Abstract
Rapid developments in the coronavirus disease 2019 (COVID-19) mRNA vaccine showcased the power of lipid nanoparticle (LNP) delivery systems in fighting infectious diseases. In addition, mRNA therapeutics are also in development for cancer immunotherapy. Recently, mRNA therapy has been expanded to induce immune tolerance, the opposite of immune-boosting effects, to treat diseases involving enhanced immune responses including allergies and autoimmune diseases. mRNA LNPs have been used to treat peanut allergy by us and autoimmune experimental autoimmune encephalomyelitis by Ugur Sahin. It is expected that more and more research is going to delve into the immune tolerance field for allergies and autoimmune diseases, where effective therapies are in short supply.
Keywords: allergy, autoimmune diseases, mRNA lipid nanoparticle, nanomedicine
The coronavirus disease 2019 (COVID-19) pandemic accelerated the application of mRNA lipid nanoparticle (LNP) technology to develop successful mRNA vaccines that saved millions of lives. This showcased the power of this technology for the treatment of infectious diseases, not limited to the SARS-CoV-2, but also the rapid development of mRNA vaccines for a range of viruses including varicella-zoster virus (VSV), herpes simplex virus (HSV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), human immunodeficiency virus (HIV) [1]. In addition to infectious diseases, there is mRNA immunotherapy targeting cancer. Cancer cells often escape recognition by the immune system due to the low levels of neoantigen and the presence of immune checkpoints, which are proteins that inhibit immune responses. New mRNA-based cancer vaccine encoding for neoantigens and checkpoint proteins that are highly expressed on cancer cells such as programmed death-ligand 1 (PD-L1) and Indoleamine 2,3-dioxygenase (IDO), could expand the neoantigen-or checkpoint protein-recognizing T cells and induce clearance of the tumor cells by the immune system [2].
Most mRNA LNPs are focused on immune-boosting effects, which lead to enhanced immune recognition and effects, few studies have been carried out on using mRNA LNPs to induce tolerance or suppress immune responses. This is mainly because unmodified mRNA could induce proinflammatory cytokines including IFN-γ, TNF-α, IL-6, GM-CSF, and IL-2 inflammatory cytokines, while pseudouridine-modified mRNA did not, even at high doses [3]. Unmodified mRNA could induce type I interferon production through the activation of endosomal toll-like receptors (e.g., TLR-7) or inflammasomes (e.g., MDA5, RIG-I, NOD2, and PKR), making them de facto effective adjuvant in boosting immune responses. In addition, during in vitro transcription of mRNA, it is inevitable to produce short RNA that forms small double-strand RNAs (dsRNA), which can activate TLR-3. To develop mRNA for immune tolerance, it is necessary to reduce the production of short RNAs or purify the mRNA to remove the dsRNA and replace uridine with N1-methyl-pseudouridine in the nucleotide backbone to avoid skewing of the immune response [1, 2].
Immune tolerance is required for allergy and autoimmune diseases because they involve overreactive immune systems to antigens [3], [4], [5], [6]. Allergy and autoimmune diseases are common to major global health concerns, affecting millions of people, much more than cancer patients. Allergic diseases occur when the immune system is overly active after being exposed to foreign proteins or allergens, producing antigen-specific IgE antibodies, which could induce mast cell degranulation, releasing histamine and proteases causing symptoms such as itching, redness, swelling, and pain. In the case of autoimmune diseases, the immune system recognizes “self” antigen, leading to anti-self antibody production and self-responsive T cells that attack healthy cells and tissues, resulting in serious and sometimes life-threatening conditions, e.g., type 1 diabetes, lupus, rheumatoid arthritis, etc. The conventional treatment for these diseases involves the use of immunosuppressive drugs, which tackle the symptoms but not the root cause and can have severe side effects. There is an urgent need for antigen-specific immune therapies that target the immune cells responsible for these diseases without compromising the rest of the immune system [3], [4], [5], [6].
mRNA LNP technology has the potential to transform the treatment of allergies and autoimmune diseases by offering a safe and effective alternative to immunosuppressive drugs and antigen or peptide-based immunotherapy platforms (Figure 1). The advantages of developing a tolerogenic mRNA platform instead of using a peptide-delivery platform include facile carrier design, reducing the cost of peptide synthesis, and ease of obtaining nucleic acid incorporation into the nanocarrier, in contradistinction to the challenge of loading peptides with heterogeneous charge and solubility. Moreover, mRNA presents a versatile platform for the co-expression and loading of multiple antigens or epitopes because it is easy to design mRNA constructs linking the antigens or epitopes and synthesize mRNA via in vitro transcription. Importantly, the physical and chemical properties of mRNA do not change much even though the nucleotide sequences vary greatly, meaning that the conditions and formulations required to load mRNA into LNPs only need minor adjustments [3, 7].
