Abstract
A total of 129 clinical isolates of Mycobacterium tuberculosis representing 91 patients were typed by a combination of direct-repeat (DR)-based spoligotyping and an inter-IS6110–PGRS (polymorphic GC-rich region)–PCR, also designated double-repetitive-element PCR (DRE-PCR). During the first phase of this investigation, 72 clinical strains representing 52 patients were initially typed by IS6110-restriction fragment length polymorphism (RFLP) and DR-RFLP, followed by spoligotyping and DRE-PCR. In the second phase of this investigation, the discriminating ability of spoligotyping plus DRE-PCR was studied for 57 isolates from 39 patients who were suspected to be epidemiologically linked, and the typing results were later confirmed by IS6110-RFLP and DR-RFLP analyses. The molecular clustering of the isolates remained identical irrespective of the methods used. These results show that the association of two PCR-based fingerprinting techniques for molecular epidemiology of tuberculosis has a discriminating ability similar to the IS6110-RFLP reference method.
Molecular fingerprinting of Mycobacterium tuberculosis with the transposon IS6110 (12) is useful for epidemiological studies (10), and an internationally accepted restriction fragment length polymorphism (RFLP) procedure has been described previously (13). However, the development of rapid typing methods remains important, as IS6110-RFLP requires culturing, DNA extraction, and Southern hybridization, and may take as long as 4 to 5 weeks. Moreover, IS6110 fingerprinting may be limited, as some strains may not harbor a copy of IS6110 whereas others may contain only 1 to 5 copies (14). In this context, alternative PCR-based techniques seem particularly promising, as they may help with both rapid diagnosis and molecular typing of tuberculosis; however, the methods described may not be sufficiently discriminatory when used alone (2, 6, 8). For this reason, a combination of rapid methods with spoligotyping as a first-line test was recently hypothesized as a potential alternative to IS6110-RFLP (3). In this paper, we describe a combination of spoligotyping (6) followed by double-repetitive-element (DRE)-PCR (2) as a rapid alternative strategy for M. tuberculosis typing.
A total of 129 clinical isolates, representing 91 patients, that were isolated at the Pasteur Institute of Guadeloupe from 1994 to 1996 and identified as M. tuberculosis by classical mycobacteriology procedures were the subject of the present investigation. DNA was prepared by the CTAB (cetyltrimethylammonium bromide) method (13) for the IS6110-RFLP, direct-repeat (DR)-RFLP, and DRE-PCR procedures. For spoligotyping experiments, DNA was prepared by a microbead disruption method (1). IS6110-RFLP and DR-RFLP analyses were performed as reported previously (11, 13). Spoligotyping was performed with membranes that were prepared locally (6). DRE-PCR was performed as reported previously (2); a 20 25-μl aliquot of the PCR mixture was analyzed on 2% agarose gels, and pictures were taken after ethidium bromide staining by using a video-copy system and Gel-Analyst software (Bioprobe, Montreuil, France). IS6110-RFLP, DR-RFLP, and DRE-PCR results were analyzed with Taxotron software (Taxolab, Institut Pasteur, Paris, France), as reported previously (11). Spoligotyping results were entered in a spreadsheet (Excel) file and ordered for cluster identification.
When 72 clinical strains representing 52 patients were typed by IS6110-RFLP and DR-RFLP analyses, followed by spoligotyping and DRE-PCR, during the first phase of this investigation, a total of 17 patient isolates were grouped in three clusters; specific spoligotyping and DRE-PCR patterns are illustrated in Table 1. In the second phase of the investigation, the discriminating ability of spoligotyping followed by DRE-PCR was confirmed for 57 isolates from 39 patients who were suspected to be epidemiologically linked; a total of 28 patient isolates were grouped in 10 clusters, whereas the remaining patient isolates were unrelated upon DRE-PCR (Table 2). Concordant typing results were later obtained when the IS6110 reference method was used independently (Table 2).
TABLE 1.
