Abstract
Probiotics, like lactic acid bacteria, are non-pathogenic microbes that exert health benefits to the host when administered in adequate quantity. Currently, research is being conducted on the molecular events and applications of probiotics. The suggested mechanisms by which probiotics exert their action include; competitive exclusion of pathogens for adhesion sites, improvement of the intestinal mucosal barrier, gut immunomodulation, and neurotransmitter synthesis. This review emphasizes the recent advances in the health benefits of probiotics and the emerging applications of probiotics in the food industry. Due to their capability to modulate gut microbiota and attenuate the immune system, probiotics could be used as an adjuvant in hypertension, hypercholesterolemia, cancer, and gastrointestinal diseases. Considering the functional properties, probiotics are being used in the dairy, beverage, and baking industries. After developing the latest techniques by researchers, probiotics can now survive within harsh processing conditions and withstand GI stresses quite effectively. Thus, the potential of probiotics can efficiently be utilized on a commercial scale in food processing industries.
Keywords: probiotics, lactic acid bacteria, immunomodulation, anti-allergic and gastrointestinal diseases, functional foods
1. Introduction
Probiotics, in the form of supplements or food products, have emerged as the most prominent ingredient in the era of functional foods. Probiotics have always been a vital component and commercial target for providing potential health benefits (Sanz et al., 2016; Hamad et al., 2022). The term “probiotic” was first presented by Werner Kollath in 1953, which is known to be a derivative of the Latin word pro and the Greek word βιο meaning “for life.” Kollath defined probiotics as active bodies with essential functions for promoting various health aspects (Gasbarrini et al., 2016). Food and Agriculture Organization (FAO) and World Health Organization (WHO) described them as “live microbes when administered in adequate quantities, confer health benefits on host organisms” (Munir et al., 2022). Several bacteria belonging to the genera Pediococcus, Lactococcus, Enterococcus, Streptococcus, Propionibacterium, and Bacillus are considered potential microbes for probiotic status (de Brito Alves et al., 2016; Hamad et al., 2022).
The frequently used strains belong to the divergent group of Bifidobacterium and Lactobacillus that significantly affect health with various actions. They detoxify xenobiotics and environmental pollutants (Reid, 2015), bio-transform mycotoxins in foods (Hamad et al., 2022), synthesize vitamin K, riboflavin, and folate (Reid, 2015; Hamad et al., 2022), and ferment undigested fiber in the colon (Warman et al., 2022). Probiotics prevent pathogenic bacteria by restricting binding sites on mucosal epithelial cells and modulating the host immune response, thus improving intestinal barrier integrity (Fusco et al., 2023). The advantages of probiotics are related to the modulation of gut microbiota, mitigation of nutritional intolerances (lactose intolerance), increase in bioavailability of macro and micronutrients, and alleviation of allergic incidences in susceptible individuals (Roobab et al., 2020).
Probiotics can be consumed either by incorporating them into foods or drinks in the form of dairy or non-dairy foodstuffs or as supplements (Fenster et al., 2019). Various fermented foods have active microbes genetically similar to the strains utilized as probiotics. It has been observed that fermented foods enhance the functional and nutritional aspects by transforming substrates and producing bioactive and bioavailable end-products (Marco et al., 2017). The approximate consumption of 109 colony-forming unit (CFU)/day have been revealed as an effective dose (Hill et al., 2014). By keeping in view, the effective dosage, probiotics are being incorporated into many foods like beverages, ice cream, yogurt, bread, and many others by the food industry. The most significant barrier associated with probiotics in the food industry is their susceptibility to processing conditions and sensitivity to gastrointestinal (GI) stresses. However, regarding their health benefits, the consumer always showed an inclined interest in probiotic products (Konuray and Erginkaya, 2018). Now scientists have developed new and innovative methods like nanoencapsulation and genetic modification, which enable probiotics to withstand harsh conditions of both processing and GI stresses in the body (Putta et al., 2018). This review paper provides a profound insight into the mechanistic approach and current perspective on the beneficial aspects of probiotics in preventing and treating various diseases. The application and safe utilization of probiotics in major food industries have also been described.
2. Mechanisms of action
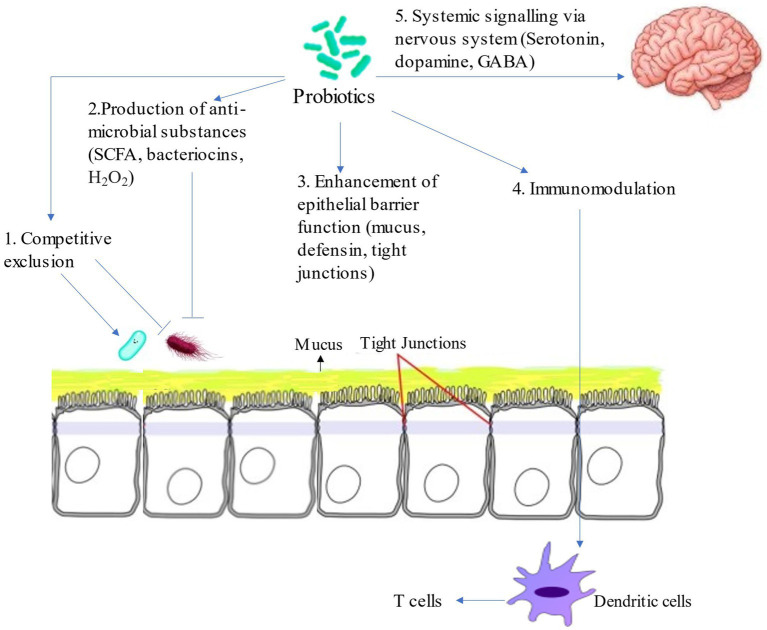
Outstanding advances have been made in the field of probiotics, but there has yet to be a key breakthrough in the documentation of their mechanism of action. Probiotics possibly exert a positive potential on the human body through these main mechanisms; competitive exclusion of pathogens, improvement in intestinal barrier functions, immunomodulation in the host’s body, and production of neurotransmitters (Figure 1; Plaza-Diaz et al., 2019). Probiotics compete with pathogens for nutrients and receptor-binding sites, making their survival difficult in the gut (Plaza-Diaz et al., 2019). Probiotics also act as anti-microbial agents by producing substances; short chain fatty acids (SCFA), organic acids, hydrogen peroxide (Ahire et al., 2021), and bacteriocins (Fantinato et al., 2019) thus decreasing pathogenic bacteria in the gut. Moreover, probiotics improve the intestinal barrier function by stimulating the production of mucin proteins (Chang et al., 2021), regulating the expression of tight junction proteins, including occluding and claudin 1, and regulating the immune response in the gut (Bu et al., 2022; Ma et al., 2022).
Figure 1.
Mechanism of action of probiotics. 1. Probiotics perform their function by competing with pathogens for nutrients and receptors for binding thereby making their survival and adherence to gut mucosa difficult. 2. Probiotics produce anti-microbial substances which inhibit pathogens growth. 3. Probiotics promote epithelial barrier function by enhancing mucus production and increasing the expression of tight junction proteins which prevents the translocation of pathogens from intestine into the blood. 4. Probiotics regulate immunity of the host by modulating maturation and function of dendritic cells subsequently increasing the activity of T cells which play important role in immune homeostasis. 5. Probiotics also regulate the production of neurotransmitters including serotonin, dopamine and gamma aminobutyric acid (GABA).
Probiotics also regulate the innate and adaptive immune response modulating dendritic cells (DC), macrophages B and T lymphocytes. Probiotics also increase the production of anti-inflammatory cytokines while interacting with intestinal epithelial cells and attracting macrophages and mononuclear cells (Petruzziello et al., 2023). Furthermore, probiotics can produce neurotransmitters in the gut through the gut-brain axis. Specific probiotic stains can modulate the serotonin, gamma-aminobutyric acid (GABA), and dopamine levels, affecting mood, behavior, gut motility, and stress-related pathways (Srivastav et al., 2019; Sajedi et al., 2021; Gangaraju et al., 2022).
3. Health attributes of probiotics
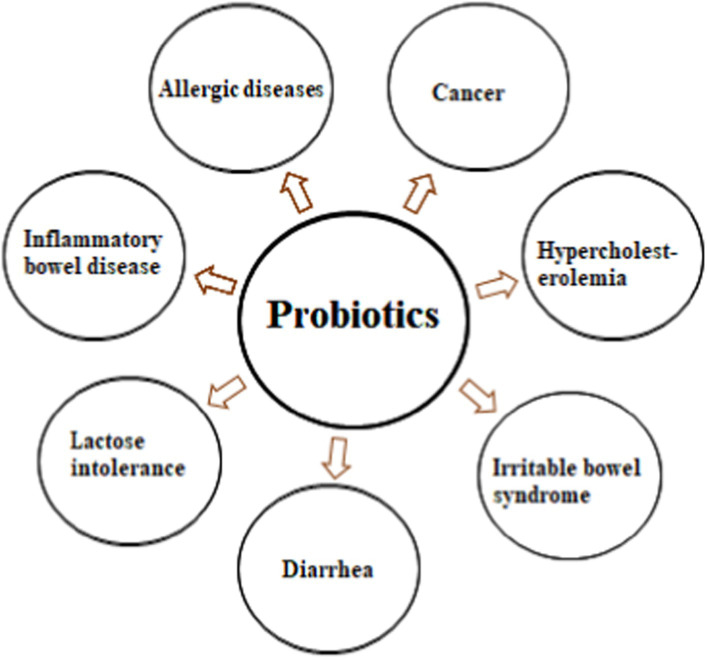
The health benefits of probiotics are associated with preventing and reducing many diseases, i.e., allergic diseases, cancer, hypercholesterolemia, lactose intolerance, inflammatory bowel disease, diarrhea, and irritable bowel syndrome (Grom et al., 2020), as shown in Figure 2. Table 1 shows different studies regarding the application of probiotics in different diseases.
Figure 2.
Health attributes of probiotics. Probiotics help in the prevention and management of allergic diseases, cancer, hypercholesterolemia, irritable bowel syndrome, diarrhea, lactose intolerance, inflammatory bowel disease.
Table 1.
Therapeutic effect of probiotics in gastrointestinal disorders.
