Abstract
The population worldwide is getting older as a result of advances in public health, medicine, and technology. Older individuals are living longer with a higher prevalence of subclinical and clinical cardiovascular disease (CVD). In 2010, the American Heart Association introduced a list of key prevention targets, known as “Life’s Simple 7’’ to increase CVD-free survival, longevity, and quality of life. In 2022, sleep health was added to expand the recommendations to “Life’s Essential 8’’ (eat better, be more active, stop smoking, get adequate sleep, manage weight, manage cholesterol, manage blood pressure, and manage diabetes). These prevention targets are intended to apply regardless of chronologic age. During this same time, the understanding of aging biology and goals of care for older adults further enhanced the relevance of prevention across the range of functions. From a biological perspective, aging is a complex cellular process characterized by genomic instability, telomere attrition, loss of proteostasis, inflammation, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. These aging hallmarks are triggered by and enhanced by traditional CVD risk factors leading to geriatric syndromes (eg, frailty, sarcopenia, functional limitation, and cognitive impairment) which complicate efforts toward prevention. Therefore, we review Life’s Essential 8 through the lens of aging biology, geroscience, and geriatric precepts to guide clinicians taking care of older adults.
Key words: cardiovascular disease prevention, cardiovascular health, geriatric cardiology, geroscience, Life’s Essential 8
Central Illustration
Highlights
-
•
Biological aging is driven by a complex molecular and cellular process characterized by key aging hallmarks that are often accelerated by traditional CVD risk factors leading to development of not only CVD but also various geriatric syndromes that possess unique challenge.
-
•
Optimization of Life Essential 8 components impact aging process at multiple molecular and cellular levels and contribute to healthy aging, increased lifespan, and health span.
-
•
Future studies of gero-therapeutics may identify interventions that can improve cardiovascular health, as well as healthy aging and longevity.
Chronological age is the single most important risk factor for the development of chronic diseases, including cardiovascular disease (CVD).1 In the last few decades, population worldwide has seen unprecedented growth, with resultant increase in the prevalence of clinical and subclinical CVD.1, 2, 3, 4 In 2010, the American Heart Association (AHA) introduced the concept of “Life’s Simple 7’’ to provide key prevention targets for improved cardiovascular (CV) health: physical activity, dietary quality, smoking, weight, blood glucose, cholesterol, and blood pressure (BP).5 In 2022, it was expanded to “Life’s Essential 8” with the addition of sleep health.6 Implementation of Life’s Essential 8 in older adults has unique challenges due to heterogeneity of biological aging, multimorbidity, and coexisting geriatric syndromes such as frailty, sarcopenia, functional limitation, and cognitive impairment. In addition, despite optimization of these essentials, there is also interest in identification of strategies informed by geroscience.
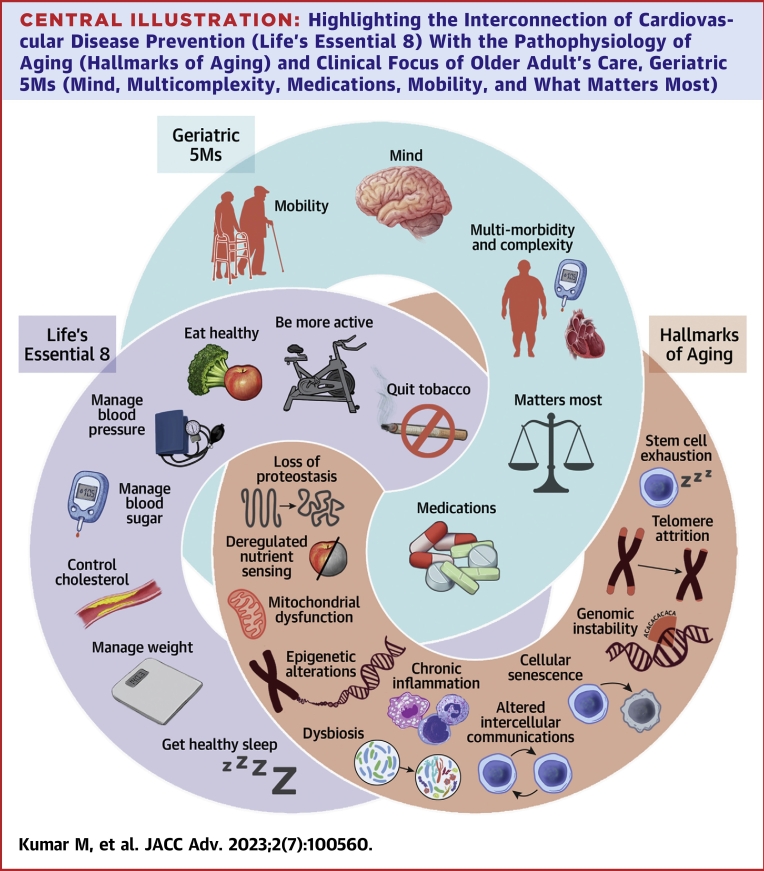
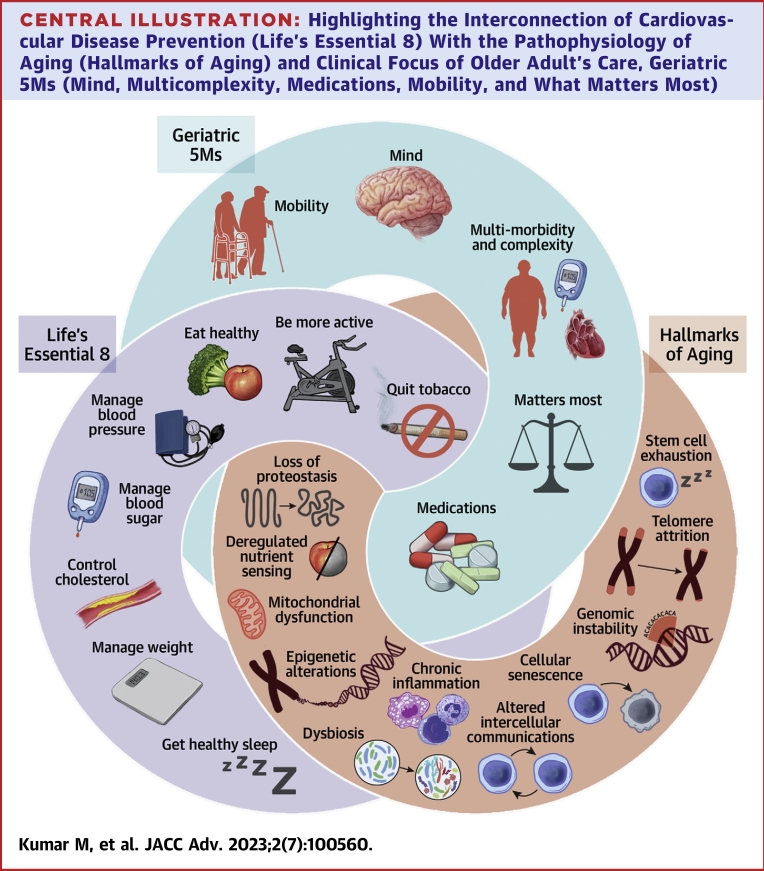
Aging is a multifactorial, complex, biologically malleable processes that entails key molecular signals, identified as “aging hallmarks”: genomic instability, telomere attrition, loss of proteostasis, inflammation, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, systemic inflammation, gut dysbiosis, and altered intercellular communication.7 These aging hallmarks collectively contribute to each individual’s ‘aging phenotype’ with associated decline in the ability of physiological systems to respond to stressors.8 As a consequence, these pathways contribute to geriatric syndromes and chronic conditions including CVD (Central Illustration).9,10 Therefore, prevention of CVD can be considered foundational for healthy aging. Recently, geroscience insights suggest ways to slow decline and delay development of chronic diseases from its physiologic foundations.8
Central Illustration.
Highlighting the Interconnection of Cardiovascular Disease Prevention (Life’s Essential 8) With the Pathophysiology of Aging (Hallmarks of Aging) and Clinical Focus of Older Adult's Care, Geriatric 5Ms (Mind, Multicomplexity, Medications, Mobility, and What Matters Most)
Often improving one node will lead to improvements in another and vice versa.
We review AHA’s “Life’s Essential 8’’ through the lens of aging physiology, geroscience, and unique challenges older adults face. Moreover, we consider prevention as it applies across the geriatric framework referred to as ‘Geriatric 5Ms.’ Geriatric 5Ms include: 1) mind; 2) mobility; 3) medications; 4) multicomplexity; and 5) what matters most.10
Be more active (exercise)
Staying active has health benefits irrespective of age, exercise capacity, or functional status, yet, older adults, especially women, are most likely to be sedentary.11 The prevalence of inactivity increases from 26.9% in those aged 65 to 74 years to 35.3% in those ≥75 years.12 On average, older adults spend 9 hours or more being sedentary, accounting for 65 to 80% of waking hours per day.13 Physical inactivity has detrimental consequences in older adults beyond the CV system. It exacerbates sarcopenia, frailty, metabolic syndrome leading to loss of independence, and poor quality of life (QOL).14, 15, 16 Furthermore, sedentary behavior accelerates the hallmarks of aging in muscle cells.17 Although exercise is important for health, restoration of age-appropriate physiology may not occur even after resuming a physically active routine.16
Physical activity considerations for older adults
Multiple factors contribute to physical inactivity in older adults. Aging reduces exercise capacity and capability due to age-related changes in multiple organ systems.18 In addition, multimorbidity, cognitive impairment, and polypharmacy worsen physical function and vice versa.19,20
Benefits of physical activity
Exercise reduces CVD and related mortality through improvement of risk factors by reducing systemic inflammation, improve cardiorespiratory fitness, and mitochondrial function.21,22 More fundamentally, aerobic and resistance exercise attenuates biological aging and pathological reduction in physical fitness.17,23,24 As a result, exercise improves functional status, independence, and QOL while decreasing multimorbidity, and polypharmacy.21,25,26 Moreover, use of senolytics, drugs with the ability to clear senescent cells, mimic some of the properties of exercise and may therefore also reduce musculoskeletal decline associated with aging.27,28
Recommendations for physical activity in older adults
“Life’s Essential 8’’ recommends 150 minutes of moderate or 75 minutes of vigorous physical activity per week regardless of age, sex, or race.5 However, achieving these goals may not always be feasible or safe. Careful consideration of individual characteristics such as preferences, functional status, cognitive limitation, exercise capacity, and fall risk can determine best type and duration of exercise (Table 1).
