Abstract
Recovery of function following a complete spinal cord injury (SCI) or an incomplete SCI where recovery has plateaued still eludes us despite extensive research. Epidural spinal cord stimulation (SCS) was initially used for managing neuropathic pain. It has subsequently demonstrated improvement in motor function in otherwise non-recovering chronic spinal cord injury in animal and human trials. The mechanisms of how it is precisely effective in doing so will need further research, which would help refine the technology for broader application. Transcutaneous spinal cord stimulation (TSCS) is also emerging as a modality to improve the functional outcome in SCI individuals, especially when coupled with appropriate rehabilitation. Apart from motor recovery, ESCS and TSCS have also shown improvement in autonomic, metabolic, genitourinary, and pulmonary function. Since the literature on this is still in its infancy, with no large-scale randomised trials and different studies using different protocols in a wide range of patients, a review of the present literature is imperative to better understand the latest developments in this field. This article examines the existing literature on the use of SCS for SCI individuals with the purpose of enabling functional recovery. It also examines the voids in the present research, thus providing future directions.
Keywords: Spinal cord injury, Spinal cord stimulation, Epidural stimulation, Transcutaneous stimulation
1. Introduction
In spite of tremendous progress in modern medicine, spinal cord injury (SCI) can wreck the lives of its victims and their families. It is a catastrophic event that culminates in a deficiency of sensorimotor and/or autonomic functions. Broad classification into complete and incomplete injuries helps in prognosticating the outcome. In addition to the loss of several essential functions, morbidity in patients with SCI can increase due to infections of the lower respiratory or urinary tract, pressure injuries, and deep venous thrombosis. Research so far has helped in improving functional outcomes, limiting complications, and improving quality of life but has not been able to help with motor recovery significantly.1 Pharmacological agents and stem cell transplantation have shown promising outcomes with regard to neurological recovery in animal models though these interventions were not able to achieve positive results in human studies.2,3
In most SCI, there is the presence of intact tissue at the level of injury, which is functionally silent.4 At the level of SCI lesion, the afferent signals to upper motor neurons are lost. The efferent signals travelling rostrocaudally terminate at the level of SCI lesion. Remnant proprioceptive connections may act as a subsidiary channel for descending signals.5 Contemporary advances have demonstrated the recovery of coordinated purposeful movements with the aid of extant neural linkages.6 Of late, there has been increased interest in newer modalities of research, such as Spinal cord Stimulation (SCS). It is majorly divided into Epidural spinal cord stimulation (ESCS) and transcutaneous spinal cord stimulation (TSCS).7 SCS has been used for the management of pain (failed back syndrome, radicular pain), stable angina, peripheral arterial disease, complex regional pain syndrome, and neuropathic pain.7 Its use is being explored in Parkinson's disease, spasticity, and SCI rehabilitation.7, 8, 9 SCS has been found to produce rhythmic motor movements in animal models and has gained interest for translation into human trials.10 In this narrative review, we discuss the various human studies conducted on ESCS and TSCS, the present status and the future prospects.
2. Methods
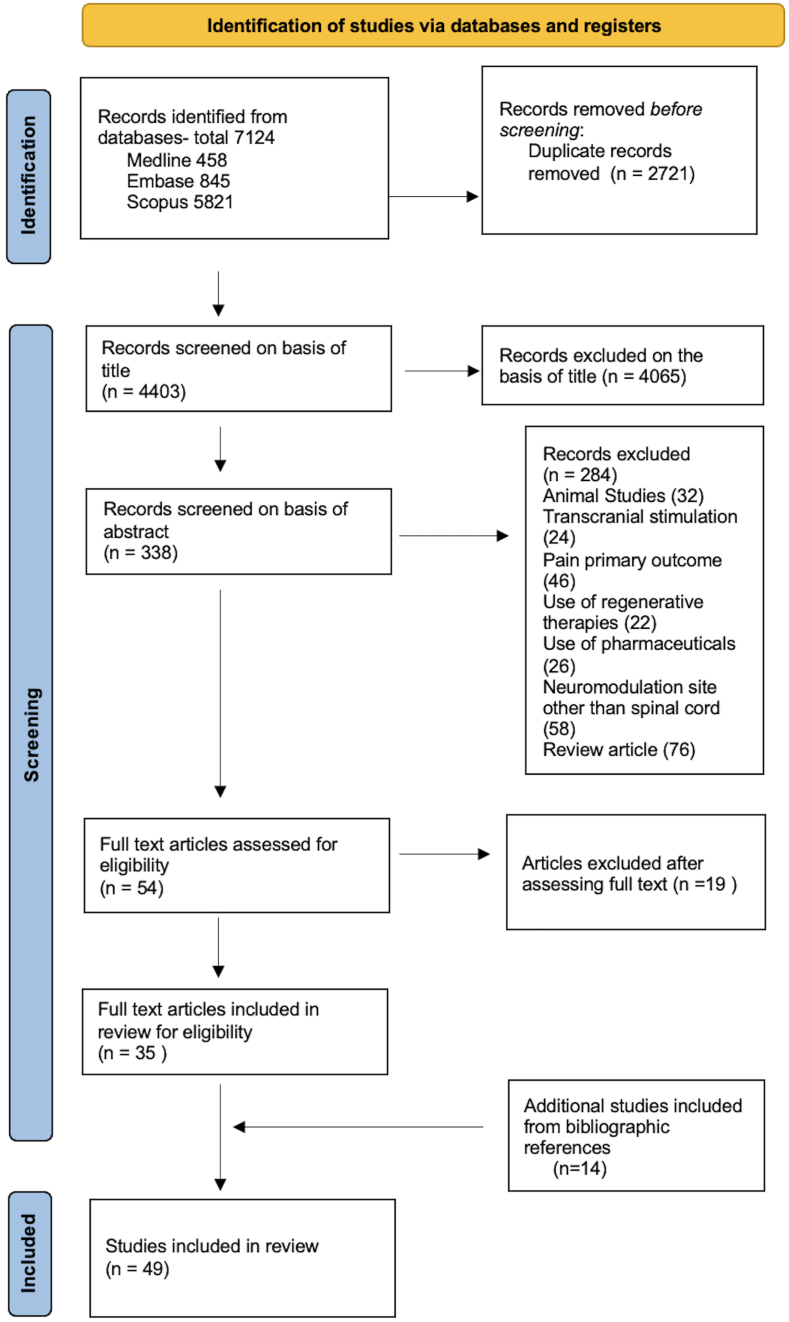
We used the search terms (Spinal Cord Injury OR Spinal Injury OR Tetrapleg∗ OR Parapleg∗) AND (Spinal cord stimulat∗ OR Epidural Stimulat∗ OR Transcutaneous Stimulat∗ OR Neuromodulat∗) and conducted a literature search in Medline, Embase and Scopus. Relevant articles in the English language searched for Title/Abstract with available full text (free full text or access available to the authors) were included. Only clinical studies focusing on neurological, autonomic, and functional improvement were included in the review. Studies were included irrespective of the stage of care following SCI. Studies simultaneously involving other interventions or therapies, such as pharmaceuticals, regenerative therapies, and brain stimulation were excluded. Articles discussing sacral neuromodulation and tibial nerve stimulation were excluded. A total of 7124 research studies were identified in the above databases, including 458 Medline, 845 Embase, and 5821 Scopus results. After rejecting duplicates and screening of titles and abstracts, 54 studies were chosen for a detailed reading of the text. After full-text reading, 19 articles were excluded and 14 additional studies were identified through the review of bibliographic references. After screening the articles, a total of 49 articles were included for data extraction and assessment (Fig. 1).
Fig. 1.
Prisma Flowchart
3. Epidural spinal cord Stimulation (ESCS)
ESCS helps deliver stimulation to the spinal cord following a surgical procedure. For ESCS, an array of electrodes are implanted over the dura mater, within a few millimetre distance of the dorsal aspect of the spinal cord. The stimulating interfaces can be placed either percutaneously or by open surgery in which the dura is accessed following the removal of the lamina. Whereas in the former technique, electrode placement is in a single line, in the latter technique, electrodes can be organised in an array of rows and columns on a paddle. The electrodes are linked through lead wires tunnelled under the skin to an often rechargeable programmed rhythm generator that can be surgically placed subcutaneously and its activation can be modulated using a programmer over the skin surface.11 ESCS for the purpose of motor recovery in SCI usually involves implantation of an array of 12–16 electrodes over the dura mater around L1 to S1 spinal cord level (T10 to L1 vertebral level).
3.1. Evolution of ESCS
Gate control theory, developed in 1959, helped create a new understanding of pain perception mechanisms. It postulates that stimulus to large-diameter fibres (touch, pressure, vibration) suppresses pain signals in small-diameter nerve fibres.12 This was the scientific basis for the development of the first SCS device in 1968 by Shealy and Mortimer.2,7 The potential of SCS to improve function in motor impairments was recognised rather unexpectedly. A multiple sclerosis patient with paraparesis, when managed for intractable pain with SCS, surprisingly developed significant improvement in volitional motor power in her legs.13 The first article on the use of ESCS in SCI individuals was published in 1985.14 It was discovered by chance quite later that ESCS could help restore voluntary movement in patients with complete SCI.15,16 Dimitrijevic et al. reported the optimal location of electrodes for reducing spasticity.16 They found dorsal placement caudal to the site of the lesion as most suitable. They reported rhythmic muscle activity in paralysed legs with tonic ESCS in the supine position in 6 persons with chronic complete SCI. A few of these even exhibited coordinated flexion and extension movements which seemed similar to stepping. These vital observations were one of the earliest hints at the presence of a central pattern generator in human beings for gait.17 All studies for improvement of function in lower limbs place epidural electrodes at similar levels. Despite these significant effects, the appeal of SCS in motor recovery in SCI waned in the 1990s. Inconsistency mainly was due to a lack of benchmarks that could predict SCS outcomes in patients with differing neurology and lesions along with contention on site of electrode implantations.11,18 Studies pertaining to motor recovery with ESCS are listed in Table 1.
Table 1.
