Abstract
Genomic subtractive hybridization of closely related Pasteurella multocida isolates has generated clones useful in distinguishing hemorrhagic septicemia-causing type B strains from other P. multocida serotypes. Oligonucleotide primers designed during the sequencing of these clones have proved valuable in the development of PCR assays for rapid species- and type-specific detection of P. multocida and of type B:2 in particular. This study demonstrated that the primer pair designed from the sequence of the clone 6b (KTT72 and KTSP61) specifically amplified a DNA fragment from types B:2, B:5, and B:2,5 P. multocida and that the primers KMT1T7 and KMT1SP6 produced an amplification product unique to all P. multocida isolates analyzed. It was also shown that PCR amplification performed directly on bacterial colonies or cultures represents an extremely rapid, sensitive method of P. multocida identification.
Hemorrhagic septicemia (HS) is a peracute disease of cattle and buffalo, and recently swine, that is endemic in most parts of tropical Asia, Africa, and India (5, 6). Definitive diagnosis of HS is presently made by laboratory identification of the causative agent, Pasteurella multocida serotype B:2 or E:2, although some isolates demonstrate cross-reactivity with type 5 antisera. In recent years, group B isolates possessing somatic antigens other than serotype 2 or 5 have been implicated in causing HS-like disease (or septicemic pasteurellosis) in wild ruminants (17, 18). In addition, reexamination of P. multocida strains isolated from outbreaks of HS in North America demonstrated that certain strains presumed to be serotype B:2 were in fact serotype B:3,4 (20). These findings emphasize the necessity of employing both capsular and somatic typing methods for definitive serological characterization of P. multocida. The identification of serotypes other than B:2 and E:2 from reported HS outbreaks clearly indicates that the definition of HS and its distinction, if any, from septicemic pasteurellosis require reevaluation.
Accurate laboratory detection of P. multocida depends on the isolation and identification of suspect bacterial colonies by microscopy and biochemical tests. Samples taken immediately from animals that died of suspected pasteurellosis yield almost pure cultures of P. multocida from, e.g., heart blood, liver, spleen, bone marrow, or lung. However, isolation of P. multocida can prove difficult during field surveys of carrier status when samples are taken from a contaminated site on the animal, such as the nose or throat. Extensive subculturing is then required to obtain a pure culture of the causative organism. In addition, difficulties experienced in the preparation of antisera and the time required for current P. multocida serotyping procedures have meant that definitive serological determination is impractical for most laboratories in countries where HS is endemic (19). This may lead to an increased lag between the collection of animal material and serotype identification if lengthy transportation is required for the material to reach a laboratory able to perform definitive serotyping procedures.
In recent years, genotypic methods of bacterial identification have proved beneficial in overcoming some limitations of traditional phenotypic procedures. Nucleic acid-based assays allow the detection of organisms directly from clinical samples or from small amounts of cultured bacterial cells, thus dramatically improving the sensitivity and decreasing the time required for bacterial identification. PCR has been particularly useful in this regard, with the use of primer sequences designed to facilitate identification at any level of specificity: strain, species, genus, or all members of a domain (16).
Genomic subtractive hybridization has been of great value in the identification of unique DNA sequences, with its recent application to the identification of differences between closely related bacterial genomes (3, 7, 24). The original subtractive hybridization method described was designed to isolate and clone differentially expressed mRNA sequences (21). Modifications to include the use of genomic DNA have expanded the application of the technique in molecular biology. In recent years, there have been an increasing number of reports of differential cloning of genomic DNA, particularly from prokaryotic genomes. Genomic subtraction has proved effective in isolating DNA fragments for direct use as probes for strain identification (3, 4, 7, 8).
The incorporation of streptavidin-coated paramagnetic particles and a low-background cloning strategy (9, 24) has exponentially increased the efficiency of the subtraction procedure and remains applicable to the employment of competitive reassociation of DNA fragments of any cell types to identify unique DNA sequences. This report details the replacement of Streptavidin Magnesphere Paramagnetic Particles (Promega, Sydney, Australia) with Dynabeads M-280 streptavidin (Dynal), allowing the addition of solid-phase driver fragments to ensure the enrichment of unique tester DNA sequences following magnetic separation.
