Abstract
Mitochondrial dysfunction plays an important role in the occurrence and development of different liver diseases. Oxidative phosphorylation (OXPHOS) dysfunction and production of reactive oxygen species are closely related to mitochondrial dysfunction, forcing glycolysis to become the main source of energy metabolism of liver cells. Moreover, glycolysis is also enhanced to varying degrees in different liver diseases, especially in liver cancer. Therefore, targeting the glycolytic signaling pathway provides a new strategy for the treatment of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis associated with liver cancer. Natural products regulate many steps of glycolysis, and targeting glycolysis with natural products is a promising cancer treatment. In this review, we have mainly illustrated the relationship between glycolysis and liver disease, natural products can work by targeting key enzymes in glycolysis and their associated proteins, so understanding how natural products regulate glycolysis can help clarify the therapeutic mechanisms these drugs use to inhibit liver disease.
Keywords: glycolysis, liver disease, liver, metabolism, natural products
1 Introduction
The incidence of liver disease remains increasing (Yu et al., 2014b), it ranges from simple steatosis or non-alcoholic fatty liver disease (NAFLD) or non-alcoholic steatohepatitis (NASH) to cirrhosis and liver cancer (Li et al., 2019). As the core organ of nutrient storage, synthesis and metabolism, the liver has a remarkable ability to maintain metabolic homeostasis (Petersen et al., 2017). However, under various adverse conditions, liver metabolic homeostasis is destroyed, especially glycolysis and other glucose metabolic functions. In recent years, abnormal of glycolysis has received increasing attention due to its interaction with liver disease.
Glycolysis refers to the process in which glucose is catalyzed to pyruvate and provides 2 reduced nicotinamide adenine dinucleotides (NADH) and 2 adenosine triphosphates (ATP). Pyruvate can be oxidized to acetyl-CoA by pyruvate dehydrogenase (PDH) or converted to oxaloacetic acid by pyruvate carboxylase. In the absence of oxygen, pyruvate is reduced to lactate by lactate dehydrogenase (LDH), or to acetaldehyde by pyruvate decarboxylase (Nishikawa et al., 2014). When the liver is in a pathological state, there is an adaptive metabolic transition from oxidative phosphorylation (OXPHOS), which preferentially produces energy, to glycolysis, in which pyruvate is partially converted to lactic acid (Nishikawa et al., 2014). It was found that glycolysis activity was enhanced and lactic acid levels increased in NAFLD and NASH (Ye et al., 2016). Some glycolytic enzyme levels are elevated in cirrhotic precancerous lesions and are associated with an increase in hepatocellular carcinoma (HCC) (Lee et al., 2018). NAFLD is considered a new major risk factor for HCC, and the increase in serum BCAA levels in NAFLD patients affects glucose metabolism through mTOR signaling (Chalasani et al., 2018; Gaggini et al., 2018; Chakravarthy and Neuschwander-Tetri, 2020). In addition, studies have shown that aerobic glycolysis exists in HCC, and the degree of aerobic glycolysis of cancer cells in HCC is enhanced, and cancer cells preferentially metabolize glucose into lactic acid even under aerobic conditions (Li et al., 2017b). These findings suggested that abnormal glycolysis might promote the development of liver disease.
Traditional cancer treatment methods, such as surgery, chemoradiotherapy and immunotherapy, bring heavy psychological and physical pressure and financial burden to patients [Zhao et al. (2015)]. Studies have found that natural products inhibit glycolysis process and disrupt the proliferation and invasion of cancer by targeting glycolytic or metabolic phenotype [Zhao et al. (2015)]. Furthermore, there are currently no clinically approved drugs to treat NAFLD, which is mainly treated with lifestyle changes through diet and exercise. However, people with NAFLD often have difficulty maintaining an improved lifestyle. Therefore, it is of great practical significance to strengthen the research on the pathogenesis of NAFLD and find safe and effective drugs to prevent and treat NAFLD (Guo et al., 2022a). Many studies have shown that compared with traditional therapies, natural products have obvious advantages in terms of fewer side effects, low toxicity, and light economic burden (Efferth et al., 2007; Hsiao and Liu, 2010).
This paper reviews the important factors and related mechanisms affecting glycolysis in liver diseases. We discuss the role of key glycolytic enzymes and proteins, including hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase (PK), lactate dehydrogenase (LDH), glucose transporters (GLUTs), in liver disease. We also reviewed the role of relevant signaling pathways, including phosphoinositide 3-kinase (PI3K), Wnt/β-catenin, adenosine monophosphate-activated protein kinase (AMPK), in liver disease. Finally, we discuss the role of oncogenes, including c-MYC, HIF-1α, and ROS, in liver disease. We also review the effects of natural products and their active ingredients on metabolic reprogramming in liver disease. Through this review, we hope to identify a number of metabolites with ideal efficacy and low side effects from natural products, and to provide promising therapeutic drugs for metabolic reprogramming of various liver diseases.
1.1 NAFLD and glycolysis
When the liver is in a pathological state, particularly in their ability to process excess fatty acids via OXPHOS) the resulting mitochondrial dysfunction due to elevated ROS is a main cause of liver dysfunction, which forces hepatocytes to rely on glycolysis as an alternative energy source. Energy metabolism preferentially switches from OXPHOS to glycolysis, with the result that part of the pyruvate is converted to lactate (Nishikawa et al., 2014). NAFLD is driven by inflammatory processes, oxidative stress and insulin resistance triggered by multiple pathways, including NASH, which can lead to liver fibrosis, cirrhosis and HCC (Kanda et al., 2020; Alshehade et al., 2022). The different stages of development of liver disease (Figure 1). Studies have shown that the mechanism of altered glycolysis processes can promote the progression of NAFLD to NASH, and eventually to cirrhosis and HCC (Go et al., 2016). Liver macrophages, neutrophils, dendritic cells, and NK cells are all involved in the development of NASH (Nati et al., 2016). Moreover, macrophages, including Kupffer cells and infiltrating monocytes, play an important role in the progression of NASH. Annexin A5 regulates liver macrophages through interaction with PKM2, improving steatosis, inflammation, and fibrosis in NASH mice (Xu et al., 2020). The activation of glycolysis in Kupffer cells during NASH is induced in part by inhibition of PKM2 upregulation by miR-122-5p (Inomata et al., 2022). Some metabolic pathways involved in glycolysis may be potential therapeutic targets for NAFLD. It was found that the expression of key glycolytic enzyme HK2 was increased in the liver of mice fed high-fat diet (HFD). HFD increased glycolysis by down-regulating the expression of geranylgeranyl diphosphate synthase (GGPPS), accelerating the NAFLD fibrosis process (Liu et al., 2018). Studies have shown that down-regulating NOD-like receptor (NLR) X1 (NLRX1) can inhibit glycolysis, enhance fat oxidation and reduce hepatic steatosis (Kors et al., 2018). Therefore, NLRX1 may be an attractive new therapeutic target for NAFLD and metabolic syndrome. A study has found that hyperacetylation of LDHB is associated with lactic acid accumulation in the liver of NAFLD and NASH in humans and mice. p300/CBP-associated factor (PCAF) mediated acetylation of LDHB K82 significantly decreases LDHB activity, affected lactate clearance in the liver, leading to lactate accumulation. In HFD-induced NASH, acetylated LDHB induces lactic acid accumulation by activating histone hyperacetylation, which intensifies lipid deposition and inflammatory responses (Wang T. et al., 2021). Therefore, targeting the glycolytic pathway may be a treatment option for NAFLD.
FIGURE 1.
The different stages of development of liver disease.
1.2 Natural products regulate glycolysis in NAFLD
Natural products regulate glycolysis in NAFLD (Table 1). HFD caused a significant decrease in levels of anaerobic (lactic acid) and aerobic glycolytic metabolites (pyruvate) as well as an increase in blood sugar and insulin levels (Chao et al., 2014). Gallic acid (GA) is a natural plant phenolic metabolite isolated from Cornus officinalis. It is found in vegetables, tea, grapes, berries and wine and has anti-inflammatory, anti-oxidant and other therapeutic effects (Tanaka et al., 2018). Levels of metabolites associated with anaerobic (lactic acid) and aerobic glycolysis, such as pyruvate and lactic acid, recovered significantly in the GA treatment group. The results indicated that GA had a protective effect on the liver of NAFLD mice, which was partly achieved by improving glycolysis (Chao et al., 2014). Antrodan (Ant), a β-glucan purified from Antrodia cinnamomea, has many functions, including anti-cancer, liver protection, and anti-inflammatory effects (Chen et al., 2007; Chiu et al., 2013; Ker et al., 2014; Fa et al., 2015). Ant effectively inhibited glucose and insulin levels, and effectively alleviated glucose metabolism abnormalities in NAFLD through adenosine monophosphate-activated protein kinase (AMPK)/Sirt1/SREBP-1c/PPARγ pathway (Chyau et al., 2020). Vine tea (VT), a tea traditionally used in Chinese botanical drugs, which is derived from Ampelopsis grossedentata. It is rich in the natural anti-oxidant dihydromyricetin (ampelopsin). In addition to its many health benefits, rattan tea extract is considered to be a potential natural anti-oxidant (Carneiro et al., 2020). VT decreased serum glucose, decreased the area under the glucose curve in insulin tolerance tests, and decreased fructose-1, 6-phosphate (F1,6P), glucose-6-phosphate (G6P), 6-phospho-gluconate (6PG). Moreover, with low levels of the important intermediates of the phosphor gluconate pathway, pyruvate, G6P, and fructose 6-phosphate (F6P), VT improved HFD-induced glucose metabolism disorders (Wan et al., 2017). Caralluma fimbriata (Family: Apocynaceae; CFE), is one such medicinal plant that is becoming increasingly popular, with a variety of bioactive ingredients and properties such as anti-oxidants, liver protection and cancer prevention (Anwar et al., 2022). Extract of CFE promoted the recovery of liver glycolysis (HK, PK, PFK) in HFD rats, suggesting that combined administration of CFE/Met partially corrected the deficiency of glycolysis caused by HFD diet in rats (Gujjala et al., 2017).
TABLE 1.
Natural products regulate glycolysis in NAFLD.
| Name | Origin | Regulatory mechanism | Dose | Cell line/Experiment | References |
|---|---|---|---|---|---|
| Gallic acid | Cornus officinalis | Increased levels of pyruvate and lactic acid | 50 and 100 mg/kg | HFD fed mice | Chao et al. (2014) |
| Antrodan | A. cinnamomea | Inhibition of glucose and insulin levels via AMPK/Sirt1/SREBP-1c/PPARγ pathway | 20 and 40 mg/kg | HFD fed mice | Chyau et al. (2020) |
| Vine tea | Ampelopsis grossedentata | Inhibition of F1,6P, G6P,6PG, F6P, pyruvate | 500 and 2000 mg/L | HFD fed rats | Wan et al. (2017) |
| Extract of Caralluma fimbriata | Caralluma fimbriata | Increased levels of HK, PK, PFK | 200 mg/kg | HFD fed rats | Gujjala et al. (2017) |
| Saskatoon berry | Amelanchier alnifolia Nutt | Increased levels of HK1 | 8 mg/kg | HFD fed rats | (du Preez et al., 2020) |
Saskatoon berry (Amelanchier alnifolia Nutt.) is a potential functional food containing phenolic acids, anthocyanins, ellagtannins and flavonols (Nile and Park, 2014; Lachowicz et al., 2017). Saskatoon berry treatment normalized the expression of HK1 and glycogen phosphorylase in liver and increased the expression of G6Pase. These results suggested that saskatoon berry regulates glycolysis, gluconeogenesis, and glycogenesis, improving metabolic syndrome (du Preez et al., 2020).
In summary, most of the experimental studies on the improvement of NAFLD by natural products through glycolysis are mostly studies on complex natural products, which will cause certain uncertainty to the results of the studies. Future studies should identify active natural products and conduct individual natural product studies. In addition, the research on the mechanism of action is not deep enough, and it is necessary to carry out research on the pharmacological action targets of natural products to improve NAFLD on the existing basis, to provide theoretical support for the development of drugs with clear curative effects and clear targets.
1.3 Liver fibrosis and glycolysis
Fibrosis is the result of advanced liver damage, it is also closely associated with cirrhosis and liver cancer (Vilar-Gomez et al., 2018). Therefore, the improvement of liver fibrosis has become an important indicator to evaluate the efficacy of NAFLD drugs (Heyens et al., 2021). Moreover, chronic inflammatory environment and fibrotic deposition play a key role in the pathogenesis of HCC (Zhang et al., 2020). However, current drugs to treat liver fibrosis have limited effectiveness and it is important to develop drugs to prevent and reverse fibrosis (Schuppan et al., 2018). Liver fibrosis is characterized by the activation, proliferation, and migration of hepatic stellate cell (HSC) (Jiang et al., 2021). Activated HSCS further promote the formation of excess collagen and the accumulation of extracellular matrix (ECM), leading to persistent chronic liver injury. Without timely intervention, which gradually worsens to cirrhosis and eventually liver cancer (Shan et al., 2019). Activated macrophages release various cytokines, directly damage liver parenchymal cells, promote inflammatory cell infiltration, and activate HSC (Tacke and Zimmermann, 2014). Macrophages follistatin-like protein 1 (FSTL1) binds to PKM2, induces M1 polarization and inflammation, and promotes the progression of liver fibrosis (Rao et al., 2022). Tetramerization of PKM2 can reverse liver fibrosis, and inducing tetramerization of PKM2 to reduce the level of PKM2 dimer may be a potential therapeutic strategy for liver fibrosis (Satyanarayana et al., 2021). It has been found that the expression of GLUT1 and PKM2 is upregulated in liver fibrosis. And the expression of GLUT1 and PKM2 is significantly increased in activated HSC exosomes, suggesting that the exosomes released by HSC are related to HSC activation and glucose uptake (Wan et al., 2019). Activated HSC exosomes affect the metabolism of liver non-parenchymal cells through the transfer of glycolytic-related proteins (Wan et al., 2019). TGF-β1 was found to promote the development of liver fibrosis in mice by activating the Smad, p38 MAPK and PI3K/AKT signaling pathways, causing an increase in aerobic glycolysis in HSC and inducing GLUT1 expression in HSC. After GLUT1 inhibitors were administered, liver inflammation and the degree of liver fibrosis were significantly reduced in mice with liver fibrosis (Zhou et al., 2021). Focal adhesion kinase (FAK) promoted the aerobic glycolysis of cancer cells and fibroblasts, while FAK-related non-kinase kinase (FRNK) inhibited the aerobic glycolysis of HSC by inhibiting the FAK/Ras/c-MYC/ENO1 pathway, thereby improving liver fibrosis. FRNK might be a potential target for treatment of liver fibrosis (Huang et al., 2022). HSC activation is the core process of liver fibrosis, and glycolysis is one of its metabolic markers. Therefore, blocking glycolysis may become a new treatment option for liver fibrosis.
