Abstract
Macrophage-arbitrated inflammation is associated with the regulation of rheumatoid arthritis (RA). Low risk and better efficiency are steered herbal drugs more credible than conventional medicines in RA management. Bhadradarvadi (BDK) concoction has been traditionally used for rheumatism in Ayurveda. However, the mechanisms at the molecular level are still elusive. This study was designed to inspect the process of immunomodulation and anti-inflammatory properties of BDK in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages for the first time. BDK concoction was prepared and evaluated with the stimulated murine macrophage-like RAW 264.7 cell lines. TNF-α, IL6, and PGE2 were quantified by ELISA. The normalization of the fold change in the expression of the target gene mRNA was done by comparing the values of the β-actin housekeeping gene using the 2−ΔΔCt comparative cycle threshold. The expression of TNF-α, IL6, iNOS, and COX-2 in the RAW 264.7 macrophage cells was analyzed using flow cytometry. Our results showed that BDK (150–350 μl/ml) treatment significantly decreased the inflammatory cytokines (TNF-α, and IL-6) and inflammatory mediators (PGE2) in LPS-stimulated RAW 264.7 macrophage cells. The pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) expression, inflammatory enzymes (iNOS and COX-2), and NF-κBp65 were significantly downregulated at transcriptome level in LPS-stimulated RAW 264.7 macrophage cells. The flow cytometry analysis revealed that BDK treatment diminished the TNF-α, IL-6, iNOS, and COX-2 expression at the proteome level, as well as obstruction of NF-κB-p65 nuclear translocation was observed by immunofluorescence analysis in LPS-stimulated RAW 264.7 macrophage cells. Collectively, BDK can intensely augment the anti-inflammatory activities via inhibiting the NF-κB signaling pathway trigger for treating autoimmune disorders including RA.
Keywords: Bhadradarvadi, Immunomodulation, Inflammatory response, NF-κB signaling, Rheumatoid arthritis
Graphical abstract
1. Introduction
Inflammation is a physiological condition triggered by detrimental stimuli such as infection, trauma or lipopolysaccharide endotoxin (LPS), tissue damage, or acquaintance with allergens to recoup tissue homeostasis which may lead to the manifestation of chronic inflammation [[1], [2], [3]]. Such chronic, substandard inflammatory conditions may rankle over the long term and lead to various age-related disorders (ARD), including rheumatoid arthritis (RA) [[4], [5], [6]]. Therefore, several studies on excessive inflammatory responses have been documented over the past decades [[7], [8], [9]]. RA is a chronic inflammatory autoimmune arthropathy that affects approximately 0.5–2% of the world population, which is usually multiple immune cells and their associated inflammatory mediators [10,11]. The originating reason for RA has not been completely understood yet, however, dysregulation of the immune system has been long-established to contribute a significant part to the progression of the disease [10]. Several pro-inflammatory mediators are secreted by infiltrating T cells, B cells and macrophages in the synovial tissues and fluids, which contribute to cartilage damage and joint inflammation [12,13].
Macrophages play vital roles in the stimulation and advancement of inflammatory processes via acting as a barrier against invading mediators (bacteria, fungi and viruses), reacting to pathogenic attacks like infection as well as immune regulatory functions [14]. Activated macrophages can drive the disease progression critically by stimulating inflammatory cascades that are treated as mediators promoting inflammation, such as tumor necrosis factor-alpha (TNF-α), interleukin IL-1, IL-1β, IL-6, IL-8, IL-10, IL-12, chemokine and interferon families [15,16], which are mainly regulated by NF-κB and MAPK pathways [17]. Hence, targeting the suppression of these pro-inflammatory mediators by blocking the activation of macrophages could be a productive way of managing chronic inflammatory responses that were extensively studied using stimulated RAW 264.7 macrophages [[18], [19], [20], [21], [22], [23]].
Immunomodulation is the way of transforming an immune response, either positively or negatively by providing a drug or compound. Immunomodulatory agents are those that regulate or modulate the pathophysiological processes rather than being primarily stimulatory or suppressive [24]. In recent years, the therapeutic approaches to RA have improved abundantly, but still, there is lagging in managing or at least lowering disease activity [25]. Various medications like non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), steroids and novel biologics have shown possible improving disease responses but, they come up with notable side effects. Therefore, the search for efficient alternative therapies for this disease continues [26,27]. Growing shreds of evidence have exhibited that the Indian traditional system of medicines, including Ayurveda, Siddha and Unani formulas are increasing exponentially and they are considered a precious alternative and complementary source to cure unmanageable diseases like diabetes and RA. Bioactives from natural sources have significant therapeutic capability and lesser side effects [28]. Studies have stated that herbal medicine-based products for RA treatment have exposed optimistic outcomes in the attenuation of the disease [29].
Bhadradarvadi (BDK) is an herbal concoction composed of Radix of Valeriana jatamansi, Radix of Oroxylum indicum, Lignum of Cedrus deodara, Radix of Saussurea costus, Radix of Desmodium gangeticum, Radix of Gmelina arborea, Radix of Aegle marmelos, Radix of Stereospermum colais, Radix of Solanum surattense, Fructus of Tribulus terrestris, Radix of Abutilon indicum, Radix of Premna corymbosa, Radix of Pseudarthria viscida, Radix of Solanum anguivi, Radix of Sida cordifolia, is precisely used to treat RA in Ayurveda, also for asthma, other pain or inflammatory joint diseases. Collective evidence has shown that the key herbs and their active ingredients available in BDK can enhance the immunomodulatory effect as well as suppress the inflammatory response and arthritic activity from altered mechanisms [[30], [31], [32], [33]]. Nevertheless, there remains no scientific information has been investigated to determine the efficacy of BDK against RA through enhancing the immunomodulatory activity. Consequently, the motive of the study discussed here is aimed at finding how the inflammatory process initialized by the 264.7 macrophages, that are stimulated by LPS is controlled by the anti-inflammatory property of BDK. The current study findings could provide insights into the molecular mechanism of BDK against diseases like RA which has inflammation as the core pathology.