Oligonucleotide primers designed from cloned subtracted fragments have contributed to the development of PCR-based assays for species- and type-specific identification of P. multocida and of P. multocida type B, the causal agent of HS. Rapid identification of P. multocida and presumptive confirmation of the HS-causing serotype have the potential to reform HS diagnosis in Southeast Asia, as this technique could be implemented in regional laboratories that are currently not able to perform serological determination.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in the genomic subtraction and determination of species specificity are listed in Table 1. All bacteria were grown overnight at 37°C on sheep blood agar plates, except for Actinobacillus pleuropneumoniae and Haemophilus influenzae, which were grown on 8% sheep blood chocolate agar with a Vitox supplement (Oxoid) overnight at 37°C in 5% CO2.
TABLE 1.
Strains used in this studya
| Strain | Sourceb |
|---|---|
| P. multocida | |
| VP17 (A:4) | VP |
| VP21 (A:3) | VP |
| VP161 (A:1) | VP |
| VP130 (B:2) | VP |
| VP145 (B:2,5) | VP |
| VP164 (B:5) | VP |
| P1511 (B:1) | RR |
| P5226 (B:3,4) | RR |
| 0140 (B:3,4) | RVL |
| P5325 (B:4) | RR |
| VP170 (D:1) | VP |
| 0349 (D) | RVL |
| P4218 (F:3) | RR |
| P. multocida subsp. multocida NCTC 10322 | PB |
| P. multocida subsp. gallicida NCTC 10204 | PB |
| P. multocida subsp. septica CIP A125 | PB |
| P. dagmatis NCTC 11617 | PB |
| P. canis biotype 1 NCTC 11621 | PB |
| P. canis biotype 2 HIM 843-5 | PB |
| P. stomatis NCTC 11623 | PB |
| P. anatis NCTC 11413 | PB |
| P. langaa NCTC 11411 | PB |
| Pasteurella sp. B strain SSI P6835 | PB |
| P. haemolytica | |
| 0155 (A5) | RVL |
| 0158 (T10) | RVL |
| H. influenzae type b ATCC 10211 | CP |
| A. pleuropneumoniae FD131 | VP |
| Actinobacillus sp. strain 0134 | RVLc |
| E. coli K-12 | VP |
| P. aeruginosa FD28 | VP |
| S. typhimurium FD27 | VP |
| S. aureus Oxford FD32 | VP |
| S. faecalis FD72 | VP |
| B. cereus FD8 | VP |
Known serotypes of P. multocida and P. haemolytica are given in parentheses following each isolate identification number.
VP, Department of Veterinary Pathology, The University of Queensland, Brisbane, Queensland, Australia. RR, R. Rimler, U.S. Department of Agriculture, National Animal Disease Center, Ames, Iowa. RVL, Regional Veterinary Laboratory, Benalla, Victoria, Australia. PB, P. Blackall, Queensland Department of Primary Industries, Animal Research Institute, Yeerongpilly, Queensland, Australia. CP, J. J. Sullivan, N. J. Nicolaides, and Partners, Consulting Pathologists, Brisbane, Queensland, Australia.
Originally obtained from RVL as P. multocida, this strain, upon subculture, was determined to be an Actinobacillus species by the Medvet Microbact 24E system (Medvet, Adelaide, Australia).
Subtractive hybridization and nucleotide sequence analysis.
