Abstract
Background
Traditional and natural immunomodulators are increasingly used as supplements in animal feeds and as interventions in the prevention and treatment of disease in animals.
Objective
The aim of this study was to examine the immunomodulatory characteristics of distilled cow urine in vivo using two mouse models, a normal mouse model and an immunosuppressive mouse model.
Methodology
We divided 144 Swiss Albino mice weighing between 15 g and 30 g, aged between two and three months, into two groups of 72 mice each. In the first group, we subdivided the animals into six subgroups of 12 each. In this group paramerters such as, body weight, organ weights of liver and kidney, haemagglutination titre, Jerne plaque-forming assay, and bone marrow cellularity were measured. We divided the second group into six subgroups for the assessment of delayed-type hypersensitivity (DTH).
Results
As compared to normal control mice, immunocompetent and immunosuppressed mice (given cow urine distillate) had significant increases in body weight, spleen weight, liver weight, total leucocyte count, lymphocyte count, serum protein, and globulin contents. In the treatment groups, the titre of antibodies, the number of antibody- producing cells, the cellularity of bone marrow, and foot pad thickness also increased. In the treatment group, both humoral and cellular immunity were altered compared to the control group, suggesting cow urine distillate to be a potential animal feed ingredient for immunoregulation.
Conclusion
This study was able to demonstrate the experimental validity of natural compounds as immunomodulators that can be used in feed supplements for animals. Various compounds could be tested for immunomodulatory effects using this technique in experimental animals.
Keywords: Nutrition, Immunomodulation, Natural feed additive, Cellular immunity, Humoral immunity, Swiss albino mice, Distilled cow urine
1. Introduction
Animal feed supplements containing immunomodulators are becoming increasingly popular [[1], [2], [3]]. It is widely recognized that antibiotics as feed additives are linked to the development of antimicrobial resistance and the emergence of superbugs, which are detrimental to livestock, poultry, and aquaculture. In the livestock industry, acquired resistance to antibiotics plagues the industry, making it imperative to continue exploring alternative options [6]. There has been considerable evidence that immunomodulatory agents positively influence animal immunity and are crucial for the host's innate immune system [7]. A growing body of evidence indicates that immunomodulators protect livestock against invasive pathogens and reduce the need for antibiotics as feed additives [[4], [5], [6], [7]]. There are some countries that restrict the use of antibiotics in feed supplements. To solve this problem, alternative feed supplements derived from natural sources are being studied [8,9]. Animal and plant-derived natural products may be used as immune modulatory feed additives [[10], [11], [12], [13]]. An example would be a study conducted by Park et al., 2011 examining the effect of porcine placental extract on immune modulation in weaned piglets in a mouse model and recommending its use as a feed supplement [14].
Despite the fact that urine is biowaste, it contains a number of bioactive compounds with therapeutic properties [[15], [16], [17], [18], [19], [20]]. In Ayurvedic medicine, urine-derived formulations are prescribed to treat many diseases [13]. In the murine colitis model, human urine-derived stem cells have been shown to have immunomodulatory properties [16]. These cells are used to treat a variety of ailments [17]. Based on physiological pathway analysis, Kumar et al. [18] applied peptide profiling and in-silico analysis to predict the bioactive properties of cow urine. Novel feed supplements have been identified as suitable for use in a range of climates, including those with extreme conditions [13]. Cow urine has been patented in the U.S. for its medicinal properties, including its use to treat bacterial infections and cancer [20,21]. Urine contains several therapeutic compounds that can be used for both human and veterinary medicine [[22], [23], [24]].
In this study, immunosuppressed Swiss albino mice as well as normal Swiss albino mice were tested to see if cow urine could be used as a feed supplement to modulate immune function.
2. Materials and methods
In immunocompetent and immunosuppressed mice, Vechur distillate and crossbred cow urine distillate were administered at a dose rate of 10.8 mlkg-1 body weight [25]. On days 9, 10, and 11, cyclophosphamide was administered intraperitoneally to six animals, then to another six animals on days 16, 17, and 18 [26]. Cyclophosphamide was injected 1 h after giving urine distillate or distilled water (see supplementary material).
2.1. Experimental animals and experimental design
A total of 144 male Swiss Albino mice aged between two and three months weighing between 15 and 30 g were used for this study. We obtained the mice in the small animal breeding station at the College of Veterinary and Animal Sciences, Mannuthy. In the week prior to the experiment, the animals were kept in the shelter and were fed and managed similarly.
A total of 72 mice were used for testing haemagglutination (HA) titre, the Jerne plaque-forming assay, and bone marrow cellularity, while the remaining 72 mice were tested for delayed-type hypersensitivity (DTH). To begin, the experimental set was randomly divided into six groups of 12 each, denoted AI, AII, AIII, AIV, AV, and AVI. In addition, 72 mice were randomly divided into six groups of 12 mice each, identified as BI, BII, BIII, BIV, BV, and BVI. A summary of the experimental protocol is shown in Table 1.
Table 1.
Experimental protocol.
| Group | Treatment |
|---|---|
| A1&BI (normal control) | For 19 days, distilled water alone was administered at a dose of 10.8 ml kg−1 body weight. |
| AII&BII (cyclophosphamide control) | On the 9th, 10th, and 11th day, distilled water was given at a dose rate of 10.8 ml/kg body weight to the first six animals. On the 16th, 17th, and 18th day, cyclophosphamide was given at a dose rate of 30 mg kg−1 body weight to the remaining six animals, 1 h after distilled water administration. |
| AIII&BIII | A dose of 10.8 ml kg−1 body weight was administered orally for 19 days with crossbred cow urine distillate. |
| AIV&BIV | Oral administration of crossbred cow urine distillate at a dose rate of 10.8 ml kg−1 body weight for 19 days and intraperitoneal injection of cyclophosphamide at a dose rate of 30 mg k−1 g body weight was administered during the 9th, 10th, and 11th days for the first six animals and on the 16th, 17th and 18th days for the remaining six animals, 1 h after the urine distillate was administered. |
| AV&BV | Vechur cow urine distillate was administered orally for 19 days at a dose rate of 10.8 ml kg−1 body weight. |
| AVI&BVI | Orally administered Vechur cow urine distillate at a dose rate of 10.8 ml kg−1 body weight for 19 days and intraperitoneally injected cyclophosphamide at a dose rate of 30 mgkg-1 body weight on 9th, 10th, and 11th days for the first six animals and on 16th, 17th, and 18th days for the remaining six animals, 1 h after urine distillate administration. |
A dose of 0.2 ml of sheep RBC (SRBC) antigen containing 1 × 108 cells ml−1 per 100 g of body weight was administered intraperitoneally on day five to all groups (see Table 2). For this study, cow urine distillates were given at a dose of 10.8 ml kg−1 body weight, and distilled water was given as a control. Cyclophosphamide was administered intraperitoneally at 30 mg kg−1 of body weight [26]. On the 12th day, the first six animals in each group were sacrificed. The rest were sacrificed on the 19th day.
Table 2.
Constituents of Vechur and crossbred cow urine distillate.
| SI. No | Component | Vechur urine distillate | Crossbred urine distillate |
|---|---|---|---|
| 1 | Protein | Not Detected | Not Detected |
| 2 | Glucose | Not Detected | Not Detected |
| 3 | Ketone bodies | Not Detected | Not Detected |
| 4 | Bile salt | Not Detected | Not Detected |
| 5 | Urobilinogen | Not Detected | Not Detected |
| 6 | Bilirubin | Not Detected | Not Detected |
| 7 | Urea | Present (71 mg %) | Present (58 mg %) |
| 8 | Uric acid | Present (0.7 mg %) | Present (0.5 mg %) |
| 9 | Creatinine | Present (3 mg %) | Present (1.8 mg %) |
2.2. Evaluation of immunomodulatory status
To evaluate immune modulatory function, a variety of physiological, haematological, biochemical, and immunological parameters were used. In addition to assessing physiological parameters such as body weight and relative organ weight, we assessed haematological parameters such as leukocyte count and differential leukocyte count. Biochemical parameters assessed included serum total protein, serum globulin, albumin-globulin ratio, and haemagglutination test. Utilizing both the Jerne plaque-forming assay and bone marrow cellularity, we evaluated both humoral and cellular immune responses.
