Abstract
Fuel interactions in contracting muscle represent a complex interplay between enzymes regulating carbohydrate and fatty acid catabolism, converging in the mitochondrial matrix. While increasing exercise intensity promotes carbohydrate use at the expense of fatty acid oxidation, the mechanisms underlying this effect remain poorly elucidated. As a potential explanation, we investigated whether exercise-induced reductions in intramuscular pH (acidosis) attenuate carnitine palmitoyltransferase-I (CPT-I)-supported bioenergetics, the rate-limiting step for fatty acid oxidation within mitochondria. Specifically, we assessed the effect of a physiologically relevant reduction in pH (pH 7.2 versus 6.8) on single and mixed substrate respiratory responses in murine skeletal muscle isolated mitochondria and permeabilized fibers. While pH did not influence oxidative phosphorylation stoichiometry (ADP/O ratios), coupling efficiency, oxygen affinity, or ADP respiratory responses, acidosis impaired lipid bioenergetics by attenuating respiration with L-carnitine and palmitoyl-CoA, while enhancing the inhibitory effect of malonyl-CoA on CPT-I. These acidotic effects were largely retained following a single bout of intense exercise. At rest, pyruvate and succinate-supported respiration were also impaired by acidosis. However, providing more pyruvate and ADP at pH 6.8 to model increases in glycolytic flux and ATP turnover with intense exercise overcame the acidotic attenuation of carbohydrate-linked oxidative phosphorylation. Importantly, this situation is fundamentally different from lipids where CPT-I substrate sensitivity and availability is impaired at higher power outputs suggesting lipid metabolism may be more susceptible to the effects of acidosis, possibly contributing to fuel shifts with increasing exercise intensity.
Keywords: mitochondrial bioenergetics, skeletal muscle, pH, CPT-I, lipid metabolism, fuel metabolism, exercise
Decades of research have been devoted to understanding the regulation of carbohydrate and lipid metabolism within skeletal muscle in different metabolic contexts. It is understood that both lipids and carbohydrates are oxidized at rest and during exercise; however, the relative contribution of these substrates to ATP provision is reciprocally regulated, whereby a shift in fuel preference toward carbohydrates occurs beyond exercise intensities of ∼60 to 65% VO2max (1, 2). The mechanisms within skeletal muscle contributing to these fuel interactions remain incompletely understood; however, it is clear that intramuscular conditions during intense exercise impose some limitation on lipid oxidation. Within this context, carnitine palmitoyltransferase-I (CPT-I) has been proposed as a key control point, considering this enzyme is rate limiting for the oxidation of lipids within mitochondria and is highly regulated by substrate (L-carnitine and fatty acyl-CoA [e.g., palmitoyl-CoA (P-CoA)]) and allosteric inhibitor (malonyl-CoA [M-CoA]) content and sensitivity.
Historically, the content of M-CoA has been considered fundamental to understand fuel selection during exercise. However, in humans, M-CoA content is not sufficiently reduced with exercise to account for absolute increases in lipid oxidation (3, 4, 5), nor does M-CoA content display intensity-dependent responses despite marked differences in rates of lipid oxidation (4). In addition, while exercise has been shown to attenuate M-CoA sensitivity as a mechanism to promote CPT-I flux (6, 7, 8), this does not appear to be influenced by exercise intensity (7) and therefore cannot account for the reduction in lipid oxidation that occurs during intense exercise (1). Alternatively, reductions in the sensitivity of CPT-I to L-carnitine, as well as L-carnitine availability, are intensity dependent and have been proposed as possible mechanisms for the apparent reduction in CPT-I flux with increasing intensity (1, 7), responses likely exacerbated by reduced lipolytic rates (1) and acidotic inhibition of maximal CPT-I activity (9, 10). Alternatively, unlike lipid metabolism, during intense exercise glycolytic/glycogenolytic rates increase the provision of pyruvate to the mitochondrial matrix despite marked reductions in intramuscular pH (as low as ∼6.3 during maximal exercise in humans from values at rest of ∼7.0–7.2) (11, 12, 13). For this reason, understanding the influence of pH on CPT-I, as well as overall oxidative phosphorylation (OXPHOS), is warranted, as it is possible that lipid metabolism is more susceptible to the effects of acidosis.
Therefore, the aim of the current study was to examine how pH may differentially influence mitochondrial bioenergetics supported by lipids and carbohydrates. Herein, we provide evidence that acidosis attenuates lipid-supported bioenergetics by two independent mechanisms—a reduction in substrate sensitivity (P-CoA and L-carnitine) and an enhanced ability of M-CoA to inhibit CPT-I, responses largely retained in skeletal muscle immediately following a bout of intense exercise. Alternatively, while pyruvate and succinate-supported bioenergetics were attenuated by acidosis in resting muscle, providing more pyruvate and ADP to model increases in glycolytic flux/ATP turnover with intense exercise was sufficient to overcome this effect. Notably, unlike substrate provision from carbohydrate, CPT-I substrate sensitivity and availability are limited rather than enhanced at higher power outputs, suggesting lipid metabolism may be more susceptible to the attenuating effects of acidosis.
