Abstract
SARS-CoV-2 (CoV2) infected, asymptomatic individuals are an important contributor to COVID transmission. CoV2-specific immunoglobulin (Ig)—as generated by the immune system following infection or vaccination—has helped limit CoV2 transmission from asymptomatic individuals to susceptible populations (e.g. elderly). Here, we describe the relationships between COVID incidence and CoV2 lineage, viral load, saliva Ig levels (CoV2-specific IgM, IgA and IgG), and ACE2 binding inhibition capacity in asymptomatic individuals between January 2021 and May 2022. These data were generated as part of a large university COVID monitoring program in Ohio, United States of America, and demonstrate that COVID incidence among asymptomatic individuals occurred in waves which mirrored those in surrounding regions, with saliva CoV2 viral loads becoming progressively higher in our community until vaccine mandates were established. Among the unvaccinated, infection with each CoV2 lineage (pre-Omicron) resulted in saliva Spike-specific IgM, IgA, and IgG responses, the latter increasing significantly post-infection and being more pronounced than N-specific IgG responses. Vaccination resulted in significantly higher Spike-specific IgG levels compared to unvaccinated infected individuals, and uninfected vaccinees’ saliva was more capable of inhibiting Spike function. Vaccinees with breakthrough Delta infections had Spike-specific IgG levels comparable to those of uninfected vaccinees; however, their ability to inhibit Spike binding was diminished. These data are consistent with COVID vaccines having achieved hoped-for effects in our community, including the generation of mucosal antibodies that inhibit Spike and lower community viral loads, and suggest breakthrough Delta infections were not due to an absence of vaccine-elicited Ig, but instead limited Spike binding activity in the face of high community viral loads.
Author summary
Our study identified relationships between specific SARS-CoV-2 (CoV2) variants of concern (VOCs) and CoV2-specific immunoglobulin (Ig) among asymptomatic young adults in our university community. Asymptomatic young adults are important source of CoV2 transmission in the United States of America and other countries. Major findings from our study which we believe inform our understanding of CoV2 transmission and immunity, and may potentially influence COVID monitoring policies at other universities include the following: (1) CoV2 positivity occurred in waves which mirrored those in regions surrounding our university campuses, and were driven by newly emerged VOCs. (2) Only after university vaccine requirements went into effect did net viral loads among all community members decline. (3) Breakthrough infections among vaccinees were not due to an absence of vaccine-elicited Ig, but rather diminished inhibitory capacity during a period when community viral loads peaked. In other words, vaccination efforts achieved their intended goal of increasing CoV2-specific Ig; in individuals with breakthrough infections, however, the capacity of this Ig to inhibit Spike function was limited and corresponded to when community viral loads were at their highest.
Introduction
Coronaviruses are single-stranded RNA viruses that cause respiratory disease in a range of mammalian hosts [1]. The Coronavirus Disease 2019 (COVID-19, or COVID) pandemic began in December 2019, after transmission of a novel coronavirus to individuals living in China [2,3]. The sequence homology of this novel coronavirus to severe acute respiratory syndrome associated coronavirus (SARS-CoV) led to its being named SARS-CoV-2 (CoV2) [4]. CoV2 spreads via aerosol and respiratory droplets [5], causing either an asymptomatic infection or a flu-like illness that affects multiple organ systems and presents as fever, cough, dyspnea, malaise, delirium, and death [6]. International spread of CoV2 was rapid, and by February 2020 it had spread to nearly every country in the world [1]. Now, nearly 4 years after its emergence, CoV2 is estimated to have infected ~760 million individuals and killed >6.9 million individuals worldwide [7]. The United States of America (US) has reported more deaths than any other country [7].
Viruses mutate to varying degrees depending on the nature of their genome and the proofreading activity (or lack thereof) of associated polymerases [8]. CoV2 is no exception to this, and within a year of its emergence multiple lineage variants of concern (VOCs) appeared in numerous countries. B.1.1.7 (now called Alpha) and B.1.351 (now called Beta) were the first VOCs to be identified in September 2020 (Alpha, in United Kingdom) and October 2020 (Beta, in South Africa), and contained numerous missense mutations affecting the Spike protein [9,10]. The Spike protein is essential for CoV2 infection of target cells and contains a receptor-binding domain (RBD) which recognizes and binds the host receptor angiotensin-converting enzyme 2 (ACE2) [11]. The Alpha and Beta lineage RBD mutations lead to tighter Spike:ACE2 structural interactions [12] and increased the transmissibility of CoV2 [13,14]. In January 2021, the P.1. (now called Gamma) lineage was reported in Brazil to contain even more missense mutations in more genes, including Spike [15]. As with Alpha, the mutations inherent to the Gamma lineage increased its transmissibility [15]. Two additional lineages emerged in March 2021 and November 2021, respectively, and in time would supplant all prior lineages in the speed with which they spread: the Delta lineage, which was first reported in India [16], and the Omicron lineage, reported in southern Africa [17]. CoV2 continues to evolve, and deaths due to COVID continue to cause overall declines in life expectancy for many countries, including the US [18,19].
After previous coronavirus disease outbreaks, such as those caused by SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), animal models and other experimental systems demonstrated that coronavirus-specific antibodies are generated soon after infection [20,21], and can block viral entry by interfering with the Spike:ACE2 interaction [22–27]. In the upper respiratory tract and oral cavity, antibodies are generated by B cells in mucosa-associated lymphoid tissue and regional draining lymph nodes, typically within several days of antigen encounter, and comprise several isotypes (IgM, IgA and IgG) which differ in their secretion kinetics and effector mechanisms [28,29]. IgM is often the first isotype to appear following antigen exposure, and eliminates viruses by precipitating the membrane attack complex on virus-infected cells (i.e. the classical complement pathway) [29]. In the context of CoV2 infection, however, IgA dominates the early neutralizing antibody response at mucosal sites [30]. IgA, a weak inducer of the complement pathway, protects mucosal sites by blocking and sterically hindering antigen interaction with the epithelial surface, trapping it in mucus which is eventually cleared via peristalsis [29]. IgG is often the last isotype to appear following antigen exposure, mostly appears in the oral cavity due to passive leakage from the blood circulation via gingival crevicular epithelium, and is the most versatile in terms of effector mechanisms and durability [29].
The fact that coronavirus-specific Ig is secreted following natural infection, long-lived, and able to disrupt Spike:ACE2 interactions are the foundations on which multiple monitoring, therapeutic, and vaccine strategies against CoV2 have been built. Prior to mass PCR testing, CoV2-reactive Ig in sera was the only biomarker for monitoring CoV2 prevalence at a population level [31]. The discovery that plasma of COVID-convalescent individuals contains polyclonal Ig with CoV2-neutralizing activity [32] paved the way for multiple clinical trials testing the efficacy of convalescent plasma therapy against COVID [33]. In the US, the first COVID vaccines available comprised either a two-dose encapsulated mRNA formulation (BNT162b2 or mRNA-1273) or a single-dose adenovirus vector formulation (Ad26.COV2.S). The US Food & Drug Administration (FDA) granted emergency use authorizations (EUA) for BNT162b2 and mRNA-1273 on December 11 2020 and December 18 2020, respectively [34,35]; the FDA EUA for Ad26.COV2.S was granted on February 27 2021 [36]. The advent of these and other COVID vaccines led to dramatic declines in COVID morbidity and mortality [37], and—relative to vaccinated individuals—unvaccinated individuals are more likely to need hospitalization or die following CoV2 infection [38].
Since interrupting the Spike:ACE2 interaction was the goal of now-approved vaccines [39, 40], and remains a goal of potential COVID therapies [41,42], the continual emergence of new CoV2 lineages with numerous and diverse Spike mutations threatens our ability to prevent and treat future CoV2 infections. It is therefore important to understand the relationships between CoV2 lineage emergence, CoV2-specific Ig levels—as elicited by either natural infection or vaccination—and their neutralization capacity. This is especially true of asymptomatic individuals who are PCR positive (PCRPOS), as they are estimated to account for 50–65% of all transmission [43,44]. Here, we describe the relationships between COVID incidence, CoV2 lineage, viral load, CoV2-specific Ig responses (IgM, IgA, & IgG), and inhibitory capacity in the saliva of asymptomatic PCRPOS individuals, as the oral cavity and saliva—in addition to being readily accessible—are important sites of CoV2 infection and transmission [45] (especially newer Omicron VOCs [46–50]). CoV2-specific Ig responses were similarly assessed in PCRNEG individuals with a history of CoV2 infection and/or COVID vaccination with pre-Omicron vaccines. These data were generated as part of a large university COVID monitoring program which occurred between August 2020 and June 2022.
Methods
Ethics statement
This work was reviewed and approved by The Ohio State University Biomedical Sciences Institutional Review Board (IRB, ID #2021H0080). This work was also reviewed and approved by the Ohio State Institutional Biosafety Committee (IBC) (ID #2020R00000046). Each participant provided formal, electronic consent to the following HIPAA AUTHORIZATION TO DISCLOSE PROTECTED HEALTH INFORMATION statement: ““I voluntarily authorize OSUWMC to use and/or disclose my COVID-19 test results to The Ohio State University as part of the ongoing surveillance testing related to COVID-19 community spread. I understand that my COVID-19 test results are considered Protected Health Information (PHI) and no payment will be exchanged for disclosure of my test results. I further understand that I have the right to revoke this authorization, in writing, by sending written notification to: Office of Compliance and Integrity-Privacy, 650 Ackerman Road, Columbus, Ohio 43202. I understand that PHI used or disclosed pursuant to this authorization may be redisclosed by the recipient and its confidentiality may no longer be protected by federal or state law. I consent to the use of electronic signature and understand that my documenting consent below, I have affirmatively executed this authorization.” Per our IRB-approved Waiver of Consent Process, we did not seek additional consent beyond that which participants had already agreed (i.e. the above HIPAA AUTHORIZATION TO DISCLOSE PROTECTED HEALTH INFORMATION statement) for the following reasons: (1) our study used leftover human specimens that were not individually identifiable; (2) the use of each sample posed no additional risk to the original donor than that to which they are already aware (i.e. the potential loss of privacy), and the intent of our study also related to surveillance of COVID community spread, to which donors have already consented per the statement above.
Saliva specimen collection and handling
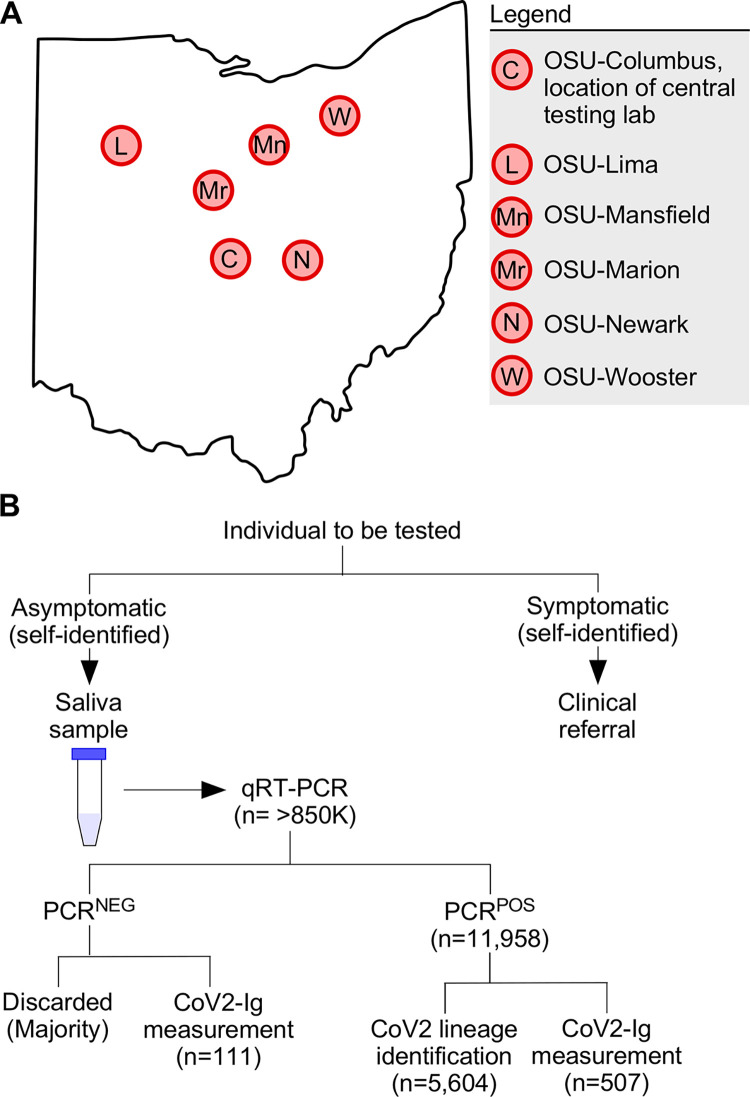
The Ohio State COVID monitoring program was active from August 2020 through June 2022. As part of this program, saliva specimens were collected on a weekly basis from students, staff, and faculty who self-reported as being asymptomatic at the time of specimen collection. At the time of specimen collection each participant provided formal, electronic consent per our Ethics Statement. On and prior to the day of saliva collection at one of several mass testing sites (Fig 1A), individuals were instructed to define themselves symptomatic if they had at least one or more of the following: fever, chills, shortness of breath, difficulty breathing, fatigue, muscle aches, body aches, headache, new loss of taste, new loss of smell, sore throat, congestion, runny nose, nausea, vomiting, or diarrhea. To prevent contagion, symptomatic individuals were instructed not to come to the mass testing site and were instead referred to a healthcare provider for follow-up (e.g. the campus student health clinic). Individuals were defined as asymptomatic if they had none of the symptomatic conditions listed above. On the day of testing, individuals were instructed to refrain from food or drink for 30 minutes prior to collection, and to gently eject saliva into the collection tube, swallowing first and keeping saliva free from mucus, until the 1 mL mark on a sterile conical was reached (i.e. passive drool method). Specimens from asymptomatic individuals were collected at each of the six Ohio State campuses in Franklin county (OSU-Columbus), Licking county (OSU-Newark), Richland county (OSU-Mansfield), Allen county (OSU-Lima), Marion county (OSU-Marion) and Wayne county (OSU-Wooster). Specimens were then couriered to the CLIA-approved Applied Microbiology Services Lab (AMSL) of the Ohio State Infectious Disease Institute (IDI) and analyzed in accordance with the SalivaDirect assay, a clinical diagnostic test that is Emergency Use Authorization (EUA) approved by the US Food & Drug Administration (FDA) for SARS-COV-2 detection [51]. While performing the SalivaDirect real time polymerase chain reaction (PCR), saliva samples were stored in a 4°C cold room until they were deemed either PCR negative (PCRNEG) or PCR positive (PCRPOS) for CoV2. Per the SalivaDirect method [52], any sample with a CT value ≤ 40 was considered PCRPOS for CoV2. The positive or negative status of the sample was reported to the individual and regional public health authorities (Columbus Public Health, Ohio Department of Health, ODH) per state and federal policies at the time. PCRPOS saliva samples and select PCRNEG saliva samples were then removed from the 4°C cold room, aliquoted into microcentrifuge tubes, frozen (-20°C), and analyzed for viral genome sequencing and lineage identification, as well as host antibody response characterization.
