Abstract
The optimization of an arbitrarily primed PCR method for typing 96 methicillin-resistant Staphylococcus aureus (MRSA) isolates was compared with pulsed-field gel electrophoresis. Identical results in the differentiation of MRSA clones and identification of the main cluster that included 82 strains (88% of patients) were obtained by both techniques.
Outbreaks of infections caused by methicillin-resistant Staphylococcus aureus (MRSA) continue to be a major problem for patients admitted to intensive care units. Since 1993, there has been an increase in the incidence of nosocomial outbreaks of MRSA strains in different geographic areas in Spain (4, 17), with an incidence of close to 50% at our institution for the period 1994 to 1995, which is covered in the present study. Pulsed-field gel electrophoresis (PFGE) is a highly discriminatory method for typing MRSA isolates because it can distinguish among several concurrent epidemic strains (12). However, it is a time-consuming and expensive typing method not well suited for screening a large number of isolates by a diagnostic laboratory (5). By arbitrarily amplifying variable regions in the bacterial genome (arbitrarily primed PCR [AP-PCR]), an isolate-specific DNA fingerprint can be obtained in a rapid and reproducible manner (11). Although AP-PCR is a cost-effective procedure as well, its discriminatory ability for typing MRSA isolates is lower than that of PFGE (6, 14, 16). Amplification of fragments of the 16S to 23S rRNA intergenic spacer region by ribosome spacer PCR (RS-PCR) (5) or with the nested-PCR-amplified ribosomal DNA spacer region (13) have been proposed recently as being highly reproducible and almost as discriminatory as PFGE for the local investigation of MRSA outbreaks.
We describe here the optimization of AP-PCR for typing isolates of MRSA and compared the effectiveness of this rapid assay with that of PFGE.
Ninety-six MRSA isolates from 51 patients (31 men and 20 women between 22 and 90 years of age) with nosocomial infection collected from 1994 to 1995 at our institution were included (Table 1), as well as other Staphylococcus isolates from 29 men and 17 women between 27 and 89 years of age (26 methicillin-susceptible S. aureus [MSSA] strains, 17 coagulase-negative strains, and 3 unrelated [community-acquired] MRSA strains), which served as controls (Table 2). Reference strains were S. aureus CECT435 and CECT4630. Methicillin resistance was assessed with 1-μg methicillin disks at 35°C for 24 h on Mueller-Hinton agar supplemented with 4% NaCl, according to standard MICs (≤4 mg/liter) (6) and E-Test-oxacillin (AB Biodisk, Solna, Sweden). The mecA gene was confirmed by PCR with the RSM2647 and RSM2648 primers (8).
TABLE 1.
Clinical features of 51 patients with nosocomial infection and results obtained with isolates analyzed by PFGE and AP-PCR
| Patient | Infection typea | Wardb | Date (wk/yr) | Isolate | Sitec | Typing pattern by:
|
|
|---|---|---|---|---|---|---|---|
| PFGE | AP-PCR | ||||||
| 1 | IC | NEPH (SUR) | 1/94 | 1.1 | VC | A1 | A |
| 2 | NP | ICU | 1/94 | 2.1 | BC | A1 | A |
| 3 | SWI | NER (SUR) | 2/94 | 3.1 | WE | A2 | A |
| 4 | IC + NP | ICU | 2/94 | 4.1 | BS | A2 | A |
