Abstract
Background
Aluminum phosphide is a highly toxic pesticide that results in high mortality. To date, there is neither a definitive antidote nor a unified protocol for managing acute aluminum phosphide poisoning.
Objectives
This cross-sectional questionnaire-based study aims to explore different management approaches and rely on the expertise of Egyptian medical professionals to enhance the prognosis for acute aluminum phosphide poisoning.
Subjects and methods
A self-administered questionnaire was formulated and electronically distributed according to published literature and experience of senior physicians.
Results and conclusions
Responses were received from 151 physicians from 10 governorates. Management modalities were variable among respondents. Noradrenaline was used by 90.7% of respondents with no fixed-dose regimen. In all, 84.1% of participants utilized oil in gastrointestinal decontamination; paraffin oil was the most used solution. Overall, 92.1, 61.6, 46.4, and 34.4% of participants used sodium bicarbonate, proton pump inhibitors, IV magnesium sulfate, and antioxidants, respectively. Regarding the frequency of acute aluminum phosphide poisoning, 47% of participants managed these cases daily or a few times a week. Participants' responses denoted a poor prognosis of acute aluminum phosphide poisoning, and high percentages attributed the prognosis to exposure factors rather than treatment modalities. Statistical analysis revealed that using oil in gastrointestinal decontamination improved the outcome by 4.62-fold. Clinical toxicologists were more likely to rescue ≥ 30% of the cases about 3-fold (2.97) than other specialties. Clinical toxicologists used oil in gastrointestinal decontamination, magnesium sulfate, and antioxidant therapy and calculated base deficit before administration of sodium bicarbonate by 7.70-, 5.30-, 3.26-, and 2.08-fold than other specialties.
Keywords: aluminum phosphide, acute poisoning, clinical toxicologist, Egypt
1. Introduction
Aluminum phosphide (AlP) is a highly toxic pesticide that exerts its effect by liberating deadly phosphine gas. AlP is extensively used in agricultural countries with limited resources because of its low cost and potency in controlling various pests. AlP has become a popular suicidal agent in recent years, with an alarming increase in related morbidities and mortalities.1
After oral intake of AlP, phosphine is liberated under gastric acidity. The cytotoxic phosphine is immediately absorbed with subsequent disruption of mitochondrial respiration and energy production all over the body.2 AlP-related toxicity is presented by unresponsive cardiogenic shock and metabolic acidosis with rapid deterioration of the patient’s conditions. Acute AlP poisoning is often associated with poor prognosis as adequate supportive measures often fail to rescue the patients; even survivors might suffer from life-long sequelae. Thus, treating patients with acute AlP poisoning is challenging and frustrating.3
High AlP-related fatalities encourage physicians to exert the maximum effort to save patients’ lives. To date, there is neither a definitive antidote nor a unified protocol for managing acute AlP poisoning.4 Acute phosphide poisoning is managed empirically from the experience of physicians in this context. Subsequently, acute AlP poisoning management strategies are variable or even controversial among healthcare providers.
Egypt is the highest agricultural country in the Middle East, considering its population, which inhabits more than 102 million in 20225,6; thus, there are increasing cases of acute pesticide poisoning, including metal phosphides. In Egypt, clinical toxicology is recognized as a separate clinical specialty in which clinical toxicologists are specialized in managing various toxicological emergencies.7 This study highlights different management strategies for acute AlP toxicity in Egypt. Also, the current research aimed to use Egyptian physicians’ experiences to improve acute AlP poisoning prognosis.
2. Materials and methods
2.1 Study design and setting
The current research is a cross-sectional questionnaire-based study. This study was conducted on Egyptian physicians who were engaged in managing AlP-intoxicated patients in Egypt.
2.2 Sample size calculation
The sample size was calculated using the Epi Info-7 program8 by adjusting power at 80%, confidence level 95.0%, and incidence of AlP poisoning at 7.12%.9 The minimum estimated sample size was 103 participants; it was increased to 151 participants to account for nonresponse and to increase the power of the study.
The following formula was used: S = Z2 × P × (1 − P)/M.
S = sample size for infinite population
Z = Z score (1.96)
P = population proportion (0.0712)
M = Margin of error (0.5)
2.3 Inclusion and exclusion criteria
The study included Egyptian physicians registered in the Egyptian Medical Syndicate who were involved in the management of intoxicated patients in the Egyptian healthcare system. The study did not include non-Egyptian physicians or those practicing medicine outside Egypt. Also, those who did not manage acute AlP poisoning before or provided incomplete responses were excluded from the study.
2.4 Piloting
Before starting data collection, a pilot study that included 15 clinical toxicologists was conducted. The pilot study was carried out to ensure that the adopted questionnaire was well-formulated and clearly understood. Also, the pilot study aimed to anticipate any probable obstacles that might interfere with the completion of the study.
The pilot study feedback denoted that the questionnaire was well-prepared. Participants needed about 15 min to respond to the questionnaire, which consisted of 26 questions. The responses obtained from the pilot study were not included in the results of the current study.
2.5 Data collection tool
A self-administered questionnaire was formulated after a comprehensive review of acute AlP poisoning treatment modalities in published literature.4,10–17 Also, the questionnaire was enriched with the experience of senior physicians with extended clinical practice in managing AlP-intoxicated patients.
Participants were personally invited to provide their responses to web-based questionnaires and encourage their colleagues to participate. Also, the questionnaire was distributed through medical web pages that Egyptian physicians frequently accessed. The aim of the current study was clearly demonstrated.
A structured questionnaire comprises the following sections:
2.5.1 Personal and professional characteristics of participants (8 questions)
Personal data: age, gender, and governorate.
Professional data: specialty, experience duration, qualifications, job level, and affiliated healthcare institute.
2.5.2 Treatment modalities of AlP-intoxicated patients (14 questions)
Cardiovascular (CVS) supportive measures: fluid therapy, management of refractory hypotension, and noradrenaline dosage (3 questions).
Cardiopulmonary resuscitation (CPR): conduction of CPR and its duration (2 questions).
Gastrointestinal tract (GIT) decontamination: methods, types of used oily solutions, and factors affecting the physician’s decisions regarding decontamination (3 questions).
Additional supportive measures: administration of adjuvant agents (sodium bicarbonate, proton pump inhibitor, IV magnesium sulfate, antioxidant therapy, IV lipid emulsion, and anti-arrhythmic drugs), type of used antioxidant, sodium bicarbonate administration regimens, and N-acetyl cysteine (NAC) administration regimens (6 questions).
2.5.3 Frequency and outcome of AlP-intoxicated patients (4 questions)
Frequency of cases in a healthcare institute.
The percentage of successfully managed cases (number of cases survived out of 10 intoxicated patients), if it could be estimated. Successful management of 30% or more of AlP-intoxicated patients was considered a favorable outcome.
Participants’ observations regarding the factors affecting the outcome.
Decision for discharging of survived cases.
The survey questions were designed in 2 forms: single correct answer per question and multiple correct answers per question. Four clinical toxicology consultants assessed the content validity of the questions. Google Forms was used as a tool for questionnaire formulation; then, it was electronically distributed.
2.6 Ethical considerations
Before the study commencement, approval was obtained from the Research Ethics Committee of the Faculty of Medicine, Alexandria University (IRB Number: 00012098, FWA Number: 00018699, Approval Serial Number: 0305302). The submission of responses was considered as implied consent for participation. The authors guarantee the maintenance of the confidentiality of participants’ data.
