Abstract
Background
Time to symptom resolution measures were used in outpatient coronavirus disease 2019 (COVID-19) treatment trials without prior validation.
Methods
ACTIV-2/A5401 trial participants completed a COVID-19 diary assessing 13 targeted symptoms and global experience (overall COVID-19 symptoms, return to pre–COVID-19 health) daily for 29 days. We evaluated concordance of time to sustained (2 days) resolution of all targeted symptoms (TSR) with resolution of overall symptoms and return to health in participants receiving placebo.
Results
The analysis included 77 high-risk and 81 standard-risk participants with overall median 6 days of symptoms at entry and median age 47 years, 50% female, 82% white, and 31% Hispanic/Latino. Correlation between TSR and resolution of overall symptoms was 0.80 and 0.68, and TSR and return to health, 0.66 and 0.57 for high- and standard-risk groups, respectively. Of the high- and standard-risk participants, 61% and 79%, respectively, achieved targeted symptom resolution, of which 47% and 43%, respectively, reported symptom recurrence. Requiring >2 days to define sustained resolution reduced the frequency of recurrences.
Conclusions
There was good internal consistency between TSR and COVID-19–specific global outcomes, supporting TSR as a trial end point. Requiring >2 days of symptom resolution better addresses natural symptom fluctuations but must be balanced against the potential influence of non-COVID-19 symptoms.
Clinical Trials Registration
Keywords: ACTIV-2, COVID-19, internal validity, patient-reported outcomes, symptom diary, symptom outcomes, symptom rebound, symptom recurrence, time to symptom resolution
The emergence of the global coronavirus disease 2019 (COVID-19) pandemic prompted the rapid development of clinical trials to evaluate potential therapeutic interventions. Early outpatient phase 2 and 3 trials and US Food and Drug Administration (FDA) emergency use authorization approvals of COVID-19 therapeutics for nonhospitalized persons focused on demonstrating efficacy in reducing hospitalizations and deaths [1–4]. Time to symptom resolution or alleviation/improvement measures were often included as key symptom-based outcome measures [1, 2, 5–7]. The importance of symptom-based outcomes has increased as hospitalization and death rates have declined with widespread vaccination, prior infection, and current Omicron subvariants [8]. Time to symptom resolution or improvement measures are recommended patient-reported outcome measures in FDA guidance for drug development for COVID-19 and other respiratory diseases [9–13], but there has been limited validation of these measures for COVID-19 treatment trials.
Using data from a symptom diary completed daily by participants in the ACTIV2/A5401 trial, we sought to describe characteristics of time to symptom resolution or improvement measures, now commonly the primary outcome in outpatient COVID-19 clinical trials, and to assess the internal validity of these measures. For these analyses, we focused on a time to sustained symptom resolution measure, but also explored a measure of sustained symptom improvement. We additionally explored the frequency of symptom recurrences after achieving sustained symptom resolution and examined how this varied based on the definition of sustained. The analyses were conducted separately for participants at high risk versus standard risk of progression to severe COVID-19 (risk groups defined in “Methods” section) given that recent and current trials often address these populations separately (both in defining trial eligibility and primary analysis populations). It is important to understand the performance of these measures among patients with different levels of risk for progression of COVID-19 as the pandemic has evolved to have lower rates of hospitalization and death and future drug development in this area will likely need to rely on symptom end points. At the time these measures were developed, there were no data to inform the design of such measures for outpatient COVID-19 treatment trials. The ACTIV-2/A5401 data presented in this manuscript underpinned the selection of the primary and secondary end points in the ACTIV-2/A5401 platform trial for subsequent arms, as well as the EPIC-HR (NCT04960202) and EPIC-SR (NCT05011513) trials of ritonavir-boosted nirmatrelvir and the National Institutes of Health-funded ACTIV-6 platform trial (NCT04885530). Even today, no standard exists for symptom outcome assessment in COVID-19 clinical trials. Our findings inform the design of symptom-based outcome measures for outpatient COVID-19 therapeutics trials, which is a considerable challenge for drug development today.
