Abstract
The complexity of the tumor microenvironment (TME) severely hinders the therapeutic effects of various cancer treatment modalities. The TME differs from normal tissues owing to the presence of hypoxia, low pH, and immune-suppressive characteristics. Modulation of the TME to reverse tumor growth equilibrium is considered an effective way to treat tumors. Recently, polymeric nanomedicines have been widely used in cancer therapy, because their synthesis can be controlled and they are highly modifiable, and have demonstrated great potential to remodel the TME. In this review, we outline the application of various stimuli responsive polymeric nanomedicines to modulate the TME, aiming to provide insights for the design of the next generation of polymeric nanomedicines and promote the development of polymeric nanomedicines for cancer therapy.
Keywords: cancer therapy, polymeric nanomedicines, stimuli responsive, tumor microenvironment
Introduction
Cancer is among the leading cause of death worldwide [1]. A major obstacle to effective cancer treatment is the extreme complicated tumor microenvironment (TME), which greatly contributes to the growth, progression, and invasion of solid tumors [2]. The TME is unique and complex, containing proliferating tumor cells, endothelial cells, vasculature, extracellular matrix (ECM), tumor-associated fibroblasts (TAFs), lymphocytes, bone marrow-derived inflammatory cells, and signaling molecules [3]. The rapid proliferation of tumor cells and dysfunctional vascular growth lead to tumor hypoxia and acidosis and decrease the efficacy of various anti-tumor therapies, including chemotherapy and radiotherapy [4]. In addition, the tumor ECM consists of a network of macromolecules, including glycoproteins and fibrous proteins [5]. The physical properties, composition, and morphology of the ECM affect the migration and invasion of tumor cells [6]. Moreover, the TME is immune suppressive, containing very high levels of suppressive immune cells. For example, tumor-associated macrophages (TAMs) support cell invasion and expansion by producing various molecules that promote tissue remodeling (e.g., matrix metalloproteinases [MMPs]) and pro-inflammatory molecules (e.g., tumor necrosis factor alpha [TNF-α]) [7]. Regulatory T cells (Tregs) suppress the anti-tumor immune responses of effector T cells by activating transforming growth factor-β (TGF-β) produced by cancer cells, thereby promoting cancer immune escape [8]. Numerous studies have shown that modulation of the TME is crucial for effective cancer treatment [9].
An increasing number of approaches have been developed to modulate the TME [10], [11], [12], [13]. For example, tumor hypoxia can be alleviated by improving tumor blood flow [14], delivering oxygen to the tumor site [15], generating oxygen in situ [16], or reducing oxygen consumption [17]. Many research groups have explored pH interference therapies that target pH-regulated membrane transport proteins or vacuolar-type H+-ATPase (V-ATPase), using proton pump inhibitors such as V-ATPase inhibitors to reverse the development of the acidic TME [18, 19]. In addition, the TME can be modulated by disrupting the tumor ECM. By decreasing the number of TAFs or the intratumoral collagen content, the interstitial flow pressure within the tumor can be decreased and blood perfusion inside the tumor can be increased, thereby improving drug accumulation and penetration [20]. Furthermore, the development of immunotherapy has enabled the modulation of the immune-suppressive microenvironment [21]. For instance, effective strategies have been developed to polarize TAMs from M2-like macrophages to M1-like macrophages to enhance anti-tumor immune responses [22]. Other approaches including delivering immunomodulators, promoting dendritic cell (DC) activation and antigen cross-presentation, enhancing the effector function of T cells, and attenuating immunosuppressive activities such as those of Tregs and myeloid-derived suppressor cells (MDSCs) have also been used to remodel the tumor immune-suppressive microenvironment [23, 24].
Nanomedicines are widely used in cancer therapy because their prolonged blood circulation time and enriched tumor accumulation through the enhanced permeability and retention (EPR) effect [25, 26]. In particular, advances in polymer chemistry have made it possible to synthesize a variety of smart polymeric nanocarriers with versatile functionalities [27]. Polymeric nanomedicines could be prepared from natural or synthetic polymers with precise control of their structure and properties, including size, charge, and morphology [28]. Therapeutic agents are encapsulated within the nanoparticle core, or chemically coupled to the polymer backbone or the nanoparticle surface. This allows polymeric nanomedicines to deliver a variety of payloads, including hydrophobic and hydrophilic small-molecule drugs and biomacromolecules (proteins or genes) [25].
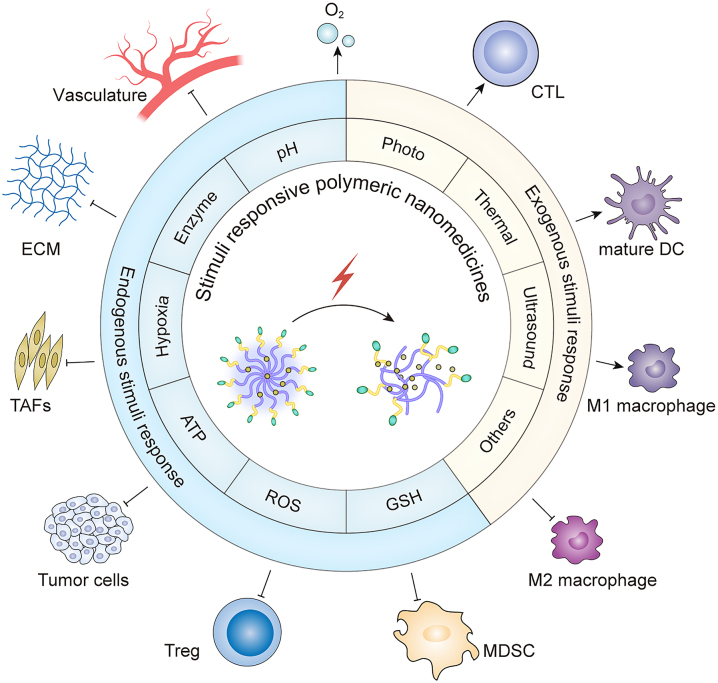
Here, we summarize the recent progress in the development of stimuli responsive polymeric nanomedicines (including those responding to endogenous stimuli such as pH, enzymes, hypoxia, redox, and ATP, as well as exogenous stimuli such as photo, thermal, and ultrasound) to modulate the TME (Figure 1). These nanomedicines typically contain responsive chemical groups/bonds that are summarized and can achieve stimuli-triggered responses to achieve the desired properties (Table 1). Such stimuli responsive polymeric nanomedicines modulate the TME by disrupting and interfering with the tumor ECM, disrupting TAFs, causing tumor vascular rupture or normalization, modulating tumor hypoxia, or changing the suppressive immune microenvironment. The aim of this review is to provide insight into the next generation of polymeric nanomedicines that may help to improve cancer therapy in the future.
Figure 1:
Schematic diagram of stimuli responsive polymeric nanomedicines modulating the tumor microenvironment to improve cancer therapy. Reactive oxygen species (ROS); Glutathione (GSH); Extracellular matrix (ECM); Tumor-associated fibroblasts (TAFs); Regulatory T cell (Treg); Dendritic cell (DC); Myeloid-derived suppressor cell (MDSC); Cytotoxic T lymphocyte (CTL).
Table 1:
Summary of stimuli responsive groups/bonds.
| Type of responsive | Responsive group/bond | Chemical structure | Ref. |
|---|---|---|---|
| pH | Imidazole |
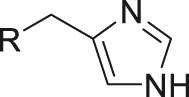
|
[12] |
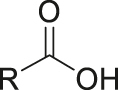
| pH | Carboxylic acid |
|
[29] |
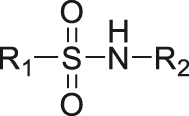
| pH | Sulphonamide |
|
[30] |
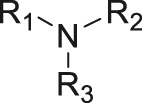
| pH | Amine |
|
[31, 32] |
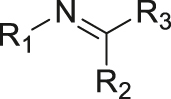
| pH | Imine |
|
[33] |
| pH | Acetal/ketal |
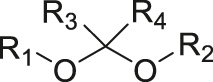
|
[34] |
| pH | Boronate ester |
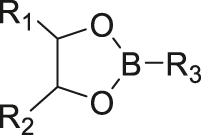
|
[35, 36] |
| pH | Hydrazone |
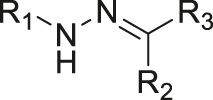
|
[37] |
| pH | Enamine |
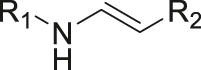
|
[38] |
| pH | Oxime |
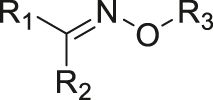
|
[39] |
| pH | Amide |
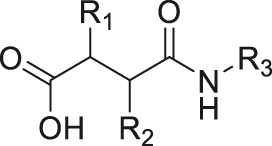
|
[40] |
| pH | Orthoester |
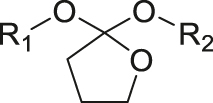
|
[41] |
| Enzyme (γ-glutamyl transpeptidase) | γ-glutamylamide |
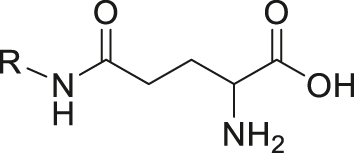
|
[42] |
| Enzyme (esterase) | Ester |
|
[43] |
| Enzyme (cathepsin B) | Peptide | LL/RR/AL/FR/FK/GFLG/ALAL | [44] |
| Enzyme (MMP) | Peptide | GPLGV/PVGLIG | [45] |
| Enzyme (Caspase-3) | Peptide | DEVD | [46] |
| Enzyme (legumain) | Peptide | AADL | [47] |
| Hypoxia | Azobenzene |
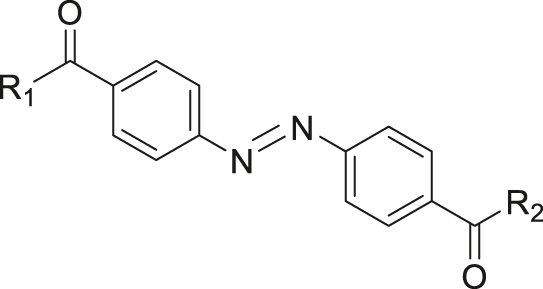
|
[48] |
| Hypoxia | 2-Nitroimidazole |
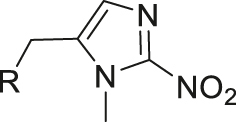
|
[49] |
| ROS | Thioketal |
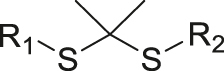
|
[50, 51] |
| ROS | Phenylborate ester |
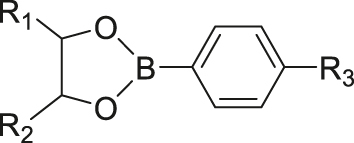
|
[52] |
| ROS | Thioether |
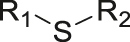
|
[53] |
| ROS | Selenoether |

|
[54] |
| ROS | Aminoacrylate |
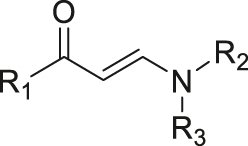
|
[55] |
| ROS | Peroxalate ester |
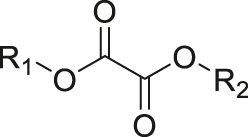
|
[56] |
| GSH | Disulfide |
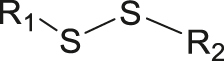
|
[57] |
| GSH | Thiol-pyridazinedione |
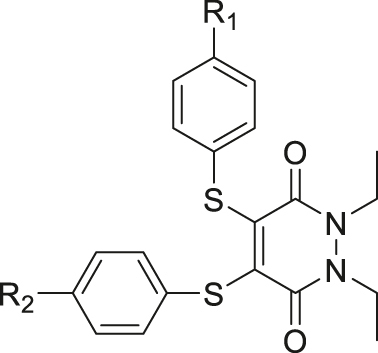
|
[58] |
| GSH | 2,4-Dinitrobenzenesulfonyl |
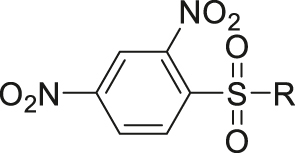
|
[59] |
| GSH | Trisulfide |
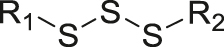
|
[60] |
| GSH | Platinum (IV) |
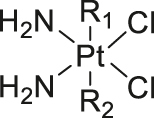
|
[61] |
| ROS/GSH | Diselenide |
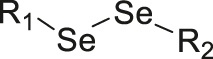
|
[62] |
| ATP | Host-guest interaction |
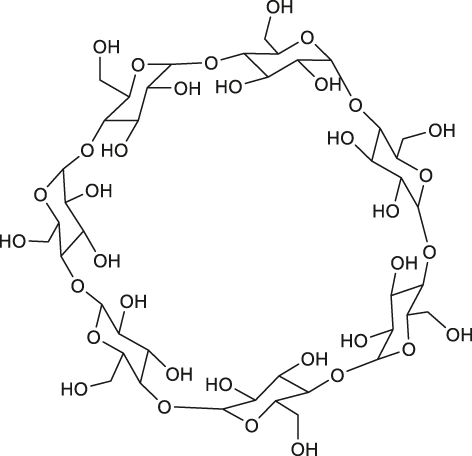
|
[63] |
| ATP | DNA/RNA (hydrogen bond) | ACCTGGGGGAGTATTGCGGAGGAAGGT | [64] |
| ATP | Metal coordination (coordination competition) | Zn2+ | [65] |
| ATP | Phenylborate ester (ester exchange reaction) |
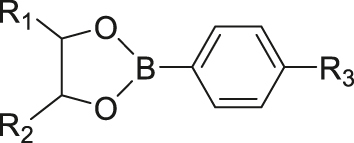
|
[66] |
| Photo | o-nitrobenzyl |
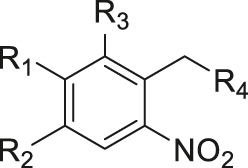
|
[67] |
| Photo | Coumarin |
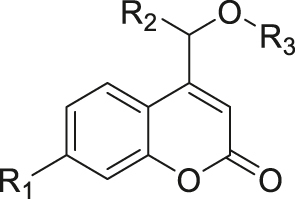
|
[68] |
| Photo | Boron-dipyrromethene |
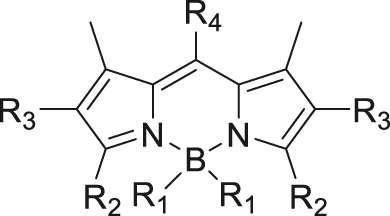
|
[69] |
| Thermal | Azo |

|
[70] |
| Thermal | Poly(N-isopropylacrylamide) |
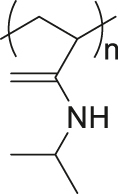
|
[71] |
| Thermal | Elastin-like polypeptide | VPGXG (X is any amino acid other than proline) | [72] |
| Ultrasound | Disulfide |
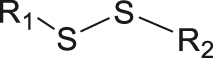
|
[73, 74] |
| Ultrasound | Poly(methoxyethyl methacrylate) |
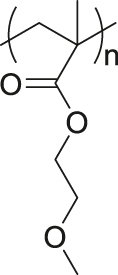
|
[75] |
| Magnetic | Superparamagnetic iron oxide | Fe3O4 | [76] |
| X-ray | Diselenide |
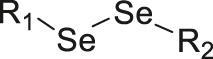
|
[77] |
Characteristics of the tumor microenvironment
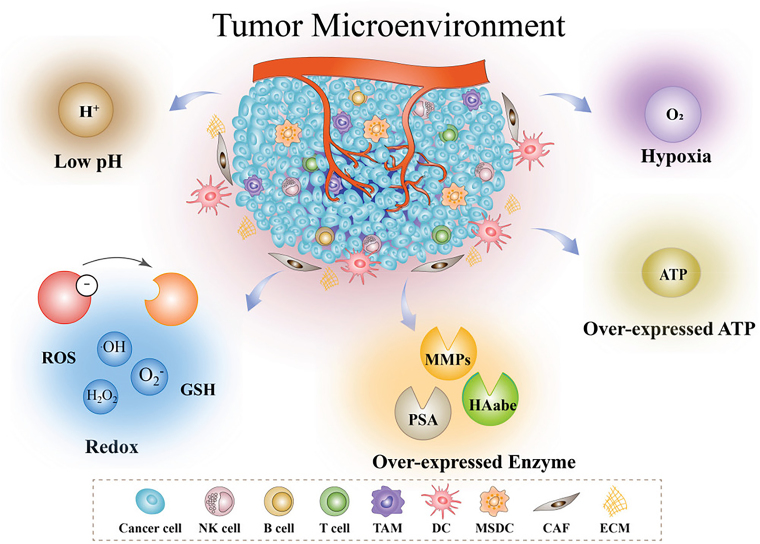
The physical heterogeneity of a solid tumor leads to a complex system inside the tumor, including not only tumor cells but also vasculature, ECM, infiltrating immune cells, TAFs, multiple signaling molecules, and an abnormal metabolic environment [78]. A major feature of the TME is the inhibition of tumor suppressor genes and the activation of pro-oncogenes, resulting in the proliferation and invasion of tumor cells. Compared with normal tissues, the TME usually exhibits an acidic, hypoxic, redox environment with high levels of reactive oxygen species (ROS), glutathione (GSH), enzymes, and ATP (Figure 2) [79].
