27 (Stemcell, USA) and 1% Penicillin-Streptomycin (Gibco, USA). After one day, the medium was changed to new complete medium with 4 μg/mL Cytosine β-D-arabinoside (Macklin, Shanghai, China) for suppressing the growth of neuroglia.
Bacterial strain and culture condition
The B. thetaiotaomicron VPI 5482 was from Gao’s Lab (ATCC 29148, Manassas, USA). B. thetaiotaomicron VPI 5482 was grown in brain heart infusion medium (BHIS) supplemented with 0.5% hemin solution and 1 mg/mL L-Cysteine in anaerobic chamber at 37°C for 24 h. The analysis of growth curve for B.thetaiotaomicron was performed as previously described.54
Colitis and CAC model
BGN (-/0) mice and littermate WT mice received 2.5% DSS (36–50 kDa, MP Biomedicals, UK) in drinking water for 7 days, followed by 7 days normal drinking water and the untreated control mice drunk water freely. After 14 days, the animals were sacrificed and specimen were collected. The establishment of AOM/DSS model was performed according to previous study.55 Briefly, all mice were divided into four groups including AOM/DSS-treated BGN (-/0) mice group, AOM/DSS-treated WT mice groups and AOM/DSS-treated littermate WT mice with B. thetaiotaomicron administration group. Mice were intraperitoneally injected with AOM (10 mg/kg, Sigma-Aldrich, USA) dissolved in PBS at day 1 for only one time. After 7 days, the mice were supplied with 2.5% DSS in drinking water for a week, followed by regular drinking water for two weeks. This cycle was repeated for four times and the concentration of DSS was replaced to 1.5% at the fourth cycle. B. thetaiotaomicron or PBS was administrated by gavage at dose of 1 × 108 CFU in 200 μL/mouse every other day from day 1 to the end of experiment. After 17 weeks, the animals were sacrificed and specimen were collected.
Allograft mouse models
The combination of 4 × 105 MC38 cells and 5 × 104 enteric neurons that derived from WT mice or BGN (-/0) mice and the only 4 × 105 MC38 cells were injected subcutaneously into the upper left flank of C57BL/6 mice. For allograft mouse models of sodium propionate (SP) treatment, 1 × 106 MC38 were used. SP (100 mM) was performed at 100 μL intra-tumoral injection after the volume of tumor to 300 mm3 twice a week. For allograft mouse models of B. thetaiotaomicron treatment, 5 × 105 MC38 were used. The gavage of B. thetaiotaomicron (1 × 10ˆ9 CFU) was performed at 200 μL daily. MC38 cells Tumor size was measured with digital caliper once a week and tumor volume was calculated by the formula (length×width2)/2.
Cell transfection
Enteric neurons from BGN (-/0) mice were seeded into 12-well plates and the cells reached 80% confluence after 24 h. GenMute siRNA transfection reagent (SignaGen, China) were used to transfect negative control (NC) and IL-10 siRNA (GenePharma, China) to BGN (-/0) and WT mice enteric neurons according to the protocol. The vector TK-PCDH-copGFP-T2A-Puro (Tsingke, China) was used to construct the overexpression plasmid of BGN. HCT116 cells were seeded into 6-well plates and the cells reached 60% confluence after 24 h. The mixture of lipidosome Polyjet DNA in vitro transfection reagent (SignaGen, China) and the plasmid of BGN were transfected to HCT116 cells according to manufacturer’s protocol.
Transwell migration assay
Cell migration assays were performed with transwell inserts (8 μm pore size, Corning, Life Sciences, USA) in 24-well plates. For co-culture, MC38 cells were seeded in the upper chambers with 200 μL fresh medium containing 10% FBS. The bottom chambers were filled with 500 μL enteric neurons (WT/BGN (-/0)) or enteric neurons (BGN (-/0)-NC/BGN (-/0)-IL-10 siRNA/WT-NC/WT- IL-10 siRNA) suspension containing 20% FBS. After 48 h incubation, non-migration cell on the top of chamber were cleared with swab. The migration cell on the underside of the chamber were fixed with methanol for 15 min, stained with 0.1% crystal violet for 20 min, rinsed with water and counted in four-five random fields under microscope.