2.3. Measurement of physiological parameters
The weight of the individual mouse was recorded before and after the experiment. The weight of the organs like the spleen, liver, and kidney was also recorded at the time of sacrifice.
2.4. Measurement of haematological parameters
Mice from the groups AI-AVI were bled on days 0, 5, 12, and 19 of the experiment by puncturing the retro-orbital plexus with plastic capillaries. Anticoagulants used in this study were heparinized capillary tubes and ethylene diamine tetraacetic acid, and total and differential counts of leukocytes were measured [27].
2.5. Measurement of biochemical parameters
Blood was collected from AI - AVI in a test tube without anticoagulant using plastic capillaries [27]. The blood was centrifuged for 10 min at 2500 rpm, and the serum was separated. The serum total protein content was determined by the Biuret method [28] and the bromocresol green dye method [29]. By subtracting serum albumin from serum total protein, serum globulin is calculated.
2.6. Evaluation of humoral and cell-mediated immune response
2.6.1. Haemagglutination test
Blood samples were collected from mice from groups AI-AVI on day 0, day 12, and day 19 of the experiment, and haemagglutination tests were performed [30]. Two-fold dilutions of sera were prepared in 0.15 M PBS (pH 7.2), and 50 ml from each of the dilutions was transferred to 96-well microtitre plates. To each well, 1 ml of SRBC suspension was added in 99 ml of PBS and thoroughly mixed. After 1 h of incubation at 37 °C, the plates were tested for haemagglutination. As a measure of HA titre, the inverse of the peak dilution of sample serum that agglutinated 50% is used.
2.6.2. Determination of antibody-forming cells (splenic plaque-forming cells)
Following sterile procedures, rabbit blood was collected from the University Rabbit Farm in Mannuthy. The serum was stored at −20 °C after separation. The number of antibody-producing cells was calculated, as described in Ref. [31]. Six mice from each group AI - AVI were overdosed with ether on day 12 and sacrificed. Spleen cells were isolated and suspended in the RPMI-1640 medium (Rosewell Park Memorial Institute-1640, Himedia, Bombay). 1 ml of spleen cells from treated mice (1 × 106 cells in RPMI-1640 medium) and 0.1 mL of fresh 20 percent SRBC suspension in PBS (20 ml of SRBC in 80 ml of PBS) were added to 2ml of agarose (0.6%) taken in tubes kept at 45 °C and mixed well. In a grease-free slide, the contents of the tubes were poured and spread over a 1″ × 2″ area until solidified. Using adhesive tapes, a second slide was placed over the first. The emptiness between the two slides was filled with the complement. Fresh rabbit serum (1:10 diluted in PBS, pH 7.2) was used as a complement source. One hour was spent incubating the slides at 37 °C on an incubation rack. Plaques formed by haemolysis, found to be empty due to hemolysis, were counted and expressed as the number of plaques per million lymphocytes (splenocytes). On day 19, the experiment was repeated in the same pattern in each of the remaining six mice.
2.6.3. Determination of bone marrow cellularity
Cells from the femur were sampled to determine the cellularity of the bone marrow [32]. Both hind legs were dissected, and the femurs were removed. The condyles of the femur were removed using sharp scissors followed by a flush with 10% foetal calf serum (Himedia in Bombay). The blood cells per femur were expressed as a million count haemocytometrically. Six mice from each group were subjected to the same experiment on day 19 of the experiment.
2.7. Delayed-type hypersensitivity (DTH)
In order to prime the animals before the DTH test, SRBC antigen was used [33]. The mice were transiently injected with SRBC antigen subcutaneously on their right hind footpads on day 12 after being primed with SRBC antigen (concentration of 1 × 108 cells per 0.025 ml in PBS) intraperitoneally. The left hind footpad was administered 0.025 ml of saline. We used Vernier calipers to determine the swelling at three different dimensions following 24 h of the challenge. The difference in footpad thickness was measured as a measure of DTH. On the 19th day, the test was repeated on the remaining six mice in each group.
2.8. Statistical analysis
To compare conditions between periods, between treatments, and between period × treatment interactions, we performed repeated measure ANOVA. To compare means between groups, a one-way ANOVA was used, and Duncan's Multiple Range Test was used if the analysis of variance was significant. It was assumed that P < 0.05 was statistically significant. All analyses were performed in R (version 4.1.0) [34].
3. Results
3.1. Physiological parameters
3.1.1. Body weight
On days 0, 12, and 19 of the experiment, the mean body weights of the AI and AVI groups are shown in Table 3. On the 19th day of the experiment, the mice in the Vechur urine distillate-treated group (AV) had significantly higher body weights than the mice in the normal control group (NC). An increase in body weight (P < 0.05) was also observed on the 19th day when Vechur urine distillate was administered to cyclophosphamide-treated animals (AVI).
Table 3.
Body and organ weight.
| Physiological parameters | Days | AI | AII | AIII | AIV | AV | AVI |
|---|---|---|---|---|---|---|---|
| Body weight (g) | |||||||
| First six animals | 0 | 23.67 ± 1.74 | 23.67 ± 1.36 | 22.42 ± 1.65 | 22.93 ± 2.16 | 23.42 ± 2.15 | 23.67 ± 1.73 |
| First six animals | 12 | 23.83 ± 1.47 | 21.55 ± 1.09 | 24.43 ± 1.84 | 22.98 ± 1.82 | 26.38 ± 1.59 | 23.7 ± 1.59 |
| Last six animals | 0 | 24.17 ± 1.42 | 23.58 ± 1.51 | 23.3 ± 1.31 | 23.27 ± 1.67 | 23.48 ± 1.28 | 23.68 ± 1.60 |
| Last six animals | 12 | 24.50 ± 1.59 | 23.77 ± 1.55 | 24.78 ± 1.65 | 24.65 ± 1.34 | 26.18 ± 1.23 | 25.73 ± 1.57 |
| Last six animals | 19 | 24.67 ± 1.49 | 21.32 ± 1.05 | 27.83 ± 1.14 | 24.70 ± 1.44 | 29.37a ± 1.08 | 25.93b ± 1.13 |
| Spleen weight (g/100 g body weight) | |||||||
| 12 | 0.30 ± 0.01 | 0.25a ± 0.01 | 0.35a ± 0.04 | 0.31b ± 0.02 | 0.39a ± 0.01 | 0.34ab ± 0.02 | |
| 19 | 0.30 ± 0.01 | 0.26a ± 0.01 | 0.26a ± 0.03 | 0.34b ± 0.01 | 0.51a ± 0.05 | 0.30ab ± 0.01 | |
| Kidney weight (g/100 g body weight) | |||||||
| 12 | 1.59 ± 0.05 | 1.50 ± 0.04 | 1.64 ± 0.08 | 1.53 ± 0.07 | 1.68 ± 0.05 | 1.60 ± 0.08 | |
| 19 | 1.59 ± 0.08 | 1.51 ± 0.02 | 1.69 ± 0.19 | 1.58 ± 0.06 | 1.73 ± 0.23 | 1.59 ± 0.06 | |
| Liver weight (g/100 g body weight) | |||||||
| 12 | 5.70 ± 0.33 | 5.16 ± 0.14 | 6.61 ± 0.54 | 5.81 ± 0.26 | 6.99a ± 0.43 | 6.30b ± 0.31 | |
| 19 | 5.84 ± 0.39 | 5.19 ± 0.12 | 7.02a ± 0.38 | 6.31b ± 0.54 | 8.04ac ± 0.15 | 6.96ab ± 0.31 | |
First six animals- Cyclophosphamide 30 mg kg−1 body weight administered intraperitoneally on days 9, 10, and 11. Last six animals- Cyclophosphamide 30 mg kg−1 body weight was administered intraperitoneally on days 16, 17, and 18. AI-normal control, AII-cyclophosphamide control, AIII- crossbred urine, AIV-crossbred urine + cyclophosphamide, AV- Vechur urine, AVI-Vechur urine + cyclophosphamide. Values are expressed in mean ± SE for six observations. Means bearing superscript a (comparison of AI versus AII to AVI) differ significantly (P < 0.05). Means bearing superscript b (comparison of AII versus AIV and AVI) differ significantly (P < 0.05). Means bearing superscript c (comparison of AIII versus AV) differ significantly (P < 0. 05). Means bearing superscript d (comparison of AIV versus AVI) differ significantly (P < 0.05).