Figure 2:
Characteristics of the TME include hypoxia, low pH, redox, overexpression of enzymes, overexpression of ATP, and an immune-suppressive microenvironment. Reproduced with permission from Peng et al. [79]. Copyright 2022, Wiley‐VCH GmbH.
The proliferation of tumor cells requires more oxygen than that of normal cells and produces large amounts of lactic acid, resulting in an imbalance in oxygen supply and causing a low pH and hypoxic microenvironment [80]. Furthermore, the production and regulation of ROS in cancer plays an important part in determining the progression of cancer [81]. Tumor cells promote their own proliferation, metastasis, and epithelial–mesenchymal transition by ROS production in the TME. Moreover, elevated ROS leads to the adaptive development of an enhanced antioxidant defense system in tumor cells that scavenges ROS by elevating the concentration of GSH [82]. GSH is the primary ROS quencher and is considered to be another key regulator of tumor initiation, progression, and metastasis. High GSH concentration in tumors has been associated with resistance to chemotherapy and radiotherapy. Therefore, eliminating intracellular GSH to enhance cancer therapy is considered an attractive approach [83].
An increase in extracellular ATP is a hallmark of the TME [84]. Elevated extracellular ATP is mainly from intratumoral cells, including stromal and parenchymal cells. Tumor tissue is relatively hypoxic owing to its excessive growth rate. In addition, there is an immune response in the TME with infiltration of interstitial inflammatory cells leading to massive tumor and stromal cells necrosis. Release of intracellular ATP results in ATP enrichment within the TME, which in turn promotes cancer cell growth by activating purinergic receptors (e.g., P2X7) [85].
Enzyme expression and secretion in the TME is also different from that in normal tissues [86]. In particular, the TME overexpresses multiple enzymes, including MMP, hyaluronidase, and esterase [87]. For example, MMPs are hydrolytic enzymes that are highly expressed in the ECM of tumors and are closely related to the development and metastasis of tumors. Therefore, these enzymes are attractive therapeutic targets for cancer treatment strategies [88].
Solid tumors use negative regulatory mechanisms to suppress the anti-tumor activity of the immune system. The TME contains many immunosuppressive cells and cytokines [89]. MDSCs, Tregs, and M2-like macrophages are three major immunosuppressive cell types that exert their suppressive function by secreting negative immunoregulatory cytokines such as interleukin-10 and TGF-β [90, 91]. This immunosuppressive environment not only promotes tumor cell growth and metastasis but also impedes DC maturation and antigen presentation, further reducing the immune efficacy of T cells and exacerbating immunosuppression. In addition, tumor cells express immune checkpoint proteins such as programmed death ligand 1 and the “don’t eat me” signal CD47, which inhibit the attack of effector T cells and phagocytes for immune escape [92, 93].
Nanomedicine systems are currently used to deliver anti-tumor drugs to tumor sites by active or passive targeting; this approach is more effective and safer than traditional treatment methods [94]. To achieve better antitumor efficacy, responsive nanomedicines have been widely designed. According to their type of responsiveness, polymeric nanomedicines can be classified into two types: endogenous stimuli responsive and exogenous stimuli responsive nanomedicines, and we summarize these polymeric nanomedicines separately according to different types of responsiveness in this review.
Endogenous stimuli responsive polymeric nanomedicines
pH responsive polymeric nanomedicines
Since the concept of pH-sensitive linkage release of drugs was first proposed in 1981 [95], large numbers of acidity-triggered polymeric nanomedicines have been developed for cancer treatment [96], [97], [98], [99], [100]. The TME (pH 6.5–7.2) and the endosome/lysosomal envelope (pH 4.5–6.5) are weakly acidic, in contrast to the neutral pH level of normal tissue (pH 7.4) [101]. pH responsive linkers including hydrazone bonds, acetal bonds, cis-aconityl groups, Schiff bases, and β-thiopropionate linkages have been used in the design of polymeric nanomedicines [102]. The incorporation of pH responsive linkers between polymers and drugs enables acidity-triggered release of therapeutic agents in the ECM or after intracellularization in endosomes/lysosomes in tumor tissues. Numerous studies have shown that such pH responsive nanomedicines can effectively modulate the TME to treat refractory cancer [75, 103], [104], [105], [106], [107].
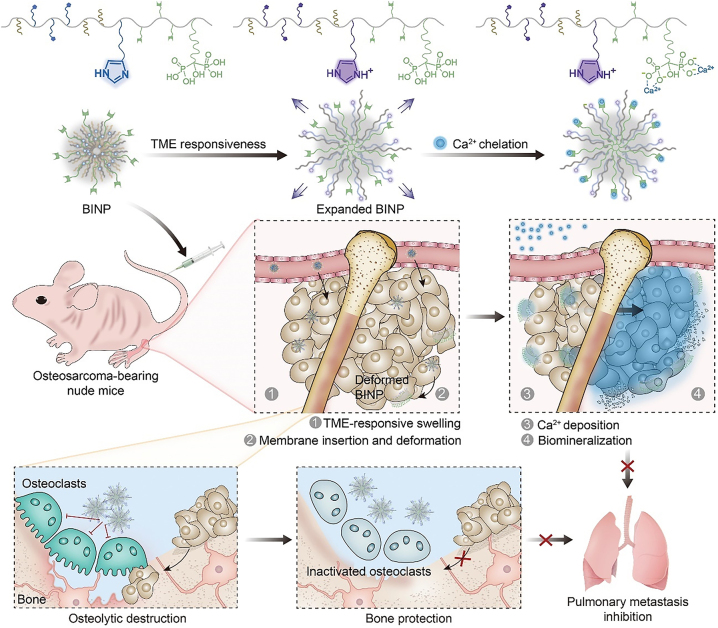
For instance, Chen and Ding group designed a pH responsive nanomedicine for the biomineralization therapy of osteosarcoma [12]. They synthesized novel biomineralization-inducing nanoparticles (BINP) composed of dodecylaminepoly([γ-dodecyl-L-glutamate]-co-L-histidine)-block-poly(L-glutamate-graft-alendronate) (DDA-P[DLG-co-LH]-b-P[LG-g-ALN]). BINP assembled into nanoparticles under normal physiological conditions, but the self-assembly process was disturbed and the internal aliphatic chains were exposed under weakly acidic conditions. After injection of the nanoparticles into osteosarcoma tumor-bearing mice, BINP was cleaved in the weakly acidic TME, exposing the dodecyl groups on the surface of the nanoparticles, which promoted insertion of the nanoparticles into the cell membrane. Owing to the subsequent continuing ion deposition triggered by the protruding bisphosphonic acid group, a mineralized barrier that could block substance exchange between the tumor and surrounding normal tissues was constructed. The progression of osteosarcoma was suppressed with minimal side effects (Figure 3).
Figure 3:
Schematic illustrating the TME responsive BINP for osteosarcoma therapy. Reproduced with permission from Liu et al. [12]. Copyright 2022, Wiley-VCH GmbH.
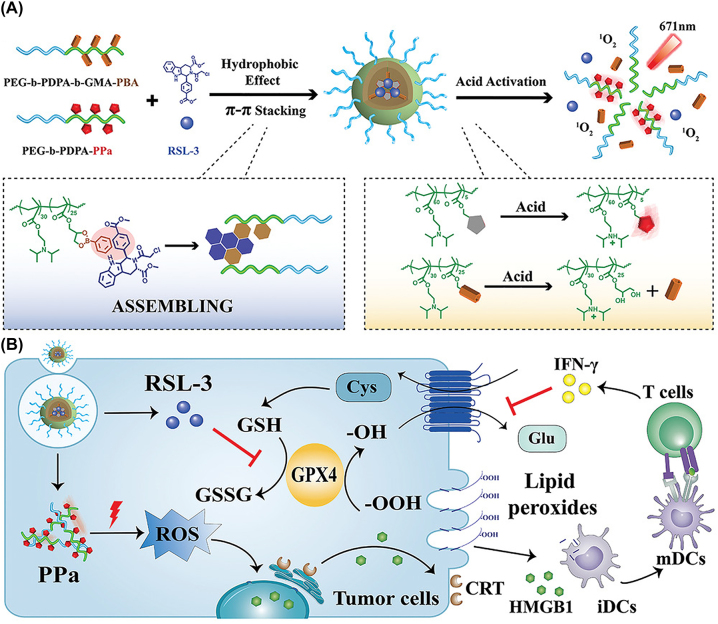
Yu group engineered intracellular pH responsive nanomedicines which could promote ferroptosis of tumor cells and generate an anti-tumor immune response [108]. At neutral pH (pH 7.4), the nanoparticles could stably encapsulate pyropheophorbide-a (PPa) to mediate photodynamic therapy (PDT), as well as ferroptosis inducer RSL-3. However, in an endocytic vesicle (pH 5.8–6.2), the payload was released via acid-triggered cleavage of the dynamic covalent bond of the phenylboronate ester (Figure 4). PDT treatment resulted in infiltration of IFN-γ-positive T cells, which further synergized with RSL-3 to promote ferrogenesis at the tumor site, producing an efficient anti-tumor immune response.
Figure 4:
Schematic illustration of the induction of immunogenicity by pH responsive nanomedicines. (A) Preparation and activation of PPa-conjugated nanoparticles. (B) Boosting of ferroptotic death by nanoparticles. Reproduced with permission from Song et al. [108]. Copyright 2021, Wiley‐VCH GmbH.
In addition, Ding and Nie group developed a stimuli responsive DNA based nanovaccine for cancer immunotherapy [109]. Antigens and multiple adjuvants were accurately loaded on the nanodevice to stimulate tumor-specific T cell responses that efficiently inhibited tumor growth. With a size suitable to efficient draining to and retention in draining lymph nodes, the nanodevice achieved enhanced delivery to antigen-presenting cells (APCs) and exposed its active payloads in a pH responsive manner. Moreover, Liang and Li group developed a proton-driven nanotransformer-based vaccine that could induce a strong immune response [34]. In an acidic environment, the particles transformed into bigger structures, which caused endosomal membrane disruption and thus transported antigenic peptides (APs) to the cytoplasm. As well as promoting cytosolic delivery and cross-presentation of APs, this nanovaccine could activate the NLRP3-inflammasome pathway and thus strengthen antitumor immunity.
Enzyme responsive polymeric nanomedicines
Enzymes have essential roles in current nanotechnology because of their excellent biorecognition abilities and preeminent catalytic properties [88, 110, 111]. The abnormal enzyme expression observed in the TME facilitates strategies using enzyme responsive polymeric nanomedicines for precise delivery and triggered release of therapeutic agents at the tumor site to enhance cancer therapy [87, 112], [113], [114], [115], [116], [117], [118], [119].
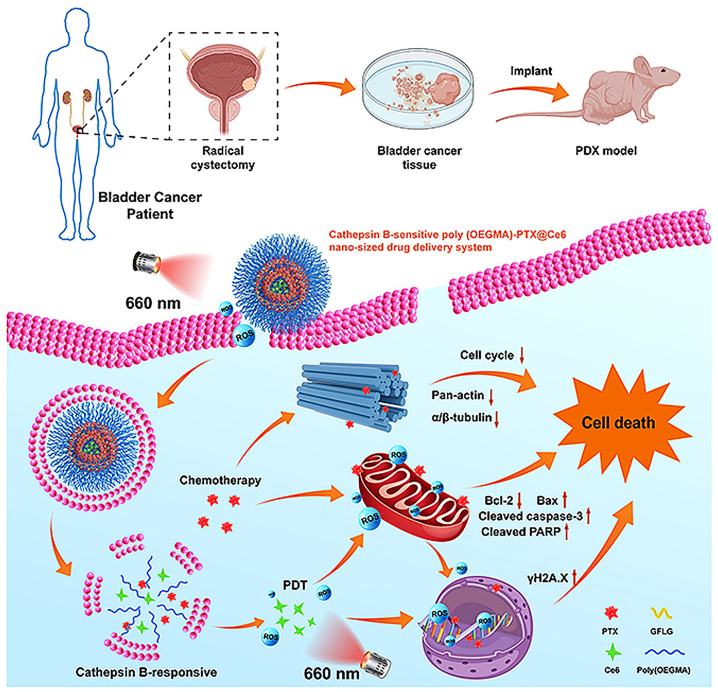
For example, cathepsin B is a proteolytic enzyme secreted and overexpressed in the TME of many solid tumors including breast cancer, colorectal cancer, melanoma, and, prostate cancer [120]. The use of cathepsin B responsive linkers in construction of nanomedicines for effective treatment of tumors has been widely explored [121], [122], [123], [124]. Pu and Huang group reported a semiconducting polymer nano-proteolysis targeted chimeras (SPNpro) with phototherapeutic and activatable protein degradation abilities for photo-immunometabolic cancer therapy [125]. SPNpro was specifically activated by cathepsin B to trigger targeted proteolysis of immunosuppressive indoleamine 2,3-dioxygenase in tumor-bearing mice, thereby promoting the activation of effector T cells. Furthermore, Luo group synthesized poly (OEGMA)-PTX@Ce6 (NPs@Ce6) composed of a photosensitizer, chlorin e6 (Ce6), and a cathepsin B responsive polymer-paclitaxel (PTX) prodrug (Figure 5) [126]. The hydrophilic fragment of the polymer prodrug was prepared by reversible addition fragmentation chain transfer polymerization of OEGMA, and PTX was covalently linked to the polymer backbone through a cathepsin B responsive tetrapeptide Gly-Phe-Leu-Gly (GFLG). The combination of PTX-based chemotherapy with PDT upregulated oxidative phosphorylation and ROS production, blocked the cell cycle and proliferation, and downregulated genes associated with tumor progression, invasion, and metastasis.