Fig 1. Overview of our university COVID monitoring program and workflow.
(A) Map of Ohio with locations of the six university campuses which participated in the COVID monitoring program. Original map source: Wikimedia Commons [111]. (B) On and prior to the day of testing, each individual assessed themselves for one or more COVID symptoms (see Methods). If symptomatic, the individual was given a clinical referral and instructed to not go to their on-campus testing facility, to prevent contagion. If asymptomatic, the individual provided a saliva sample which was tested (typically within 24 hours of sample provision) via qRT-PCR for the presence of the CoV2 N gene. Individuals were notified as soon as possible as to whether their sample was negative (PCRNEG) or positive (PCRPOS) for the virus, a positive result being a CT ≤ 40. PCRPOS samples were subsequently aliquoted and used for both CoV2 lineage identification and measuring the concentrations of immunoglobulin against specific CoV2 antigens (CoV2-Ig). The vast majority of PCRNEG samples were discarded; however, a minority were retained and used for CoV2-Ig measurements. PCRPOS and PCRNEG samples were otherwise treated identically.
Sequencing and lineage identification
PCRPOS saliva samples with a CT ≤ 33 had their whole CoV2 viral genome sequenced and lineage assigned per the methods described in our previous work [53] (samples with a CT> 33 had insufficient viral RNA for sequencing). There was only one exception to this in September 2021, when a single sample with a CT of >35 was sequenced. CoV2 genome copy numbers were calculated via linear regression analysis, by comparison to the CT values of SalivaDirect reference standards. CoV2 genome sequences were submitted to the Global Initiative on Sharing Avian Influenza Data (GISAID) database in a manner consistent with ODH expectations and policies at that time, in as close to real time as possible. The abbreviations we use for each lineage in this study and associated figures are as follows: CoV2Anc, the ancestral lineage of CoV2 which emerged from Wuhan, China; CoV2US, the B.1.2 lineage which was among the first detected in our region of the US [53–55]; CoV2Alpha, the B.1.1.7 lineage or Alpha VOC which was first reported by the UK in December 2020 [9]; CoV2Beta, the B.1.351 lineage or Beta VOC which was first reported in South Africa in December 2020 [10]; CoV2Gamma, the P.1 lineage or Gamma VOC which was first reported in Brazil in January 2021 [15]; CoV2Delta, the B.1.617.2 lineage or Delta VOC which was first reported in India in December 2020 [16]; CoV2Omicron, the B.1.1.529 lineage or Omicron VOC which was first reported in South Africa in Nov 2021 [17]; CoV2O-BA.1, the BA.1 subvariant of CoV2Omicron; CoV2O-BA.2, the BA.2 subvariant of CoV2Omicron; CoV2O-BA.4, the BA.4 subvariant of CoV2Omicron; CoV2O-BA.5, the BA.5 subvariant of CoV2Omicron. The nonsynonymous Spike mutations which distinguish these lineages are depicted in supplemental S1 Fig. Any lineage which was not a VOC or otherwise not mentioned above (e.g. Epsilon) is labeled “Non-VOC.”
COVID wave designations and comparisons
We defined a COVID wave within our university community as when new PCRPOS case counts rose above the overall period median for ≥ 3 weeks in a row (the overall period being January 2021 through June 2022). For comparisons to COVID incidence in surrounding counties, we accessed publicly available ODH data via their public-facing dashboard (accessed November 14 2022).
Measuring binding antibody levels in saliva
After PCR results were reported (typically within 24 hours of specimen collection), PCRPOS and select PCRNEG specimens were removed from the 4°C cold room, aliquoted into microcentrifuge tubes containing Triton X-100 to inactivate CoV2 (final concentration: 1% Triton X-100) [56]. PCRNEG samples were selected based on the donors’ having had either a prior CoV2 infection (allowing us to measure durability of the antibody response following natural infection) or their having been vaccinated against COVID (allowing us to compare the antibody responses of uninfected vaccinated individuals to those of infected vaccinated individuals, a.k.a. breakthrough infections). All samples were treated identically regardless of whether they were PCRPOS or PCRNEG. Following the addition of Triton X-100, samples were vortexed and allowed to incubate for 1 hour at room temperature [56]. Samples were subsequently stored at -80°C until the antibody levels in all samples could be measured at the same time, thus eliminating batch effects. Meso Scale Diagnostics (MSD) V-Plex platform assays Panel 1 (#K15375U), Panel 5 (#K15383U, #K14384U, #K15385U), Panel 6 (#K15433U) and Panel 13 (#K15463U, #K15464U, #K15465U) were used to measure the concentration of CoV2 antigen specific immunoglobulin (IgM, IgA and/or IgG) in PCRPOS and PCRNEG samples. Briefly, the MSD V-Plex assay comprises a 96-well plate which, within each well, contains multiple spots that are coated with defined antigens. For our study, these antigens included recombinant forms of three CoV2Anc lineage proteins (Nucleocapsid [N], Spike, and the Spike Receptor Binding Domain [RBD]), as well as CoV2Alpha Spike, CoV2Beta Spike, CoV2Gamma Spike, and CoV2Delta Spike (S1 Fig). The Spike antigens consisted of the trimerized form of the ectodomain; the N antigen consisted of the full-length protein. Antibodies in the sample bind to the antigens, and reporter-conjugated secondary antibodies were used for detection. Saliva samples were thawed on ice and diluted by a factor of 10 in the diluent provided in the V-Plex assay kit for each assay. The V-Plex assays were performed according to manufacturer instructions, and plates were read on an MSD instrument which measures light emitted from reporter-conjugated secondary antibodies. Using MSD’s analysis software, the light signal measured by the MSD instrument was converted into arbitrary units (AU) representing amount of antibody present relative to the standard curve of the assay. The AU values for IgM, IgA, and IgG binding to CoV2Anc N, Spike, and Spike RBD were transformed to WHO binding antibody units (BAU) via validated WHO standards and conversion factors provided by MSD. The AU values for IgM, IgA, and IgG binding to other forms of N or Spike (i.e., those of VOC) cannot be converted to WHO BAU, as there are no WHO standards for these recombinant proteins. For this reason, the levels of each Ig isotype which bind to CoV2Alpha, CoV2Beta, CoV2Gamma, and CoV2Delta forms of Spike are expressed as AU.
Spike inhibition assay
The capacity of saliva specimens to inhibit Spike activity was quantified using a commercially available ACE2 displacement assay (MSD COVID-19 ACE2 Neutralization Kit method). Plate-bound Spike was incubated with diluted saliva (the same specimens used for Ig measurements) per manufacturer protocols, followed by washing and addition of a luminescent probe-conjugated, recombinant form of human ACE2. The extent to which luminescence declined relative to non-saliva (i.e., diluent only) treated wells was used to derive a percent inhibition value for each individual sample, using the following formula: % inhibition = 1 –(saliva sample luminescence value / diluent only luminescence value) × 100.
Graphing and statistics
Graphs were generated in RStudio or GraphPad. All statistical tests were performed in RStudio. Data was tested for normality using the Kolmogorov-Smirnov test and for equal variance using the Bartlett test of homogeneity of variances. For data that did not have normal distribution, the Kruskal-Wallis rank sum test was used to determine if there were significant differences between groups in unpaired datasets, and the Friedman rank sum test was used in paired datasets. Within those datasets, the significant differences between groups were identified via an unpaired or paired Wilcoxon rank sum test as appropriate with Benjamini-Hochberg p value adjustment method. For the neutralization data which contained several zero values, the Shapiro-Wilk normality test was used, followed by the Bartlett test of homogeneity of variances. The Kruskal-Wallis rank sum test was used to determine if significant differences were present, followed by Dunn’s test with Benjamini-Hochberg p value adjustment method to identify which groups were significantly different. Differences between groups were considered significant if P < 0.05 and are graphically indicated by 1 or more asterisks (*P < 0.05; **P < 0.005; ***P < 0.0005).
Abbreviations
The following abbreviations are used throughout our manuscript: PCRPOS, an individual or saliva specimen that was PCR positive for CoV2 (CT value ≤ 40); PCRNEG, an individual or saliva specimen that was PCR negative for CoV2; Spike and N, unless otherwise stated the Spike and N proteins of CoV2 (not any other coronavirus); CoV2-Ig, immunoglobulin of any isotype that recognizes any CoV2 antigen; IgMSpike, IgM that recognizes Spike; IgASpike, IgA that recognizes Spike; IgGSpike, IgG that recognizes Spike; IgGRBD, IgG that recognizes the Spike Receptor Binding Domain; IgGN, IgG that recognizes the N protein; VaxPOS, an individual who was fully vaccinated against COVID prior to saliva specimen collection; VaxNEG, an individual who was not fully vaccinated against COVID prior to saliva specimen collection; NewPOS, an individual who at the time of saliva collection was PCRPOS for the first time; PriorPOS, an individual who at the time of saliva collection was PCRNEG but who had a prior CoV2 infection (i.e. the individual had been PCRPOS 2–37 weeks prior).
Vaccination status
For the purposes of our study, an individual was defined as “VaxPOS” if they had been fully vaccinated against COVID prior to the date of saliva specimen collection, with either of the following vaccines: BNT162b2 (both doses), mRNA-1273 (both doses), Ad26.COV2.2. Among the VaxPOS individuals in our study, the aggregate representations of each vaccine are as follows: ~75% were vaccinated with BNT162b2, ~17% with mRNA-1273, and ~8% with Ad26.COV2.2. An individual was defined as “VaxNEG” if they were not fully vaccinated against COVID prior to saliva specimen collection. This includes individuals who had only received one dose of either BNT162b2 or mRNA-1273, without receiving the second dose. All samples were identically treated regardless of whether they came from someone who was VaxPOS or VaxNEG.
Results
I. Study overview
The first confirmed cases of COVID in the state of Ohio were reported on March 9 2020 [57]. The Ohio State University suspended on campus activities the same day [58] and subsequently developed a campus wide plan to monitor the incidence of CoV2 infection among its students, staff and faculty [59]. Individuals participating in this monitoring program, which formally began in August 2020, provided saliva on a weekly basis for COVID testing. Prior to testing, individuals who self-reported as being symptomatic were not tested and were instead given a clinical referral (see Methods for additional details). Individuals who self-reported as being asymptomatic provided a saliva specimen via a passive drool method at each of our six university campuses (Fig 1A). Specimens were assessed by our CLIA-certified lab for the presence of CoV2 using real-time quantitative reverse transcription PCR (qRT-PCR). Specimens were not pooled prior to testing. qRT-PCR results were reported to the individual and the regional public health authority per state and federal policies at the time. If a specimen had a CT value ≤40 it was considered positive for CoV2 virus (PCRPOS). Per our Institutional Review Board (IRB) approved protocol and workflow (Fig 1B), PCRPOS saliva samples were subsequently used for CoV2 lineage identification and CoV2-specific immunoglobulin (CoV2-Ig) measurements. In some instances, select saliva samples that were negative for CoV2 virus (PCRNEG) were also collected, the reasons for which will be made clear in sections below. The relationships between these molecular and immunological readouts to one another, as well as to coded data concerning the prior infection status and vaccination status of the saliva donor, are described below for the period spanning January 2021 (before COVID vaccines were widely available to students in our university community) to June 2022, when the monitoring program ended. See Methods for details regarding saliva collection, symptomatic versus asymptomatic designation, qRT-PCR, CoV2 lineage identification, CoV2-Ig measurements, and statistical analyses. In total, >850,000 diagnostic PCR tests were performed by our lab during this monitoring program.