Results
Mitochondrial purity, coupling efficiency, ADP bioenergetics, and O2 affinity
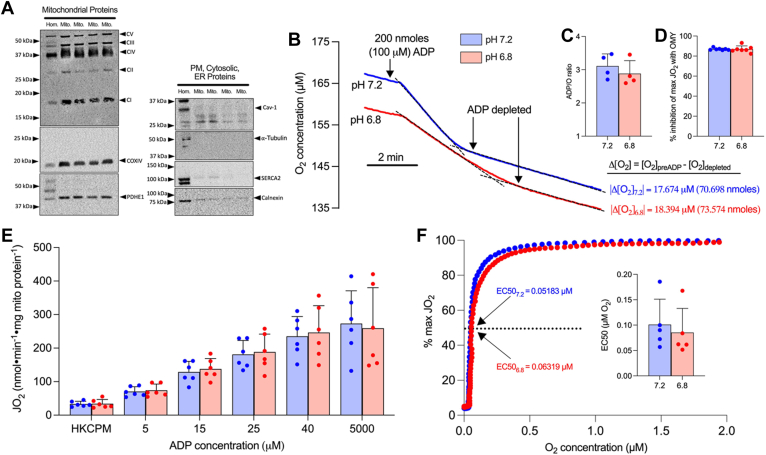
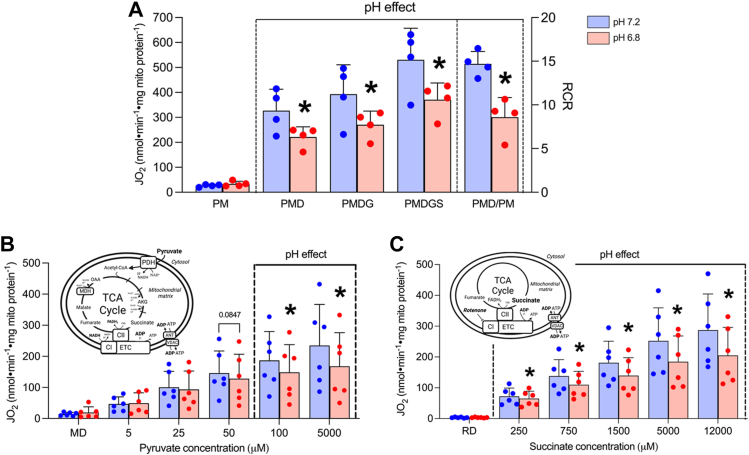
We first assessed the purity and quality of our in vitro isolated mitochondrial preparation. Mitochondrial isolation concentrated mitochondrial proteins (OXPHOS, cytochrome c oxidase subunit IV, pyruvate dehydrogenase [PDH] E1) while the presence of non-mitochondrial proteins was negligible (caveolin-1, alpha-tubulin, sarcoendoplasmic reticulum calcium ATPase [SERCA2], calnexin) (Fig. 1A). Mitochondrial isolation yielded coupled mitochondria assessed by ADP/O ratios (Fig. 1, B and C) and inhibition of maximal respiration with oligomycin (OMY), an inhibitor of F1/F0 ATPase (Fig. 1D). Importantly, pH had no effect on ADP/O ratios (Fig. 1C, pH 7.2, 3.11 ± 0.36; pH 6.8, 2.88 ± 0.39; p = 0.286) or the respiratory response to OMY (Fig. 1D, pH 7.2, 86.90 ± 1.08% inhibition; pH 6.8, 86.85 ± 3.30% inhibition; p = 0.959) suggesting mild acidosis does not affect OXPHOS coupling, a finding further supported by the observed consistent respiratory response during an ADP titration (Fig. 1E). To further assess the impact of acidosis on mitochondrial quality we assessed mitochondrial oxygen (O2) affinity, as O2 provision to mitochondria is a key determinant of OXPHOS rate. Similar to the absence of a pH effect on ADP responses, acidosis did not alter the kinetic assessment of O2 affinity (Fig. 1F). These initial experiments verified our in vitro model to subsequently investigate the impact of pH on CPT-I.
Figure 1.
Acidosis does not affect coupling efficiency, respiratory responses to ADP, or mitochondrial oxygen affinity.A, representative blots of mitochondrial and non-mitochondrial proteins in red gastrocnemius muscle homogenate (Hom.) and purified muscle mitochondria (Mito.). B, representative trace (single experiment) of O2 utilization following the addition of 200 nmoles ADP. The change in O2 content (nmoles O2) is used to calculate (C) ADP/O ratios. D, percent inhibition of maximal CI/CII-supported respiration following the addition of 0.5 μM oligomycin. E, respiration with titrating ADP in the presence of a hexokinase-ADP clamp (HKC), 5 mM pyruvate, and 2 mM malate. F, representative curve of respiration/oxygen kinetic relationship utilized to quantify (inset) mitochondrial oxygen affinity (O2 EC50). Data are expressed as mean + SD (n = 4–7 per group) and analyzed with a paired Student’s t test (∗p ≤ 0.05). ADP, adenosine diphosphate; Cav-1, caveolin-1; CI-V, complexes I-V; COXIV, cytochrome C oxidase subunit IV; D, ADP; ER, endoplasmic reticulum; HKC, hexokinase-ADP clamp; JO2, respiratory flux rate; M, malate; OMY, oligomycin; PM, plasma membrane; P, pyruvate; PDH, pyruvate dehydrogenase; SERCA2, sarcoendoplasmic reticulum calcium ATPase.
Effects of acidosis on lipid bioenergetics
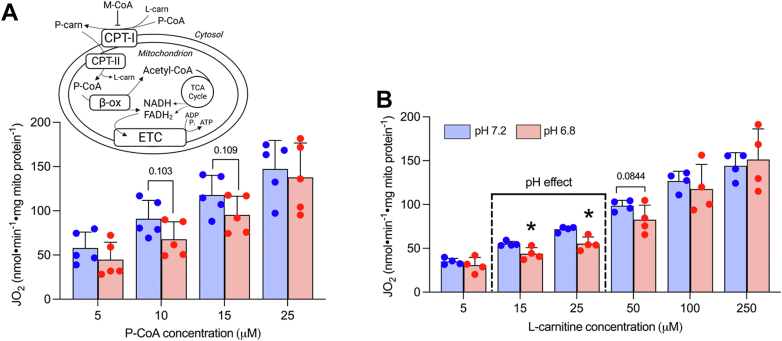
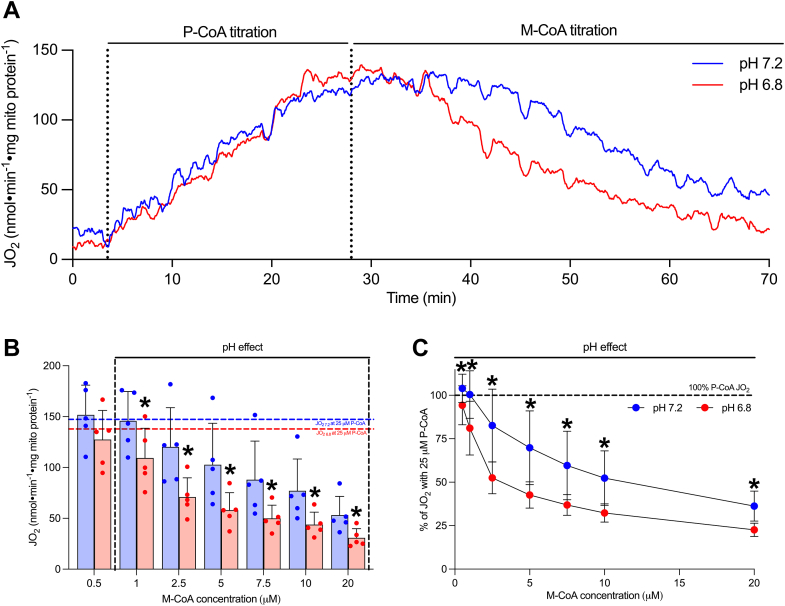
Our understanding of the mechanisms whereby acidosis may inhibit lipid-supported bioenergetics is limited; therefore, we examined the impact of acidosis on respiratory responses with CPT-I substrates (P-CoA and L-carnitine) across a range of concentrations (Fig. 2A, inset). Respiration supported by submaximal P-CoA trended to be lower (Fig. 2A, p = 0.10), while acidosis attenuated submaximal L-carnitine-supported respiration (Fig. 2B). Combined, these data suggest that acidosis may attenuate lipid provision to the mitochondrial matrix in situations where substrate content is limiting (i.e., during intense exercise). However, in addition to P-CoA and L-carnitine provision/responsiveness, CPT-I is also subject to allosteric control by M-CoA, which limits lipid flux through CPT-I. Therefore, we examined whether pH affected the ability of M-CoA to suppress lipid-supported respiration in our model. Specifically, P-CoA was titrated in the presence of saturating L-carnitine to stimulate CPT-I-dependent respiration, and subsequently M-CoA was titrated to examine its inhibitory effects (Fig. 3A). This approach revealed that CPT-I was more sensitive to the inhibition exerted by M-CoA in acidotic conditions, as both the absolute (Fig. 3B) and relative (Fig. 3C) inhibition of CPT-I-dependent respiration was enhanced at pH 6.8 compared with pH 7.2. Together, these data provide two independent mechanisms whereby acidosis may be attenuating lipid-supported bioenergetics—a reduction in substrate-supported respiration, especially when L-carnitine availability is limiting, and an enhanced ability of M-CoA to inhibit CPT-I.
