Abstract
The tumor ecosystem with heterogeneous cellular compositions and the tumor microenvironment has increasingly become the focus of cancer research in recent years. The extracellular matrix (ECM), the major component of the tumor microenvironment, and its interactions with the tumor cells and stromal cells have also enjoyed tremendously increased attention. Like the other components of the tumor microenvironment, the ECM in solid tumors differs significantly from that in normal organs and tissues. We review recent studies of the complex roles the tumor ECM plays in cancer progression, from tumor initiation, growth to angiogenesis and invasion. We highlight that the biomolecular, biophysical, and mechanochemical interactions between the ECM and cells not only regulate the steps of cancer progression, but also affect the efficacy of systemic cancer treatment. We further discuss the strategies to target and modify the tumor ECM to improve cancer therapy.
Keywords: cancer invasion, cancer metabolism, cancer progression, cancer therapy, cell-ECM interaction, extracellular matrix, metastasis
Introduction
The study of solid tumors has traditionally centered on the individual tumor cells, the processes leading to their transformation or progression in their malignancy. In recent decades the tumor ecosystem with heterogeneous cellular compositions and the tumor microenvironment (TME) increasingly became the focus of cancer research. All components of the TME, including the tumor vasculature, infiltrating immune cells, tumor-associated fibroblasts and other cell types, and the extracellular matrix (ECM), have received increased attention. Many novel discoveries of the TME have led to new successful and potential cancer treatments.
The ECM provides critical signals to preserve tissue architecture, polarity, and homeostasis and to regulate cell growth and apoptosis. During cancer progression, alterations in tumor cell – ECM interactions drive malignant transformation, invasion, and metastasis, as well as treatment resistance [1], [2], [3], [4], [5], [6]. The rapid expansion of tumor cells leads to the compression of adjacent tissue and the accumulation of stress within the tumor microenvironment [7]. This solid stress is compounded by dysregulated ECM deposition and remodeling. Degradation of the pericellular matrix and loss of basement membrane integrity due to both soluble matrix metalloproteinases (MMPs) [8] and membrane-localized MMPs [9] define a hallmark of invasive lesions.
In this review, we focus on the functional roles of the ECM in cancer initiation, growth, angiogenesis, invasion, and metastasis, as well as the ECM in the current cutting-edge tools to diagnose and treat invasive cancer. We will start by describing how tumor ECM differs from that in normal tissue.
The normal tissue ECM comprises a network of biochemically distinct components, including water, minerals, glycoproteins, proteoglycans, polysaccharides, and fibrous proteins such as collagen, elastin, and laminin, secreted by the resident cells. The ECM composition and structure are unique in every tissue to serve the tissue-specific functional needs. For example, the ECM in most glandular epithelial tissues, including breast, lung, and prostate, is composed of a relaxed meshwork of type I and II collagens, elastin, and fibronectin that allows for a mechanically compliant tensional homeostasis. By contrast, the ECM in ductal epithelial tissues is rich in proteoglycans, decorin, biglycan, and lumican associated with collagen to generate a structure that is essential for mechanical buffering and hydration [10, 11]. This unique composition arises through dynamic biophysical and biochemical feedback between cellular components and their evolving microenvironment [12]. The crosslinked fiber network of the ECM provides key tissue scaffolding structures so cells can adhere, grow, migrate within ECM. The cell-ECM cross-interaction is regulated in multiple scales, from genomic and epigenomic regulation to biochemical signaling from cell to ECM, and then biomechanical signaling from ECM to cell. The ECM is a highly dynamic structure, constantly undergoing a remodeling process where ECM components are deposited, degraded, or modified [13]. The ECM production by residence cells and degradation through metalloproteinases (MMPs) secreted by matrix modifying cells are balanced under normal conditions [14, 15], which is responsible for tensional homeostasis of the tissues.