Role of ESCS in motor recovery in persons with SCI.
| Role of ESCS in lower limb motor recovery and ambulation in persons with SCI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. No. | Study | No. of Subjects | AIS; Neurological level | Place | Primary Outcome | Device | Stimulation site | Stimulation parameters | Intervention and Follow Up | Concurrent Therapy |
| 1 | Barolat 1986 19 | 1 | C; C5 |
|
|
|
|
|
|
None |
| 2 | Dimitrijevic et al., 1998 17 | 6 | A; C5-T8 |
|
|
|
|
|
|
None |
| 3 | Herman et al., 2002 20 | 1 | C; C6 |
|
|
|
|
|
|
Weight supported treadmill walking |
| 4 | Carhart et al., 2004 21 | 1 | C; C5–C6 |
|
|
|
|
|
|
Weight supported treadmill and over ground walking |
| 5 | Minassian et al., 2004 22 | 10 | 8A,2B; C4-T10 |
|
|
|
|
|
|
None |
| 6 | Ganley et al., 2005 23 | 2 | C; C6-T8 |
|
|
|
|
|
|
Weight supported treadmill walking |
| 7 | Huang et al., 2006 24 | 2 | C; C5-T8 |
|
|
|
|
|
|
Weight supported treadmill walking |
| 8 | Harkema et al., 2011 25 | 1 | B; C7 |
|
|
|
|
|
|
Weight supported treadmill walking and parallel bar functional training |
| 9 | Angeli et al., 2014 26 | 4 | 2A, 2B; C5-T4 |
|
|
|
|
|
|
None |
| 10 | Sayenko et al., 2014 27 | 3 | 1A, 2B; C7-T4 |
|
|
|
|
|
|
None |
| 11 | Rejc et al., 2015 28 | 4 | 2A, 2B; C7-T4 |
|
|
|
|
|
|
Parallel bar full weight bearing standing training |
| 12 | Grahn et al., 2017 29 | 1 | A; T6 |
|
|
|
|
|
Weight supported treadmill training | |
| 13 | Rejc et al., 2017 30 | 1 | C7; B |
|
|
|
|
|
|
Activity based training in lab and home |
| 14 | Rejc et al., 2017 31 | 4 | 2A, 2B; C7-T4 |
|
|
|
|
|
|
Full weight bearing standing training |
| 15 | Angeli et al., 2018 32 | 4 | 2A, 2B; C5-T4 |
|
|
|
|
|
|
Over ground walking training |
| 16 | Gill et al., 2020 33 | 2 | A; T3-T6 |
|
|
|
|
|
|
Weight supported treadmill training |
| 17 | Peña Pino et al., 2020 34 | 7 | 6A,1 B; T4-T8 |
|
|
|
|
|
|
None |
|
||||||||||
| 1 | Lu et al., 2016 35 | 2 | B; C5–C6 |
|
|
|
|
|
|
Hand and arm activity |
Abbreviations: AIS American Spinal Injury Association Impairment Scale; C, Cervical vertebrae; L, Lumbar vertebrae; T, Thoracic vertebrae; S, Sacral vertebrae.
3.2. Impact of ESCS on lower limb function
The impact of ESCS on the lower limb has been more widely studied as compared to its effect on upper limb function. Herman et al. initially established that epidural stimulators that were being used for pain management could as well be used in individuals with SCI to augment motor recovery and ambulation.19 In a subject with 3.5 years old C5-6 SCI with AIS –C neurology, they reported with ESCS more effective stepping, less metabolic stress, and the ability to ambulate but with considerable difficulty.19,20 Harkema et al. (2011) found that a participant with SCI(AIS-B) could achieve full weight-bearing and supraspinal control of a few leg movements with a combination of training and ESCS.21 They also noted improvement in the bladder along with sexual wellness and temperature regulation after epidural stimulation.21 Grahn et al. (2017) reported that their participant with SCI (AIS-A) was able to stand, do intentional step-like movements, and later do overground stepping when ESCS was added with task-specific training (multi-modal rehabilitation).22
Rejc et al. (2017) reported significant improvement in standing ability in four participants (2 AIS-A, 2 AIS-B). The participants had gone through prolonged training in locomotion before the implantation of the epidural stimulator. They hypothesised that standing and stepping were varied functions and would need different programs.23 Two studies in 2018 were the first to demonstrate independently that AIS A subjects with SCI were able to walk overground with tonic SCS.24,25 Angeli et al. noted that two out of four AIS B subjects with SCI developed the ability to walk overground after rigorous locomotor training with ESCS and use of assistive devices. The other two participants with complete SCI were able to accomplish stepping partially with body weight support on the treadmill but not overground walking. Voluntary limb movement upon command was observed in the participants only in the presence of ESCS.25
SCS has evolved over time. Lead positioning, which was earlier subdural, was shifted to epidural space. Progress in engineering has ushered in the usage of battery-dependent implantable devices.26 Since the initial applications of SCS were derived from its usage in pain management, the use of tonic SCS was virtually universal until 2018 for motor recovery in individuals with SCI. Formento et al. hypothesised that perpetual stimulation by ESCS would lead to the collision of signals from stimulus to afferent orthodromic impulses, thereby negating each other and rendering tonic SCS ineffective, limiting the utility of applicable stimulation intensities and frequencies of tonic SCS strategies.10 The firing rates of action potentials in proprioceptive fibres are lower in human beings as compared to rats. In contrast, the transit time is higher. They demonstrated the obliteration of proprioceptive information in humans following antidromic collisions, but similar abolition was not observed in rats.
With this background, Courtine and colleagues developed a new approach of spatiotemporal ESCS in pre-clinical studies.27, 28, 29 They conceptualised timely recruitment of proprioceptive circuits in the spinal cord to reproduce natural, task-specific activation of motor neurons.30 They designed “targeted SCS” or “biomimetic stimulation” to deliver stimulation in a phase-based manner at targeted areas in contrast to continuous nonpatterned stimulation.31 Use of spatiotemporal ESCS was initially established in rats.29 The first reported use in human participants was in three cervical incomplete SCI individuals (two AIS-C and one AIS-D) who were wheelchair users for four years and more.31 A spatiotemporal map of the activity of the motor neuron pool of ambulatory individuals with intact neurology was obtained through Electromyography (EMG) recordings. This included three key areas successively controlling weight bearing, swing, and propulsion. Analogous to pre-clinical studies, ESCS protocols for the SCI participants were exclusively designed to independently aim at dorsal roots innervating respective hot spots.29 For achievement of desired activity in the open-loop mode, subjects need to coordinate their volitional intent with the predetermined sequence of stimuli. Closed-loop mode used a controller analysing foot trajectory wirelessly in real-time to trigger spatiotemporal stimulation. While being rehabilitated, the three subjects were able to walk better over the ground with ESCS and even without it. Results from the STIMO (Stimulation Movement Overground) clinical trial involving spatiotemporal ESCS with rehabilitation aided by Robot in SCI patients (6 incomplete and 3 complete) revealed the improved ability to walk while supported by a robotic interface.32 The positive outcomes of the aforementioned research have brought us to the brink of a breakthrough in the rehabilitation of SCI individuals.
3.3. Impact of ESCS on upper extremity motor recovery
The use of ESCS for upper extremity motor recovery is considered even more challenging as compared to the lower limb.33 Waltz et al., in 1987 reported the use of stimulation of the spinal cord in the cervical region in SCI in 112 patients and reported progress in motor function in 65% of cases though they have not detailed the severity and chronicity of injury.34
Lu et al. reported improvement in motor score and improvement in grip strength one week after ESCS in two subjects with cervical SCI (AIS-B). Authors demonstrated improved grip strength even when tested without SCS following repeated stimulation sessions. Both the participants of the study suffered from intractable pain in the shoulder and upper limbs. Results reported for the first participant were from the permanent implant phase, and for the second participant were from a temporary implant phase. Authors theorised that volitional movements were enabled by neuromodulation of the cervical pre-motor group of neurons. EMG findings were also suggestive of the transmission of motor impulses via proprioceptive fibres.35 Currently, several ongoing pieces of research are targeting upper limb functional improvement, which should be able to guide rehabilitation protocols and lead placement.
3.4. Mechanisms of ESCS for motor recovery
The mechanisms triggering the facilitating effect of ESCS on voluntary movements are not yet precise. The following mechanisms have been proposed.
3.4.1. Recruitment and stimulation of afferent fibres of large-diameter
Computational modelling and electromyography-based studies in SCI subjects support that the SCS triggers larger proprioception fibres. Stimulation generates an afferent signal to the thecal sac that transsynaptically recruits neurocircuits crucial in the management of information from proprioceptive fibres and of motor neuronal activation, mono- and polysynaptic spinal reflex, along with spinal neural networks controlling various components of lower extremity synergistic movements.11 Lumbosacral nerve roots that are dorsally located have membrane properties favourable for electrical depolarisation. Myelinated axons with large diameters have higher excitability.36 Differential stimulation owing to axon diameter/fibre size as an isolated hypothesis is insufficient to explain immensely complex motor behavioural consequences of spinal cord stimulation.2
3.4.2. Central pattern generators (CPGs)
CPGs are specialised circuitry in the spinal cord that elicit synchronised rhythmic movement of various muscles in the absence of sensory feedback or activation from descending neurons to produce characteristic motor activities in invertebrates and vertebrates such as breathing, chewing, deglutition, crawling, flying, swimming, locomotion and micturition.37(38) In rats, systematic stimulation of CPGs has been shown to result in adaptive plasticity, promoting spinal cord learning.39 Neural plastic remodelling in the spinal cord has been extensively researched. ESCS may enable the induction of neuroplasticity in the local region involving afferent fibres, internuncial neurons, and motor neurons.40 Large-diameter afferent fibres exhibit extensive branching and can thereby modulate multiple locations, including motor agonists and antagonists. They provide inputs to CPGs and may likely influence locomotion. They may also transmit sensory information to supraspinal centres.41 Most importantly CPGs are instrumental in the generation of movements that may be enabled even without supraspinal signals.20 Numerous studies are underway to identify CPG neurons consisting of electrophysiological, anatomical, biochemical, genetic, and pharmacological studies.2,38,42 Research has revealed that CPGs are housed in the anterior aspect of the spinal cord.43,44 Several animal studies aimed at identifying the CPG interneurons based on the expression of distinctive transcription factors have found specific domains classified into 11 main interneuronal classes (dI1–dI6, V0–V3) in the spinal cord. These neuronal populations exhibit rhythmic activity during locomotion.45 Reflex pathways formed by interneuronal networks in the spine may be associated with signal transduction. Conspicuous examples have been characterised as intrasegmental, intersegmental, and commissural levels.46,47 Conventional reflexes consist of Ia- (muscle spindle, myotactic reflex), Ib- (inverse myotatic reflex, Golgi tendon organ), flexor-reflex-afferent (FRA, nocifensive reflex, withdrawal reflex), II-reflex (myotactic polysynaptic).38 Various muscle groups exhibit differential activation patterns, i.e. monosynaptic pathways activate extensors, whereas indirect polysynaptic reflex pathways activate flexors.54 These different pathways are probably activated in a phase-based manner.48,49 Certain attributes of processes involved in weight acceptance, like standing and ambulation, which help to maintain stability in an erect position, are incorporated directly within the motor neuronal networks of the spinal cord.50 As load bearing increases, extensor tone increases while the tone of flexor muscles diminishes. Coordination of phases of the gait cycle is enabled by real-time integration of the signals from proprioceptive inputs, such as baseline angles of joints and axial loading with spatiotemporal dynamic activation programs.2,31
3.4.3. Role of supraspinal pathways
3.4.3.1. Increasing excitability
Evidence supports the involvement of supraspinal centres in influencing function following electrical stimulation. One of the mechanisms of the traumatic spinal lesion is the disruption of connections of efferent tracts results in the loss of signals triggering movement to downstream motor neurons leading to physiological obtundation into a suppressed state that prohibits functional and volitional motor production.21 Even in several complete SCI patients, some supraspinal connections are preserved.51 These connections are considered to be inactive functionally.25 Angeli et al. have observed the influence of audiovisual feedback modulating motor movements and subsequently demonstrated that overground walking with EES was under wilful command.25,52 Asboth et al., in 2018, in a mouse model of SCI, combined motor cortex stimulation with electrochemical spinal cord stimulation, which restored locomotion and on abandoning motor cortex stimulation, locomotion was disabled.53 ESCS may increase the net excitability of the spinal cord, thus allowing the attainment of a threshold, enabling functional outcomes, and highlighting the importance of supraspinal pathways. This theory does not explain the persistent long-term functional improvement in SCI patients, even in the absence of spinal cord stimulation, as has been demonstrated in rats as well as human studies.23,53