METHODS
Study Design and Participants
ACTIV-2/A5401 is a phase 2/3 randomized, controlled platform trial. This exploratory analysis includes data from participants who received blinded saline placebo by intravenous infusion at 38 sites in the United States during the evaluation of the monoclonal antibody bamlanivimab [14]. Participants were 18 years of age or older with documented severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection by an FDA-authorized antigen or nucleic acid test within 7 days prior to study entry, no more than 10 days of COVID-19 symptoms, and ongoing symptoms within 48 hours prior to study entry (see Supplementary Material for qualifying symptoms), without the need for hospitalization. Participants were categorized at study entry as high risk for progression to severe COVID-19 if aged ≥55 years and/or having 1 or more predefined comorbidities (see Supplementary Material for protocol definitions); those not meeting these criteria were categorized as being at standard risk. Details of the ACTIV-2/A5401 study design have previously been described [14]. The protocol was approved by a central institutional review board (IRB), Advarra (Pro00045266), with additional local IRB approval as required by participating sites. All participants provided written informed consent.
Participant Study Diary
Participants completed a paper symptom diary (Supplementary Material) once each day for 29 days from day 0 (prior to infusion) to day 28. They were instructed to complete the diary at approximately the same time each day and received daily reminders (such as by text, telephone, email, or other method for which the participant provided permission) to complete the diary. Diaries were also reviewed by site staff at each study visit (days 0, 2, 3, 7, 10, 14, 21, and 28). The diary included 2 COVID-19–specific global assessment questions: “Overall, how bad are your COVID-19 symptoms TODAY?” (response options of “no symptoms,” “mild,” “moderate,” “severe,” and “very severe”) and “Have you returned to your usual (pre–COVID-19) health today?” (response options of “yes” or “no”). These were followed by self-assessment of 13 targeted symptoms: “cough,” “shortness of breath or difficulty breathing at rest or with activity,” “feeling feverish,” “chills,” “fatigue (low energy),” “body pain or muscle pain or aches,” “diarrhea,” “nausea,” “vomiting,” “headache,” “sore throat,” “nasal obstruction or congestion (stuffy nose),” and “nasal discharge (runny nose).” Participants were asked to assess each targeted symptom as “absent,” “mild,” “moderate,” or “severe,” and made no assessment of attribution to COVID-19. The content validity of the diary has been established [15].
Outcomes
We focused on time to sustained resolution of all targeted symptoms, defined as the number of days from start of study treatment to the first of 2 consecutive days when all 13 targeted symptoms were scored as absent. Participants were assumed to have symptoms during hospitalization. Measures based on the global assessment questions were also constructed: time to sustained resolution of overall COVID-19 symptoms and time to sustained return to usual (pre–COVID-19) health, again defining sustained as 2 consecutive days. Details on these measures and their definitions, as well as an alternative measure of time to sustained improvement in all targeted symptoms, which we consider briefly, are summarized in Supplementary Table 1. We considered alternative definitions of sustained symptom resolution, which varied the number of required days from 1 day to 7 days. Symptom recurrence was defined as any of the 13 targeted symptoms recorded in the study diary as present with mild or worse severity at any time after meeting the outcome of all targeted symptoms resolved. Similarly, overall COVID-19 symptom recurrence was defined as overall COVID-19 symptoms recorded as present with mild or worse severity at any time after meeting the outcome of overall COVID-19 symptoms resolved, and relapse of return to health status as participants recording that they had not returned to health on at least 1 day after recording that they had returned to health.
Statistical Analysis
All analyses were conducted separately in high-risk and standard-risk participant groups. Descriptive summaries provided an overview of targeted symptom and global symptom severity over time. Kaplan-Meier methods were used to estimate the median time to each symptom outcome and percentage of participants not meeting the outcome by 28 days. Censoring of follow-up for a given symptom measure was on the last day that the symptom measure could have been achieved. For example, if 2 consecutive days of symptoms were required to meet the outcome, for participants completing the diary through to day 28, censoring occurred on day 27 as that was the last day the symptom measure could have been met (requiring the outcome be met on days 27 and 28). A similar approach was used if participants were lost to follow-up before day 28. Scatterplots and Spearman correlations were used to characterize associations between symptom outcome measures for assessing internal validity (ranking participants who did not meet the outcome by end of follow-up as worse than those who did meet the outcome). Specifically, the validity of the time to sustained targeted symptom resolution or improvement measures (ie, clinical recovery as measured using participant report on multiple individual symptoms, which may or may not be COVID-19 specific but are known potential COVID-19 symptoms—an approach commonly used now in COVID-19 clinical trials) was assessed against the same individual's report of their general sense of recovery from COVID-19 (whether or not they felt they had ongoing COVID-19 symptoms overall or had returned to their usual pre–COVID-19 health).