2. Materials and methods
2.1. Drugs and chemicals
LPS (Escherichia coli strain 0111: B4), Diclofenac sodium and TRIzol were procured from Sigma chemical co. (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), trypsin, and antibiotics (penicillin and streptomycin) were purchased from HiMedia Laboratories (Mumbai, India). ELISA kits for TNF-α, IL6, and PGE2 were purchased respectively, from PeproTech (NJ, USA) and Cayman Chemical, Ann Arbor, MI). The procurement of the high-capacity reverse transcriptase kit for cDNA was through Applied Biosystems (Foster City, NY, USA) and the master mix used (EvaGreen master mix) was obtained from G-Biosciences (St. Louis, MO, USA). Antibodies against NF-κB-p65, TNF-α, IL-6, iNOS and COX-2 were obtained from Cell Signaling Technology (Beverly, MA) and β-actin was purchased from Bioss Antibodies (Woburn, MA, USA). Secondary horseradish peroxidase (HRP)-conjugated antibody was also acquired from Cell Signaling Technology (Beverly, MA, USA). All the other chemicals used in this study were purchased from HiMedia Laboratories (Mumbai, India).
2.2. Preparation of BDK concoction
The amalgamation of the beneficial parts of 15 plants of medicinal value formulates BDK (Table 1) as recorded in the Ashtanga Hridayam (Ancient root text of Ayurveda). Assortment of raw materials and preparation of concoction was accomplished by following classical guidelines as well as by the guidance of an Ayurvedic physician from Kottakal Arya Vaidyasala, Kerala. All the plant parts were delicately, collected, gloom dried, crudely powdered and preserved. From this specific lot, concoctions were blended for the whole research and the stages were followed: one part of crude powder was drenched in 16 parts of water, boiled until it was decreased to 1/4th its actual volume, then filtered and utilized for the study.
Table 1.
The medicinal plant components for Bhadradarvadi decoction preparation (Each 10 ml prepared out of the given quantities of plant parts).
| Official Name | Scientific Name | Family Name | Part used |
|---|---|---|---|
| Bhadradaru | Cedrus deodara | Pinaceae | Heart Wood |
| Natam | Valeriana jatamansi | Valerianaceae | Root |
| Kushtha | Saussurea costus | Asteraceae | Root |
| Kasmari | Gmelina arborea | Lamiaceae | Root |
| Bilwa | Aegle marmelos | Rutaceae | Root |
| Patala | Stereospermum colais | Bignoniaceae | Root |
| Syonaka | Oroxylum indicum | Bignoniaceae | Root |
| Agnimandha | Premna corymbosa | Verbenaceae | Root |
| Salaparni | Desmodium gangeticum | Fabaceae | Root |
| Prisniparni | Pseudarthria viscida | Fabaceae | Root |
| Brihati | Solanum anguivi | Solanaceae | Root |
| Nidigdhika | Solanum surattense | Solanaceae | Root |
| Gokshura | Tribulus terrestris | Zygophyllaceae | Fruit |
| Bala | Sida cordifolia | Malvaceae | Root |
| Atibala | Abutilon indicum | Malvaceae | Root |
2.3. LC-MS analysis
LC-MS/MS analysis was performed using a Dionex UltiMate 3000 RSLCnano LC system from Thermo Scientific (Waltham, MA, USA) with C18-SB analytical column (2.0 mm × 50 mm; particle size 1.8 μm) with an injection volume of 10 μl and 40 °C temperature maintenance. The mobile phase comprises solvent A (H2O/0.1% formic acid) and solvent B (acetonitrile) eluent in a gradient mode (flow rate of 0.5 mL/min). The parameters for analysis were fixed using positive ion mode along with spectra obtained over a mass range from m/z 100 to 1000. The MS data were studied utilizing the Empower software (version 3.0, Waters Corporation, Milford, MA, USA), and the compounds were screened using METLIN metabolomics and the MassBank database.
2.4. Cell culture
The murine macrophage-like RAW 264.7 cell line was obtained from the National Centre for Cell Sciences (NCCS), (Pune, India) and cultured in DMEM supplemented with 10% FBS and antibiotics (100 U penicillin/ml and 10 mg streptomycin/ml) were maintained at 37 °C in a humidified atmosphere of 95% O2 and 5% CO2. Cells were permitted to grow up to a confluency of 90–95%, whereupon phosphate-buffered saline (PBS, pH 7.4) wash of the cells was performed. Except as otherwise specified, in every analysis, RAW 264.7 cells with a count of 1.5 × 106 cells/well were seeded in six-well plates and after that, the former were BDK (150–350 μl/ml) or DCF (1 μg/ml) treated for 24 h and then with LPS (1 μg/ml).
2.5. Cell viability assessment
The effect of BDK on the viability of RAW 264.7 cells was determined by MTT assay. RAW 264.7 cells were seeded into a 96-well plate at a density of 5 × 104 cells/well. The cells were treated with deferring concentrations of BDK (150, 250, 350, 450, 550, and 650 μl/ml) and incubated for 24 h. Then the cells were treated with LPS (1 μg/ml) and incubated for 1 day again. Later to the decantation of the medium, 20 μl of MTT reagent was discharged to each well and followed by incubation for 4 h. After that, the supernatant was pipetted out and 200 μl of DMSO was pipetted into all the wells for the resulting formazan crystals to become dissolvable, an incubation with gentle shaking was carried out for 15 min. The relative cell viability of untreated cells was defined as 100%. Absorbance at 570 nm using a microplate reader (Bio Tek Instruments Inc., Winooski, USA) was measured to account for the former. All experiments were performed in triplicates.
2.6. Enzyme-linked immunosorbent assays (ELISA)
RAW 264.7 cells from the culture were added with varying concentrations of DCF or BDK and subjected to incubation for 24 h, later to which it was collected and stored at −80 °C until the experiment was performed. The levels of TNF-α, IL6, and PGE2 were determined using ELISA kits (Peprotech, Rocky Hill, USA) according to the manufacturer's instructions.
2.7. RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
RAW 264.7 macrophages had been seeded and treated respectively with both BDK or DCF and LPS for 24h each. The cells seeded were given a PBS buffer wash and TRIzol was used to extract the total RNA from each group as per the manufacturer's protocol. The total RNA quantity/quality was verified by utilizing Nanodrop (Thermo Scientific Nanodrop one C, version 1.3.1) analyzer and estimated at 260 nm and 280 nm, and the proportionate absorbance ratio of 260/280 was applied to obtain the purity of RNA.