3.4.3.2. Role of the reticulospinal tract
Kathe et al. observed a decrease in metabolic activity in lumbar segments following ESCS and rehabilitation.32 They identified a group of excitatory lumbar interneurons SCVsx2:Hoxa10 that were especially responsive to ESCS post-SCI and transcriptional activity increased two-fold following ESCS and rehabilitation. These neurons receive input from large-diameter afferents and also connect to the ventral part of the spinal cord. These neurons also receive input from descending reticulospinal tracts which may facilitate supraspinal influence. Asboth et al. emphasised the role of reticulospinal tracts in recovery following spinal cord contusion injury in rats.53 Despite the abolition of corticospinal tracts, the voluntary movement was possible following electrochemical stimulation. Along with this, there was the preservation of supraspinal connections through reticulospinal tracts to ventral gigantocellular reticular nuclei. Drug-mediated inhibition of these nuclei resulted in the abolishment of neuromodulation. These findings highlight the role of the reticulospinal tract and SCVsx2:Hoxa10 neurons in long-term functional recovery following neuromodulation. Propriospinal interneurons, which are limited to the spinal cord, have close connections with the reticulospinal tract and can help in relaying descending and ascending signals.54 Courtine et al., in a study of mice with SCI, demonstrated that propriospinal interneurons facilitated supraspinal control bypassing staggered hemisection at T7 and T12.5
3.4.4. Axonal regeneration and upregulation of neurotrophic factors
There is limited evidence to favour that electrical spinal cord stimulation may enhance axonal regeneration across the injury site and further research is required.55,56 Several studies have demonstrated that following electrical stimulation, there is upregulation of brain-derived neurotrophic factor (BDNF), which is instrumental in promoting neuroplasticity.57, 58, 59 Electrical stimulation may also influence glial cells and modulate inflammation and myelination.60
3.5. Issues with ESCS
The electrode array gets engulfed by scar tissue in the epidural space and thus becomes more or less fixed. However, the thecal sac and vertebral column are mobile. Open-loop controllers are not able to execute real-time adjustments to make up for inconsistency in motor recruitment. The absence of integration of reaction from the propriospinal system to the neuroprosthetic controller creates a considerable challenge in the maintenance of balance and stable posture, thus resulting in unsteady and turbulent motion.2 Closed-loop controllers have the capability to perform integration with feedback signals to implement concurrent stimulus alterations aligned toward the intentional motor output.31 Volitional control may be enhanced by controlled loop feedback enabled by either physiological activity from the dorsal root ganglion or peripherally implanted sensors.2,61, 62, 63
Intraoperative imaging with fluoroscopy can guide device implantation by providing gross information about the location. It can be complemented by a neurophysiological assessment during surgery using evoked segmental myotomal monosynaptic EMG-response patterns to direct optimal placement of the array, both in proximal to distal and mediolateral alignment along the neuroaxis.38 It is yet to be confirmed whether reliable activation of optimal stimulation sites in an awake state is feasible or not. Following implantation, programming of the device has been found to be challenging with multiple trial-and-error evaluations to determine the optimal configuration for stimulation, stimulation parameters, orientations of anode and cathode orientations, exact permutations of pulse frequencies, durations and current. The most efficient configurations of electrodes and settings for stimulators can be different, are individual-specific, and may show task-based variability with time.21,22,24,25,65, 66, 67 A few studies report greater efficiency with phasic stimulation programs in comparison with programs utilising tonic stimulation for motor recovery as they allow synergistic incorporation of necessary proprioception afferent inputs.10,24,31,66
4. Transcutaneous spinal cord Stimulation (TSCS)
After its first publication in 2009, TSCS is now being explored more and more in patients with SCI.68 TSCS is minimally invasive and less costly, being devoid of surgical intervention. Being the most subject-friendly of the current types of SCS, it is under investigation for its impact on rehabilitation and neurological recovery in SCI patients. TSCS involves the placement of electrodes on of skin-surface, as compared to the dura in ESCS, in specific arrangements to generate a flow of current that somewhat crosses the dura stimulating the spinal cord and roots.69,70 Such currents are permitted by the spine's transversal conductivity but are reduced substantially by the intervertebral discs and ligaments. Manifestation of tingling sensations and activity in muscles in the lower extremities reflects stimulation of deeply placed afferent neural structures in the thecal sac.49,70,71 By use of distinct waveforms, TSCS allows stimulation with high electric current to access the spinal cord without causing discomfort.72 Once the skin is stimulated with any of the techniques, it may cause increased neural activity.73 The primary afferent fibres make up most of the dorsal roots in the spinal cord. These proprioceptive sensory fibres have the minimum activation threshold owing to their larger diameter and are recruited preferentially during stimulation.74 Studies relevant to motor recovery with TSCS are listed in Table 2.
Table 2.
Use of TSCS in motor recovery in persons with SCI.
| Transcutaneous Spinal Cord Stimulation- Motor recovery and ambulation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. No. | Study | No. of subjects | AIS; Neurological level | Place | Primary Outcome | Device | Stimulation site/Electrode Placement | Stimulation Parameters | Intervention & Follow Up | Concurrent Therapy |
| 1 | Gerasimenko et al., 2015 65 | 5 | NA |
|
|
|
|
|
|
|
| 2 | Hofstoetter et al., 2015 68 | 3 | D; C5-T9 |
|
|
|
|
|
|
|
| 3 | Minassian et al., 2016 69 | 4 | A; C8-T8 |
|
|
|
|
|
|
|
| 4 | Powell et al., 2016 70 | 6 | 4C, 2D; C6-L1 |
|
|
|
|
|
|
|
| 5 | Gad et al., 2017 71 | 1 | A; T9 |
|
|
|
|
|
|
|
| 6 | Powell et al., 2018 72 | 6 | 4C, 2D; C6-L1 |
|
|
|
|
|
|
|
| 7 | Rath et al., 2018 73 | 8 | 6A, 2C; C4-T9 |
|
|
|
|
|
|
|
| 8 | Sayenko et al., 2019 74 | 15 | 11A, 1B, 3C; C4-T12 |
|
|
|
|
|
|
|
| 9 | Meyer et al., 2020 75 | 10 | D; C3-T10 |
|
|
|
|
|
|
|
| 10 | Shapkova et al., 2020 76 | 19 | 12A, 4B, 3C; C5-T12 |
|
|
|
|
|
|
|
| 11 | Wiesener et al., 2020 77 | 2 | A; T5-T6 |
|
|
|
|
|
|
|
| 12 | McHugh et al., 2020 78 | 10 | 4C, 6D; C4-T9 |
|
|
|
|
|
|
|
|
||||||||||
| 1 | Gad et al., 2018 79 | 6 | 2B, 4C; C4–C8 |
|
|
|
|
|
|
|
| 2 | Inanici et al., 2018 66 | 1 | D; C3-4 |
|
|
|
|
|
|
|
| 3 | Inanici et al., 2021 80 | 6 | 2B, 2C, 2D; C3–C5 |
|
|
|
|
|
|
|
| 4 | Zhang et al., 2020 81 | 1 | A; C5 |
|
|
|
|
|
|
|
Abbreviations: AIS, American Spinal Injury Association Impairment Scale; C, Cervical vertebrae L, Lumbar vertebrae; T, Thoracic vertebrae; S, Sacral vertebrae.
4.1. Effect on lower extremity function
In most studies, stimulation was given via electrodes over T11-T12 or L1-L2 intervertebral space with anodes over the bilateral iliac crests. Rath et al., in 2018 reported the efficaciousness of TSCS in regaining postural control during sitting following long-standing SCI in 8 (6 AIS-A and 2 AIS-C; injury level C4-T9) participants. EMG revealed increased erector spinae, rectus abdominis, and external oblique activity, thereby helping with stability in the seated position.75 Sayenko et al., in 2019 reported the use of TSCS in 15 participants (11 AIS-A and 4 incomplete SCI). With stimulation, participants could maintain an upright position without external support (7 of 15 participants) and some with minimal external assistance. No participant could stand with sham stimulation or without stimulation.76 During TSCS, there was a reduced threshold for motor activation, increased muscle activity in the lower limb observed via surface EMG and increased weight tolerance. Meyer et al., in 2020, demonstrated mildly improved ankle mobility with TSCS in 10 participants with AIS-D neurology.77 Shapkova et al., in 2020 reported results of exoskeleton-based training with (19 individuals) and without (16 individuals).78 TSCS was applied at T12 vertebral level. The stimulation magnitude (0.5 ms monophasic square-wave pulses) was around 1.3–1.4 times of threshold of leg muscles as determined by EMG. Participants reported improved coordination, voluntary control, and weight acceptance during stepping. The authors also reported the impact of higher frequencies on improved walking ability with the aid of exoskeleton and spasticity.
Various studies have assessed treadmill-based and over-ground walking with TSCS and reported statistically significant improvements in walking speed, quality and endurance.79, 80, 81 McHugh et al. assessed TSCS with walking-based therapy in a clinical setting in a prospective case series of 10 participants with chronic incomplete SCI.67 A commercial stimulator was used for stimulation with a biphasic waveform with 1 ms pulses at 50 Hz. The authors concluded that the combined training approach improved walking ability and reported it to be clinically feasible. Estes et al. published results comparing locomotor training combined with TSCS or sham. In participants receiving experimental stimulation, walking speed, as well as the distance, improved.81
4.2. Effect on upper limb function
Gad et al., in 2018 assessed EMG recordings and hand grip in 8 participants (incomplete SCI) following TSCS.82 They observed greater grip strength, improved trunk control and hand dexterity. Activation of large interneuronal networks projecting to more distal motor pools was observed in EMG. Inanici et al., in 2018 reported dramatic improvement in upper extremity function in a participant having central cord syndrome (AIS-D) following cervical TSCS and physiotherapy. Upper extremity strength improved to 75% stronger than baseline at 3 months.73 Zhang et al. reported improvement in Graded Redefined Assessment of Strength, Sensibility, and Prehension (GRASSP) score and hand grip in a participant with chronic complete SCI following 18 TSCS sessions combined with task-specific hand training for 8 weeks.83
Inanici et al., in 2021 studied 6 participants with chronic incomplete SCI. TSCS was done by placing one of the electrodes over the cervical disc space level adjacent to the injury level. Anodes are placed over the iliac crest. Primarily upper extremity functions were assessed involving grip along with lateral pinch force, spasticity and neurological recovery. The speed and magnitude of changes were evaluated with and without stimulation. Dexterity and grip strength improved with TSCS. Some cases had benefits on spasticity in the upper extremity.84
Huang et. concluded that TSCS combined with task-specific training improves manual dexterity and hand strength in SCI subjects who have a minimum grip strength of more than 0.1 N. As per the results, they hypothesised that some residual hand motor power is essential for improvement after TSCS and rehabilitation.85
The main focus of neurophysiological investigations has been on evoked motor potential production and assessment of the characteristics of these reactions as well as elements affecting response modulation. Stimulation is commonly given with paired or singular pulses at low frequencies to assess an evoked response or attain a motor threshold.86, 87, 88 In contrast, therapeutic investigations aim to improve motor responses in SCI individuals by neuromodulation using TSCS. Consequently, TSCS is usually given for extended periods in adjunct to physical therapy. Due to this difference in the targeted outcome, stimulation parameters may vary and may rationalise the choice of multiple stimulation sites by several therapeutic studies.89 Although in clinical scenarios, very limited studies demonstrate motor improvement with TSCS, the evidence does favour its practicality and utility, along with it being more consumer-friendly and cost-effective with low risk in comparison to available interventions. Effective TSCS applications generally use moderate frequencies of range 30–50 Hz and long duration pulse of range 0.5 μs–1 ms.80,81
5. Utility of spinal cord stimulation (ESCS and TSCS) in non-motor issues faced by SCI patients (relevant studies in Table 3)
Table 3.
