Abstract
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is one of the most prominent housekeeping proteins and is widely used as an internal control in some semi-quantitative assays. In addition to glycolysis, GAPDH is involved in several cancer-related biological processes and has been reported to be commonly dysregulated in multiple cancer types. Therefore, its role in the physiological process of cancer needs to be urgently elucidated. Pan-cancer analysis indicated that GAPDH is ubiquitously highly expressed in most cancer types, and that patients with a high GAPDH expression of in tumor tissues have a poor prognosis. The concordance of GAPDH expression in tumors with the infiltration of immune cells and immune checkpoints implies a certain association between GAPDH and the tumor microenvironment as well as tumor development. Gene Set Enrichment Analysis revealed that GAPDH may contribute to multiple important cancer-related pathways and biological processes. Multi-omics analysis and in vitro cell experiments revealed that GAPDH overexpression is regulated by DNA copy number amplification and promoter methylation modification. Importantly, a transcription factor, forkhead box M1 (FOXM1), which is capable of regulating GAPDH expression, was also identified and was confirmed to be an oncogene and ubiquitously highly expressed in multiple cancer types. Semi-quantitative chromatin immunoprecipitation, quantitative PCR, and dual-luciferase assays showed that FOXM1 mainly binds to the promoter region of GAPDH in two cancer cell lines. The present findings revealed the implication of GAPDH in tumor development, thus bringing attention to this important molecule and casting doubts on its role as an internal reference gene in cancer studies.
Keywords: GAPDH, Pan-cancer, Immunological, Prognostic, FOXM1, DNA methylation
Graphical abstract
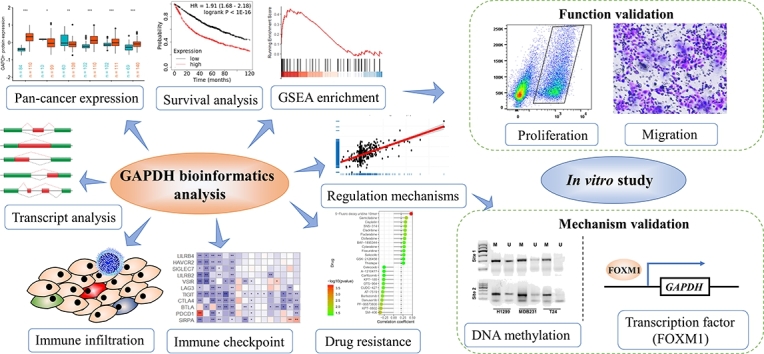
Highlights
-
•
GAPDH was ubiquitously overexpressed in most cancer types.
-
•
GAPDH overexpression is associated with poor prognosis.
-
•
GAPDH correlated with immune cells infiltration and immune checkpoint gene expression.
-
•
GAPDH is regulated by DNA copy number, methylation and the transcription factor FOXM1.
1. Introduction
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is one of the most prominent housekeeping proteins and is known for its function in cellular metabolism. During glycolysis, GAPDH catalyzes the oxidative phosphorylation of glyceraldehyde 3-phosphate to glyceryl 1,3-bisphosphate with the release of reduced nicotinamide adenine dinucleotide [1], [2]. Such high concentrations render GAPDH more available to interact with other proteins as well as with DNA and RNA species, thus enabling its functional diversity [3]. In addition to glycolysis, GAPDH is involved in biological processes such as RNA transport, DNA replication and repair, endocytosis, exocytosis, cytoskeletal organization, iron metabolism, carcinogenesis, and cell death [4], [5]. These non-glycolytic functions are regulated through different post-translational modifications (PTMs) of GAPDH, including phosphorylation, acetylation, sulfenylation and nitrosylation [6], [7], [8], [9], [10].
Since the GAPDH expression level is usually not affected under experimental or physiological conditions, GAPDH is widely used as an internal control in numerous studies. However, it is worth noting that the expression status of this enzyme varies in different human cell lines [11] as well as under stress and other conditions [12], [13]. In addition, a previous study that analyzed the expression changes of glycolysis-related genes using the National Institutes of Health (NIH)'s public database dbEST found that GAPDH was also ubiquitously overexpressed in all cancer types [14]. Other studies also revealed that GAPDH was potentially required for cancer cell proliferation and tumor formation in multiple cancer types [15]. Numerous studies have also found that GAPDH is commonly dysregulated in multiple types of cancer, including lung [16], renal [17], breast [18], prostate [19] and liver [20] cancer, implying that it plays a vital regulatory role in cancer cell proliferation and tumor formation. However, the cancer-related mechanisms involved in GAPDH regulation remain unclear.
Therefore, GAPDH should not be considered a simple housekeeping gene to be used as an internal control, particularly in tumor studies. The aim of this study was to explore the expression and biological functions of GAPDH in multiple tumors through bioinformatics analyses, and to examine the molecular mechanisms responsible for the differences in its expression. The function and regulatory mechanism of GAPDH were further verified by in vitro experiments. The present findings may provide useful insights for future studies on GAPDH.
2. Materials and methods
2.1. Dataset collection
Three cancer-related datasets were obtained from the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) database, namely the lung cancer dataset GSE19804 [21], the endometrial cancer dataset GSE17025 [22] and the prostate cancer dataset GSE6919 [23]. In addition, the datasets of 7 different exposure condition studies (GSE40795 [24], GSE33520 [25], GSE12792 [26], GSE31286 [27], GSE6907 [28], GSE6873 [29] and GSE6878 [30]) were also obtained for GAPDH expression analysis.
The log2 (TPM + 0.001) transformed normalized expression profiles, copy number variations on gene expression were estimated using the GISTIC2.0 method, DNA methylation profiles and phenotype data of pan-cancer in The Cancer Genome Atlas (TCGA) and normal tissues in Genotype-Tissue Expression (GTEx) database were downloaded from the UCSC Xena Browser (https://xenabrowser.net/). It should be pointed out that the data from two different databases, TCGA and GTEx, were integrated by the UCSC team and batch effects were removed [31]. Furthermore, the proteomics data of multiple cancer types were obtained from the Clinical Proteomic Tumor Analysis Consortium (CPTAC) database (https://proteomics.cancer.gov/programs/cptac). In addition, the DNA methylation-based (DNAss) and RNA expression-based (RNAss) tumor stemness score of each tumor were obtained from a previous study for correlation analysis with GAPDH expression. The expression data of cell lines were downloaded from the Cancer Cell Line Encyclopedia (CCLE, https://sites.broadinstitute.org/ccle/). The pan-cancer immune cell infiltration data were downloaded from Tumor Immune Estimation Resource 2.0 (TIMER2.0, http://timer.cistrome.org/). Five web tools, namely hTFTarget (http://bioinfo.life.hust.edu.cn/hTFtarget/), ENCODE (http://amp.pharm.mssm.edu/Harmonizome/dataset/ENCODE+Transcription+Factor+Targets), KnockTF (http://www.licpathway.net/KnockTF/), Contra v3 (http://bioit2.irc.ugent.be/contra/v3/) and AnimalTFDB (http://bioinfo.life.hust.edu.cn/AnimalTFDB/) were used to analyze the upstream transcription factor regulating GAPDH.
2.2. Prognosis analysis
The survival information of pan-cancer, including overall survival (OS), progression-free interval (PFI), disease-free interval (DFI) and disease-specific survival (DSS), was downloaded from TCGA database for evaluating the prognostic significance of GAPDH. All patients with each cancer type were divided into the GAPDH-high and GAPDH-low groups based on the median expression level of GAPDH. The R packages ‘survival’ and ‘survminer’ were used to perform Cox analysis and to generate Kaplan-Meier (KM) survival curves to analyze the association between the expression of GAPDH and patient prognosis.
2.3. Immune-related analysis
TIMER2.0 is a database that comprehensively characterizes molecular tumor-immune interactions [32]. The TIMER2.0 database was used to explore the abundance of different cell types in the tumor microenvironment of 33 cancer types. In total, 11 immune checkpoint genes (including PDCD1, CTLA4, C10orf54, HAVCR2, LAG3, TIGIT, SIRPA, BTLA, SIGLEC7, LILRB2 and LILRB4) [33] were extracted from TCGA datasets for immune checkpoint gene correlation analysis. In addition, immune checkpoint molecules were collected from the study conducted by Charoentong et al. [34], who reported 24 immunoinhibitory genes and 45 immunostimulatory genes.
2.4. Drug sensitivity analysis
The Genomics of Drug Sensitivity in Cancer (GDSC) database was developed by the Sanger Research Institute to collect data on the sensitivity and response of tumor cells to drugs [35]. The ‘oncoPredict’ tool was used to calculate the drug sensitivity of each sample in the training and validation datasets based on the GDSC V2 database [36]. In addition, the CellMiner database was used to evaluate the association between anti-tumor drug sensitivity and GAPDH expression [37].
2.5. Gene Set Enrichment Analysis (GSEA) and correlation analysis
To assess the biological function of a single gene in tumors, Pearson's correlation analysis of the association between the expression of GAPDH and other mRNAs retrieved from TCGA transcriptome data was conducted. According to the level of the association index between genes and GAPDH, the genes that were most associated with GAPDH expression were selected for enrichment analysis. GSEA [38] based on predefined gene sets from the Molecular Signatures Database v5.0 (http://software.broad0institute.org/gsea/msigdb/index.jsp) was conducted using the R package ‘clusterProfiler’ [39]. In the present study the ‘c2.cp.kegg.v7.5.1.entrez.gmt’ and ‘c5.go.bp.v7.5.1.entrez.gmt’ collection sets were used for GSEA.
2.6. Measurement of reference gene stability
A total of 10 commonly used reference genes (ACTB, UBC, YWHAZ, GAPDH, SDHA, RPL13A, TBP, B2M, HPRT1 and HMBS) were examined for their stability in 33 cancer types using the R package ‘NormqPCR’ [40], which works according to the geNorm algorithm [41]. Coefficient of variation (CV%) was used to evaluate the stability of the 10 genes [42].
2.7. Cell culture
Considering lung cancer and breast cancer (BC) are among the cancers with the highest incidence rate and mortality, as well as the results of the GSEA analysis, cell lines of both cancers were selected for functional validation. Human lung adenocarcinoma cell line NCI-H1299 (Cat. no. TCHu160), bladder cancer cell line MDA-MB-231 (Cat. no. TCHu227) and bladder cancer cell line T24 (Cat. no. TCHu 55) were obtained from China Center for Type Culture Collection (China). Cells were cultured in Dulbecco's modified Eagle medium (DMEM; Cat. no. 11960044; Life Technologies, USA) supplemented with 10% fetal bovine serum (FBS; Cat. no. 26010074; Life Technologies, USA) at 37 ∘C in a humidified 5% CO2 incubator (Thermo Fisher Scientific, Inc., USA).