2.7 Data analysis
All 2-sided statistical tests were judged at a 0.05 significance level and performed using the IBM SPSS statistics program version 28. The questions that had multiple answers per question were treated as multiple responses. Categorical variables were summarized by frequency and percent. The chi-square test was performed to study the significant association between different categorical variables. Fischer’s exact and Monte Carlo’s significance was used if more than 20% of the total expected cell counts < 5.
3. Results
The responses were received from 151 physicians who managed acute AlP poisoning in 10 Egyptian governorates (Cairo, Alexandria, Beheira, Gharbia, Qalyubiyya, Dakahlia Damietta, Ismailia, Kafr El-Shaikh, and Asyut).
Table 1 illustrates that more than half (52.3%) of participants were aged 30–40 years old, and 43% were aged 25–30 years old. Females constituted around three-quarters (71.5%) of the respondents. Clinical toxicologists constituted more than half (55%) of the participants, whereas the rest belonged to other specialties.
Table 1.
Personal and professional characteristics of participants (n = 151) engaged in managing AlP-intoxicated patients.
| Personal and professional characteristics of participants | Frequency (n = 151) | % |
|---|---|---|
| Age | ||
| 25 to < 30 | 65 | 43.0 |
| 30 to < 40 | 79 | 52.3 |
| 40 to < 50 | 6 | 4.0 |
| 50–60 | 1 | 0.7 |
| Gender | ||
| Male | 43 | 28.5 |
| Female | 108 | 71.5 |
| Specialty | ||
| Clinical toxicologist | 83 | 55.0 |
| Emergency medicine doctors | 31 | 20.5 |
| ICU doctors | 22 | 14.6 |
| Pediatricians | 11 | 7.3 |
| General practitioners | 4 | 2.6 |
| Years of experience | ||
| <5 | 65 | 43.0 |
| 5 to < 10 | 46 | 30.5 |
| 10 to < 20 | 38 | 25.2 |
| ≥20 | 2 | 1.3 |
| Academic degree | ||
| Bachelor | 44 | 29.1 |
| Master | 68 | 45.0 |
| Doctorate | 39 | 25.8 |
| Job level | ||
| Resident/demonstrator | 65 | 43.0 |
| Specialist | 53 | 35.1 |
| Consultant | 33 | 21.9 |
| Affiliated healthcare institute | ||
| University hospital | 127 | 84.1 |
| Ministry of health hospital | 21 | 13.9 |
| Private sector | 3 | 2.0 |
ICU, intensive care unit.
Considering experience duration, 43% of participants had <5 years of experience, and 30.5% with experience duration ranging from 5 to 10 years. Regarding qualification, 45% of participants had a master’s degree. Forty-three percent of respondents were residents/demonstrators, whereas specialists constituted 35.1%. Most physicians (84.1%) managed cases of acute AlP poisoning in university hospitals.
3.1 Treatment modalities of AlP-intoxicated patients
3.1.1 CVS supportive measures
Table 2 reveals that fluid therapy was empirically initiated by more than half (57%) of participants, whereas 43 and 39.1% administrated fluids under the guidance of central venous pressure (CVP) measurement and echocardiography (ECHO) assessment, respectively.
Table 2.
Responses of participating physicians (n = 151) regarding CVS supportive measures in the management of AlP-intoxicated patients.
| Questions addressing CVS supportive measures in AlP-intoxicated patients | Frequency (n = 151) | % |
|---|---|---|
| Q In your practice, fluid therapy for the management of acute AlP poisoning is:a | ||
| Initiated Empirically IV Crystalloids | 86 | 57.0 |
| Guided with CVP measurement | 65 | 43.0 |
| Guided with ECHO to assess ejection fraction and myocardial contractility | 59 | 39.1 |
| Q In your practice, management of AlP-induced refractory hypotension include:a | ||
| Noradrenaline | 137 | 90.7 |
| Dobutamine and/or dopamine | 69 | 45.7 |
| IV Hydrocortisone | 38 | 25.2 |
| Aggressive CVS supportive measures such as IABP/ECMO | 22 | 14.6 |
| Q When you administer noradrenaline infusion in AlP cases the dose regimen is:a | ||
| No fixed regimen for noradrenaline infusion in these cases. | 55 | 36.4 |
| Start with rate of infusion 5 ml/h of single dose. | 44 | 29.1 |
| Start with rate of infusion 7.4 ml/h of single dose. | 8 | 5.3 |
| Dose readjusted every 30 min or 1 h according to perfusion status. | 71 | 47.0 |
| Maximum rate of infusion (≥30 ml/h single dose) could be applied in severe cases. | 38 | 25.2 |
ECHO, echocardiogram.
aMultiple response question, percent is calculated out of total (n = 151) per each answer.
Noradrenaline was the commonest therapeutic agent, 90.7% administered it to manage AlP-induced refractory hypotension, followed by dobutamine (45.7%) and hydrocortisone (25.2%). In all, 36.4% of the respondents declared that there is no fixed-dose regimen of noradrenaline administration in treating phosphide-induced refractory hypotension. CPR for ≥20 min was done by 58% of participants, whereas 22% performed CPR for <20 min while managing these cases. Overall, 20% of respondents declared that they did not perform CPR on arrested patients because of acute phosphide poisoning, as illustrated in Fig. 1.
Fig. 1.
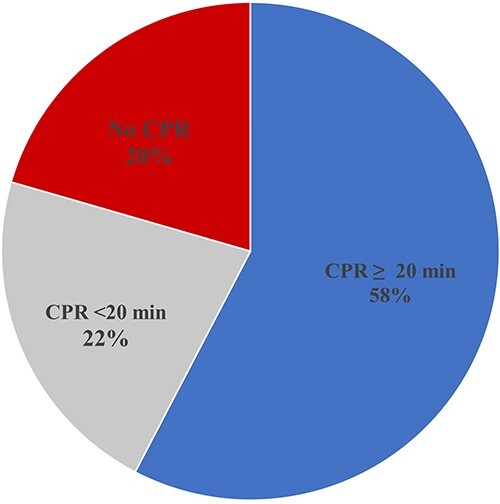
CPR in acute AlP-intoxicated patients.
No significant association was found between nonconduction of CPR and treating physicians’ characteristics, including age, experience duration, academic degree, job level, affiliated institutes, and specialty with P-values of 0.632, 0.752, 0.126, 0.784, 0.227, and 0.956, respectively. Similarly, the duration of CPR had no significant association with age, specialty, experience duration, academic degree, job level, and affiliated institutes of treating physicians with P-values of 0.886, 0.834, 0.954, 0.702, 0.784, and 0.169, respectively.
3.1.2 GIT decontamination methods
Table 3 shows that more than three-quarters (84.1%) of participants utilized oil in GIT decontamination, whereas 11.9% used aqueous-based solutions. A tiny fraction (4%) of the participants did not perform any GIT decontamination. Paraffin oil was the most used oily solution in GIT decontamination (74.2%). Coconut oil was used by 10.6% of respondents; these physicians managed AlP-intoxicated patients in Alexandria, Sharqia, Dakahlia, Gharbia, and Cairo Governorates.
Table 3.
