ABSTRACT
Sexual reproduction of the malaria parasites is critical for their transmission to a mosquito vector. Several signaling molecules, such as kinases and phosphatases, are known to regulate this process. We previously demonstrated that Plasmodium falciparum (Pf) Ca2+-dependent protein kinase 4 (CDPK4) and serine/arginine-rich protein kinase 1 (SRPK1) are critical for axoneme formation during male gametogenesis, with genetic deletion of either gene causing a complete block in parasite transmission to the mosquito. A comparative phospho-proteome analysis of Pfcdpk4− and RNA-seq analysis of Pfsrpk1− gametocytes showed that these kinases regulate similar biological processes linked to both microtubule (MT) dynamics and cell motility. One of these proteins was a nuclear MT-associated End Binding protein 1 (EB1), which was hypophosphorylated in Pfcdpk4− gametocytes. To study the functional relevance of EB1, we created gene deletion parasites for EB1. We further demonstrate that Pfeb1− parasites like WT NF54 parasites proliferate normally as asexuals and undergo gametocytogenesis and gametogenesis. Strikingly, these parasites suffer a severe defect in nuclear segregation and partitioning of nuclei into emerging microgametes. Further genetic crosses utilizing male- and female-sterile parasites revealed that Pfeb1− parasites only suffer a male fertility defect. Overall, our study reveals an essential function for PfEB1 in male gamete nuclear segregation and suggests a potential therapeutic avenue in the design of transmission-blocking drugs to prevent malaria transmission from humans to mosquito.
IMPORTANCE
Gametogenesis and subsequent gamete fusion are central to successful transmission of the malaria parasites to a female Anopheles mosquito vector and completion of the sexual phase of the parasite life cycle. Male gametogenesis involves the formation of axonemes inside male gametes from male gametocytes via active cytoskeleton remodeling. The tubulin and tubulin-binding proteins are, thus, attractive anti-malarial drug targets. In the present study, we demonstrate that a microtubule-binding protein PfEB1 is essential for male gamete fertility, specifically for the inheritance of nuclei from activated male gametocytes. Targeting PfEB1 function may provide new avenues into designing interventions to prevent malaria transmission and disease spread.
KEYWORDS: Plasmodium, microtubules, gamete, nucleus, mosquito, transmission
INTRODUCTION
Plasmodium parasites utilize both vertebrate hosts and female Anopheles mosquito vectors for the completion of their life cycle. Sporozoites are transmitted by mosquito bite which infect hepatocytes and replicate asexually to form and release merozoites, which infect red blood cells (RBCs). Blood-stage parasites replicate asexually within RBCs, but some of these parasites commit to sexual-stage differentiation called gametocytogenesis, which proceeds through five distinct stages (stages I–V), and in the human malaria parasite Plasmodium falciparum, takes up to 14 days. Stage V gametocytes rapidly undergo gametogenesis when ingested by a mosquito during a blood meal. Mosquitoes are infected only by the parasite stages that arise after fertilization, and thus, malaria transmission is dependent on successful gamete development.
The morphologically distinct changes during gametocytogenesis require active cytoskeletal remodeling, and male gametes depend on microtubule (MT)-driven motility to fertilize females. MTs are cytoskeletal filaments with a diameter of ~25 nm, composed of alternating rings of α- and β-tubulin dimers (1). Plasmodium tubulin is more similar to that of plants than to mammalian tubulin, and inhibitors targeting parasite tubulin specifically disrupt their growth without affecting human MTs (2). More recently, antibodies targeting Pf α-tubulin I have shown strong transmission reducing efficacy (3). Therefore, cytoskeletal proteins such as MTs and their associated proteins (MAPs) are attractive drug or vaccine targets, especially during sexual-stage development. Tubulin-binding proteins in eukaryotic cells facilitate the assembly and disassembly of MTs, which drive cell division, differentiation, and motility (1, 4, 5). In eukaryotes, MTs also form the scaffolds of the mitotic and meiotic spindles of dividing cells (6). Plasmodium invasive stages and gametocytes maintain cell shape and rigidity with a pellicle undergirding the plasma membrane, which is composed of a network of subpellicular microtubules (SPMTs) (7) and associated proteins (8) underneath a double membrane known as the inner membrane complex (9, 10). Two additional populations of microtubules are found in the gametocyte nuclear spindle or hemi-spindle (11) and in the cytoplasm. While the cytoplasmic microtubules are short lived and only appear in early-stage gametocytes on the opposite side of the cell from the developing pellicle (10), the nuclear microtubules are present in stages III/IV gametocyte (7, 12).
Male gametogenesis is a rapid process and culminates in the formation of eight flagellated microgametes that egress from the infected RBC (exflagellation) (13). Microtubule-binding proteins impact flagellar formation and length via involvement in the equilibrium of assembly and disassembly of tubulin at the plus end (14, 15). In Apicomplexan parasites, the MT-binding apicortin protein complex comprise of a doublecortin domain and a partial p25α domain, which exhibit MAP-like functions (16). In rodent malaria parasite, Plasmodium yoelii (Py) (Pyp25α) is crucial for exflagellation and, thus, essential for microgametogenesis (17). A recent study reported the nucleation of SPMTs in P. falciparum gametocytes at the outer centriolar plaque, a non-mitotic microtubule organizing center (MTOC) embedded in the nuclear membrane of the parasite (12). This study also reported that classical mitotic machinery components, including centriolar plaque proteins, Pfcentrin-1 and -4, microtubule-associated protein, end-binding protein-1 (EB1), a kinetochore protein, PfNDC80, and centromere-associated protein, PfCENH3, are involved in the nuclear microtubule assembly/disassembly (12).
End-binding EB1 proteins are microtubule plus-end-tracking proteins (+TIPs) (18), which play a crucial role in regulating MT dynamics by binding growing MT ends and interacting with a network of +TIPs (18). In budding yeast mutants, BIM1 (an EB1 homolog) plays a role in MT search and capture, cell polarization, and chromosome stability (19). BIM1 gene deletion results in increased net polymerization (19). EB1 demonstrates a critical role in mitosis by aiding in kinetochore clustering and MT stability during segregation of chromosomes toward cell poles (20). However, in plant cells, EB1 colocalizes with MTs, demonstrates +TIP abilities, and influences MT dynamics by impacting polymerization rates (21). In the Apicomplexan parasite, Toxoplasma gondii (Tg), TgEB1 is localized to the nucleus and tightly regulates MT dynamics (22). EB1 has recently been localized to the full length of the nuclear MT bundles of P. falciparum during asexual and sexual erythrocytic stages (12), suggesting a possible role in chromosomal segregation during these developmental stages.