| Disease | Strain | Dosage | Subjects | Results | References |
|---|---|---|---|---|---|
| Allergic reactions | L. plantarum | 5 × 1010 cells once a week for 4 weeks | Mice sensitized with peanut allergen | ↓ Interleukin-10 ↑ Interferon-γ | Yang et al. (2021) |
| Allergic reactions | Lactobacillus multiple strains | 109 CFU lactobacilli every day for 28 days | 30 BALB/c mice model of soybean sensitization | ↑ Interferon-γ and IL-2 ↓ IL-4, IL-6 Promoted Tregs | Yang et al. (2021) |
| Cancer | Lactobacillus fermentum | – | CCD18-Co, HCT-116, and HT-29 cell lines | Activation of intrinsic apoptosis | Lee et al. (2019) |
| Cancer | Pediococcus acidilactici TMAB26 | – | HT-29 and Caco-2 cell lines | Significant toxicity on cancer cells | Barigela and Bhukya (2021) |
| Hypercholesterolemia | L. casei pWQH01 L. plantarum AR113 | 1 × 109 CFU for 5 weeks | 30 male C57BL/6J mice | Have Bile Salt Hydrolase activity ↓ hepatic levels of TC and LDL-C ↑cholesterol 7α-hydroxylase (CYP7A1) gene | |
| Hypercholesterolemia | L. fermentum MJM60397 | 5 × 1010 CFU | Male mice | ↓ cholesterol and low-density lipoprotein (LDL) cholesterol levels ↑ LDLR gene | Palaniyandi et al. (2020) |
| Ulcerative colitis | Bifidobacterium longum 536 (BB536) | 2–3 × 1011 three times daily for 8 weeks | 56 patients with mild to moderate UC | ↓ Mayo subscore ↓Rachmilewitz endoscopic index (EI) | Tamaki et al. (2016) |
| Ulcerative colitis | L. lactis NCDO 2118 | 2.5 × 106 CFU/g | 36 mice | ↓ Severity of colitis ↓ disease activity index ↑ gene expression of tight junction proteins (zo-1, zo-2) | Cordeiro et al. (2021) |
| Lactose intolerance | L. acidophilus | 1 × 1010 once daily for 4 weeks | 60 human participants | ↓Abdominal pain ↓Abdominal cramping ↓Vomiting | Pakdaman et al. (2015) |
| IBS | L. delbruekii and L. fermentum | 10 billion bacteria twice daily for 4 weeks | 90 human subjects | ↓Abdominal pain ↓IL-8 Restore normal intestinal flora | Husein et al. (2017) |
| Radiation-induced diarrhea | L. acidophilus and B. animalis | 1.75 billion lyophilized live bacteria three times daily | 53 patients receiving external beam pelvic radiotherapy | ↓Moderate and severe diarrhea ↓Grade II abdominal pain | Linn et al. (2019) |
| Chronic diarrhea | L. plantarum CCFM1143 | 3.52 × 109 CFU per day | 55 human patients with chronic diarrhea | Improved clinical symptoms of diarrhea Improved immune response Modulated gut microbiota | Yang et al. (2021) |
| Antibiotic associated diarrhea | Lactobacillus and Bifidobacterium strains | 1 × 109 CFU once a day | 36 human subjects | Delayed recurrence of diarrhea (5.39 days) ↓ Average no. of daily stools 45% positive evaluation | Trallero et al. (2019) |
| Chron’s disease | B. longum and inulin/oligofructose | 2 × 1011 freeze-dried viable B. longum twice daily for 6 months | 35 human subjects | ↓Crohn disease activity indices ↓Histological scores ↓TNF-α expression | Steed et al. (2010) |
↓ shows the reduction in different parameters while ↑ shows increasing trend.
3.1. Antiallergic effect of probiotics
Allergy is a hypersensitive disorder of the immune system, termed as type I hypersensitivity and defined as a “disease following a response by the immune system to an antigen.” With escalating incidence rate, allergies affect nearly half of the population of Europe and North America. These allergic reactions occur due to one or more common environmental substances or antigens (Prakash et al., 2014). The most common allergic reactions include asthma, rhinitis, atopic eczema, dermatitis, urticaria, angioedema, hay fever, and food, drug, and insect hypersensitivity (Lopez-Santamarina et al., 2021). The gut microbiome is a viable therapeutic target for managing allergic diseases (Harata et al., 2016), as they modulate the immunological and inflammatory response that consequently affects the development of sensitization and allergy (Fiocchi et al., 2015).
Allergic diseases are characterized by an imbalance in lymphocyte-governed immunity in which the immune response becomes overly biased toward T helper 2 lymphocytes dominated response (Th2 cells) (Di Costanzo et al., 2016). Allergen-sensitized Th2 cells produce various interleukins such as IL-1, IL-4, and IL-5, thus recruiting granular effector cells, i.e., mast cells, eosinophils, and basophils toward the site of allergic inflammation. In addition, the interleukins switch B lymphocyte immunoglobulin isotype, which upsurges the circulating level of total and allergen-specific IgE (Galli et al., 2020). Although the precise mechanism is not entirely known, it is expected that the probiotics improve mucosal barrier functions, stimulate the immune system, reduce leakage of antigen through the mucosa, produce anti-inflammatory cytokines, increase the production of secretory IgA (exclude antigens from intestinal mucosa), degrade dietary antigen and up-regulate anti-inflammatory cytokines as IL-10 (Liang et al., 2022).
The proposed mechanism for the antiallergic effect of probiotics is the augmentation of T helper cells (Th)1/Th2 immune balance by suppressing Th2 skewed immune response and favoring Th1 cell response (Di Costanzo et al., 2016). Ma et al. (2019) explain that probiotics modulate the function of dendritic cells, which in turn have the ability peripheral Tregs. Tregs control the excess immune response and maintain a balance between Th1 and Th2 cells (Figure 3). Besides, lactobacilli stimulate regulatory T cells which play a paramount role in balancing immune response through the production of immunosuppressive cytokines and modulation of IgE, IgA, and IgG production (Owaga et al., 2014).
Figure 3.
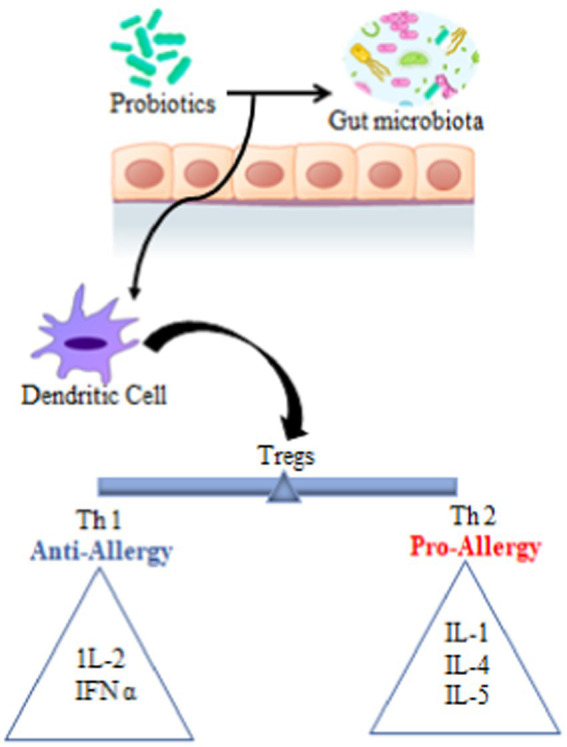
Anti-allergic effect of probiotics. Tregs, T regulatory cells; Th 1, T helper cells type 1; Th 2, T helper cell type 2; IL, interleukin; IFN α, interferon α. Probiotics help in the migration and maturation of dendritic cells via modulating the composition of gut microbiota. Dendritic cells in the gut-associated lymphoid tissues have the ability to induce the development of peripheral Tregs and to play a central role in the development of immune homeostasis. Tregs maintain the proper level of Th 1, Th 2 cells as well as anti-allergy and pro-allergy cytokines.
The antiallergic effect of Lactiplantibacillus plantarum SY12 and L. plantarum SY11 was studied using RAW 264.7 (murine macrophage) cell line. Both species showed a reduction in the production of nitric oxide, T helper 2 linked cytokines, tumor necrosis factor-α, and cyclooxygenase-2 as well as inducible nitric oxide synthase compared to the control group (Lee et al., 2014). In this regard, the Limosilactobacillus reuteri effect was also investigated against the food allergy in ovalbumin (OVA)-sensitized BALB/c mice. Oral intake of L. reuteri helped restore the deteriorated profile of colonic microflora and attenuated allergic diarrhea. It also increased the activation of mast cells, enhanced the production of serum immunoglobulin E (IgE), suppressed the T helper 1 and 2 cytokines production, down-regulated the GATA3 expression, and increased the expression of TGF-b, IL-10, and Foxp3. The findings confirmed the anti-allergic activities of L. reuteri promoted by the modulation of enteric flora and enhancement of tolerogenic immune responses (Huang et al., 2017).
3.2. Cancer suppressor activity of probiotics
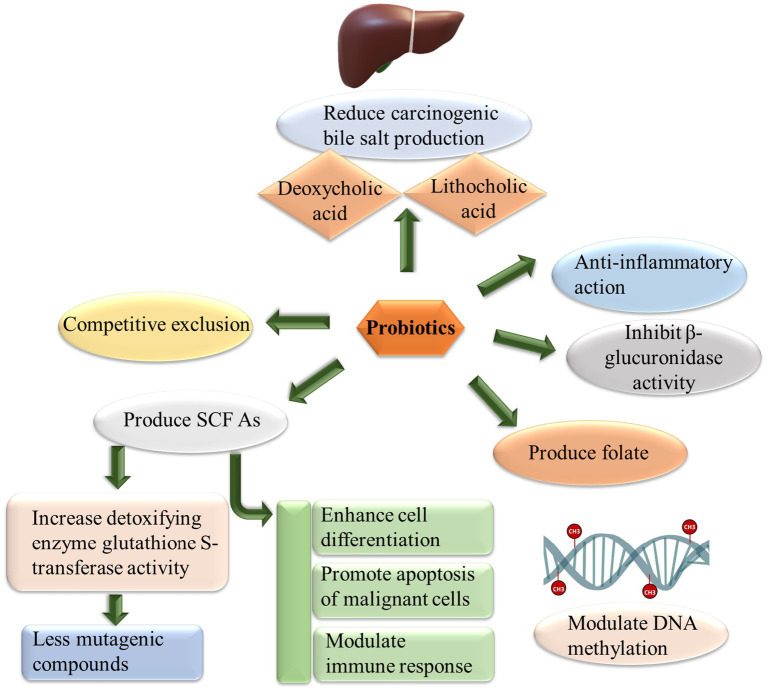
Probiotics could be used as an adjuvant for various types of cancers based on their potential to modulate enteric flora and enhance local and systematic immunity. They prevent the initiation, progression, and metastasis of transplantable or chemically induced tumors (Samanta, 2022). The effect of probiotics can be observed in suppressing both intestinal and extraintestinal cancers (So et al., 2017). The interaction of probiotics and their metabolites (bacteriocin, peptides, and organic acids) with critical metabolic pathways such as cellular proliferation, inflammation, apoptosis, angiogenesis, and metastasis has been revealed by many researchers (Harikumar et al., 2013). Moreover, the probiotics inhibit carcinogenesis by inhibiting pathogens through competitive exclusion, increasing short-chain fatty acid production (Chong, 2014), reducing carcinogenic bile salts production, binding carcinogens and mutagens, down-regulating NF-kappa B dependent genes products for cell proliferation (Cox-2, cyclin D1) and cell survivability (Bcl-3, Bcl-xL) and enhancing apoptosis (Konishi et al., 2016). Probiotics also upregulate TNF-related apoptosis-inducing ligand (TRAIL) (Klłonowska-Olejnik, 2004), modulate cell cycle by rapamycin (mTOR)/4EBP1 (Islam et al., 2014) and inhibit the formation of aberrant crypt foci (Yu and Li, 2016). Figure 4 describes the anti-cancer effect of probiotics.
Figure 4.
Cancer suppressor activity of probiotics. Probiotics use different pathways to fight against cancer. Probiotics inhibit β glucuronidase activity, produce folate which ultimately modulate DNA methylation patterns protecting the integrity of genome, produce short chain fatty acids (SCFA) enhancing cell differentiation and apoptosis of cancerous cells, exclude pathogens involved in chronic inflammation which may lead to cancer development.
Previous studies have scrutinized that the ERK1/2 pathway modulates cell survival, proliferation, differentiation, and cell motility by regulating the BCL-2 protein family in mitochondria (Passaniti et al., 2022). Saccharomyces boulardii, both in vitro and in vivo, inhibited the activation of ERK1/2 mitogen-associated protein kinase. In the same way, probiotic L. reuteri induced apoptosis in human myeloid leukemia-derived cells by modulating NF-kappa B and MAPK signaling pathways (Saber et al., 2017). The colonic microflora has also been related to the development of liver disorders such as liver fibrosis (De Minicis et al., 2014), nonalcoholic fatty liver diseases (Zhuge et al., 2022), and more recently, liver cancer (So et al., 2017). Probiotics have been demonstrated to inhibit hepatocellular carcinoma (HCC) progression by reducing liver tumor size and down-regulating angiogenic factors. The mechanistic approach to this is the level of T helper (Th) 17 cells in the gut and its recruitment to tumor sites was lower in probiotic-treated mice (Li et al., 2016). In breast cancer apart from immunomodulation, the hypoxia-inducible factor (HIF) pathway was also reported to be significantly suppressed by Lactobacillus cultures supernatant (Esfandiary et al., 2016).