Responses of participating physicians (n = 151) to questions addressing GIT decontamination the management of AlP-intoxicated patients.
| Questions addressing GIT decontamination in AlP-intoxicated patients | n = 151 | % |
|---|---|---|
| Q GIT decontamination that you do to manage acute AlP poisoning include: | ||
| Oil | 127 | 84.1 |
| Administration of oil only | 47 | 31.1 |
| Gastric lavage with oil | 25 | 16.6 |
| Gastric lavage with oil + sodium bicarbonate | 40 | 26.5 |
| Suction followed by administration of oil. | 15 | 9.9 |
| Water | 18 | 11.9 |
| Gastric lavage with water/saline | 2 | 1.3 |
| Gastric lavage with water/saline + potassium permanganate | 2 | 1.3 |
| Gastric lavage with water/saline + Charcoal | 7 | 4.6 |
| Gastric lavage with water/saline + sodium bicarbonate | 7 | 4.6 |
| No decontamination | 6 | 4.0 |
| Q If you use oil in GIT decontamination in acute AlP poisoning, which type is administrated: | ||
| Any available oil | 10 | 6.6 |
| Paraffin oil | 112 | 74.2 |
| Coconut oil | 16 | 10.6 |
| Sunflower oil | 11 | 7.3 |
| Castor oil | 1 | 0.7 |
| Olive oil | 1 | 0.7 |
| Q Your choice regarding the manner of GIT decontamination of AlP is governed by:a | ||
| GIT decontamination is the same in all cases of acute AlP poisoning. | 45 | 29.8 |
| Time since ingestion | 72 | 47.7 |
| Route of exposure (inhalational/ingestion) | 69 | 45.7 |
| General condition of the patient (GCS, BP, acid–base status) | 49 | 32.5 |
| Mode of ingestion (dissolved tablet in water or swallowed intact tablet). | 24 | 15.9 |
| Amount ingested | 22 | 14.6 |
GCS, Glasgow Coma Scale.
BP, blood pressure.
aMultiple response question, percent is calculated out of total (n = 151) per each answer.
The responses of 47.7 and 45.7% of participants denoted that the choice GIT decontamination governed by time since ingestion of AlP and route of exposure, respectively. Whereas, 29.8% of the participants declared that GIT decontamination was the same in all cases.
3.1.3 Additional supportive measures
Table 4 reveals that a large majority (92.1%) of participants used sodium bicarbonate infusion in managing AlP-intoxicated patients. Proton pump inhibitors, IV magnesium sulfate, and antioxidants are administered by 61.6, 46.4, and 34.4% of respondents, respectively.
Table 4.
Responses of participating physicians (n = 151) regarding additional supportive measures the management of AlP-intoxicated patients.
| Questions addressing additional supportive measures in management of AlP-intoxicated patients. | n = 151 | % |
|---|---|---|
| Q Which of the following supportive measures do you routinely use in the management of acute AlP poisoning?a | ||
| Sodium bicarbonate infusion | 139 | 92.1 |
| Proton pump inhibitor | 93 | 61.6 |
| IV Magnesium sulfate | 70 | 46.4 |
| Antioxidant therapy | 52 | 34.4 |
| IV lipid emulsion | 16 | 10.6 |
| Prophylactic anti-arrhythmic, e.g. amiodarone | 13 | 8.6 |
| Q In your practice, sodium bicarbonate infusion (1 mEq/Kg) in management of acute AlP poisoningb | ||
| Initiated following calculation of base deficit. | 88 | 58.3 |
| Empirically initiated before calculation of base deficit. | 63 | 41.7 |
| Q In your practice, sodium bicarbonate infusion is administered to the patients with acute AlP poisoning to achieve.b | ||
| Full correction of metabolic acidosis according to the calculated base deficit. | 66 | 43.7 |
| Partial correction of metabolic acidosis according to the calculated base deficit | 54 | 35.8 |
| Elevation of bicarbonate level recorded in ABG without calculation of base deficit | 31 | 20.5 |
| Q In management of acute AlP poisoning, which type of antioxidant(s) administrated:a,b | ||
| NAC | 124 | 82.1 |
| L-carnitine | 19 | 12.6 |
| CO-enzyme Q10 | 16 | 10.6 |
| Vit C | 12 | 7.9 |
| Vit E | 7 | 4.6 |
| Others | 2 | 1.3 |
| Q If you give IV NAC in management of AlP poisoning:b | ||
| Administrated only in cases with elevated liver enzymes | 70 | 46.4 |
| Initiated immediately on admission to all cases | 51 | 33.8 |
| Q If you administer IV NAC the dosing regimen is:b | ||
| IV administration with loading 150 mg/kg over 1 h then 50 mg/kg over 4 h followed by 100 mg/kg over 16 h. | 85 | 56.3 |
| No fixed regimen for NAC in these cases | 22 | 14.6 |
| IV administration of 300 mg/kg infusion for 20 h | 9 | 6.0 |
aMultiple response question, percent is calculated out of total (n = 151) per each answer.
bOptional question.
More than half (58.3%) of respondents initiated sodium bicarbonate infusion following the calculation of base deficit, whereas the rest initiated it empirically. The goals of 43.7 and 35.8% of respondents were to achieve full and partial correction of metabolic acidosis, respectively. Nevertheless, the rest of the participants aimed to elevate the recorded bicarbonate level in arterial blood gasses (ABG).
Regarding antioxidants, NAC was the most used antioxidant, followed by L-carnitine and CO-enzyme Q10. Variable NAC treatment regimens were followed in managing acute phosphide poisoning.
3.2 Frequency and outcome of AlP-intoxicated patients
Figure 2 illustrates that 24% of respondents manage acute AlP poisoning cases daily, and 23% of respondents stated that these cases attend a few times weekly.
Fig. 2.
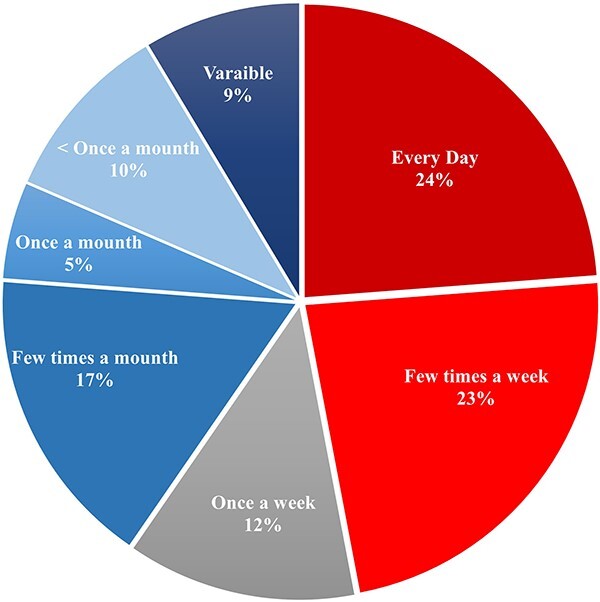
Frequency of acute AlP poisoning cases managed by participating physicians (n = 151).
Regarding the percentage of successfully managed cases, approximately half (47%) of the participating physicians could not determine how many cases of acute AlP poisoning survived out of 10. Nearly two-thirds (63%) of the respondents who determined the percentage of successfully managed cases mentioned that 20% or less of patients survived following AlP intoxication (Fig. 3).
Fig. 3.