Molecular description of clusters observed for 72 M. tuberculosis clinical isolates from 52 patients by IS6110-RFLP and DR-RFLP analyses, followed by spoligotyping and DRE-PCR
| Cluster | No. of IS6110-RFLP bands | No. of DR-RFLP bands (size [bp]) | Spoligotype | No. of major DRE-PCR bands (size [bp]) | No. of strainsa |
|---|---|---|---|---|---|
| A | 4–5 | 2 (4,800; 3,000) | ▪□▪□□□□□□□□□▪▪▪▪▪□▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪▪▪▪▪▪▪ | 1 (180) | 12 |
| B | 13 | 1 (5,100) | ▪▪▪▪▪□□□□□□□□□□□□▪▪□□□□□□□□□□□□□□□□□□□▪▪▪▪▪ | 1 (625) | 3 |
| C | 9–11 | 2 (4,750; 3,000) | ▪□▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□□□□□▪▪▪▪□□□□▪▪▪▪▪▪▪ | 1 (500) | 2 |
The number of clustered isolates was based on IS6110-RFLP analysis followed by DR-RFLP analysis.
TABLE 2.
Molecular description of clusters observed for 57 M. tuberculosis clinical isolates from 39 patients suspected to be epidemiologically linked by spoligotyping, followed by DRE-PCRa
| Cluster | Spoligotype | No. of major DRE-PCR bands (size [bp]) | No. of IS6110-RFLP bands | No. of DR-RFLP bands (size [bp]) | No. of strainsb |
|---|---|---|---|---|---|
| A | ▪□▪□□□□□□□□□▪▪▪▪▪□▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪▪▪▪▪▪▪ | 1 (180) | 4–5 | 2 (4,800; 3,000) | 3 |
| B | ▪▪▪▪▪□□□□□□□□□□□□▪▪□□□□□□□□□□□□□□□□□□□▪▪▪▪▪ | 1 (625) | 13 | 1 (5,100) | 1 |
| D | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪▪▪□□□□ | 1 (1,400) | 8 | 2 (4,300; 3,600) | 2 |
| E | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□▪□□□□▪▪▪▪▪▪▪ | 2 (3,700; 2,600) | 9 | 3 (4,300; 3,500; 1,900) | 2 |
| F | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□▪□□□□▪▪▪▪▪▪▪ | 3 (3,700; 2,600; 1,100) | 7–8 | 3 (4,300; 3,500; 1,900) | 3 |
| H | ▪▪▪▪▪▪□▪▪▪▪▪▪▪▪▪▪□▪▪□□□□▪▪▪▪▪▪▪▪□□□□▪▪▪▪▪▪▪ | 1 (440) | 11 | 2 (4,750; 3,400) | 2 |
| J | □□□□□□□□□□□□□□□□□□□□□□□□▪□□□□□□▪□□□□▪▪▪▪▪▪▪ | 2 (2,700; 1,100) | 5–6 | 1 (4,300) | 8 |
| K | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□□□□▪▪▪▪▪▪▪ | 1 (950) | 5 | 2 (4,800; 3,600) | 2 |
| M | ▪▪▪▪▪▪▪▪▪▪▪▪▪▪□▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪□▪□□□□▪▪▪▪▪▪▪ | 2 (600; 180) | 5–6c | NDd | 3 |
| N | ▪▪▪▪▪▪▪▪▪▪▪▪□▪▪▪▪▪▪▪□□□□▪▪▪▪▪▪▪▪□□□□▪▪▪▪▪▪▪ | 3 (1,500; 700; 300) | 10 | ND | 2 |
Clustering results were later confirmed by IS6110-RFLP and DR-RFLP analyses.
The number of clustered isolates was based on spoligotyping followed by DRE-PCR and remained unaltered upon IS6110-RFLP and DR-RFLP analyses.
M was not identical to J, based on the positions of bands upon IS6110-RFLP analysis.
ND, not done.