Figure 2.
Acidosis attenuates submaximal respiration with L-carnitine.A, respiration with titrating P-CoA in the presence of 2 mM malate, 5 mM ADP, and 750 μM L-carnitine. Inset: Schematic outlining the contribution of P-CoA and L-carnitine as CPT-I substrates to lipid-supported bioenergetics and M-CoA as an allosteric inhibitor of CPT-I. B, respiration with titrating L-carnitine in the presence of 2 mM malate, 5 mM ADP, and 25 μM P-CoA. Data are expressed as mean + SD (n = 4–5 per group) and analyzed with a paired Student’s t test (∗p ≤ 0.05)). B-ox, beta-oxidation; ETC, electron transport chain; FADH2, flavin adenine dinucleotide, JO2, respiratory flux rate; L-carn, L-carnitine; M, malate; M-CoA, malonyl-CoA; P-carn, palmitoyl-carnitine; P-CoA, palmitoyl-CoA.
Figure 3.
Acidosis enhances the inhibitory effect of M-CoA on CPT-I-supported bioenergetics.A, representative trace of protocol used to assess M-CoA inhibitory response where P-CoA was titrated in the presence of 2 mM malate, 5 mM ADP, and 750 μM L-carnitine followed by a titration of M-CoA (0.5–20 μM). B, inhibition of maximal respiration (JO2) with 25 μM P-CoA with titrating M-CoA. C, relative inhibition of maximal respiration (100% P-CoA JO2) with titrating M-CoA. Data are expressed as mean + SD (n = 5 per group) and analyzed with a paired Student’s t test (∗p ≤ 0.05). JO2, respiratory flux rate; M-CoA, malonyl-CoA; P-CoA, palmitoyl-CoA.
The effect of a single bout of intense exercise on lipid-supported bioenergetics and malonyl-CoA inhibition of CPT-I
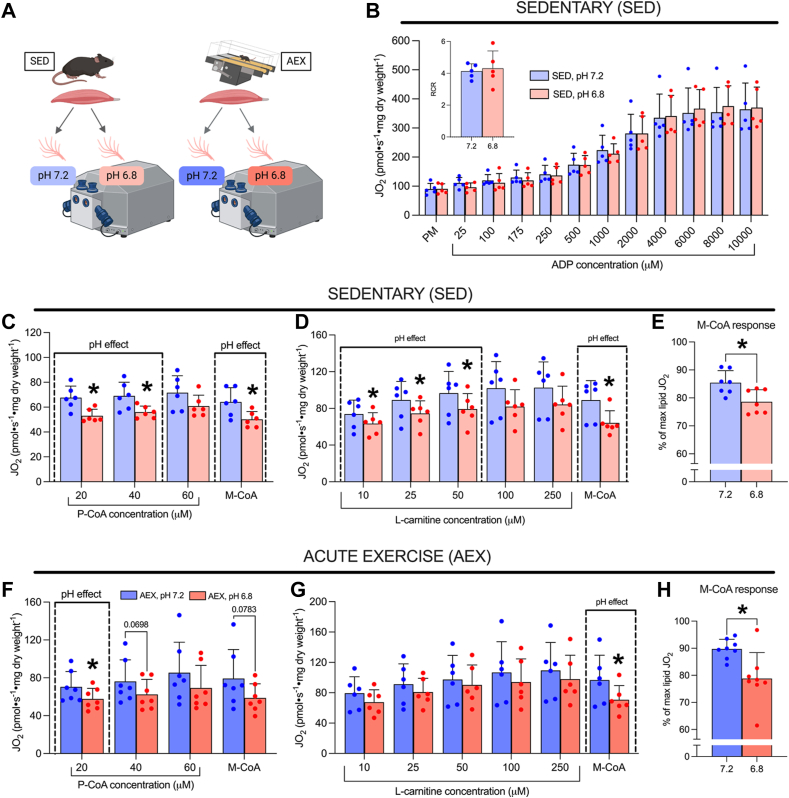
While the current data suggest mild acidosis enhances M-CoA inhibition of CPT-I, in contrast, M-CoA inhibition of CPT-I is impaired following a single bout of exercise when assessed in neutral buffers (6, 7, 8). Therefore, we also aimed to understand the possible interaction between exercise and acidosis by determining if our previous observation in resting muscle (enhanced M-CoA inhibition of CPT-I by acidosis) would persist following a single bout of intense exercise. To investigate this, we utilized permeabilized skeletal muscle fibers (PmFbs) in sedentary mice (SED) and in mice immediately following one bout of intense exercise (acute exercise, AEX) (Fig. 4A). We transitioned to PmFb as the cytosolic/cytoskeletal components retained in this model may influence CPT-I respiratory responses (14) and have been shown to alter M-CoA inhibition of CPT-I in the context of exercise (6). However, given the change in model, we first aimed to replicate certain respiratory responses to pH in sedentary animals to ensure the pH effects of interest (in particular M-CoA inhibitory responses) were maintained (Fig. 4, B–E). Respiration with titrating ADP revealed no pH effect (Fig. 4B), while submaximal P-CoA and L-carnitine-supported respiration were attenuated by acidosis (Fig. 4, C and D), largely replicating responses in isolated mitochondria (Figs. 1E and 2B). In addition, in resting muscle, the effect of acidosis to enhance M-CoA inhibition of CPT-I-supported bioenergetics was replicated when expressed both as absolute respiration in the presence of 7 μM M-CoA (Fig. 4, C and D) and as a percentage of maximal lipid-supported respiration (Fig. 4E). These experiments in sedentary animals provided initial validation that the respiratory responses to acidosis are generally maintained between PmFb and isolated mitochondria; thus, we next evaluated the same pH responses in mice following a single bout of intense exercise.
Figure 4.