RNA was then reverse transcribed into cDNA (Applied Biosystems, CA, USA). qRT-PCR was performed using QuantiTect SYBR® Green PCR kit (QIAGEN, Valencia, CA, USA) following the manufacturer's procedure on an ABI One-step sequence detection system (Applied Biosystems, Foster City, CA, USA). Gene-specific primer pairs were designed manually with the help of online NCBI/Primer3-BLAST and were purchased from Sigma-Aldrich (St. Louis, MO, USA) (Table 2). Thermal cycling conditions were as follows: denaturation (15 s at 94 °C), annealing (30 s at 60 °C), and the extension step (72 °C for 30 s). The normalization of the fold change in the expression of the target gene mRNA was done by comparing the values of the β-actin housekeeping gene using the 2−ΔΔCt comparative cycle threshold.
Table 2.
Primer sequences used for quantitative real-time PCR analysis.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| TNF-α | 5′-TGGAACTGGCAGAAGAGGCACT-3′ | 5′-AGAGGCTGAGACATAGGCACCG-3′ |
| IL-6 | 5′-GTTCTCTGGGAAATCGTGGA-3′ | 5′-GGAAATTGGGGTAGGAAGGA-3′ |
| IL-1β | 5′-ACCTGGGCTGTCCTGATGAGAG-3′ | 5′-TGTTGATGTGCTGCTGCGAGAT-3′ |
| COX-2 | 5′-CTGGTGCCTGGTCTGATGATGTATG-3′ | 5′-TCTCCTATGAGTATGAGTCTGCTGGTT-3′ |
| iNOS | 5′-CAGCGGAGTGACGGCAAACAT-3′ | 5′-GCAAGACCAGAGGCAGCACATC-3′ |
| NFκB-p65 | 5′-GCTCCTAAGGTGCTGACA-3′ | 5′-ACCTCCGAAAGCGAGATA-3′ |
| GAPDH | 5′-ACTCCACTCACGGCAAATTC-3′ | 5′-GTCATGAGCCCTTCCACAAT-3′ |
2.8. Flow cytometric analyses for protein expression
The expression of TNF-α, IL6, iNOS, and COX-2 in the RAW 264.7 macrophage cells was analyzed using flow cytometry. The fluorescence intensity was quantified in the cells. In brief, the cells after the prior treatments were harvested, rinsed twice with cold PBS and incubated for 30 min with monoclonal antibodies against TNF-α, IL6, iNOS, and COX-2 at 4 °C in the unlit condition. The PBS resuspended cells after the cold PBS wash was delivered to the FACS Caliber system (Becton-Dickson, San Diego, USA) for the flow cytometric analysis at an excitation and emission wavelength of 488 nm and 525 nm, respectively. For the analysis of the data, 10,000 events per sample were acquired. The intensity of the signal fluorescence was analyzed by CellQuest software (Becton-Dickson, USA).
2.9. Immunofluorescence staining for NF-κB nuclear translocation
The cells treated as per given in the RNA extraction protocol were then transferred to gelatin-coated round glass slides whereon they were permeabilized with 100% cold methanol for 10 min at −2 °C. Thereafter, rinsing of the cells with PBS was performed followed by 30 min of blocking with the blocking buffer (3% (w/v) bovine serum albumin in PBS). An overnight staining of samples with rabbit polyclonal antibodies targeting the NF-κB-p65 at 4 °C and incubation for 2 h at room temperature using a specific Alexa Fluor 488 conjugated secondary goat-anti-rabbit antibody (1:200 dilution in PBS/1% BSA) (Cell Signaling Technology, Beverly, USA) was performed later to the over-night staining. The counterstained of the nuclei was carried out for 10 min at room temperature with 4′6-diamino-2-phenylindole (DAPI; Sigma Aldrich, St Louis, USA) and viewed using a confocal microscope (Olympus America, Melville, NY, USA) and the mean intensity of fluorescence was measured using ImageJ software.
2.10. Statistical analysis
The results were illustrated as mean ± standard error mean (SEM) for the analysis that was performed by GraphPad Prism (version 5.0). The intergroup assessments of statistical comparison were assessed using ANOVA, and Bonferroni's multiple comparison post-test. The statistical significance was inferred with P < 0.05.
3. Results
3.1. Metabolite profiling of Bhadradarvadi concoction
The LC-MS analysis of Bhadradarvadi concoction displayed the presence of various phenolics, flavonoids, tannins, and so on. The total ion chromatogram was further processed using Empower software, which furnished the list of 7 compounds (Table 3) with their corresponding elemental details by incorporating with libraries. Fig. 1 (A-F) illustrates the mass spectra and structures of identified phytoconstituents in BDK.
Table 3.
The main compounds present in the Bhadradarvadi concoction identified by LC-MS analysis.
| S. No | Compound name | Retention time | m/z value | Mass | Chemical Formula | Abundance |
|---|---|---|---|---|---|---|
| 1. | beta-Bisabolol | 1.055 | 117.77 | 222.4 | C15H26O | 164,977 |
| 2. | beta-Elemene | 5.903 | 226.1 | 192.3 | C15H24 | NA |
| 3. | Kanokonol | 5.903 | 534.81 | 238.4 | C15H26O2 | 139,055 |
| 4. | Deodarone | 6.141 | 534.89 | 236.4 | C25H24O2 | 589,814 |
| 5. | Kaempferol | 6.453 | 332.8 | 286.2 | C15H10O6 | 68,215 |
| 6. | Biochanin A | 6.623 | 514.9 | 284.3 | C16H12O5 | 309,902 |
| 7. | Nerolidol | 7.147 | 137.10 | 222.37 | C15H26O | 500,356 |
Fig. 1.
The mass spectra and structures of seven active compounds in BDK; (A) beta-Bisabolol; (B) beta-Elemene and kanokonol; (C) deodarone; (D) kaempferol (E) biocahnin A (F) nerolidol.
3.2. Effect of BDK on the cell viability of RAW 264.7 macrophages
The MTT assay was used for measuring the cytotoxicity that is caused by the BDK treatment to the 264.7 macrophage cells. As presented in Fig. 2, the viability of the cells was not significantly changed upon the BDK treatment with varying concentrations up to 350 μl/ml. Hence, in the subsequent experiments, BDK was used at the levels of 150–350 μl/ml, however, BDK concentration of 350 μl/ml only was used for flow cytometry and Immunofluorescence analysis.
Fig. 2.
Effect of BDK on the cell viability of RAW 264.7 macrophage cells. Cells were seeded on 96-well plate and treated with different concentration of BDK (150–350 μl/ml) for 24 h. The results represents mean ± SEM of three independent experiments.