In a normal wound healing process, ECM remodeling plays a critical role in not only providing structural integrity and scaffolding but also as storage and delivery of growth factors and cytokines that are important to tissue repair. E.g., degradation of ECM proteins by MMPs in response to wounds can locally release growth factors, including transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), and epithelial growth factor (EGF), from their anchorage and modulate wound healing [16]. Normal skin wound healing ends with scars that have a denser and more rigid ECM, characterized by thicker aligned collagen fibers, excessive dermal fibrosis, lack of elastic fibers, and impaired tissue functions. Interestingly, early developing fetus can heal wounds by regenerating skin and restoring the normal ECM structure, strength, and function. The mechanisms of fetal scarless wound healing remains unknown, but are believed to involve the ECM [17]. Abnormal ECM reconstruction during wound healing results in the formation of pathological scars, including hypertrophic scars that are raised, painful, rigid, disfiguring, and functionally limiting form of dermal fibrosis, and keloid scars that are red, raised, fibrous, and can grow into large, tumorous (benign) neoplasms [18]. Similar to normal scars, both hypertrophic and keloid scars have collagen fibers parallel to the epithelial surface instead of a 3D network seen in normal skin tissue. There are structural and composition differences between normotrophic and abnormal scars and between hypertrophic and keloid scars. For example, in keloid scars, collagen forms disorganized, larger, thicker bundles than normal scars, which is absent in hypertrophic scars [19].
Tumors have been considered as wounds that do not heal [20, 21]. Under stress, tumors co-opted the wound healing response to induce the stroma they required for maintenance and growth, resulting in the formation of dense fibrous connective tissue, which is called scar tissue in wounds and desmoplasia in cancer. In essence, the tumor ECM is the worst form of abnormal scar, much denser and more rigid, and functionally less desirable than its counterpart in normal tissue. The tumor ECM differs from the normal tissue ECM not only in the amount of deposition but also in composition, organization, and post-translational modification. Many solid tumors express high levels of various ECM molecules like fibrillar collagens, fibronectin, elastin, and laminins [22, 23]. Pancreatic ductal adenocarcinomas are rich in hyaluronan [24]. These excess ECM molecules come from the tumor cells and cancer-associated fibroblasts (CAFs) [25, 26]. Increases in ECM deposition without the appropriately balanced degradation result in increased stromal density, including both fibrillar collagen [22, 27], which contributes to the overall increase in stiffness in mammary tumors [28], and fibronectin, which influences tumor growth, invasion, metastasis, and therapy resistance. Increased fibrillar collagen density and changes in architecture have been appreciated in various solid and malignant tumors [13, 22, 29], [30], [31], [32]. For example, the increased mammographic density is associated with a 4- to 6-fold increased risk of breast cancer [33], and increased stromal collagen in mouse mammary tissue significantly increases tumor formation and results in a significantly more invasive phenotype with lung metastasis [22]. In addition to the changes in collagen density, changes in collagen compositions are indicative of cancer progression. Collagen production is shifted toward collagen type I (Col I) and type III (Col III) in invasive ductal carcinomas compared to benign mammary lesions [34]. Experiments using second harmonic generation microscopy show that increasing the Col V/Col I ratio reduces the length and organization of collagen fibers [35]. In melanoma, increased Col I expression is correlated with invasiveness, angiogenesis, and reduced survival [36]. Furthermore, the increased invasive phenotype of tumor cells that arose within collagen-dense mammary tissues remains after tumor explants are cultured within reconstituted 3D collagen gels [22]. These characteristic changes in collagen fiber organization have a demonstrated potential as an early diagnostic and prognostic marker in breast tumors [31].
The tumor ECM also is rich in matrix fragments resulting from the activation of proteases during cell migration and invasion. The matrix fragments act directly on cancer cells influencing their viability, apoptotic rate, and metastatic potential. These ECM fragments can often display opposite effects compared to the intact molecules of origin [37], making the study on the ECM microenvironment even more complex. Adding to the mix, tumor-associated hypoxia induces lysyl oxidase expression [38], along with other ECM modifying enzymes, resulting in the fibrillar collagen crosslinking within the ECM and the synergy of biophysical and biochemical changes that allow cancer cells to more efficaciously invade and metastasize [1].