We recently demonstrated that both Pfcdpk4− parasites (23) and Pfsrpk1− parasites (24) exhibit severe defects in exflagellation and microgametogenesis. Also, our transcriptomic and proteomic studies on both these parasite gene knockout lines revealed that they have a strong dysregulation in the expression of genes encoding transcripts for biological processes linked to MT and cell motility (23, 24). Interestingly, PfEB1 was hypo-phosphorylated in Pfcdpk4− parasites at S15 (23), and its transcripts were severely downregulated in Pfsrpk1− parasites (24). Therefore, we sought to determine PfEB1 function during sexual stages. We created gene deletion parasites (Pfeb1−) via CRISPR/Cas9-mediated transgenesis. Pfeb1− parasites showed normal asexual blood-stage growth and underwent normal gametocytogenesis. Strikingly, Pfeb1− parasites exhibited a robust block in transmission to mosquitoes. Careful examination of microgametes revealed a severe defect in partitioning of nuclei in microgametes in Pfeb1− parasites, while macrogametes formed normally. Further genetic crosses involving male-only and female-only sterile parasite lines demonstrated that PfEB1 is critical for male gamete fertility only.
RESULTS
PfEB1 is not required for intra-erythrocytic parasite development
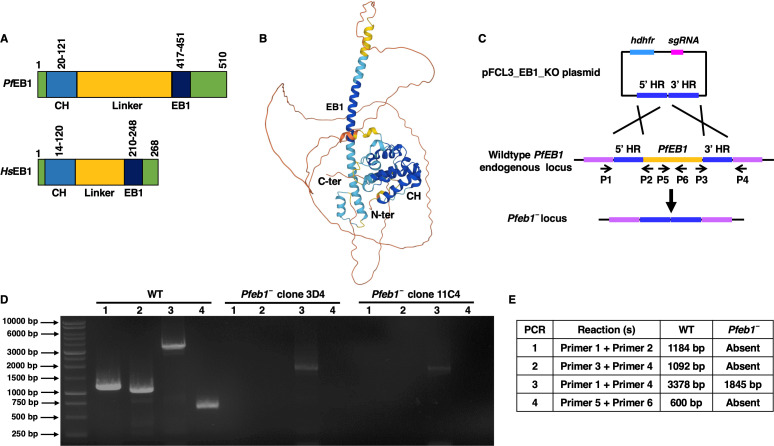
PfEB1 is encoded on chromosome 3 with gene identifier PF3D7_0307300 (https://plasmodb.org/plasmo/app/record/gene/PF3D7_0307300). PfEB1 domain analysis using SMART (http://smart.embl-heidelberg.de/) revealed that it contains a calponin homology (CH) domain at the N terminus and a namegiving EB1 domain at the C terminus, separated by a linker region (Fig. 1A). The linker region of PfEB1 is longer than that of Human EB1 (HsEB1) (Fig. 1A). Further structural analysis using Alphafold (https://alphafold.ebi.ac.uk/) revealed a conserved EB1 structure for PfEB1 (Fig. 1B). A sequence alignment of the various Plasmodium spp. revealed that EB1 shows high degree of conservation in its CH domain and EB1 domain including a conserved serine residue at amino acid position 15 (S15) (Fig. S1). The CH domain of HsEB1 is responsible for the MT binding observed from EB1 localization to MT plus ends (25) and while the EB1 domain binds to adenomatous polyposis coli-binding domain (26, 27). For studying the role of EB1 in parasite development, endogenous PfEB1 gene deletion was achieved using CRISPR/Cas9 (Fig. 1C). A set of diagnostic PCRs with oligonucleotides specific to the PfEB1 locus and its upstream (5′) and downstream (3′) regions were used for confirmation of gene deletion parasites (Pfeb1−) (Fig. 1C to E). Two clones for Pfeb1− parasites (clones 3D4 and 11C4) were used for phenotypic analysis. Pfeb1− parasites (clones 3D4 and 11C4) as well as wild-type (WT) NF54 parasites were used for a comparative asexual parasite growth assay. Growth of the parasites was monitored over two continuous asexual replication cycles with Giemsa-stained thin smears prepared from in vitro cultures every 48 hours. Microscopic enumeration of parasitemias indicated that Pfeb1− parasites grow similarly to WT PfNF54 parasites (Fig. 2A), demonstrating that PfEB1 is not critical for asexual blood-stage development and replication.
Fig 1.
PfEB1 and generation of Pfeb1− parasites. (A) Domain architecture of PfEB1 (upper cartoon) and HsEB1 (lower cartoon) showing the CH domains in blue and the EB1 domains in dark blue. The EB1 domain extends from 417 to 451 amino acids (aa) in Pf and from 210 to 248 aa in Hs. The linker region is shown in yellow. (B) Structural model of PfEB1 created using Alphafold (https://alphafold.ebi.ac.uk/). (C) The schematic of strategy for PfEB1 deletion. Oligonucleotides were designed from outside 5′HR and 3′HR. The arrows indicate their positions inside PfEB1 locus and outside homology arms. (D) Confirmation of Pfeb1− clonal parasites by a set of diagnostic PCRs. (E) The expected sizes for different sets of PCRs are shown.
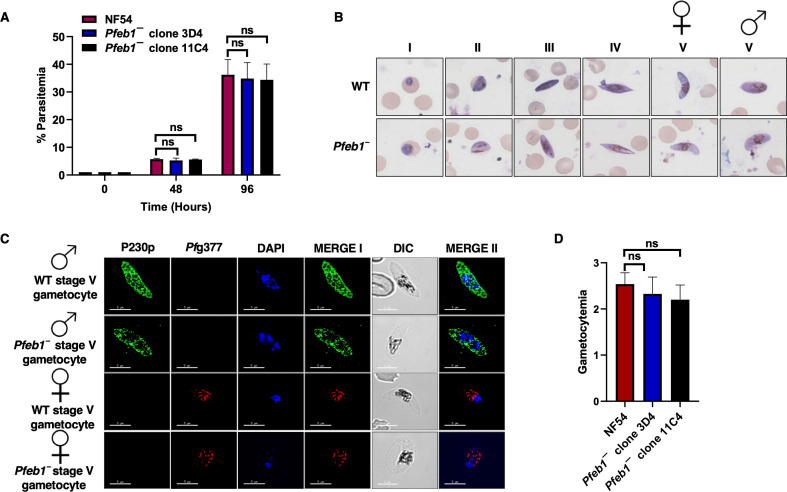
Fig 2.