3.3. Effect of BDK on pro-inflammatory cytokine production in LPS-stimulated RAW 264.7 macrophages
Fig. 3(A) represents that, LPS treatment has significantly increased the production of PGE2 in RAW 264.7 macrophage cells. However, previous treatment with BDK exhibited remarkable inhibition of PGE2 production, in a concentration-dependent manner in comparison to the cells that are not treated. As depicted in Fig. 3(B) and (C), under normal conditions, the macrophages produce reduced levels of cytokines that can promote inflammation (TNF-α, and IL6), while contrary to this, a notable increase in secretion was found on LPS stimulation. Similar to the pattern observed on treating the RAW 264.7 cells with DCF, prior treatment with BDK (150–350 μl/ml) also significantly inhibited the secretion of the cytokines that can promote inflammation (TNF-α and IL-6) in a dose-dependent manner.
Fig. 3.
Effect of BDK on the production of selective inflammatory proteins on LPS-stimulated RAW 264.7 macrophage cells The levels of inflammatory enzyme (A) PGE2, and pro-inflammatory cytokines (B) TNF-α, (C) IL-6 were quantified using ELISA kits. The values are represented as mean ± SEM from three individual experiments. Comparisons as follows: # - control vs LPS-induced, $ - LPS induced vs LPS and treatment with BDK and Diclofenac (DCF). *P < 0.05 statistically significant, one-way ANOVA with the Bonferroni multiple comparison post-test.
3.4. Effect of BDK on mRNA expression of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages
To investigate the effect of BDK on the expression of NF-κB target inflammatory gene encoding cytokines (TNF-α, IL-6, and IL-1β), inflammatory enzymes (COX-2 and iNOS) and the transcription factor (NFκB-p65) in RAW 264.7 macrophage cells stimulated by LPS were examined by qRT-PCR. Shown in Fig. 4(A-F) are the LPS-treated cells showing upregulation of expression levels of the target gene when compared to untreated cells. On the other hand, treatment of the cells with either BDK (150–350 μl/ml) or DCF showed a dose-dependent down-regulation of the expression levels of mRNA of these particular genes.
Fig. 4.
Effect of BDK on the mRNA expression levels of selective inflammatory mediators on LPS-stimulated RAW 264.7 macrophage cells. The mRNA expression of (A) TNF-α, (B) IL-1β, (C) IL-6, (D) COX-2, (E) iNOS, (F) NFκB-p65 were measured with RT-PCR. RQ values were calculated with reference to the GAPDH gene. The values are represented as mean ± SEM from three individual experiments. Comparisons as follows: # - control vs LPS-induced, treatment with BDK and DCF, $ - LPS induced vs LPS and treatment with BDK and DCF. *P < 0.05 statistically significant, one-way ANOVA with the Bonferroni multiple comparison post-test.
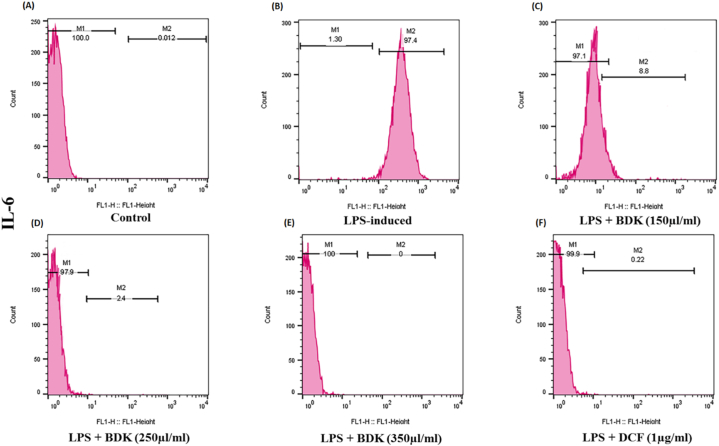
3.5. Effect of BDK on protein expression of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages
Fig. 5, Fig. 6, Fig. 7, Fig. 8 (A-F) represent the result of the flow cytometric analysis performed to find the effect of BDK in protein expression of TNF-α, IL-6, COX-2, and iNOS and it was observed to increase after LPS stimulation. In comparison to the control group, an increased percentage of expression of TNF-α (nearly 89.9%), IL-6 (97.4%), iNOS (97.7%), and COX-2 (91.4%), was found respectively in LPS-stimulated group. However, upon the BDK treatment in RAW 264.7 macrophage cells (150–350 μl/ml) that are stimulated by LPS, notably decreased the expression of TNF-α (64.7%–0.016%), IL-6 (8.8%–0.1%), iNOS (97.3%–0.15%), and COX-2 (68.2%–0.014%), respectively in a concentration-dependent manner. Also, pre-treatment with DCF (1 μg/ml) slightly decreased the percentage expression of TNF-α (56.1%), IL-6 (0.22%), iNOS (88.8%), and COX-2 (64%), respectively in LPS-stimulated cells.
Fig. 5.
Effect of BDK on the protein expression levels of TNF-α on LPS-stimulated RAW 264.7 macrophage cells were performed by flow cytometry analysis (a representative flow cytometry peak figure). In comparison to the (A) control group, an increased percentage of expression of (B) TNF-α (nearly 89.9%) was found in the LPS-stimulated group. But upon the BDK treatment in RAW 264.7 macrophage cells (C–E) (150–350 μl/ml) that are stimulated by LPS, a notable decrease in the expression of TNF-α (64.7%–0.016%) was observed. Whereas, the (F) pre-treatment of the LPS-stimulated cells with DCF (1 μg/ml) showed a slight decrease in the TNF-α expression.
Fig. 6.
Effect of BDK on the protein expression levels of IL-6 on LPS-stimulated RAW 264.7 macrophage cells were performed by flow cytometry analysis (a representative flow cytometry peak figure). In comparison to the (A) control group, an increased percentage of expression of (B) IL-6 (nearly 97.4%) was found in the LPS-stimulated group. On BDK treatment in RAW 264.7 macrophage cells (C–E) (150–350 μl/ml) that are stimulated by LPS, a notable decrease in the expression of IL-6 (8.8%–0.1%) was observed. Whereas, the (F) pre-treatment of the LPS-stimulated cells with DCF (1 μg/ml) only showed a slight decrease in the IL-6 expression.
Fig. 7.
Effect of BDK on the protein expression levels of COX-2 on LPS-stimulated RAW 264.7 macrophage cells were performed by flow cytometry analysis (a representative flow cytometry peak figure). In comparison to the (A) control group, an increased percentage of expression of (B) COX-2 (nearly 91.4%) was found in the LPS-stimulated group. On BDK treatment in RAW 264.7 macrophage cells (C–E) (150–350 μl/ml) that are stimulated by LPS, a notable decrease in the expression of COX-2 (68.2%–0.014%) was observed. Whereas, the (F) pre-treatment of the LPS-stimulated cells with DCF (1 μg/ml) only showed a slight decrease in the COX-2 expression.