RNA extraction and real-time quantitative PCR analysis
Total RNA from human colon tissues and enteric neurons was isolated by TRIzol reagent (Invitrogen, CA, USA) following the manufacturer’s protocol, respectively. cDNA was synthesized using the HiScript II Q Select RT SuperMix for qPCR (+gDNA wiper) (Vazyme, R233-01, China) according to the manufacturer’s instructions. The RT-qPCR was performed using the Hieff qPCR SYBR Green Master Mix (Low Rox Plus) (YEASEN, 11202ES08, China) with the Applied Biosystems, Quant Studio5 (Applied Biosystems, CA, USA). The primers used for qPCR were listed in Table S1. The qPCR data were calculated by the 2−ΔΔCt method, normalizing to 18S rRNA gene expression.
Genotyping
The genomic DNA was extracted from the toe of mouse and lysed, subsequently RT-PCR using One Step Mouse Genotyping Kit (Vazyme, PD101-01, China) according to the manufacturer’s instructions. The primers were as follows: BGN Forward: AGAGAGAACCCTAGCTACTACTGCTGG Reverse: CCCATCTCACAGGGTAGCAAATGT. The PCR product was detected by Sanger sequencing (Tsingke, China) and on 2% agarose gel by electrophoresis.
Histopathological analysis
The distal colon from DSS-induced colitis mice and the Swiss-rolled whole colon from AOM/DSS-treated mice were collected and fixed in 4% paraformaldehyde, embedded in paraffin, cut into the thickness of 5 μm sections and stained with hematoxylin-eosin (H&E). The pathological scores of colon tissue were assessed blindly as described previously.32,56,57 The scores of histological lesions were assessed based on inflammation severity, edema, ulceration, hyperplasia and lesions areas. Inflammation severity was assigned as follow: 0 = normal; 1 = mild (small, focal, or widely separated, mostly limited to lamina propria); 2 = moderate (multifocal mucosal inflammation, often extending to submucosa); 3 = marked (multifocal and coalescing to extensive inflammation with occasional transmural foci); 4 = severe (diffuse inflammation with transmural inflammation). Scores for ulceration were assigned as follow: 0 = normal (no ulcers); 1 = mild (1–2 ulcers, involving up to a total of 20 crypts); 2 = moderate (1–4 ulcers, involving a total of 20–40 crypts); 3 = severe (more than 4 ulcers or over 40 crypts). Hyperplasia scores were assessed as follows: 0 = normal; 1 = mild (Crypts elongated, normal epithelium); 2 = moderate (Crypts elongated with crowding of hyperchromatic epithelium, no branching of crypts); 3 = marked (Crypts 2–3 times normal thickness, hyperchromatic epithelium, reduced goblet cells, scattered arborization); 4 = severe (Crypts 2–3 times normal thickness, marked hyperchromasia, few to no goblet cells, high mitotic index, frequent arborization). Scoring for lesion areas were assessed as follows: 0 = normal (0% involvement); 1 = minimal (less than 10% involvement); 2 = mild (10%–25% involvement); 3 = moderate (25%–50% involvement); 4 = marked (50%–75% involvement); 5 = severe (over 75% involvement).