Table 1.
Highlights of Each Life's Essential 8 Component Pertaining to the Care of Older Adults
| Eat better |
|
| Be more active |
|
| Get more sleep |
|
| Quit tobacco |
|
| Manage weight |
|
| Control cholesterol |
|
| Manage blood sugar |
|
| Manage blood pressure |
|
CVD = cardiovascular disease; DASH = Dietary Approaches to Stop Hypertension; SGLT-2 = sodium-glucose cotransporter-2.
The benefits of physical activity are driven by exercise intensity.22,29 However, the most important goal for older adults is to avoid inactivity. The greatest benefit is seen in those who go from a sedentary lifestyle to any activity, that is, walking for 5 minutes several times a day.29,30 In order to perform aerobic activity safely, other modes of exercises focusing on strength, balance, and mobility are often required.31,32 Physical therapists, exercise physiologists, and nurses play an important role in implementation of progressive exercise. For those with mobility limitations, seated range of motion exercise, controlled breathing, stretching, and yoga can be safe and helpful.33,34 Strength (eg, stretch bands), balance (eg, chair stands, standing on 1 foot,), and flexibility training improves gait, speed/power of movement, and reduce risk of falls by improving muscle mass and strength, balance, and bone strength.35, 36, 37 These are usually initiated at low intensity for short durations (ie, 5-10 minutes) and then advanced in length and then intensity as tolerated.
Get healthy sleep
Healthy sleep is important for maintaining health at all ages. Sleep has distinct dimensions: timing, continuity or efficiency, duration, and satisfaction/quality.38 Sleep architecture undergoes several changes with aging. Sleep in later years is characterized by increased latency, reduced efficiency, fragmentation, awakening, phase advancement, and periodic limb movements.39, 40, 41
Sleep duration between 7 and 9 hours is associated with reduced risk of coronary artery disease (CAD), stroke, CVD, and all-cause mortality.42,43 Poor sleep quality is associated with CV risk factors such as metabolic syndrome, frailty, functional impairment, falls, cognitive decline, depression and poor QOL, diet quality and physical inactivity.44, 45, 46, 47, 48, 49 The effects of poor sleep may be mediated through impaired autonomic tone, endothelial dysfunction, inflammation, altered systemic/cardiac hemodynamics, and pro-coagulation milieu.50,51
Sleep considerations for older adults
Normal age-related changes in sleep patterns predispose to sleeping disturbances that impact more than 50% of older adults.39,52 Social identities and statuses, sociocultural factors, and physical/built environment factors that intersect with age also affect sleep health.53 Sleep health can additionally be compromised by precipitating factors such as primary sleep disorders (eg, sleep apnea syndromes, insomnia), medical illnesses, medications, alcohol use, psychosocial (eg, bereavement, caregiving roles, social isolation, lifestyle transitions), and physical stressors.45,54
Senescent cell accumulation seen with aging, obesity, and comorbidities contribute to changes in circadian rhythm, resulting in impaired cellular homeostasis.55,56 Declines in sleep quantity and quality accelerate biological aging.55,57 Therefore, efforts to restore and maintain sleep quality and age-appropriate duration can have important effects on the health, function, and QOL.43,45
Recommendations to promote sleep health in older adults
Life’s Essential 8 recommends 7 to 9 hours of sleep.5,6 Similar to younger adults, recommendations for sleep health-promoting behaviors include: avoid substances (eg, limiting caffeine after lunch time, avoid nicotine and alcohol within 3-4 hours of bedtime), avoid evening fluids or diuretics, engage in regular physical activity, manage stress, make environmental changes (eg, dark bedroom at cool temperature), regularize sleep-wake timing, and avoid naps or be intentional about their timing and duration.58 In older adults, review of medications can identify those that can affect sleep quality and/or architecture (eg, central nervous system stimulants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, lipophilic beta-adrenergic blockers, centrally acting alpha adrenergic agonists, and glucocorticoids).59
Healthy sleep habits are often not enough treatment.60 Cognitive behavioral therapy is the first-line of treatment for insomnia at all ages.60,61 Other modalities such as bright light therapy, acupuncture, and mindfulness-based stress reduction techniques may be helpful.62, 63, 64 Behavioral interventions carry fewer risks of adverse effects than pharmacological interventions. In older adults, pharmacological interventions should be avoided. Specifically, benzodiazepines and “Z-drugs” such as zolpidem should be avoided due to the high risk of falls, fracture, and dementia.65 There has been increasing use of melatonin to address sleep difficulties in older adults, however evidence is limited.66 (Table 1) There is early interest in the role of senolytics targeting cellular senescence to improve circadian rhythm and prevent age-related decline in sleep duration and quality.56
Eat better (nutrition)
The healthy eating index of older adults ranges from 47.7 to 65.8 (out of 100), reflecting suboptimal diet.67,68 Adherence to a healthy eating pattern is associated with reduced CVD and mortality, as well as improving frailty, sarcopenia, polypharmacy, mobility limitations, cognitive, and mental health.69, 70, 71, 72, 73
In older adults, poor dietary quality and malnutrition are key issues. Malnutrition, defined as a deficiency of energy and nutrients, affects 6% of older adults in the community and 50% in rehabilitation facilities.74,75 Aging increases the risk for malnutrition through complex factors including loss of appetite, impaired smell and taste, impaired dentition, difficulty swallowing, loss of mobility necessary to acquire and prepare healthy food, cognitive decline, social isolation, depression, financial food insecurity, and diminished nutrition absorption.76,77 Therefore, dietary interventions in older adults range from emphasis on dietary quality to prioritizing adequate protein-energy intake.
Recommendations to improve dietary quality for older adults
A healthy balanced diet consists of whole grains, fruits and vegetables, lean protein, nuts, seeds, and cooking in nontropical oils such as olive oil.5 Various diets have beneficial impacts on life span. Some have focused on the content, such as Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) diet, plant-based diet, Ketogenic diet, Baltic Sea diet, Nordic diet, and others on the timing of food intake, such as intermittent fasting.78 Diets rich in fibers and antioxidants have been associated with more favorable biological aging in the form of telomere shortening, DNA responses to oxidative damage, DNA methylation patterns, mitochondrial function, stem cell survival, and inflammation.78,79 While more intervention studies are needed, greater adherence to any healthy eating pattern over long term is required to lower the risk of total and cause-specific mortality.80,81
Improved dietary quality may be achieved by counseling on beneficial dietary patterns. The Mediterranean and DASH diets have the most robust evidence for prevention of CVD, cancer, and diabetes mellitus (DM).82, 83, 84 Both of these diets are very similar, with the exception of emphasis on reduction of sodium, sugar, and saturated fats in DASH diet.85 Certain vegetarian eating patterns such as lacto-ovo vegetarian and pescatarian diets, which both exclude meat and poultry, also positively impact CV health.86
Supplements
Older adults are at risk for micronutrient deficiencies, however, routine multivitamin supplementation for CVD prevention is not recommended.87,88 Similarly, over the counter, N-3 polyunsaturated fatty acid (omega-3 fatty acid) supplementation, is not effective in CVD prevention.89,90 However, a prescription-only omega-3 fatty acids (eicosapentaenoic acid ethyl ester) reduces CV events in individuals with established CVD and elevated triglyceride levels.91 Finally, despite the popularity of vitamin D supplementation, it has not been shown to reduce risk of bone fractures, falls, frailty, or CV events.92, 93, 94
Quit tobacco (smoking cessation)
Nicotine use is lowest among people aged 65 years and older (11.8%) as compared to the individuals aged 25 to 44 years (22.9%) and 45 to 64 years (20.4%), nevertheless, remains a cause of excess mortality and morbidity at advancing age.95,96 The effects of smoking on the CV system are mediated via promotion of atherogenesis through a complex interplay of inflammation, oxidation of lipids, pro-thrombosis, insulin resistance, and increased release of catecholamines.96
Smoking considerations for older adults
Smoking affects older adults disproportionately because of the longer duration of cumulative injury, leading to higher associated rates of CVD, pulmonary disease, and cancers.97,98 Smoking accelerates the aging process through free radical damage, shortening of telomere length, development of concurrent pathologies, and therefore, contributes to age-related syndromes such as frailty, functional impairment, cognitive decline, and poor QOL.98,99
At all ages, quitting tobacco dramatically reduces CVD risk, cognitive decline, pulmonary disease, and cancer.100,101 The magnitude of reduction of smoking-related morbidity and mortality directly relates to duration and amount of tobacco use.97,102 The CV benefits of smoking cessation begin within 20 minutes.103 The risk of myocardial infarction decreases within 24 hours, and excess risk of CAD is half that of a smoker at 1 year. At 5 years, stroke risk decreases to same as nonsmokers and risk of CAD becomes same as nonsmokers at 15 years.103
Quitting tobacco can be challenging for older adults because it has been a part of their lifestyle for decades. Recognition of the immediate benefits of smoking cessation, dangers of smoking, including fire risk, should be emphasized. For those with cognitive impairment, involvement of caregivers is key to successful smoking cessation. Other barriers include previous failed attempts, lack of awareness, lack of resources, and support.104 Short-term declines in motor abilities and cognition following cessation may impair QOL and contribute to continued tobacco dependence.105 As a result, older adults are half as likely to quit compared to younger adults, however, remain abstinent at similar rates following cessation.106
Recommendations for smoking cessation in older adults
Effective interventions include counseling by a physician, nurse, pharmacists or cessation specialist, (multiple sessions better than a single session), group behavioral interventions, and telephone counseling.107 Cognitive behavioral therapy is successful in cessation and maintenance of abstinence in older adults and can be used in conjunction with pharmacotherapy108 (Table 1).