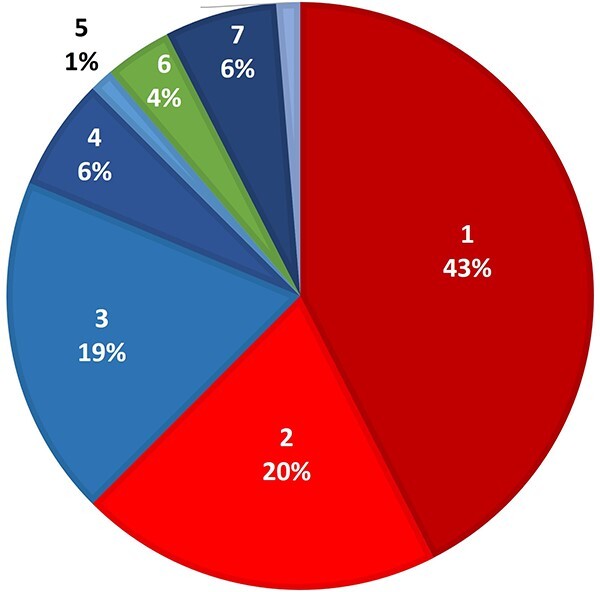
Percentage of successfully managed AlP-intoxicated patients determined by participating physicians (n = 80).
3.3 Outcome analysis of AlP-intoxicated patients
3.3.1 Participants’ observations
Figure 4 demonstrates that high percentages of the respondents associated the patients’ prognosis with exposure factors, whereas fewer percentages associated the treatment options with patients’ prognosis.
Fig. 4.
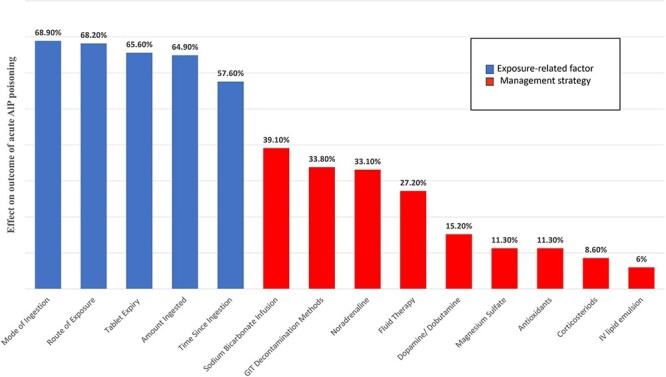
Participants’ responses regarding exposure-related factors and management strategies affecting the outcome of AlP-intoxicated patients (n = 151).
3.3.2 Association between management and percentage of successfully managed cases
The personal and professional characteristics of the participating physicians and their responses regarding different treatment options were carefully analyzed in relation to the percentage of successfully managed cases of AlP-intoxicated patients (Table 5).
Table 5.
Association between management and percentage of successfully managed AlP-intoxicated patients (n = 80)a.
| Survived cases | Sig. | ||||
|---|---|---|---|---|---|
| n = 50 | ≤20% | n = 30 | ≥30% | ||
| 1. Personal and professional data of participants | |||||
| Age | |||||
| 25 to <30 | 22 | 44.0 | 13 | 43.3 | .954 |
| ≥30 | 28 | 56.0 | 17 | 56.7 | |
| Specialty | |||||
| Clinical toxicologist | 22 | 44.0 | 21 | 70.0 | .024b |
| Other specialties | 28 | 56.0 | 9 | 30.0 | |
| Years of experience | |||||
| <5 | 21 | 42.0 | 15 | 50.0 | .486 |
| ≥5 | 29 | 58.0 | 15 | 50.0 | |
| Academic degree | |||||
| Bachelor | 15 | 30.0 | 11 | 36.7 | .537 |
| Master | 23 | 46.0 | 10 | 33.3 | |
| Doctorate | 12 | 24.0 | 9 | 30.0 | |
| Job description | |||||
| Resident/demonstrator | 19 | 38.0 | 17 | 56.7 | 0.098 |
| Specialist | 22 | 44.0 | 6 | 20.0 | |
| Consultant | 9 | 18.0 | 7 | 23.3 | |
| Affiliated institute | |||||
| University hospitals | 39 | 78.0 | 27 | 90.0 | 0.171 |
| Other healthcare institutes | 11 | 22.0 | 3 | 10.0 | |
| 2. CVS supportive measures | |||||
| Fluid therapyc | |||||
| Initiated Empirically IV Crystalloids | 30 | 60.0 | 18 | 60.0 | 1 |
| Guided with CVP measurement | 19 | 38.0 | 17 | 56.7 | 0.104 |
| Guided with ECHO | 20 | 40.8 | 9 | 30.0 | 0.333 |
| Management of ALP-induced refractory hypotensionc | |||||
| Noradrenaline | 47 | 94.0 | 25 | 83.3 | 0.144 |
| Dobutamine and/or dopamine | 20 | 40.8 | 17 | 56.7 | 0.171 |
| IV Hydrocortisone | 11 | 22.0 | 7 | 23.3 | 0.890 |
| Aggressive CVS supportive measures | 7 | 14.0 | 5 | 16.7 | 0.756 |
| Noradrenaline infusion dose regimen isc | |||||
| No fixed regimen for noradrenaline infusion in these cases. | 17 | 34.0 | 9 | 30.0 | 0.712 |
| Start with rate of infusion 5 ml/h of single dose | 15 | 30.0 | 10 | 33.3 | 0.755 |
| Start with rate of infusion 7.4 ml/h of single dose | 2 | 4.0 | 3 | 10.0 | 0.358 |
| Dose readjusted every 30 min or 1 h according to perfusion status. | 29 | 58.0 | 13 | 43.3 | 0.203 |
| Maximum rate of infusion (≥30 ml/h single dose) applied in severe cases. | 14 | 28.0 | 8 | 26.7 | 0.897 |
| 3. GIT decontamination | |||||
| GIT decontamination that you do to manage acute AlP poisoning include | |||||
| No decontamination | 1 | 2.0 | 2 | 6.7 | 0.015b |
| Oil | 40 | 80.0 | 28 | 93.3 | |
| Aqueous | 9 | 18.0 | 0 | 0.0 | |
| 4. Other supportive measures | |||||
| Supportive measuresc | |||||
| Sodium bicarbonate infusion | 44 | 88.0 | 27 | 90.0 | 1 |
| IV Magnesium sulfate | 19 | 38.0 | 16 | 53.3 | 0.181 |
| Prophylactic anti-arrhythmic, e.g. amiodarone | 3 | 6.0 | 5 | 16.7 | 0.144 |
| IV lipid emulsion | 5 | 10.0 | 1 | 3.3 | 0.402 |
| Antioxidant therapy | 15 | 30.0 | 12 | 40.0 | 0.360 |
| Proton pump inhibitor | 31 | 62.0 | 22 | 73.3 | 0.299 |
| Sodium bicarbonate infusion (1 mEq/kg) regimen | |||||
| Empirically initiated before calculation of base deficit. | 20 | 40.0 | 11 | 36.7 | 0.767 |
| Initiated following calculation of base deficit. | 30 | 60.0 | 19 | 63.3 | |
ECHO, echocardiogram.
aIn all, 71 participants (47%) could not determine the percentage of successfully managed cases.
bSignificant results ≤ 0.05
cMultiple response question.
3.3.2.1 Personal and professional characteristics of participants
There was a significant association between being a clinical toxicologist and achieving a favorable outcome (P = 0.024). It was observed that approximately three-quarters (70%) of those who succeeded in rescuing ≥30% of the patients were clinical toxicologists. In other words, nearly half (48.8%) of clinical toxicologists reported saving the lives of ≥30% of patients, whereas 24.3% of other specialties achieved the same outcome. By calculation of the odds ratio (OR), clinical toxicologists were more likely to rescue ≥ 30% of the cases about 3-fold (2.97) than other specialties with 95% CI (1.137–7.756).
3.3.2.2 CVS supportive measures
There was no significant association between various CVS supportive measures and the percentage of successfully managed cases (P-values > 0.05).