Figure 5:
Schematic illustration of the cathepsin B responsive PTX prodrug. Reproduced with permission from Tan et al. [126]. Copyright 2021, Elsevier.
Overexpression of various MMPs has been reported in multiple types of tumors, and MMP-2 and MMP-9 in particular have been explored with respect to applications in enzyme responsive drug delivery [127, 128]. For instance, Yu and Li group designed an enzyme activatable prodrug vesicles (EAPVs) for combination photodynamic immunotherapy [129]. To construct the TME sheddable prodrug vesicles, PPa was conjugated with methoxy poly(ethylene glycol) (mPEG-NH2) via an MMP-2-liable peptide spacer to obtain mPEG-GALGLPG-PPa. EAPV-mediated PDT resulted in an immune-promoting environment with enhanced intratumoral infiltration of IFN-γ-positive T cells, inhibited tryptophan degradation in the tumor tissues, and finally reduced infiltration of intratumoral Tregs.
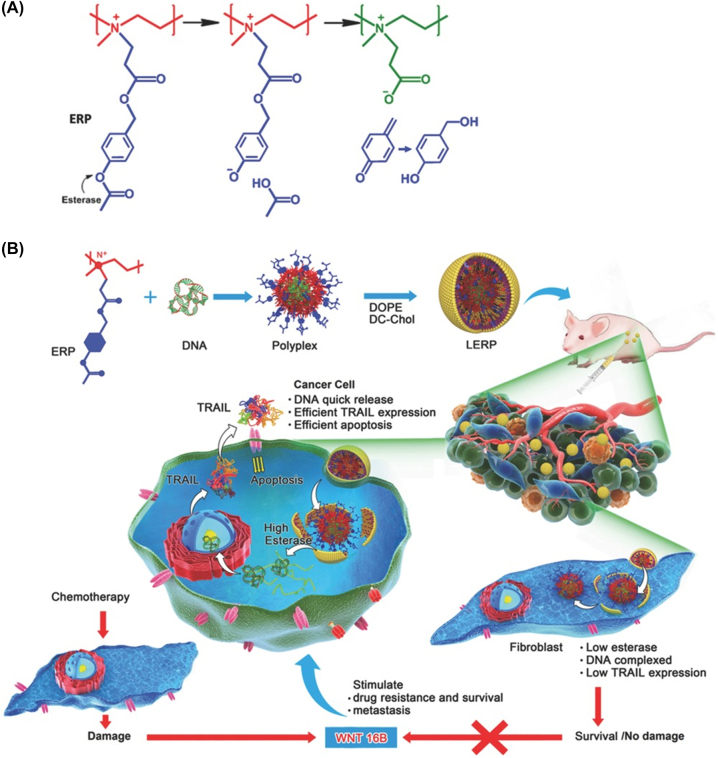
Many nanomedicines have been designed based on enzymes responsive structures. These nanomedicines not only kill tumor cells but also prevent excessive activation of fibroblasts, thereby reducing drug resistance and tumor metastasis. For instance, Shen group designed an esterase responsive charge-reversal polymer (ERP) [87]. Alkyl esters are stable and difficult to hydrolyze even in the presence of enzymes, whereas phenolic acetates are easily hydrolyzed by esterase. Hence, 4-acetoxybenzyl esters were employed as triggers for ester-catalyzed charge reversal. The ERP polyplexes showed selective gene expression in cancer cells with high levels of esterase but conserved fibroblasts owing to their low esterase activity. Such polyplexes effectively induced apoptosis of HeLa cells but did not activate fibroblasts to secrete WNT16B which caused the surviving cancer cells to be drug resistant and metastatic, enabling potent anticancer activity with few side effects (Figure 6).
Figure 6:
Schematic illustration of esterase responsive nanomedicines for cancer gene therapy. (A) The charge-reversal mechanism of ERP in the presence of esterase. (B) Application of the ERP mechanism in cancer gene therapy. Reproduced with permission from Qiu et al. [87]. Copyright 2016, Wiley-VCH GmbH.
Hypoxia responsive polymeric nanomedicines
The unlimited proliferation and metabolism of tumor cells leads to hypoxia in the TME, which causes drug resistance and tumor metastasis [130]. Malignant tumors are difficult to cure and prone to relapse after surgery, largely owing to the hypoxic TME. Therefore, changing the hypoxic environment at the tumor site is an important aspect of tumor treatment [131]. In addition, to utilize tumor hypoxia, various hypoxia responsive nanomedicines have also been proposed. There are three main strategies to treat hypoxic tumors: improving the hypoxic TME [132], hypoxia responsive nanomedicines [133], and hypoxia activated prodrug [134].
You group reported a multifunctional nanomedicine co-loaded with hemoglobin and doxorubicin (DOX) to change the hypoxic TME and enhance the therapeutic effect of chemotherapy medicines [135]. This nanomedicine could be efficiently loaded with oxygen and deliver oxygen and chemotherapeutic drugs to the tumor site, causing them to accumulate at the tumor site. It not only reversed the hypoxia of the TME but also improved the utilization rate of chemotherapy drugs. Compared with commonly used oxygen delivery strategies, such as hyperbaric oxygen and perfluorinated carbon nanoparticles, this hemoglobin and DOX co-loaded nanomedicine overcame the problems of limited oxygen-loading capacity and inefficient drug delivery to hypoxic tumors.
Abnormal proliferation of tumor cells, an inadequate blood supply, and insufficient endogenous oxygen lead to hypoxia of tumor tissues [136]. A common strategy is to engineer hypoxia responsive release through hypoxia-sensitive linkages [137]. Guo group reported a calixarene based hypoxia responsive molecular container called CAC4A, which showed strong binding to a range of chemotherapy medicines [138]. Formation of a complex with CAC4A significantly improved the solubility and stability of drugs, and, more importantly, the host-guest complex dissociated easily under low oxygen conditions. Under these conditions, the reduction of the azo group of CAC4A led to a decrease in the affinity between the drug and calixarene, with eventual targeted release of the medicine within the tumor.
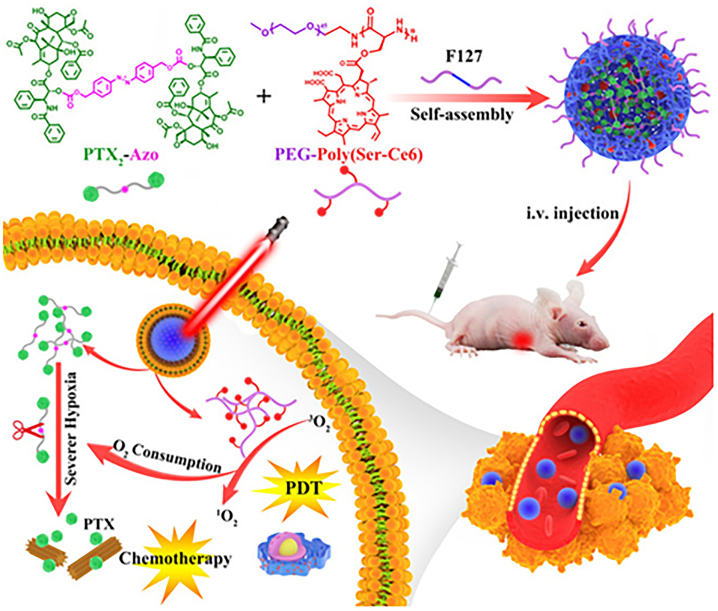
Hypoxia responsive prodrug have been designed primarily for combination with PDT and vascular occlusion to enhance the therapeutic effect of the prodrug [131, 139]. With increasing tumor volume and continuous proliferation, many blood vessels are established near the tumor, which indicates a new way to alter the TME [140]. Disruption of the established tumor vasculature effectively blocks the blood supply to the tumor, thereby preventing exponential tumor growth and metastasis [141]. Jing group reported a PTX prodrug that could be activated in a hypoxic environment [142]. They first modified the photosensitizer Ce6 in the peptide copolymer and then encapsulated the PTX autolytic medicine (PTX2-Azo) in the copolymer (Figure 7). This design achieved the precise release of PTX under the tumor hypoxic environment.
Figure 7:
Design of hypoxia responsive nanomedicine Ce6/PTX2-Azo NP and its release under hypoxic conditions in vivo. Reproduced with permission from Zhou et al. [142]. Copyright 2020, Wiley-VCH GmbH.
Cancer stem cells (CSCs) are highly resistant in the hypoxic region of tumors and are also highly carcinogenic; these are the main causes of tumor proliferation and recurrence [143]. Mo group reported a nanomedicine loaded with differentiation inducer all-trans retinoic acid (ATRA) and chemotherapy medicine camptothecin (CPT) [144]. First, ATRA was released in the hypoxic TME, and the nanoparticles dissociated. Then, ROS levels increased in the vicinity of tumor cells, leading to the release of CPT. This strategy not only achieved controlled drug release but also reduced CSC-related drug resistance.
Redox responsive polymeric nanomedicines
ROS is a general term for oxygen-containing chemically reactive substances, including singlet oxygen (1O2), hydrogen peroxide (H2O2), superoxide anion (O2-), and hydroxyl radical (–OH) [145]. There are many mechanisms by which cells produce ROS, but endogenous ROS are mainly generated through mitochondrial metabolism, or by peroxidases and members of the transmembrane NaDPH oxidase family [81]. Owing to the activation of oncogenes, tumor suppressor function is lost and mitochondrial activity is changed, resulting in significantly higher levels of ROS in tumor cells compared with normal tissue cells. The process of tumor spread further increases the production of ROS [146]. Therefore, ROS responsive nanomedicines can achieve more precise drug delivery at the tumor site [147, 148].
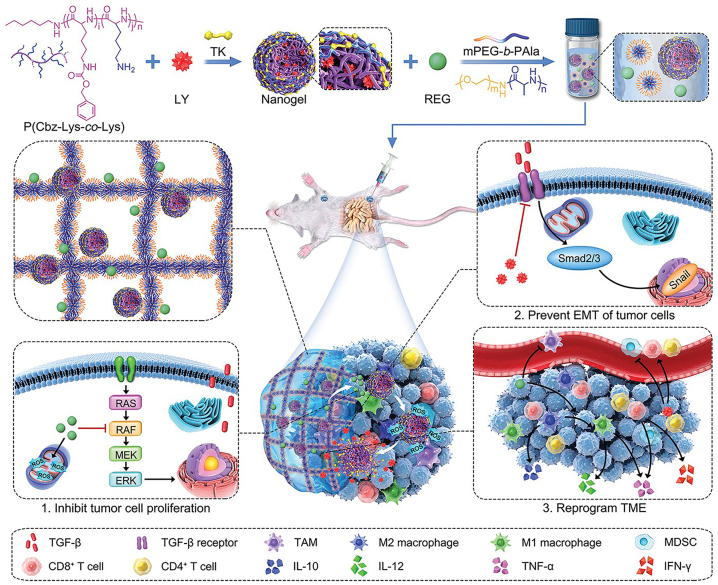
Chen group reported a ROS responsive nanomedicine consisting of β-lapafenone (Lap), ferric ions, and a ROS responsive PEG-block-poly thioketal DOX prodrug. Lap catalyzed the generation of H2O2, which killed tumor cells by producing toxic –OH through the Fenton reaction [97]. DOX was released in response to a high concentration of ROS levels when the nanomedicine entered the TME. Furthermore, Ding group reported a TME responsive composite gel consisting of a temperature responsive hydrogel and a ROS responsive nanogel, which enabled precise drug release while enhancing nanomedicine targeting and immune system activation [149]. Regorafenib (REG) was preferentially released from the hydrogel after in situ formation, generated ROS and inhibited tumor growth. Subsequently, excess ROS enabled LY3200882 (a selective TGF-β inhibitor) to be released on demand, thereby reversing the tumor proliferation and immune escape of tumor cells induced by TGF-β (Figure 8).
Figure 8:
Schematic illustration of the preparation and in vivo antitumor effects of gel/(REG+NG/LY). After hydrogel formation in situ, REG and LY are released sequentially to induce ICD and enhance immune effects. Reproduced with permission from Li et al. [149]. Copyright 2022, Wiley-VCH GmbH.
Shuai group reported a ROS responsive nanomedicine in which oleic acid was modified with manganese oxide and incorporated with temozolomide (TMZ) into polyethylene glycol-polyethyl methacrylate polymer micelles containing internalized arginine-glycine-aspartic acid (iRGD) [150]. iRGD enhanced the targeting and tumor penetration of the nanomedicine. TMZ, Mn2+, and O2 were released in the TME after aggregation of the nanomedicine within glioma. Large amounts of Mn2+ caused intracellular oxidative stress and killed tumor cells through toxic ·OH generated by the Fenton reaction. TMZ induced immunogenic cell death (ICD) and O2 generation, which changed the hypoxic TME. In summary, this nanomedicine exerts a promising anti-tumor effect through synergistic effects on multiple pathways.
Kataoka group reported a strategy to construct reactive nanomedicines based on multi-ion composite vesicles by incorporation of ROS responsive groups into a cross-linked membrane network [151]. After H2O2 exposure, the ROS reactive linkers were gradually split, and the vesicle structure underwent stable expansion. Owing to the decrease in membrane cross-linking density, the membrane changed from hydrophobic to hydrophilic, leading to the swelling of vesicles and therefore an increase in the permeability of the membrane.
Song group reported a nanomedicine constructed using host-guest interactions between poly-([N-2-hydroxyethyl]-asparagine)-Pt(IV)/β-cyclodextrin, CpG/polyamidoamine-thioketal-adamantane, and methoxy poly(ethylene glycol)-thioketal-adamantane [152]. The nanomedicine accumulated around the tumor after intravenous injection and released antigens and immunoadjuvant CpG upon stimulation with high levels of ROS. This resulted in the activation and antigen presentation of APCs and generated a potent anti-tumor immune response.
GSH removes excessive ROS and maintains the redox balance in the vicinity of cells. However, the presence of excessive levels of GSH in the vicinity of tumor cells disrupts the balance of redox homeostasis [153]. Levels of GSH in tumor cells can be as high as 2–10 mM, about 100–1000 times higher than that in the ECM (2–10 μM) and 7–10 times higher than that in normal tissues [83]. This makes GSH a preferred target for endogenously responsive nanomedicines.