| ICU | 3/94 | 4.2 | S | A2 | A | ||
| ICU | 3/94 | 4.3 | S | A2 | A | ||
| ICU | 3/94 | 4.4 | S | A2 | A | ||
| ICU | 4/94 | 4.5 | VC | A2 | A | ||
| ICU | 4/94 | 4.6 | VC | A2 | A | ||
| ICU | 5/94 | 4.7 | S | A2 | A | ||
| ICU | 5/94 | 4.8 | S | A1 | A | ||
| PNE | 6/94 | 4.9 | S | A1 | A | ||
| PNE | 7/94 | 4.10 | P | B | B | ||
| 5 | SWI + NP | ICU | 2/94 | 5.1 | VC | A2 | A |
| ICU | 2/94 | 5.2 | P | A2 | A | ||
| ICU | 2/94 | 5.3 | S | A2 | A | ||
| ICU | 3/94 | 5.4 | PF | A2 | A | ||
| ICU | 3/94 | 5.5 | S | A2 | A | ||
| ICU | 5/94 | 5.6 | S | A2 | A | ||
| 6d | SWI | TRA | 2/94 | 6.1 | SF | C | C |
| TRA | 3/94 | 6.2 | WE | C | C | ||
| TRA | 5/94 | 6.3 | WE | C | C | ||
| TRA | 6/94 | 6.4 | WE | C | C | ||
| TRA | 7/94 | 6.5 | WE | C | C | ||
| 7 | NP | ICU | 3/94 | 7.1 | S | A2 | A |
| 8 | PR | ICU | 3/94 | 8.1 | PE | A2 | A |
| 9e | SWI | SUR | 4/94 | 9.1 | P | D | D |
| 10 | SWI | TRA | 4/94 | 10.1 | WE | A2 | A |
| TRA | 34/94 | 10.2 | WE | A2 | A | ||
| TRA | 35/94 | 10.3 | WE | A2 | A | ||
| 11 | SI + NP | ICU | 4/94 | 11.1 | BA | A2 | A |
| ICU | 4/94 | 11.2 | S | A2 | A | ||
| 12 | PN | ICU | 4/94 | 12.1 | S | A2 | A |
| 13 | SWI | ICU | 5/94 | 13.1 | VC | A1 | A |
| 14 | NP | IM (ICU) | 6/94 | 14.1 | S | A1 | A |
| 15 | NP | ORL (SUR) | 8/94 | 15.1 | WE | A1 | A |
| 16 | SWI | URO (ICU) | 8/94 | 16.1 | WE | A2 | A |
| 17 | SWI | SUR | 9/94 | 17.1 | VC | A2 | A |
| 18 | SWI | SUR | 12/94 | 18.1 | WE | A2 | A |
| 19 | SWI | SUR | 12/94 | 19.1 | WE | A2 | A |
| 20 | IC + SWI + NP | CIV | 12/94 | 20.1 | S | A1 | A |
| NEPH | 15/94 | 20.2 | WE | A1 | A | ||
| NEPH | 15/94 | 20.3 | VC | A1 | A | ||
| 21 | B | IM | 15/94 | 21.1 | BC | A1 | A |
| 22 | NP | PNE (ICU) | 16/94 | 22.1 | S | A3 | A |
| 23 | SWI | ORL (SUR) | 16/94 | 23.1 | WE | A1 | A |
| ORL | 23/94 | 23.2 | WE | A1 | A | ||
| 24 | NP | ICU | 16/94 | 24.1 | S | A1 | A |
| 25 | NP | ICU | 16/94 | 25.1 | BF | A3 | A |
| 26 | NP | ICU | 17/94 | 26.1 | BF | A1 | A |
| 27 | NP | ICU | 18/94 | 27.1 | BF | A1 | A |
| 28 | NP | ICU | 19/94 | 28.1 | S | A1 | A |
| 29 | SWI | URO (SUR) | 21/94 | 29.1 | WE | A2 | A |
| 30 | SWI | SUR | 24/94 | 30.1 | WE | A1 | A |
| 31 | NP | NEPH (ICU) | 31/94 | 31.1 | S | A1 | A |
| 32 | B | ICU | 48/94 | 32.1 | BC | A1 | A |
| 33 | B | ICU | 49/94 | 33.1 | BC | A1 | A |
| ICU | 49/94 | 33.2 | BC | A1 | A | ||
| ICU | 50/94 | 33.3 | BC | A1 | A | ||
| ICU | 51/94 | 33.4 | WE | A1 | A | ||
| 34 | B | ICU | 51/94 | 34.1 | BC | A1 | A |
| 35 | B | ICU | 52/94 | 35.1 | BC | A1 | A |
| 36 | B | SUR | 3/95 | 36.1 | VC | A1 | A |
| SUR | 4/95 | 36.2 | BC | A1 | A | ||
| 37 | B | IM | 4/95 | 37.1 | BC | A1 | A |
| IM | 4/95 | 37.2 | WE | A1 | A | ||
| IM | 4/95 | 37.3 | WE | A1 | A | ||
| IM | 4/95 | 37.4 | WE | A1 | A | ||
| IM | 4/95 | 37.5 | WE | A1 | A | ||
| 38 | SWI + NP | ICU | 6/95 | 38.1 | TS | A1 | A |
| ICU | 6/95 | 38.2 | VC | A1 | A | ||
| ICU | 6/95 | 38.3 | AF | A1 | A | ||
| 39 | NP | ICU | 11/95 | 39.1 | TS | A1 | A |
| 40 | NP | ICU | 13/95 | 40.1 | S | A1 | A |
| 41 | SWI | TRA | 13/95 | 41.1 | WE | A1 | A |
| TRA | 14/95 | 41.2 | WE | A1 | A | ||
| TRA | 15/95 | 41.3 | WE | A1 | A | ||
| TRA | 16/95 | 41.4 | WE | A1 | A | ||
| TRA | 17/95 | 41.5 | WE | A1 | A | ||