RESULTS
Participant Characteristics
The study population was composed of 158 participants enrolled between August and November 2020 (prior to the emergence of the Delta and Omicron variants), including 77 in the high-risk and 81 in the standard-risk groups. Participant characteristics are summarized in Table 1. As expected, the high-risk group was older (median 55 vs 38 years). Median time with symptoms at study entry was 6 days in both risk groups. Through day 28, among high-risk participants, 8 (10%) were hospitalized, none died, and 4 (5%) were lost to follow-up. Of the hospitalizations, 4 were deemed COVID-19–related and 4 not COVID-19–related (1 each of severe headache, severe anemia, hyperglycemia, and pyelonephritis) by the blinded site investigator. None of the standard-risk participants were hospitalized, died, or lost to follow-up through day 28.
Table 1.
Baseline Characteristics by Risk Group
| Characteristic | High Risk (n = 77) |
Standard Risk (n = 81) |
Overall (n = 158) |
|---|---|---|---|
| Age, y, median (IQR) | 55 (46–62) | 38 (29–47) | 47 (34–55) |
| Sex, n (%) | |||
| Female | 33 (43) | 46 (57) | 79 (50) |
| Male | 44 (57) | 35 (43) | 79 (50) |
| Gender identity, cis-gender, n (%) | 77 (100) | 81 (100) | 158 (100) |
| Race, n (%) | |||
| White | 60 (79) | 69 (85) | 129 (82) |
| Black | 8 (11) | 4 (5) | 12 (8) |
| Asian | 5 (7) | 4 (5) | 9 (6) |
| Other | 3 (4) | 4 (5) | 7 (4) |
| Missing | 1 | 0 | 1 |
| Ethnicity, n (%) | |||
| Hispanic/Latino | 19 (25) | 29 (36) | 48 (31) |
| Not Hispanic/Latino | 56 (75) | 51 (64) | 107 (69) |
| Missing | 2 | 1 | 3 |
| High-risk characteristic, n (%) | |||
| Age 55 y or older | 42 (55) | … | … |
| Chronic lung disease | 4 (5) | … | … |
| Moderate to severe asthma | 6 (8) | … | … |
| Body mass index >35 kg/m2 | 19 (25) | … | … |
| Hypertension | 44 (57) | … | … |
| Cardiovascular disease, including history of stroke | 12 (16) | … | … |
| Diabetes | 15 (19) | … | … |
| Chronic kidney disease | 3 (4) | … | … |
| Chronic liver disease | 3 (4) | … | … |
| Days from symptom onset at study entry, median (IQR) | 6 (5–7) | 6 (4–7) | 6 (4–7) |
| ≤5 d, n (%) | 30 (39) | 36 (44) | 66 (42) |
| >5 d, n (%) | 47 (61) | 45 (56) | 92 (58) |
Abbreviation: IQR, interquartile range.
Profile of Symptoms and Global Assessments Over Time
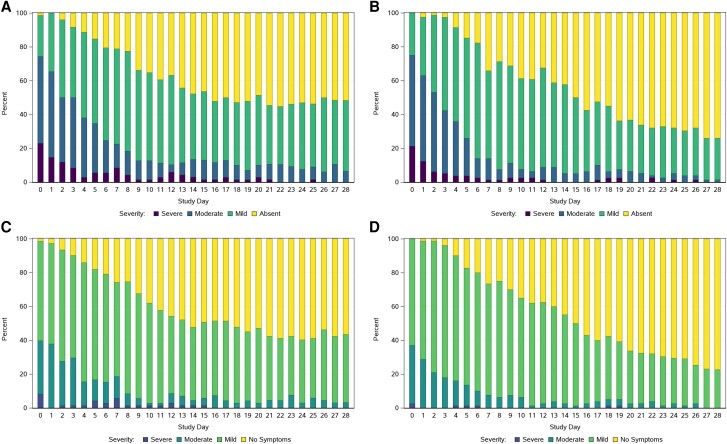
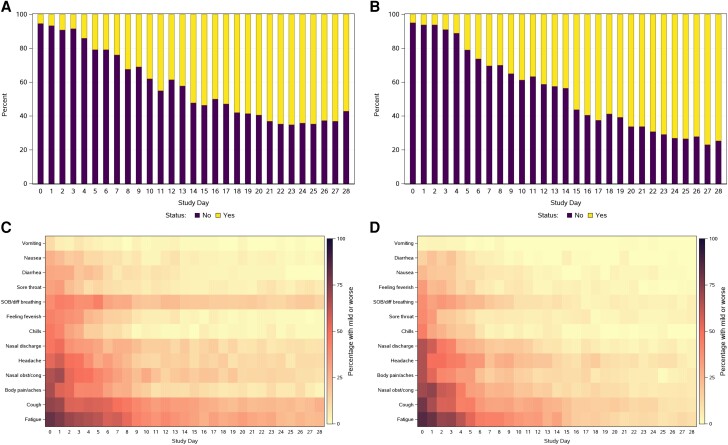
Diary completion rates each day between day 0 and day 28 were generally about 90% among high-risk participants and over 95% among standard-risk participants (Supplementary Table 2). For both the high- and standard-risk groups, the temporal profiles of the proportion of participants reporting any of the targeted symptoms, reporting overall COVID-19 symptoms, and reporting not having returned to pre–COVID-19 health were very similar (Figure 1 and Figure 2). Many participants in both risk groups, however, graded individual symptoms as being of worse severity than they graded their overall COVID-19 symptoms on a given day (Figure 1).