Nicotine replacement therapy has the best data in older adults, with cessation rates ranging from 10% to 25%.109 Nicotine is available in multiple modalities (ie, transdermal patches, gums, lozenges, sublingual tablets, inhalers, and nasal sprays). Caution should be taken for patients with dysphagia and aspiration risk as gums and lozenges may not be tolerated.
Bupropion is equally efficacious and can be used alone or in combination with nicotine replacement therapy. However, there are insufficient data for smoking cessation in older adults and should be used with caution, especially with impaired renal or liver function, due to the risk of side effects and mild anticholinergic properties.65,109 Varenicline is a partial agonist of alpha4-beta2 neuronal nicotinic acetylcholine receptor. It is safe, well-tolerated, and most effective smoking cessation pharmacotherapy in older adults.98,107 The most common adverse effects include nausea, sleep disturbances, and abnormal dreams.110
Manage weight (obesity)
Nearly 40% of men and women aged >60 years are obese and the prevalence, especially in women, continues to rise.111 Older adults are particularly susceptible to sarcopenic obesity wherein fat mass increases with concomitant reduction in muscle mass and strength.112 Sarcopenic obesity affects 12 to 48% of older adults.113 Although epidemiologic studies suggest obesity may be protective in older adults, this “obesity paradox” may be misleading. Worse outcomes with lower body weight may reflect confounding from smoking, diseases causing weight loss (reverse causation, eg, cancer), and varying periods of follow-up.114 Obesity is known to accelerate biological aging including telomere shortening and an altered epigenetic landscape typical of age-related dysfunction.115,116
Weight considerations for older adults
Aging is associated with changes in body composition. Fat mass increases with the accumulation of visceral and intermuscular adipose tissue, while fat-free mass decreases.117,118 This ectopic fat is associated with elevated inflammatory molecules such as IL-6 and TNF-α and contributes to inflammation of aging and insulin resistance.119,120 The most important contributor to the accumulation of body fat is decrease in major components of total energy expenditure (resting metabolic rate, thermal effect of food, physical activity). Approximately 50% of the reduction is due to physical inactivity.118 Age-related hormonal changes such as decreased growth hormone and testosterone secretion, reduced responsiveness to thyroid hormone, and resistance to leptin with decreased ability to down-regulate appetite also account for shifts in proportion of fat and fat-free mass.118,121
Recommendations for managing weight in older adults
Life’s Essential 8 recommends maintenance of a healthy body weight with a body mass index <25 kg/m2.5 Weight loss in individuals with obesity, regardless of age, sex, or race improves physical function and QOL.118 Lifestyle interventions are effective at all ages.122 A realistic weight loss goal for older adults is a 5 to 10% reduction in body weight. The combination of an energy-deficit diet (energy deficit ∼500-750 kcal/day), rich in high-quality protein (1 g/kg/day) with increased physical activity results in moderate weight loss.118,123 Interventions aimed at weight loss should consider worsening body composition from baseline and focus on loss of adiposity with maintenance or increase of bone and muscle.124 (Table 1) Tailored programs that account for biologic sex (eg, greater protein needs for men) should be considered. A specific behavioral strategy includes self-monitoring, goal setting, social support, and stimulus control.125 The program should be nutritionally adequate and applicable to ethnic and cultural background, and physical and cognitive capabilities of the individual.
In preclinical models, energetic restriction has demonstrated reduced risk for all-cause mortality and CVD due to favorable effects on aging-related molecular mechanisms.126,127 Intermittent fasting is a popular approach to lose weight by reduction in calorie intake.128,129 Safety and CV outcomes of intermittent fasting have not been evaluated in older adults, and it may have a negative effect on lean mass retention.129,130
Clinical trials studying pharmacotherapy in obesity treatment have enrolled few older adults (2%-8.8%).131, 132, 133, 134 There is insufficient evidence to determine efficacy and safety of weight loss medications in this population. Weight loss medications, in addition, may have detrimental adverse effects and add to the burden of polypharmacy. When selecting a weight management medication, several factors must be considered including each drug’s efficacy, side effects, cautions, warnings, patient’s comorbidities, and should be a shared decision with a comprehensive lifestyle intervention. Bariatric surgery is the most effective intervention especially in patients with severe obesity, however, evidence concerning its efficacy and safety in older adults is limited.135 Preclinical data suggest that senolytics may alleviate the development of obesity and metabolic syndrome by promoting favorable deposition of fat to subcutaneous region rather than visceral deposition, thereby increasing insulin sensitivity and reduction of metabolic syndrome in mice.136
Control cholesterol (hyperlipidemia)
The role of lipids, in particular, low-density lipoprotein cholesterol (LDL-C), in the development of atherosclerosis is well-established.137 However, there is conflicting evidence for the association of LDL-C with CVD in older adults.138,139
Cholesterol physiology considerations for older adults
With aging, lipid metabolism becomes dysregulated via complex multifaceted mechanisms.140 Total cholesterol and LDL-C levels initially increase with age until the early 50s followed by a plateau or even decrease in subsequent years.141, 142, 143 Significant sex differences in plasma lipid levels are observed with aging, with decreased estrogen levels in postmenopausal women leading to increased triglyceride levels compared with men.144,145 Changes in body composition associated with aging, insulin resistance, decreased circulating levels of growth hormone contribute to changes in lipid profiles over time.142,146 Lower cholesterol levels in older adults may also reflect survivorship bias: individuals with lower cholesterol levels live longer. Moreover, low LDL-C may also be confounded by a catabolic condition such as cancer, renal disease, or dementia.
Recommendations for controlling cholesterol in older adults
Most recently, 2018 American College of Cardiology/AHA guideline recommended statin use for primary prevention in patients with LDL-C ≥190 mg/dL, DM, or a 10-year atherosclerotic CVD risk ≥7.5% with risk enhancers.147 Atherosclerotic CVD risk is validated for age 20 to 79 years.147 On the other hand, cardiovascular risk score-3 risk calculator is valid up to the age of 85 years.148
Coronary artery calcium score is a valuable prognostic tool in older adults. The negative predictive value of zero coronary artery calcium score for predicting CAD events and mortality increases with age and can be used as an impactful prognostic marker for de-risking at older ages.149
Adherence to a healthy lifestyle aids in cholesterol lowering. Statins are important for secondary prevention, and to reduce first CVD event at least to age 75 years.150, 151, 152 Observational data suggest lower risk for CV events for primary prevention over age 75 years with statin.152,153 Few trials have included individuals aged 75 and older. The PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) trial, the first dedicated trial to older individuals (70-82 years), demonstrated an overall improvement in CVD outcomes with pravastatin treatment in a mixed primary and secondary prevention population.154 Subsequent analysis of primary prevention data from Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin and Heart Outcomes Prevention Evaluation-3 demonstrated a significant 26% relative reduction in CVD events and death among individuals aged ≥70 years.155 Moreover, meta-analysis of 28 major statin trials that included over 186,000 participants (both primary and secondary prevention) and found a 12% reduction in vascular mortality per 1.0 mmol/L reduction in LDL-C, though benefits were attenuated in those over age 75 without pre-existing CVD.151 Two ongoing randomized controlled trials, the STAREE (Statin Therapy for Reducing Events in the Elderly; NCT02099123) trial and the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults; NCT04262206) trial will address the role of statins for primary prevention in those aged 75 years and older. In addition to lowering cholesterol, statins may have antiaging properties through modulation of telomerase activity.156 That being said, in older adults with life-limiting illnesses and polypharmacy concerns, the potential net benefits of statin treatment for primary prevention are likely attenuated.
The utility of non-statin drugs, ezetimibe added to simvastatin, or PCSK9 inhibitor, extend to older adults as well.157,158 Novel therapeutics such as inclisiran and bempedoic acid offer additional treatment options, though evidence in older adults is limited.159,160
Statins are safe and well tolerated even at advanced ages.151,161 Less serious muscle symptoms are commonly observed with statin treatment >90% of which can be attributable to the so-called “nocebo effect.”162 Nevertheless, it is important to consider how such ‘aches and pains’ may impact an older adult with mobility limitations and careful follow-up for side effects, considering dose adjustment, or rotating to another statin if these should occur.
Manage blood sugar (DM)
Life’s Essential 8 recommends controlling blood sugar for optimum CV health, which is relevant to older adults as well.5,163 About 50% and 30% of older adults >65 years have prediabetes and diabetes, respectively.164 Older adults with diabetes are more likely to be Blacks and Hispanics, and as a result, have increased prevalence of CVD.165, 166, 167, 168 In addition, DM increases the risk of geriatric syndromes such as urinary incontinence, falls, sarcopenia, frailty, chronic pain, dementia, and polypharmacy.167 Furthermore, older adults have the highest rates of hospitalization due to hyperglycemia and hypoglycemia, visual impairment, and diabetes-related renal disease.163,165,169
Blood sugar considerations for older adults
Aging is associated with dysregulated glucose metabolism manifesting as elevated fasting and postprandial glycemic levels irrespective of presence or absence of diabetes.170,171 Body composition changes throughout life, and as mentioned above, is characterized by increased ectopic fat deposition. Accumulation of visceral fat, especially intra-abdominal, is the major driver of insulin resistance through increased pro-inflammatory cytokines.119,170 In addition, age-related impaired insulin secretion, reduced insulin sensitivity, and promotion of pancreatic B-cell death contribute to the development of DM.170
Recommendations for managing blood sugar in older adults
Glycemic control in older adults brings unique challenges due to comorbidities, cognitive, and functional heterogeneity. Studies have demonstrated increased risk of CV events, frailty, disability, cognitive impairment, and mortality with intensive glycemic control.166 Moreover, tight glycemic control contributes to falls and fractures. Therefore, the goal for diabetes treatment in older adults is a simplified regimen that avoids hypoglycemia and hyperglycemia, and involves caregivers (5Ms medications) with a focus on health status and life expectancy.163 Current guidelines from American Diabetes Association recommend an HbA1c of <7.5% in older adults with few chronic illness and intact cognitive and physical function who do not have a life-limiting illness.172 Higher HbA1c goals may be most relevant to nursing home populations and those with very limited life expectancy (Table 1).
