Abstract
Introduction:
Osimertinib, a third-generation, irreversible, epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), selectively inhibits both EGFR-TKI sensitizing (EGFRm) and EGFR T790M resistance mutations and has demonstrated efficacy in non-small cell lung cancer (NSCLC) CNS metastases. Most patients with EGFRm NSCLC treated with osimertinib will eventually develop resistance. ORCHARD (NCT03944772) is a phase II study aiming to characterize first-line osimertinib resistance and identify post-progression treatments.
Methods:
Adults aged ≥ 18 years (Japan ≥ 20 years), with EGFRm locally advanced/metastatic NSCLC will be allocated to one of three groups after first-line osimertinib progression, based on molecular profiling from a post-progression tumor biopsy. Group A will evaluate patients with protocol-determined biomarkers of resistance treated with novel osimertinib combination therapies, Group B will evaluate patients without a detectable protocol-determined biomarker treated with non-biomarker selected therapies that are chemotherapy- or EGFR-TKI-based, and Group C (observational) includes patients with histologically transformed disease, and/or a biomarker with an available therapy not investigated in ORCHARD. Group C patients will be treated as per local practice and followed to assess overall survival. The study’s platform design allows for adaptability to include emerging treatments related to novel resistance mechanisms. The primary endpoint is confirmed objective response rate (investigator assessed). Other endpoints are progression-free survival, duration of response, overall survival, pharmacokinetics and safety.
Conclusions:
ORCHARD aims to characterize mechanisms of resistance to first-line osimertinib and explore treatments to overcome acquired resistance. The modular design allows for additional biomarker-directed cohorts and treatment options as understanding of osimertinib resistance mechanisms evolves.
Microabstract
Osimertinib is an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) effective for the treatment of EGFR mutation-positive locally advanced/metastatic non-small cell lung cancer (NSCLC). Most patients develop resistance to EGFR-TKIs, including osimertinib. ORCHARD, a phase II study, utilizes an expandable platform-based study design to identify resistance mechanisms to first-line osimertinib and explore effective post-progression treatment combinations.
Keywords: Acquired resistance, EGFRm, EGFR-TKI, NSCLC, osimertinib
Introduction
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) are the recommended treatment for patients with advanced NSCLC harboring EGFR TKI-sensitizing mutations (EGFRm), 1 Osimertinib, a third-generation, irreversible, EGFR-TKI, selectively inhibits both EGFRm and EGFR T790M resistance mutations, and has demonstrated efficacy in NSCLC central nervous system (CNS) metastases.2–4 In the phase III FLAURA study, first-line osimertinib demonstrated superiority in progression-free survival (PFS) and overall survival (OS) versus comparator EGFR-TKIs in patients with EGFRm advanced/metastatic NSCLC.3,4 Even so, most patients with EGFRm NSCLC treated with a first-line EGFR-TKI, including osimertinib, eventually develop resistance.1
Optimal treatment following progression may depend on the mechanism underlying the resistance. In FLAURA, using next-generation sequencing (NGS) of circulating tumor DNA (ctDNA), a heterogeneous profile of resistance mechanisms to first-line osimertinib was observed; the most common resistance mechanisms identified were MET amplification (16%) and EGFR C797S mutation (6%).5 Targeting acquired resistance alterations can be an effective treatment strategy, such as MET amplification with savolitinib, a potent and highly selective MET-TKI.6 Due to tumor heterogeneity, continuation of osimertinib post-progression may provide additional clinical benefit versus immediate discontinuation,7,8 and combination therapy post-progression with other targeted treatments may continue to suppress EGFR signaling while also targeting the resistance mechanism.
The ORCHARD study aims to define the resistance spectrum following first-line osimertinib progression, and to evaluate optimal treatment based on the identified resistance mechanism(s).
Materials and Methods
ORCHARD (NCT03944772) is an open-label, multicenter, multi-drug, biomarker-directed, phase II platform study in patients with EGFRm-positive advanced NSCLC with disease progression following first-line osimertinib monotherapy. The anticipated enrollment is ~182 patients at ~43 sites across Europe, Asia and North America, although the study design enables cohorts to be added as relevant data emerge, subject to regulatory and ethics approval. The first patient was enrolled in June 2019, and first dose administered in August 2019. The estimate for study completion is Q4 2023. This may change as further cohorts are added. For recent updates, visit https://www.clinicaltrials.gov/ct2/show/NCT03944772.