Pfeb1− asexual stages grow normally and undergo gametocytogenesis. (A) Ring-stage synchronous cultures for WT PfNF54 and Pfeb1− parasites (clones 3D4 and 11C4) were plated, and parasite growth was measured over the course of two asexual replication cycles. Data from three biological replicates were averaged and presented as mean ± standard deviation. ns, not significant. (B) Ring-stage synchronous cultures for WT PfNF54 and Pfeb1− parasites (clones 3D4 and 11C4) were assessed for their ability to form gametocytes. Light microscopy images of Giemsa-stained smears at 100× magnification showed development of WT PfNF54 and Pfeb1− parasites through the five (I–V) distinct morphological stages. Female and male gametocytes are indicated using sex symbols shown on top of stage V gametocytes. (C) Immunofluorescence assays were performed on thin culture smears of mature stage V gametocytes for WT PfNF54 and Pfeb1− parasites and were stained using anti-PfP230p (green) and anti-Pfg377 antisera (red), markers for stage V male and female gametocytes, respectively. Representative images are shown. Parasite DNA was visualized with DAPI (blue). Scale bar = 5 µm. Merge I indicates the merged images for red and green panels. Merge II indicates the merged images for red, green, and blue panels. DAPI, 4′,6-diamidino-2-phenylindole; DIC, differential interest contrast. Male and female gametocytes are indicated using sex symbols shown on the left side of the image panels. (D) On day 15 of in vitro culture, gametocytemia were measured using thin Giemsa-stained smears. Data from three biological replicates were averaged and presented as mean ± standard deviation. ns, not significant.
Pfeb1− parasites undergo gametocytogenesis and exhibit normal exflagellation
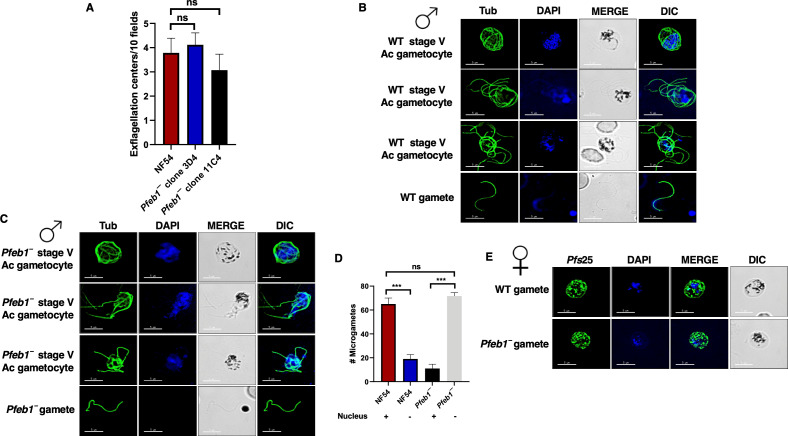
Pfeb1− parasites were next analyzed for their ability to undergo gametocytogenesis. On day 15 of in vitro culture, gametocytemia for both Pfeb1− parasites (clones 3D4 and 11C4) and WT PfNF54 parasites were scored using Giemsa-stained culture smears and microscopic inspection. Pfeb1− gametocytemias were similar to WT PfNF54 (Fig. 2D), indicated by their ability to undergo gametocytogenesis, develop through all five gametocyte stages, and further develop into mature male and female gametocytes (Fig. 2B and C). Immunofluorescence assays (IFAs) were performed to better visualize male and female stage V gametocytes using anti-PfP230p (28) and anti-Pfg377 antibodies (29), respectively. This revealed normal differentiation of Pfeb1− parasites into both genders and further confirmed results obtained from Giemsa-stained smears (Fig. 2C). To next analyze the ability of Pfeb1− mature gametocytes to undergo gametogenesis, day 15 WT PfNF54 and Pfeb1− parasites were activated by addition of O+ human serum and a decrease in temperature from 37°C to room temperature. Wet mounts were prepared using activated gametocyte cultures and then viewed under bright-field microscopic illumination at 40× magnification to measure the exflagellation centers. Pfeb1− parasites exhibited similar numbers of exflagellation centers as WT parasites, indicating no exflagellation defects (Fig. 3A). We next performed IFAs on activated gametocytes and free microgametes using anti-tubulin antibodies. This revealed that microgamete formation in Pfeb1− parasites was similar to WT PfNF54 parasites (Fig. 3B and C). During microgametogenesis, male gametocytes rapidly undergo three rounds of DNA replication (8N), which is allocated in eight flagellar microgametes along with axoneme formation. Therefore, we next examined the DNA allocation in free microgametes (Fig. 3B and C). Strikingly, however, examination of free microgametes for WT PfNF54 and Pfeb1− parasites for DNA staining revealed that the majority of Pfeb1− microgametes were DAPI negative (Fig. 3D), suggesting a possible male fertility defect. To determine a possible defect in female gametogenesis, we performed IFAs using Pfs25 antibodies, which mark activated female gametocytes and macrogametes. These revealed that Pfeb1− parasites form normal macrogametes like WT PfNF54 parasites (Fig. 3E).
Fig 3.
The Pfeb1− parasites exhibit defect in nuclear segregation during microgametogenesis. (A) Exflagellation centers per field were quantified at 15-minute post-activation. Data from three biological replicates were averaged and presented as mean ± standard deviation. Pfeb1− male gametocytes formed similar exflagellation centers as WT NF54 parasites. (B) and (C) IFAs were performed on mature stage V microgametocyte thin culture smears. Cultures were activated for 20 minutes in vitro for WT PfNF54 or Pfeb1− (clones 3D4 and 11C4) and stained for α-tubulin (green), a marker for male gametocytes in an IFA. Microgametes emerged from an exflagellating male gametocyte in the WT PfNF54 and Pfeb1− male gametocytes, shown via α-tubulin staining. (D) IFAs were performed on free microgametes for WT PfNF54 and Pfeb1− using α-tubulin, and parasite DNA was stained with DAPI. Quantitation of microgametes showed normal nuclear segregation for WT NF54 parasites, while the majority of Pfeb1−-free microgametes did not inherit nuclei. (F) IFAs were performed on free macrogametes for WT PfNF54 and Pfeb1− parasites using α-Pfs25, and parasite DNA was stained with DAPI. Pfeb1− female gametes formed normally like WT NF54 parasites. ns, not significant.
