Abstract
In 7 of 18 solid-organ transplant recipients with primary human cytomegalovirus (HCMV) infection, HCMV antigenemia levels were unexpectedly found to rise significantly (P = 0.018) during a mean time of 7.3 ± 3.2 days after initiation of specific antiviral treatment, whereas corresponding levels of viremia dropped significantly (P = 0.043). Thus, shifting to an alternative antiviral drug based solely on increasing antigenemia levels is not justified in this group of patients.
Several methods have been developed to detect and quantitate virus or viral components in blood of immunocompromised patients with human cytomegalovirus (HCMV) infection, such as viremia (7), antigenemia (6, 13, 17), leukocyte DNAemia (L-DNAemia), and plasma DNAemia (2–4). According to a preemptive therapy approach (14), treatment of primary HCMV infections is currently started in several transplant centers upon the first detection of antigenemia (1, 9, 10). This strategy led us to observe a paradoxical phenomenon in some transplant patients, in whom antigenemia levels were unexpectedly found to rise during the first week of antiviral treatment (9).
(This paper was presented in part [abstract 194] at the VI International Cytomegalovirus Workshop, Orange Beach, Ala., on March 5 to 7, 1997.)
In the period 1990 to 1995, 249 patients underwent heart transplantation (HT), 22 underwent heart-lung transplantation, and 17 underwent double-lung and 21 underwent single-lung transplantation (SLT) at the Cardiac Surgery Department of IRCCS Policlinico San Matteo, Pavia, Italy. Of these, 20 patients (16 HT, 2 heart-lung transplantation, and 2 SLT patients) developed a primary HCMV infection (17 males and 3 females; median age, 38.5; range, 13 to 65 years), and 18 of them received antiviral treatment. Clinical and virological follow-up lasted a median time of 138 (47 to 347) days. The immunosuppressive regimen was based on cyclosporine, azathioprine, and steroids supplemented by a course of antithymocyte globulin (10).
All patients were prospectively monitored for clinical evidence of HCMV-related symptoms or signs. Disseminated HCMV disease (in the absence of overt organ localization) was diagnosed based on the presence of fever and/or thrombocytopenia and/or leukopenia associated with a high viral load in the blood. High HCMV load was defined by levels of viremia of >10 infected fibroblasts/2 × 105 peripheral blood leukocytes (PBL) inoculated (7), antigenemia of >100 pp65-positive/2 × 105 PBL (6), and L-DNAemia of >1,000 genome equivalents (GE)/105 PBL (4, 12). Diagnostic criteria for HCMV end-organ disease followed recommendations made by participants in a workshop on HCMV disease (11).
Ganciclovir was administered intravenously at a standard dosage of 10 mg/kg of body weight/day for at least 14 days or until antigenemia clearance. Alternatively, foscarnet was administered intravenously at a dosage of 180 mg/kg/day for 21 days. In the 18 treated patients with primary HCMV infection, indications for initiation of antiviral therapy changed with time, i.e., appearance of HCMV-related clinical symptoms in 13 patients, HCMV antigenemia of >100 in 2 patients, and an antigenemia level of 48 in one patient, whereas in 2 patients treatment was started upon the first observation of antigenemia.
All patients were virologically monitored by prospective quantitation of pp65 antigenemia and viremia and retrospective quantitation of L-DNAemia in PBL. PBL were obtained from buffy coat samples, which were processed within 4 h after bleeding. The level of viremia was measured according to the shell vial technique (7). The level of antigenemia was measured by using a pool of three HCMV pp65-specific monoclonal antibodies reactive with three different epitopes of the protein (6). L-DNAemia was quantitated by PCR by using external standards (pCM2) and an internal amplification control (pAC2), which were coamplified (12). DNA extraction was performed by proteinase K lysis (20 mg/ml for 1 h at 55°C) followed by DNA precipitation. The method allowed reproducible quantification in the range of 10 to 10,000 GE by the single-step PCR, whereas samples containing 1 to 10 GE could be identified by the nested PCR protocol. The outer and the inner set of primers have been reported (12).
Differences in means of nonparametric data were tested by the Wilcoxon signed-rank test for paired data and the Kolmogorow-Smirnov test for unpaired data. In addition, Fisher’s exact test was used to test differences in proportions when the total sample size was less than 30. All tests were two-tailed.