1.4 Natural products regulate glycolysis in liver fibrosis
In recent years, many studies have shown that natural products and their active ingredients have anti-fibrosis effects (Table 2). HSC activation was the central event of liver fibrosis. Costunolide is a natural sesquiterpene lactone extracted from Radix Aucklandiae and exhibits a variety of biological activities, including anti-fibrotic, anti-oxidant, anti-inflammatory, and anti-cancer properties (Tian et al., 2022; Saraswati et al., 2018; Niu et al., 2021). Costunolide reduced HSC activity by inhibiting the expression of two key markers of HSC activation, a-smooth muscle actin (a-SMA) and Collagen alpha-1(I) chain (COL1A1). It also reduced glucose uptake and consumption, and reduced the level of lactic acid and inhibited HK2 expression and activity, inhibiting glycolysis (Ban et al., 2019). Deoxyelephantopin is a sesquiterpene lactone extracted from Compositae Elephantopus scaber L., which has good anti-oxidant and anti-carcinogenic properties (Mehmood et al., 2017). Deoxyelephantopin decreased the expression of a-SMA and α1(I) procollagen II (pro-COL1A1) and inhibited liver fibrosis. In addition, it decreased the expression of HK, PFK2, GLUT4 through the hedgehog pathway, and reduced the production of lactic acid in HSC, inhibiting the production of aerobic glycolysis in HSC (Gao et al., 2019). Curcumin is a bright yellow metabolite isolated from Curcuma longa L. (turmeric) plants and has a variety of therapeutic applications, showing liver protection, anti-cancer, anti-inflammatory, anti-oxidant, anti-proliferation effects (Tagde et al., 2021). Curcumin inhibited glycolysis in HSC by decreasing the expression of HK, PFK2, and GLUT4, and lactic acid depending on AMPK activation. Curcumin inhibited the expression of α-SMA and pro-COL1A1, and inhibited liver fibrosis (Lian et al., 2016).
TABLE 2.
Natural products regulate glycolysis in liver fibrosis.
| Name | Origin | Regulatory mechanism | Dose | Cell line/Experiment | References |
|---|---|---|---|---|---|
| Costunolide | Radix Aucklandiae | Inhibition of HK2 | 10, 20 and 30 μM | Primary HSCs were isolated from rats | Ban et al. (2019) |
| Deoxyelephantopin | Elephantopus scaber L | Inhibition of HK, PFK2, GLUT4 levels via hedgehog pathway | 2.5, 5 and 10 μM | Primary rat HSCs (HSC-T6) | Gao et al. (2019) |
| Curcumin | Curcuma longa L | Inhibition of HK, PFK2, GLUT4 levels via AMPK pathway | 20 μM | Primary rat HSCs | Lian et al. (2016) |
In conclusion, there are few researches on glycolytic treatment of liver fibrosis based on natural products, and the content of the research is not deep enough. The signaling pathways closely related to liver fibrosis should be associated with glycolysis, such as TGF-β, PDGF, Wnt/β-catenin and Hedgehog signaling pathways to increase the depth of glycolytic treatment of liver fibrosis. In addition, most studies mainly focus on the cellular level, these cannot completely simulate the pathological characteristics of human liver fibrosis, and its drug activity needs to be further studied and confirmed. At present, there is still a lack of specific anti-fibrosis drugs in clinical practice, which leads to no control drugs in animal, cell and clinical experiments, making the natural drug anti-fibrosis research lack of unified and clear efficacy standards. In addition, there are many causes of liver fibrosis, including viral hepatitis, non-alcoholic steatohepatitis and cholestatic liver disease, so it is more meaningful to study cell models and animal models based on different etiology.
1.5 Liver cancer and glycolysis
Normal cells produce ATP mainly through OXPHOS under aerobic conditions. In the absence of oxygen, ATP is produced mainly by glycolysis. In the process of rapid proliferation of tumor cells, the demand for energy increases, resulting in a reprogramming process of tumor cell metabolism. Therefore, cancer cells produce ATP mainly through glycolysis, even under aerobic conditions, which is called aerobic glycolysis and also known as the Warburg effect (Koppenol et al., 2011). Tumor cells eventually metabolize glucose into lactic acid through glycolysis, a process that produces energy, but the energy produced by this pathway is much lower than the energy produced by each cycle of tricarboxylic acid. In order to obtain high efficiency glycolysis, tumor cells increase glucose transporters or various key enzymes to promote the efficient entry of nutrients into cells and participate in metabolism (Reckzeh et al., 2019).
The basic way to regulate glycolysis is to change the activity of key glycolytic enzymes, including HK, PFK1 and PK, whose activity directly affects the speed and direction of the entire metabolic pathway (Cui et al., 2022). HK as an important glycolytic enzyme, HKs is responsible for the first rate-limiting step in glucose metabolism, the phosphorylation of glucose to G6P (Tan and Miyamoto, 2015). Currently, there are four different types of HK, namely, HK1-4 (Perrin-Cocon et al., 2018). Among them, HK1 and HK2 are located mainly on the outer membrane of mitochondria, HK3 is located in the perinuclear compartment, and HK4 is located in the cytoplasm. Localization in the mitochondrial outer membrane gives HK2 the advantage of escaping product inhibition and preferentially obtaining mitochondrial ATP (Pedersen, 2008). Among these different HKs, HK2 was upregulated in a variety of cancers and played a key role in the development of Warburg (Xu et al., 2017). Therefore, based on the key role of HK in HCC, HK2 may become a target for the development of new therapies for liver cancer. PFK1 is the second rate-limiting enzyme involved in glycolysis, whose activity is regulated by phosphofructokinase-2/ructose-2, 6-diphosphatase 3 (PFKFB3) (Zuo et al., 2021). PFKFB3 does not directly participate in the catalytic process of glycolysis, but instead produces fructose 2, 6-diphosphate by catalyzing F6P. Fructose 2, 6-diphosphate is an allosteric activator of PFK1 and can significantly enhance the catalytic activity of PFK-1 (Boutard et al., 2019). It was found that the combination of aspirin and sorafenib after inhibiting the expression of PFKFB3 can overcome the resistance of sorafenib by inducing apoptosis of HCC cells, so as to enhance the therapeutic effect of HCC (Li et al., 2017a). Therefore, PFKFB3 is also the key to regulate glycolysis and is another important target for tumor therapy. Another rate-limiting enzyme in glycolysis is PK, which has 4 subtypes, including PKL, PKR, PKM1, and PKM2 (Dong et al., 2016). PKM2 was highly expressed in liver cancer and was associated with poor prognosis (Li et al., 2020). PKM2 existed in different forms, mainly in the form of low activity dimer, which promoted tumor growth. Studies have shown that PKM2 drives HCC progression by inducing immunosuppressive microenvironment and upregulation of PD-L1. Overexpression of PKM2 makes HCC sensitive to immune checkpoint blocking, thereby enhancing IFN-γ-positive CD8 T cells in mouse models of liver cancer (Li et al., 2020). These results indicated that PKM2 was expected to be another therapeutic target in the treatment of liver cancer. In addition, LDH catalyzes the final step in the glycolysis process and is responsible for the mutual conversion of lactic acid and pyruvate (Sharma et al., 2022). There are three subtypes of LDH, including LDHA, LDHB and LDHC, of which LDHA is responsible for converting pyruvate into lactic acid (Feng et al., 2018) and LDHB is responsible for converting lactic acid into pyruvate (Urbańska and Orzechowski, 2019). LDHA was mainly expressed in tumor cells. The expression of LDHA was increased in liver cancer, and a large amount of energy wasrapidly generated through glycolysis to support cancer cell growth (Feng et al., 2018). In addition, it is methylated at R112 and is essential for PRMT3-induced glycolysis and HCC growth (Lei et al., 2022). Therefore, LDHA is a promising therapeutic target. Regulation of glycolysis is also regulated by GLUTs. Of the 14 members of the GLUTs family, only GLUT1-5 is currently the most intensively studied, and they all act as glucose and/or fructose transporters in a variety of tissues and cell types (Pyla et al., 2013; Cui et al., 2022). Hypoxia induced abnormal activation of hypoxia-inducible factor-1α (HIF-1α) in the immune microenvironment, and also upregulated LDHA and GLUT1 to cause glycolysis, which promoted the progression of HCC and led to enhanced drug resistance of cancer cells (Zhou et al., 2022). Therefore, various key enzymes and transporters of glycolysis are expected to become drug targets for liver cancer treatment.
Lactate transport from the extracellular depends on monocarboxylate transporters, MCT family currently consists of 14 members, glycolysis speed is fast, may lead to the increase of lactate production in cancer cells, affect the development and proliferation of tumor cells (Halestrap, 2012). If cancer cells are unable to process lactic acid, it may lead to tumor cell death (Cui et al., 2022). Studies have shown that MCT1 and MCT4 are the main transporters of lactic acid excretion in tumor cells (Ruzzolini et al., 2020; Soni et al., 2020), therefore, MCT may become the target of liver cancer treatment.
Glycolysis also affects the tumor immune microenvironment. The tumor microenvironment is a complex cellular environment. Inducing differentiation of naive CD8+T cells is the main anti-tumor mechanism of immune cells. Naive CD8+T cells are usually present in a quiescent state. Maintenance of this state is mediated primarily by two molecules: sphingosine 1-phosphate (S1P) and interleukin (IL)-7 (Goronzy et al., 2015; Mendoza et al., 2017). S1P is important for OXPHOS (Mendoza et al., 2017), while IL-7 mainly promotes glucose uptake by GLUT-1, affecting the glycolysis process (Niu et al., 2023). Glycolysis is primarily used to activate T cells, but OXPHOS are also important, and their absence prevents T cell proliferation (Tarasenko et al., 2017). In addition, high levels of lactic acid can cause macrophages to develop into M2 macrophages, inhibit T cell activation and proliferation, and exert their immunosuppressive function by expressing arginase 1 (ARG1) protein (Andrejeva and Rathmell, 2017). Macrophages that ingested glucose can develop into M1 macrophages after receiving interferon gamma (IFN-γ) secreted by Th1 cells, and the anti-tumor ability of M1-macrophages can be enhanced (Andrejeva and Rathmell, 2017). Activation of NK cells require a shift in their metabolism from mitochondrial oxidation to glycolysis (Assmann et al., 2017).
1.6 Natural products regulate glycolysis in liver cancer
1.6.1 Targeting glycolytic enzymes
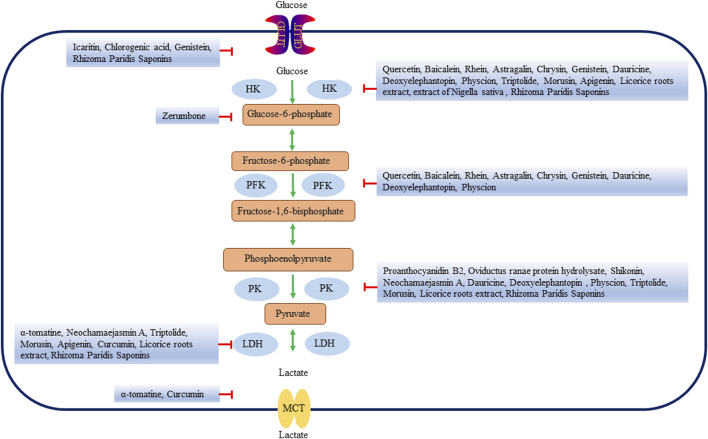
Many natural products affect glycolysis by directly or indirectly regulating key enzymes of glycolysis (Table 3; Figure 2). Quercetin is a flavonoid that is present in a variety of vegetables and fruits (Di Petrillo et al., 2022) and has anti-inflammatory, anti-oxidant and anti-cancer effects (Xu et al., 2019; Saeedi-Boroujeni and Mahmoudian-Sani, 2021; Wang et al., 2022c). Studies have shown that in vivo and in vitro experiments, quercetin decreases the expression of HK2 in HCC cells (Wu et al., 2019a). Baicalein, one of the main bioactive metabolites isolated from Scutellaria baicalensis, has anti-inflammatory, anti-lipogenesis, anti-viral and cardiovascular protective effects (He et al., 2021). Baicalein inhibited the activity and energy metabolism of liver cancer cells. Baicalein also significantly inhibited the expression of HK2 and decreased the glycolysis ability of liver cancer cells (郭舜 et al., 2021). In glycolytic-dependent cells, the binding of HK and VDAC was an important factor in the maintenance of glycolysis and could inhibit mitochondrial energy metabolism (Todisco et al., 2016). Rhein is an anthraquinone metabolite extracted from the Traditional Chinese Medicine (TCM) rhubarb [Huang et al. (2022)], which has anti-cancer, anti-inflammatory and anti-viral effects (Wang et al., 2018; Zhuang et al., 2019; Bu et al., 2020). Rhein dissociated the binding of VDAC and HK, inhibited glycolysis, reduced ATP in liver cancer, inducing apoptosis of liver cancer cells (Wu et al., 2019b). Oviductus Ranae, derived from the dried tubular product of Rana temporaria chensinensis David, has anti-fatigue and increased immune biological activity (Xiao et al., 2019). Oviductus Ranae is a precious natural product in northeast China, which has been developed into a series of health food and TCM (Wang et al., 2021a). Oviductus ranae protein hydrolysate (ORPH) treatment decreased the expression of PKM2 by upregulating miR-491-5p in a post-transcriptional manner, and inhibited the growth, metastasis and glycolysis of mouse liver cancer cells (Xu et al., 2018). Astragalin (ASG) is a flavonoid that was found in a variety of botanical drugs, such as Radix astragali, Morus alba and Cassia alata, as well as in some fruits and vegetables (Liu et al., 2019). ASG has been widely used in various pharmacological fields because of its anti-inflammatory, anti-oxidant and inhibitory effect on malignant tumor cells (Harikrishnan et al., 2020). ASG could inhibit HCC cell proliferation by promoting microRNA-125b (miR-125b) and metabolic reprogramming, reducing HK2 expression and inhibiting glycolysis in HCC cells (Li et al., 2017c). Chrysin is a bioactive flavonoid derived from plant extracts, found in blue passion flower, propolis and honey, and is widely used as a Chinese herbal medicine in China. Chrysin not only has anti-oxidant, anti-inflammatory and other biological activities, but also has anti-cancer effect (Yao et al., 2014; Zhang et al., 2015; Tang et al., 2016). Chrysin or its derivatives significantly inhibited glucose uptake and lactate production in HCC cells by decreasing HK2 expression. Reduced expression of HK2 bond to voltage-dependent anion channel 1 on mitochondria, leading to transport of Bcl-2-associated X protein (Bax) from cytoplasm to mitochondria and inducing apoptosis (Xu et al., 2017). Shikonin is a naturally occurring naphthoquinone isolated from the root of the plant Lithospermum erythrorhizon. Studies have shown that comfrey and its derivatives have anti-cancer effects on many types of tumors (Lee et al., 2014; Ruan et al., 2021). By inhibiting PKM2, Shikonin decreased the expression of cyclinD1, inhibited liver cancer glycolysis and cell proliferation, and induced cell apoptosis. The effect of Shikonin on the proliferation, apoptosis and glycolysis of HCC cells will make it a promising drug for the treatment of HCC (Liu et al., 2020). Icaritin is an active component of Chinese botanical drug Epimedium. It has a wide range of biological and pharmacological functions, including anti-oxidant and anti-cancer (He et al., 2010; Zhou et al., 2011; Qin et al., 2020). Icaritin induced upregulation of FAM99A expression in HCC cells, blocked JAK2/STAT3 pathway, and inhibited GLUT1-mediated glycolysis and HCC cell viability (Zheng et al., 2021). Chlorogenic acid (CGA) is a dietary phenolic acid produced by a variety of plants, such as Sonchus oleraceus Linn. CGA is the most prevalent metabolite in the phenolic acid group, which is also found in tea and coffee extracts (Gupta et al., 2022). CGA has a wide range of effects, such as anti-cancer, anti-bacterial, anti-oxidant and so on (Zeng et al., 2021). The hepatoprotective effect of CGA might be related to increasing the production of ATP, stimulating mitochondrial OXPHOS and inhibiting glycolysis (Zhou et al., 2016). CGA also prevented the glucose-induced decline in GLUT4 levels and regulated glucose uptake and transport in HepG2 cells (Chen et al., 2019). Oleuropein is an iridoid phenolic metabolite composed of three structural subunits: hydroxytyrosol, enolic acid and glucose molecules. It is also reported to be a chemical classification marker for olives. Oleuropein has been reported to have a variety of biological activities, including anti-dyslipidemia, anti-atherosclerosis, anti-inflammatory, anti-diabetes, and liver protective effects (Ahamad et al., 2019). Glucose-6-phosphate isomerase (GPI) was a key enzyme in glycolysis. The expression of GPI in tumor cells affected different physiological functions and signal transduction. GPI was the direct target of oleuropein, which could inhibit liver cancer glycolysis by inhibiting GPI, and it showed good anti-tumor activity in vivo without adverse side effects (Hong et al., 2023). Erianin, extracted from the rare Chinese medicine Dendrobium chrysotoxum Lindl, is a small molecule natural metabolite with a wide range of anti-cancer potential in vivo and in vitro (Sun et al., 2020). Erianin effectively inhibited the enzyme activity of pyruvate carboxylase (PC), promoted mitochondrial oxidative stress, inhibited glycolysis, inducing insufficient energy required for the proliferation of liver cancer cells (Sheng et al., 2022). Zerumbone is a natural metabolite of the ginger plant Zingiber zerumbet (L.) Smith, which is used to treat a wide variety of ailments. Zerumbone’s anti-cancer properties have been reported in vitro and in vivo studies of a variety of cancers (Zainal et al., 2018; Girisa et al., 2019). Zerumbone blocked the binding of G6P through the pentose phosphate pathway, reduced glucose consumption and lactic acid production, inhibited glycolysis, inducing cell cycle arrest and apoptosis of liver cancer cells (Wani et al., 2018).