2.8. Short hairpin RNA (shRNA) construction and transfection
GAPDH shRNA sequences were generated by cloning into the pGreen vector (Cat. no. SI505A-1; System Biosciences, USA). After testing several candidate shRNAs, the target sequences 5'-CCTCAACTACATGGTTTACAT-3' (sh1) and 5'-ACCTGACCTGCCGTCTAGAAA-3' (sh2) were selected for subsequent experiments. A scrambled non-specific control shRNA (shNC) was also cloned into the same vector and used as a negative control. A total of 2 × 105 cells were seeded per well in 6-well plates and cultured for 24 h. Next, the cells were transfected with 2.5 μg GAPDH sh1/2 or shNC per well using 6000 reagent (Cat. no. C0526; Beyotime Institute of Biotechnology, China) according to the manufacturer's instructions. The cells were harvested 48 h post-transfection and used for EdU assay, as well as for RNA and protein extraction.
2.9. Soft agar colony formation assay
In total, cells from each group were seeded in 0.35% agarose on top of a 0.7%-agarose base supplemented with complete medium. The colonies were fixed 14 days later and stained with 0.5% crystal violet for 20 min at room temperature. The colony formation rate was calculated using the following equation: Colony formation rate = numbers of colonies/numbers of seeded cells × 100%.
2.10. Western blotting
Total protein was extracted with RIPA lysis buffer (Cat. No. P0013D. Beyotime Institute of Biotechnology, China) and quantified by BCA assay (Cat. No. P0010S. Beyotime Institute of Biotechnology, China). Following protein denaturation by boiling in a water bath and the addition of loading buffer, 30 μg protein was loaded and separated on SDS-PAGE, and then transferred onto a PVDF membrane (Cat. No. IPFL00010. Millipore, USA). Following blocking with 5% bovine serum albumin, the membrane was incubated at 4 ∘C overnight with specific antibodies against GAPDH (Cat. No. 60004-1-Ig, 1:5000 dilution; Proteintech Group, Inc., USA.) and β-actin (ACTB; Cat. No. 81115-1-RR, 1:5000 dilution; Proteintech Group, Inc., USA.). Following the incubation with host species-specific HRP-labeled secondary antibodies, the bands were visualized using an enhanced chemiluminescence substrate (Cat. No. P0018S. Beyotime Institute of Biotechnology, China), and images were acquired using the GeneTools GBOX system (Syngene International). The intensity of each band was quantified using ImageJ software (version 1.8.0; NIH).
2.11. EdU proliferation assay
Logarithmic growth phase cells (2 × 105) from each group were seeded into a 6-well plate with 10 μM EdU reagent for 2 h. Following fixing with 4% paraformaldehyde for 30 min, the cells were permeabilized with 0.3% Triton X-100 in PBS and incubated with Click-iT reaction solution (cat. no. C0075S. Beyotime Institute of Biotechnology, China). Images were collected at 24 h under an inverted fluorescent microscope and were quantitatively analyzed with NIH ImageJ software (version 1.8.0).
2.12. Methylation-specific PCR (MSP)
Genomic DNA was extracted for methylation analysis from cells by using a DNA Purification Kit (Cat. No. DP304. Tiangen Biotech Co., Ltd., China). Genomic DNA (1 μg) was modified with sodium bisulfite using the Epitect Bisulfite kit (Cat. No. 59104. Qiagen AB, Germany) according to the manufacturer's instructions. MSP was conducted in a total volume of 20 μl using AmpliTaq Gold (Applied Biosystems; Thermo Fisher Scientific, Inc., USA). MSP products were separated on 2% agarose gels containing GelRed® Nucleic Acid Gel Stain (Cat. No. SCT123. MilliporeSigma, USA). To confirm the successful bisulfite modification of the DNA, the GAPDH promoter region was amplified with every modified DNA sample. The following primers were used (M: methylated, U: unmethylated) and synthesized by Genewiz (China): site1_MF: 5'-CGGTGTTATTATCGTAGAGTTTCGA-3', site1_MR: 5'-ATCAAAAACGAAAACCCTTACACG-3', site1_UF: 5'-TTGGTGTTATTATTGTAGAGTTTTGA-3', site1_UR: 5'-CAAAAACAAAAACCCTTACACACT-3', site2_MF: 5'-GGAGGGATTTTCGTTTTTACGTTTC-3', site2_MR: 5'-TATATACACCAACGACTCTCTCCGA-3', site2_UF: 5'-GAGGGATTTTTGTTTTTATGTTTTG-3' and site2_UR: 5'-TATATACACCAACAACTCTCTCCAA-3'.
2.13. Quantitative PCR (qPCR) assay
Total RNA was extracted from 5 × 106 cells with 1 ml TRIzol (Cat. No. 15596026. Invitrogen, USA), according to the manufacturer's instructions. Subsequently, total RNA (~1.5 μg) was reverse transcribed into complementary DNA (cDNA) using the RevertAid First Strand cDNA Synthesis Kit (Cat. No. K1622. Thermo Fisher Scientific, Inc., USA), according to the manufacturer's instructions. qPCR was performed using the FastStart Universal SYBR Green Master (ROX) Kit (Cat. No. 04913914001. Roche Diagnostics, Switzerland) on the QuantStudioTM 6 Flex RT-qPCR System (Applied Biosystems; Thermo Fisher Scientific, Inc., USA). ACTB served as the internal reference gene. The qPCR primer sequences used are as follows: ACTB_F: CATGTACGTTGCTATCCAGGC, ACTB_R: CTCCTTAATGTCACGCACGAT, GAPDH_F: GGAGCGAGATCCCTCCAAAAT, GAPDH_R: GGCTGTTGTCATACTTCTCATGG.
2.14. Transcription factor prediction
Three web tools, namely hTFTarget, ENCODE and KnockTF, were used to identify the upstream transcription factor regulating GAPDH. In addition, GAPDH-correlated genes in TCGA lung adenocarcinoma (LUAD) and BC datasets were also analyzed for intersection with the prediction results of the aforementioned online tools. The transcription factor binding sites (TFBS) were predicted using the web tools Contra v3 and AnimalTFDB.
2.15. Genomic PCR and dual-luciferase reporter assay
The PCR primers used in the present study to clone predicted TFBS and flanking sequences are shown in Table S1. PCR was performed using standard conditions from total genomic DNA, and the PCR fragments were gel purified prior to cloning. Next, the fragments were ligated to the pGL3 plasmid backbone (Cat. No. E1751. Promega Corporation, USA) downstream of the luciferase gene open reading frame.
Aliquots of 2 × 105 exponentially growing cells were seeded into 24-well plates. Following overnight culture, cells were co-transfected with recombinant pGL3 plasmids containing TFBS, forkhead box M1 (FOXM1) overexpression plasmids, and the Renilla luciferase plasmid (pRL, as an internal control. Cat. No. E227A. Promega Corporation, USA) at a ratio of 5:5:1 by using Lipofectamine . Cell lysates were collected 48 h after transfection. Firefly and Renilla luciferase activities were measured with Firefly Luciferase Reporter Gene Assay Kit (Cat. no. C0526; Beyotime Institute of Biotechnology, China).
2.16. Chromatin immunoprecipitation (ChIP)
Cell lysates (2 × 106) were prepared for ChIP assay. The lysates were sonicated and immunoprecipitated with an anti-FOXM1 antibody (2 μg; Cat. No. 13147-1-AP, Proteintech Group, Inc., USA). Following immunoprecipitation, immune complexes were collected with protein A agarose (Cat. no. C600689-0020. BBI Corporation, China) and extracted with an extraction buffer (1% SDS and 0.1 M NaHCO2). DNA was de-crosslinked by heating at 65 ∘C for 8 h. DNA was extracted with phenol/chloroform and precipitated with ethanol. The DNA isolated from an aliquot of total nuclear extract was used as the input control for PCR. qPCR was performed as described above. Data are presented after normalizing each immunoprecipitated DNA Cq value to 10% of the input DNA Cq value.