Genomic subtractive hybridization with Dynabead magnetic separation was performed essentially as described previously (24) with minor modifications. Genomic DNA of tester and driver P. multocida strains was prepared as described by Townsend et al. (23). The tester DNA was from isolate 0113 (type I), while the cocktail driver mix was comprised of 20 μg of sonicated (two 5-min bursts), biotinylated DNA from each of three strains: P1511 (B:1), P5226 (B:3,4), and 0140 (B:3,4). The cocktail driver mix was added to 200 μg of prewashed Dynabeads M-280 streptavidin and incubated at room temperature for 30 min with constant gentle shaking. The coated driver beads were captured, alkali denatured, and washed three times in 1× B&W buffer (5 mM Tris-HCl [pH 7.5], 0.5 mM EDTA [pH 8.0], 1 M NaCl). Sau3AI-digested tester DNA was denatured by boiling, cooled on ice, and then added to the biotinylated DNA-coated beads. Hybridization of driver and tester DNA was performed in a hybridization buffer containing 40 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 6.4), 1 mM EDTA (pH 8.0), 0.4 M NaCl, and 80% deionized formamide, at 42°C for 24 to 48 h with constant rolling in a Hybaid hybridization oven (Hybaid Limited, Teddington, United Kingdom).
Following hybridization, the magnetic beads were captured, and the hybridization mixture was transferred to a new Eppendorf tube. The hybridization mixture was then denatured by heating at 95°C for 5 min and stored on ice until required. The magnetic beads were regenerated by alkali denaturation with immediate magnetic separation. The beads were washed three times, resuspended in the denatured hybridization mixture, and incubated for a further 24 to 48 h at 42°C. Following the second round of subtraction, the magnetic beads were captured, and enriched subtracted DNA was purified with the BRESA-CLEAN kit (Bresatec Ltd., Thebarton, Australia) and resuspended in 10 μl of nuclease-free water (Promega). All subsequent steps were performed as described previously (24) with additional purification of partially end-filled vector and enriched DNA with the BRESA-CLEAN kit prior to ligation to remove unincorporated nucleotides. Isolated clones successfully amplified by PCR with SP6-T7 promoter primers were examined by Southern blot hybridization with membrane-bound PstI-digested P. multocida DNA, and nucleotide sequence analysis was performed.
Amplification by PCR.
Oligonucleotide primers used to sequence the clones 6b (24) and KMT1 were synthesized by the Centre for Cell and Molecular Biology, Queen Elizabeth II Medical Centre, Nedlands, Western Australia, Australia. The primer sequences are as follows: SP6 promoter primer, 5′-TATTTAGGTGACACTATAG-3′; T7 promoter primer, 5′-d(TAATACGACTCACTATAGGG)-3′; KTSP61, 5′-ATCCGCTAACACACTCTC-3′ (internal sequencing primer for 6b); KTT72, 5′-AGGCTCGTTTGGATTATGAAG-3′ (internal sequencing primer for 6b); KMT1SP6, 5′-GCTGTAAACGAACTCGCCAC-3′ (internal sequencing primer for KMT1); and KMT1T7, 5′-ATCCGCTATTTACCCAGTGG-3′ (internal sequencing primer for KMT1).
Specificity of the PCR assays.
In order to determine the specificities of the primers KMT1SP6-KMT1T7 and KTSP61-KTT72, a broad range of bacterial species and P. multocida serotypes (Table 1) were examined. For ease and rapidity, PCR was performed directly from single colonies grown on agar plates. A pipette tip was lightly touched onto a colony, and this sample was then resuspended in PCR amplification mixture containing 10 ng of each primer per μl, 200 μM concentrations of each dNTP, 1× Expand High Fidelity buffer with 1.5 mM MgCl2, and 1 U of Expand High Fidelity PCR System enzyme mix (Boehringer Mannheim). The PCR was performed on an FTS-320 thermal sequencer (Corbett Research), with an initial denaturation at 95°C for 4 min, followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 55°C for 1 min, extension at 72°C for 1 min, and a final extension at 72°C for 9 min. Amplification products were separated by agarose gel electrophoresis (2% agarose in 1× TAE) at 4 V/cm for 1 h and stained with ethidium bromide. DNA fragments were viewed by UV illumination and photographed.
