The authors regret that there were errors in the text and published figures. The authors would like to apologise for any inconvenience caused.
The corrections are as follows:
Diagnosis, pathology and molecular biology
Figure 1
On page 3, in Figure 1, an option is added after “cT1-T2, N0”:
-
•
High gradec
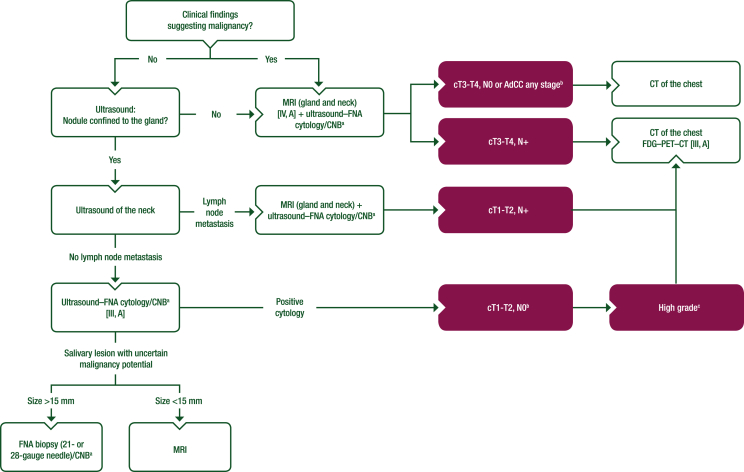
Figure 1.
Work-up of major salivary gland nodules.
Purple: general categories or stratification; white: other aspects of management.
AdCC, adenoid cystic carcinoma; CNB, core needle biopsy; CT, computed tomography; FDG–PET–CT, [18F]2-fluoro-2-deoxy-D-glucose–positron emission tomography–computed tomography; FNA, fine-needle aspiration; MRI, magnetic resonance imaging; SGC, salivary gland cancer.
aCNB considered when FNA is non-diagnostic or if more histological information is required.
bFDG–PET–CT is recommended for treatment planning in lymph node-positive or high-grade SGC; otherwise, CT of the chest can suffice.
cDefinition of high-grade tumours is described in Section 1 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100602.
This option then connects with the box containing “CT of the chest FDG–PET–CT [III, A]”.
On page 3, in Figure 1 and the figure footnote, a new footnote ‘b’ is added to the following boxes:
-
•
cT3-T4, N0 or AdCC any stageb
-
•
cT1-T2, N0b
-
b
bFDG–PET–CT is recommended for treatment planning in lymph node-positive or high-grade SGC; otherwise, CT of the chest can suffice.
On page 3, in Figure 1 and the figure footnote, a new footnote ‘c’ is added to the following box:
-
•
High gradec
-
c
cDefinition of high-grade tumours is described in Section 1 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100602.
Recommendations
On page 3:
-
•
FDG–PET–CT is recommended in high-grade SGC for the detection of distant metastases [III, A].
is replaced with:
-
•
FDG–PET–CT is recommended in high-grade or lymph-node positive SGC for the detection of distant metastases [III, A].
Management of local and locoregional disease
Figure 2
On page 5, in Figure 2:
-
•
No high-risk factors: RT to primary [IV, A]
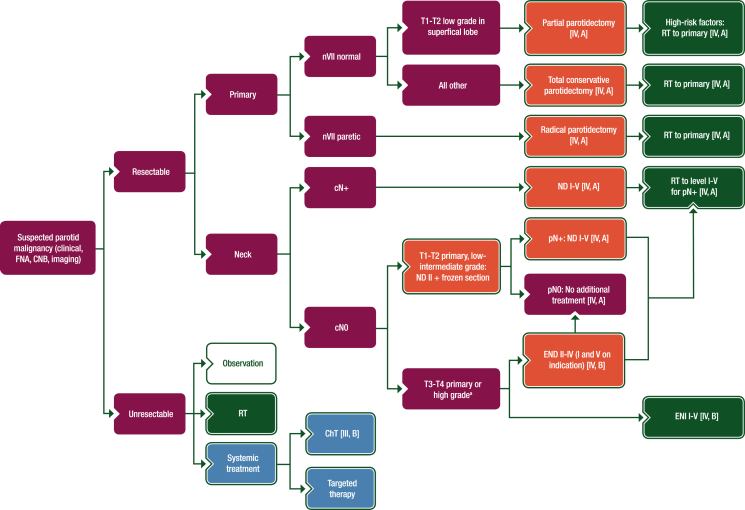
Figure 2.
Treatment algorithm for parotid gland cancer.
Purple: general categories or stratification; red: surgery; dark green: radiotherapy; white: other aspects of management; blue: systemic anticancer therapy.
ChT, chemotherapy; CNB, core needle biopsy; END, elective neck dissection; ENI, elective neck irradiation; FNA, fine-needle aspiration; ND, node dissection; nVII, seventh nerve; RT, radiotherapy.
aDefinition of high-grade tumours is described in Section 1 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100602.
is replaced with:
-
•
High-risk factors: RT to primary [IV, A]
On page 5, in Figure 2:
-
•
RT to neck
is replaced with:
-
•
RT to level I-V for pN+ [IV, A]
and an arrow is added between the box containing “END II-IV (I and V on indication) [IV, B] and the box containing “pN0”.