The values are expressed as mean ± SE for six observations. AI-normal control, AII-cyclophosphamide-control, AIII- crossbred urine, AIV-crossbred urine plus cyclophosphamide, AV- Vechur urine, AVI-Vechur urine plus cyclophosphamide. The means with superscript a (comparison of AI versus AII to AVI) differ significantly (P < 0.05). The means bearing superscript b (comparison of AII versus AIV and AVI) differ significantly (P < 0.05). The means bearing superscript c (comparison of A1 versus AV) differ significantly (P < 0.05). The means bearing superscript d (comparison of AII versus AVI) differ significantly (P < 0.05).
3.1.2. Organ weight
The weight of internal organs such as liver, kidney, and spleen was recorded on days 12 and 19 of the experiment and is given in Table 3. When compared to the normal control, the cyclophosphamide control group (AII) had lower spleen weights (P < 0.05) on days 12 and 19. During the experimental period, the spleen weight of the urine-treated and crossbred animals increased significantly relative to the control. On the 12th and 19th days of the experiment, immunosuppressed animals (AVI) treated with Vechur urine had significantly greater spleen weights (P < 0.05) than control animals (AI). Compared to cyclophosphamide controls (Group AII), immunosuppressed animals from both crossbred and Vechur urine groups (Group AIV and AVI) showed an increase in spleen weight on days 12 and 19. The kidney weights did not differ significantly between the treatment groups on the 12th and 19th days. The liver weight of only the Vechur urine (AV) treated animals increased significantly (P < 0.05) in comparison to the normal controls. Immunosuppressed animals (AVI) treated with Vechur urine showed a significant increase compared to cyclophosphamide-treated controls.
3.1.3. Haematological parameters
3.1.3.1. Total leukocyte count
For all mice in groups AI to AVI, Table 4 shows the total leukocyte count on the 0th, 5th, 12th, and 19th day of the experiment. Crossbred and Vechur urine-administered mice (AIII and AV) had significantly higher leukocyte counts on the 12th day than control mice. Both crossbred and Vechur urine-treated animals (AIV and AVI) had higher leukocyte counts in comparison to cyclophosphamide controls. The crossbred and Vechur urine-treated animals (AIII and AV) showed a significant increase in total leukocyte count on the 19th day (P < 0.05) relative to normal controls. Leukocyte counts increased significantly in crossbred and Vechur urine -treated mice (AIV and AVI) compared to normal and cyclophosphamide-injected mice. When Vechur urine-treated immunocompetent animals (AV) were compared to crossbred urine-fed animals (AIII), AV had a higher value.
Table 4.
Haematological parameters.
| Haematological parameters | Days | AI | AII | AIII | AIV | AV | AVI |
|---|---|---|---|---|---|---|---|
| Total Leukocyte Count (103/mm3) | |||||||
| First six animals | 0 | 6.25 ± 0.41 | 6.40 ± 0.47 | 6.45 ± 0.41 | 6.23 ± 0.67 | 6.38 ± 0.38 | 6.48 ± 0.31 |
| First six animals | 5 | 6.30 ± 0.26 | 6.50 ± 0.43 | 6.85 ± 0.24 | 6.58 ± 0.40 | 7.02 ± 0.5 | 6.98 ± 0.18 |
| First six animals | 12 | 6.50 ± 0.23 | 5.38a ± 0.49 | 7.75a ± 0.55 | 6.61b ± 0.27 | 8.10a ± 0.13 | 7.08b ± 0.29 |
| Last six animals | 0 | 6.75 ± 0.09 | 6.70 ± 0.09 | 6.78 ± 0.42 | 6.92 ± 0.13 | 7.03 ± 0.11 | 6.68 ± 0.21 |
| Last six animals | 5 | 6.78 ± 0.29 | 6.97 ± 0.36 | 7.35 ± 0.33 | 7.48 ± 0.2 | 7.73 ± 0.19 | 7.30 ± 0.40 |
| Last six animals | 12 | 6.67 ± 0.19 | 6.98 ± 0.36 | 8.02a ± 0.15 | 7.97ab ± .23 | 8.67a ± 0.13 | 8.00ab ± 0.33 |
| Last six animals | 19 | 6.67 ± 0.19 | 6.98 ± 0.36 | 8.02a ± 0.15 | 7.97ab ± .23 | 8.67a ± 0.13 | 8.00ab ± 0.33 |
| Lymphocyte count (%) | |||||||
| First six animals | 0 | 74.83 ± 1.6 | 73.83 ± 1.2 | 74.83 ± 1.2 | 74.83 ± 1.2 | 74.83 ± 1.7 | 75.5 ± 1.2 |
| First six animals | 5 | 74.67 ± 0.8 | 73.50 ± 1.5 | 75.67 ± 1.2 | 75.5 ± 1.4 | 76.33 ± 1.2 | 76 ± 0.7 |
| First six animals | 12 | 75 ± 1.1 | 72 a ± 0.5 | 77.5 ± 0.8 | 76b ± 1.1 | 79a ± 1.1 | 78ab ± 0.36 |
| Last six animals | 0 | 75 ± 1.3 | 74 ± 1.5 | 74 ± 1.8 | 75 ± 1.6 | 75 ± 1.4 | 75 ± 1.4 |
| Last six animals | 5 | 75.5 ± 0.9 | 74.8 ± 1.4 | 75.3 ± 1.2 | 75.8 ± 0.8 | 76.2 ± 0.7 | 76 ± 1.0 |
| Last six animals | 12 | 75 ± 1.1 | 74 ± 0.9 | 77 ± 1.4 | 76 ± 0.7 | 79a ± 1.3 | 79 b ± 1.1 |
| Last six animals | 19 | 76 ± 1.1 | 73 ± 1.2 | 79a ± 0.7 | 77b ± 1.1 | 84ac ± 0.5 | 80abd ± 0.4 |
| Neutrophil count (%) | |||||||
| First six animals | 0 | 24.83 ± 1.49 | 25.33 ± 1.38 | 24.83 ± 1.4 | 24.83 ± 1.4 | 24.83 ± 1.99 | 24 ± 1.57 |
| First six animals | 5 | 24.67 ± 0.92 | 25.83 ± 1.66 | 24 ± 1.32 | 24.17 ± 1.54 | 23.5 ± 1.28 | 23.17 ± 0.79 |
| First six animals | 12 | 24.33 ± 1.41 | 27.17 ± 0.83 | 22.17 ± 0.95 | 23.17b ± 1.47 | 19.7a ± 1.43 | 21.67b ± 0.33 |
| Last six animals | 0 | 24.3 ± 1.3 | 25 ± 1.8 | 24.8 ± 2.1 | 24.5 ± 1.9 | 24.8 ± 1.5 | 23.8 ± 1.6 |
| Last six animals | 5 | 24.2 ± 1.0 | 24 ± 1.5 | 23.7 ± 1.3 | 23.8 ± 0.9 | 23.7 ± 0.8 | 23.5 ± 1.3 |
| Last six animals | 12 | 24 ± 1.5 | 25 ± 1.2 | 22.5 ± 1.7 | 22.8 ± 0.6 | 19.8 ± 1.5 | 21 ± 1.1 |
| Last six animals | 19 | 23.7 ± 1.2 | 25.8 ± 1.4 | 19.3a ± 1.1 | 22.2b ± 1.2 | 15.5ac ± 0.7 | 19.2ab ± 0.4 |
First six animals- Cyclophosphamide 30 mg kg−1 body weight administered intraperitoneally on days 9, 10, and 11. Last six animals- Cyclophosphamide 30 mg kg−1 body weight was administered intraperitoneally on days 16, 17, and 18. AI-normal control, AII-cyclophosphamide control, AIII- crossbred urine, AIV-crossbred urine + cyclophosphamide, AV- Vechur urine, AVI-Vechur urine + cyclophosphamide. Values are expressed in mean ± SE for six observations. Means bearing superscript a (comparison of AI versus AII to AVI) differ significantly (P < 0.05). Means bearing superscript b (comparison of AII versus AIV and AVI) differ significantly (P < 0.05). Means bearing superscript c (comparison of AIII versus AV) differ significantly (P < 0. 05). Means bearing superscript d (comparison of AIV versus AVI) differ significantly (P < 0.05).