3.3.2.3 GIT decontamination
The majority (93.3%) of the physicians who rescued ≥30% of patients used oil in GIT decontamination. None of the physicians who used an aqueous solution in GIT decontamination achieved the same outcome. GIT decontamination using oil was significantly associated with better outcome (P = 0.015). By calculation of OR, when oil was used in GIT decontamination, rescuing ≥30% of patients was more likely to be achieved 4.62-fold with 95% CI (1.716–12.435).
3.3.2.4 Additional supportive measures
Magnesium sulfate, anti-arrhythmics, antioxidants, and proton pump inhibitors were used with greater percentages by those who achieved better outcome (53.3, 16.7, 40, and 73.3%, respectively) in relation to the others who reported worse outcome (38, 6, 30, and 62%, respectively). However, the differences did not reach statistical significance (P-values > 0.05).
3.3.3 Differences between clinical toxicologists and other specialties in the management of AlP-intoxicated patients (Table 6)
Table 6.
Differences between clinical toxicologists and other specialties in management of AlP-intoxicated patients (n = 151).
| Clinical toxicology | Other specialties | Sig. | |||
|---|---|---|---|---|---|
| n = 83 | % | n = 58 | % | ||
| 1. CVS supportive measures | |||||
| In your practice, fluid therapy for the management of acute AlP poisoning is:a | |||||
| Initiated Empirically IV Crystalloids | 42 | 50.6 | 44 | 64.7 | 0.082 |
| Guided with CVP measurement | 42 | 50.6 | 23 | 33.8 | 0.038 |
| Guided with ECHO | 32 | 38.6 | 27 | 40.9 | 0.770 |
| In your practice, management of AlP-induced refractory hypotension include:a | |||||
| Noradrenaline | 72 | 86.7 | 65 | 95.6 | 0.062 |
| Dobutamine and/or dopamine | 41 | 50.0 | 28 | 42.4 | 0.358 |
| IV Hydrocortisone | 23 | 28.0 | 15 | 22.1 | 0.401 |
| Aggressive CVS supportive measures | 14 | 16.9 | 8 | 11.8 | 0.377 |
| When you administer noradrenaline infusion in AlP cases the dose regimen is:a | |||||
| No fixed regimen for noradrenaline infusion in these cases. | 28 | 33.7 | 27 | 39.7 | 0.448 |
| Start with rate of infusion 5 ml/h of single dose | 24 | 28.9 | 20 | 29.4 | 0.947 |
| Start with rate of infusion 7.4 ml/h of single dose | 4 | 4.8 | 4 | 5.9 | 1 |
| Dose readjusted every 30 min or 1 h according to perfusion status. | 40 | 48.2 | 31 | 45.6 | 0.750 |
| Maximum rate of infusion (≥30 ml/h single dose) applied in severe cases. | 19 | 22.9 | 19 | 27.9 | 0.477 |
| 2. GIT decontamination | |||||
| GIT decontamination that you do to manage acute AlP poisoning include: | |||||
| No decontamination | 1 | 1.2 | 5 | 7.4 | 0.005b |
| Oil | 77 | 92.8 | 50 | 73.5 | |
| Aqueous | 5 | 6.0 | 13 | 19.1 | |
| 3. Other supportive measures | |||||
| Which of the following supportive measures do you use in the management of acute AlP poisoning?a | |||||
| Sodium bicarbonate infusion | 76 | 91.6 | 63 | 92.6 | 0.807 |
| IV Magnesium sulfate | 53 | 63.9 | 17 | 25.0 | <0.001b |
| Prophylactic anti-arrhythmic, e.g. amiodarone | 5 | 6.0 | 8 | 11.8 | 0.211 |
| IV lipid emulsion | 12 | 14.5 | 4 | 5.9 | 0.088 |
| Antioxidant therapy | 38 | 45.8 | 14 | 20.6 | 0.011b |
| Proton pump inhibitor | 55 | 66.3 | 39 | 57.4 | 0.208 |
| In your practice, Sodium bicarbonate infusion (1 mEq/kg) in management of acute AlP poisoning | |||||
| Empirically initiated before calculation of base deficit. | 28 | 33.7 | 35 | 51.5 | 0.028b |
| Initiated following calculation of base deficit. | 55 | 66.3 | 33 | 48.5 | |
ECHO, echocardiogram.
aMultiple response question.
bSignificant results ≤ 0.05.
3.3.3.1 CVS supportive measures
There were no significant differences between clinical toxicologists and other specialties regarding various CVS supportive measures in managing acute AlP poisoning (P-values > 0.05).
3.3.3.2 GIT decontamination
There was a significant difference between clinical toxicologists and others regarding GIT decontamination (P = 0.005). A large majority (92.8%) of clinical toxicologists administered oil for GIT decontamination compared with 73.5% in other specialties. Most of physicians used an aqueous solution in GIT decontaminating (72.3%), or those who did not perform decontamination at all (83.3%) were not specialized in clinical toxicology. By calculating the OR, clinical toxicologists were more likely to use oil in the GIT decontamination about 8-fold (7.70) than other specialties with 95% CI (0.87–67.87). Nevertheless, non-specialized physicians performed GIT decontamination with an aqueous solution about 2-fold (1.92) than clinical toxicologists with 95% CI (0.18–20.82).
3.3.3.3 Additional supportive measures
There were significant differences between clinical toxicologists and other specialties regarding the administration of intravenous magnesium sulfate (P < 0.001) and antioxidants (P = 0.011). By calculating the OR, clinical toxicologists were more likely to give magnesium sulfate more than 5-fold (5.30) than other specialties with 95% CI (2.61–1.76). In addition, toxicologists gave antioxidant therapy 3.26-fold than others with 95% CI (1.57–6.75).
There was a significant difference between clinical toxicologists and others considering the regimen of administration of sodium bicarbonate (P = 0.028). Clinical toxicologists tended to calculate base deficit before administration of sodium bicarbonate 2.08-fold than other specialties with 95% CI (1.08–4.02).
3.4 Decision for discharging of survived cases
More than two-thirds (67.5%) of the treating physicians attained a fixed follow-up time after stabilizing patients’ condition according to internal regulations in their healthcare facilities. In comparison, 29.1, 28.5, and 15.9% of physicians depended on vital signs, cardiac, and liver functions, respectively, as shown in Table 7.
Table 7.
Responses of participating physicians (n = 151) regarding the decision of hospital discharge of AlP-intoxicated patients.
| Q In your practice, the decision of hospital discharge of acute AlP-intoxicated patients depends on:a | ||
|---|---|---|
| n = 151 | % | |
| • Vital signs | 44 | 29.1 |
| • Cardiac function (ECG findings, ECHO parameters) and cardiac enzymes (troponin, CPK, CK-MB) | 43 | 28.5 |
| • Liver function tests (bilirubin, ALT, AST) | 24 | 15.9 |
| • Fixed follow-up time after stabilization of patient’s condition (internal regulation in your institute) | 102 | 67.5 |
ALT, alanine aminotransferase.
AST, aspartate aminotransferase.
CK-MB, creatine kinase-muscle/brain.
CPK, creatine phosphokinase.
ECHO, echocardiogram.
ECG, electrocardiogram.
aMultiple response question, percent is calculated out of total (n = 151) per each answer.