| 42 | B | PNE (ICU) | 15/95 | 42.1 | BC | A1 | A |
| ICU | 16/95 | 42.2 | TS | A1 | A | ||
| ICU | 20/95 | 42.3 | BC | A1 | A | ||
| 43 | SWI + NP | ICU | 20/95 | 43.1 | S | A1 | A |
| ICU | 21/95 | 43.2 | PHE | A1 | A | ||
| ICU | 22/95 | 43.3 | VC | A1 | A | ||
| 44 | IC + NP | NER (ICU) | 37/95 | 44.1 | TS | A1 | A |
| 45 | UTI | PNE (ICU) | 42/95 | 45.1 | WE | A2 | A |
| 46 | UTI | IM (ICU) | 48/95 | 46.1 | WE | A1 | A |
| 47 | SWI | CIV | 49/95 | 47.1 | WE | E | E |
| 48 | SWI | CIV | 49/95 | 48.1 | WE | E | E |
| 49 | SWI | CIV | 50/95 | 49.1 | WE | E | E |
| 50 | SWI | TRA | 50/95 | 50.1 | WE | F | F |
| TRA | 50/95 | 50.2 | WE | F | F | ||
| TRA | 50/95 | 50.3 | WE | F | F | ||
| TRA | 51/95 | 50.4 | WE | F | F | ||
| 51 | B | ICU | 51/95 | 51.1 | BC | A1 | A |
B, bacteremia; PR, peritonitis; IC, infection by catheter; NP, nosocomial pneumonia; SWI, surgery wound infection; SI, skin infection; UTI, urinary tract infection.
NEPH, nephrology; SUR, general surgery; NER, neurology; ICU, intensive care unit; PNE, pneumology; TRA, traumatology; IM, internal medicine; ORL, otorhinolaryngology; URO, urology; CIV, vein surgery. If there was a previous department to which patients had been admitted (1 to 5 days before), it is given in parentheses.
VC, venous catheter; BC, blood culture; WE, wound exudate; BS, bronchial secretion; S, sputum; P, pus; TS, tracheal secretion; PF, pleural fluid; SF, sinovial fluid; PE, peritoneal exudate; AF, ascitic fluid; PHE, pharyngeal exudate.
Patient transferred from another district hospital (La Fe).
Patient transferred from another district hospital (Arnau de Vilanova).
TABLE 2.
Features of the control isolates tested and results of the characterization by PFGE and AP-PCR
| Patient | Isolate | Warda | Date (wk/yr) | Siteb | Identification by:
|
|
|---|---|---|---|---|---|---|
| PFGE | AP-PCR | |||||
| A | MSSA1 | ICU | 1/94 | BC | CO1a | CO1 |
| B | MSSA2 | NER | 2/94 | P | CO2 | CO2 |
| C | MSSA3 | CIR | 4/94 | WE | CO3 | CO3 |
| D | MSSA4 | ICU | 4/94 | BC | CO4 | CO4 |
| E | MSSA5 | ICU | 5/94 | PHE | CO5 | CO5 |
| F | MSSA6 | TRA | 5/94 | WE | CO6 | CO6 |
| G | MSSA7 | ICU | 12/94 | VC | CO7 | CO7 |
| H | MSSA8 | ICU | 20/94 | BC | CO8a | CO8 |
| I | MSSA9 | CIR | 24/94 | P | CO9 | CO9 |
| J | MSSA10 | NEPH | 31/94 | P | CO10 | CO10 |
| H | MSSA11 | ICU | 20/94 | S | CO8b | CO8 |
| A | MSSA12 | ICU | 1/94 | BC | CO1b | CO1 |
| K | MSSA13 | CIR | 3/95 | WE | CO11 | CO11 |
| L | MSSA14 | MI | 4/95 | TS | CO12 | CO12 |
| M | MSSA15 | ICU | 6/95 | BC | CO13 | CO13 |
| N | MSSA16 | ICU | 16/95 | BC | CO14a | CO14 |
| O | MSSA17 | NEU | 42/95 | TS | CO15 | CO15 |
| P | MSSA18 | CIV | 49/95 | VC | CO16 | CO16 |
| Q | MSSA19 | TRA | 50/95 | WE | CO17 | CO17 |
| R | MSSA20 | NEU | 12/94 | WE | CO18 | CO18 |
| S | MSSA21 | ICU | 24/94 | VC | CO19 | CO19 |
| T | MSSA22 | ICU | 31/95 | P | CO20 | CO20 |
| U | MSSA23 | ICU | 18/95 | BC | CO21 | CO21 |
| V | MSSA24 | ICU | 14/95 | BC | CO22 | CO22 |
| N | MSSA25 | ICU | 16/95 | BC | CO14b | CO14 |
| W | MSSA26 | ICU | 51/95 | BC | CO23 | CO23 |
| I | CNS1 | CIR | 4/94 | WE | CO24a | CO24a |
| II | CNS2 | CIR | 12/94 | WE | CO25a | CO25 |
| III | CNS3 | URO | 21/94 | WE | CO26 | CO26 |