Figure 1.
Reported symptom severity on each study day: (A) worst symptom severity recorded across all 13 targeted symptoms among high-risk participants; (B) worst symptom severity recorded across all 13 targeted symptoms among standard-risk participants; (C) symptom severity by overall COVID-19 symptoms assessment among high-risk participants; and (D) symptom severity by overall COVID-19 symptoms assessment among standard-risk participants. C and D, Response options were “no symptoms,” “mild,” “moderate,” “severe,” and “very severe.” No participants recorded “very severe” symptoms.
Figure 2.
Reported return to health status on each study day among (A) high-risk and (B) standard-risk participants, and percentage of participants reporting presence of each of the 13 targeted symptoms among (C) high-risk and (D) standard-risk participants. C and D, Symptoms are ordered by ascending prevalence at day 0. Abbreviations: SOB, shortness of breath; diff, difficulty; obst/cong, obstruction/congestion.
On day 0, similar proportions of high- and standard-risk participants reported at least 1 of the 13 targeted symptoms as moderate or severe (74% [55/74] of high-risk and 75% [60/80] of standard-risk participants) and reported overall COVID-19 symptoms as being moderate or severe (40% [29/73] of high-risk and 37% [30/81] of standard-risk participants). However, the high-risk group appeared to have a slower rate of decline in proportion reporting symptoms than the standard-risk group from day 15 through day 28, although most symptoms were mild during this period (Figure 1A and 1B). This longitudinal pattern of a higher proportion of high-risk compared to standard-risk participants reporting ongoing symptoms from about day 15 onwards was also observed for the global assessments of overall COVID-19 symptoms (Figure 1C and 1D) and return to pre–COVID-19 health (Figure 2A and 2B).
Fatigue and cough were the most common symptoms at day 0 and also tended to be the most persistent symptoms through day 28 in both risk groups (Figure 2C and 2D). Conversely, vomiting, nausea, diarrhea, sore throat, feeling feverish, and chills were less frequent at day 0 and less persistent during follow-up in both groups. Besides fatigue and cough, the greater proportion in the high-risk group with symptoms persisting from about day 15 onwards appeared to also be driven by greater persistence of shortness of breath, headache, nasal discharge, and nasal obstruction.
Relationships Between Time to Resolution of All Targeted Symptoms and Global Measures
The distributions of time to resolution of all targeted symptoms, time to overall COVID-19 symptom resolution, and time to return to pre–COVID-19 health (each required to be sustained for 2 consecutive days) showed very similar features in both the high-risk and standard-risk groups (Supplementary Table 3). The median time to achieving each of the 3 outcomes varied very little: 16 days for the high-risk group and 15 days for the standard-risk group for time to all targeted symptoms resolved; 15 days for both groups for time to overall COVID-19 symptoms resolved; and 14 days for the high-risk group and 15 days for the standard-risk group for time to return to pre–COVID-19 health. Among standard-risk participants, the proportion of participants achieving each outcome by day 27 (the last day that the requirement for 2 consecutive days of symptoms absent could be assessed) was similar for the 3 outcomes: 79% for all targeted symptoms resolved, 81% for overall COVID-19 symptoms resolved, and 77% for return to pre–COVID-19 health. The proportions achieving each outcome were lower in the high-risk group and somewhat more varied: 61%, 66%, and 70%, respectively.
