Abstract
Pancreatic ductal adenocarcinomas are distinguished by their robust desmoplasia, or fibroinflammatory response. Dominated by non-malignant cells, the mutated epithelium must therefore combat, cooperate with or co-opt the surrounding cells and signalling processes in its microenvironment. It is proposed that an invasive pancreatic ductal adenocarcinoma represents the coordinated evolution of malignant and non-malignant cells and mechanisms that subvert and repurpose normal tissue composition, architecture and physiology to foster tumorigenesis. The complex kinetics and stepwise development of pancreatic cancer suggests that it is governed by a discrete set of organizing rules and principles, and repeated attempts to target specific components within the microenvironment reveal self-regulating mechanisms of resistance. The histopathological and genetic progression models of the transforming ductal epithelium must therefore be considered together with a programme of stromal progression to create a comprehensive picture of pancreatic cancer evolution. Understanding the underlying organizational logic of the tumour to anticipate and pre-empt the almost inevitable compensatory mechanisms will be essential to eradicate the disease.
Introduction
Pancreatic ductal adenocarcinoma (PDA) has the highest 1-year, 5-year and 10-year mortalities of any cancer type and is projected to be the second-leading cause of cancer-related death by 2030 (ref.1). Most patients with PDA present with locally advanced or overt metastatic disease, precluding the chance for surgical resection and any hope of cure2,3. However, this does not entirely explain the dismal prognosis. In the fortunate few for whom surgery is possible and who successfully undergo a pancreaticoduodenectomy, or Whipple procedure, treatment success is still not assured; even after supplementing surgery with adjuvant systemic chemotherapy and/or radiotherapy, most of these patients will nevertheless succumb to disseminated disease4. Both the primary tumours and metastases display unusual resistance to essentially all forms of chemotherapy and radiotherapy as well as attempts to engage immunity.
The robust desmoplasia of PDA is its distinguishing feature, and non-malignant components constitute the bulk of the tumour mass5. This stromal reaction includes myofibroblasts, inflammatory fibroblasts, endothelial cells, pericytes and various immune cell subsets all embedded within a dense and complex extracellular matrix (ECM). Many of these cells infiltrate, differentiate and operate under the influence of oncogenic KRAS, the signature genetic mutation in PDA. These cells, in turn, support and shape the evolving malignancy. The exquisite choreography of events and processes in pancreatic cancer neo-organogenesis calls upon, and is no less complex than, the same capabilities that gave rise to the normal organ during embryogenesis.
It is frequently debated as to whether the stroma in pancreatic cancer as well as other cancer types is tumour constraining or tumour promoting. However, the stroma is neither uniform nor unchanging and the question, therefore, is misguided. Stromal influences are more nuanced: some elements overtly accelerate and others attenuate disease progression, but most of the cells and processes found in an evolving pancreatic cancer are adaptive at some point during its development6. Collectively, these elements conspire to create a drug- and immune-privileged sanctuary for PDA progression. A complex matrix biology with unusual physicomechanical properties shields PDAs from drugs and contributes to treatment resistance. Furthermore, pancreatic cancers appear to largely bypass immune editing in the classical sense because of a coordinated recruitment and reprogramming of suppressive immune cells that begins at the earliest precursor stages7. PDAs have been sheltered from, rather than being forced to engage with, and ultimately overcome, adaptive immunity. Even transiently surmounting these immune and mechanical barriers has revealed unanticipated vulnerabilities that can, in principle, be exploited.
To fully overcome the therapeutic resistance of this cancer will require parsing the myriad interactions and interdependencies among the various stromal elements and the epithelial PDA cell. Understanding how to combine strategies against these distinct components and processes — which ones to target, in what order and for how long — will be critical, and perhaps necessary, for success and to avoid making matters worse8. For example, recent studies revealing unintended consequences of prolonged chemical9 and genetic10 abrogation of myofibroblast activity in PDA provide sobering reminders that this therapeutic landscape harbours both risks and opportunities. Extrapolation from the concept of oncogene dependence suggests there may also be stromal components and mechanisms that not only support tumorigenesis but are absolutely essential for its initiation, progression and/or maintenance. The therapeutic window in targeting such an event lies in a newly created dependency where none existed before.
As there are a number of excellent recent reviews on various aspects of the pancreatic cancer microenvironment, including metabolic dependencies11,12, fibroblast heterogeneity13,14 and the immune response15-18, in this Review, I instead focus mainly on important representative examples of epithelial and stromal reciprocity, and critical interdependencies, in the hope of identifying axes of ‘stromal addiction’ that may be exploited therapeutically.
Initiating pancreatic cancer
The adenoma-to-carcinoma sequence for pancreatic cancer begins in microscopic precursor lesions, termed ‘pancreatic intraepithelial neoplasias’ (PanINs), that arise in the terminal ductules of the gland19,20. Activating mutations in the KRAS proto-oncogene lock the protein product, a small GTPase, in a constitutively ‘on’ conformation, and are the earliest and most frequent mutations in PDA21. Targeting endogenous expression of KrasG12D to tissue progenitor cells of the embryonic mouse pancreas established that the mutation was both necessary and sufficient to initiate the stochastic development and spontaneous progression of PanINs22. Specific signalling pathways were aberrantly activated in these early lesions, including potent morphogens such as sonic Hedgehog (SHH)23 and Notch22,24 and pro-inflammatory and tissue-remodelling enzymes such as cyclooxygenases and matrix metalloproteinases22. Introducing cooperating mutations in tumour suppressor genes implicated in the human disease25-28 accelerates disease progression along distinct biological and histopathological trajectories. Tumour suppressor gene mutations help shape the disease that mutant KRAS initiates, including perhaps subtler features of the tumour microenvironment (TME)29,30.
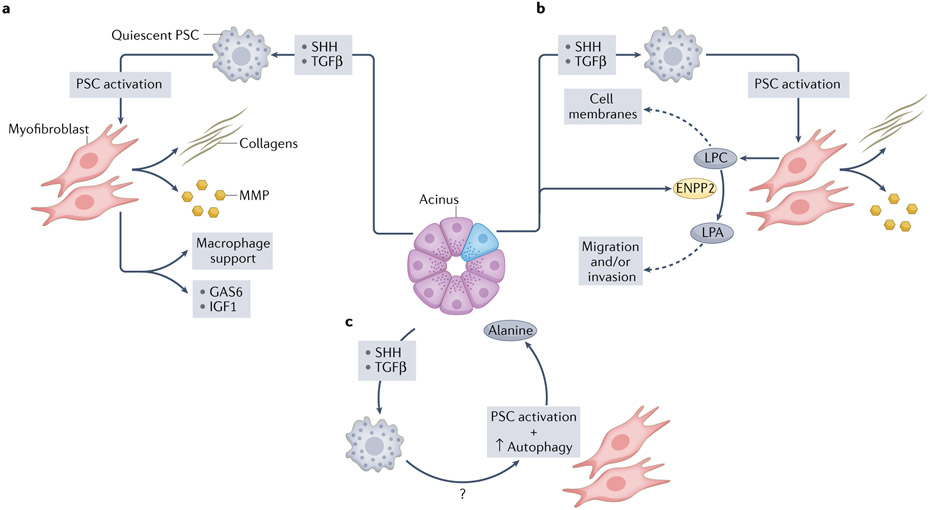
Mutant KRAS also initiates a cytokine, chemokine and growth factor storm that acts locally and systemically to shape the mesenchymal and immune response31,32. Put succinctly, mutant KRAS talks to the stroma and the stroma talks back. In a prototypical example of such heterotypic ‘oncogenic reciprocal signalling’, KRAS-G12D expression in epithelial cells induces aberrant secretion of SHH, which activates adjacent pancreatic stellate cells (PSCs)33 (Fig. 1). The activated fibroblasts respond by initiating the processes that drive desmoplasia, as well as providing growth factors and other factors to augment signalling and proliferative capacities in the initiating epithelium.
Fig. 1 ∣. Epithelial–mesenchymal reciprocity in pancreatic ductal adenocarcinoma.
Continuing the communication begun in development, the epithelial and mesenchymal compartments in pancreatic cancer engage in reciprocal signalling, shaping and evolving with each other as disease progresses. The aberrant signalling appears to start with a spontaneous activating mutation in the KRAS proto-oncogene. Mutant KRAS engages numerous signalling pathways, including the potent morphogens sonic Hedgehog (SHH) and transforming growth factor-β (TGFβ). Mesenchymal cells in close apposition with the activated epithelium are impacted by these morphogenetic gradients and respond by changing their phenotype and secretome as well. a, ‘Oncogenic reciprocal signalling’ relationships were revealed in experiments using cell-specific proteomics and multivariate phosphoproteomics analyses of epithelial KRAS-G12D signalling in conjunction with pancreatic stellate cells (PSCs)33, which are quiescent resident fibroblasts in the pancreas. Oncogenic KRAS-G12D signalling in epithelial cells induced secretion of SHH, which activated PSCs, while the epithelium remained insensitive to any potential autocrine effects. The activated fibroblasts, in turn, secreted a concerted set of molecules and enzymes (for example, collagens and matrix metalloproteinases (MMPs)) that initiated the pathognomonic desmoplasia of pancreatic ductal adenocarcinoma (PDA), and also provided growth factors, such as growth arrest-specific protein 6 (GAS6) and insulin-like growth factor 1 (IGF1), that stimulated additional receptor tyrosine kinase signalling pathways in the initiating epithelial cells. Reciprocal signalling also activated non-cell-autonomous AKT responses, promoted proliferation and survival, and increased the mitochondrial respiratory capacity in KRAS-G12D-expressing epithelial cells33. b, Activation of PSCs induced by mutant epithelial cells leads to rewiring of their lipid metabolism85. The activated myofibroblasts produce and secrete lysophosphatidylcholines (LPCs), which are taken up by newly proliferating epithelial cells and incorporated into cell membranes. The mutant epithelial cells also secrete an enzyme, ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP2; also known as ATX), that catalyses the conversion of LPCs into lysophosphatidic acid (LPA), a serum phospholipid with mitogen-like properties that signals through G protein-coupled surface receptors to stimulate proliferation, migration and invasion in target cells. c, Profoundly rewired metabolism induced by mutant KRAS in epithelial cells shunts glucose for use in glycolysis and biosynthetic pathways. To keep mitochondrial oxidative phosphorylation (OXPHOS) fuelled, the tumour epithelial cells activate PSCs and induce the myofibroblasts to increase their own autophagy and release alanine into the microenvironment, where it is scavenged by the tumour epithelial cells to be shunted into OXPHOS.
Epithelial and mesenchymal reciprocity
The mesenchyme helps instruct epithelial morphogenesis during development, maintains the architectural and functional integrity of those epithelia in the adult, and assists in the repair of epithelial tissues after injury (Box 1). The same mesenchymal programmes can also promote transformation when corrupted.
Box 1. Development of the pancreas.
The epithelial sheet, the basic building block from which all other tissues arise, is the earliest tissue to develop186. Gastrulation represents the first epithelial-to-mesenchymal transition (EMT) in the development of higher vertebrates, and results in the formation of mesoderm and endoderm germ layers. A subsequent EMT gives rise to the sclerotome mesenchyme and the ability to generate a vertebral column. Thus, mesenchymal cells both derive from and provide material and structural support to epithelia. They also respond to cues from those epithelial cells. This fundamental intimacy between epithelia and mesenchyme is essential to complex multi-organ life.
To better understand their influences in disease states, it is helpful to briefly review the forces driving the normal embryologic development of the pancreas213. The pancreas develops from dorsal and ventral evaginations of the foregut endodermal anlage. The two epithelial buds branch into the surrounding mesenchyme and later fuse as the gut tube rotates. Sequential expression of several transcription factors, including pancreas/duodenum homeobox protein 1 (PDX1; also known as IPF1), pancreas transcription factor 1 subunit-α (PTF1A; also known as p48), and SRY-box 9 (SOX9), begins at embryonic day 8.5 in the mouse and is essential for establishing pancreatic identity. However, these anlage also receive inductive influences from surrounding mesenchyme to distinguish hepatic and pancreatic fate specification213. Cessation of Hedgehog (Hh) signalling in both dorsal and ventral buds must follow to prevent hepatic differentiation. This is accomplished by distinct mechanisms in the two buds: the aorta and notochord overlying the dorsal bud secrete fibroblast growth factor 2 (FGF2) and activin to suppress Hh expression214, and the transcription factors GATA4 and GATA6 accomplish the same in the ventral bud, in conjunction with inductive signals from the adjacent cardiac mesenchyme and vitelline veins.
Rotation of the foregut tube allows the ventral and dorsal buds to then fuse and generate a unified gland. WNT215 and Notch216 signalling, respectively, are required at critical junctures to promote sufficient expansion of pancreatic exocrine progenitors and to segregate and define acinar and multiple endocrine lineages. Thus, the stepwise movements of germ layers and evolving anlage are exposed to an exquisitely timed and spatially defined programme of potent morphogenetic signalling gradients that give rise to a fully developed and differentiated gland of sufficient mass to support the adult organism. Many of these same morphogens and developmental pathways are re-engaged in pancreatic cancer, albeit with altered spatial and temporal control.
As the epithelium begins to emerge during development, neighbouring cells in the epithelial sheet and then the gut tube are bound by adherens junctions217, which are reinforced to become macula adherens (containing cadherins and catenins) as the epithelium is placed under tension218. At the basal surface of the elaborating sheet, the cells attach through surface integrins to an underlying matrix of collagen and laminin. The traction applied by the matrix signals through, and is counterpoised by, an intracellular actomyosin contractile apparatus. Force and form evolve coordinately, and ultimately give rise to function219,220. Thus, cells can be ‘pulled’ into altered states, either as part of normal developmental programmes or by ‘corrupted influences’ that drive disease.
Functional and phenotypic heterogeneity of mesenchymal cells in PDA
The study of cancer-associated fibroblasts (CAFs) or mesenchymal cells is confounded by their ability to arise from multiple tissue sources and differentiation states34. Mesenchymal cell types and their functional consequences reflect both their lineage (relatively fixed) and their plasticity (highly dynamic), and differences in the contributions of each of these sources of diversity may help explain some of the apparent inconsistencies in the literature. Numerous markers have been proposed to define the mesenchymal state in health and disease, including vimentin, desmin, fibronectin, α-smooth muscle actin (αSMA; also known as ACTA2) and fibroblast activation protein-α (FAPα), but many of these proteins are also expressed by other cell types and none represents a uniquely identifying marker. Moreover, it is becoming clear that PDA, and likely other cancer types, is populated by multiple phenotypically, functionally and spatially distinct types of mesenchymal cells (Fig. 2), and that considering the fibroblasts in a carcinoma as a monomorphic entity is not only overly simplistic but potentially dangerous in terms of its implications for therapy34,35.