2.17. Statistical analysis
Spearman's correlation coefficients were calculated to investigate the correlation between i) GAPDH expression and immune cell infiltration score in pan-cancer in TCGA database; ii) the expression of GAPDH and immune checkpoints in pan-cancer in TCGA database; and iii) GAPDH expression and stem cell index in pan-cancer in TCGA database. Pearson correlation coefficients were calculated to investigate the correlation between i) GAPDH expression and methylation probes value; and ii) the pan-cancer expression of GAPDH and FOXM1 in TCGA and CCLE databases, and in normal tissues in the GTEx database. The results from in vitro experiments are presented as means ± standard deviation, and were analyzed using SPSS 22.0 software (IBM Corp.). Differences between groups were analyzed using Student's t-test or one-way ANOVA as appropriate. P < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. GAPDH is upregulated in multiple cancer types
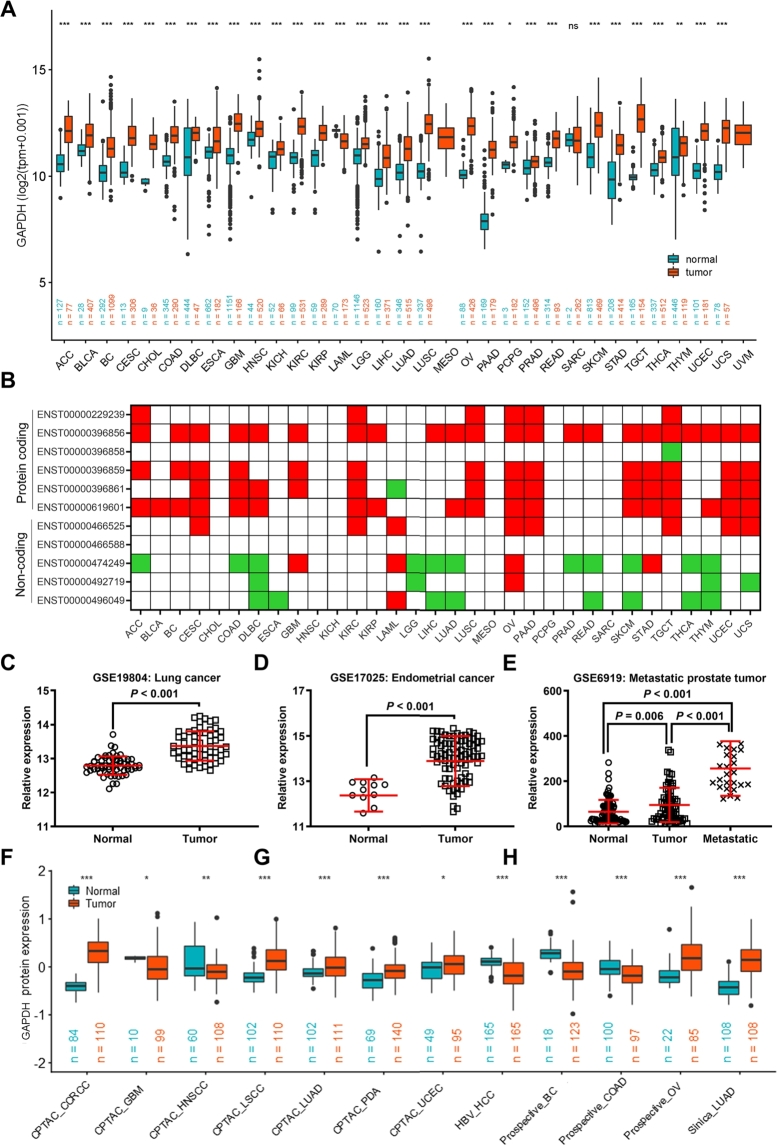
When using the TCGA pan-cancer dataset only for differential analysis, GAPDH was found to be upregulated in 17 cancer types with log2 (fold change) > 0.5 and in 9 cancer types with log2 (fold change) > 1 (Table S2). After merging GTEx and TCGA samples, the results demonstrated a significant upregulation of GAPDH expression in multiple tumors with a fold change of > 2 in more than half of the tumor types, including pancreatic cancer (PAAD), testicular cancer (TGCT), lung squamous cell carcinoma (LUSC), ovarian serous cystadenocarcinoma (OV), uterine carcinosarcoma (UCS), bile duct cancer (CHOL), stomach adenocarcinoma (STAD), uterine corpus endometrial carcinoma (UCEC), kidney renal clear cell carcinoma (KIRC), glioblastoma multiforme (GBM), adrenocortical carcinoma (ACC), kidney renal papillary cell carcinoma (KIRP), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), colon adenocarcinoma (COAD), skin cutaneous melanoma (SKCM), rectal cancer (READ), BC, LUAD, pheochromocytoma & paraganglioma (PCPG) and kidney chromophobe (KICH) (Fig. 1A and Table S3). The paired Student's t-test also suggested a significant upregulation of GAPDH in multiple tumor tissues compared with the levels in paired normal adjacent tissues (Figure S1). To further verify the overexpression of GAPDH in pan-cancer, the expression of 11 transcripts of GAPDH, including 5 non-coding transcripts and 6 protein-coding transcripts (Figure S2A), was also analyzed. Of note, the results demonstrated that the expression of all protein-coding transcripts was significantly increased in multiple cancer types, while the non-coding transcripts were significantly reduced in several cancer types (Fig. 1B). The present study also analyzed the GAPDH expression in three GEO datasets, and the results showed an increased GAPDH expression in the lung cancer dataset GSE19804 (Fig. 1C), endometrial cancer dataset GSE17025 (Fig. 1D) and metastatic prostate tumor dataset GSE6919 (Fig. 1E). At the protein expression level, GAPDH upregulation was found in 12 datasets with 11 cancer types in the CPTAC database, including clear cell renal cell carcinoma, GBM, head and neck squamous cell carcinoma (HNSCC), lung squamous cell carcinoma, LUAD, pancreatic ductal adenocarcinoma, UCEC, hepatocellular carcinoma, BC, COAD and OV (Fig. 1F). These results suggested that GAPDH was upregulated in multiple cancer types, suggesting that it may have potential roles in cancer development. In addition, GAPDH dysregulation was identified in several exposure conditions (Figure S3), including several chemicals, hypoxia, radiation and heavy metals, based on the datasets evaluated in the GEO database. These findings indicate that GAPDH needs to be carefully considered as a mere housekeeping gene in several studies.
Fig. 1.
GAPDH is upregulated in multiple cancer types. (A) GAPDH mRNA expression in 33 cancer types based on TCGA and GTEx database. The red and blue dots refer to tumor (T) and normal tissues (N), respectively. (B) Protein-coding transcripts of GAPDH were upregulated in 33 cancer types based on TCGA and GTEx databases. Red and green squares represent upregulation and downregulation, respectively. GAPDH expression in three Gene Expression Omnibus datasets, including (C) the lung cancer dataset GSE19804, the (D) endometrial cancer dataset GSE17025 and (E) the metastatic prostate cancer dataset GSE6919. (F) GAPDH protein expression in 12 cancer types based on the Clinical Proteomic Tumor Analysis Consortium database. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TCGA, The Cancer Genome Atlas.
3.2. GAPDH is associated with prognosis, drug sensitivity, tumor immunity and tumor stemness
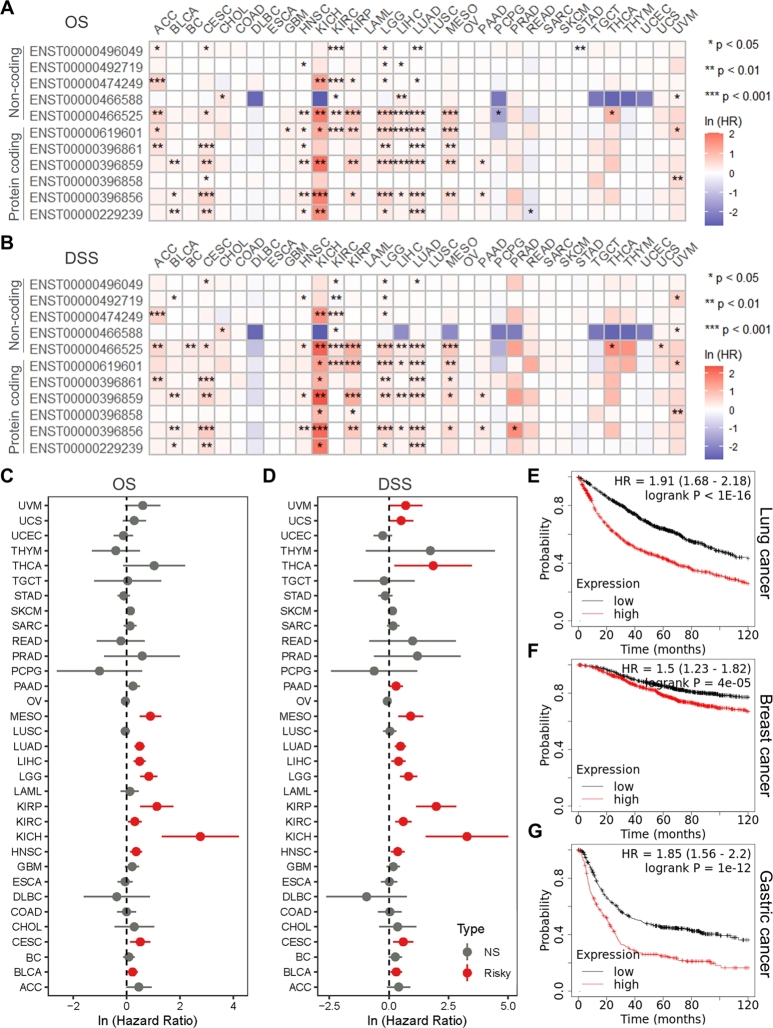
To explore the clinical significance of GAPDH expression, the association between GAPDH expression and patient OS, DSS, DFI and PFI was analyzed in 33 cancer types in the TCGA database. It was found that almost all GAPDH transcripts were risk factors for patients' OS and DSS in multiple cancer types (Fig. 2A-B). Regardless of transcripts, using gene expression data, the high GAPDH expression was also found to be associated with poor OS and DSS in a variety of cancer types, mainly in SARC, MESO, LUSC, LUAD, LGG, KIRP, KIRC and kidney chromophobe (KICH) (Fig. 2C-D). In addition, the KM plots demonstrated that GAPDH was a significant risk factor for OS in multiple cancer types (Figure S4A-H). The KM-plotter web tool revealed that high GAPDH mRNA expression was associated with poor OS in lung (Fig. 2E), breast (Fig. 2F) and gastric (Fig. 2G) cancer. Transcript and gene expression data revealed that high expression of GAPDH was a risk factor for DFI and PFI in numerous cancer types (Figure S5A-D). Using the ‘OncoPredict’ package and the GDSC v2 database, the sensitivity of 198 antitumor drugs was calculated. Further correlation analysis suggested that GAPDH expression was correlated with multiple drugs in numerous cancer types and this correlation appeared to be cancer-type dependent (Figure S6).
Fig. 2.
GAPDH is associated with poor OS and DSS. Heatmap shows univariable Cox regression analysis for (A) OS and (B) DSS of 11 GAPDH transcripts in 33 TCGA cancer types. Color ranges from blue to red representing logarithmic-scaled hazard ratios. Forest plots show univariable Cox regression analysis for (C) OS and (D) DSS of GAPDH expression in 33 TCGA cancer types. Kaplan-Meier analysis of OS for GAPDH mRNA expression in patients with (E) lung, (F) breast and (G) gastric cancer based on the KMplotter online tool. OS, overall survival; DSS, disease-specific survival; NS, not significant; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TCGA, The Cancer Genome Atlas.
