ABSTRACT
Little is known regarding the effectiveness of tixagevimab/cilgavimab in preventing SARS-CoV-2 infection in vaccinated immunocompromised patients, particularly after the emergence of the Omicron variant. In this retrospective cohort study with exact matching and propensity score adjustment within the U.S. Department of Veterans Affairs (VA) healthcare system, we selected immunocompromised veterans age ≥18 years as of 1 January 2022, receiving VA healthcare. We compared a cohort of 1,878 patients treated with at least one dose of intramuscular tixagevimab/cilgavimab to 7,014 matched controls selected from patients who met study criteria but were not treated. Patients were followed through 15 June 2022, or until death, whichever occurred earlier. The primary outcome was a composite of SARS-CoV-2 infection, COVID-19-related hospitalization, and all-cause mortality. We used Cox proportional hazards modeling to estimate the hazard ratios (HRs) and 95% CI for the association between receipt of tixagevimab/cilgavimab and outcomes. Most (73%) tixagevimab/cilgavimab recipients were ≥65 years old, and 80% had ≥3 mRNA vaccine doses or two doses of Ad26.COV2. Compared to matched controls, recipients had a lower incidence of the composite COVID-19 outcome (49/1,878 [2.6%] versus 312/7,014 [4.4%]; HR 0.35; 95% CI, 0.24–0.52), and individually SARS-CoV-2 infection (HR 0.44; 95% CI, 0.22–0.88), COVID-19 hospitalization (HR 0.24; 95% CI, 0.10–0.59), and all-cause mortality (HR 0.32; 95% CI, 0.19–0.55). In conclusion, tixagevimab/cilgavimab was associated with lower rates of SARS-CoV-2 infection and severe COVID-19 during the Omicron BA.1, BA.2, and BA.2.12.1 surge.
IMPORTANCE
SARS-CoV-2 remains an ongoing global health crisis that justifies continued efforts to validate and expand, when possible, knowledge on the efficacy of available vaccines and treatments. Clinical trials have been limited due to fast tracking of medications for mitigation of the COVID-19 pandemic for the general population. We present a real-world analysis, using electronic health record data, of the effectiveness of tixagevimab/cilgavimab for the prevention of COVID-19 infection in the unique population of U.S. veterans. Unlike those in the PROVENT clinical trial from which the emergency use authorization for tixagevimab/cilgavimab as a preventative treatment arose, the veterans population is highly immunocompromised and nearly 96% totally vaccinated. These demographics allowed us to analyze the effectiveness of tixagevimab/cilgavimab in preventing COVID-19 under different conditions in a more fragile population than that of the initial clinical trial.
KEYWORDS: COVID-19, SARS-CoV-2, monoclonal antibodies, prevention, real-world data, propensity score matching
INTRODUCTION
Immunocompromised individuals are at high risk for morbidity and mortality due to COVID-19 (1). While vaccines have helped to prevent the spread of SARS-CoV-2 and decrease the risk of severe disease in the general population, immunocompromised individuals remain at higher risk for breakthrough infections and persistent viral replication (2 - 5).
The PROVENT study, a phase 3, multicenter, randomized, and placebo-controlled trial, demonstrated that a single dose of intramuscular tixagevimab/cilgavimab (Evusheld, AstraZeneca) significantly reduced the incidence of symptomatic SARS-CoV-2 infection by 76.7% after 90 days in a broad population of adults with an increased risk of inadequate response to vaccination and/or increased risk of exposure to SARS-CoV-2 (6). Tixagevimab/cilgavimab is effective because it binds to non-overlapping portions of the SARS-CoV-2 spike protein, preventing the virus from interacting with the human angiotensin converting enzyme-2 receptor. Based on these findings, on 8 December 2021, the U.S. Food and Drug Administration (FDA) granted an emergency use authorization (EUA) of tixagevimab/cilgavimab as pre-exposure prophylaxis for moderate-to-severe immune compromised individuals or for whom vaccination with any available COVID-19 vaccine is not recommended due to a history of severe adverse reaction (7). The PROVENT trial also included those with chronic health conditions that could predispose individuals to COVID-19 complications.
Importantly, questions remain regarding the effectiveness of tixagevimab/cilgavimab for the prevention of COVID-19. Only a small proportion (11%) of participants in the PROVENT trial were immunocompromised (i.e., receipt of immunosuppressive therapy, have an immunosuppressive disease or cancer), and treatment effectiveness in this crucial subgroup could not be estimated in the trial. Furthermore, all participants in the PROVENT trial were unvaccinated at the time of trial entry; therefore, indications for tixagevimab/cilgavimab among vaccinated persons remain unknown. Finally, the follow-up of participants in the PROVENT trial ended in September 2021; therefore, an analysis regarding “real-world” effectiveness is needed for tixagevimab/cilgavimab among vaccinated immunocompromised Veterans after the emergence of the Omicron variant (December 2021 in the United States) (8).
The objective of this study was to assess the effectiveness of tixagevimab/cilgavimab for the prevention of COVID-19 during the Omicron surge using electronic data from the U.S. Veterans Health Administration (VHA), the largest integrated healthcare system in the U.S. Using exact matching and propensity score matching, we estimated the “real-world” effectiveness of tixagevimab/cilgavimab among immunocompromised Veterans for the prevention of SARS-CoV-2 infection, COVID-19-related hospitalization, and all-cause mortality, with secondary outcomes including emergency room (ER) visits, urgent care visits, and intensive care unit (ICU) stays.
MATERIALS AND METHODS
Study setting and data sources
The Veterans Health Administration (VHA) provides care to nearly 9 million veterans at 171 medical centers, and 1,112 outpatient clinics across the United States. Electronic health records (EHR) were analyzed using the VA Corporate Data Warehouse, which contains patient-level information on all patient encounters within VA medical facilities, including treatments, prescriptions, vaccinations, laboratory results, healthcare utilization, and vital status (9, 10). We identified tixagevimab/cilgavimab use through the VA Pharmacy Benefits Management (PBM) EUA prescription dashboard, which captures and links records of recipients, date, and dosage of tixagevimab/cilgavimab administered in medical facilities across the VA (11). The first dose of tixagevimab/cilgavimab was given at VA on 13 January 2022.
Before data collection, this study was approved by the institutional review board of the VA Medical Center in White River Junction, VT, and was granted a waiver of informed consent because the study was deemed a minimal risk and consent impractical to acquire. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study population and ascertainment of tixagevimab/cilgavimab administration
Veterans who were ≥18 years and had received VHA benefits for at least 2 years as of 1 January 2022 were included when tixagevimab/cilgavimab first became available at the VHA. All included veterans met the definition of immunocompromised at that date: either treatment with an immunosuppressant (Table S1) in the previous 30 days and/or by having at least one inpatient or two outpatient visits with an International Classification of Diseases, Tenth Revision (ICD-10) diagnosis code for an immunocompromising condition (Table S2) in the year prior to 1 January 2022, which would make them eligible for tixagevimab/cilgavimab. Individuals who had a SARS-CoV-2 infection within 90 days prior to receiving tixagevimab/cilgavimab treatment were excluded.