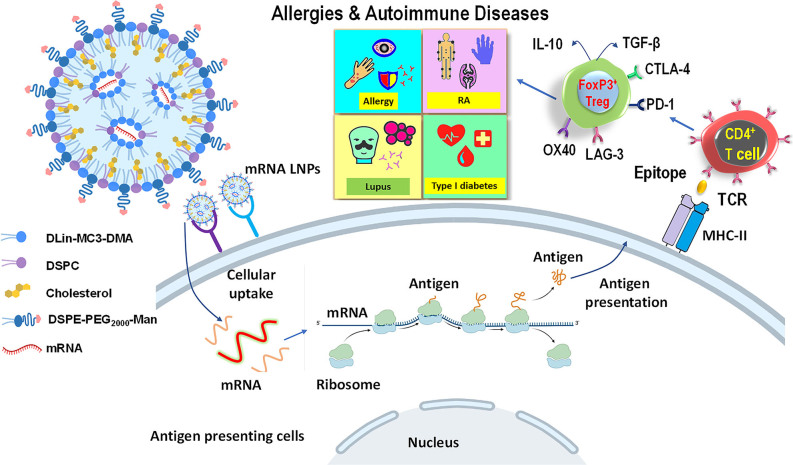
Figure 1:
mRNA lipid particles for induction of immune tolerance. mRNA LNPs have been widely used to boost immune responses in infectious diseases and cancer immunotherapy, however, fewer attempts have been on induction of immune tolerance. mRNA LNPs are formed by protonated ionizable cationic lipids (e.g., MC3) enveloping mRNA and forming inverted micellar structures that provide shielding effects. Helper lipids (DSPC and cholesterol) bind to inverted micelles and increase LNP stability and fluidity. PEG-lipids locate on the LNP surface and regulate LNP size and colloidal stability. Recently, studies have shown that mRNA LNPs could be used to treat allergies and autoimmune diseases in animal models. After cellular uptake of mRNA LNPs by APC, mRNA is released and expressed by ribosomes to synthesize proteins as antigens. Antigens are presented to the cell surface after endosomal processing to generate epitopes, which are loaded onto MHC-II molecules and presented on the APC surface. T cell receptors on naïve T cells recognize the epitopes binding to MHC-II molecule and differentiate into regulatory T cells by expressing Foxp3, checkpoint proteins (CTLA-4, PD-1, LAG-3, OX40, etc.), and secreting immunosuppressive cytokines such as IL-10 and TGF-β. These cells can induce immune tolerance, which is promising to treat allergies and autoimmune diseases including rheumatoid arthritis, systemic lupus erythematosus, and type I diabetes. LNP, lipid nanoparticle; APC, Antigen-presenting cells.
Because of these advantages, mRNA lipid nanoparticle (LNP) technology offers a promising solution for the treatment of allergies and autoimmune diseases. mRNA LNPs are small particles made up of lipids and mRNA that can deliver antigens or epitopes, small fragments of a protein, to antigen-presenting cells (APCs) to generate immune tolerance. When the mRNA LNP enters a cell, the mRNA is translated into the antigen or epitope, which is then presented to the immune system. In the absence of an adjuvant, the epitopes that are presented on the surface of APCs train the immune system to recognize the epitope and produce regulatory T cells (Treg), which is an important cell type that specializes in calming overactive immune responses to allergens or self-antigens through various ways [8, 9]. A recent study showed the feasibility of the approach for the treatment of autoimmune diseases. It has been demonstrated that an mRNA-based construct expressing a myelin oligodendrocyte glycoprotein peptide, MOG35–55, is capable of inducing Tregs that prevent the generation of experimental autoimmune encephalomyelitis (EAE) in response to the same peptide in a mouse model resembling multiple sclerosis. These results also agree with the previous demonstration that the delivery of a MOG35-55 nucleic acid construct to the liver by an adenovirus-associated vector can prevent EAE or reverse neurological damage in animals challenged with the immune epitope [3].
Against this background, we recently demonstrated the possibility of targeted delivery of epitope-delivering mRNA LNP to liver sinusoidal endothelial cells (LSECs), capable of suppressing anaphylaxis to a crude peanut protein extract [10]. We developed codon-optimized mRNA constructs for the expression of immune dominant Ara h 2 epitopes, designed to be routed to the MHC-II compartment by the invariant chain (Ii) and presented on the surface of LSECs, which leads to the induction of CD4+CD25+ T cells to Foxp3+ Tregs that secrets Interleukin-10 (IL-10), Interleukin-35 (IL-35), and transforming growth factor-beta (TGF-β) [7]. We demonstrated successful encapsulation of in vitro transcriptionally synthesized mRNA constructs encoding immunodominant Ara h 2 epitopes in LNPs, designed for liver biodistribution and LSEC uptake by introducing mannose ligands on the LNP surface. Administration of these tolerogenic NP was successful in preventing anaphylaxis in a murine peanut anaphylaxis model. Alleviation of the anaphylaxis response was accompanied by evidence of reduced symptom score, suppressed Th2 immunity, allergen sensitization, IgE production, and mast cell protease release (Table 1). These results demonstrate the feasibility of the approach that is promising not only for peanut allergy but also for additional allergic disorders, e.g., allergies induced by dust mites, pollen, egg white, milk, seafood, etc. [7].
