Abstract
The first reported human case of possible disseminated infection with the insect pathogen Metarrhizium anisopliae var. anisopliae, a fungus which has been used commercially for biocontrol of insects, is described. The patient, a 9-year-old boy, had a 5-year history of pre-B-cell acute lymphoblastic leukemia and had been on chemotherapy throughout this period. After 10 days of profound neutropenia, lesions consistent with ecthyma gangrenosum appeared on his arms and legs. M. anisopliae was grown from specimens from three separate sites, collected at different times over a period of 1 month: a skin biopsy, a swab from the base of a lesion, and the core of another skin lesion which spontaneously discharged. The initial skin biopsy also showed histological evidence of epidermal necrosis and dermal invasion with fungal hyphae. A computed-tomography (CT) scan of the chest demonstrated a lesion in the superior segment of the lower lobe of the left lung. A CT scan of the brain revealed a lesion in the left temporoparietal region of the brain, consistent with an abscess. Despite antifungal treatment including liposomal amphotericin and 5-flucytosine, the patient eventually died. The initial portal of entry is unknown, but hematogenous dissemination to the skin appears likely because of the multiple ecthymic lesions, and the appearances of the brain lesion on the CT scan are consistent with a hematogenous fungal abscess.
Advances in the treatment of many childhood malignancies have improved prognosis, but intensive chemotherapy regimens have increased susceptibility to opportunistic infections, particularly to deep fungal infections (14). Such infections are increasingly important causes of morbidity and mortality in neutropenic children (9). The fungi most often encountered are Candida and Aspergillus (14).
We describe the first reported case of possible disseminated infection with the insect pathogen Metarrhizium anisopliae (Metschnikow) Sorokin var. anisopliae. This fungus has been used commercially for biocontrol of insects.
Case report.
The patient, a 9-year-old boy, had a 5-year history of pre-B-cell acute lymphoblastic leukemia and had been on chemotherapy throughout this period. The condition had relapsed twice, with bone marrow involvement but without central nervous system involvement. Since the most recent relapse, the boy had been receiving vincristine, methotrexate (intrathecal), etoposide, asparaginase, cyclophosphamide, and dexamethasone in a high-dose reinduction protocol.
He presented, on day 11 of reinduction, with fever to 39°C and neutropenia (neutrophil count = 0.3 × 109/liter). Blood cultures grew Escherichia coli and Staphylococcus aureus, and the patient was treated by the standard oncology neutropenia protocol with ticarcillin-clavulanic acid (250 mg/kg of body weight/day), vancomycin (40 mg/kg/day), and gentamicin (7 mg/kg/day), the actual dosages adjusted for renal function as necessary, and granulocyte colony-stimulating factor. He subsequently became afebrile.
Two weeks after this presentation, and after a period of 10 days of profound neutropenia (neutrophil count < 0.05 × 109/liter), lesions consistent with ecthyma gangrenosum appeared on the boy’s arms and legs. His neutrophil count at this time was 1.5 × 109/liter, and he was afebrile. Because of the possibility of fungal infection, amphotericin B and itraconazole (400 mg/day orally) were added to his treatment regimen and chemotherapy was ceased.
A skin biopsy specimen from a lesion showed histological evidence of epidermal necrosis, and dermal invasion with fungal hyphae was seen in a periodic acid-Schiff-stained section. No leukemic infiltration of the skin was seen. The skin biopsy specimen was processed for detection of bacteria, fungi, and mycobacteria. Acid-fast bacilli were not detected by microscopy. Slivers of tissue were inoculated onto Sabouraud’s agar, which was incubated at 35°C in air for 48 h and subsequently at room temperature. Ground tissue was cultured on horse blood agar (in 5% carbon dioxide and anaerobically), chocolate agar (in 5% carbon dioxide), MacConkey agar, and cooked-meat medium. It was also inoculated onto a Lowenstein-Jensen slope incubated in air at 35°C. Diphtheroids were cultured from the cooked-meat medium. No mycobacteria were isolated.
After 7 days, one colony of fungus grew on the original Sabouraud’s agar. No fungi grew on any of the bacteriological media. Because the fungus was not readily identifiable, it was sent to the Australian National Reference Laboratory in Medical Mycology, where it was provisionally identified as M. anisopliae. Because of the unusual nature of the isolate and the fact that it had not previously been described as a human pathogen, it was initially considered a contaminant, although all processing was performed in a class II safety cabinet. However, when the same fungus was isolated from separate lesions, a skin swab taken 23 days later and the tissue core of a lesion taken 3 days after that, it became clear that it was an invasive pathogen. The organism grew from the skin swab specimen after 6 days in Sabouraud’s broth and from the tissue core specimen after 3 days on Sabouraud’s agar and chocolate agar (incubated at 35°C in 5% CO2) and in Sabouraud’s broth.
