Immune checkpoint blockade has represented a breakthrough for the treatment of human cancers, but only a minority of patients obtain clinical benefits from this therapy. A critical issue is how to shape the “cold” tumor, characterized by desert or suppressive immunity, into a “hot” phenotype [1]. The cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) pathway is a fundamental regulator of innate immune sensing, with potential to enhance tumor rejection by activating type I interferon (IFN)-dependent responses in multiple cell types, including dendritic cell, monocyte and even tumor cell [2], illustrated in many preclinical murine tumor models treated with stimulator of interferon genes (STING)-activating therapies. A plethora of STING agonists have been developed and dozens of them are forwarded to clinical trials, whereas none of them show promising outcomes by now, even in combination with anti-programmed death 1 (PD-1). The underlying mechanisms are largely unknown.
Now, Li et al. show us their encouraging findings in recent Nature research article that systemic delivery of STING agonist suppresses natural killer (NK) cell-mediated anti-tumor responses via B cell-derived interleukin (IL)-35 in an interferon regulatory factor 3 (IRF3)-dependent but type I interferon independent manner [3].
They started with systemic delivery of multiple STING agonists in mice bearing pancreatic ductal adenocarcinoma (PDAC), and found that 2′3′-cyclic GMP-AMP (cGAMP) increased the percentage and absolute number of CD19+ B cells in the tumor and inhibition of STING signaling in B cells limited tumor burden. To better understand the impact of cGAMP on B cells, RNA-seq analysis revealed that cGAMP increased IL-10 and Ebi3 mRNA (a subunit of IL-35) in wildtype (WT) but not in STING-deficient B cells. Moreover, STING activation contributed to the expansion of IL-35+ and IL-10+ regulatory B (Breg) cells. Mechanistically, activation of STING in B cells induced the direct binding of IRF3 to the promoter of Ebi3, p35 and Il10 while interferon is not involved, a novel outcome of STING-IRF3 signal pathway. It is generally believed that both IL-10 and IL-35 act as negative regulators of immune responses. However, B cell-specific deletion of Ebi3 or p35 but not IL-10 increased tumor-infiltrated CD8+ T cells and NK cells, indicating IL-35, but not IL-10, plays a major role in suppressing antitumor immune responses.
Consistent with the findings in the mouse study, the authors found that STING agonists promoted IL-35 expression in peripheral blood B cells from pancreatic cancer patients. Therefore, this new axis of B cell-IL-35-NK cell contributes to understanding the puzzle of clinical failure in STING agonist-based immunotherapy, meanwhile, provides a therapeutic strategy to block the negative regulator IL-35 during STING agonist treatment. In several mouse tumor models, cGAMP combined with IL-35-neutralizing antibody reduced tumor burden. For immune cells profiling, intratumoral Breg were decreased while effector NK cells and T cells are increased. To explore the cellular contributor in the control of PDAC model, NK cells or CD8+ T cells were depleted by antibodies, and most surprisingly, cGAMP plus anti-IL-35 mediated tumor control was abolished in the absence of NK cells but not CD8+ T cells, with a mechanism given in this study that IL-35 inhibits TRAIL expression in NK cells. As CD8+ T cells are essential for STING agonists-mediated tumor regression in many studies, it is necessary to confirm such NK cell-dependency in additional more tumor models.
In addition to B cells, activation of STING in T cells also leads to impaired anti-tumor immunity due to ER stress-mediated T cells apoptosis [4, 5]. Therefore, the outcomes of STING activation are cell type dependent, with opposing effects in different immune cell subsets (Figure 1). The story of STING agonist is reminiscent of immune checkpoints blockade therapy. Though programmed death-ligand-1 (PD-L1) in tumor tissue is a biomarker for effective anti-PD-L1/PD-1 therapy in clinic, increasing evidence reveals that it is dendritic cell derived PD-L1 that plays a critical role in tolerating T cells through interaction with PD-1. T cell immunoglobulin and mucin-containing molecule 3 (TIM-3) correlates with T cell exhaustion, and anti-TIM-3 antibody can enhance T cell responses and synergizes with anti-PD-1 in regressing mouse tumors. However, general blocking of TIM-3 has limited efficiency in clinic. Recent studies have shown that loss of TIM-3 on dendritic cells but not on CD4+ or CD8+ T cells—promotes strong anti-tumor immunity [6]. Similarly, systemically dual blocking of PD-L1 and TGF-β signaling shows very promising anti-tumor evidence in phase I clinical trial, but fails in phase III ones. Mechanistic studies reveal that TGF-β acts on CD4+ T cells, but not CD8+ cell, to foster tumor growth. Blocking TGF-β signaling in CD4+ T cells induces potent anti-tumor effects [7, 8]. Together with STING, all these cases show the importance of cell type-dependent function of a given molecule, and imply the future of cancer immunotherapy: cell type-specific targeting.