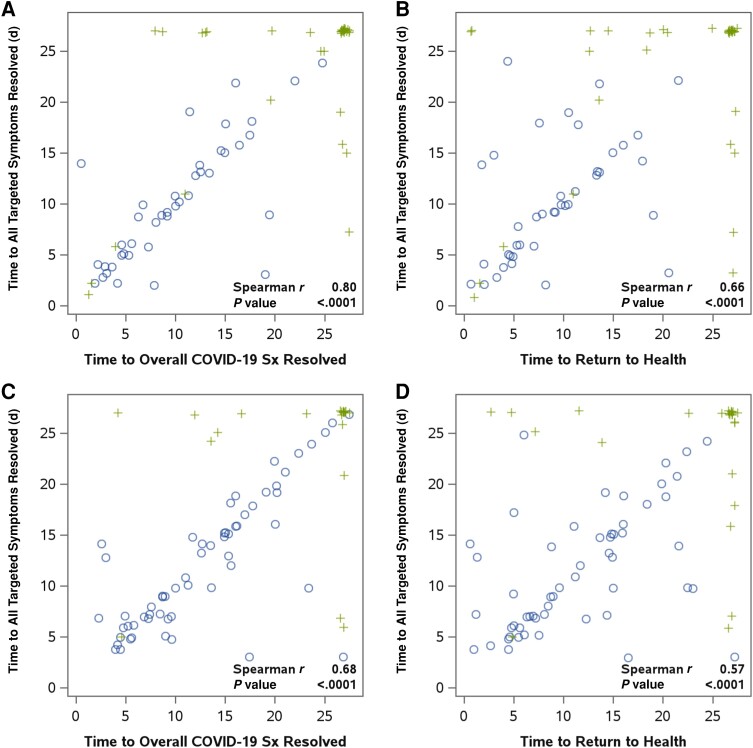
Supporting the internal validity of the primary targeted symptom resolution measure, time to sustained resolution of all targeted symptoms for 2 days was significantly correlated with both time to sustained resolution of overall COVID-19 symptoms and time to sustained return to health for both risk groups: for correlation with time to overall COVID-19 symptom resolution, r = 0.80 for high risk and r = 0.68 for standard risk, and for correlation with time to return to health, r = 0.66 for high risk and r = 0.57 for standard risk, P < .0001 for all (Figure 3). The global assessment measures (time to sustained resolution of overall COVID-19 symptoms and time to sustained return to pre–COVID-19 health) were strongly correlated for both risk groups (r = 0.77 for high and r = 0.88 for standard risk) (Supplementary Figure 1).
Figure 3.
Internal validity of time to sustained resolution of all targeted symptoms (for 2 days) measure for high- and standard-risk participants. Presented are scatterplots of time to sustained resolution of all targeted symptoms for 2 days (primary outcome measure) against time to sustained resolution of overall COVID-19 symptoms (for 2 days) and time to sustained return to health (for 2 days) for high-risk participants (A and B) and standard-risk participants (C and D). Spearman rank correlations assessed the association between the measures. Circles indicate times that were observed for both outcomes; crosses indicate times that were censored because of end of diary follow-up (values of 27 for 1 or both outcomes) or premature discontinuation of diary completion (values less than 27 for 1 or both outcomes).
Examining parallel time to sustained symptom improvement (rather than resolution) measures, sustained improvement in all targeted symptoms occurred earlier than sustained resolution of all targeted symptoms (median 12 vs 16 days for high risk, and 7 vs 15 days for standard risk, respectively; Supplementary Table 4), largely reflecting persistence of mild symptoms. The correlation of time to sustained improvement of all targeted symptoms with time to return to health was r = 0.54 for high-risk and r = 0.46 for standard-risk participants (P < .0001 for both; Supplementary Figure 2). The correlations of the global time to sustained improvement in overall COVID-19 symptoms measure with time to return to health were weaker (r = 0.42 [P = .0002] and r = 0.16 [P = .16] for high- and standard-risk participants, respectively; Supplementary Figure 3) than correlations of the global time to sustained resolution in overall COVID-19 symptoms measure with time to return to health.