II. The incidence of CoV2 positivity in our university community occurred in waves which reflected those occurring in surrounding regions
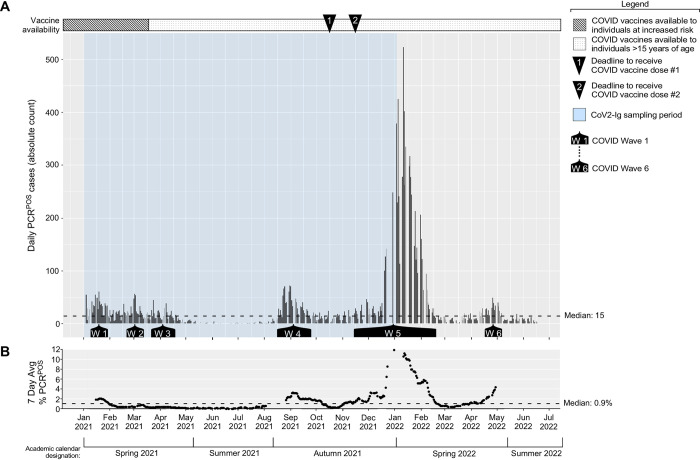
The incidence of new PCRPOS cases among asymptomatic individuals in our university community, for the period spanning January 2021 to June 2022, is shown in Fig 2A along with the seven day average PCR positivity rate (Fig 2B). COVID monitoring occurred before January 2021; however, because the bulk of PCR testing at that time was contracted to a commercial entity, our access to the raw PCR data before January 2021 is limited. Above these data are two timelines relevant to data interpretation, indicating when Ohio COVID vaccination policies shifted from prioritizing at risk populations (e.g. elderly) to anyone ≥16 years of ages well as the deadlines for all our community members (i.e. university students, faculty, and staff) to have received their first and second COVID doses (October 15 2021 and November 15 2021, respectively) [60]. Indicated below the data are corresponding intervals in the academic calendar, which will be referred to in subsequent sections. We identified 11,958 PCRPOS individuals between January 2021 to June 2022; the median, mean and maximum new PCRPOS cases per test day were 15, 34 and 523, respectively. There were, however, six time periods when the new case counts rose above the overall period median for ≥ 3 weeks in a row. These six time periods are hereafter referred to as Waves 1–6 and spanned the following dates: Wave 1, January 11 2021 to January 29 2021; Wave 2, February 22 2021 to March 12 2021; Wave 3, March 22 2021 to April 16 2021; Wave 4, August 16 2021 to September 24 2021; Wave 5, November 15 2021 to February 18 2022; Wave 6, April 18 2022 to May 6 2022. The waves of COVID incidence amongst asymptomatic individuals in our university community mirrored (rather than preceded) the waves of COVID incidence in the counties surrounding each university campus [61] (S2 Fig).
Fig 2. The incidence of PCR positivity among asymptomatic members of our university community.
Saliva samples from asymptomatic individuals were collected on a daily basis and tested by qRT-PCR for the presence of the CoV2 N gene. Shown are (A) the number of PCRPOS saliva samples identified each day during the period spanning January 2021 to May 2022, with each bar representing a single day, as well as (B) the corresponding seven day average PCR positivity rate. Above the graph is a timeline depicting when COVID vaccine availability shifted in Ohio (i.e. when the national vaccination priority expanded from vulnerable populations to encompass anyone >15 years of age), as well as indications of the deadlines by which all university community members were required to have received their first and second vaccine dose of either the BNT162b2 or mRNA-1273 vaccines. Below the graph are indications of the periods we refer to as Waves 1–6, a wave being defined as when the daily PCRPOS case count exceeded the period median (15) for ≥3 weeks, as well Blue shading indicates when the samples we used for CoV2 Ig measurements were collected.
III. Prior to community vaccine requirements being established, CoV2 was becoming progressively more concentrated in the saliva of asymptomatic individuals
The emergence of CoV2 VOCs in multiple Ohio communities [62–68] with potential for greater infectivity and/or transmissibility led us to assess the relationship between CoV2 abundance in saliva and VOC identity. We used the qRT-PCR cycle threshold (CT) value as a readout of CoV2 abundance, as the SalivaDirect CT value is inversely proportional to CoV2 viral load (i.e. a lower CT value corresponds to higher CoV2 RNA levels in the tested sample) [52], and a commonly used as a proxy for probability of transmission (i.e. a lower CT value correspond to higher transmission probability) [69–73]. VOC identity was determined by next generation sequencing of the entire CoV2 genome and subsequent alignment with Global Initiative on Sharing Avian Influenza Data (GISAID) reference sequences. During the entire monitoring period, CoV2 genome sequences were submitted to the GISAID database in a manner consistent with ODH expectations and policies at that time, in as close to real time as possible.
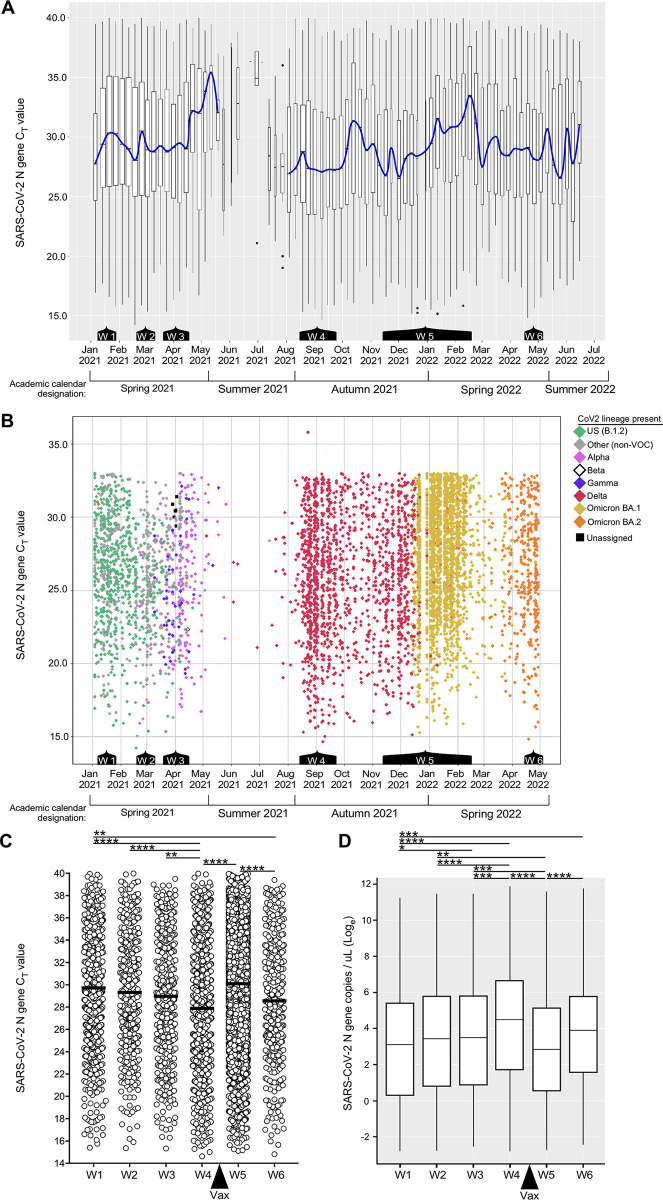
The weekly composite CT values of all PCRPOS samples, and daily individual CT values of sequenced samples are shown in Fig 3A and 3B, respectively, with color annotations in Fig 3B indicating the lineage identity. The same data are also presented as wave composites (Fig 3C) in order to best illustrate the following trends: During Wave 1 (Week 2 of January 2021 to Week 4 of January 2021), the mean CT value of all PCRPOS saliva samples was 29.8 (Fig 3C). During Wave 2 (Week 4 of February 2021 to Week 2 of March 2021) the mean CT value was 29.3 (Fig 3C). The mean CT value lowered to 29.0 during Wave 3 (Week 4 of March 2021 to Week 2 of April 2021) (Fig 3C). The number of tests performed fell precipitously during June 2021 and July 2021, as the campus population is minimal during the summer months; therefore, we are reluctant to draw conclusions from or otherwise compare Summer 2021 CT value data to the prior semester, when testing volume was higher. Upon resumption of high-volume testing during the later weeks of August 2021, which marked the beginning of the Autumn 2021 semester and Wave 4 (Week 3 of August 2021 to Week 3 of September 2021), we noted the lowest mean CT value of all waves (27.9) (Fig 3C). The extrapolated CoV2 genome copy concentrations (Fig 3D) are consistent with Wave 4 saliva samples having the highest virus concentrations of all waves. Wave 5 was the longest wave (Week 3 of November 2021 to Week 2 of February 2022) with daily PCRPOS cases reaching a maximum of 523 on January 11, 2022. The mean CT value of Wave 5, which followed our community deadline for vaccine requirements, was 30.1 and significantly higher than that of Wave 4 (Fig 3C). The last wave before the COVID monitoring program ended, Wave 6 (Week 2 of April 2022 to Week 1 of May 2022), had a lower mean CT value (28.6) than Wave 5 (Fig 3C). The lowest CT value we ever observed was on February 18 2021 (CT = 14.2).
Fig 3. Saliva CoV2 viral loads among asymptomatic members of our university community.
(A) Box plot representation of all the CT values of all the PCRPOS saliva samples during each week of the period spanning January 2021 to June 2022 (n = 11,958). The blue line passes through the median CT value of each week. Below the graph are indications of the periods corresponding to Waves 1–6 of the prior figure. (B) Scatter plot representation of the same CT value data as in (A) above, the exceptions being daily data are shown (as opposed to weekly composites) and samples with a CT >33 are omitted (with one exception in September 2021, these could not be sequenced due to insufficient amounts of genetic material). Each diamond represents an individual sample (n = 5604); the color of each diamond indicates the CoV2 lineage present (Green, CoV2US; Pink, CoV2Alpha; White, CoV2Beta; Blue, CoV2Gamma; Red, CoV2Delta; Gold, CoV2O-BA.1; Orange, CoV2O-BA.2). Gray diamonds indicate samples whose lineage was not a VOC. Black squares indicate a sequence that did not align to known lineages and thus could not be assigned. Note that CoV2Beta only appeared once in our university community, on April 15 2021. (C) The CT value and (D) calculated CoV2 genome copy concentration in of each positive sample during Wave 1 (n = 638), Wave 2 (n = 442), Wave 3 (n = 453), Wave 4 (n = 1041), Wave 5 (n = 7129), and Wave 6 (n = 422). In (C), the mean of each Wave is indicated by a line. The “Vax” arrow indicates when community vaccine requirements went into effect (after Wave 4, before Wave 5). Asterisks indicate those inter-wave differences that were statistically significant, as determined by one way ANOVA (* p≤ 0.05, ** p≤ 0.005, *** p≤ 0.0005, *** p≤ 0.00005).
IV. Each wave of CoV2 positivity corresponded to the emergence of a new CoV2 lineage within our community
The CoV2 lineages present in each individual PCRPOS sample during the same time periods as above are shown in Fig 3B, exceptions being samples with a CT value of >33 as these could not be sequenced due to the viral RNA levels being too low. Males were more likely to meet sequencing criteria (i.e. a CT ≤ 33) than females during Waves 1–3 (S3A Fig); this was not true of later Waves, however, and female representation was higher during the entire monitoring period overall (S3A Fig). Among sequenced samples, the median and mean ages of individuals were 21 and 23, respectively, and varied minimally during the monitoring period (S3B Fig). During the period spanning January 2021 to mid-February 2021, the predominant lineage was B.1.2, which we hereafter refer to as CoV2US since it was among the first detected in our region of the US [53–55]. The period of CoV2US lineage predominance corresponds to Wave 1 in our community (Figs 2 and 3B). Beginning mid-February 2021 and extending to mid-March 2021 was a period of time when an array of lineages which we collectively refer to as “non-VOC” were predominant, as they were more diverse compared to earlier and later testing periods and were never considered to be VOCs. Although the CoV2US lineage was still being detected, Wave 2 primarily comprised of non-VOCs (Fig 3B). As the Ides of March approached in 2021, so too did two VOCs begin appearing with increasing frequency: the Alpha VOC (CoV2Alpha) and Gamma VOCs (CoV2Gamma). CoV2Alpha and CoV2Gamma were widely considered at that time to be more transmissible than previous lineages [15,74]. CoV2Alpha and CoV2Gamma were the primary lineages detected during Wave 3 (Fig 3B), and continued to predominate among the few positive samples collected during May 2021. The Beta VOC (CoV2Beta) only appeared once in our university community (April 15 2021). Beginning June 2021 and continuing through December 2021 the new Delta VOC (CoV2Delta) made up the vast majority of PCRPOS saliva samples (Fig 3B). CoV2Delta is more transmissible than CoV2Alpha and CoV2Gamma [75], and the period in which CoV2Delta predominated coincided with COVID Wave 4 in our community (Fig 2). Wave 5, the penultimate and largest COVID wave, coincided with the emergence and dominance of the Omicron VOC (CoV2Omicron) subvariant, BA.1 (CoV2O-BA.1). Wave 6, final wave before our COVID monitoring program ended, was dominated by the CoV2Omicron subvariant BA.2 (CoV2O-BA.2) (Fig 3B). When considered alongside the CT values and CoV2 genome copy numbers that characterized each wave (Fig 3C and 3D), the above data demonstrate that the shift from CoV2US to CoV2Alpha/CoV2Gamma to CoV2Delta coincided with the virus becoming progressively more concentrated in the saliva of asymptomatic individuals, this trend ending after community vaccine requirements were established.