Fig. 8.
Effect of BDK on the protein expression levels of iNOS on LPS-stimulated RAW 264.7 macrophage cells were performed by flow cytometry analysis (a representative flow cytometry peak figure). In comparison to the (A) control group, an increased percentage of expression of (B) iNOS (nearly 97.7%) was found in the LPS-stimulated group. On BDK treatment in RAW 264.7 macrophage cells (C–E) (150–350 μl/ml) that are stimulated by LPS, a notable decrease in the expression of iNOS (97.3%–0.15%) was observed. Whereas, the (F) pre-treatment of the LPS-stimulated cells with DCF (1 μg/ml) only showed a slight decrease in the iNOS expression.
3.6. Effect of BDK on NFκB-p65 expression in LPS-stimulated RAW 264.7 macrophages
Fig. 9(A) represents the confocal microscopic analysis result of the effect of BDK on the NFκB-p65 protein expression assessed using immunofluorescence staining and it was found that in comparison to the cells that are not treated, there was a pronounced cellular expression of NFκB-p65 in LPS-treated cells. However, BDK administration (150–350 μl/ml) in LPS-stimulated RAW 264.7 macrophage cells substantially reduced the NFκB-p65 in a dose-reliant manner (Fig. 9(B)).
Fig. 9.
(A) Effect of BDK on NFκB-p65 nuclear translocation in LPS-stimulated RAW 264.7 macrophage cells was determined by immunofluorescence analysis. (B) The mean fluorescence intensity was calculated using ImageJ software, scale bars = 10 μm. All the data's are represented as mean ± SEM. Comparisons as follows: # - control vs LPS-induced, treatment with BDK and DCF, $ - LPS induced vs LPS and treatment with BDK and DCF. *P < 0.05 statistically significant.
4. Discussion
The contribution of the immune system is one of the most important aspects in the pathogenesis of rheumatoid arthritis and it is a generally acknowledged hypothesis. Immunomodulatory drugs are typically used to treat autoimmune diseases and have the potential to diminish unwanted functions. Therefore, this study offered us evidence that BDK influences immunomodulatory and anti-inflammatory effects by regulating the serum and cytokine levels which are closely associated with rheumatoid arthritis.
Chronic or systemic inflammation is the primary cause of RA, which can create people more prone to complicated life associated with aging and diseases [25,34]. Though there is a ground-breaking development made in recent years for the treatment of RA including NSAIDs, DMARDs, and novel biologics, still, there is no satisfactory approach in managing or curing the disease activity as well as they uncover notable side effects [26,35]. Currently, many people are moving towards complementary and alternative medicines including the traditional Indian system of medicines (Ayurveda, Siddha, and Unani formulas) to treat unmanageable inflammatory diseases like RA. Kashayams or herbal decoctions have been considered the most significant complementary and alternative medicines for various diseases owing to their lesser side effects and therapeutic ability. To explore its molecular mechanisms of anti-inflammatory actions, the present study was undertaken to determine the inhibitory effects of BDK with respect to the synthesis and expression of pro-inflammatory mediators, inflammatory enzymes, and intracellular ROS levels in RAW 264.7 macrophage cells that are LPS-stimulated.
Insight into the bioactive ingredients present in polyherbal formulations remains a vital target in the pharmacological sector. In this study, LC-MS analysis of BDK validates the presence of 7 bioactive ingredients (beta-Bisabolol, beta-Elemene, deodarone, biochanin A, kaempferol, and nerolidol). Numerous pharmacological and biological properties such as anti-inflammatory, anti-arthritic, and anti-oxidant activities elucidated in earlier studies for bioactive ingredients of BDK including gallic acid [36], solavetivone [37], kaempferol [38,39], scyllo-inositol [40], choline [41], deodarone [42], linalool [43], and squalene [44]. Therefore, it might be promising to speculate on the anti-inflammatory activity of BDK ingredients.
Concerning the active ingredients, gallic acid can help to diminish inflammation by decreasing the affinity of NF-κB to DNA in LPS-treated cells, which leads to a subsequently reduced expression of pro-inflammatory mediators. Also, TNFα and IL-6 formation/release can be suppressed by gallic acid via induced suppression of p65 acetylation of secondary human monocytes [45,46]. Similarly, Kaempferol inhibited the NF-κB activation promoted by IL-1β, as well as phosphorylation of p38, ERK-1/2, and JNK [46,47]. Therefore, active ingredients within the BDK extract had various means to influence genes/pathways that contribute to inflammation by activated macrophages.
Generally, when there is an LPS exposure, numerous cytokines which are involved in inflammation (IL-1β, IL-6, and TNFα) and mediators like PGE2 (produced by COX-2 and iNOS) are activated downstream which are induced by macrophages. Several prostaglandins like PGE2, play a prominent role in the inflammatory process [48]. Hence, excessive PGE2 production inhibition by blocking iNOS and/or COX-2 could be an effective approach to treat diseases with underlying inflammation. Our results were also suggestive of the fact that BDK-treated macrophages reduced the PGE2 production at the transcriptional level.
The present study also reported that BDK markedly lowers the expression of TNFα, IL-1β, IL-6 at mRNA level, and NF-κB p65 which are activated by the macrophages. Subsequent to the activation of the macrophages, the signaling mediated by NF-κB intensely modulates the further activation of various cytokines/mediators of inflammation [49]. The immunofluorescence analyses in the positive control revealed that LPS-stimulated RAW 264.7 cells showed an increased NF-κB p65 expression and the flow cytometry analysis also showed the higher protein expression of the cytokines in quest. But the BDK treatment suppressed the LPS-stimulated expression of NF-κB p65 and subsequently reduced the expression of TNFα, IL-6, COX-2 and iNOS in RAW 264.7 macrophages. These outcomes were as per the earlier reports that kaempferol and gallic acid mediates anti-inflammatory responses by activating NF-κB pathway which is by suppressing the TNFα, IL-1β and COX-2 expression [[50], [51], [52]].