Immunofluorescence
Immunofluorescence staining was carried out as previously reported.57 In brief, put the paraffin-embedded slides in 60°C ovens for 2 h and then deparaffinized in xylene and rehydrated in graded ethanol. The slides were heated in 0.01 M sodium citric buffer (pH = 6.0) for antigen retrieval, blocked with 5% donkey serum (Solarbio, Beijing, China) for reducing nonspecific background and incubated with primary antibodies overnight at 4°C. Primary antibodies were used as follows: mouse anti-TUBB3 (1:500, Abcam, Cambridge, UK) and anti-IL-10 (1:200, abcam, Cambridge, UK). After washing 3 times with TBST, the slides were incubated with secondary antibodies including goat anti-rat IgG H&L (Alexa Fluor 488) and Alexa Fluor 647 AffiniPure Rabbit Anti-Mouse IgG (H + L) (1:500, Jackson ImmunoResearch, USA) for 1 h at room temperature. After washing 5 times with TBST, Hoechst 33258 (1:5000, Invitrogen, CA, USA) was used to stain cell nucleus and then sealed with cover glass. Images were obtained by laser scanning confocal microscope Leica TCS SP8 (Leica Biosystems, Germany) and quantified by LEICA SP8 software. Isolation and culture of enteric neurons from WT and BGN (-/0) mice were fixed in 4% paraformaldehyde at room temperature for 30 min, washed with PBS for 3 times and blocked with 5% donkey serum for reducing nonspecific background. The remaining steps were identical with the process of tissues staining mentioned above. The length of neurite was analyzed using ImageJ 1.53k with Neuron J 1.4.3.
Western blot
HCT116 cells were transfected with overexpression plasmid of BGN. The medium of cell culture was digested with ChABC (Sigma, USA) for Western Blot according to previous report.58 Besides, HCT116 cells with overexpression of BGN were incubated with B. thetaiotaomicron at multiplicity of infection (MOI) of 100:1, while control cells with overexpression of BGN were treated with PBS. After 8 h, the cell culture medium was collected for Western Blot, as reported by previous study.58,59 The following antibodies were used: Biglycan Monoclonal antibody (67275-1-Ig, proteintech, China), GAPDH Monoclonal antibody (60004-1-Ig, proteintech, China), goat anti-mouse IgG-HRP (sc-2005, Santa Cruz Biotechnology, USA). The band intensity was quantified using Image JA 1.45b.
Chondroitin sulfate (CS) concentration assay
HCT116 cells were transfected with overexpression plasmid of BGN, followed by B. thetaiotaomicron at MOI of 100:1, while control cells with overexpression of BGN were treated with PBS. The medium of cell culture was collected for dissociative CS measurement by ELISA (CLOUD-CLONE CORP, USA) assay according to the manufacturer’s protocol.
Cytokine assay
The serum was collected from AOM/DSS-treated mice and the serum cytokines GM-CSF, IL-10, TNF-α, IL-1β, IL-6 and IFN-γ were measured by enzyme-linked immunosorbent assay (ELISA, R&D System, USA) according to the manufacture’s protocol. This detection was conducted by the company ColorfulGene Biological Technology Co., LTD (WuHan, China).
Gas chromatography/mass spectrometry (GC/MS)
20 mL supernatant of B. thetaiotaomicron medium and control medium were collected for detecting the SCFAs using GC/MS. The standard curve of mixture including acetic acid, isovaleric acid, propionic acid, isobutyric acid, butyric acid and caproic acid were established. The extract of metabolite, testing and analysis were performed by Bioprofile Company (Shanghai, China).