Nucleotide sequence accession number.
The GenBank accession numbers for the subtracted clones KMT1 and 6b are AF016259 and AF016260, respectively.
RESULTS
Genomic subtraction utilizing Dynabead magnetic separation produced three candidate clones, of which one clone (KMT1) was amplified successfully with SP6-T7 promoter primers. The amplified product was radioactively labelled and used to probe membrane-bound PstI-digested P. multocida DNA. Hybridization of the clone KMT1 revealed binding to all serotypes of P. multocida; however, type B and type E isolates could be distinguished from other strains on the basis of fragment size (data not shown). In addition, the clone KMT1 was able to distinguish HS-causing P. multocida B:2 from type B strains possessing other somatic serotypes.
Nucleotide sequence analysis of the clone KMT1 was performed, and the size of the subtracted fragment was determined to be 866 nucleotides (nt) after allowances were made for the partial end-fill of both the fragment and the vector (Fig. 1). Analysis of open reading frame (ORF) location demonstrated a large ORF of >260 amino acids with a termination at +778 in reading frame 2 of the sequence obtained with the T7 promoter primer. Multiple terminations were demonstrated in all reading frames of the sequence by means of the SP6 promoter primer. Therefore, it was assumed that the strand obtained with the T7 promoter sequence was more likely to be the coding strand, and this primer was used for subsequent database similarity searches. While a search of the Haemophilus influenzae Rd genome (http://www.tigr.org/) did not demonstrate significant identity between the latter and KMT1, a GenBank database search (November, 1995) revealed a degree of identity (59.1% of 115-nt overlap with the T7 sequence) with bexB of the Haemophilus influenzae type b capsulation locus (11). Identity (56.0%) in 243 overlapping nt was also observed with an ORF adjacent to the Escherichia coli crp divergent RNA (1). However, recent analysis (November, 1997) of the nucleotide and partial amino acid sequences did not reveal any significant homology to published DNA or protein sequences in either the GenBank or the Swiss-Prot database.
FIG. 1.
Predicted nucleotide sequence of the clone KMT1. Predicted nucleotide sequence of the clone KMT1 from the T7 promoter primer (GenBank accession number AF016259). The sequence contains an ORF of >260 amino acids in frame 2 before reaching a termination codon at +781 (marked in bold and underlined). The oligonucleotide primers used for sequencing and the PM-PCR assay are underlined and marked KMT1T7 and KMT1SP6.
Specificities of PCR primers.
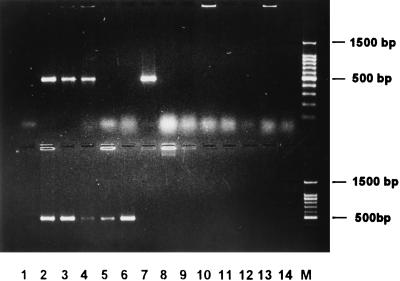
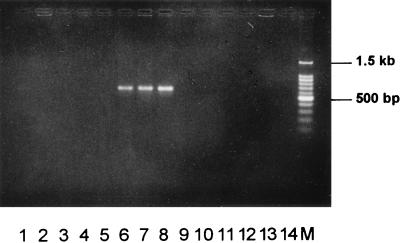
In order to determine the specificities of the regions encoded by clones 6b and KMT1, the internal sequencing primers from each fragment were used to amplify DNA sequences from a broad range of P. multocida isolates, other members of the Pasteurellaceae family, and unrelated bacteria. The primer pair KMT1SP6-KMT1T7 amplified a product of approximately 460 bp from all strains of P. multocida, from the three P. multocida subspecies reference strains (subsp. multocida, subsp. gallicida, and subsp. septica), and from Pasteurella canis biotype 2 (Fig. 2). No product was detected from any of the remaining cultures. Some variation in the intensity of the amplified product was observed, illustrating the inconsistency of the DNA concentration used in each PCR by the pipette tip method. However, a positive result is still easily determined. PCR amplification with the primer pair designed during the sequencing of clone 6b (KTSP61-KTT72) specifically produced a product of approximately 590 bp from HS-causing type B isolates of P. multocida (Fig. 3). These primers were unable to amplify DNA from other P. multocida serotypes, other Pasteurella species, other members of the Pasteurellaceae family, or unrelated bacteria. It was also clearly evident that no product was amplified from type B P. multocida isolates possessing somatic serotypes other than type 2, type 5, or type 2,5.