Advances in pharmacotherapeutics with improved CV outcomes have reinvigorated the management of DM. With these newer agents, lower HbA1C levels can be targeted in healthy older adults without comorbidities.173 Metformin is the first-line agent for treatment but is contraindicated in renal dysfunction (estimated glomerular filtration rate <30 mL/min/1.73 m2). Insulin and insulin secretagogues such as sulfonylureas should be used with caution due to the risk of hypoglycemia. Oral dipeptidyl peptidase 4 inhibitors are safe with low risk of hypoglycemia, though are costly and do not impact CVD outcomes. Glucagon-like peptide-1 receptor agonists have demonstrated CV benefits in patients with diabetes and established CVD.174 These drugs are injectable and require adequate visual, motor, and cognitive skills for proper use. Sodium-glucose transporter-2 inhibitors are oral drugs that demonstrated reduction in CVD in patients with and without diabetes.175
Senolytics have shown promise in reducing senescent cell burden, macrophages, and crown-like structures in adipose tissues in diabetics with kidney disease, suggesting that onset or progression of diabetes might be delayed with these drugs, though large-scale studies are needed.176 On the other hand, existing antidiabetic drugs, metformin, and sodium-glucose cotransporter-2 inhibitors have shown antiaging properties by attenuating multiple aspects of biological aging raising promise for their use as gero-therapeutics.177,178
Manage blood pressure (hypertension)
Hypertension is highly prevalent in older adults, affecting nearly 80% of older adults ≥75 years, with highest prevalence in men and non-Hispanic Blacks.2 Isolated systolic hypertension, defined as elevated systolic pressure with normal or reduced diastolic pressure is the predominant form of hypertension seen in older adults.179
Blood pressure considerations for older adults
Aging is characterized by generalized endothelial dysfunction and arterial stiffening which occurs due to a loss of elastin, increase in collagen and calcification.180,181 These changes are accentuated by age-induced chronic low-grade inflammation, irreversible mitochondrial oxidative stress from accumulation of reactive oxygen species, and metabolic syndrome.179,182 Arterial stiffness, especially of large vessels, causes diminished baroreflex sensitivity leading to neurohormonal dysregulation and sympathetic activation.183 Loss of distensibility of major central vessels and increased vascular resistance cause augmented reflected waves and elevated systolic pressure. Additionally, reduced arterial reservoir capacity and altered blood flow dynamics lead to low diastolic pressure and elevated pulse pressure.179,181
Age-related increase in BP also arises from decline in renal function, increased salt sensitivity, and upregulation of ENaC channels, reduced nitric oxide bioavailability, increased endothelin-1, and reduced levels of aldosterone and renin.184, 185, 186 Environmental and lifestyle factors including low physical activity, poor diet, high salt intake, and weight gain further contribute to elevated BP. Common comorbidities, such as obstructive sleep apnea, renal dysfunction, and thyroid disorders, may present as secondary causes of hypertension and increase the likelihood of treatment resistant hypertension.187
Hypertension affects brain structure leading to cognitive impairment, neurogenerative, and mood disorders. This in turn sets up a vicious cycle of poor insight, low treatment adherence, increased risk of polypharmacy, worsening cognition, functional status, and mortality.188,189 Hypertension control in older adults reduces CVD risk and associated mortality and has beneficial impact on delaying the onset and progression of cognitive impairment.190,191 Aging hallmarks are not well studied in the context of hypertension, and a few initial studies have demonstrated increased biomarkers of accelerated aging in these patients.192 Senolytics, such as navitoclax, dasatinib, and quercetin have demonstrated some efficacy in reducing myocardial senescent cells, vascular calcification, and interstitial fibrosis that may have a role in prevention of development of hypertension in future in humans.193,194
Recommendations for managing blood pressure in older adults
Lifestyle modification should be encouraged and emphasized to prevent development and aid in BP management at every visit. Several trials (SHEP [Systolic Hypertension in the Elderly Program], HYET [Hypertension in Very Elderly Trial], STEP [Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients], SPRINT [Systolic Blood Pressure Intervention Trial]) have studied hypertension management in older adults with targets as low as systolic BP <120. Overall, these trials demonstrated benefit for CV events, mortality, and dementia.190,195 Yet results remain inconclusive among those with advanced multimorbidity, frailty, cognitive decline, or institutionalization as these individuals were not included in the trials. Nevertheless, the 2017 American College of Cardiology/AHA hypertension guidelines recommend a target systolic BP of <130/80 mm Hg for most adults aged 65 years and older.196 On the other hand, the 2018 European Society of Cardiology/European Society of Hypertension suggest a higher systolic BP goal of 130 to 139 mm Hg and diastolic BP of 70 to 79 mm Hg in adults between ages 65 and 80 years.197
For patients with preserved functional status, BP targets in older adults should be similar to those in younger adults. For those who have significant cognitive and/or functional decline, frailty and limited life expectancy, a BP target of systolic BP 130 to 150 mm Hg may be reasonable if lower BP causes hypotension or other adverse reactions. The pharmacologic maxim of “start low, go slow, but get there” should be followed. Intensive BP lowering is most appropriate for older adults with high CV risk burden and life expectancy of 3 or more years as those with limited life expectancy may not live long enough to derive benefit102,198 (Table 1).
There are multiple first-line agents for BP treatment: thiazide diuretics, angiotensin-converting enzyme inhibitor, angiotensin II receptor blockers, and calcium-channel blocker. Thiazides have the highest risk of adverse events such as falls, acute renal injury, and should be used with caution.199 Calcium channel blocker and angiotensin-converting enzyme inhibitor/angiotensin II receptor blockers may be beneficial in frail older patients, with the latter having a role in increasing lower limb muscle mass.200, 201, 202 Alpha blockers, such as those commonly used for benign prostatic hyperplasia in men, and diuretics can induce or worsen orthostatic hypotension, increase risk of falls, and hip fracture.65,203 Other drugs to be used with caution are clonidine, methyldopa, nifedipine, and reserpine.65
Social determinants of health in older adults
In addition to Life’s Essential 8 components, potentially modifiable social determinants of health also play an important role in preservation of CV health. Negative psychological conditions such as depression, anxiety, stress, social isolation and loneliness, pessimism, and so on adversely impact CV health, whereas positive psychological well-being improves CVH and associated mortality.204 Poor mental health in older adults may cause medication non adherence, substance misuse, inequalities in access to health care, behavioral changes such as lack of motivation for exercise, and increased risk of medication adverse effects that may impact CVH directly or indirectly. Therefore, to improve CVH, a comprehensive and holistic approach addressing social determinants is necessary.205 Further studies exploring the impact of social determinants of health and potential intervention specifically in older adults are needed.
Conclusions
The risk of developing CVD varies considerably between individuals of the same chronological age. There is growing evidence indicating that variability in biological aging and reduced efficiency of homeostatic mechanisms that oppose aging may contribute to this clinical heterogeneity. A comprehensive, holistic, and individualized approach is required to treat the whole person with consideration of Geriatric 5Ms to ensure the extension of not only lifespan but also health span. Future studies of gero-therapeutics may identify interventions that can improve CV health, as well as healthy aging and longevity.