Tixagevimab/cilgavimab (150 mg/150 mg) was first administered on 13 January 2022 at VA. In response to concerns regarding the effectiveness of tixagevimab/cilgavimab against the Omicron variant, the FDA revised the EUA on 24 February 2022 to double the initial dose of tixagevimab/cilgavimab (300 mg/300 mg). Veterans who received the previously authorized (lower) dose were advised to receive an additional dose (12). Among 234,604 qualifying individuals, we identified 2,083 veterans who were administered at least one dose of tixagevimab/cilgavimab for the treatment group and designated the first date of tixagevimab/cilgavimab administration as the index date for each veteran (Fig. 1). If tixagevimab/cilgavimab was administered before the end of the study period (15 June 2022), the patient is included in the analysis and contributes to time of exposure to tixagevimab/cilgavimab.
Fig 1.
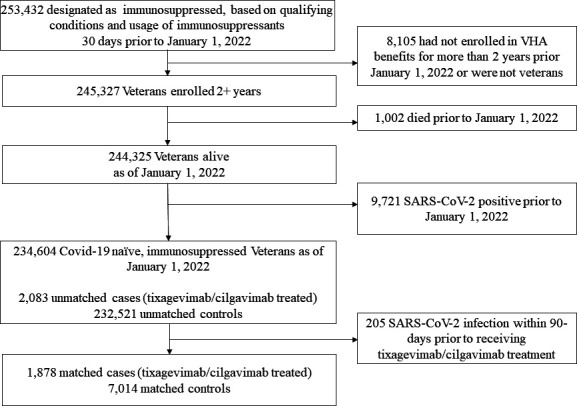
Selection of veterans for matched cohort analysis of the effectiveness of tixagevimab/cilgavimab.
We included baseline characteristics (e.g., demographics, significant comorbidities, and healthcare utilization) documented within 2 years prior to the index date. We used a VA-assigned priority group for healthcare to serve as a surrogate measure for socioeconomic status (13). Information regarding comorbidities was abstracted from diagnosis codes recorded in VA electronic data for healthcare encounters; significant comorbidities were defined according to an adaptation of Deyo-Charlson comorbidity index (DCCI) (14). We defined severely immunocompromised as those who had a solid organ transplant or received anti-rejection medication for transplant or chemotherapy for cancer treatment in the prior month.
Outcomes
The primary outcome was the composite of SARS-CoV-2 infections confirmed by the presence of SARS-CoV-2 virus detected by reverse transcriptase-polymerase chain reaction (RT-PCR) or antigen testing performed at or recorded by the VHA. Infections could also be confirmed by a record of COVID-19 treatment, including antivirals and antiviral monoclonal antibodies, because per EUA guidelines, early COVID-19 treatment can only be given to veterans with confirmed COVID-19 infection as some veterans may have had a positive test detecting SARS-CoV-2 at home. Second, COVID-19 hospitalizations, defined as having both an admission and discharge diagnosis (ICD-10 U07.1) for COVID-19 from an admission date within 30 days of a positive SARS-CoV-2 RT-PCR result or antigen test was an additional endpoint. The final endpoint of the primary composite outcome was all-cause mortality, defined as having a date of death during the follow-up. Clinical visits with urinary tract infection (UTI) as the primary discharge diagnosis were added as a fourth outcome in the falsification test as for a negative control (15). The reason UTI was chosen is because there is no causal mechanism for an association between tixagevimab/cilgavimab and UTI, but some of the same biases may exist for that association, and the association between tixagevimab/cilgavimab and COVID-19 outcomes (16). Secondary outcomes included COVID-19-related ER visits, urgent care visits, and ICU stays following the index date. Survival (e.g., SARS-CoV-2 infection) analyses were calculated using the time from the index date (either the first administration date for treated patients or the matched date for controls) to the first occurrence of the event of interest. Individuals without the event of interest were censored at the end of the follow-up.
Statistical analysis
Matching
We used both exact matching and propensity score adjustment in models to account for observable baseline differences between patients who received tixagevimab/cilgavimab and up to four matched controls. Exact matching was implemented for the following variables: sex, race/ethnicity category (non-Hispanic White, non-Hispanic Black, Hispanic any race, and others), VA-assigned priority group (1 to 4 or other priority), index date for cases and controls within 1 week, age (for 5-year age bands, see Table S3), vaccination status (two-dose mRNA COVID-19 vaccine/one-dose J&J/Janssen COVID-19 vaccine or not vaccinated), body mass index (BMI) category (missing, normal as ≤26, and overweight/obese as >26), and certain underlying conditions (asthma, chronic obstructive pulmonary disease [COPD], human immunodeficiency virus [HIV], rheumatoid arthritis, and renal disease). A propensity score was estimated from additional variables (Table S3) that were not included in the exact matching. All covariates in the exact matching and propensity score (Table S3) were measured before the initiation of tixagevimab/cilgavimab to avoid adjustment for potential mediators. Indicator variables were generated to capture missing or unknown values for any of the matching criteria to retain patients in the study. To assess the robustness of the matching, we calculated the standardized mean difference (SMD); a successful match was estimated when at least 90% of the covariates included in the propensity score model had SMD of ≤10 (17).
Cox proportional hazards regression was used to compare outcomes through 15 June 2022 for Veterans who received tixagevimab/cilgavimab and the matched untreated veterans. Hazard ratios (HRs) were estimated for primary and secondary outcomes. Matching was incorporated into the Cox model via stratification on sets of matched subjects. A subgroup analysis for severely immunocompromised individuals was performed. Cumulative risk curves for each group were estimated with Nelson-Aalen cumulative hazard function. Also, 24-week risks of outcomes, with accompanying confidence intervals, for treated and untreated groups, respectively, were calculated.