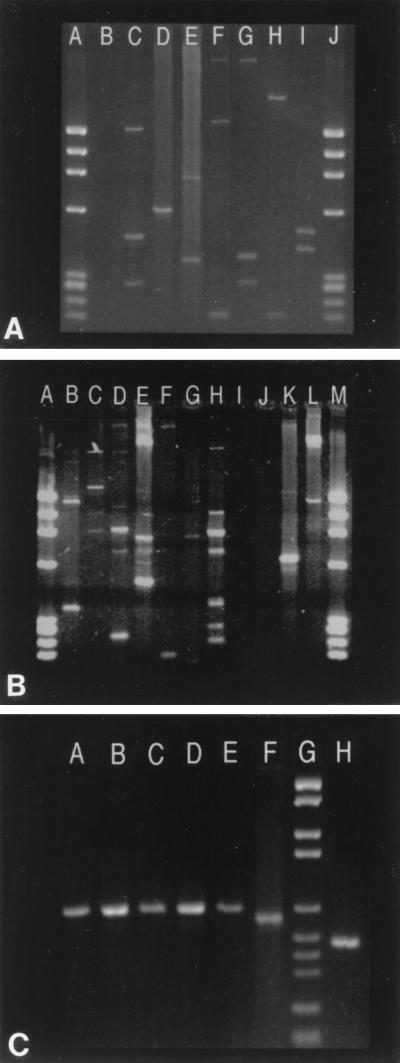
Spoligotyping alone overestimated the number of clustered isolates in our region by about 16%, compared to IS6110-RFLP analysis, which was mainly due to the presence of common spoligotypes, such as those shown for clusters E, F, and K (Table 2), present around the world. This discrepancy was overcome following DRE-PCR, with final clustering results identical to those observed with IS6110-RFLP analysis (Table 2). The diversity of DRE-PCR patterns generated for unclustered isolates is represented in Fig. 1A and B, whereas the representative profiles for some of the clustered isolates are illustrated in Fig. 1C. These results confirmed that spoligotyping plus DRE-PCR showed a discriminatory power equal to that of IS6110-RFLP.
FIG. 1.
Representative patterns obtained following DRE-PCR analysis of M. tuberculosis clinical isolates. (A and B) Results for isolates that were clustered by neither IS6110-RFLP nor spoligotyping. (A) Lanes: A, molecular weight (MW) marker; B, negative control; C to I, various unclustered isolates (GP14, GP35, GP16, GP5, GP25, GP33, and GP10); J, MW marker. (B) Lanes: A, MW marker; B to H, various unclustered isolates (GP1, PMON, GP34, GP9, A11, GP39, and GP15); I, negative control; J, an unclustered isolate, GP29, that did not generate any bands upon DRE-PCR; K, isolate B4 from cluster B; L, unclustered isolate GP37; M, MW marker. (C) DRE-PCR analysis of isolates that were clustered by IS6110-RFLP analysis followed by spoligotyping. Lanes: A to E, five isolates from four patients of cluster B; F, an unclustered isolate (GP20); G, MW marker; H, an isolate of cluster C. DNA MW markers IX (A and B) and VI (C), from Boehringer Mannheim, Meylan, France, were used.
Consequently, the present investigation validates a new strategy for M. tuberculosis typing which associates two PCR-based fingerprinting techniques for molecular epidemiology of tuberculosis with excellent discriminating ability: ultimately, all the results obtained were confirmed by the IS6110-RFLP reference methodology. By using spoligotyping in association with DRE-PCR, we studied the polymorphisms contained inside the DR locus (5) and between the IS6110 and PGRS (polymorphic GC-rich region) (9) loci. This study demonstrates that spoligotyping is useful as a first-line screening method; however, clustering should be reconfirmed by DRE-PCR to retain only epidemiologically linked isolates. Based on the available genetic information for the type strain, H37Rv (7), seven potential loci may be amplified by DRE-PCR alone, showing the discriminatory potential of this method as a second-line test. We suggest that the current combination of spoligotyping followed by IS6110-RFLP analysis (4) could be satisfactorily replaced by spoligotyping followed by DRE-PCR, which may help gain a minimum of 2 weeks if the procedure begins upon receipt of a patient’s specimen. Unlike IS6110-RFLP analysis, which requires about 5 μg of bacterial DNA in a nonradioactive hybridization format, spoligotyping and DRE-PCR require only about 10 and 100 ng of DNA, respectively. Thus, in the latter case, it is possible to proceed with the microcolonies that are visible within 2 weeks on Middlebrook agar medium instead of the 3 to 4 weeks necessary for a fully grown culture for IS6110-RFLP analysis. A further gain of about 4 to 5 days is achieved due to the simplicity of the spoligotyping plus DRE-PCR procedures over IS6110-RFLP analysis. Although most of the experience we acquired on this technique was done with CTAB-prepared DNA, it has been shown that DRE-PCR can also be performed with less-purified DNA (2). We noticed that amplification yields were lower when DNA prepared by microbeads was used, and weak amplification bands were sometimes lost.