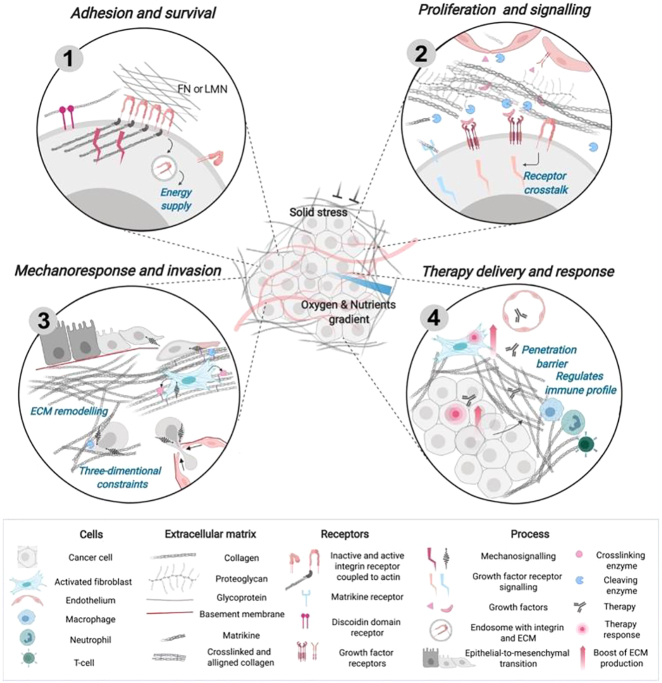
The dense and rigid tumor ECM not only serves as a diffusion barrier for drug molecules and therapeutic immune cells [39, 40], but also reduces the diffusion of nutrients and oxygen, resulting in a hypoxic and stressful TME such that tumor cells are less responsive to drugs [41]. The increased cell-ECM contact activates signaling pathways that increase epithelial-to-mesenchymal transition (EMT) for tumor cells, making cells more chemo-resistant [41]. These effects of tumor ECM on tumor progression and therapy (Figure 1 [42]) will be discussed in more detail below.
Figure 1:
The ECM as a regulator of tumor progression and therapy.
(1) Adhesion to the ECM through the receptors (e.g., via integrin or discoidin domain receptor) promotes signaling that supports cancer cell survival. To supply metabolic pathways, cancer cells can use the ECM (laminin and fibronectin), internalized with an integrin. (2) Growth factors that are released upon cleavage by ECM proteases (mainly MMPs) can regulate cancer cells and other cells of the TME. Biologically active ECM fragments (peptides) released upon proteolysis can promote cell signaling in the TME and spread with the blood flow. (3) Recruitment of activated fibroblasts stimulates ECM production, alignment, and cross‐linking (e.g., by LOX) of collagen fibrils resulting in fibrosis. Changes in mechanical properties can prompt cancer cells to adapt their signaling, gene expression, and cytoskeleton polarization, acquiring invasive phenotype (EMT). (4) Dense and rigid ECM creates a barrier to drug and T-cell penetration. Targeted cancer cells and fibroblasts can increase their resistance by promoting ECM production and deposition. Changes in the tumor ECM are associated with the formation of a tumor‐permissive immune profile (Figure reproduced with permission [42]).
ECM in cancer progression
Tumorigenesis and growth
The ECM provides critical signals to maintain tissue architecture, polarity, and homeostasis, and regulate cell growth and apoptosis. In the omics era, matrisome is defined as the ensemble of 1,000+ genes encoding ECM and ECM-associated proteins [43]. Matrisome has two interactome layers: the core-matrisome, genes for the ECM building proteins including ECM glycoproteins, collagens, and proteoglycans, and the ECM-associated genes including ECM-affiliated proteins (e.g., mucins, glypicans), and ECM regulators, including ECM-crosslinking and modifying enzymes and their inhibitors (e.g., LOX, MMP, and tissue inhibitors of MMP or TIMPs), and other secreted factors as signaling proteins (e.g., Wnts, cytokines) [42, 44]. Mutations in ECM genes are causal of musculoskeletal, cardiovascular, renal, ocular, and skin diseases [45]. Conversely, excessive deposition or destruction of ECM is a hallmark of pathologies such as fibrosis, osteoarthritis, and cancer [11]. High ECM deposition and increased stiffness combined with integrin overexpression in various cancers trigger tumor promotion [46], [47], [48].
Dysregulation of the ECM genes has been found to relate to tumor initiation. ECM gene mutations and protein signatures were identified in many tumors, including breast, ovarian, colon cancer, and melanoma [49]. Recent studies also discovered more frequent copy number alteration and mutations in matrisome than that in the rest of the genome based on TCGA [50]. In addition, biologically active ECM fragments (peptides) released upon ECM proteolysis, termed matricryptins or matrikines, can promote cell signaling in the TME [37, 42], further promoting cancer progression.