The Pfeb1− parasites do not transmit to the mosquito vector due to reduced male fertility
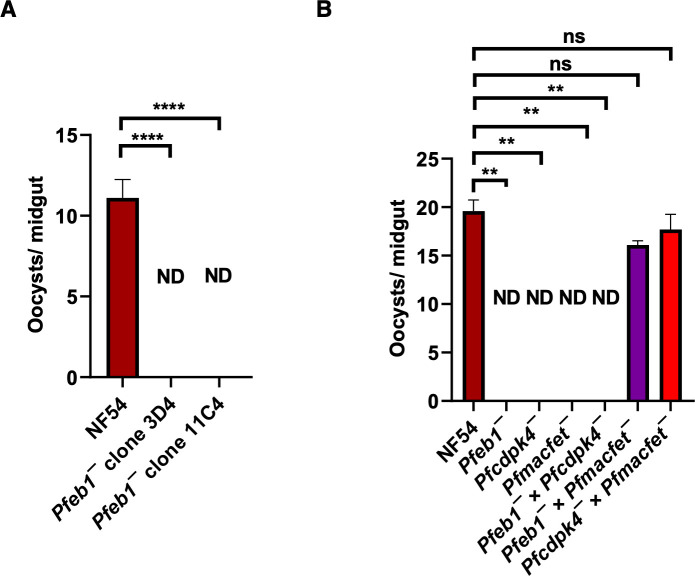
Next, Pfeb1− gametocytes were examined for their transmissibility to female Anopheles stephensi mosquitoes. Infectious blood meals were prepared for WT PfNF54 and Pfeb1− stage V gametocytes using standard procedures, and gametocytes were then fed to mosquitoes through membrane feeders. Mosquito midguts, dissected and analyzed under bright-field microscope at 10× magnification on day 7 post-feed, revealed a severe reduction in oocyst numbers for Pfeb1− parasite infections in comparison to well-infected WT controls (Fig. 4A). Since P. falciparum male and female gametocytes exist together, it is not feasible to determine a sex-specific fertility and transmission defect; genetic crosses were performed between Pfeb1− parasites and male-sterile Pfcdpk4− parasites (23) and female-sterile Pfmacfet− parasites (30). For this, the stage V gametocytes from both Pfeb1− parasites and Pfcdpk4− parasites or Pfeb1− parasites and Pfmacfet− parasites were mixed and fed to the same mosquitoes. The genetic cross between Pfeb1− parasites and Pfcdpk4− parasites showed no transmission, while the genetic cross between Pfeb1− parasites and Pfmacfet− parasites showed productive transmission. This demonstrated a male-gamete-specific fertility defect in Pfeb1− gametocytes (Fig. 4B). Taken together, the data show that PfEB1 is critical for parasite transmission to the mosquito vector via a crucial role during microgametogenesis.
Fig 4.
The Pfeb1− parasites exhibit a robust defect in parasite transmission to the mosquitos. (A) On day 7 post-feed, A. stephensi mosquitos were dissected, and the number of oocysts per midgut were measured. The Pfeb1− parasites did not transmit to the mosquitoes. Data from three biological replicates with a minimum of 50 midguts were averaged and presented as mean ± standard deviation. (B) On day 7 post-feed, A. stephensi mosquitos were dissected, and the number of oocysts per midgut were measured for WT PfNF54, Pfeb1−, Pfcdpk4−, Pfmacfet−, Pfeb1− × Pfcdpk4−, Pfeb1− × Pfmacfet−, and Pfcdpk4− × Pfmacfet−. In vitro genetic crosses revealed a microgamete fertility defect in Pfeb1− parasites. ND, not detected; ns, not significant.
DISCUSSION
To complete the sexual phase of the P. falciparum life cycle, a subset of asexually replicating parasites commit to the sexual pathway, followed by differentiation into gametocytes and uptake by Anopheline mosquitoes. Environmental triggers within the mosquito midgut activate male gametocyte differentiation into eight flagellated microgametes in a rapid process called exflagellation (13). Microgametes show vigorous motility and, upon encountering a female, initiate fertilization to enable the union of the two haploid genomes and sexual recombination. In Plasmodium, the microtubules play important structural roles in the invasive forms (31, 32) as well as in gametocytes, power the motility of microgametes, and play critical role in mitosis (33, 34). During mitosis in Plasmodium, the chromosomes are present in an uncondensed state, and the nuclear membrane remains intact as nuclear microtubules capture the kinetochores (34, 35). An electron-dense acentriolar centrosome called the centriolar plaque, which is embedded in a pore in the nuclear envelope, serves as MTOC and is likely present throughout the cell cycle (34, 36, 37). Exflagellation is an astounding cellular process producing eight motile exflagellae, each with a full genome equivalent, from a 1N haploid microgamete following the assembly and stabilization of the nuclei with their associated nuclear envelope non-mitotic MTOC, outer centriolar basal body, and axoneme (38). Proteins involved specifically with basal body function during male gametogenesis of Plasmodium berghei (Pb) and most likely for P. falciparum include SAS6 (39), SAS4 (40), and Kinesin-8B (41). Our study reveals a critical function for nuclear MAP EB1 in nuclear partitioning during P. falciparum microgametogenesis, which is crucial for male gamete fertility.