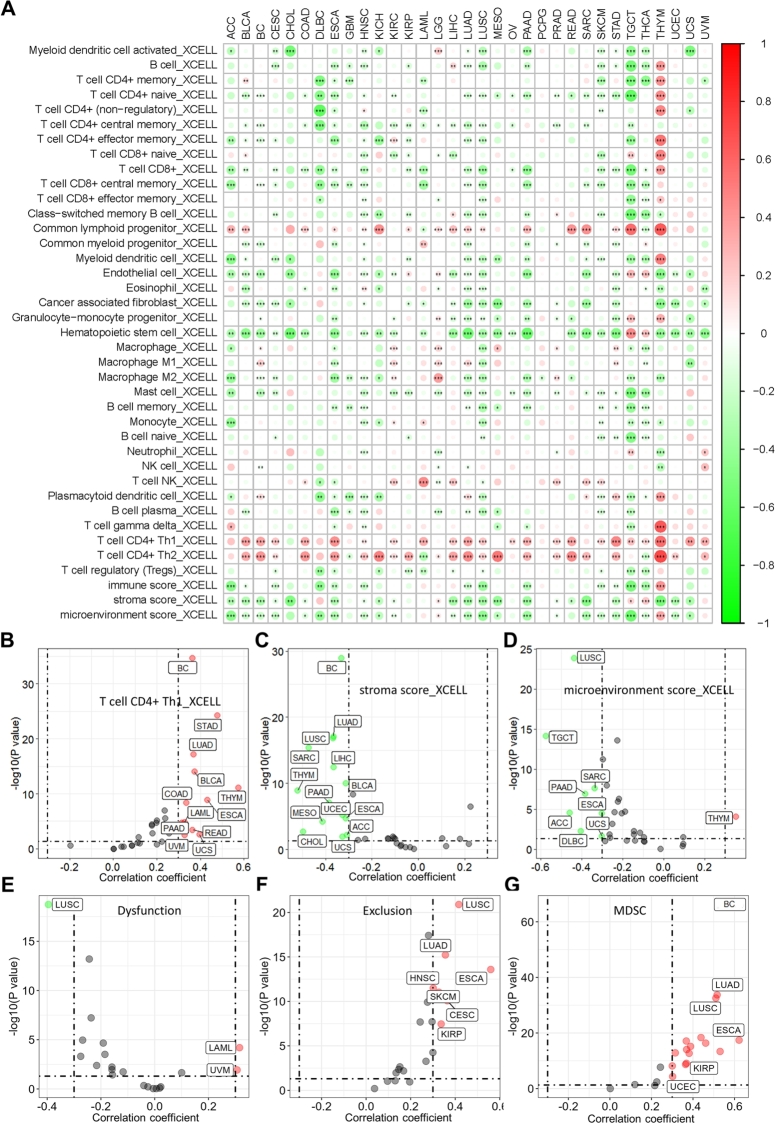
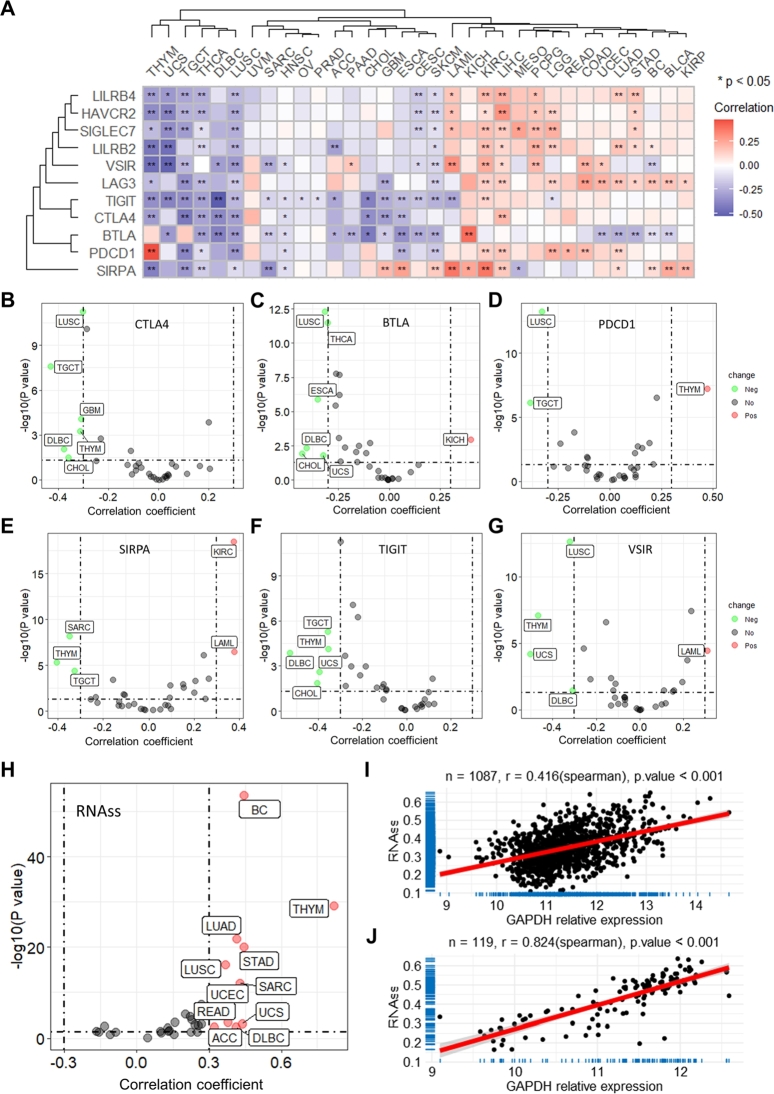
In addition, the association between GAPDH expression and immune cell infiltration was analyzed to determine its pan-cancer immunological role. The results revealed that GAPDH is negatively correlated with the infiltration of numerous types of immune cells in multiple cancer types based on the XCELL algorithm (Fig. 3A). Among them, CD4+ T helper (Th) 1 was found to be positively correlated with the GAPDH expression (Fig. 3B), as well as the stroma and microenvironment scores were found to be negatively correlated with GAPDH expression (Fig. 3C-D) in various cancer types. Besides, GAPDH expression was found positively correlated with Tumor immune dysfunction and exclusion (TIDE) score (Fig. 3E-F), as well as myeloid-derived suppressor cell (MDSC) infiltration (Fig. 3G) in multiple cancer types based on the TIDE online tool (Table S4). In addition, GAPDH was significantly correlated with the expression of several immune checkpoints (Fig. 4A), including CTLA4 (Fig. 4B), BTLA (Fig. 4C), PDCD1 (Fig. 4D), SIRPA (Fig. 4E), TIGIT (Fig. 4F) and VSIR (Fig. 4G), in multiple cancer types. GAPDH was also found to be significantly positively correlated with the expression of several immunomodulatory molecules (Figure S7), including TGFB1 (Figure S8A), CD276 (Figure S8B) and PVR (Figure S8C), in multiple cancer types. By contrast, GAPDH was negatively correlated with the expression of other immunomodulatory molecules (Figure S7), including CD40LG (Figure S8D), CD160 (Figure S8E) and SELP (Figure S8F) in the majority of cancer types.
Fig. 3.
GAPDH contributes to multiple immune cell infiltration in pan-cancer. (A) The correlation heatmap shows the correlation between GAPDH expression and multiple immune cell infiltration based on the XCELL algorithm. Color ranging from green to red represents the correlation coefficient. Scatter plots show the results of Spearman correlation analysis between the expression of GAPDH and the infiltration of (B) CD4+ Th1 cells, (C) stroma score and (D) microenvironment score by using the XCELL algorithm across pan-cancer. Scatter plots show the results of Spearman correlation analysis between the expression of GAPDH and the (E) TIDE dysfunction, (F) TIDE exclusion and (G) MDSC infiltration by using the TIDE algorithm across pan-cancer. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Th, T helper; MDSC, Myeloid-derived suppressor cell.
Fig. 4.
GAPDH is associated with immune checkpoint gene expression and tumor stemness in 33 cancer types. (A) Heatmap shows the correlation between the expression of GAPDH and 11 immune checkpoint genes. Color ranging from blue to red represents the correlation coefficient. Scatter plots show the results of Spearman's correlation analysis between the expression of GAPDH and (B) CTLA4, (C) BTLA, (D) PDCD1, (E) SIRPA, (F) TIGIT and (G) VSIR across pan-cancer. (H) Scatter plots show the results of Spearman's correlation analysis between GAPDH expression and tumor stemness score based on the RNAss algorithm. Correlation analysis of GAPDH expression and RNAss stemness score in the (I) BC and (J) THYM datasets. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; BC, breast cancer; THYM, thymoma.
Tumor stemness was found to be positively correlated with GAPDH expression in various cancer types (Fig. 4H), including BC (Fig. 4I), THYM (Fig. 4J), LUAD, STAD and LUSC. These results suggested that GAPDH may have prognostic significance in multiple cancer types, and may contribute to tumor microenvironment status and stemness.
3.3. GAPDH serves as an oncogene in multiple cancer types
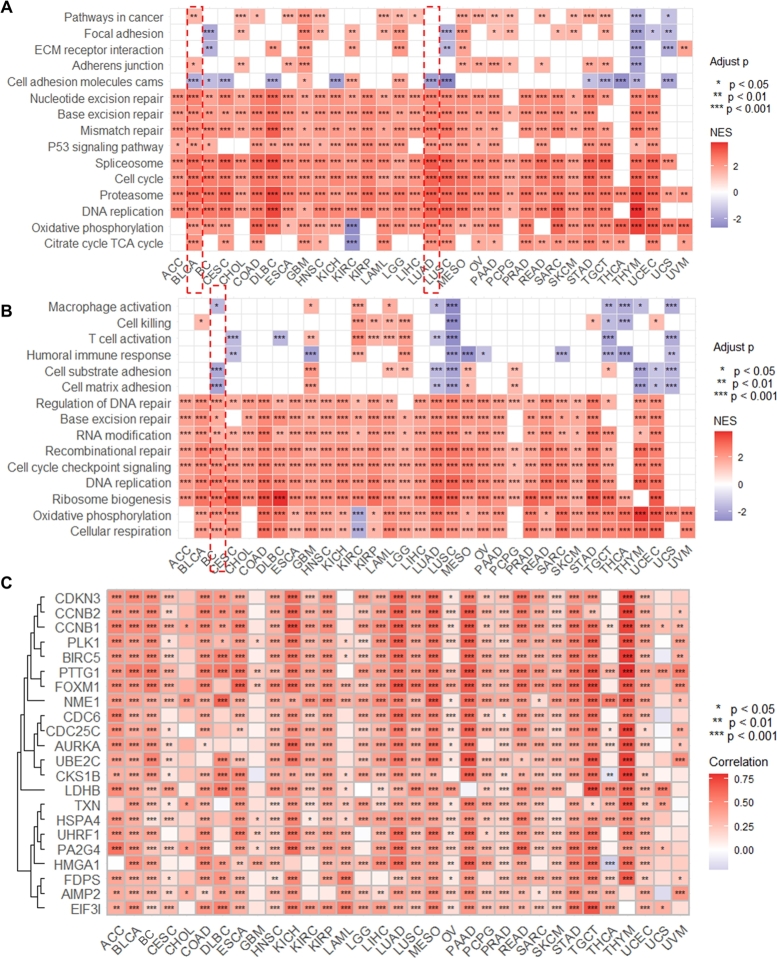
To predict the potential function and mechanism of GAPDH in pan-cancer, GSEA was used to enrich GAPDH-related Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and biological processes. As shown in Fig. 5A, several cancer-related pathways were significantly enriched, including cell cycle (Figure S9A), base excision repair (Figure S9B), spliceosome (Figure S9C), pathways in cancer, mismatch repair, DNA replication, as well as focal adhesion and cell adhesion molecules. In addition, the GSEA results of the biological process indicated that GAPDH was associated with multiple vital biological processes (Fig. 5B), including DNA replication (Figure S9D), cell matrix adhesion (Figure S9E), cell cycle checkpoint signaling, base excision repair, combinational repair and RNA modification. The correlation analysis revealed that GAPDH expression was also associated with several oncogenes (Fig. 5C), including well-known oncogenes PLK1 (Figure S9F), CCNB1 (Figure S9G), CCNB2 (Figure S9H) and BIRC5 (Figure S9I). Moreover, the correlation results showed that the majority of cell cycle pathway-related genes were positively correlated with GAPDH expression in LUAD (Figure S10A) and BC (Figure S10B), including cyclin (CCN) protein, cell division cycle (CDC) protein, minichromosomal maintenance (MCM) protein and E2F transcription factor family members. In addition, the majority of cell matrix adhesion-related genes were negatively correlated with GAPDH in LUAD (Figure S10C) and BC (Figure S10D).