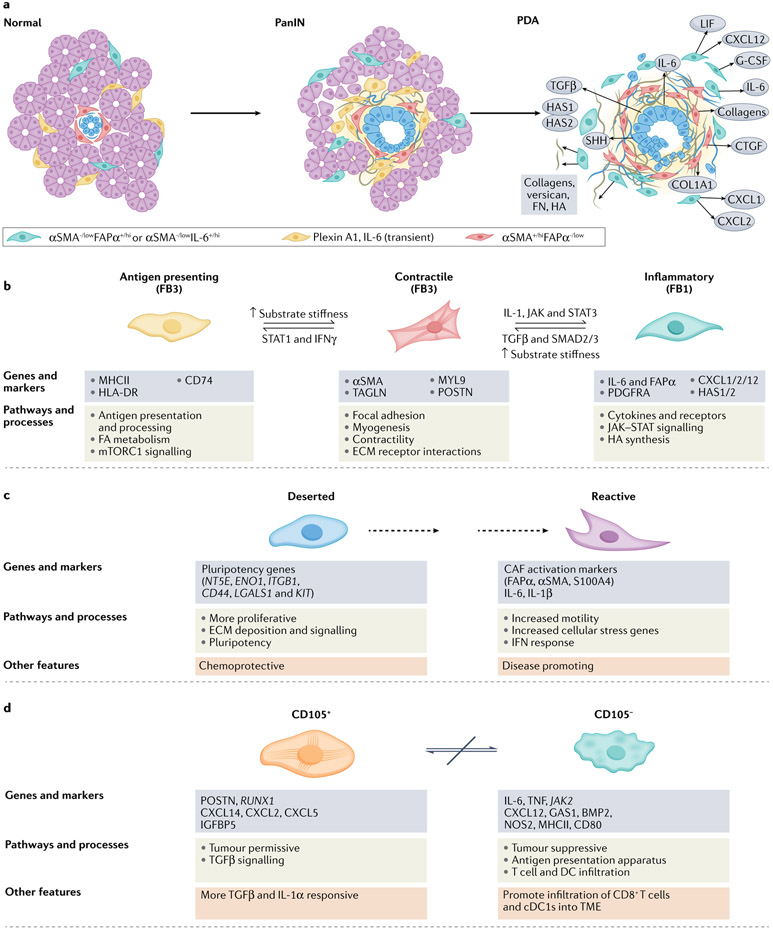
Fig. 2 ∣. Mesenchymal cell lineages and diversity in pancreatic cancer.
Our understanding of fibroblast heterogeneity is beginning to progress beyond a simple binary designation of ‘quiescent’ versus ‘activated’ states. Instead, phenotypically, functionally and spatially distinct cancer-associated fibroblast (CAF) subsets are emerging. Mesenchymal cells, derived from both resident pancreatic stellate cells (PSCs) and bone marrow-derived stem cells, respond to cues from the epithelium and infiltrating immune cells and evolve coordinately in a developing pancreatic neoplasm. a, Although the detailed classification schemes and lists of markers for these subsets continue to evolve with advancing technologies, the integrated information from several early studies suggested two broad classes of CAF subsets68-70,75,77. One subset of CAFs appear to have a more contractile phenotype, participate in juxtacrine signalling with tumour epithelial cells through close apposition and typically overexpress αSMA. Although signalling and metabolic support (see below) together with the onset of increased tension associated with matrix stiffening can promote transformation of early epithelia, the sustained activation, increasing fibrosis and increased contractility of these adjacent myofibroblasts may also act as a counter and constrain invasion and migration of epithelial cells in later stages of disease. A separate set of fibroblasts may be more removed from epithelial cells, express lower levels of αSMA and higher levels of FAPα, and possess an immunosuppressive phenotype characterized by secretion of IL-6, CXCL12 and other key inflammatory chemokines and cytokines. These two subtypes appear to be interconvertible depending on exposure to key signals from the tumour epithelial cells, such as TGFβ and IL-1, as well as mechanical cues from the underlying matrix. Another study described a third population in mouse pancreatic ductal adenocarcinoma (PDA) that expresses plexin A1 and IL-6 and exists transiently in the normal pancreas and in a pancreas with pre-invasive ductal neoplasms but is lost in the setting of invasive disease51. The spatial distribution of these functional subtypes may therefore be defined in part by gradients of signalling molecules (indicated by background yellow shading) from the tumour epithelium. b, The relatively recent ability to perform massive parallel transcriptional analyses of single cells followed by iterative unsupervised clustering analyses has provided unprecedented resolution of the subtypes, numbers and activities of cells in normal tissues, tumours and other disease states. Several such analyses have been performed on human pancreatic cancers and genetically engineered mouse models (GEMMs) of the disease51-56. Each of these studies has broadly confirmed the existence of two functional classes of fibroblasts, with the possibility of a third, while also providing new insights into their evolution during disease progression. Elyada et al. compared analyses of GEMMs with human pancreatic cancers and normal adjacent tissues from resected specimens52. Their studies on human CAFs confirmed their prior designations of inflammatory and myofibroblastic subtypes, with the former expressing immune and redox regulatory genes and hyaluronan synthases, and the latter expressing genes involved in contractility and the mesenchymal state. The initial analyses of mouse CAFs recapitulated the human findings; however, to further increase the sensitivity of the analysis, the investigators performed negative selection followed by selection for podoplanin (PDPN) expression (as a pan-CAF marker) to enrich for CAFs from their single-cell suspension. These studies permitted the identification of a potential third subtype of CAFs characterized by expression of MHCII genes, including Cd74, but not co-stimulatory molecules, and were designated antigen-presenting CAFs. Interestingly, this population also expressed mesothelin. The possibility that fibroblasts might take up and/or express mesothelin on their cell surface and present it to T cells was raised in an earlier study of adoptive T cell therapy in a KrasLSL–G12D/+;Trp53LSL–R175H/+;Cre(KPC) GEMM of PDA in which not only tumour epithelial cells but also some fibroblasts appeared to undergo T cell-mediated apoptosis209. On returning to their human dataset, the authors could discern a population of CAFs expressing modest levels of MHCII genes, not as a separate subcluster but, rather, that were more diffusively distributed across the other two CAF clusters and, perhaps to a greater extent, with inflammatory CAFs. Imaging mass cytometry of human pancreatic tumours confirmed the presence of cells co-expressing HLA-DR and CD74. Hosein et al.51 analysed principally a KrasLSL–G12D/+; Ink4aflox/flox;Cre (KIIC) model, complemented with some analyses of KPC mice, and identified three transcriptionally distinct fibroblast subtypes (FB1, FB2 and FB3) in the normal mouse pancreas, of which two persisted through to invasive disease. The FB2 cluster expanded during progression to pre-invasive disease before disappearing altogether, leaving only the FB1 (inflammatory-like) and FB3 (myofibroblast-like) populations in roughly equal proportions in advanced disease. In their description of FB3 myofibroblasts, this subset also expressed MHCII, a mark of professional antigen-presenting cells (APCs), and genes associated with the mesothelial state51. Single-cell RNA sequencing complemented by cytometry time-of-flight (CyTOF) analyses of human and mouse specimens in a study by Zhang et al.55 was broadly consistent with the picture presented thus far. A subsequent study confirmed the mesothelial cell origin of the MHCII-expressing CAFs210 and found that the transition to an antigen-presenting CAF phenotype was induced by IL-1 and TGFβ, molecules also implicated in inducing inflammatory and myofibroblastic CAFs, respectively77. These CAFs also promoted the expansion of immunosuppressive regulatory T cells. How exactly these mesothelial cell-derived fibroblasts and dermatopontin-positive universal fibroblasts relate to each other, and to the respective PDA CAF populations they give rise to, remains to be clarified. c, A competing conceptualization and radical departure for understanding CAF diversity was instead rooted in distinct phenotypic and functional properties of CAFs isolated from two unique histopathological subdomains identified in resected human PDAs53. These subdomains, or sub-tumour microenvironments (subTMEs), were distinguished by their cellularity and ECM abundance: the ‘deserted’ subTME was paucicellular but had extensive ECM deposition, whereas ‘reactive’ regions were populated with more fibroblasts, endothelial cells and immune cells, including evidence of direct contact between T cells and tumour epithelial cells. Deserted CAFs were more proliferative and expressed genes associated with pluripotency; reactive CAFs were more motile and expressed signatures associated with EMT, activation and inflammation. The relative abundance of these subTMEs varied with respect to disease evolution and response to therapy. Deserted subTMEs increased after exposure to cytotoxic chemotherapy and were therefore described as being chemoresistant, whereas reactive subTMEs became sparse; conditioned media from deserted CAFs were chemoprotective for patient-derived organoids (PDOs) in culture. Reactive subTMEs increased overall as disease progressed, and conditioned media from reactive CAFs increased PDO proliferation. These results therefore suggest that the phenotypic behaviours of the malignant epithelium in PDA are defined, or at least strongly influenced, by factors from their adjoining CAF subpopulations. If this classification endures further validation, it could have profound implications for prognosis and for informing treatment course. d, A more simplified depiction of CAF heterogeneity in PDA emerged from mass cytometry of 19 individual pancreatic cancers from the KPC GEMM of PDA (and was later confirmed in other models and human PDAs)54. These analyses found that PDPN, CD90, desmin and CD63 were expressed on most CAFs and that other common markers, including αSMA, PDGFRα and/or PDGFRβ, ICAM1, integrin α5 and CD73, showed graded expression across several subclusters, revealing a spectrum of phenotypic states. Expression of αSMA and expression of PDGFRα were inversely related: αSMAhiPDGFRαlow CAFs and αSMAlowPDGFRαhi CAFs largely formed separate groups of clusters, and were most closely reminiscent of myofibroblastic CAFs and inflammatory CAFs, respectively. Expression of CD105 (part of the TGFβ receptor complex) cleanly delineated two distinct CAF populations, each again containing both contractile and inflammatory signatures (that is, αSMA and PDGFRα showed graded expression across both populations). CD105+ CAFs were described as tumour permissive on the basis of subcutaneous co-injection experiments with tumour epithelial cells, and CD105− CAFs were tumour suppressive in a manner that depended on an intact adaptive immunity. Similar populations were found in human PDAs and in normal pancreatic tissue, defining CD105 as a key cell surface determinant of distinct fibroblast lineages in human and mouse pancreas. CD105+ CAFs tended to be more intra-acinar, and CD105− CAFs tended to be more inter-acinar. Each population manifested a great deal of plasticity in terms of responses to various stimuli, with each class capable of engaging the same signalling nodes, although with distinct outputs. The two populations were not interconvertible, which is consistent with them representing distinct lineages, albeit of currently unknown provenance. This newly presented conceptualization of two fixed lineages with dynamic plasticity within each lineage and distinct abilities to influence disease trajectory could also have enormous implications for patient management. PanIN, pancreatic intraepithelial neoplasias.
CAFs can evolve from cells residing in the pancreas or from those recruited from the bone marrow, each possessing an unadulterated genome35, or they can even be misconstrued as such when an epithelial cell undergoes a postneoplastic epithelial-to-mesenchymal transition (EMT)36. The PSC, the resident fibroblast in the pancreas, is a star-shaped cell laden with vitamin A-containing lipid granules in its quiescent state that are rapidly depleted upon activation37,38. Mesenchymal stem cells (MSCs) are another potential source of fibroblasts in the normal pancreas and in malignancy39, and they demonstrate remarkable plasticity40. A population of MSCs was recently characterized in resected human PDAs on the basis of prototypical expression of surface markers, including αSMA, FAPα and vimentin; these cells were shown to secrete granulocyte–macrophage colony-stimulating factor (GM-CSF), promoting tumour cell proliferation, invasion and metastasis, but their precise origin remains unclear41. MSCs arising from bone marrow (also known as bone marrow stromal cells (BMSCs)) were serendipitously discovered to express FAPα after an adoptive T cell strategy targeting this serine protease caused cachexia and lethal bone marrow toxicity in mice42,43. Adipose-derived MSCs have been shown to be able to differentiate into both contractile-like and inflammatory-like CAFs in vitro44 and into additional, as yet not fully characterized, subtypes in xenografts45.
A study of transplantable fibrosarcoma and colon cancer models concluded that most CAFs derive from local, not circulating, precursor cells46. In contrast, lineage-tracing studies in infiltrating PDAs revealed surprisingly that resident PSCs contributed to only 10–15% of the total CAFs present47. Complementing these findings, a very recent study found that 85–90% of CAFs in transplantable models of mouse PDA cells were marked by expression of leucine-rich repeat-containing protein 15 (LRRC15) and were derived from transforming growth factor-β (TGFβ) stimulation of so-called pan-tissue universal fibroblasts (marked by dermatopontin expression)48. Targeted deletion of the minor PSC-derived CAF population in autochthonous PDAs established the central role of CAFs in the mechanical properties and metastatic potential of the primary tumours. PSC-derived CAFs produced elevated levels of the proteoglycan perlecan, contributing to chemoresistance, and stimulated phosphorylated signal transducer and activator of transcription 3 (STAT3) signalling, likely through secretion of interleukin-6 (IL-6)47. LRRC15+ myofibroblasts instead directly suppressed the effector functions of CD8+ T cells and induced the expression of exhaustion markers48. Thus, the phenotypic diversity and functional consequences of distinct CAF populations in PDA may reflect not only the local signalling microenvironment but also the specific sources of the precursors. That both of these CAF subpopulations, which together appear to account for ~100% of CAFs, at least in mouse PDAs, seem to be tumour promoting from the targeted depletion studies, and we know that not all CAFs are (see later), suggests either that embedded within one or the other of these subpopulations is a further restricted subset that is tumour constraining or that these CAF subtypes also regulate the activity of each other.
Earlier work in the stromal biology field described the expression of FAPα, αSMA, vitamin D receptor49 and/or IL-650 to define both unique and overlapping classes of fibroblasts in pancreatic cancers13,30 (Fig. 2). These classifications have since been further refined and expanded through several recent large-scale sequencing and multi-omics efforts51-56 (Fig. 2b-2d). Despite the plasticity and behavioural complexity of these cell subtypes, experiments attempting to target distinct phenotypic or functional properties of these cell subtypes have revealed distinguishable features that contribute in fundamentally different ways to pancreatic carcinogenesis. An early notable and unexpected example emerged from attempts to inhibit paracrine Hedgehog (Hh) signalling between the epithelial and mesenchymal compartments in PDA57,58. Hh signalling is essential in the early development of the alimentary tract, and plays a decisive role in hepatopancreatobiliary fate specification (Box 1). In PDA, paracrine Hh signalling from the ductal epithelium supports the survival and proliferation of αSMA+ myofibroblasts59, while apparently suppressing — directly or indirectly — endothelial cell proliferation57. Short-term (2 weeks) inhibition of this signalling axis with IPI-926 (later called ‘vismodegib’) in the prototypical KrasLSL–G12D/+;Trp53LSL–R172H/+;Cre (KPC) mouse model25 depleted intratumoural myofibroblasts, decreased fibrosis and promoted angiogenesis, collectively enabling increased delivery of cytotoxic chemotherapy to the tumour and inducing tumour regression57. However, patients did no better and sometimes fared worse with the combination regimens of Hh inhibitor plus chemotherapy58,60. In retrospect, the initial preclinical study revealed only short-lived stromal remodelling, and showed that fibroblasts and the associated fibrosis reappeared as resistance emerged57. Sustained chemical inhibition of SHH signalling, endogenous genetic ablation of the ligand9,61 or deletion of αSMA+ cells10 (which would also include BMSCs and pericytes) instead unleashed a more aggressive, poorly differentiated disease, revealing an unanticipated constraint on disease progression by these cells.