The EB1 family proteins are evolutionarily conserved and have been identified in every organism and nearly all cell types (18). EB proteins comprise of ~300 amino acids with an N-terminal CH domain, a linker region, C-terminal domain, and overlapping coiled-coil and EBH domains, with the CH and coiled-coil domains being conserved in EB1 proteins from diverse organisms (20). However, EB1 is the only member of the +TIP family with an ortholog in P. falciparum (12). PfEB1 contains a CH domain and an EB1 domain separated by a linker region. The linker region between the CH and EB1 domains in P. falciparum is larger compared to that in HsEB1, despite having similar respective positioning of their EB1 domains and CH domains of similar sizes. HsEB1 is heavily modified at numerous sites (Y6, S7, S9, S40, K60, K66, Y71, K76, K83, K89, K95, K100, Y119, K122, Y124, S140, N147, K148, K150, K151, T154, S156, S155, S165, T166, K174, K182, T206, K212, Y217, K220, Y247, and Y268) (PhosphoSitePlus v6.7.0.1). Notably, K66 of HsEB1 is acetylated, ubiquitylated, and crotonylated, with crotonylation of K66 functioning in spindle orientation during mitosis (42). So far, no PTMs have been reported for PfEB1, although in our previous data sets, we found S15 is phosphorylated in gametocyte stages and hypo-phosphorylated in Pfcdpk4− parasites, which exhibited a severe male gametogenesis defect (23). The Pfcdpk4− gametocytes also show hypo-phosphorylation of several parasite kinases, including ARK2, RIO1, and SRPK2 (23). Yeast EB1 (BIM1) is phosphorylated by Aurora kinase homolog lpl1 (43), while HsEB1 co-immunoprecipitates with Aurora kinase B (44). Also, HsEB1 is required to enrich Aurora kinase B at inner centromeres in an MT-dependent manner, resulting in phosphorylation of both kinetochore and other chromatin substrates (45). A recent study in P. berghei also demonstrated the interaction between ARK2 and EB1 (46). It is reasonable to propose that PfCDPK4-mediated phosphorylation of ARK2 and/or ARK2-mediated phosphorylation of EB1 at S15 may be regulating its microgametogenesis function. This hypothesis is further supported by a recent study demonstrating the role of EB1 S15 in spindle-kinetochore attachment during microgametogenesis in P. yoelii (47). It will be interesting to explore the role of S15 phosphorylation on PfEB1 for its relevance during parasite transmission.
PfEB1 contributes to the functions of an MTOC embedded in the nuclear membrane, which stabilizes and controls the elaborate MT network in gametocytes (12). PfEB1 associates with nuclear MTs, where they bind along the full length of the MT bundles, rather than being strictly localized to the plus end (12). Captured chromatin on the nuclear MT bundles in gametocytes has been indicated by the elongated localization of EB1 in conjunction with the localized regions of chromatin to the nuclear MTs (12). Further heterologous expression experiments have demonstrated that PyEB1 has an intrinsic MT-lattice binding property (47). The Pfeb1− parasites described herein showed that PfEB1 is not required for asexual blood-stage proliferation and gametocytogenesis. Strikingly, the majority of the male Pfeb1− gametes lack a nucleus, indicating a defect in nuclear division in the absence of EB1, which may result in a motility defect or early death in these microgametes. It is possible that some parasites can compensate for the defects in MT stabilization in the absence of EB1, but there is a delay in mitosis completion, which results in the absence of nuclei in majority of the microgametes. This would be in stark contrast to PbEB1 as Pbeb1− parasites form normal microgametes and do transmit to the mosquitoes (46). Interestingly, the Pyeb1− parasites like Pfeb1− also exhibit a nuclear segregation defect in microgametes which is restored upon complementation with PfEB1 (47). This reflects a species-specific function for EB1 in different malaria parasites.
Human Kinesin Family Member 18B (KIF18B) is an EB1-binding protein localized to the nucleus during interphase, where it is enriched on astral MT plus ends (48). Furthermore, Kif18B regulates astral MT organization and length during early mitosis (48). Similarly, P. berghei kinesin-8B is essential for microgamete formation due to its key role in basal body formation and assembly, as well as structural organization of the axoneme (41). Taken together, kinesin-8B is predicted to interact with EB1 during microgametogenesis. Proper kinetochore assembly is essential for ensuring chromosome segregation during mitosis and, thus, is an essential step in parasite transmission. This process is regulated by centromere proteins CENP-A and CENP-C, kinesins, and MAPs (49). Kinesin-8s, specifically, are associated with MTs and influence MT dynamics (41). The Plasmodium genome encodes kinesin-8X and kinesin-8B, the latter of which is essential in microgamete development due to its role in the formation of basal bodies and axoneme development (41). In related Apicomplexan parasite, T. gondii, TgEB1 regulates nuclear divisions by controlling spindle dynamics and its association to the kinetochore NDC80 complex (22). Indirectly, TgEB1 secures kinetochore organization and promotes accurate chromosome segregation (22). Both in P. falciparum (12) and P. berghei (46), EB1 associates with kinetochore marker NDC80 (46), while PbEB1 also associates with basal body marker SAS4 (46). Another basal body marker PbSAS6 is known to have role in nuclear allocation in microgametes (39). Since PfEB1 associates with kinetochore (12) and Pfeb1− parasites show an abnormal nuclear allocation, we hypothesize that PfEB1 may function in endomitosis during microgametogenesis. The high-resolution microscopy and live-cell imaging experiments would be required to confirm this hypothesis.
In summary, our study demonstrates a crucial role for PfEB1 during male gametogenesis and parasite transmission to the mosquito and links a nuclear microtubule-binding protein to DNA segregation during microgametogenesis.
MATERIALS AND METHODS
Reagents and antibodies
The molecular biology reagents were purchased from either MilliporeSigma, USA or Thermo Fisher Scientific, USA, unless otherwise stated. All the restriction enzymes and DNA polymerases were purchased from New England Biolabs, and all the oligonucleotides were purchased from Integrated DNA Technologies, Inc., USA. The following primary antibodies, antisera, and dilutions were utilized: mouse α-tubulin (1:250; Sigma-Aldrich; catalog# T5168), rabbit α-Pfg377 (1:250; kindly gifted by Prof. Pietro Alano at Istituto Superiore di Sanità, Rome, Italy) (29), and mouse α-PfP230p (1:200, kindly gifted by Prof. Kim C. Williamson, Uniformed Services University of the Health Sciences, USA (28). Reagents obtained through BEI Resources, NIAID, and NIH include: hybridoma 4B7 α-Pfs25-kilodalton gamete surface protein (Pfs25), MRA-315, contributed by Louis H. Miller and Allan Saul (1:1 in 3% bovine serum albumin/phosphate-buffered saline, mouse). The Alexa Fluor-conjugated secondary antibodies utilized for IFAs were procured from Thermo Fisher Scientific, USA.