Emulation of a target trial
In our sensitivity analysis, we employed a target trial emulation design (18) to evaluate the effectiveness of tixagevimab/cilgavimab. This time, eligible veterans who received tixagevimab/cilgavimab were matched in a 1:1 ratio to eligible veterans who did not receive tixagevimab/cilgavimab. To ensure matching, cohorts of treated and untreated veterans were anchored around the same calendar date, and the study observation period was divided into 1-week periods (19). At the start of each 1-week period, all veterans who were alive, were SARS-CoV-2-naive, and had not received tixagevimab/cilgavimab were identified. Those who received tixagevimab/cilgavimab during the week could potentially be allocated to the treatment arm in the target trial, and those who had not received tixagevimab/cilgavimab at the start of the week could potentially be allocated to the comparison arm. Using the baseline characteristics for all veterans and their vaccination status at the beginning of the week, we matched an untreated veteran to each treated veteran, this time using exact matching only. These exactly matched variables included sex, race/ethnicity category (non-Hispanic White, non-Hispanic Black, and Hispanic any race), VHA-assigned priority group (1 to 4 or other priority), age (65 or older), vaccination status (at least primary series—two mRNA vaccine doses or one dose of Ad26.COV2 versus no record of vaccination), BMI category (normal as <26 versus overweight/obese as >26), and certain underlying conditions (asthma, heart disease [including hypertension and diabetes], COPD, HIV, rheumatoid arthritis, renal disease, severely immunocompromised [veterans with a solid organ transplant or hematologic cancer, or other cancer, veterans on immunosuppressants]), and VHA facility.
For each veteran initiating treatment in any given week, one matched untreated individual was randomly selected from all eligible veterans based on the above exact matching criteria, provided that the veteran was still alive, untreated, and uninfected on the index date of the treated veteran. Matched treated and untreated veterans were followed from the index date (i.e., date of treatment for tixagevimab/cilgavimab recipients) until the earliest of the following: occurrence of the outcome of interest, death, initiation of tixagevimab/cilgavimab (for untreated veterans), inconsistent contact with the VHA, or the end of the study period (15 June 2022).
Analyses were performed with Stata 17 software (StataCorp) and SAS software, version 8.2 (SAS Institute).
RESULTS
Study population
We identified 2,083 recipients of tixagevimab/cilgavimab and 232,521 control patients who were immunocompromised (Fig. 1). After exact matching, 1,878 remained in the treatment group and 7,014 in the control group, which were balanced across baseline characteristics (Table 1). Among treated patients, 1,753 (93%) were male, 273 (15%) were Black, 59 (3%) were Hispanic, and 1,427 (76%) were non-Hispanic White. Most (1,362 [73%]) of the tixagevimab/cilgavimab recipients were ≥65 years old, and 1,332 (71%) lived in urban areas (20). Among recipients, 208 (11.1%) received only a single dose, 10 (0.6%) had another visit to receive their second single dose, and 1,660 (88.4%) received double doses on the same visit. Common comorbidities and immunocompromising conditions among cases included hypertension (1,174; 63%), dyslipidemia (694; 37%), and cancer (745; 40%), specifically hematological cancer (657; 35%). Most (96%) tixagevimab/cilgavimab recipients had received two doses of mRNA vaccines or one dose of Ad26.COV2 (Janssen) or ≥3 vaccine doses or two doses of Ad26.COV2 (80%). Prior to matching, only 87 (4%) tixagevimab/cilgavimab recipients did not have any record of COVID-19 vaccination, compared to 53,195 (23%) among controls; this imbalance was resolved with matching.
TABLE 1.
Selecteda baseline characteristics
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Controls | Recipients | Controls | Recipients | |||
| (N = 232,521) | (N = 2,083) | SMD | (N = 7,014) | (N = 1,878) | SMD | |
| Age on 31 Dec 2021 | ||||||
| Mean (SD) | 65 (14.1) | 68 (10.5) | 28.1 | 69 (10.5) | 69 (10.3) | −2.5 |
| Sex | ||||||
| Male | 203,678 (88%) | 1,909 (92%) | 13.3 | 6,620 (94%) | 1,753 (93%) | −4.3 |
| Race/ethnicity | ||||||
| Black: non-Hispanic Black | 48,372 (21%) | 310 (15%) | −15.5 | 996 (14%) | 273 (15%) | 1 |
| Hispanic any race | 15,044 (6%) | 89 (4%) | −9.8 | 179 (3%) | 59 (3%) | 3.5 |
| Other | 16,517 (7%) | 148 (7%) | 0 | 399 (6%) | 119 (6%) | 2.7 |
| White: non-Hispanic White | 152,588 (66%) | 1,536 (74%) | 17.7 | 5,440 (78%) | 1,427 (76%) | −3.7 |
| Number of vaccinations | ||||||
| Zero-dose vaccine | 53,195 (23%) | 87 (4%) | −56.8 | 260 (4%) | 71 (4%) | 0.4 |
| Two-dose vaccineb | 179,326 (77%) | 1,996 (96%) | 56.8 | 6,754 (96%) | 1,807 (96%) | −0.4 |
| Third dose of vaccine | 96,330 (41%) | 1,650 (79%) | 83.7 | 3,989 (57%) | 1,501 (80%) | 51.2 |
| BMI category | ||||||
| Missing | 11,262 (5%) | 54 (3%) | −11.9 | 110 (2%) | 35 (2%) | 2.3 |
| Normal | 59,953 (26%) | 603 (29%) | 7.1 | 1,935 (28%) | 529 (28%) | 1.3 |
| Overweight/obese | 161,306 (69%) | 1,426 (68%) | −2 | 4,969 (71%) | 1,314 (70%) | −1.9 |
| DCCI | ||||||
| Mean (SD) | 1.7 (2.09) | 2.8 (2.326) | 53.1 | 2.2 (2.26) | 2.7 (2.16) | 24.1 |
| Immunocompromised status | ||||||
| Diagnoses of immunocompromising condition or use of immunosuppressants | 232,521 (100%) | 2,083 (100%) | 0 | 7,014 (100%) | 1,878 (100%) | 0 |
| Specific immunocompromising conditions | ||||||
| Cancerc | 32,708 (14%) | 804 (39%) | 58 | 1,187 (17%) | 745 (40%) | 52.2 |
| Cancer, metastaticc | 7,281 (3%) | 56 (3%) | −2.6 | 268 (4%) | 53 (3%) | −5.6 |
| HIVc | 1,178 (1%) | 32 (2%) | 10.3 | S | S | S |
| Hematologic cancerc | 9,177 (4%) | 702 (34%) | 82.3 | 361 (5%) | 657 (35%) | 80.3 |
| Rheumatoid arthritisc | 18,412 (8%) | 269 (13%) | 16.4 | 668 (10%) | 208 (11%) | 5.1 |
| Solid organ transplantc | 9,014 (4%) | 437 (21%) | 53.7 | 575 (8%) | 400 (21%) | 37.6 |
| Any immunocompromisingcondition | 85,268 (37%) | 1,619 (78%) | 91.2 | 3,302 (47%) | 1,449 (77%) | 65.2 |
| Other underlying conditions | ||||||
| Coronary artery disease | 33,459 (14%) | 357 (17%) | 7.5 | 1,329 (19%) | 322 (17%) | −4.7 |
| Chronic kidney disease | 24,759 (11%) | 512 (25%) | 37.2 | 1,528 (22%) | 432 (23%) | 2.9 |
| Chronic obstructive pulmonary disease | 44,534 (19%) | 398 (19%) | −0.1 | 1,088 (16%) | 322 (17%) | 4.4 |
| Diabetes mellitus w/complications | 25,257 (11%) | 340 (16%) | 16 | 1,132 (16%) | 305 (16%) | 0.3 |
| Diabetes mellitus w/ocomplications | 38,284 (16%) | 353 (17%) | 1.3 | 1,299 (19%) | 326 (17%) | −3 |
| Hypertension | 125,278 (54%) | 1,308 (63%) | 18.2 | 4,468 (64%) | 1,174 (63%) | −2.5 |
See Table S2 for complete definitions of variables and Table S3 for distribution of all the baseline characteristics.