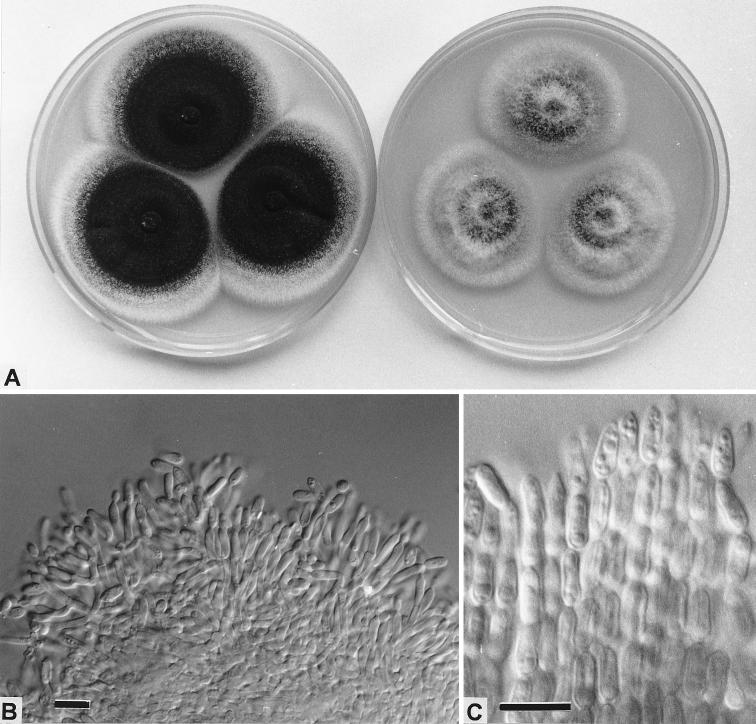
The identification was confirmed by the Division of Food Science and Technology, Commonwealth Scientific and Industrial Research Organisation (CSIRO), North Ryde, and the Biological and Chemical Research Institute of the Department of Agriculture, Sydney, New South Wales, Australia, as M. anisopliae (Metschnikow) Sorokin var. anisopliae by the culture methods outlined previously (13), with reference to descriptions contained in references 5 and 16 (Fig. 1). The isolate was inoculated onto Czapek yeast extract agar (CYA) and malt extract agar (MEA) and grown at 25 and 37°C. Later, its ability to grow at temperatures between 35 and 37°C was examined in more detail.
FIG. 1.
M. anisopliae. (A) Colonies on CYA (left) and MEA (right) after 14 days at 25°C; (B) aggregated conidiophores, showing cylindroidal phialides producing ellipsoidal conidia; (C) adherent chains of ellipsoidal conidia. Bars = 10 μm.
The isolate produced long, dry, adherent chains of dark-olive ellipsoidal conidia (Fig. 1C) from phialides in aggregated conidiophores (Fig. 1B). The aggregation of the conidial chains into adherent columns is typical of M. anisopliae (5). This isolate compared well with many reference cultures held at the DAR Plant Pathology Herbarium at NSW Agriculture, Orange, Australia. The fungus grew well at 25°C and very weakly at 37°C. This isolate has been deposited in the culture collection of the CSIRO Division of Food Science and Technology, North Ryde, New South Wales, Australia, as isolate FRR 4834.
To determine its potential as a pathogen, the isolate was grown at 35, 36, and 37°C. Temperatures were accurate to within ± 0.2°C. After 7 days, growth at 35°C was 12 to 14 mm in diameter on CYA and 8 to 10 mm on MEA. At 36°C, growth was 4 to 5 mm (CYA) and 3 to 4 mm (MEA), and at 37°C, the isolate formed microcolonies on both media but growth on CYA was slightly stronger. There was no sporulation at 37°C after 7 days.
By in vitro antifungal susceptibility testing, by a tablet diffusion method (20), carried out at the Australian National Reference Laboratory in Medical Mycology, it was found that the organism was resistant to itraconazole, fluconazole, ketoconazole, and 5-flucytosine but sensitive to amphotericin B.