Study Design
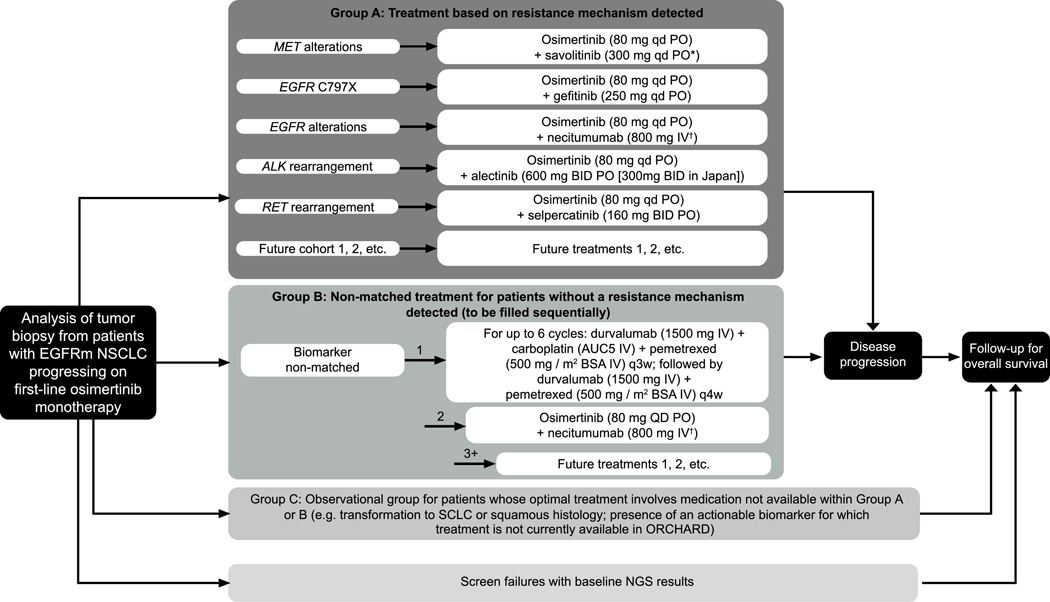
The study comprises three groups (Group A, B and C); treatment allocation and rationale are described in Figure 1. Key eligibility criteria for Groups A and B are included in Table 1. Patient allocation to treatment is based on NGS analysis from a post-progression tumor biopsy. Local NGS analysis is accepted but retrospective central NGS confirmation is required.
Figure 1.
ORCHARD Study Design
*Following a protocol amendment, all newly enrolled patients with MET alterations will receive savolitinib 300 mg qd, replacing the previous weight-based dosing regimen (300 mg for patients weighing ≤55 kg at screening, or 600 mg once daily for patients >55 kg at screening).
†On days 1 and 8 of each 3-week cycle.
Group A: Patients who are positive for protocol-determined biomarker; Group B: patients without an available protocol-determined biomarker match, allocated sequentially, once first cohort cap has been reached, the next cohort allocation will begin; Group C: observational cohort, treated in accordance with local practice. Most treatments will be provided by the sponsor. In some participating countries, pemetrexed and carboplatin are sourced locally.
Abbreviations: ALK = anaplastic lymphoma kinase; AUC= area under curve; BID = twice daily; EGFR = epidermal growth factor; EGFRm = EGFR-tyrosine kinase inhibitor sensitizing mutation-positive; IV = intravenous; NGS = next-generation sequencing; NSCLC = non-small cell lung cancer; PO = orally; q3(4)w = once every 3(4)-week cycle; QD = once daily; SCLC = small cell lung cancer.
Table 1.
Key Eligibility Criteria for Groups A and B
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adults aged ≥ 18 years (≥ 20 years in Japan) | Patients whose disease has progressed within the first 3 months of osimertinib treatment |
| Locally advanced/metastatic NSCLC not amenable to curative surgery or radiotherapy at study entry | Prior/concurrent treatment with any systemic anticancer therapy for advanced/metastatic NSCLC (except osimertinib) |
| Evidence of radiological disease progression on first-line monotherapy with osimertinib 80 mg once daily | History of diagnosis with another primary malignancy (e.g. SCLC or SCC) |
| Suitable for mandatory biopsy | Inadequate bone marrow reserve or organ function |
| Measurable disease per RECIST 1.1 | Past medical history of ILD, drug induced ILD, radiation pneumonitis requiring steroid treatment, or any evidence of clinically active ILD |
| Histologically/cytologically confirmed adenocarcinoma of the lung harboring EGFR mutation(s) known to be associated with EGFR-TKI sensitivity at diagnosis | Patients with spinal cord compression, symptomatic and unstable brain metastases, except those who have completed definitive therapy, are not on steroids and have a stable neurologic status for at least 2 weeks after completion of the definitive therapy and steroids |
| WHO performance status of 0/1 |
Abbreviations: EGFR = epidermal growth factor receptor; EGFR-TKI = epidermal growth factor receptor tyrosine kinase inhibitor; ILD = interstitial lung disease; NSCLC = non-small cell lung cancer; RECIST = Response Evaluation Criteria in Solid Tumours; SCC = squamous cell carcinoma; SCLC = small cell lung cancer; WHO = World Health Organization
Group A.