Acidosis enhances M-CoA inhibition of CPT-I in permeabilized muscle fibers from sedentary mice, which persists following a single bout of intense exercise.A, schematic representation of respiration experiments in permeabilized skeletal muscle fibers from SED and AEX mice. B, ADP titration in SED mice, inset: RCR. C, P-CoA titration in SED mice. D, L-carnitine titration in SED mice. E, percent of maximal lipid-supported respiration with M-CoA in SED mice. F, P-CoA titration in AEX mice. G, L-carnitine titration in AEX mice. H, percent of maximal lipid-supported respiration with M-CoA in AEX mice. All L-carnitine titrations were performed in the presence of 1 mM malate, 5 mM ADP, and 60 μM P-CoA. All P-CoA titrations were performed in the presence of 1 mM malate, 5 mM ADP, and 1 mM L-carnitine. M-CoA inhibition of CPT-I was assessed in the presence of 7 μM M-CoA, and relative respiration with M-CoA was calculated by averaging relative JO2 values (% of maximal P-CoA) and L-carnitine respiration as technical replicates (quadruplicate or duplicate). Data are expressed as mean + SD (n = 5–8 per group) and analyzed with a paired Student’s t test (∗p ≤ 0.05). ADP, adenosine diphosphate; AEX, acute exercise; JO2, respiratory flux rate; M-CoA, malonyl-CoA; P-CoA, palmitoyl-CoA; RCR, respiratory control ratio; SED, sedentary.
Following a single bout of exercise, P-CoA-supported respiration was attenuated by acidosis at 20 μM; however, no pH effect was observed at 40 μM or 60 μM (Fig. 4F). L-carnitine-supported respiration was unaffected by pH; however, strikingly, the ability of M-CoA to inhibit lipid-supported bioenergetics remained significantly enhanced by acidosis when expressed as absolute L-carnitine respiration in the presence of 7 μM M-CoA (Fig. 4G) and as a percentage of maximal lipid-supported respiration (Fig. 4H), indicating that the effect of acidosis to attenuate lipid metabolism at rest is not overcome with exercise.
Effect of pH on carbohydrate and electron transport chain–specific bioenergetics
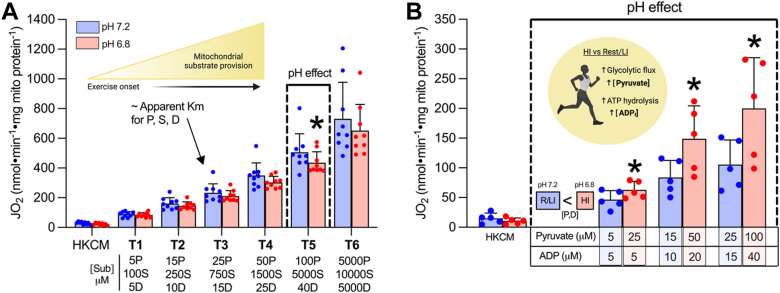
Unlike the limitation on CPT-I substrate availability at higher power outputs, carbohydrate provision is enhanced. We therefore aimed to determine the impact of acidosis on respiration with carbohydrate-linked substrates, as well as the possibility that increased substrate provision could override any inhibitory effects. Acidosis generally reduced respiration by ∼20 to 30% in the presence of complex I and II linked substrates (Fig. 5, A–C). However, unlike reductions in lipolysis and mitochondrial fatty acid provision with increasing exercise intensity (1), glycolytic/glycogenolytic flux rates and ATP hydrolysis are both upregulated, increasing free ADP and pyruvate provision to mitochondria (13, 15). To model this concept, we titrated pyruvate, succinate, and ADP in combination (Fig. 6A) with the third titration (T3) approximating the apparent Km for each substrate in isolated mitochondria. Using this approach, respiration was no longer consistently lower with acidosis (significant only at the fifth titration (T5) p = 0.0492), suggesting the pH-independent nature of titrating ADP may partially offset the inhibitory effect of acidosis on carbohydrate-linked OXPHOS. In addition, we titrated lower concentrations of ADP and pyruvate at pH 7.2 (representative of low-moderate intensity exercise) and higher concentrations of ADP and pyruvate at pH 6.8 (representative of higher intensity exercise when intramuscular pH drops but substrate provision is increased). Utilizing this approach, we found that respiration stimulated by higher substrate concentrations at pH 6.8 was ∼135 to 190% that of respiration stimulated with lower substrate concentrations at pH 7.2 (Fig. 6B, T1 p = 0.00114, T2 p = 0.00692, T3 p = 0.0103). These data support the concept that while respiration with pyruvate may be limited by acidosis at rest, providing more substrates, as occurs with greater rates of glycogenolytic/glycolytic flux at higher power outputs, can outcompete this inhibitory effect.
Figure 5.
Carbohydrate and ETC-specific respiration is attenuated by acidosis in resting muscle.A, respiration supported by saturating substrates (5 mM pyruvate, 2 mM malate, 5 mM ADP, 10 mM glutamate, 10 mM succinate, and RCR [state 3/state 4]). B, pyruvate titration in the presence of 2 mM malate and 5 mM ADP. C, succinate titration in the presence of 0.5 μM rotenone (R) and 5 mM ADP (D). Data are expressed as mean + SD (n = 4–6 per group) and analyzed with a paired Student’s t test (∗p ≤ 0.05). ANT, adenine nucleotide translocase; CI/CII, complex 1/II; D, ADP; ETC, electron transport chain; JO2, respiratory flux rate; M, malate; MDH, malate dehydrogenase; OAA, oxaloacetate; PDH, pyruvate dehydrogenase; R, rotenone; TCA cycle, tricarboxylic acid cycle; VDAC, voltage-dependent anion channel.
Figure 6.
Respiration supported by combined carbohydrate-linked substrates is mildly attenuated by acidosis but overcome when pyruvate and ADP provision is increased.A, combined titration of pyruvate, succinate, and ADP. B, titration of pyruvate and ADP at different concentrations (higher provision at pH 6.8 and lower provision at pH 7.2). Both titration protocols were conducted in the presence of HKC constituents and 2 mM malate. Data are expressed as mean + SD (n = 5–9 per group) and analyzed with a paired Student’s t test (∗ p ≤ 0.05). D, ADP; HI, high intensity (exercise); HKC, hexokinase-ADP clamp; JO2, respiratory flux rate; M, malate; P, pyruvate; R/LI, rest/low intensity (exercise); S, succinate.
Discussion
The focus of the current study was to investigate the mechanisms whereby acidosis modulates mitochondrial bioenergetics in skeletal muscle at rest and following a single bout of intense exercise. In particular, we were interested in leveraging the control of an in vitro approach to investigate possible mechanisms contributing to the reciprocal response of lipid and carbohydrate oxidation with increasing exercise intensity in vivo. We provide evidence suggesting that a physiological reduction in pH attenuates mitochondrial lipid metabolism by at least two distinct mechanisms—a reduction in P-CoA/L-carnitine respiration and an enhanced ability of M-CoA to inhibit CPT-I. In contrast, despite an attenuation of pyruvate and succinate-supported respiration by acidosis when comparing similar substrate concentrations, increasing pyruvate and ADP provision in acidotic conditions, such as what occurs during intense exercise, is sufficient to overcome the attenuating effect of acidosis on carbohydrate-linked respiration.