Symptom Recurrence After Resolution
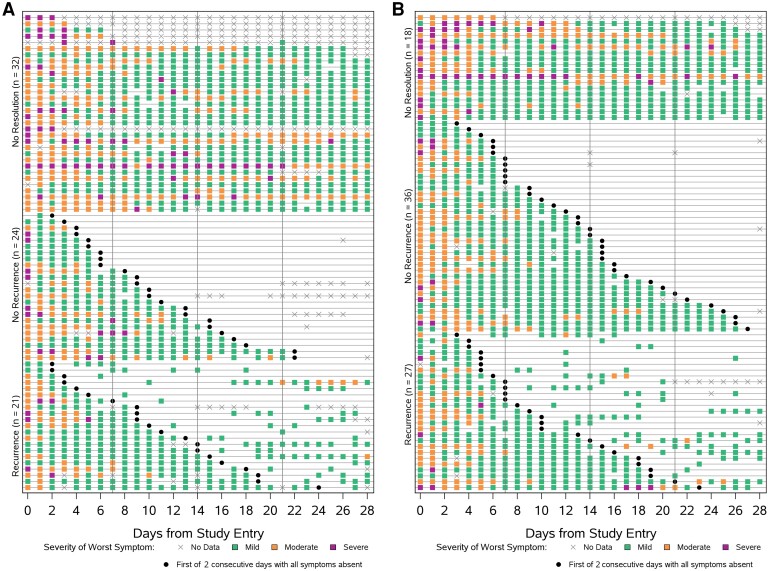
Among participants who met the outcome of sustained (2 days) resolution of all targeted symptoms, symptom recurrence occurred in 47% (21/45) of all high-risk participants and 43% (27/63) of all standard-risk participants (Supplementary Table 5). The recurrent symptoms reported most frequently by both high- and standard-risk participants were cough, fatigue, headache, and nasal obstruction (Supplementary Figure 4). Figure 4 summarizes symptom reporting for each participant. Corresponding figures for recurrent symptoms or relapse in return to health for the outcomes of sustained (2 days) resolution of overall COVID-19 symptoms and return to health are given in Supplementary Figures 5 and 6.
Figure 4.
Pattern of daily diary entries for the outcome of time to sustained (2 days) targeted symptom resolution for (A) high-risk and (B) standard-risk groups. Each row represents a participant. Meeting the outcome of all targeted symptoms resolved is given by a black dot. Green, orange, and purple boxes indicate days on which participants recorded at least 1 of 13 targeted symptoms being present (self-reported severity of mild, moderate, or severe) in their symptom diary. The color of the box reflects the worst symptom severity reported of all symptoms recorded as being present (green box if the worst severity reported was mild, orange box if the worst severity was moderate, and purple box if the worst severity was severe). An x indicates days when no data were recorded. Participants are grouped by outcome, from the top of the figure down: participants who never met the outcome, participants who met the outcome and did not report recurrent symptoms (ie, stayed resolved), and participants who met the outcome but subsequently reported recurrence of at least 1 targeted symptom.
The percentage of participants with symptom recurrence or relapse assessed by the global COVID-19 health status questions was lower than for recurrences of targeted symptoms after sustained resolution all targeted symptoms for both risk groups. Thirty-three percent (16/49) of high-risk and 26% (17/65) of standard-risk participants reported relapse in their overall COVID-19 symptom assessment on at least 1 day after achieving sustained resolution of overall COVID-19 symptoms for at least 2 days. The findings were similar in examining relapse following sustained return to pre–COVID-19 health (ie, answering “no” at least once following 2 consecutive days of answering “yes” about return to pre–COVID-19 health): 31% (16/52) of high-risk participants and 23% (14/62) of standard-risk participants reporting relapse on at least 1 day by day 28, after achieving sustained return to health for at least 2 days (Supplementary Table 5).
Exploring a Requirement for More Days of Sustained Symptom Resolution in Defining Outcomes
Requiring more than 2 consecutive days of symptoms being reported as absent to define a symptom resolution outcome measure led to fewer participants meeting the outcome within the 28-day follow-up period assessed by the diary. The percentage of high-risk participants who did not meet the targeted symptom resolution outcome increased from 39% with a 2-day requirement to 40% with a 4-day requirement and 47% with a 7-day requirement; the corresponding increase among standard-risk participants was from 21% to 31% and 37% (Supplementary Table 3). However, among both high- and standard-risk participants, the proportion with symptom recurrences after symptom resolution declined with increasing stringency in days defining sustained, from 47% with a 2-day requirement to 24% with a 7-day requirement for high-risk participants, and from 43% to 22% for standard-risk participants (Supplementary Table 5). Rates of recurrence of nonreturn to health declined similarly (Supplementary Table 5).