In addition to this, experimental studies were carried out to reduce the mutagenic potential of a powerful carcinogen; N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) by Lacticaseibacillus rhamnosus Vc. Oral feeding of L. rhamnosus Vc (109 CFU) to Gallus gallus (chicks) for 30 days significantly detoxified the parent compound reducing its mutagenicity (61%) and genotoxicity (69%) (Pithva et al., 2015). In another study, the role of Saccharomyces cerevisiae on the activation of apoptotic pathway Akt/NF-kB was explored in cancer. Heat-killed S. cerevisiae induced apoptosis in cancer cells, the SW480 cell line, by up-regulating Bax, cleaved caspase 3 and cleaved caspase 9, and down-regulating p-Akt1, Bcl-XL, Rel A, procaspase 3 and procaspase 9 expressions. Hence, it was concluded that probiotics modulate Akt/NF-kB pathway following the apoptotic cascade and play an essential role in cancer prevention (Shamekhi et al., 2020).
3.3. Hypocholesterolemic effect of probiotics
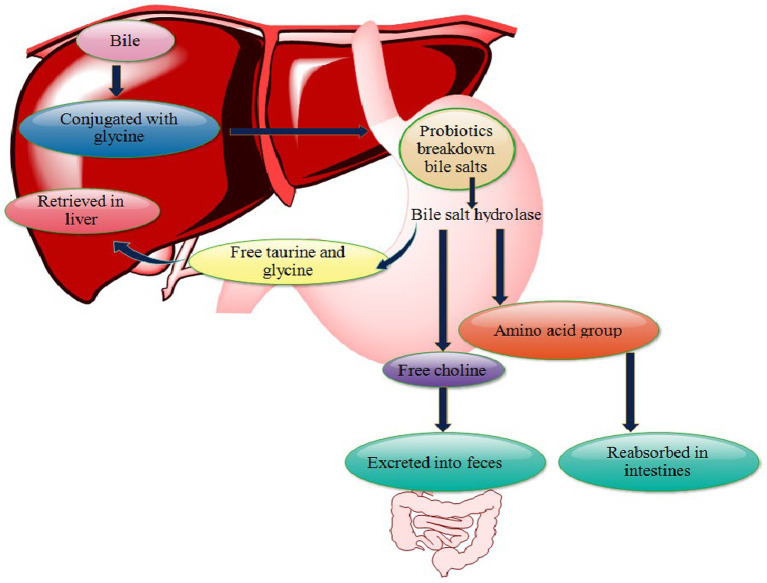
Probiotics can be used as an effective tool for lowering blood cholesterol levels. They can act directly or indirectly to decrease cholesterol levels in the body. The direct mechanism includes the inhibition of de novo synthesis of cholesterol by hypocholesterolemia factors like uric acid, lactose, orotic acid, and whey protein as well as the reduction in intestinal absorption of dietary cholesterol in three ways- assimilation, binding, and degradation (Thakkar et al., 2016). The indirect mechanism for curtailing cholesterol by probiotics is deconjugating bile salts (conjugated glycodeoxycholic acid and taurodeoxycholic acid) via bile salt hydrolase (BSH) production. Deconjugated bile salts are less reabsorbed through the intestine, thus inhibiting enterohepatic circulation of the bile and higher excretion in the feces (Figure 5; Rezaei et al., 2017).
Figure 5.
Mechanism of lowering cholesterol level by probiotics. Probiotics breakdown or deconjugate bile salts into free choline, glycine and amino group by synthesizing bile salt hydrolase. Free choline excreted via choline, amino acid group is absorbed in the intestine, and free taurine and glycine return back to the liver. This increases the elimination of bile from body and more cholesterol is used to synthesize bile thereby, reducing the cholesterol level in the blood.
Human and animal studies have provided evidence for the hypocholesterolemic properties of probiotics. In a study, the hypocholesterolemic properties of Levilactobacillus brevis MT950194 and L. brevis MW365351 were observed both in vitro and in vivo. The strains reduced cholesterol content, increased fecal cholesterol excretion, and converted bile into free cholic acid (Munir et al., 2022). The potential of a probiotic complex comprising Pediococcus, Lactobacillus, and Bifidobacteria was also investigated in lipid metabolism. After 10 weeks of the experimental period, the results showed significantly reduced cholesterol levels in medium and high-dose groups (Galli et al., 2020). The cholesterol reduction potential of a new strain, L. plantarum DMDL 9010, was investigated by using in vivo model. The intake of strain resulted in the reduction of serum cholesterol, hepatic cholesterol, triglycerides, and an increase in fecal excretion of bile acids. A significant decrease in total cholesterol, low-density lipoprotein, and atherosclerosis index by 23.03, 28.00, and 34.03%, respectively was observed with the use of L. plantarum DMDL 9010 (109 cells per day) (Liu et al., 2017).
Recently, research regarding gene expression by probiotics in hypercholesterolemia was conducted by Dehkohneh and his colleagues. The role of Lacticaseibacillus paracasei TD3 was examined in modulating two significant genes involved in cholesterol metabolism; 3-hydroxy-3-methyl glutaryl coenzyme (HMGCR) and cytochrome P450 7A1 (CYP7A1). A dose of 1 × 1010 CFU was given to male Wistar rats for 21 days. The cholesterol level was significantly decreased along with the reduction of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes. The dramatic decline of HMGCR and CYP7A1 genes in adipose tissues was also observed using real-time polymerase chain reaction (Dehkohneh et al., 2019).
3.4. Impact of probiotics on intestinal diseases
The gut plays a pivotal role in the digestion and absorption of nutrients and maintains mucosal barrier integrity. Numerous commensal bacteria reside in the human GI tract constituting an active community, which strongly affects human physiology (Shehata et al., 2022). The modification in intestinal microflora can be achieved by administering antibiotics, probiotics, prebiotics, and fecal transplant (Shahverdi, 2016).
The metabolic activity of the intestinal microbiome affects the host’s health, both favorably and unfavorably (Saber et al., 2017). The exact balance in the microflora (eubiosis), when disturbed, results in acute and chronic clinical disorders like antibiotic-associated diarrhea (AAD), ulcers, inflammatory bowel disease (IBD), and irritable bowel syndrome (IBS) (Saber et al., 2017). In addition, several researchers have supported the theory that microbial dysbiosis participates in the etiology of some human cancers (Su et al., 2021), especially GI cancers (Pereira-Marques et al., 2019). Restoring healthy gut microbiota can be used as a practical approach to managing intestinal diseases. Probiotics can increase microbial richness and diversity, increase enzyme (Lactase) production, improve immune micro-environment (Jang et al., 2019), and improves intestinal permeability (Stratiki et al., 2007). In this way, probiotics can alleviate intestinal diseases. Studies regarding the use of probiotics in intestinal diseases are given in Table 1.
4. Application of probiotics in the food industry
The public awareness of diet-related issues and ever-increasing evidence about probiotic health benefits have increased consumer interest in probiotic foods. A large number of food items, including yogurt, powdered milk, frozen fermented dairy desserts, cheese and cheese products, ice creams, baby foods, cereals, and fruit juices, are among numerous probiotic foods (Papademas and Kotsaki, 2019). The most prominent barrier to using probiotics in the food industry is their sensitivity toward heat treatments during processing and GI stresses in the human body. However, researchers and food industries are trying to find new and innovative methods and techniques to overcome the issues (Zhang et al., 2022). The global increase in sales of probiotics-based products is estimated to reach 75 billion dollars by 2025. This exponential growth in sales of probiotic products has already gained much interest from food producers to develop new products with probiotics. Probiotics are commonly used in dairy, beverage, baking, and edible film industries (Reque and Brandelli, 2021).
4.1. Probiotics in the dairy industry
Food producers have been showing great interest in developing new probiotics products due to their large acceptability among consumers. Dairy-based products are prepared as natural products to promote health and prevent diseases (Nami et al., 2019). Lactic acid bacteria (LAB) in dairy products help increase the shelf life of fermented products. LAB act as antimicrobial agents against many pathogens living inside the human body, thus improving human health (de Souza da Motta et al., 2022). Table 2 refers to the application of probiotics in the dairy industry. Considering the demand for functional dairy products in markets, it has been estimated and forecasted that the industry will jump up to a market value of 64.3 billion USD globally by the end of 2023, apart from traditional dairy products (Iqbal et al., 2017; FAO, 2022).
Table 2.
Application of probiotics in food industries.
| Food industry | Product | Probiotic strain | Storage time | Viability at the end of storage | References |
|---|---|---|---|---|---|
| Dairy | Ricotta cheese | B. animalis subsp. lactis (Bb-12) L. acidophilus (La-05) | 7 days at 7°C | ∼106 CFU/g | Meira et al. (2015) |
| Yogurt | B. Lactis | 29 days at 4°C | 106–107 CFU/g | Danielle (2015) | |
| L. acidophilus B. animalis subsp. lactis | 45 days at 5 ± 1°C | 8.84 log CFU/g 8.01 log CFU/g | Lucatto et al. (2020) | ||
| Cheddar cheese | L. lactis subsp. lactis L. helvetics S. thermophilus L. rhamnosus | 4 weeks at 16°C | 108 CFU/g | Ulpathakumbura et al. (2016) | |
| Mango juice enriched dairy drink | L. acidophilus | 5 weeks at 4 °C | 7.72 log CFU/mL | Leaf et al. (2016) | |
| Beverages-fruit based | Pineapple juice | L. acidophilus, L. plantarum, and L. lactis | 60 days at 4°C | 9–10 log CFU/mL | Nguyen et al. (2019) |
| Orange juice | P. acidilactici | 35 days at 4°C and 30°C | 7.2–8.5 log CFU/mL | Cristiny de Oliveira Vieira et al. (2020) | |
| Pomegranate | L. plantarum ATCC 14917 | 28 days at 4°C | 8.8 log CFU/mL | Mantzourani et al. (2018a) | |
| Cornelian cherry juice | L. plantarum | 4 weeks at 4°C | 9.95 log CFU/mL | Mantzourani et al. (2018b) | |
| Beverages-vegetable based | Carrot blended with orange juice | L. plantarum CECT 220 | 30 days at 4°C | 108–109 CFU/mL | Al-Sheraji et al. (2013) |
| Beet | L. plantarum | 21 days at 4°C | 7–8 log CFU/mL | Barbu et al. (2020) | |
| Melon, carrot | L. plantarum CICC22696 and L. acidophilus CICC20710 | 28 days at 4°C | 108–109 CFU/mL | Do and Fan (2019) | |
| Bakery | Pan bread | Sodium alginate and 2% whey protein concentrate L. rhamnosus GG | 7 days at room temperature | 7.57–8.98 and 6.55–6.91 log CFU/portion | Lu et al. (2018) |
| Bread | Encapsulating L. acidophilus and L. casei in calcium alginate | 4 days at ambient temperature | 7.2 × 108 CFU/g | Seyedain-Ardabili et al. (2016) |
Many products, such as pasteurized milk, infant formula, fermented milk, and ice creams are being produced and consumed worldwide as probiotic-based dairy products. Some products like cheese and fermented milk are preferred as probiotics carriers because their pH buffering capacity and fat contents give additional protection to probiotics while passing through the GI tract (Meybodi and Mortazavian, 2017). Yogurt, including reduced lactose or lactose-free, functional ingredient-supplemented yogurts such as vitamins, minerals, sterols, stanols, conjugated linoleic acids, prebiotics, and probiotics have also gained good market success for quite a long period (Fernandez and Marette, 2017).