FAPα appears to identify a distinct, perhaps overlapping, subset of fibroblasts, and targeting this subpopulation may provide benefit. FAPα+ stromal cells62,63 suppress immunity, and their depletion caused rapid necrosis in Lewis lung carcinomas in mice64. These stromal cells inhibit immunity in PDA by secretion of the chemokine CXC-chemokine ligand 12 (CXCL12; also known as SDF1), a CXC-chemokine receptor 4 (CXCR4) ligand, and the subsequent coating of tumour epithelial cells. CXCR4 stimulation appears to interfere with the directed migration mediated by other chemokine receptors that are broadly expressed on immune cells that participate in an integrated immune response65. CXCL12 also attracts CXCR4-expressing myeloid cells to the tumour. Antagonism of CXCR4 in the KPC mouse model promoted T cell infiltration and responsiveness to anti-programmed cell death 1 ligand 1 (anti-PDL1) therapy66. Collectively, these findings describe one of many tumour cell–immune cell–CAF (TIC) circuits in PDA (discussed further later). However, CXCR4 can also be expressed on epithelial cells. As a cautionary reminder that even highly selective agents may have a wider targeting pattern than anticipated, conditional deletion of Cxcr4 in the pancreatic epithelium of KPC mice decreased fibroblast αSMA expression and the associated fibrosis, and also slowed early PanIN progression, but ultimately gave rise to more undifferentiated tumours67. These findings also suggest that αSMA+ fibroblasts may be essential to establish early disease even if they later become tumour constraining.
Separate studies of CAF heterogeneity in autochthonous PDA mouse models have substantiated the presence of two subpopulations distinguished by binary states of FAPα and αSMA expression68 (Fig. 2a). Targeting FAPαhiαSMAlow cells with specific chimeric antigen receptor (CAR) T cells depleted stromal collagen and hyaluronan (HA) content and prolonged survival, albeit in an immune-independent manner69. Interestingly, most αSMA+ cells were also lost, suggesting either hierarchical differentiation to generate the two populations or a dependency of αSMA+ cells on FAPαhiαSMAlow cells for survival. In the study described above with genetic ablation of αSMA-expressing cells10, neither the FAPα+ cells nor the intratumoural HA content was affected, consistent with directionality to this relationship. In vitro studies suggest that these CAF subpopulations can indeed convert between FAPαhiαSMAlow and FAPαlowαSMAhi states depending not only on exposure to signalling gradients but also on the composition and stiffness (elastic modulus) of the underlying substratum70 (Fig. 2b).
These states may therefore be more akin to distinct polarized states in macrophages in their respective relationships to tumorigenesis and their inherent plasticity, and it may be preferable therefore to try to shift the balance from one state towards the other rather than attempting to deplete one subtype specifically49,71,72. More generally, it is also frequently suggested that fibroblasts switch between ‘quiescent’ and ‘activated’ states. However, quiescence suggests a state of dormancy or inactivity, whereas normal resident fibroblasts undoubtedly serve important homeostatic roles that help preserve epithelial integrity and function. Indeed, simply disrupting resident fibroblast function in the gastrointestinal tract can remove constraints and unleash neoplasia73. Designating them as ‘homeostatic’ fibroblasts may more accurately capture and reflect the essential tumour-constraining properties of these cells, rather than their being defined solely by the absence of tumour-promoting activity until awakened.
In several transplantable mouse carcinoma models and corroborating studies of human cancers, FAPα co-expression marked a subset of αSMA+ fibroblasts and induced an inflammatory phenotype by activating a focal adhesion kinase (FAK)–phospho-STAT3 signalling axis to secrete CC-chemokine ligand 2 (CCL2); subsequent recruitment of CC-chemokine receptor 2 (CCR2)-expressing myeloid-derived suppressor cells (MDSCs) to the TME promoted immune suppression74. Studies in mouse and human PDA similarly identified an inflammatory subset of CAFs characterized by IL-6 secretion, referred to as ‘inflammatory CAFs’, and these cells both induced and responded to Janus kinase (JAK)–STAT signalling; a distinct subpopulation of αSMA-expressing fibroblasts with a contractile phenotype, myofibroblastic CAFs, was also described75. Although FAPα was not specifically identified or used to discriminate the two subpopulations in these studies, expression profiling did reveal an order of magnitude higher FAPα level in the inflammatory subset; thus, it may be useful to think of these populations as lying along a spectrum of FAPαhiαSMAlow and FAPαlowαSMAhi states. Fibroblast subpopulations also appeared to manifest distinct spatial distributions within the tumour: inflammatory CAFs were found somewhat removed from tumour epithelial cells both in vivo and in vitro but nevertheless promoted the proliferation and propagation of tumour epithelial organoid cultures; the αSMA+ CAFs were more tightly apposed to the tumour cells and appeared to participate in juxtacrine signalling only75 (Fig. 2). The distinct distributions of cell subtypes suggests the possibility of superimposed gradients of signalling molecules that generate a spatially complex structure, not unlike morphogen and other signalling gradients observed in embryogenesis76. Indeed, the distinct inflammatory (FAPαhiαSMAlow) and myofibroblastic (FAPαlowαSMAhi) CAF populations may be supported by tumour cell secretion of IL-1 and TGFβ, respectively, with phenotypic outcomes dependent on the distance from the source77.
Consideration of these two phenotypes provides an alternative potential explanation for the conflicting results seen with Hh inhibition; namely, that submaximal inhibition — and then, too, of SHH only — may have contributed to the worsening of response78. Short-term treatment with the Hh signalling pathway inhibitor sonidegib (LDE225), which in comparison with other Hh signalling pathway inhibitors such as vismodegib achieves greater inhibition that also covers signalling via Indian Hedgehog (IHH), produced a strong antitumour response. However, it also saw the emergence of more poorly differentiated tumours, along with increased levels of inflammatory CAFs, monocytic MDSCs (Mo-MDSCs) and CD206+ (M2-like) macrophages. The intratumoural T cell landscape was also skewed from CD8+ T cells to more CD4+ T cells and, specifically, CD25+CD4+ T cells78. Collectively, these changes in the TME were associated with more aggressive disease, and the results provide further insight into the earlier preclinical work with vismodegib (ref.57). The balance between contractile and inflammatory fibroblasts appears to be pivotal, and the investigators emphasized the expansion of the latter rather than depletion of the former as the defining feature of a more aggressive disease78. This may seem like a distinction without a difference but implies at its root that both populations of CAFs have protumorigenic potential, albeit by different means, and that the short-term benefit of increased chemotherapy efficacy in depleting myofibroblasts57 comes at the expense of a more aggressive biology from unimpeded inflammatory CAF propagation. One could speculate that depleting inflammatory CAFs in the long term might allow myofibroblasts unfettered control and, if so, whether that would also create a more aggressive disease, albeit with a different organizational logic. Finally, it should be noted that longer-term exposure to sonidegib was never performed in the study, leaving open the question of whether treatment duration was the critical difference.
An alternative classification scheme for CAF diversity is based on identification and integrated multi-omics analyses of histopathological subdomains within invasive human PDAs (Fig. 2c). Two phenotypic and functional classes of CAFs were found in distinct histopathological ‘subTMEs’ identified as ‘deserted subTMEs’ (D-subTMEs) and ‘reactive subTMEs’ (R-subTMEs)53. The deserted CAFs are more proliferative and express genes associated with pluripotency, and ECM secretion and signalling. Reactive CAFs are more motile and express classic CAF activation markers and cellular stress genes. The investigators also observed that inflammatory CAF and myofibroblast gene signatures were identifiable in the single-cell sequencing analyses but were segregated across the subclusters in both subTMEs. Thus, they could not corroborate the previously proposed anatomic distribution separating inflammatory CAFs and myofibroblasts. They also noted an inverse relationship in the degree of differentiation between the CAFs and the tumour epithelium in each subTME. D-subTMEs had fewer well-differentiated CAFs and more well-differentiated tumour epithelial cells, whereas R-subTMEs had the converse, suggesting a coordinated, reciprocal evolution of differentiation states in the two compartments.
Finally, a very recent model simplifies things further, while explicitly incorporating the concepts of lineage and plasticity into the schema (Fig. 2d). Hutton et al. applied mass cytometry to 19 PDAs from KPC mice, focusing on single-cell immunophenotyping of the mesenchymal cells54. They identified CD105 (also known as endoglin) as a marker that cleanly segregated two lineages of fibroblasts that were not interconvertible in vitro. CD105 is a component of the TGFβ receptor complex but did not appear itself to influence the distinct CAF behaviours. CD105+ CAFs were tumour permissive in subcutaneous co-injection experiments with pancreatic tumour cells, and CD105− CAFs were tumour suppressive when placed in an immune-competent context. CD105+ CAFs and CD105− CAFs each expressed signatures associated with both contractile and inflammatory phenotypes in vivo. CD105+ CAFs and CD105− CAFs were also able to respond to the same wide range of molecular stimuli and accessed similar signalling nodes to do so but responded with somewhat different outputs.
The specific classification schemes that emerged from each of these and other studies reflects the experimental model or models of disease studied, the specific techniques used to generate the single-cell information and the specific methods applied to analyse the higher-order structure in the resulting enormous datasets. Common themes run through the findings with, nevertheless, sometimes widely differing implications. The power of any of these conceptual frameworks will ultimately lie in the ability to rationally inform and tailor treatment strategies and significantly change outcomes.
Metabolic complicity of the mesenchyme
Mesenchymal cells also provide material support to the developing tumour epithelium. Mutant KRAS stimulates cell proliferation79, creating increased demand for energy, biomass, reducing equivalents and maintenance of redox balance. The extensive desmoplasia, unusually high interstitial pressures, increased tissue stiffness and hypoperfused state of pancreatic cancer severely limit the delivery of oxygen and nutrients80 that would ideally be available to support the increased energetic and biosynthetic needs of the tumour. The malignant cells respond, in part, by scavenging for both protein81 and lipid82, activating autophagy to cannibalize and recycle critical building blocks83 and co-opting surrounding cells to provide molecules that sustain growth84. Thus, mesenchymal cells both create and help the epithelium survive the extreme microenvironment of PDA.
Paracrine activation of myofibroblasts by SHH and TGFβ promotes their survival and rewires their metabolism49,85 (Fig. 1). Myofibroblasts, in turn, release pyrimidines and lysophosphatidylcholines (LPCs) into the extracellular space, where they are taken up by tumour epithelial cells. The former include deoxycytidine, which can compete with the chemotherapy gemcitabine for incorporation into nucleic acids and contribute to resistance86, whereas LPCs are incorporated into proliferating cell membranes85. The tumour cells also secrete ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP2; also known as ATX), an enzyme that metabolizes LPCs to lysophosphatidic acid (LPA), which activates AKT and promotes the migration and invasion of the tumour epithelial cells85.
Mutant KRAS also rewires intracellular metabolism to increase glucose uptake, glycolytic flux and non-oxidative pentose pathway activity87, reprioritizing the primary purpose of mitochondrial oxidative phosphorylation from ATP production to the biosynthesis of macromolecules88. In this seemingly paradoxical switch to aerobic glycolysis, first highlighted by Warburg as an essential feature of cancer cells, glucose metabolites are shunted into essential pathways involving one-carbon metabolism instead of being oxidized completely to CO2 for maximum energy retrieval89. Pancreatic cancer cells can stimulate PSCs to catabolize their own protein through increased autophagy, releasing alanine for use by the tumour epithelium as a carbon source to fuel the tricarboxylic acid (TCA) cycle90 and thereby freeing up glucose and glutamine for other biosynthetic functions, including pyrimidine, serine and glycine biosynthesis (Fig. 1).
Non-essential amino acids (NEAAs), such as serine and glycine, are critical for numerous one-carbon reactions, and can become limiting in nutrient-deprived conditions91,92. More generally, amino acid starvation can lead to ribosomal stalling due to accumulated uncharged transfer RNAs (tRNAs). Indeed, the pivotal role of serine in PDA metabolism was underscored by the illuminating discovery that peripheral nerves supply this NEAA to the tumour cells to overcome translational arrest84. This finding may also help explain the basis for one of the most challenging aspects of managing patients with pancreatic cancer; namely, the intractable pain they can develop93. Pancreatic cancers readily invade surrounding nerve plexuses, and neurons extend their axons into the tumour mass5. Pancreatic cancer cells also demonstrate an unexpectedly high prevalence of somatic mutations in axon guidance genes94, which may drive these processes, but inducing pain cannot be the primary purpose of tumour innervation. Instead, understanding that neuronally supplied serine prevents the ribosomal stalling at specific serine codons that would otherwise occur in tumour epithelial cells in the absence of an exogenous supply provides an alternative explanation84. Moreover, the glial cells supporting these peripheral neurons secrete high levels of TGFβ that can further promote disease aggressiveness95.
Proliferating cells also require a sufficient supply of electron acceptors to support oxidized biomass production96,97. Datta et al.98 found that PDA cells, both in vivo and in co-culture with PSCs, were in a more reduced redox state relative to surrounding non-malignant cells, imposing a growth limitation due to the inability of tumour epithelial cells to regenerate NAD+. Pyruvate, acting as an electron acceptor supplied by PSCs, together with as yet unexplained direct interactions, helped tumour epithelial cells achieve a more oxidized state — at the expense of a more reduced state in the PSCs — and promoted tumour epithelial cell growth98,99. This metabolite may even cycle back and forth in heterotypic cell cultures as fibroblasts have also been shown to take up pyruvate from mouse PDA cell-conditioned media32. Co-culture of tumour epithelial cells and PSCs enhances the growth of both cell types through both paracrine and direct effects75,98.
Immune microenvironment
Chronic inflammation of the pancreas, or chronic pancreatitis, remains among the highest risk factors for PDA100, surpassed only by age101 and certain heritable syndromes102, including heritable chronic pancreatitis103. Inflammation appears essential to initiate transformation of the ductal epithelium in the adult pancreas104,105. Inflammatory injury promotes malignant disease in the pancreas either by awakening a latent plasticity in a differentiated acinus from which, in the context of an oncogenic co-insult, it cannot recover, or by preventing a tissue progenitor cell from accurately differentiating or maturing106.
Many of the same cells that respond to chemical injury and reflux injury in the normal pancreas107,108, including macrophages, neutrophils and fibroblasts109,110, are also engaged by the oncogenic stimulus of mutant KRAS. The secretome is dramatically altered almost immediately after mutant KRAS activation and before the development of pre-invasive disease32. Thus, cells are being actively recruited in rather than sensing and responding to histological atypia. Despite a total cellular mass representing half or more of an invasive PDA5, the immune system is remarkably ineffective at productively engaging the mutated tumour cell. Pancreatic cancers manifest multiple mechanisms of immune suppression and evasion, and this profoundly immunosuppressive microenvironment is established from seemingly the earliest step in neoplastic transformation7, effectively shielding the emerging disease from immunity. The influx of multiple immune cell subtypes is also highly ordered, further supporting the idea that they play essential and specific roles in disease progression7. Macrophages and regulatory T cells (Treg cells) infiltrate early in response to mutant KRAS activation and surround a nascent neoplasm, and the transition from pre-invasive disease to invasive disease is marked by a tremendous influx of immunosuppressive immature myeloid cells7, the numbers of which increase still further in metastases. The tight apposition of these immune cells to incipient neoplasia concentrates and localizes the signalling molecules they release.