V. Among pre-Omicron lineages, CoV2Delta elicited the highest levels of Spike-specific IgA and IgG in unvaccinated, asymptomatic individuals
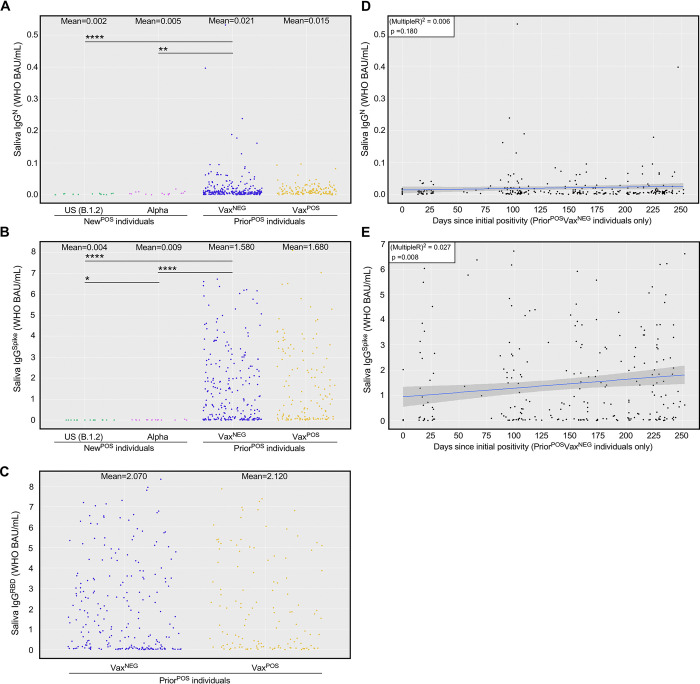
To assess whether CoV2-specific Ig was detectable in the saliva of asymptomatic CoV2 PCRPOS individuals, as well as whether levels of the same Ig varied depending on the CoV2 lineage present, we used the same samples described above (i.e. those used for lineage identification) to measure saliva levels of CoV2 Spike-specific IgM (IgMSpike), CoV2 Spike-specific IgA (IgASpike), and CoV2 Spike-specific IgG (IgGSpike) (Fig 4). Individuals vaccinated against COVID were excluded from this analysis (the vaccination record of each person in our university community was closely monitored during this time period), and saliva samples from individuals infected with CoV2Anc, CoV2Alpha and CoV2Gamma were collected prior to COVID vaccines being widely available in our community; therefore, no vaccine-elicited antibody responses would be expected in these samples. Among individuals infected with CoV2Delta, only unvaccinated individuals were included in the Fig 4 analysis. To eliminate viral load as a confounding variable, only PCRPOS saliva samples with similar CT range were used for Ig comparisons (CT range = 22–26). PCRNEG saliva collected in early 2020 from healthy individuals living in the US and no COVID history were used to estimate “pre-pandemic” levels of IgMSpike, IgASpike, and IgGSpike binding.
Fig 4. Spike-specific Ig levels in the saliva of newly positive, asymptomatic individuals at the time of PCR testing.
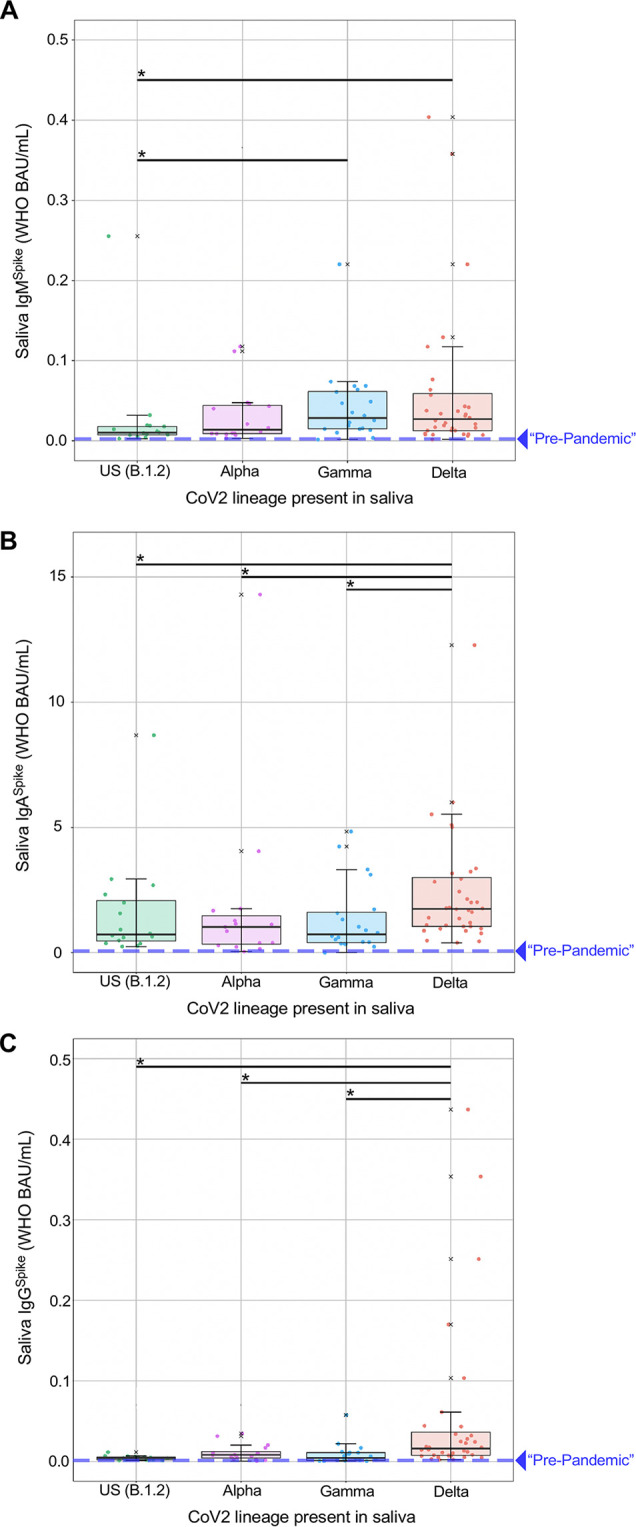
Saliva samples from individuals who were newly positive (NewPOS, PCR positive for the first time ever) for either the CoV2US (n = 16), CoV2Alpha (n = 15), CoV2Gamma (n = 21), or CoV2Delta (n = 36) lineage were used to measure the concentrations of (A) IgMSpike, (B) IgASpike and (C) IgGSpike. The CoV2Anc Spike was used as the capture antigen in each case, and concentrations are expressed as World Health Organization (WHO) binding antibody units (BAU) per mL. PCRNEG saliva collected in early 2020, from healthy individuals living in the US with no COVID history, was tested in the same manner used to estimate “pre-pandemic” levels of IgMSpike, IgASpike and IgGSpike binding, which are represented by the dashed lines on each graph. X, values that were considered outliers but are nevertheless shown for completeness and are included in all statistical group comparisons. * p≤ 0.05, as determined by unpaired Wilcoxon tests with Benjamini-Hochberg adjustment. The dilution adjusted lower limit of quantification for each isotype were as follows (LLOQ values in WHO BAU/mL): IgMSpike, 0.026691; IgASpike, 0.38378; IgGSpike, 0.044149.
Saliva IgMSpike (Fig 4A), IgASpike (Fig 4B), and IgGSpike (Fig 4C) data are shown relative to which CoV2 lineage was detected in the same saliva donor (CoV2US, CoV2Alpha, CoV2Gamma, or CoV2Delta) and are expressed as WHO binding antibody units, or BAUs. As shown in Fig 4A–4C, respectively, nearly all PCRPOS individuals had saliva IgMSpike, IgASpike, and IgGSpike levels that were above “pre-pandemic” levels, regardless of whether they were infected with CoV2US, CoV2Alpha, CoV2Gamma, or CoV2Delta. There were, however, three noteworthy differences between PCRPOS individuals depending on the lineage present. First, whereas individuals infected with CoV2US and CoV2Alpha had similar IgMSpike levels, those infected with CoV2Gamma and CoV2Delta had higher IgMSpike levels relative to those infected with CoV2US (Fig 4A). Second, saliva IgASpike levels were similar between individuals infected with CoV2US, CoV2Alpha and CoV2Gamma; CoV2Delta infected individuals, on the other hand, had significantly higher IgASpike levels compared to those infected with CoV2US, CoV2Alpha, or CoV2Gamma (Fig 4B). Third and analogous to IgASpike differences (Fig 4B), CoV2Delta-infected individuals had significantly higher IgGSpike levels compared to those infected with CoV2US, CoV2Alpha, or CoV2Gamma (Fig 4C). For IgMSpike, IgASpike, and IgGSpike measurements, the recombinant Spike antigen used for Ig detection was identical to that of CoV2Anc, as this enabled data transformation to WHO BAU (see Methods); the same patterns were observed, however, when the same saliva samples were tested against recombinant CoV2Alpha, CoV2Beta, and CoV2Gamma Spike antigens (S4 Fig).
VI. Following infection of unvaccinated individuals, IgGSpike and IgGRBD persisted at higher levels in saliva than IgGN
To determine the extent to which CoV2-specific IgG in saliva was sustained over time, we performed the analysis shown in Fig 5 wherein saliva IgGSpike levels, as well as Nucleocapsid (N)-specific IgG (IgGN) levels, were compared across two groups of individuals: “NewPOS” individuals who, at the time of saliva collection, were positive for either CoV2US or CoV2Alpha; “PriorPOS” individuals who were uninfected at the time of saliva collection, but had been PCRPOS 14–252 days earlier. In this instance, saliva samples from PriorPOS individuals were collected in May 2021. Most individuals in our PriorPOS cohort were infected during the Autumn 2020 semester, before COVID vaccines were available; however, since a portion of these individuals did go on to receive the COVID vaccine prior to May 2021, we subdivided the data from PriorPOS individuals into those who did not receive the vaccine (VaxNEG) prior to May 2021, and those who did receive the vaccine (VaxPOS) prior to May 2021.
Fig 5. Nucleocapsid- and Spike-specific IgG levels in saliva of newly positive, asymptomatic individuals versus prior positive, asymptomatic individuals.
Saliva from NewPOS individuals infected with either CoV2US (n = 16) or CoV2Alpha (n = 15), as well as PCRNEG saliva from individuals who had been infected 2–37 weeks prior (PriorPOS, n = 402) with either CoV2US, CoV2Alpha, or a non-VOC, were used to measure the concentrations of (A) Nucleocapsid-specific IgG, (B) Spike- specific IgG, and (C) Spike RBD-specific IgG. Data from PriorPOS individuals are subdivided based on whether the individual remained unvaccinated up until the day of saliva collection (VaxNEG, n = 257) or was vaccinated prior to the day of saliva collection (VaxPOS, n = 145). * p≤ 0.05, as determined by unpaired Wilcoxon test with Benjamini-Hochberg adjustment. (D-E) For those individuals who were PriorPOSVaxNEG, the relationship between time since original positivity (i.e. the number of days since the individual was first deemed PCRPOS by our program) and their current (D) saliva IgGN level and (E) saliva IgGSpike level at the time of sampling. Graph insets indicates the Multiple R-squared value associated with the linear regression model of the respective data set (i.e. the % variation in either saliva IgGN or IgGSpike that can be explained by the indicated time since positivity), as well as its p-value (i.e. the significance of the linear model as a whole).
Among NewPOS individuals, saliva IgGN levels were similar regardless of whether they were infected with CoV2US or CoV2Alpha (Fig 5A), as were saliva IgGSpike levels (Fig 5B, CoV2Alpha having slightly higher levels than CoV2US). Relative to NewPOS individuals, saliva IgGN levels in PriorPOS individuals were higher (Fig 5A); however, the difference in saliva IgGSpike levels between NewPOS versus PriorPOS individuals was more pronounced (Fig 5B). Among PriorPOS individuals who did not receive a vaccine, saliva IgGN and IgGSpike levels persisted at average concentrations of 0.0212 WHO BAU/mL and 1.58 WHO BAU/mL, respectively for up to 252 days after their initial positivity date (Fig 5D and 5E). Interestingly, mean saliva IgGSpike levels were only slightly higher in PriorPOS individuals who were VaxPOS compared to those who were VaxNEG (Fig 5B), as were the levels of IgGRBD (Fig 5C). These results indicate that although IgGN and IgGSpike both persist in saliva following natural infection, IgGSpike persists at higher levels and reacts against Spike regions that are essential for ACE2 binding (i.e., the RBD).