This organism was grown from specimens from three separate sites collected at different times over a period of 1 month: the initial biopsy, a swab from the base of a lesion, and the core of another of the skin lesions which spontaneously discharged. Within 2 weeks of the appearance of the first lesions, a total of 65 lesions of ecthyma gangrenosum appeared over the patient’s limbs, trunk, and scalp. Thirty-eight separate blood cultures (with blood taken from the time of the appearance of the first lesion until the time of death, by using Hemoline diphasic bottles) did not yield M. anisopliae. Twenty-eight of the 38 blood cultures underwent prolonged incubation for 21 days, to maximize the chance of recovering the fungus if it were present.
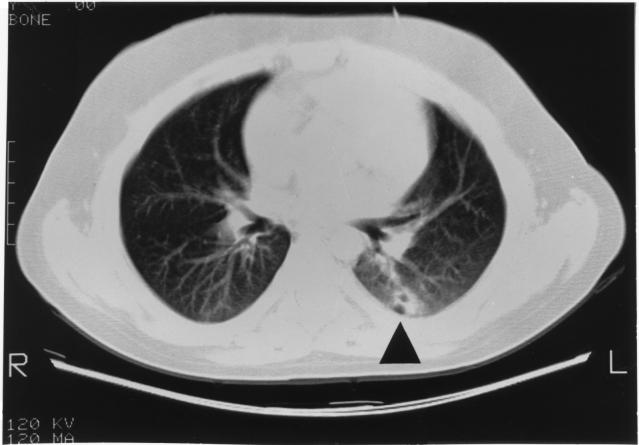
A chest radiograph taken at the onset of the ecthyma gangrenosum showed an opacity in the left lung, and a computed-tomography (CT) scan of the chest demonstrated this lesion to be in the superior segment of the lower lobe. The lesion demonstrated areas of central lucency consistent with small areas of cavitation (Fig. 2). An abdominal CT scan was normal, and an echocardiogram did not demonstrate fungal lesions within the heart or associated with the central venous catheter.
FIG. 2.
CT scan of the patient’s chest demonstrating a lesion (arrowhead) with areas consistent with small areas of cavitation in the superior portion of the lower lobe of the left lung.
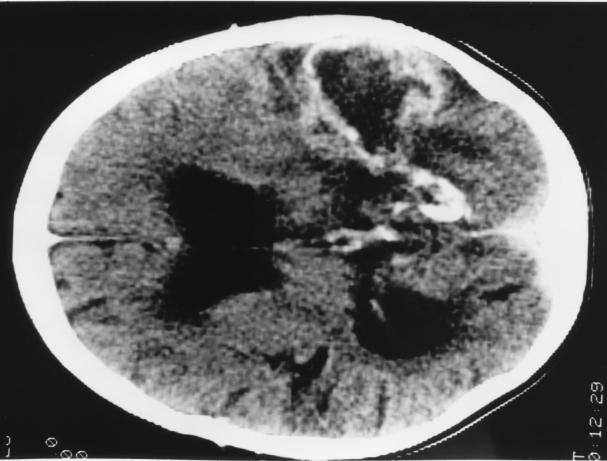
Six days after the appearance of ecthyma gangrenosum, the patient complained of headache and photophobia. A CT scan of the brain showed a wedge-shaped area of low density in the left temporoparietal region; this resembled an infarct and had no associated mass effect or edema. Over the next week, he became increasingly drowsy and confused and had a prolonged generalized seizure. A further brain CT scan indicated that the lesion had increased in size and that there was surrounding edema. Postictally the patient remained deeply obtunded. His antifungal treatment was changed to liposomal amphotericin, and 5-flucytosine (150 mg/kg/day, adjusted for renal function) was added. His parents did not grant permission for a brain biopsy. A further CT scan of the brain suggested organization and probable cavitation of the lesion in the parietal region, with surrounding edema, consistent with an abscess (Fig. 3). The patient remained comatose for a month. No new skin lesions appeared during this period, and antifungal and antibiotic therapy was continued.
FIG. 3.
CT scan of the patient’s brain demonstrating the lesion in the left parietal region. The lesion had an appearance consistent with an abscess, with probable cavitation and surrounding edema.