| I | CNS4 | CIR | 4/94 | WE | CO24b | CO24b |
| II | CNS5 | CIR | 12/94 | WE | CO25b | CO25 |
| VI | CNS6 | ICU | 21/95 | BC | CO27 | CO27 |
| VII | CNS7 | ORL | 8/94 | P | CO28 | CO28 |
| VIII | CNS8 | NEU | 16/94 | BF | CO29 | CO29 |
| IX | CNS9 | ICU | 17/94 | WE | CO30 | CO30 |
| X | CNS10 | MI | 6/94 | PHE | CO31 | CO31 |
| XI | CNS11 | CIV | 12/94 | VC | CO32 | CO32 |
| XII | CNS12 | ICU | 18/94 | BC | CO33 | CO33 |
| XIII | CNS13 | ICU | 19/94 | BC | CO34 | CO34 |
| XIV | CNS14 | CIR | 24/94 | S | CO35 | CO35 |
| XV | CNS15 | ICU | 51/94 | S | CO36 | CO36 |
| XVI | CNS16 | TRA | 15/95 | WE | CO37 | CO37 |
| XVII | CNS17 | ICU | 20/95 | BC | CO38 | CO38 |
| C1 | COM1 | E | 14/94 | PHE | CO39 | CO39 |
| C2 | COM2 | E | 21/94 | PHE | CO40 | CO40 |
| C3 | COM3 | E | 34/95 | WE | CO41 | CO41 |
NEPH, nephrology; SUR, general surgery; NER, neurology; ICU, intensive care unit; PNE, pneumology; TRA, traumatology; IM, internal medicine; ORL, otorhinolaryngology; URO, urology; CIV, vein surgery; E, communitary.
VC, venous catheter; BC, blood culture; WE, wound exudate; BS, bronchial secretion; S, sputum; P, pus; TS, tracheal secretion; PHE, pharyngeal exudate.
The PFGE method of Prévost et al. (12) was used, with minor modifications according to the instructions of a different manufacturer (Bio-Rad, Richmond, Calif.). Electrophoresis was performed with the CHEF DRIII electrophoresis system (Bio-Rad) with a 5-s initial pulse, 60-s final pulse, voltage of 6 V/cm, angle of 120°, time of 23 h, and temperature of 13°C (9). Gels were stained with ethidium bromide and photographed. Banding patterns were interpreted according to the method of Bannerman et al. (1), considering as isolates of the same strain (modal pattern) those that appeared identical in size and number of bands and considering as subtypes those with three or fewer band differences.
For the AP-PCR protocol, the method of DNA extraction described by Pitcher et al. (10) was used, except that the suspension time of S. aureus colonies in enzymatic solutions was reduced to 15 min, the incubation time was reduced to 15 min, and protein precipitation was reduced to 5 min. The DNA concentration was measured with a GeneQuant II RNA/DNA calculator (Pharmacia Biotech, Uppsala, Sweden). To determine the optimal conditions for the reliability and reproducibility of AP-PCR, the effects of different DNA template concentrations (5, 25, 50, 75, and 100 ng), DNA polymerases (RedHot, an enzyme from Thermus “Icelandicus,” a new strain [Advanced Biotechnologies, Surrey, United Kingdom]; GIBCO-BRL Taq polymerase [Life Technologies, Gaithersburg, Md.]; Boehringer Mannheim Taq polymerase [Boehringer Mannheim, Mannheim, Germany]; and Promega Taq polymerase [Promega, Madison, Wis.], at concentrations of 0.5, 1, 3, 5, 7, and 10 U), arbitrary primers (a total of 20 included in the kit from Operon Technologies, Alameda, Calif., at concentrations of 0.1 to 1 μM), MgCl2 concentrations (0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4 mM), annealing temperatures (25 to 45°C), and multiplication factors per degree centigrade in ramps of the thermocycling conditions (1, 2, 3, and 4) were tested in triplicate for 11 MRSA strains previously characterized by PFGE.