Includes one dose of Janssen.
These conditions are included in the definition of immunocompromising conditions.
Propensity score analysis
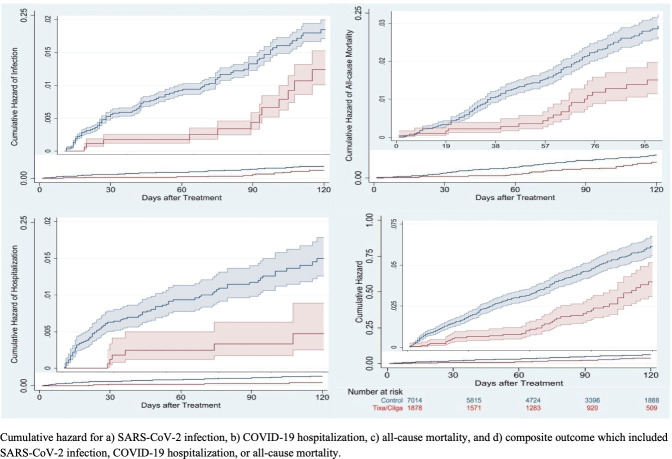
Estimated from propensity-score-adjusted survival analyses, tixagevimab/cilgavimab recipients had a lower incidence of the composite of COVID-19 outcomes versus control patients overall (49/1,878 [2.6%] versus 312/7,014 [4.4%]; HR 0.35; 95% CI, 0.24–0.52), which was similar among the severely immunocompromised (HR 0.23; 95% CI, 0.07–0.82). The association in the overall cohort was similar across each of the individual COVID-19 outcomes, including SARS-CoV-2 infections (HR 0.44; 95% CI, 0.22–0.88), COVID-19 hospitalization (HR 0.24; 95% CI, 0.10–0.59), and all-cause mortality (HR 0.32; 95% CI, 0.19–0.55) (Table 2; Fig. 2). An upward inflection in SARS-CoV-2 infections and COVID-19 hospitalizations was observed at day 90 and 60, respectively (Fig. 2A and B). A higher proportion of tixagevimab/cilgavimab recipients had secondary outcomes compared with control patients (Table 2). The proportion of tixagevimab/cilgavimab recipients and control patients who were SARS-CoV-2 positive and were treated with antivirals or monoclonal antibodies was 21% and 11% (P = 0.288), respectively.
TABLE 2.
Relative effectiveness of tixagevimab/cilgavimab versus untreated controls using exact matching and propensity-score-adjusted analysis
| Matched controls (N = 7,014) | Tixagevimab/cilgavimab recipients (N = 1,878) | Exact matched and propensity-score-adjusted survival analysis | |
|---|---|---|---|
| Number of events (%) | Number of events (%) | Hazard ratio (95% CI) | |
| Composite outcome (SARS-CoV-2 infection, COVID-19 hospitalization, and all-cause mortality) | |||
| Overall cohort | 312 (4.4%) | 49 (2.6%) | 0.35 (0.24–0.52) |
| Severely immunocompromisedc | 92 (5.4%) | 28 (2.7%) | 0.23 (0.07–0.82) |
| Individual outcome (overall cohort) | |||
| SARS-CoV-2 infection | 68 (.97%) | 13 (0.7%) | 0.44 (0.22–0.88) |
| COVID-19 hospitalization | 72 (1.0%) | <11 (<0.5%)a | 0.24 (0.10–0.59) |
| All-cause mortality | 172 (2.45%) | 29 (1.5%) | 0.32 (0.19–0.55) |
| Urinary tract infectionb | 197 (2.8%) | 44 (2.3%) | 0.96 (0.65–1.43) |
Numbers not shown to protect patient information.
Numbers not shown to protect patient information.
There were 1,048 matched cases and 1,695 matched controls for the analysis of severely immunocompromised.
Fig 2.
Cumulative risk of COVID-19 outcomes for tixagevimab/cilgavimab recipients compared to untreated controls.
Lastly, we were able to examine the impact of tixagevimab/cilgavimab with and without concomitant vaccination. Those fully vaccinated with at least three doses of any vaccine or two doses of Ad26.COV2, but without receiving tixagevimab/cilgavimab, had an incidence rate of 4.0% of composite outcome versus a rate of 5.2% among those neither vaccinated nor received tixagevimab/cilgavimab. Those not fully vaccinated but treated with tixagevimab/cilgavimab had an incidence rate of 3.8%. Most dramatically, those who were both fully vaccinated and received tixagevimab/cilgavimab had an incidence rate of 2.3%.
Falsification analysis
Healthcare encounters with UTI as the primary discharge diagnosis were unlikely to be associated with tixagevimab/cilgavimab and, therefore, served as a falsification test. A total of 241 UTI visits were observed during the follow-up period. Matched propensity-score-adjusted analysis demonstrated a similar effectiveness of tixagevimab/cilgavimab versus control against UTI (HR 0.96; 95% CI, 0.65–1.43) (Table 2). This lack of association between UTI and the treatment is reassuring that the protective effects associated with the treatment of tixagevimab/cilgavimab were unlikely due to bias or other major methodological flaws.
Sensitivity analysis
In our target trial emulation, 2,083 recipients of tixagevimab/cilgavimab and 232,521 untreated veterans who were immunocompromised met the study eligibility (Fig. S1). Overall, 382 (18%) treated veterans were excluded due to SARS-CoV-2 infection between 1 January 2022 and the index date or because the veteran could not be matched; 160,420 (69%) of untreated veterans were excluded because they did not receive medical care at the same VA facilities as treated veterans so could not serve as matches. After exact matching, 1,701 remained in the treatment group and 1,701 in the untreated group. Baseline characteristics are shown in Table 3. As exact matching was implemented, proportions of veterans with specific characteristics were equivalent for treated and untreated veterans: 95% were male, 16% were non-Hispanic Black, 3% were Hispanic, and 76% were non-Hispanic White. Most (73%) were ≥65 years old, and 71% lived in urban areas (20). Most of the exact matched population were severely immunocompromised (1,105, 65%) with additional common comorbidities, including heart disease (64%), renal disease (26%), and COPD (18%). Tixagevimab/cilgavimab recipients predominantly (96%) had received at least two doses of mRNA vaccines or one dose of Ad26.COV2 (Janssen) or a booster (at least three vaccine doses or three doses of Ad26.COV2, 79%). Among recipients, 191 (11.2%) received only a single dose of tixagevimab/cilgavimab, 9 (0.5%) had another visit to receive their second single dose, and 1,501 (88.3%) received double doses on the same visit.