Recalcitrant immunity
Despite being infiltrated by a preponderance of immune cells, human pancreatic cancers are nevertheless considered immunologically ‘cold’ because of their relative lack of effector immunity111-113. Although mouse PDAs do exhibit heterogeneity, this surprising overall dearth of effector T cells was first noted in the KrasLSL–G12D/+;Cre (KC) genetically engineered mouse model (GEMM) of PDA and later confirmed114 in the KPC model7. The relative absence of CD8+ T cells was attributed, at least in part, to the presence of suppressive neutrophils (polymorphonuclear MDSCs (PMN-MDSCs) or granulocytic MDSCs (G-MDSCs)) with cytolytic activity against effector T cells7. The inverse ratio observed between these neutrophils and CD8+ T cells was accompanied by a trend towards shorter survival in animals with larger numbers of tumour-infiltrating neutrophils. Additional mechanisms of T cell exclusion observed in PDAs from KPC mice include CXCL12 secretion from FAPα+ mesenchymal cells66 and circulating F4/80+ macrophages115.
Human PDA is also characterized by a lack of effective antitumour immunity55,113,116,117, if perhaps less extreme than in mice and mediated by distinct but overlapping mechanisms, including a relatively modest number118 and/or quality119 of neoepitopes generated by the mutated epithelium. Human PDAs enriched in CD4+ T cells and/or CD8+ T cells120, higher CD8+ to CD68+ (monocyte–macrophage marker) ratios116 and specific spatial distributions of T cells across the tumour bed121 portend more favourable prognoses. However, even when present, CD8+ effector T cells appear to be largely excluded from most of the tumour bed, are frequently relegated to the periphery112,122,123 or concentrated in intratumoural lymphoid structures113, and typically do not show signs of productive antigen engagement (that is, exhibiting immune checkpoint activation)116. Moreover, the T cells that are present tend to be more CD4+ than CD8+ and skewed towards a T helper 2 (TH2) immune tolerant phenotype rather than TH1 (refs.120,124).
Human PDA has also been resistant to a number of T cell-based therapies, including CAR T cells125 and therapeutic vaccines126. Not surprisingly, human pancreatic cancers are also notably refractory to immune checkpoint inhibition127,128, although studies in mouse neoantigen-expressing orthotopic tumours also suggest that the therapeutic focus should perhaps be on immune checkpoints other than, or at least in addition to, programmed cell death protein 1 (PD1) and/or PDL1 and cytotoxic T lymphocyte-associated antigen 4 (CTLA4)129. A massive multimodal analysis of the immune landscape in human primary PDA and peripheral blood identified a wide range of sometimes surprising interactions between cell types involving several immune checkpoint receptor–ligand pairs117. Receptors of natural killer (NK) and T cells were linked to myeloid cell immune checkpoint ligands, as previously suggested in mouse models of PDA130, and T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT)–poliovirus receptor (PVR) interactions were increased between macrophages and CD4+ T cells and CD8+ T cells, as well as NK cells, and even between endothelial and epithelial cells in the tumour.
As proposed earlier7, these findings also imply that some pancreatic cancers may never have been subjected to, nor had to overcome, the selection pressures of immunity (that is, they have not undergone ‘immune editing’), but have instead been protected from them131. Consistent with this hypothesis, depletion of CD8+ T cells in mice with implanted PDA cells did not affect tumour growth130, presumably because they were already maximally shielded from immunity. The therapeutic implications are profound as the endogenous immune response may therefore represent an as yet untapped resource if it can be successfully engaged. Several studies suggest that breaking tolerance in this setting can awaken endogenous immunity114,132, and finding ways to prolong and deepen this response is an increasing focus. The challenge is only compounded by the discovery of an expanding array of tumour-infiltrating lymphocyte states in PDAs which can differentiate still further ex vivo133. In the following subsections, an overview of the major immune cell subsets that infiltrate and cooperate with pancreatic cancers is presented along with some important implications.
Myeloid-derived suppressor cells
MDSCs have been described as immature myeloid cells having either granulocytic morphology (G-MDSCs) or polymorphonuclear morphology (PMN-MDSCs), or Mo-MDSC morphology. More recently, most of these cells have instead been proposed to reflect the pathological activation of neutrophils and monocytes, respectively, to adopt immunosuppressive phenotypes134,135. Both tissue-resident myeloid cells deposited during embryogenesis and newly recruited cells from the bone marrow are exploited by the transforming epithelium during pancreatic tumorigenesis136,137. The profoundly altered myelopoiesis, which occurs in the bone marrow, provides a prime example of pancreatic cancer as a systemic disease137,138. Immature myelocytes are expanded and recruited into the circulation by growth factors and cytokines secreted by the tumour epithelium and are further instructed after tumour infiltration to become fully immunosuppressive139. The transition from pre-invasive disease to invasive disease is marked by a dramatic increase in the number of MDSCs7,114. PMN-MDSCs appear to predominate in PDA, but may be in equilibrium with Mo-MDSCs114.
Mutant KRAS activation can stimulate the expression of the full range of CXC chemokines140,141. Myeloid cells, in turn, express numerous receptors that engage and are influenced by the cytokines and chemokines secreted by pre-invasive and invasive PDA cells, including GM-CSF, granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor 1 (CSF1), CXCL1, CXCL2 and CCL2 (refs.31,32,114,142,143). GM-CSF appears principally responsible for the recruitment and/or persistence114,142,143 of suppressive neutrophils, and GM-CSF levels increase severalfold more in metastatic tumour epithelial cells than in primary tumour epithelial cells114. Genetic deletion of GM-CSF from mutant KRAS-expressing pancreatic ductal cells prevents their ability to establish colonies upon transplantation into mice, and this barrier is T cell dependent142. Targeted depletion of G-MDSCs in an established autochthonous mouse PDA stimulates the infiltration of CD8+ T cells in a previously immunologically ‘cold’ tumour. These CD8+ T cells show markers of activation, release granzyme B and significantly increase tumour cell apoptosis114. Interestingly, G-MDSC depletion was accompanied by a corresponding increase in the monocytic subtype, suggesting homeostatic regulation between the two populations114 (and see144). It also caused a marked reduction in stromal fibrosis and a more patent vasculature. Inhibition of CXCR2 signalling in autochthonous primary disease also abrogates metastasis, stimulating the entry of T cells whose activity could be further potentiated by immune checkpoint inhibition145.
The mouse model of reversibly inducible KrasG12D (iKras*) was also used to explore the role of CD11b+ myeloid cells, which include monocytes, granulocytes and macrophages, in initiation and progression of pancreatic cancer130. The inability of the pancreas to recover from inflammatory injury in the setting of oncogenic Kras expression was overcome by depletion of myeloid cells, which aborted the initiation of pancreatic cancer. In already established invasive disease, depletion of CD11b-expressing cells arrested tumour growth and, in some cases, induced regressions, accompanied by an infiltration of CD8+ T cells and a loss of CD4+ forkhead box P3-positive (FOXP3+) Treg cells130.
Macrophages
Macrophages are found in all tissues of the body and are either sourced from the yolk sac during embryogenesis (and sustained locally) or replenished by bone marrow-derived inflammatory monocytes. Different tissues possess different proportions of yolk sac-derived and bone marrow-derived macrophages. In the normal pancreas, macrophages are largely derived from the yolk sac146. Under normal conditions, monocytes recruited from the bone marrow by CCL2–CCR2 signalling differentiate in the periphery into macrophages, dendritic cells and resident monocytes, which participate in immune surveillance and the resolution of inflammation147. Secretion of CCL2 by pancreatic cancers also mobilizes inflammatory monocytes from the bone marrow, and increased numbers in the circulation correlate with poorer survival in patients148. Upon recruitment to the tumour site, they give rise to tumour-associated macrophages (TAMs) that adopt an ‘activated’ or M2-like protumorigenic phenotype and support tumour growth and metastasis148. Macrophages can contribute to chemotherapy resistance in PDA cells by causing the upregulation of cytidine deaminase149, which metabolizes gemcitabine, or by providing pyrimidine nucleosides (including deoxycytidine) to tumour cells in response to macrophage-polarizing secreted factors150. In an orthotopic model of PDA, a reciprocal relationship between two myeloid cell types was noted by depletion of TAMs versus suppressive neutrophils (specifically, G-MDSCs). Targeting either population alone was modestly effective, but combined inhibition of CCR2+ macrophages and CXCR2+ neutrophils produced the most robust antitumour response151.
Antagonizing the CSF1–CSF1 receptor (CSF1R) signalling axis also inhibited the recruitment and maturation of inflammatory monocytes into activated TAMs, as well as reprogramming already present macrophages towards an immunostimulatory phenotype152. The productive immune response that followed was further evidenced by activation of the PD1 and CTLA4 checkpoints, and effector T cell function could be potentiated by a newfound sensitivity to immune checkpoint inhibition. Macrophage depletion through diphtheria toxin-mediated ablation of CD11b+ cells also decreased tumour cell PDL1 expression130. TAM depletion induced a further influx of G-MDSCs153, reinforcing the theme of homeostatic regulation of immune suppression, and suggesting that simultaneous inhibition of multiple axes may act synergistically and pre-empt a compensatory response.
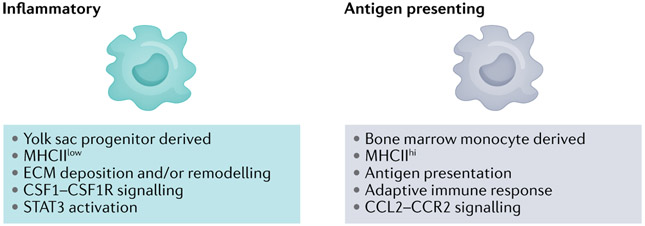
More recent studies in an autochthonous model of PDA suggested a different relationship between these macrophage populations and pancreatic cancer pathogenesis154. Both bone marrow-derived major histocompatibility complex II-high (MHCIIhi) and yolk sac-derived MHCIIlow macrophage populations were described and demonstrated unique signalling dependencies for survival and proliferation (Fig. 3). In this context, depletion of bone marrow-derived macrophages by inhibition of CCR2 signalling yielded higher-grade tumours, whereas transient ablation of embryologically derived resident macrophages inhibited disease progression154. The resident macrophages also appeared to promote ECM deposition and fibrosis. These seemingly conflicting findings may further underscore the notion of a critical homeostatic balance between distinct immune cell populations, and the differing experimental results may reflect shifting preponderances in the different model systems.
Fig. 3 ∣. Macrophage lineages in pancreatic cancer.
Macrophages found in tissues can be tissue resident, yolk sac derived or differentiate from bone marrow-derived inflammatory monocytes. Single-cell transcriptomics identified three macrophage subclusters in the normal pancreatic gland, ultimately resolving into two populations – inflammatory-like and major histocompatibility complex II (MHCII)-rich – in both pre-invasive disease and invasive disease that blended and divided the combined transcriptional repertoires between them (that is, the two classes that emerged in invasive cancer were distinct from the three that preceded them in the normal gland)51. In the normal gland, the three cell populations appear to retain some degree of fluidity and do not fully adopt their ultimate phenotypes until later in disease progression. The inflammatory signature also appeared to increase with disease progression51, and a recent study has identified the yolk sac-derived macrophages to be tumour promoting and, therefore, more like the so-called M2 phenotype154. CCL2, CC-chemokine ligand 2; CCR2, CC-chemokine receptor 2; CSF1, macrophage colony-stimulating factor 1; CSF1R, macrophage colony-stimulating factor 1 receptor; ECM, extracellular matrix; STAT3, signal transducer and activator of transcription 3.
Myeloid cell re-education
The reciprocal relationships between various arms of immune suppression in pancreatic cancer also invite a distinct therapeutic approach involving re-education rather than inhibition of myeloid cell subsets. In addition to potentially being less disruptive to homeostatic set points in the TME, this strategy has the virtue of possibly preserving and engaging the beneficial effects of a specific cell phenotype rather than eliminating the cell type outright. Agonist CD40 antibody was shown in both the KPC model and patients with pancreatic cancer to cause tumour regression without the use of cytotoxic chemotherapy155. More surprising was that the effects were dependent solely on infiltrating macrophages that were induced towards a tumoricidal phenotype155. Although the precise mechanism of action remains under debate156, this fundamental observation is the basis for numerous clinical trials currently under way. CD40 antibody also performed nominally better than CSF1R inhibition in an adoptive T cell strategy for autochthonous disease but was not sufficient to fully rescue T cell function157.
Treg cells
CD4+CD25+FOXP3+ Treg cells158 are abundant in invasive human PDA159, where their number correlates inversely with patient survival160, and they are among the earliest immune cells to infiltrate pre-invasive ductal neoplasms7,161. They tightly colocalize with developing PanINs in both mice and humans, homing via a CCL5–CCR5 signalling axis162. Conditioned medium from human PDA cell lines stimulates Treg cell expansion and inhibits CD8+ T cell proliferation in vitro159, and depletion of Treg cells with a CD25 monoclonal antibody in transplantable pancreatic cancer models slows tumour growth and prolongs host survival162-164. As with many other immune cell populations, Treg cells in malignancies may arise from developmentally assigned cells or by the conversion of a non-suppressive phenotype to a suppressive one165.
In an elegant autochthonous model of inducible oncogenic Kras expression placed in the background of constitutive CD4+ T cell deletion (iKras*;Cd4−/−), chemically induced parenchymal damage and associated acinar-to-ductal metaplasia (ADM) were largely healed within a few weeks and completely resolved within a few months despite the continued expression of oncogenic Kras166. Under similar conditions, disease in control iKras* mice instead progressed to frank carcinoma. Simultaneously depletion of CD8+ T cells in iKras*;Cd4−/− mice reversed the former effects, perhaps demonstrating the ability of immune surveillance to recognize and remove abnormal cells and structures if permitted. The implication was that abundant Treg cells ordinarily present in iKras*;Cd4+/+ mice enabled evolving disease to evade immunity. However, CD4+ T cells include several subsets (TH1 cells, TH2 cells, TH17 cells, T follicular helper (TFH) cells and Treg cells) and any one, or a combination of these, may have been responsible for the observed effects. The investigators therefore specifically deleted FOXP3+ Treg cells in the same context but unexpectedly found increased parenchymal injury, ADM and PanIN formation that essentially replaced the entire gland55. These results appear to contradict the earlier observations162-164 and may reflect differences between transplantable and autochthonous disease, differences in oncogenic Kras expression levels and the associated consequences, or differences between endogenous versus Tet on–Tet off inducible systems of oncogene expression. However, the results may also be reconciled by previously unrecognized interactions between Treg cells and other important CD4+ T cell subsets and, perhaps, CAF subtypes (see Fig. 4). Ablation of Treg cells in the iKras* model also appeared to be compensated for by increased influx of immature myeloid cells and F4/80+ macrophages55, suggesting the possibility of a homeostatic re-establishment of a set point of immune suppression in PDA that is maintained by multiple cooperating elements (Fig. 4). A reprogramming and redistribution of αSMA+ stromal cells (myofibroblasts) in pre-invasive disease was also observed with a concomitant decrease in TGFβ expression and ECM components. These findings confirm Treg cell infiltration and fibroblast activation as very early events in PDA pathogenesis and identify cooperativity between them55. Finally, the data collectively suggest that the autochthonous disease that develops by spontaneous progression of precursor lesions may have an organizational composition and operating rules different from those of transplantable tumours generated by implantation of established invasive cell lines, with important implications for the development and testing of treatment strategies for translation to the clinic.