Nowadays, probiotics-based dairy products have been recommended as safe and healthy due to their beneficial effects on health, such as aiding mineral absorptions in the body, being efficient against Helicobacter pylori infection, and preventing diarrhea and constipation (Gao et al., 2021). Nami and his team (Nami et al., 2019) found the hypocholesterolemic effects of L. plantarum from homemade yogurt. They found the most substantial cholesterol-removing potential in growing cells (84%), moderate removal of cholesterol in the resting cell (41.1%), and the lowest in dead cells (32.7%). L. plantarum showed a positive potential for controlling serum cholesterol. At the same time, it was found that L. plantarum was resistant to BSH activity, antibiotics, and hemolytic activity (Nami et al., 2019). Lee et al. (2020) prepared L. plantarum B710 containing fermented milk, which showed bone-protective effects. Moreover, Prezzi et al. (2020) examined that the addition of L. rhamnosus inhibited the growth of Listeria monocytogenes in Minas Frescal cheese. L. rhamnosus showed no negative effect on the textural and physiochemical properties of cheese and survived during storage and after simulated gastrointestinal conditions.
Arbex et al. (2018) investigated six Leuconostoc mesenteroides strains from three different sources of dairy and non-dairy products provided each sample showing probiotic properties. One strain of L. mesenteroids from camel milk coded as CM9 showed high dextran production and the best resistance to intestinal stresses. CM9 had a strong antimicrobial potential against Staphylococcus aureus and Escherichia coli (Arbex et al., 2018; Azam et al., 2021). In another research, the effect of Lactobacillus acidophilus and L. rhamnosus were investigated on soft cheese. It was found that L. acidophilus had good overall quality with a better immune-modulation response in mice. At the same time, they also controlled pro-inflammatory cytokines and interleukin regulation and enhanced the secretion of secretory immunoglobulin A (Cuffia et al., 2019). In a study, Nguyen et al. (2019) and Riaz et al. (2019) investigated the survival of Bifidobacterium bifidum encapsulated in zein. The results suggested that probiotic bacteria survived well after 32 days of storage (Nguyen et al., 2019).
4.2. Probiotics in the beverage industry
The demand for non-dairy probiotic foods has been increasing steadily, especially when the consumer has become aware of the side effects associated with medicine. Consuming probiotic food is more readily acceptable to consumers as it is a more natural way of receiving their daily dose of probiotics (Reque and Brandelli, 2021). Fruit juices supplemented with probiotics have been reported as a more unique and appropriate method in the probiotic beverage industry. Fruit juices have been accepted widely among all consumers regardless of age, gender, and geographic region around the globe due to the presence of essential nutrients (Mantzourani et al., 2018a,b). The viability of probiotics is shorter in non-dairy foods when compared to dietary supplements due to the harsh environments faced by probiotics in beverages. Processors must consider many factors in the production of probiotic juices, such as pH, temperature, anthocyanins, and most importantly a vegetative form of probiotics (Min et al., 2019; Azam et al., 2022).
To overcome these complexities, microencapsulation techniques have been introduced. Using these techniques, probiotics can be employed as an essential ingredient in the functional food industry. The micro or nanoencapsulation of probiotics allows them to withstand harsh processing and storage environments due to the protective coating around them (Afzaal et al., 2022). It was reported that the acid sensitivity of Bifidobacterium and Lactobacillus was improved after their microencapsulation with gelatin or plant gums (Ozturk et al., 2021). Besides this, low-temperature processing is also an effective strategy to control metabolic activity and protect probiotic cell viability throughout the shelf life of juices so that an adequate and safe dose of microbes is delivered to the consumer (Tyutkov et al., 2022). Some studies regarding probiotics in the beverage industry are shown in Table 2.
Miranda et al. (2019) have investigated the direct addition of an activated and microencapsulated form of probiotics in orange juice to check their effect on physical, chemical, rheological, microbial, and sensory parameters. They found that in the inactivated state, the level of organic acids was increased, but the essential volatile compounds were decreased. On the other hand, the encapsulated probiotics showed improved consistency and rheological parameters but their sensory attributes were not up to the mark due to changes in taste. The most optimum treatment was found to be the direct addition of probiotics to juice based on good physicochemical and sensory acceptance that was more similar to the natural pure product having many essential volatile compounds (octanol, o-cymene, α-cubebene, and 1-hexanol, etc.) (Miranda et al., 2019). Secondary packaging is another important technique used to produce shelf-stable beverage products. In this technique, the probiotics are in a separate compartment from food, i.e., bottle cap or straw, and are released only into juices immediately before consumption (Fenster et al., 2019).
In another research, water kefir grains were used to ferment soy whey (a byproduct of tofu) to prepare a functional beverage. After 2 days of fermentation, the polyphenol contents and antioxidant properties increased significantly, supported by good sensory scores and overall acceptability (Fenster et al., 2019). Laali et al. (2018) used L. plantarum to make a beverage from coconut water after fermentation. This process not only enhanced the vitamin and mineral (potassium, calcium, and sodium) contents but also improved anti-hypertensive, antioxidant, and antimicrobial properties making it suitable for use (Laali et al., 2018). The beverage prepared from whey, germinated millet flour, and barley extract was treated with L. acidophilus in another study, and it was found to be effective in controlling the pathogenicity induced by Shigella in mice models. The beverage stimulated the immune response and enhanced the IgA level, thus controlling pathogenicity (Ganguly et al., 2019).
4.3. Probiotics in bakery
Bakery products (bread, biscuits, doughnuts, cookies, etc.) contribute to several major food components such as carbohydrates, proteins, fats, dietary fiber, vitamins, and minerals in varying amounts (Niesche and Haase, 2012; El-Sohaimy et al., 2019). Researchers have been trying to incorporate probiotics in baked products by developing new techniques to deliver thermo-durable bioactive materials so that probiotics can survive high temperatures during baking (Mirzamani et al., 2021).
The microencapsulation technique and the sourdough method have been studied as an alternative to increasing the nutritional value and cell viability of probiotics in bread during baking (Ganguly et al., 2019) and in GI conditions (Champagne et al., 2018; Ashraf et al., 2022). In a study, L. rhamnosus was encapsulated in sodium alginate, and higher cell viability was observed during the baking of pan bread and in simulated gastrointestinal conditions (Hauser and Matthes, 2017). Zhang et al. (2018) analyzed the encapsulation of L. plantarum into bread-making using different matrices (reconstituted skim milk, gum arabic, maltodextrin, and inulin). The results suggested that bacterial survival was better in gum arabic and reconstituted skim milk than in the other two heating methods (Zhang et al., 2018). Another research studied the incorporation of L. plantarum under different baking temperatures (175, 205, and 235°C) and its survival during storage. The bacterial cell viability was counted every 2 min during baking and a decline from 109 CFU/g to 104–5 CFU/g was observed after baking. The storage results were remarkable as the probiotic viability was increased by 2–3 logarithmic cycles to 108, which was attributed to the decline in the pH of bread during storage (Zhang et al., 2018). Table 2 illustrates the use of probiotics using different strains in the baking industry.
4.4. Probiotics in edible food coatings
Bioactive food packaging is the latest approach promoting the concept of functional foods due to its extraordinary health-promoting benefits. This technique is quite helpful in overcoming the stability and GIT stresses faced by probiotics (Khodaei and Hamidi-Esfahani, 2019). Studies on the use of probiotics with some biopolymers for edible coating are illustrated in Table 3.
Table 3.
Use of probiotics in edible film.
| Application matrix | Probiotic | Biopolymer material | Viability | References |
|---|---|---|---|---|
| Baked cereal products | L. rhamnosus GG | Sodium alginate | 7.57–8.98 log CFU/portion | Lu et al. (2018) |
| L. acidophilus L. rhamnosus | Carboxymethylcellulose (CMC) | 107 CFU/g | Ebrahimi et al. (2018) | |
| Hake fillets | B. animalis spp. lactis, L. paracasei spp. paracasei | Agar | – | De Lacey et al. (2014) |
| L. rhamnosus GG | Sodium alginate/Pectin/κ-Carrageenan-Locust bean gum/Gelatine/Whey protein concentrate | 0.87–3.06 log CFU/g | Soukoulis et al. (2017) | |
| L. reuteri ATCC 55730 L. rhamnosus GG ATCC 53103 L. acidophilus DSM 20079 | Pullulan and starches (from potato, tapioca, and corn) | 12.9 log CFU/mL | Kanmani and Lim (2013) |
The encapsulation of probiotics into edible films protects them from premature degradation and increases their viability in the human body (Singh et al., 2019). The technique of edible films is being used nowadays as a tool for the effective delivery of probiotics to consumers. Still, at the same time, it also enhances the stability and safety of food by inhibiting the growth of spoilage microorganisms (Pavli et al., 2018). The prime difference between active packaging and edible coating or bioactive packaging is that active packaging is usually done to enhance the safety and quality of packaged food, while on the other hand, bioactive packaging affects the health of consumers directly generating healthier packaged foods through edible coated bioactive material which upon consumption promote health (Gagliarini et al., 2019).
Many researchers have shown keen interest in film-forming materials, for instance, biopolymers including cellulose, zein, seaweed extracts, pectins, alginates, and chitosan for entrapping probiotics to enhance the nutritional values of foods (Pop et al., 2019). Therefore, bacterial microorganisms are being incorporated into films and coatings to confer probiotics’ ability to the food products or act as antimicrobial agents (Afsah-Hejri et al., 2013). As an example, the fabricated cellulose-based edible films in combination with L. rhamnosus using sodium carboxymethyl cellulose (CMC) and hydroxymethyl cellulose (HEC) with citric acid as a crosslinker to control the consistency of film loaded with L. rhamnosus (Singh et al., 2019). Moreover, cellulose-based edible films showed the therapeutic effects of probiotics (Singh et al., 2019). The film effect provides a suitable environment to encapsulate bacteria from transport to delivery in the GIT system effectively.
Four probiotic strains (L. acidophilus, L. casei, L. rhamnosus, and B. bifidum) were investigated using CMC-based edible coatings in this regard and their effects on storage under refrigerated conditions were also checked. The results suggested that L. acidophilus showed the highest viable count during storage with more water vapor permeability and opacity and decreased tensile strength and elongation at break values of film structure. The physical and mechanical properties of edible films remained the same (Ebrahimi et al., 2018). Another research found that after incorporating L. plantarum into CMC-based edible coating, the physicochemical properties and microbial characteristics of fresh strawberries were significantly improved. The probiotics population remained constant throughout the storage period, which controlled mold and yeast growth and helped to improve the shelf life of strawberries (Khodaei and Hamidi-Esfahani, 2019).
Bambace et al. (2019) incorporated L. rhamnosus into an alginate prebiotic fiber solution to enhance the shelf life of minimally processed and ready-to-eat blueberries by fourteen days. L. rhamnosus showed good antimicrobial properties with alginate and sensory acceptability for coated food (Bambace et al., 2019). In another work, kefiran polysaccharides-based films were used to deliver probiotics (L. paracasei and Kluyveromyces marxianus) to the gut. These films exhibited good antimicrobial properties and protected the probiotics from GIT stresses. L. paracasei showed better mechanical properties and good viable count than K. marxianus (Gagliarini et al., 2019).
5. Delivery systems and the strategies to extend viability
The association between probiotics and human health has been well-known for an extended period. When consumed orally, probiotics can regulate the composition of intestinal microbiota (Sharma et al., 2023). However, the severe physicochemical stresses (high temperatures and acidity during processing, storage, and passage to the large intestine) can drastically reduce the viability of probiotics. Researchers have used different encapsulating techniques to overcome these stresses and enhance the viability of probiotics within the human body (Luo et al., 2022). The traditional and most widely used technique is microencapsulation. Microencapsulation is classified into four methods, namely; spray drying, freeze drying, emulsification, and extrusion. One can improve the ability of probiotics to withstand the harsh environment of processing and the human body. Still, these methods have certain limitations, such as extreme temperatures and acidity can ultimately affect the size, stability, and ultimately viability of microstructures of microcapsules (Razavi et al., 2021).