Fig. 5.
GAPDH is associated with cancer-related pathways and biological processes. (A) Heatmap showing the Kyoto Encyclopedia of Genes and Genomes pathways related to GAPDH based on GSEA. Color ranging from blue to red represents the NES value. (B) Heatmap showing the biological processes related to GAPDH based on GSEA. Color ranging from blue to red represents the NES value. (C) Heatmap showing the oncogenes related to GAPDH based on correlation analysis. Color ranging from blue to red represents the correlation coefficient. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GSEA, Gene Set Enrichment Analysis; NES, normalized enrichment score.
3.4. GAPDH regulates cancer cell proliferation and migration
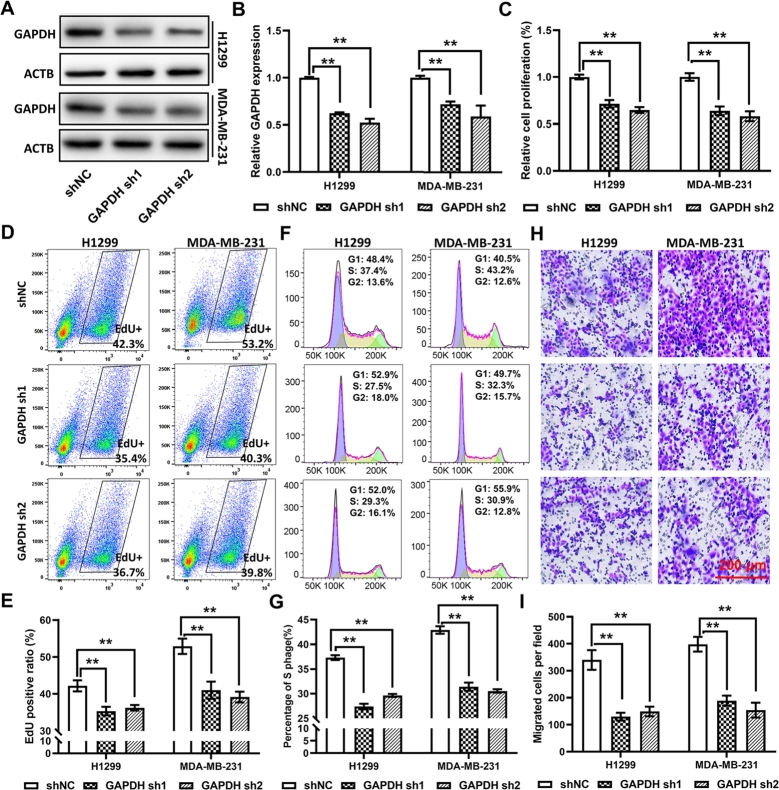
To validate the potential biological function of GAPDH predicted by GSEA, two cell lines, namely the LUAD cell line H1299 and the BC cell line MDA-MB-231, were employed to analyze the function of GAPDH. To select the best suitable reference gene, the ‘GeNorm’ algorithm and CV% were used to analyze the ranking of 10 reference genes. The results indicated that ACTB was the top-ranked reference gene in 50% types of tumors based on both criteria (Figure S11A-B). In addition, ACTB was differentially expressed in only 7 cancer types (Figure S10E), showing its superiority over most other genes; thus, it was selected as the reference gene in the present study. It is noteworthy that GAPDH ranked first in only 1 tumor based on CV%, and the even worse performance was found based on “GeNorm” (Figure S11A-B). In normal tissue datasets, ACTB no longer seems to be the most stable internal reference gene, while RPL13A seems to perform better (Figure S11C-D). Western blotting indicated a significant reduction in GAPDH protein expression in cells transfected with GAPDH sh1/2 (Fig. 6A-B). The results of the CCK-8 assay showed that GAPDH deficiency could significantly inhibit cell proliferation when compared with the results exhibited by cells transfected with empty vector (shNC) in both cell types (Fig. 6C). In addition, the percentage of EdU-positive cells and S-phase cells was significantly reduced in GAPDH-knocked down cells, indicating the inhibition of cell cycle and proliferation (Fig. 6D-G). The results of Transwell assay revealed the reduced migration ability of GAPDH-knocked down cells (Fig. 6H-I). These results showed that GAPDH contributed to multiple cancer-related pathways and biological processes, particularly cell cycle and cell adhesion, indicating that it may serve as an oncogene in multiple cancer types.
Fig. 6.
GAPDH reduction inhibits cell proliferation and migration. (A) Western blotting and (B) statistical analysis show GAPDH protein expression in the lung cancer cell line H1299 and the breast cancer cell line MDA-MB231 transfected with GAPDH shRNAs. (C) Cell Counting Kit-8, (D and E) EdU proliferation and (F and G) propidium iodide staining assays show the inhibited cell proliferation ability of cells transfected with shRNAs. (H) Representative images and (I) statistical analysis of the results of Transwell cell migration assay showing the inhibited cell migration ability of cells transfected with shRNAs. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; shRNA, short hairpin RNA.
3.5. GAPDH is regulated by copy number amplification and DNA methylation
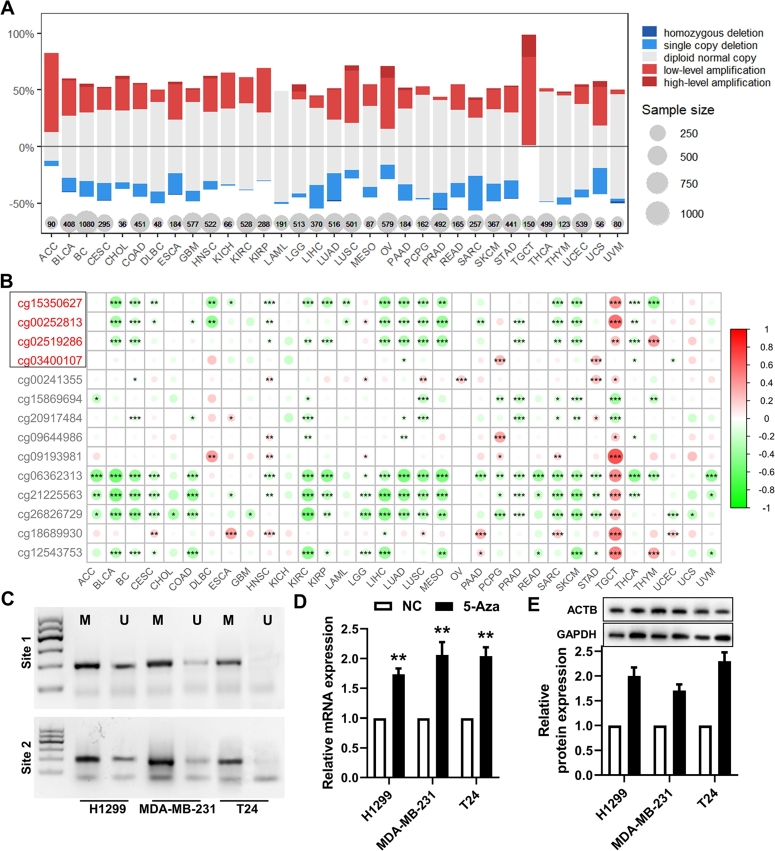
To reveal the mechanism that contributes to an increased GAPDH expression, the mutations and copy number variation of the GAPDH gene, as well as DNA methylation alteration in the GAPDH promotor region, were analyzed. At the genetic level, the mutations found in multiple cancer types were evenly distributed over the entire coding sequence of GAPDH. The proportion of mutations was generally low across tumor types. The three tumors with the highest proportion of mutations were SKCM (n = 102; two samples with a missense mutation and one with a nonsense mutation), UCS (n = 57; one sample with missense mutation) and UCEC (n = 175; one sample with nonsense mutation, missense mutation and splice site) (Figure S12A). Regarding copy number variation, copy number gains were identified in a larger proportion of GAPDH genes across the majority of tumor types and copy number deletions in a small subset of samples (Fig. 7A). Moreover, a significantly positive correlation (r > 0.3; P < 0.05) was also found between GAPDH mRNA expression and copy number variation in the majority of tumor types (Figure S12B). The top four most correlated cancer types were BC (r = 0.544; Figure S12C), HNSC (r = 0.592; Figure S12D), LUSC (r = 0.495; Fig. 2) and LUAD (r = 0.499; Figure S12F).
Fig. 7.
GAPDH is regulated by copy number amplification and DNA methylation. (A) DNA copy number variation analysis in 33 cancer types. (B) DNA methylation analysis in 33 cancer types. A red ID probe indicates that the probe targeted to the promotor region. (C) Methylation-specific PCR analysis of the GAPDH promoter in H1299, MDA-MB-231 and T24 cells. (D) mRNA and (E) protein expression of GAPDH in H1299, MDA-MB-231 and T24 cells treated with 5-aza-2'-deoxycytidine. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
At the epigenetic level, methylation levels of multiple sites within the GAPDH promoter region and gene body were significantly inversely correlated with mRNA expression levels in >50% of the tumor types, including BLCA, BC, CESC, DLBC, HNSC, KIRC, KIRP, LAML, LIHC, LUAD, LUSC, MESO, SARC, SKCM, THCA and THYM (Fig. 7B). Furthermore, the methylation values of cg15350627 and cg02519286 in tumor tissues were significantly higher than those in normal tissues in 50% of the tumor types (Figure S13A-B), including KIRP, KIRC, THCA, LUAD, LUSC, UCEC, SKCM, HNSC and GBM. Based on the methPrimer tool, CpG sites and CpG islands were predicted among the promotor of GAPDH, and the primers for MSP were designed (Figure S13C). Three cell lines, namely H1299, MDA-MB-231 and the bladder cancer cell line T24, were used to analyze the methylation levels in the promoter region. The MSP results demonstrated the presence of methylation modifications in the promoter region of GAPDH in all three cell lines (Fig. 7C). In addition, the downregulated methylation level of GAPDH promoter (Figure S14) and increased mRNA and protein expression of GAPDH was found in 5-aza-2'-deoxycytidine (5-Aza, a methyltransferase inhibitor)-treated H1299, MDA-MB-231 and T24 cells (Fig. 7D-E). These results mainly revealed two regulatory mechanisms that contributed to GAPDH upregulation, i.e. DNA copy number amplification and DNA demethylation, in multiple cancer types.