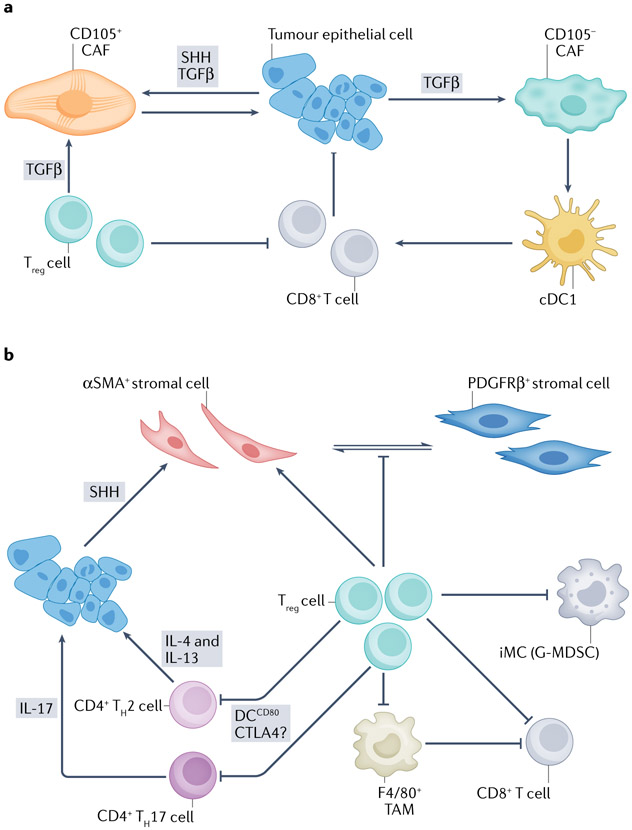
Fig. 4 ∣. Tumour cell–immune cell–cancer-associated fibroblast circuits in pancreatic ductal adenocarcinoma.
In the proposed model of the coordinated evolution of the tumour cell, immune cell and mesenchymal cell compartments (and others) in the developing pancreatic cancer neo-organ, significant crosstalk is continuous and adaptive. Numerous circuits exist, in this nevertheless simplified model, involving interactions of the tumour epithelial cells, immune cells and cancer-associated fibroblasts (CAFs), with two representative examples shown here. a, Hutton and colleagues54 identified two lineages of fibroblasts in both normal pancreas and pancreatic ductal adenocarcinoma (PDA) defined by CD105 expression. CD105− CAFs are strongly tumour suppressive when grown together with tumour epithelial cells in an immunocompetent host, an effect that was lost in non-obese diabetic (NOD)–severe combined immunodeficiency (SCID)–/Il2rg−/−, Rag−/− or Batf3−/− mice (the latter implicating the type 1 conventional dendritic cell (cDC1) subset). These CAFs are also likely responsive to and stimulated by transforming growth factor-β (TGFβ), albeit less so than CD105+ CAFs. Tumour-permissive CD105+ CAFs are generally more abundant than their counterparts (with a wide range of heterogeneity across and within tumours) and are more responsive to TGFβ provided by both tumour epithelial cells and regulatory T cells (Treg cells). b, Studies of the reversibly inducible KrasG12D (iKras*) genetically engineered mouse model (GEMM) of PDA in the context of global loss of CD4+ T cells (that is, Cd4−/−)166 versus targeted depletion of CD25+CD4+ forkhead box P3-positive (FOXP3+) Treg cells (either with CD25 antibodies or ablation in a Foxp3DTR system)55 in Cd4+/+ iKras* mice appeared to give contradictory results. In the former system, Treg cell depletion (along with depletion of other CD4+ T cell subpopulations) led to regression of disease in a CD8+ T cell-dependent manner, implying that abundant Treg cells are a primary reason for ineffective immune clearance and that this can be reversed by removing this cell type. However, targeted Treg cell depletion in an otherwise CD4+ T cell wild type context led to an unexpected and significant worsening of disease. These results may potentially be reconciled by a surprising finding in KrasLSL–G12D/+;Trp53LSL–R172H/+;Cre (KPC) mice that Treg cells primarily inhibited infiltration of CD4+ T cells, but not CD8+ T cells, into tumours, in a manner dependent on CD80+ DCs. Treg cell depletion in that context was also not sufficient to engender productive immunity (although it also did not exacerbate disease)207. Further details on which specific CD4+ T cell subpopulation or subpopulations were inhibited were not provided. However, it remains possible that the more restricted depletion of Treg cells, specifically55, may have allowed increased influx of T helper 17 (TH17) and/or TH2 CD4+ cells. TH17 cells were previously shown to accelerate disease progression via interleukin-17 (IL-17) signalling211. Also, the oncoprotein KRAS causes upregulation of interleukin-4 receptor subunit-α (IL-4Rα) and IL-13Rα in tumour epithelial cells, which can then respond to IL-4 and IL-13 secreted by infiltrating TH2 cells. The subsequent activation of Janus kinase 1 (JAK1)–signal transducer and activator of transcription 6 (STAT6) in the tumour epithelium induces MYC activity, which further stimulates glycolysis212. Thus, permitting TH2 cells to accumulate further would likely be tumour promoting. Ablation of Treg cells in the iKras* model also appeared to be compensated for by increased numbers of immature myeloid cells (iMCs) and, specifically, the granulocytic (or neutrophil-like) CC-chemokine receptor 1-positive (CCR1+) subtype as well as F4/80+ macrophages55, suggesting the potential for, and re-establishment of, a homeostatic set point of immune suppression in PDA that is maintained by multiple cooperating elements. In addition, a reprogramming and redistribution of α-smooth muscle actin-positive (αSMA+) stromal cells (myofibroblasts) in pre-invasive disease towards a platelet-derived growth factor receptor-β-positive (PDFGRβ+) phenotype with a concomitant decrease in expression of TGFβ and extracellular matrix (ECM) components was observed. In principle, all of these effects could have converged to paradoxically accelerate disease progression. However, the findings also serve as a reminder of the likely existence of multiple, interacting protumorigenic and antitumorigenic effects in PDA and that any perturbation we apply to this multicellular ecosystem of the carcinoma may have unintended consequences because of our incomplete map of all of the critical interacting nodes. CTLA4, cytotoxic T lymphocyte-associated antigen 4; G-MDSC, granulocytic myeloid-derived suppressor cell; SHH, sonic Hedgehog; TAM, tumour-associated macrophage.
The biophysical microenvironment
The dense and complex ECM deposited by activated fibroblasts and tumour epithelium167,168 is not simply an inert barrier to perfusion and diffusion. Collagens169 and HA170 feed the metabolic needs of the proliferating epithelium and activate signalling pathways in numerous cell types of the developing neoplasm. The inordinately high interstitial pressures171,172 not only cause widespread vascular collapse171,173, but the applied tensional load on cells tethered to the matrix also drives mechanosignalling174-176.
Pancreatic cancers are hypoperfused and mechanically unbalanced
Ductal adenocarcinomas of the pancreas can be distinguished radiographically from neuroendocrine tumours of the gland by contrast enhancement: Pancreatic neuroendocrine tumours (PanNETs) are hyper-vascular and take up more intravenous contrast medium and appear brighter than the surrounding normal tissues; conversely, PDAs take up less contrast medium and appear darker than adjacent tissues. In contradistinction to many solid tumours, PDAs decrease — not increase — their blood supply. They possess fewer terminal blood vessels per unit volume than the normal gland57,171,173, and most of these appear collapsed57,171.
So what drives this state? The densely fibrotic ECM in PDA is also rich in glycosaminoglycans (GAGs) and proteoglycans (PGs). The principal GAG in PDA is HA. High molecular weight (HMW) HA (more than 10–15 MDa)177 represents up to 0.1% by mass of the tumour172. HMW HA binds water avidly, and this binding energy is applied to its highly negatively charged surface to generate large swelling pressures that induce widespread vascular collapse172,178 (Supplementary Fig. 1). Targeted enzymatic degradation of HA in heterotopic implanted tumours179 and in KPC mice with established autochthonous disease decreased interstitial pressures, induced tumour regressions and increased survival171,173. Despite some success seen with short-term end points in early-phase clinical trials of the strategy180,181, phase III trials failed to show an overall survival benefit182,183. There are two principal reasons for the failure to successfully translate this strategy: (1) the clinical trials used a drug dose almost three orders of magnitude lower than that used in the preclinical models; (2) the strategy was developed for use in conjunction with agents with very short half-lives in the circulation because their rapid clearance suggested they would be unlikely to achieve significant intratumoural drug concentrations without interventions to decrease the extreme pressure barrier171. However, regimens incorporating longer-acting agents, such as continuous infusion of 5-fluorouracil (5FU) in the drug combination FOLFIRINOX (folinic acid, 5FU, irinotecan and oxaliplatin) or nanoparticle albumin-bound paclitaxel (nab-paclitaxel) in a doublet with gemcitabine, would eventually achieve those concentrations because of a more favourable pharmacokinetics–pharmacodynamics (PK–PD) profile.
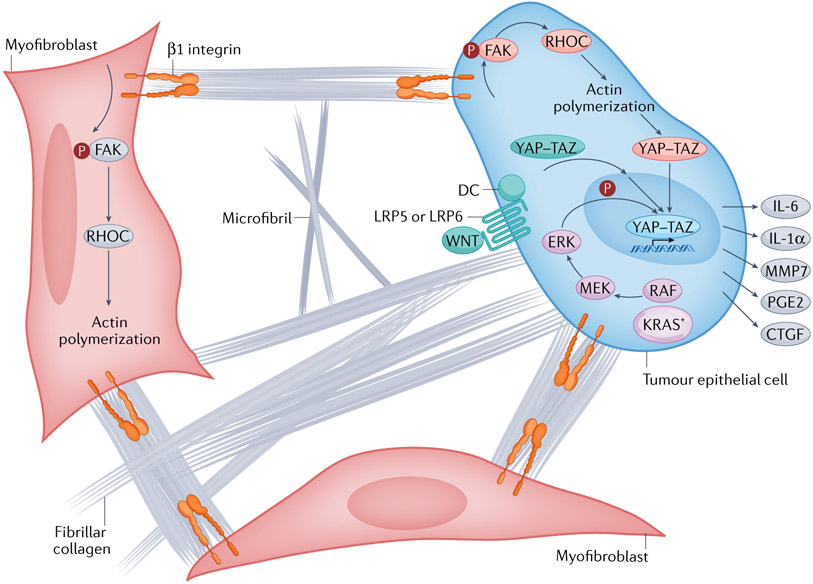
Non-covalent interactions, or ‘entanglement’, between the soluble hydrated HA polymers and insoluble collagen fibrils enable the expansile gel–fluid pressure to apply a tensional load to the collagen fibres tethered to surface β1 integrin receptors on fibroblasts and epithelial cells184,185. Traction applied to a cell activates signalling: a cell pulls on a surface and the surface pulls back186. The stiffer the substrate, the greater the strain, and the more potent the signal transduced to the cell. This force transduction activates the intracellular actomyosin contractile apparatus and, ultimately, Yes-associated protein (YAP)–transcriptional co-activator with PDZ-binding motif (TAZ)-mediated mechanosignalling (Fig. 5). In this context, nuclear translocation and activation of YAP–TAZ transcriptional activity downstream of force activation inside cells occurs independently of the Hippo-mediated sensing of cell size traditionally associated with YAP175.
Fig. 5 ∣. Activation of intracellular mechanisms of force transduction in pancreatic cancer by tensile loading of the collagen network.
Surface binding of fibrillar collagen to β1 integrins of tumour epithelial cells and myofibroblasts activates a cascade of focal adhesion complex maturation, RHO–RHO-associated kinase (ROCK) activation, phosphorylation of myosin light chain 2 (MLC2; not shown), actin polymerization and myosin-induced contractility. This feeds back to further enhance focal adhesion formation until intracellular force generation matches the traction forces applied by the extracellular matrix (ECM) (that is, the cell pulls back). In this manner, matrix stiffness and intracellular contractility are tuned to maintain tensional homeostasis. Increased fibrosis (fibrillar collagen deposition), increased collagen and microfibril crosslinking, and increased swelling pressures (from hydrated hyaluronan (HA)) can each augment the applied load and transmitted force through the surface-bound integrins. This concerted mechanism of signal transduction also suggests there may be additional targets for interventions to disrupt the feedforward loop, decrease interstitial pressures, decrease force generation and decrease mechanosignalling. The downstream consequence of this mechanosignalling is nuclear translocation of the Yes-associated protein (YAP)–transcriptional co-activator with PDZ-binding motif (TAZ) transcriptional complex, which, together with TEA domain family member (TEAD), drives a unique transcriptional programme of cell-autonomous and non-cell-autonomous behaviours, including a secretory programme promoting ECM remodelling and the influx of various immune cell subsets. The oncoprotein KRAS further shapes the YAZ–TAZ–TEAD transcriptional repertoire by activating MAPK signalling to induce distinct phosphorylation events on the DNA-binding complex. Finally, aberrant WNT signalling leads to the dissolution of the destruction complex (DC) and release of bound YAP–TAZ, which can then translocate to the nucleus. In this manner, numerous signalling pathways and biophysical stimuli converge to amplify YAP–TAZ signalling, perhaps providing the ability to even substitute for KRAS-G12D signalling once a sufficient threshold of activity is achieved. CTGF, connective tissue growth factor (also known as CCN2); FAK, focal adhesion kinase; IL, interleukin; KRAS*, mutant KRAS; LRP, low-density lipoprotein receptor-related protein; MMP7, matrix metalloproteinase 7; PGE2, prostaglandin E2.
Multiple paths converge on a common escape mechanism
Increased secretion of TGFβ by fibroblasts and tumour epithelial cells in PDA promotes collagen deposition, as well as proliferation and EMT of the tumour epithelial cells, establishing a feedforward mechanism of increasing fibrosis and matrix stiffness followed by increased tension in tumour epithelial cells, which stimulates further TGFβ secretion by the tumour epithelium and so on187. Matrix stiffening is also associated with increased secretion of key cytokines found in PDA, including GM-CSF and CXCL12. Loss of SMAD4, as occurs commonly in PDA20, exacerbates this cycle, likely by further unleashing TGFβ secretion. This ‘ratcheting up’ of tensile stress also provides potent stimuli to cell proliferation and migration mediated by hyperactivation of YAP–TAZ transcriptional programmes175 (Fig. 5), and the same mechanisms underlie the establishment and maintenance of the activated state in CAFs188. Tumour cells and fibroblasts work in concert to create an extreme microenvironment that promotes their mutual survival and activities.
YAP and TAZ are also critical mediators of the autocrine and paracrine secretory programmes induced by the oncoprotein KRAS in pancreatic cancer189,190, as well as the sensitivity of tumour epithelial cells to stromal-derived iL-6, and their genetic deletion in mice inhibited PanIN progression191. Disruption of Yap (also known as Yap1) and Taz (also known as Wwtr1) in adult mouse acinar cells also prevented inflammation-induced metaplasia (ADM)192. The YAP–TAZ complex confers resistance to MAPK pathway inhibition, and, perhaps most importantly, reactivation of the complex can bypass KRAS addiction in PDA193. TP53-mediated mechanisms of tumour suppression in PDA also appear to converge on YAP inhibition194, perhaps explaining the cooperativity between oncogenic KRAS and inactivation of TP53 in promoting PDA. Activated KRAS alters the transcriptional programme regulated by the YAP–TAZ–TEA domain family member (TEAD) nuclear complex by changing the specific phosphorylation events on YAP, and YAP–TAZ further increases KRAS activity, establishing a positive feedback loop191. Finally, many of the microenvironmental sequalae that support PDA progression also converge on YAP–TAZ signalling, including the cytokine and chemokine secretory programme that induces the recruitment of myeloid cells189.