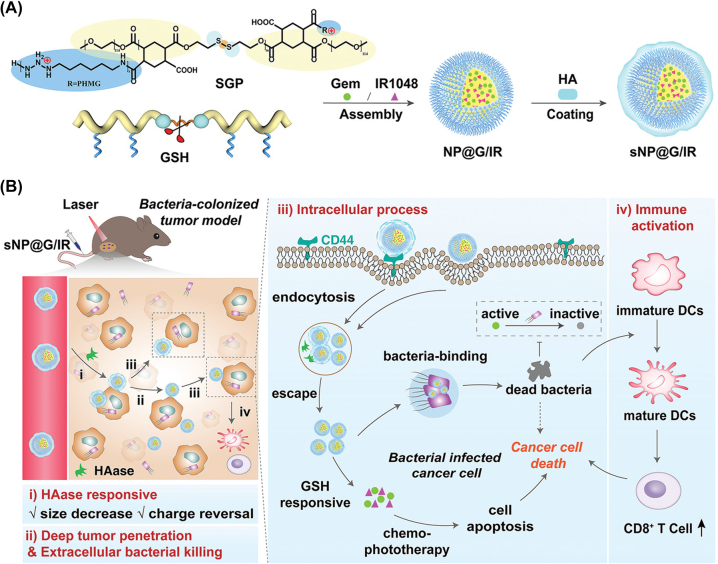
To date, various GSH responsive nanomedicines have been developed, including nanoparticles containing disulfide bonds (S-S), diene bonds (Se-Se), carbon diselenoic bonds (C-Se), and sulfonyl groups [154]. GSH react with these bonds, leading to the cleavage of nanoparticles and the precise release of chemotherapy drugs at the TME [155]. Wang group reported a GSH responsive nanomedicine (sNP@G/IR) synthesized with a disulfide containing polymer (poly[CHTA-co-HD]-PEG) that enhanced the tumor permeability of the drug and inhibited the growth of bacteria inside the tumor [156]. The outer shell of the nanomedicine was degraded by hyaluronidase in the ECM near the tumor, and the small size of the nanoparticles facilitated deep penetration into the tumor. Subsequently, the drug was released in the tumor under the action of GSH (Figure 9). The dead intratumoral bacteria (killed by the drug) led to the maturation of DCs and activation of CD8+ T cells. Furthermore, GSH responsive nanomedicines can also be designed to disrupt tumor vasculature to block tumor blood supply [157]. Chen group reported a GSH responsive polymer, PEGylated poly(alpha-lipoic acid), conjugated with vascular blocker Combretastatin A4 [158]. The selective release of Combretastatin A4 was induced by the high concentration of glutathione at the tumor site, cutting off the supply of blood (and therefore of nutrition and oxygen) to the tumor tissue, leading to tumor internal necrosis.
Figure 9:
Schematic illustration of the synthesis of GSH responsive nanomedicines as well as their actions in vivo. (A) Preparation of responsive nanomedicines with core–shell structure. (B) Nanomedicines inhibited bacteria-colonized tumor growth by killing of intratumoral bacteria and precision drug release. Reproduced with permission from Kang et al. [156]. Copyright 2022, Wiley-VCH GmbH.
ATP responsive polymeric nanomedicines
In tumor tissues, large amounts of intracellular ATP are released from cells owing to the influence of inflammation, necrosis, hypoxia, and other factors. This results in an increased concentration of ATP in the TME, which has a non-negligible impact on tumor proliferation, invasion, and metastasis [159, 160].
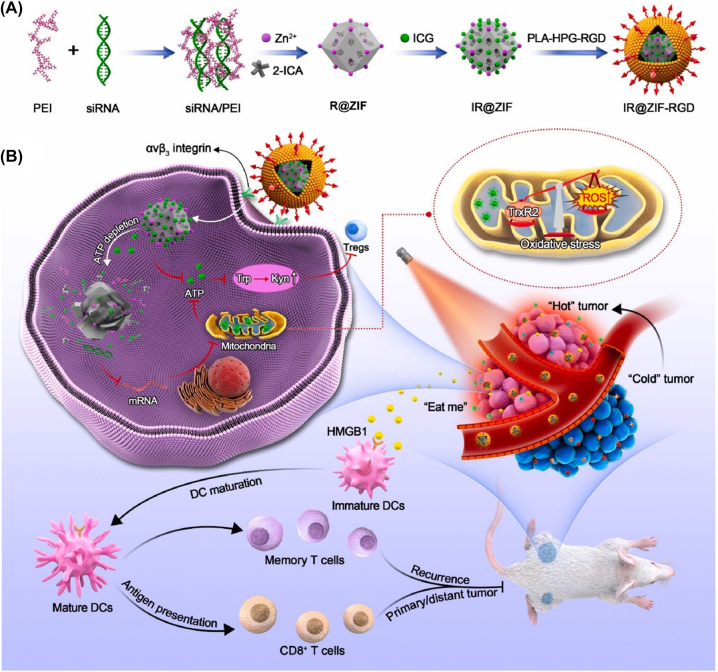
Owing to the high ATP concentration inside tumors, ATP responsive nanomedicines delivering therapeutic drugs have been designed. As intracellular and extracellular ATP concentrations are very different (1–10 mM intracellularly; 0.4 mM extracellularly), using ATP could be a practical way to modulate medicine release [161]. Kataoka group reported an ATP responsive nanomedicine in which mRNA was complexed with a phenylboronic-acid-derived poly(ethylene glycol)-poly(cationic block copolymer) and polyol by spontaneously formed phenylboronic acid esters [66]. The phenylboronic acid ester bound to high concentrations of mRNA released in response to ATP, and the released mRNA compensated for the aberrant expression of oncogenes around tumor cells. This strategy represents a new approach to designing ATP responsive nanomedicines. Moreover, Deng group developed ATP responsive nanomedicines (IR@ZIF-RGD) that affected tumor ATP metabolism and modulated the TME [65]. IR@ZIF-RGD was synthesized by self-assembly of a short interfering RNA/polyethylenimine complex, followed by electrostatic adsorption of indocyanine green and surface coating of RGD-modified polylactic acid-hyperrambrated polyglycerol. This nanomedicine depleted intracellular ATP and inhibited ATP synthesis (Figure 10). IR@ZIF-RGD released the drug in response to ATP, elicited robust ICD, and promoted intratumoral infiltration of effector T cells, thereby enhancing anti-tumor immune response.
Figure 10:
Schematic synthesis of IR@ZIF-RGD with ATP responsive properties, nanoparticle-mediated intratumoral metabolic intervention and immune response. (A) Preparation of IR@ZIF-RGD. (B) IR@ZIF-RGD mechanisms that mediate metabolic remodeling and enhance photoimmunotherapy in the TME. Reproduced with permission from Yu et al. [65]. Copyright 2022, Elsevier.
Exogenous stimuli responsive polymeric nanomedicines
Photo responsive polymeric nanomedicines
Photo responsive polymeric nanomedicines make use of photochemical reactions or photothermal effects and can be used in photoisomerization-mediated therapy [162]. Benefiting from spatiotemporal adjustability, photo responsive nanomedicines allow site-specific non-invasive delivery of therapeutics [163], [164], [165].
Near-infrared light has advantages including high tissue permeability and low side effects. Qian group reported a thioketal bond linked CPT and photosensitizer-2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a, constituting a photo responsive platinum nanoenzyme (PtNP) [166]. Under 660 nm laser irradiation, ROS were produced by PtNP, which effectively enhanced PDT and controlled the release of CPT. Furthermore, PtNP effectively catalyzed the decomposition of H2O2 into O2 to alleviate hypoxia in the TME and inhibit tumor growth.
Photoisomerization is a change in the conformation of a molecule that allows the structure to open upon absorption of light energy to trigger drug release. Light irradiation can induce reversible photoisomerization of selected organic compounds, including spiropyran and azobenzene [162]. Feng group reported a strategy for the integration of photochromic spiropyran into biocompatible cationic polymers, which involved the assembly of nucleic acids into functional nanoparticles without the introduction of other photosensitizers and imaging agents [167]. Upon UV irradiation, Borrelia bound copolymers changed from hydrophobic to hydrophilic, which facilitated controlled release of the drug. In addition, the production of ROS during light exposure could also alleviate hypoxic TME.
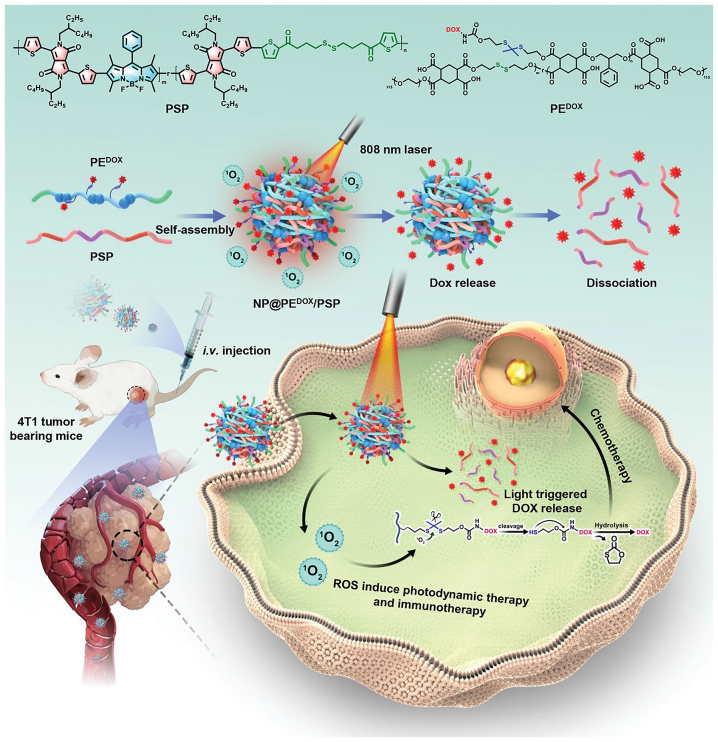
The ability to control changes in the physical and chemical structures of nanomedicine carriers plays a key part in drug delivery. Wang group reported a photo responsive donor-acceptor Stenhouse adduct and folic acid units conjugated to form a polymeric nanomedicine [168]. Drug release could be precisely controlled under light irradiation (λ = 550 nm). The photo responsive property led to simultaneous swelling and opening of nanoparticles to release the drug. In addition, the ROS produced due to light also helped to kill tumor cells and relieved the hypoxic TME. Furthermore, Xiao group reported co-assembly of a semiconductor polymer (PSP) and an amphiphilic polymer (PEDOX) with DOX conjugated through a thioketal bond [169]. Irradiation at 808 nm resulted in rapid release of DOX, and the sacrificial degradation of PEDOX and PSP by intracellular GSH resulted in dissociation of the nanomedicine (Figure 11). Furthermore, light at 808 nm induced tumor ICD and increased tumor immunogenicity. As a result, infiltration of DCs and cytotoxic T lymphocyte (CTLs) increased in the TME, and M2 macrophages were converted to M1 macrophages, resulting in the release anti-tumor cytokines and generating a potent anti-tumor immune response.
Figure 11:
Schematic illustration of NP@PEDOX/PSP and the mechanism of the photo responsive release of the drug by the nanoparticles in vivo. Reproduced with permission from Tang et al. [169]. Copyright 2022, Wiley-VCH GmbH.
Thermal responsive polymeric nanomedicines
Thermal stimulation has the advantages of a wide temperature control range and precise control of the action area [170]. Thermal responsive polymeric nanomedicines are relatively stable at a certain temperature, but undergo structural changes when the temperature increases or decreases [171]. Therefore, thermal responsive polymeric nanomedicines can be prepared for controlled drug release. In addition, it is possible to promote the release of drugs after elevation of temperature by adding thermally unstable materials to the carrier [172].
The thermal response characteristics of polymeric nanomedicines are usually determined by the upper critical solution temperature (UCST) or lower critical solution temperature (LCST) of the material [173]. When the ambient temperature is lower than the UCST of the material or higher than its LCST, the carrier will change from a hydrophilic state to a hydrophobic state, thereby facilitating drug loading or release [174]. For example, Alem group reported a nanoparticle with a core–shell structure consisting of Fe3O4, 2-(2-methoxy) ethyl methacrylate (MEO2MA) and oligo (ethylene glycol)-methacrylate (OEGMA) [175]. By adjusting the ratio between the MEO2MA and OEGMA blocks, a polymer with a LCST of 41°C was obtained and successfully loaded with the hydrophobic drug DOX. In vitro studies found that almost all of the DOX was released within 52 h at a temperature slightly higher than the LCST of the polymer. At an ultra-low DOX concentration of 0.07 μg mL−1, the killing rate of the nanomedicine against human ovarian cancer SKOV-3 cells was more than 70%; in addition, long-term drug infiltration of the TME was achieved, the immune escape of tumor cells was prevented.
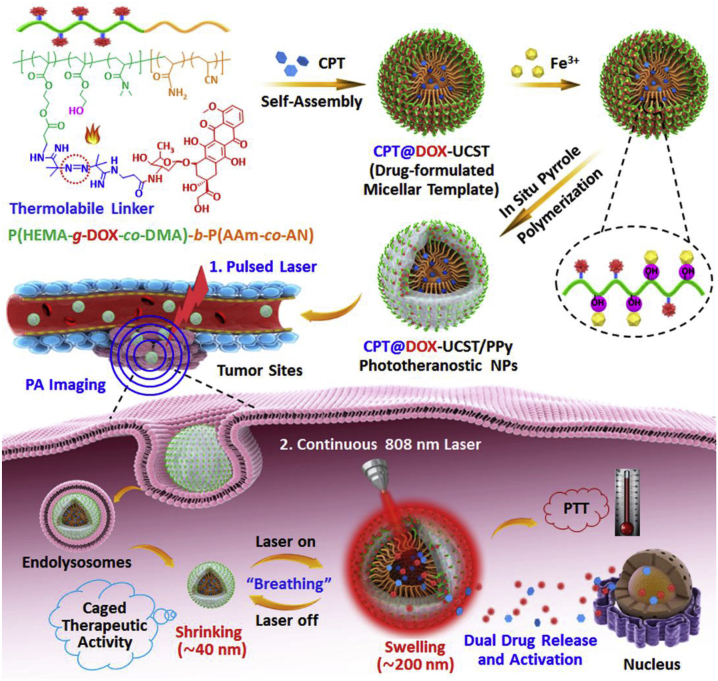
Du group reported a thermal responsive and liver tumor cell-targeting peptide A54 modified polymer [176]. The A54-poly(ethylene glycol)-g-poly(acrylamide-co-acrylonitrile) could self-assemble into an 80 nm micellar form, which exhibited thermal responsive behavior with an UCST of 43°C. DOX and magnetic nanoparticles (MNPs) were co-encapsulated at temperatures below 43°C. There was greater accumulation on the tumor under navigation with the A54 targeting peptide. The temperature increased due to the thermal effect of the MNPs not only activated drug release within tumor cells but also increased the effectiveness of thermal therapy. The thermal treatment also caused ICD of tumor cells, exposed large numbers of antigens, accelerated DC maturation, and regulated the TME. Similarly, Hu group reported multifunctional polypyrrole (PPy) nanoparticles, the inner core with UCST characteristics was loaded with CPT, whereas the outer corona was connected to the heat-lytic DOX prodrug, with a further in situ coating of PPy to obtain CPT@DOX-UCST/PPy nanoparticles (Figure 12). Upon 808 nm continuous laser irradiation, the PPy absorbed light to generate a large amount of heat, which lead to DOX prodrug cleavage and a considerable size increase, and facilitated simultaneous release of both drugs, thereby triggering the combined activation of PTT and chemotherapy. The drug was maintained tumor infiltration for a long period of time (more than 24 h) and the tumor remained in a state of long-term immune activation, which was more conducive to DC maturation and T cell activation [70].