Table 1:
mRNA lipid nanoparticles for immune tolerance applications.
| Nanoparticles | Delivery route | Targeting site | mRNA | Disease | Refs. |
|---|---|---|---|---|---|
| mRNA LPX | IV | Not specified | MOG 35–55, myelin PLP 139–151 | EAE | [3] |
| mRNA LNP | IV | LSECs | Ara H 2 epitopes (59–73, 1–15, 10–24, 145–159) | Peanut allergy and anaphylaxis | [7] |
LPX, liposome; MOG, myelin oligodendrocyte glycoprotein; PLP, proteolipid protein; EAE, experimental autoimmune encephalomyelitis; LNP, lipid nanoparticle; LSECs, liver sinusoidal endothelial cells.
In addition, mRNA LNP technology is versatile so that it can be personalized, as different patients may have different antigens or epitopes associated with their diseases. mRNA can be easily designed and produced to deliver the relevant antigens or epitopes to the individual patient via LNPs. This is relevant to allergies because people are often allergic to several types of allergens that are different from one another. For autoimmune diseases, the self-antigens are also different among people. Encouragingly, this personalized approach has been tested in clinical trials to treat cancers using individualized mRNA vaccines [2]. This approach has not been tested for the treatment of allergies and autoimmune diseases, however, it demonstrates the potential of the mRNA LNP platform for the development of future treatments for these diseases.
In conclusion, mRNA LNP technology offers a promising solution for the treatment of allergies and autoimmune diseases that have been under-researched to date [3, 7]. Its facile design and synthesis allow wider use to deliver specific antigens or epitopes to the immune system, which can lead to the induction of regulatory T cells to reduce the severity of these diseases [8, 9]. Moreover, mRNA LNP technology is safe and well-tolerated and can be tailored to the specific needs of each patient. Despite some challenges, the future of mRNA LNP technology for the treatment of allergy and autoimmune diseases is bright, and further research is needed to fully realize its potential.
Acknowledgments
We thank Angelina Lin from Brentwood School and Naomi M. Xia from Santa Monica High School for preparing the artwork in the figure and proofreading the manuscript.
Footnotes
Research funding: Support to Dr. Xia was provided by the Noble Family Innovation award by CNSI.
Author contributions: Wei Cao and Tian Xia wrote this perspective together. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Not applicable.
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
References
- 1.Kumar A, Blum J, Thanh Le T, Havelange N, Magini D, Yoon IK. The mRNA vaccine development landscape for infectious diseases. Nat Rev Drug Discov. 2022;21:333–4. doi: 10.1038/d41573-022-00035-z. [DOI] [PubMed] [Google Scholar]
- 2.Lorentzen CL, Haanen JB, Met O, Svane IM. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022;23:e450–8. doi: 10.1016/s1470-2045(22)00372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–53. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Wang X, Liao YP, Chang CH, Li J, Xia T, Nel AE. Use of a liver-targeting nanoparticle platform to intervene in peanut-induced anaphylaxis through delivery of an Ara h2 T-cell epitope. Nano Today. 2022;42:101370. doi: 10.1016/j.nantod.2021.101370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Q, Wang X, Liu X, Kumar S, Gochman G, Ji Y, et al. Use of polymeric nanoparticle platform targeting the liver to induce Treg-mediated antigen-specific immune tolerance in a pulmonary allergen sensitization model. ACS Nano. 2019;13:4778–94. doi: 10.1021/acsnano.9b01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Wang X, Liu X, Liao YP, Chang CH, Mei KC, et al. Antigen- and epitope-delivering nanoparticles targeting liver induce comparable immunotolerance in allergic airway disease and anaphylaxis as nanoparticle-delivering pharmaceuticals. ACS Nano. 2021;15:1608–26. doi: 10.1021/acsnano.0c09206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Wang X, Liao YP, Luo L, Xia T, Nel AE. Use of a liver-targeting immune-tolerogenic mRNA lipid nanoparticle platform to treat peanut-induced anaphylaxis by single and multi-epitope nucleoside sequence delivery. ACS Nano. 2023;17:4942–57. doi: 10.1021/acsnano.2c12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–66. doi: 10.1146/annurev-immunol-042718-041717. [DOI] [PubMed] [Google Scholar]
- 9.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Chen C, Xia T. Understanding nanomaterial-liver interactions to facilitate the development of safer nanoapplications. Adv Mater. 2022;34:e2106456. doi: 10.1002/adma.202106456. [DOI] [PMC free article] [PubMed] [Google Scholar]