TABLE 3.
Natural products regulate glycolysis via glycolytic enzymes in liver cancer.
| Name | Origin | Target glycolytic enzymes | Regulatory mechanism | Dose | Cell line/Experiment | References | |
|---|---|---|---|---|---|---|---|
| Quercetin | Vegetables and fruits | HK2 | downregulate | 12.5,25,50 μM and 50 mg/kg | SMMG-7721, BEL-7402 cells and BALB/c nude mice | Wu et al. (2019a) | |
| Baicalein | Scutellaria baicalensis | HK2 | downregulate | 5,10 and 20 μM | SMMC-7721 and HepG2 cells | (郭舜 et al., 2021) | |
| Rhein | rhubarb | HK2 | downregulate | 5,10,20,40,60,80 μM | SMMC-7721 and SMMC-7721/DOX cells | Wu et al. (2019b) | |
| Proanthocyanidin B2 | Vegetables and fruits | PKM2 | downregulate | 10,20,40,60,80,100,120 and 140 μM | HCC-LM3, SMMC-7721, Bel-7402, Huh-7 and HepG2 cells | Feng et al. (2019) | |
| Oviductus ranae protein hydrolysate | Rana temporaria chensinensis David | PKM2 | downregulate | 400 ug/mL for HepG2 cells and 450 ug/mL for Hep3B cells | Hep3B, HepG2 cells and clinical samples | Xu et al. (2018) | |
| Astragalin | Botanical drugs, vegetables and fruits | HK2 | downregulate | 11,33 μM and 10,20 mg/kg | HepG2, Huh-7, HL-7702, H22 cells and Kunming mice, athymic nude mice | Li et al. (2017c) | |
| Chrysin | Botanical drugs | HK2 | downregulate | 15,30,60 μM and 30 mg/kg | HepG2, Hep3B, Huh-7, HCC-LM3, Bel-7402, SMMC-7721 and nu/nu athymic nude mice | Xu et al. (2017) | |
| Shikonin | Lithospermum erythrorhizon | PKM2 | downregulate | 1,2,3 μM and 5 mg/kg | HCC-LM3, SMMC-7721, Huh-7, HepG2 cells and BALB/c nude mice | Liu et al. (2020) | |
| Icaritin | Epimedium | GLUT1 | downregulate | 2.5,5 and 10 μM | HepG2, HCC-LM3 cells and nude mice | Zheng et al. (2021) | |
| Chlorogenic acid | Plants | GLUT4 | downregulate | 25,50 and 100 μg/mL | HepG2 cells | Chen et al. (2019) | |
| Oleuropein | Olives | GPI | downregulate | 1,10,25,100,250 μM and 200 mg/kg | HepG2, HuH-7 cells and Balb/c mouse | Hong et al. (2023) | |
| Erianin | Dendrobium chrysotoxum Lindl | PC | downregulate | 10,20,30,40,50,100,200 and 400 nM | HepG2, MHCC97, SK-Hep-1 and HCC-LM3 cells | Sheng et al. (2022) | |
| Zerumbone | Zingiber zerumbet (L.) Smith | Glucose-6-phosphate | downregulate | 50,100,150,200 µM and 20 mg/kg | HepG2, Hep3B, Sk-Hep-1, SNU-182, SNU-449, HCC-LM3 cells and NSG mice | Wani et al. (2018) | |
| α-tomatine | Tomato | LDHA, MCT4 | downregulate | 0.5,1,15,2 and 2.5 µM | HuH-7 cells | (何志龙 et al., 2022) | |
| Neochamaejasmin A | Stellera chamaejasme | PK, LDH | downregulate | 10,20,40,80 μg/mL | HepG2 cells | (丁杨芳 et al., 2019) | |
| Genistein | Soybeans | HK2, GLUT1 | downregulate | 20,40,60,80,100,140 µM and 20,40,80 mg/kg | HCC-LM3, SMMC-7721, Hep3B, Bel-7402, Huh-7 cells and athymic BALB/c nu/nu mice | Li et al. (2017b) | |
| Dauricine | Menispermum dauricum DC. | HK2, PKM2 | downregulate | 2 μg/mL and 10 mg/kg | HepG2, Huh-7, Hep3B and athymic nude mice | Li et al. (2018) | |
| Deoxyelephantopin | Elephantopus scaber L | HK2, PFK1, PKM2 | downregulate | 0.625,1.25,2.5,5,10,20,40 and 80 µM | HepG2 cells | (吴红雁 et al., 2023) | |
| Physcion | Rheum officinale Baill | HK2, PFKFB3, PKM2 | downregulate | 1.25,2.5,5,10,20 and 40 µM | HepG2 cells | (陶正娣 et al., 2022) | |
| Triptolide | Tripterygium wilfordii Hook f | HK2, PKM2, LDHA | downregulate | 10,40,60 and 100 nM | SMMC-7721 cells | (李恬 et al., 2020) | |
| Morusin | Morus alba | HK2, PKM2, LDH | downregulate | 2.5,5,10,20 and 40 μM | Huh7 and Hep3B cells | Cho et al. (2021) | |
| Apigenin | Vegetables and fruits | HK2, LDHA, PDHK1 | downregulate | 20,40,80 μM and 400 mg/kg | HepG2 cells and athymic nude mice | (张睿 et al., 2020) | |
| Curcumin | Curcuma longa L | LDHA, MCT1 | downregulate | 1,2,5,10 μM | HepG2 and HuT78 cells | Soni et al. (2020) | |
| Licorice roots extract | Glycyrrhiza glabra L | HK2, PKM2, LDHA | downregulate | 1.562–200 mg/mL | HepG2 cells | Abdel-Wahab et al. (2021) | |
| Scopolin | Smilax china L | GPI, GPD2, PGK2 | downregulate | 25,50,100,200 μM and 20,50,100 mg/kg | HepG2 cells and BALB/c mice | Wang et al. (2022a) | |
| Extract of Nigella sativa | Nigella sativa | HK, GAPDH | downregulate | 1 g/kg | Wister Albino rats | Abdel-Hamid et al. (2013) | |
| Rhizoma Paridis Saponins | Rhizoma Paridis | HK2, PKM2, LDHA, GLUT1 | downregulate | 100 mg/kg | Kunming mice | Qiu et al. (2016) | |
FIGURE 2.
Natural products affect the glycolysis of liver cancer by directly regulating glycolysis enzymes. HK, hexokinase; PFK, Phosphofructokinase; PK, pyruvate kinase; LDH, lactate dehydrogenase; GLUT, glucose transporters; MCTs, Monocarboxylate transporters.
Neochamaejasmin A is a major component in the stem root of Stellera chamaejasme, which has anti-cancer effects on tumor cells (Liu et al., 2008). Neochamaejasmin A inhibited the proliferation of tumor cells by inhibiting glucose uptake and lactate production in HepG2 cells, and reducing the expression of glycolytic related proteins PK and LDH (丁杨芳 et al., 2019). Genistein is an isoflavone found in soybeans. It plays an important role in the occurrence and development of cancer and the prevention and treatment of common diseases such as metabolic syndrome by inhibiting inflammation and regulating metabolic pathways (Xu et al., 2022). Genistein inhibited aerobic glycolysis by decreasing GLUT1 and HK2 (Li et al., 2017b). Dauricine (Dau), an alkaloid metabolite isolated from the roots of Menispermum dauricum DC., inhibits tumor growth. By increasing miR-199a, Dau directly downregulated HK2 and PKM2, inhibited glycolysis and increased OXPHOS, inhibited liver cancer cell proliferation, and sensitized sorafenib therapy (Li et al., 2018).
Physcion extracted from Rheum officinale Baill., is a naturally occurring anthraquinone derivative with anti-cancer, anti-bacterial, anti-viral, anti-inflammatory and other biological activities (Zhang et al., 2021). Physcion significantly downregulated the expression of HK2, PFKFB3, PKM2, and inhibited glucose uptake and lactic acid production. These results showed that Physcion inhibited the proliferation of liver cancer cells by interfering with the process of glycolytic energy metabolism (陶正娣 et al., 2022). Triptolide is a natural epoxy diterpenoid metabolite derived from botanical drug Tripterygium wilfordii Hook f, which has strong anti-cancer properties (Chen et al., 2018; Cai et al., 2021). Licorice plant, (Glycyrrhiza glabra L.), have been widely used in TCM for the treatment of different diseases, for its role in nourishing qi, tonifying spleen and stomach, and harmonizing prescriptions (Abdel-Wahab et al., 2021; Shikov et al., 2022). Licorice roots extract induced apoptosis and cycle arrest of liver cancer cells, inhibited HK2, PKM2, and LDHA enzymes, and inhibited glycolysis by up-regulating multiple tumor suppressor genes miRNAs (Abdel-Wahab et al., 2021). Smilax china L. is a well-known Chinese medicine used as an anti-inflammatory, anti-cancer and analgesic (Yu et al., 2014a; Feng et al., 2020). Scopolin obtained from Smilax china L. plays the role of anti-HCC. Scopolin regulated glycolysis-related proteins glucose-6-phosphate isomerase (GPI), glycerol-3-phosphate dehydrogenase, mitochondrial (GPD2) and phosphoglycerate kinase 2 (PGK2) expression and inhibited protein-protein interaction, reduced energy metabolism in liver cancer tissue, inhibiting tumor growth (Wang et al., 2022a). Nigella sativa (NS), commonly known as the black seed or black cumin “Al-Habba Al-Sauda”, is the seed of an enveloped plant belonging to the Ranunculaceae family (Amin and Hosseinzadeh, 2016). It has been used as a spice and food preservative, as well as a protective and therapeutic drug for many diseases (Zielińska et al., 2021). Serum HK and GAPDH were increased in liver cancer group. Extract of Nigella sativa (MENS) inhibited glycolysis by reducing the expression of these enzymes and has chemical preventive effects on the progression of liver cancer (Abdel-Hamid et al., 2013). Rhizoma Paridis Saponins (RPS), a natural product purified from the commonly used Chinese medicine Rhizoma Paridis, is not only inhibits liver fibrosis and cirrhosis, but also inhibits the growth of a variety of cancers (Man et al., 2014a; Man et al., 2014b). RPS decreased the expression of GLUT1, HK2, PKM2, and LDHA. RPS also reversed aerobic glycolysis by activating tumor suppressor genes p53 and PTEN, and inhibited the proliferation of mouse liver cancer H22 tumors (Qiu et al., 2016). In summary, as a rich resource, natural products show potential as glycolysis inhibitors in the future clinical treatment of liver cancer.