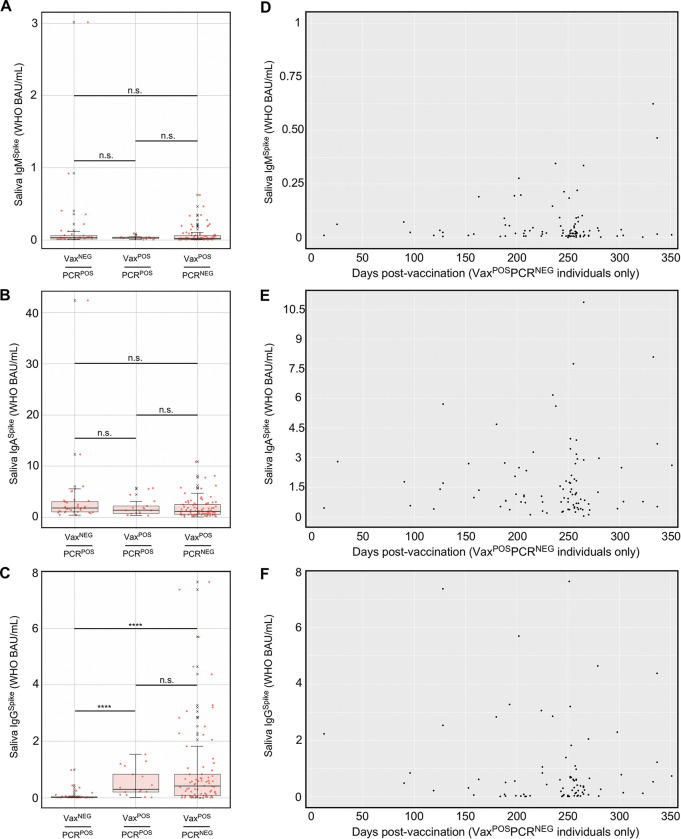
VII. Individuals with breakthrough CoVDelta infections had comparable saliva IgGSpike levels to those of uninfected, vaccinated individuals
During the period of December 2020 to March 2021, COVID vaccination was prioritized and available to the elderly and other individuals at increased risk of severe disease (e.g. healthcare workers, first responders). In Ohio, beginning on March 22 2021, individuals who were 16 years or older could receive a COVID vaccine, including all college students [76]. Despite the widespread availability of vaccines by our Autumn 2021 semester, CoV2Delta lineage infections occurred among unvaccinated (VaxNEG) individuals and vaccinated (VaxPOS) individuals. The term “breakthrough infection” is older than COVID [77] but is now commonly applied to individuals who are PCRPOS despite their being VaxPOS. Since BNT162b2, mRNA-1273 and Ad26.COV2.S were each designed to elicit an Ig response against CoV2 Spike (since it is essential for CoV2 infection of ACE2-expressing cells), we assessed whether breakthrough infections with CoV2Delta were associated with lower levels of Spike-specific Ig in saliva compared to PCR (neg) vaccinees. Shown in Fig 6 are saliva levels of IgMSpike, IgASpike, and IgGSpike in three groups of individuals: VAXNEGPCRPOS individuals infected with CoV2Delta, VAXPOSPCRPOS individuals infected with CoV2Delta, and VAXPOSPCRNEG individuals. Saliva from VAXNEGPCRPOS and VAXPOSPCRPOS individuals was collected during Wave 4 (Fig 2), when community viral burdens were their highest (Fig 3D). Saliva from VAXPOSPCRNEG individuals was collected shortly after Wave 4 had passed; however, the time between vaccination to saliva sample collection for VAXNEGPCRPOS and VAXPOSPCRPOS cohorts were comparable (S5 Fig). These results demonstrate that VAXPOSPCRPOS and VAXPOSPCRNEG groups each had significantly higher saliva IgGSpike levels than VAXNEGPCRPOS individuals (Fig 6C). Furthermore, the saliva IgGSpike levels of VAXPOSPCRPOS and VAXPOSPCRNEG groups did not significantly differ from one another (Fig 6C). Notably, saliva IgMSpike levels were indistinguishable across groups (Fig 6A), as were saliva IgASpike levels (Fig 6B). Among VAXPOSPCRNEG individuals, saliva IgGSpike could be detected up to 352 days post-vaccination (Fig 6D–6F). Similar trends were observed using recombinant CoV2Alpha, CoV2Beta, CoV2Gamma, and CoV2Delta Spike as capture antigens (S6 Fig). We conclude from this that COVID vaccination increased saliva IgGSpike levels in our university community as intended, the saliva IgGSpike levels in all vaccinees being comparable (regardless of whether they had a breakthrough CoVDelta infection) and significantly higher than the saliva IgGSpike levels of unvaccinated, infected individuals.
Fig 6. Spike-specific Ig levels in saliva of CoV2Delta-infected unvaccinated individuals, CoV2Delta-infected vaccinees, and uninfected vaccinees.
During and shortly after COVID Wave 4 (i.e. that which was caused by CoV2Delta), saliva from three groups of individuals were collected and used for Ig measurements: those who had not been fully vaccinated and were positive for the CoV2Delta lineage (VaxNEGPCRPOS, n = 36), those who had been fully vaccinated and were positive for the CoV2Delta lineage (VaxPOSPCRPOS, n = 17), and those who had been fully vaccinated and were negative for any CoV2 lineage (VaxPOSPCRNEG, n = 111). Shown are the (A) IgMSpike, (B) IgASpike, and (C) IgGSpike levels in each individual sample per group. X, values that were considered outliers but are nevertheless shown for completeness and are included in all statistical group comparisons. * p≤ 0.05, as determined by unpaired Wilcoxon tests with Benjamini-Hochberg adjustment. (D-F) For those individuals who were VaxPOSPCRNEG, the relationship between time since being vaccinated (i.e. for those who received an mRNA-based vaccine, the number of days since their second dose) and their current (D) saliva IgMSpike, (E) saliva IgASpike, and (F) saliva IgGSpike level at time of sampling.
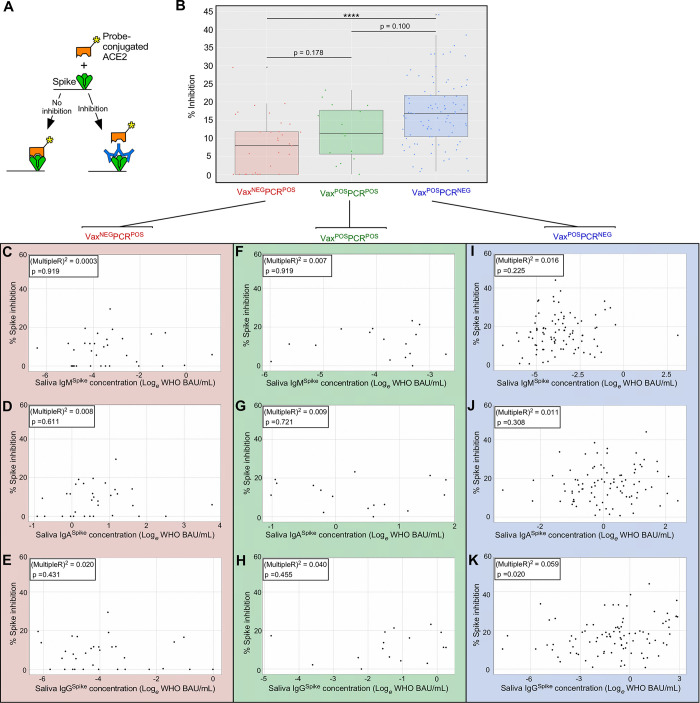
VIII. Despite comparable Spike-specific Ig levels, CoV2Delta-infected vaccinee saliva was less capable of Spike:ACE2 inhibition, relative to uninfected vaccinees
Since the presence of CoV2-specific Ig does not equate to its having neutralization capacity [78], we next compared the ability of VaxNEGPCRPOS, VaxPOSPCRPOS and VaxPOSPCRNEG saliva samples to inhibit Spike:ACE2 interactions. We quantified inhibitory activity using an ACE2 displacement assay (Fig 7A), wherein plate-bound Spike was incubated with the same saliva samples above (i.e., those of Fig 6), followed by washing and addition of a luminescent probe-conjugated, recombinant form of human ACE2. The extent to which luminescence declined relative to non-saliva treated wells was used to derive a percent inhibition value for each individual sample (see Methods for additional details). The results of this analysis are shown in Fig 7B and demonstrate that there were differences between cohorts, the inhibitory activity of VAXPOSPCRNEG saliva being significantly higher than that of VaxNEGPCRPOS saliva (Fig 7B). The inhibitory activity of VaxPOSPCRPOS saliva (median = 12) was 50% higher than that of VaxNEGPCRPOS saliva (median = 8), but 25% lower than that of VaxPOSPCRNEG saliva (median = 16); as a whole, however, the inhibitory activity of VaxPOSPCRPOS saliva did not significantly differ from that of VaxNEGPCRPOS saliva, nor did it significantly differ from VaxPOSPCRNEG saliva (Fig 7B). Within the VaxNEGPCRPOS cohort, there were no significant correlations between these samples’ inhibitory activity and their IgMSpike (Fig 7C), IgASpike (Fig 7D), or IgGSpike concentrations (Fig 7E) within linear regression models. This was also true of the VaxPOSPCRPOS cohort, as no significant correlations were observed between these samples’ inhibitory activity and their IgMSpike (Fig 7F), IgASpike (Fig 7G), or IgGSpike concentrations (Fig 7H). Within the VaxPOSPCRNEG cohort, although the linear regression models were significant between samples’ inhibitory activity and their IgASpike concentration (Fig 7J), as well as their IgGSpike concentration (Fig 7K), but not their IgMSpike concentration (Fig 7I), the Multiple R-squared values were too low to suggest strong correlation. When considered alongside the data shown in Fig 6, we conclude COVID vaccination led to increases in saliva IgGSpike concentrations, the levels being similar between vaccinees who had a breakthrough CoV2Delta infection (VaxPOSPCRPOS) and vaccinees who did not (VaxPOSPCRNEG), but that during Wave 4 the antibodies in VaxPOSPCRPOS saliva were limited in their ability to inhibit Spike, the inhibition values being intermediate between VaxPOSPCRNEG saliva (which had the highest inhibition values) and VaxNEGPCRPOS controls (which had the lowest inhibition values).
Fig 7. Inhibition of Spike function by saliva of CoV2Delta-infected unvaccinated individuals, CoV2Delta-infected vaccinees, and uninfected vaccinees.
(A) Depiction of the probe-conjugated ACE2 displacement assay used to measure saliva samples’ ability to inhibit CoV2 Spike binding to its human receptor, ACE2. The samples in this case were from VAXNEGPCRPOS (n = 33), VAXPOSPCRPOS (n = 37), and VAXPOSPCRNEG individuals (n = 91) (the same samples used for IgMSpike, IgASpike, and IgGSpike measurements in Fig 6 above). (B) The percent inhibition value of each individual sample in each group. Within the (C-E) VAXNEGPCRPOS group, (F-H) VAXPOSPCRPOS group, and (I-K) VAXPOSPCRNEG group, the relationship between an individual samples’ inhibition value and cognate (C,F,H) IgMSpike concentration, (D,G,I) IgMSpike concentration, and (E,H,J) IgGSpike concentration. Graph insets indicates the Multiple R-squared value associated with the linear regression model of the respective data set (i.e. the % variation in inhibition that can be explained by the indicated Ig concentration), as well as its p-value (i.e. the significance of the linear model as a whole).
Discussion
The spread of CoV2 to the US marked the beginning of an extraordinary period wherein a novel respiratory virus transmitted and evolved in a population with no prior immunity, our primary defenses being behavioral changes (e.g., masking and physical distancing) until the advent of effective vaccines. The first COVID case in the US occurred in January 2020 [79]. It was soon discovered that CoV2 caused both symptomatic and asymptomatic infections (the latter being more common in young adults), that asymptomatic individuals could transmit CoV2 [80,81], and that isolation of symptomatic individuals alone would not sufficiently “flatten the curve” of COVID incidence [44,82]. By April 2020, most US universities shut down on-campus activities so as to limit CoV2 transmission among their students, staff, and faculty. Many universities established COVID monitoring programs prior to campus reopening as a means of identifying symptomatic and asymptomatic individuals. These monitoring programs varied in their testing modalities (PCR- or antigen-based), cadence (weekly versus biweekly testing), and sample pooling practices (pooled versus individual testing); all monitoring programs, however, had the same goal in mind: enabling safe resumption of on-campus classes and activities. Now that mass COVID testing programs have ended in US, enabling time for processing and reflection, we are sharing the results of our monitoring program which we believe are most relevant to the ongoing issues of community spread, the longevity of mucosal Ig following natural infection, breakthrough infections, and the utility of saliva for assessing Ig responses to newer Omicron subvariants and booster vaccines.
That the COVID waves in our campus community mirrored those which occurred in surrounding counties, instead of preceding the surrounding county waves, touches on an important question at the time regarding campus reopening: what, if any, contribution would the influx of students have on COVID incidence in surrounding communities. In January 2021, student returns to university campuses were a contentious subject in the US due to the potential risk of contracting the virus and subsequent transmission to surrounding communities. COVID vaccines were not yet widely available to young adults, and—fairly or unfairly—university students were perceived as being more cavalier in their adherence to masking protocols and social distancing. Whether or not the reopening of a given college or university contributed to higher off-campus COVID transmission will depend on several variables (e.g. whether a school was in a state that mandated mask-wearing) [83], but in our case the COVID wave that occurred in our university in January 2021 (Wave 1) peaked during the tail end of one which had been ongoing in surrounding counties (compare Fig 2 and S2 Fig). This was also true in August 2022, when our campus reopened after summer break and experienced Wave 4, which followed the Delta wave that had already begun in surrounding counties. The timing of Wave 1 and Wave 4 in relation to those in surrounding counties is inconsistent with the argument that our university reopening contributed to COVID incidence in the surrounding communities. Studies at other large universities with COVID policies and monitoring programs similar to our own support this conclusion [84–86].
Early in the COVID pandemic, it was unknown whether natural infection would give rise to Ig responses that were durable and protective, as those against common seasonal coronaviruses are short-lived (only 6 months in some cases) [87], or worse still whether the Ig response would actually enhance infection or disease [88–91]. Regarding the durability and protective capacity of the antibody response to natural CoV2 infection, current knowledge on this subject was recently reviewed [78]. In our study of asymptomatic individuals, at the time of initial PCR positivity we could already detect elevations in CoV2-specific Ig (IgM, IgA, and to a lesser extent IgG) in the saliva, the degree to which varied by lineage, CoV2Delta being the most immunogenic of the lineages we assessed. Up to 252 days after initial PCR positivity, saliva levels of CoV2-specific IgG were substantially higher in PriorPOS individuals compared to NewPOS individuals, were directed against Spike, Spike RBD, and (to a lesser extent) the N protein. Potential reasons why Spike-specific IgG (IgGSpike) levels were higher than those of N-specific IgG (IgGN) include Spike being more antigenic, or alternatively it may reflect an inherent inability of IgGN to persist in saliva relative to IgGSpike, as is the case in plasma [92]. The N protein of CoV2 strongly resembles those of other coronaviruses that can infect humans [93]. For this reason, and because the US National Institutes of Health states that false positives in serological tests for CoV2 may occur due to cross-reactivity from pre-existing antibodies to other coronaviruses [94], it is possible that our sample population could have nucleocapsid-binding antibodies from a previous coronavirus infection.