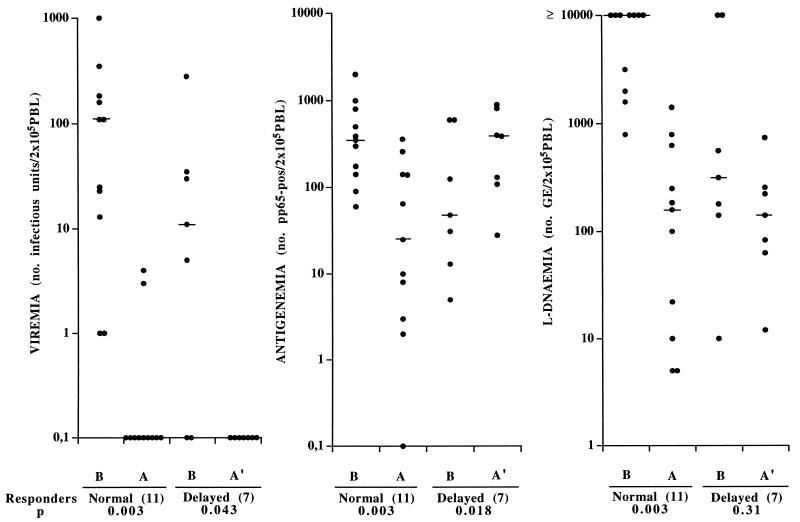
During follow-up of 18 solid organ transplant recipients with primary HCMV infection, the mean time to a ≥90% reduction of viral load in the blood, following initiation of antiviral treatment, was 3.5 (0 to 8) days when viral load was measured by viremia, 6.9 (2 to 22) days when it was measured by L-DNAemia, and 14.6 (3 to 34) days when it was quantified by antigenemia. More detailed analysis of these data unexpectedly showed that, while viremia and most L-DNAemia levels rapidly decreased after onset of antiviral therapy, antigenemia levels increased in 7 of 18 (38.9%) patients. As shown in Fig. 1, the 18 patients belonged to two groups on the basis of antigenemia response to treatment during the first week of therapy: the group of “normal” responders (n = 11), in whom viremia, L-DNAemia, and antigenemia levels decreased, and the group of “delayed” responders in whom decreasing levels of viremia and (mostly) L-DNAemia were associated with increasing levels of antigenemia. Subsequently, a progressive decrease in antigenemia levels was observed also among delayed responders. Thus, the group of normal responders presented significant (P < 0.01) decreases in median levels of viremia (from 110 [1 to 1,000] to 0 [0 to 4]), antigenemia (from 350 [60 to 2,000] to 25 [0 to 362]), and L-DNAemia (from >10,000 [794 to >10,000] to 159 [5 to 1,412] GE). On the other hand, in the group of delayed responders, while the median level of viremia dropped significantly (P < 0.05) from 11.0 (0 to 280) to 0 and level of L-DNAemia did not change significantly (316, range 10 to >10,000 versus 141, range 12 to 742 GE), the level of antigenemia rose significantly (P = 0.018) from 48 (5 to 600) to 390 (28 to 900). The mean time of antiviral treatment during this observation was 5.5 ± 1.8 days for normal responders and 7.3 ± 3.2 days for delayed responders.
FIG. 1.
Distribution of HCMV viremia, antigenemia, and L-DNAemia levels in a group of 18 solid-organ transplant recipients with primary HCMV infection, including 11 with normal and 7 with delayed responses to antiviral treatment. Bars indicate median values. B, values before onset of treatment. A, reported antigenemia values referring to the earliest blood sample showing ≥90% progressive reduction in antigenemia level after onset of treatment; viremia and L-DNAemia were determined with the same sample. A′, reported antigenemia values referring to peaks of increasing antigenemia levels detected after onset of treatment; viremia and L-DNAemia values were determined with the same blood samples.