1.6.2 Targeting multiple signaling pathway in liver cancer
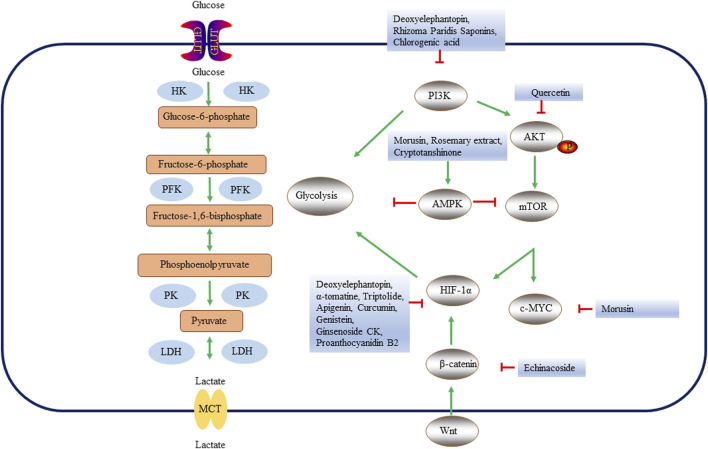
Various signaling pathways play an important role in the glycolysis of liver cancer (Table 4; Figure 3). Studies have shown that the activated PI3K/AKT signaling pathway stimulates glucose uptake by regulating GLUT1 expression, enhances glycolysis, drives lactic acid production in cancer cells, inhibits macromolecular degradation, and affects tumor cell metabolism (Wasik and Lehtonen, 2018; Jin et al., 2021). Quercetin has been found to reduce HK2 levels and inhibit the AKT/mTOR pathway in liver cancer cells in vivo and in vitro (Wu et al., 2019a). In addition to its role in liver fibrosis, Deoxyelephantopin also plays an important role in liver cancer. Deoxyelephantopin reduced glucose uptake and lactic acid production and inhibited glycolysis through PI3K/Akt/mTOR/HIF-1α signaling pathway, thus inhibiting the proliferation and migration of HepG2 cells (吴红雁 et al., 2023). It was found that the combination of RPS sorafenib increased the anti-cancer effect, overcoming the tolerance of sorafenib by protecting mitochondrial damage, inhibiting anaerobic glycolytic through PI3K/AKT/mTOR pathway (Yao et al., 2018). CGA regulated glucose uptake and transport in HepG2 cells through the PI3K/AKT pathway (Chen et al., 2019). Prunella vulgaris is dried fruit spike of Lamiacea plant P. vulgaris L., which is an important medicinal plant mainly found in Europe and Asia (Chang et al., 2023). Prunella vulgaris total flavonoids inhibit the proliferation of liver cancer (Song et al., 2021). The Prunella vulgaris total flavonoids activated Bcl-2/Bax protein to induce apoptosis of liver cancer cells, and the mechanism might be related to the inhibition of aerobic glycolysis and OXPHOS levels of liver cancer cells (宋亚刚 et al., 2020). The Wnt/β-catenin signaling pathway stimulates glycolysis by up-regulating the expression of HK2, LDHA and pyruvate dehydrogenase kinase 1 (PDK1) (Fang et al., 2019). The Wnt/β-catenin pathway stimulates the downstream PI3K/AKT pathway and HIF-1α, thereby indirectly activating aerobic glycolysis (Vallée et al., 2017). Therefore, targeting the Wnt/β-catenin signaling pathway can regulate glycolysis. Echinacoside (ECH) is an active component of Cistanche salsa, which has strong anti-proliferation and pro-apoptotic activities in various cancers including HCC (Ye et al., 2019; Wang et al., 2022b). Studies have shown that ubiquitin protein ligase E3 component N-recognin 5 (UBR5) expression is associated with decreased apoptosis and increased glycolysis of hepatoma cells through β-catenin signaling pathway. AMPG nanocomposites have low cytotoxicity and good biosafety. The ECH of AMPG effectively reduced glycolysis and promoted the apoptosis of liver cancer cells (Wang et al., 2022b). HIF-1α and AMPK signaling pathways are major regulators of glycolysis and OXPHOS and are critical for metabolic reprogramming of tumor cells (Chen et al., 2022). AMPK is a key sensor and regulator of cellular metabolism (González et al., 2020). AMPK is a heterotrimer complex consisting of a catalytic α subunit and two regulatory β and γ subunits (Herzig and Shaw, 2018). Morusin is a kind of naturally existing prenylated flavonoid isolated from the root bark of M. alba, which has the effect of inhibiting cancer (Wang et al., 2020). Morusin inhibited HK2, PKM2 and LDH expression and reduced lactic acid, glucose and c-MYC, activated AMPK through AMPK pathway, which played an important role in anti-HCC (Cho et al., 2021). Rosemary extract from the plant Rosmarinus officinalis L., has anti-oxidant, anti-cancer, anti-bacterial and other effects (Jaglanian and Tsiani, 2020). Rosemary extract significantly increased glucose consumption in HepG2 cells and promoted liver glycolysis and fatty acid oxidation by activating AMPK and PPAR pathways (Tu et al., 2013). Cryptotanshinone is a liposoluble soluble diterpene derivative mainly found in the genus Salvia, among which S. miltiorrhiza Bunge, is a diterpene-rich plant (Dalil et al., 2022). Arsenic trioxide cooperate cryptotanshinone (ACCS) inhibited liver cancer by increasing AMPK phosphorylation and activating AMPK signaling pathway, which enhanced glucose utilization and glycolysis of macrophages (Jiang et al., 2022).
TABLE 4.
Natural products regulate glycolysis via multiple signaling pathways in liver cancer.
| Name | Origin | Regulatory mechanism | Dose | Cell line/Experiment | References |
|---|---|---|---|---|---|
| Quercetin | Vegetables and fruits | AKT, mTOR | 12.5,25,50 μM and 50 mg/kg | SMMG-7721, BEL-7402 cells and BALB/c nude mice | Wu et al. (2019a) |
| Deoxyelephantopin | Elephantopus scaber L | PI3K, Akt, mTOR | 0.625,1.25,2.5,5,10,20,40 and 80 µM | HepG2 cells | (吴红雁 et al., 2023) |
| Rhizoma Paridis Saponins | Rhizoma Paridis | PI3K, Akt, mTOR | 80 mg/kg | Kunming mice | Yao et al. (2018) |
| Chlorogenic acid | Plants | PI3K, AKT | 25,50 and 100 μg/mL | HepG2 cells | Chen et al. (2019) |
| Prunella vulgaris total flavonoids | Prunella vulgaris L | Bcl-2, Bax | 50,100,200,400 and 800 μg/μL | SMMG7721 cells | (宋亚刚 et al., 2020) |
| Echinacoside | Cistanche salsa | β-catenin | 1,2,5,10 and 20 μg/mL | HepG2 and Huh7 cells and clinical samples | Wang et al. (2022b) |
| Morusin | Morus alba | AMPK, p-mTOR | 2.5,5,10,20 and 40 μM | Huh7 and Hep3B cells | Cho et al. (2021) |
| Rosemary extract | Rosmarinus officinalis L | AMPK | 2,10 and 50 μg/mL | HepG2 cells | Tu et al. (2013) |
| Cryptotanshinone | S. miltiorrhiza Bunge | AMPK | 5,10,15,20,25 μM and 2.5 mg/kg | H22, Hepa1-6 cells and C57BL/6J mice | Jiang et al. (2022) |
FIGURE 3.
Key enzymes, proteins and pathways of natural products regulating glycolysis process in liver cancer. Natural products regulate glycolysis in three ways. First, natural products affect the glycolysis of liver cancer cells by directly regulating glycolytic enzymes. Secondly, natural products can inhibit liver cancer cell glycolysis through PI3K, Wnt/β-catenin or AMPK pathways. Finally, natural products may regulate genes related to glycolysis by regulating oncogenes c-MYC, HIF-1α, etc., thus changing the metabolic pathway of liver cancer. HK, hexokinase; PFK, Phosphofructokinase; PK, pyruvate kinase; LDH, lactate dehydrogenase; GLUT, glucose transporters; MCTs, Monocarboxylate transporters; HIF-1α, Hypoxia Inducible Factor 1 Subunit Alpha; AMPK, AMP-dependent protein kinase; PI3K, Phosphatidylinositol 3-kinase; AKT, protein kinase B; mTOR, mammalian target of rapamycin.
1.6.3 Targeting multiple oncogenes in liver cancer
Multiple oncogenes play an important role in the glycolysis of liver cancer (Table 5). Oncogenes maintained the survival and development of cancer cells through reprogramming of glycolytic metabolism [Mukhopadhyay et al. (2021)]. Studies have found that abnormal expression of MYC exists in cancer, and the expression of MYC is closely related to genes regulating glucose metabolism, such as GLUT1, HK2, PFKM, etc. (Wokolorczyk et al., 2008; Dang et al., 2009). Cancer cells also used activation of HIF-1α to increase glucose uptake and glycolysis flux, promoted glucose catabolism and adapted to low oxygen environment to ensure tumor growth (Lee et al., 2020). ROS stimulated carcinogenic signaling, specifically HIF-1α. ROS stabilized HIF-1α protein by inhibiting propyl hydroxylase protein D (PHD) (Ren et al., 2022). In recent years, more and more natural products targeting HIF-1α and ROS expression and inhibiting liver cancer glycolysis have been studied for the treatment of liver cancer. Deoxyelephantopin reduced the glucose uptake and lactic acid production of liver cancer cells, downregulated the key glycolysis enzymes HK2, PFK1, and PKM2, inhibited the glycolysis of liver cancer through the PI3K/AKT/mTOR/HIF-1α signaling pathway, inhibiting the proliferation and migration of HepG2 cells (吴红雁 et al., 2023). α-tomatine, a steroid sugar alkaloid, is abundant in the flowers, leaves, calyx and immature fruits of tomato. It has various biological activities, such as anti-cancer, anti-inflammatory and anti-viral (Zhao et al., 2015; Fujimaki et al., 2022). α-tomatine reduced the expression of LDHA and MCT4, inhibited the uptake of glucose, reduced lactic acid and intracellular ATP, reduce the expression of HIF-1α, inhibiting glycolysis, proliferation and metastasis of Huh-7 cells (何志龙 et al., 2022). Triptolide could reduce the expression of HIF-1α, inhibit the production of glucose and lactic acid, inhibiting the glycolysis and proliferation and metastasis of liver cancer cells (李恬 et al., 2020). Apigenin is a natural flavonoid that is found in a variety of natural plants, including most vegetables and fruits and exhibits many beneficial effects, including anti-cancer, anti-oxidant and anti-bacterial (Ginwala et al., 2019; Wang et al., 2021b; Kashyap et al., 2022). Apigenin inhibited HIF-1α in HepG2 cells, and decreased the expression of glycolytic related proteins (HK2, LDHA, PDHK1), thereby inhibiting the anti-cancer effect of glycolysis (张睿 et al., 2020). Curcumin inhibited anaerobic glycolysis by inhibiting the expression of LDH and HIF-1α, which strengthened the anti-HCC effect of sorafenib (Man et al., 2020). Curcumin also reduced the expression of HIF-1α, inhibited glucose consumption and lactic acid production, alleviating the drug resistance of liver cancer cells to chemotherapy (Soni et al., 2020). By directly down-regulating HIF-1α, Genistein made aerobic glycolytic HCC cells sensitive to apoptosis, and thus inactivated GLUT1 and HK2 to inhibit aerobic glycolysis (Li et al., 2017b). Proanthocyanidin B2 (PB2) is widely exists in natural product, such as fruits and vegetables and has strong anti-oxidant activity because the phenolic hydrogen atoms can effectively intercept free radicals in the free radical chain reaction (Snow et al., 2019). The anti-cancer properties of these metabolites have been well documented, and these effects are mainly attributable to their powerful anti-oxidant and anti-inflammatory effects (Al-Ishaq et al., 2020). PB2 inhibited the expression and nuclear translocation of PKM2, thereby disrupting the interaction between PKM2/HSP90/HIF-1α, and inhibiting the aerobic glycolysis and proliferation of liver cancer cells (Feng et al., 2019). Ginseng (Panax ginseng C. A. Meyer, Family Araliaceae) is one of the major medicinal and nutraceutical plants (Murthy et al., 2018). Ginsenoside CK is one of the most abundant intestinal metabolites of ginsenoside prototype saponins (Guo et al., 2020). It has anti-cancer and anti-inflammation effects, among others (Zhang et al., 2013; Li et al., 2014). Ginsenoside CK inhibited the expression of HIF-1α under hypoxia condition, promoted the ubiquitination degradation of HIF-1α, thus inhibiting the glycolysis and proliferation of hepatoma cells (苏杰琳 et al., 2021).
TABLE 5.
Natural products regulate glycolysis via oncogenes in liver cancer.
| Name | Origin | Regulatory mechanism | Dose | Cell line/Experiment | References |
|---|---|---|---|---|---|
| Morusin | Morus alba | c-MYC | 2.5,5,10,20 and 40 μM | Huh7 and Hep3B cells | Cho et al. (2021) |
| Deoxyelephantopin | Elephantopus scaber L | HIF-1α | 0.625,1.25,2.5,5,10,20,40 and 80 µM | HepG2 cells | (吴红雁 et al., 2023) |
| α-tomatine | Tomato | HIF-1α | 0.5,1,15,2 and 2.5 µM | HuH-7 cells | (何志龙 et al., 2022) |
| Triptolide | Tripterygium wilfordii Hook f | HIF-1α | 10,40,60 and 100 nM | SMMC-7721 cells | (李恬 et al., 2020) |
| Apigenin | Vegetables and fruits | HIF-1α | 20,40,80 μM and 400 mg/kg | HepG2 cells and athymic nude mice | (张睿 et al., 2020) |
| Curcumin | Curcuma longa L | HIF-1α | 1,2,5,10 μM | HepG2 and HuT78 cells | Soni et al. (2020) |
| Genistein | Soybeans | HIF-1α | 20,40,60,80,100,140 µM and 20,40,80 mg/kg | HCC-LM3, SMMC-7721, Hep3B, Bel-7402, Huh-7 cells and athymic BALB/c nu/nu mice | Li et al. (2017b) |
| Proanthocyanidin B2 | Vegetables and fruits | HIF-1α | 10,20,40,60,80,100,120 and 140 μM | HCC-LM3, SMMC-7721, Bel-7402, Huh-7 and HepG2 cells | Feng et al. (2019) |
| Ginsenoside CK | Panax ginseng C. A. Meyer | HIF-1α | 20,40,60 μM | Bel-7404 cells | (苏杰琳 et al., 2021) |
| Prunella vulgaris total flavonoids | Prunella vulgaris L | ROS | 50,100,200,400 and 800 μg/μL | SMMG7721 cells | (宋亚刚 et al., 2020) |
At present, there have been many studies on natural products regulating glycolysis in the treatment of liver disease, but most of the studies focused on the changes in glycolysis of liver cancer cells. However, with the in-depth study of liver cancer in recent years, we found that it is far from enough to study single cells of liver cancer cells. Glycolysis products in liver cancer cells also play an important role in the microenvironment of liver cancer. We should also study how glycolysis affects other cell types in the liver cancer microenvironment. For example, glycolysis product lactic acid has a profound effect on macrophage activation and T cell exhaustion. The further study of glycolysis also provides an alternative target for the existing combination therapy of liver cancer.