Regulation of lipid bioenergetics by pH
While absolute rates of lipid oxidation increase from rest to exercise, maximal rates peak at ∼65% VO2max and decline thereafter (16), aligning with the relative reduction in lipid oxidation in favor of carbohydrate use (17). Many factors likely contribute to this downregulation, but multiple theories implicate the rate-limiting reaction catalyzed by CPT-I as a key control point (1, 3, 8). Both L-carnitine and P-CoA are obligatory substrates for CPT-I, and, while M-CoA does not directly participate in this transesterification reaction, it is a potent allosteric inhibitor of this enzyme. Thus, mechanisms limiting the availability or potency of CPT-I substrates (L-carnitine and P-CoA) and/or enhancing the inhibitory effects of M-CoA would limit CPT-I flux and subsequently lipid oxidation.
In the current study, we observed an attenuation of P-CoA and L-carnitine-supported respiration in an acidic environment. Intense exercise, which reduces intramuscular pH, has been shown to reduce both L-carnitine content (1) and sensitivity (7) when assessed in a neutral buffer. Thus, it is possible that acidosis could impose an additional limitation on CPT-I during intense exercise, especially when L-carnitine availability is reduced, as we only observed a reduction in respiration when the concentration of L-carnitine was limiting. While this is in contrast to previous reports examining maximal CPT-I activity in isolated mitochondria (9, 10, 18), important methodological differences exist. For instance, the current work uses intact respiring mitochondria, while CPT-I maximal activity assays assess flux to palmitoylcarnitine while inhibiting the electron transport chain (8). Furthermore, maximal CPT-I activity assays are supported by supraphysiological concentrations of P-CoA (300 μM) and L-carnitine (5 mM), which are ∼5- to 100-fold greater than concentrations where we detected pH effects in the current study (<60 μM P-CoA, <50 μM L-carnitine). Interestingly, unlike L-carnitine kinetics, P-CoA sensitivity remains unchanged following acute exercise in rodents (7) and humans (8), suggesting CPT-I may be more susceptible to acute changes in L-carnitine. Indeed, a reduction in P-CoA respiration may not be required to limit CPT-I flux in this context, considering the transesterification reaction catalyzed by CPT-I relies on both P-CoA (fatty acyl-CoA) and L-carnitine; thus, reducing the content or sensitivity of one substrate would likely limit CPT-I flux. Nevertheless, in the present study acidosis also attenuated submaximal P-CoA-supported respiration, but only when assessed in permeabilized fibers, possibly suggesting a role for intermediate filaments/microtubules, which are known to influence CPT-I substrate responses (6, 19). In support of this, early work by Mills et al. (1984) reported the absence of a change in P-CoA sensitivity across a range of pH conditions in isolated mitochondria. Regardless of these methodological differences, there is consistency with the finding that CPT-I is inhibited by acidosis, which may contribute to the well-established reduction in lipid oxidation during intense exercise.
In addition to substrate-dependent control, M-CoA localization, content, and responsiveness present as independent mechanisms regulating CPT-I activity. While reductions in M-CoA content in rat skeletal muscle occur during exercise (20), in human skeletal muscle M-CoA content is not sufficiently reduced to fully account for the absolute increase in lipid oxidation from rest to exercise (3, 4). While the sequestration of M-CoA in a bound/intramitochondrial form may present as an alternative mechanism limiting M-CoA availability at the level of CPT-I (21), changes in M-CoA responsiveness/sensitivity have also been investigated, where studies in rodents (6, 7) and humans (8) have indicated the CPT-I sensitivity to M-CoA is reduced following acute exercise, which would also promote lipid use. However, these acute exercise responses have only been evaluated in neutral buffers; thus, the possible influence of acidosis in this context was unknown. In the current study, we observed a robust ability for acidosis to enhance the inhibitory effects of M-CoA on OXPHOS across a wide range of concentrations in isolated mitochondria (1–20 μM) and PmFb (with 7 μM M-CoA) from resting skeletal muscle. While it is tempting to directly extrapolate these findings to in vivo biology given the overlap with M-CoA concentrations in human skeletal muscle (∼2 μM (4)), caution is warranted with this thought process given the absence of possible M-CoA binding proteins, microfilaments (6) and microtubules (19) in an isolated mitochondrial preparation, proteins all present in PmFb. Nevertheless, in the present study, M-CoA sensitivity in respiring mitochondria appears to be lower than historical assessments directly evaluating CPT-I enzymatic activity (18, 22) which would predict almost complete inhibition of fat oxidation in resting muscle given the known content of M-CoA within this tissue (4). Regardless of these differences, importantly, all methodologies support the notion that CPT-I sensitivity to M-CoA is enhanced in acidotic conditions, which would limit lipid oxidation. Moreover, we extend upon these findings in resting muscle, showing that this response is maintained following a single bout of intense exercise, suggesting that the alterations in CPT-I activity/kinetics conveyed by exercise may be overcome by those induced by acidosis. While it remains unknown how acute exercise alters CPT-I substrate/inhibitor sensitivity, classical exercise-related allosteric metabolites do not alter CPT-I activity in vitro (10), and it is quite striking that acute exercise-mediated reductions in M-CoA sensitivity are retained through muscle fiber permeabilization and differential centrifugation to obtain isolated mitochondria, suggesting exercise may induce a lasting (perhaps structural) change at the level of CPT-I. Although the mechanism(s) by which exercise and pH affect CPT-I remain elusive, several possibilities exist including alterations in N-C termini spatial interactions (23), protonation of membrane lipids and alterations in membrane fluidity (24, 25), and protonation of key histidine residues within the catalytic domain of CPT-I (26). Alternatively, the effect of pH may be more transient by altering M-CoA-CPT-I interactions, as work by Stephens et al. (1983) and Mills et al. (1984) suggest that reductions in M-CoA binding (lower Kd) in acidotic conditions may be explained by transient protonation of M-CoA or amino acid residues in the M-CoA-binding site of CPT-I (18, 27). Altogether, while these data implicate both substrate and allosteric inhibitor (M-CoA) responses as possible explanations for the observed reduction in CPT-I activity by acidosis (9, 10), the mechanisms of action remain to be fully delineated.