The patient’s level of consciousness gradually improved, but he had right hemiparesis and nominal dysphasia. Further antileukemic chemotherapy was withheld because of continuing fungal sepsis. Three months after presentation, the leukemia returned again, with 50% lymphoblasts in his peripheral blood. After consultation with his parents, in view of the poor prognosis and lack of a suitable bone marrow donor, the decision was made not to recommence further chemotherapy. The child’s peripheral lymphoblast count continued to rise, and he became neutropenic. The initial ecthyma lesions never resolved, in spite of the antifungal therapy, and further ecthyma gangrenosum lesions appeared on his limbs when he again became neutropenic despite continuing treatment with amphotericin. The new lesions were not cultured. He died 4 days later. Permission for a postmortem examination was refused. Neither lumbar puncture nor bronchoscopy was performed at any time.
Discussion.
M. anisopliae was cultured from this patient from three different cutaneous lesions on three separate occasions, and clinical, histological, and radiographic findings were consistent with a disseminated fungal infection. Although the patient eventually died from his underlying malignancy, the fungal infection caused severe morbidity and contributed to his death, as disseminated M. anisopliae infection precluded further chemotherapy. While the initial portal of entry is unknown, hematogenous dissemination of the organism to the skin and possibly the brain appears likely.
M. anisopliae is well recognized as an insect pathogen with a worldwide distribution. It is being used for biological control of insects belonging to the orders Coleoptera and Orthoptera, (beetles [21] and locusts [8]) and is currently registered for control of the redheaded pasture cockchafer in Australia under the trade name Biogreen. It is also relatively common in forest and cultivated soils throughout the world (5). The optimum temperature for growth is 25°C, with a generally recognized maximum near 35 to 36°C. In a study of 204 isolates of M. anisopliae, Yip et al. (21) were able to recognize three groups based upon their ability to grow at 5 or 37°C. At 37°C, several isolates produced colonies up to 6 mm in diameter after 7 days. The pH range for growth is 3.3 to 8.5.
M. anisopliae is capable of decomposing wool and chitin, and some isolates may be weakly lipolytic and proteolytic (5). It has been reported to produce a range of toxic metabolites, including cytochalasins C and D, which are highly toxic to mouse fibroblasts in culture (5), and destructins (cyclic peptides), which have been suggested to suppress cellular immune response to the pathogen in insects (12).
Ecthyma gangrenosum is indicative of disseminated sepsis. The lesions typically begin as erythematous or purpuric macules and progress to form indurated painless necrotic or hemorrhagic bullae (2). The lesions may be single or multiple and typically occur on the trunk and the limbs. Histologically, there is invasion of the dermis with necrosis of the epidermis and dermis. Invading organisms may be seen in the dermal blood vessels, and skin biopsy culture is often positive (2). Ecthyma gangrenosum is described to occur in infection with Pseudomonas aeruginosa (2, 6), Stenotrophomonas (Xanthomonas) maltophilia (17), and Klebsiella pneumoniae (15). Fungal infection with Fusarium species (10) and Scytalidium dimidiatum (1) has also been associated with the development of ecthyma gangrenosum, but as this case illustrates, other fungi can cause the characteristic lesions.
While there is no direct evidence that this patient had a cerebral abscess due to M. anisopliae, the presence of multiple ecthymic skin lesions is strongly suggestive of hematogenous dissemination, and the cerebral lesion seen on the CT scan is consistent with a fungal abscess. Fungi are common causes of brain abscesses in immunocompromised patients, especially in those with neutropenia or following marrow transplantation (7, 19). Occasional cases occur in the apparently immunocompetent (19). Aspergillus and Candida are the most common causes (7), although a variety of other opportunistic fungi have been described, including Rhizopus species (7), phaeohyphomycetes (7, 11), and, particularly, Cryptococcus species (19). The outcome is poor, with a very high mortality rate (19). To the best of our knowledge, M. anisopliae has not previously been reported as a cause of brain abscess or invasive disease in humans. One human isolate was apparently collected from sputum and deposited in the Centralbureau voor Schimmelcultures in 1952 (3). M. anisopliae has also recently been reported as a cause of keratitis in a patient from Colombia (4).
We have no evidence to suggest that the infection in this patient was connected with commercial usage of M. anisopliae.