Utility of SCS (Epidural and Transcutaneous) in non-motor functions and spasticity in persons with SCI.
| S. No. | Study | No. of Subjects | AIS; Neurological level | Place | Primary Outcome | Device | Stimulation Site | Stimulation parameters | Intervention, Follow Up and outcome | Concurrent Therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| ESCS-Genitourinary function | ||||||||||
| 1 | Katz et al., 1991 89 | 23 |
|
|
|
|
|
|
|
None |
| 2 | Herrity AN. et al., 2021 90 | 10 |
|
|
|
|
|
|
|
Active recovery-based training |
|
||||||||||
| 1 | DiMarco et al., 2020 91 | 10 |
|
|
|
|
|
|
|
None |
| 2 | DiMarco et al., 2006 92 | 1 |
|
|
|
|
|
|
|
None |
| 3 | DiMarco et al., 2009 93 | 9 |
|
|
|
|
|
|
|
None |
|
||||||||||
| 1 | Gad et a., 2020 94 | 1 |
|
|
|
|
|
|
|
None |
|
||||||||||
| 1 | Aslan SC. et al., 2018 95 | 7 |
|
|
|
|
|
|
|
None |
| 2 | West et al., 2018 96 | 1 |
|
|
|
|
|
|
|
None |
| 3 | Darrow et al., 2019 97 | 2 |
|
|
|
|
|
|
None | |
| 4 | Nightingale et al., 2019 98 | 1 |
|
|
|
|
|
|
|
None |
|
||||||||||
| 1 | Phillips et al., 2018 99 | 5 |
|
|
|
|
|
|
|
None |
| 2 | Knikou et al., 2019 100 | 10 |
|
|
|
|
|
|
|
None |
| 3 | Sachdeva et al., 2021 101 | 1 |
|
|
|
|
|
|
|
None |
|
||||||||||
| 1 | Hofstoetter et al., 2014 64 | 3 |
|
|
|
|
|
|
|
None |
| 2 | Estes et al., 2017 102 | 18 |
|
|
|
|
|
|
|
None |
Abbreviations: AIS American Spinal Injury Association Impairment Scale; C, Cervical vertebrae; L, Lumbar vertebrae; T, Thoracic vertebrae; S, Sacral vertebrae.
5.1. Genitourinary function
Current strategies to manage bladder function in a patient with SCI accompany several side effects. Sacral neuromodulation and percutaneous tibial nerve stimulation (PTNS) relieve symptoms related to bladder overactivity.90 Though PTNS is non-invasive as compared to sacral neuromodulation, the location of stimulation is distant from the spinal cord and needs multiple sittings and thus having issues with sustainability.91
Harkema et. have shown in previous studies that continence, sexual and bowel function in human subjects improve with ESCS.21 Herrity et al. concluded that ESCS focusing on the parasympathetic outflow to the bladder was adequate to enable efficient micturition. They hypothesised that ESCS influences detrusor contraction strength and external urethral sphincter relaxation.92 Various trials have reported improvement in bowel and bladder function in SCI patients with ESCS at T11 to L1 and L1 to S2 and TSCS around T11 to L3-L4.93,94 Darrow et al. reported improvement in bowel and bladder synergy after ESCS in two SCI participants with complete injury. One participant achieved orgasm for the first time since trauma after ESCS. One participant was able to regain volitional urination following ESCS.93 SCS is currently hypothesised to improve genitourinary function by enabling volitional sphincter control, which is facilitated by access of supraspinal micturition centres to the micturition circuitry in the sacral region. SCS may also help increase storage and voiding reflexes.95
Gad et al., in 2018 concluded that TSCS could help normalise the urethral and bladder function in individuals with SCI. They assessed 7 SCI participants and observed increased bladder capacity and enabled voiding, reduced detrusor overactivity and decreased detrusor-sphincter dyssynergia.96 Kreydin et al. reported in 5 SCI patients (3 AIS-A and 2 AIS-C) decreased detrusor overactivity, improved continence, and enhanced lower urinary tract sensation following TSCS.97 Niu et al. reported the use of Transcutaneous Magnetic SCS (TMSCS), which is a non-invasive and painless technique for stimulation of the lumbar region of the spine in an attempt to enhance bladder efficiency in five participants with traumatic spinal cord lesion (AIS-A/B).95 All the participants lacked the ability to self-void. They were given TMSCS every week for 16 weeks. These were followed by sham stimulations given at similar intervals for 6 weeks. Every participant underwent bladder function monitoring. All subjects reported improvement in bladder function while being on TMSCS therapy. All 5 subjects were able to achieve volitional micturition. The urine volume voided voluntarily increased with a decrease in the frequency of self-catheterisation. The bladder capacity increased to 404 mL from 244 mL. So far, the research has supported the role of TMSCS with regard to genitourinary issues only.
5.2. Pulmonary function & cough
SCS has been investigated and found to enhance chest function in individuals with SCI. Sustained use of ESCS around T9 to L1 level (stimulation parameters; 40 V, 30–55 Hz) can allow an increase in positive end-expiratory pressure over 10–20 weeks, thereby helping restore cough.98, 99, 100 The ability to cough is paramount to the prevention of lower respiratory tract infections. It is realised principally by the intercostal muscles and the abdominal muscles (T4-L2).101 Individuals with injury above T4 develop an ineffective cough, thereby predisposing them to various respiratory infections.102,103 Spinal cord stimulation at lower thoracic levels (T9-T11) results in triggering of key muscles and restoration of an effective cough.100 Enhanced cough and breathing were observed in a patient with tonic TSCS (carrier pulse 10-kHz and burst pulse 30-Hz over C3–4, C5–6, and T1–12, with improvements enduring for some days even on discontinuation of TSCS. Recruitment of trunk and intercostal muscles following induction into an excitatory state is considered to facilitate improvement in lung function.104
5.3. Autonomic & metabolic issues in SCI
Autonomic dysfunction increases morbidity in SCI individuals.105 They are prone to develop metabolic issues, coronary artery disease, type 2 diabetes and obesity. This is in part due to decreased potential for mobility as well as deranged autonomic reflexes to exercise in cervical SCI. Sympathetic activation which helps elevate blood pressure (BP), heart rate (HR), mediate sweating response, and appropriate substrate breakdown, is inappropriate in individuals with cervical SCI.105 In athletes with traumatic spinal cord lesions rostral to T1, the catecholamine levels are very low.106 A low cervical injury is not significantly different from a high thoracic injury (i.e., T4) from a motor control standpoint. Injury at C8 is differentiated from T4 by the autonomic response to exercise.105 ESCS and TSCS have been reported to have a positive impact on autonomic functions though how this is made possible remains to be elucidated.105,107
5.3.1. Effect of SCS on blood pressure
In those with orthostatic intolerance, blood pressure can be increased by triggering at several sites (e.g., T6 or T11 to L1) and with various frequencies (e.g., 30 vs 120 Hz).108 Stimulation of peripheral nerve, either in the presence or absence of contraction in muscles can also raise BP by 10–30 mmHg, comparable to that seen with TSCS or ESCS.108 Rise in BP is mediated by the constriction of vessels in the splanchnic region. This is mediated by sympathetic activation from T6-T12. Therefore it is pretty surprising that even stimulus given at caudal levels (L1-S1) attains adequate blood pressure in spite of a lack of lower limb muscle activity. These observations support the presence of internal spinal links ascending to thoracic spinal preganglionic circuits.105 Individuals with traumatic spinal lesions at or below T6 and complete motor weakness maintain autonomic responses comparable to normal subjects.109
5.3.2. Effects of SCS on metabolism
Several isolated reports suggest that SCS can improve autonomic nervous system control of sweat gland activity in chronic SCI. An appropriate assessment is needed to define the role of SCS and its outcome in this regard. The adrenal gland is innervated by T6 to T10 spinal segments. Cervical-level SCI individuals have low catecholamine and free fatty acid (FFA) levels.110, 111, 112 The above factors add up to reducing resting and activity-associated energy consumption in these subsets of SCI. Even vigorous exercise will burn calories similar to a sedentary state in comparison with neurologically intact controls.113 SCS enhanced FFA breakdown as opposed to glucose. It is associated with improvement in maximal exercise response and endurance.106,114
5.3.3. Mechanisms influencing autonomic & metabolic system via SCS
SCS at lumbar levels is known to improve blood pressure in orthostatic intolerance via splanchnic vasoconstriction, which derives its nerve supply from T6-T12. Mechanisms influencing metabolism at the spinal level would likely involve the triggering of ascending tracts and their linkages with thoracic sympathetic circuits.105 SCS at a similar spinal location with the same parameters of stimulation can result in a contradictory impact on BP. Transcutaneous stimulation at T7 or T8 diminished the increase in BP provoked by stimulus in the rectum, whereas transcutaneous stimulation at T7 or T8 raised BP in response to orthostatic hypotension.115,116 Sachdeva et al. theorised that irritable triggers such as digital stimulus were suppressed by spinal cord stimulation that recruited large diameter fibres.116 All studies reported the use of pulsed stimulation except Shelyakin et al.,117 who stimulated using direct current and reported a surge in HR of subjects along with improved lower extremity movements that they ascribed to thoracic paravertebral ganglia activation. Retrograde labelling using injectable pseudorabies virus (PRV) and electrophysiological assessments have consistently shown descending signals to spinal neurons innervating adrenal, heart, blood vessels, temperature regulation, adipose and skeletal muscle tissues. In this framework triggering of locomotion occurs in tandem with a surge in sympathetic activity to metabolic and homeostatic systems.118 This helps explain the improvement in sympathetic functions when SCS is used for the activation of lower limb motor abilities.