During pre-tumorigenesis, diverse stromal cell types are activated [51]. Notably, the irreversible activation of the pro-carcinogenic fibroblasts (termed cancer-associated fibroblasts or CAFs) contributes to the ECM remodeling and the TME reprogramming [52], [53], [54]. Despite the origins, the CAFs are recognized as an essential driver of ECM alternation during tumor progression [55], [56], [57], [58], [59]. In pancreatic ductal adenocarcinoma (PDAC), CAFs near the tumor cells show active TGF-β signaling and deposit collagen. In contrast, CAFs at a greater distance are also activated by the tumor cells but are unresponsive to TGF-β, deposit hyaluronic acid, and establish a tumorigenic, pro-inflammatory environment through the expression of cytokines such as IL-6 or antigen presentation [60, 61]. This pro-inflammatory environment fosters the recruitment and activation of immune cells, contributing to the establishment of the cancer niche. Some CAFs additionally support tumor growth through mechanisms independent of ECM remodeling, such as promoting cancer stemness or preventing cancer cell recognition by T-cells [62, 63].
In addition to biochemically and biomechanically controlling a plethora of cellular functions, the ECM has also been shown to impinge on the cancer metabolism, as the cancer metabolism reprograms the ECM of the TME [64, 65]. The cell-ECM interactions via mechanotransduction pathways are found to regulate the metabolic interactions of cancer cells with the TME. In pancreatic tumors, the ATP/ADP and ATP/AMP ratios are modulated, leading to invasive migration, chemotaxis, and enhanced liver metastasis by tuning the creatine–phosphagen ATP-recycling system [66]. Furthermore, the cell-ECM adhesion transduces the neutral lipid synthesis. It enhances glycolysis while dysregulated glutamine metabolism in both cancer and stromal cells facilitates aspartate–glutamate exchange, supporting tumor growth and metastasis [66, 67]. In addition, increased ECM may promote fatty acid oxidation in ovarian cancer [68]. Reciprocally, cancer cells accumulate metabolic alterations that allow them to gain access to conventional nutrient sources as well as to unconventional nutrient sources, utilize these nutrients towards the creation of new biomass to sustain deregulated proliferation, and take advantage of the ability to select metabolites to affect the fate of cancer cells themselves as well as a variety of normal cell types within the TME [69].
Accompanying the secreted growth factors and alterations to the extracellular matrix and cell-cell interactions, proliferating cancer cells also alter the metabolic composition of the extracellular milieu. The high utilization of extracellular glucose and glutamine by cancer cells results in the accumulation of extracellular lactate, which was shown to affect a number of cell types within the TME. For example, the tumor-derived lactic acid promotes the emergence of an immune-permissive microenvironment by attenuating dendritic and T cell activation and monocyte migration [70], [71], [72]. Furthermore, increased lactate levels also stimulate hyaluronic acid production by fibroblasts, which may contribute to tumor invasiveness [73].
Tumorigenesis and tumor growth require stroma that the host provides. In this respect, tumors behave like parasites that prey on the host, leaving behind a messed-up microenvironment that is easier for tumor cells to survive and progress but stressful for host cells to survive.
ECM in tumor angiogenesis
Solid tumors need the vascularized stroma to survive, grow, and metastasize. A hallmark of cancer is angiogenesis, the formation of new blood capillaries from pre-existing vessels that enables oxygen and nutrients from the surrounding microenvironment to reach the tumor [74]. The list of ECM molecules that have been shown to influence angiogenesis has grown over the last two decades [75]. Several ECM components have been shown to play a critical role in cancer angiogenesis. A recent review presented a detailed list of these molecular players [75]. Here we highlight only a few major ones: fibronectin, collagen, and thrombospondins.
Collagen as a major component of the ECM is intimately involved in angiogenesis. Type I collagen interacts with α1β1, α2β1, ανβ3 and ανβ5 integrins and induces the activation of MAP kinase pathway supporting endothelial survival. The binding of type I collagen to α1β1 and α2β1 integrins suppresses cAMP-dependent protein kinase A, resulting in the actin cytoskeleton remodeling and cell shape changes [76]. Type I collagen is also important for lumen formation during angiogenesis [77]. Type IV collagen is one of the major components of the basement membrane [78]. Fragments of type IV collagen promote endothelial cell adhesion and migration in the early stages of tumor development [79] but inhibit angiogenesis later in tumor development. Type XV collagen and type XVIII collagen are also components of the vascular basement membrane important in stabilizing microvessels; their fragments have various anti-angiogenic effects [75].