DISCUSSION
In this analysis, we evaluated the outcome of time to sustained resolution of 13 targeted COVID-19 symptoms for 2 consecutive days, based on participant responses in a 29-day daily symptom diary. Participant symptom assessment followed FDA recommendations [12]. We found that time to sustained resolution of all targeted symptoms was strongly correlated with time to sustained resolution of overall COVID-19 symptoms and with time to sustained return to pre–COVID-19 health in both high- and standard-risk populations, suggesting good internal validity of the outcomes based on symptom diary responses. Consistent with this, in the standard-risk group, the median time to all 3 of these outcomes was the same and the proportion of participants not meeting each outcome very similar. In the high-risk group, there was more variability between the measures. The medians varied from 16 days for targeted symptom resolution, to 15 days for overall symptom resolution, and 14 days for return to pre–COVID-19 health, with a corresponding decreasing trend in proportion not meeting each outcome by day 27: 39%, 34%, and 30%, respectively. This pattern in the high-risk group could reflect participants self-assessing a return to pre–COVID-19 health and resolution of overall COVID-19 symptoms earlier than is measured by sustained resolution of targeted symptoms because the latter is capturing symptoms associated with comorbidities. Alternatively, the symptoms were not felt to be significant enough to impact their sense of recovery. Furthermore, the proportion of participants in each risk group experiencing relapses by the 2 COVID-19–specific global assessments was lower than the proportion experiencing recurrence of targeted symptoms. These observations suggest that global measures of overall COVID-19 symptom resolution or return to health may be more specific measures of recovery from COVID-19 than targeted symptom measures. Assessment of the presence and severity of targeted symptoms prior to COVID-19 onset could be considered in defining symptom recovery (ie, requiring return to pre–COVID-19 baseline symptom status and not complete absence of all symptoms), with the recognized limitation that such reports are subject to recall bias.
The proportion of high-risk participants not achieving targeted symptom resolution was high (39%). This is consistent with other trials, including EPIC-HR, conducted before the appearance of Omicron variants, and PANORAMIC, conducted in a largely vaccinated population early following the emergence of Omicron variants [16, 17]. It is also consistent with the long-term symptoms and sequelae following acute COVID-19 (long COVID) with reported rates of 45% or more [18]. Among standard-risk participants, the proportion not achieving targeted symptom resolution by day 27 was lower but still significant (21%) and comparable with other trials that enrolled later, including during the Omicron period [19–21]. The differences in temporal profiles of symptomology according to risk group and symptom profile/severity at study entry are therefore important considerations in designing trials, for example affecting power and sample size considerations.
Recurrence of symptoms was common, affecting 47% of high-risk and 43% of standard-risk participants who achieved sustained resolution of all targeted symptoms for 2 days. This likely reflects natural fluctuations in COVID-19, which have been underappreciated, but could also reflect occurrence of symptoms due to comorbidities. Requiring more than 2 consecutive days of sustained resolution decreased the frequency of recurrences, but also led to a lower proportion of participants meeting the outcome (resolved symptoms). Requiring 4 days of sustained symptom resolution might offer a good balance with lower rates of recurrence, while not being so stringent that the outcome cannot be observed in a large proportion of participants. We note that this exploratory analysis contributed to the use of a 4-day requirement in primary or secondary symptom outcome measures in the phase 3 nirmatrelvir/ritonavir EPIC-SR and EPIC-HR trials [16, 22]. For the common practice of using 2 consecutive days as the requirement for defining sustained symptom resolution, our results (and the concerns about possible symptom recurrence after treatment) suggest the need for supportive analyses of symptom recurrence to rule out an adverse effect of treatment or symptoms unrelated to COVID-19 on recurrence rates. Use of COVID-specific global assessment questions may have value in providing supportive evidence of a participant's self-assessment of disease-specific health status.
Strengths of the analysis include blinded, standardized assessments of placebo-randomized participants within the ACTIV-2/A5401 trial, high completion rates of the diary, and prior qualitative diary content validation [15]. An important limitation is that the data in this analysis were collected earlier in the COVID-19 pandemic in an unvaccinated population with primary SARS-CoV-2 infection prior to the emergence of the current Omicron SARS-CoV-2 variants. It is unknown if the same observations would be found in vaccinated and/or previously COVID-19–experienced populations, or in populations with current or future variants. Additionally, while we observed high diary completion rates, we note that self-collected long-duration diaries such as those used in ACTIV-2/A5401 and other COVID-19 trials may be burdensome to participants and increase risk for missing data, making symptom durations challenging to determine.