These hindrances paved the way to find new encapsulation strategies to enhance the durability and viability of probiotics. In recent years, the nanoencapsulation technique has been used widely to enhance probiotics-loaded nanoparticles’ ability to face severe processing and in-vivo stresses. These techniques also facilitate the targeted delivery and control release of probiotics in the intestine (Xu et al., 2022). The unique biological and physicochemical characteristics of nanocapsules, such as smaller particle sizes, higher surface areas, and increased reactivities, improve the efficiency of encapsulated probiotics, thus, providing a logical solution to human health and safety (Singh et al., 2022). The ability of nanoencapsulation to entrap probiotics is analyzed by the potential of electrospun nanofibers, hydrogels, nanocoating, nanoliposomes, and other nanomaterials (Garcia-Brand et al., 2022).
Mojaveri and his colleagues, in their recent work, attempted to improve the viability of Bifidobacterium animalis Bb12 by using a nanofiber technique made from chitosan and poly (vinyl alcohol) and inulin as prebiotics. The simulated results of the GI tract showed that the encapsulation of probiotics in electrospun nanofibers significantly enhanced the physicochemical behavior with increased stability of nanoparticles within the human body (Mojaveri et al., 2020). In another study, Li et al. (2019) studied the cellulose-based gels for control release of encapsulated L. plantarum with better storage and concluded that cellulose-based gels provide better storage stability and much-enhanced control release pattern in simulated intestinal fluids (Li et al., 2019).
Encapsulation of probiotics with the help of biomaterial-based nanocoating can also protect these beneficial microbes from antibiotics and GI conditions, facilitating the retention of probiotics within the GI tract. It was found that metal-phenolic network-based nano-coating made from iron (III) and tannic acid can help protect probiotic microbes from the detrimental effect of antibiotics (Ashraf et al., 2023; Guo and Wu, 2023). Due to their physicochemical parameters, smaller structures, and thermodynamic properties, nanoliposomes enjoy vast applications for a wide range of products. The stability of L. rhamnosus was analyzed by loading them into chitosan-gelatin coated nanoliposomes. The characterization study suggested the successful coating of bifidobacteria with coated nanoliposomes. Further supported by the results of simulated GI fluids with a significant amount of viable cells present in the fluid guiding toward the suitability of nanoliposomes as a potential carrier of probiotics in developing nutraceutical foods (Hosseini et al., 2022).
6. Conclusion
Probiotics have well-documented physiological effects with a definitive mechanism. However, the exact mechanism of how they work to enhance health and prevent different diseases must be explored. Evidence from well-documented clinical trials has revealed that probiotics can potentially alleviate different GI and other disorders. Despite our understanding of some molecular mechanisms underlying beneficial aspects of probiotics, we are still far from clinically proven efficacy in many autoimmune and inflammatory diseases. Moreover, many studies have been done on the animal model, so there is an emergent need to translate these results into humans. Currently, genetically modified commensal lactic acid bacteria are being used to deliver special health-interest compounds. But most of the work regarding recombinant bacteria is related to vaccines. However, genetically modified bacteria can be used for exploring innovative strategies to deliver bioactive molecules to mucosal tissues. More consistent and reproducible clinical trials are required to reveal probiotics efficacy, limitations, and safety, determining their effects on the immune system. Considering all the methodologies discussed in this review, probiotics can be applied easily by food producers to make novel functional foods to promote human health.
Author contributions
All authors wrote the manuscript, read and agreed to the published version of the manuscript.
Funding
This work was based upon the work from COST Action 18101 SOURDOMICS—Sourdough biotechnology network toward novel, healthier, and sustainable food and bioprocesses (https://sourdomics.com/; https://www.cost.eu/actions/CA18101/, accessed on 12 April 2023), where TE was member of the working groups 4, 6, 7, and 8, FÖ was the leader of the working group 8, “Food safety, health-promoting, sensorial perception and consumers’ behavior” and JR was the Chair and Grant Holder Scientific Representative and is supported by COST (European Cooperation in Science and Technology) (https://www.cost.eu/, accessed on 12 April 2023). COST was a funding agency for research and innovation networks. JR also acknowledged the Universidade Católica Portuguesa, CBQF—Centro de Biotecnologia e Química Fina—Laboratório Associado, Escola Superior de Biotecnologia, Porto, Portugal, as well as the support made by LA/P/0045/2020 (Alice) and UIDB/00511/2020-UIDP/00511/2020 (LEPABE) funded by national funds through FCT/MCTES (PIDDAC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Afsah-Hejri L., Jinap S., Hajeb P., Radu S., Shakibazadeh S. (2013). A review on mycotoxins in food and feed: Malaysia case study. Compr. Rev. Food Sci. Food Saf. 12, 629–651. doi: 10.1111/1541-4337.12029, PMID: [DOI] [PubMed] [Google Scholar]
- Afzaal M., Saeed F., Hussain M., Ismail Z., Siddeeg A., al-Farga A., et al. (2022). Influence of encapsulation on the survival of probiotics in food matrix under simulated stress conditions. Saudi J. Biol. Sci. 29:103394. doi: 10.1016/j.sjbs.2022.103394, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahire J., Jakkamsetty C., Kashikar M. S., Lakshmi S. G., Madempudi R. S. (2021). In vitro evaluation of probiotic properties of Lactobacillus plantarum UBLP40 isolated from traditional indigenous fermented food. Probiotics Antimicrob Proteins 13, 1413–1424. doi: 10.1007/s12602-021-09775-7 [DOI] [PubMed] [Google Scholar]
- al-Sheraji S. H., Ismail A., Manap M. Y., Mustafa S., Yusof R. M., Hassan F. A. (2013). Prebiotics as functional foods: a review. J. Funct. Foods 5, 1542–1553. doi: 10.1016/j.jff.2013.08.009, PMID: 37536023 [DOI] [Google Scholar]
- Arbex P. M., Moreira M. E. C., Toledo R. C. L., de Morais Cardoso L., Pinheiro-Sant'ana H. M., Benjamin L. A., et al. (2018). Extruded sorghum flour (Sorghum bicolor L.) modulate adiposity and inflammation in high fat diet-induced obese rats. J. Funct. Foods 42, 346–355. doi: 10.1016/j.jff.2018.01.010 [DOI] [Google Scholar]
- Ashraf W., Latif A., Lianfu Z., Jian Z., Chenqiang W., Rehman A., et al. (2022). Technological advancement in the processing of lycopene: a review. Food Rev. Intl. 38, 857–883. doi: 10.1080/87559129.2020.1749653, PMID: 33086895 [DOI] [Google Scholar]
- Ashraf W., Rehman A., Hussain A., Karim A., Sharif H. R., Siddiquy M., et al. (2023). Optimization of extraction process and estimation of flavonoids from Fenugreek using green extracting deep eutectic solvents coupled with ultrasonication. Food Bioprocess Technol. doi: 10.1007/s11947-023-03170-6 [DOI] [Google Scholar]
- Azam M., Saeed M., Ahmad T., Yamin I., Khan W. A., Iqbal M. W., et al. (2022). Correction to: characterization of biopolymeric encapsulation system for improved survival of Lactobacillus brevis. Food Meas. 16:2604. doi: 10.1007/s11694-022-01383-5 [DOI] [Google Scholar]
- Azam M., Saeed M., Yasmin I., Afzaal M., Ahmed S., Khan W. A., et al. (2021). Microencapsulation and invitro characterization of Bifidobacterium animalis for improved survival. J. Food Meas. Charact. 15, 2591–2600. doi: 10.1007/s11694-021-00839-4 [DOI] [Google Scholar]
- Bambace M. F., Alvarez M. V., del Rosario Moreira M. (2019). Novel functional blueberries: Fructo-oligosaccharides and probiotic lactobacilli incorporated into alginate edible coatings. Food Res. Int. 122, 653–660. doi: 10.1016/j.foodres.2019.01.040, PMID: [DOI] [PubMed] [Google Scholar]
- Barbu V., Cotârleț M., Bolea C. A., Cantaragiu A., Andronoiu D. G., Bahrim G. E., et al. (2020). Three types of beetroot products enriched with lactic acid bacteria. Foods 9:786. doi: 10.3390/foods9060786, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barigela A., Bhukya B. J. B. (2021). Probiotic Pediococcus acidilactici strain from tomato pickle displays anti-cancer activity and alleviates gut inflammation in vitro. 3 Biotech. 11, 1–11. doi: 10.1007/s13205-020-02570-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Y., Liu Y., Liu Y., Wang S., Liu Q., Hao H., et al. (2022). Screening and probiotic potential evaluation of bacteriocin-producing Lactiplantibacillus plantarum in vitro. Foods 11:1575. doi: 10.3390/foods11111575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne C. P., da Cruz A. G., Daga M. (2018). Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 22, 160–166. doi: 10.1016/j.cofs.2018.04.008, PMID: 37513721 [DOI] [Google Scholar]
- Chang Y., Jeong C. H., Cheng W. N., Choi Y., Shin D. M., Lee S., et al. (2021). Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J. Dairy Sci. 104, 7415–7425. doi: 10.3168/jds.2020-19820 [DOI] [PubMed] [Google Scholar]
- Chong E. S. L. (2014). A potential role of probiotics in colorectal cancer prevention: review of possible mechanisms of action. World J. Microbiol. Biotechnol. 30, 351–374. doi: 10.1007/s11274-013-1499-6, PMID: [DOI] [PubMed] [Google Scholar]
- Cordeiro B. F., Alves J. L., Belo G. A., Oliveira E. R., Braga M. P., da Silva S. H., et al. (2021). Therapeutic effects of probiotic minas frescal cheese on the attenuation of ulcerative colitis in a murine model. Front. Microbiol. 12:623920. doi: 10.3389/fmicb.2021.623920, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiny de Oliveira Vieira K., da Silva Ferreira C., Toso Bueno E. B., de Moraes Y. A., Campagnolo Gonçalves Toledo A. C., Nakagaki W. R., et al. (2020). Development and viability of probiotic orange juice supplemented by Pediococcus acidilactici CE51. LWT 130:109637. doi: 10.1016/j.lwt.2020.109637 [DOI] [Google Scholar]
- Cuffia F., George G., Godoy L., Vinderola G., Reinheimer J., Burns P. (2019). In vivo study of the immunomodulatory capacity and the impact of probiotic strains on physicochemical and sensory characteristics: case of pasta filata soft cheeses. Food Res. Int. 125:108606. doi: 10.1016/j.foodres.2019.108606, PMID: [DOI] [PubMed] [Google Scholar]
- Danielle C. G. D. S. (2015). Effect of the addition of water-soluble soybean extract and probiotic culture on chemical characteristics and folate concentration in yogurts produced with goats milk. Afr. J. Microbiol. Res. 9, 1268–1274. doi: 10.5897/AJMR2015.7394 [DOI] [Google Scholar]