FIG. 2.
P. multocida-specific PCR assay. This figure illustrates fragments specifically amplified by PCR in all P. multocida subspecies and serotypes by means of the primers KMT1SP6 and KMT1T7. The upper panel shows the following: lane 1, negative control; lane 2, P. multocida subsp. multocida; lane 3, P. multocida subsp. gallicida; lane 4, P. multocida subsp. septica; lane 5, Pasteurella dagmatis; lane 6, P. canis biotype 1; lane 7, P. canis biotype 2; lane 8, Pasteurella stomatis; lane 9, Pasteurella anatis; lane 10, Pasteurella langaa; lane 11, Pasteurella species B; lane 12, Pasteurella haemolytica A5; lane 13, Pasteurella haemolytica T10; lane 14, Actinobacillus species 0134; and lane 15, 100-bp DNA marker (Promega). The lower panel shows the following: lane 1, negative control; lane 2, P. multocida Carter type A; lane 3, type B; lane 4, type D; lane 5, type E; lane 6, type F; lane 7, H. influenzae type b; lane 8, A. pleuropneumoniae; lane 9, E. coli; lane 10, Pseudomonas aeruginosa; lane 11, Salmonella typhimurium; lane 12, Staphylococcus aureus; lane 13, Streptococcus faecalis; lane 14, Bacillus cereus; and lane M, 100-bp DNA marker. Samples were electrophoresed at 2 V/cm for 2 h in a 2% agarose gel (1× TAE), stained with ethidium bromide, visualized by UV illumination, and photographed.
FIG. 3.
HS-causing type B P. multocida-specific PCR assay. This figure illustrates fragments specifically amplified by PCR from type B P. multocida organisms that cause hemorrhagic septicemia by means of the primers KTSP61 and KTT72. It can be seen that only P. multocida B:2, B:5, and B:2,5 produced amplification products. This gel shows a negative control (lane 1), P. multocida strain VP161, serotype A:1 (lane 2), VP21, A:3 (lane 3), VP17, A:4 (lane 4), P1511, B:1 (lane 5), 0332, B:2 (lane 6), VP164, B:5 (lane 7), VP145, B:2,5 (lane 8), P5226, B:3,4 (lane 9), P5325, B:4 (lane 10), 0349, D (lane 11), VP170, D:1 (lane 12), 0350, E (lane 13), P4218, F:3 (lane 14), and a 100-bp DNA marker (Promega) (lane M). Samples were electrophoresed at 2 V/cm for 2 h in a 2% agarose gel (1× TAE), stained with ethidium bromide, visualized by UV illumination, and photographed.
DISCUSSION
The development of genomic subtractive hybridization has revolutionized the search for virulence genes in pathogenic bacteria with the use of virulent and related avirulent strains to enhance the isolation of DNA fragments related to pathogenicity. In addition, this technique is capable of isolating species-specific sequences useful for identification of bacterial species. A modified magnetic cloning strategy incorporating the use of Dynabeads has produced a cloned fragment (KMT1) that, with subsequent hybridization analysis, is capable of distinguishing type B:2 P. multocida from other serotypes. Oligonucleotide primers designed from the nucleotide sequence of this clone and a previously isolated subtracted DNA fragment arbitrarily named 6b (24) have formed the basis for two PCR assays that specifically identify P. multocida, and in particular type B isolates that cause HS.