Fibronectin binds to integrin receptors and, in particular, to integrin α5β1, which is markedly up-regulated during tumor-associated angiogenesis [80]. The deposition of fibronectin is necessary for matrix assembly and vascular morphogenesis [81]. Fibronectin promotes endothelial survival, whereas the blockage of its polymerization impairs endothelial cell proliferation and tube formation in vitro and in vivo [82, 83].
Thrombospondins (TSPs) are a family of large ECM glycoproteins, including five members (TSP-1 to TSP-5) [84]. TSP-1 and TSP-2 have been extensively studied for their anti-angiogenic properties. Accordingly, the over-expression of these two molecules by tumor cells is associated with impaired tumor growth in mice [85, 86].
The role of matrix metalloproteinases (MMPs) in angiogenesis is more complex than that in the remodeling of basement membranes by degrading ECM components. Specific MMPs have been shown to enhance angiogenesis by helping to detach pericytes from vessels undergoing angiogenesis, by releasing ECM-bound angiogenic growth factors, by exposing cryptic proangiogenic integrin binding sites in the ECM, by generating pro-migratory ECM component fragments, by activating other MMPs, and by cleaving endothelial cell-cell adhesions. MMPs can also contribute negatively to angiogenesis by generating endogenous angiogenesis inhibitors by proteolytic cleavage of certain collagen chains and plasminogen, modulating cell receptor signaling, and cleaving off their ligand-binding domains. A number of inhibitors of MMPs that show antiangiogenic activity are already in the early stages of clinical trial. A rather complete summary of these roles of MMPs in facilitating and inhibiting angiogenesis can be found in this classic review [87]. A more recent review on MMPs in cancer angiogenesis [88] emphasized the role of cytokines and growth factors inducing EMT in various types of cancer together with the role of MMPs.
In addition to matrix components and their fragments, matrix remodeling also leads to the release of growth factors and cytokines, of which the ECM serves as a reservoir [89]. Moreover, cell-matrix mechanics in the mechanical regulation of angiogenesis have been increasingly recognized. The motility, metabolism, proliferation, and differentiation of anchorage-dependent cells, including endothelial cells and pericytes, are highly regulated by mechanical stimuli received by cells via mechanotransduction through the ECM [90], [91], [92], [93]. Angiogenic neovessels remodel the ECM through traction, proteolytic degradation, and cell-matrix adhesion, and the ECM properties influence vascular growth and alignment. The cell-ECM mechanical interaction was also found to regulate endothelial sprouting speed and proliferation, thereby regulate sprout stalk diameter [94].
Tumor angiogenesis has been a fertile ground for mathematical models since the early 1970s. Some of the first models used differential equations to represent a generic growth factor produced and released by a tumor, which as chemoattractant triggered endothelial cell growth and migration into the tumor [95], [96], [97]. More mathematical models have since included detailed vessel network formation [98], molecular-level interactions of VEGF and its receptors [99], rule-based cellular automaton models of vascular network growth [100], cell-based cellular Potts models of angiogenic sprouting [101, 102], a finite-element/level-set method for topological changes including tumor splitting [103], among many others [104, 105]. Together they give insight into the multiscale, multi-faceted conditions affecting tumor angiogenesis. The roles of ECM in tumor angiogenesis have also been modeled respectively and in combination: 1) ECM-bound VEGF and other factors are released when MMPs degrade the ECM [101, 106], 2) ECM-alignment and density guides capillary movement through the matrix and mediates vascular morphogenesis [104, 107, 108], and 3) ECM-mechanical feedback to endothelial cells explains the behavior of individual endothelial cells and the interactions of endothelial cell pairs in compliant matrices [109] and cell-ECM mechanics, including load initiation time, magnitude, and mode regulate microvascular growth, as well as upstream angiogenic and mechanotransduction signaling pathways [110].
Angiogenesis is another process that is shared between wound healing and tumor development. Microhemorrhages are common in both healing wounds and solid tumors, emanating from newly formed, fragile blood vessels. Abnormal intravascular hemostasis has a long historical association with cancer [111]. The biomechanical and biochemical roles the ECM plays in angiogenesis are similar in wound healing and in tumor development. The major differences are that the normal microenvironment allows the wound to heal, and the stressful tumor microenvironment renders tumors as wounds never heal.