It should be emphasized that spoligotyping plus DRE-PCR gave essentially the same typing results as those obtained by IS6110-RFLP analysis; the results were identical (the same number of clusters, the same isolates in each cluster, and the same unclustered isolates) for 87 of 91 patients studied (4 patient isolates did not give visible bands upon DRE-PCR, which involved 4 unclustered isolates in this study). The use of DRE-PCR as a first-line test cannot be recommended, as it is not sufficiently discriminatory when used alone and both the low (below 200 bp)- and high (above 3,500 bp)-molecular-size bands may be difficult to interpret and compare. Furthermore, interpretation of the results may be tedious when hundreds of isolates on separate gels are compared. In this sense, initial screening by spoligotyping limits the number of potentially linked isolates prior to DRE-PCR. In conclusion, the strategy described in this article is easily applicable to the handling of a very large number of samples and would be well suited to developing countries and/or countries with high prevalences of tuberculosis.
Acknowledgments
We are grateful to the “Délégation générale du Réseau International des Instituts Pasteur” for organizing a workshop on spoligotyping under the expert guidance of B. Gicquel and Y. Goguet de la Salmonière, Institut Pasteur, Paris.
This work was financed by a research grant provided by the Fondation Française Raoul Follereau, Paris, France.
REFERENCES
- 1.Devallois A, Goh K S, Rastogi N. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J Clin Microbiol. 1997;35:2969–2973. doi: 10.1128/jcm.35.11.2969-2973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman C R, Stoeckle M Y, Johnson W D, Jr, Riley L W. Double-repetitive-element PCR Method for subtyping Mycobacterium tuberculosis clinical isolates. J Clin Microbiol. 1995;33:1383–1384. doi: 10.1128/jcm.33.5.1383-1384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goguet Y O, Li H M, Torrea G, Bunschoten A, van Embden J D A, Gicquel B. Evaluation of spoligotyping in a study of the transmission of Mycobacterium tuberculosis. J Clin Microbiol. 1997;35:2210–2214. doi: 10.1128/jcm.35.9.2210-2214.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal M, Saunders N A, van Embden J D A, Young D B, Shaw R J. Differentiation of Mycobacterium tuberculosis isolates by spoligotyping and IS6110 restriction fragment length polymorphism. J Clin Microbiol. 1997;35:647–651. doi: 10.1128/jcm.35.3.647-651.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groenen P M A, Bunschoten A E, van Soolingen D, van Embden J D A. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis: application for strain differentiation by a novel typing method. Mol Microbiol. 1993;10:1057–1065. doi: 10.1111/j.1365-2958.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 6.Kamerbeek J, Schouls L, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philipp W J, Poulet S, Eiglmeier K, Pascopella L, Balasubramanian V, Heym B, Bergh S, Bloom B R, Jacobs W, Cole S T. An integrated map of the genome of the tubercle bacillus, Mycobacterium tuberculosis H37Rv, and comparison with Mycobacterium leprae. Proc Natl Acad Sci USA. 1996;93:3132–3137. doi: 10.1073/pnas.93.7.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plykaitis B P, Crawford J T, Woodley C L, Butler W R, Eisenach K D, Cave M D, Shinnick T M. Rapid, amplification-based fingerprinting of Mycobacterium tuberculosis. J Gen Microbiol. 1993;139:1537–1542. doi: 10.1099/00221287-139-7-1537. [DOI] [PubMed] [Google Scholar]
- 9.Ross B C, Raios K, Jackson K, Dwyer B. Molecular cloning of a highly repeated DNA element from Mycobacterium tuberculosis and its use as an epidemiological tool. J Clin Microbiol. 1992;30:942–946. doi: 10.1128/jcm.30.4.942-946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Small P M, van Embden J D A. Molecular epidemiology of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: American Society for Microbiology; 1994. pp. 569–582. [Google Scholar]
- 11.Sola C, Horgen L, Goh K S, Rastogi N. Molecular fingerprinting of Mycobacterium tuberculosis on a Caribbean island with IS6110 and DRr probes. J Clin Microbiol. 1997;35:843–846. doi: 10.1128/jcm.35.4.843-846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thierry D, Brisson-Noël A, Vincent-Levy-Frébault V, Nguyen S, Guesdon J L, Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990;28:2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuen L K W, Ross B C, Jackson K M, Dwyer B. Characterization of Mycobacterium tuberculosis strains from Vietnamiese patients by Southern blot hybridization. J Clin Microbiol. 1993;31:1615–1618. doi: 10.1128/jcm.31.6.1615-1618.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]