3.6. GAPDH is regulated by the transcription factor FOXM1
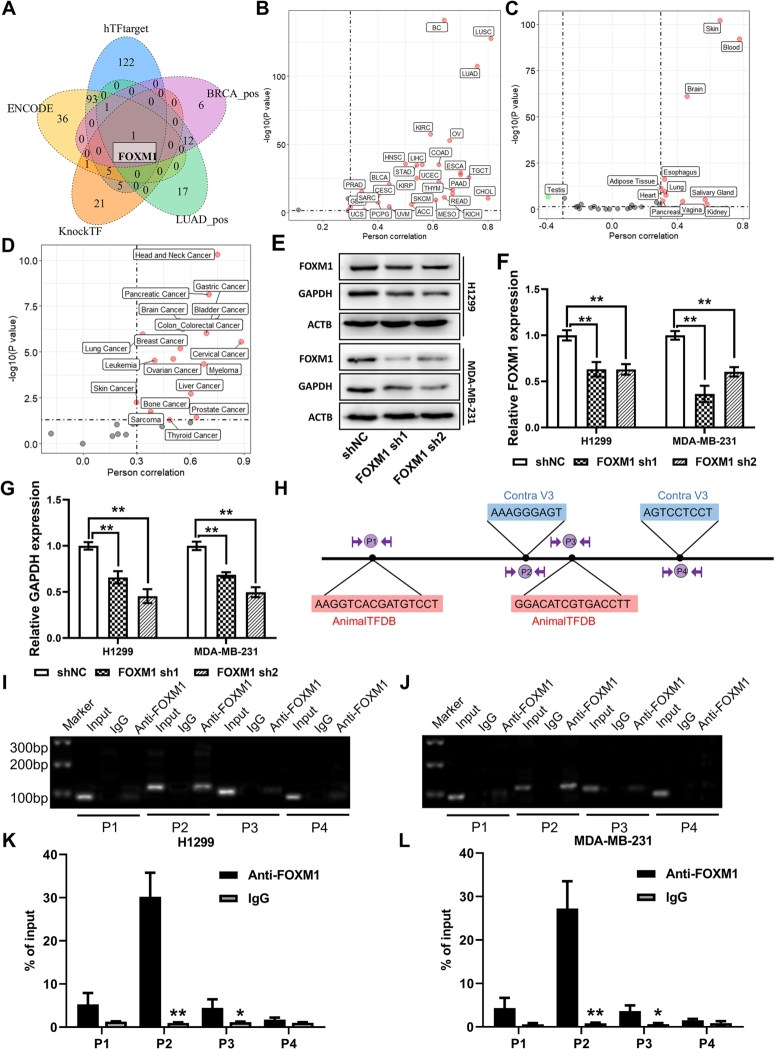
To explore the more comprehensive upstream regulatory mechanism of GAPDH, the transcription factors that may regulate it were further analyzed. Based on three transcription factor prediction web tools and the results of correlation analysis of GAPDH in the BC and LUAD datasets, one intersection gene, FOXM1, was identified (Fig. 8A). Correlation analysis of GAPDH and FOXM1 indicated a highly significant positive correlation between them in the majority of datasets in three databases, i.e. TCGA (Fig. 8B and Figure S15E), GTEx (Fig. 8C) and CCLE (Fig. 8D). In the TCGA database, the top 3 correlated datasets were LUSC (r = 0.809; Figure S15F), LUAD (r = 0.758; Figure S155G) and BC (r = 0.644; Figure S15H). In the GTEx database, the top 3 correlated datasets were skin (r = 0.661; Figure S15I), blood (r = 782; Figure S15J) and brain (r = 0.463; Figure S15K). Moreover, three GEO datasets were also used to explore the FOXM1-GAPDH regulatory mechanism, including the GSE186682 dataset, which used the FOXM1 inhibitor NB73 to treat MDA-MB-231 and MCF7 cells, thereby inhibiting FOXM1 expression, and the GSE142567 and GSE25741 datasets, which used small interfering RNA to treat the human ovarian cancer cell line A2780 and MDA-MB-231 cells, respectively. The results revealed that GAPDH expression was significantly reduced under both drug- and genetically- induced FOXM1 deficiency (Figure S15A-D). The present study constructed FOXM1-deficient H1299 and MDA-MB-231 cells through transfection with shRNAs (Fig. 8E-F). Consistently, GAPDH protein expression was significantly reduced in shRNA-transfected cells compared with that in empty vector-transfected cells (Fig. 8G).
Fig. 8.
GAPDH is regulated by the transcription factor FOXM1. (A) GAPDH upstream transcription factors prediction based on three web tools and correlation analysis. Correlation analysis between GAPDH and FOXM1 expression in (B) The Cancer Genome Atlas, (C) Genotype-Tissue Expression and (D) Cancer Cell Line Encyclopedia databases. (E and F) Western blotting analysis showing reduced GAPDH expression in GAPDH short hairpin RNA-transfected cells. (G and H) Transcription factor binding sites were predicted using the AnimalTFDB and Contra v3 web tools. Chromatin immunoprecipitation was performed on H1299 or MDA-MB-231 cells using anti-FOXM1 antibody or IgG as control, and the precipitated chromatin DNA was subjected to (I and J) PCR or (K and L) quantitative PCR using primers to amplify the regions of the GAPDH promoter. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; FOXM1, forkhead box M1.
Next, ChIP-PCR/qPCR and luciferase assays were used to identify whether FOXM1 binds to the binding sites in the GAPDH promoter. A total of 4 TFBS were predicted by using AnimalTFDB and Contra v3 web tools, and the corresponding primer pairs were designed (Fig. 8H). Semiquantitative ChIP RT-PCR/qPCR assays showed that FOXM1 is mainly bounded to one site, P2, in the H1299 or MDA-MB-231 cells (Fig. 8I-L). Moreover, dual-luciferase assay further confirmed the potential ability of FOXM1 to bind to the P2 site in the promoter region of GAPDH (Figure S16A-C).
4. Discussion
The use of inappropriate internal reference genes can lead to unreliable data and offset or even reverse the high performance of qPCR technology in evaluating differential gene expression. As an internal reference gene, in addition to having stable expression levels in different samples, it should also show a low mutation rate and as few transcripts and pseudogenes as possible [43]. However, the GAPDH gene has 11 transcripts, but only 2 of them encode functional canonical protein, while the rest can pose a challenge for its use as a reference gene. Also, it is important to note that GAPDH has over 60 pseudogenes, which have a very similar to that of GAPDH and could lead to interference when GAPDH is used as a reference gene in qPCR. The present study demonstrated an elevated expression of one commonly used internal reference gene, GAPDH, in most cancers, as well as the significance of this change for patient prognosis and immune response. Specifically, the current study explored its upstream regulatory mechanism through multi-omics analysis and in vitro experiments. The present study thus brought attention to GAPDH as a culprit in tumorigenesis, casting doubts on its suitability as an internal reference gene in several conditions.
Previous studies have found that GAPDH is involved in a variety of biological functions in cells and participates in important chemical cascades as a typical moonlighting protein, which is a class of proteins that exhibit various physiologically relevant biochemical or biophysical functions within a polypeptide chain. In addition to executing the glycolytic processes necessary for normal cell survival, GAPDH is involved in the cellular response to various stresses, such as oxidative stress [44], [45], hypoxia [46] and toxicity of chemical agents [47]. Significantly increased GAPDH expression levels were observed in numerous human cancer types and were generally involved in cancer development, metastasis and proliferation, as well as poor patient prognosis [14], [15]. However, this gene is still commonly used as an internal reference in numerous studies, including cancer-related studies. The present findings, based on pan-cancer analysis of TCGA database, indicated that GAPDH was ubiquitously highly expressed in the majority of cancer types, and that patients with high GAPDH expression in tumor tissues have a poor prognosis. In addition, the increase was present in multiple protein-coding transcripts as well as protein products, implying that the elevation was regulated at the transcriptional or gene level. It is worth mentioning that, contrary to the high expression of GAPDH in solid tumors, it exhibited a reduction in acute myeloid leukemia (LAML), which may be associated with the different growth patterns of LAML and solid tumor cells.
It is known that tumor cells influence and co-evolve with each other with their microenvironment, thus facilitating tumor development. An increasing number of effective immunotherapies for the treatment of patients with cancer are currently emerging clinically. In the present study, GAPDH was found to correlate with the infiltration of multiple immune cells in the majority of tumors, including B, CD8+ T, CD4+ T, regulatory T (Treg), cancer-associated fibroblast (CAF) and macrophage cells. Treg cells are essential for preventing autoimmunity and can also suppress effective tumor immunity. Massive infiltration of Treg cells into tumor tissues is generally associated with poor prognosis in patients with cancer [48]. The fibroblast population found in primary and metastatic cancer types, collectively referred to as CAFs, has been widely studied, and is associated with the occurrence, progression and metastasis of tumors [49], [50]. In addition, GAPDH is significantly correlated with the expression of multiple immune checkpoints and immunomodulatory molecules. Due to the unique role in immune escape, immune checkpoint has become a main target in drug research, and increasing evidence shows that blocking it is the most promising method in cancer immunotherapy [51], [52]. The present study revealed that GAPDH is significantly correlated with the expression of multiple immune checkpoints and immunomodulatory molecules. In most cancer types, the expression of GAPDH is positively correlated with tumor dryness, which has been reported to promote tumor occurrence, metastasis, and drug resistance. These results imply a certain association between GAPDH and tumor immunity, which further indicates that GAPDH is associated with tumor occurrence and progression, and may influence the efficacy of immunotherapy.
In terms of function and mechanism, GSEA revealed that GAPDH may contribute to numerous important cancer-related pathways and biological processes. In particular, it was found that GAPDH had important effects on cell cycle and DNA replication. Previous studies have identified GAPDH as a binding protein of oncoprotein SET, a cyclin B1 binding protein that has been shown to promote cancer initiation and progression [53], [54]. GAPDH depletion in lung cancer cells (A549) was able to inhibit glycolysis and impede the cell cycle through a 5'-AMP activated protein kinase-mediated pathway [55]. A recent study has found that tumor cells utilize specific post-translational modifications of GAPDH to prevent its function during programmed cell death and thereby promote cancer cell survival [56]. In addition, GAPDH upregulation was shown to promote tumor angiogenesis development as well as cancer invasion and metastasis [57], [58]. The present in vitro experiments further validated the promoting effect of GAPDH on cancer behavior, including enhancing cell proliferation and migration. Mechanistically, GAPDH upregulation is regulated by DNA copy number amplification and modification of promoter methylation based on multi-omics analysis. Of note, the transcription factor FOXM1 was identified as capable of transcriptionally regulating GAPDH expression, and was confirmed to be an oncogene ubiquitously highly expressed in multiple cancer types [59]. Increased FOXM1 expression could significantly contribute to tumorigenesis and cancer progression by modulating G1/S and G2/M transition as well as M phase progression [60].