On page 5, in Figure 2:
-
•
pN+ and no high-risk factors: RT to level I-V [IV, A]
is replaced with an arrow to the box containing “RT to level I-V for pN+ [IV, A]”.
Figure 3
On page 6, in Figure 3, an additional option is added following “Open approach [IV, A]” and “Selected transoral/endoscopic/robotic [V, A]”:
-
•
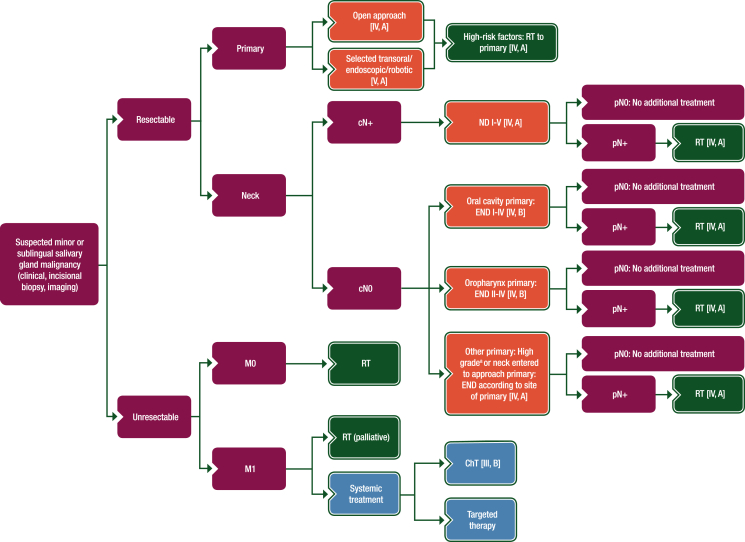
High-risk factors: RT to primary [IV, A]
Figure 3.
Treatment algorithm for minor or sublingual SGC.
Purple: general categories or stratification; red: surgery; dark green: radiotherapy; white: other aspects of management; blue: systemic anticancer therapy.
ChT, chemotherapy; END, elective neck dissection; ND, node dissection; RT, radiotherapy; SGC, salivary gland cancer.
aDefinition of high-grade tumours is described in Section 1 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100602.
Figure 4
On page 8, in Figure 4, an additional option is added following “Resection of submandibular gland and level Ib [IV, B]”:
-
•
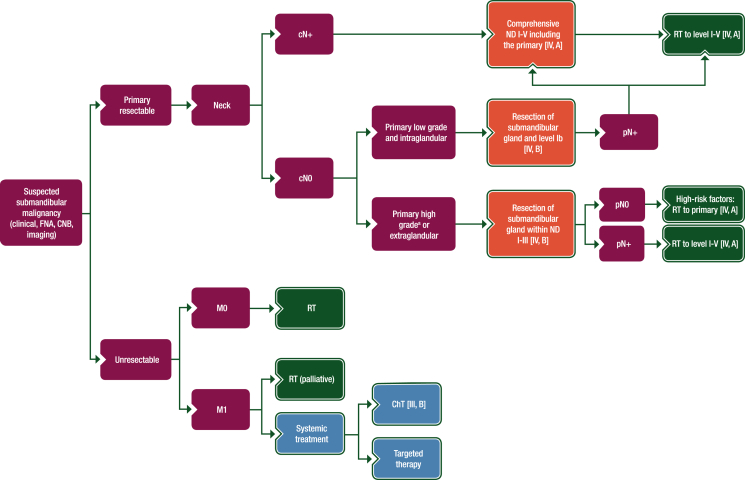
pN+
Figure 4.
Treatment algorithm for submandibular gland cancer.
Purple: general categories or stratification; red: surgery; dark green: radiotherapy; white: other aspects of management; blue: systemic anticancer therapy.
ChT, chemotherapy; CNB, core needle biopsy; FNA, fine-needle aspiration; ND, node dissection; RT, radiotherapy.
aDefinition of high-grade tumours is described in Section 1 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2022.100602.
with arrows connecting to “Comprehensive ND I-V including the primary [IV, A]” and “RT to level I-V [IV, A]”.
On page 8, in Figure 4:
-
•
pN0: No additional treatment
is replaced with:
-
•
pN0
with an arrow connecting to:
-
•
High-risk factors: RT to primary [IV, A]
Recommendations
On page 9, before the recommendations of “Surgical management of the primary: submandibular gland cancer” the following text is added:
-
•Surgical management of the primary: minor SGC and cancer of the sublingual gland
-
oDepending on the anatomical site of origin, a classical open approach [IV, A] or endoscopic, transoral or combined transoral-endoscopic resection [V, A] are recommended in selected patients, with the aim of achieving free margins.
-
o
Management of locally recurrent and metastatic disease
Systemic treatment for recurrent and/or metastatic disease
On page 10:
-
•
In case of R/M disease, systemic treatment is challenging but can be urgent, depending on tumour subtype and behaviour. For all types of SGC with distant metastases (71% of patients will present or develop R/M disease), median OS is 15 months and 1-, 3- and 5-year OS rates are 54.5%, 28.4% and 14.8%, respectively.
is replaced with:
-
•
In case of R/M disease, systemic treatment is challenging but can be urgent, depending on tumour subtype and behaviour. For all types of SGC with distant metastases (up to 60% of patients will present or develop R/M disease), median OS is 15 months and 1-, 3- and 5-year OS rates are 54.5%, 28.4% and 14.8%, respectively.