Increased mechanosignalling, and the associated remodelling and stiffening of the ECM, may also explain the increased risk of PDA associated with chronic pancreatitis, quite apart from the other protumorigenic aspects of chronic inflammation. In this context, inflammatory injury provokes the requisite sensitivity or reprogramming of acinar cells required for ADM, while enhancing the potent and sustained oncogenic programme driven by YAP–TAZ signalling. In its ability to respond to diverse and extreme inputs in a developing pancreatic cancer and evolving TME, as well as the myriad functions and signalling pathways it in turn activates, the YAP–TAZ transcriptional complex is perfectly poised to promote epithelial transformation.
The path forward
Even as our understanding of disease pathophysiology deepens and our therapeutic armamentarium expands, pancreatic cancers continue to confound us with endlessly surprising and challenging mechanisms of treatment evasion and resistance. The complex, interconnected, heterogeneous community of cell types, matrix deposition, rewired metabolism and extreme biophysical properties is constructed in a stepwise manner such that malignant and non-malignant cells and their interrelationships evolve coordinately during disease progression. The result is an ingrained organizational logic that we must fully comprehend, not only to achieve durable impact but also to avoid therapeutic catastrophes. The realization that disrupting one node in the ‘logic board’ can activate many others in often unanticipated ways adds urgency to the imperative to complete the puzzle. Nevertheless, some rules and principles of stromal biology in PDA are beginning to emerge that should inform our overarching approach and bring greater clarity to newer treatment strategies (Box 2).
Box 2. Organizing principles and predictions from a model of stromal and epithelial co-evolution in pancreatic cancer.
The stroma and transforming epithelium co-evolve in pancreatic cancer.
Intratumouraland intertumoural heterogeneity will therefore be reflected not only in the tumour epithelial cells (for example, genetic or differentiation state heterogeneity) but also in the stroma (for example, architectural or microenvironmental heterogeneity).
Mutated epithelial cells secrete signals that modify stromal fibroblasts, which respond by expanding the signalling capacity of the tumour epithelial cells as well as offering material support in the form of amino acids, nucleotides and lipids, redox reciprocity and even their own autophagy under conditions where passive nutrient delivery is compromised by excessive interstitial pressures and poor perfusion.
Inevitable regional variations in the type and extent of selection pressures (for example, blood flow, nutrient delivery and local injury) will create subniches for continued evolution underlying the development of intratumoural (and intertumoural) heterogeneity.
Each stage in disease progression, from pancreatic intraepithelial neoplasia 1 (PanIN-1) to PanIN-2 to PanIN-3 (or carcinoma in situ) and then on to invasive and metastatic pancreatic adenocarcinoma (PDA), can be conceptualized as a semi-stable state comprising accumulating genetic mutations, cellular subtypes, matrix components, and morphogen, cytokine and chemokine signalling cascades that collectively define that stage.
Disruption of the most critical of the various elements in the evolving neoplasm will disrupt the relative homeostasis of that state; such a forced transition can, in principle, result in the emergence of a more ordered (less aggressive) or less ordered (more aggressive) state, although the second law of thermodynamics suggests that the latter is more likely (Supplementary Fig. 2). For example, powerful morphogenetic gradients are reactivated in an emerging pancreatic cancer, and disrupting these to date has been deleterious for the most part.
There will be ‘bystander’ events and processes in the stroma just as there are in the genetic evolution of a tumour. That is, some elements in the stroma are critical for maintenance and/or progression of PDA and others are not.
- It is overly simplistic to strictly classify particular elements as either protumorigenic or antitumorigenic for several reasons:
- All of the events are likely adaptive at some point in disease evolution. Cells or processes that, when targeted in invasive disease, appear to result in increased aggressiveness — and would therefore be classified as tumour constraining — likely facilitated disease initiation or progression at earlier stages.
- Most of the elements are in dialogue with each other, and sometimes are involved in multiple dialogues, and it may not always be possible to discern or disentangle a direct effect on a given target from its cascading effect or effects on other interconnecting nodes.
- Targeting one node may reveal a new and unexpected vulnerability that is more amenable to exploitation with current therapies.
- Many of the cell states in question are highly fluid, and cell subtypes can sometimes more safely and more readily be pushed in a particular direction rather than eliminated altogether.
It would seem axiomatic yet is frequently forgotten that the investigation of processes involving complex, direct and exquisitely regulated communication between an epithelium and its microenvironment would require study of the native setting in which the autochthonous condition arises. Repeated experience suggests this provides the best chance of accurately capturing the essential features of these critical relationships.
The concept of combination therapy is one approach that is ready for revision. The advent of combination cytotoxic chemotherapy regimens was a major advance in mid-twentieth-century clinical oncology, even if its premises were not fully developed and its promises never fully realized195. We need to reconsider and expand the concept of combination chemotherapy from that of targeting multiple processes within a single cell type — the mutated tumour cell — to instead include targeting multiple cell types and interconnected nodes within a complex neoplasm. All of the same cells and processes complicit in the genesis of a pancreatic cancer are also available, in principle, to help it resist treatment and even reorganize in response to an applied selection pressure. We will have to apply several stressors to the system, in the correct order, and anticipate the most likely routes of escape.
The challenge lies in distinguishing which of the multiple events and processes are critical (that is, dependent), which are supportive or permissive, and which are incidental for tumour progression. Indeed, there are multiple examples of compensatory responses not just within a cell or signalling pathway but across heterogeneous cell types in a unified ecosystem. Most worrisome are strategies that interfere with powerful morphogenetic gradients, such as those associated with Hh or TGFβ. The abilities of the five key morphogens (TGFβ, Hh, Notch, WNT and the epithelial growth factor (EGF) family) to affect cell behaviour are so extraordinary that they are the very basis for pattern formation in metazoan embryogenesis196, and all of them are aberrantly activated in PDA. The reorganizing forces they impose on a cancer are likely as deeply ingrained, and disrupting them indiscriminately could be catastrophic not only for the tumour but also for the host, if it unleashes an even more aggressive disease state.
So, where does opportunity lie? Pancreatic cancer cells are under inordinate stress. They are nutrient deprived, oxygen deprived, redox imbalanced and under extreme interstitial pressures and tensional strain. Enormous energy and numbers of distinct cell types are required to survive the hostile environment of a PDA, and pushing the tumour epithelial cell further down its stress-response capacity may be lethal for the malignant cell. Targeting unique aspects of PDA metabolism with, for example, inhibitors of autophagy and other nutrient scavenging mechanisms, together perhaps with inhibition of key cytokines that recruit the most important cell types, might be productive. As noted glucose is shunted by PDA cells in part to fuel the hexosamine biosynthetic pathway (HBP), which supports critical glycosylation reactions However, although ablation of the gene encoding the rate-limiting enzyme glutamine–fructose 6-phosphate amidotransferase 1 (GFAT1 also known as GFPT1) in the pathway caused pancreatic cancer cell death in vitro, it was far less effective in vivo90. It was recently found that PDA cells can leverage the abundant HA from the TME to fuel the HBP via the N-acetylglucosamine (NAG) salvage pathway170. Thus, although singularly targeting GFAT1 would appear to be an attractive strategy from in vitro studies, it would fail in vivo. Perhaps resurrecting methods to deplete intratumoural HA would have traction in combination with GFAT1 inhibition or other disruptors of the HBP.
TGFβ inhibition197 can be used (cautiously) to deactivate fibroblasts, fibrosis and Treg cell-mediated immune suppression; it should, of course, be combined with measures such as CXCR2 inhibition to deplete the anticipated compensatory increase in the number of myeloid cells, and further complemented with CCR2 inhibition. Alternatively, efforts to re-educate or reprogramme6,198 rather than inhibit or deplete the stroma, as in the examples of various CAF and myeloid cell populations, should also continue to be pursued — with the caveat to be mindful about potential biphasic dose–response relationships199 — in the hope of leveraging their potential beneficial properties, while suppressing the deleterious ones. Vitamin D49 (NCT03472833 and NCT03520790) to reprogramme PSCs and anti-CD40 (ref.200) (NCT03214250 and NCT04888312) to re-educate macrophages are being tested in exactly this way, and they could be combined with additional agents that capitalize on the lost support those cells provided to the tumour epithelial cells.
With the long-awaited advent of RAS inhibitors now at hand201, we should anticipate the need to combine these with inhibitors of YAP–TAZ signalling to prevent the inevitable bypass pathway and other mechanisms of acquired resistance202, and perhaps together with inhibitors of activated PSCs to prevent residual reciprocal signalling mechanisms that activate receptor tyrosine kinase (RTK) pathways33 (Fig. 1).
Efforts to soften the ECM (that is, decrease tissue stiffness) and reduce mechanotransduction may also be especially potent in undermining this highly desmoplastic disease. Indeed, some of the suggestive results seen with agents such as FAK inhibitors203,204 and angiotensin receptor blockers205 may result from disruption of force generation and inhibition of mechanosignalling. The enormous expenditure of energy devoted by tumour epithelial cells and stromal fibroblasts in PDA to generating and remodelling the matrix suggests that removing this ‘foundational platform’ should reveal new vulnerabilities that can be exploited by multicomponent ECM-targeting and multicompartment TME-targeting strategies.
So, is there a critical dependence in the stroma for pancreatic cancer? Given the deep involvement of stromal elements, it seems more likely that some combination of processes will need to be targeted to achieve efficacy in pancreatic cancers. PDA cells do become addicted to one obvious candidate, oncogenic KRAS, although, worryingly, escape from this dependency has already been demonstrated193,206. These and other studies also suggest that disrupting YAP might abort disease either at initiation of pre-invasive lesions192 or at progression to invasion and metastasis191. Similarly, inhibition of GM-CSF signalling results not only in arrest of pre-invasive disease progression but also in regression or ‘reversion’ of the lesion, suggesting that myeloid cell infiltration may be absolutely required for the maintenance and/or transition of advanced PanINs to invasive PDA142. Depletion of FAPα+ cells also induced tumour epithelial cell apoptosis in vivo, revealing another potentially dependent cell type in PDA maintenance69. Conversely, targeted depletion of Treg cells alone was insufficient to stimulate effector immunity207, and, at least in one study55, instead unleashed a more aggressive disease, and it would therefore need to be coupled to additional, as yet not fully clarified, mechanisms to have therapeutic value.
Conclusions
The long-established genetic progression scheme for pancreatic cancer has provided a powerful blueprint for the stepwise evolution of disease and guided research, including efforts to create faithful models of the disease. It cannot, however, explain the heterogeneity in disease biology observed. Transcriptional and epigenetic analyses have provided additional detail but are still not complete. A stromal progression map to supplement the genetic model is coming into focus, deepening our understanding of the organizational logic of pancreatic cancer and providing an entirely new terrain of therapeutic targets. Constructing a complete picture robust enough to fully capture the range of disease presentations and optimal therapeutic targets will likely require enumerating and integrating all of the cell types present208, analysing their transcriptional states at single-cell resolution and superimposing these data onto the positional information of these elements within the autochthonous disease, together with 3D maps of overlapping and interacting morphogen signalling gradients. This level of systems biology analytics is within reach and should enable the construction of a platform, perhaps in conjunction with artificial intelligence (AI) tools, that models the organizational and systems logic of the cancer such that inputting various combinations of perturbations should reveal a predicted outcome. In this manner, in silico experiments can be executed iteratively to identify and inform the most productive preclinical investigations to perform that will, in turn, translate into meaningful therapies in the clinic.
Supplementary Material
Acknowledgements
The author thanks J. Potter, C. Ghajar and T. Hollingsworth for helpful comments on the manuscript, S. Thorsen for help with preparation of the manuscript, M. Whittle and C. Dufort for help with figure preparation, and a great many colleagues for stimulating discussions. Work in the author’s laboratory is supported in part by NIH/NCI R01 CA161112, R01 CA223483 and U01 CA224193, Cancer Center Support Grant P30 CA015704 and Pancreatic Cancer Action Network Precision Medicine Targeted Grant 17-85-HING.
Glossary
- Acinar-to-ductal metaplasia
(ADM). A postulated transdifferentiation of a mature acinar cell into an abnormal ductal-like cell in response to an oncogenic stimulus with or without a superimposed injury that may give rise to the precursors (pancreatic intraepithelial neoplasias) of pancreatic cancer.
- Anlage
A classical term in embryology used to define an area or collection of cells that serve as a foundation, or primordium, for a subsequent developmental event.
- Contrast enhancement
The differential uptake of systemically introduced radiographic contrast material by an organ over time or in relation to another adjacent organ.
- Ductules
The terminal portions of the branching pancreatic ductal system, which gets progressively smaller in calibre as the tree spreads from the centre of the gland to the periphery.
- Elastic modulus
Also called ‘Young’s modulus’, a measure of stiffness and formally the slope of the stress–strain curve (that is, a plot of the extent to which a material is reversibly (elastically) deformed in response to an applied force).
- Evaginations
Extensions of a sheet of cells while maintaining contiguity to form a protrusion or outpouching from the surface layer (as opposed to invagination, or inward migration of a sheet of cells, to form a dimple or tunnel within a cell mass).
- Hedgehog (Hh) signalling
A critical embryonic signalling pathway that figures prominently in the development and differentiation of many tissues and organs of the body and that is regulated by three ligands, Indian Hedgehog (IHH), sonic Hedgehog (SHH) and desert Hedgehog (DHH).
- Hexosamine biosynthetic pathway
(HBP). This pathway integrates metabolites from glycolysis with those from amino acid, nucleic acid and lipid breakdown to generate uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) for use in critical glycosylation reactions.
- Hyaluronan
(HA). A highly soluble, negatively charged, linear unbranched sugar polymer comprising repeating disaccharide units of N-acetylglucosamine (NAG) and d-glucuronic acid that avidly binds water and swells, providing turgor to many tissues in the body.
- Myelocytes
Precursors to granulocytes (basophils, neutrophils and eosinophils).
- Myelopoiesis
The generation of myeloid (as opposed to lymphoid) cells in the bone marrow, including red blood cells, platelets, mast cells, granulocytes and monocytes (macrophages and dendritic cells).
- Nerve plexuses
Confluences or bundles of afferent and efferent nerve fibres from several levels of the spine as they emerge from the foramina.
- Non-oxidative pentose pathway
The non-oxidative phase of the pentose phosphate pathway; it generates ribose 5-phosphate for nucleotide synthesis, and this is further metabolized to generate glycolytic intermediates. The oxidative phase generates reducing equivalents in the form of NADPH for reductive biosynthetic reactions.
- One-carbon metabolism
A series of elemental reactions built upon folate biochemistry that involve one-carbon molecules (methyl groups) to provide the building blocks for the most fundamental reactions in cellular metabolism.
- Pancreaticoduodenectomy
Also called ‘Whipple procedure’ (after its pioneer), a complex surgical procedure involving removal of the head of the pancreas, the adjoining portions (first and second parts) of the small bowel, the extrahepatic biliary ducts and the gall bladder, and previously the pylorus and antrum of the stomach as well, in the attempt to achieve cure of a pancreatic cancer.