NF-κB plays a major role in synchronizing the inflammatory immune responses exclusively in RA pathogenesis, likely to influence the activation of various cytokines and chemokines involved in the process of inflammation [53,54]. This study showed that the BDK treatment group inhibited the expression of respective proteins that trigger the NF-κB pathway activation in the RAW 264.7 macrophage cells. The outcome of the result delivers adequately showcases our hypothesis of BDK suppressing the production of mediators of inflammation in situ, probably via NF-κB signaling pathways inhibition. However, ruling out the property of individual bioactive principles in the polyherbal formulation will be a challenging future study due to their synergistic potentials.
Author contribution statement
Vino Sundararajan: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools, or data; Wrote the paper. </p>
Mohamed Thoufic Ali A M and Sagnik Nag: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools, or data; Wrote the paper. </p>
Devi Soorya Narayana S: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools, or data; Wrote the paper. </p>
Sajitha Lulu S: Conceived and designed the experiments, Analyzed and interpreted the data; Contributed reagents, materials, analysis tools, or data; Wrote the paper. </p>
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are grateful to the management of VIT for providing the facilities to carry out this research work.
References
- 1.Scrivo R., Vasile M., Bartosiewicz I., Valesini G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev. 2011;10(7):369. doi: 10.1016/j.autrev.2010.12.006. https://pubmed.ncbi.nlm.nih.gov/21195808/ May[cited 2023 Mar 15] 74. Available from: [DOI] [PubMed] [Google Scholar]
- 2.Newton K., Dixit V.M. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4(3) doi: 10.1101/cshperspect.a006049. https://pubmed.ncbi.nlm.nih.gov/22296764/ Mar [cited 2023 Mar 15] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borges R.S., Ortiz B.L.S., Pereira A.C.M., Keita H., Carvalho J.C.T. Rosmarinus officinalis essential oil: a review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J Ethnopharmacol. 2019;229:29–45. doi: 10.1016/j.jep.2018.09.038. https://pubmed.ncbi.nlm.nih.gov/30287195/ Jan 30 [cited 2023 Mar 15] Available from: [DOI] [PubMed] [Google Scholar]
- 4.Kurowska-Stolarska M., Alivernini S. Synovial tissue macrophages: friend or foe? RMD Open. 2017;3(2) doi: 10.1136/rmdopen-2017-000527. https://pubmed.ncbi.nlm.nih.gov/29299338/ Dec 6[cited 2023 Mar 15] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017:2. doi: 10.1038/sigtrans.2017.23. https://pubmed.ncbi.nlm.nih.gov/29158945/ [cited 2023 Mar 15] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siouti E., Andreakos E. The many facets of macrophages in rheumatoid arthritis. Biochem Pharmacol. 2019;165:152. doi: 10.1016/j.bcp.2019.03.029. https://pubmed.ncbi.nlm.nih.gov/30910693/ Jul 1 [cited 2023 Mar 15] 69. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Ryan G.B., Majno G. Acute inflammation. A review. Am. J. Pathol. 1977;86(1):183–276. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2032041/ [cited 2023 Mar 15]. Available from: [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson S.L., Parrish M.E., Springer J.E., Doty K., Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998;151(1):77–88. doi: 10.1006/exnr.1998.6785. https://pubmed.ncbi.nlm.nih.gov/9582256/ [cited 2023 Mar 15] Available from: [DOI] [PubMed] [Google Scholar]
- 9.Ko W.K., Lee S.H., Kim S.J., Jo M.J., Kumar H., Han I.B., et al. Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0180673. https://pubmed.ncbi.nlm.nih.gov/28665991/ Jun 1[cited 2023 Mar 15] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McInnes I.B., Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. https://pubmed.ncbi.nlm.nih.gov/22150039/ [cited 2023 Mar 15]. Available from: [DOI] [PubMed] [Google Scholar]
- 11.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388(10055):2023. doi: 10.1016/S0140-6736(16)30173-8. https://pubmed.ncbi.nlm.nih.gov/27156434/ Oct 22 [cited 2023 Mar 15] 38. Available from: [DOI] [PubMed] [Google Scholar]
- 12.Choy E.H.S., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001;344(12):907–916. doi: 10.1056/NEJM200103223441207. https://pubmed.ncbi.nlm.nih.gov/11259725/ Mar 22 [cited 2023 Mar 15]; Available from: [DOI] [PubMed] [Google Scholar]
- 13.Asif Amin M., Fox D.A., Ruth J.H. Synovial cellular and molecular markers in rheumatoid arthritis. Semin. Immunopathol. 2017;39(4):385. doi: 10.1007/s00281-017-0631-3. https://pubmed.ncbi.nlm.nih.gov/28497350/ Jun 1 [cited 2023 Mar 15] 93. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginhoux F., Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014;14(6):392–404. doi: 10.1038/nri3671. https://pubmed.ncbi.nlm.nih.gov/24854589/ [cited 2023 Mar 15] Available from: [DOI] [PubMed] [Google Scholar]
- 15.Pope R.M. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat. Rev. Immunol. 2002;2(7):527. doi: 10.1038/nri846. https://pubmed.ncbi.nlm.nih.gov/12094227/ [cited 2023 Mar 15] 35. Available from: [DOI] [PubMed] [Google Scholar]
- 16.Noack M., Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin. Immunopathol. 2017;39(4):365. doi: 10.1007/s00281-017-0619-z. https://pubmed.ncbi.nlm.nih.gov/28213794/ Jun 1 [cited 2023 Mar 15] 83. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Makarov S.S. NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation. hyperplasia, and tissue destruction. Arthritis Res. 2001;3(4):200. doi: 10.1186/ar300. https://pubmed.ncbi.nlm.nih.gov/11438035/ [cited 2023 Mar 15] 6. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furst D.E., Emery P. Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatology. 2014;53(9):1560. doi: 10.1093/rheumatology/ket414. https://pubmed.ncbi.nlm.nih.gov/24402580/ [cited 2023 Mar 15] 9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Hsouna A., Ben Saad R., Dhifi W., Mnif W., Brini F. Novel non-specific lipid-transfer protein (TdLTP4) isolated from durum wheat: antimicrobial activities and anti-inflammatory properties in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Microb. Pathog. 2021:154. doi: 10.1016/j.micpath.2021.104869. May 1. [DOI] [PubMed] [Google Scholar]