TABLE 3.
Baseline characteristics of immunocompromised veterans treated with at least one dose of intramuscular tixagevimab/cilgavimab between 1 January and 15 June 2022 and untreated matched patients
| Matched patients without treatment (n = 1,701) | Tixagevimab/cilgavimab-treated patients (n = 1,701) | |||
|---|---|---|---|---|
| Male | 1,610 (95%) | 1,610 (95%) | ||
| Mean age (SD) yr | 71 (10.8) | 69 (10.2) | ||
| Age 65+ | 1,248 (73%) | 1,248 (73%) | ||
| Race/ethnicity | ||||
| White | 1,301 (76%) | 1,301 (76%) | ||
| Black | 272 (16%) | 272 (16%) | ||
| Hispanic | 42 (2.5%) | 42 (2.5%) | ||
| Rurality | ||||
| Highly rural | 17 (1%) | 17 (1%) | ||
| Rural | 425 (25%) | 425 (25%) | ||
| Urban | 1,259 (74%) | 1,259 (74%) | ||
| Vaccination status | ||||
| No vaccination record | 62 (4%) | 62 (4%) | ||
| >2 doses vaccine or 1 dose of Ad26.COV2 | 1,639 (96%) | 1,639 (96%) | ||
| ≥3 vaccine doses or 2 doses of Ad26.COV2 | 1,138 (67%) | 1,351 (79%) | ||
| BMI | ||||
| Mean index (SD) | 29 (6.4) | 29 (12.1) | ||
| Normal | 521 (31%) | 521 (31%) | ||
| Overweight/obese | 1,180 (69%) | 1,180 (69%) | ||
| Priority level (1–4) | 729 (43%) | 729 (43%) | ||
| DCCI | 4 (2) | 3(2) | ||
| 0 | 279 (16%) | 275 (16%) | ||
| 1 | 143 (8%) | 144 (8%) | ||
| 2+ | 1,279 (75%) | 1,282 (75%) | ||
| Diagnosis of immunocompromising condition or use of immunosuppressants |
1,701 (100%) | 1,701 (100%) | ||
| Underlying conditionsa | ||||
| Asthma | 54 (3%) | 54 (3%) | ||
| Heart diseaseb | 1,088 (64%) | 1,088 (64%) | ||
| Chronic obstructive pulmonary disease | 299 (18%) | 299 (18%) | ||
| HIV | 6 (.4%) | 6 (.4%) | ||
| Immunocompromised diagnoses | 1,295 (76%) | 1,309 (77%) | ||
| Renal disease | 443 (26%) | 443 (26%) | ||
| Chronic kidney disease | 439 (26%) | 401 (24%) | ||
| Rheumatoid arthritis | 160 (9%) | 160 (9%) | ||
| Severely immunocompromised | 1,105 (65%) | 1,105 (65%) | ||
| Solid organ transplant | 185 (11%) | 367 (22%) | ||
| Cancer, including hematologic | 920 (54%) | 738 (44%) | ||
These conditions are included in the definition of immunocompromising conditions.
Includes hypertension and diabetes mellitus.
The median follow-up time was 12 weeks (interquartile range 6, 16) with a maximum of 24 weeks. Compared with untreated veterans, tixagevimab/cilgavimab recipients had a lower 24-week incidence rate of the composite outcome and 61% overall lower risk (HR 0.39; 95% CI, 0.27–0.57), which were similar among the severely immunocompromised (HR 0.43; 95% CI, 0.25–0.76). The findings were similar across each of the individual COVID-19 outcomes, including SARS-CoV-2 infections (HR 0.34; 95% CI, 0.17–0.68), COVID-19 hospitalization (HR 0.20; 95% CI, 0.09–0.47), and all-cause mortality (HR 0.48; 95% CI, 0.29–0.80). Tixagevimab/cilgavimab recipients also had a lower risk (HR 0.41; 95% CI, 0.24–0.69) of secondary outcomes compared with untreated veterans. In short, results from the sensitivity analysis were similar to those from the main analysis (Table 4).
TABLE 4.
Relative effectiveness of tixagevimab/cilgavimab versus untreated controls using exact matching
| Matched patients without treatment (N = 1,701) | Tixagevimab/cilgavimab-treated patients (N = 1,701) | ||
|---|---|---|---|
| Number of events, 24-wk riska (95% CI) | Number of events, 24-wk risk (95% CI) | ||
| Composite outcome (SARS-CoV-2 infection, COVID-19 hospitalization, and all-cause mortality) | |||
| Overall cohort | 86, 51 (41–63) | 44, 26 (19–35) | |
| Severely immunocompromised | 58, 52 (35–79) | 34, 30 (21–42) | |
| Individual outcome (overall cohort) | |||
| SARS-CoV-2 infection | 30, 18 (12–25) | 12, 7 (4–12) | |
| COVID-19-related hospitalization | 27, 16 (11–23) | <11, 4 (2–9)a | |
| All-cause mortality | 41, 24 (17–32) | 26, 15 (10–22) | |
| Secondary outcome (overall cohort) | |||
| Emergency department, urgent or intensive care | 48, 15 (11–20) | 20, 6 (4–9) | |
Exact number not shown, to protect patient information.
DISCUSSION
In this retrospective cohort study using data from veterans across the VHA, the largest integrated healthcare system in the United States, administration of tixagevimab/cilgavimab was associated with a significant reduction in the risk of SARS-CoV-2 infection, COVID-19 hospitalization, and all-cause mortality among recipients, compared with untreated, matched controls. These findings were observed among severely immunocompromised veterans, further supporting the EUA criteria for the use of tixagevimab/cilgavimab in this population. In addition, evidence of augmented protection against SARS-CoV-2 infections among vaccinated immunocompromised veterans who received tixagevimab/cilgavimab was found, akin to that of the population of fully boosted adults who were not immunocompromised (21).
This study of tixagevimab/cilgavimab in a real-world setting provides important insights regarding the patient population who have received tixagevimab/cilgavimab for the prevention of COVID-19 . Of note, the EUA encourages the use of tixagevimab/cilgavimab primarily among fully vaccinated immunocompromised patients; however, none of the participants in the PROVENT trial were vaccinated. In comparison, nearly all (96%) of our study population received at least two doses of a COVID-19 mRNA vaccine or one dose of Ad26.COV2 before receiving tixagevimab/cilgavimab. In PROVENT, only 11% of trial participants were immunocompromised, compared to all veterans in the current analysis. Furthermore, patients aged 65 years and older accounted for a small proportion (24%) of patients included in the PROVENT trial, compared to 73% of those in the current study. However, only a small proportion of eligible veterans received treatment, indicating that enhanced education and outreach are paramount to ensure that more immunocompromised veterans across the VHA healthcare system receive this medication, specifically during COVID-19 surges.