Patients who are positive for a protocol-determined biomarker are allocated to osimertinib 80 mg daily in combination with a targeted therapy for the resistance mechanisms identified. Current active osimertinib combination cohorts include savolitinib 300 mg once daily targeting MET alterations, gefitinib 250 mg once daily targeting EGFR C797X mutations, necitumumab 800 mg on days 1 and 8 of a 3-week cycle targeting other EGFR alterations, alectinib 600 mg twice daily (300 mg twice daily in Japan) targeting ALK rearrangement and selpercatinib 160 mg twice daily to target RET rearrangements. Cohorts will be added depending on future biomarkers and combination treatments identified.
Group B.
Patients without an identifiable resistance mechanism will be allocated to a cohort in Group B; treatment allocation will be made to balance adverse prognostic features based on prior osimertinib treatment response and presence of CNS metastases. There are currently two cohorts in Group B: 1) up to 6 cycles of durvalumab 1500 mg plus carboplatin target area under the curve 5 plus pemetrexed 500 mg/m2 body surface area (BSA) every 3 weeks (Q3W), followed by durvalumab 1500 mg plus pemetrexed 500 mg/m2 BSA Q4W, and 2) osimertinib 80 mg daily plus necitumumab 800 mg days 1 and 8 of a 3-week cycle. Cohorts can be added upon identification of future treatment options.
Group C.
In this observational group, patients will not be allocated to a treatment and will be managed by the investigator or healthcare professional responsible for their care, in accordance with local practice guidelines.
Study Assessments
Treatment will be administered until objective disease progression (Response Evaluation Criteria in Solid Tumours [RECIST] 1.1) unless the patient is still receiving clinical benefit (investigator assessed), and in the absence of discontinuation criteria (patient/investigator decision, severe non-compliance with the clinical study protocol or development of any adverse events that meet the study treatment-specific criteria for permanent discontinuation of study treatment), or until withdrawal of consent.
The primary endpoint is confirmed objective response rate (ORR) using RECIST 1.1 (investigator assessed). Secondary endpoints are PFS, duration of response, OS and pharmacokinetics. Exploratory endpoints include ctDNA analysis of tumor burden in ctDNA derived from longitudinal plasma samples, time to progression and understanding mechanisms of resistance to second-line therapies. Safety and tolerability will also be assessed. Patients in Groups A and B will undergo tumor evaluation (RECIST 1.1) every 6 weeks (±1 week) for the first 24 weeks, and Q9W thereafter until progression. Survival status and information on anti-cancer therapies (start/stop date, best overall response, date of progression) for patients who discontinue treatment permanently, or who are in Group C, will be collected at follow-up assessments Q12W unless consent is withdrawn.
Statistical Analysis
Sample Size Calculation and Interim Analysis.
A lower reference value of 30% and a target value of 45% will be used to assess ORR according to RECIST 1.1. Up to 40 patients will be recruited into each cohort with an interim analysis using data from 16 patients who have had the opportunity for two post-baseline radiological assessments. If ≤ 4 of the first 16 evaluable patients in a cohort have confirmed responses, recruitment to that cohort may be stopped as this translates to a < 10% probability that the ORR will be above the target of 45%.
Safety Run-in.
As there is little information available about some of the treatment combinations, a safety run-in phase will be performed using a ‘3 + 3’ design to confirm the recommended dose of the combination. After 3 weeks, the Safety Review Committee will evaluate the safety, tolerability and pharmacokinetics of the combination to confirm recommended doses; if a dose is not tolerated, recruitment to that combination cohort will be stopped. Efficacy and safety analyses will be descriptive with no formal statistical hypothesis testing.