16S rRNA sequence and analysis
The 16S rRNA sequence and analysis on mice fecal DNA was performed as our previous study.60 The DNA was extracted from mice fecal using the TIANGEN Fecal Genomic DNA extraction kit (TIANGEN, China), following the manufacturer’s instruction. The 16S rRNA V4 region was amplified by PCR using the Phusion High-Fidelity PCR Master Mix (New England Biolabs, USA). 16S rRNA V4 primers are as follows: 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806A (5′-GGACTACHVGGGTWTCTAAT-3′). Sequencing libraries were generated using Illumina TruSeq DNA PCR-Free Library Preparation Kit (Illumina, USA) following the manufacturer’s instructions, and index codes were added. The sequencing was performed by Illumina Hiseq platform (NovoGene, China). The sequence reads were processed in QIIME 2 2020.2 platform.41 Taxonomy was assigned to ASVs using the SILVA naive Bayes taxonomy classifier.61,62 PICRUSt 242 was used for predicting the functional abundances and STAMP 2.1.343 to conduct the differential functional abundances plot. For B. thetaiotaomicron abundance analysis, we obtained the 16S ribosomal RNA sequence of B. thetaiotaomicron and other species listed in Table S2 from NCBI database. The unique 16s reads of stool samples were extracted by usearch v11.0.667_i86linux3244 and matched to Table S2 listed species’ 16S ribosomal RNA sequence with 100% identity and 100% qcov by vsearch v2.17.1_linux_x86_64.45
Enzyme chondroitinase ABC conservatism analysis
Over 40000 bacteria genome were derived from GTDB24 and we annotated the genome using eggNOG 5.0.46 Totally 667 proteins functioning as chondroitinase ABC encoded by 470 taxon species were found, in which 92 species colonized in human gut according to the human gut microbiome reference database.63 We then analyzed the motif pattern on the 161 chondroitinase ABC proteins encoded by the 92 speceies using MEME Suite.47 The sequence protein distance and conservatism analysis at the lyase_catalyt domain (from 221th-580th amino acid) identified by Pfam48 were conducted by MEGA software49 and Clustal X2.50
RNA sequencing and analysis
The RNA-sequencing was conducted as our previous report.60 The enteric neurons from the BGN (-/0) mice or littermate WT mice were treated with LPS (100 μg/L) for 24 h. Then the RNA of control and LPS-treated enteric neurons was extracted with TRIzol reagent. cDNA libraries were prepared used by an Illumina TruSeq RNA sample prep kit (New England Biolabs, USA). RNA-seq was conducted by Novogene Company (Tianjin, China). RNA-Seq reads from the FASTQ files were mapped to the Mus musculus (assembly GRCm39) using HISAT2, and RSeQC package was used to inspect sequence quality. The output files in BAM format were analyzed by HTSeq to quantify gene expression. After that, we used R package DESeq2 for differential expression analysis, R package ggplot2, VennDiagram, and pheatmap were used for visualization.
Single-cell sequencing data analysis
We used R package Seurat64 to analyze the dataset downloaded from UCSC Cell Browser (Cell Browser dataset ID: mouse-nervous-system/enteric).65 Cells with more than 200 and less than 6000 genes are retained, then with more than 10% of mitochondrion-derived genes were filtered out. Genes detected in less than 3 cells were also excluded. Using vst method, we selected 2000 highly variable genes, which showed a significant difference across cells. After ScaleData operation, principal component analysis (PCA) was performed for linear dimensionality reduction and to identify significantly available dimensions of datasets. We examined PCA results in a few different ways including JackStrawand Elbow method. Then, t-distributed stochastic neighbor embedding (tSNE) algorithm was used to visualize cell clusters. The marker genes of each cluster were identified by FindAllMarkers function, using adjusted p value <0.05 and |logFC| > 0.5 standard of truncation. Enteric neurons with widely expressing Ret were mainly divided into cholinergic neurons and nitrogenic neurons, and the markers were Chat and Nos1, respectively. FeaturePlot was used to visualize BGN expression. Based on co-expression of featured genes, we identified 5 putative neurons including putative excitatory motor neurons (PEMNs), putative interneurons (PINs), putative secretomotor/vasodilator neurons (PSVNs), putative sensory neurons (PSNs) and putative inhibitory motor neurons (PIMNs).
Quantification and statistical analysis
Data were determined by the two-sided Student’s t test and the Mann–Whitney U test were used for distinguishing parametric data from nonparametric data. GraphPad Prism Version 6 software (GraphPad Software, USA) was used for graphing and statistical analysis. Data are presented as mean ± SEM and p value <0.05 was considered statistically significant. ∗p < 0.05, ∗∗p < 0.01 and n.s. indicates not significant (p > 0.05).
Acknowledgments
This work was supported by National Key Research and Development Program, 2022YFA1303900, National Natural Science Foundation of China (NSFC) grant 82172288 to XL and Key society development project of Jiangsu Province, BE2021721 to XL. Key development project of innovation of Suzhou in Nanjing Medical University to XL. Youth Research Project of Wuxi Municipal Health Commission, Q202224 to FW.