4. Discussion
AlP gained popularity as a suicidal poison, resulting in high mortalities in different countries worldwide.9 This study explored different modalities for treating acute AlP toxicity in Egypt and potentially effective strategies in this context. Therefore, a comprehensive questionnaire was formulated to cover the prevailing AlP-management strategies either mentioned in the literature or done in real settings. A total of 151 Egyptian physicians shared their experience in managing acute AlP poisoning. Toxicological emergencies in Egypt are often managed by new generations of physicians who cover 24 × 7 shifts in hospitals.18 Thus, resident physicians and specialists constituted more than three-quarters (78.1%) of the study population, and nearly all participants (95.3%) were aged < 40 years. Most of the participants (84.1%) managed AlP-intoxicated patients in university hospitals that possess experienced staff and adequate equipment to deal with intoxicated patients. Nearly, two-thirds (70.8%) of participants had postgraduate qualifications. Also, more than half (55%) of respondents were clinical toxicologists who specialized in managing cases of acute poisoning.
The participants’ responses denoted the high frequency of acute AlP poisoning as nearly half (47%) of participants managing cases with acute AlP poisoning daily or a few times a week. Similarly, Mwaheb and Hassan19 and Deraz et al.9 pointed to the escalating trend of AlP-related morbidities and mortalities in Egypt. Also, the agricultural countries, such as Iran20,21 and India,22 reported high incidence of acute AlP poisoning.
Now, supportive measures remain the mainstay treatment of acute AlP poisoning because of the absence of a specific antidote. AlP toxicity could be ameliorated by minimizing the amount of released phosphine through GIT decontamination or conversing with the toxic effects of absorbed phosphine.23 GIT decontamination in AlP-intoxicated patients is a special consideration because it is water-soluble and extensively liberates phosphine in an aqueous medium. Thus, oily solutions were proposed as alternatives to traditional gastric lavage in acute AlP poisoning.16
Clinically, acute AlP poisoning is manifested by a cardiogenic shock, and metabolic acidosis.24 The participants’ responses revealed that CVS supportive measures in managing AlP-induced cardiogenic shock were highly variable. It was observed that more than half (57%) of participants empirically administered IV fluids. Also, it was evident that a large majority of 90.7% of participants used noradrenaline; however, there was no consensus regarding the regimen of noradrenaline administration. The current study revealed that intra-aortic balloon pump (IABP) and extracorporeal membrane oxygenation (ECMO) were uncommonly used to rescue AlP-intoxicated patients in Egypt, which could be attributed to the limited availability of these expensive measures.25
Generally, CPR should be performed for at least 20 min.26 However, in the current study, 20% of respondents declared that they did not perform CPR in AlP-intoxicated patients. In addition, 22% performed CPR in these cases for <20 min, which is considered inadequate CPR. Only 58 of the participants performed CPR in these cases for 20 min or more. It was found that CPR practices did not get influenced by the personal or professional data of treating physicians. Thus, none or inadequate conduction of CPR could be explained by the frustration of physicians who considered acute AlP poisoning an inevitable death. Nonconduction of CPR is illegal and unethical regardless of the gravity of the condition.27–29
Considering GIT decontamination, the oily solutions were used by more than three-quarters (84.1%) of participants that was in agreement with Egyptian studies.4,16,30,31 Nearly three-quarters (74.2%) of respondents used paraffin oil for GIT decontamination, which could be attributed to its availability as a pharmaceutical preparation in Egypt.16
Coconut oil is vegetable oil with antioxidant properties, and some physicians might consider it more effective and safer than petroleum-based paraffin oil, as mentioned by Elbastawesy and Elmansy30 that might explain the preference of 10.6% of participants to use the coconut oil in the GIT decontamination of AlP-intoxicated patients.
Severe metabolic acidosis is one of the features of acute AlP poisoning; therefore, sodium bicarbonate infusion was used by almost all (92.1%) respondents that are in concordance with the published literature.32 However, it was observed that the regimens of administration of sodium bicarbonate and the rationale of its use were variable among physicians.
Various antioxidants were hypothesized to be promising medications that counteract AlP-induced oxidative stress.33 In the current research, NAC was the most used antioxidant, followed by L-carnitine and CO-enzyme Q10. Previous studies conducted by Tehrani et al.34, Agarwal et al.35, Bahalla et al.12, and El-Ebiary and Abuelfadl36 pointed to the benefits of NAC in the treatment of acute AlP poisoning. In addition, Elgazzar et al.15 recommended using L-carnitine as an adjuvant in managing acute AlP poisoning. Darwish et al.16 provided CO-enzyme Q10 as an antioxidant of choice, which could selectively enhance myocardial functions.
Regarding prognosis, nearly half of the participants could not determine the percentage of successfully managed cases, which denoted controversy regarding AlP outcome that agrees with Proudfoot37, who reviewed the literature and reported that AlP mortality ranged from 40 to 91%.
In the current study, nearly two-thirds of the participants who determined the percentage of successfully managed cases mentioned that no more than 20% of AlP-intoxicated patients survived, which coincided with the results of El-Ebiary and Abuelfad36 and Elgazzar et al.15.
High percentages of the respondents attributed the patients’ prognosis to exposure factors, including mode of ingestion, route of exposure, tablet expiry, ingested amount, and time passed since ingestion. Less respondents attributed patients’ prognosis to treatment modalities that might explain the nonconduction of CPR and GIT decontamination by some participants.
It is worth mentioning that successful management of ≥30% of AlP-intoxicated patients was considered a favorable outcome in intervention arms of clinical trials.15,35,36 Thus, the current study analyzed participants’ responses who denoted successful rescue of 3 or more AlP-intoxicated patients out of 10.
It was found that the possibility of successful management increases by 4.62-fold when oil is used in GIT decontamination, which is in agreement with Darwish et al.16, Helal et al.4, Elbastawesy and Elmansy30, and Abdelhamid et al.31. In addition, it was observed that none of the physicians who used an aqueous solution in GIT decontamination rescued ≥30% of cases. Sanaei-Zadeh and Marashi38 also proved the disastrous effect of GIT decontamination using aqueous solutions that enhance phosphine release from AlP; thus, the use of any aqueous solution in gastric decontamination of AlP-intoxicated patients must be forbidden.
The study pointed to the adjuvant effects of magnesium sulfate, anti-arrhythmics, antioxidants, and proton pump inhibitors in improving the prognosis of acute AlP poisoning. Magnesium sulfate and anti-arrhythmics might improve the prognosis through mitigation of AlP cardiotoxic effects, in agreement with Hassan et al.11 and Hallaj et al.39, respectively. Antioxidants could improve the prognosis of AlP-intoxicated patients by counteracting AlP-induced oxidative stress.40 Proton pump inhibitors decrease gastric acidity and phosphine release from AlP tablets with subsequent less toxicity and better prognosis.41
Clinical toxicologists could rescue ≥30% of AlP-intoxicated patients, about 3-fold compared with other specialties. Therefore, management strategies followed by clinical toxicologists were carefully analyzed to explore the best practices that stand behind improving prognosis.
Oil administration in managing acute AlP poisoning was the most apparent difference between clinical toxicologists and other specialties. All clinical toxicologists administered oil for GIT decontamination, and statistical analysis revealed that clinical toxicologists used oil about 8-fold than others. Also, clinical toxicologists applied additional supportive measures that have potentially beneficial effects in managing acute AlP poisoning; they administered magnesium sulfate and antioxidants by 5- and 3-fold than other specialties, respectively. In addition, clinical toxicologists administered fluid therapy with the guidance of CVP and sodium bicarbonate after calculating the base deficit. Nevertheless, other specialties were more likely to empirically apply these supportive measures in managing acute AlP poisoning.