- 20.Hsouna A Ben, Boye A., Ackacha B Ben, Dhifi W., Saad R Ben, Brini F., et al. Thiamine demonstrates bio-preservative and anti-microbial effects in minced beef meat storage and lipopolysaccharide (LPS)-Stimulated RAW 264.7 macrophages. Animals. 2022;12(13) doi: 10.3390/ani12131646. Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsouna A Ben, Dhibi S., Dhifi W., Saad R Ben, Brini F., Hfaidh N., et al. Essential oil from halophyte: lobularia maritima: protective effects against CCl4-induced hepatic oxidative damage in rats and inhibition of the production of proinflammatory gene expression by lipopolysaccharide-stimulated RAW 264.7 macrophages. RSC Adv. 2019;9(63):36758–36770. doi: 10.1039/c9ra05885k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben Hsouna A., Gargouri M., Dhifi W., Ben Saad R., Sayahi N., Mnif W., et al. Potential anti-inflammatory and antioxidant effects of Citrus aurantium essential oil against carbon tetrachloride-mediated hepatotoxicity: a biochemical, molecular and histopathological changes in adult rats. Environ Toxicol [Internet. 2019 doi: 10.1002/tox.22693. https://onlinelibrary.wiley.com/doi/full/10.1002/tox.22693 Apr 1 [cited 2023 Jun 27];34(4):388–400. Available from: [DOI] [PubMed] [Google Scholar]
- 23.Ben Hsouna A., Michalak M., Ben Akacha B., Dhifi W., Ben Saad R., Brini F., et al. Assessment of the phytochemical composition, antimicrobial activity and anti-inflammatory effects of Lobularia maritima extracts on lipopolysaccharide-stimulated RAW 264.7 cells and their capacity to extend the shelf life of raw minced beef. J. Funct.Foods. 2022;99 Dec 1. [Google Scholar]
- 24.Tzianabos A.O. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin. Microbiol. Rev. 2000;13(4):523. doi: 10.1128/cmr.13.4.523-533.2000. https://pubmed.ncbi.nlm.nih.gov/11023954/ Oct[cited 2023 Mar 15] 33. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aletaha D., Smolen J.S. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360. doi: 10.1001/jama.2018.13103. https://pubmed.ncbi.nlm.nih.gov/30285183/ Oct 2 [cited 2023 Mar 15] 72. Available from: [DOI] [PubMed] [Google Scholar]
- 26.Deane K.D., Norris J.M., Holers V.M. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am. 2010;36(2):213. doi: 10.1016/j.rdc.2010.02.001. https://pubmed.ncbi.nlm.nih.gov/20510231/ May [cited 2023 Mar 15] 41. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furst D.E., Manorama M.V., McGann Mary, Manohar P. Ram, Booth-LaForce Cathryn, Sarin Reshmi, et al. Double-blind, randomized, controlled, pilot study comparing classic ayurvedic medicine, methotrexate, and their combination in rheumatoid arthritis. J. Clin. Rheumatol. 2011;17(4):185. doi: 10.1097/RHU.0b013e31821c0310. https://pubmed.ncbi.nlm.nih.gov/21617554/ Jun [cited 2023 Mar 15] 92. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Ben Hsouna A., Dhibi S., Dhifi W., Mnif W., Ben Nasr hmed, Hfaiedh N. Chemical composition and hepatoprotective effect of essential oil from Myrtus communis L. flowers against CCL 4 -induced acute hepatotoxicity in rats. RSC Adv. 2019;9(7):3777–3787. doi: 10.1039/c8ra08204a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatesha S.H., Berman B.M., Moudgil K.D. Herbal medicinal products target defined biochemical and molecular mediators of inflammatory autoimmune arthritis. Bioorg. Med. Chem. 2011 Jan 1;19(1):21. doi: 10.1016/j.bmc.2010.10.053. https://pubmed.ncbi.nlm.nih.gov/21115252/ [cited 2023 Mar 15] 9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripathi P., Chauhan N.S., Patel J.R. Anti-inflammatory activity of Abutilon indicum extract. Nat. Prod. Res. 2012;26(17):1659. doi: 10.1080/14786419.2011.616508. https://pubmed.ncbi.nlm.nih.gov/21999427/ Sep 1 [cited 2023 Mar 15] 61. Available from: [DOI] [PubMed] [Google Scholar]
- 31.Wadekar J.B., Sawant R.L., Patel U.B. Rheumatoid arthritis and herbal drugs: a review. J Phytopharm. 2015;4(6):311. www.phytopharmajournal.com [cited 2023 Mar 15] 8. Available from: [Google Scholar]
- 32.Lee H.H., Ahn E.K., Hong S.S., Oh J.S. Anti-inflammatory effect of tribulusamide D isolated from Tribulus terrestris in lipopolysaccharide-stimulated RAW264.7 macrophages. Mol. Med. Rep. 2017;16(4):4421. doi: 10.3892/mmr.2017.7208. https://pubmed.ncbi.nlm.nih.gov/28849109/ Oct 1 [cited 2023 Mar 15] 8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A., Arora P. Anti-cancer activity of Cedrus deodara in 1,2- dimethly hydrazine (DMH) induced anti cancer model in rats. Asian J. Pharmaceut. Res. Dev. 2018;6(2):82. https://ajprd.com/index.php/journal/article/view/348 Jun 20 [cited 2023 Mar 15] 6. Available from: [Google Scholar]
- 34.Boissier M.C., Semerano L., Challal S., Saidenberg-Kermanac’h N., Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J. Autoimmun. 2012;39(3):222. doi: 10.1016/j.jaut.2012.05.021. https://pubmed.ncbi.nlm.nih.gov/22704962/ Sep[cited 2023 Mar 15] 8. Available from: [DOI] [PubMed] [Google Scholar]
- 35.Burmester G.R., Pope J.E. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338. doi: 10.1016/S0140-6736(17)31491-5. https://pubmed.ncbi.nlm.nih.gov/28612748/ Jun 10 [cited 2023 Mar 15] 48. Available from: [DOI] [PubMed] [Google Scholar]
- 36.Yoon C.H., Chung S.J., Lee S.W., Park Y.B., Lee S.K., Park M.C. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Joint Bone Spine. 2013;80(3):274. doi: 10.1016/j.jbspin.2012.08.010. https://pubmed.ncbi.nlm.nih.gov/23058179/ May[cited 2023 Mar 15] 9. Available from: [DOI] [PubMed] [Google Scholar]