REFERENCES
- 1.Benne C A, Neelman C, Bruin M, de Hoog G S, Fleer A. Disseminating infection with Scytalidium dimidiatum in a granulocytopenic child. Eur J Clin Microbiol Infect Dis. 1993;12:118–121. doi: 10.1007/BF01967587. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal N C, Sood U R, Aronsen P J, Hashimoto K. Facial ulcerations in an immunocompromised patient. Arch Dermatol. 1990;126:527–532. doi: 10.1001/archderm.126.4.529. [DOI] [PubMed] [Google Scholar]
- 3.Centralbureau voor Schimmelcultures Baarn. List of cultures. 33rd ed. Baarn, The Netherlands: Centralbureau voor Schimmelcultures; 1994. [Google Scholar]
- 4.Cepero de Garcia M C, Arboleda M L, Barraquer F, Grose E. Fungal keratitis caused by Metarrhizium anisopliae var. anisopliae. J Med Vet Mycol. 1997;35:361–363. [PubMed] [Google Scholar]
- 5.Domsch K H, Gams W, Anderson T-H. Compendium of soil fungi. London, United Kingdom: Academic Press; 1980. [Google Scholar]
- 6.Fergie J E, Patrick C C, Lott L. Pseudomonas aeruginosa cellulitis and ecthyma gangrenosum in immunocompromised children. Pediatr Infect Dis J. 1991;10:496–500. doi: 10.1097/00006454-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hagensee M E, Bauwens J E, Kjos B, Bowden R A. Brain abscess following marrow transplantation: experience at the Fred Hutchinson Cancer Research Center, 1984–1992. Clin Infect Dis. 1994;19:402–408. doi: 10.1093/clinids/19.3.402. [DOI] [PubMed] [Google Scholar]
- 8.Hall R A, Papierok B. Fungi as biological control agents of arthropods of agricultural and medical importance. Parasitology. 1982;84:205–240. [Google Scholar]
- 9.Koll B S, Brown A E. Changing patterns of infections in the immunocompromised patient with cancer. Hematol Oncol Clin North Am. 1993;7:753–769. [PubMed] [Google Scholar]
- 10.Martino, P., R. Gastaldi, R. Raccah, and C. Girmenia. 1994. Clinical patterns of Fusarium infections in immunocompromised patients. J. Infect. 28(Suppl. 1):7–15. [DOI] [PubMed]
- 11.Palaoglu S, Sav A, Basak T, Yalcinlar Y, Scheithauer B W. Cerebral phaeohyphomycosis. Neurosurgery. 1993;33:894–897. doi: 10.1227/00006123-199311000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Parry M. A review of mycochemical and insect interactions. Biocontrol News Inf. 1995;16:27N–33N. [Google Scholar]
- 13.Pitt J I, Hocking A D. Fungi and food spoilage. 2nd ed. London, United Kingdom: Blackie Academic and Professional; 1997. [Google Scholar]
- 14.Pizzo, P. A., and T. J. Walsh. 1990. Fungal infections in the paediatric cancer patient. Semin. Oncol. 17(Suppl 6):6–9. [PubMed]
- 15.Stotka J L, Rupp M E. Klebsiella pneumoniae urinary tract infection complicated by endophthalmitis, perinephric abscess, and ecthyma gangrenosum. South Med J. 1991;84:790–793. doi: 10.1097/00007611-199106000-00032. [DOI] [PubMed] [Google Scholar]
- 16.Tulloch M. The genus Metarhizium. Trans Br Mycol Soc. 1976;66:407–411. [Google Scholar]
- 17.Vartivarian S E, Papadakis K A, Palacios J A, Manning J T, Anaissie E J. Mucocutaneous and soft tissue infections caused by Xanthomonas maltophilia. A new spectrum. Ann Intern Med. 1994;121:969–973. doi: 10.7326/0003-4819-121-12-199412150-00011. [DOI] [PubMed] [Google Scholar]
- 18.Wilson E. Cerebral abscess caused by Cladosporium bantianum. Case report. Pathology. 1982;14:91–96. doi: 10.3109/00313028209069050. [DOI] [PubMed] [Google Scholar]
- 19.Wispelwey B, Scheld M W. Brain abscess. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious disease. 4th ed. New York, N.Y: Churchill; 1995. p. 890. [Google Scholar]
- 20.Wood G, McCormack J, Muir D, Ellis D, Ridley M, Pritchard R, Harrison M. Clinical features of human infection with Scedosporium inflatum. Clin Infect Dis. 1992;14:1027–1033. doi: 10.1093/clinids/14.5.1027. [DOI] [PubMed] [Google Scholar]
- 21.Yip H Y, Rath A C, Koen T B. Characterization of Metarhizium anisopliae isolated from Tasmanian pasture soils and their pathogenicity to redheaded pasture cockchafer (Coleoptera: Scarabaeidae: Adoryphous couloni) Mycol Res. 1992;96:92–96. [Google Scholar]