Major differences between the two groups were the following: (i) the mean time of antigenemia positivity prior to antiviral therapy was found to be significantly shorter in the group of delayed responders (5.7 ± 6.4 versus 9.7 ± 5.2 days) (P = 0.046); (ii) mean pretreatment levels of viremia, antigenemia, and L-DNAemia were lower in the group of delayed responders, even though only L-DNAemia reached the level of significance (P = 0.046); and (iii) all four patients in whom treatment was started with antigenemia levels of <50 had increasing antigenemia levels, whereas only 3 of 14 (21%) patients starting treatment with a higher antigenemia level showed the same type of response (P < 0.05). There was no correlation between time elapsed after transplantation and clinical symptoms or type of antiviral drug.
Clinical and virological consequences of the delayed antigenemia response to antiviral treatment were as follows: (i) the proportion of patients with secondary episodes of HCMV infection (reactivations) following the primary episode was higher (although not significantly) in the group of delayed (5 of 7; 71%) compared to normal (4 of 11; 36%) responders; in addition, the overall incidence of secondary episodes during follow-up was higher among delayed responders (12 versus 6 episodes); (ii) the mean times to ≥90% antigenemia level reduction after onset of antiviral treatment were 20.8 ± 7.7 days for delayed and 9.9 ± 6.7 days for normal responders (P = 0.18).
Table 1 reports the follow-up of three normal responders (patients 5, 10, and 12) and three delayed responders (patients 16, 22, and 26). In patient 16 the increasing antigenemia level prompted clinicians to shift from ganciclovir to foscarnet following the first 8 days of antiviral treatment.
TABLE 1.
Correlation of onset of antiviral treatment and antigenemia, viremia, and L-DNAemia level in solid-organ transplant patients with primary HCMV infection
| Patient | Type of transplant | Sexa | Age (yr) | Days after transplant | HCMV quantitation in blood
|
Antiviral drug (days of administration)b | Onset of treatment after 1st positive antigenemia (day) | HCMV-related clinical symptomsc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Viremia | Antigenemia | L-DNAemia | ||||||||
| 5 | HT | M | 37 | 3 | 0 | 0 | 0 | |||
| 10 | 0 | 0 | 0 | |||||||
| 17 | 0 | 0 | 10 | |||||||
| 24 | 0 | 0 | 12 | |||||||
| 28 | 3 | 5 | 147 | |||||||
| 31 | 4 | 26 | 1,122 | |||||||
| 33 | 155 | 127 | >10,000 | |||||||
| 35 | 184 | 390 | >10,000 | G (35–48) | 7 | + | ||||
| 38 | 0 | 85 | 114 | |||||||
| 40 | 0 | 25 | 185 | |||||||
| 42 | 0 | 6 | 10 | |||||||
| 47 | 0 | 1 | NDd | |||||||
| 10 | HT | M | 18 | 2 | 0 | 0 | 0 | |||
| 9 | 0 | 0 | 11 | |||||||
| 16 | 0 | 0 | 18 | |||||||
| 18 | 32 | 17 | 562 | |||||||
| 23 | 0 | 33 | 79 | |||||||
| 26 | 23 | 53 | ND | |||||||
| 30 | ND | 142 | 1,995 | G (31–44) | 13 | + | ||||
| 37 | 0 | 10 | 251 | |||||||
| 40 | 0 | 0 | 20 | |||||||
| 12 | HT | M | 27 | 16 | 0 | 0 | 0 | |||
| 19 | 0 | 4 | 19 | |||||||
| 26 | 70 | 180 | 194 | |||||||
| 30 | 136 | 400 | >10,000 | |||||||
| 33 | 110 | 300 | >10,000 | F (34–48) | 15 | + | ||||
| 37 | 0 | 3 | 5 | |||||||
| 16 | SLT | M | 46 | 20 | 0 | 0 | 10 | |||
| 23 | 0 | 8 | 208 | |||||||
| 25 | 30 | 125 | 316 | G (25–33) | 2 | − | ||||
| 27 | 0 | 323 | 225 | |||||||
| 30 | 0 | 400 | 141 | |||||||
| 32 | 5 | 390 | 105 | |||||||
| 34 | 3 | 360 | 101 | F (34–47) | ||||||
| 36 | 0 | 10 | 11 | |||||||
| 39 | 0 | 5 | 5 | |||||||
| 41 | 0 | 0 | 12 | |||||||
| 44 | 0 | 0 | 0 | |||||||
| 22 | SLT | F | 24 | 20 | 0 | 0 | 0 | |||
| 25 | ND | 13 | 10 | |||||||
| 32 | 0 | 66 | 37 | G (26–41) | 1 | − | ||||
| 35 | 0 | 109 | 256 | |||||||
| 39 | 0 | 23 | 5 | |||||||
| 41 | 0 | 8 | 5 | |||||||
| 50 | 0 | 1 | 5 | |||||||
| 55 | 0 | 0 | 5 | |||||||
| 26 | HT | M | 30 | 21 | 0 | 0 | 0 | |||
| 28 | 0 | 0 | 0 | |||||||
| 33 | 0 | 0 | 5 | |||||||
| 35 | 5 | 5 | 141 | |||||||
| 40 | 0 | 28 | 742 | G (36–57) | 1 | − | ||||
| 47 | 0 | 22 | 5 | |||||||
| 54 | 0 | 2 | 5 | |||||||
| 57 | 0 | 0 | 0 | |||||||
M, male; F, female.