Author contributions
X.L. conceived and design this project; Y.X., F.W., K.M., Q.Z., X.Z., X.W., D.W., Y.L., and J.W. performed all of experiments. K.M., Q.Z., and Z.L. performed 16S rRNA and RNA-seq analysis. X.L. and Y.X. wrote the manuscript. All the authors approved the final manuscript.
Declaration of interests
The authors declare no conflicts of interest.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: August 2, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107515.
Supplemental information
Data and code availability
-
•
All sequencing data have been deposited at NCBI website and are publicly available as of the date of publication. Accession number is listed in the key resources table. All of unprocessed data and code have been deposited at Mendeley data and are publicly available as of the date of publication. The DOI is listed in the key resources table.
-
•
All original code has been deposited at github website and is publicly available as of the date of publication. The website is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 3.Yu A.I., Zhao L., Eaton K.A., Ho S., Chen J., Poe S., Becker J., Gonzalez A., McKinstry D., Hasso M., et al. Gut Microbiota Modulate CD8 T Cell Responses to Influence Colitis-Associated Tumorigenesis. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kvorjak M., Ahmed Y., Miller M.L., Sriram R., Coronnello C., Hashash J.G., Hartman D.J., Telmer C.A., Miskov-Zivanov N., Finn O.J., Cascio S. Cross-talk between Colon Cells and Macrophages Increases ST6GALNAC1 and MUC1-sTn Expression in Ulcerative Colitis and Colitis-Associated Colon Cancer. Cancer Immunol. Res. 2020;8:167–178. doi: 10.1158/2326-6066.CIR-19-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X., Yang H., Chen X., Gao J., Duan Y., Wei D., Zhang J., Ge K., Liang X.J., Huang Y., et al. Nano-herb medicine and PDT induced synergistic immunotherapy for colon cancer treatment. Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2021.120654. [DOI] [PubMed] [Google Scholar]
- 6.Schonkeren S.L., Thijssen M.S., Vaes N., Boesmans W., Melotte V. The Emerging Role of Nerves and Glia in Colorectal Cancer. Cancers. 2021;13 doi: 10.3390/cancers13010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schledwitz A., Sundel M.H., Alizadeh M., Hu S., Xie G., Raufman J.P. Differential Actions of Muscarinic Receptor Subtypes in Gastric, Pancreatic, and Colon Cancer. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222313153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaes N., Schonkeren S.L., Rademakers G., Holland A.M., Koch A., Gijbels M.J., Keulers T.G., de Wit M., Moonen L., Van der Meer J.R.M., et al. Loss of enteric neuronal Ndrg4 promotes colorectal cancer via increased release of Nid1 and Fbln2. EMBO Rep. 2021;22 doi: 10.15252/embr.202051913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaes N., Idris M., Boesmans W., Alves M.M., Melotte V. Nerves in gastrointestinal cancer: from mechanism to modulations. Nat. Rev. Gastroenterol. Hepatol. 2022;19:768–784. doi: 10.1038/s41575-022-00669-9. [DOI] [PubMed] [Google Scholar]
- 10.Jarret A., Jackson R., Duizer C., Healy M.E., Zhao J., Rone J.M., Bielecki P., Sefik E., Roulis M., Rice T., et al. Enteric Nervous System-Derived IL-18 Orchestrates Mucosal Barrier Immunity. Cell. 2020;180:813–814. doi: 10.1016/j.cell.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Cienfuegos J.A., Martínez P., Baixauli J., Beorlegui C., Rosenstone S., Sola J.J., Rodríguez J., Hernández-Lizoáin J.L. Perineural Invasion is a Major Prognostic and Predictive Factor of Response to Adjuvant Chemotherapy in Stage I-II Colon Cancer. Ann. Surg Oncol. 2017;24:1077–1084. doi: 10.1245/s10434-016-5561-0. [DOI] [PubMed] [Google Scholar]
- 12.Duchalais E., Guilluy C., Nedellec S., Touvron M., Bessard A., Touchefeu Y., Bossard C., Boudin H., Louarn G., Neunlist M., Van Landeghem L. Colorectal Cancer Cells Adhere to and Migrate Along the Neurons of the Enteric Nervous System. Cell. Mol. Gastroenterol. Hepatol. 2018;5:31–49. doi: 10.1016/j.jcmgh.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadighparvar S., Darband S.G., Ghaderi-Pakdel F., Mihanfar A., Majidinia M. Parasympathetic, but not sympathetic denervation, suppressed colorectal cancer progression. Eur. J. Pharmacol. 2021;913 doi: 10.1016/j.ejphar.2021.174626. [DOI] [PubMed] [Google Scholar]