Knowledge of the identity and function of the gene partially encoded by KMT1 would enhance our understanding of the distinction between HS and septicemic-pasteurellosis-causing isolates. However, recent analysis (November, 1997) of sequences in the GenBank database did not reveal any significant identity. While the initial search of the GenBank database (November, 1995) demonstrated a degree of identity between clone KMT1 and bexB from H. influenzae type b and also with crp divergent RNA from E. coli, the failure of primers KMT1SP6 and KMT1T7 to produce an amplification product with either species suggests that either this fragment is unique to P. multocida or the primer sequences are not conserved.
The positive amplification of DNA from P. canis biotype 2 was of some interest, as this strain was originally classified as a P. multocida-like strain, designated Taxon 13, isolated from a pneumonic calf lung (13). DNA-DNA hybridization studies by Mutters et al. (14) indicated high homology of this strain to isolates now designated as P. canis biotype 1 (previously known as P. multocida biovar 6). At the time of submission of this report, there had not been any published studies documenting the use of specific primers for the detection of P. multocida. Therefore, it is not known whether other laboratories have also observed false-positive amplification of P. canis biotype 2 DNA when testing the specificity of PCR assays for the detection of P. multocida. These results may, however, indicate a higher degree of genomic relatedness of P. canis biotype 2 to P. multocida than was previously seen by DNA-DNA hybridization analysis. Alternatively, the distinction of P. canis biotype 2 (Orn−) from P. multocida (Orn+) by DNA-DNA hybridization could reflect the findings of Bisgaard et al. (2), in which ornithine-positive and -negative strains of P. multocida subsp. septica showed only 44% DNA binding. Comparison of the 16S rRNA sequences from P. multocida and P. canis biotype 2 could provide clarification of the phylogenetic relationship between these two strains and determine whether these strains represent two species or ornithine variants of P. multocida.
In order to assess accurately the impact of pasteurellosis on the poultry and livestock industries, a rapid diagnostic method specific for the detection of P. multocida is essential. The development of a P. multocida-specific PCR assay will provide rapid species identification without relying on phenotypic differentiation, which could require up to 2 weeks before definitive biotype results are obtained. This assay will also assist in the rapid detection of P. multocida from mixed cultures, a common activity when the clinical sample is obtained from a contaminated area of the animal such as the nose or throat. Recently developed PCR assays have been directed at the identification of toxigenic P. multocida for clinical diagnosis of atrophic rhinitis (10, 12, 25), with one report detailing the use of arbitrary primers to differentiate P. multocida subsp. multocida (2).
The present study describes the development of a PCR assay that will detect all subspecies of P. multocida, a technique useful for the identification of P. multocida directly from bacterial cultures without extraction and purification of genomic DNA. As isolation of P. canis biotype 2 has only been reported with pneumonic calves and swine (15, 22), it is unlikely that a false-positive reaction due to this species will hinder field trials aimed at ascertaining the level of carriage or infection with P. multocida in poultry. Therefore, protocols to detect P. multocida in chicken blood and feces by means of P. multocida-specific PCR (PM-PCR) are currently being developed, with the aim of providing a rapid, sensitive method for the detection of clinically infected birds. It is hoped that future optimization of this protocol will either eliminate false-positive amplification from P. canis biotype 2 or clarify the phylogenetic relationship between these two species, thus permitting the use of this technique in field studies of cattle and swine.
Discrimination of the B:2 serotype with the clone KMT1 requires additional hybridization analysis. However, this study has shown that oligonucleotide primers designed during nucleotide sequencing analysis of the clone 6b (24) can be used to identify type B P. multocida that causes HS (types B:2, B:5, and B:2,5). It is understood that this assay will not identify all HS-causing strains of P. multocida, as these primers do not amplify DNA from type E:2 strains that cause HS in Africa. Nor will this assay identify type B strains of other somatic serotypes that have been implicated in septicemic pasteurellosis of wild ruminants. However, the ability of the PCR assays described in this study to provide rapid identification of P. multocida and confirmation of the HS-causing serotype has the potential to reform HS diagnosis in Southeast Asia. This technique could be implemented in regional laboratories that are currently not able to perform serological determination and be used to rapidly confirm a field diagnosis of HS without the need to obtain pure cultures and perform extensive biochemical tests.