P. falciparum culture and transfection
Standard procedures were followed to culture P. falciparum parasites (WT PfNF54 and Pfeb1−) as asexual blood stages, which received complete RPMI 1640 media, supplemented with 10% (vol/vol) human serum every 24 hours. O+ human RBCs (Valley Biomedical, VA, USA) and O+ human serum (Valley Biomedical, VA, USA or Interstate Blood Bank, TN, USA) were used to generate in vitro gametocytes using methods published elsewhere (50). PfEB1 Gene deletion parasites, Pfeb1−, were generated via CRISPR/Cas9 strategy. Recombinant parasites were drug selected, PCR genotyped, and then cloned by limiting dilution. The clonal parasites were confirmed by a set of genotyping PCRs (Fig. 1D and E). Functional assays used two individual clones for Pfeb1− parasites (clones 3D4 and 11C4).
Measurement of asexual blood-stage growth and gametocyte development
WT PfNF54 and Pfeb1− parasites were synchronized at ring stages using 5% sorbitol, and plating was done at equal parasitemia (1%) in six-well plates for comparative analysis of growth rates. Parasite growth for two consecutive asexual replication cycles was quantified by scoring parasitemia on thin Giemsa-stained smears. Likewise, gametocytemia was quantified on day 15 of in vitro culture on thin Giemsa-stained smears for comparison of sexual development.
Indirect immunofluorescence assays
IFAs were performed on asexual and sexual blood-stage parasites and exflagellating microgametocytes using thin smears prepared on Teflon-coated slides as described elsewhere (50). Antigens were visualized using anti-species antibodies. Images were acquired using a 100× 1.4 NA objective 90 (Olympus) on a Delta Vision Elite High-Resolution Microscope (GE Healthcare Life Sciences).
Exflagellation, standard membrane feeding assay, genetic crosses, and oocyst measurements
Exflagellation, standard membrane feeding assay, and mosquito midgut oocyst measurements for comparative assessments were performed as described elsewhere (51). For performing genetic crosses, Pfcdpk4−, Pfmacfet−, and Pfeb1− parasites were cultured as gametocytes in vitro. The day 15 gametocytes from Pfcdpk4− and Pfmacfet− were mixed to perform a positive control genetic cross (Pfcdpk4− × Pfmacfet−). The day 15 gametocytes for Pfcdpk4− and Pfeb1− were mixed to determine a male-specific function (Pfcdpk4− × Pfeb1−), while gametocytes for Pfmacfet− and Pfeb1− were mixed to determine a female-specific function (Pfmacfet− × Pfeb1−) for PfEB1, respectively. These gametocyte mixes were fed to female A. stephensi mosquitoes, and day 7 midgut oocysts were enumerated for parasite transmission.
Statistical analysis
Data collected were expressed as mean ± SD. One-way analysis of variance with post hoc Bonferroni multiple comparison test or unpaired two-tailed Student’s t test were used, as indicated, to determine statistical differences. Statistical significances were calculated using GraphPad Prism 9.4, with values of P < 0.05 being considered significant. Significance is represented in the figures as follows: ns, not significant, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
ACKNOWLEDGMENTS
The authors acknowledge and thank William W. Betz, Kenza M.Z. Oualim, and Cecilia Kalthoff for maintaining insectaries at Center for Global Infectious Disease Research, Seattle Children’s Research Institute and timely providing uninfected mosquitoes for this research.
Seed funds were received from Seattle Children’s to S.H.I.K. via LAN code 24010119.
Conceptualization: S.K. Methodology: S.M. and S.K. Investigation: S.M., N.C., B.A.A., O.R.G., and S.K. Visualization: S.M., B.A.A., O.R.G., and S.K. Resources: S.H.I.K. Funding acquisition: S.H.I.K. Supervision: S.H.I.K. Writing—original draft: S.M. and S.K. Writing—review and editing: S.M., S.K., and S.H.I.K.
The authors declare no competing financial or non-financial interests.
Contributor Information
Sudhir Kumar, Email: sudhir.kumar@seattlechildrens.org.
Louis M. Weiss, Albert Einstein College of Medicine, Bronx, New York, USA
DATA AVAILABILITY
All other relevant data are available from the authors upon reasonable request.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.00822-23.
The EB1 proteins are highly conserved in Plasmodium.
Supplemental material descriptions.
Oligonucleotides used in the study.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Goodson HV, Jonasson EM. 2018. Microtubules and microtubule-associated proteins. Cold Spring Harb Perspect Biol 10:a022608. doi: 10.1101/cshperspect.a022608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hirst WG, Fachet D, Kuropka B, Weise C, Saliba KJ, Reber S. 2022. Purification of functional Plasmodium falciparum tubulin allows for the identification of parasite-specific microtubule inhibitors. Curr Biol 32:919–926. doi: 10.1016/j.cub.2021.12.049 [DOI] [PubMed] [Google Scholar]
- 3. Zhang G, Niu G, Hooker-Romera D, Shabani S, Ramelow J, Wang X, Butler NS, James AA, Li J. 2023. Targeting plasmodium α-tubulin-1 to block malaria transmission to mosquitoes. Front Cell Infect Microbiol 13:1132647. doi: 10.3389/fcimb.2023.1132647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pollard TD. 2016. Actin and actin-binding proteins. Cold Spring Harb Perspect Biol 8:a018226. doi: 10.1101/cshperspect.a018226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fletcher DA, Mullins RD. 2010. Cell mechanics and the cytoskeleton. Nature 463:485–492. doi: 10.1038/nature08908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vleugel M, Kok M, Dogterom M. 2016. Understanding force-generating microtubule systems through in vitro reconstitution. Cell Adh Migr 10:475–494. doi: 10.1080/19336918.2016.1241923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreira JL, Pražák V, Vasishtan D, Siggel M, Hentzschel F, Binder AM, Pietsch E, Kosinski J, Frischknecht F, Gilberger TW, Grünewald K. 2023. Variable microtubule architecture in the malaria parasite. Nat Commun 14:1216. doi: 10.1038/s41467-023-36627-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wichers-Misterek JS, Binder