- Reflux injury
Parenchymal damage in the pancreas caused by forced exudation of digestive enzymes across the ductal barrier because of a downstream obstruction.
- Tricarboxylic acid (TCA) cycle
Also called the ‘citric acid cycle’ or the ‘Krebs cycle’, oxidizes acetate (linked to coenzyme A) to generate NADH, which is then used by the electron transport chain in the mitochondria to generate ATP.
Footnotes
Competing interests
The author declares no competing interests.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41568-022-00530-w.
References
- 1.Rahib L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N. Engl. J. Med 362, 1605–1617 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Kleeff J. et al. Pancreatic cancer. Nat. Rev. Dis. Prim 2, 16022 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Allison DC et al. DNA content and other factors associated with ten-year survival after resection of pancreatic carcinoma. J. Surgical Oncol 67, 151–159 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Hruban RH in Atlas of Tumor Pathology (eds Hruban RH, Pitman MB & Klimstra DS) (Armed Forces Institute of Pathology, 2007). [Google Scholar]
- 6.Stromnes IM, DelGiorno KE, Greenberg PD & Hingorani SR Stromal reengineering to treat pancreas cancer. Carcinogenesis 35, 1451–1460 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark CE et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 67, 9518–9527 (2007). This study provides the first evidence that multiple mechanisms of immune suppression become operative beginning at the earliest stages of pre-invasive pancreatic cancer.
- 8.Bijlsma MF & van Laarhoven HW The conflicting roles of tumor stroma in pancreatic cancer and their contribution to the failure of clinical trials: a systematic review and critical appraisal. Cancer Metastasis Rev. 34, 97–114 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Rhim AD et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ozdemir BC et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014). Together with Rhim et al. (2014), this study demonstrates that some fibroblast populations in PDA can be tumour constraining.
- 11.Lyssiotis CA & Kimmelman AC Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Encarnacion-Rosado J & Kimmelman AC Harnessing metabolic dependencies in pancreatic cancers. Nat. Rev. Gastroenterol. Hepatol 18, 482–492 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helms E, Onate MK & Sherman MH Fibroblast heterogeneity in the pancreatic tumor microenvironment. Cancer Discov. 10, 648–656 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neesse A. et al. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut 68, 159–171 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Stone ML & Beatty GL Cellular determinants and therapeutic implications of inflammation in pancreatic cancer. Pharmacol. Ther 201, 202–213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balachandran VP, Beatty GL & Dougan SK Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology 156, 2056–2072 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Crawford HC & Pasca di Magliano M Epithelial-stromal interactions in pancreatic cancer. Annu. Rev. Physiol 81, 211–233 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Kemp SB, Pasca di Magliano M & Crawford HC Myeloid cell mediated immune suppression in pancreatic cancer. Cell Mol. Gastroenterol. Hepatol 12, 1531–1542 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hruban RH, Goggins M, Parsons J & Kern SE Progression model for pancreatic cancer. Clin. Cancer Res 6, 2969–2972 (2000). This article presents a proposed histological blueprint for progression of precursor ductal lesions from PanIN-1 to PanIN-2 to PanIN-3 (carcinoma in situ) and culminating in invasive PDA.
- 20. Hruban RH, Wilentz RE & Kern SE Genetic progression in the pancreatic ducts. Am. J. Pathol 156, 1821–1825 (2000). This study correlates the stepwise accumulation of key genetic mutations with the histopathological depiction of PanIN progression to create a more comprehensive model.
- 21.Waters AM & Der CJ KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Perspect. Med 10.1101/cshperspect.a031435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hingorani SR et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 (2003). This article describes the first genetically engineered model to faithfully model human PanIN progression and provides the first definitive proof that oncogenic Kras mutations drive the stochastic formation of these lesions, which progress histologically to give rise to invasive and metastatic PDA.
- 23. Thayer SP et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 425, 851–856 (2003). This study demonstrates that aberrant expression of SHH, a potent morphogen, alone can induce PanIN-like lesions in the ductal epithelium of the pancreas.
- 24. Miyamoto Y. et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 3, 565–576 (2003). This study demonstrates the role of Notch (another potent developmental morphogen) signalling in inducing ADM.
- 25. Hingorani SR et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005). This article describes the first genetically engineered model of widely metastatic PDA, which faithfully models the full spectrum of the human disease, including a high degree of both intratumoural and intertumoural heterogeneity and complex (structural) genomic instability.
- 26. Aguirre AJ et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 17, 3112–3126 (2003). This article describes the first genetically engineered model of locally aggressive PDA, which demonstrates the less common sarcomatoid or spindle cell-like histology.
- 27. Izeradjene K. et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell 11, 229–243 (2007). This article describes the first genetically engineered model of mucinous cystic neoplasms (MCNs), including the faithful recapitulation of an ‘ovarian stroma’, and also provides evidence that the chronological order in which otherwise identical genetic mutations arise can dramatically influence the phenotype and functional behaviours of pancreatic cancers.
- 28.Bardeesy N. et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 20, 3130–3146 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moffitt RA et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet 47, 1168–1178 (2015). This study identifies tumour cell-specific and stromal cell-specific transcriptional subtypes of PDA that do not, however, necessarily correlate with each other.
- 30.Whittle MC & Hingorani SR Fibroblasts in pancreatic ductal adenocarcinoma: biological mechanisms and therapeutic targets. Gastroenterology 156, 2085–2096 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purohit A. et al. CXCR2 signaling regulates KRAS(G12D)-induced autocrine growth of pancreatic cancer. Oncotarget 7, 7280–7296 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velez-Delgado A. et al. Extrinsic KRAS signaling shapes the pancreatic microenvironment through fibroblast reprogramming. Cell Mol. Gastroenterol. Hepatol 13, 1673–1699 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tape CJ et al. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 165, 910–920 (2016). This study provides the first clear demonstration of reciprocal signalling between the tumour epithelial cells and stromal fibroblasts in PDA, and shows that the latter can expand the available signalling repertoire in the former.
- 34.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Sahai E et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rhim AD et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012). This article describes the possibility of EMT and systemic dissemination of pre-invasive pancreatic ductal cells very early in disease progression through delamination.
- 37.Apte MV et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43, 128–133 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apte MV, Wilson JS, Lugea A & Pandol SJ A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 144, 1210–1219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLean K et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J. Clin. Invest 121, 3206–3219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seeberger KL et al. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab. Invest 86, 141–153 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Waghray M. et al. GM-CSF mediates mesenchymal-epithelial cross-talk in pancreatic cancer. Cancer Discov. 6, 886–899 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kidd S. et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS ONE 7, e30563 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran E. et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med 210, 1125–1135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyazaki Y, Oda T, Mori N & Kida YS Adipose-derived mesenchymal stem cells differentiate into pancreatic cancer-associated fibroblasts in vitro. FEBS Open Bio 10, 2268–2281 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyazaki Y. et al. Adipose-derived mesenchymal stem cells differentiate into heterogeneous cancer-associated fibroblasts in a stroma-rich xenograft model. Sci. Rep 11, 4690 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arina A. et al. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl Acad. Sci. USA 113, 7551–7556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Helms EJ et al. Mesenchymal lineage heterogeneity underlies non-redundant functions of pancreatic cancer-associated fibroblasts. Cancer Discov. 10.1158/2159-8290.CD-21-0601 (2021). Through genetic deletion and lineage-tracing studies in mouse models, this study demonstrates that only 10–15% of PDA CAFs originate from resident PSCs and that this minor subpopulation figured prominently in the unusual biophysical properties and metastatic drive of the cancers.
- 48. Krishnamurty AT et al. LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature 10.1038/s41586-022-05272-1 (2022). This study uses transplantable mouse tumour models to identify dermatopontin-positive pan-tissue ‘universal’ fibroblasts as the source of 85–90% of PDA CAFs and reveals their ability to directly inhibit CD8+ effector T cell function.
- 49. Sherman MH et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014). This study describes a hormone-based strategy to cause activated myofibroblasts to revert to a ‘quiescent’ state that also leads to tumour growth inhibition in a GEMM of PDA.
- 50.Mace TA et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 73, 3007–3018 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosein AN et al. Cellular heterogeneity during mouse pancreatic ductal adenocarcinoma progression at single-cell resolution. JCI Insight 10.1172/jci.insight.129212 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elyada E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grunwald BT et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 184, 5577–5592 e5518 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Hutton C. et al. Single-cell analysis defines a pancreatic fibroblast lineage that supports anti-tumor immunity. Cancer Cell 39, 1227–1244 e1220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang Y. et al. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discov. 10, 422–439 (2020). This study demonstrates the surprising finding that targeting some presumptively suppressive elements of immunity may paradoxically exacerbate disease aggressiveness through unanticipated crosstalk in the pancreatic cancer neo-organ.
- 56.Neuzillet C. et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J. Pathol 248, 51–65 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Olive KP et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009). This study demonstrates that disrupting paracrine Hh signalling from tumour cells depletes stromal fibroblasts and the associated fibrosis and also stimulates angiogenesis to promote therapeutic drug delivery in a GEMM of PDA.
- 58.Ko AH et al. A phase I study of FOLFIRINOX plus IPI-926, a Hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas 45, 370–375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yauch RL et al. A paracrine requirement for hedgehog signalling in cancer. Nature 455, 406–410 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Kim EJ et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin. Cancer Res 20, 5937–5945 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JJ et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc. Natl Acad. Sci. USA 111, E3091–E3100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park JE et al. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J. Biol. Chem 274, 36505–36512 (1999). [DOI] [PubMed] [Google Scholar]
- 63.Garin-Chesa P, Old LJ & Rettig WJ Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl Acad. Sci. USA 87, 7235–7239 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kraman M. et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330, 827–830 (2010). This study provides a clear demonstration of an immunosuppressive axis in PDA mediated by FAPα-expressing CAFs.
- 65.Biasci D. et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl Acad. Sci. USA 117, 28960–28970 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feig C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morita T. et al. CXCR4 in tumor epithelial cells mediates desmoplastic reaction in pancreatic ductal adenocarcinoma. Cancer Res. 80, 4058–4070 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Pure E & Hingorani SR Mesenchymal cell plasticity and perfidy in epithelial malignancy. Trends Cancer 4, 273–277 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Lo A. et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 75, 2800–2810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Avery D. et al. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol. 10.1016/j.matbio.2017.12.003 (2017). This article presents a mechanism for class switching between FAPαhiαSMAlow CAFs and FAPαlowαSMAhi CAFs based on underlying matrix stiffness and stimulation of mechanosignalling.
- 71.Froeling FE et al. Retinoic acid–induced pancreatic stellate cell quiescence reduces paracrine Wnt–β-catenin signaling to slow tumor progression. Gastroenterology 141, 1486–1497.e14 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Chronopoulos A. et al. ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat. Commun 7, 12630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhowmick NA, Neilson EG & Moses HL Stromal fibroblasts in cancer initiation and progression. Nature 432, 332–337 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang X et al. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 76, 4124–4135 (2016). [DOI] [PubMed] [Google Scholar]
- 75. Ohlund D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med 214, 579–596 (2017). This study presents a two-state model of inflammatory and contractile CAFs in PDA based on spatial topography and differential sensitivity to gradients of IL-1 and TGFβ.
- 76.Potter JD Morphogens, morphostats, microarchitecture and malignancy. Nat. Rev. Cancer 7, 464–474 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Biffi G. et al. IL1-induced JAK/STAT signaling is antagonized by TGFbeta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9, 282–301 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Steele NG et al. Inhibition of Hedgehog signaling alters fibroblast composition in pancreatic cancer. Clin. Cancer Res 27, 2023–2037 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tuveson DA et al. Endogenous oncogenic K-rasG12D stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375–387 (2004). This study demonstrates that endogenous (physiologic) levels of oncogenic KRAS signalling can serve as an initiating event in tumorigenesis as opposed to inducing senescence as seen with overexpression of the oncogene.
- 80.Kamphorst JJ et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Commisso C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013). This study reveals the critical role of oncogenic KRAS-induced macropinocytosis as a mechanism to support tumour cell survival in a nutrient-poor microenvironment.
- 82.Kamphorst JJ et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl Acad. Sci. USA 110, 8882–8887 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang S. et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 25, 717–729 (2011). This study reveals the critical role of autophagy in supporting PDA tumour cell survival in a nutrient-poor microenvironment.
- 84.Banh RS et al. Neurons release serine to support mRNA translation in pancreatic cancer. Cell 183, 1202–1218 e1225 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Auciello FR et al. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov. 9, 617–627 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dalin S. et al. Deoxycytidine release from pancreatic stellate cells promotes gemcitabine resistance. Cancer Res. 79, 5723–5733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sousa CM et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ward PS & Thompson CB Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Warburg O. On respiratory impairment in cancer cells. Science 124, 269–270 (1956). [PubMed] [Google Scholar]
- 90. Ying H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012). In this study, a reversibly inducible mouse model of PDA is used to demonstrate that pancreatic cancers are addicted to mutant KRAS through its ability to alter glucose metabolism.
- 91.Yang M & Vousden KH Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 16, 650–662 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Newman AC & Maddocks ODK Serine and functional metabolites in cancer. Trends Cell Biol. 27, 645–657 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Koulouris AI, Banim P & Hart AR Pain in patients with pancreatic cancer: prevalence, mechanisms, management and future developments. Dig. Dis. Sci 62, 861–870 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Biankin AV et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491, 399–405 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roger E. et al. Schwann cells support oncogenic potential of pancreatic cancer cells through TGFbeta signaling. Cell Death Dis. 10, 886 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sullivan LB et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Diehl FF, Lewis CA, Fiske BP & Vander Heiden MG Cellular redox state constrains serine synthesis and nucleotide production to impact cell proliferation. Nat. Metab 1, 861–867 (2019). This article demonstrates a novel mechanism of stromal support by PSCs for PDA tumour epithelial cells in helping them achieve a more oxidized state to promote proliferation.
- 98.Datta R. et al. Interactions with stromal cells promote a more oxidized cancer cell redox state in pancreatic tumors. Sci. Adv 8, eabg6383 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kerk SA et al. The pancreatic tumor microenvironment compensates for loss of GOT2. eLife 10.7554/eLife.73245 (2022). [DOI] [Google Scholar]
- 100.Lowenfels AB et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N. Engl. J. Med 328, 1433–1437 (1993). [DOI] [PubMed] [Google Scholar]
- 101.Meza R, Jeon J, Moolgavkar SH & Luebeck EG Age-specific incidence of cancer: phases, transitions, and biological implications. Proc. Natl Acad. Sci. USA 105, 16284–16289 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yabar CS & Winter JM Pancreatic cancer: a review. Gastroenterol. Clin. North Am 45, 429–445 (2016). [DOI] [PubMed] [Google Scholar]
- 103.Lowenfels AB et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J. Natl Cancer Inst 89, 442–446 (1997). [DOI] [PubMed] [Google Scholar]
- 104. Guerra C. et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302 (2007). This study in a GEMM suggests that, in contrast to embryonic activation of oncogenic Kras, somatic activation could only effectively initiate pancreatic cancer in the setting of concomitant inflammatory injury.
- 105. Collins MA et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Invest 122, 639–653 (2012). Through the use of a novel reversibly inducible allele, this study shows that extinguishing oncogenic Kras expression results in widespread regression of pancreatic cancer.