The optimization of the technique was as follows. The PCR mixture consisted of 5 U of RedHot DNA polymerase (Advanced Biotechnologies); 15 mM Tris-HCl (pH 8.8 at 25°C); 2.5 mM MgCl2; 50 mM KCl; 250 μM (each) dATP, dGTP, dCTP, and dTTP; 1 μM primer OPA11 (5′-CAATCGTCCGT-3′); and 50 ng of template DNA for a final volume of 25 μl. Cycling was performed in a Perkin-Elmer PCR machine (model Gene Amp 9600) and consisted of the following steps: denaturation at 94°C for 5 min, followed by 44 cycles of 94°C for 1 min and a ramp of 3 min 52 s to 36°C for 1 min, a ramp of 2 min 24 s to 72°C for 1 min, and a ramp of 1 min 18 s to 94°C, followed by 10 min of extension at 72°C. Five strains were used as internal controls in each AP-PCR assay.
For the analysis of reproducibility and similarity, each new PFGE and AP-PCR pattern was included in subsequent tests for comparison. The similarity between PFGE and AP-PCR patterns was assessed by using the Dice coefficient for each pair of isolates. The GelPrinter and Lane Manager 2.0 software (TDI, S.A., Madrid, Spain) were used for the analysis. A coefficient of similarity higher than 95% was required to establish a same-clone origin.
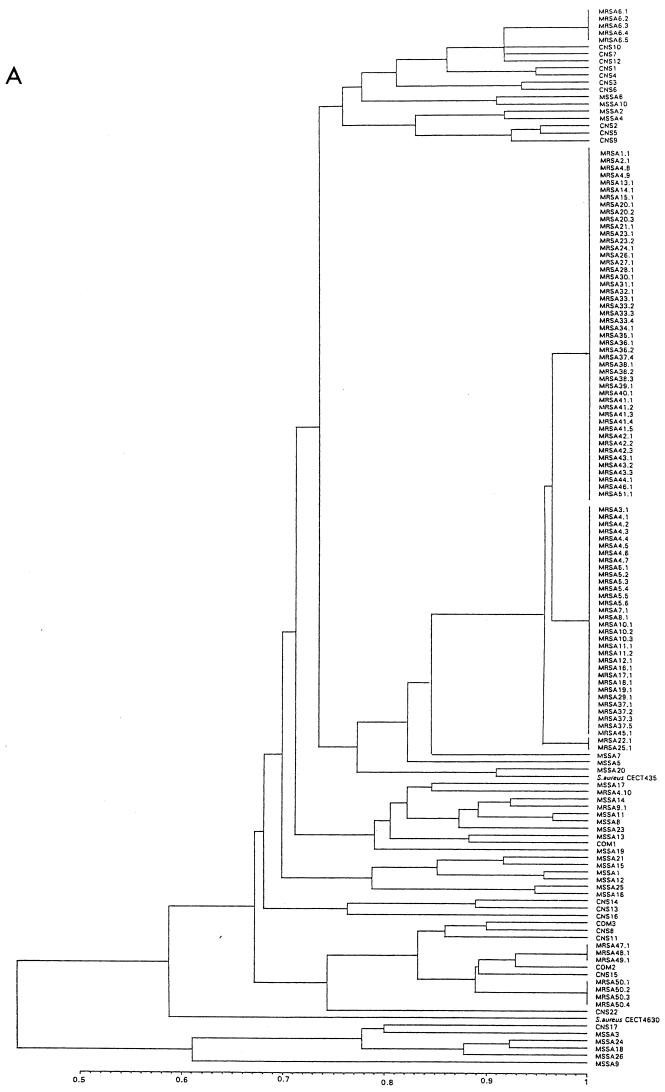
PFGE after restriction with SmaI resolved the chromosomal DNA of the 96 MRSA isolates into six main distinct PFGE patterns (A to F). PFGE type A contained 82 strains (88% of patients), which could be further classified into three subtypes (A.1 to A.3) (Fig. 1), of which subtype A.1 represented the majority (48 strains [50%]), followed by A.2 (32 strains [33.3%] and A.3 (2 strains [2.1%]). The remaining PFGE patterns had no subtypes. PFGE types B, C, D, E, and F contained one, five, one, three, and four isolates, respectively (Table 1). The Dice coefficient of similarity among major patterns had an average value of 67%. The subtype variants showed similarity values higher than 95%. Control isolates showed different PFGE patterns (Table 2), with average similarity values ranging between 43 and 84%. Figure 2A shows the epidemiologic clustering according to all PFGE banding patterns of all S. aureus strains included in the study.