Discussion
The platform-based design of the ORCHARD study enables identification of resistance mechanisms to first-line osimertinib, and exploration of the safety and efficacy of treatment combinations that might overcome resistance. This design allows for the inclusion of new cohorts based on identification of emerging resistance mechanisms through real-time analysis of translational biospecimens and from the ever-expanding scientific literature. Since study initiation, the protocol has been amended to include treatment with the RET and ALK inhibitors selpercatinib and alectinib, respectively. The addition of selpercatinib is based on data from a proof-of-concept study showing that RET inhibition may overcome resistance to EGFR-TKIs in patients with acquired RET fusions and supported by recent data from a study evaluating safety and preliminary efficacy of the osimertinib with selpercatinib combination.9,10 Although there is limited information available regarding the combination of EGFR-TKIs with ALK inhibitors, phase I studies have reported safety profiles consistent with the individual treatments.11,12 It has been suggested that the more manageable toxicity profile of osimertinib monotherapy versus comparator EGFR-TKIs may lead to osimertinib being more suitable for combination strategies, and this is supported by anecdotal reports of efficacy from case reports.12–14
In addition, based on emerging data, the MET alterations cohort has been expanded from MET amplification to include MET exon 14 skipping, and the EGFR alterations cohort to include mutations at the EGFR L718/G724 residue, or exon 20 insertion; EGFR C797X mutations are treated in a separate cohort for distinct efficacy analyses. These are rare alterations with an estimated prevalence of < 5%,5 making individual studies to evaluate them not feasible to run. Therefore, this platform will provide clinical data to corroborate the current case studies available in the literature.
The prevalence of resistance mechanisms will be comprehensively examined via tissue-based testing, including investigating the concordance with plasma-based testing. Baseline plasma analysis may provide insights into the value of utilizing non-invasive plasma testing versus tumor biopsies for cohort allocation, as well as to track responses and resistance to treatment. The observational Group C will enable investigation of targetable mutations not currently being treated in ORCHARD and may inform future cohorts for the study, as well as providing further information on the outcomes of patients with histologic transformation.
A potential limitation of this study is the relatively short time that first-line osimertinib has been reimbursed (April 2018, USA), which may lead to inclusion bias of patients whose disease progresses early on osimertinib. To control for this, biomarker non-matched cohorts will be capped when a pre-determined number of patients with CNS metastases and with early disease progression (< 12 months on first-line osimertinib) have been allocated to treatment. This study does not include a standard-of-care cohort and has relatively small cohort sizes, thus limiting the capability to draw definitive conclusions. However, the design allows for data to be continuously collected and new treatment cohorts to be added based on emerging results, with the potential for cohorts to be expanded into independent confirmatory studies in the future.
Summary
The ORCHARD study will investigate novel treatment options for patients with EGFRm NSCLC and disease progression on first-line osimertinib. The flexible, platform-based design has opportunity for adaptability and inclusion of patient cohorts or treatment options based on emerging scientific evidence.
Acknowledgments
The authors would like to thank the ORCHARD study team, the site staff and the patients involved in this study. The study (NCT03944772) was funded by AstraZeneca, Cambridge, United Kingdom, the manufacturer of osimertinib, savolitinib, durvalumab and gefitinib. The authors would like to acknowledge Eli Lilly and Company, the manufacturer of necitumumab and selpercatinib, and Roche, the manufacturer of alectinib. The authors would like to acknowledge Eleanor Thomas, BSc, and Alexandra Webster, MSc, of Ashfield MedComms, part of Ashfield Health for medical writing support that was funded by AstraZeneca, Cambridge, UK in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author disclosures
H Yu has received research funding to her institution from AstraZeneca, Pfizer, Cullinan, Daiichi, Novartis, Lilly and advisory board fees from AstraZeneca, Blueprint Medicine and Janssen. S Goldberg has received research funding from AstraZeneca and Boehringer Ingelheim, received advisory board/personal fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Genentech and Spectrum. Z Piotrowska has received research funding to her institution from Novartis, Takeda, Spectrum, AstraZeneca, Tesaro, Cullinan, Daiichi and AbbVie and has received advisory board fees from AstraZeneca, Blueprint Medicines, Janssen, Takeda, Jazz Pharmaceuticals and C4 Therapeutics. X Le receives consulting/advisory fees from EMD Serono (Merck KGaA), AstraZeneca, Spectrum Pharmaceutics, Eli Lilly, Boehringer Ingelheim, and Research Funding from Eli Lilly and Boehringer Ingelheim. JW Riess has received research funding from AstraZeneca, Merck, Spectrum, Revolution Medicines and Novartis, received advisory board/personal fees from Novartis, Medtronic, Blueprint Medicines, Boehringer Ingelheim and Spectrum. G Patel is an employee of AstraZeneca. H Ambrose, J Chmielecki, G Doughton, S Kraljevic, X Li-Sucholeiki, J Maidment and P Szekeres are employees of AstraZeneca and hold stock/stock options.