G, ganciclovir; F, foscarnet.
+, presence of clinical symptoms; −, absence of clinical symptoms.
ND, not determined.
Results of the present study indicated that, in solid-organ transplant recipients with primary HCMV infection, early initiation of treatment may be followed by a significant rise in antigenemia level during the first week of treatment, delayed antigenemia clearance, or a higher incidence of HCMV reactivation episodes after discontinuation of treatment. The significantly earlier initiation of treatment based on first antigenemia positivity along with the significantly lower absolute antigenemia level in the group of delayed responders represented the major factors associated with the rise in antigenemia level after onset of therapy. These conclusions are in keeping with two recent studies reporting an increase in quantitative antigenemia in liver (9) and allogeneic bone marrow (1) transplant recipients.
The reported increase in antigenemia level is often the critical factor prompting the clinician to shift to an alternative drug (patient 16 [Table 1]) due to the suspicion that a drug-resistant strain is emerging (15). To avoid such an erroneous therapeutic approach, other assays should be performed in parallel, e.g., measurement of viremia, which in the presence of a sensitive HCMV strain, drops sharply within 24 to 48 h.
Although early replicative events have been shown to occur in both mononuclear (16) and polymorphonuclear leukocytes (8), it should be noted that virus or viral material detected in blood by different assays has been assumed to be mostly taken up by PBL by phagocytosis (5). Thus, it appears reasonable to hypothesize that while antiviral treatment quickly blocks virus replication, previously synthetized pp65 may still be phagocytized by PBL for several days after discontinuation of treatment.
In conclusion, antigenemia-guided antiviral treatment in solid-organ transplant recipients with primary HCMV infection could be reasonably started with antigenemia levels of >50. This could avoid erroneous treatment modifications as well as partially prevent secondary episodes of HCMV reactivation and result in a faster virus clearance from blood.
Acknowledgments
We thank Linda D’Arrigo for revision of the English. We are also indebted to Lucia Chezzi for excellent technical assistance and to Barbara Ferrara for typing the manuscript.
This work was supported by Ministero della Sanità, Ricerca Corrente IRCCS Policlinico San Matteo, grant 820RCR96/01.