Funding support and author disclosures
Dr Orkaby is supported by VA CSR&D CDA-2 award IK2-CX001800 and NIA GEMSSTAR R03-AG060169; and has received consulting fees from Anthos Therapeutics. Dr Goyal is supported by the American Heart Association grant 20CDA35310455 and National Institute on Aging grant K76AG064428; and has received consulting fees from Sensorum Health. Dr Rich has received support from the NIH (R01 AG060499, R01 AG078153, R01 HL147862, R01 HL151431). Dr Tighe is supported by Career Development/Capacity Building Award Number IK2 RX003393 from the U.S. Department of Veterans Affairs Rehabilitation R&D (Rehab RD) Service; resources and the use of facilities at the Veterans Integrated Service Network 4 Mental Illness Research, Education, and Clinical Center at the VA Pittsburgh Healthcare System, Pittsburgh, Pennsylvania; and is PI of Clinical Trial NCT04506112 and has grant funding through the Veterans Health Foundation, Pittsburgh, PA. Dr Billingsley is supported by the National Institute of Aging NIH/NIA T32AG062403. Dr Villareal is supported by the National Institutes of Health (RO1-AG031176, RO1-DK109950) and U.S. Department of Veterans Affairs (CX000906, CX002161). The contents do not represent the views of the U.S. Government or the U.S. Department of Veterans Affairs. Dr Nanna has received research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), and the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award). Dr Kuchel is supported by NIA P30 AG067988; R33AG061456; R25AG073119; U54AG075941 and the Travelers Chair in Geriatrics and Gerontology. Dr Hummel is supported by support from the American Heart Association 20-SFRN35370008, National Institutes of Health, NIH/NIA R01-AG062582, R01-AG078153, NIH/NHLBI R61-HL155498 and Veterans Affairs RDC-2017-1066. Dr Forman is funded by NIA R01 AG060499, R01 AG05883, 1U19AG065188, P30AG024827 and VA HSR&D Merit 1I01 HX003518, VHA RR&D SPIRE 1I21RX004409. Dr Alexander is funded by NIA 1U19AG065188. Views expressed in this article are those of the authors and do not necessarily represent the position or policy of the Department of Veterans Affairs or the U.S. Government. This work is not subject to U.S. copyright as several of the authors of this manuscript are employees of the U.S. Government. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Yazdanyar A., Newman A.B. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med. 2009;25:563–577. doi: 10.1016/j.cger.2009.07.007. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart Disease and Stroke Statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease control and Prevention. Life stages and Populations. Older Person's Health. 2019. https://www.cdc.gov/nchs/fastats/older-american-health.htm
- 4.Ageing and Health. World Health Organization; 2022. [Google Scholar]
- 5.Life's Essential 8™. Your Checklist for Lifelong Good Health. World Health Organization; 2022. [Google Scholar]
- 6.Lloyd-Jones D.M., Allen N.B., Anderson C.A.M., et al. Life's Essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy B.K., Berger S.L., Brunet A., et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marengoni A., Angleman S., Melis R., et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Goyal P., Kwak M.J., Malouf C.A., et al. Geriatric cardiology: coming of age. JACC: Adv. 2022;1 doi: 10.1016/j.jacadv.2022.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W., Procter-Gray E., Churchill L., et al. Gender and age differences in levels, types and locations of physical activity among older adults living in car-dependent neighborhoods. J Frailty Aging. 2017;6:129–135. doi: 10.14283/jfa.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watson K.B., Carlson S.A., Gunn J.P., et al. Physical inactivity among adults aged 50 years and older - United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954–958. doi: 10.15585/mmwr.mm6536a3. [DOI] [PubMed] [Google Scholar]
- 13.Harvey J.A., Chastin S.F., Skelton D.A. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471–487. doi: 10.1123/japa.2014-0164. [DOI] [PubMed] [Google Scholar]
- 14.Bowden Davies K.A., Pickles S., Sprung V.S., et al. Reduced physical activity in young and older adults: metabolic and musculoskeletal implications. Ther Adv Endocrinol Metab. 2019;10 doi: 10.1177/2042018819888824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham C., O'Sullivan R., Caserotti P., Tully M.A. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports. 2020;30:816–827. doi: 10.1111/sms.13616. [DOI] [PubMed] [Google Scholar]
- 16.Verlaan S., Aspray T.J., Bauer J.M., et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: a case-control study. Clin Nutr. 2017;36:267–274. doi: 10.1016/j.clnu.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Raffin J., de Souto Barreto P., Le Traon A.P., Vellas B., Aubertin-Leheudre M., Rolland Y. Sedentary behavior and the biological hallmarks of aging. Ageing Res Rev. 2023;83 doi: 10.1016/j.arr.2022.101807. [DOI] [PubMed] [Google Scholar]
- 18.Shilpa A., Kalyani S., Manisha S. In: Gerontology. Grazia D.O., Antonio G., Daniele S., editors. IntechOpen; 2018. Ageing process and physiological changes. Ch. 1. [Google Scholar]
- 19.Ryan A., Murphy C., Boland F., Galvin R., Smith S.M. What is the impact of physical activity and physical function on the development of multimorbidity in older adults over time? A population-based cohort study. J Gerontol A Biol Sci Med Sci. 2018;73:1538–1544. doi: 10.1093/gerona/glx251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsimpris A., Linseisen J., Meisinger C., Volaklis K. The association between polypharmacy and physical function in older adults: a systematic review. J Gen Intern Med. 2019;34:1865–1873. doi: 10.1007/s11606-019-05106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piercy K.L., Troiano R.P., Ballard R.M., et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinckard K., Baskin K.K., Stanford K.I. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med. 2019;6:69. doi: 10.3389/fcvm.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu Y., Pan X., Chen Y., Xiao J. Hallmarks of exercised heart. J Mol Cell Cardiol. 2022;164:126–135. doi: 10.1016/j.yjmcc.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Garatachea N., Pareja-Galeano H., Sanchis-Gomar F., et al. Exercise attenuates the major hallmarks of aging. Rejuvenation Res. 2015;18:57–89. doi: 10.1089/rej.2014.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erickson K.I., Hillman C., Stillman C.M., et al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. 2019;51:1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delpino F.M., de Lima A.P.M., da Silva B.G.C., Nunes B.P., Caputo E.L., Bielemann R.M. Physical activity and multimorbidity among community-dwelling older adults: a systematic review with meta-analysis. Am J Health Promot. 2022;36:1371–1385. doi: 10.1177/08901171221104458. [DOI] [PubMed] [Google Scholar]
- 27.Chaib S., Tchkonia T., Kirkland J.L. Cellular senescence and senolytics: the path to the clinic. Nat Med. 2022;28:1556–1568. doi: 10.1038/s41591-022-01923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Justice J.N., Nambiar A.M., Tchkonia T., et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hupin D., Roche F., Gremeaux V., et al. Even a low-dose of moderate-to-vigorous physical activity reduces mortality by 22% in adults aged >/=60 years: a systematic review and meta-analysis. Br J Sports Med. 2015;49:1262–1267. doi: 10.1136/bjsports-2014-094306. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Nie J., Ferrari G., Rey-Lopez J.P., Rezende L.F.M. Association of physical activity intensity with mortality: a national cohort study of 403 681 US adults. JAMA Intern Med. 2021;181:203–211. doi: 10.1001/jamainternmed.2020.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitzman D.W., Whellan D.J., Duncan P., et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385:203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liew J.M., Teo S.P. Physical activity in older people with cardiac co-morbidities. J Geriatr Cardiol. 2018;15:557–558. doi: 10.11909/j.issn.1671-5411.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordes T., Schoene D., Kemmler W., Wollesen B. Chair-based exercise interventions for nursing home residents: a systematic review. J Am Med Dir Assoc. 2021;22:733–740. doi: 10.1016/j.jamda.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 34.Loewenthal J., Innes K.E., Mitzner M., Mita C., Orkaby A.R. Effect of yoga on frailty in older adults: a systematic review. Ann Intern Med. 2023;176:524–535. doi: 10.7326/M22-2553. [DOI] [PubMed] [Google Scholar]
- 35.Copeland J.L., Good J., Dogra S. Strength training is associated with better functional fitness and perceived healthy aging among physically active older adults: a cross-sectional analysis of the Canadian Longitudinal Study on Aging. Aging Clin Exp Res. 2019;31:1257–1263. doi: 10.1007/s40520-018-1079-6. [DOI] [PubMed] [Google Scholar]
- 36.Lesinski M., Hortobagyi T., Muehlbauer T., Gollhofer A., Granacher U. Effects of balance training on balance performance in healthy older adults: a systematic review and meta-analysis. Sports Med. 2015;45:1721–1738. doi: 10.1007/s40279-015-0375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bird M.L., Hill K., Ball M., Williams A.D. Effects of resistance- and flexibility-exercise interventions on balance and related measures in older adults. J Aging Phys Act. 2009;17:444–454. doi: 10.1123/japa.17.4.444. [DOI] [PubMed] [Google Scholar]
- 38.Makarem N., Castro-Diehl C., St-Onge M.P., et al. Redefining cardiovascular health to include sleep: prospective associations with cardiovascular disease in the MESA sleep study. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.122.025252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mander B.A., Winer J.R., Walker M.P. Sleep and human aging. Neuron. 2017;94:19–36. doi: 10.1016/j.neuron.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohayon M.M., Carskadon M.A., Guilleminault C., Vitiello M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 41.Evans M.A., Buysse D.J., Marsland A.L., et al. Meta-analysis of age and actigraphy-assessed sleep characteristics across the lifespan. Sleep. 2021;44 doi: 10.1093/sleep/zsab088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krittanawong C., Tunhasiriwet A., Wang Z., et al. Association between short and long sleep durations and cardiovascular outcomes: a systematic review and meta-analysis. Eur Heart J Acute Cardiovasc Care. 2019;8:762–770. doi: 10.1177/2048872617741733. [DOI] [PubMed] [Google Scholar]
- 43.Yin J., Jin X., Shan Z., et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun R., Xie Y., Jiang W., Wang E., Li X. Effects of different sleep disorders on frailty in the elderly: a systematic review and meta-analysis of observational studies. Sleep Breath. 2023;27:91–101. doi: 10.1007/s11325-022-02610-5. [DOI] [PubMed] [Google Scholar]