ECM in cancer invasion and metastasis
Cancer metastasis is a multistep process that starts with invasion whereby cancer cells penetrate neighboring tissues and escape the primary compartment, intravasate and ride the bloodstream, extravasate to establish growth at distant sites [112]. The acquisition of invasive phenotype requires the abrogation of cell-cell contacts, remodeling of the ECM, and cell-ECM interactions. A recent review [113] summarized four distinguishing features of ECM as hallmarks of cancer for metastasis [114]: 1) motility and invasion; 2) the ability to modulate secondary sites and/or local microenvironments; 3) phenotypic plasticity; 4) the ability to colonize secondary tissues. Extensive research during the last decade has unraveled the significance of ECM remodeling at each stage of metastasis, from invasion and surviving in circulation to forming the pre-metastatic and metastatic niches.
The increased tissue stiffness in many carcinomas modifies the local forces experienced by the tumor cells through the ECM [115]. Tumor cells respond to this altered mechanical microenvironment by converting mechanical stimuli into downstream biochemical signaling changes in cytoskeletal dynamics, integrin signaling, and various mechano-sensitive signaling molecules such as the nuclear localization of b-catenin or Yes-associated protein 1 (Yap) [116]. In turn, cell-generated forces respond to this altered microenvironment by modifying the cellular response to growth factor cues, increasing cell growth and survival, destabilizing cell-cell adhesions, and compromising tissue integrity [117]. This destabilization of cell-cell adhesions and tissue integrity is necessary for cancer cells to break free from the confinements of the primary tumor [118], [119], [120]. Most carcinomas are epithelial in origin, and transition to an invasive phenotype requires drastic reprogramming on a genetic and physiological level, known as epithelial-to-mesenchymal transition (EMT). An epithelial cell in a normal situation is laterally linked to its akin neighbors via cell adhesion molecules (e.g., E-cadherin) and basally rooted in the ECM via integrins.
On the other hand, mesenchymal cells exhibit strong matrix interactions, the ability to migrate through ECM, and increased resistance to chemotherapeutics, facilitating cancer dissemination. Cancer cells or CAFs can also tunnel through the ECM and create micro-tracks for the follower cancer cells facilitating the migration through the ECM [121] before intravasation into the blood or lymphatic systems. Several lines of evidence revealed that CAFs produce a broad spectrum of MMPs [8] and plasminogen activators to degrade ECM directly [122], facilitating mesenchymal cell invasion. Other tumor cells going through EMT also have altered signaling pathways involving growth factors, cytokines, cell adhesion molecules, leading to upregulated motilities [123], [124], [125], [126], [127]. Moreover, MMPs can also trigger a cascade of molecular alterations that leads to EMT [125], [126], [127].
Traditionally, the mesenchymal cells are considered to migrate individually, appear as single cells in the circulation and directly initiate metastatic lesions by reverting to a proliferating epithelial phenotype [128]. Collective cancer invasion with leader-follower organization is increasingly recognized as a predominant mechanism in the metastatic cascade, e.g., in non-small-cell lung cancer (NSCLC) spheroids [129], head and neck squamous cell carcinomas (HNSCC) [130]. Leader cells support cancer invasion by creating invasion tracks, sensing environmental cues, and coordinating with follower cells biochemically and biomechanically (recently reviewed in Ref. [131]). This review proposed a set of definitions for leaders in collective cancer invasion and a framework to unify the cellular and molecular programs adopted by each leader cell subtype [131]. A recent study [132] on clinical invasive ductal carcinomas (IDC) and invasive lobular carcinoma (ILC) showed that the downregulation of E-cadherin and p120-catenin caused a transition from coordinated to uncoordinated collective movement along extracellular boundaries. In contrast, single-cell escape depended on locally free tissue space. These results provide a new understanding of 3D invasion plasticity on how cell-cell adhesion and cell confinement affect collective cell motion.