REFERENCES
- 1.Boeckh M, Godey T A, Myerson D, Cunningham T, Schoch G, Bowden R A. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88:4063–4071. [PubMed] [Google Scholar]
- 2.Boivin G, Olson C A, Quirk M R, St.-Cyr S M, Jordan M C. Quantitation of cytomegalovirus glycoprotein H gene in cells using competitive PCR and a rapid fluorescent-based detection system. J Virol Methods. 1995;51:329–342. doi: 10.1016/0166-0934(94)00128-4. [DOI] [PubMed] [Google Scholar]
- 3.Fox J C, Griffiths P D, Emery V C. Quantitation of human cytomegalovirus DNA using the polymerase chain reaction. J Gen Virol. 1992;73:2405–2408. doi: 10.1099/0022-1317-73-9-2405. [DOI] [PubMed] [Google Scholar]
- 4.Gerna G, Furione M, Baldanti F, Sarasini A. Comparative quantitation of human cytomegalovirus DNA in blood leukocytes and plasma of transplant and AIDS patients. J Clin Microbiol. 1994;32:2709–2717. doi: 10.1128/jcm.32.11.2709-2717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerna G, Percivalle E, Revello M G, Morini F. Correlation of quantitative human cytomegalovirus pp65-, p72- and p150-antigenemia, viremia and circulating endothelial giant cells with clinical symptoms and antiviral treatment in immunocompromised patients. Clin Diagn Virol. 1993;1:47–59. doi: 10.1016/0928-0197(93)90033-2. [DOI] [PubMed] [Google Scholar]
- 6.Gerna G, Revello M G, Percivalle E, Morini F. Comparison of different immunostaining techniques and monoclonal antibodies to the lower matrix phosphoprotein (pp65) for optimal quantitation of human cytomegalovirus antigenemia. J Clin Microbiol. 1992;30:1232–1237. doi: 10.1128/jcm.30.5.1232-1237.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerna G, Revello M G, Percivalle E, Zavattoni M, Parea M, Battaglia M. Quantification of human cytomegalovirus viremia by using monoclonal antibodies to different viral proteins. J Clin Microbiol. 1990;28:2681–2688. doi: 10.1128/jcm.28.12.2681-2688.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerna G, Zipeto D, Percivalle E, Parea M, Revello M G, Maccario R, Peri G, Milanesi G. Human cytomegalovirus infection of the major leukocyte subpopulations and evidence for initial viral replication in polymorphonuclear leukocytes from viremic patients. J Infect Dis. 1992;166:1236–1244. doi: 10.1093/infdis/166.6.1236. [DOI] [PubMed] [Google Scholar]
- 9.Grossi P, Kusne S, Rinaldo C, St. George K, Magnone M, Rakela J, Fung J, Starzl T E. Guidance of ganciclovir therapy with pp65 antigenemia in cytomegalovirus-free recipients of livers from seropositive donors. Transplantation. 1996;61:1659–1660. doi: 10.1097/00007890-199606150-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossi P, Minoli L, Percivalle E, Irish W, Viganò M, Gerna G. Clinical and virological monitoring of human cytomegalovirus infection in 294 heart transplant recipients. Transplantation. 1995;59:847–851. [PubMed] [Google Scholar]
- 11.Ljungman P, Plotkin S A. Workshop on CMV disease; definitions, clinical severity scores, and new syndromes. Scand J Infect Dis Suppl. 1995;99:87–89. [Google Scholar]
- 12.Revello M G, Baldanti F, Furione M, Sarasini A, Percivalle E, Zavattoni M, Gerna G. Polymerase chain reaction for prenatal diagnosis of congenital human cytomegalovirus infection. J Med Virol. 1995;47:462–466. doi: 10.1002/jmv.1890470428. [DOI] [PubMed] [Google Scholar]
- 13.Revello M G, Percivalle E, Zavattoni M, Parea M, Grossi P, Gerna G. Detection of human cytomegalovirus immediate-early antigen in leukocytes as a marker of viremia in immunocompromised patients. J Med Virol. 1989;29:88–93. doi: 10.1002/jmv.1890290204. [DOI] [PubMed] [Google Scholar]
- 14.Rubin R H. Preemptive therapy in immunocompromised hosts. N Engl J Med. 1991;324:1057–1059. doi: 10.1056/NEJM199104113241509. [DOI] [PubMed] [Google Scholar]
- 15.Sarasini A, Baldanti F, Furione M, Percivalle E, Brerra R, Barbi M, Gerna G. Double resistance to ganciclovir and foscarnet of four human cytomegalovirus strains recovered from AIDS patients. J Med Virol. 1995;47:237–244. doi: 10.1002/jmv.1890470309. [DOI] [PubMed] [Google Scholar]
- 16.Schrier R D, Nelson J A, Oldstone M B A. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985;230:1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- 17.van der Bij W, Schirm J, Torensma R, van Son W J, Tegzess A M, The T H. Comparison between viremia and antigenemia for detection of cytomegalovirus in blood. J Clin Microbiol. 1988;26:2531–2535. doi: 10.1128/jcm.26.12.2531-2535.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]