- de Brito Alves J. L., de Sousa V. P., Cavalcanti Neto M. P., Magnani M., Braga V. A., da Costa-Silva J. H., et al. (2016). New insights on the use of dietary polyphenols or probiotics for the management of arterial hypertension. Front. Physiol. 7:448. doi: 10.3389/fphys.2016.00448, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lacey A. L., López-Caballero M., Montero P. (2014). Agar films containing green tea extract and probiotic bacteria for extending fish shelf-life. LWT 55, 559–564. doi: 10.1016/j.lwt.2013.09.028 [DOI] [Google Scholar]
- de Minicis S., Rychlicki C., Agostinelli L., Saccomanno S., Candelaresi C., Trozzi L., et al. (2014). Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology 59, 1738–1749. doi: 10.1002/hep.26695, PMID: [DOI] [PubMed] [Google Scholar]
- de Souza da Motta A., Nespolo C. R., Breyer G. M. (2022). “Probiotics in milk and dairy foods” in Probiotics (Oxford, UK: Elsevier Academic Press; ), 103–128. [Google Scholar]
- Dehkohneh A., Jafari P., Fahimi H. (2019). Effects of probiotic Lactobacillus paracasei TD3 on moderation of cholesterol biosynthesis pathway in rats. Iran. J. Basic Med. Sci. 22:1004. doi: 10.22038/ijbms.2019.33933.8073, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Costanzo M., Amoroso A., Canani R. B. (2016). Gut microbiota as a target for food allergy. J. Pediatr. Gastroenterol. Nutr. 63, S48–S13. doi: 10.1097/01.mpg.0000489618.97688.b8 [DOI] [PubMed] [Google Scholar]
- Do T. V. T., Fan L. (2019). Probiotic viability, qualitative characteristics, and sensory acceptability of vegetable juice mixture fermented with lactobacillus strains. Food Nutr. Sci. 10:412. doi: 10.4236/fns.2019.104031 [DOI] [Google Scholar]
- Ebrahimi B., Mohammadi R., Rouhi M., Mortazavian A. M., Shojaee-Aliabadi S., Koushki M. R. (2018). Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT 87, 54–60. doi: 10.1016/j.lwt.2017.08.066 [DOI] [Google Scholar]
- el-Sohaimy S. A., Shehata M. G., Mehany T., Zeitoun M. A. (2019). Nutritional, physicochemical, and sensorial evaluation of flat bread supplemented with quinoa flour. Int. J. Food Sci. 2019, 1–15. doi: 10.1155/2019/4686727, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfandiary A., Taherian-Esfahani Z., Abedin-do A., Mirfakhraie R., Shirzad M., Ghafouri-Fard S., et al. (2016). Lactobacilli modulate hypoxia-inducible factor (HIF)-1 regulatory pathway in triple negative breast cancer cell line. Yakhteh 18, 237–244. doi: 10.22074/cellj.2016.4319, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantinato V., Camargo H. R., Sousa A. L. O. P. (2019). Probiotics study with Streptococcus salivarius and its ability to produce bacteriocins and adherence to KB cells. Rev Odontol UNESP 48, 1–9. doi: 10.1590/1807-2577.02919 [DOI] [Google Scholar]
- FAO , World food and agriculture - statistical yearbook 2022. (2022). Rome. [Google Scholar]
- Fenster K., Freeburg B., Hollard C., Wong C., Rønhave Laursen R., Ouwehand A. (2019). The production and delivery of probiotics: a review of a practical approach. Microorganisms 7:83. doi: 10.3390/microorganisms7030083, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M. A., Marette A. J. A. I. N. (2017). Potential health benefits of combining yogurt and fruits based on their probiotic and prebiotic properties. Adv. Nutr. 8, 155S–164S. doi: 10.3945/an.115.011114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocchi A., Pawankar R., Cuello-Garcia C., Ahn K., al-Hammadi S., Agarwal A., et al. (2015). World allergy organization-McMaster University guidelines for allergic disease prevention (GLAD-P): probiotics. World Allergy Organ. J. 8, 4–13. doi: 10.1186/s40413-015-0055-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco A., Savio V., Cimini D., D’Ambrosio S., Chiaromonte A., Schiraldi C., et al. (2023). In vitro evaluation of the most active probiotic strains able to improve the intestinal barrier functions and to prevent inflammatory diseases of the gastrointestinal system. Biomedicine 11:865. doi: 10.3390/biomedicines11030865, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliarini N., Diosma G., Garrote G. L., Abraham A. G., Piermaria J. (2019). Whey protein-kefiran films as driver of probiotics to the gut. LWT 105, 321–328. doi: 10.1016/j.lwt.2019.02.023 [DOI] [Google Scholar]
- Galli S. J., Metz M., Starkl P., Marichal T., Tsai M. (2020). Mast cells and IgE in defense against lethality of venoms: possible “benefit” of allergy. Allergo J. Int. 29, 46–62. doi: 10.1007/s40629-020-00118-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangaraju D., Raghu A. V., Siddalingaiya Gurudutt P. J. N. S. (2022). Green synthesis of γ-aminobutyric acid using permeabilized probiotic Enterococcus faecium for biocatalytic application. Nano Select 3, 1436–1447. doi: 10.1002/nano.202200059 [DOI] [Google Scholar]
- Ganguly S., Sabikhi L., Singh A. K. (2019). Effect of whey-pearl millet-barley based probiotic beverage on Shigella-induced pathogenicity in murine model. J. Funct. Foods 54, 498–505. doi: 10.1016/j.jff.2019.01.049 [DOI] [Google Scholar]
- Gao J., Li X., Zhang G., Sadiq F. A., Simal-Gandara J., Xiao J., et al. (2021). Probiotics in the dairy industry—advances and opportunities. Compr. Rev. Food Sci. Food Saf. 20, 3937–3982. doi: 10.1111/1541-4337.12755, PMID: [DOI] [PubMed] [Google Scholar]
- Garcia-Brand A. J., Quezada V., Gonzalez-Melo C., Bolaños-Barbosa A. D., Cruz J. C., Reyes L. H. (2022). Novel developments on stimuli-responsive probiotic encapsulates: from smart hydrogels to nanostructured platforms. Fermentation 8:117. doi: 10.3390/fermentation8030117 [DOI] [Google Scholar]
- Gasbarrini G., Bonvicini F., Gramenzi A. (2016). Probiotics history. J. Clin. Gastroenterol. 50, S116–S119. doi: 10.1097/MCG.0000000000000697, PMID: [DOI] [PubMed] [Google Scholar]
- Grom L. C., Coutinho N. M., Guimarães J. T., Balthazar C. F., Silva R., Rocha R. S., et al. (2020). Probiotic dairy foods and postprandial glycemia: a mini-review. Trends Food Sci. Technol. 101, 165–171. doi: 10.1016/j.tifs.2020.05.012 [DOI] [Google Scholar]
- Guo Y., Wu F.-G. (2023). Probiotics armored with metal-phenolic network-based nanocoatings for gut microbiome modulation. Matter 6, 23–25. doi: 10.1016/j.matt.2022.12.006 [DOI] [Google Scholar]
- Hamad G. M., Amer A., el-Nogoumy B., Ibrahim M., Hassan S., Siddiqui S. A., et al. (2022). Evaluation of the effectiveness of charcoal, Lactobacillus rhamnosus, and Saccharomyces cerevisiae as aflatoxin adsorbents in chocolate. Toxins 15:21. doi: 10.3390/toxins15010021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad G. M., Omar S. A., Mostafa A. G. M., Cacciotti I., Saleh S. M., Allam M. G., et al. (2022). Binding and removal of polycyclic aromatic hydrocarbons in cold smoked sausage and beef using probiotic strains. Food Res. Int. 161:111793. doi: 10.1016/j.foodres.2022.111793, PMID: [DOI] [PubMed] [Google Scholar]
- Hamad G., Ombarak R. A., Eskander M., Mehany T., Anees F. R., Elfayoumy R. A., et al. (2022). Detection and inhibition of Clostridium botulinum in some Egyptian fish products by probiotics cell-free supernatants as bio-preservation agents. LWT 163:113603. doi: 10.1016/j.lwt.2022.113603 [DOI] [Google Scholar]
- Harata G., He F., Takahashi K., Hosono A., Miyazawa K., Yoda K., et al. (2016). Human Lactobacillus strains from the intestine can suppress IgE-mediated degranulation of rat basophilic leukaemia (RBL-2H3) cells. Microorganisms 4:40. doi: 10.3390/microorganisms4040040, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar K., Ramunaik M., Suvarna C. (2013). A review on hyperlipidemic. Int. J. Novel Trends Pharm. Sci. 3, 59–71. doi: 10.7759/cureus.16412 [DOI] [Google Scholar]
- Hauser K., Matthes J. (2017). Medical students’ medication communication skills regarding drug prescription—a qualitative analysis of simulated physician-patient consultations. Eur. J. Clin. Pharmacol. 73, 429–435. doi: 10.1007/s00228-016-2192-0, PMID: [DOI] [PubMed] [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G. R., Merenstein D. J., Pot B., et al. (2014). Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66, PMID: [DOI] [PubMed] [Google Scholar]
- Hosseini S. F., Ansari B., Gharsallaoui A. (2022). Polyelectrolytes-stabilized liposomes for efficient encapsulation of Lactobacillus rhamnosus and improvement of its survivability under adverse conditions. Food Chem. 372:131358. doi: 10.1016/j.foodchem.2021.131358, PMID: [DOI] [PubMed] [Google Scholar]
- Huang C.-H., Lin Y.-C., Jan T.-R. (2017). Lactobacillus reuteri induces intestinal immune tolerance against food allergy in mice. J. Funct. Foods 31, 44–51. doi: 10.1016/j.jff.2017.01.034 [DOI] [Google Scholar]
- Husein N. A., Rashad N. M., Shaheen A. A. (2017). The effect of probiotics on interleukin-8 and intestinal Flora in irritable bowel syndrome in Hospital of Zagazig University. Egypt. J. Med. Microbiol. 26, 33–40. doi: 10.12816/0046270 [DOI] [Google Scholar]
- Iqbal M. W., Mu W., Khan I. M., Mohsin A., Rehman A., Koko M. Y. F. (2017). Development of probiotic soft cheese with Lactobacillus casei as adjunct culture. J. Acad. Industr. Res. 6:1. [Google Scholar]
- Islam M. S., Ahmed M. K., Habibullah-al-Mamun M., Islam K. N., Ibrahim M., Masunaga S. (2014). Arsenic and lead in foods: a potential threat to human health in Bangladesh. Food Addit. Contam. A 31, 1982–1992. doi: 10.1080/19440049.2014.974686 [DOI] [PubMed] [Google Scholar]
- Jang W. J., Lee J. M., Hasan M. T., Lee B. J., Lim S. G., Kong I. S. (2019). Effects of probiotic supplementation of a plant-based protein diet on intestinal microbial diversity, digestive enzyme activity, intestinal structure, and immunity in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 92, 719–727. doi: 10.1016/j.fsi.2019.06.056, PMID: [DOI] [PubMed] [Google Scholar]
- Kanmani P., Lim S. T. (2013). Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 141, 1041–1049. doi: 10.1016/j.foodchem.2013.03.103, PMID: [DOI] [PubMed] [Google Scholar]
- Khodaei D., Hamidi-Esfahani Z. (2019). Influence of bioactive edible coatings loaded with Lactobacillus plantarum on physicochemical properties of fresh strawberries. Postharvest Biol. Technol. 156:110944. doi: 10.1016/j.postharvbio.2019.110944 [DOI] [Google Scholar]
- Klłonowska-Olejnik M. (2004). Redescription of Electrogena quadrilineata (Landa, 1969) from type material (Ephemeroptera, Heptageniidae). Aquat. Insects 26, 85–95. doi: 10.1080/01650420412331325828 [DOI] [Google Scholar]