ACKNOWLEDGMENT
This work was supported in part by the Australian Centre for International Agricultural Research.
REFERENCES
- 1.Bhasin R, Freundlich M. The nucleotide sequence of the Escherichia coli crp divergent RNA and an overlapping ORF. Biochim Biophys Acta. 1991;1129:109–111. doi: 10.1016/0167-4781(91)90222-8. [DOI] [PubMed] [Google Scholar]
- 2.Bisgaard M, Houghton S B, Mutters R, Stenzel A. Reclassification of German, British and Dutch isolates of so-called Pasteurella multocida obtained from pneumonic calf lungs. Vet Microbiol. 1991;26:115–124. doi: 10.1016/0378-1135(91)90048-k. [DOI] [PubMed] [Google Scholar]
- 3.Bjourson A J, Stone C E, Cooper J E. Combined subtraction hybridization and polymerase chain reaction amplification procedure for isolation of strain-specific Rhizobium DNA sequences. Appl Environ Microbiol. 1992;58:2296–2301. doi: 10.1128/aem.58.7.2296-2301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjourson A J, Cooper J E. Isolation of Rhizobium loti strain-specific DNA sequences by subtraction hybridization. Appl Environ Microbiol. 1988;54:2852–2855. doi: 10.1128/aem.54.11.2852-2855.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter G R, De Alwis M C L. Haemorrhagic septicaemia. In: Adlam C, Rutter J M, editors. Pasteurella and pasteurellosis. London, England: Academic Press Limited; 1989. pp. 131–160. [Google Scholar]
- 6.Chanter N, Rutter J M. Pasteurellosis in pigs and the determinants of virulence of toxigenic Pasteurella multocida. In: Adlam C, Rutter J M, editors. Pasteurella and pasteurellosis. London, England: Academic Press Limited; 1989. pp. 161–195. [Google Scholar]
- 7.Chen J, Brosch R, Luchansky J B. Isolation and characterization of Listeria monocytogenes-specific nucleotide sequences. Appl Environ Microbiol. 1993;59:4367–4370. doi: 10.1128/aem.59.12.4367-4370.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darrasse A, Kotoujansky A, Bertheau Y. Isolation by genomic subtraction of DNA probes specific for Erwinia carotovora subsp. atroseptica. Appl Environ Microbiol. 1994;60:298–306. doi: 10.1128/aem.60.1.298-306.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher S, Darragh D, Fan Y, Grounds M D, Fisher C J, Beilharz M W. Specific cloning of DNA fragments unique to the dog Y chromosome. Genet Anal Tech Appl. 1993;10:77–83. doi: 10.1016/1050-3862(93)90038-k. [DOI] [PubMed] [Google Scholar]
- 10.Kamp E M, Bokken G C, Vermeulen T M, de Jong M F, Buys H E, Reek F H, Smits M A. A specific and sensitive PCR assay suitable for large-scale detection of toxigenic Pasteurella multocida in nasal and tonsillar swab specimens of pigs. J Vet Diagn Invest. 1996;8:304–309. doi: 10.1177/104063879600800305. [DOI] [PubMed] [Google Scholar]
- 11.Kroll J S, Loynds B, Brophy L N, Moxon E R. The bex locus in encapsulated Haemophilus influenzae: a chromosomal region involved in capsule polysaccharide export. Mol Microbiol. 1990;4:1853–1862. doi: 10.1111/j.1365-2958.1990.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 12.Lichtensteiger C A, Steenbergen S M, Lee R M, Polson D D, Vimr E R. Direct PCR analysis for toxigenic Pasteurella multocida. J Clin Microbiol. 1996;34:3035–3039. doi: 10.1128/jcm.34.12.3035-3039.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madsen E B, Bisgaard M, Mutters R, Pedersen K B. Characterization of Pasteurella species isolated from lungs of calves with pneumonia. Can J Comp Med. 1985;49:63–67. [PMC free article] [PubMed] [Google Scholar]