In comparison to the PROVENT trial that was conducted prior to the emergence of the Omicron variants, the observation period of our analysis coincided with the Omicron BA.1, BA.2, and BA.2.12.1 surges across the United States and provides important clinical data in this latest evolution of the pandemic. While tixagevimab/cilgavimab maintained neutralization against the Delta variant of SARS-CoV-2, tixagevimab/cilgavimab was shown to have decreased neutralizing activity against the Omicron BA.1 variant, prompting the FDA’s revision of EUA to increase the initial dose of tixagevimab/cilgavimab. Current data indicate that tixagevimab/cilgavimab maintains neutralization against Omicron BA.2 and BA.2.12.1, but this effect may be significantly attenuated with Omicron BA.4 and BA.5 (22 - 26) and newer Omicron subvariants such as BQ.1.1 and XBB (27). Effectiveness of tixagevimab/cilgavimab against these subvariants will be the focus of ongoing follow-up and future longitudinal analyses. Albeit with a median length of follow-up of 3 months, our data still showed some indication that the effect could be waning against SARS-CoV-2 infection and COVID-19 hospitalizations (Fig. 2A and B). Other studies have also shown a waning effect after 3 months, which could be influenced by patient characteristics and variant type (28, 29). These observations warrant further investigation.
Our study had several notable strengths. We analyzed 2,019 patient-years of observation, making our study one of the largest ever conducted. The rich and comprehensive real-time, real-world VHA clinical data to assess tixagevimab/cilgavimab’s effectiveness while being distributed to combat a concurrent surge of the pandemic is a unique feature. The large VHA veteran population provided an opportunity to narrow down to the precise study population while still adjusting for potential confounding variables by matching. Previous studies demonstrated that EHR data were more likely to be complete in capturing medical conditions and have a lower risk of up-coding (30, 31). Nevertheless, conventional analytical strategies such as stratification, matching (with or without propensity score), and multivariate regression analysis cannot adequately adjust for unobserved confounders (31 - 33). To address lingering confounding, we conducted a robust sensitivity analysis using another study design—target trial emulation—to ensure all eligible veterans were alive, SARS-CoV-2 infection-free, and alike both demographically and clinically, at the beginning of each week before a portion of them went on to receive tixagevimab/cilgavimab.
Limitations
First, VA data include only healthcare encounters that occur in VA medical centers, and some infections and hospitalizations that occurred outside the VA could have been missed; assuming that missed events were as likely among those treated and untreated this non-differential misclassification would bias the results toward the null. While mortality data in the VHA are updated readily due to the need to update benefits information, cause of death data is not updated as readily. Therefore, the analysis was restricted to all-cause mortality rather than focusing on COVID-19-related mortality. The significantly lower rate of all-cause mortality among those treated with tixagevimab/cilgavimab versus untreated controls may be driven by COVID-19 specifically and not ascertainable by the current data; the stronger association for death versus infection may be because all-cause mortality outcome was better captured in the VHA data versus infection. We relied on vaccination data captured by the VA to assess individuals’ vaccination status. Some veterans may have been vaccinated outside the VHA, particularly older veterans with Medicare coverage who made up most of the study population. Based on our earlier analyses which showed >99% of Medicare vaccinations were captured in VHA records for individuals who were tested for SARS-CoV-2 at the VHA during the last quarter of 2021, we are confident that we captured all vaccination records. Second, the VHA has a unique population (mostly male and older), and our results may not be generalizable to a larger population of Veterans that were not treated at the VHA (34). Third, ICD-10 codes have been shown to inadequately capture comorbidity and functional status (35). Fourth, the sequencing data for this study to determine the variants of the SARS-CoV-2 infections included in this analysis were not available, and effectiveness of tixagevimab/cilgavimab may differ according to the variant type as evidenced by various studies on antibody neutralization (21 - 26). While our study did report COVID-19 treatments given following a positive SARS-CoV-2, the impact of these treatments on the estimated association of tixagevimab/cilgavimab and COVID-19 outcomes was not assessed, and it is possible that those differences may have affected differences in hospitalization and mortality between the groups.
Because only 238 (10%) of patients in our tixagevimab/cilgavimab cohort received a single dose of 150 mg/150 mg tixagevimab/cilgavimab, we did not have sufficient sample size to compare the original dosage of 150 mg/150 mg to the revised dosage of 300 mg/300 mg to assess the optimal dosing of tixagevimab/cilgavimab in the current analysis. In addition, the number of tixagevimab/cilgavimab eligible veterans who were treated at VA was low. In our sensitivity analysis, we matched treated and untreated veterans at facilities where tixagevimab/cilgavimab was given to ensure comparability between groups for this study. This matching requirement excluded 69% of eligible untreated veterans in the current analysis. Further research on drivers of prescribing differences is needed as to why providers and veterans may not realize potential benefits and eligibility for this and other treatments (36). Finally, we could not assess the optimal timing of tixagevimab/cilgavimab in relation to COVID-19 vaccine administration nor could we identify a target population who would be optimal to receive tixagevimab/cilgavimab.
Conclusion
Using national data from vaccinated, immunocompromised veterans, these analyses show that administration of tixagevimab/cilgavimab was associated with lower rates of SARS-CoV-2 infection, COVID-19 hospitalization, and all-cause mortality, compared with matched untreated veterans during the Omicron surge. Our results suggest that tixagevimab/cilgavimab administration, in addition to vaccination, protects vulnerable veterans from SARS-CoV-2 infection and severe COVID-19 in a contemporary phase of the pandemic. Ongoing real-world data will help to understand the effectiveness of tixagevimab/cilgavimab for pre-exposure prophylaxis over time and against emerging variants.
ACKNOWLEDGMENTS
The authors thank Dr. Hector Izurieta, for his insight on observational study methods and guidance on real-world data. Medical writing assistance was provided by Tara Krause, BA of the Clinical Epidemiology Program at the White River Junction VA Medical Center, Vermont, USA. The authors are grateful to collaborators at VA SHIELD (Science and Health Initiative to Combat Infections and Emerging Life-threatening Diseases) for providing insights on the results.
This work was supported by the Department of Veterans Affairs (VA) Office of Research and Development, the VA Office of Rural Health, Clinical Epidemiology Program at the White River Junction VA Medical Center, by resources and the use of facilities at the White River Junction VA Medical Center, and VA Informatics and Computing Infrastructure, and data from the VA COVID-19 Shared Data Resource. The funders did not influence the study design, conduct, or reporting.