Figure 12:
Schematic illustration of polymeric nanomedicines for photothermal synergistic therapy of cancer. Reproduced with permission from Yang et al. [70]. Copyright 2018, Elsevier.
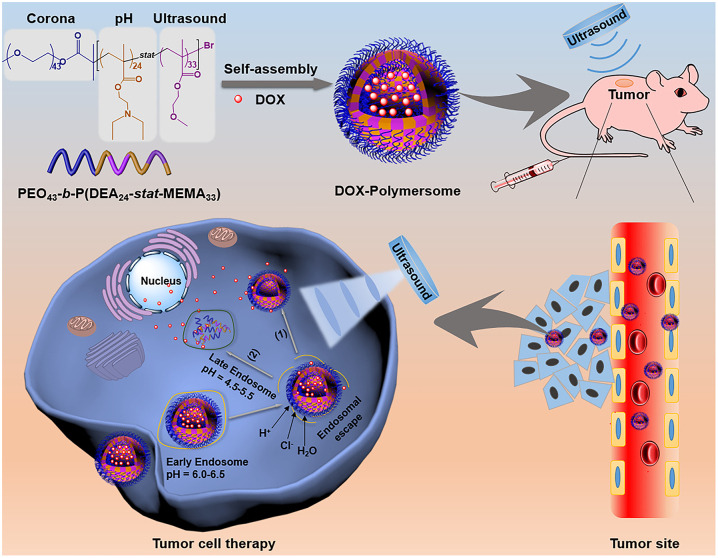
Ultrasound responsive polymeric nanomedicines
In recent years, ultrasound responsive polymeric nanomedicines have attracted considerable attention owing to their non-invasiveness, high tissue penetration depth, and spatiotemporal controllability [177]. Such systems typically encapsulate drugs in ultrasound responsive nanomedicines, making them stable in physiological environments before accumulating at tumor sites through the EPR effect. Then, under the action of ultrasound, the drugs are released to kill tumor tissues selectively [178], [179], [180]. For instance, Pu group reported a series of semiconducting polymers as sonosensitizers. Then they conjugated anti cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies onto the surface of the nanoparticles that delivered sonosensitizers. Under the action of ultrasound, nanomedicines elevated the level of 1O2 in TME and induced ICD. Increased 1O2 also promoted the release of anti-CTLA-4 antibodies conjugated to the polymers so that triggered checkpoint blockade [181]. Jiang group developed a polymer for ultrasound responsive ultrasound therapy through the Stella coupling reaction and used it to prepare a nanomedicine based on 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(carboxy[polyethylene glycol]-2,000). Catalase was coupled to the surface of the nanomedicine to relieve hypoxia in the TME. The polymeric nanomedicine converted H2O2 into 1O2 under ultrasound and effectively inhibited tumor growth. This strategy simultaneously addressed the issues of poor tumor site penetration and hypoxia, thereby enhancing cancer therapy [182].
In addition, Du group synthesized a triblock dual-response copolymer using atom transfer radical polymerization on the basis of their previous research (Figure 13) [75, 183]. The 2-(diethylamino)ethyl methacrylate segment could be protonated under acidic conditions to enhance the endosome escape ability, and the methoxyethyl methacrylate segment was hydrophobic and ultrasound responsive. In vivo anti-tumor experiments showed that under the action of ultrasound, the loaded DOX was released from the polymeric nanomedicines at an accelerated rate and achieved tumor-inhibiting efficiency of more than 95%, which further confirmed the applicability of ultrasound responsive polymeric nanomedicines in the field of tumor treatment. Ultrasound triggered drug release from the DOX-multimers and promoted drug release in the tumor, which led to more drug diffusing into the surrounding or deeper tumor tissues. Ultrasound treatment increased vascular permeability and promoted drug accumulation in tumors.
Figure 13:
In vivo mechanism of ultrasound responsive polymeric nanomedicines. Reproduced with permission from Wei et al. [75]. Copyright 2020, Elsevier.
Other types of responsive polymeric nanomedicines
Magnetic responsive polymeric nanomedicines have also been used to modulate the TME. Charushin group reported a magnetic responsive nanomedicine consisting of ([3-triethoxysilyl]propyl)succinic acid-polyethylene-glycol and silica co-modified Fe3O4 magnetic nanoparticles, which were loaded with DOX [76]. DOX was released under an alternating magnetic field at a frequency of 230 kHz at 0.27 kOe, which resulted in a 2.5-fold increase in the cytotoxic effect of the nanomedicine against 4T1 tumor cells within 1 h.
Begum group reported a pH and magnetic field responsive casein‐calcium ferrite hybrid biopolymeric carrier conjugated with biotin for the lung carcinoma treatment [184]. Cinnamaldehyde (CNA) reached 85.5% release within 4 h in response to magnetic field stimulus. The nanomedicine significantly increased the anticancer potential of CNA with an 18‐fold reduction in the IC50 value compared with the pure drug against A549 cell lines.
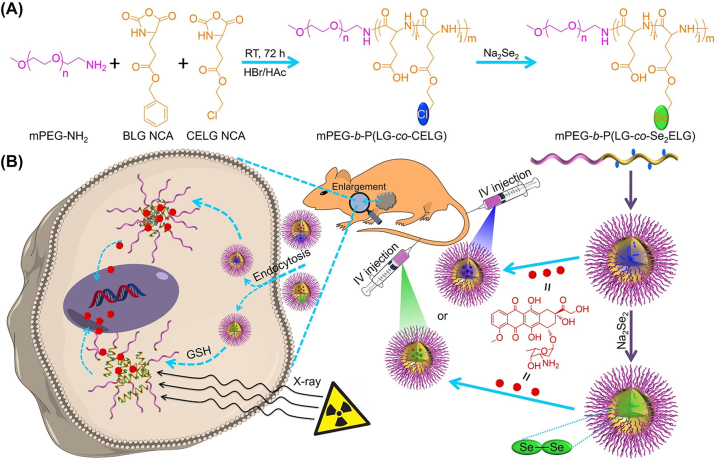
X-ray have also been used as stimuli to trigger drug release [185, 186]. Chen group reported a smart nanomedicine formed by crosslinking methoxy poly(ethylene glycol)-block-poly(L-glutamic acid-co-γ-2-chloroethyl-L-glutamate) (mPEG-b-P[LG-co-CELG]) with a Se-Se bond [187]. Polypeptide nanogels loaded with DOX (PNG/DOX) showed accelerated drug release due to degradation of Se-Se bonds when exposed to X-ray irradiation (Figure 14).
Figure 14:
Preparation and in vivo metabolism of mPEG-b-P(LG-co-CELG) micelle and mPEG-b-P(LG-co-Se2ELG) nanogel. (A) Synthesis of mPEG-b-P(LG-co-CELG) and mPEG-b-P(LG-co-Se2ELG). (B) Responsive release of DOX at the TME. Reproduced with permission from Wang et al. [187]. Copyright 2021, Elsevier.
Multimodal responsive polymeric nanomedicines
In order to achieve better control for delivery of anti-tumor drugs, multiple responsive polymeric nanomedicines with high efficiency, intelligence, and collaborative therapeutic effects were developed; these conferred the ability not only to accurately control the release of different drugs under different conditions but also to reduce the dosage of different drugs while maintaining a synergistic effect [188].
PDT and magnetothermal combination have synergistic anti-tumor activity and induce ICD, resulting in tumor-specific immune response. Dong group synergistically enhanced the anti-metastatic efficacy of immunotherapy by combining immunogenic-nanoparticle-mediated PDT with magnetic hyperthermia [189]. Based on bullet magnetic mesoporous organic silica nanoparticles (M-MONs) embedded with photosensitizer Ce6, mesoporous organic silica rods with disulfide bonds grew asymmetrically on Fe3O4 nanospheres. The obtained M-MON@Ce6 nanoparticles were further coated with breast cancer cell membranes to generate CM@M-MON@Ce6 for effective PDT and magnetothermal therapy. This treatment effectively triggered a strong anti-tumor immune response, which inhibited the distant metastasis combined with CTLA-4 checkpoint blockade.
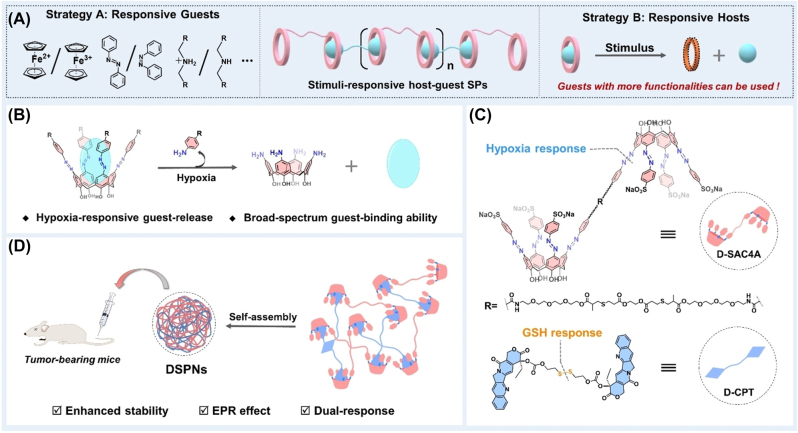
Shi group developed upconversion nanoparticles (UCNP) as intelligent pH/ROS-responsive polymeric nanomedicines (Fe2+@UCM-BBD) [190]. The acidic microenvironment of tumors induced DOX release and subsequent apoptosis of tumor cells and promoted the production of H2O2. Furthermore, the UCNP could convert 980 nm light to ultraviolet/visible light, which activated Ce6 and produced 1O2. The ROS responsive diallyl sulfur olefins were cleaved by 1O2, resulting in the release of Fenton reagent. Moreover, Guo group reported GSH and hypoxia dual-responsive nanomedicines. Hypoxia responsive dimeric azocalixarene (D-SAC4A) was mixed with GSH responsive disulfide-linked CPT (D-CPT) in equal proportions in water to assemble a polymeric nanomedicine (DSPNs) [191]. The tumor suppression rate of 16.7% with CPT alone was increased to 87.2% with this nanomedicine. Therefore, this GSH and hypoxia dual responsive nanomedicine greatly improved the anti-tumor efficiency of the chemotherapeutic drug (Figure 15).
Figure 15:
The design of dual responsive nanomedicines. (A) Two strategies for constructing nanomedicines. (B) Hypoxia responsive release behavior of azocalixarenes. (C) Structural formula of hypoxia responsive D-SAC4A and GSH responsive D-CPT. (D) Self-assembly of anti-tumor dual responsive DSPNs. Reproduced with permission from Yao et al. [191]. Copyright 2022, Wiley‐VCH GmbH.
Lei group designed a polymeric nanomedicine based on Ganoderma lucidum polysaccharides with pH and redox dual responsiveness [192]. In acidic environments, rutin was released to inhibit the activity of MMP. Dihydroartemisinin and 10-hydroxy CPT were released in a redox environment with a high concentration of GSH. The nanomedicine effectively suppressed tumor growth with reduced side effects.
With the development of precision nanomedicine, responsive polymeric nanomedicines have increasingly been used to remodulate the complex TME. In the future, polymeric nanomedicines will be designed more precisely to achieve high levels of targeting and hypersensitive responsiveness while modulating multiple TME components to achieve efficient cancer therapy and avoid toxicity to normal tissues.
Conclusions
The TME plays a key part in tumor progression by contributing to tumor growth, metastasis, immunosuppression, and resistance to multiple therapies. Therefore, modulation of the TME is crucial for tumor therapy. In this review, we have summarized the stimuli responsive polymeric nanomedicines developed in recent years to modulate the TME for improved cancer therapy, aiming to promote the design and innovation of polymeric nanomedicines for cancer therapy. As understanding of the TME increases, we expect that more potent polymeric nanomedicines will enter clinic settings in the future, providing new opportunities for cancer therapy.
Footnotes
Research funding: This work was financially supported by the National Natural Science Foundation of China (Nos. 51988102, 51833010, and 52273114), the Fundamental Research Funds for the Central Universities (No. PKU2022XGK008).
Author contributions: Yuanzhen Su: Conceptualization, Investigation, Writing – Original Draft, Validation, Writing – Review & Editing. Guanyu Jin: Investigation, Validation, Writing – Review & Editing. Huicong Zhou: Investigation, Validation, Writing – Review & Editing. Zhaofan Yang: Writing – Review & Editing. Lanqing Wang: Writing – Review & Editing. Zi Mei: Writing – Review & Editing. Qionghua Jin: Writing – Review & Editing. Shixian Lv: Conceptualization, Writing – Review & Editing, Supervision, Project administration, Funding acquisition. Xuesi Chen: Conceptualization, Writing – Review & Editing, Supervision, Project administration, Funding acquisition.
Competing interests: All authors declare that they have contributed to the manuscript and have no conflict of interest.
Ethical approval: Not applicable.
Contributor Information
Shixian Lv, Email: lvshixian@pku.edu.cn.
Xuesi Chen, Email: xschen@ciac.ac.cn.