- 14.Yarandi S.S., Kulkarni S., Saha M., Sylvia K.E., Sears C.L., Pasricha P.J. Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice. Gastroenterology. 2020;159:200–213.e8. doi: 10.1053/j.gastro.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aktar R., Parkar N., Stentz R., Baumard L., Parker A., Goldson A., Brion A., Carding S., Blackshaw A., Peiris M. Human resident gut microbe Bacteroides thetaiotaomicron regulates colonic neuronal innervation and neurogenic function. Gut Microb. 2020;11:1745–1757. doi: 10.1080/19490976.2020.1766936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber C.K., Sommer G., Michl P., Fensterer H., Weimer M., Gansauge F., Leder G., Adler G., Gress T.M. Biglycan is overexpressed in pancreatic cancer and induces G1-arrest in pancreatic cancer cell lines. Gastroenterology. 2001;121:657–667. doi: 10.1053/gast.2001.27222. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y.S., Huang T., Zhong X.M., Zhang H.W., Cong X.L., Xu H., Lu G.X., Yu F., Xue S.B., Lv Z.W., Fu D. Proteogenomic characterization and comprehensive integrative genomic analysis of human colorectal cancer liver metastasis. Mol. Cancer. 2018;17:139. doi: 10.1186/s12943-018-0890-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H.C., Cai B.H., Suen C.S., Lee H.Y., Hwang M.J., Liu F.T., Kannagi R. BGN/TLR4/NF-B Mediates Epigenetic Silencing of Immunosuppressive Siglec Ligands in Colon Cancer Cells. Cells. 2020;9:397. doi: 10.3390/cells9020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S., Guo D., Lei B., Bi J., Yang H. Biglycan protects human neuroblastoma cells from nitric oxide-induced death by inhibiting AMPK-mTOR mediated autophagy and intracellular ROS level. Biotechnol. Lett. 2020;42:657–668. doi: 10.1007/s10529-020-02818-z. [DOI] [PubMed] [Google Scholar]
- 20.Rawat P.S., Seyed Hameed A.S., Meng X., Liu W. Utilization of glycosaminoglycans by the human gut microbiota: participating bacteria and their enzymatic machineries. Gut Microb. 2022;14 doi: 10.1080/19490976.2022.2068367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns M.B., Blekhman R. Integrating tumor genomics into studies of the microbiome in colorectal cancer. Gut Microb. 2019;10:547–552. doi: 10.1080/19490976.2018.1549421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J.X., Tao Z.H., Qian Y., Yu C.Y., Li J., Kang Z.R., Lu S., Xie Y., Hong J., Chen H., et al. ZFP90 drives the initiation of colitis-associated colorectal cancer via a microbiota-dependent strategy. Gut Microb. 2021;13:1–20. doi: 10.1080/19490976.2021.1917269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45 doi: 10.1093/nar/gkw1092. D353-d361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parks D.H., Chuvochina M., Rinke C., Mussig A.J., Chaumeil P.-A., Hugenholtz P. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022;50:D785–D794. doi: 10.1093/nar/gkab776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delday M., Mulder I., Logan E.T., Grant G. Bacteroides thetaiotaomicron Ameliorates Colon Inflammation in Preclinical Models of Crohn's Disease. Inflamm. Bowel Dis. 2019;25:85–96. doi: 10.1093/ibd/izy281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang C.J., Lin T.L., Tsai Y.L., Wu T.R., Lai W.F., Lu C.C., Lai H.C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019;27:615–622. doi: 10.1016/j.jfda.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UniProt Consortium UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49:D480–D489. doi: 10.1093/nar/gkaa1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng H., Umar S., Rust B., Lazarova D., Bordonaro M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojas J.M., Avia M., Martín V., Sevilla N. IL-10: A Multifunctional Cytokine in Viral Infections. J. Immunol. Res. 2017;2017 doi: 10.1155/2017/6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang W., O'Garra A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity. 2019;50:871–891. doi: 10.1016/j.immuni.