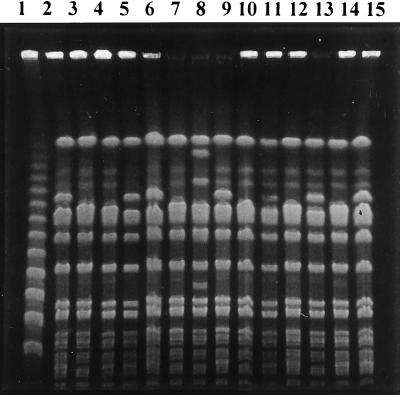
FIG. 1.
The principal PFGE pattern (type A). Lanes: 1, PFGE marker I, λ ladder (Boehringer Mannheim); 2, 5, 6, 9, 13, and 15, SmaI macrorestriction pattern A1; 3, 4, 7, 10, 11, 12, and 14, SmaI macrorestriction pattern A2; 8, SmaI macrorestriction pattern A3.
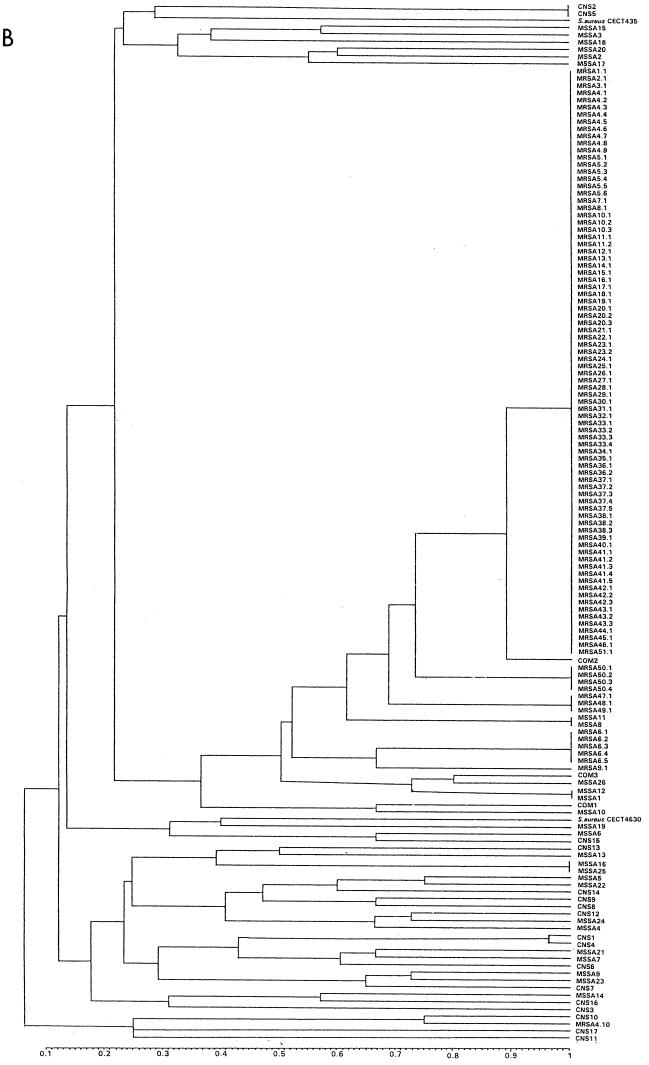
FIG. 2.
Unweighted pair group method with arithmetic mean Tree. (A) PFGE. (B) AP-PCR. Results are from analysis of similarity by Dice’s coefficient, corresponding to all strains included in the study.