When COVID vaccine doses were in short supply (early 2021), university students were generally not considered a vaccine priority by national public health agencies. By the time COVID vaccines were widely available, non-trivial levels of vaccine hesitancy had arisen among university students in many countries for many reasons [95]. Vaccine hesitancy was reinforced by the occurrence of breakthrough infections with CoV2Delta [96, 97], the first lineage to emerge after vaccines had become more widely available in Summer 2021. If vaccines were effective, conventional logic at the time being, how then could a vaccinated individual still become PCRPOS? Our current understanding is that a combination of three factors affects susceptibility to breakthrough infections: (1) antibody levels at the time of virus exposure, (2) the neutralizing capacity of these antibodies, and (3) the amount of virus to which a vaccinee is exposed. Our data demonstrate that saliva IgGSpike levels were comparable between CoVDelta-infected vaccinees (VaxPOSPCRPOS) and uninfected vaccinees (VaxPOSPCRNEG), but that the collective inhibitory capacity of this IgGSpike and other saliva antibodies differed between groups, with VaxPOSPCRPOS saliva being less inhibitory than VaxPOSPCRNEG saliva (Fig 7B). If the saliva Ig response is representative of that which occurs in other parts of the upper airway, then the combination of weak neutralization capacity and higher viral loads, which were typical of the Delta wave (Wave 4 of Fig 3D), created conditions that were conducive to CoV2Delta breakthrough infections. Our observation that CoV2Delta was more concentrated in saliva of asymptomatic individuals is consistent with work showing CoV2Delta-infected individuals were more likely to transmit virus before developing symptoms, compared to individuals infected with pre-Delta lineages [98].
The largest COVID wave our university community experienced was caused by the Omicron lineage. The Omicron lineage spread rapidly after its first detection in southern Africa in November 2021 [17]; the >30 amino acid substitutions in Spike enabled Omicron to bind ACE2 with higher affinity, as well as escape the anti-Spike antibody response elicited by either natural infection or vaccination with pre-Omicron lineages or vaccines [99–101]. The immunoevasive properties of Omicron are consistent with its causing a COVID wave in our community after vaccine mandates had been established. The rapidity with which Omicron took over was observed in other university settings which, like ours, were highly vaccinated at the time [102]. Compared to infections caused by the Delta lineage, those by Omicron tend to cause less severe disease [103], which may be due in whole or part to its being enriched in upper airways (including the oral cavity) as opposed to the lower airways [46–50]. Omicron subvariants BA.1 and BA.2 were the last lineages detected in our university community before our testing program ended in May 2022. At that time, which corresponded to Wave 6, saliva CT values were again trending lower than the prior wave that began ~6 months earlier (Fig 3D). We are reluctant to conclude this reflected waning immunity, however, for two reasons: (1) First and from a virological perspective, Wave 6 was due to an Omicron VOC and—relative to pre-Omicron VOCs, which had a lower airway tropism—Omicron VOCs had a greater tropism for the upper airways, including the oral cavity [46–50]; the lower CT values may be a reflection of this upper airway tropism. (2) Second, from a molecular diagnostic perspective, there are preprint studies demonstrating the SalivaDirect PCR assay we used amplifies the Omicron variants with modestly higher efficiency than pre-Omicron VOCs [104, 105]; the lower CT values may be a reflection of this higher amplification efficiency. Since then CoV2 has continued to evolve, there now being additional Omicron subvariants (BA.4, BA.5, BA.2.12.1, BA.2.75, XBB) and “Scrabble” subvariants (BQ.1 and BQ1.1) with Spike protein sequences that further desensitize the virus to in vitro neutralization by many (but not all) monoclonal therapies [106–109], as well as convalescent plasma [110]. Since Omicron has a higher tropism for the nasopharyngeal and oral cavities than that of pre-Omicron lineages [46–50], saliva antibodies may be more important inhibitors of Omicron transmission than plasma or lower airway antibodies, and saliva—the collection of which is far easier than blood—may be more suitable for rapid determination of whether someone has neutralizing capacity against future CoV2 VOCs that have yet to emerge.
The limitations of our study are as follows: (1) Since participants in our monitoring program provided saliva on a weekly basis, we cannot know the exact date on which someone was infected, rather only that they were infected 0–7 days prior to their scheduled test; (2) By only measuring CoV2-specific Ig in individuals whose CT values fell within a narrow range (thus normalizing for viral load), we cannot make any statements regarding the relationship between lower or higher CT values and CoV2-specific Ig levels; (3) Although we can correlate saliva samples’ Spike inhibition capacity with their corresponding IgMSpike, IgASpike, and IgGSpike levels, we cannot definitively state which of these isotypes most contributed to inhibition, nor did we test saliva using a neutralizing assay, which is the gold standard for evaluating the effectiveness of antibodies against CoV2 (this would need to occur in a BSL3 laboratory); (4) We did not measure CoV2-specific Ig levels in individuals infected with CoV2O-BA.1 or CoV2O-BA.2, a reason being at that stage in the pandemic (i.e. Waves 5–6 in our community) vaccine mandates were in place, and boosters were becoming available, making it difficult if not impossible to discern what levels of IgMSpike, IgASpike, and IgGSpike were due to vaccination versus boosters versus Omicron infection; (5) Finally, our study was not designed to take into account temporal biases due to changing policies and behaviors, such as the closing of dormitories, closing of classrooms and shift to remote learning, closing of indoor eating areas, on-campus social distancing requirements or masking requirements, nor can we account for the effect of prior infection with common cold coronaviruses that existed prior to the COVID pandemic. It is beyond the scope and ability of our study to measure the extent to which each of these changes—either individually or synergistically—affected CT value differences across time.
Supporting information
(A) Depiction of CoV2 and its RNA genome, nucleocapsid (N, yellow) and Spike proteins, the latter being differentially colored to indicate the Receptor Binding Domain (RBD, blue) and non-RBD regions (green). (B) The amino acids which distinguish the CoV2Anc Spike protein from CoV2US (also known as B.1.2), CoV2Alpha, CoV2Gamma, and CoV2Delta, as well as the Omicron lineages CoV2O-BA.1, CoV2O-BA.2, CoV2O-BA.4, and CoV2O-BA.5.
(TIF)
Daily COVID cases in the counties surrounding each campus of our university, as reported by the Ohio Department of Health (ODH), for the period spanning January 2021 to May 2022. Shown are the data for (A) Franklin County, which surrounds the OSU-Columbus campus; (B) Licking County, which surrounds the OSU-Newark campus; (C) Richland County, which surrounds the OSU-Mansfield campus; (D) Allen County, which surrounds the OSU-Lima campus; (E) Marion County, which surrounds the OSU-Marion campus; and (F) Wayne County, which surrounds the OSU-Wooster campus. Overlaid onto each graph are the dates which correspond to the six COVID waves (W1-W6) that occurred in our campus community (see Fig 2).
(TIF)
(A) The percent of males, females and undefined sex among individuals whose saliva was PCRPOS and sequenced for lineage identification for each week of our study period, the criteria for sequencing being a CT≤33. The average values for each sex across the entire study period are indicated by the hatched lines. (B) The age range of individuals whose saliva was PCRPOS and sequenced throughout the monitoring period. Overlaid onto each graph in gray are the periods corresponding to Waves 1–6 in our university community along with the academic calendar beginning and end dates.
(TIF)
Saliva samples that were positive for either the CoV2US, CoV2Alpha, CoV2Gamma or CoV2Delta lineage were used to measure the concentrations of (A) IgMSpike, (B) IgASpike, and (C) IgGSpike. Varying by column were the coating antigens (Ag) used for each measurement, the Ag being recombinant forms of either the CoVAnc Spike (Column 1), CoV2Alpha Spike (Column 2), CoV2Beta Spike (Column 3), and CoV2Gamma Spike (Column 4). Antibody levels are expressed in arbitrary units of luminescence. Note that the CoVAnc -specific IgM, IgA, and IgG values in (A-C) Column 1 were transformed into WHO Binding Antibody Units (BAUs) for Fig 4.
(TIF)
To generate the data shown in Fig 6, we analyzed saliva from individuals with a breakthrough Delta infection (VaxPOSPCRPOS individuals) and those who were vaccinated but PCR negative around the same time (VaxPOSPCRNEG individuals). Shown for both groups are the time in days since receiving the final dose of their vaccine series, with each dot representing a single individual.
(TIF)
During and after COVID Wave 4 (i.e. that which was caused by CoV2Delta), saliva from three groups of individuals were collected and used for Ig measurements: those who had not been fully vaccinated and were positive for CoV2Delta (VaxNEG PCRPOS), those who had been fully vaccinated and were positive for the CoV2Delta (VaxPOS PCRPOS), and those who had been fully vaccinated and were negative for any CoV2 lineage (VaxPOS PCRNEG). Shown for each individual in each group are the levels of (A) IgMSpike, (B) IgASpike, and (C) IgGSpike which bind to four different coating antigens (Ag), the Ag being recombinant forms of either the CoVAnc Spike (Column 1), CoV2Alpha Spike (Column 2), CoV2Beta Spike (Column 3), CoV2Gamma Spike (Column 4), and CoV2Delta Spike (Column 5). Antibody levels are expressed in arbitrary units of luminescence. Note that for (A) six outliers are not shown, and that the CoVAnc -specific IgM, IgA, and IgG values in (A-C) Column 1 were transformed into WHO Binding Antibody Units (BAUs) for Fig 6.
(TIF)
Acknowledgments
We would like to acknowledge the hundreds of Ohio State staff and student employees who collected, transported and processed saliva during the period that our campus testing program was active. We would also like to acknowledge the research compliance expertise of Tish Denlinger (Office of Responsible Research Practices) and Angela Emerson (Office of Research), the technical expertise of Dr. Arpita Agrawal, Brian Gribble, and Trina Wemlinger (Center for Clinical and Translational Science), the general lab assistance of Sam Hagenbaugh and Elizabeth Griffin (TOPS Program), and documentation assistance of Marie Klever (Infectious Disease Institute). Finally, we acknowledge the outstanding leadership of OSU President Dr. Kristina Johnson during what was an extremely difficult and challenging period for our university community. This work was supported by the Analytical and Development Laboratory of the Clinical Research Center/Center for Clinical Research Management of Wexner Medical Center, the OSU College of Medicine (Microbial Infection & Immunity Department), OSU College of Public Health (Epidemiology Division), and the OSU Infectious Disease Institute.
Data Availability
All the data underlying our study findings are reported in the manuscript and available.
Funding Statement
Funding for this work was provided by The Ohio State University (OSU) (MO, ANT, MQ, RTR). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. As all authors were/are employees of OSU, all authors received a salary from the funder (MRM, AM, NM, MRF, JS, JJ, LWA, JAB, AN, ANT, MO, SAF, MBQ, RTR).