2 Conclusion and future perspectives
In NAFLD, glycolysis is significantly enhanced, resulting in increased levels of pyruvate. Pyruvate is enhanced in NAFLD by conversion to oxaloacetic acid or lactic acid (Wang et al., 2021c). The enhancement of glycolytic activity will promote the production of mitochondrial ROS, leading to the progression of NAFLD to NASH, and inhibiting mtROS to maintain mitochondrial homeostasis may be a potential treatment for NAFLD and prevent the further development of the disease (Shimada et al., 2012). In addition, the 2020 proposal to change NAFLD to metabolic (dysfunction) associated fatty liver disease (MAFLD) puts more emphasis on the importance of metabolism (Eslam et al., 2020). Although the metabolic abnormalities of NAFLD are not only the abnormalities of glycolysis and oxidative phosphorylation, glycolysis plays a more important role in the progression of NAFLD disease. More and more evidences showed that HSC played an important role in the process of liver fibrosis. Glucose metabolic reprogramming played an important role in the activation of HSC, mainly through upregulation of glycolysis to meet the energy requirements of HSC activation (Guo et al., 2022b). Therefore, natural products blocking glycolysis through some metabolic pathways may become a new treatment option for liver fibrosis. The occurrence of various types of liver cancer is closely related to liver fibrosis and cirrhosis, and the abnormal state of glycolysis in liver cancer has also aroused our attention to the changes of glycolysis in liver cirrhosis in the early stage of liver cancer.
Metabolic reprogramming is a core marker of cancer and is crucial for tumorigenesis and progression (Liu et al., 2021). Glycolysis plays an important role in promoting the progression of liver cancer, including proliferation, migration and drug resistance.
Natural products may be important for overcoming limitations in liver cancer treatment by targeting key enzymes contained in glycolysis (such as HK2, PFK, or PKM2) and other signaling pathways. Importantly, natural products inhibit key glycolytic enzymes and proteins, and inhibit oncogenes c-MYC, HIF-1α, and ROS-mediated metabolic reprogramming toward glycolytic phenotypes. Moreover, it has advantages in improving metabolic reprogramming of tumor cells, and in glycolytic signaling pathways, such as PI3K, Wnt/β-catenin and AMPK may be the main targets of natural products. The multi-target and multi-pathway therapeutic effect of natural products on liver disease is its advantage, but the intensity of the effect on the target is not enough. In addition, the rate-limiting enzymes in the process of glycolysis have a variety of isoenzymes, such as HK, HK1-2 plays a role in the glycolysis of liver cancer, but glucokinase (HK4) plays a role in NAFLD. Therefore, the study of natural products should select different enzymes according to different liver diseases (Pusec et al., 2019). The depth of research is more limited to the study of hepatocyte glycolysis abnormalities, and more attention should be paid to the study of non-hepatocyte glycolysis in different liver diseases, such as fibroblasts, Kupffer cells, macrophages and T cells. In addition, many studies on natural products are in the pre-clinical stage, and more clinical data are needed to support the safety and effectiveness of natural products. In addition, the research on the treatment of natural products for liver disease should not only stay in pre-clinical research, but also strive to be transformed into clinical drugs. Since natural products have the potential to treat diseases, they should show their advantages.
The occurrence of liver diseases is closely related to the metabolic dysfunction of liver cells, and the abnormal glycolysis function concerned in this paper is only a part of the metabolic abnormalities, and the abnormal glycolysis function is closely related to and mutually influenced by the abnormal oxidative phosphorylation. Due to the limitation of the length of the study, it is difficult for us to introduce different kinds of metabolic abnormalities in different liver disease at the same time in one article. Therefore, we selected the dysfunction of glycolysis in liver disease to introduce it. Glycolysis is an important part of liver cells to provide energy, and it is also an important metabolic mode of liver cancer. Therefore, we choose glycolysis to review.
Funding Statement
The present study was financially supported by the National Natural Science Foundation of China (81973840 and 81273748); National science and Technology major projects of the 13th Five-Year Plan (2018ZX10303502); Science and Technology Program of Hebei (223777156D); Sichuan Provincial Administration of Traditional Chinese Medicine Major science and technology projects (2021XYCZ004).
Author contributions
XH designed research; SL and LH wrote the manuscript with contributions from all authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdel-Hamid N. M., Abdel-Ghany M. I., Nazmy M. H., Amgad S. W. (2013). Can methanolic extract of Nigella sativa seed affect glyco-regulatory enzymes in experimental hepatocellular carcinoma? Environ. Health Prev. Med. 18 (1), 49–56. 10.1007/s12199-012-0292-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Wahab A. A., Effat H., Mahrous E. A., Ali M. A., Al-Shafie T. A. (2021). A licorice roots extract induces apoptosis and cell cycle arrest and improves metabolism via regulating MiRNAs in liver cancer cells. Nutr. Cancer 73 (6), 1047–1058. 10.1080/01635581.2020.1783329 [DOI] [PubMed] [Google Scholar]
- Ahamad J., Toufeeq I., Khan M. A., Ameen M. S. M., Anwer E. T., Uthirapathy S., et al. (2019). Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 33 (12), 3112–3128. 10.1002/ptr.6511 [DOI] [PubMed] [Google Scholar]
- Al-Ishaq R. K., Overy A. J., Büsselberg D. (2020). Phytochemicals and gastrointestinal cancer: cellular mechanisms and effects to change cancer progression. Biomolecules 10 (1), 105. 10.3390/biom10010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehade S., Alshawsh M. A., Murugaiyah V., Asif M., Alshehade O., Almoustafa H., et al. (2022). The role of protein kinases as key drivers of metabolic dysfunction-associated fatty liver disease progression: new insights and future directions. Life Sci. 305 (120732), 120732. 10.1016/j.lfs.2022.120732 [DOI] [PubMed] [Google Scholar]
- Amin B., Hosseinzadeh H. (2016). Black cumin (Nigella sativa) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects. Planta Med. 82 (1-2), 8–16. 10.1055/s-0035-1557838 [DOI] [PubMed] [Google Scholar]
- Andrejeva G., Rathmell J. C. (2017). Similarities and distinctions of cancer and immune metabolism in inflammation and tumors. Cell. Metab. 26 (1), 49–70. 10.1016/j.cmet.2017.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar R., Rabail R., Rakha A., Bryla M., Roszko M., Aadil R. M., et al. (2022). Delving the role of caralluma fimbriata: an edible wild plant to mitigate the biomarkers of metabolic syndrome. Oxid. Med. Cell. Longev. 20 (5720372), 5720372. 10.1155/2022/5720372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann N., O'Brien K. L., Donnelly R. P., Dyck L., Zaiatz-Bittencourt V., Loftus R. M., et al. (2017). Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. 18 (11), 1197–1206. 10.1038/ni.3838 [DOI] [PubMed] [Google Scholar]
- Ban D., Hua S., Zhang W., Shen C., Miao X., Liu W. (2019). Costunolide reduces glycolysis-associated activation of hepatic stellate cells via inhibition of hexokinase-2. Cell. Mol. Biol. Lett. 24 (52), 52–0179. 10.1186/s11658-019-0179-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutard N., Białas A., Sabiniarz A., Guzik P., Banaszak K., Biela A., et al. (2019). Synthesis of amide and sulfonamide substituted N-aryl 6-aminoquinoxalines as PFKFB3 inhibitors with improved physicochemical properties. Bioorg Med. Chem. Lett. 29 (4), 646–653. 10.1016/j.bmcl.2018.12.034 [DOI] [PubMed] [Google Scholar]
- Bu T., Wang C., Jin H., Meng Q., Huo X., Sun H., et al. (2020). Organic anion transporters and PI3K-AKT-mTOR pathway mediate the synergistic anticancer effect of pemetrexed and rhein. J. Cell. Physiol. 235 (4), 3309–3319. 10.1002/jcp.29218 [DOI] [PubMed] [Google Scholar]
- Cai J., Yi M., Tan Y., Li X., Li G., Zeng Z., et al. (2021). Natural product triptolide induces GSDME-mediated pyroptosis in head and neck cancer through suppressing mitochondrial hexokinase-ΙΙ. J. Exp. Clin. Cancer Res. 40 (1), 190–01995. 10.1186/s13046-021-01995-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro R. C. V., Wang H., Duncan S. E., O'Keefe S. F. (2020). Flavor compounds in vine tea (Ampelopsis grossedentata) infusions. Food Sci. Nutr. 8 (8), 4505–4511. 10.1002/fsn3.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy M. V., Neuschwander-Tetri B. A. (2020). The metabolic basis of nonalcoholic steatohepatitis. Endocrinol. Diabetes Metab. 3 (4), e00112. 10.1002/edm2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani N., Younossi Z., Lavine J. E., Charlton M., Cusi K., Rinella M., et al. (2018). The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67 (1), 328–357. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- Chang Q., Zhang L., Chen S., Gong M., Liu L., Hou X., et al. (2023). Exogenous melatonin enhances the yield and secondary metabolite contents of Prunella vulgaris by modulating antioxidant system, root architecture and photosynthetic capacity. Plants 12 (5), 1129. 10.3390/plants12051129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J., Huo T. I., Cheng H. Y., Tsai J. C., Liao J. W., Lee M. S., et al. (2014). Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS One 9 (2), e96969. 10.1371/journal.pone.0096969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Liu Y. W., Ker Y. B., Wu Y. Y., Lai E. Y., Chyau C. C., et al. (2007). Chemical characterization and anti-inflammatory effect of polysaccharides fractionated from submerge-cultured Antrodia camphorata mycelia. J. Agric. Food Chem. 55 (13), 5007–5012. 10.1021/jf063484c [DOI] [PubMed] [Google Scholar]
- Chen L., Teng H., Cao H. (2019). Chlorogenic acid and caffeic acid from Sonchus oleraceus Linn synergistically attenuate insulin resistance and modulate glucose uptake in HepG2 cells. Food Chem. Toxicol. 127, 182–187. 10.1016/j.fct.2019.03.038 [DOI] [PubMed] [Google Scholar]
- Chen S. R., Dai Y., Zhao J., Lin L., Wang Y. (2018). A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front. Pharmacol. 9 (104), 104. 10.3389/fphar.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu L., Xia L., Wu N., Wang Y., Li H., et al. (2022). TRPM7 silencing modulates glucose metabolic reprogramming to inhibit the growth of ovarian cancer by enhancing AMPK activation to promote HIF-1α degradation. J. Exp. Clin. Cancer Res. 41 (1), 022–02252. 10.1186/s13046-022-02252-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. H., Peng C. C., Ker Y. B., Chen C. C., Lee A., Chang W. L., et al. (2013). Physicochemical characteristics and anti-inflammatory activities of antrodan, a novel glycoprotein isolated from Antrodia cinnamomea mycelia. Molecules 19 (1), 22–40. 10.3390/molecules19010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho A. R., Park W. Y., Lee H. J., Sim D. Y., Im E., Park J. E., et al. (2021). Antitumor effect of morusin via G1 arrest and antiglycolysis by AMPK activation in hepatocellular cancer. Int. J. Mol. Sci. 22 (19), 10619. 10.3390/ijms221910619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyau C. C., Wang H. F., Zhang W. J., Chen C. C., Huang S. H., Chang C. C., et al. (2020). Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57bl/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int. J. Mol. Sci. 21 (1), 360. 10.3390/ijms21010360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Li C., Sang F., Cao W., Qin Z., Zhang P. (2022). Natural products targeting glycolytic signaling pathways-an updated review on anti-cancer therapy. Front. Pharmacol. 13, 1035882. 10.3389/fphar.2022.1035882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalil D., Iranzadeh S., Kohansal S. (2022). Anticancer potential of cryptotanshinone on breast cancer treatment; A narrative review. Front. Pharmacol. 13 (979634), 979634. 10.3389/fphar.2022.979634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Le A., Gao P. (2009). MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin. Cancer Res. 15 (21), 6479–6483. 10.1158/1078-0432.CCR-09-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Petrillo A., Orrù G., Fais A., Fantini M. C. (2022). Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 36 (1), 266–278. 10.1002/ptr.7309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Ren B., Zhao W., Li D., Chen X., Zheng Q. (2019). Effect of Neochamaejasmin A on glycolysis of human hepatocellular carcinoma Hep G2 cells. J. Shihezi Univ. Sci. 37 (03), 377–381. (in Chinese). 10.13880/j.cnki.65-1174/n.2019.03.016 [DOI] [Google Scholar]
- Dong G., Mao Q., Xia W., Xu Y., Wang J., Xu L., et al. (2016). PKM2 and cancer: the function of PKM2 beyond glycolysis. Oncol. Lett. 11(3), 931–1986. 10.3390/nu12040931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Preez R., Wanyonyi S., Mouatt P., Panchal S. K., Brown L. (2020). saskatoon berry amelanchier alnifolia regulates glucose metabolism and improves cardiovascular and liver signs of diet-induced metabolic syndrome in rats. Nutrients 12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T., Li P. C., Konkimalla V. S., Kaina B. (2007). From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 13 (8), 353–361. 10.1016/j.molmed.2007.07.001 [DOI] [PubMed] [Google Scholar]