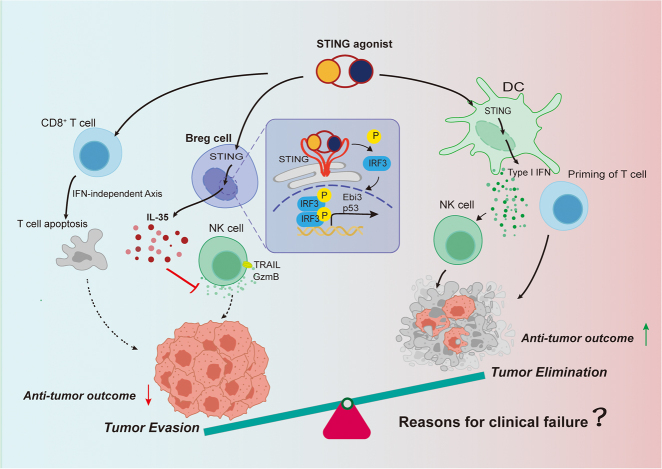
Figure 1:
Upon different immune cell subsets, STING agonists have distinct impacts on tumor. Canonically, STING agonist can induce the expression of type I IFN via dendritic cells to promote the anti-tumor effect. However, STING activation also can cause the opposite impacts in other cell types. According to Li et al. [3] in the B cells, STING agonists including cGAMP increase the production of IL-35 in an IRF3-dependent manner and further suppress NK cell-mediated anti-tumor responses. Additionally, in the T cells, cGAMP also can impair anti-tumor immunity by inducing T cells apoptosis in an IFN-independent manner. STING, stimulator of interferon genes; IFN, interferon; cGAMP, cyclic GMP-AMP; IL-35, interleukin-35; IRF3, interferon regulatory factor 3; NK, natural killer; DC, dendritic cell; Breg, regulatory B; TRAIL, TNF-related apoptosis-inducing ligand; GzmB, Granzyme B.
Footnotes
Research funding: None declared.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Informed consent was obtained from all individuals included in this study.
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
References
- 1.Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yum S, Li M, Chen ZJ. Old dogs, new trick: classic cancer therapies activate cGAS. Cell Res. 2020;30:639–48. doi: 10.1038/s41422-020-0346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Mirlekar B, Johnson BM, Brickey WJ, Wrobel JA, Yang N, et al. STING-induced regulatory B cells compromise NK function in cancer immunity. Nature. 2022;610:373–80. doi: 10.1038/s41586-022-05254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Chen YJ, Dobbs N, Sakai T, Liou J, Miner JJ, et al. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J Exp Med. 2019;216:867–83. doi: 10.1084/jem.20182192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J, Dobbs N, Yang K, Yan N. Interferon-independent activities of mammalian STING mediate antiviral response and tumor immune evasion. Immunity. 2020;53:115–26. doi: 10.1016/j.immuni.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon KO, Tabaka M, Schramm MA, Xiao S, Tang R, Dionne D, et al. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature. 2021;595:101–6. doi: 10.1038/s41586-021-03626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu M, Kuo F, Capistrano KJ, Kang D, Nixon BG, Shi W, et al. TGF-beta suppresses type 2 immunity to cancer. Nature. 2020;587:115–20. doi: 10.1038/s41586-020-2836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Liu M, Do MH, Chou C, Stamatiades EG, Nixon BG, et al. Cancer immunotherapy via targeted TGF-beta signalling blockade in TH cells. Nature. 2020;587:121–5. doi: 10.1038/s41586-020-2850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]