References
- 1.Keusch GT, Amuasi JH, Anderson DE, Daszak P, Eckerle I, Field H, et al. Pandemic origins and a One Health approach to preparedness and prevention: Solutions based on SARS-CoV-2 and other RNA viruses. Proc Natl Acad Sci U S A. 2022;119(42):e2202871119. Epub 2022/10/11. doi: 10.1073/pnas.2202871119 ; PubMed Central PMCID: PMC9586299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worobey M. Dissecting the early COVID-19 cases in Wuhan. Science. 2021;374(6572):1202–4. Epub 2021/11/19. doi: 10.1126/science.abm4454 . [DOI] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. Epub 2020/01/25. doi: 10.1056/NEJMoa2001017 ; PubMed Central PMCID: PMC7092803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Ho W, Huang Y, Jin DY, Li S, Liu SL, et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet. 2020;395(10228):949–50. Epub 2020/03/11. doi: 10.1016/S0140-6736(20)30557-2 ; PubMed Central PMCID: PMC7133598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tellier R. COVID-19: the case for aerosol transmission. Interface Focus. 2022;12(2):20210072. Epub 2022/03/10. doi: 10.1098/rsfs.2021.0072 ; PubMed Central PMCID: PMC8831082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Perlman S. COVID-19: Inflammatory Profile. Annu Rev Med. 2022;73:65–80. Epub 2021/08/27. doi: 10.1146/annurev-med-042220-012417 . [DOI] [PubMed] [Google Scholar]
- 7.https://covid19.who.int. [Google Scholar]
- 8.Domingo E, Holland JJ. RNA virus mutations and fitness for survival. Annu Rev Microbiol. 1997;51:151–78. Epub 1997/01/01. doi: 10.1146/annurev.micro.51.1.151 . [DOI] [PubMed] [Google Scholar]
- 9.Galloway SE, Paul P, MacCannell DR, Johansson MA, Brooks JT, MacNeil A, et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage—United States, December 29, 2020-January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(3):95–9. Epub 2021/01/22. doi: 10.15585/mmwr.mm7003e2 ; PubMed Central PMCID: PMC7821772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854):438–43. Epub 2021/03/11. doi: 10.1038/s41586-021-03402-9 . [DOI] [PubMed] [Google Scholar]
- 11.Evans JP, Liu SL. Role of host factors in SARS-CoV-2 entry. J Biol Chem. 2021;297(1):100847. Epub 2021/06/01. doi: 10.1016/j.jbc.2021.100847 ; PubMed Central PMCID: PMC8160279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou D, Dejnirattisai W, Supasa P, Liu C, Mentzer AJ, Ginn HM, et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–61 e6. Epub 2021/03/18. doi: 10.1016/j.cell.2021.02.037 ; PubMed Central PMCID: PMC7901269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021;593(7858):266–9. Epub 2021/03/27. doi: 10.1038/s41586-021-03470-x . [DOI] [PubMed] [Google Scholar]
- 14.Davies NG, Jarvis CI, Group CC-W, Edmunds WJ, Jewell NP, Diaz-Ordaz K, et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593(7858):270–4. Epub 2021/03/17. doi: 10.1038/s41586-021-03426-1 ; PubMed Central PMCID: PMC9170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DDS, Mishra S, et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science. 2021;372(6544):815–21. Epub 2021/04/16. doi: 10.1126/science.abh2644 ; PubMed Central PMCID: PMC8139423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira I, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114–9. Epub 2021/09/07. doi: 10.1038/s41586-021-03944-y ; PubMed Central PMCID: PMC8566220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679–86. Epub 2022/01/19. doi: 10.1038/s41586-022-04411-y ; PubMed Central PMCID: PMC8942855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholey J, Aburto JM, Kashnitsky I, Kniffka MS, Zhang L, Jaadla H, et al. Life expectancy changes since COVID-19. Nat Hum Behav. 2022. Epub 2022/10/18. doi: 10.1038/s41562-022-01450-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu JQ, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2021. NCHS Data Brief, no 456 Hyattsville, MD: National Center for Health Statistics; 2022. 10.15620/cdc:122516. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Zhao J, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111(13):4970–5. Epub 2014/03/07. doi: 10.1073/pnas.1323279111 ; PubMed Central PMCID: PMC3977243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Y, Bao L, Deng W, Xu L, Li F, Lv Q, et al. An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J Infect Dis. 2014;209(2):236–42. Epub 2013/11/13. doi: 10.1093/infdis/jit590 ; PubMed Central PMCID: PMC7107340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang TH, Park WJ, Lee H, Woo HM, Lee SY, Kim KC, et al. The structure of a novel antibody against the spike protein inhibits Middle East respiratory syndrome coronavirus infections. Sci Rep. 2022;12(1):1260. Epub 2022/01/26. doi: 10.1038/s41598-022-05318-4 ; PubMed Central PMCID: PMC8786824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du L, Zhao G, Yang Y, Qiu H, Wang L, Kou Z, et al. A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein. J Virol. 2014;88(12):7045–53. Epub 2014/04/11. doi: 10.1128/JVI.00433-14 ; PubMed Central PMCID: PMC4054355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma C, Li Y, Wang L, Zhao G, Tao X, Tseng CT, et al. Intranasal vaccination with recombinant receptor-binding domain of MERS-CoV spike protein induces much stronger local mucosal immune responses than subcutaneous immunization: Implication for designing novel mucosal MERS vaccines. Vaccine. 2014;32(18):2100–8. Epub 2014/02/25. doi: 10.1016/j.vaccine.2014.02.004 ; PubMed Central PMCID: PMC4194189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song F, Fux R, Provacia LB, Volz A, Eickmann M, Becker S, et al. Middle East respiratory syndrome coronavirus spike protein delivered by modified vaccinia virus Ankara efficiently induces virus-neutralizing antibodies. J Virol. 2013;87(21):11950–4. Epub 2013/08/30. doi: 10.1128/JVI.01672-13 ; PubMed Central PMCID: PMC3807317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du L, Zhao G, Kou Z, Ma C, Sun S, Poon VK, et al. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J Virol. 2013;87(17):9939–42. Epub 2013/07/05. doi: 10.1128/JVI.01048-13 ; PubMed Central PMCID: PMC3754113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mou H, Raj VS, van Kuppeveld FJ, Rottier PJ, Haagmans BL, Bosch BJ. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J Virol. 2013;87(16):9379–83. Epub 2013/06/21. doi: 10.1128/JVI.01277-13 ; PubMed Central PMCID: PMC3754068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ptasiewicz M, Bebnowska D, Malkowska P, Sierawska O, Poniewierska-Baran A, Hrynkiewicz R, et al. Immunoglobulin Disorders and the Oral Cavity: A Narrative Review. J Clin Med. 2022;11(16). Epub 2022/08/27. doi: 10.3390/jcm11164873 ; PubMed Central PMCID: PMC9409910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandtzaeg P. Secretory immunity with special reference to the oral cavity. J Oral Microbiol. 2013;5. Epub 2013/03/15. doi: 10.3402/jom.v5i0.20401 ; PubMed Central PMCID: PMC3595421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claer L, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577). Epub 2020/12/09. doi: 10.1126/scitranslmed.abd2223 ; PubMed Central PMCID: PMC7857408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kline D, Li Z, Chu Y, Wakefield J, Miller WC, Norris Turner A, et al. Estimating seroprevalence of SARS-CoV-2 in Ohio: A Bayesian multilevel poststratification approach with multiple diagnostic tests. Proc Natl Acad Sci U S A. 2021;118(26). Epub 2021/06/27. doi: 10.1073/pnas.2023947118 ; PubMed Central PMCID: PMC8255994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–42. Epub 2020/06/20. doi: 10.1038/s41586-020-2456-9 ; PubMed Central PMCID: PMC7442695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757–65. Epub 2020/04/08. doi: 10.1172/JCI138745 ; PubMed Central PMCID: PMC7259988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19.
- 35.https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid.
- 36.https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine.
- 37.Barouch DH. Covid-19 Vaccines—Immunity, Variants, Boosters. N Engl J Med. 2022;387(11):1011–20. Epub 2022/09/01. doi: 10.1056/NEJMra2206573 ; PubMed Central PMCID: PMC9454645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson AG, Amin AB, Ali AR, Hoots B, Cadwell BL, Arora S, et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence—25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):132–8. Epub 2022/01/28. doi: 10.15585/mmwr.mm7104e2 ; PubMed Central PMCID: PMC9351531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–16. Epub 2020/12/31. doi: 10.1056/NEJMoa2035389 ; PubMed Central PMCID: PMC7787219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–15. Epub 2020/12/11. doi: 10.1056/NEJMoa2034577 ; PubMed Central PMCID: PMC7745181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bojadzic D, Alcazar O, Chen J, Chuang ST, Condor Capcha JM, Shehadeh LA, et al. Small-Molecule Inhibitors of the Coronavirus Spike: ACE2 Protein-Protein Interaction as Blockers of Viral Attachment and Entry for SARS-CoV-2. ACS Infect Dis. 2021;7(6):1519–34. Epub 2021/05/13. doi: 10.1021/acsinfecdis.1c00070 ; PubMed Central PMCID: PMC8130611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41(9):1141–9. Epub 2020/08/05. doi: 10.1038/s41401-020-0485-4 ; PubMed Central PMCID: PMC7396720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart WS, Maini PK, Thompson RN. High infectiousness immediately before COVID-19 symptom onset highlights the importance of continued contact tracing. Elife. 2021;10. Epub 2021/04/27. doi: 10.7554/eLife.65534 ; PubMed Central PMCID: PMC8195606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, et al. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw Open. 2021;4(1):e2035057. Epub 2021/01/08. doi: 10.1001/jamanetworkopen.2020.35057 ; PubMed Central PMCID: PMC7791354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang N, Perez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27(5):892–903. Epub 2021/03/27. doi: 10.1038/s41591-021-01296-8 ; PubMed Central PMCID: PMC8240394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Migueres M, Mansuy JM, Vasseur S, Claverie N, Lougarre C, Soulier F, et al. Omicron Wave SARS-CoV-2 Diagnosis: Evaluation of Saliva, Anterior Nasal, and Nasopharyngeal Swab Samples. Microbiol Spectr. 2022:e0252122. Epub 2022/11/02. doi: 10.1128/spectrum.02521-22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marais G, Hsiao NY, Iranzadeh A, Doolabh D, Joseph R, Enoch A, et al. Improved oral detection is a characteristic of Omicron infection and has implications for clinical sampling and tissue tropism. J Clin Virol. 2022;152:105170. Epub 2022/05/08. doi: 10.1016/j.jcv.2022.105170 . [DOI] [PubMed] [Google Scholar]
- 48.Suzuki R, Yamasoba D, Kimura I, Wang L, Kishimoto M, Ito J, et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature. 2022;603(7902):700–5. Epub 2022/02/02. doi: 10.1038/s41586-022-04462-1 ; PubMed Central PMCID: PMC8942852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hui KPY, Ng KC, Ho JCW, Yeung HW, Ching RHH, Gu H, et al. Replication of SARS-CoV-2 Omicron BA.2 variant in ex vivo cultures of the human upper and lower respiratory tract. EBioMedicine. 2022;83:104232. Epub 2022/08/22. doi: 10.1016/j.ebiom.2022.104232 ; PubMed Central PMCID: PMC9387351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng B, Abdullahi A, Ferreira I, Goonawardane N, Saito A, Kimura I, et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature. 2022;603(7902):706–14. Epub 2022/02/02. doi: 10.1038/s41586-022-04474-x ; PubMed Central PMCID: PMC8942856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.https://www.fda.gov/media/141192/download.
- 52.Vogels CBF, Watkins AE, Harden CA, Brackney DE, Shafer J, Wang J, et al. SalivaDirect: A simplified and flexible platform to enhance SARS-CoV-2 testing capacity. Med (N Y). 2021;2(3):263–80 e6. Epub 2021/02/02. doi: 10.1016/j.medj.2020.12.010 ; PubMed Central PMCID: PMC7836249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hale VL, Dennis PM, McBride DS, Nolting JM, Madden C, Huey D, et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602(7897):481–6. Epub 2021/12/24. doi: 10.1038/s41586-021-04353-x ; PubMed Central PMCID: PMC8857059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rambaut A, Holmes EC, O’Toole A, Hill V, McCrone JT, Ruis C, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5(11):1403–7. Epub 2020/07/17. doi: 10.1038/s41564-020-0770-5 ; PubMed Central PMCID: PMC7610519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodcroft EB, Domman DB, Snyder DJ, Oguntuyo KY, Van Diest M, Densmore KH, et al. Emergence in late 2020 of multiple lineages of SARS-CoV-2 Spike protein variants affecting amino acid position 677. medRxiv. 2021. Epub 2021/02/18. doi: 10.1101/2021.02.12.21251658 ; PubMed Central PMCID: PMC7885944 Sequencing Center, LLC. All other authors declare no conflicts of interest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patterson EI, Prince T, Anderson ER, Casas-Sanchez A, Smith SL, Cansado-Utrilla C, et al. Methods of Inactivation of SARS-CoV-2 for Downstream Biological Assays. J Infect Dis. 2020;222(9):1462–7. Epub 2020/08/17. doi: 10.1093/infdis/jiaa507 ; PubMed Central PMCID: PMC7529010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.https://www.dispatch.com/story/news/2020/03/09/3-ohioans-test-positive-for/1553817007/.
- 58.https://www.thelantern.com/2020/03/ohio-state-suspends-classes-until-march-30-due-to-coronavirus-outbreak/.
- 59.https://www.thelantern.com/2020/07/ohio-state-announces-proactive-covid-19-testing-measures-no-specific-guidance-on-when-to-close-campus/.
- 60.https://news.osu.edu/ohio-state-announces-vaccination-requirement/.
- 61.Data were obtained from https://coronavirus.ohio.gov/dashboards/overview accessed 11.14.22.
- 62.Ohio Department of Health COVID-19 Dashboard. https://coronavirusohiogov/dashboards.
- 63.https://www.who.int/news/item/15-01-2021-statement-on-the-sixth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic.
- 64.https://www.daytondailynews.com/local/delta-variant-in-ohio-what-you-should-know/WQ3EI57ZPNA67OC346RKSDCKAU/.
- 65.https://governor.ohio.gov/media/news-and-media/COVID-19-Update-Vaccinations-Increasing-Delta-Variant-Local-Efforts-Encouraged-08062021.
- 66.https://www.10tv.com/article/news/health/coronavirus/covid-variant-dashboard/530-0a10581c-cf0b-4255-b2f0-46984b0cbd30.
- 67.https://odh.ohio.gov/media-center/odh-news-releases/odh-news-release-12-11-21.
- 68.https://www.thelantern.com/2021/12/first-cases-of-omicron-covid-19-variant-detected-in-ohio/.
- 69.Woodbridge Y, Amit S, Huppert A, Kopelman NM. Viral load dynamics of SARS-CoV-2 Delta and Omicron variants following multiple vaccine doses and previous infection. Nat Commun. 2022;13(1):6706. Epub 2022/11/08. doi: 10.1038/s41467-022-33096-0 ; PubMed Central PMCID: PMC9640564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trunfio M, Richiardi L, Alladio F, Staffilano E, Longo B, Venuti F, et al. Determinants of SARS-CoV-2 Contagiousness in Household Contacts of Symptomatic Adult Index Cases. Front Microbiol. 2022;13:829393. Epub 2022/04/19. doi: 10.3389/fmicb.2022.829393 ; PubMed Central PMCID: PMC9010948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levine-Tiefenbrun M, Yelin I, Alapi H, Katz R, Herzel E, Kuint J, et al. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med. 2021;27(12):2108–10. Epub 2021/11/04. doi: 10.1038/s41591-021-01575-4 . [DOI] [PubMed] [Google Scholar]
- 72.Al Bayat S, Mundodan J, Hasnain S, Sallam M, Khogali H, Ali D, et al. Can the cycle threshold (Ct) value of RT-PCR test for SARS CoV2 predict infectivity among close contacts? J Infect Public Health. 2021;14(9):1201–5. Epub 2021/08/21. doi: 10.1016/j.jiph.2021.08.013 ; PubMed Central PMCID: PMC8362640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawasuji H, Takegoshi Y, Kaneda M, Ueno A, Miyajima Y, Kawago K, et al. Transmissibility of COVID-19 depends on the viral load around onset in adult and symptomatic patients. PLoS One. 2020;15(12):e0243597. Epub 2020/12/10. doi: 10.1371/journal.pone.0243597 ; PubMed Central PMCID: PMC7725311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(6538). Epub 2021/03/05. doi: 10.1126/science.abg3055 ; PubMed Central PMCID: PMC8128288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hart WS, Miller E, Andrews NJ, Waight P, Maini PK, Funk S, et al. Generation time of the alpha and delta SARS-CoV-2 variants: an epidemiological analysis. Lancet Infect Dis. 2022;22(5):603–10. Epub 2022/02/18. doi: 10.1016/S1473-3099(22)00001-9 ; PubMed Central PMCID: PMC8843191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.https://www.thelantern.com/2021/04/dewine-all-ohio-college-students-will-have-access-to-single-dose-covid-vaccine/.