An increase in fatty acid metabolism following upper lumbar SCS is attributed to adrenergic targets to white adipose tissue. It results in higher endurance and fatty acid utilisation as opposed to glucose oxidation.106,114 Activation of proprioceptive afferents may lead to increased activation of spinal preganglionic neurons via interneurons innervating the cardiovascular, metabolic and sudomotor tissues at various spinal levels.119 These findings support the application of neuromodulation strategies targeting the autonomic system for homeostasis under various stresses and enhanced exercise tolerance, thereby avert or defer vascular and metabolic diseases frequently observed in chronic SCI.
5.4. Neuropathic pain in SCI patients
SCI has several disturbing consequences, including among them high rates of chronic pain and altered sensory function. According to some estimates, 30%–80% of SCI patients suffer from long-standing pain that develops after an injury.120 The neuropathic pain is often described as shooting, stabbing, sharp or burning pain. It occurs in a dermatomal distribution and is, at times, intractable in spite of extensive medicinal intake.121 Severe pain distributed at the neurological level of injury or adjacent to it is frequently seen in SCI patients.122 Conventional SCS may suppress pain by acting at the spinal or peripheral level. Relief in neuropathic pain in SCI patients has been observed to be less with conventional SCS when compared to those patients with pain due to failed back syndrome or peripheral neuropathic origin. Several studies and a review in 2009 have reported that conventional SCS has unsatisfactory results with only a 30–40% success rate. As of now, the evidence does not support SCS use for neuropathic pain.123
6. Technical parameters in SCS
6.1. Lead placement
Leads have been placed in various combinations for TSCS, with the highest at C2 and the lowest at Co1.84,104 For voluntary muscle activity in the lower extremities, the most frequent and efficient placement of leads was in the range of T10-L2. For voluntary muscle activity in the upper limbs, the most frequent and efficient level for placement of leads ranged from C4–6. Placement of leads at T11 to L1 has proven efficacious in decreasing orthostatic hypotension.104,124, 125, 126, 127 Although for effectively managing cardiovascular function, placement of leads at L1-S1 and T7–8 was also effective. The most frequent and efficient placement of leads for bladder function was L1-S1. In most studies assessing pulmonary function, leads were placed at T9-11.64,84,115,128
6.2. Stimulation parameters
Stimulation parameters can be divided into 2 types: tonic stimulation involving the firing of uniform pulses or spatiotemporal modulation, wherein targeted stimulation is optimized to produce intended movements. Most studies have used tonic stimulation.31,129 Pulse widths were in the range of 150 microsec to 2 msec. Current intensities were in the range of 0.1–15 mA/1 to 40 V in studies on ESCS. In studies on TSCS, current intensities were in the range of 2.5–210 mA/18 V, although many studies used higher intensities almost up to the participant's tolerance threshold.
Stimulation to induce voluntary activity in lower limbs was not efficacious in enhancing bladder function. In order to improve voiding and bladder storage, tonic stimulation ranging from 2 to 60 Hz was found to be effective. For pulmonary functions as well, tonic stimulation at 2–60 Hz was effective. Most studies on TSCS used a continuous pulse mode, with the rest of them using train/burst modes. While some had used an alternating or biphasic waveform, others used a direct current or monophasic waveform. There was an absence of consistency across studies with respect to the specificity of stimulation parameters used for certain outcomes.130 In humans, continuous ESCS fails to facilitate meaningful locomotion without rehabilitation. This is hypothesised due to the cancellation of proprioception feedback. Burst stimulations and Spatiotemporal stimulations mitigate this issue. Spatial and temporal adjustment of the amplitude and frequency according to the profile of the proprioceptive feedback would facilitate the modulating activity of motor neurons. Using Computational modelling, Formento et al. concluded that high frequency and low amplitude (5 pulses at 600 Hz) ESCS would prevent the cancellation of proprioceptive feedback at the same time allowing the activation of motor neurons.10 Several scores and formulas are hypothesised to be helpful in delineating patient-specific stimulation profiles. Further research will help broaden the role of spatiotemporal stimulation.131
7. ESCS vs TSCS
Both techniques have basic differences in methodology that have an impact on the respective areas of application and efficacy. ESCS, placed over dura mater, yields a focused electric field leading to greater segmental selectivity in recruiting posterior roots, a characteristic that allows the generation of nonvolitional movements.48,70,132 TSCS, acting via skin surface electrodes, creates a more far-off and diffuse electric field, albeit with reduced segmental selectivity. By uniformly stimulating a number of segments of the spinal cord, TSCS increases the global sensitivity of the spinal cord to effect voluntary movement when combined with physiotherapy.133,134 Different electrode models and their selective placement may permit some rostrocaudal discrimination,135 while the side-to-side selectivity of dorsal root stimulation is yet to be proved. Moreover, because it involves the usage of skin surface electrodes besides variation in stimulation conditions depending on body position, TSCS cannot be applied chronically.136 ESCS hence seems to be a convincing method to increase or prompt, purposeful motion after SCI, which may lead to better outcomes in gait therapy.29 Being simple and non-invasive, TSCS may be beneficial in issues where uniform spinal cord segment stimulation is helpful such as lower limb spasticity, or to enhance outcomes of various locomotor therapies.71,137
8. Adverse events
Sivaganesan et al. analysed retrospectively and found a 13% complication rate with SCS. Spinal cord stimulators are considered amongst frontrunners of injury among all used medical devices following hip prostheses and implantable insulin pumps.138 An economic analysis performed by the United Kingdom's National Institute of Health and Clinical Excellence assessing the application of SCS for neuropathic pain management has shown the treatment to be cost-effective.139 Though similar studies assessing the cost-benefit ratio of SCS in neurological recovery in SCI individuals are not available.
The need for the removal of epidural electrodes is not uncommon, which is a challenging surgical procedure as, with time, electrodes get encapsulated in epidural fibrosis.140
Infection is the most common complication, with incidence up to 5%. It may be managed by antibiotics and require even removal of the device and reimplantation following healing from sepsis.141 Rate of infection is higher than other implantable electronic devices, for instance, cardiac pacemakers (infection rate 0.5–2.2%).142
Lead migration incidence, which earlier used to be very high (13–22%), has gone down (1.4–2.1%) as techniques of implantation have evolved.143,144 If the lead migrates, it may need relocation or substitution of the electrode surgically. Reported adverse events of TSCS to include pain or discomfort following stimulation of nociceptive receptors in the skin, increased spasticity seen in one participant, initial intolerance of the procedure, and skin fissuring due to the concentration of current under the electrodes. TSCS may induce autonomic dysreflexia in SCI individuals, and therefore its translation in home-based programs will need caution.130,145
9. SCS-where do we stand and what are future prospects?
The demographic distribution and SCI characteristics of the population with SCI represented by the studies included match that of the general populace, with the majority being incomplete injuries and male participants. Multicentre trials are potent ways to increase the sample size for relatively rare health conditions such as SCI since most studies had less than 10 participants.
To deal with the diverseness of SCI, it is essential for upcoming research to sort and classify SCI subjects by their injury grade and level to inform treatment protocols better. Furthermore, the inclusion of other markers relevant to spinal cord injuries, such as radiology and other clinical assessments, may better classify participants to appreciate possible differences in outcomes of SCS. Recovery outcomes following SCS are directly linked to the severity of the lesion. Many studies have mentioned the neurology as motor incomplete or complete and proper subgrouping according to the AIS classification will shed more light on the effectiveness of SCS in complete SCI. Anatomical and physiological proof of remnant descending white matter and voluntary signals have been realised in an increasing number of AIS A subjects.146,147 McKay et al. analysed 41 patients with AIS-A neurology and found 68% of participants to have subclinical evidence of translesional motor connections using surface electromyography.148 Therefore, integration of injury severity with MRI would help establish the role of SCS in patients with a complete cut-off of the spinal cord.
Several studies did not distinctly report the device used or the stimulation details. This will help systematise research further. Different stages of research can be planned in such a way as to engage individuals with lived experiences of SCI to make sure that the results of SCS studies resonate with the critical concerns of the SCI community.149 There is a requirement of comparative studies of two types of SCS. As per available literature there is limited evidence of multi-modal studies. Only two studies have assessed TSCS and ESCS together.74,150 The results should report subject-reported outcomes as well.
Increasingly several studies suggest that persistent neuromodulation appears to persist following long-term ESCS use and TSCS interventions as short-term as two months, even after termination of active stimulation by ESCS or TSCS. In spite of several unanswered issues and a minimal understanding of the mechanisms of SCS, electrical neuromodulation has tremendous potential in the treatment of those living with SCI.
10. Study limitations
This review was limited to original clinical research available in the English language. To improve the data quality, we excluded conference proceedings, reviews, proposed research or protocol description, theses, abstracts, lectures, commentaries and editorials from this review. Due to the fragmentary nature of information, along with the variable use of terms, in particular, related to the devices used and stimulation characteristics, the data extracted are incomplete.
11. Conclusion
Significant advances in the investigation of the role of spinal cord electrical stimulation in the motor improvement of SCI patients have been achieved. However, high-quality studies have to be conducted to establish it as a standard of care. Further research has to be performed on the mechanism of SCS for other issues faced by SCI patients, such as pulmonary, genitourinary, and autonomic functions. Support of government agencies, funding agencies, and collaborative activities could go a long way in carrying out further research on SCS, which has immense potential to drastically change the lives of individuals with SCI.