- Eslam M., Sanyal A. J., George J., and International Consensus Panel (2020). Mafld: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 158 (7), 1999–2014.e1. 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- Fa K. N., Yang C. M., Chen P. C., Lee Y. Y., Chyau C. C., Hu M. L. (2015). Anti-metastatic effects of antrodan, the Antrodia cinnamomea mycelia glycoprotein, in lung carcinoma cells. Int. J. Biol. Macromol. 74, 476–482. 10.1016/j.ijbiomac.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Fang Y., Shen Z. Y., Zhan Y. Z., Feng X. C., Chen K. L., Li Y. S., et al. (2019). CD36 inhibits β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat. Commun. 10 (1), 3981–11662. 10.1038/s41467-019-11662-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H., He Y., La L., Hou C., Song L., Yang Q., et al. (2020). The flavonoid-enriched extract from the root of Smilax China L. inhibits inflammatory responses via the TLR-4-mediated signaling pathway. J. Ethnopharmacol. 256 (112785), 112785. 10.1016/j.jep.2020.112785 [DOI] [PubMed] [Google Scholar]
- Feng J., Wu L., Ji J., Chen K., Yu Q., Zhang J., et al. (2019). PKM2 is the target of proanthocyanidin B2 during the inhibition of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 38 (1), 204–1194. 10.1186/s13046-019-1194-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Xiong Y., Qiao T., Li X., Jia L., Han Y. (2018). Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 7 (12), 6124–6136. 10.1002/cam4.1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki J., Sayama N., Shiotani S., Suzuki T., Nonaka M., Uezono Y., et al. (2022). The steroidal alkaloid tomatidine and tomatidine-rich tomato leaf extract suppress the human gastric cancer-derived 85As2 cells in vitro and in vivo via modulation of interferon-stimulated genes. Nutrients 14 (5), 1023. 10.3390/nu14051023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggini M., Carli F., Rosso C., Buzzigoli E., Marietti M., Della Latta V., et al. (2018). Altered amino acid concentrations in NAFLD: impact of obesity and insulin resistance. Hepatology 67 (1), 145–158. 10.1002/hep.29465 [DOI] [PubMed] [Google Scholar]
- Gao W., Sun J., Wang F., Lu Y., Wen C., Bian Q., et al. (2019). Deoxyelephantopin suppresses hepatic stellate cells activation associated with inhibition of aerobic glycolysis via hedgehog pathway. Biochem. Biophys. Res. Commun. 516 (4), 1222–1228. 10.1016/j.bbrc.2019.07.015 [DOI] [PubMed] [Google Scholar]
- Ginwala R., Bhavsar R., Chigbu D. I., Jain P., Khan Z. K. (2019). Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 8 (2), 35. 10.3390/antiox8020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girisa S., Shabnam B., Monisha J., Fan L., Halim C. E., Arfuso F., et al. (2019). Potential of zerumbone as an anti-cancer agent. Molecules 24 (4), 734. 10.3390/molecules24040734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y., Jeong J. Y., Jeoung N. H., Jeon J. H., Park B. Y., Kang H. J., et al. (2016). Inhibition of pyruvate dehydrogenase kinase 2 protects against hepatic steatosis through modulation of tricarboxylic acid cycle anaplerosis and ketogenesis. Diabetes 65 (10), 2876–2887. 10.2337/db16-0223 [DOI] [PubMed] [Google Scholar]
- González A., Hall M. N., Lin S. C., Hardie D. G. (2020). AMPK and TOR: the yin and yang of cellular nutrient sensing and growth control. Cell. Metab. 31 (3), 472–492. 10.1016/j.cmet.2020.01.015 [DOI] [PubMed] [Google Scholar]
- Goronzy J. J., Fang F., Cavanagh M. M., Qi Q., Weyand C. M. (2015). Naive T cell maintenance and function in human aging. J. Immunol. 194 (9), 4073–4080. 10.4049/jimmunol.1500046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujjala S., Putakala M., Nukala S., Bangeppagari M., Rajendran R., Desireddy S. (2017). Modulatory effects of Caralluma fimbriata extract against high-fat diet induced abnormalities in carbohydrate metabolism in Wistar rats. Biomed. Pharmacother. 92, 1062–1072. 10.1016/j.biopha.2017.06.016 [DOI] [PubMed] [Google Scholar]
- Guo S., Shi L., Zhang S., Liu J., Wei J., Zhao X. (2021). Baicalein inhibiting the energy metabolism of hepatoma cells by regulating glycolysis and glutamine metabolism. China Pharm. 24 (12), 2154–2159. (in Chinese). 10.19962/j.cnki.issn1008-049X.2021.12.003 [DOI] [Google Scholar]
- Guo X., Yin X., Liu Z., Wang J. (2022a). Non-alcoholic fatty liver disease (NAFLD) pathogenesis and natural products for prevention and treatment. Int. J. Mol. Sci. 23 (24), 15489. 10.3390/ijms232415489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Zheng B., Wang J., Zhao T., Zheng Y. (2022b). Exploring the mechanism of action of Chinese medicine in regulating liver fibrosis based on the alteration of glucose metabolic pathways. Phytother. Res. 25 (10). 10.1002/ptr.7667 [DOI] [PubMed] [Google Scholar]
- Guo Y. P., Shao L., Chen M. Y., Qiao R. F., Zhang W., Yuan J. B., et al. (2020). In vivo metabolic profiles of panax notoginseng saponins mediated by gut microbiota in rats. J. Agric. Food Chem. 68 (25), 6835–6844. 10.1021/acs.jafc.0c01857 [DOI] [PubMed] [Google Scholar]
- Gupta A., Atanasov A. G., Li Y., Kumar N., Bishayee A. (2022). Chlorogenic acid for cancer prevention and therapy: current status on efficacy and mechanisms of action. Pharmacol. Res. 186 (106505), 106505. 10.1016/j.phrs.2022.106505 [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. (2012). The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life 64 (1), 1–9. 10.1002/iub.573 [DOI] [PubMed] [Google Scholar]
- Harikrishnan H., Jantan I., Alagan A., Haque M. A. (2020). Modulation of cell signaling pathways by Phyllanthus amarus and its major constituents: potential role in the prevention and treatment of inflammation and cancer. Inflammopharmacology 28 (1), 1–18. 10.1007/s10787-019-00671-9 [DOI] [PubMed] [Google Scholar]
- He J., Wang Y., Duan F., Jiang H., Chen M. F., Tang S. Y. (2010). Icaritin induces apoptosis of HepG2 cells via the JNK1 signaling pathway independent of the estrogen receptor. Planta Med. 76 (16), 1834–1839. 10.1055/s-0030-1250042 [DOI] [PubMed] [Google Scholar]
- He S., Wang S., Liu S., Li Z., Liu X., Wu J. (2021). Baicalein potentiated M1 macrophage polarization in cancer through targeting PI3Kγ/NF-κB signaling. Front. Pharmacol. 12 (743837). 10.3389/fphar.2021.743837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Lin Y., Deng Y., Jiang L. (2022). Effects of alpha-tomatine on proliferation, migration and glycolytic activity of human hepatocellular carcinoma cells HuH-7. Chin. Tradit. Pat. Med. 44 (06), 2005–2010. (in Chinese). 10.3969/j.issn.1001-1528.2022.06.051 [DOI] [Google Scholar]
- Herzig S., Shaw R. J. (2018). Ampk: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 19 (2), 121–135. 10.1038/nrm.2017.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyens L. J. M., Busschots D., Koek G. H., Robaeys G., Francque S. (2021). Liver fibrosis in non-alcoholic fatty liver disease: from liver biopsy to non-invasive biomarkers in diagnosis and treatment. Front. Med. 8 (615978), 615978. 10.3389/fmed.2021.615978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z., Lu Y., Liu B., Ran C., Lei X., Wang M., et al. (2023). Glycolysis, a new mechanism of oleuropein against liver tumor. Phytomedicine 114 (154770), 154770. 10.1016/j.phymed.2023.154770 [DOI] [PubMed] [Google Scholar]
- Hsiao W. L., Liu L. (2010). The role of traditional Chinese herbal medicines in cancer therapy-from TCM theory to mechanistic insights. Planta Med. 76 (11), 1118–1131. 10.1055/s-0030-1250186 [DOI] [PubMed] [Google Scholar]
- Hu H. C., Zheng L. T., Yin H. Y., Tao Y., Luo X. Q., Wei K. S., et al. (2019). A significant association between rhein and diabetic nephropathy in animals: A systematic review and meta-analysis. Front. Pharmacol. 10, 1473. 10.3389/fphar.2019.01473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Li Y. Q., Zhou M. Y., Hu R. H., Zou G. L., Li J. C., et al. (2022). Focal adhesion kinase-related non-kinase ameliorates liver fibrosis by inhibiting aerobic glycolysis via the FAK/Ras/c-myc/ENO1 pathway. World J. Gastroenterol. 28 (1), 123–139. 10.3748/wjg.v28.i1.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata Y., Oh J. W., Taniguchi K., Sugito N., Kawaguchi N., Hirokawa F., et al. (2022). Downregulation of miR-122-5p activates glycolysis via PKM2 in kupffer cells of rat and mouse models of non-alcoholic steatohepatitis. Int. J. Mol. Sci. 23 (9), 5230. 10.3390/ijms23095230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglanian A., Tsiani E. (2020). Rosemary extract inhibits proliferation, survival, akt, and mTOR signaling in triple-negative breast cancer cells. Int. J. Mol. Sci. 21 (3), 810. 10.3390/ijms21030810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Huang J. B., Xu C. Y., Lv Y. L., Lu J., Zhao Z. Q., et al. (2022). Arsenic trioxide cooperate cryptotanshinone exerts antitumor effect by medicating macrophage polarization through glycolysis. J. Immunol. Res. 8 (2619781), 2619781. 10.1155/2022/2619781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xiang C., Zhong F., Zhang Y., Wang L., Zhao Y., et al. (2021). Histone H3K27 methyltransferase EZH2 and demethylase JMJD3 regulate hepatic stellate cells activation and liver fibrosis. Theranostics 11 (1), 361–378. 10.7150/thno.46360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Chang X. C., Wen J., Yang J., Ao N., Zhang K. Y., et al. (2021). Decarboxylated osteocalcin, a possible drug for type 2 diabetes, triggers glucose uptake in MG63 cells. World J. Diabetes 12 (7), 1102–1115. 10.4239/wjd.v12.i7.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Goto T., Hirotsu Y., Masuzaki R., Moriyama M., Omata M. (2020). Molecular mechanisms: connections between nonalcoholic fatty liver disease, steatohepatitis and hepatocellular carcinoma. Int. J. Mol. Sci. 21 (4), 1525. 10.3390/ijms21041525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap P., Shikha D., Thakur M., Aneja A. (2022). Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: A review. J. Food Biochem. 46 (4), e13950. 10.1111/jfbc.13950 [DOI] [PubMed] [Google Scholar]
- Ker Y. B., Peng C. C., Chang W. L., Chyau C. C., Peng R. Y. (2014). Hepatoprotective bioactivity of the glycoprotein, antrodan, isolated from Antrodia cinnamomea mycelia. PLoS One 9 (4), e93191. 10.1371/journal.pone.0093191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenol W. H., Bounds P. L., Dang C. V. (2011). Otto Warburg's contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11 (5), 325–337. 10.1038/nrc3038 [DOI] [PubMed] [Google Scholar]
- Kors L., Rampanelli E., Stokman G., Butter L. M., Held N. M., Claessen N., et al. (2018). Deletion of NLRX1 increases fatty acid metabolism and prevents diet-induced hepatic steatosis and metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis 5 (10), 1883–1895. 10.1016/j.bbadis.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Lachowicz S., Oszmiański J., Pluta S. (2017). The composition of bioactive compounds and antioxidant activity of Saskatoon berry (Amelanchier alnifolia Nutt) genotypes grown in central Poland. Food Chem. 235, 234–243. 10.1016/j.foodchem.2017.05.050 [DOI] [PubMed] [Google Scholar]
- Lee M. J., Kao S. H., Hunag J. E., Sheu G. T., Yeh C. W., Hseu Y. C., et al. (2014). Shikonin time-dependently induced necrosis or apoptosis in gastric cancer cells via generation of reactive oxygen species. Chem. Biol. Interact. 211, 44–53. 10.1016/j.cbi.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Lee N. C. W., Carella M. A., Papa S., Bubici C. (2018). High expression of glycolytic genes in cirrhosis correlates with the risk of developing liver cancer. Front. Cell. Dev. Biol. 6 (138), 138. 10.3389/fcell.2018.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., Chandel N. S., Simon M. C. (2020). Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell. Biol. 21 (5), 268–283. 10.1038/s41580-020-0227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Han P., Chen Y., Wang H., Wang S., Wang M., et al. (2022). Protein arginine methyltransferase 3 promotes glycolysis and hepatocellular carcinoma growth by enhancing arginine methylation of lactate dehydrogenase A. Clin. Transl. Med. 12 (1), 686. 10.1002/ctm2.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang T., Xia J., Yao W., Huang F. (2019). Enzymatic and nonenzymatic protein acetylations control glycolysis process in liver diseases. Faseb J. 33 (11), 11640–11654. 10.1096/fj.201901175R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhong W., Wang W., Hu S., Yuan J., Zhang B., et al. (2014). Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-κB activation. PLoS One 9 (2), e87810. 10.1371/journal.pone.0087810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Dai W., Mo W., Li J., Feng J., Wu L., et al. (2017a). By inhibiting PFKFB3, aspirin overcomes sorafenib resistance in hepatocellular carcinoma. Int. J. Cancer 141 (12), 2571–2584. 10.1002/ijc.31022 [DOI] [PubMed] [Google Scholar]
- Li S., Li J., Dai W., Zhang Q., Feng J., Wu L., et al. (2017b). Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br. J. Cancer 117 (10), 1518–1528. 10.1038/bjc.2017.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. E., Wang S., Shen X. T., Zhang Z., Chen M., Wang H., et al. (2020). PKM2 drives hepatocellular carcinoma progression by inducing immunosuppressive microenvironment. Front. Immunol. 11 (589997), 589997. 10.3389/fimmu.2020.589997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Jin M., Song S., Huang G. (2020). Triptolide inhibits human hepatocarcinoma SMMC-7721 cells by regulating glycolysis. World J. Integr. Traditional West. Med. 15 (06), 981–985+990. (in Chinese). 10.13935/j.cnki.sjzx.200601 [DOI] [Google Scholar]
- Li W., Hao J., Zhang L., Cheng Z., Deng X., Shu G. (2017c). Astragalin reduces hexokinase 2 through increasing miR-125b to inhibit the proliferation of hepatocellular carcinoma cells in vitro and in vivo . J. Agric. Food Chem. 65 (29), 5961–5972. 10.1021/acs.jafc.7b02120 [DOI] [PubMed] [Google Scholar]
- Li W., Qiu Y., Hao J., Zhao C., Deng X., Shu G. (2018). Dauricine upregulates the chemosensitivity of hepatocellular carcinoma cells: role of repressing glycolysis via miR-199a:HK2/PKM2 modulation. Food Chem. Toxicol. 121, 156–165. 10.1016/j.fct.2018.08.030 [DOI] [PubMed] [Google Scholar]