In conclusion, our results support the validity of time to sustained symptom resolution outcome measures constructed from participant-reported responses in a 29-day symptom diary that has established content validity. Confirmation of our findings in the evolving nonhospitalized populations with mild-to-moderate COVID-19 from current and future variant infections will be important.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Kara W Chew, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA.
Carlee Moser, Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Eunice Yeh, Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
David A Wohl, Department of Medicine, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Eric S Daar, Division of HIV Medicine, The Lundquist Institute, Harbor-University of California, Los Angeles Medical Center, Torrance, California, USA.
Justin Ritz, Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Arzhang Cyrus Javan, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, Maryland, USA.
Joseph J Eron, Department of Medicine, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Judith S Currier, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, USA.
Davey M Smith, Department of Medicine, University of California, San Diego, La Jolla, California, USA.
Michael D Hughes, Department of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Notes
Acknowledgments. We thank the study participants, site staff, site investigators, and the entire ACTIV-2/A5401 study team; the AIDS Clinical Trials Group; the Harvard Center for Biostatistics in AIDS Research and ACTG Statistical and Data Analysis Center; the ACTIV-2 Community Advisory Board; the National Institute of Allergy and Infectious Diseases/Division of AIDS; the Foundation for the National Institutes of Health; the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership; and the PPD clinical research business of Thermo Fisher Scientific.
Disclaimer . The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers UM1AI068636, UM1AI068634, and UM1AI106701).
Supplement sponsorship . This article appears as part of the supplement “Findings From the ACTIV-2/AIDS Clinical Trials Group A5401 Adaptive Platform Trial of Investigational Therapies for Mild-to-Moderate COVID-19,” sponsored by the National Institutes of Health through a grant to the University of California, Los Angeles.
References
- 1. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med 2021; 384:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med 2021; 385:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med 2022; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med 2021; 385:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gottlieb RL, Nirula A, Chen P, et al. Effect of Bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2021; 325:632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernal A J, da Silva MM G, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor CA, Whitaker M, Anglin O, et al. COVID-19-associated hospitalizations among adults during SARS-CoV-2 Delta and Omicron variant predominance, by race/ethnicity and vaccination status—COVID-NET; 14 states, July 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US oral neuraminidase study group. JAMA 2000; 283:1016–24. [DOI] [PubMed] [Google Scholar]
- 10. Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 11. US Department of Health and Human Services, Food and Drug Administration . COVID-19: developing drugs and biological products for treatment or prevention, guidance for industry. February 2021. https://www.fda.gov/media/167274/download.
- 12. US Department of Health and Human Services, Food and Drug Administration . Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment, guidance for industry. September 2020. https://www.fda.gov/media/142143/download.
- 13. US Department of Health and Human Services, Food and Drug Administration . Community-acquired bacterial pneumonia: developing drugs for treatment, guidance for industry. June 2020. https://www.fda.gov/media/75149/download.
- 14. Chew KW, Moser C, Daar ES, et al. Antiviral and clinical activity of bamlanivimab in a randomized trial of non-hospitalized adults with COVID-19. Nat Commun 2022; 13:4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matza LS, Stewart KD, Naegeli AN, et al. Qualitative interviews to evaluate content validity of the ACTIV-2 COVID-19 symptom diary (ACSD). J Patient Rep Outcomes 2023; 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hammond J, Leister-Tebbe H, Gardner A, et al. 1156 Sustained alleviation and resolution of targeted COVID-19 symptoms with nirmatrelvir/ritonavir versus placebo. Open Forum Infect Dis 2022; 9(Suppl 2):ofac492.994. [Google Scholar]
- 17. Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet 2023; 401:281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Mahoney LL, Routen A, Gillies C, et al. The prevalence and long-term health effects of long COVID among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine 2023; 55:101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naggie S, Boulware DR, Lindsell CJ, et al. Effect of ivermectin vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2022; 328:1595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCarthy MW, Naggie S, Boulware DR, et al. Effect of fluvoxamine vs placebo on time to sustained recovery in outpatients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2023; 329:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naggie S, Boulware DR, Lindsell CJ, et al. Effect of higher-dose ivermectin for 6 days vs placebo on time to sustained recovery in outpatients with COVID-19: a randomized clinical trial. JAMA 2023; 329:888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pfizer, Inc . Press release: Pfizer reports additional data on PAXLOVID supporting upcoming new drug application submission to U.S. FDA. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-reports-additional-data-paxlovidtm-supporting. Accessed 10 March 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.