Integrating in vitro findings in the context of fuel utilization with increasing exercise intensity
In vitro assessments of glycogen phosphorylase and phosphofructokinase activity indicate that acidosis in isolation may limit the activity of these enzymes (28, 29); however, since carbohydrate breakdown and pyruvate/lactate accumulation are enhanced in vivo during intense exercise despite reduced intramuscular pH (11), the provision of pyruvate to the mitochondrial matrix is likely not limiting. Indeed, we observed a reduction in pyruvate and succinate-supported respiration when comparing equal concentrations of these substrates between pH conditions; however, this does not recapitulate differences in substrate supply with intense exercise where glycolytic flux/ATP turnover is enhanced concomitantly with reduced intramuscular pH (12, 13, 15, 30). When modeling this concept by providing higher concentrations of ADP and pyruvate at pH 6.8 compared with pH 7.2, the previously observed acidotic depression of carbohydrate-linked OXPHOS was overcome. We acknowledge that substrate interactions are difficult to directly evaluate using mitochondrial respiration methodologies and titrations with multiple substrates/concentrations assess but one factor (substrate availability) changing in muscle during intense exercise. However, these data suggest that enhanced pyruvate and ADP availability associated with increased glycolytic/glycogenolytic flux is sufficient to enhance carbohydrate use despite the acidotic environment, but interestingly the opposite may occur for lipids. With high glycolytic flux rates, the formation of acetyl-CoA by PDH can occur in excess of what can be metabolized by the tricarboxylic acid cycle cycle, leading to PDH back-inhibition. Excess acetyl-CoA can be dissipated by forming acetylcarnitine to alleviate this backpressure, but at the expense of L-carnitine availability to CPT-I (1, 31). Therefore, pH-dependent reductions in L-carnitine respiration may be exacerbated in the presence of high rates of glycolytic flux. In addition, considering M-CoA binding occurs at a distinct site from CPT-I substrates (32), the observed enhancement of M-CoA inhibition of CPT-I by acidosis may attenuate lipid use in a compounding manner. Thus, when considering the whole-muscle environment during intense exercise, mitochondrial lipid flux/oxidation may be more susceptible to the attenuating effects of low pH compared with the aerobic oxidation of carbohydrate.
Conclusion
We provide evidence that, while acidosis attenuates L-carnitine, palmitoyl-CoA, pyruvate, and succinate-supported respiration, increasing non-lipid substrate provision (pyruvate) and ADP, as occurs during high intensity exercise, is sufficient to overcome the inhibitory effect of acidosis on carbohydrate-linked OXPHOS. However, since acidosis attenuates lipid bioenergetics by two independent mechanisms, a reduction in L-carnitine-supported respiration and enhanced M-CoA inhibition of CPT-I, we provide new insights into the mechanisms whereby acidosis may contribute to the reduction in whole-body lipid oxidation rates at higher power outputs.
Experimental procedures
Rodents
All rodent experiments were conducted in accordance with institutional guidelines approved by the Animal Care Committee at the University of Guelph. Female C57BL/6N mice were bred in house (University of Guelph) and group-housed (2–4 mice/cage) for all sedentary experiments and single-housed for acute exercise experiments. All mice were kept on a 12:12 light–dark cycle at room temperature with ad libitum access to standard chow diet and water.
Skeletal muscle mitochondrial isolation and respirometry
Hindlimb skeletal muscles (red gastrocnemius, plantaris, tibialis anterior, soleus, and quadriceps) were excised and immediately utilized to isolate mitochondria using temperature-controlled (4 °C) differential centrifugation as described (33). Isolated subsarcolemmal and intermyofibrillar mitochondria were pooled to increase protein recovery and resuspended in 200 μl of Mg2+-absent MiR05 (0.5 mM EGTA, 60 mM potassium lactobionate, 10 mM KH2PO4, 20 mM Hepes, 110 mM sucrose, 1 g/L fatty acid–free bovine serum albumin, pH 7.2). A Bradford protein assay was utilized to quantify the amount of mitochondrial protein (μg/μl) in each sample prior to conducting mitochondrial respiration.
Mitochondrial respiration experiments were performed using the Oroboros Oxygraph-2k systems at 37 °C with constant stirring and room air saturation (starting at ∼180 μM O2). A total of 30 or 50 μg of mitochondrial protein was loaded per 2-ml chamber, and each experiment was conducted as a technical replicate (duplicate). Depending on the protocol, respiration was conducted in buffers (Mg2+-absent MiR05, or MiR05 [Mg2+ absent MiR05 + 3 mM MgCl26H2O]) at pH 7.2 or 6.8 (Mg2+-absent unless specified). Randomly, mitochondria remaining following respiration were frozen at −80 °C prior to purification utilizing a Percoll density gradient and subsequent Western blotting (n = 4 independent mitochondrial samples) as described (33, 34). Commercially available antibodies were used to detect OXPHOS proteins (1:500, Abcam Ab110413), cytochrome c oxidase subunit IV (1:30,000, Invitrogen A21347), PDHE1 (1:1000, Invitrogen 459400), Caveolin-1 (1:1000, BD Biosciences 610406), Alpha-tubulin (1:1000, Abcam Ab7291), SERCA2 (1:1000, Abcam Ab2861), and Calnexin (1:2000, Sigma C4731).
ADP/O ratios were calculated utilizing the ADP pulse method (35), using the change in oxygen concentration following the addition of 200 nmoles ADP in the presence of 5 mM pyruvate and 2 mM malate. Maximal respiration was assessed with subsequent additions of 5 mM ADP (PMD, State 3), 10 mM glutamate (PMDG, maximal complex I–supported respiration), and 10 mM succinate (PMDGS, maximal complex I/II–supported respiration). Respiratory control ratios were quantified as the ratio of state 3 to state 4 (presence of pyruvate and malate but absence of ADP) respiration.
Mitochondrial oxygen affinity was evaluated as previously described (36) with minor modifications. Briefly, respiration was assessed in MiR05 in the presence of a hexokinase-ADP clamp (2-deoxyglucose [3 mM] and hexokinase [0.5 U/ml]) to prevent ADP depletion. Following the addition of saturating substrates (5 mM pyruvate, 2 mM malate, 5 mM ADP) mitochondria respired until oxygen was depleted (anoxia). Respiratory flux was corrected for instrumental background and the EC50 (μM O2) was calculated from a sigmoidal dose–response curve (variable slope) fitted over a low oxygen range using GraphPad Prism software.
To assess lipid bioenergetics palmitoyl-CoA (P-CoA) titrations (5, 10, 15, 25 μM) were performed in the presence of 2 mM malate, 5 mM ADP, and 750 μM L-carnitine. Malonyl-CoA (M-CoA) was subsequently titrated (0.5, 1, 2.5, 5, 7.5, 10, 20 μM) to assess M-CoA inhibition of P-CoA-supported respiration. L-carnitine titrations (5, 15, 25, 50, 100, 250 μM) were performed in the presence of 2 mM malate, 5 mM ADP, and 25 μM P-CoA.
To assess non-lipid-supported biogenetics, pyruvate titrations (5, 25, 50, 100, 5000 μM) were performed in the presence of 2 mM malate and 5 mM ADP. Succinate titrations (250, 750, 1500, 5000, 12,000 μM) were performed in the presence of rotenone (rotenone, 0.5 μM) and 5 mM ADP. ADP titrations, combined pyruvate/succinate/ADP and combined pyruvate/ADP titrations were performed in MiR05 with a hexokinase-ADP clamp. ADP titrations (5, 15, 25, 40, 5000 μM) were performed in the presence of 5 mM pyruvate and 2 mM malate. Combined pyruvate/succinate/ADP titrations (Titration 1 [T1]: 5 μM pyruvate, 100 μM succinate, 5 μM ADP; T2: 15 μM pyruvate, 250 μM succinate, 10 μM ADP; T3: 25 μM pyruvate, 750 μM succinate, 15 μM ADP; T4: 50 μM pyruvate, 1500 μM succinate, 25 μM ADP; T5: 100 μM pyruvate, 5000 μM succinate, 40 μM ADP; T6: 5000 μM pyruvate, 10,000 μM succinate, 5000 μM ADP) were performed in the presence of 2 mM malate. OMY (0.5 μM) was added following the measurement of maximal respiration (T6) to assess coupling efficiency. For combined pyruvate/ADP titrations, substrates were titrated at different concentrations depending on the buffer pH, in the presence of 2 mM malate. At pH 7.2, T1: 5 μM pyruvate, 5 μM ADP; T2:15 μM pyruvate, 10 μM ADP; T3: 25 μM pyruvate, 15 μM ADP. At pH 6.8, T1: 25 μM pyruvate, 5 μM ADP; T2: 50 μM pyruvate, 20 μM ADP; T3: 100 μM pyruvate, 40 μM ADP.