- 14.Mutters R, Ihm P, Pohl S, Fredericksen W, Mannheim W. Reclassification of the genus Pasteurella Trevisan 1887 on the basis of deoxyribonucleic acid homology, with proposals for the new species Pasteurella dagmatis, Pasteurella canis, Pasteurella stomatis, Pasteurella anatis, and Pasteurella langaa. Int J Syst Bacteriol. 1985;35:309–322. [Google Scholar]
- 15.Nagai S, Someno S, Yagihashi T. Differentiation of toxigenic from nontoxigenic isolates of Pasteurella multocida by PCR. J Clin Microbiol. 1994;32:1004–1010. doi: 10.1128/jcm.32.4.1004-1010.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Relman D A, Persing D H. Genotypic methods for microbial identification. In: Persing D H, editor. PCR protocols for emerging infectious diseases: a supplement to Diagnostic Molecular Microbiology: Principles and Applications. Washington, D.C: ASM Press; 1996. pp. 3–31. [Google Scholar]
- 17.Rhoades K R, Rimler R B. Serological characterisation of Pasteurella multocida strains isolated from wild ruminants as capsular serogroup B. Vet Rec. 1992;130:331–332. doi: 10.1136/vr.130.15.331. [DOI] [PubMed] [Google Scholar]
- 18.Rimler R B. Passive immune cross-protection in mice produced by rabbit antisera against different serotypes of Pasteurella multocida. J Comp Pathol. 1996;114:347–360. doi: 10.1016/s0021-9975(96)80011-9. [DOI] [PubMed] [Google Scholar]
- 19.Rimler R B, Rhoades K R. Pasteurella multocida. In: Adlam C, Rutter J M, editors. Pasteurella and pasteurellosis. London, England: Academic Press Limited; 1989. pp. 37–73. [Google Scholar]
- 20.Rimler R B, Wilson M A. Re-examination of Pasteurella multocida serotypes that caused haemorrhagic septicaemia in North America. Vet Rec. 1994;134:256. doi: 10.1136/vr.134.10.256. [DOI] [PubMed] [Google Scholar]
- 21.Sargent T D, Dawid I B. Differential gene expression in the gastrula of Xenopus laevis. Science. 1983;222:135–139. doi: 10.1126/science.6688681. [DOI] [PubMed] [Google Scholar]
- 22.Schimmel D, Sachse K. Classification of Pasteurella field strains isolated from farms in Germany using traditional methods and DNA-DNA hybridization. Zentbl Bakteriol. 1993;279:125–130. doi: 10.1016/s0934-8840(11)80498-6. [DOI] [PubMed] [Google Scholar]
- 23.Townsend K M, Dawkins H J S, Papadimitriou J M. Analysis of haemorrhagic septicaemia-causing isolates of Pasteurella multocida by ribotyping and field alteration gel electrophoresis (FAGE) Vet Microbiol. 1997;57:383–395. doi: 10.1016/s0378-1135(97)00121-1. [DOI] [PubMed] [Google Scholar]
- 24.Townsend K M, Dawkins H J S, Zeng B J, Watson M W, Papadimitriou J M. Cloning of a unique sequence specific to type B:2 Pasteurella multocida isolates. Res Vet Sci. 1996;61:199–205. doi: 10.1016/s0034-5288(96)90063-6. [DOI] [PubMed] [Google Scholar]
- 25.Zucker B, Kruger M, Horsch F. Differentiation of Pasteurella multocida subspecies multocida isolates from the respiratory system of pigs using polymerase chain reaction fingerprinting techniques. Zentbl Vetmed Reihe B. 1996;43:585–591. doi: 10.1111/j.1439-0450.1996.tb00357.x. [DOI] [PubMed] [Google Scholar]