References
- 1.Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135:584–90. doi: 10.1097/cm9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–8. doi: 10.1016/j.copbio.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arneth B. Tumor microenvironment. Medicina. 2020;56:15. doi: 10.3390/medicina56010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–49. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Zhang L, Wan D, Zhou L, Zheng S, Lin S, et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Targeted Ther. 2021;6:153. doi: 10.1038/s41392-021-00544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lainé A, Labiad O, Hernandez-Vargas H, This S, Sanlaville A, Léon S, et al. Regulatory T cells promote cancer immune-escape through integrin αvβ8-mediated TGF-β activation. Nat Commun. 2021;12:6228. doi: 10.1038/s41467-021-26352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Chen Q, Feng L, Liu Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today. 2018;21:55–73. doi: 10.1016/j.nantod.2018.06.008. [DOI] [Google Scholar]
- 10.Nielsen SR, Strøbech JE, Horton ER, Jackstadt R, Laitala A, Bravo MC, et al. Suppression of tumor-associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade. Nat Commun. 2021;12:3414. doi: 10.1038/s41467-021-23731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karin M, Shalapour S. Regulation of antitumor immunity by inflammation-induced epigenetic alterations. Cell Mol Immunol. 2022;19:59–66. doi: 10.1038/s41423-021-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Jiang Z, Tong S, Sun Y, Zhang Y, Zhang J, et al. Acidity-triggered transformable polypeptide self-assembly to initiate tumor-specific biomineralization. Adv Mater. 2022;n/a:2203291. doi: 10.1002/adma.202203291. [DOI] [PubMed] [Google Scholar]
- 13.Lam KC, Araya RE, Huang A, Chen Q, Di Modica M, Rodrigues RR, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184:5338–56. doi: 10.1016/j.cell.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mpekris F, Baish JW, Stylianopoulos T, Jain RK. Role of vascular normalization in benefit from metronomic chemotherapy. Proc Natl Acad Sci USA. 2017;114:1994–9. doi: 10.1073/pnas.1700340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu B, Sun Z, Wu J, Ruan J, Zhao P, Liu K, et al. Nanoparticle-stabilized oxygen microcapsules prepared by interfacial polymerization for enhanced oxygen delivery. Angew Chem Int Ed. 2021;60:9284–9. doi: 10.1002/ange.202100752. [DOI] [PubMed] [Google Scholar]
- 16.Fu LH, Wan Y, Qi C, He J, Li C, Yang C, et al. Nanocatalytic theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient cancer therapy. Adv Mater. 2021;33:2006892. doi: 10.1002/adma.202006892. [DOI] [PubMed] [Google Scholar]
- 17.Yan J. Antitumor γδ T cells need oxygen to function. Nat Immunol. 2021;22:268–9. doi: 10.1038/s41590-021-00874-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767–77. doi: 10.1038/nrd3554. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Wang J, Hassan A, Lee CH, Xie XS, Li X. Molecular basis of V-ATPase inhibition by bafilomycin A1. Nat Commun. 2021;12:1782. doi: 10.1038/s41467-021-22111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Wu Y, Qian X, Wang Y, Wang J, Li J, et al. Nanomedicine strategies to circumvent intratumor extracellular matrix barriers for cancer therapy. Adv Healthc Mater. 2022;11:2101428. doi: 10.1002/adhm.202101428. [DOI] [PubMed] [Google Scholar]
- 21.Irvine DJ, Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20:321–34. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2:578–88. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong N, Sheppard NC, Billingsley MM, June CH, Mitchell MJ. Nanomaterials for T-cell cancer immunotherapy. Nat Nanotechnol. 2021;16:25–36. doi: 10.1038/s41565-020-00822-y. [DOI] [PubMed] [Google Scholar]
- 24.Jiang W, Wang Y, Wargo JA, Lang FF, Kim BYS. Considerations for designing preclinical cancer immune nanomedicine studies. Nat Nanotechnol. 2021;16:6–15. doi: 10.1038/s41565-020-00817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–24. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14:1007–17. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong R, Tang L, Ma L, Tu C, Baumgartner R, Cheng J. Smart chemistry in polymeric nanomedicine. Chem Soc Rev. 2014;43:6982–7012. doi: 10.1039/c4cs00133h. [DOI] [PubMed] [Google Scholar]
- 28.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo YL, Yu W, Xu F. pH-responsive PMAA-b-PEG-b-PMAA triblock copolymer micelles for prednisone drug release and release kinetics. Polym Bull. 2012;69:597–620. doi: 10.1007/s00289-012-0774-2. [DOI] [Google Scholar]
- 30.Hu J, Miura S, Na K, Bae YH. pH-responsive and charge shielded cationic micelle of poly(L-histidine)-block-short branched PEI for acidic cancer treatment. J Contr Release. 2013;172:69–76. doi: 10.1016/j.jconrel.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Huang L, Zhang W, Wang X, Geng Y, Zhang Y, et al. A transistor-like pH-sensitive nanodetergent for selective cancer therapy. Nat Nanotechnol. 2022;17:541–51. doi: 10.1038/s41565-022-01085-5. [DOI] [PubMed] [Google Scholar]
- 32.Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12:648–54. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R, Xu X, Li D, Zhang W, Shi X, Xu H, et al. Smart pH-responsive polyhydralazine/bortezomib nanoparticles for remodeling tumor microenvironment and enhancing chemotherapy. Biomaterials. 2022;288:121737. doi: 10.1016/j.biomaterials.2022.121737. [DOI] [PubMed] [Google Scholar]
- 34.Gong N, Zhang Y, Teng X, Wang Y, Huo S, Qing G, et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2020;15:1053–64. doi: 10.1038/s41565-020-00782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su J, Chen F, Cryns VL, Messersmith PB. Catechol polymers for pH-responsive, targeted drug delivery to cancer cells. J Am Chem Soc. 2011;133:11850–3. doi: 10.1021/ja203077x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Wu R, Gong S, Xiao H, Thayumanavan S. In situ formation of polymeric nanoassemblies using an efficient reversible click reaction. Angew Chem Int Ed. 2020;59:15247–52. doi: 10.1002/ange.202004017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo L, Xu F, Peng H, Luo Y, Tian X, Battaglia G, et al. Stimuli-responsive polymeric prodrug-based nanomedicine delivering nifuroxazide and doxorubicin against primary breast cancer and pulmonary metastasis. J Contr Release. 2020;318:124–35. doi: 10.1016/j.jconrel.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Song Y, He J, Zhang M, Ni P. Polymer–Doxorubicin prodrug with biocompatibility, pH response, and main chain breakability prepared by catalyst-free click reaction. ACS Biomater Sci Eng. 2019;5:2307–15. doi: 10.1021/acsbiomaterials.9b00301. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Yang C, Wang W, Liu J, Liu Q, Huang F, et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci Rep. 2016;6:21225. doi: 10.1038/srep21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du JZ, Li HJ, Wang J. Tumor-acidity-cleavable maleic acid amide (TACMAA): a powerful tool for designing smart nanoparticles to overcome delivery barriers in cancer nanomedicine. Accounts Chem Res. 2018;51:2848–56. doi: 10.1021/acs.accounts.8b00195. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Song Y, Ma J, Chen X, Guan Y, Peng H, et al. Dynamic crosslinked polymeric nano-prodrugs for highly selective synergistic chemotherapy. Asian J Pharm Sci. 2022;17:880–91. doi: 10.1016/j.ajps.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Q, Shao S, Wang J, Xu C, Xiang J, Piao Y, et al. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat Nanotechnol. 2019;14:799–809. doi: 10.1038/s41565-019-0485-z. [DOI] [PubMed] [Google Scholar]
- 43.Dong H, Pang L, Cong H, Shen Y, Yu B. Application and design of esterase-responsive nanoparticles for cancer therapy. Drug Deliv. 2019;26:416–32. doi: 10.1080/10717544.2019.1588424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quader S, Van Guyse JFR. Bioresponsive polymers for nanomedicine—expectations and reality. Polymers. 2022;14:3659. doi: 10.3390/polym14173659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hua D, Tang L, Wang W, Tang S, Yu L, Zhou X, et al. Improved antiglioblastoma activity and BBB permeability by conjugation of paclitaxel to a cell-penetrative MMP-2-cleavable peptide. Adv Sci. 2021;8:2001960. doi: 10.1002/advs.202001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung SW, Choi JU, Cho YS, Kim HR, Won TH, Dimitrion P, et al. Self-triggered apoptosis enzyme prodrug therapy (STAEPT): enhancing targeted therapies via recurrent bystander killing effect by exploiting caspase-cleavable linker. Adv Sci. 2018;5:1800368. doi: 10.1002/advs.201800368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poreba M. Recent advances in the development of legumain-selective chemical probes and peptide prodrugs. Biol Chem. 2019;400:1529–50. doi: 10.1515/hsz-2019-0135. [DOI] [PubMed] [Google Scholar]
- 48.Kumari R, Sunil D, Ningthoujam RS. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: an up-to-date review. J Contr Release. 2020;319:135–56. doi: 10.1016/j.jconrel.2019.12.041. [DOI] [PubMed] [Google Scholar]
- 49.Cui D, Huang J, Zhen X, Li J, Jiang Y, Pu K. A semiconducting polymer nano‐prodrug for hypoxia‐activated photodynamic cancer therapy. Angew Chem Int Ed. 2019;131:5981–5. doi: 10.1002/ange.201814730. [DOI] [PubMed] [Google Scholar]
- 50.Rinaldi A, Caraffi R, Grazioli MV, Oddone N, Giardino L, Tosi G, et al. Applications of the ROS-responsive thioketal linker for the production of smart nanomedicines. Polymers. 2022;14:687. doi: 10.3390/polym14040687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang K, Qi S, Yu X, Bai B, Zhang X, Mao Z, et al. A hybrid supramolecular polymeric nanomedicine for cascade‐amplified synergetic cancer therapy. Angew Chem Int Ed. 2022;134:e202203786. doi: 10.1002/ange.202203786. [DOI] [PubMed] [Google Scholar]
- 52.Zheng M, Liu Y, Wang Y, Zhang D, Zou Y, Ruan W, et al. ROS-responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv Mater. 2019;31:1903277. doi: 10.1002/adma.201903277. [DOI] [PubMed] [Google Scholar]
- 53.Liu B, Wang D, Liu Y, Zhang Q, Meng L, Chi H, et al. Hydrogen peroxide-responsive anticancer hyperbranched polymer micelles for enhanced cell apoptosis. Polym Chem. 2015;6:3460–71. doi: 10.1039/c5py00257e. [DOI] [Google Scholar]
- 54.Ding C, Chen C, Zeng X, Chen H, Zhao Y. Emerging strategies in stimuli-responsive prodrug nanosystems for cancer therapy. ACS Nano. 2022;16:13513–53. doi: 10.1021/acsnano.2c05379. [DOI] [PubMed] [Google Scholar]
- 55.Chen H, Zeng X, Tham HP, Phua SZF, Cheng W, Zeng W, et al. NIR-light-activated combination therapy with a precise ratio of photosensitizer and prodrug using a host–guest strategy. Angew Chem Int Ed. 2019;58:7641–6. doi: 10.1002/ange.201900886. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Wang Z, Yu G, Zhou Z, Jacobson O, Liu Y, et al. Tumor-specific drug release and reactive oxygen species generation for cancer chemo/chemodynamic combination therapy. Adv Sci. 2019;6:1801986. doi: 10.1002/advs.201801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng X, Xu W, Liu J, Li D, Li G, Ding J, et al. Polypeptide nanoformulation-induced immunogenic cell death and remission of immunosuppression for enhanced chemoimmunotherapy. Sci Bull. 2021;66:362–73. doi: 10.1016/j.scib.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Fernández M, Shamsabadi A, Chudasama V. Fine-tuning thio-pyridazinediones as SMDC scaffolds (with intracellular thiol release via a novel self-immolative linker) Chem Commun. 2020;56:1125–8. doi: 10.1039/c9cc08744c. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Huang Y, Zhou S, Sun M, Chen L, Wang J, et al. Tailored chemodynamic nanomedicine improves pancreatic cancer treatment via controllable damaging neoplastic cells and reprogramming tumor microenvironment. Nano Lett. 2020;20:6780–90. doi: 10.1021/acs.nanolett.0c02622. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y, Sun B, Zuo S, Li X, Zhou S, Li L, et al. Trisulfide bond–mediated doxorubicin dimeric prodrug nanoassemblies with high drug loading, high self-assembly stability, and high tumor selectivity. Sci Adv. 2020;6:eabc1725. doi: 10.1126/sciadv.abc1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang W, Cai J, Wen J, Li X, Yu Y, Zhang L, et al. Boosting ferroptosis via abplatin(iv) for treatment of platinum-resistant recurrent ovarian cancer. Nano Today. 2022;44:101459. doi: 10.1016/j.nantod.2022.101459. [DOI] [Google Scholar]
- 62.Xue Y, Bai H, Peng B, Fang B, Baell J, Li L, et al. Stimulus-cleavable chemistry in the field of controlled drug delivery. Chem Soc Rev. 2021;50:4872–931. doi: 10.1039/d0cs01061h. [DOI] [PubMed] [Google Scholar]
- 63.Deng J, Walther A. ATP-responsive and ATP-fueled self-assembling systems and materials. Adv Mater. 2020;32:202002629. doi: 10.1002/adma.202002629. [DOI] [PubMed] [Google Scholar]
- 64.Sun L, Shen F, Tian L, Tao H, Xiong Z, Xu J, et al. ATP-responsive smart hydrogel releasing immune adjuvant synchronized with repeated chemotherapy or radiotherapy to boost antitumor immunity. Adv Mater. 2021;33:2007910. doi: 10.1002/adma.202007910. [DOI] [PubMed] [Google Scholar]
- 65.Yu M, Zeng W, Ouyang Y, Liang S, Yi Y, Hao H, et al. ATP-exhausted nanocomplexes for intratumoral metabolic intervention and photoimmunotherapy. Biomaterials. 2022;284:121503. doi: 10.1016/j.biomaterials.2022.121503. [DOI] [PubMed] [Google Scholar]
- 66.Yoshinaga N, Uchida S, Dirisala A, Naito M, Osada K, Cabral H, et al. mRNA loading into ATP-responsive polyplex micelles with optimal density of phenylboronate ester crosslinking to balance robustness in the biological milieu and intracellular translational efficiency. J Contr Release. 2021;330:317–28. doi: 10.1016/j.jconrel.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 67.Klán P, Šolomek T, Bochet CG, Blanc A, Givens R, Rubina M, et al. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem Rev. 2013;113:119–91. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang W, Ji T, Li Y, Zheng Y, Mehta M, Zhao C, et al. Light-triggered release of conventional local anesthetics from a macromolecular prodrug for on-demand local anesthesia. Nat Commun. 2020;11:2323. doi: 10.1038/s41467-020-16177-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kand D, Liu P, Navarro MX, Fischer LJ, Rousso-Noori L, Friedmann-Morvinski D, et al. Water-soluble BODIPY photocages with tunable cellular localization. J Am Chem Soc. 2020;142:4970–4. doi: 10.1021/jacs.9b13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J, Zhai S, Qin H, Yan H, Xing D, Hu X. NIR-controlled morphology transformation and pulsatile drug delivery based on multifunctional phototheranostic nanoparticles for photoacoustic imaging-guided photothermal-chemotherapy. Biomaterials. 2018;176:1–12. doi: 10.1016/j.biomaterials.2018.05.033. [DOI] [PubMed] [Google Scholar]