- Konishi H., Fujiya M., Tanaka H., Ueno N., Moriichi K., Sasajima J., et al. (2016). Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 7:12365. doi: 10.1038/ncomms12365, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konuray G., Erginkaya Z. (2018). Potential use of Bacillus coagulans in the food industry. Foods 7:92. doi: 10.3390/foods7060092, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laali K. K., Greves W. J., Correa-Smits S. J., Zwarycz A. T., Bunge S. D., Borosky G. L., et al. (2018). Novel fluorinated curcuminoids and their pyrazole and isoxazole derivatives: synthesis, structural studies, computational/docking and in-vitro bioassay. J. Fluor. Chem. 206, 82–98. doi: 10.1016/j.jfluchem.2017.11.013 [DOI] [Google Scholar]
- Leaf J. B., Alcalay A., Leaf J. A., Tsuji K., Kassardjian A., Dale S., et al. (2016). Comparison of most-to-least to error correction for teaching receptive labelling for two children diagnosed with autism. J. Res. Spec. Educ. Needs 16, 217–225. doi: 10.1111/1471-3802.12067 [DOI] [Google Scholar]
- Lee N.-K., Kim S. Y., Han K. J., Eom S. J., Paik H. D. (2014). Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starters. LWT 58, 130–134. doi: 10.1016/j.lwt.2014.02.028 [DOI] [Google Scholar]
- Lee C. S., Lee S. H., Kim S. H. (2020). Bone-protective effects of Lactobacillus plantarum B719-fermented milk product. Int. J. Dairy Technol. 73, 706–717. doi: 10.1111/1471-0307.12701 [DOI] [Google Scholar]
- Lee J.-E., Lee J., Kim J. H., Cho N., Lee S. H., Park S. B., et al. (2019). Characterization of the anti-cancer activity of the probiotic bacterium Lactobacillus fermentum using 2D vs. 3D culture in colorectal cancer cells. Biomolecules 9:557. doi: 10.3390/biom9100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Liu L., Tian H., Luo X., Liu S. (2019). Encapsulation of Lactobacillus plantarum in cellulose based microgel with controlled release behavior and increased long-term storage stability. Carbohydr. Polym. 223:115065. doi: 10.1016/j.carbpol.2019.115065, PMID: [DOI] [PubMed] [Google Scholar]
- Li J., Sung C. Y. J., Lee N., Ni Y., Pihlajamäki J., Panagiotou G., et al. (2016). Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. U. S. A. 113, E1306–E1315. doi: 10.1073/pnas.1518189113, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Zhang Y., Miao Z., Cheng R., Jiang F., Ze X., et al. (2022). Anti-allergic effects of two potential probiotic strains isolated from infant feces in China. J. Funct. Foods 92:105070. doi: 10.1016/j.jff.2022.105070 [DOI] [Google Scholar]
- Linn Y. H., Thu K. K., Win N. H. H. (2019). Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiot. Antimicrob. Proteins 11, 638–647. doi: 10.1007/s12602-018-9408-9, PMID: [DOI] [PubMed] [Google Scholar]
- Liu D. M., Guo J., Zeng X. A., Sun D. W., Brennan C. S., Zhou Q. X., et al. (2017). The probiotic role of Lactobacillus plantarum in reducing risks associated with cardiovascular disease. Int. J. Food Sci. Technol. 52, 127–136. doi: 10.1111/ijfs.13234 [DOI] [Google Scholar]
- Lopez-Santamarina A., Gonzalez E. G., Lamas A., Mondragon A. C., Regal P., Miranda J. M. (2021). Probiotics as a possible strategy for the prevention and treatment of allergies. A narrative review. Foods 10:701. doi: 10.3390/foods10040701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Zhang H., Li Y., Huang Q. (2018). Fabrication of milled cellulose particles-stabilized Pickering emulsions. Food Hydrocoll. 77, 427–435. doi: 10.1016/j.foodhyd.2017.10.019 [DOI] [Google Scholar]
- Lucatto J. N., Silva-Buzanello R. A., Mendonça S. N. T. G., Lazarotto T. C., Sanchez J. L., Bona E., et al. (2020). Performance of different microbial cultures in potentially probiotic and prebiotic yoghurts from cow and goat milks. Int. J. Dairy Technol. 73, 144–156. doi: 10.1111/1471-0307.12655 [DOI] [Google Scholar]
- Luo Y., de Souza C., Ramachandran M., Wang S., Yi H., Ma Z., et al. (2022). Precise oral delivery systems for probiotics: a review. J. Control. Release 352, 371–384. doi: 10.1016/j.jconrel.2022.10.030, PMID: [DOI] [PubMed] [Google Scholar]
- Ma J., Zhang J., Li Q., Shi Z., Wu H., Zhang H., et al. (2019). Oral administration of a mixture of probiotics protects against food allergy via induction of CD103+ dendritic cells and modulates the intestinal microbiota. J. Funct. Foods 55, 65–75. doi: 10.1016/j.jff.2019.02.010 [DOI] [Google Scholar]
- Ma X.-Y., Son Y.-H., Yoo J.-W., Joo M.-K., Kim D.-H. (2022). Tight junction protein expression-inducing probiotics alleviate TNBS-induced cognitive impairment with colitis in mice. Nutrients 14:2975. doi: 10.3390/nu14142975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzourani I., Kazakos S., Terpou A., Alexopoulos A., Bezirtzoglou E., Bekatorou A., et al. (2018a). Potential of the probiotic Lactobacillus plantarum ATCC 14917 strain to produce functional fermented pomegranate juice. Foods 8:4. doi: 10.3390/foods8010004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzourani I., Nouska C., Terpou A., Alexopoulos A., Bezirtzoglou E., Panayiotidis M., et al. (2018b). Production of a novel functional fruit beverage consisting of cornelian cherry juice and probiotic bacteria. Antioxidants 7:163. doi: 10.3390/antiox7110163, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco M. L., Heeney D., Binda S., Cifelli C. J., Cotter P. D., Foligné B., et al. (2017). Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 44, 94–102. doi: 10.1016/j.copbio.2016.11.010, PMID: [DOI] [PubMed] [Google Scholar]
- Meira Q. G. S., Magnani M., de Medeiros Júnior F. C., Queiroga R. C. R. E., Madruga M. S., Gullón B., et al. (2015). Effects of added Lactobacillus acidophilus and Bifidobacterium lactis probiotics on the quality characteristics of goat ricotta and their survival under simulated gastrointestinal conditions. Food Res. Int. 76, 828–838. doi: 10.1016/j.foodres.2015.08.002, PMID: [DOI] [PubMed] [Google Scholar]
- Meybodi N., Mortazavian A. (2017). Probiotic supplements and food products: a comparative approach. Biochem. Pharmacol. 6:1000227. doi: 10.4172/2167-0501.1000227 [DOI] [Google Scholar]
- Min M., Bunt C. R., Mason S. L., Hussain M. A. (2019). Non-dairy probiotic food products: an emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 59, 2626–2641. doi: 10.1080/10408398.2018.1462760, PMID: [DOI] [PubMed] [Google Scholar]
- Miranda R. F., de Paula M. M., da Costa G. M., Barão C. E., da Silva A. C. R., Raices R. S. L., et al. (2019). Orange juice added with L. casei: is there an impact of the probiotic addition methodology on the quality parameters? LWT 106, 186–193. doi: 10.1016/j.lwt.2019.02.047 [DOI] [Google Scholar]
- Mirzamani S., Bassiri A. R., Tavakolipour H., Azizi M. H., Kargozari M. (2021). Survival of fluidized bed encapsulated Lactobacillus acidophilus under simulated gastro-intestinal conditions and heat treatment during bread baking. J. Food Meas. Charact. 15, 5477–5484. doi: 10.1007/s11694-021-01108-0 [DOI] [Google Scholar]
- Mojaveri S. J., Hosseini S. F., Gharsallaoui A. (2020). Viability improvement of Bifidobacterium animalis Bb12 by encapsulation in chitosan/poly(vinyl alcohol) hybrid electrospun fiber mats. Carbohydr. Polym. 241:116278. doi: 10.1016/j.carbpol.2020.116278, PMID: [DOI] [PubMed] [Google Scholar]
- Munir A., Javed G. A., Javed S., Arshad N. (2022). Levilactobacillus brevis from carnivores can ameliorate hypercholesterolemia: in vitro and in vivo mechanistic evidence. J. Appl. Microbiol. 133, 1725–1742. doi: 10.1111/jam.15678, PMID: [DOI] [PubMed] [Google Scholar]
- Nami Y., Vaseghi Bakhshayesh R., Manafi M., Hejazi M. A. (2019). Hypocholesterolaemic activity of a novel autochthonous potential probiotic Lactobacillus plantarum YS5 isolated from yogurt. LWT 111, 876–882. doi: 10.1016/j.lwt.2019.05.057 [DOI] [Google Scholar]
- Nguyen B. T., Bujna E., Fekete N., Tran A. T. M., Rezessy-Szabo J. M., Prasad R., et al. (2019). Probiotic beverage from pineapple juice fermented with Lactobacillus and Bifidobacterium strains. Front. Nutr. 6:54. doi: 10.3389/fnut.2019.00054, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M.-H., Vu N. B. D., Nguyen T. H. N., le H. S., le H. T., Tran T. T., et al. (2019). In vivo comparison of wound healing and scar treatment effect between curcumin–oligochitosan nanoparticle complex and oligochitosan-coated curcumin-loaded-liposome. J. Microencapsul. 36, 156–168. doi: 10.1080/02652048.2019.1612476, PMID: [DOI] [PubMed] [Google Scholar]
- Niesche R., Haase M. (2012). Emotions and ethics: a Foucauldian framework for becoming an ethical educator. Educ. Philos. Theory 44, 276–288. doi: 10.1111/j.1469-5812.2010.00655.x [DOI] [Google Scholar]
- Owaga E. E., Elbakkoush A., MS L. R. K. S. (2014). Antiallergic effects of probiotic lactobacilli–cellular and molecular mechanisms. J. Microbiol. Res. 4, 92–97. doi: 10.5923/j.microbiology.20140402.08 [DOI] [Google Scholar]
- Ozturk B., Elvan M., Özer M., Tellioğlu Harsa Ş. (2021). Effect of different microencapsulating materials on the viability of S. thermophilus CCM4757 incorporated into dark and milk chocolates. Food Biosci. 44:101413. doi: 10.1016/j.fbio.2021.101413 [DOI] [Google Scholar]
- Pakdaman M. N., Udani J. K., Molina J. P., Shahani M. (2015). The effects of the DDS-1 strain of lactobacillus on symptomatic relief for lactose intolerance-a randomized, double-blind, placebo-controlled, crossover clinical trial. Nutr. J. 15, 1–11. doi: 10.1186/s12937-016-0172-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyandi S. A., Damodharan K., Suh J.-W., Yang S. H. (2020). Probiotic characterization of cholesterol-lowering Lactobacillus fermentum MJM60397. Probiotics Antimicrob. Proteins 12, 1161–1172. doi: 10.1007/s12602-019-09585-y [DOI] [PubMed] [Google Scholar]
- Papademas P., Kotsaki P. (2019). Technological utilization of whey towards sustainable exploitation. J. Adv. Dairy Res. 7:231. doi: 10.35248/2329-888X.7.4.231 [DOI] [Google Scholar]
- Passaniti A., Kim M. S., Polster B. M., Shapiro P. (2022). Targeting mitochondrial metabolism for metastatic cancer therapy. Mol. Carcinog. 61, 827–838. doi: 10.1002/mc.23436, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavli F., Tassou C., Nychas G. J., Chorianopoulos N. (2018). Probiotic incorporation in edible films and coatings: bioactive solution for functional foods. Int. J. Mol. Sci. 19:150. doi: 10.3390/ijms19010150, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Marques J., Ferreira R. M., Pinto-Ribeiro I., Figueiredo C. (2019). Helicobacter pylori infection, the gastric microbiome and gastric cancer. Adv. Exp. Med. Biol 1149, 195–210. doi: 10.1007/5584_2019_366 [DOI] [PubMed] [Google Scholar]
- Petruzziello C., Saviano A., Ojetti V. J. V. (2023). Probiotics, the immune response and acute appendicitis: a review. Vaccines 11:1170. doi: 10.3390/vaccines11071170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pithva S. P., Ambalam P. S., Ramoliya J. M., Dave J. M., Vyas B. R. M. (2015). Antigenotoxic and antimutagenic activities of probiotic Lactobacillus rhamnosus Vc against N-methyl-N′-nitro-N-nitrosoguanidine. Nutr. Cancer 67, 1142–1150. doi: 10.1080/01635581.2015.1073751, PMID: [DOI] [PubMed] [Google Scholar]