The contents of this article do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Food and Drug Administration, as well as any other agency of the U.S. Government. Assumptions made within and interpretations from the analysis do not necessarily reflect the position of any U.S. Government entity.
V.C.M. has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences and ViiV. Y.Y.-X., G.Z., C.K., and J.S. reported receiving grants from the U.S. Food and Drug Administration through an interagency agreement with the Veterans Health Administration and from the U.S. Department of Veterans Affairs Office of Rural Health. Y.Y.-X., G.Z., C.K, and J.S. also reported receiving funding from Pfizer to the U.S. Department of Veterans Affairs for other research projects outside the submitted work. R.A.B. is supported by VA/BLRD VA SHIELD (821-SD-null-41942); Y.Y.-X., V.C.M., and R.A.B. are supported by VA/BLRD VA SEQCURE (821-SD-ID-42403). A.A.G. received COVID-19 research project funding from the National Institutes of Health, Department of Defense, Centers for Disease Control and Prevention, AbbVie, and Faron Pharmaceuticals, outside the submitted work.
Conception and design: Y.Y.-X., L.E., V.C.M., and A.A.G. Analysis and interpretation of the data: Y.Y.-X., L.E., V.C.M., V.D., G.Z., J.S., C.K., F.C., R.A.B., and A.A.G. Drafting of the article: Y.Y.-X., L.E., V.C.M., V.D., and A.A.G. Critical revision for important intellectual content: Y.Y.-X., L.E., V.C.M., V. D., G.Z., J.S., C.K., F.C., R.A.B., and A.A.G. Final approval of the article: Y.Y.-X., L.E., V.C.M., V.D., G.Z., J.S., C.K., F.C., R.A.B., and A.A.G. Provision of study materials or veterans: Y.Y.-X. and F.C. Statistical expertise: Y.Y.-X. and C.K. Administrative, technical, or logistic support: Y.Y.-X. Collection and assembly of data: Y.Y.-X., G.Z., and J.S.
Contributor Information
Yinong Young-Xu, Email: Yinong.Young-Xu@VA.gov.
Dimitrios Paraskevis, Medical School, National and Kapodistrian University of Athens, Athens, Greece .
DATA AVAILABILITY
Requests for access to the deidentified study data can be made via e-mail to Yinong.Young-Xu@va.gov. The data will be made available to qualified scientific researchers for specific purposes outlined in a proposal after the researcher enters into a standard data sharing agreement and the proposal is approved.
ETHICS APPROVAL
Before data collection, this study was approved by the institutional review board of the VA Medical Center in White River Junction, VT, and was granted a waiver of informed consent because the study was deemed a minimal risk and consent impractical to acquire. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.01024-23.
Exact matching attrition.
Immunosuppressants.
Definitions of patient characteristics.
All baseline characteristics.
Abbreviations.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. 2021. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med 385:562–566. doi: 10.1056/NEJMsb2104756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Embi PJ, Levy ME, Naleway AL, Patel P, Gaglani M, Natarajan K, Dascomb K, Ong TC, Klein NP, Liao I-C, Grannis SJ, Han J, Stenehjem E, Dunne MM, Lewis N, Irving SA, Rao S, McEvoy C, Bozio CH, Murthy K, Dixon BE, Grisel N, Yang D-H, Goddard K, Kharbanda AB, Reynolds S, Raiyani C, Fadel WF, Arndorfer J, Rowley EA, Fireman B, Ferdinands J, Valvi NR, Ball SW, Zerbo O, Griggs EP, Mitchell PK, Porter RM, Kiduko SA, Blanton L, Zhuang Y, Steffens A, Reese SE, Olson N, Williams J, Dickerson M, McMorrow M, Schrag SJ, Verani JR, Fry AM, Azziz-Baumgartner E, Barron MA, Thompson MG, DeSilva MB. 2021. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - nine states, January-September 2021. MMWR Morb Mortal Wkly Rep 70:1553–1559. doi: 10.15585/mmwr.mm7044e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. 2021. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 385:661–662. doi: 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kennedy NA, Lin S, Goodhand JR, Chanchlani N, Hamilton B, Bewshea C, Nice R, Chee D, Cummings JF, Fraser A, Irving PM, Kamperidis N, Kok KB, Lamb CA, Macdonald J, Mehta S, Pollok RC, Raine T, Smith PJ, Verma AM, Jochum S, McDonald TJ, Sebastian S, Lees CW, Powell N, Ahmad T, Contributors to the CLARITY IBD study . 2021. Infliximab is associated with attenuated Immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 70:1884–1893. doi: 10.1136/gutjnl-2021-324789 [DOI] [PubMed] [Google Scholar]
- 5. Agha ME, Blake M, Chilleo C, Wells A, Haidar G. 2021. Suboptimal response to Coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: A need for vigilance in the Postmasking era. Open Forum Infect Dis 8:ofab353. doi: 10.1093/ofid/ofab353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, Yuan Y, Seegobin S, Ellery A, Levinson DJ, Ambery P, Arends RH, Beavon R, Dey K, Garbes P, Kelly EJ, Koh GCKW, Near KA, Padilla KW, Psachoulia K, Sharbaugh A, Streicher K, Pangalos MN, Esser MT, PROVENT Study Group . 2022. Intramuscular AZD7442 (Tixagevimab–cilgavimab) for prevention of COVID-19. N Engl J Med 386:2188–2200. doi: 10.1056/NEJMoa2116620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fact sheet for Healthcare providers: Emergency use authorization for Evushel (Tixagevimab Co-packaged with Cilgavimab). n.d. Food and Drug Administration. Available from: https://www.fda.gov/media/154701/download [Google Scholar]
- 8. Paneth N, Joyner MJ, Casadevall A. 2022. Finding evidence for treatment decisions in a pandemic. Trends Mol Med 28:536–541. doi: 10.1016/j.molmed.2022.04.008 [DOI] [PubMed] [Google Scholar]
- 9. Corporate data warehouse, health services research and development, U.S. Department of Veterans Affairs. Available from: https://www.hsrd.research.va.gov/for_researchers/cdw.cfm. Retrieved 2424 SepSeptember 2022. Accessed , 2424 SepSeptember 2022 [Google Scholar]
- 10. De Groot MPH, Kristin . Ascertaining veterans’ vital status: VA data source for mortality ascertainment and cause of death, database & methods Cyberseminar series. VA Information Resource Center. Available from: https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3544-notes.pdf. Retrieved 2424 SepSeptember 2022. Accessed , 2424 SepSeptember 2022 [Google Scholar]
- 11. Pharmacy benefits management services. U.S. Department of Veterans Affairs. Available from: https://www.pbm.va.gov/. Retrieved . 24 September 2022 [Google Scholar]
- 12. FDA authorizes revisions to evusheld dosing. Food and Drug Administration. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-revisions-evusheld-dosing. Retrieved . 24 September 2022 [Google Scholar]