- 45.Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21:41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- 46.Fan L., Xu W., Cai Y., Hu Y., Wu C. Sleep duration and the risk of dementia: a systematic review and meta-analysis of prospective cohort studies. J Am Med Dir Assoc. 2019;20:1480–1487.e5. doi: 10.1016/j.jamda.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Stone K.L., Ancoli-Israel S., Blackwell T., et al. Actigraphy-measured sleep characteristics and risk of falls in older women. Arch Intern Med. 2008;168:1768–1775. doi: 10.1001/archinte.168.16.1768. [DOI] [PubMed] [Google Scholar]
- 48.Sindi S., Perez L.M., Vetrano D.L., et al. Sleep disturbances and the speed of multimorbidity development in old age: results from a longitudinal population-based study. BMC Med. 2020;18:382. doi: 10.1186/s12916-020-01846-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St-Onge M.P., Grandner M.A., Brown D., et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tobaldini E., Fiorelli E.M., Solbiati M., Costantino G., Nobili L., Montano N. Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nat Rev Cardiol. 2019;16:213–224. doi: 10.1038/s41569-018-0109-6. [DOI] [PubMed] [Google Scholar]
- 51.Cesari M., Cherubini A., Guralnik J.M., et al. Early detection of accelerated aging and cellular decline (AACD): a consensus statement. Exp Gerontol. 2021;146 doi: 10.1016/j.exger.2021.111242. [DOI] [PubMed] [Google Scholar]
- 52.Boulos M.I., Jairam T., Kendzerska T., Im J., Mekhael A., Murray B.J. Normal polysomnography parameters in healthy adults: a systematic review and meta-analysis. Lancet Respir Med. 2019;7:533–543. doi: 10.1016/S2213-2600(19)30057-8. [DOI] [PubMed] [Google Scholar]
- 53.Jackson C.L., Walker J.R., Brown M.K., Das R., Jones N.L. A workshop report on the causes and consequences of sleep health disparities. Sleep. 2020;43 doi: 10.1093/sleep/zsaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaz Fragoso C.A., Gill T.M. Sleep complaints in community-living older persons: a multifactorial geriatric syndrome. J Am Geriatr Soc. 2007;55:1853–1866. doi: 10.1111/j.1532-5415.2007.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carvalhas-Almeida C., Cavadas C., Alvaro A.R. The impact of insomnia on frailty and the hallmarks of aging. Aging Clin Exp Res. 2023;35:253–269. doi: 10.1007/s40520-022-02310-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed R., Nakahata Y., Shinohara K., Bessho Y. Cellular senescence triggers altered circadian clocks with a prolonged period and delayed phases. Front Neurosci. 2021;15 doi: 10.3389/fnins.2021.638122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carroll J.E., Prather A.A. Sleep and biological aging: a short review. Curr Opin Endocr Metab Res. 2021;18:159–164. doi: 10.1016/j.coemr.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irish L.A., Kline C.E., Gunn H.E., Buysse D.J., Hall M.H. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23–36. doi: 10.1016/j.smrv.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roehrs T., Roth T. UpToDate; 2023. The Effects of Medications on Sleep Quality and Sleep Architecture. [Google Scholar]
- 60.Edinger J.D., Arnedt J.T., Bertisch S.M., et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17:255–262. doi: 10.5664/jcsm.8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qaseem A., Kansagara D., Forciea M.A., Cooke M., Denberg T.D., Clinical Guidelines Committee of the American College of Physicians Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 62.Sloane P.D., Figueiro M., Cohen L. Light as therapy for sleep disorders and depression in older adults. Clin Geriatr. 2008;16:25–31. [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J.X., Liu X.H., Xie X.H., et al. Mindfulness-based stress reduction for chronic insomnia in adults older than 75 years: a randomized, controlled, single-blind clinical trial. Explore. 2015;11:180–185. doi: 10.1016/j.explore.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Kwok T., Leung P.C., Wing Y.K., et al. The effectiveness of acupuncture on the sleep quality of elderly with dementia: a within-subjects trial. Clin Interv Aging. 2013;8:923–929. doi: 10.2147/CIA.S45611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.By the American Geriatrics Society Beers Criteria Update Expert Panel American Geriatrics Society 2019 updated AGS beers criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 66.Should melatonin be used as a sleeping aid for elderly people? Can J Hosp Pharm. 2019;72:327–329. [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao H., Andreyeva T. Diet quality and health in older Americans. Nutrients. 2022;14:1198. doi: 10.3390/nu14061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lloyd-Jones D.M., Ning H., Labarthe D., et al. Status of cardiovascular health in US adults and children using the American Heart Association's new “Life's Essential 8” metrics: prevalence estimates from the National Health and Nutrition Examination Survey (NHANES), 2013 through 2018. Circulation. 2022;146:822–835. doi: 10.1161/CIRCULATIONAHA.122.060911. [DOI] [PubMed] [Google Scholar]
- 69.Chen X., Maguire B., Brodaty H., O'Leary F. Dietary patterns and cognitive health in older adults: a systematic review. J Alzheimers Dis. 2019;67:583–619. doi: 10.3233/JAD-180468. [DOI] [PubMed] [Google Scholar]
- 70.Zhong V.W., Ning H., Van Horn L., et al. Diet quality and long-term absolute risks for incident cardiovascular disease and mortality. Am J Med. 2020;134:490–498. doi: 10.1016/j.amjmed.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bollwein J., Diekmann R., Kaiser M.J., et al. Dietary quality is related to frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2013;68:483–489. doi: 10.1093/gerona/gls204. [DOI] [PubMed] [Google Scholar]
- 72.Bishop N.J., Ullevig S.L., Wang K., Zuniga K.E. Dietary quality modifies the association between multimorbidity and change in mobility limitations among older Americans. Prev Med. 2021;153 doi: 10.1016/j.ypmed.2021.106721. [DOI] [PubMed] [Google Scholar]
- 73.Martinez-Gomez D., Guallar-Castillon P., Higueras-Fresnillo S., Banegas J.R., Sadarangani K.P., Rodriguez-Artalejo F. A healthy lifestyle attenuates the effect of polypharmacy on total and cardiovascular mortality: a national prospective cohort study. Sci Rep. 2018;8 doi: 10.1038/s41598-018-30840-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dent E., Wright O.R.L., Woo J., Hoogendijk E.O. Malnutrition in older adults. Lancet. 2023;401(10380):951–966. doi: 10.1016/s0140-6736(22)02612-5. [DOI] [PubMed] [Google Scholar]
- 75.Kaiser M.J., Bauer J.M., Rämsch C., et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58:1734–1738. doi: 10.1111/j.1532-5415.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 76.Volkert D., Beck A.M., Cederholm T., et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38:10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 77.Saffel-Shrier S., Johnson M.A., Francis S.L. Position of the Academy of Nutrition and Dietetics and the Society for Nutrition Education and Behavior: food and nutrition programs for community-residing older adults. J Nutr Educ Behav. 2019;51:781–797. doi: 10.1016/j.jneb.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 78.Leitao C., Mignano A., Estrela M., et al. The effect of nutrition on aging-a systematic review focusing on aging-related biomarkers. Nutrients. 2022;14:554. doi: 10.3390/nu14030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Virecoulon Giudici K. Editorial: nutrition and the hallmarks of aging. J Nutr Health Aging. 2021;25:1039–1041. doi: 10.1007/s12603-021-1686-3. [DOI] [PubMed] [Google Scholar]
- 80.Shan Z., Wang F., Li Y., et al. Healthy eating patterns and risk of total and cause-specific mortality. JAMA Intern Med. 2023;183:142–153. doi: 10.1001/jamainternmed.2022.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Naggar I.M., Newman J.C., Kuchel G.A. Healthy eating patterns: a stealthy geroscience-guided approach to enhancing the human healthspan. J Nutr Health Aging. 2023;27:238–239. doi: 10.1007/s12603-023-1897-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwingshackl L., Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med. 2015;4:1933–1947. doi: 10.1002/cam4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ali Mohsenpour M., Fallah-Moshkani R., Ghiasvand R., et al. Adherence to dietary approaches to stop hypertension (DASH)-style diet and the risk of cancer: a systematic review and meta-analysis of cohort studies. J Am Coll Nutr. 2019;38:513–525. doi: 10.1080/07315724.2018.1554460. [DOI] [PubMed] [Google Scholar]
- 84.Ntanasi E., Yannakoulia M., Kosmidis M.H., et al. Adherence to mediterranean diet and frailty. J Am Med Dir Assoc. 2018;19:315–322.e2. doi: 10.1016/j.jamda.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 85.Steinberg D., Bennett G.G., Svetkey L. The DASH diet, 20 years later. JAMA. 2017;317:1529–1530. doi: 10.1001/jama.2017.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gardner C.D., Vadiveloo M.K., Petersen K.S., et al. Popular dietary patterns: alignment with American Heart Association 2021 dietary guidance: a scientific statement from the American Heart Association. Circulation. 2023;147:1715–1730. doi: 10.1161/CIR.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 87.ter Borg S., Verlaan S., Hemsworth J., et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195–1206. doi: 10.1017/S0007114515000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.USPSTF Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer: US Preventive Services task force recommendation statement. JAMA. 2022;327:2326–2333. doi: 10.1001/jama.2022.8970. [DOI] [PubMed] [Google Scholar]
- 89.Manson J.E., Cook N.R., Lee I.M., et al. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2018;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nicholls S.J., Lincoff A.M., Garcia M., et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268–2280. doi: 10.1001/jama.2020.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhatt D.L., Steg P.G., Miller M., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2018;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 92.LeBoff M.S., Chou S.H., Ratliff K.A., et al. Supplemental vitamin D and incident fractures in midlife and older adults. N Engl J Med. 2022;387:299–309. doi: 10.1056/NEJMoa2202106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.LeBoff M.S., Murata E.M., Cook N.R., et al. VITamin D and OmegA-3 TriaL (VITAL): effects of vitamin D supplements on risk of falls in the US population. J Clin Endocrinol Metab. 2020;105:2929–2938. doi: 10.1210/clinem/dgaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orkaby A.R., Dushkes R., Ward R., et al. Effect of vitamin D3 and omega-3 fatty acid supplementation on risk of frailty: an Ancillary study of a randomized clinical trial. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.31206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cornelius M.E., Loretan C.G., Wang T.W., Jamal A., Homa D.M. Tobacco product use among adults - United States, 2020. MMWR Morb Mortal Wkly Rep. 2022;71:397–405. doi: 10.15585/mmwr.mm7111a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); 2014. [PubMed] [Google Scholar]