Ethical approval
No ethical approval was required for this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lin A., Shaaya E., Calvert J.S., Parker S.R., Borton D.A., Fridley J.S. A review of functional restoration from spinal cord stimulation in patients with spinal cord injury. Neurospine. 2022;19(3):703–734. doi: 10.14245/ns.2244652.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hachmann J.T., Yousak A., Wallner J.J., Gad P.N., Edgerton V.R., Gorgey A.S. Epidural spinal cord stimulation as an intervention for motor recovery after motor complete spinal cord injury. J Neurophysiol. 2021;126(6):1843–1859. doi: 10.1152/jn.00020.2021. [DOI] [PubMed] [Google Scholar]
- 3.Choi E., Gattas S., Brown N., et al. Epidural electrical stimulation for spinal cord injury. Neural Regen Res. 2021;16(12):2367. doi: 10.4103/1673-5374.313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonizzato M., James N.D., Pidpruzhnykova G., et al. Multi-pronged neuromodulation intervention engages the residual motor circuitry to facilitate walking in a rat model of spinal cord injury. Nat Commun. 2021;12(1):1925. doi: 10.1038/s41467-021-22137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtine G., Song B., Roy R.R., et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14(1):69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taccola G., Sayenko D., Gad P., Gerasimenko Y., Edgerton V.R. And yet it moves: recovery of volitional control after spinal cord injury. Prog Neurobiol. 2018;160:64–81. doi: 10.1016/j.pneurobio.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tapias Pérez J.H., Medula N., Dolor Spinal Cord Stimulation: Beyond Pain Management PALABRAS CLAVE [Internet] Neurología. 2022;37 doi: 10.1016/j.nrleng.2019.05.007. www.elsevier.es/neurologia Available from: [DOI] [PubMed] [Google Scholar]
- 8.Cai Y., Reddy R.D., Varshney V., Chakravarthy K v. Spinal cord stimulation in Parkinson's disease: a review of the preclinical and clinical data and future prospects. Bioelectron Med. 2020;6(1):5. doi: 10.1186/s42234-020-00041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagel S.J., Wilson S., Johnson M.D., et al. Spinal cord stimulation for spasticity: historical approaches, current status, and future directions. Neuromodu: Technol Neural Interface. 2017;20(4):307–321. doi: 10.1111/ner.12591. [DOI] [PubMed] [Google Scholar]
- 10.Formento E., Minassian K., Wagner F., et al. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci. 2018;21(12):1728–1741. doi: 10.1038/s41593-018-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minassian K., Perret I., Hofstoetter U.S. Neuroprosthetics and Brain-Computer Interfaces in Spinal Cord Injury. Springer International Publishing; Cham: 2021. Epidural and transcutaneous spinal cord stimulation strategies for motor recovery after spinal cord injury; pp. 167–190. [Google Scholar]
- 12.Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 13.Cook A.W., Weinstein S.P. Chronic dorsal column stimulation in multiple sclerosis. Preliminary report. N Y State J Med. 1973;73(24):2868–2872. [PubMed] [Google Scholar]
- 14.Barolat-Romana G., Myklebust J.B., Hemmy D.C., Myklebust B., Wenninger W. Immediate effects of spinal cord stimulation in spinal spasticity. J Neurosurg. 1985;62(4):558–562. doi: 10.3171/jns.1985.62.4.0558. [DOI] [PubMed] [Google Scholar]
- 15.Barolat G., Myklebust J.B., Wenninger W. Effects of spinal cord stimulation on spasticity and spasms secondary to myelopathy. Stereotact Funct Neurosurg. 1988;51(1):29–44. doi: 10.1159/000099381. [DOI] [PubMed] [Google Scholar]
- 16.Dimitrijevic M.R., Illis L.S., Nakajima K., Sharkey P.C., Sherwood A.M. Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: II. Neurophysiologic observations. Cent Nerv Syst Trauma. 1986;3(2):145–152. doi: 10.1089/cns.1986.3.145. [DOI] [PubMed] [Google Scholar]
- 17.Dimitrijevic M.R., Gerasimenko Y., Pinter M.M. Evidence for a spinal central pattern generator in humansa. Ann N Y Acad Sci. 1998;860(1 NEURONAL MECH):360–376. doi: 10.1111/j.1749-6632.1998.tb09062.x. [DOI] [PubMed] [Google Scholar]
- 18.Nagel S.J., Wilson S., Johnson M.D., et al. Spinal cord stimulation for spasticity: historical approaches, current status, and future directions. Neuromodul: Technol Neural Interface. 2017;20(4):307–321. doi: 10.1111/ner.12591. [DOI] [PubMed] [Google Scholar]
- 19.Barolat G., Myklebust J.B., Wenninger W. Enhancement of voluntary motor function following spinal cord stimulation - case study. Stereotact Funct Neurosurg. 1986;49(6):307–314. doi: 10.1159/000100160. [DOI] [PubMed] [Google Scholar]
- 20.Herman R., He J., D'Luzansky S., Willis W., Dilli S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord. 2002;40(2):65–68. doi: 10.1038/sj.sc.3101263. [DOI] [PubMed] [Google Scholar]
- 21.Carhart M.R., He Jiping, Herman R., D'Luzansky S., Willis W.T. Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12(1):32–42. doi: 10.1109/TNSRE.2003.822763. [DOI] [PubMed] [Google Scholar]
- 22.Minassian K., Jilge B., Rattay F., et al. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004;42(7):401–416. doi: 10.1038/sj.sc.3101615. [DOI] [PubMed] [Google Scholar]
- 23.Ganley K., Willis W., Carhart M., He J., Herman R. Epidural spinal cord stimulation improves locomotor performance in low ASIA C, wheelchair-dependent, spinal cord-injured individuals: insights from metabolic response. Top Spinal Cord Inj Rehabil. 2005;11(2):50–63. [Google Scholar]
- 24.Huang He, He Jiping, Herman R., Carhart M.R. Modulation effects of epidural spinal cord stimulation on muscle activities during walking. IEEE Trans Neural Syst Rehabil Eng. 2006;14(1):14–23. doi: 10.1109/TNSRE.2005.862694. [DOI] [PubMed] [Google Scholar]
- 25.Harkema S., Gerasimenko Y., Hodes J., et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angeli C.A., Edgerton V.R., Gerasimenko Y.P., Harkema S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137(5):1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayenko D.G., Angeli C., Harkema S.J., Edgerton V.R., Gerasimenko Y.P. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J Neurophysiol. 2014;111(5):1088–1099. doi: 10.1152/jn.00489.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rejc E., Angeli C., Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grahn P.J., Lavrov I.A., Sayenko D.G., et al. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc. 2017;92(4):544–554. doi: 10.1016/j.mayocp.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Rejc E., Angeli C.A., Atkinson D., Harkema S.J. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-14003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rejc E., Angeli C.A., Bryant N., Harkema S.J. Effects of stand and step training with epidural stimulation on motor function for standing in chronic complete paraplegics. J Neurotrauma. 2017;34(9):1787–1802. doi: 10.1089/neu.2016.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angeli C.A., Boakye M., Morton R.A., et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379(13):1244–1250. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- 33.Gill M., Linde M., Fautsch K., et al. Epidural electrical stimulation of the lumbosacral spinal cord improves trunk stability during seated reaching in two humans with severe thoracic spinal cord injury. Front Syst Neurosci. 2020:14. doi: 10.3389/fnsys.2020.569337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peña Pino I., Hoover C., Venkatesh S., et al. Long-term spinal cord stimulation after chronic complete spinal cord injury enables volitional movement in the absence of stimulation. Front Syst Neurosci. 2020:14. doi: 10.3389/fnsys.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu D.C., Edgerton V.R., Modaber M., et al. Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients. Neurorehabilitation Neural Repair. 2016;30(10):951–962. doi: 10.1177/1545968316644344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill M.L., Grahn P.J., Calvert J.S., et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24(11):1677–1682. doi: 10.1038/s41591-018-0175-7. [DOI] [PubMed] [Google Scholar]
- 37.Gildenberg P.L. Evolution of neuromodulation. Stereotact Funct Neurosurg. 2005;83(2–3):71–79. doi: 10.1159/000086865. [DOI] [PubMed] [Google Scholar]
- 38.Courtine G., Sofroniew M v. Spinal cord repair: advances in biology and technology. Nat Med. 2019;25(6):898–908. doi: 10.1038/s41591-019-0475-6. [DOI] [PubMed] [Google Scholar]
- 39.Capogrosso M., Milekovic T., Borton D., et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539(7628):284–288. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wenger N., Moraud E.M., Gandar J., et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med. 2016;22(2):138–145. doi: 10.1038/nm.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capogrosso M., Wagner F.B., Gandar J., et al. Configuration of electrical spinal cord stimulation through real-time processing of gait kinematics. Nat Protoc. 2018;13(9):2031–2061. doi: 10.1038/s41596-018-0030-9. [DOI] [PubMed] [Google Scholar]
- 42.Wagner F.B., Mignardot J.B., le Goff-Mignardot C.G., et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563(7729):65–71. doi: 10.1038/s41586-018-0649-2. [DOI] [PubMed] [Google Scholar]
- 43.Kathe C., Skinnider M.A., Hutson T.H., et al. The neurons that restore walking after paralysis. Nature. 2022;611(7936):540–547. doi: 10.1038/s41586-022-05385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietz V., Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain. 2014;137(3):654–667. doi: 10.1093/brain/awt262. [DOI] [PubMed] [Google Scholar]
- 45.Waltz J.M., Andreesen W.H., Hunt D.P. Spinal cord stimulation and motor disorders. Pacing Clin Electrophysiol. 1987;10(1):180–204. doi: 10.1111/j.1540-8159.1987.tb05947.x. [DOI] [PubMed] [Google Scholar]
- 46.McIntyre C.C., Grill W.M. Excitation of central nervous system neurons by nonuniform electric fields. Biophys J. 1999;76(2):878–888. doi: 10.1016/S0006-3495(99)77251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman A.G., Levin M.F., Garofolini A., Piscitelli D., Zhang L. Central pattern generator and human locomotion in the context of referent control of motor actions. Clin Neurophysiol. 2021;132(11):2870–2889. doi: 10.1016/j.clinph.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Guertin P.A. Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front Neurol. 2013;3 doi: 10.3389/fneur.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumbauer K.M., Huie J.R., Hughes A.J., Grau J.W. Timing in the absence of supraspinal input II: regularly spaced stimulation induces a lasting alteration in spinal function that depends on the NMDA receptor, BDNF release, and protein synthesis. J Neurosci. 2009;29(46):14383–14393. doi: 10.1523/JNEUROSCI.3583-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisdorfer J.T., Smit R.D., Keefe K.M., Lemay M.A., Smith G.M., Spence A.J. Epidural electrical stimulation: a review of plasticity mechanisms that are hypothesized to underlie enhanced recovery from spinal cord injury with stimulation. Front Mol Neurosci. 2020:13. doi: 10.3389/fnmol.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guertin P.A. Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front Neurol. 2013;3 doi: 10.3389/fneur.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cina C., Hochman S. Diffuse distribution of sulforhodamine-labeled neurons during serotonin-evoked locomotion in the neonatal rat thoracolumbar spinal cord. J Comp Neurol. 2000;423(4):590–602. doi: 10.1002/1096-9861(20000807)423:4<590::aid-cne5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 53.Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 2016;17(4):224–238. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grillner S., Wallén P. Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci. 1985;8(1):233–261. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- 55.Gosgnach S., Bikoff J.B., Dougherty K.J., El Manira A., Lanuza G.M., Zhang Y. Delineating the diversity of spinal interneurons in locomotor circuits. J Neurosci. 2017;37(45):10835–10841. doi: 10.1523/JNEUROSCI.1829-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaunt R.A., Prochazka A., Mushahwar V.K., Guevremont L., Ellaway P.H. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local alpha-motoneuron responses. J Neurophysiol. 2006;96(6):2995–3005. doi: 10.1152/jn.00061.2006. [DOI] [PubMed] [Google Scholar]