Acute exercise protocol
A separate subset of mice (n = 16, female C57BL/6N) either remained sedentary (SED, n = 8) or underwent a single bout of intense exercise to exhaustion (acute exercise [AEX] n = 8, 20 m/min, 15% grade). All mice (SED and AEX) were acclimatized to motorized treadmill running (15% grade, variable speed for 10 min) over 4 to 5 days with a standardized 48-h rest period preceding the exercise bout to ensure no residual effect of prior exercise. Exhaustion was considered reached when the mouse was unable to maintain the set running speed for 5 s despite mechanical prodding. In the mice that were exercised, surgery was performed immediately after exhaustion.
Respirometry in permeabilized skeletal muscle fibers
All mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg/kg) and the red gastrocnemius was excised and placed in ice-cold BIOPS preservation buffer (50 mM Mes, 7.23 mM K2EGTA, 2.77 mM CaK2EGTA, 20 mM imidazole, 0.5 mM dithiothreitol, 20 mM taurine, 5.77 mM Na2ATP, 15 mM PCr, and 6.56 mM MgCl26H2O; pH 7.1) to prepare permeabilized skeletal muscle fibers as previously described (7). Chamber conditions were identical to experiments using isolated mitochondria; however, all respiration was conducted in MiR05 respiration buffer at pH 7.2 or 6.8. To prevent muscle fiber contraction, 5 μM of blebbistatin was added to the chamber prior to each experiment. Following each protocol, fiber bundles were recovered and freeze-dried and respiration data were normalized to fiber bundle weight (dry weight).
ADP titrations (25, 100, 175, 250, 500, 1000, 2000, 4000, 6000, 8000, 10,000 μM) were performed in the presence of 5 mM pyruvate and 1 mM malate. P-CoA titrations were performed (20, 40, 60 μM) in the presence of 1 mM malate, 5 mM ADP, and 1 mM L-carnitine. L-carnitine titrations were performed (10, 25, 50, 100, 250 μM) in the presence of 1 mM malate, 5 mM ADP, and 60 μM P-CoA. The subsequent addition of 7 μM M-CoA in both lipid-supported protocols was used to assess the inhibitory effects of M-CoA on CPT-I-dependent bioenergetics. M-CoA inhibition was assessed both as absolute respiration (JO2 following 60 μM P-CoA or 250 μM L-carnitine in the presence of 7 μM M-CoA) and as relative respiration (% of maximal lipid JO2). Relative respiration with M-CoA was calculated by averaging relative JO2 values (% of maximal P-CoA or L-carnitine respiration) as technical replicates (quadruplicate). If a measurement was not present for one protocol, a duplicate was utilized.
Statistics
Statistical analyses were completed in Microsoft Excel and GraphPad Prism software. Unless specified, respiration experiments were conducted using technical replicates (duplicate), which were averaged at each JO2 value. As our primary outcome was the effect of pH, all comparisons were conducted as a within-animal comparison (i.e., mitochondrial aliquot or permeabilized muscle fibers from one mouse assessed at two pH conditions) using a paired Student’s t test at each substrate concentration. Data are expressed as means + SD. Findings were considered statistically significant if p 0.05.
Data availability
The full dataset is available from the corresponding authors upon reasonable request.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
S. M. F., G. P. H. conceptualization; S. M. F., G. P. H. methodology; S. M. F., G. J. D., G. P. H. validation; S. M. F., G. P. H. formal analysis; S. M. F., G. J. D. investigation; S. M. F., G. P. H. writing – original draft; S. M. F., G. J. D., G. P. H. writing – review & editing; G. P. H. supervision; G. P. H. project administration; G. P. H. funding acquisition.
Funding and additional information
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC) grant to G. P. H. (400362). S. M. F. and G. J. D. are funded by NSERG CGS-D scholarships.
Reviewed by members of the JBC Editorial Board. Edited by Qi-Qun Tang
Contributor Information
Sara M. Frangos, Email: sfrangos@uoguelph.ca.
Graham P. Holloway, Email: ghollowa@uoguelph.ca.
References
- 1.Loon L.J.C.V., Greenhaff P.L., Saris W.H.M., Wagenmakers A.J.M. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romijn J.A., Coyle E.F., Sidossis L.S., Gastaldelli A., Horowitz J.F., Endert E., et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. Endocrinol. Metab. 1993;265:380–391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 3.Roepstorff C., Halberg N., Hillig T., Saha A.K., Ruderman N.B., Wojtaszewski J.F.P., et al. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am. J. Physiol. Endocrinol. Metab. 2005;288:133–142. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- 4.Odland L.M., Howlett R.A., Heigenhauser G.J.F., Hultman E., Spriet L.L. Skeletal muscle malonyl-CoA content at the onset of exercise at varying power outputs in humans. Am. J. Physiol. Endocrinol. Metab. 1998 doi: 10.1152/ajpendo.1998.274.6.e1080. [DOI] [PubMed] [Google Scholar]
- 5.Odland L.M., Heigenhauser G.J., Lopaschuk G.D., Spriet L.L. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am. J. Physiol. 1996;270:E541–544. doi: 10.1152/ajpendo.1996.270.3.E541. [DOI] [PubMed] [Google Scholar]
- 6.Miotto P.M., Steinberg G.R., Holloway G.P. Controlling skeletal muscle CPT-I malonyl-CoA sensitivity: the importance of AMPK-independent regulation of intermediate filaments during exercise. Biochem. J. 2017;474:557–569. doi: 10.1042/BCJ20160913. [DOI] [PubMed] [Google Scholar]
- 7.Petrick H.L., Holloway G.P. High intensity exercise inhibits carnitine palmitoyltransferase-I sensitivity to L-carnitine. Biochem. J. 2019;476:547–558. doi: 10.1042/BCJ20180849. [DOI] [PubMed] [Google Scholar]
- 8.Holloway G.P., Bezaire V., Heigenhauser G.J.F., Tandon N.N., Glatz J.F.C., Luiken J.J.F.P., et al. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J. Physiol. 2006;571:201–210. doi: 10.1113/jphysiol.2005.102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starritt E.C., Howlett R.A., Heigenhauser G.J.F., Spriet L.L. Sensitivity of CPT I to malonyl-CoA in trained and untrained human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2000;278:462–468. doi: 10.1152/ajpendo.2000.278.3.E462. [DOI] [PubMed] [Google Scholar]
- 10.Bezaire V., Heigenhauser G.J.F., Spriet L.L. Regulation of CPT I activity in intermyofibrillar and subsarcolemmal mitochondria from human and rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004;286:85–91. doi: 10.1152/ajpendo.00237.2003. [DOI] [PubMed] [Google Scholar]