- Lian N., Jin H., Zhang F., Wu L., Shao J., Lu Y., et al. (2016). Curcumin inhibits aerobic glycolysis in hepatic stellate cells associated with activation of adenosine monophosphate-activated protein kinase. IUBMB Life 68 (7), 589–596. 10.1002/iub.1518 [DOI] [PubMed] [Google Scholar]
- Liu D., Jin X., Yu G., Wang M., Liu L., Zhang W., et al. (2021). Oleanolic acid blocks the purine salvage pathway for cancer therapy by inactivating SOD1 and stimulating lysosomal proteolysis. Mol. Ther. Oncolytics 23, 107–123. 10.1016/j.omto.2021.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Jiang S., Zhao Y., Sun Q., Zhang J., Shen D., et al. (2018). Geranylgeranyl diphosphate synthase (GGPPS) regulates non-alcoholic fatty liver disease (NAFLD)-fibrosis progression by determining hepatic glucose/fatty acid preference under high-fat diet conditions. J. Pathol. 246 (3), 277–288. 10.1002/path.5131 [DOI] [PubMed] [Google Scholar]
- Liu L., Wang D., Qin Y., Xu M., Zhou L., Xu W., et al. (2019). Astragalin promotes osteoblastic differentiation in mc3t3-E1 cells and bone formation in vivo . Front. Endocrinol. 10 (228), 228. 10.3389/fendo.2019.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Li S., Wu L., Yu Q., Li J., Feng J., et al. (2020). Experimental study of hepatocellular carcinoma treatment by Shikonin through regulating PKM2. J. Hepatocell. Carcinoma 7, 19–31. 10.2147/JHC.S237614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W. K., Cheung F. W., Liu B. P., Li C., Ye W., Che C. T. (2008). Involvement of p21 and FasL in induction of cell cycle arrest and apoptosis by neochamaejasmin A in human prostate LNCaP cancer cells. J. Nat. Prod. 71 (5), 842–846. 10.1021/np8001223 [DOI] [PubMed] [Google Scholar]
- Man S., Fan W., Gao W., Li Y., Wang Y., Liu Z., et al. (2014a). Anti-fibrosis and anti-cirrhosis effects of Rhizoma paridis saponins on diethylnitrosamine induced rats. J. Ethnopharmacol. 151 (1), 407–412. 10.1016/j.jep.2013.10.051 [DOI] [PubMed] [Google Scholar]
- Man S., Fan W., Liu Z., Gao W., Li Y., Zhang L., et al. (2014b). Antitumor pathway of Rhizoma Paridis Saponins based on the metabolic regulatory network alterations in H22 hepatocarcinoma mice. Steroids 84, 17–21. 10.1016/j.steroids.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Man S., Yao J., Lv P., Liu Y., Yang L., Ma L. (2020). Curcumin-enhanced antitumor effects of sorafenib via regulating the metabolism and tumor microenvironment. Food Funct. 11 (7), 6422–6432. 10.1039/c9fo01901d [DOI] [PubMed] [Google Scholar]
- Mehmood T., Maryam A., Zhang H., Li Y., Khan M., Ma T. (2017). Deoxyelephantopin induces apoptosis in HepG2 cells via oxidative stress, NF-κB inhibition and mitochondrial dysfunction. Biofactors 43 (1), 63–72. 10.1002/biof.1324 [DOI] [PubMed] [Google Scholar]
- Mendoza A., Fang V., Chen C., Serasinghe M., Verma A., Muller J., et al. (2017). Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature 546 (7656), 158–161. 10.1038/nature22352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S., Vander Heiden M. G., McCormick F. (2021). The metabolic landscape of RAS-driven cancers from biology to therapy. Nat. Cancer 2 (3), 271–283. 10.1038/s43018-021-00184-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy H. N., Dandin V. S., Park S. Y., Paek K. Y. (2018). Quality, safety and efficacy profiling of ginseng adventitious roots produced in vitro . Appl. Microbiol. Biotechnol. 102 (17), 7309–7317. 10.1007/s00253-018-9188-x [DOI] [PubMed] [Google Scholar]
- Nati M., Haddad D., Birkenfeld A. L., Koch C. A., Chavakis T., Chatzigeorgiou A. (2016). The role of immune cells in metabolism-related liver inflammation and development of non-alcoholic steatohepatitis (NASH). Rev. Endocr. Metab. Disord. 17 (1), 29–39. 10.1007/s11154-016-9339-2 [DOI] [PubMed] [Google Scholar]
- Nile S. H., Park S. W. (2014). Edible berries: bioactive components and their effect on human health. Nutrition 30 (2), 134–144. 10.1016/j.nut.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Nishikawa T., Bellance N., Damm A., Bing H., Zhu Z., Handa K., et al. (2014). A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease. J. Hepatol. 60 (6), 1203–1211. 10.1016/j.jhep.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu N., Ye J., Hu Z., Zhang J., Wang Y. (2023). Regulative roles of metabolic plasticity caused by mitochondrial oxidative phosphorylation and glycolysis on the initiation and progression of tumorigenesis. Int. J. Mol. Sci. 24 (8), 7076. 10.3390/ijms24087076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X., Wang X., Niu B., Meng Y., He H., Wang Y., et al. (2021). Costunolide loaded in pH-responsive mesoporous silica nanoparticles for increased stability and an enhanced anti-fibrotic effect. Pharmaceuticals 14 (10), 951. 10.3390/ph14100951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen P. L. (2008). Voltage dependent anion channels (VDACs): A brief introduction with a focus on the outer mitochondrial compartment's roles together with hexokinase-2 in the "Warburg effect" in cancer. J. Bioenerg. Biomembr. 40 (3), 123–126. 10.1007/s10863-008-9165-7 [DOI] [PubMed] [Google Scholar]
- Perrin-Cocon L., Aublin-Gex A., Diaz O., Ramière C., Peri F., André P., et al. (2018). Toll-like receptor 4-induced glycolytic burst in human monocyte-derived dendritic cells results from p38-dependent stabilization of HIF-1α and increased hexokinase II expression. J. Immunol. 201 (5), 1510–1521. 10.4049/jimmunol.1701522 [DOI] [PubMed] [Google Scholar]
- Petersen M. C., Vatner D. F., Shulman G. I. (2017). Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13 (10), 572–587. 10.1038/nrendo.2017.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusec C. M., De Jesus A., Khan M. W., Terry A. R., Ludvik A. E., Xu K., et al. (2019). Hepatic HKDC1 expression contributes to liver metabolism. Endocrinology 160 (2), 313–330. 10.1210/en.2018-00887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyla R., Poulose N., Jun J. Y., Segar L. (2013). Expression of conventional and novel glucose transporters, GLUT1, -9, -10, and -12, in vascular smooth muscle cells. Am. J. Physiol. Cell. Physiol. 304 (6), C574–C589. 10.1152/ajpcell.00275.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S. K., Li Q., Ming Xu J., Liang J., Cheng Y., Fan Y., et al. (2020). Icaritin-induced immunomodulatory efficacy in advanced hepatitis B virus-related hepatocellular carcinoma: immunodynamic biomarkers and overall survival. Cancer Sci. 111 (11), 4218–4231. 10.1111/cas.14641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu P., Man S., Yang H., Fan W., Yu P., Gao W. (2016). Utilization of metabonomics to identify serum biomarkers in murine H22 hepatocarcinoma and deduce antitumor mechanism of Rhizoma Paridis saponins. Chem. Biol. Interact. 256, 55–63. 10.1016/j.cbi.2016.06.026 [DOI] [PubMed] [Google Scholar]
- Rao J., Wang H., Ni M., Wang Z., Wei S., Liu M., et al. (2022). FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut 71 (12), 2539–2550. 10.1136/gutjnl-2021-325150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reckzeh E. S., Karageorgis G., Schwalfenberg M., Ceballos J., Nowacki J., Stroet M. C. M., et al. (2019). Inhibition of glucose transporters and glutaminase synergistically impairs tumor cell growth. Cell. Chem. Biol. 26 (9), 1214–1228. 10.1016/j.chembiol.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Ren Z., Liang H., Galbo P. M., Jr., Dharmaratne M., Kulkarni A. S., Fard A. T., et al. (2022). Redox signaling by glutathione peroxidase 2 links vascular modulation to metabolic plasticity of breast cancer. Proc. Natl. Acad. Sci. U. S. A. 119 (8), 2107266119. 10.1073/pnas.2107266119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Z., Liang M., Shang L., Lai M., Deng X., Su X. (2021). Shikonin-mediated PD-L1 degradation suppresses immune evasion in pancreatic cancer by inhibiting NF-κB/STAT3 and NF-κB/CSN5 signaling pathways. Pancreatology 21 (3), 630–641. 10.1016/j.pan.2021.01.023 [DOI] [PubMed] [Google Scholar]
- Ruzzolini J., Peppicelli S., Bianchini F., Andreucci E., Urciuoli S., Romani A., et al. (2020). Cancer glycolytic dependence as a new target of olive leaf extract. Cancers 12 (2), 317. 10.3390/cancers12020317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi-Boroujeni A., Mahmoudian-Sani M. R. (2021). Anti-inflammatory potential of Quercetin in COVID-19 treatment. J. Inflamm. 18 (1), 3–00268. 10.1186/s12950-021-00268-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswati S., Alhaider A. A., Abdelgadir A. M. (2018). Costunolide suppresses an inflammatory angiogenic response in a subcutaneous murine sponge model. Apmis 126 (3), 257–266. 10.1111/apm.12808 [DOI] [PubMed] [Google Scholar]
- Satyanarayana G., Turaga R. C., Sharma M., Wang S., Mishra F., Peng G., et al. (2021). Pyruvate kinase M2 regulates fibrosis development and progression by controlling glycine auxotrophy in myofibroblasts. Theranostics 11 (19), 9331–9341. 10.7150/thno.60385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D., Ashfaq-Khan M., Yang A. T., Kim Y. O. (2018). Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol. 69, 435–451. 10.1016/j.matbio.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Shan L., Liu Z., Ci L., Shuai C., Lv X., Li J. (2019). Research progress on the anti-hepatic fibrosis action and mechanism of natural products. Int. Immunopharmacol. 75 (105765), 105765. 10.1016/j.intimp.2019.105765 [DOI] [PubMed] [Google Scholar]
- Sharma D., Singh M., Rani R. (2022). Role of LDH in tumor glycolysis: regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 87, 184–195. 10.1016/j.semcancer.2022.11.007 [DOI] [PubMed] [Google Scholar]
- Sheng Y., Chen Y., Zeng Z., Wu W., Wang J., Ma Y., et al. (2022). Identification of pyruvate carboxylase as the cellular target of natural bibenzyls with potent anticancer activity against hepatocellular carcinoma via metabolic reprogramming. J. Med. Chem. 65 (1), 460–484. 10.1021/acs.jmedchem.1c01605 [DOI] [PubMed] [Google Scholar]
- Shikov A. N., Shikova V. A., Whaley A. O., Burakova M. A., Flisyuk E. V., Whaley A. K., et al. (2022). The ability of acid-based natural deep eutectic solvents to Co-extract elements from the roots of Glycyrrhiza glabra L. And associated health risks. Molecules 27 (22), 7690. 10.3390/molecules27227690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Crother T. R., Karlin J., Dagvadorj J., Chiba N., Chen S., et al. (2012). Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36 (3), 401–414. 10.1016/j.immuni.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Castillo G. M., Nguyen B. P., Choi P. Y., Cummings J. A., Cam J., et al. (2019). The Amazon rain forest plant Uncaria tomentosa (cat's claw) and its specific proanthocyanidin constituents are potent inhibitors and reducers of both brain plaques and tangles. Sci. Rep. 9 (1), 561–38645. 10.1038/s41598-019-38645-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Fang X., Bai M., Li Y., Miao M. (2020). Total flavones from Prunus subtilis induced apoptosis of SMMC-7721 cells by inhibiting oxidative phosphorylation and glycolysis. Pharmacol. Clin. Chin. Materia Medica 36 (06), 109–113. (in Chinese). 10.13412/j.cnki.zyyl.2020.06.015 [DOI] [Google Scholar]
- Song Y. G., Kang L., Tian S., Cui L. L., Li Y., Bai M., et al. (2021). Study on the anti-hepatocarcinoma effect and molecular mechanism of Prunella vulgaris total flavonoids. J. Ethnopharmacol. 273 (113891), 113891. 10.1016/j.jep.2021.113891 [DOI] [PubMed] [Google Scholar]
- Soni V. K., Shukla D., Kumar A., Vishvakarma N. K. (2020). Curcumin circumvent lactate-induced chemoresistance in hepatic cancer cells through modulation of hydroxycarboxylic acid receptor-1. Int. J. Biochem. Cell. Biol. 123 (105752), 105752. 10.1016/j.biocel.2020.105752 [DOI] [PubMed] [Google Scholar]
- Su J., Zhang S., Zhao J., Chen J., Zhang X. (2021). Studies on the mechanism of Ginsenoside CK regulating HIF-1-mediated glycolysis inhibiting proliferation of human hepatocellular carcinoma cells. Lishizhen Med. Materia Medica Res. 32 (07), 1623–1626. (in Chinese). [Google Scholar]
- Sun Y., Li G., Zhou Q., Shao D., Lv J., Zhou J. (2020). Dual targeting of cell growth and phagocytosis by erianin for human colorectal cancer. Drug Des. Devel Ther. 14, 3301–3313. 10.2147/DDDT.S259006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F., Zimmermann H. W. (2014). Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 60 (5), 1090–1096. 10.1016/j.jhep.2013.12.025 [DOI] [PubMed] [Google Scholar]
- Tagde P., Islam F., Tagde S., Shah M., Hussain Z. D., Rahman M. H., et al. (2021). The multifaceted role of curcumin in advanced nanocurcumin form in the treatment and management of chronic disorders. Molecules 26 (23), 7109. 10.3390/molecules26237109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan V. P., Miyamoto S. (2015). HK2/hexokinase-II integrates glycolysis and autophagy to confer cellular protection. Autophagy 11 (6), 963–964. 10.1080/15548627.2015.1042195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Kishimoto Y., Sasaki M., Sato A., Kamiya T., Kondo K., et al. (2018). Terminalia bellirica (gaertn) roxb. Extract and gallic acid attenuate LPS-induced inflammation and oxidative stress via MAPK/NF-κB and akt/AMPK/Nrf2 pathways. Oxid. Med. Cell. Longev. 8 (9364364), 9364364. 10.1155/2018/9364364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Ji F., Guo J., Wang J., Li Y., Bao Y. (2016). Directional modification of chrysin for exerting apoptosis and enhancing significantly anti-cancer effects of 10-hydroxy camptothecin. Biomed. Pharmacother. 82, 693–703. 10.1016/j.biopha.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Tao Z., Wu Q., Lan X., Gao M., Wei W., Xie X., et al. (2022). Effect of physcion on proliferation and glycolysis of liver cancer cells and its mechanism. Chin. Clin. Oncol. 27 (09), 769–775. (in Chinese). 10.3969/j.issn.1009-0460.2022.09.001 [DOI] [Google Scholar]