3.1.3.1.1. Lymphocytes
On days 0, 5, 12, and 19 of the experiment, the lymphocyte counts for all the AI to AVI groups are shown in Table 4. The lymphocyte count of the Vechur urine-treated group (AV) was significantly higher than that of the control group. The lymphocyte count increased significantly (P < 0.05) in immunosuppressed animals treated with Vechur urine when compared with normal control. There was a significant difference in lymphocyte number between the cyclophosphamide control group and the crossbred and Vechur urine-treated groups (AIV and AVI) (P0.05).
3.1.3.1.2. Neutrophils
On days 0, 5, 12, and 19 of the experiment, the neutrophil counts for all AI to AVI groups are reported in Table 4. As compared to the control group, the Vechur urine-treated group (AV) had lower neutrophil counts (P < 0.05). AVI and AVI of crossbred and Vechur urine-treated immunosuppressed animals showed significantly (P < 0.05) lower counts than the cyclophosphamide control group. On the 19th day, the neutrophil count of both crossbred and Vechur urine-treated groups (AIII and AV) decreased significantly compared to that of the normal control group.
3.2. Biochemical parameters
As shown in Table 5, the mean serum protein concentrations during the 0th, 5th, 12th, and 19th days of the experiment. On day 5, only the Vechur urine-treated group (AV) had a significantly higher total protein level than the control group. Both Vechur urine-treated mice (AIII and AV) had significantly higher serum protein concentrations on days 12, and 19. Using data from experiment days 0, 5, 12, and 19, Table 5 shows globulin concentrations on each of these days. The globulin concentrations in crossbred and Vechur-treated mice (AIII and AV) increased significantly on days 12, and 19, compared to the control. Crossbred and Vechur urine-treated immunosuppressed animals (AIV and AVI) developed higher globulin levels on days 12 and 19 compared to cyclophosphamide-treated control animals. In Table 5, we show the albumin-globulin ratios of the experimental animals on days 0, 5, 12, and 19.
Table 5.
Biochemical parameters.
| Biochemical parameters | Days | AI | AII | AIII | AIV | AV | AVI |
|---|---|---|---|---|---|---|---|
| Serum protein (g/dl) | |||||||
| First six animals | 0 | 3.62 ± 0.15 | 3.75 ± 0.09 | 3.69 ± 0.11 | 3.79 ± 0.13 | 3.74 ± 0.09 | 3.77 ± 0.20 |
| First six animals | 5 | 3.64 ± 0.14 | 3.79 ± 0.06 | 3.89 ± 0.14 | 3.87 ± 0.15 | 4.10 ± 0.10 | 3.91 ± 0.25 |
| First six animals | 12 | 3.70 ± 0.15 | 2.94 a ± 0.15 | 4.29a ± 0.15 | 3.97b ± 0.19 | 4.63a ± 0.18 | 4.11b ± 0.11 |
| Last six animals | 0 | 3.70 ± 0.13 | 3.80 ± 0.13 | 3.70 ± 0.11 | 3.80 ± 0.12 | 3.90 ± 0.11 | 3.80 ± 0.14 |
| Last six animals | 5 | 3.80 ± 0.17 | 3.80 ± 0.11 | 3.90 ± 0.14 | 3.90 ± 0.08 | 4.30a ± 0.10 | 4.10 ± 0.16 |
| Last six animals | 12 | 3.80 ± 0.13 | 3.70 ± 0.13 | 4.50a ± 0.18 | 4.30 ab ± 0.10 | 4.90a ± 0.22 | 4.60 ab ± .17 |
| Last six animals | 19 | 3.90 ± 0.09 | 2.90a ± 0.15 | 5.20a ± 0.19 | 4.50ab ± 0.16 | 6.00ac ± 0.29 | 4.90ab ± 0.17 |
| Serum globulin (g/dl) | |||||||
| First six animals | 0 | 1.34 ± 0.03 | 1.36 ± 0.11 | 1.35 ± 0.06 | 1.34 ± 0.06 | 1.33 ± 0.08 | 1.36 ± 0.03 |
| First six animals | 5 | 1.35 ± 0.08 | 1.36 ± 0.07 | 1.47 ± 0.11 | 1.45 ± 0.05 | 1.55 ± 0.05 | 1.55 ± 0.04 |
| First six animals | 12 | 1.35 ± 0.07 | 1.02a ± 0.03 | 1.64a ± 0.10 | 1.49b ± 0.08 | 1.76a ± 0.07 | 1.61ab ± 0.05 |
| Last six animals | 0 | 1.37 ± 0.04 | 1.33 ± 0.06 | 1.38 ± 0.06 | 1.32 ± 0.05 | 1.35 ± 0.05 | 1.45 ± 0.04 |
| Last six animals | 5 | 1.32 ± 0.05 | 1.34 ± 0.07 | 1.33 ± 0.05 | 1.47 ± 0.05 | 1.54a ± 0.09 | 1.57ab ± 0.05 |
| Last six animals | 12 | 1.62 ± 0.04 | 1.59 ± 0.06 | 1.74a ± 0.05 | 1.73ab ± 0.04 | 1.36 a ± 0.02 | 1.33 ab ± 0.03 |
| Last six animals | 19 | 1.36 ± 0.05 | 1.01a ± 0.04 | 1.82a ± 0.03 | 1.67ab ± 0.07 | 1.97ac ± 0.06 | 1.82abd ± 0.05 |
| Albumin-globulin ratio | |||||||
| First six animals | 0 | 1.72 ± 0.15 | 1.82 ± 0.16 | 1.76 ± 0.17 | 1.85 ± 0.15 | 1.87 ± 0.18 | 1.78 ± 0.19 |
| First six animals | 5 | 1.72 ± 0.13 | 1.84 ± 0.19 | 1.69 ± 0.13 | 1.68 ± 0.10 | 1.66 ± 0.09 | 1.53 ± 0.17 |
| First six animals | 12 | 1.77 ± 0.10 | 1.87 ± 0.15 | 1.64 ± 0.10 | 1.68 ± 0.15 | 1.66 ± 0.16 | 1.55 ± 0.06 |
| Last six animals | 0 | 1.86 ± 0.21 | 1.83 ± 0.09 | 1.83 ± 0.17 | 1.77 ± 0.11 | 1.94 ± 0.09 | 1.76 ± 0.16 |
| Last six animals | 5 | 1.89 ± 0.15 | 1.82 ± 0.10 | 1.69 ± 0.08 | 1.69 ± 0.13 | 1.84 ± 0.17 | 1.63 ± 0.16 |
| Last six animals | 12 | 1.87 ± 0.14 | 1.77 ± 0.16 | 1.76 ± 0.10 | 1.76 ± 0.15 | 1.85 ± 0.17 | 1.67 ± 0.09 |
| Last six animals | 19 | 1.87 ± 0.06 | 1.84 ± 0.13 | 1.87 ± 0.09 | 1.69 ± 0.16 | 2.04 ± 0.15 | 1.71 ± 0.12 |
First six animals- Cyclophosphamide 30 mg kg−1 body weight administered intraperitoneally on days 9, 10, and 11. Last six animals- Cyclophosphamide 30 mg kg−1 body weight was administered intraperitoneally on days 16, 17, and 18. AI-normal control, AII-cyclophosphamide control, AIII- crossbred urine, AIV-crossbred urine + cyclophosphamide, AV- Vechur urine, AVI-Vechur urine + cyclophosphamide. Values are expressed in mean ± SE for six observations. Means bearing superscript a (comparison of AI versus AII to AVI) differ significantly (P < 0.05). Means bearing superscript b (comparison of AII versus AIV and AVI) differ significantly (P < 0.05). Means bearing superscript c (comparison of AIII versus AV) differ significantly (P < 0. 05). Means bearing superscript d (comparison of AIV versus AVI) differ significantly (P < 0.05).
3.3. Immunological parameters
3.3.1. Haemagglutination
The haemagglutination titre (HA) values obtained are presented in Table 6. The results are shown in Fig. 1. Vechur urine-treated mice showed significantly higher titres than the normal control mice. The titre values of crossbred and Vechur urine-treated immunosuppressed mice (AIV and AVI) were significantly higher than those of cyclophosphamide-injected controls. Compared to crossbred urine-treated mice, immunocompetent mice treated with Vechur urine (AV) showed significantly higher values on both 12th and 19th days.