In conclusion, the present study illustrates the function of GAPDH in tumor regulation, which mainly includes the following: i) GAPDH is upregulated in the majority of tumor types; ii) high GAPDH expression is regulated by DNA copy number, methylation, and the transcription factor FOXM1; and iii) GAPDH expression has important clinical implications in patients with tumors in terms of immunotherapy and prognosis. Therefore, it can be suggested that, particularly in tumor studies, the use of GAPDH as an internal reference gene for gene/protein level normalization requires great caution, and its role in tumor development warrants further investigation.
CRediT authorship contribution statement
JW, JL, and RC contributed to the conception and design of the study. JW organized the database. JW and XY performed the experiments. XC, LT and BJ performed the statistical analysis. JW wrote the first draft of the manuscript. XY and XC wrote sections of the manuscript. JL and RC revised the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) do not used generative AI and AI-assisted technologies in the writing process.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81573178 and 82070095). The study was also supported by Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, MOE Key Laboratory of Geriatric Diseases and Immunology as well as the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Supplementary material related to this article can be found online at https://doi.org/10.1016/j.csbj.2023.07.034.
Contributor Information
Rui Chen, Email: chenruigood@126.com.
Jianxiang Li, Email: aljxcr@suda.edu.cn.
Appendix A. Supplementary material
The following is the Supplementary material related to this article.
The supplementary data file further provides analysis of differential expression and correlation of GAPDH and regulation of DNA methylation in multiple cancer types based on TCGA pan-cancer and GTEx datasets. GAPDH is upregulated in many cancer types. The expression, mutation and copy number variation of GAPDH are associated with several important biological processes and oncogenes. Meanwhile, the expression changes of glyceraldehyde 3-phosphate dehydrogenase under different exposure conditions were analyzed based on Gene expression Omnibus database. Prognostic analysis of tumor patients with high and low GAPDH expression.
Data availability
Publicly available datasets were analyzed in this study. These data can be found as follows. The RNA sequencing data, somatic mutation data, copy number variation data, methylation data, as well as clinicopathological and survival data of 33 cancers in TCGA database were downloaded from UCSC Xena browser (https://xenabrowser.net/datapages/?cohort=TCGA%20Pan-Cancer%20(PANCAN)&removeHub=https%3A%2F%2Fxena.treehouse.gi.ucsc.edu%3A443). Tumor cell line data were downloaded from the CCLE database (https://portals.broadinstitute.org/ccle/). GAPDH and FOXM1 expressions in 31 various tissues were downloaded from GTEx (https://commonfund.nih.gov/GTEx). GAPDH expression in different GEO datasets were downloaded from GEO database (https://www.ncbi.nlm.nih.gov/geo/).
References
- 1.Singh R., Green M.R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993;259(5093):365–368. doi: 10.1126/science.8420004. https://www.ncbi.nlm.nih.gov/pubmed/8420004 [DOI] [PubMed] [Google Scholar]
- 2.Weber J.P., Bernhard S.A. Transfer of 1, 3-diphosphoglycerate between glyceraldehyde-3-phosphate dehydrogenase and 3-phosphoglycerate kinase via an enzyme-substrate-enzyme complex. Biochemistry. 1982;21(17):4189–4194. doi: 10.1021/bi00260a042. [DOI] [PubMed] [Google Scholar]
- 3.Seidler N.W. vol. 985. 2013. Gapdh: biological properties and diversity introduction. (Gapdh: Biological Properties and Diversity). [DOI] [Google Scholar]
- 4.Sheokand N., Malhotra H., Kumar S., Tillu V.A., Chauhan A.S., Raje C.I., et al. Moonlighting cell-surface GAPDH recruits apotransferrin to effect iron egress from mammalian cells. J Cell Sci. 2014;127(Pt 19):4279–4291. doi: 10.1242/jcs.154005. https://www.ncbi.nlm.nih.gov/pubmed/25074810 [DOI] [PubMed] [Google Scholar]
- 5.Colell A., Green D.R., Ricci J.E. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009;16(12):1573–1581. doi: 10.1038/cdd.2009.137. https://www.ncbi.nlm.nih.gov/pubmed/19779498 [DOI] [PubMed] [Google Scholar]
- 6.Tossounian M.A., Zhang B., Gout I. The writers, readers, and erasers in redox regulation of GAPDH. Antioxid (Basel) 2020;9(12) doi: 10.3390/antiox9121288. https://www.ncbi.nlm.nih.gov/pubmed/33339386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant C.M., Quinn K.A., Dawes I.W. Differential protein s-thiolation of glyceraldehyde-3-phosphate dehydrogenase isoenzymes influences sensitivity to oxidative stress. Mol Cell Biol. 1999;19(4):2650–2656. doi: 10.1128/MCB.19.4.2650. https://www.ncbi.nlm.nih.gov/pubmed/10082531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baba T., Kobayashi H., Kawasaki H., Mineki R., Naito H., Ohmori D. Glyceraldehyde-3-phosphate dehydrogenase interacts with phosphorylated akt resulting from increased blood glucose in rat cardiac muscle. FEBS Lett. 2010;584(13):2796–2800. doi: 10.1016/j.febslet.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Ventura M., Mateo F., Serratosa J., Salaet I., Carujo S., Bachs O., et al. Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase is regulated by acetylation. Int J Biochem Cell Biol. 2010;42(10):1672–1680. doi: 10.1016/j.biocel.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Hara M.R., Agrawal N., Kim S.F., Cascio M.B., Fujimuro M., Ozeki Y., et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following siah1 binding. Nat Cell Biol. 2005;7(7):665–U40. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 11.Caradec J., Sirab N., Revaud D., Keumeugni C., Loric S. Is GAPDH a relevant housekeeping gene for normalisation in colorectal cancer experiments? Br J Cancer. 2010;103(9):1475–1476. doi: 10.1038/sj.bjc.6605851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comajoan P., Gubern C., Huguet G., Serena J., Kádár E., Castellanos M. Evaluation of common housekeeping proteins under ischemic conditions and/or rt-pa treatment in bend. 3 cells. J Proteomics. 2018;184:10–15. doi: 10.1016/j.jprot.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Goasdoue K., Awabdy D., Bjorkman S.T., Miller S. Standard loading controls are not reliable for western blot quantification across brain development or in pathological conditions. Electrophoresis. 2016;37(4):630–634. doi: 10.1002/elps.201500385. [DOI] [PubMed] [Google Scholar]
- 14.Altenberg B., Greulich K.O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014–1020. doi: 10.1016/j.ygeno.2004.08.010. https://www.ncbi.nlm.nih.gov/pubmed/15533718 [DOI] [PubMed] [Google Scholar]
- 15.Guo C.M., Liu S.Q., Sun M.Z. Novel insight into the role of GAPDH playing in tumor. Clin Transl Oncol. 2013;15(3):167–172. doi: 10.1007/s12094-012-0924-x. [DOI] [PubMed] [Google Scholar]
- 16.Tokunaga K., Nakamura Y., Sakata K., Fujimori K., Ohkubo M., Sawada K., et al. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47(21):5616–5619. https://www.ncbi.nlm.nih.gov/pubmed/3664468 [PubMed] [Google Scholar]
- 17.Vila M.R., Nicolas A., Morote J., de I., Meseguer A. Increased glyceraldehyde-3-phosphate dehydrogenase expression in renal cell carcinoma identified by RNA-based, arbitrarily primed polymerase chain reaction. Cancer. 2000;89(1):152–164. https://www.ncbi.nlm.nih.gov/pubmed/10897012 [PubMed] [Google Scholar]
- 18.Revillion F., Pawlowski V., Hornez L., Peyrat J.P. Glyceraldehyde-3-phosphate dehydrogenase gene expression in human breast cancer. Eur J Cancer. 2000;36(8):1038–1042. doi: 10.1016/s0959-8049(00)00051-4. https://www.ncbi.nlm.nih.gov/pubmed/10885609 [DOI] [PubMed] [Google Scholar]
- 19.Epner D.E., Partin A.W., Schalken J.A., Isaacs J.T., Coffey D.S. Association of glyceraldehyde-3-phosphate dehydrogenase expression with cell motility and metastatic potential of rat prostatic adenocarcinoma. Cancer Res. 1993;53(9):1995–1997. https://www.ncbi.nlm.nih.gov/pubmed/8481901 [PubMed] [Google Scholar]
- 20.Blanquicett C., Johnson M.R., Heslin M., Diasio R.B. Housekeeping gene variability in normal and carcinomatous colorectal and liver tissues: applications in pharmacogenomic gene expression studies. Anal Biochem. 2002;303(2):209–214. doi: 10.1006/abio.2001.5570. https://www.ncbi.nlm.nih.gov/pubmed/11950223 [DOI] [PubMed] [Google Scholar]
- 21.Lu T.P., Tsai M.H., Lee J.M., Hsu C.P., Chen P.C., Lin C.W., et al. Identification of a novel biomarker, sema5a, for non-small cell lung carcinoma in nonsmoking women. Cancer Epidemiol Biomark Prev. 2010;19(10):2590–2597. doi: 10.1158/1055-9965.EPI-10-0332. https://www.ncbi.nlm.nih.gov/pubmed/20802022 [DOI] [PubMed] [Google Scholar]
- 22.Day R.S., McDade K.K., Chandran U.R., Lisovich A., Conrads T.P., Hood B.L., et al. Identifier mapping performance for integrating transcriptomics and proteomics experimental results. BMC Bioinform. 2011;12:213. doi: 10.1186/1471-2105-12-213. https://www.ncbi.nlm.nih.gov/pubmed/21619611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y.P., Landsittel D., Jing L., Nelson J., Ren B., Liu L., et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22(14):2790–2799. doi: 10.1200/JCO.2004.05.158. https://www.ncbi.nlm.nih.gov/pubmed/15254046 [DOI] [PubMed] [Google Scholar]
- 24.Thomas R.S., Himmelstein M.W., Clewell H.J., 3rd., Yang Y., Healy E., Black M.B., et al. Cross-species transcriptomic analysis of mouse and rat lung exposed to chloroprene. Toxicol Sci. 2013;131(2):629–640. doi: 10.1093/toxsci/kfs314. https://www.ncbi.nlm.nih.gov/pubmed/23125180 [DOI] [PubMed] [Google Scholar]