- 106.Morris JPT, Cano DA, Sekine S, Wang SC & Hebrok M Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J. Clin. Invest 120, 508–520 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lampel M & Kern HF Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch. A Pathol. Anat. Histol 373, 97–117 (1977). [DOI] [PubMed] [Google Scholar]
- 108.Niederau C, Ferrell LD & Grendell JH Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology 88, 1192–1204 (1985). [DOI] [PubMed] [Google Scholar]
- 109.Gukovsky I, Li N, Todoric J, Gukovskaya A & Karin M Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 144, 1199–1209 e1194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng C, Li Z & Yu X The role of pancreatic infiltrating innate immune cells in acute pancreatitis. Int. J. Med. Sci 18, 534–545 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lutz ER et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol. Res 2, 616–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Joyce JA & Fearon DT T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015). [DOI] [PubMed] [Google Scholar]
- 113.Stromnes IM, Hulbert A, Pierce RH, Greenberg PD & Hingorani SR T-cell localization, activation, and clonal expansion in human pancreatic ductal adenocarcinoma. Cancer Immunol. Res 5, 978–991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Stromnes IM et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63, 1769–1781 (2014). This study demonstrates that depletion of immunosuppressive neutrophils (G-MDSCs) in the setting of established PDA in a GEMM enables endogenous CD8+ effector T cells to infiltrate and kill tumour epithelial cells.
- 115.Beatty GL et al. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6Clow F4/80+ extratumoral macrophages. Gastroenterology 149, 201–210 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liudahl SM et al. Leukocyte heterogeneity in pancreatic ductal adenocarcinoma: phenotypic and spatial features associated with clinical outcome. Cancer Discov. 10.1158/2159-8290.CD-20-0841 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steele NG et al. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nat. Cancer 1, 1097–1112 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lawrence MS et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Balachandran VP et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516 (2017). This article establishes the principle that in addition to, or perhaps more importantly than, the number of neoepitopes generated by a PDA, the quality of an epitope in terms of its ability to engage productive immunity can influence long-term survival in patients.
- 120.Ino Y. et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 108, 914–923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carstens JL et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun 8, 15095 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.von Bernstorff W et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin. Cancer Res. 7, 925s–932s (2001). [PubMed] [Google Scholar]
- 123.Ene-Obong A. et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 145, 1121–1132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ajina R & Weiner LM T-cell immunity in pancreatic cancer. Pancreas 49, 1014–1023 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beatty GL et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res 2, 112–120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Le DT et al. Safety and survival with GVAX pancreas prime and Listeria monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol 33, 1325–1333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Le DT et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med 372, 2509–2520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Le DT et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother 36, 382–389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Freed-Pastor WA et al. The CD155/TIGIT axis promotes and maintains immune evasion in neoantigen-expressing pancreatic cancer. Cancer Cell 39, 1342–1360 e1314 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang Y. et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 66, 124–136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Evans RA et al. Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight 10.1172/jci.insight.88328 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mitchem JB et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 73, 1128–1141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schalck A. et al. Single cell sequencing reveals trajectory of tumor-infiltrating lymphocyte states in pancreatic cancer. Cancer Discov. 10.1158/2159-8290.CD-21-1248 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tcyganov E, Mastio J, Chen E & Gabrilovich DI Plasticity of myeloid-derived suppressor cells in cancer. Curr. Opin. Immunol 51, 76–82 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Veglia F, Sanseviero E & Gabrilovich DI Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol 21, 485–498 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stromnes IM, Greenberg PD & Hingorani SR Molecular pathways: myeloid complicity in cancer. Clin. Cancer Res 20, 5157–5170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Markowitz J. et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol. Immunother 64, 149–159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Porembka MR et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol. Immunother 61, 1373–1385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ostrand-Rosenberg S & Fenselau C Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J. Immunol 200, 422–431 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim BY, Gaynor RB, Song K, Dritschilo A & Jung M Constitutive activation of NF-kappaB in Ki-ras-transformed prostate epithelial cells. Oncogene 21, 4490–4497 (2002). [DOI] [PubMed] [Google Scholar]
- 141.Hamarsheh S, Gross O, Brummer T & Zeiser R Immune modulatory effects of oncogenic KRAS in cancer. Nat. Commun 11, 5439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G & Bar-Sagi D Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21, 836–847 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bayne LJ et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21, 822–835 (2012). Together with Pylayeva-Gupta et al. (2012), this study establishes that GM-CSF-mediated recruitment of suppressive myeloid cells is essential for pancreatic cancer progression in mouse models.
- 144.Mastio J. et al. Identification of monocyte-like precursors of granulocytes in cancer as a mechanism for accumulation of PMN-MDSCs. J. Exp. Med 216, 2150–2169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Steele CW et al. CXCR2 Inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29, 832–845 (2016). Extending prior studies on the essential role of suppressive myeloid cells in PDA progression, this study demonstrates that their recruitment via CXCR2-mediated signalling is also required to develop and maintain metastatic disease.
- 146.Schulz C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Shi C & Pamer EG Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol 11, 762–774 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanford DE et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin. Cancer Res 19, 3404–3415 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weizman N. et al. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene 33, 3812–3819 (2014). [DOI] [PubMed] [Google Scholar]
- 150.Halbrook CJ et al. Macrophage-released pyrimidines inhibit gemcitabine therapy in pancreatic cancer. Cell Metab. 29, 1390–1399 e1396 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Nywening TM et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 67, 1112–1123 (2018). This study supports the notion of a homeostatic set point of immune suppression maintained by a community of immune cell subsets that can compensate for each other when one population is targeted.
- 152.Zhu Y. et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pahler JC et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia 10, 329–340 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhu Y. et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 47, 597 (2017). This study delineates the sources of macrophages in a GEMM of PDA and defines their respective roles in disease pathogenesis.
- 155. Beatty GL et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011). This study demonstrates the ability of an immunomodulatory agent to re-educate intratumoural macrophages towards a tumour-suppressive phenotype in both mouse and human PDA.
- 156.Wattenberg MM et al. Systemic inflammation is a determinant of outcomes of CD40 agonist-based therapy in pancreatic cancer patients. JCI Insight 10.1172/jci.insight.145389 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stromnes IM et al. Differential effects of depleting versus programming tumor-associated macrophages on engineered T cells in pancreatic ductal adenocarcinoma. Cancer Immunol. Res 7, 977–989 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wing JB, Tanaka A & Sakaguchi S Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 50, 302–316 (2019). [DOI] [PubMed] [Google Scholar]
- 159.Linehan DC & Goedegebuure PS CD25+CD4+ regulatory T-cells in cancer. Immunol. Res 32, 155–168 (2005). [DOI] [PubMed] [Google Scholar]
- 160.Tang Y. et al. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS ONE 9, e91551 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hiraoka N, Onozato K, Kosuge T & Hirohashi S Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin. Cancer Res 12, 5423–5434 (2006). [DOI] [PubMed] [Google Scholar]
- 162.Tan MC et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J. Immunol 182, 1746–1755 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jang JE et al. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep. 20, 558–571 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Viehl CT et al. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann. Surg. Oncol 13, 1252–1258 (2006). [DOI] [PubMed] [Google Scholar]
- 165.Plitas G & Rudensky A Regulatory T cells in cancer. Annu. Rev. Cancer Biol 4, 457–477 (2020). [Google Scholar]
- 166.Zhang Y. et al. CD4+ T lymphocyte ablation prevents pancreatic carcinogenesis in mice. Cancer Immunol. Res 2, 423–435 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mahlbacher V, Sewing A, Elsasser HP & Kern HF Hyaluronan is a secretory product of human pancreatic adenocarcinoma cells. Eur. J. Cell Biol 58, 28–34 (1992). [PubMed] [Google Scholar]
- 168.Tian C et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc. Natl Acad. Sci. Us 116, 19609–19618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Olivares O. et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat. Commun 8, 16031 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim PK et al. Hyaluronic acid fuels pancreatic cancer cell growth. Elife 10.7554/eLife.62645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Provenzano PP et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012). This study first identified the presence of exorbitantly elevated interstitial pressures (more than 100 mmHg) in a GEMM of autochthonous PDA and their dependence on HMW HA and then, together with Jacobetz et al. (2013), showed that specifically degrading intratumoural HA reverses vascular collapse and enables effective drug delivery to the tumour bed.
- 172. DuFort CC et al. Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel-fluid phase. Biophys. J 110, 2106–2119 (2016). This article describes the unusual properties of an HA-dependent gel-fluid phase in PDA that generates large swelling pressures that are counterpoised by entanglement with a tethered fibrillar collagen network, and together result in enormous interstitial pressures.
- 173.Jacobetz MA et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bosveld F. et al. Modulation of junction tension by tumor suppressors and proto-oncogenes regulates cell-cell contacts. Development 143, 623–634 (2016). [DOI] [PubMed] [Google Scholar]
- 175.Dupont S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). [DOI] [PubMed] [Google Scholar]
- 176.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- 177.Toole BP Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer 4, 528–539 (2004). [DOI] [PubMed] [Google Scholar]
- 178.Katchalsky A Polyelectrolytes and their biological interactions. Biophys. J 4, 9–41 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Thompson CB et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther 9, 3052–3064 (2010). This study provides a proof of principle that systemically delivered PEGylated hyaluronidase can deplete interstitial HA and lower interstitial pressures in implanted tumour models.
- 180.Hingorani SR et al. A phase Ib study of gemcitabine plus PEGPH20 (pegylated recombinant human hyaluronidase) in patients with stage IV previously untreated pancreatic cancer. J. Clin. Oncol 31(15 Suppl.), 4010 (2013). [Google Scholar]
- 181.Hingorani SR et al. Randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine (PAG) vs AG in patients (Pts) with untreated, metastatic pancreatic ductal adenocarcinoma (mPDA). J. Clin. Oncol 10.1200/JCO.2017.35.15_suppl.4008 (2017). [DOI] [PubMed] [Google Scholar]
- 182.Van Cutsem E et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J. Clin. Oncol 38, 3185–3194 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ramanathan RK et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J. Clin. Oncol 37, 1062–1069 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Meyer FA Macromolecular basis of globular protein exclusion and of swelling pressure in loose connective tissue (umbilical cord). Biochim. Biophys. Acta 755, 388–399 (1983). [DOI] [PubMed] [Google Scholar]
- 185.Meyer FA, Laver-Rudich Z & Tanenbaum R Evidence for a mechanical coupling of glycoprotein microfibrils with collagen fibrils in Wharton’s jelly. Biochim. Biophys. Acta 755, 376–387 (1983). [DOI] [PubMed] [Google Scholar]
- 186.Trinkaus JP Cells into Organs: The Forces That Shape the Embryo (Prentice-Hall, 1984). [Google Scholar]
- 187.Laklai H. et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med 22, 497–505 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Calvo F et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol 15, 637–646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Murakami S. et al. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene 36, 1232–1244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jiang Z. et al. Inhibiting YAP expression suppresses pancreatic cancer progression by disrupting tumor-stromal interactions. J. Exp. Clin. Cancer Res 37, 69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang W. et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci. Signal 7, ra42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gruber R. et al. YAP1 and TAZ control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterology 151, 526–539 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Kapoor A. et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell 158, 185–197 (2014). This study identifies aberrant YAP activation as an escape mechanism for PDA cells to cause relapse after prior tumour regressions induced by extinguishing oncogenic Kras expression.
- 194.Mello SS et al. A p53 super-tumor suppressor reveals a tumor suppressive p53-Ptpn14-Yap axis in pancreatic cancer. Cancer Cell 32, 460–473 e466 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.DeVita VT Jr & Chu E A history of cancer chemotherapy. Cancer Res. 68, 8643–8653 (2008). [DOI] [PubMed] [Google Scholar]
- 196.Rogers KW & Schier AF Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol 27, 377–407 (2011). [DOI] [PubMed] [Google Scholar]
- 197.Alvarez MA, Freitas JP, Mazher Hussain S & Glazer ES TGF-beta inhibitors in metastatic pancreatic ductal adenocarcinoma. J. Gastrointest. Cancer 50, 207–213 (2019). [DOI] [PubMed] [Google Scholar]
- 198.Mpekris F et al. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc. Natl Acad. Sci. USA 117, 3728–3737 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Waterhouse M. et al. Effect of vitamin D supplementation on selected inflammatory biomarkers in older adults: a secondary analysis of data from a randomised, placebo-controlled trial. Br. J. Nutr 114, 693–699 (2015). [DOI] [PubMed] [Google Scholar]
- 200.Vonderheide RH CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med 71, 47–58 (2020). [DOI] [PubMed] [Google Scholar]
- 201.Stalnecker CA & Der CJ RAS, wanted dead or alive: advances in targeting RAS mutant cancers. Sci. Signal 10.1126/scisignal.aay6013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tanaka N. et al. Clinical acquired resistance to KRASG12C inhibition through a novel KRAS switch-II pocket mutation and polyclonal alterations converging on RAS-MAPK reactivation. Cancer Discov. 11, 1913–1922 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jiang H. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med 22, 851–860 (2016). This study reveals the role of activated FAK signalling in a GEMM of PDA in supporting an immunosuppressive state.
- 204.Jiang H. et al. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut 69, 122–132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhao Y. et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc. Natl Acad. Sci. USA 116, 2210–2219 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shao DD et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 158, 171–184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bengsch F, Knoblock DM, Liu A, McAllister F & Beatty GL CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol. Immunother 66, 1609–1617 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Thorsson V. et al. The immune landscape of cancer. Immunity 48, 812–830 e814 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Stromnes IM et al. T Cells engineered against a native antigen can surmount immunologic and physical barriers to treat pancreatic ductal adenocarcinoma. Cancer Cell 28, 638–652 (2015). This study provides a proof of principle for a strategy of serial infusions of tumour-targeting engineered T cells to overcome interstitial pressures and mechanisms of immune suppression in a GEMM of PDA.
- 210. Huang H. et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell 40, 656–673 e657 (2022). This study identifies mesothelial cells as another source of tumour-promoting PDA CAFs with a unique antigen-presenting-like phenotype that promote the expansion of Treg cells.
- 211.McAllister F. et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 25, 621–637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dey P. et al. Oncogenic KRAS-driven metabolic reprogramming in pancreatic cancer cells utilizes cytokines from the tumor microenvironment. Cancer Discov. 10, 608–625 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gittes GK Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 326, 4–35 (2009). [DOI] [PubMed] [Google Scholar]
- 214.Hebrok M, Kim SK & Melton DA Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 12, 1705–1713 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Murtaugh LC, Law AC, Dor Y & Melton DA Beta-catenin is essential for pancreatic acinar but not islet development. Development 132, 4663–4674 (2005). [DOI] [PubMed] [Google Scholar]
- 216.Jensen J. et al. Control of endodermal endocrine development by Hes-1. Nat. Genet. 24, 36–44 (2000). [DOI] [PubMed] [Google Scholar]
- 217.Harris TJ & Tepass U Adherens junctions: from molecules to morphogenesis. Nat. Rev 11, 502–514 (2010). [DOI] [PubMed] [Google Scholar]
- 218.Gumbiner BM Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev 6, 622–634 (2005). [DOI] [PubMed] [Google Scholar]
- 219.Keller R. Developmental biology. Physical biology returns to morphogenesis. Science 338, 201–203 (2012). [DOI] [PubMed] [Google Scholar]
- 220.Krieg M. et al. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol 10, 429–436 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.