Table 6.
Immunological parameters.
| Immunological parameters | Days | AI | AII | AIII | AIV | AV | AVI |
|---|---|---|---|---|---|---|---|
| Haemagglutination titer | |||||||
| First six animals | 0 | 2.33 ± 0.33 | 3 ± 0.45 | 3 ± 0.45 | 2.67 ± 0.42 | 2.67 ± 0.42 | 3 ± 0.45 |
| First six animals | 12 | 42.67 ± 6.7 | 12a ± 2.5 | 8 ± 1.6 | 42.67b ± 6.7 | 149.3ac ± 3.6 | 69.3b ± 12.8 |
| Last six animals | 0 | 3 ± 0.45 | 3 ± 0.45 | 2.67 ± 0.42 | 3 ± 0.45 | 3 ± 0.45 | 3 ± 0.45 |
| Last six animals | 12 | 42.67 ± 6.7 | 42.67 ± 6.7 | 85.3 ± 13.5 | 80 ± 16.00 | 160ac ± 32 | 149.3abd ± 35.7 |
| Last six animals | 19 | 48 ± 7.1 | 18.67 ± 2.7 | 192a ± 28.6 | 96 ± 14.3 | 341.3ac ± 53.9 | 192abd ± 28.6 |
| Plaque forming cells | |||||||
| First six animals | 12 | 124.30 ± 1.40 | 93a ± 2.02 | 372.67a ± 1.61 | 215.3ab ± 9 | 432.8ac ± 2.5 | 346.5abd ± 2.7 |
| Last six animals | 19 | 132.17 ± 2.75 | 94a ± 2.53 | 422a ± 2.71 | 235.20ab ± 4 | 561.5ac ± 6.35 | 385abd ± 2.49 |
| Bone marrow cellularity (millions) | |||||||
| First six animals | 12 | 10.38 ± 0.17 | 6.72a ± 0.14 | 13.25a ± 0.14 | 11.85ab ± 0.18 | 14.19ac ± 0.25 | 12.25ab ± 0.25 |
| Last six animals | 19 | 10.41 ± 0.09 | 6.88a ± 0.09 | 14.39a ± 0.16 | 12.75ab ± 0.15 | 15.65ac ± 0.19 | 13.24abd ± 0.12 |
| Foot pad thickness (mm) | |||||||
| First six animals | 12 | 0.48 ± 0.06 | 0.49 ± 0.06 | 1.30a ± 0.04 | 1.29ab ± 0.05 | 1.58ac ± 0.04 | 1.45abd ± 0.05 |
| Last six animals | 19 | 0.56 ± 0.04 | 0.54 ± 0.05 | 1.47a ± 0.06 | 1.32ab ± 0.06 | 2.03ac ± 0.18 | 1.66abd ± 0.09 |
First six animals- Cyclophosphamide 30 mg kg−1 body weight administered intraperitoneally on days 9, 10, and 11. Last six animals- Cyclophosphamide 30 mg kg−1 body weight was administered intraperitoneally on days 16, 17, and 18. AI-normal control, AII-cyclophosphamide control, AIII- crossbred urine, AIV-crossbred urine + cyclophosphamide, AV- Vechur urine, AVI-Vechur urine + cyclophosphamide. Values are expressed in mean ± SE for six observations. Means bearing superscript a (comparison of AI versus AII to AVI) differ significantly (P < 0.05). Means bearing superscript b (comparison of AII versus AIV and AVI) differ significantly (P < 0.05). Means bearing superscript c (comparison of AIII versus AV) differ significantly (P < 0. 05). Means bearing superscript d (comparison of AIV versus AVI) differ significantly (P < 0.05).
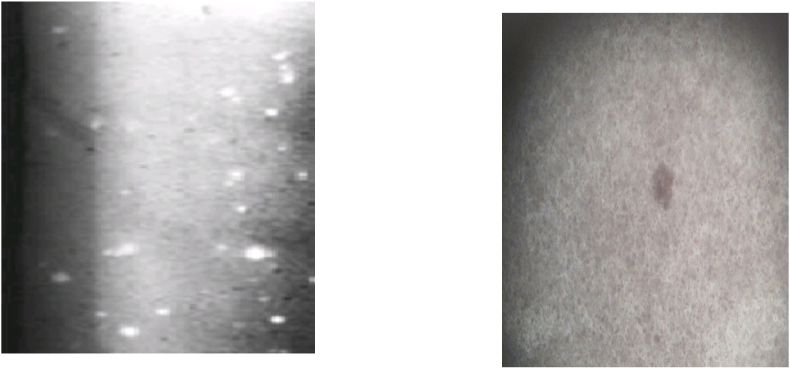
Fig. 1.
Splenic plaque-forming assay (macroscopic and microscopic).
3.3.2. Splenic plaque-forming assay
We measured the number of plaque-forming cells in the spleen on the 12th and 19th days of the experiment. Table 6 shows their mean values. Fig. 1 shows macroscopic and microscopic views of splenic plaques. The number of plaque-forming cells on days 12 and 19 in immunocompetent and immunosuppressed Vechur and crossbred urine-treated animals was significantly higher than that in control animals. The increases on days 12 and 19 were significant for both crossbred and Vechur urine-treated immunosuppressed animals (AIV and AVI) in comparison with the cyclophosphamide control.
3.3.3. Bone marrow cellularity
The total bone marrow cellularity was measured on days 12 and 19 of the experiment. The mean value can be seen in Table 6. On days 12 and 19, both Vechur-treated and crossbred urine-treated immunocompetent and immunosuppressed animals had significantly increased bone marrow cellularity compared to normal controls.
3.3.4. Delayed-type hypersensitivity
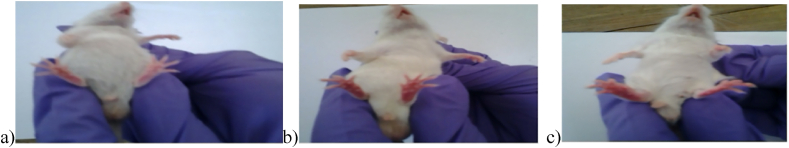
The delayed-type hypersensitivity reaction on the footpad of mice is shown in Fig. 2. Table 6 shows the change in footpad thickness on the 12th and 19th days for all experimental animals. On days 12 and 19 after treatment with Vechur and crossbred urine, footpad thickness increased significantly in both immunocompetent and immunosuppressed mice.
Fig. 2.
Delayed-type hypersensitivity reaction on footpad of mice (a- Control, b- Crossbred urine distillate group, c- Vechur urine distillate group).
The values are expressed as mean ± SE for six observations. The BI-normal control, the BII-cyclophosphamide-control, BIII- crossbred urine, BIV-crossbred urine plus cyclophosphamide, the BV- Vechur urine, the BVI-Vechur urine plus cyclophosphamide. The means with superscript a (comparison of BI versus BII to BVI) differ significantly (P < 0.05). The means bearing superscript b (comparison of BII versus BIV and BVI) differ significantly (P < 0.05). The means bearing superscript c (comparison of BI versus BV) differ significantly (P < 0.05). The means bearing superscript d (comparison of BII versus BVI) differ significantly (P < 0.05).
4. Discussion
The urine distillates of Vechur and crossbred animals contained urea, uric acid, and creatinine. It was found that neither Vechur nor crossbred urine distillates contained glucose, proteins, ketone bodies, bile salts, or bile pigments. Vechur urine distillate has higher levels of urea, uric acid, and creatinine than crossbred cow urine distillate. The main component of urine is urea, a by-product of protein metabolism. Purines are broken down to form uric acid [35]. Creatinine, which is a breakdown product of creatine, is completely removed from the body by the kidneys. Because cow urine contains urea, uric acid, and creatinine, it is germicidal. A study by Bristow et al. (1992) found that cow's urine contained nitrogen-containing compounds, of which 69% were urea [36].
Cyclophosphamide, a nitrogen mustard alkylating agent, suppresses the immune system by inhibiting DNA synthesis and function. Better utilization of feed may contribute to the increase in body weight since it protects the liver and regulates gastric function [37]. Additionally, levamisole was found to increase body weight in broiler chicks [38,39]. The lymphoid organs facilitate antigen trapping (by phagocytes) and enable antigen-sensitized cells (T and B lymphocytes) to find antigens. Spleen is a secondary lymphoid organs that contain B cells, T cells, macrophages, dendritic cells, natural killer cells, and red blood cells. As the spleen filters blood directly, it removes antigens, while circulating macrophages and dendritic cells deliver the antigens to the spleen. An immune response is triggered when macrophages or dendritic cells present antigen to B or T cells. When B cells become activated in the spleen, they produce large quantities of antibodies [40].