- 97.Muezzinler A., Mons U., Gellert C., et al. Smoking and all-cause mortality in older adults: results from the CHANCES Consortium. Am J Prev Med. 2015;49:e53–e63. doi: 10.1016/j.amepre.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 98.Bassil N.K., Ohanian M.L.K., Bou Saba T.G. Nicotine use disorder in older adults. Clin Geriatr Med. 2022;38:119–131. doi: 10.1016/j.cger.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 99.Nogami E., Miyai N., Zhang Y., et al. [Association of cigarette smoking with muscle mass reduction and low muscle strength in community-dwelling elderly men] Nihon Eiseigaku Zasshi. 2021;76 doi: 10.1265/jjh.21003. [DOI] [PubMed] [Google Scholar]
- 100.Kivipelto M., Mangialasche F., Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 101.Mons U., Muezzinler A., Gellert C., et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551. doi: 10.1136/bmj.h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coll P.P., Roche V., Olsen J.S., Voit J.H., Bowen E., Kumar M. The prevention of cardiovascular disease in older adults. J Am Geriatr Soc. 2020;68:1098–1106. doi: 10.1111/jgs.16353. [DOI] [PubMed] [Google Scholar]
- 103.Roy A., Rawal I., Jabbour S., Prabhakaran D. In: Cardiovascular, Respiratory, and Related Disorders. 3rd ed. Prabhakaran D., Anand S., Gaziano T.A., Mbanya J.C., Wu Y., Nugent R., editors. The International Bank for Reconstruction and Development/The World Bank; 2017. Tobacco and cardiovascular disease: a summary of evidence. Chapter 4. [PubMed] [Google Scholar]
- 104.Kerr S., Watson H., Tolson D., Lough M., Brown M. Smoking after the age of 65 years: a qualitative exploration of older current and former smokers' views on smoking, stopping smoking, and smoking cessation resources and services. Health Soc Care Community. 2006;14:572–582. doi: 10.1111/j.1365-2524.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 105.Heishman S.J.K.B., Singleton E.G. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeremias E., Chatkin J.M., Chatkin G., Seibert J., Martins M., Wagner M. Smoking cessation in older adults. Int J Tuberc Lung Dis. 2012;16:273–278. doi: 10.5588/ijtld.11.0312. [DOI] [PubMed] [Google Scholar]
- 107.US Preventive Services Task Force. Krist A.H., Davidson K.W., et al. Interventions for tobacco smoking cessation in adults, including pregnant persons: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:265–279. doi: 10.1001/jama.2020.25019. [DOI] [PubMed] [Google Scholar]
- 108.Barua R.S., Rigotti N.A., Benowitz N.L., et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72:3332–3365. doi: 10.1016/j.jacc.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 109.Cawkwell P.B., Blaum C., Sherman S.E. Pharmacological smoking cessation therapies in older adults: a review of the evidence. Drugs Aging. 2015;32:443–451. doi: 10.1007/s40266-015-0274-9. [DOI] [PubMed] [Google Scholar]
- 110.Burstein A.H., Fullerton T., Clark D.J., Faessel H.M. Pharmacokinetics, safety, and tolerability after single and multiple oral doses of varenicline in elderly smokers. J Clin Pharmacol. 2006;46:1234–1240. doi: 10.1177/0091270006291837. [DOI] [PubMed] [Google Scholar]
- 111.Flegal K.M., Kruszon-Moran D., Carroll M.D., Fryar C.D., Ogden C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Villareal D.T., Banks M., Siener C., Sinacore D.R., Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 113.Batsis J.A., Villareal D.T. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–537. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bowman K., Delgado J., Henley W.E., et al. Obesity in older people with and without conditions associated with weight loss: follow-up of 955,000 primary care patients. J Gerontol A Biol Sci Med Sci. 2017;72:203–209. doi: 10.1093/gerona/glw147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mundstock E., Sarria E.E., Zatti H., et al. Effect of obesity on telomere length: systematic review and meta-analysis. Obesity. 2015;23:2165–2174. doi: 10.1002/oby.21183. [DOI] [PubMed] [Google Scholar]
- 116.Salvestrini V., Sell C., Lorenzini A. Obesity may accelerate the aging process. Front Endocrinol. 2019;10:266. doi: 10.3389/fendo.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beaufrere B., Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr. 2000;54 Suppl 3:S48–S53. doi: 10.1038/sj.ejcn.1601025. [DOI] [PubMed] [Google Scholar]
- 118.Villareal D.T., Apovian C.M., Kushner R.F., Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, the Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 119.Colleluori G., Villareal D.T. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp Gerontol. 2021;155 doi: 10.1016/j.exger.2021.111561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kahn S.E., Prigeon R.L., Schwartz R.S., et al. Obesity, body fat distribution, insulin sensitivity and Islet beta-cell function as explanations for metabolic diversity. J Nutr. 2001;131:354S–360S. doi: 10.1093/jn/131.2.354S. [DOI] [PubMed] [Google Scholar]
- 121.Lamberts S.W., van den Beld A.W., van der Lely A.J. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 122.Colleluori G., Villareal D.T. Weight strategy in older adults with obesity: calorie restriction or not? Curr Opin Clin Nutr Metab Care. 2023;26:17–22. doi: 10.1097/MCO.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Villareal D.T., Chode S., Parimi N., et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chmelo E.A., Beavers D.P., Lyles M.F., Marsh A.P., Nicklas B.J., Beavers K.M. Legacy effects of short-term intentional weight loss on total body and thigh composition in overweight and obese older adults. Nutr Diabetes. 2016;6 doi: 10.1038/nutd.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Butryn M.L., Webb V., Wadden T.A. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34:841–859. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weindruch R., Walford R.L., Fligiel S., Guthrie D. The Retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 127.Colman R.J., Anderson R.M., Johnson S.C., et al. Caloric restriction delays disease onset and mortality in Rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu D., Huang Y., Huang C., et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386:1495–1504. doi: 10.1056/NEJMoa2114833. [DOI] [PubMed] [Google Scholar]
- 129.Santos H.O., Genario R., Tinsley G.M., et al. A scoping review of intermittent fasting, chronobiology, and metabolism. Am J Clin Nutr. 2022;115:991–1004. doi: 10.1093/ajcn/nqab433. [DOI] [PubMed] [Google Scholar]
- 130.Chow L.S., Manoogian E.N.C., Alvear A., et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity. 2020;28:860–869. doi: 10.1002/oby.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qsymia (Phentermine and Topiramate Extended-Release) [Package Insert]. Vivus IC. 2012 [Google Scholar]
- 132.Contrave (Naltrexone HCl and Bupropion HCl Extended Release) [Package Insert]. Orexigen Therapeutics IC. 2014 [Google Scholar]
- 133.Saxenda (Liraglutide) Injection [Package Insert] Novo Nordisk Inc. 2014 [Google Scholar]
- 134.Wegovy (Semaglutide Injection 2.4mg) [Package Insert] Novo Nordisk Inc. 2021 [Google Scholar]
- 135.Mathus-Vliegen E.M.H., Obesity Management Task Force of the European Association for the Study of Obesity Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts. 2012;5:460–483. doi: 10.1159/000341193. [DOI] [PubMed] [Google Scholar]
- 136.Palmer A.K., Xu M., Zhu Y., et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18 doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ference B.A., Ginsberg H.N., Graham I., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nanna M.G., Navar A.M., Wojdyla D., Peterson E.D. The association between low-density lipoprotein cholesterol and incident atherosclerotic cardiovascular disease in older adults: results from the National Institutes of Health pooled cohorts. J Am Geriatr Soc. 2019;67:2560–2567. doi: 10.1111/jgs.16123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ravnskov U., Diamond D.M., Hama R., et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mc Auley M.T., Mooney K.M. Computationally modeling lipid metabolism and aging: a mini-review. Comput Struct Biotechnol J. 2015;13:38–46. doi: 10.1016/j.csbj.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hubacek J.A., Nikitin Y., Ragino Y., et al. Longitudinal trajectories of blood lipid levels in an ageing population sample of Russian Western-Siberian urban population. PLoS One. 2021;16 doi: 10.1371/journal.pone.0260229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang P., Su Q., Ye X., et al. Trends in LDL-C and non-HDL-C levels with age. Aging Dis. 2020;11:1046–1057. doi: 10.14336/AD.2019.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ettinger W.H., Wahl P.W., Kuller L.H., et al. Lipoprotein lipids in older people. Results from the cardiovascular health study. The CHS Collaborative Research Group. Circulation. 1992;86:858–869. doi: 10.1161/01.cir.86.3.858. [DOI] [PubMed] [Google Scholar]
- 144.Audano M., Maldini M., De Fabiani E., Mitro N., Caruso D. Gender-related metabolomics and lipidomics: from experimental animal models to clinical evidence. J Proteonomics. 2018;178:82–91. doi: 10.1016/j.jprot.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 145.Slade E., Irvin M.R., Xie K., et al. Age and sex are associated with the plasma lipidome: findings from the GOLDN study. Lipids Health Dis. 2021;20:30. doi: 10.1186/s12944-021-01456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barzilai N., Huffman D.M., Muzumdar R.H., Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 148.Hippisley-Cox J., Coupland C., Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357 doi: 10.1136/bmj.j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tota-Maharaj R., Blaha M.J., McEvoy J.W., et al. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33:2955–2962. doi: 10.1093/eurheartj/ehs230. [DOI] [PubMed] [Google Scholar]
- 150.Gencer B., Marston N.A., Im K., et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2020;396:1637–1643. doi: 10.1016/S0140-6736(20)32332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Armitage J., Baigent C., Barnes E., et al. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Orkaby A.R., Driver J.A., Ho Y.L., et al. Association of statin use with all-cause and cardiovascular mortality in US Veterans 75 years and older. JAMA. 2020;324:68–78. doi: 10.1001/jama.2020.7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ramos R., Comas-Cufí M., Martí-Lluch R., et al. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ. 2018;362 doi: 10.1136/bmj.k3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shepherd J., Blauw G.J., Murphy M.B., et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 155.Ridker P.M., Lonn E., Paynter N.P., Glynn R., Yusuf S. Primary prevention with statin therapy in the elderly. Circulation. 2017;135:1979–1981. doi: 10.1161/CIRCULATIONAHA.117.028271. [DOI] [PubMed] [Google Scholar]