AM, Mesén-Ramírez P, Dorner LP, Safavi S, Fuchs G, Lenz TL, Bachmann A, Wilson D, Frischknecht F, Gilberger T-W. 2023. A microtubule-associated protein is essential for malaria parasite transmission. mBio 14:e0331822. doi: 10.1128/mbio.03318-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferreira JL, Heincke D, Wichers JS, Liffner B, Wilson DW, Gilberger TW. 2020. The dynamic roles of the inner membrane complex in the multiple stages of the malaria parasite. Front Cell Infect Microbiol 10:611801. doi: 10.3389/fcimb.2020.611801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parkyn Schneider M, Liu B, Glock P, Suttie A, McHugh E, Andrew D, Batinovic S, Williamson N, Hanssen E, McMillan P, Hliscs M, Tilley L, Dixon MWA. 2017. Disrupting assembly of the inner membrane complex blocks Plasmodium falciparum sexual stage development. PLoS Pathog 13:e1006659. doi: 10.1371/journal.ppat.1006659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sinden RE, Canning EU, Bray RS, Smalley ME. 1978. Gametocyte and gamete development in Plasmodium falciparum. Proc R Soc Lond B Biol Sci 201:375–399. doi: 10.1098/rspb.1978.0051 [DOI] [PubMed] [Google Scholar]
- 12. Li J, Shami GJ, Cho E, Liu B, Hanssen E, Dixon MWA, Tilley L. 2022. Repurposing the mitotic machinery to drive cellular elongation and chromatin reorganisation in Plasmodium falciparum gametocytes. Nat Commun 13:5054. doi: 10.1038/s41467-022-32579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arai M, Billker O, Morris HR, Panico M, Delcroix M, Dixon D, Ley SV, Sinden RE. 2001. Both mosquito-derived xanthurenic acid and a host blood-derived factor regulate gametogenesis of Plasmodium in the midgut of the mosquito. Mol Biochem Parasitol 116:17–24. doi: 10.1016/s0166-6851(01)00299-7 [DOI] [PubMed] [Google Scholar]
- 14. Gunes S, Sengupta P, Henkel R, Alguraigari A, Sinigaglia MM, Kayal M, Joumah A, Agarwal A. 2020. Microtubular dysfunction and male infertility. World J Mens Health 38:9–23. doi: 10.5534/wjmh.180066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akhmanova A, Hoogenraad CC. 2005. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr Opin Cell Biol 17:47–54. doi: 10.1016/j.ceb.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 16. Orosz F. 2009. Apicortin, a unique protein, with a putative cytoskeletal role, shared only by apicomplexan parasites and the placozoan Trichoplax adhaerens. Infect Genet Evol 9:1275–1286. doi: 10.1016/j.meegid.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 17. Zhang C, Li D, Meng Z, Zhou J, Min Z, Deng S, Shen J, Liu M. 2022. Pyp25α is required for male gametocyte exflagellation. Pathog Dis 80:ftac043. doi: 10.1093/femspd/ftac043 [DOI] [PubMed] [Google Scholar]
- 18. Tirnauer JS, Bierer BE. 2000. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J Cell Biol 149:761–766. doi: 10.1083/jcb.149.4.761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz K, Richards K, Botstein D. 1997. BIM1 encodes a microtubule-binding protein in yeast. Mol Biol Cell 8:2677–2691. doi: 10.1091/mbc.8.12.2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nehlig A, Molina A, Rodrigues-Ferreira S, Honoré S, Nahmias C. 2017. Regulation of end-binding protein EB1 in the control of microtubule dynamics. Cell Mol Life Sci 74:2381–2393. doi: 10.1007/s00018-017-2476-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bisgrove SR, Lee Y-R, Liu B, Peters NT, Kropf DL. 2008. The microtubule plus-end binding protein EB1 functions in root responses to touch and gravity signals in Arabidopsis. Plant Cell 20:396–410. doi: 10.1105/tpc.107.056846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen C-T, Kelly M, Leon J de, Nwagbara B, Ebbert P, Ferguson DJP, Lowery LA, Morrissette N, Gubbels M-J. 2015. Compartmentalized Toxoplasma EB1 bundles spindle microtubules to secure accurate chromosome segregation. Mol Biol Cell 26:4562–4576. doi: 10.1091/mbc.E15-06-0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar S, Haile MT, Hoopmann MR, Tran LT, Michaels SA, Morrone SR, Ojo KK, Reynolds LM, Kusebauch U, Vaughan AM, Moritz RL, Kappe SHI, Swearingen KE, Weiss LM. 2021. Plasmodium falciparum calcium-dependent protein kinase 4 is critical for male gametogenesis and transmission to the mosquito vector. mBio 12:e0257521. doi: 10.1128/mBio.02575-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar S, Baranwal VK, Leeb AS, Haile MT, Oualim KMZ, Hertoghs N, Vaughan AM, Kappe SHI. 2022. PfSRPK1 regulates asexual blood stage schizogony and is essential for male gamete formation. Microbiol Spectr 10:e0214122. doi: 10.1128/spectrum.02141-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayashi I, Ikura M. 2003. Crystal structure of the amino-terminal microtubule-binding domain of end-binding protein 1 (EB1). J Biol Chem 278:36430–36434. doi: 10.1074/jbc.M305773200 [DOI] [PubMed] [Google Scholar]
- 26. Barth AIM, Siemers KA, Nelson WJ. 2002. Dissecting interactions between EB1, microtubules and APC in cortical clusters at the plasma membrane. J Cell Sci 115:1583–1590. doi: 10.1242/jcs.115.8.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Askham JM, Vaughan KT, Goodson HV, Morrison EE. 2002. Evidence that an interaction between EB1 and P150(glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol Biol Cell 13:3627–3645. doi: 10.1091/mbc.e02-01-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eksi S, Williamson KC. 2002. Male-specific expression of the paralog of malaria transmission-blocking target antigen Pfs230, PfB0400w. Mol Biochem Parasitol 122:127–130. doi: 10.1016/s0166-6851(02)00091-9 [DOI] [PubMed] [Google Scholar]