Increases in spleen weight in the present study could be viewed as indicators of increased immunocompetence in the treated animals [42]. Lymphocytes and bone marrow hematopoietic cells contribute to the weight of the spleen [43]. When lymphoid organs such as the spleen are treated with urine distillate, their relative organ weight increases, which indicates that the immune system is activated.Immunocompetent and immunosuppressed animals responded similarly to Vechur and crossbred urine distillate in terms of the number of leukocytes. A significant increase in total leukocyte count was observed following the simultaneous administration of urine distillate and cyclophosphamide, indicating that urine distillate counteracts the immunosuppressive effects of this drug. Activating macrophages, which secrete colony-stimulating factor and interleukin, might prevent leukopenia in urine distillate-treated mice [44].
The increase in total leukocyte count seen in the present study is consistent with a previous study [44] showing freeze-dried cow urine stimulates the proliferation of leukocytes in rats. Following levamisole administration, broilers exhibited a similar increase in leukocyte counts [45]. Cyclophosphamide-induced immunotoxicity may be responsible for the reduction in lymphocyte counts in the control group. A similar increase in lymphocytes was observed in another study [47] which administered cow urine to white leghorn hens. It may be that the neutrophilia in the cyclophosphamide control group compensates for the drop in lymphocytes. After concomitant administration of cyclophosphamide and cow urine distillate, a significant drop in neutrophil count was observed, suggesting that cow urine plays a role in preventing cyclophosphamide-induced neutrophilia. As phagocytic cells are marginalized, neutrophil counts increase, signaling a more effective defensive response [48,49]. A key innate defense reaction against pathogens is phagocytosis. As neutrophils consume pathogens, they undergo phagocytosis. Phagocytosis helps the immune system work. Thus, the present study indicates stimulation of the hematopoietic system by an increase in lymphocytes and a decrease in neutrophils in the treated mice.
There are several different kinds of proteins in serum, each with its own properties and functions. Furthermore, they maintain homeostasis by controlling inflammation and preventing infection [50]. There is an increase in protein concentrations due primarily to an increase in total globulins, typically those of the γ-globulin type, with levels of albumin remaining the same or decreasing marginally [44]. A ratio of serum protein to serum albumin globulin is one of the first indicators of normal serum chemistry. Changing serum protein concentrations or albumin ratios may indicate an altered immune response [[51], [52], [53]]. The group receiving Vechur cow urine distillate had the highest serum protein levels, according to the current study. On the basis of the findings regarding serum protein, we can speculate that increased immunoglobulins and other humoral factors contributed to the serum protein [45]. Getting a sense of humoral immunity is achieved using haemagglutination antibody titre assays since antigens trigger the production of antibodies in response to them. SRBCs were used in the present study to test whether antibodies were produced against RBCs. The level of antibodies can be expected to be higher in an individual who has a primed immune system. Among the serum molecules are serum immunoglobulins, produced by B lymphocytes. In addition to being called an antibody, it is produced to the maximum level to protect the body from an invasion by an antigen. According to a study on mice, macrophages and B lymphocyte subsets were involved in antibody synthesis, resulting in an enhanced humoral response as evidenced by an enhanced antibody response to SRBC [46]. A rise in antibody titre might be due to the proliferation of B lymphocytes and their transformation into plasmocytes, which suggests a humoral immune response [47]. As evidenced by these secondary immune responses, the antibody-forming system is capable of recollecting past antigen exposures from earlier periods.
At neutral pH, RBCs possess a cloud of negative ions that repel one another. This repellent force is the zeta potential. A large molecule of IgM with a pentameric structure will cross the electric barrier and crosslink to RBCs, leading to subsequent agglutination [54]. Antigens are recognized by a lymphoid cell and become activated as part of the immune response. The antibodies produced by B cells bind to pathogens and act as defence mechanisms against infection by binding to antigens. During T helper or B lymphocyte differentiation, cytokines produced by T cells react with antigens on the surface of antigen-presenting cells. T helper cell response and macrophage stimulation, cell-mediated immunity, and antibody production are all associated with T helper cells [11].
Observed higher levels of antibody titre in urine distillate treated mice may indicate greater cell-to-cell communication is a cause of the better immune response. A positive antibody response was also observed against SRBC in rats given Panchagavya and Haridradi Ghrita, which contain cow urine [[55], [56], [57]]. The serum IgG levels of white leghorn layers also increased when cow urine was given to them [47]. The haemagglutination titre also increased in broiler chicks and goats after levamisole administration [47,58].
Generally speaking, cells that produce antibodies are called plaque-forming cells. Immune cells release antibodies, and these antibodies bond with antigens on adjacent red blood cells, which then lyse in response to complement, forming a clear plaque around each antibody-producing cell [31]. There is a strong correlation between increased antibody titres and increased splenic plaque formation. This could be explained by the spleen producing more antibodies [58]. Levamisole was also found to be effective in goats and in mice when immunostimulation herbal extracts were administered [59].
Bone marrow is affected the most by immunosuppressive therapy, especially with cyclophosphamide [46]. In general, the cells of the immune system that are active both cyclically and intermitotically are depleted by its action [48]. The bone marrow is a particularly sensitive target for cytotoxic drugs due to its high level of cell proliferation. Leukopenia is characterized by a failure to generate new blood cells or the loss of stem cells in the bone marrow [60]. Hemopoietic cells primarily make up bone marrow. Body fat fills any spaces left over.
All major cell types of the immune system are derived from the hematopoietic stem cells in the bone marrow. Several different kinds of immune cells are differentiated from stem cells by cytokines. Furthermore, the bone marrow also provides an antigen-independent microenvironment for the differentiation of B cells [59]. The bone marrow continually produces a wide variety of blood cells, including granulocytes, erythrocytes, and platelets as hematopoietic stem cells divide. All immune system cells originate in the bone marrow. As a result of differentiation into mature cells or precursors, bone marrow stem cells also become adult cells in other tissues [59]. Lymphocytes, granulocytes (white blood cells, platelets, and monocytes), and T cells (including B cells and immature T cells) are all produced in the bone marrow. An increase in bone marrow cellularity is correlated with an increase in blood cell count.
Increasing bone marrow cells shows that Vechur urine distillate enhances the immune response. With or without cyclophosphamide, Vechur urine distillate stimulated bone marrow cell proliferation. Apparently, the presence of urine distillate has a positive impact on stem cell differentiation and immunity. Similar results have been observed in mice treated with an immunostimulatory herbal extract [59]. T lymphocytes and lymphokines are responsible for cell-mediated immunity. It is possible to determine whether antigen-specific cellular immune response has been affected by assessing the degree of DTH reaction in experimental animals using the footpad swelling test. Proliferated T lymphocytes are able to recognize antigens and secrete cytokines. Cytokines pressure blood vessels and activate macrophages, which leads to inflammation. Phagocytosis is triggered as a result. The release of cytokines and the production of DTH is a result of T helper cells responding to specific antigens such as SRBCs [41,61]. The Type IV hypersensitivity reaction DTH is caused when an antigen triggers the activation of sensitized T cells. The release of cytokines by T lymphocytes attracts scavenger cells to the site of the reaction. When subsequent exposure to SRBC antigen results in the activation of T cells, DTH releases cytokines and other inflammatory mediators. Activated macrophages and cytokines release cause a delay in response [46,62]. The treatment group had an increased DTH response as a result of stimulating the T cells with urine distillate. Thus, increased DTH responses indicate that the immunomodulator promotes lymphocyte proliferation and the proliferation of accessory cells [63]. As previously reported, when whole freeze-dried cow urine and its fractions or levamisole were administered to rats and broilers, DTH responses were improved [44,45]. Further studies at the molecular level may reveal the precise mechanism of this activity.