- 25.Stueckle T.A., Lu Y., Davis M.E., Wang L., Jiang B.H., Holaskova I., et al. Chronic occupational exposure to arsenic induces carcinogenic gene signaling networks and neoplastic transformation in human lung epithelial cells. Toxicol Appl Pharmacol. 2012;261(2):204–216. doi: 10.1016/j.taap.2012.04.003. https://www.ncbi.nlm.nih.gov/pubmed/22521957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beyer S., Kristensen M.M., Jensen K.S., Johansen J.V., Staller P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J Biol Chem. 2008;283(52):36542–36552. doi: 10.1074/jbc.M804578200. https://www.ncbi.nlm.nih.gov/pubmed/18984585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabbri M., Urani C., Sacco M.G., Procaccianti C., Gribaldo L. Whole genome analysis and microRNAs regulation in HepG2 cells exposed to cadmium. ALTEX. 2012;29(2):173–182. doi: 10.14573/altex.2012.2.173. https://www.ncbi.nlm.nih.gov/pubmed/22562489 [DOI] [PubMed] [Google Scholar]
- 28.Kawata K., Yokoo H., Shimazaki R., Okabe S. Classification of heavy-metal toxicity by human dna microarray analysis. Environ Sci Technol. 2007;41(10):3769–3774. doi: 10.1021/es062717d. https://www.ncbi.nlm.nih.gov/pubmed/17547211 [DOI] [PubMed] [Google Scholar]
- 29.Dressman H.K., Muramoto G.G., Chao N.J., Meadows S., Marshall D., Ginsburg G.S., et al. Gene expression signatures that predict radiation exposure in mice and humans. PLoS Med. 2007;4(4):e106. doi: 10.1371/journal.pmed.0040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De S., Ghosh S., Chatterjee R., Chen Y.Q., Moses L., Kesari A., et al. Pcb congener specific oxidative stress response by microarray analysis using human liver cell line. Environ Int. 2010;36(8):907–917. doi: 10.1016/j.envint.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vivian J., Rao A.A., Nothaft F.A., Ketchum C., Armstrong J., Novak A., et al. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol. 2017;35(4):314–316. doi: 10.1038/nbt.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li T., Fu J., Zeng Z., Cohen D., Li J., Chen Q., et al. Timer2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–W514. doi: 10.1093/nar/gkaa407. https://www.ncbi.nlm.nih.gov/pubmed/32442275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibru B., Fey K., Fricke S., Blaudszun A.R., Furst F., Weise M., et al. Detection of immune checkpoint receptors - a current challenge in clinical flow cytometry. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.694055. https://www.ncbi.nlm.nih.gov/pubmed/34276685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charoentong P., Finotello F., Angelova M., Mayer C., Efremova M., Rieder D., et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18(1):248–262. doi: 10.1016/j.celrep.2016.12.019. https://www.ncbi.nlm.nih.gov/pubmed/28052254 [DOI] [PubMed] [Google Scholar]
- 35.Yang W., Soares J., Greninger P., Edelman E.J., Lightfoot H., Forbes S., et al. Genomics of drug sensitivity in cancer (gdsc): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41:D955–D961. doi: 10.1093/nar/gks1111. https://www.ncbi.nlm.nih.gov/pubmed/23180760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeser D., Gruener R.F., Huang R.S. Oncopredict: an R package for predicting in vivo or cancer patient drug response and biomarkers from cell line screening data. Brief Bioinform. 2021;22(6) doi: 10.1093/bib/bbab260. https://www.ncbi.nlm.nih.gov/pubmed/34260682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinhold W.C., Sunshine M., Liu H., Varma S., Kohn K.W., Morris J., et al. Cellminer: a web-based suite of genomic and pharmacologic tools to explore transcript and drug patterns in the nci-60 cell line set. Cancer Res. 2012;72(14):3499–3511. doi: 10.1158/0008-5472.CAN-12-1370. https://www.ncbi.nlm.nih.gov/pubmed/22802077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. https://www.ncbi.nlm.nih.gov/pubmed/16199517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu G., Wang L.G., Han Y., He Q.Y. Clusterprofiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins J.R., Dawes J.M., McMahon S.B., Bennett D.L., Orengo C., Kohl M. Readqpcr and normqpcr: R packages for the reading, quality checking and normalisation of rt-qpcr quantification cycle (cq) data. BMC Genomics. 2012;13:296. doi: 10.1186/1471-2164-13-296. https://www.ncbi.nlm.nih.gov/pubmed/22748112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. https://www.ncbi.nlm.nih.gov/pubmed/12184808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jonge H.J., Fehrmann R.S., de Bont E.S., Hofstra R.M., Gerbens F., Kamps W.A., et al. Evidence based selection of housekeeping genes. PLoS ONE. 2007;2(9):e898. doi: 10.1371/journal.pone.0000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krasnov G.S., Kudryavtseva A.V., Snezhkina A.V., Lakunina V.A., Beniaminov A.D., Melnikova N.V., et al. Pan-cancer analysis of tcga data revealed promising reference genes for qpcr normalization. Front Genet. 2019;10 doi: 10.3389/fgene.2019.00097. https://www.frontiersin.org/articles/10.3389/fgene.2019.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang S., Figtree G., Aiqun M., Ping Z. GAPDH-knockdown reduce rotenone-induced H9C2 cells death via autophagy and anti-oxidative stress pathway. Toxicol Lett. 2015;234(3):162–171. doi: 10.1016/j.toxlet.2015.02.017. https://www.ncbi.nlm.nih.gov/pubmed/25725130 [DOI] [PubMed] [Google Scholar]
- 45.Schneider M., Knuesting J., Birkholz O., Heinisch J.J., Scheibe R. Cytosolic GAPDH as a redox-dependent regulator of energy metabolism. BMC Plant Biol. 2018;18(1):184. doi: 10.1186/s12870-018-1390-6. https://www.ncbi.nlm.nih.gov/pubmed/30189844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong H., Simons J.W. Direct comparison of GAPDH, beta-actin, cyclophilin, and 28s rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem Biophys Res Commun. 1999;259(3):523–526. doi: 10.1006/bbrc.1999.0815. [DOI] [PubMed] [Google Scholar]
- 47.Steinritz D., Weber J., Balszuweit F., Thiermann H., Schmidt A. Sulfur mustard induced nuclear translocation of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) Chem-Biol Interact. 2013;206(3):529–535. doi: 10.1016/j.cbi.2013.06.015. https://www.ncbi.nlm.nih.gov/pubmed/23827652 [DOI] [PubMed] [Google Scholar]
- 48.Tanaka A., Sakaguchi S. Targeting treg cells in cancer immunotherapy. Eur J Immunol. 2019;49(8):1140–1146. doi: 10.1002/eji.201847659. https://www.ncbi.nlm.nih.gov/pubmed/31257581 [DOI] [PubMed] [Google Scholar]
- 49.Ford K., Hanley C.J., Mellone M., Szyndralewiez C., Heitz F., Wiesel P., et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Res. 2020;80(9):1846–1860. doi: 10.1158/0008-5472.CAN-19-3158. https://www.ncbi.nlm.nih.gov/pubmed/32122909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–598. doi: 10.1038/nrc.2016.73. https://www.ncbi.nlm.nih.gov/pubmed/27550820 [DOI] [PubMed] [Google Scholar]
- 51.Postow M.A., Callahan M.K., Wolchok J.D. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi: 10.1200/jco.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dacol E.C., Wang S., Chen Y., Lepique A.P. The interaction of set and protein phosphatase 2a as target for cancer therapy. Biochim Biophys Acta, Rev Cancer. 2021;1876(1) doi: 10.1016/j.bbcan.2021.188578. [DOI] [PubMed] [Google Scholar]
- 54.Liang X., Bao X., Chen G. Set protein in cancer: a potential therapeutic target. Mini Rev Med Chem. 2021;21(16):2290–2299. doi: 10.2174/1389557521666210114163318. https://www.ncbi.nlm.nih.gov/pubmed/33459234 [DOI] [PubMed] [Google Scholar]
- 55.Phadke M., Krynetskaia N., Mishra A., Krynetskiy E. Accelerated cellular senescence phenotype of GAPDH-depleted human lung carcinoma cells. Biochem Biophys Res Commun. 2011;411(2):409–415. doi: 10.1016/j.bbrc.2011.06.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sirover M.A. Academic Press; 2017. Chapter 11 - GAPDH and tumorigenesis: molecular mechanisms of cancer development and survival; pp. 181–197.https://www.sciencedirect.com/science/article/pii/B978012809852300011X [DOI] [Google Scholar]
- 57.Liu K.Y., Tang Z.J., Huang A.M., Chen P., Liu P.P., Yang J., et al. Glyceraldehyde-3-phosphate dehydrogenase promotes cancer growth and metastasis through upregulation of snail expression. Int J Oncol. 2017;50(1):252–262. doi: 10.3892/ijo.2016.3774. [DOI] [PubMed] [Google Scholar]
- 58.Chiche J., Pommier S., Beneteau M., Mondragon L., Meynet O., Zunino B., et al. GAPDH enhances the aggressiveness and the vascularization of non-Hodgkin's b lymphomas via nf-kappab-dependent induction of HIF-1alpha. Leukemia. 2015;29(5):1163–1176. doi: 10.1038/leu.2014.324. https://www.ncbi.nlm.nih.gov/pubmed/25394713 [DOI] [PubMed] [Google Scholar]
- 59.Gartel A.L. Foxm1 in cancer: interactions and vulnerabilities. Cancer Res. 2017;77(12):3135–3139. doi: 10.1158/0008-5472.Can-16-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y., Wu F., Tan Q., Guo M., Ma P., Wang X., et al. The multifaceted roles of foxm1 in pulmonary disease. Cell Commun Signal. 2019;17(1):35. doi: 10.1186/s12964-019-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary data file further provides analysis of differential expression and correlation of GAPDH and regulation of DNA methylation in multiple cancer types based on TCGA pan-cancer and GTEx datasets. GAPDH is upregulated in many cancer types. The expression, mutation and copy number variation of GAPDH are associated with several important biological processes and oncogenes. Meanwhile, the expression changes of glyceraldehyde 3-phosphate dehydrogenase under different exposure conditions were analyzed based on Gene expression Omnibus database. Prognostic analysis of tumor patients with high and low GAPDH expression.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found as follows. The RNA sequencing data, somatic mutation data, copy number variation data, methylation data, as well as clinicopathological and survival data of 33 cancers in TCGA database were downloaded from UCSC Xena browser (https://xenabrowser.net/datapages/?cohort=TCGA%20Pan-Cancer%20(PANCAN)&removeHub=https%3A%2F%2Fxena.treehouse.gi.ucsc.edu%3A443). Tumor cell line data were downloaded from the CCLE database (https://portals.broadinstitute.org/ccle/). GAPDH and FOXM1 expressions in 31 various tissues were downloaded from GTEx (https://commonfund.nih.gov/GTEx). GAPDH expression in different GEO datasets were downloaded from GEO database (https://www.ncbi.nlm.nih.gov/geo/).