- 71.Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol. 2015;33:1201–10. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater. 2016;2:16075. doi: 10.1038/natrevmats.2016.75. [DOI] [Google Scholar]
- 73.Huo S, Zhao P, Shi Z, Zou M, Yang X, Warszawik E, et al. Mechanochemical bond scission for the activation of drugs. Nat Chem. 2021;13:131–9. doi: 10.1038/s41557-020-00624-8. [DOI] [PubMed] [Google Scholar]
- 74.Garcia-Manyes S, Beedle AEM. Steering chemical reactions with force. Nat Rev Chem. 2017;1:0083. doi: 10.1038/s41570-017-0083. [DOI] [Google Scholar]
- 75.Wei P, Sun M, Yang B, Xiao J, Du J. Ultrasound-responsive polymersomes capable of endosomal escape for efficient cancer therapy. J Contr Release. 2020;322:81–94. doi: 10.1016/j.jconrel.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Demin AM, Vakhrushev AV, Pershina AG, Valova MS, Efimova LV, Syomchina AA, et al. Magnetic-responsive doxorubicin-containing materials based on Fe3O4 nanoparticles with a SiO2/PEG shell and study of their effects on cancer cell lines. Int J Mol Sci. 2022;23:9093. doi: 10.3390/ijms23169093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L, Zhang S, Xu J, Li Y, He J, Yang Y, et al. Low-dose X-ray-responsive diselenide nanocarriers for effective delivery of anticancer agents. ACS Appl Mater Interfaces. 2020;12:43398–407. doi: 10.1021/acsami.0c11627. [DOI] [PubMed] [Google Scholar]
- 78.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.cd-21-1059. [DOI] [PubMed] [Google Scholar]
- 79.Peng S, Xiao F, Chen M, Gao H. Tumor-microenvironment-responsive nanomedicine for enhanced cancer immunotherapy. Adv Sci. 2022;9:2103836. doi: 10.1002/advs.202103836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Cheung EC, Vousden KH. The role of ROS in tumour development and progression. Nat Rev Cancer. 2022;22:280–97. doi: 10.1038/s41568-021-00435-0. [DOI] [PubMed] [Google Scholar]
- 82.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 83.Xiong Y, Xiao C, Li Z, Yang X. Engineering nanomedicine for glutathione depletion-augmented cancer therapy. Chem Soc Rev. 2021;50:6013–41. doi: 10.1039/d0cs00718h. [DOI] [PubMed] [Google Scholar]
- 84.Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18:601–18. doi: 10.1038/s41568-018-0037-0. [DOI] [PubMed] [Google Scholar]
- 85.Mimoto F, Tatsumi K, Shimizu S, Kadono S, Haraya K, Nagayasu M, et al. Exploitation of elevated extracellular ATP to specifically direct antibody to tumor microenvironment. Cell Rep. 2020;33:108542. doi: 10.1016/j.celrep.2020.108542. [DOI] [PubMed] [Google Scholar]
- 86.Shimoda M, Ohtsuka T, Okada Y, Kanai Y. Stromal metalloproteinases: crucial contributors to the tumor microenvironment. Pathol Int. 2021;71:1–14. doi: 10.1111/pin.13033. [DOI] [PubMed] [Google Scholar]
- 87.Qiu N, Liu X, Zhong Y, Zhou Z, Piao Y, Miao L, et al. Esterase-activated charge-reversal polymer for fibroblast-exempt cancer gene therapy. Adv Mater. 2016;28:10613–22. doi: 10.1002/adma.201603095. [DOI] [PubMed] [Google Scholar]
- 88.Shahriari M, Zahiri M, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M. Enzyme responsive drug delivery systems in cancer treatment. J Contr Release. 2019;308:172–89. doi: 10.1016/j.jconrel.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 89.Wu Y, Yi M, Niu M, Mei Q, Wu K. Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol Cancer. 2022;21:184. doi: 10.1186/s12943-022-01657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nixon BG, Gao S, Wang X, Li MO. TGF-β control of immune responses in cancer: a holistic immuno-oncology perspective. Nat Rev Immunol. 2022 doi: 10.1038/s41577-022-00796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17:251–66. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345–62. doi: 10.1038/s41571-021-00473-5. [DOI] [PubMed] [Google Scholar]
- 93.Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19:568–86. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhatia SN, Chen X, Dobrovolskaia MA, Lammers T. Cancer nanomedicine. Nat Rev Cancer. 2022;22:550–6. doi: 10.1038/s41568-022-00496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen WC, Ryser HJP. Cis-aconityl spacer between daunomycin and macromolecular carriers: a model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem Biophys Res Commun. 1981;102:1048–54. doi: 10.1016/0006-291x(81)91644-2. [DOI] [PubMed] [Google Scholar]
- 96.Ji X, Kong N, Wang J, Li W, Xiao Y, Gan ST, et al. A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv Mater. 2018;30:e1803031. doi: 10.1002/adma.201803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang S, Yu G, Wang Z, Jacobson O, Lin LS, Yang W, et al. Enhanced antitumor efficacy by a cascade of reactive oxygen species generation and drug release. Angew Chem Int Ed. 2019;58:14758–63. doi: 10.1002/ange.201908997. [DOI] [PubMed] [Google Scholar]
- 98.Qu Y, Chu B, Wei X, Lei M, Hu D, Zha R, et al. Redox/pH dual-stimuli responsive camptothecin prodrug nanogels for “on-demand” drug delivery. J Contr Release. 2019;296:93–106. doi: 10.1016/j.jconrel.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 99.Li S, Zou Q, Li Y, Yuan C, Xing R, Yan X. Smart peptide-based supramolecular photodynamic metallo-nanodrugs designed by multicomponent coordination self-assembly. J Am Chem Soc. 2018;140:10794–802. doi: 10.1021/jacs.8b04912. [DOI] [PubMed] [Google Scholar]
- 100.Yang G, Xu L, Xu J, Zhang R, Song G, Chao Y, et al. Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 2018;18:2475–84. doi: 10.1021/acs.nanolett.8b00040. [DOI] [PubMed] [Google Scholar]
- 101.Binauld S, Stenzel MH. Acid-degradable polymers for drug delivery: a decade of innovation. Chem Commun. 2013;49:2082–102. doi: 10.1039/c2cc36589h. [DOI] [PubMed] [Google Scholar]
- 102.Pang X, Jiang Y, Xiao Q, Leung AW, Hua H, Xu C. pH-responsive polymer-drug conjugates: design and progress. J Contr Release. 2016;222:116–29. doi: 10.1016/j.jconrel.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 103.Jia L, Li X, Liu H, Xia J, Shi X, Shen M. Ultrasound-enhanced precision tumor theranostics using cell membrane-coated and pH-responsive nanoclusters assembled from ultrasmall iron oxide nanoparticles. Nano Today. 2021;36:101022. doi: 10.1016/j.nantod.2020.101022. [DOI] [Google Scholar]
- 104.Ma W, Yang Y, Zhu J, Jia W, Zhang T, Liu Z, et al. Biomimetic nanoerythrosome-coated aptamer-DNA tetrahedron/maytansine conjugates: pH-responsive and targeted cytotoxicity for HER2-positive breast cancer. Adv Mater. 2022;34:e2109609. doi: 10.1002/adma.202109609. [DOI] [PubMed] [Google Scholar]
- 105.Yang W, Zhang F, Deng H, Lin L, Wang S, Kang F, et al. Smart nanovesicle-mediated immunogenic cell death through tumor microenvironment modulation for effective photodynamic immunotherapy. ACS Nano. 2020;14:620–31. doi: 10.1021/acsnano.9b07212. [DOI] [PubMed] [Google Scholar]
- 106.Wang K, Jiang M, Zhou J, Liu Y, Zong Q, Yuan Y. Tumor-acidity and bioorthogonal chemistry-mediated on-site size transformation clustered nanosystem to overcome hypoxic resistance and enhance chemoimmunotherapy. ACS Nano. 2022;16:721–35. doi: 10.1021/acsnano.1c08232. [DOI] [PubMed] [Google Scholar]
- 107.Ling X, Han W, Jiang X, Chen X, Rodriguez M, Zhu P, et al. Point-source burst of coordination polymer nanoparticles for tri-modality cancer therapy. Biomaterials. 2021;270:120690. doi: 10.1016/j.biomaterials.2021.120690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song R, Li T, Ye J, Sun F, Hou B, Saeed M, et al. Acidity-activatable dynamic nanoparticles boosting ferroptotic cell death for immunotherapy of cancer. Adv Mater. 2021;33:e2101155. doi: 10.1002/adma.202101155. [DOI] [PubMed] [Google Scholar]
- 109.Liu S, Jiang Q, Zhao X, Zhao R, Wang Y, Wang Y, et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat Mater. 2021;20:421–30. doi: 10.1038/s41563-020-0793-6. [DOI] [PubMed] [Google Scholar]
- 110.Sloane BF, Dunn JR, Honn KV. Lysosomal cathepsin B: correlation with metastatic potential. Science. 1981;212:1151–3. doi: 10.1126/science.7233209. [DOI] [PubMed] [Google Scholar]
- 111.Yao Q, Kou L, Tu Y, Zhu L. MMP-responsive ‘smart’ drug delivery and tumor targeting. Trends Pharmacol Sci. 2018;39:766–81. doi: 10.1016/j.tips.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 112.Herceg V, Bouilloux J, Janikowska K, Allemann E, Lange N. Cathepsin B-cleavable cyclopeptidic chemotherapeutic prodrugs. Molecules. 2020;25:4285. doi: 10.3390/molecules25184285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hashemkhani M, Demirci G, Bayir A, Muti A, Sennaroglu A, Mohammad Hadi L, et al. Cetuximab-Ag2S quantum dots for fluorescence imaging and highly effective combination of ALA-based photodynamic/chemo-therapy of colorectal cancer cells. Nanoscale. 2021;13:14879–99. doi: 10.1039/d1nr03507j. [DOI] [PubMed] [Google Scholar]
- 114.Del Genio V, Falanga A, Allard-Vannier E, Herve-Aubert K, Leone M, Bellavita R, et al. Design and validation of nanofibers made of self-assembled peptides to become multifunctional stimuli-sensitive nanovectors of anticancer drug doxorubicin. Pharmaceutics. 2022;14:1544. doi: 10.3390/pharmaceutics14081544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pyataev NA, Petrov PS, Minaeva OV, Zharkov MN, Kulikov OA, Kokorev AV, et al. Amylase-sensitive polymeric nanoparticles based on dextran sulfate and doxorubicin with anticoagulant activity. Polymers. 2019;11:921. doi: 10.3390/polym11050921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He Y, Lei L, Cao J, Yang X, Cai S, Tong F, et al. A combinational chemo-immune therapy using an enzyme-sensitive nanoplatform for dual-drug delivery to specific sites by cascade targeting. Sci Adv. 2021;7:eaba0776. doi: 10.1126/sciadv.aba0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Zhang W, Sun P, Cai Y, Xu W, Fan Q, et al. A novel multimodal NIR-II nanoprobe for the detection of metastatic lymph nodes and targeting chemo-photothermal therapy in oral squamous cell carcinoma. Theranostics. 2019;9:391–404. doi: 10.7150/thno.30268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Z, Wang Y, Jia X, Han Q, Qian Y, Li Q, et al. MMP-2-controlled transforming micelles for heterogeneic targeting and programmable cancer therapy. Theranostics. 2019;9:1728–40. doi: 10.7150/thno.30915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou H, Sun H, Lv S, Zhang D, Zhang X, Tang Z, et al. Legumain-cleavable 4-arm poly(ethylene glycol)-doxorubicin conjugate for tumor specific delivery and release. Acta Biomater. 2017;54:227–38. doi: 10.1016/j.actbio.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 120.Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15:712–29. doi: 10.1038/nrc4027. [DOI] [PubMed] [Google Scholar]
- 121.Cai H, Xiang Y, Zeng Y, Li Z, Zheng X, Luo Q, et al. Cathepsin B-responsive and gadolinium-labeled branched glycopolymer-PTX conjugate-derived nanotheranostics for cancer treatment. Acta Pharm Sin B. 2021;11:544–59. doi: 10.1016/j.apsb.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cho H, Shim MK, Yang S, Song S, Moon Y, Kim J, et al. Cathepsin B-overexpressed tumor cell activatable albumin-binding doxorubicin prodrug for cancer-targeted therapy. Pharmaceutics. 2022;14:83. doi: 10.3390/pharmaceutics14010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moon Y, Shim MK, Choi J, Yang S, Kim J, Yun WS, et al. Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death. Theranostics. 2022;12:1999–2014. doi: 10.7150/thno.69119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luo Q, Duan Z, Li X, Gu L, Ren L, Zhu H, et al. Branched polymer-based redox/enzyme-activatable photodynamic nanoagent to trigger STING-dependent immune responses for enhanced therapeutic effect. Adv Funct Mater. 2022;32:2110408. doi: 10.1002/adfm.202110408. [DOI] [Google Scholar]
- 125.Zhang C, Zeng Z, Cui D, He S, Jiang Y, Li J, et al. Semiconducting polymer nano-PROTACs for activatable photo-immunometabolic cancer therapy. Nat Commun. 2021;12:2934. doi: 10.1038/s41467-021-23194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tan P, Cai H, Wei Q, Tang X, Zhang Q, Kopytynski M, et al. Enhanced chemo-photodynamic therapy of an enzyme-responsive prodrug in bladder cancer patient-derived xenograft models. Biomaterials. 2021;277:121061. doi: 10.1016/j.biomaterials.2021.121061. [DOI] [PubMed] [Google Scholar]
- 127.Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol. 2019;137:57–83. doi: 10.1016/j.critrevonc.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 128.Huang J, Xiao Z, An Y, Han S, Wu W, Wang Y, et al. Nanodrug with dual-sensitivity to tumor microenvironment for immuno-sonodynamic anti-cancer therapy. Biomaterials. 2021;269:120636. doi: 10.1016/j.biomaterials.2020.120636. [DOI] [PubMed] [Google Scholar]
- 129.Gao A, Chen B, Gao J, Zhou F, Saeed M, Hou B, et al. Sheddable prodrug vesicles combating adaptive immune resistance for improved photodynamic immunotherapy of cancer. Nano Lett. 2020;20:353–62. doi: 10.1021/acs.nanolett.9b04012. [DOI] [PubMed] [Google Scholar]
- 130.Cook AB, Decuzzi P. Harnessing endogenous stimuli for responsive materials in theranostics. ACS Nano. 2021;15:2068–98. doi: 10.1021/acsnano.0c09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu X, Chen S, Yi N, Li X, Chen S, Lei Z, et al. Research progress on tumor hypoxia-associative nanomedicine. J Contr Release. 2022;350:829–40. doi: 10.1016/j.jconrel.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 132.Won M, Koo S, Li H, Sessler JL, Lee JY, Sharma A, et al. An ethacrynic acid-brominated BODIPY photosensitizer (EA-BPS) construct enhances the lethality of reactive oxygen species in hypoxic tumor-targeted photodynamic therapy. Angew Chem Int Ed. 2021;60:3196–204. doi: 10.1002/ange.202012687. [DOI] [PubMed] [Google Scholar]
- 133.Wang H, Xue K, Yang Y, Hu H, Xu J, Zhang X. In situ hypoxia-induced supramolecular perylene diimide radical anions in tumors for photothermal therapy with improved specificity. J Am Chem Soc. 2022;133:2360–7. doi: 10.1021/jacs.1c13067. [DOI] [PubMed] [Google Scholar]