AP-PCR analysis generated between 8 and 17 DNA products from 0.4 to 3.2 kb (Fig. 3) and also characterized the 96 MRSA isolates into six main distinct clusters (A to F). Eighty-two MRSA isolates (88% of patients) were grouped into main cluster A. The remaining AP-PCR clusters, B, C, D, E, and F, contained one, five, one, three, and four isolates, respectively (Table 1). The Dice coefficient of similarity among major patterns had an average value of 50%. Control isolates showed different AP-PCR patterns (Table 2), with an average similarity value lower than 50%, except for two MSSA strains and one unrelated MRSA strain. Figure 2B shows the epidemiologic clustering according to all AP-PCR banding patterns of all S. aureus strains included in the study.
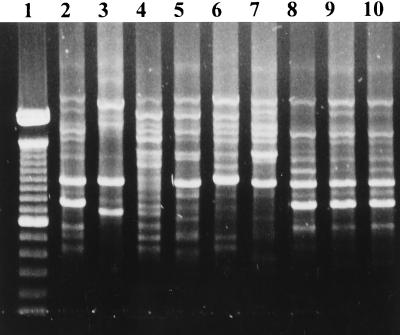
FIG. 3.
OPA11-PCR patterns belonging to different clonal groups. Lanes: 1, 100-bp DNA ladder (GIBCO BRL); 2 to 10, different PCR fingerprinting profiles (2, 8, 9, and 10, pattern A; 3, pattern CNS2; 4, pattern C; 5, pattern MSSA9; 6, pattern D; 7, pattern MSSA18).
Complete coincidence between PFGE and AP-PCR in differentiation of MRSA clones and in identification of the main cluster that included 82 MRSA strains was obtained.
During the past few years, MRSA has become an increasing source of nosocomial infections causing therapeutic and control problems in hospitals in many parts of the world (3, 7). Accurate epidemiological typing by reproducible and rapid methods is a major step in determining MRSA clones and sources of transmission. Although PFGE was developed as an epidemiological marker for typing S. aureus strains (10), different PCR-based methods have been introduced to overcome the complexity of this technique. Among these relatively novel techniques, AP-PCR has been one of the most used because of its simplicity and rapidity (a few hours versus about 3 days for PFGE). Recently, RS-PCR (5) and the nested-PCR-amplified ribosomal DNA spacer region (13) have been proposed as alternatives to PFGE for typing MRSA isolates in local investigations of MRSA outbreaks (5, 13). However, PFGE detects minor variations among the isolates and could identify variants that are not discriminated by RS-PCR (5). Moreover, the nested-PCR-amplified ribosomal DNA spacer region increases the time-consuming aspects of detection and enhances the risks of contamination. Based on our previous experience of AP-PCR with commercially available primers (2), we selected this simple fast technique as an epidemiological molecular marker. In contrast to the study of Saulnier et al. (14), in which PFGE was superior to the randomly amplified polymorphic DNA assay for typing MRSA strains, we obtained a complete correlation between PFGE and the optimization of AP-PCR. This included 1 μM OPA11 primer, 50 ng of DNA template, 5 U of RedHot DNA polymerase, 2.5 mM MgCl2, a 36°C annealing temperature, and ramps in the thermocycling conditions. The optimized AP-PCR technique was a useful tool for the discrimination and surveillance of MRSA strains associated with an outbreak localized in the intensive care and surgery wards that spread to other departments. Reproducible patterns for different MRSA, MSSA, and coagulase-negative S. aureus clones confirmed the validity of this method. The comparative typing of the 136 strains (40 as controls) by SmaI macrorestriction patterns and OPA11-PCR resulted in the same clustering by similarity in dendrograms and disclosed the most persistent local MRSA clone in the hospital. In conclusion, together with a simple method of template DNA extraction, OPA11-PCR seems to be a very rapid, inexpensive, and useful technique for epidemiological typing of S. aureus strains in routine surveillance.
Acknowledgments
We thank the staff of the Department of Microbiology of Hospital “Dr. Peset” for useful comments, M. Cambra and M. J. Asins from the “Instituto Valenciano de Investigaciones Agrarias” for providing the CHEF DRIII electrophoresis system, and Marta Pulido for editing the manuscript and editorial assistance.