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Kou M., Lu W., Zhu M., Qu K., Wang L., Yu Y. Massively recruited sTLR9(+) neutrophils in rapidly formed nodules at the site of tumor cell inoculation and their contribution to a pro-tumor microenvironment. Cancer Immunol. Immunother. 2023;72:2671–2686. doi: 10.1007/s00262-023-03451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man S.M., Zhu Q., Zhu L., Liu Z., Karki R., Malik A., Sharma D., Li L., Malireddi R.K.S., Gurung P., et al. Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell. 2015;162:45–58. doi: 10.1016/j.cell.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaxevanis C.K., Bauer M., Subbarayan K., Friedrich M., Massa C., Biehl K., Al-Ali H.K., Wickenhauser C., Seliger B. Biglycan as a mediator of proinflammatory response and target for MDS and sAML therapy. OncoImmunology. 2023;12 doi: 10.1080/2162402X.2022.2152998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei J., Hu M., Huang K., Lin S., Du H. Roles of Proteoglycans and Glycosaminoglycans in Cancer Development and Progression. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21175983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Z.X., Zhao S.B., Fang X., E J.F., Fu H.Y., Song Y.H., Wu J.Y., Pan P., Gu L., Xia T., et al. Prognostic and Predictive Value of BGN in Colon Cancer Outcomes and Response to Immunotherapy. Front. Oncol. 2022;11 doi: 10.3389/fonc.2021.761030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ndeh D., Baslé A., Strahl H., Yates E.A., McClurgg U.L., Henrissat B., Terrapon N., Cartmell A. Metabolism of multiple glycosaminoglycans by Bacteroides thetaiotaomicron is orchestrated by a versatile core genetic locus. Nat. Commun. 2020;11:646. doi: 10.1038/s41467-020-14509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers T.E., Pudlo N.A., Koropatkin N.M., Bell J.S.K., Moya Balasch M., Jasker K., Martens E.C. Dynamic responses of Bacteroides thetaiotaomicron during growth on glycan mixtures. Mol. Microbiol. 2013;88:876–890. doi: 10.1111/mmi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuncil Y.E., Xiao Y., Porter N.T., Reuhs B.L., Martens E.C., Hamaker B.R. Reciprocal Prioritization to Dietary Glycans by Gut Bacteria in a Competitive Environment Promotes Stable Coexistence. mBio. 2017;8 doi: 10.1128/mBio.01068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryu T.Y., Kim K., Han T.S., Lee M.O., Lee J., Choi J., Jung K.B., Jeong E.J., An D.M., Jung C.R., et al. Human gut-microbiome-derived propionate coordinates proteasomal degradation via HECTD2 upregulation to target EHMT2 in colorectal cancer. ISME J. 2022;16:1205–1221. doi: 10.1038/s41396-021-01119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian Y., Xu Q., Sun L., Ye Y., Ji G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J. Nutr. Biochem. 2018;57:103–109. doi: 10.1016/j.jnutbio.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douglas G.M., Maffei V.J., Zaneveld J.R., Yurgel S.N., Brown J.R., Taylor C.M., Huttenhower C., Langille M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020;38:685–688. doi: 10.1038/s41587-020-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 45.Rognes T., Flouri T., Nichols B., Quince C., Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4 doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huerta-Cepas J., Szklarczyk D., Heller D., Hernández-Plaza A., Forslund S.K., Cook H., Mende D.R., Letunic I., Rattei T., Jensen L.J., et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019;47 doi: 10.1093/nar/gky1085. D309-d314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey T.L., Johnson J., Grant C.E., Noble W.S. The MEME Suite. Nucleic Acids Res. 2015;43:W39–W49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mistry J., Chuguransky S., Williams L., Qureshi M., Salazar G.A., Sonnhammer