5. Conclusions
Increasing the immunity of farm animals to diseases is becoming increasingly important since the European Union banned growth-promoting antibiotics in feed. Animals exposed to major stressful events can become more vulnerable to pathogens, as a result of stress. To prevent the potentially harmful effects of pathogens in the gastrointestinal tract, innate and adaptive immunity must be developed at the mucosal surface. The purpose of several in vivo nutritional studies is to modulate immune function and boost the immune system using bioactive components derived from natural sources. Therefore, screening and validating immunomodulators for use as animal feed additives is necessary. As part of this study, cow urine was tested to determine its traditional use for modulating immunity as well as its potential to be used as an animal feed supplement, specifically for pigs, poultry, and aquaculture. Moreover, the experimental methods used in the present study could be replicated in the future to identify the effects of natural compounds on the immune system.
Author contributions
Conceptualization, NKT., UPTA., SN., NNS., PMP., and MEM; methodology, NKT., UPTA., SN., NNS., PMP., and MEM; software, SO., N.P.P. and M.S.K.; validation, NKT., UPTA., SN., NNS., PMP., and MEM; formal analysis, NKT., UPTA., SN., NNS., PMP., and MEM; investigation, NKT., UPTA., SN., NNS., PMP., and MEM; resources, NKT., UPTA., SN., NNS., PMP., PPN., MSK., SO and MEM; data curation, PPN., MSK., SO writing—original draft preparation, NKT., UPTA., SN., NNS., PMP and MEM; writing—review and editing, NKT., UPTA., SN., NNS., PMP., PPN., MSK., SO and MEM; visualization, NKT and E.M.M.; supervision, UPTA., SN., NNS., PMP; project administration, NKT; funding acquisition, NKT., UPTA., SN., NNS and PMP. All authors have read and agreed to the published version of the manuscript.
Sources of funding
None.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of the Kerala Veterinary and Animal Sciences University (No. 1684/2013 dated 15-07-2013).
Data availability statement
Data can be made available upon reasonable request to the corresponding author.
Declaration of competing interest
None.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through the Research Group Program under Grant No: RGP2/348/44.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2023.100784.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Gallois M., Rothkotter H.J., Bailey M., Stokes C.R., Oswald I.P. Natural alternatives to in-feed antibiotics in pig production: can immunomodulators play a role? Animal. 2009;3:1644–1661. doi: 10.1017/S1751731109004236. [DOI] [PubMed] [Google Scholar]
- 2.Valdivieso-Ugarte M., Gomez-Llorente C., Plaza-Diaz J., Gil A. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: a systematic review. Nutrients. 2019;11:2786. doi: 10.3390/nu11112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandner G., Heckmann M., Weghuber J. Immunomodulatory activities of selected essential oils. Biomolecules. 2020;10:1139. doi: 10.3390/biom10081139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandyopadhyay S., Samanta I. Antimicrobial resistance in agri-food chain and companion animals as a re-emerging menace in post-COVID epoch: low-and middle-income countries perspective and mitigation strategies. Front Vet Sci. 2020;7:620. doi: 10.3389/fvets.2020.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadeem S.F., Gohar U.F., Tahir S.F., Mukhtar H., Pornpukdeewattana S., Nukthamna P., et al. Antimicrobial resistance: more than 70 years of war between humans and bacteria. Crit Rev Microbiol. 2020;46:578–599. doi: 10.1080/1040841X.2020.1813687. [DOI] [PubMed] [Google Scholar]
- 6.Monger X.C., Gilbert A.A., Saucier L., Vincent A.T. Antibiotic resistance: from pig to meat. Antibiotics. 2021;10:1209. doi: 10.3390/antibiotics10101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirchhelle C. Pharming animals: a global history of antibiotics in food production (1935–2017) Palgrave Commun. 2018;4:1–13. [Google Scholar]
- 8.Brussow H. Adjuncts and alternatives in the time of antibiotic resistance and in-feed antibiotic bans. Microb Biotechnol. 2017;10:674–677. doi: 10.1111/1751-7915.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silveira R.F., Roque-Borda C.A., Vicente E.F. Antimicrobial peptides as a feed additive alternative to animal production, food safety and public health implications: an overview. Anim Nutr. 2021;11:1–9. doi: 10.1016/j.aninu.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adedokun S.A., Olojede O.C. Optimizing gastrointestinal integrity in poultry: the role of nutrients and feed additives. Front Vet Sci. 2019;5:1–11. doi: 10.3389/fvets.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kothari D., Lee W.D., Niu K.M., Kim S.K. The genus Allium as poultry feed additive: a review. Animals. 2019;9:1–21. doi: 10.3390/ani9121032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y., Zhu C.P., Zhang Y., Li Y., Sun J.R. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int J Biol Macromol. 2019;137:504–511. doi: 10.1016/j.ijbiomac.2019.06.139. [DOI] [PubMed] [Google Scholar]
- 13.Randhawa G.K. Cow urine distillate as bioenhancer. J Ayurveda Integr Med. 2010;1:240–241. doi: 10.4103/0975-9476.74089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park H.J., Suh H.G., Kim J.H., Jang A.R., Jung H.J., Lee S.D., et al. Immune modulation effect of pig placenta extracts in a mouse model: putative use as a functional food supplement. Food Sci Anim. 2011;31:701–709. [Google Scholar]
- 15.Vaidya A.D. Urine therapy in Ayurveda: ancient insights to modern discoveries for cancer regression. J Ayurveda Integr Med. 2018;9:221–224. doi: 10.1016/j.jaim.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou C., Wu X.R., Liu H.S., Liu X.H., Liu G.H., Zheng X.B., et al. Immunomodulatory effect of urine-derived stem cells on inflammatory bowel diseases via downregulating Th1/Th17 immune responses in a PGE2-dependent manner. J Crohns Colitis. 2020;14:654–668. doi: 10.1093/ecco-jcc/jjz200. [DOI] [PubMed] [Google Scholar]
- 17.Liu G., Wang X., Sun X., Deng C., Atala A., Zhang Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials. 2013;34:8617–8629. doi: 10.1016/j.biomaterials.2013.07.077. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R., Ali S.A., Singh S.K., Bhushan V., Kaushik J.K., Mohanty A.K., et al. Peptide profiling in cow urine reveals molecular signature of physiology-driven pathways and in-silico predicted bioactive properties. Sci Rep. 2021;11:1–16. doi: 10.1038/s41598-021-91684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoh J.M., Dhanashree B. Antifungal effect of cow's urine distillate on Candida species. J Ayurveda Integr Med. 2017;8:233–237. doi: 10.1016/j.jaim.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanuja S.P. 2002. Pharmaceutical composition containing cow urine distillate and an antibiotic. US Patent No. 6410059. [Google Scholar]
- 21.Khanuja S.P. 2005. Use of bioactive fraction from cow urine distillate as a bioenhancer of anti-infective, anti-cancer agents and nutrients. US Patent No. 6896907. [Google Scholar]
- 22.Sarsar V., Selwal K.K., Selwal M.K., Pannu R., Tyagi P.K. Evaluation of antibacterial activity of photoactivated cow urine against human pathogenic strains. Environ Exp Biol. 2013;11:201–203. [Google Scholar]
- 23.Guan X., Mack D.L., Moreno C.M., Strande J.L., Mathieu J., Shi Y. Dystrophin-deficient cardiomyocytes derived from human urine: new biologic reagents for drug discovery. Stem Cell Res. 2014;12:467–480. doi: 10.1016/j.scr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee K.I., Kim H.T., Hwang D.Y. Footprint-and xeno-free human iPSCs derived from urine cells using extracellular matrix-based culture conditions. Biomaterials. 2014;35:8330–8338. doi: 10.1016/j.biomaterials.2014.05.059. [DOI] [PubMed] [Google Scholar]