Regarding hospital discharge of patients, more than two-thirds of participants declared this decision is governed by healthcare institutes regulations that determine a fixed patient follow-up time. Setting internal policies that discharge hemodynamically stable patients could be related to the mission of Egyptian poison centers concerned only with managing life-threatening acute toxicities.42
The study limitations included incomplete responses that were excluded from the study. Also, there was a probability of non-accuracy in the question that investigated the percentage of successfully managed AlP-intoxicated patients. Thus, the response to this question included an option not to determine the percentage of rescued cases, to minimize the probability of non-accuracy, and to ensure that those who answered this question had a reasonable degree of confidence regarding their responses.
Egypt is a model of a developing agricultural country that suffers from acute AlP poisoning tragedy. To date, no standardized management protocol for phosphide poisoning. Thus, the current study explored various treatment modalities of acute AlP poisoning and their respective outcomes, emphasizing the management approaches of clinical toxicologists. This research highlighted the importance of adopting a standardized protocol for AlP poisoning management and emphasizing the performance of CPR, which is a medicolegal responsibility in toxicological emergencies. Also, it is recommended to conduct similar studies in other countries with high incidences of acute AlP poisoning, such as India and Iran, so that the comparability of the results with that of other nations will be possible.
5. Conclusions
This study pointed to the high frequency of acute AlP poisoning in Egypt and its poor prognosis. The participants’ responses revealed that managing acute AlP poisoning was highly variable. However, a large majority of participants used sodium bicarbonate infusion. More than three-quarters of participants utilized oil in GIT decontamination, especially paraffin oil. Also, a high percentage of the participants were administered antioxidants, especially NAC.
The current results elucidated that using oil in GIT decontamination improved survival by 4.62-fold. Interestingly, clinical toxicologists succeeded in managing 30% or more of the cases by about 3-fold compared with other specialties. By analysis of clinical toxicologists’ practices, they used oil in the GIT decontamination about 8-fold than others. Also, they administered magnesium sulfate and antioxidants 5- and 3-fold, respectively, in relation to other specialties. In addition, they calculated base deficit before administering sodium bicarbonate 2-fold than others. Therefore, the current study mandates the use of oil in GIT decontamination of AlP-intoxicated patients. Administration of magnesium sulfate, antioxidants, and sodium bicarbonate after calculation of the base deficit is recommended as they were associated with a better prognosis.
Acknowledgments
The authors express their deep gratitude to Prof. Dr Somaya Madkour, Dr Maii Farag, Dr Maram Atef, Dr Israa Sanad, and Dr Mai Elgendy for their valuable critiques of the study questionnaire. The authors acknowledge Dr Hend Mostafa for her contribution to statistical analysis. Also, the authors thank all the participants who shared their experience; this study could only be performed with their cooperation.
Contributor Information
Zahraa K Sobh, Forensic Medicine and Clinical Toxicology Department, Faculty of Medicine, Alexandria University, Alexandria 21517, Egypt.
Maha Ghanem, Forensic Medicine and Clinical Toxicology Department, Faculty of Medicine, Alexandria University, Alexandria 21517, Egypt.
Marwa Kholief, Forensic Medicine and Clinical Toxicology Department, Faculty of Medicine, Alexandria University, Alexandria 21517, Egypt; Center of Excellence for Research in Regenerative Medicine and Applications (CERRMA) in Faculty of Medicine, Alexandria University, Alexandria 21517, Egypt.
Author contributions
Zahraa K. Sobh contributed to the search concept and study design. All authors contributed equally to data analysis, results interpretation, writing the manuscript, and reviewing the final draft.
Funding
None.
Conflict of interest statement
None declared.
Data availability
Data are available upon reasonable request from the corresponding author.
References
- 1. Elgazzar FM, Shama MA, Shoeib O, Hafez AS. The role of echocardiographic findings in estimating survival probability of intensive care unit admitted aluminum phosphide poisoned patients. J Med Toxicol. 2022:18(2):128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bogale DE, Ejigu BD, Muche TA. Clinical profile and treatment outcome of aluminum phosphide poisoning in Felege Hiwot referral hospital, Northwest Ethiopia: a retrospective study. Open Access Emerg Med. 2021:13:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armandeh M, Bameri B, Baeeri M, Haghi-Aminjan H, Rahimifard M, Hassani S, Hooshangi Shayesteh MR, Khalid M, Samadi M, Hosseini R, et al. . The role of levosimendan in phosphine-induced cardiotoxicity: evaluation of electrocardiographic, echocardiographic, and biochemical parameters. Toxicol Mech Methods. 2021:31(9):631–643. [DOI] [PubMed] [Google Scholar]
- 4. Helal N, Lashin H, Nagy A, Shama M, Mostafa T, Wahdan A. Potential role of paraffin oil gastric lavage in acute aluminum phosphide poisoning: a randomized controlled trial. Environ Sci Pollut Res. 2022:29(22):33844–33855. [DOI] [PubMed] [Google Scholar]
- 5. Zaki MK, Sobh ZK. Optimum standardization of healthcare medicolegal reports in Egypt: a forensic medicine initiative. Forensic Sci Int Rep. 2022:5:100255. [Google Scholar]
- 6. Sobh ZK, Oraby EHA, Abdelaziz SAM. Experience of obstetricians and gynecologists in the management of medicolegal cases in Egypt. BMC Womens Health. 2022:22(1):544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fayed MM, Abdo SA, Sharif AF. Preclinical and clinical medical students' perception of the learning environment: a reference to the forensic medicine and clinical toxicology course. Adv Med Educ Pract. 2022:13:369–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dean A, Arner T, Sunki G, Friedman R, Lantinga M, Sangam S, et al. . Epi Info™, a database and statistics program for public health professionals. Centers for disease control and prevention (CDC); 2011. www.cdc.gov/epiinfo (accessed 28 June 2023). [Google Scholar]
- 9. Deraz RH, Elrafey DS, Mesallam DIA. Acute aluminium phosphide poisoning in East Delta, Egypt: a growing public health problem over the last five years. ESCTJ. 2022:10(1):49–61. [Google Scholar]
- 10. Mostafazadeh B, Farzaneh E. A novel protocol for gastric lavage in patients with aluminum phosphide poisoning: a double-blind study. Acta Med Iran. 2012:50(8):530–534. [PubMed] [Google Scholar]
- 11. Hassan AW, Meo MH, Saqib N, Saleem NM. Efficacy of high dose of magnesium sulphate for cardiac arrhythmias in patients of wheat pill poisoning. J Postgrad Med Inst. 2013:27(3):257–261. [Google Scholar]