E.L.L., Tosatto S.C.E., Paladin L., Raj S., Richardson L.J., et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021;49:D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 51.Wang H., Yang H., Shivalila C.S., Dawlaty M.M., Cheng A.W., Zhang F., Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Hu W. Mouse enteric neuronal cell culture. Methods Mol. Biol. 2013;1078:55–63. doi: 10.1007/978-1-62703-640-5_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith T.H., Ngwainmbi J., Grider J.R., Dewey W.L., Akbarali H.I. An in-vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. J. Vis. Exp. 2013;78:50688. doi: 10.3791/50688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwalm N.D., 3rd, Townsend G.E., 2nd, Groisman E.A. Prioritization of polysaccharide utilization and control of regulator activation in Bacteroides thetaiotaomicron. Mol. Microbiol. 2017;104:32–45. doi: 10.1111/mmi.13609. [DOI] [PubMed] [Google Scholar]
- 55.Parang B., Barrett C.W., Williams C.S. AOM/DSS Model of Colitis-Associated Cancer. Methods Mol. Biol. 2016;1422:297–307. doi: 10.1007/978-1-4939-3603-8_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaki M.H., Vogel P., Malireddi R.K.S., Body-Malapel M., Anand P.K., Bertin J., Green D.R., Lamkanfi M., Kanneganti T.D. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malik A., Sharma D., Malireddi R.K.S., Guy C.S., Chang T.C., Olsen S.R., Neale G., Vogel P., Kanneganti T.D. SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity. 2018;49:515–530.e5. doi: 10.1016/j.immuni.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin C.L., Oh J.H., Han M., Shin M.K., Yao C., Park C.H., Jin Z.H., Chung J.H. UV irradiation-induced production of monoglycosylated biglycan through downregulation of xylosyltransferase 1 in cultured human dermal fibroblasts. J. Dermatol. Sci. 2015;79:20–29. doi: 10.1016/j.jdermsci.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 59.Wang J., Lu R., Fu X., Dan Z., Zhang Y.G., Chang X., Liu Q., Xia Y., Liu X., Sun J. Novel Regulatory Roles of Wnt1 in Infection-Associated Colorectal Cancer. Neoplasia. 2018;20:499–509. doi: 10.1016/j.neo.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen K., Luan X., Liu Q., Wang J., Chang X., Snijders A.M., Mao J.H., Secombe J., Dan Z., Chen J.H., et al. Drosophila Histone Demethylase KDM5 Regulates Social Behavior through Immune Control and Gut Microbiota Maintenance. Cell Host Microbe. 2019;25:537–552.e8. doi: 10.1016/j.chom.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bokulich N.A., Kaehler B.D., Rideout J.R., Dillon M., Bolyen E., Knight R., Huttley G.A., Gregory Caporaso J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2's q2-feature-classifier plugin. Microbiome. 2018;6:90. doi: 10.1186/s40168-018-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almeida A., Nayfach S., Boland M., Strozzi F., Beracochea M., Shi Z.J., Pollard K.S., Sakharova E., Parks D.H., Hugenholtz P., et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol. 2021;39:105–114. doi: 10.1038/s41587-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hao Y., Hao S., Andersen-Nissen E., Mauck W.M., 3rd, Zheng S., Butler A., Lee M.J., Wilk A.J., Darby C., Zager M., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drokhlyansky E., Smillie C.S., Van Wittenberghe N., Ericsson M., Griffin G.K., Eraslan G., Dionne D., Cuoco M.S., Goder-Reiser M.N., Sharova T., et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell. 2020;182:1606–1622.e23. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All sequencing data have been deposited at NCBI website and are publicly available as of the date of publication. Accession number is listed in the key resources table. All of unprocessed data and code have been deposited at Mendeley data and are publicly available as of the date of publication. The DOI is listed in the key resources table.
-
•
All original code has been deposited at github website and is publicly available as of the date of publication. The website is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.