- Tarasenko T. N., Pacheco S. E., Koenig M. K., Gomez-Rodriguez J., Kapnick S. M., Diaz F., et al. (2017). Cytochrome c oxidase activity is a metabolic checkpoint that regulates cell fate decisions during T cell activation and differentiation. Cell. Metab. 25 (6), 1254–1268. 10.1016/j.cmet.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Wang R., Gu T., Ma F., Laster K. V., Li X., et al. (2022). Costunolide is a dual inhibitor of MEK1 and AKT1/2 that overcomes osimertinib resistance in lung cancer. Mol. Cancer 21 (1), 193. 10.1186/s12943-022-01662-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todisco S., Di Noia M. A., Onofrio A., Parisi G., Punzi G., Redavid G., et al. (2016). Identification of new highly selective inhibitors of the human ADP/ATP carriers by molecular docking and in vitro transport assays. Biochem. Pharmacol. 100, 112–132. 10.1016/j.bcp.2015.11.019 [DOI] [PubMed] [Google Scholar]
- Tu Z., Moss-Pierce T., Ford P., Jiang T. A. (2013). Rosemary (Rosmarinus officinalis L) extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in HepG2 cells. J. Agric. Food Chem. 61 (11), 2803–2810. 10.1021/jf400298c [DOI] [PubMed] [Google Scholar]
- Urbańska K., Orzechowski A. (2019). Unappreciated role of LDHA and LDHB to control apoptosis and autophagy in tumor cells. Int. J. Mol. Sci. 20 (9), 2085. 10.3390/ijms20092085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée A., Lecarpentier Y., Guillevin R., Vallée J. N. (2017). Aerobic glycolysis hypothesis through WNT/Beta-Catenin pathway in exudative age-related macular degeneration. J. Mol. Neurosci. 62 (3-4), 368–379. 10.1007/s12031-017-0947-4 [DOI] [PubMed] [Google Scholar]
- Vilar-Gomez E., Calzadilla-Bertot L., Wai-Sun Wong V., Castellanos M., Aller-de la Fuente R., Metwally M., et al. (2018). Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: A multi-national cohort study. Gastroenterology 155 (2), 443–457. 10.1053/j.gastro.2018.04.034 [DOI] [PubMed] [Google Scholar]
- Wan L., Xia T., Du Y., Liu J., Xie Y., Zhang Y., et al. (2019). Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: A role for exosomes in metabolic switch of liver nonparenchymal cells. Faseb J. 33 (7), 8530–8542. 10.1096/fj.201802675R [DOI] [PubMed] [Google Scholar]
- Wan W., Jiang B., Sun L., Xu L., Xiao P. (2017). Metabolomics reveals that vine tea (Ampelopsis grossedentata) prevents high-fat-diet-induced metabolism disorder by improving glucose homeostasis in rats. PLoS One 12 (8), e0182830. 10.1371/journal.pone.0182830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhou Q., Wu S. T., Li G., Wu Y., Wang Z., et al. (2022a). Scopolin obtained from Smilax China L. against hepatocellular carcinoma by inhibiting glycolysis: A network pharmacology and experimental study. J. Ethnopharmacol. 296 (115469), 16–23. 10.1016/j.biologicals.2022.05.003 [DOI] [PubMed] [Google Scholar]
- Wang J., Liu X., Zheng H., Liu Q., Zhang H., Wang X., et al. (2020). Morusin induces apoptosis and autophagy via JNK, ERK and PI3K/Akt signaling in human lung carcinoma cells. Chem. Biol. Interact. 331, 7, 109279. 10.1016/j.cbi.2020.109279 [DOI] [PubMed] [Google Scholar]
- Wang M., Ma X., Wang G., Song Y., Zhang M., Mai Z., et al. (2022b). Targeting UBR5 in hepatocellular carcinoma cells and precise treatment via echinacoside nanodelivery. Cell. Mol. Biol. Lett. 27 (1), 92–00394. 10.1186/s11658-022-00394-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. W., Su Y., Sheng J. T., Gu L. M., Zhao Y., Chen X. X., et al. (2018). Anti-influenza A virus activity of rhein through regulating oxidative stress, TLR4, Akt, MAPK, and NF-κB signal pathways. PLoS One 13 (1), e0191793. 10.1371/journal.pone.0191793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Gan Y., Kan H., Mao X., Wang Y. (2021a). Exploitation of HPLC analytical method for simultaneous determination of six principal unsaturated fatty acids in oviductus ranae based on quantitative analysis of multi-components by single-marker (QAMS). Molecules 26 (2), 479. 10.3390/molecules26020479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. M., Yang P. W., Feng X. J., Zhu Y. W., Qiu F. J., Hu X. D., et al. (2021b). Apigenin inhibits the growth of hepatocellular carcinoma cells by affecting the expression of microRNA transcriptome. Front. Oncol. 11 (657665), 657665. 10.3389/fonc.2021.657665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Chen K., Yao W., Zheng R., He Q., Xia J., et al. (2021c). Acetylation of lactate dehydrogenase B drives NAFLD progression by impairing lactate clearance. J. Hepatol. 74 (5), 1038–1052. 10.1016/j.jhep.2020.11.028 [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen X., Li J., Xia C. (2022c). Quercetin antagonizes esophagus cancer by modulating miR-1-3p/TAGLN2 pathway-dependent growth and metastasis. Nutr. Cancer 74 (5), 1872–1881. 10.1080/01635581.2021.1972125 [DOI] [PubMed] [Google Scholar]
- Wani N. A., Zhang B., Teng K. Y., Barajas J. M., Motiwala T., Hu P., et al. (2018). Reprograming of glucose metabolism by zerumbone suppresses hepatocarcinogenesis. Mol. Cancer Res. 16 (2), 256–268. 10.1158/1541-7786.MCR-17-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasik A. A., Lehtonen S. (2018). Glucose transporters in diabetic kidney disease-friends or foes? Front. Endocrinol. 9 (155), 155. 10.3389/fendo.2018.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wokolorczyk D., Gliniewicz B., Sikorski A., Zlowocka E., Masojc B., Debniak T., et al. (2008). A range of cancers is associated with the rs6983267 marker on chromosome 8. Cancer Res. 68 (23), 9982–9986. 10.1158/0008-5472.CAN-08-1838 [DOI] [PubMed] [Google Scholar]
- Wu H., Pan L., Gao C., Xu H., Li Y., Zhang L., et al. (2019a). Quercetin inhibits the proliferation of glycolysis-addicted HCC cells by reducing hexokinase 2 and akt-mTOR pathway. Molecules 24 (10), 1993. 10.3390/molecules24101993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Zhou S., Wang C., Zhou H., Li F., Chen X., et al. (2023). Effects of deoxydibilin on proliferation, migration and glycolysis of hepatocellular carcinoma HepG2 cells. Chin. Tradit. Pat. Med. 45 (01), 208–212. (in Chinese). [Google Scholar]
- Wu L., Cao K., Ni Z., Wang S., Li W., Liu X., et al. (2019b). Rhein reverses doxorubicin resistance in SMMC-7721 liver cancer cells by inhibiting energy metabolism and inducing mitochondrial permeability transition pore opening. Biofactors 45 (1), 85–96. 10.1002/biof.1462 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Ni S., Wang S., Gan Y., Zhou Y., Guo H., et al. (2019). Environmental influences on quality features of oviductus ranae in the changbai mountains. RSC Adv. 9 (62), 36050–36057. 10.1039/c9ra04823e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Hu M. J., Wang Y. Q., Cui Y. L. (2019). Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 24 (6), 1123. 10.3390/molecules24061123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Jin J., Yu H., Zhao Z., Ma D., Zhang C., et al. (2017). Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J. Exp. Clin. Cancer Res. 36 (1), 44–0514. 10.1186/s13046-017-0514-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Guo M., Huang W., Feng L., Zhu J., Luo K., et al. (2020). Annexin A5 regulates hepatic macrophage polarization via directly targeting PKM2 and ameliorates NASH. Redox Biol. 36 (101634), 101634. 10.1016/j.redox.2020.101634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Ma H., Zha L., Li Q., Pan H., Zhang L. (2022). Genistein promotes apoptosis of lung cancer cells through the IMPDH2/AKT1 pathway. Am. J. Transl. Res. 14 (10), 7040–7051. [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Dou C., Liu X., Yang L., Ni C., Wang J., et al. (2018). Oviductus ranae protein hydrolysate (ORPH) inhibits the growth, metastasis and glycolysis of HCC by targeting miR-491-5p/PKM2 axis. Biomed. Pharmacother. 107, 1692–1704. 10.1016/j.biopha.2018.07.071 [DOI] [PubMed] [Google Scholar]
- Yao J., Man S., Dong H., Yang L., Ma L., Gao W. (2018). Combinatorial treatment of Rhizoma Paridis saponins and sorafenib overcomes the intolerance of sorafenib. J. Steroid Biochem. Mol. Biol. 183, 159–166. 10.1016/j.jsbmb.2018.06.010 [DOI] [PubMed] [Google Scholar]
- Yao Y., Chen L., Xiao J., Wang C., Jiang W., Zhang R., et al. (2014). Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. Int. J. Mol. Sci. 15 (11), 20913–20926. 10.3390/ijms151120913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J. H., Chao J., Chang M. L., Peng W. H., Cheng H. Y., Liao J. W., et al. (2016). Pentoxifylline ameliorates non-alcoholic fatty liver disease in hyperglycaemic and dyslipidaemic mice by upregulating fatty acid β-oxidation. Sci. Rep. 6 (33102), 33102. 10.1038/srep33102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Song Y., Zhuang J., Wang G., Ni J., Xia W. (2019). Anticancer effects of echinacoside in hepatocellular carcinoma mouse model and HepG2 cells. J. Cell. Physiol. 234 (2), 1880–1888. 10.1002/jcp.27063 [DOI] [PubMed] [Google Scholar]
- Yu H. J., Shin J. A., Lee S. O., Kwon K. H., Cho S. D. (2014a). Extracellular signal-regulated kinase inhibition is required for methanol extract of Smilax China L.-induced apoptosis through death receptor 5 in human oral mucoepidermoid carcinoma cells. Mol. Med. Rep. 9 (2), 663–668. 10.3892/mmr.2013.1826 [DOI] [PubMed] [Google Scholar]
- Yu Y., Wang X., Nyberg S. L. (2014b). Application of induced pluripotent stem cells in liver diseases. Cell. Med. 7 (1), 1–13. 10.3727/215517914X680056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal N. S., Gan C. P., Lau B. F., Yee P. S., Tiong K. H., Abdul Rahman Z. A., et al. (2018). Zerumbone targets the CXCR4-RhoA and PI3K-mTOR signaling axis to reduce motility and proliferation of oral cancer cells. Phytomedicine 39, 33–41. 10.1016/j.phymed.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Zeng A., Liang X., Zhu S., Liu C., Wang S., Zhang Q., et al. (2021). Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 45 (2), 717–727. 10.3892/or.2020.7891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Gu C., Song Q., Zhu M., Xu Y., Xiao M., et al. (2020). Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell. Biosci. 10 (1), 127–00488. 10.1186/s13578-020-00488-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Ge Z., Xue Z., Huang W., Mei M., Zhang Q., et al. (2015). Chrysin suppresses human CD14(+) monocyte-derived dendritic cells and ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 288, 13–20. 10.1016/j.jneuroim.2015.08.017 [DOI] [PubMed] [Google Scholar]
- Zhang L., Dong R., Wang Y., Wang L., Zhou T., Jia D., et al. (2021). The anti-breast cancer property of physcion via oxidative stress-mediated mitochondrial apoptosis and immune response. Pharm. Biol. 59 (1), 303–310. 10.1080/13880209.2021.1889002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Chen R., Li W., Cao Y., Zhu J., Zhang J., et al. (2020). Inhibition of PIM1 kinase attenuates bleomycin-induced pulmonary fibrosis in mice by modulating the ZEB1/E-cadherin pathway in alveolar epithelial cells. Asia-Pacific Tradit. Med. 16 (04), 15–22. (in Chinese). 10.1016/j.molimm.2020.06.013 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Du G. J., Wang C. Z., Wen X. D., Calway T., Li Z., et al. (2013). Compound K, a ginsenoside metabolite, inhibits colon cancer growth via multiple pathways including p53-p21 interactions. Int. J. Mol. Sci. 14 (2), 2980–2995. 10.3390/ijms14022980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Zhou B., Bao L., Yang Y., Guo K. (2015). Alpha-tomatine exhibits anti-inflammatory activity in lipopolysaccharide-activated macrophages. Inflammation 38 (5), 1769–1776. 10.1007/s10753-015-0154-9 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chard Dunmall L. S., Cheng Z., Wang Y., Si L. (2022). Natural products targeting glycolysis in cancer. Front. Pharmacol. 13, 1036502. 10.3389/fphar.2022.1036502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Gou Y., Jiang Z., Yang A., Yang Z., Qin S. (2021). Icaritin-induced FAM99A affects GLUT1-mediated glycolysis via regulating the JAK2/STAT3 pathway in hepatocellular carcinoma. Front. Oncol. 11 (740557), 740557. 10.3389/fonc.2021.740557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Wu J., Chen X., Fortenbery N., Eksioglu E., Kodumudi K. N., et al. (2011). Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int. Immunopharmacol. 11 (7), 890–898. 10.1016/j.intimp.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Zhao Y., Pan L. C., Wang J., Shi X. J., Du G. S., et al. (2022). Sirolimus increases the anti-cancer effect of Huai Er by regulating hypoxia inducible factor-1α-mediated glycolysis in hepatocellular carcinoma. World J. Gastroenterol. 28 (32), 4600–4619. 10.3748/wjg.v28.i32.4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M. Y., Cheng M. L., Huang T., Hu R. H., Zou G. L., Li H., et al. (2021). Transforming growth factor beta-1 upregulates glucose transporter 1 and glycolysis through canonical and noncanonical pathways in hepatic stellate cells. World J. Gastroenterol. 27 (40), 6908–6926. 10.3748/wjg.v27.i40.6908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Ruan Z., Zhou L., Shu X., Sun X., Mi S., et al. (2016). Chlorogenic acid ameliorates endotoxin-induced liver injury by promoting mitochondrial oxidative phosphorylation. Biochem. Biophys. Res. Commun. 469 (4), 1083–1089. 10.1016/j.bbrc.2015.12.094 [DOI] [PubMed] [Google Scholar]
- Zhuang S., Zhong J., Zhou Q., Zhong Y., Liu P., Liu Z. (2019). Rhein protects against barrier disruption and inhibits inflammation in intestinal epithelial cells. Int. Immunopharmacol. 71, 321–327. 10.1016/j.intimp.2019.03.030 [DOI] [PubMed] [Google Scholar]
- Zielińska M., Dereń K., Polak-Szczybyło E., Stępień A. E. (2021). The role of bioactive compounds of Nigella sativa in rheumatoid arthritis therapy-current reports. Nutrients 13 (10), 3369. 10.3390/nu13103369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Tang J., Lu M., Zhou Z., Li Y., Tian H., et al. (2021). Glycolysis rate-limiting enzymes: novel potential regulators of rheumatoid arthritis pathogenesis. Front. Immunol. 12 (779787), 779787. 10.3389/fimmu.2021.779787 [DOI] [PMC free article] [PubMed] [Google Scholar]