- 57.Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38(4):335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- 58.Sayenko D.G., Angeli C., Harkema S.J., Edgerton V.R., Gerasimenko Y.P. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J Neurophysiol. 2014;111(5):1088–1099. doi: 10.1152/jn.00489.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Courtine G., Harkema S.J., Dy C.J., Gerasimenko Y.P., Dyhre-Poulsen P. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J Physiol. 2007;582(Pt 3):1125–1139. doi: 10.1113/jphysiol.2007.128447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dorrian R.M., Berryman C.F., Lauto A., Leonard A.V. Electrical stimulation for the treatment of spinal cord injuries: a review of the cellular and molecular mechanisms that drive functional improvements. Front Cell Neurosci. 2023:17. doi: 10.3389/fncel.2023.1095259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angeli C.A., Edgerton V.R., Gerasimenko Y.P., Harkema S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137(5):1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asboth L., Friedli L., Beauparlant J., et al. Cortico–reticulo–spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018;21(4):576–588. doi: 10.1038/s41593-018-0093-5. [DOI] [PubMed] [Google Scholar]
- 63.Flynn J.R., Graham B.A., Galea M.P., Callister R.J. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60(5):809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 64.Goganau I., Sandner B., Weidner N., Fouad K., Blesch A. Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury. Exp Neurol. 2018;300:247–258. doi: 10.1016/j.expneurol.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thornton M.A., Mehta M.D., Morad T.T., et al. Evidence of axon connectivity across a spinal cord transection in rats treated with epidural stimulation and motor training combined with olfactory ensheathing cell transplantation. Exp Neurol. 2018;309:119–133. doi: 10.1016/j.expneurol.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin Y. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177(1):265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- 67.Ghorbani M., Shahabi P., Karimi P., et al. Impacts of epidural electrical stimulation on Wnt signaling, FAAH, and BDNF following thoracic spinal cord injury in rat. J Cell Physiol. 2020;235(12):9795–9805. doi: 10.1002/jcp.29793. [DOI] [PubMed] [Google Scholar]
- 68.Hayashi N., Himi N., Nakamura-Maruyama E., et al. Improvement of motor function induced by skeletal muscle contraction in spinal cord-injured rats. Spine J. 2019;19(6):1094–1105. doi: 10.1016/j.spinee.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Li G., Fan Z.K., Gu G.F., et al. Epidural spinal cord stimulation promotes motor functional recovery by enhancing oligodendrocyte survival and differentiation and by protecting myelin after spinal cord injury in rats. Neurosci Bull. 2020;36(4):372–384. doi: 10.1007/s12264-019-00442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prochazka A., Ellaway P. Comprehensive Physiology. Wiley; 2012. Sensory systems in the control of movement; pp. 2615–2627. [DOI] [PubMed] [Google Scholar]
- 71.Rascoe A., Sharma P., Shah P.K. Development of an activity-dependent epidural stimulation system in freely moving spinal cord injured rats: a proof of concept study. Front Neurosci. 2018:12. doi: 10.3389/fnins.2018.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holinski B.J., Everaert D.G., Mushahwar V.K., Stein R.B. Real-time control of walking using recordings from dorsal root ganglia. J Neural Eng. 2013;10(5) doi: 10.1088/1741-2560/10/5/056008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calvert J.S., Grahn P.J., Strommen J.A., et al. Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis. J Neurotrauma. 2019;36(9):1451–1460. doi: 10.1089/neu.2018.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beck L., Veith D., Linde M., et al. Impact of long-term epidural electrical stimulation enabled task-specific training on secondary conditions of chronic paraplegia in two humans. J Spinal Cord Med. 2021;44(5):800–805. doi: 10.1080/10790268.2020.1739894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Norrbrink C. Transcutaneous electrical nerve stimulation for treatment of spinal cord injury neuropathic pain. J Rehabil Res Dev. 2009;46(1):85–93. [PubMed] [Google Scholar]
- 76.Ladenbauer J., Minassian K., Hofstoetter U.S., Dimitrijevic M.R., Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehabil Eng. 2010;18(6):637–645. doi: 10.1109/TNSRE.2010.2054112. [DOI] [PubMed] [Google Scholar]
- 77.Minassian K., Persy I., Rattay F., Dimitrijevic M.R., Hofer C., Kern H. Posterior root–muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve. 2007;35(3):327–336. doi: 10.1002/mus.20700. [DOI] [PubMed] [Google Scholar]
- 78.Hofstoetter U.S., McKay W.B., Tansey K.E., Mayr W., Kern H., Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2014;37(2):202–211. doi: 10.1179/2045772313Y.0000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gerasimenko Y., Gorodnichev R., Moshonkina T., Sayenko D., Gad P., Reggie Edgerton V. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med. 2015;58(4):225–231. doi: 10.1016/j.rehab.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inanici F., Samejima S., Gad P., Edgerton V.R., Hofstetter C.P., Moritz C.T. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng. 2018;26(6):1272–1278. doi: 10.1109/TNSRE.2018.2834339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hofstoetter U.S., Freundl B., Binder H., Minassian K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: elicitation of posterior root-muscle reflexes. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0192013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hofstoetter U.S., Krenn M., Danner S.M., et al. Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals. Artif Organs. 2015;39(10):E176–E186. doi: 10.1111/aor.12615. [DOI] [PubMed] [Google Scholar]
- 83.Minassian K., Hofstoetter U.S., Danner S.M., et al. Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord–injured individuals. Neurorehabilitation Neural Repair. 2016;30(3):233–243. doi: 10.1177/1545968315591706. [DOI] [PubMed] [Google Scholar]
- 84.Powell E.S., Carrico C., Westgate P.M., et al. Time configuration of combined neuromodulation and motor training after stroke: a proof-of-concept study. NeuroRehabilitation. 2016;39(3):439–449. doi: 10.3233/NRE-161375. [DOI] [PubMed] [Google Scholar]
- 85.Gad P., Gerasimenko Y., Zdunowski S., et al. Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front Neurosci. 2017:11. doi: 10.3389/fnins.2017.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Powell E.S., Carrico C., Salyers E., Westgate P.M., Sawaki L. The effect of transcutaneous spinal direct current stimulation on corticospinal excitability in chronic incomplete spinal cord injury. NeuroRehabilitation. 2018;43(2):125–134. doi: 10.3233/NRE-172369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rath M., Vette A.H., Ramasubramaniam S., et al. Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma. 2018;35(21):2540–2553. doi: 10.1089/neu.2017.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sayenko D.G., Rath M., Ferguson A.R., et al. Self-Assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J Neurotrauma. 2019;36(9):1435–1450. doi: 10.1089/neu.2018.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyer C., Hofstoetter U.S., Hubli M., et al. Immediate effects of transcutaneous spinal cord stimulation on motor function in chronic, sensorimotor incomplete spinal cord injury. J Clin Med. 2020;9(11):3541. doi: 10.3390/jcm9113541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shapkova E.Y., Pismennaya E.V., Emelyannikov D.V., Ivanenko Y. Exoskeleton walk training in paralyzed individuals benefits from transcutaneous lumbar cord tonic electrical stimulation. Front Neurosci. 2020:14. doi: 10.3389/fnins.2020.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiesener C., Spieker L., Axelgaard J., et al. Supporting front crawl swimming in paraplegics using electrical stimulation: a feasibility study. J NeuroEng Rehabil. 2020;17(1):51. doi: 10.1186/s12984-020-00682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McHugh L.v., Miller A.A., Leech K.A., Salorio C., Martin R.H. Feasibility and utility of transcutaneous spinal cord stimulation combined with walking-based therapy for people with motor incomplete spinal cord injury. Spinal Cord Ser Cases. 2020;6(1):104. doi: 10.1038/s41394-020-00359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gad P., Lee S., Terrafranca N., et al. Non-invasive activation of cervical spinal networks after severe paralysis. J Neurotrauma. 2018;35(18):2145–2158. doi: 10.1089/neu.2017.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inanici F., Brighton L.N., Samejima S., Hofstetter C.P., Moritz C.T. Transcutaneous spinal cord stimulation restores hand and arm function after spinal cord injury. IEEE Trans Neural Syst Rehabil Eng. 2021;29:310–319. doi: 10.1109/TNSRE.2021.3049133. [DOI] [PubMed] [Google Scholar]
- 95.Zhang F., Momeni K., Ramanujam A., et al. Cervical spinal cord transcutaneous stimulation improves upper extremity and hand function in people with complete tetraplegia: a case study. IEEE Trans Neural Syst Rehabil Eng. 2020;28(12):3167–3174. doi: 10.1109/TNSRE.2020.3048592. [DOI] [PubMed] [Google Scholar]
- 96.Alam M., Ling Y.T., Wong A.Y.L., Zhong H., Edgerton V.R., Zheng Y. Reversing 21 years of chronic paralysis via non-invasive spinal cord neuromodulation: a case study. Ann Clin Transl Neurol. 2020;7(5):829–838. doi: 10.1002/acn3.51051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Estes S., Zarkou A., Hope J.M., Suri C., Field-Fote E.C. Combined transcutaneous spinal stimulation and locomotor training to improve walking function and reduce spasticity in subacute spinal cord injury: a randomized study of clinical feasibility and efficacy. J Clin Med. 2021;10(6):1167. doi: 10.3390/jcm10061167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang R., Nikooyan A.A., Moore L.D., et al. Minimal handgrip force is needed for transcutaneous electrical stimulation to improve hand functions of patients with severe spinal cord injury. Sci Rep. 2022;12(1):7733. doi: 10.1038/s41598-022-11306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dy C.J., Gerasimenko Y.P., Edgerton V.R., Dyhre-Poulsen P., Courtine G., Harkema S.J. Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J Neurophysiol. 2010;103(5):2808–2820. doi: 10.1152/jn.00316.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Militskova A., Mukhametova E., Fatykhova E., et al. Supraspinal and afferent signaling facilitate spinal sensorimotor network excitability after discomplete spinal cord injury: a case report. Front Neurosci. 2020:14. doi: 10.3389/fnins.2020.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Atkinson D.A., Sayenko D.G., D'Amico J.M., et al. Interlimb conditioning of lumbosacral spinally evoked motor responses after spinal cord injury. Clin Neurophysiol. 2020;131(7):1519–1532. doi: 10.1016/j.clinph.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 102.Megía García A., Serrano-Muñoz D., Taylor J., Avendaño-Coy J., Gómez-Soriano J. Transcutaneous spinal cord stimulation and motor rehabilitation in spinal cord injury: a systematic review. Neurorehabilitation Neural Repair. 2020;34(1):3–12. doi: 10.1177/1545968319893298. [DOI] [PubMed] [Google Scholar]
- 103.Sahni K.S., Zampieri T.A., Severs S.L., Greenstein A., Katz P.G. Effect of implanted epidural stimulator on lower urinary tract function in spinal-cord-injured patients. Eur Urol. 1991;20(2):103–106. doi: 10.1159/000471675. [DOI] [PubMed] [Google Scholar]