- 13. Petersen LA, Byrne MM, Daw CN, Hasche J, Reis B, Pietz K. 2010. Relationship between clinical conditions and use of veterans affairs healthcare among medicare-enrolled veterans. Health Serv Res 45:762–791. doi: 10.1111/j.1475-6773.2010.01107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deyo RA, Cherkin DC, Ciol MA. 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 15. Pizer SD. 2016. Falsification testing of instrumental variables methods for comparative effectiveness research. Health Serv Res 51:790–811. doi: 10.1111/1475-6773.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lipsitch M, Tchetgen Tchetgen E, Cohen T. 2010. A tool for detecting confounding and bias in observational studies. Epidemiology 21:383–388. doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Austin PC. 2009. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernán MA, Robins JM. 2016. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 183:758–764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. 2016. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol 79:70–75. doi: 10.1016/j.jclinepi.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cromartie J. n.d. Rural urban commuting codes, U.S. Department of Agriculture. Available from: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/ [Google Scholar]
- 21. VanBlargan LA, Errico JM, Halfmann PJ, Zost SJ, Crowe JE, Purcell LA, Kawaoka Y, Corti D, Fremont DH, Diamond MS. 2022. S an infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med 28:490–495. doi: 10.1038/s41591-021-01678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takashita Emi, Yamayoshi S, Simon V, van Bakel H, Sordillo EM, Pekosz A, Fukushi S, Suzuki T, Maeda K, Halfmann P, Sakai-Tagawa Y, Ito M, Watanabe S, Imai M, Hasegawa H, Kawaoka Y. 2022. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med 387:468–470. doi: 10.1056/NEJMc2207519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takashita Emi, Yamayoshi S, Halfmann P, Wilson N, Ries H, Richardson A, Bobholz M, Vuyk W, Maddox R, Baker DA, Friedrich TC, O’Connor DH, Uraki R, Ito M, Sakai-Tagawa Y, Adachi E, Saito M, Koga M, Tsutsumi T, Iwatsuki-Horimoto K, Kiso M, Yotsuyanagi H, Watanabe S, Hasegawa H, Imai M, Kawaoka Y. 2022. Efficacy of antiviral agents against the Omicron subvariant BA.2.75. N Engl J Med 387:2094–2097. doi: 10.1056/NEJMc2211845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, Iwatsuki-Horimoto K, Halfmann P, Watanabe S, Maeda K, Imai M, Mitsuya H, Ohmagari N, Takeda M, Hasegawa H, Kawaoka Y. 2022. Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N Engl J Med 386:1475–1477. doi: 10.1056/NEJMc2201933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, Bolland W-H, Porrot F, Staropoli I, Lemoine F, Péré H, Veyer D, Puech J, Rodary J, Baele G, Dellicour S, Raymenants J, Gorissen S, Geenen C, Vanmechelen B, Wawina-Bokalanga T, Martí-Carreras J, Cuypers L, Sève A, Hocqueloux L, Prazuck T, Rey FA, Simon-Loriere E, Bruel T, Mouquet H, André E, Schwartz O. 2022. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 602:671–675. doi: 10.1038/s41586-021-04389-z [DOI] [PubMed] [Google Scholar]
- 26. Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, Nehlmeier I, Graichen L, Moldenhauer A-S, Winkler MS, Lier M, Dopfer-Jablonka A, Jäck H-M, Behrens GMN, Pöhlmann S. 2022. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell 185:447–456. doi: 10.1016/j.cell.2021.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imai M, Ito M, Kiso M, Yamayoshi S, Uraki R, Fukushi S, Watanabe S, Suzuki T, Maeda K, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Halfmann PJ, Kawaoka Y. 2023. Efficacy of antiviral agents against Omicron subvariants BQ.1.1 and XBB. N Engl J Med 388:89–91. doi: 10.1056/NEJMc2214302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karaba AH, Kim JD, Chiang TP-Y, Alejo JL, Sitaras I, Abedon AT, Eby Y, Johnston TS, Li M, Aytenfisu T, Hussey C, Jefferis A, Fortune N, Abedon R, Thomas L, Habtehyimer F, Ruff J, Warren DS, Avery RK, Clarke WA, Pekosz A, Massie AB, Tobian AAR, Segev DL, Werbel WA. 2023. Neutralizing activity and 3-month durability of tixagevimab and cilgavimab prophylaxis against Omicron sublineages in transplant recipients. Am J Transplant 23:423–428. doi: 10.1016/j.ajt.2022.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Najjar-Debbiny R, Gronich N, Weber G, Stein N, Saliba W. 2023. Effectiveness of evusheld in immunocompromised patients: propensity score–matched analysis. Clin Infect Dis. 76:1067–1073. doi: 10.1093/cid/ciac855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adler-Milstein J, Jha AK. 2014. No evidence found that hospitals are using new electronic health records to increase Medicare reimbursements. Health Aff 33:1271–1277. doi: 10.1377/hlthaff.2014.0023 [DOI] [PubMed] [Google Scholar]
- 31. Devoe JE, Gold R, McIntire P, Puro J, Chauvie S, Gallia CA. 2011. Electronic health records vs medicaid claims: completeness of diabetes preventive care data in community health centers. Ann Fam Med 9:351–358. doi: 10.1370/afm.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. 2012. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 33. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group . 2001. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–56. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 34. Rogers WH, Kazis LE, Miller DR, Skinner KM, Clark JA, Spiro A, Fincke RG. 2004. Comparing the health status of VA and non-VA ambulatory veterans: the veterans' health and medical outcomes studies. J Ambul Care Manage 27:249–262. doi: 10.1097/00004479-200407000-00009 [DOI] [PubMed] [Google Scholar]
- 35. Mazzali C, Duca P. 2015. Use of administrative data in healthcare research. Intern Emerg Med 10:517–524. doi: 10.1007/s11739-015-1213-9 [DOI] [PubMed] [Google Scholar]
- 36. Kotton CN. 2022. Belt and suspenders: vaccines and tixagevimab/cilgavimab for prevention of COVID-19 in immunocompromised patients. Ann Intern Med 175:892–894. doi: 10.7326/M22-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Exact matching attrition.
Immunosuppressants.
Definitions of patient characteristics.
All baseline characteristics.
Abbreviations.
Data Availability Statement
Requests for access to the deidentified study data can be made via e-mail to Yinong.Young-Xu@va.gov. The data will be made available to qualified scientific researchers for specific purposes outlined in a proposal after the researcher enters into a standard data sharing agreement and the proposal is approved.