- Plaza-Diaz J., Ruiz-Ojeda F. J., Gil-Campos M., Gil A. (2019). Mechanisms of action of probiotics. Adv. Nutr. 10, S49–S66. doi: 10.1093/advances/nmy063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop O. L., Pop C. R., Dufrechou M., Vodnar D. C., Socaci S. A., Dulf F. V., et al. (2019). Edible films and coatings functionalization by probiotic incorporation: a review. Polymers 12:12. doi: 10.3390/polym12010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Tomaro-Duchesneau C., Saha S., Rodes L., Kahouli I., Malhotra M. (2014). Probiotics for the prevention and treatment of allergies, with an emphasis on mode of delivery and mechanism of action. Curr. Pharm. Des. 20, 1025–1037. doi: 10.2174/138161282006140220145154, PMID: [DOI] [PubMed] [Google Scholar]
- Prezzi L. E., Lee S. H. I., Nunes V. M. R., Corassin C. H., Pimentel T. C., Rocha R. S., et al. (2020). Effect of Lactobacillus rhamnosus on growth of listeria monocytogenes and Staphylococcus aureus in a probiotic Minas Frescal cheese. Food Microbiol. 92:103557. doi: 10.1016/j.fm.2020.103557, PMID: [DOI] [PubMed] [Google Scholar]
- Putta S., Yarla N. S., Lakkappa D. B., Imandi S. B., Malla R. R., Chaitanya A. K., et al. (2018). “Probiotics: supplements, food, pharmaceutical industry” in Therapeutic, probiotic, and unconventional foods (Cambridge, Massachusetts, United States: Elsevier Academic Press; ), 15–25. [Google Scholar]
- Razavi S., Janfaza S., Tasnim N., Gibson D. L., Hoorfar M. (2021). Microencapsulating polymers for probiotics delivery systems: preparation, characterization, and applications. Food Hydrocoll. 120:106882. doi: 10.1016/j.foodhyd.2021.106882 [DOI] [Google Scholar]
- Reid G. (2015). The growth potential for dairy probiotics. Int. Dairy J. 49, 16–22. doi: 10.1016/j.idairyj.2015.04.004, PMID: 37512891 [DOI] [Google Scholar]
- Reque P. M., Brandelli A. (2021). Encapsulation of probiotics and nutraceuticals: applications in functional food industry. Trends Food Sci. Technol. 114, 1–10. doi: 10.1016/j.tifs.2021.05.022, PMID: 37033316 [DOI] [Google Scholar]
- Rezaei M., Sanagoo A., Jouybari L., Behnampoo N. (2017). The effect of probiotic yogurt on blood glucose and cardiovascular biomarkers in patients with type II diabetes: a randomized controlled trial. Evid. Based Care 6, 26–35. doi: 10.22038/EBCJ.2016.7984 [DOI] [Google Scholar]
- Riaz T., Iqbal M. W., Saeed M., Yasmin I., Hassanin H. A. M., Mahmood S., et al. (2019). In vitro survival of Bifidobacterium bifidum microencapsulated in zein-coated alginate hydrogel microbeads. J. Microencapsul. 36, 192–203. doi: 10.1080/02652048.2019.1618403, PMID: [DOI] [PubMed] [Google Scholar]
- Roobab U., Batool Z., Manzoor M. F., Shabbir M. A., Khan M. R., Aadil R. M. (2020). Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 32, 17–28. doi: 10.1016/j.cofs.2020.01.003 [DOI] [Google Scholar]
- Saber A., Alipour B., Faghfoori Z., Yari Khosroushahi A. (2017). Cellular and molecular effects of yeast probiotics on cancer. Crit. Rev. Microbiol. 43, 96–115. doi: 10.1080/1040841X.2016.1179622, PMID: [DOI] [PubMed] [Google Scholar]
- Sajedi D., Shabani R., Elmieh A. J. C. (2021). Changes in leptin, serotonin, and cortisol after eight weeks of aerobic exercise with probiotic intake in a cuprizone-induced demyelination mouse model of multiple sclerosis. Cytokine 144:155590. doi: 10.1016/j.cyto.2021.155590 [DOI] [PubMed] [Google Scholar]
- Samanta S. (2022). Potential impacts of prebiotics and probiotics on cancer prevention. Anti Cancer Agents Med. Chem. 22, 605–628. doi: 10.2174/1871520621999201210220442 [DOI] [PubMed] [Google Scholar]
- Sanz Y., Portune K., Gómez del Pulgar E. M., Benítez-Páez A. (2016). “Targeting the microbiota: considerations for developing probiotics as functional foods” in The gut-brain Axis (Oxford, UK: Elsevier Science Ltd.), 17–30. [Google Scholar]
- Seyedain-Ardabili M., Sharifan A., Ghiassi Tarzi B. (2016). Proizvodnja kruha s dodatkom mikroinkapsuliranih sinbiotika. Food Technol. Biotechnol. 54, 52–59. doi: 10.17113/ftb.54.01.16.4234, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahverdi E. (2016). Probiotics and gastrointestinal diseases. Int. J. Dig. Dis. 2, 1–2. doi: 10.4172/2472-1891.100022 [DOI] [Google Scholar]
- Shamekhi S., Abdolalizadeh J., Ostadrahimi A., Mohammadi S. A., Barzegari A., Lotfi H., et al. (2020). Apoptotic effect of Saccharomyces cerevisiae on human colon cancer SW480 cells by regulation of Akt/NF-ĸB signaling pathway. Probiotics Antimicrob. Proteins 12, 311–319. doi: 10.1007/s12602-019-09528-7, PMID: [DOI] [PubMed] [Google Scholar]
- Sharma H., Sharma S., Bajwa J., Chugh R., Kumar D. (2023). Polymeric carriers in probiotic delivery system. Carbohyd. Polym. Technol. Appl. 5:100301. doi: 10.1016/j.carpta.2023.100301, PMID: 37172705 [DOI] [Google Scholar]
- Shehata A. A., Yalçın S., Latorre J. D., Basiouni S., Attia Y. A., Abd el-Wahab A., et al. (2022). Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 10:395. doi: 10.3390/microorganisms10020395, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Magalhães S., Alves L., Antunes F., Miguel M., Lindman B., et al. (2019). Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 88, 68–74. doi: 10.1016/j.foodhyd.2018.08.057 [DOI] [Google Scholar]
- Singh V., Singh N., Verma M., Praveena S. M., Verma M. K., Bilal M., et al. (2022). “Nanotechnology in agriculture and bioencapsulation of probiotics/food additives” in Smart nanomaterials for bioencapsulation. eds. Castro G., Nadda A. K., Nguyen T. A., Sharma S., Gupta R. K. (New York, USA: Elsevier Science Inc.), 213–223. [Google Scholar]
- So S. S., Wan M. L., El-Nezami H. (2017). Probiotics-mediated suppression of cancer. Curr. Opin. Oncol. 29, 62–72. doi: 10.1097/CCO.0000000000000342, PMID: [DOI] [PubMed] [Google Scholar]
- Soukoulis C., Behboudi-Jobbehdar S., Macnaughtan W., Parmenter C., Fisk I. D. (2017). Stability of Lactobacillus rhamnosus GG incorporated in edible films: impact of anionic biopolymers and whey protein concentrate. Food Hydrocoll. 70, 345–355. doi: 10.1016/j.foodhyd.2017.04.014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastav S., Neupane S., Bhurtel S., Katila N., Maharjan S., Choi H., et al. (2019). Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem 69, 73–86. doi: 10.1016/j.jnutbio.2019.03.021 [DOI] [PubMed] [Google Scholar]
- Steed H., Macfarlane G. T., Blackett K. L., Bahrami B., Reynolds N., Walsh S. V., et al. (2010). Clinical trial: the microbiological and immunological effects of synbiotic consumption–a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment. Pharmacol. Ther. 32, 872–883. doi: 10.1111/j.1365-2036.2010.04417.x, PMID: [DOI] [PubMed] [Google Scholar]
- Stratiki Z., Costalos C., Sevastiadou S., Kastanidou O., Skouroliakou M., Giakoumatou A., et al. (2007). The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 83, 575–579. doi: 10.1016/j.earlhumdev.2006.12.002, PMID: [DOI] [PubMed] [Google Scholar]
- Su S.-C., Chang L. C., Huang H. D., Peng C. Y., Chuang C. Y., Chen Y. T., et al. (2021). Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 42, 127–135. doi: 10.1093/carcin/bgaa062, PMID: [DOI] [PubMed] [Google Scholar]
- Tamaki H., Nakase H., Inoue S., Kawanami C., Itani T., Ohana M., et al. (2016). Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: a randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 28, 67–74. doi: 10.1111/den.12553, PMID: [DOI] [PubMed] [Google Scholar]
- Thakkar P. N., Modi H. A., Prajapati J. (2016). Therapeutic impacts of probiotics--as magic bullet. Am. J. Biomed. Sci. 8, 97–113. doi: 10.5099/aj160200097 [DOI] [Google Scholar]
- Trallero O. G., Serrano L. H., Inglés M. B., Vallés D. R., Rodríguez A. M. (2019). Effect of the administration of a probiotic with a combination of Lactobacillus and Bifidobacterium strains on antibiotic-associated diarrhea. Rev. Esp. Quimioter. 32:268. [PMC free article] [PubMed] [Google Scholar]
- Tyutkov N., Zhernyakova A., Birchenko A., Eminova E., Nadtochii L., Baranenko D. (2022). Probiotics viability in frozen food products. Food Biosci. 50:101996. doi: 10.1016/j.fbio.2022.101996, PMID: 36240011 [DOI] [Google Scholar]
- Ulpathakumbura C., Ranadheera C. S., Senavirathne N. D., Jayawardene L. P. I. N. P., Prasanna P. H. P., Vidanarachchi J. K. (2016). Effect of biopreservatives on microbial, physico-chemical and sensory properties of Cheddar cheese. Food Biosci. 13, 21–25. doi: 10.1016/j.fbio.2015.12.003 [DOI] [Google Scholar]
- Warman D. J., Jia H., Kato H. (2022). The potential roles of probiotics, resistant starch, and resistant proteins in ameliorating inflammation during aging (inflammaging). Nutrients 14:747. doi: 10.3390/nu14040747, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Ban Q., Wang W., Hou J., Jiang Z. (2022). Novel nano-encapsulated probiotic agents: encapsulate materials, delivery, and encapsulation systems. J. Control. Release 349, 184–205. doi: 10.1016/j.jconrel.2022.06.061, PMID: [DOI] [PubMed] [Google Scholar]
- Yang B., Yue Y., Chen Y., Ding M., Li B., Wang L., et al. (2021). Lactobacillus plantarum CCFM1143 alleviates chronic diarrhea via inflammation regulation and gut microbiota modulation: a double-blind, randomized, placebo-controlled study. Front. Immunol. 12:746585. doi: 10.3389/fimmu.2021.746585, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A., Liao Y., Zhu J., Zhang J. (2021). Screening of anti-allergy Lactobacillus and its effect on allergic reactions in BALB/c mice sensitized by soybean protein. J. Funct. Foods 87:104858. doi: 10.1016/j.jff.2021.104858 [DOI] [Google Scholar]
- Yu A.-Q., Li L. (2016). The potential role of probiotics in cancer prevention and treatment. Nutr. Cancer 68, 535–544. doi: 10.1080/01635581.2016.1158300, PMID: [DOI] [PubMed] [Google Scholar]
- Zhang L., Taal M. A., Boom R. M., Chen X. D., Schutyser M. A. I. (2018). Effect of baking conditions and storage on the viability of Lactobacillus plantarum supplemented to bread. LWT 87, 318–325. doi: 10.1016/j.lwt.2017.09.005 [DOI] [Google Scholar]
- Zhang Y., Xie Y., Liu H., McClements D. J., Cheng C., Zou L., et al. (2022). Probiotic encapsulation in water-in-oil high internal phase emulsions: enhancement of viability under food and gastrointestinal conditions. LWT 163:113499. doi: 10.1016/j.lwt.2022.113499 [DOI] [Google Scholar]
- Zhuge A., Li S., Lou P., Wu W., Wang K., Yuan Y., et al. (2022). Longitudinal 16S rRNA sequencing reveals relationships among alterations of gut microbiota and nonalcoholic fatty liver disease progression in mice. Microbiol. Spectr. 10:e00047-22. doi: 10.1128/spectrum.00047-22 [DOI] [PMC free article] [PubMed] [Google Scholar]