- 77.Mahoney JL. Malaria ’breakthroughs’ and resistance to chloroquine in Africa. Case reports. S Afr Med J. 1981;60(20):786–8. Epub 1981/11/14. . [PubMed] [Google Scholar]
- 78.Sette A, Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev. 2022;310(1):27–46. Epub 2022/06/24. doi: 10.1111/imr.13089 ; PubMed Central PMCID: PMC9349657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–36. Epub 2020/02/01. doi: 10.1056/NEJMoa2001191 ; PubMed Central PMCID: PMC7092802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323(14):1406–7. Epub 2020/02/23. doi: 10.1001/jama.2020.2565 ; PubMed Central PMCID: PMC7042844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382(10):970–1. Epub 2020/02/01. doi: 10.1056/NEJMc2001468 ; PubMed Central PMCID: PMC7120970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nussbaumer-Streit B, Mayr V, Dobrescu AI, Chapman A, Persad E, Klerings I, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;9(9):CD013574. Epub 2021/05/08. doi: 10.1002/14651858.CD013574.pub2 ; PubMed Central PMCID: PMC8133397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang CN, Chien HY, Malagon-Palacios L. College reopening and community spread of COVID-19 in the United States. Public Health. 2022;204:70–5. Epub 2022/02/18. doi: 10.1016/j.puhe.2022.01.001 ; PubMed Central PMCID: PMC8747949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arnold CRK, Srinivasan S, Rodriguez S, Rydzak N, Herzog CM, Gontu A, et al. A longitudinal study of the impact of university student return to campus on the SARS-CoV-2 seroprevalence among the community members. Sci Rep. 2022;12(1):8586. Epub 2022/05/22. doi: 10.1038/s41598-022-12499-5 ; PubMed Central PMCID: PMC9124192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bharti N, Lambert B, Exten C, Faust C, Ferrari M, Robinson A. Large university with high COVID-19 incidence is not associated with excess cases in non-student population. Sci Rep. 2022;12(1):3313. Epub 2022/03/02. doi: 10.1038/s41598-022-07155-x ; PubMed Central PMCID: PMC8885693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pollock BH, Kilpatrick AM, Eisenman DP, Elton KL, Rutherford GW, Boden-Albala BM, et al. Safe reopening of college campuses during COVID-19: The University of California experience in Fall 2020. PLoS One. 2021;16(11):e0258738. Epub 2021/11/05. doi: 10.1371/journal.pone.0258738 ; PubMed Central PMCID: PMC8568179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Edridge AWD, Kaczorowska J, Hoste ACR, Bakker M, Klein M, Loens K, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26(11):1691–3. Epub 2020/09/16. doi: 10.1038/s41591-020-1083-1 . [DOI] [PubMed] [Google Scholar]
- 88.Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5(10):1185–91. Epub 2020/09/11. doi: 10.1038/s41564-020-00789-5 . [DOI] [PubMed] [Google Scholar]
- 89.Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. 2020;584(7821):353–63. Epub 2020/07/14. doi: 10.1038/s41586-020-2538-8 . [DOI] [PubMed] [Google Scholar]
- 90.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–6. Epub 2020/05/10. doi: 10.1126/science.abb8923 . [DOI] [PubMed] [Google Scholar]
- 91.Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020;20(6):339–41. Epub 2020/04/23. doi: 10.1038/s41577-020-0321-6 ; PubMed Central PMCID: PMC7187142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Van Elslande J, Oyaert M, Lorent N, Vande Weygaerde Y, Van Pottelbergh G, Godderis L, et al. Lower persistence of anti-nucleocapsid compared to anti-spike antibodies up to one year after SARS-CoV-2 infection. Diagn Microbiol Infect Dis. 2022;103(1):115659. Epub 2022/03/13. doi: 10.1016/j.diagmicrobio.2022.115659 ; PubMed Central PMCID: PMC8837483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng Y, Du N, Lei Y, Dorje S, Qi J, Luo T, et al. Structures of the SARS-CoV-2 nucleocapsid and their perspectives for drug design. EMBO J. 2020;39(20):e105938. Epub 2020/09/12. doi: 10.15252/embj.2020105938 ; PubMed Central PMCID: PMC7560215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.https://www.covid19treatmentguidelines.nih.gov/overview/sars-cov-2-testing/. [Google Scholar]
- 95.Schafer M, Stark B, Werner AM, Mulder LM, Heller S, Reichel JL, et al. Determinants of university students’ COVID-19 vaccination intentions and behavior. Sci Rep. 2022;12(1):18067. Epub 2022/10/28. doi: 10.1038/s41598-022-23044-9 ; PubMed Central PMCID: PMC9610342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dzinamarira T, Tungwarara N, Chitungo I, Chimene M, Iradukunda PG, Mashora M, et al. Unpacking the Implications of SARS-CoV-2 Breakthrough Infections on COVID-19 Vaccination Programs. Vaccines (Basel). 2022;10(2). Epub 2022/02/27. doi: 10.3390/vaccines10020252 ; PubMed Central PMCID: PMC8879800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haque A, Pant AB. Mitigating Covid-19 in the face of emerging virus variants, breakthrough infections and vaccine hesitancy. J Autoimmun. 2022;127:102792. Epub 2022/01/08. doi: 10.1016/j.jaut.2021.102792 ; PubMed Central PMCID: PMC8719928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang M, Xin H, Yuan J, Ali ST, Liang Z, Zhang J, et al. Transmission dynamics and epidemiological characteristics of SARS-CoV-2 Delta variant infections in Guangdong, China, May to June 2021. Euro Surveill. 2022;27(10). Epub 2022/03/12. doi: 10.2807/1560-7917.ES.2022.27.10.2100815 ; PubMed Central PMCID: PMC8915401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nutalai R, Zhou D, Tuekprakhon A, Ginn HM, Supasa P, Liu C, et al. Potent cross-reactive antibodies following Omicron breakthrough in vaccinees. Cell. 2022;185(12):2116–31 e18. Epub 2022/06/07. doi: 10.1016/j.cell.2022.05.014 ; PubMed Central PMCID: PMC9120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu L, Iketani S, Guo Y, Chan JF, Wang M, Liu L, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–81. Epub 2022/01/12. doi: 10.1038/s41586-021-04388-0 . [DOI] [PubMed] [Google Scholar]
- 101.Cameroni E, Bowen JE, Rosen LE, Saliba C, Zepeda SK, Culap K, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602(7898):664–70. Epub 2022/01/12. doi: 10.1038/s41586-021-04386-2 ; PubMed Central PMCID: PMC9531318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petros BA, Turcinovic J, Welch NL, White LF, Kolaczyk ED, Bauer MR, et al. Early introduction and rise of the Omicron SARS-CoV-2 variant in highly vaccinated university populations. Clin Infect Dis. 2022. Epub 2022/05/27. doi: 10.1093/cid/ciac413 ; PubMed Central PMCID: PMC9213864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Doremalen N, Singh M, Saturday TA, Yinda CK, Perez-Perez L, Bohler WF, et al. SARS-CoV-2 Omicron BA.1 and BA.2 are attenuated in rhesus macaques as compared to Delta. Sci Adv. 2022;8(46):eade1860. Epub 2022/11/19. doi: 10.1126/sciadv.ade1860 ; PubMed Central PMCID: PMC9674298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.https://www.medrxiv.org/content/10.1101/2021.12.16.21267734v1.
- 105.https://www.medrxiv.org/content/10.1101/2022.02.16.22271096v1.
- 106.Takashita E, Yamayoshi S, Halfmann P, Wilson N, Ries H, Richardson A, et al. In Vitro Efficacy of Antiviral Agents against Omicron Subvariant BA.4.6. N Engl J Med. 2022;387(22):2094–7. Epub 2022/11/17. doi: 10.1056/NEJMc2211845 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cox M, Peacock TP, Harvey WT, Hughes J, Wright DW, Consortium C-GU, et al. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat Rev Microbiol. 2022:1–13. Epub 2022/10/29. doi: 10.1038/s41579-022-00809-7 ; PubMed Central PMCID: PMC9616429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park YJ, Pinto D, Walls AC, Liu Z, De Marco A, Benigni F, et al. Imprinted antibody responses against SARS-CoV-2 Omicron sublineages. Science. 2022;378(6620):619–27. Epub 2022/10/21. doi: 10.1126/science.adc9127 . [DOI] [PubMed] [Google Scholar]
- 109.Hoffmann M, Kruger N, Schulz S, Cossmann A, Rocha C, Kempf A, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447–56 e11. Epub 2022/01/14. doi: 10.1016/j.cell.2021.12.032 ; PubMed Central PMCID: PMC8702401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Qu P, Evans JP, Zheng YM, Carlin C, Saif LJ, Oltz EM, et al. Evasion of neutralizing antibody responses by the SARS-CoV-2 BA.2.75 variant. Cell Host Microbe. 2022;30(11):1518–26 e4. Epub 2022/10/15. doi: 10.1016/j.chom.2022.09.015 ; PubMed Central PMCID: PMC9515334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.https://commons.wikimedia.org/wiki/File:Map_of_Ohio_highlighting_Delaware_County.svg.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Depiction of CoV2 and its RNA genome, nucleocapsid (N, yellow) and Spike proteins, the latter being differentially colored to indicate the Receptor Binding Domain (RBD, blue) and non-RBD regions (green). (B) The amino acids which distinguish the CoV2Anc Spike protein from CoV2US (also known as B.1.2), CoV2Alpha, CoV2Gamma, and CoV2Delta, as well as the Omicron lineages CoV2O-BA.1, CoV2O-BA.2, CoV2O-BA.4, and CoV2O-BA.5.
(TIF)
Daily COVID cases in the counties surrounding each campus of our university, as reported by the Ohio Department of Health (ODH), for the period spanning January 2021 to May 2022. Shown are the data for (A) Franklin County, which surrounds the OSU-Columbus campus; (B) Licking County, which surrounds the OSU-Newark campus; (C) Richland County, which surrounds the OSU-Mansfield campus; (D) Allen County, which surrounds the OSU-Lima campus; (E) Marion County, which surrounds the OSU-Marion campus; and (F) Wayne County, which surrounds the OSU-Wooster campus. Overlaid onto each graph are the dates which correspond to the six COVID waves (W1-W6) that occurred in our campus community (see Fig 2).
(TIF)
(A) The percent of males, females and undefined sex among individuals whose saliva was PCRPOS and sequenced for lineage identification for each week of our study period, the criteria for sequencing being a CT≤33. The average values for each sex across the entire study period are indicated by the hatched lines. (B) The age range of individuals whose saliva was PCRPOS and sequenced throughout the monitoring period. Overlaid onto each graph in gray are the periods corresponding to Waves 1–6 in our university community along with the academic calendar beginning and end dates.
(TIF)
Saliva samples that were positive for either the CoV2US, CoV2Alpha, CoV2Gamma or CoV2Delta lineage were used to measure the concentrations of (A) IgMSpike, (B) IgASpike, and (C) IgGSpike. Varying by column were the coating antigens (Ag) used for each measurement, the Ag being recombinant forms of either the CoVAnc Spike (Column 1), CoV2Alpha Spike (Column 2), CoV2Beta Spike (Column 3), and CoV2Gamma Spike (Column 4). Antibody levels are expressed in arbitrary units of luminescence. Note that the CoVAnc -specific IgM, IgA, and IgG values in (A-C) Column 1 were transformed into WHO Binding Antibody Units (BAUs) for Fig 4.
(TIF)
To generate the data shown in Fig 6, we analyzed saliva from individuals with a breakthrough Delta infection (VaxPOSPCRPOS individuals) and those who were vaccinated but PCR negative around the same time (VaxPOSPCRNEG individuals). Shown for both groups are the time in days since receiving the final dose of their vaccine series, with each dot representing a single individual.
(TIF)
During and after COVID Wave 4 (i.e. that which was caused by CoV2Delta), saliva from three groups of individuals were collected and used for Ig measurements: those who had not been fully vaccinated and were positive for CoV2Delta (VaxNEG PCRPOS), those who had been fully vaccinated and were positive for the CoV2Delta (VaxPOS PCRPOS), and those who had been fully vaccinated and were negative for any CoV2 lineage (VaxPOS PCRNEG). Shown for each individual in each group are the levels of (A) IgMSpike, (B) IgASpike, and (C) IgGSpike which bind to four different coating antigens (Ag), the Ag being recombinant forms of either the CoVAnc Spike (Column 1), CoV2Alpha Spike (Column 2), CoV2Beta Spike (Column 3), CoV2Gamma Spike (Column 4), and CoV2Delta Spike (Column 5). Antibody levels are expressed in arbitrary units of luminescence. Note that for (A) six outliers are not shown, and that the CoVAnc -specific IgM, IgA, and IgG values in (A-C) Column 1 were transformed into WHO Binding Antibody Units (BAUs) for Fig 6.
(TIF)
Data Availability Statement
All the data underlying our study findings are reported in the manuscript and available.