- 37.Chen Y.C., Lee H.Z., Chen H.C., Wen C.L., Kuo Y.H., Wang G.J. Anti-inflammatory components from the root of Solanum erianthum. Int. J. Mol. Sci. 2013;14(6):12581. doi: 10.3390/ijms140612581. https://pubmed.ncbi.nlm.nih.gov/23771024/ Jun[cited 2023 Mar 15] 92. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calderon-Montano M., Burgos-Moron E., Perez-Guerrero C., Lopez-Lazaro M. Mini Rev Med Chem; 2011 Apr 12. A Review on the Dietary Flavonoid Kaempferol.https://pubmed.ncbi.nlm.nih.gov/21428901/ [Internet] [cited 2023 Mar 15];11(4):298–344. Available from: [DOI] [PubMed] [Google Scholar]
- 39.Imran M., Salehi B., Sharifi-Rad J., Gondal T.A., Saeed F., Imran A., et al. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24(12) doi: 10.3390/molecules24122277. https://pubmed.ncbi.nlm.nih.gov/31248102/ [cited 2023 Mar 15] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenili D., Brown M., Rappaport R., McLaurin J.A. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J. Mol. Med. (Berl.) 2007;85(6):603. doi: 10.1007/s00109-007-0156-7. https://pubmed.ncbi.nlm.nih.gov/17279347/ Jun[cited 2023 Mar 15] 11. Available from: [DOI] [PubMed] [Google Scholar]
- 41.Rowley T.J., McKinstry A., Greenidge E., Smith W., Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br. J. Anaesth. 2010;105(2):201. doi: 10.1093/bja/aeq113. https://pubmed.ncbi.nlm.nih.gov/20511332/ [cited 2023 Mar 15] 7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhary A.K., Ahmad S., Mazumder A. Cedrus deodara (roxb.) loud.: a review on its ethnobotany, phytochemical and pharmacological profile. Phcog. J. 2011;3(23):12–17. Jul 1. [Google Scholar]
- 43.Huo M., Cui X., Xue J., Chi G., Gao R., Deng X., et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013;180(1):E47–E54. doi: 10.1016/j.jss.2012.10.050. https://pubmed.ncbi.nlm.nih.gov/23228323/ Mar [cited 2023 Mar 15]. Available from: [DOI] [PubMed] [Google Scholar]
- 44.Cárdeno A., Aparicio-Soto M., Montserrat-de la Paz S., Bermudez B., Muriana F.J.G., Alarcón-de-la-Lastra C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct.Foods. 2015;14:779. https://www.researchgate.net/publication/276452447_Squalene_targets_pro-_and_anti-inflammatory_mediators_and_pathways_to_modulate_over-activation_of_neutrophils_monocytes_and_macrophages Apr 1[cited 2023 Mar 15] 90. Available from: [Google Scholar]
- 45.Choi K.C., Lee Y.H., Jung M.G., Kwon S.H., Kim M.J., Jun W.J., et al. Gallic acid suppresses lipopolysaccharide-induced nuclear factor-kappaB signaling by preventing RelA acetylation in A549 lung cancer cells. Mol. Cancer Res. 2009;7(12):2011. doi: 10.1158/1541-7786.MCR-09-0239. https://pubmed.ncbi.nlm.nih.gov/19996305/ Dec[cited 2023 Mar 15] 21. Available from: [DOI] [PubMed] [Google Scholar]
- 46.Yoon H.Y., Lee E.G., Lee H., Cho I.J., Choi Y.J., Sung M.S., et al. Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 2013;32(4):971. doi: 10.3892/ijmm.2013.1468. https://pubmed.ncbi.nlm.nih.gov/23934131/ Oct[cited 2023 Mar 15] 7. Available from: [DOI] [PubMed] [Google Scholar]
- 47.Kadioglu O., Nass J., Saeed M.E.M., Schuler B., Efferth T. Kaempferol is an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 2015;35(5) [PubMed] [Google Scholar]
- 48.Salvemini D., Kim S.F., Mollace V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304(7):R473–R487. doi: 10.1152/ajpregu.00355.2012. https://pubmed.ncbi.nlm.nih.gov/23389111/ [cited 2023 Mar 15]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang J.M., Yu J.Y., Jang Y.O., Kim B.T., Hwang K.J., Jeon Y.M., et al. A phenolic acid phenethyl urea compound inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines in cell culture. Int. Immunopharm. 2010;10(4):526. doi: 10.1016/j.intimp.2010.01.016. https://pubmed.ncbi.nlm.nih.gov/20138247/ Apr[cited 2023 Mar 15] 32. Available from: [DOI] [PubMed] [Google Scholar]
- 50.Ho H.H., Chang C Sen, Ho W.C., Liao S.Y., Wu C.H., Wang C.J. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-kappaB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem. Toxicol. 2010;48:8–9. doi: 10.1016/j.fct.2010.06.024. https://pubmed.ncbi.nlm.nih.gov/20600540/ Aug[cited 2023 Mar 15] 2508–16. Available from: [DOI] [PubMed] [Google Scholar]
- 51.Kim S.H., Park J.G., Lee J., Yang W.S., Park G.W., Kim H.G., et al. The dietary flavonoid Kaempferol mediates anti-inflammatory responses via the Src, Syk, IRAK1, and IRAK4 molecular targets. Mediators Inflamm. 2015;2015:904142. doi: 10.1155/2015/904142. https://pubmed.ncbi.nlm.nih.gov/25922567/ [cited 2023 Mar 15]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhuang Z., Ye G., Huang B. Kaempferol alleviates the interleukin-1β-induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-κB. Med. Sci. Monit. 2017;23:3925–3931. doi: 10.12659/MSM.902491. https://pubmed.ncbi.nlm.nih.gov/28806392/ Aug 14 [cited 2023 Mar 15]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desai A., Darland G., Bland J.S., Tripp M.L., Konda V.R. 2012 Jul. META060 Attenuates TNF-α-Activated Inflammation, Endothelial-Monocyte Interactions, and Matrix Metalloproteinase-9 Expression, and Inhibits NF-Κb and AP-1 in THP-1 Monocytes.https://pubmed.ncbi.nlm.nih.gov/22658256/ Atherosclerosis [Internet] cited 2023 Mar 15];223(1):130–6. Available from: [DOI] [PubMed] [Google Scholar]
- 54.Adewoyin M., Mohsin S.M.N., Arulselvan P., Hussein M.Z., Fakurazi S. Enhanced anti-inflammatory potential of cinnamate-zinc layered hydroxide in lipopolysaccharide-stimulated RAW 264.7 macrophages. Drug Des. Dev. Ther. 2015;9:2475–2484. doi: 10.2147/DDDT.S72716. https://pubmed.ncbi.nlm.nih.gov/25995619/ Mar 30 [cited 2023 Mar 15]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.