REFERENCES
- 1.Bannerman T L, Hancock G A, Tenover F C, Miller J M. Pulsed-field electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camarena J J, Nogueira J M, Dasí M A, Moreno F, García R, Ledesma E, Llorca J, Hernández J. DNA amplification fingerprinting for subtyping Neisseria gonorrhoeae strains. Sex Transm Dis. 1995;22:128–135. doi: 10.1097/00007435-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Cotter L, Lynch M, Cryan B, Greer P, Fanning S. Investigation of a methicillin-resistant Staphylococcus aureus (MRSA) outbreak in an Irish hospital: triplex PCR and DNA amplification fingerprinting. J Hosp Infect. 1997;36:37–47. doi: 10.1016/s0195-6701(97)90089-x. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez M A, de Lencastre H, Linares J, Tomasz A. Spread and maintenance of a dominant methicillin-resistant Staphylococcus aureus (MRSA) clone during an outbreak of MRSA disease in a Spanish hospital. J Clin Microbiol. 1994;32:2081–2087. doi: 10.1128/jcm.32.9.2081-2087.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumari D N P, Keer V, Hawkey P M, Parnell P, Joseph N, Richardson J F, Cookson B. Comparison and application of ribosome spacer DNA amplicon polymorphisms and pulsed-field gel electrophoresis for differentiation of methicillin-resistant Staphylococcus aureus strains. J Clin Microbiol. 1997;35:881–885. doi: 10.1128/jcm.35.4.881-885.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leitch C, Boonlayangoor S. Tests to detect oxacillin (methicillin)-resistant staphylococci with an oxacillin screen plate. In: Isenberg H D, editor. Clinical microbiology procedures handbook. Vol. 1. Washington, D.C: American Society for Microbiology; 1992. pp. 5.5.1–5.5.7. [Google Scholar]
- 7.Mulligan M E, Murray-Leisure K A, Ribner B S, Standiford H C, John J F, Korvick J A, Kauffman C A, Yu V L. Methicillin-resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am J Med. 1993;94:313–328. doi: 10.1016/0002-9343(93)90063-u. [DOI] [PubMed] [Google Scholar]
- 8.Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S. Identification of methicillin-resistant staphylococci by polymerase chain reaction. J Clin Microbiol. 1991;29:2240–2244. doi: 10.1128/jcm.29.10.2240-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller M A, Hollis R J, Sader H S. PFGE analysis of chromosomal restriction fragment. In: Isenberg H D, editor. Clinical microbiology procedures handbook. Vol. 2. Washington, D.C: American Society for Microbiology; 1992. pp. 10.5.c.1–10.5.c.12. [Google Scholar]
- 10.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 11.Power E G M. RAPD typing in microbiology—a technical review. J Hosp Infect. 1996;34:247–265. doi: 10.1016/s0195-6701(96)90106-1. [DOI] [PubMed] [Google Scholar]
- 12.Prévost G, Pottecher B, Dahlet M, Bientz M, Mantz J M, Piemont Y. Pulsed field electrophoresis as a new epidemiological tool for monitoring methicillin-resistant Staphylococcus aureus in an intensive care unit. J Hosp Infect. 1991;17:255–269. doi: 10.1016/0195-6701(91)90270-i. [DOI] [PubMed] [Google Scholar]
- 13.Saruta K, Matsunaga T, Kono M, Hoshina S, Ikawa S, Sakai O, Machida K. Rapid identification and typing of Staphylococcus aureus by nested PCR amplified ribosomal DNA spacer region. FEMS Microbiol Lett. 1997;146:271–278. doi: 10.1111/j.1574-6968.1997.tb10204.x. [DOI] [PubMed] [Google Scholar]
- 14.Saulnier P, Bourneix C, Prévost G, Andremont A. Random amplified polymorphic DNA assay is less discriminant than pulsed-field gel electrophoresis for typing strains of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:982–985. doi: 10.1128/jcm.31.4.982-985.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyler K D, Wang G, Tyler S D, Johnson W M. Factors affecting reliability and reproducibility of amplification-based DNA fingerprinting of representative bacterial pathogens. J Clin Microbiol. 1997;35:339–346. doi: 10.1128/jcm.35.2.339-346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Belkum A, Kluytmans J, van Leeuwen W, Bax W, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, van den Brule A, Koeleman H, Melchers W, Meis J, Elaichouni A, Vaneechoutte M, Moonens F, Maes N, Struelens M, Tenover F, Verbrugh H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vindel, A., and J. A. Saez-Nieto. 1992. Caracterización de cepas de Staphylococcus aureus resistentes a meticilina causantes de brotes mediante fagotipicación. Enferm. Infecc. Microbiol. Clin. 10(Suppl. 3):36–38. [PubMed]