- 29. Suárez-Cortés P, Sharma V, Bertuccini L, Costa G, Bannerman N-L, Sannella AR, Williamson K, Klemba M, Levashina EA, Lasonder E, Alano P. 2016. Comparative proteomics and functional analysis reveal a role of Plasmodium falciparum osmiophilic bodies in malaria parasite transmission. Mol Cell Proteomics 15:3243–3255. doi: 10.1074/mcp.M116.060681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumar S, Abatiyow BA, Haile MT, Oualim KMZ, Leeb AS, Vaughan AM, Kappe SHI. 2022. A putative Plasmodium RNA-binding protein plays a critical role in female gamete fertility and parasite transmission to the mosquito vector. Front Cell Dev Biol 10:825247. doi: 10.3389/fcell.2022.825247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fowler RE, Fookes RE, Lavin F, Bannister LH, Mitchell GH. 1998. Microtubules in Plasmodium falciparum merozoites and their importance for invasion of erythrocytes. Parasitology 117:425–433. doi: 10.1017/s003118209800328x [DOI] [PubMed] [Google Scholar]
- 32. Morrissette NS, Sibley LD. 2002. Cytoskeleton of apicomplexan parasites. Microbiol Mol Biol Rev 66:21–38. doi: 10.1128/MMBR.66.1.21-38.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Francia ME, Dubremetz J-F, Morrissette NS. 2015. Basal body structure and composition in the apicomplexans Toxoplasma and Plasmodium. Cilia 5:3. doi: 10.1186/s13630-016-0025-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gerald N, Mahajan B, Kumar S. 2011. Mitosis in the human malaria parasite Plasmodium falciparum. Eukaryot Cell 10:474–482. doi: 10.1128/EC.00314-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aikawa M, Beaudoin RL. 1968. Studies on nuclear division of a malarial parasite under pyrimethamine treatment. J Cell Biol 39:749–754. doi: 10.1083/jcb.39.3.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arnot DE, Ronander E, Bengtsson DC. 2011. The progression of the intra-erythrocytic cell cycle of Plasmodium falciparum and the role of the centriolar plaques in asynchronous mitotic division during schizogony. Int J Parasitol 41:71–80. doi: 10.1016/j.ijpara.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 37. Simon CS, Funaya C, Bauer J, Voβ Y, Machado M, Penning A, Klaschka D, Cyrklaff M, Kim J, Ganter M, Guizetti J. 2021. An extended DNA-free intranuclear compartment organizes centrosome microtubules in malaria parasites. Life Sci Alliance 4:e202101199. doi: 10.26508/lsa.202101199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sinden RE. 1991. Mitosis and meiosis in malarial parasites. Acta Leiden 60:19–27. [PubMed] [Google Scholar]
- 39. Marques SR, Ramakrishnan C, Carzaniga R, Blagborough AM, Delves MJ, Talman AM, Sinden RE. 2015. An essential role of the basal body protein SAS-6 in Plasmodium male gamete development and malaria transmission. Cell Microbiol 17:191–206. doi: 10.1111/cmi.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeeshan M, Brady D, Markus R, Vaughan S, Ferguson D, Holder AA, Tewari R. 2022. Plasmodium SAS4: basal body component of male cell which is dispensable for parasite transmission. Life Sci Alliance 5:e202101329. doi: 10.26508/lsa.202101329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeeshan M, Ferguson DJ, Abel S, Burrrell A, Rea E, Brady D, Daniel E, Delves M, Vaughan S, Holder AA, Le Roch KG, Moores CA, Tewari R. 2019. Kinesin-8B controls basal body function and flagellum formation and is key to malaria transmission. Life Sci Alliance 2:e201900488. doi: 10.26508/lsa.201900488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song X, Yang F, Liu X, Xia P, Yin W, Wang Z, Wang Y, Yuan X, Dou Z, Jiang K, Ma M, Hu B, Zhang R, Xu C, Zhang Z, Ruan K, Tian R, Li L, Liu T, Hill DL, Zang J, Liu X, Li J, Cheng J, Yao X. 2021. Dynamic crotonylation of EB1 by TIP60 ensures accurate spindle positioning in mitosis. Nat Chem Biol 17:1314–1323. doi: 10.1038/s41589-021-00875-7 [DOI] [PubMed] [Google Scholar]
- 43. Zimniak T, Stengl K, Mechtler K, Westermann S. 2009. Phosphoregulation of the budding yeast EB1 homologue Bim1p by Aurora/Ipl1p. J Cell Biol 186:379–391. doi: 10.1083/jcb.200901036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun L, Gao J, Dong X, Liu M, Li D, Shi X, Dong J-T, Lu X, Liu C, Zhou J. 2008. EB1 promotes Aurora-B kinase activity through blocking its inactivation by protein phosphatase 2A. Proc Natl Acad Sci U S A 105:7153–7158. doi: 10.1073/pnas.0710018105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Banerjee B, Kestner CA, Stukenberg PT. 2014. EB1 enables spindle microtubules to regulate centromeric recruitment of Aurora B. J Cell Biol 204:947–963. doi: 10.1083/jcb.201307119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeeshan M, Rea E, Abel S, Vukusic K, Markus R, Brady D, Eze A, Rashpa R, Balestra A, Bottrill A, Brochet M, Guttery D, Tolić I, Holder A, Roch KL, Tromer E, Tewari R. 2023. Plasmodium ARK2-EB1 axis drives the unconventional spindle dynamics, scaffold formation and chromosome segregation of sexual transmission stages. Res Sq:rs.3.rs-2539372. doi: 10.21203/rs.3.rs-2539372/v1 [DOI] [PMC free article] [PubMed]
- 47. Yang S, Cai M, Huang J, Zhang S, Mo X, Jiang K, Cui H, Yuan J. 2023. EB1 decoration of microtubule lattice facilitates spindle-kinetochore lateral attachment in Plasmodium male gametogenesis. Nat Commun 14:2864. doi: 10.1038/s41467-023-38516-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stout JR, Yount AL, Powers JA, Leblanc C, Ems-McClung SC, Walczak CE. 2011. Kif18B interacts with EB1 and controls astral microtubule length during mitosis. Mol Biol Cell 22:3070–3080. doi: 10.1091/mbc.E11-04-0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Navarro AP, Cheeseman IM. 2021. Kinetochore assembly throughout the cell cycle. Semin Cell Dev Biol 117:62–74. doi: 10.1016/j.semcdb.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kumar S, Leeb AS, Vaughan AM, Kappe SHI. 2022. Plasmodium falciparum cysteine rich secretory protein uniquely localizes to one end of male gametes. Mol Biochem Parasitol 248:111447. doi: 10.1016/j.molbiopara.2022.111447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tripathi AK, Mlambo G, Kanatani S, Sinnis P, Dimopoulos G. 2020. Plasmodium falciparum gametocyte culture and mosquito infection through artificial membrane feeding. J Vis Exp. doi: 10.3791/61426 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The EB1 proteins are highly conserved in Plasmodium.
Supplemental material descriptions.
Oligonucleotides used in the study.
Data Availability Statement
All other relevant data are available from the authors upon reasonable request.