- 11.Sahlin K., Harris R.C., Nylind B., Hultman E. Lactate content and pH in muscle samples obtained after dynamic exercise. Pflüg. Arch. Eur. J. Physiol. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- 12.Spriet L.L., Soderlund K., Bergstrom M., Hultman E. Skeletal muscle glycogenolysis, glycolysis, and pH during electrical stimulation in men. J. Appl. Physiol. (1985) 1987;62:616–621. doi: 10.1152/jappl.1987.62.2.616. [DOI] [PubMed] [Google Scholar]
- 13.Howlett R.A., Parolin M.L., Dyck D.J., Hultman E., Jones N.L., Heigenhauser G.J.F., et al. Regulation of skeletal muscle glycogen phosphorylase and PDH at varying exercise power outputs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998;275:418–425. doi: 10.1152/ajpregu.1998.275.2.R418. [DOI] [PubMed] [Google Scholar]
- 14.Smith B.K., Perry C.G.R., Koves T.R., Wright D.C., Smith J.C., Neufer P.D., et al. Identification of a novel malonyl-CoA IC50 for CPT-I: implications for predicting in vivo fatty acid oxidation rates. Biochem. J. 2012;448:13–20. doi: 10.1042/BJ20121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putman C.T., Jones N.L., Lands L.C., Bragg T.M., Hollidge-Horvat M.G., Heigenhauser G.J. Skeletal muscle pyruvate dehydrogenase activity during maximal exercise in humans. Am. J. Physiol. 1995;269:E458–468. doi: 10.1152/ajpendo.1995.269.3.E458. [DOI] [PubMed] [Google Scholar]
- 16.Achten J., Gleeson M., Jeukendrup A.E. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002;34:92–97. doi: 10.1097/00005768-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Van Loon L.J.C. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J. Appl. Physiol. 2004;97:1170–1187. doi: 10.1152/japplphysiol.00368.2004. [DOI] [PubMed] [Google Scholar]
- 18.Mills S.E., Foster D.W., McGarry J.D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem. J. 1984;219:601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velasco G., Geelen M.J.H., Gómez Del Pulgar T., Guzmán M. Malonyl-CoA-independent acute control of hepatic carnitine palmitoyltransferase I activity: role of Ca2+/calmodulin-dependent protein kinase II and cytoskeletal components. J. Biol. Chem. 1998;273:21497–21504. doi: 10.1074/jbc.273.34.21497. [DOI] [PubMed] [Google Scholar]
- 20.Winder W.W., Arogyasami J., Barton R., Elayan I., Vehrs P. Muscle malonyl-CoA decreases during exercise. J. Appl. Physiol. 1989;67:2230–2233. doi: 10.1152/jappl.1989.67.6.2230. [DOI] [PubMed] [Google Scholar]
- 21.Eaton S. Control of mitochondrial beta-oxidation flux. Prog. Lipid Res. 2002;41:197–239. doi: 10.1016/s0163-7827(01)00024-8. [DOI] [PubMed] [Google Scholar]
- 22.Mills S.E., Foster D.W., McGarry J.D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem. J. 1983;214:83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faye A., Borthwick K., Esnous C., Price N.T., Gobin S., Jackson V.N., et al. Demonstration of N- and C-terminal domain intramolecular interactions in rat liver carnitine palmitoyltransferase 1 that determine its degree of malonyl-CoA sensitivity. Biochem. J. 2005;387:67. doi: 10.1042/BJ20041533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolodziej M.P., Zammit V.A. Sensitivity of inhibition of rat liver mitochondrial outer-membrane carnitine palmitoyltransferase by malonyl-CoA to chemical- and temperature-induced changes in membrane fluidity. Biochem. J. 1990;272:421–425. doi: 10.1042/bj2720421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGarry J.D., Brown N.F. Reconstitution of purified, active and malonyl-CoA-sensitive rat liver carnitine palmitoyltransferase I: relationship between membrane environment and malonyl-CoA sensitivity. Biochem. J. 2000;349:179–187. doi: 10.1042/0264-6021:3490179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jogl G., Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell. 2003;112:113–122. doi: 10.1016/s0092-8674(02)01228-x. [DOI] [PubMed] [Google Scholar]
- 27.Stephens T.W., Cook G.A., Harris R.A. Effect of pH on malonyl-CoA inhibition of carnitine palmitoyltransferase I. Biochem. J. 1983;212:521–524. doi: 10.1042/bj2120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chasiotis D. The regulation of glycogen phosphorylase and glycogen breakdown in human skeletal muscle. Acta Physiol. Scand. Suppl. 1983;518:1–68. [PubMed] [Google Scholar]
- 29.Spriet L.L. Phosphofructokinase activity and acidosis during short-term tetanic contractions. Can. J. Physiol. Pharmacol. 1991;69:298–304. doi: 10.1139/y91-046. [DOI] [PubMed] [Google Scholar]
- 30.Spriet L.L., Howlett R.A., Heigenhauser G.J.F. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med. Sci. Sports Exerc. 2000;32:756–763. doi: 10.1097/00005768-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Harris R.C., Foster C.V., Hultman E. Acetylcarnitine formation during intense muscular contraction in humans. J. Appl. Physiol. 1987;63:440–442. doi: 10.1152/jappl.1987.63.1.440. [DOI] [PubMed] [Google Scholar]
- 32.McGarry J.D., Brown N.F. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 33.Holloway G.P., Jain S.S., Bezaire V., Han X.X., Glatz J.F., Luiken J.J., et al. FAT/CD36-null mice reveal that mitochondrial FAT/CD36 is required to upregulate mitochondrial fatty acid oxidation in contracting muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:960–967. doi: 10.1152/ajpregu.91021.2008. [DOI] [PubMed] [Google Scholar]
- 34.Benton C.R., Campbell S.E., Tonouchi M., Hatta H., Bonen A. Monocarboxylate transporters in subsarcolemmal and intermyofibrillar mitochondria. Biochem. Biophys. Res. Commun. 2004;323:249–253. doi: 10.1016/j.bbrc.2004.08.084. [DOI] [PubMed] [Google Scholar]
- 35.Chance B., Williams G. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J. Biol. Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- 36.Monaco C.M.F., Miotto P.M., Huber J.S., van Loon L.J.C., Simpson J.A., Holloway G.P. Sodium nitrate supplementation alters mitochondrial H2O2 emission but does not improve mitochondrial oxidative metabolism in the heart of healthy rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R191–R204. doi: 10.1152/ajpregu.00275.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full dataset is available from the corresponding authors upon reasonable request.