- 25.Gururaja M.P., Joshi A.B., Joshi H., Sathyanarayana D., Subramanyam E.V.S., Chandrasekhar K.S. Antidiabetic potential of cow urine in streptozotocin induced diabetic rats. Asian J Tred Med. 2011;6:8–13. [Google Scholar]
- 26.Mitsuoka A., Baba M., Morikawa S. Enhancement of delayed hypersensitivity by depletion of suppressor T cells with cyclophosphamide in mice. Nature. 1976;262:77–78. doi: 10.1038/262077a0. [DOI] [PubMed] [Google Scholar]
- 27.Grill A., Kiouptsi K., Karwot C., Jurk K., Reinhardt C. Evaluation of blood collection methods and anticoagulants for platelet function analyses on C57BL/6J laboratory mice. Platelets. 2020;31:981–988. doi: 10.1080/09537104.2019.1701185. [DOI] [PubMed] [Google Scholar]
- 28.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;77:751–766. [PubMed] [Google Scholar]
- 29.Wells F.E., Addison G.M., Postlethwaite R.J. Albumin analysis in serum of haemodialysis patients: discrepancies between bromocresol purple, bromocresol green and electroimmunoassay. Ann Clin Biochem. 1985;22:304–309. doi: 10.1177/000456328502200314. [DOI] [PubMed] [Google Scholar]
- 30.Ray A., Mediratta P.K., Puri S., Sen P. Effect of stress on immuneresponsiveness, gastric ulcerogenesis and plasma corticosterone in rats: modulation by diazepam and naltrexone. Indian J Exp Biol. 1991;29:233–236. [PubMed] [Google Scholar]
- 31.Jerne N.K., Nordin K.K. Plaque formation in agar by single antibody producing cells. Science. 1963;11:140–405. [PubMed] [Google Scholar]
- 32.Mehra E., Vaidya M.C. C.B.S publishers; New Delhi: 1985. A handbook of practical and clinical immunology; p. 320. [Google Scholar]
- 33.Askenase P.W., Hayden B.J., Gershon R.K. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975;141:697–702. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: a language and environment for statistical computing.https://www.R-project.org/ URL. [Google Scholar]
- 35.Holstein S.A., McCarthy P.L. Immunomodulatory drugs in multiple myeloma: mechanisms of action and clinical experience. Drugs. 2017;77:505–520. doi: 10.1007/s40265-017-0689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bristow A.W., Whitehead D.C., Cockburn J.E. Nitrogenous constituents in the urine of cattle, sheep and goat. J Sci Food Agric. 1992;59:387–394. [Google Scholar]
- 37.Thatte U.M., Chhabria S.N., Karandikar S.M., Dahanukar S.A. Protective effects of Indian medicinal plants against cyclophosphamide neutropenia. J Postgrad Med. 1987;33:185–188. [PubMed] [Google Scholar]
- 38.Gururaja M.P., Joshi A.B., Joshi H., Sathyanarayana D., Subrahmayan E.V.S., Chandrashekhar K.S. Attenuation of carbon tetrachloride-induced hepatotoxicity by cow urine distillate in rats. Biomed Environ Sci. 2009;22:345–347. doi: 10.1016/S0895-3988(09)60066-0. [DOI] [PubMed] [Google Scholar]
- 39.Panda S.K., Rao A.T. Effect of levamisole on chicken infected with infectious bursal disease virus. Indian Vet J. 1994;71:427–439. [Google Scholar]
- 40.Todar K. 8th ed. Williams and Wilkins Company; Baltimore, U. S.A: 2008. Text book of bacteriology; p. 356. [Google Scholar]
- 41.Tizard I.R. 7th ed. WB Saunders Company; Philadelphia: 2004. Veterinary immunology, an introduction; p. 447. [Google Scholar]
- 42.Bin-Hafeez B., Haque R., Parvez S., Pandey S., Sayeed I., Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int J Immunopharm. 2003;3:257–265. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- 43.Clark Steven C., Kamen Robert. The human hematopoietic colony-stimulating factors. Science. 1987;236:1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- 44.Verma A., Kumar B., Singh M.K., Kharya M.D. Immunomodulatory potential of cow urine. Schol Res Library. 2011;3:507–513. [Google Scholar]
- 45.Bhardwaj U., Tiwary B.K., Prasad A., Ganguly S. Effect of Tinospora cordifolia extract on immune response and serum biochemical profile in broilers. Indian J Anim Sci. 2012;82:379–381. [Google Scholar]
- 46.Yadav H., Mehta A., Singh V. In vitro study of go-mutra-aloe-an ayurvedic preparation against pathogenic bacteria. Indian J Anim Sci. 2006;76:448–451. [Google Scholar]
- 47.Garg N., Chauhan R.S., Kumar A. Assessing the effect of cow urine on immunity of White Leghorn layers. ISAH. 2005;2:81–83. [Google Scholar]
- 48.Sultana R., Khanam S., Devi K. Evaluation of Immunomodulatory activity of Solanum xanthocarpum fruits aqueous extract. Der Pharm Lett. 2011;3:247–253. [Google Scholar]
- 49.Kaneko J.J. 5th ed. Academic Press, U.S.A; 1997. Serum proteins and the dysproteinemias. Clinical biochemistry of domestic animals; pp. 120–129. [Google Scholar]
- 50.Goran H.M. Harper and Row publishers; U. S. A: 2009. Clinical biochemistry; p. 283. [Google Scholar]
- 51.Pragasam S.J., Venkatesan V., Rasool M. Immunomodulatory and anti-inflammatory effect of p-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation. 2013;36:169–176. doi: 10.1007/s10753-012-9532-8. [DOI] [PubMed] [Google Scholar]
- 52.Mungantiwar A.A., Nair A.M., Shinde U.A., Dixishit V.J., Saraf M.N., Thakur V.S., et al. Studies on the immunomodulatory effects of Boerhaavia diffusa alkaloidal fraction. J Ethnopharmacol. 1999;65:125–133. doi: 10.1016/s0378-8741(98)00153-6. [DOI] [PubMed] [Google Scholar]
- 53.Kumari R., Prasad A., Tiwary B.K., Ganguly S. Oroxylum indicum possess a potential effect on humoral and cell mediated immune response in broiler chicks. Indian J Anim Sci. 2011;81:1212–1214. [Google Scholar]
- 54.Mazumder P.M., Pattnayak S., Parvani H., Sasmal D., Rathinavelusamy P. Evaluation of immunomodulatory activity of Glycyrhiza glabra L roots in combination with zing. Asian Pac J Trop Biomed. 2012;2:15–20. [Google Scholar]
- 55.McHeyzer-Williams M., McHeyzer-Williams L., Panus J., Pogue-Caley R., Bikah G., Driver D. Helper T cell- regulated B cell immunity. Microb Infect. 2003;5:205–212. doi: 10.1016/s1286-4579(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 56.Ross R.G., Selvasubramanian S., Samuel J. Effect of Punica granatum on dexamethasone induced immunosuppression in rabbits. Indian J Anim Sci. 2004;74:139–142. [Google Scholar]
- 57.Fulzele S.V., Satturwar P.M., Joshi S.B., Dorle A.K. Study of the immunomodulatory activity of Haridradigrita in rats. Indian J Pharmacol. 2003;35:51–54. [Google Scholar]
- 58.Meenakshi V.K., Paripooranaselvi M., Senthamarai S., Gomathy S., Chamundeswari K.P. Antitumor and immunomodulatory activity of Phallusia nigra savigny, 1816 against Ehrlich carcinoma. Res J Pharmaceut Sci. 2012;1:7–12. [Google Scholar]
- 59.Kuttan G. Immunomodulatory activities of punarnavine, an alkaloid from Boerhaavia diffusa. Immunopharmacol Immunotoxicol. 2009;31:377–387. doi: 10.1080/08923970802702036. [DOI] [PubMed] [Google Scholar]
- 60.Mendez-Ferrer S., Bonnet D., Steensma D.P., Hasserjian R.P., Ghobrial I.M., Gribben J.G., et al. Bone marrow niches in haematological malignancies. Nat Rev Cancer. 2020;20:285–298. doi: 10.1038/s41568-020-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sido J.M., Nagarkatti P.S., Nagarkatti M. Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed-type hypersensitivity. Eur J Immunol. 2016;46:1472–1479. doi: 10.1002/eji.201546181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith H.F., Kroes B.H., Vandenberg A.J.J., Vanderwal D., Vanderworm E., Beukelman C.J., et al. Immunomodulatory and anti-inflammatory activity of Picorrhiza scrophulariflora. J Ethnopharmacol. 2000;73:101–109. doi: 10.1016/s0378-8741(00)00268-3. [DOI] [PubMed] [Google Scholar]
- 63.Li Q., Wang H., Peng H., Huyan T., Cacalano N.A. Exosomes: versatile nano mediators of immune regulation. Cancers. 2019;11:1–21. doi: 10.3390/cancers11101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be made available upon reasonable request to the corresponding author.