Footnotes
Credit Author Statement
Helena A. Yu: Conceptualization, Methodology, Investigation, Resources, Supervision, Drafting the manuscript, Manuscript writing.: Sarah B. Goldberg: Conceptualization, Methodology, Investigation, Resources, Supervision, Drafting the manuscript, Manuscript writing.: Xiuning Le: Conceptualization, Investigation, Supervision, Drafting the manuscript.: Zofia Piotrowska: Conceptualization, Methodology, Investigation, Resources, Supervision, Drafting the manuscript.: Jonathan W. Goldman: Conceptualization, Methodology, Investigation, Data curation, Visualization, Drafting the manuscript, Manuscript writing.: Adrianus J. De Langen: Conceptualization, Manuscript writing.: Isamu Okamoto: Investigation, Resources, Data curation, Drafting the manuscript.: Byoung Chul Cho: Conceptualization, Drafting the manuscript, Interpretation, Data curation, Data analysis.: Paul Smith: Conceptualization, Methodology, Investigation, Supervision, Project administration, Funding acquisition, Drafting the manuscript, Manuscript writing.: Ilhem Mensi: Conceptualization, Methodology, Investigation, Supervision, Project administration, Funding acquisition, Drafting the manuscript, Manuscript writing.: Helen Ambrose: Conceptualization, Methodology, Investigation, Resources, Data curation, Visualization, Supervision, Drafting the manuscript, Manuscript writing.: Silvija Kraljevic: Conceptualization, Methodology, Investigation, Resources, Data curation, Visualization, Supervision, Manuscript writing.: Julie Maidment: Methodology, Manuscript writing.: Juliann Chmielecki: Conceptualization, Methodology, Data curation, Data analysis, Visualization, Drafting the manuscript.: Xiaocheng Li-Sucholeiki: Conceptualization, Methodology, Resources, Drafting the manuscript, Manuscript writing.: Gail Doughton: Data Curation, Project administration, Drafting the manuscript.: Gargi Patel: Conceptualization, Methodology, Investigation, Resources, Visualization, Supervision, Project administration, Funding acquisition, Drafting the manuscript, Manuscript writing.: Phil Jewsbury: Conceptualization, Methodology, Investigation, Supervision, Project administration, Funding acquisition, Drafting the Manuscript.: Phil Szekeres: Conceptualization, Methodology, Project administration.: Jonathan Riess: Conceptualization, Methodology, Investigation, Resources, Supervision, Manuscript writing All authors gave approval of the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Updated version published 15 September 2020 by the ESMO Guidelines Committee. Ann Oncol 2018; 29:iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 2.Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol 2018; 36:2702–2709. [DOI] [PubMed] [Google Scholar]
- 3.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020; 382:41–50. [DOI] [PubMed] [Google Scholar]
- 4.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018; 378:113–125. [DOI] [PubMed] [Google Scholar]
- 5.Ramalingam SS, Cheng Y, Zhou C, et al. LBA 50 Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol 2018; 29:LBA50. [Google Scholar]
- 6.Sequist LV, Han JY, Ahn MJ, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. Lancet Oncol 2020; 21:373–386. [DOI] [PubMed] [Google Scholar]
- 7.Le X, Puri S, Negrao MV, et al. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res 2018; 24:6195–6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mu Y, Hao X, Yang K, et al. Clinical modality of resistance and subsequent management of patients with advanced non-small cell lung cancer failing treatment with osimertinib. Target Oncol 2019; 14:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer Discov 2018; 8:1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotow J. FP14.07 Combination osimertinib plus selpercatinib for EGFR-mutant non-small cell lung cancer (NSCLC) with acquired RET fusions. IASLC 2020 World Conference on Lung Cancer. [Google Scholar]
- 11.Ou SH, Govindan R, Eaton KD, et al. Phase I results from a study of crizotinib in combination with erlotinib in patients with advanced nonsquamous non–small cell lung cancer. J Thorac Oncol 2016; 12:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janne PA, Shaw AT, Camidge DR, et al. Combined pan-HER and ALK/ROS1/MET inhibition with dacomitinib and crizotinib in advanced non-small cell lung cancer: results of a phase I study. J Thorac Oncol 2016; 11:737–747. [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld AJ, Chan JM, Kubota D, et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res 2020; 26:2654–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Offin M, Somwar R, Rekhtman N, et al. Acquired ALK and RET gene fusions as mechanisms of resistance to osimertinib in EGFR-mutant lung cancers. JCO Precis Oncol 2018; 2:PO.18.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]