- 134.Liu J, Zhang J, Song K, Du J, Wang X, Liu J, et al. Tumor microenvironment modulation platform based on composite biodegradable bismuth-manganese radiosensitizer for Inhibiting radioresistant hypoxic tumors. Small. 2021;17:e2101015. doi: 10.1002/smll.202101015. [DOI] [PubMed] [Google Scholar]
- 135.Yang J, Li W, Luo L, Jiang M, Zhu C, Qin B, et al. Hypoxic tumor therapy by hemoglobin-mediated drug delivery and reversal of hypoxia-induced chemoresistance. Biomaterials. 2018;182:145–56. doi: 10.1016/j.biomaterials.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 136.Zethoven M, Martelotto L, Pattison A, Bowen B, Balachander S, Flynn A, et al. Single-nuclei and bulk-tissue gene-expression analysis of pheochromocytoma and paraganglioma links disease subtypes with tumor microenvironment. Nat Commun. 2022;13:6262. doi: 10.1038/s41467-022-34011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qian C, Yu J, Chen Y, Hu Q, Xiao X, Sun W, et al. Light-activated hypoxia-responsive nanocarriers for enhanced anticancer therapy. Adv Mater. 2016;28:3313–20. doi: 10.1002/adma.201505869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang TX, Zhang ZZ, Yue YX, Hu XY, Huang, F, Shi L, et al. A general hypoxia-responsive molecular container for tumor-targeted therapy. Adv Mater. 2020;32:201908435. doi: 10.1002/adma.201908435. [DOI] [PubMed] [Google Scholar]
- 139.Antoniou AI, Giofre S, Seneci P, Passarella D, Pellegrino S. Stimulus-responsive liposomes for biomedical applications. Drug Discov Today. 2021;26:1794–824. doi: 10.1016/j.drudis.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 140.Pfister SM, Reyes-Mugica M, Chan JKC, Hasle H, Lazar AJ, Rossi S, et al. A summary of the inaugural WHO classification of pediatric tumors: transitioning from the optical into the molecular era. Cancer Discov. 2022;12:331–55. doi: 10.1158/2159-8290.cd-21-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–70. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou S, Hu X, Xia R, Liu S, Pei Q, Chen G, et al. A paclitaxel prodrug activatable by Irradiation in a hypoxic microenvironment. Angew Chem Int Ed. 2020;59:23198–205. doi: 10.1002/ange.202008732. [DOI] [PubMed] [Google Scholar]
- 143.Fu A, Yao B, Dong T, Chen Y, Yao J, Liu Y, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185:1356–72.e1326. doi: 10.1016/j.cell.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 144.Shen S, Xu X, Lin S, Zhang Y, Liu H, Zhang C, et al. A nanotherapeutic strategy to overcome chemotherapeutic resistance of cancer stem-like cells. Nat Nanotechnol. 2021;16:104–13. doi: 10.1038/s41565-020-00793-0. [DOI] [PubMed] [Google Scholar]
- 145.Cao Z, Li D, Wang J, Yang X. Reactive oxygen species-sensitive polymeric nanocarriers for synergistic cancer therapy. Acta Biomater. 2021;130:17–31. doi: 10.1016/j.actbio.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 146.He Y, Liu SH, Yin J, Yoon J. Sonodynamic and chemodynamic therapy based on organic/organometallic sensitizers. Coord Chem Rev. 2021;429:213610. doi: 10.1016/j.ccr.2020.213610. [DOI] [Google Scholar]
- 147.Gong Z, Dai Z. Design and challenges of sonodynamic therapy system for cancer theranostics: from equipment to sensitizers. Adv Sci. 2021;8:202002178. doi: 10.1002/advs.202002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI. Oxidative stress mitigation by antioxidants – an overview on their chemistry and influences on health status. Eur J Med Chem. 2021;209:112891. doi: 10.1016/j.ejmech.2020.112891. [DOI] [PubMed] [Google Scholar]
- 149.Li Z, Xu W, Yang J, Wang J, Wang J, Zhu G, et al. A tumor microenvironments-adapted polypeptide hydrogel/nanogel composite boosts antitumor molecularly targeted inhibition and immunoactivation. Adv Mater. 2022;34:202200449. doi: 10.1002/adma.202200449. [DOI] [PubMed] [Google Scholar]
- 150.Tan J, Duan X, Zhang F, Ban X, Mao J, Cao M, et al. Theranostic nanomedicine for dynergistic chemodynamic therapy and chemotherapy of orthotopic glioma. Adv Sci. 2020;7:202003036. doi: 10.1002/advs.202003036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li J, Anraku Y, Kataoka K. Self-boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for Initiation of immunogenic cell death by pyroptosis. Angew Chem Int Ed. 2020;59:13526–30. doi: 10.1002/ange.202004180. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Y, Ma S, Liu X, Xu Y, Zhao J, Si X, et al. Supramolecular assembled programmable nanomedicine as in situ cancer vaccine for cancer immunotherapy. Adv Mater. 2021;33:e2007293. doi: 10.1002/adma.202007293. [DOI] [PubMed] [Google Scholar]
- 153.Chen M, Liu D, Liu F, Wu Y, Peng X, Song F. Recent advances of redox-responsive nanoplatforms for tumor theranostics. J Contr Release. 2021;332:269–84. doi: 10.1016/j.jconrel.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 154.Liu Y, Zhai S, Jiang X, Liu Y, Wang K, Wang C, et al. Intracellular mutual promotion of redox homeostasis regulation and iron metabolism disruption for enduring chemodynamic therapy. Adv Funct Mater. 2021;31:202010390. doi: 10.1002/adfm.202010390. [DOI] [Google Scholar]
- 155.Sood A, Gupta A, Bharadwaj R, Ranganath P, Silverman N, Agrawal G. Biodegradable disulfide crosslinked chitosan/stearic acid nanoparticles for dual drug delivery for colorectal cancer. Carbohydr Polym. 2022;294:119833. doi: 10.1016/j.carbpol.2022.119833. [DOI] [PubMed] [Google Scholar]
- 156.Kang X, Bu F, Feng W, Liu F, Yang X, Li H, et al. Dual-cascade responsive nanoparticles enhance pancreatic cancer therapy by eliminating tumor-resident intracellular bacteria. Adv Mater. 2022;34:202206765. doi: 10.1002/adma.202206765. [DOI] [PubMed] [Google Scholar]
- 157.Chung C, Lu K, Lee W, Hsu W, Lee W, Dai J, et al. Fucoidan-based, tumor-activated nanoplatform for overcoming hypoxia and enhancing photodynamic therapy and antitumor immunity. Biomaterials. 2020;257:120227. doi: 10.1016/j.biomaterials.2020.120227. [DOI] [PubMed] [Google Scholar]
- 158.Liu Z, Shen N, Tang Z, Zhang D, Ma L, Yang C, et al. An eximious and affordable GSH stimulus-responsive poly(alpha-lipoic acid) nanocarrier bonding combretastatin A4 for tumor therapy. Biomater Sci. 2019;7:2803–11. doi: 10.1039/c9bm00002j. [DOI] [PubMed] [Google Scholar]
- 159.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, et al. Lactate metabolism in human lung tumors. Cell. 2017;171:358–71.e359. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ovais M, Mukherjee S, Pramanik A, Das D, Mukherjee A, Raza A, et al. Designing stimuli-responsive upconversion nanoparticles that exploit the tumor microenvironment. Adv Mater. 2020;32:202000055. doi: 10.1002/adma.202000055. [DOI] [PubMed] [Google Scholar]
- 161.Sameiyan E, Bagheri E, Dehghani S, Ramezani M, Alibolandi M, Abnous K, et al. Aptamer-based ATP-responsive delivery systems for cancer diagnosis and treatment. Acta Biomater. 2021;123:110–22. doi: 10.1016/j.actbio.2020.12.057. [DOI] [PubMed] [Google Scholar]
- 162.Tao Y, Chan HF, Shi B, Li M, Leong KW. Light: a magical tool for controlled drug delivery. Adv Funct Mater. 2020;30:2005029. doi: 10.1002/adfm.202005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jiang Y, Huang J, Xu C, Pu K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat Commun. 2021;12:742. doi: 10.1038/s41467-021-21047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ruskowitz ER, DeForest CA. Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nat Rev Mater. 2018;3:17087. doi: 10.1038/natrevmats.2017.87. [DOI] [Google Scholar]
- 165.Li SY, Cheng H, Xie BR, Qiu WX, Zeng JY, Li CX, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11:7006–18. doi: 10.1021/acsnano.7b02533. [DOI] [PubMed] [Google Scholar]
- 166.Hao Y, Chen Y, He X, Yu Y, Han R, Li Y, et al. Polymeric nanoparticles with ROS-responsive prodrug and platinum nanozyme for enhanced chemophotodynamic therapy of colon cancer. Adv Sci. 2020;7:202001853. doi: 10.1002/advs.202001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ji J, Li X, Wu T, Feng F. Spiropyran in nanoassemblies as a photosensitizer for photoswitchable ROS generation in living cells. Chem Sci. 2018;9:5816–21. doi: 10.1039/c8sc01148f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Senthilkumar T, Zhou L, Gu Q, Liu L, Lv F, Wang S. Conjugated polymer nanoparticles with appended photo-responsive units for controlled drug delivery, release, and imaging. Angew Chem Int Ed. 2018;57:13114–9. doi: 10.1002/anie.201807158. [DOI] [PubMed] [Google Scholar]
- 169.Tang D, Yu Y, Zhang J, Dong X, Liu C, Xiao H. Self-sacrificially degradable pseudo-semiconducting polymer nanoparticles that integrate NIR-II fluorescence bioimaging, photodynamic immunotherapy, and photo-activated chemotherapy. Adv Mater. 2022;34:e2203820. doi: 10.1002/adma.202203820. [DOI] [PubMed] [Google Scholar]
- 170.Ruan L, Chen J, Du C, Lu H, Zhang J, Cai X, et al. Mitochondrial temperature-responsive drug delivery reverses drug resistance in lung cancer. Bioact Mater. 2022;13:191–9. doi: 10.1016/j.bioactmat.2021.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10:4557–88. doi: 10.7150/thno.38069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen KJ, Liang HF, Chen HL, Wang Y, Cheng PY, Liu HL, et al. A thermoresponsive bubble-generating liposomal system for triggering localized extracellular drug delivery. ACS Nano. 2013;7:438–46. doi: 10.1021/nn304474j. [DOI] [PubMed] [Google Scholar]
- 173.Sponchioni M, Palmiero UC, Moscatelli D. Thermo-responsive polymers: applications of smart materials in drug delivery and tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;102:589–605. doi: 10.1016/j.msec.2019.04.069. [DOI] [PubMed] [Google Scholar]
- 174.Tian M, Xin X, Wu R, Guan W, Zhou W. Advances in intelligent-responsive nanocarriers for cancer therapy. Pharmacol Res. 2022;178:106184. doi: 10.1016/j.phrs.2022.106184. [DOI] [PubMed] [Google Scholar]
- 175.Ferjaoui Z, Al Dine EJ, Kulmukhamedova A, Bezdetnaya L, Soon Chang C, Schneider R, et al. Doxorubicin-loaded thermoresponsive superparamagnetic nanocarriers for controlled drug delivery and magnetic hyperthermia applications. ACS Appl Mater Interfaces. 2019;11:30610–20. doi: 10.1021/acsami.9b10444. [DOI] [PubMed] [Google Scholar]
- 176.Li WS, Wang XJ, Zhang S, Hu JB, Du YL, Kang XQ, et al. Mild microwave activated, chemo-thermal combinational tumor therapy based on a targeted, thermal-sensitive and magnetic micelle. Biomaterials. 2017;131:36–46. doi: 10.1016/j.biomaterials.2017.03.048. [DOI] [PubMed] [Google Scholar]
- 177.Tu L, Liao Z, Luo Z, Wu YL, Herrmann A, Huo S. Ultrasound‐controlled drug release and drug activation for cancer therapy. Explorations. 2021;1:20210023. doi: 10.1002/exp.20210023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu P, Jia Y, Qu F, Sun Y, Wang P, Zhang K, et al. Ultrasound-responsive polymeric micelles for sonoporation-assisted site-specific therapeutic action. ACS Appl Mater Interfaces. 2017;9:25706–16. doi: 10.1021/acsami.7b05469. [DOI] [PubMed] [Google Scholar]
- 179.Gong F, Cheng L, Yang N, Betzer O, Feng L, Zhou Q, et al. Ultrasmall oxygen-deficient bimetallic oxide MnWOX nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv Mater. 2019;31:e1900730. doi: 10.1002/adma.201900730. [DOI] [PubMed] [Google Scholar]
- 180.Ghosh B, Biswas S. Polymeric micelles in cancer therapy: state of the art. J Contr Release. 2021;332:127–47. doi: 10.1016/j.jconrel.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 181.Zeng Z, Zhang C, He S, Li J, Pu K. Activatable cancer sono-immunotherapy using semiconducting polymer nanobodies. Adv Mater. 2022;34:e2203246. doi: 10.1002/adma.202203246. [DOI] [PubMed] [Google Scholar]
- 182.Wang X, Wu M, Li H, Jiang J, Zhou S, Chen W, et al. Enhancing penetration ability of semiconducting polymer nanoparticles for sonodynamic therapy of large solid tumor. Adv Sci. 2022;9:202104125. doi: 10.1002/advs.202104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen W, Du J. Ultrasound and pH dually responsive polymer vesicles for anticancer drug delivery. Sci Rep. 2013;3:2162. doi: 10.1038/srep02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Purushothaman BK, Maheswari UP, Begum MSKM. pH and magnetic field responsive protein-inorganic nanohybrid conjugated with biotin: a biocompatible carrier system targeting lung cancer cells. J Appl Polym Sci. 2021;138:49949. doi: 10.1002/app.49949. [DOI] [Google Scholar]
- 185.Fu W, Zhang X, Mei L, Zhou R, Yin W, Wang Q, et al. Stimuli-responsive small-on-large nanoradiosensitizer for enhanced tumor penetration and radiotherapy sensitization. ACS Nano. 2020;14:10001–17. doi: 10.1021/acsnano.0c03094. [DOI] [PubMed] [Google Scholar]
- 186.Choi B, Choi H, Yu B, Kim DH. Synergistic local combination of radiation and anti-programmed death ligand 1 Immunotherapy using radiation-responsive splintery metallic nanocarriers. ACS Nano. 2020;14:13115–26. doi: 10.1021/acsnano.0c04701. [DOI] [PubMed] [Google Scholar]
- 187.Wang J, Xu W, Zhang N, Yang C, Xu H, Wang Z, et al. X-ray-responsive polypeptide nanogel for concurrent chemoradiotherapy. J Contr Release. 2021;332:1–9. doi: 10.1016/j.jconrel.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 188.Zhou Z, Vazquez-Gonzalez M, Willner I. Stimuli-responsive metal-organic framework nanoparticles for controlled drug delivery and medical applications. Chem Soc Rev. 2021;50:4541–63. doi: 10.1039/d0cs01030h. [DOI] [PubMed] [Google Scholar]
- 189.Wang Z, Zhang F, Shao D, Chang Z, Wang L, Hu H, et al. Janus nanobullets combine photodynamic therapy and magnetic hyperthermia to potentiate synergetic anti-metastatic immunotherapy. Adv Sci. 2019;6:1901690. doi: 10.1002/advs.201901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen M, Yang J, Zhou L, Hu X, Wang C, Chai K, et al. Dual-responsive and ROS-augmented nanoplatform for chemo/photodynamic/chemodynamic combination therapy of triple negative breast cancer. ACS Appl Mater Interfaces. 2022;14:57–68. doi: 10.1021/acsami.1c14135. [DOI] [PubMed] [Google Scholar]
- 191.Yao S, Yue Y, Ying A, Hu X, Li H, Cai K, et al. An antitumor dual-responsive host-guest supramolecular polymer based on hypoxia-cleavable azocalix 4 arene. Angew Chem Int Ed. 2022;62:e202213578. doi: 10.1002/anie.202213578. [DOI] [PubMed] [Google Scholar]
- 192.Zheng D, Zhao J, Tao Y, Liu J, Wang L, He J, et al. pH and glutathione dual responsive nanoparticles based on ganoderma lucidum polysaccharide for potential programmable release of three drugs. Chem Eng J. 2020;389:124418. doi: 10.1016/j.cej.2020.124418. [DOI] [Google Scholar]