- 104.Herrity A.N., Aslan S.C., Ugiliweneza B., Mohamed A.Z., Hubscher C.H., Harkema S.J. Improvements in bladder function following activity-based recovery training with epidural stimulation after chronic spinal cord injury. Front Syst Neurosci. 2021:14. doi: 10.3389/fnsys.2020.614691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DiMarco A.F., Geertman R.T., Tabbaa K., Nemunaitis G.A., Kowalski K.E. Restoration of cough via spinal cord stimulation improves pulmonary function in tetraplegics. J Spinal Cord Med. 2020;43(5):579–585. doi: 10.1080/10790268.2019.1699678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DiMarco A.F., Kowalski K.E., Geertman R.T., Hromyak D.R. Spinal cord stimulation. Am J Respir Crit Care Med. 2006;173(12):1386–1389. doi: 10.1164/rccm.200601-097CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DiMarco A.F., Kowalski K.E., Geertman R.T., et al. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a national institutes of health–sponsored clinical trial. Part II: clinical outcomes. Arch Phys Med Rehabil. 2009;90(5):726–732. doi: 10.1016/j.apmr.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gad P., Kreydin E., Zhong H., Edgerton V.R. Enabling respiratory control after severe chronic tetraplegia: an exploratory case study. J Neurophysiol. 2020;124(3):774–780. doi: 10.1152/jn.00320.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aslan S.C., Legg Ditterline B.E., Park M.C., et al. Epidural spinal cord stimulation of lumbosacral networks modulates arterial blood pressure in individuals with spinal cord injury-induced cardiovascular deficits. Front Physiol. 2018:9. doi: 10.3389/fphys.2018.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.West C.R., Phillips A.A., Squair J.W., et al. Association of epidural stimulation with cardiovascular function in an individual with spinal cord injury. JAMA Neurol. 2018;75(5):630. doi: 10.1001/jamaneurol.2017.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Darrow D., Balser D., Netoff T.I., et al. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J Neurotrauma. 2019;36(15):2325–2336. doi: 10.1089/neu.2018.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nightingale T.E., Walter M., Williams A.M.M., Lam T., Krassioukov A v. Ergogenic effects of an epidural neuroprosthesis in one individual with spinal cord injury. Neurology. 2019;92(7):338–340. doi: 10.1212/WNL.0000000000006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Phillips A.A., Squair J.W., Sayenko D.G., Edgerton V.R., Gerasimenko Y., Krassioukov A v. An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J Neurotrauma. 2018;35(3):446–451. doi: 10.1089/neu.2017.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Knikou M., Murray L.M. Repeated transspinal stimulation decreases soleus H-reflex excitability and restores spinal inhibition in human spinal cord injury. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0223135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sachdeva R., Nightingale T.E., Pawar K., et al. Noninvasive neuroprosthesis promotes cardiovascular recovery after spinal cord injury. Neurotherapeutics. 2021;18(2):1244–1256. doi: 10.1007/s13311-021-01034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Estes S.P., Iddings J.A., Field-Fote E.C. Priming neural circuits to modulate spinal reflex excitability. Front Neurol. 2017:8. doi: 10.3389/fneur.2017.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burton C., Sajja A., Latthe P.M. Effectiveness of percutaneous posterior tibial nerve stimulation for overactive bladder: a systematic review and meta-analysis. Neurourol Urodyn. 2012;31(8):1206–1216. doi: 10.1002/nau.22251. [DOI] [PubMed] [Google Scholar]
- 118.Gaziev G., Topazio L., Iacovelli V., et al. Percutaneous tibial nerve stimulation (PTNS) efficacy in the treatment of lower urinary tract dysfunctions: a systematic review. BMC Urol. 2013;13(1):61. doi: 10.1186/1471-2490-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walter M., Lee A.H.X., Kavanagh A., Phillips A.A., Krassioukov A v. Epidural spinal cord stimulation acutely modulates lower urinary tract and bowel function following spinal cord injury: a case report. Front Physiol. 2018;9:1816. doi: 10.3389/fphys.2018.01816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Niu T., Bennett C.J., Keller T.L., Leiter J.C., Lu D.C. A proof-of-concept study of transcutaneous magnetic spinal cord stimulation for neurogenic bladder. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-30232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gad P.N., Kreydin E., Zhong H., Latack K., Edgerton V.R. Non-invasive neuromodulation of spinal cord restores lower urinary tract function after paralysis. Front Neurosci. 2018:12. doi: 10.3389/fnins.2018.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kreydin E., Zhong H., Latack K., Ye S., Edgerton V.R., Gad P. Transcutaneous electrical spinal cord neuromodulator (TESCoN) improves symptoms of overactive bladder. Front Syst Neurosci. 2020:14. doi: 10.3389/fnsys.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DiMarco A.F., Geertman R.T., Tabbaa K., Polito R.R., Kowalski K.E. Case report: minimally invasive method to activate the expiratory muscles to restore cough. J Spinal Cord Med. 2018;41(5):562–566. doi: 10.1080/10790268.2017.1357916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.DiMarco A.F. Restoration of respiratory muscle function following spinal cord injury. Respir Physiol Neurobiol. 2005;147(2–3):273–287. doi: 10.1016/j.resp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 125.Brown R., DiMarco A.F., Hoit J.D., Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006;51(8):853–868. discussion 869-70. [PMC free article] [PubMed] [Google Scholar]
- 126.Zimmer M.B., Nantwi K., Goshgarian H.G. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med. 2007;30(4):319–330. doi: 10.1080/10790268.2007.11753947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Flett S., Garcia J., Cowley K.C. Spinal electrical stimulation to improve sympathetic autonomic functions needed for movement and exercise after spinal cord injury: a scoping clinical review. J Neurophysiol. 2022;128(3):649–670. doi: 10.1152/jn.00205.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cowley K.C. A new conceptual framework for the integrated neural control of locomotor and sympathetic function: implications for exercise after spinal cord injury. Appl Physiol Nutr Metabol. 2018;43(11):1140–1150. doi: 10.1139/apnm-2018-0310. [DOI] [PubMed] [Google Scholar]
- 129.Parittotokkaporn S., Varghese C., O'Grady G., Svirskis D., Subramanian S., O'Carroll S.J. Non-invasive neuromodulation for bowel, bladder and sexual restoration following spinal cord injury: a systematic review. Clin Neurol Neurosurg. 2020;194 doi: 10.1016/j.clineuro.2020.105822. [DOI] [PubMed] [Google Scholar]
- 130.Krassioukov A., Eng J.J., Warburton D.E., Teasell R. A systematic review of the management of orthostatic hypotension after spinal cord injury. Arch Phys Med Rehabil. 2009;90(5):876–885. doi: 10.1016/j.apmr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hopman M.T., Oeseburg B., Binkhorst R.A. Cardiovascular responses in persons with paraplegia to prolonged arm exercise and thermal stress. Med Sci Sports Exerc. 1993;25(5):577–583. [PubMed] [Google Scholar]
- 132.Campbell I.G., Williams C., Lakomy H.K. Physiological and metabolic responses of wheelchair athletes in different racing classes to prolonged exercise. J Sports Sci. 2004;22(5):449–456. doi: 10.1080/02640410410001675298. [DOI] [PubMed] [Google Scholar]
- 133.Gass G.C., Camp E.M. Effects of prolonged exercise in highly trained traumatic paraplegic men. J Appl Physiol. 1987;63(5):1846–1852. doi: 10.1152/jappl.1987.63.5.1846. [DOI] [PubMed] [Google Scholar]
- 134.Stallknecht B., Lorentsen J., Enevoldsen L.H., et al. Role of the sympathoadrenergic system in adipose tissue metabolism during exercise in humans. J Physiol. 2001;536(1):283–294. doi: 10.1111/j.1469-7793.2001.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shea J.R., Shay B.L., Leiter J., Cowley K.C. Energy expenditure as a function of activity level after spinal cord injury: the need for tetraplegia-specific energy balance guidelines. Front Physiol. 2018:9. doi: 10.3389/fphys.2018.01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shelyakin A.M., Preobrazhenskaya I.G., Komantsev V.N., Makarovskii A.N., Bogdanov O v. The use of micropolarization in the treatment of spinal cord lesions. Neurosci Behav Physiol. 2000;30(1):1–4. doi: 10.1007/BF02461384. [DOI] [PubMed] [Google Scholar]
- 137.Cowley K.C. A new conceptual framework for the integrated neural control of locomotor and sympathetic function: implications for exercise after spinal cord injury. Appl Physiol Nutr Metabol. 2018;43(11):1140–1150. doi: 10.1139/apnm-2018-0310. [DOI] [PubMed] [Google Scholar]
- 138.Sato A., Schmidt R.F. Somatosympathetic reflexes: afferent fibers, central pathways, discharge characteristics. Physiol Rev. 1973;53(4):916–947. doi: 10.1152/physrev.1973.53.4.916. [DOI] [PubMed] [Google Scholar]
- 139.Widerström-Noga E., Biering-Sørensen F., Bryce T.N., et al. The international spinal cord injury pain basic data set (version 2.0) Spinal Cord. 2014;52(4):282–286. doi: 10.1038/sc.2014.4. [DOI] [PubMed] [Google Scholar]
- 140.Siddall P., Loeser J. Pain following spinal cord injury. Spinal Cord. 2001;39(2):63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- 141.Bryce T.N., Biering-Sørensen F., Finnerup N.B., et al. International spinal cord injury pain classification: part I. Background and description. Spinal Cord. 2012;50(6):413–417. doi: 10.1038/sc.2011.156. [DOI] [PubMed] [Google Scholar]
- 142.Lagauche D., Facione J., Albert T., Fattal C. The chronic neuropathic pain of spinal cord injury: which efficiency of neuropathics stimulations? Ann Phys Rehabil Med. 2009;52(2):180–187. doi: 10.1016/j.rehab.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 143.Harkema S.J., Wang S., Angeli C.A., et al. Normalization of blood pressure with spinal cord epidural stimulation after severe spinal cord injury. Front Hum Neurosci. 2018:12. doi: 10.3389/fnhum.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Terson de Paleville D.G.L., Harkema S.J., Angeli C.A. Epidural stimulation with locomotor training improves body composition in individuals with cervical or upper thoracic motor complete spinal cord injury: a series of case studies. J Spinal Cord Med. 2019;42(1):32–38. doi: 10.1080/10790268.2018.1449373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rowald A., Komi S., Demesmaeker R., et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat Med. 2022;28(2):260–271. doi: 10.1038/s41591-021-01663-5. [DOI] [PubMed] [Google Scholar]
- 146.Laskin J.J., Waheed Z., Thorogood N.P., Nightingale T.E., Noonan V.K. Spinal cord stimulation research in the restoration of motor, sensory, and autonomic function for individuals living with spinal cord injuries: a scoping review. Arch Phys Med Rehabil. 2022;103(7):1387–1397. doi: 10.1016/j.apmr.2022.01.161. [DOI] [PubMed] [Google Scholar]
- 147.Moraud E.M., Capogrosso M., Formento E., et al. Mechanisms underlying the neuromodulation of spinal circuits for correcting gait and balance deficits after spinal cord injury. Neuron. 2016;89(4):814–828. doi: 10.1016/j.neuron.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 148.Krenn M., Hofstoetter U.S., Danner S.M., Minassian K., Mayr W. Multi-electrode array for transcutaneous lumbar posterior root stimulation. Artif Organs. 2015;39(10):834–840. doi: 10.1111/aor.12616. [DOI] [PubMed] [Google Scholar]
- 149.Roy F.D., Gibson G., Stein R.B. Effect of percutaneous stimulation at different spinal levels on the activation of sensory and motor roots. Exp Brain Res. 2012;223(2):281–289. doi: 10.1007/s00221-012-3258-6. [DOI] [PubMed] [Google Scholar]
- 150.Sivanesan E., Bicket M.C., Cohen S.P. Retrospective analysis of complications associated with dorsal root ganglion stimulation for pain relief in the FDA MAUDE database. Reg Anesth Pain Med. 2019;44(1):100–106. doi: 10.1136/rapm-2018-000007. [DOI] [PMC free article] [PubMed] [Google Scholar]