- 156.Boccardi V., Barbieri M., Rizzo M.R., et al. A new pleiotropic effect of statins in elderly: modulation of telomerase activity. FASEB J. 2013;27:3879–3885. doi: 10.1096/fj.13-232066. [DOI] [PubMed] [Google Scholar]
- 157.Bach R.G., Cannon C.P., Giugliano R.P., et al. Effect of simvastatin-ezetimibe compared with simvastatin monotherapy after acute coronary syndrome among patients 75 years or older: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2019;4:846–854. doi: 10.1001/jamacardio.2019.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sinnaeve P.R., Schwartz G.G., Wojdyla D.M., et al. Effect of alirocumab on cardiovascular outcomes after acute coronary syndromes according to age: an ODYSSEY OUTCOMES trial analysis. Eur Heart J. 2019;41:2248–2258. doi: 10.1093/eurheartj/ehz809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ray K.K., Wright R.S., Kallend D., et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 160.Ray K.K., Raal F.J., Kallend D.G., et al. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2022;44:129–138. doi: 10.1093/eurheartj/ehac594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Newman C.B., Preiss D., Tobert J.A., et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38–e81. doi: 10.1161/ATV.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 162.Blazing M., Braunwald E., de Lemos J., et al. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet. 2022;400:832–845. doi: 10.1016/S0140-6736(22)01545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.ElSayed N.A., Aleppo G., Aroda V.R., et al. 13. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46:S216–S229. doi: 10.2337/dc23-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention (US); 2020. National Diabetes Statistics Report website. [Google Scholar]
- 165.Bethel M.A., Sloan F.A., Belsky D., Feinglos M.N. Longitudinal incidence and prevalence of adverse outcomes of diabetes mellitus in elderly patients. Arch Intern Med. 2007;167:921–927. doi: 10.1001/archinte.167.9.921. [DOI] [PubMed] [Google Scholar]
- 166.Sinclair A.J., Abdelhafiz A.H. Challenges and strategies for diabetes management in community-living older adults. Diabetes Spectr. 2020;33:217–227. doi: 10.2337/ds20-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Laiteerapong N.H.E. In: Diabetes in America. 3rd ed. Cowie C.C., Casagrande S.S., Menke A., et al., editors. National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018. Diabetes in older adults. CHAPTER 16. [PubMed] [Google Scholar]
- 168.Racial and Ethnic Disparities in Diabetes Prevalence, Self-Management, and Health Outcomes among Medicare Beneficiaries. Centers for Medicare and Medicaid Services; 2017. [Google Scholar]
- 169.Huang E.S., Laiteerapong N., Liu J.Y., John P.M., Moffet H.H., Karter A.J. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med. 2014;174:251–258. doi: 10.1001/jamainternmed.2013.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chia C.W., Egan J.M., Ferrucci L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ Res. 2018;123:886–904. doi: 10.1161/CIRCRESAHA.118.312806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Menke A., Rust K.F., Savage P.J., Cowie C.C. Hemoglobin A1c, fasting plasma glucose, and 2-hour plasma glucose distributions in U.S. population subgroups: NHANES 2005-2010. Ann Epidemiol. 2014;24:83–89. doi: 10.1016/j.annepidem.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kirkman M.S., Briscoe V.J., Clark N., et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012;60:2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kumar M., Thompson P.D. Should hemoglobin A1c targets be re-evaluated? Am J Cardiol. 2022;173:141–142. doi: 10.1016/j.amjcard.2022.02.017. [DOI] [PubMed] [Google Scholar]
- 174.Sheahan K.H., Wahlberg E.A., Gilbert M.P. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgrad Med J. 2020;96:156–161. doi: 10.1136/postgradmedj-2019-137186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zelniker T.A., Wiviott S.D., Raz I., et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 176.Hickson L.J., Langhi Prata L.G.P., Bobart S.A., et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kulkarni A.S., Aleksic S., Berger D.M., Sierra F., Kuchel G.A., Barzilai N. Geroscience-guided repurposing of FDA-approved drugs to target aging: a proposed process and prioritization. Aging Cell. 2022;21 doi: 10.1111/acel.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Soares R.N., Ramirez-Perez F.I., Cabral-Amador F.J., et al. SGLT2 inhibition attenuates arterial dysfunction and decreases vascular F-actin content and expression of proteins associated with oxidative stress in aged mice. Geroscience. 2022;44:1657–1675. doi: 10.1007/s11357-022-00563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Paneni F., Diaz Canestro C., Libby P., Luscher T.F., Camici G.G. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. 2017;69:1952–1967. doi: 10.1016/j.jacc.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 180.Celermajer D.S., Sorensen K.E., Spiegelhalter D.J., Georgakopoulos D., Robinson J., Deanfield J.E. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 181.Cavalcante J.L., Lima J.A., Redheuil A., Al-Mallah M.H. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 182.Pietri P., Stefanadis C. Cardiovascular aging and longevity: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:189–204. doi: 10.1016/j.jacc.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 183.Okada Y., Galbreath M.M., Shibata S., et al. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Paar M., Pavenstadt H., Kusche-Vihrog K., Druppel V., Oberleithner H., Kliche K. Endothelial sodium channels trigger endothelial salt sensitivity with aging. Hypertension. 2014;64:391–396. doi: 10.1161/HYPERTENSIONAHA.114.03348. [DOI] [PubMed] [Google Scholar]
- 185.Weinberger M.H., Fineberg N.S. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 186.Bauer J.H. Age-related changes in the renin-aldosterone system. Physiological effects and clinical implications. Drugs Aging. 1993;3:238–245. doi: 10.2165/00002512-199303030-00005. [DOI] [PubMed] [Google Scholar]
- 187.Wong M.C., Wang H.H., Cheung C.S., et al. Factors associated with multimorbidity and its link with poor blood pressure control among 223,286 hypertensive patients. Int J Cardiol. 2014;177:202–208. doi: 10.1016/j.ijcard.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 188.Canavan M., O'Donnell M.J. Hypertension and cognitive impairment: a review of mechanisms and key concepts. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.821135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Turana Y., Tengkawan J., Chia Y.C., et al. Mental health problems and hypertension in the elderly: review from the HOPE Asia Network. J Clin Hypertens (Greenwich) 2021;23:504–512. doi: 10.1111/jch.14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Baffour-Awuah B., Dieberg G., Pearson M.J., Smart N.A. Blood pressure control in older adults with hypertension: a systematic review with meta-analysis and meta-regression. Int J Cardiol Hypertens. 2020;6 doi: 10.1016/j.ijchy.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hughes D., Judge C., Murphy R., et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020;323:1934–1944. doi: 10.1001/jama.2020.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xiao L., Zan G., Liu C., et al. Associations between blood pressure and accelerated DNA methylation aging. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.022257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Owens W.A., Walaszczyk A., Spyridopoulos I., Dookun E., Richardson G.D. Senescence and senolytics in cardiovascular disease: promise and potential pitfalls. Mech Ageing Dev. 2021;198 doi: 10.1016/j.mad.2021.111540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roos C.M., Zhang B., Palmer A.K., et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ding J., Davis-Plourde K.L., Sedaghat S., et al. Antihypertensive medications and risk for incident dementia and Alzheimer's disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61–70. doi: 10.1016/S1474-4422(19)30393-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Whelton P.K., Carey R.M., Aronow W.S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 197.Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 198.Chen T., Shao F., Chen K., et al. Time to clinical benefit of intensive blood pressure lowering in patients 60 years and older with hypertension: a secondary analysis of randomized clinical trials. JAMA Intern Med. 2022;182:660–667. doi: 10.1001/jamainternmed.2022.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Puttnam R., Davis B.R., Pressel S.L., et al. Association of 3 different antihypertensive medications with hip and pelvic fracture risk in older adults: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2017;177:67–76. doi: 10.1001/jamainternmed.2016.6821. [DOI] [PubMed] [Google Scholar]
- 200.Chuang S.Y., Pan W.H., Chang H.Y., Wu C.I., Chen C.Y., Hsu C.C. Protective effect of calcium channel blockers against frailty in older adults with hypertension. J Am Geriatr Soc. 2016;64:1356–1358. doi: 10.1111/jgs.14155. [DOI] [PubMed] [Google Scholar]
- 201.Di Bari M., van de Poll-Franse L.V., Onder G., et al. Antihypertensive medications and differences in muscle mass in older persons: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:961–966. doi: 10.1111/j.1532-5415.2004.52265.x. [DOI] [PubMed] [Google Scholar]
- 202.Brown J.D., Smith S.M., Strotmeyer E.S., et al. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on response to a physical activity intervention in older adults: results from the lifestyle interventions and independence for elders study. J Gerontol A Biol Sci Med Sci. 2020;75:1010–1016. doi: 10.1093/gerona/glz120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Corrao G., Mazzola P., Monzio Compagnoni M., et al. Antihypertensive medications, loop diuretics, and risk of hip fracture in the elderly: a population-based cohort study of 81,617 Italian patients newly treated between 2005 and 2009. Drugs Aging. 2015;32:927–936. doi: 10.1007/s40266-015-0306-5. [DOI] [PubMed] [Google Scholar]
- 204.Levine G.N., Cohen B.E., Commodore-Mensah Y., et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American Heart Association. Circulation. 2021;143:e763–e783. doi: 10.1161/CIR.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 205.Chin K., Ghosh S., Subramaniam H., Beishon L. Cardiovascular disease in older people with serious mental illness: current challenges and future directions. Front Psychiatry. 2023;14 doi: 10.3389/fpsyt.2023.1110361. [DOI] [PMC free article] [PubMed] [Google Scholar]