- 12. Singh S, Jyothinath P, Singh S. Antioxidant therapy in patients with severe aluminum phosphide poisoning: a pilot study. Indian J Crit Care Med. 2017:21(12):836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohan B, Gupta V, Ralhan S, Gupta D, Puri S, Mahajan R, Goyal A, Chhabra S, Tandon R, Aslam N, et al. . Impact of extra-corporeal membrane oxygenation on outcome of aluminium phosphide poisoning complicated with myocardial dysfunction. Clin Toxicol. 2019:57(11):1095–1102. [DOI] [PubMed] [Google Scholar]
- 14. Beyranvand MR, Farrokhi S, Peyvandi H, Soltaninejad K, Shadnia S. The effects of amiodarone prophylaxis on cardiac dysrhythmia in acute aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2019:70(1):49–53. [DOI] [PubMed] [Google Scholar]
- 15. Elgazzar F, Keshk W, Khalifa H. Early L-carnitine therapy in severe acute aluminum phosphide poisoning: a randomized controlled clinical trial. Egypt J Forensic Sci Appli Toxicol. 2019:19(2):147–164. [Google Scholar]
- 16. Darwish RT, Sobh ZK, Hamouda EH, Saleh EM. The efficacy of coenzyme Q10 and liquid paraffin oil in the management of acute aluminum phosphide poisoning. Toxicol Res. 2020:9(4):444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Talaab Y, Helmy M, Hassan AAE. Evaluation of the role of intravenous lipid emulsion as a putative treatment for acute aluminum phosphide poisoning. Mansoura J Forens Med Clin Toxicol. 2022:30(1):71–84. [Google Scholar]
- 18. Mostafa EMA, Tawfik AM, Abd-Elrahman KM. Egyptian perspectives on potential risk of paracetamol/acetaminophen-induced toxicities: lessons learnt during COVID-19 pandemic. Toxicol Rep. 2022:9:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mwaheb, Hassan S. Fatal aluminium phosphide poisoning in Fayoum governorate, Egypt (2012-2019). Egypt J Forensic Sci Appli Toxicol. 2021:21(2):47–58. [Google Scholar]
- 20. Etemadi-Aleagha A, Akhgari M, Iravani FS. Aluminum phosphide poisoning-related deaths in Tehran, Iran, 2006 to 2013. Medicine. 2015:94(38):e1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bagherian F, Kalani N, Rahmanian F, Abiri S, Hatami N, Foroughian M, Mehramiz NJ, Shahi B. Aluminum phosphide poisoning mortality rate in Iran; a systematic review and meta-analysis. Arch Acad Emerg Med. 2021:9(1):e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sonawane S, Peddawad R, Patil A, Manghani P. Trends and outcomes of acute poisoning cases in a tertiary care teaching hospital in Navi Mumbai. Indian J Forensic Med Toxicol. 2019:13(3):95–101. [Google Scholar]
- 23. Naddafi M, Mehrizi AA, Eghbal MA, Khansari MG, Azarmi Y, Sattari MR, Karaman C, Karimi F, Alizadeh M, Yazdani MN, et al. . Reducing the risk of death induced by aluminum phosphide poisoning: the new therapies. Chemosphere. 2022:294:133800. [DOI] [PubMed] [Google Scholar]
- 24. Sheta AA, El-Banna AS, Elmeguid RA, Mohamed HE, Gad NH. A study of the predictive factors of mortality in acute poisoning with aluminum phosphide with special reference to echocardiography and SOFA score. Environ Sci Pollut Res. 2019:26(32):33135–33145. [DOI] [PubMed] [Google Scholar]
- 25. Abdelbary A, Khaled M, Sami W, Said A, Yosri M, Abuelwafa M, Saad M, Tawfik H, Zoghbi I, Abouelgheit M, et al. . Initial Egyptian ECMO experience. Egypt J Crit Care Med. 2016:4(1):25–32. [Google Scholar]
- 26. Matos RI, Watson RS, Nadkarni VM, Huang H-H, Berg RA, Meaney PA, Carroll CL, Berens RJ, Praestgaard A, Weissfeld L, et al. . Duration of cardiopulmonary resuscitation and illness category impact survival and neurologic outcomes for in-hospital pediatric cardiac arrests. Circulation. 2013:127(4):442–451. [DOI] [PubMed] [Google Scholar]
- 27. Rubulotta F, Rubulotta G. Cardiopulmonary resuscitation and ethics. Rev Bras Ter Intensiva. 2013:25(4):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mentzelopoulos S, Haywood K, Cariou A, Mantzanas M, Bossaert L. Evolution of medical ethics in resuscitation and end of life. Trends Anaesth Crit. 2016:10:7–14. [Google Scholar]
- 29. Sobh Z, El-Banna S. COVID-19 pandemic: ethical challenges of healthcare and research. Zagazig J Forensic MedToxicol. 2021:19(1):1–10. [Google Scholar]
- 30. Elbastawesy S, Elmansy A. Comparison between gastric lavage with paraffin oil versus coconut oil in acute aluminum phosphide poisoning: a randomized controlled clinical trial. Ain Shams J Forensic Med Clin Toxicol. 2023:40(1):1–14. [Google Scholar]
- 31. Abdelhamid WG, Sakr ML, Mostafa OE, Zaafar D, Abdelwahab HM. Comparing the effectiveness of L-carnitine and paraffin oil in acute aluminum phosphide poisoning using predictive biomarkers and scores: a randomized controlled clinical trial. Hum Exp Toxicol. 2023:42:096032712211496. [DOI] [PubMed] [Google Scholar]
- 32. Marashi SM, Nasri-Nasrabadi Z. Can sodium bicarbonate really help in treating metabolic acidosis caused by aluminium phosphide poisoning? Arh Hig Rada Toksikol. 2015:66(1):83–84. [DOI] [PubMed] [Google Scholar]
- 33. Sobh Z, Abd-Elhameed A. The therapeutic benefit of antioxidants on the outcome of acute aluminum phosphide poisoning: a systemic review and meta-analysis. Toxicol Res. 2023. 10.1093/toxres/tfad035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tehrani H, Halvaie Z, Shadnia S, Soltaninejad K, Abdollahi M. Protective effects of N-acetylcysteine on aluminum phosphide-induced oxidative stress in acute human poisoning. Clin Toxicol. 2013:51(1):23–28. [DOI] [PubMed] [Google Scholar]
- 35. Agarwal A, Robo R, Jain N, Gutch M, Consil S, Kumar S. Oxidative stress determined through the levels of antioxidant enzymes and the effect of N-acetylcysteine in aluminum phosphide poisoning. Indian J Crit Care Med. 2014:18(10):666–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El-Ebiary A, Abuelfad A. N-acetylcysteine as an adjuvant in the treatment of acute aluminum phosphide poisoning: a randomized clinical trial. Ain-Shams J Forensic Med Clin Toxicol. 2017:28(1):38–46. [Google Scholar]
- 37. Proudfoot AT. Aluminium and zinc phosphide poisoning. Clin Toxicol. 2009:47(2):89–100. [DOI] [PubMed] [Google Scholar]
- 38. Sanaei-Zadeh H, Marashi SM. Gastric decontamination in aluminium phosphide poisoning: a case against the use of water-based solutions. Arh Hig Rada Toksikol. 2016:67(4):364–365. [DOI] [PubMed] [Google Scholar]
- 39. Hallaj S, Mohammadi AB, Ghorbani A, Ostadi A, Nahandi MZ. Case report: successful management of an aluminum phosphide poisoned patient following ventricular tachycardia. Int J Med Toxicol Forensic Med. 2020:10(4):30473. [Google Scholar]
- 40. Halvaei Z, Tehrani H, Soltaninejad K, Abdollahi M, Shadnia S. Vitamin E as a novel therapy in the treatment of acute aluminum phosphide poisoning. Turk J Med Sci. 2017:47(3):795–800. [DOI] [PubMed] [Google Scholar]
- 41. Abedi SH, Moazezi Z. A successful management of aluminum phosphide intoxication. Caspian J Intern Med. 2011:2(3):286–288. [PMC free article] [PubMed] [Google Scholar]
- 42. Mashali AA, Salama NH, Elsobky HA, Sobh ZK. Prediction of zinc phosphide-induced hepatotoxicity and cardiotoxicity from clinical, laboratory, and radiological indicators. Environ Sci Pollut Res Int. 2020:27(31):39547–39559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request from the corresponding author.