Next, tumor-induced angiogenesis and heightened MMP activity at the primary tumor site result in a leaky vasculature that facilitates invasive tumor cells to intravasate and extravasate. Circulating tumor cells (CTCs) in the blood upregulate the expression of common stroma-derived ECM proteins, such as collagens, TIMP-2, the proteoglycan decorin, the glycoprotein osteonectin, and fibronectin, as revealed by single-cell RNA-sequencing of pancreatic CTCs [133]. CTCs captured by neutrophil extracellular traps (NETs) have been shown to correlate with increased metastasis [134]. Potential sites of metastasis before the presence of the metastatic tumor cells, so-called pre-metastatic niches [135], involve ECM remodeling that is distinct from the primary tumor [136]. In contrast to increased collagen deposition in the primary TME, fibronectin is the leading player in the formation of the pre-metastatic niche along with glycoproteins and proteoglycans [136]. Primary tumor-derived exosomes activate stromal cells in the pre-metastatic site to secrete new ECM molecules or remodel and modify the ECM directly, creating a fibrotic and pro-inflammatory environment. Bone marrow-derived cells (BMDCs) are recruited to the pre-metastatic niche, attach to the remodeled ECM via integrins, and contribute to further ECM remodeling in preparation for the arrival of disseminated tumor cells [137]. CTCs that extravasate through the disrupted vasculature into the distant tissue may be dormant. Proteases expressed in NETs, including neutrophil elastase and MMP-9 cleave laminin, generating a specific matrikine that can awaken dormant tumor cells [138]. Together, these ECM remodeling processes support the formation of metastasis [48].
Contrary to the common impression that ‘malignent’ cancer cells are formidable armies that invade and occupy new grounds with ease, cancer cells must adapt to a relatively inhospitable microenvironment, evade immune surveillance, and progress from the micro- to the macro-metastatic stage to generate a secondary tumor. Cancer invasion and metastasis involve cell migration and proliferation that are also central to wound healing. A recent review titled “Cancer metastasis as non-healing wound” [139] focused on cellular responses to inflammation in both wound healing and metastasis. We show here that the ECM as the major component of the microenvironment and ECM remodeling as a dynamic response to environmental stresses also support the parallel between metastasis and non-healing wound.
ECM as diagnostic markers
The expression profile of ECM-related genes is, in many cancers, a valuable prognostic factor. For example, besides indicators for immune suppression, high expression of collagens Col3a1, Col4a1, and Col5a2 is correlated with poor prognosis in glioblastoma [140]. According to their ECM expression profile, breast cancer was subdivided into four groups (ECM1–4) with significant correlations with prognosis [141]. Between tumor subtypes, ECM expression and deposition also differ significantly. Triple-negative breast cancer and, to a lesser extent, Her2 tumors show not only increased deposition of collagen but also enhanced invasion with CAFs [142, 143].
In addition, the stromal architectures were indicative of tumor progress status and prognosis. In breast cancer again, tumor-associated collagen signatures (TACS) were identified as TACS-1, -2, and -3. TACS-1 is the presence of locally dense collagen within the globally increased collagen concentration surrounding tumors. TACS-1 serves as a reliable hallmark for locating small tumor regions. TACS-2 is identified as straight (taut) collagen fibers stretched tangentially around the tumor, constraining the tumor volume, indicative of a growing tumor. TACS-3 is characterized by bundles of straightened and aligned collagen fibers that are oriented perpendicular to the tumor boundary and facilitate local invasion [29]. A rigorous examination of the relationship of TACS-3 to the long-term survival rate of human patients showed that the presence of TACS-3 was associated with poor disease-specific and disease-free survival [30].
Furthermore, TACS-3 was confirmed to be an independent prognostic indicator regardless of tumor grade and size, estrogen or progesterone receptor status, human epidermal growth factor receptor-2 status, node status, and tumor subtype [30]. Interestingly, TACS-3 was positively correlated to the expression of stromal syndecan-1, a receptor for several ECM proteins, including collagens [30]. Aligned ECM in vivo serves as natural trails on which cancer cells migrate [29, 144, 145]. The deposition of fibrillar collagen and collagen-induced release of pro-inflammatory COX-2 is sufficient to induce collective invasion in otherwise benign or less aggressive breast tumor lesions [146]. These studies strongly suggest that tumor ECM morphology is a biomarker for diagnosis and prognosis.
These ECM alterations can be visualized as tumor-associated collagen signatures that can be measured using second harmonic generation imaging [29, 31, 32, 147]. Several computational methods facilitate quantification of the fiber alignment based on the second harmonic generation images of collagen and have demonstrated clear discrimination of invasiveness in breast tumor and non-small cell lung cancer spheroids [32, 148, 149].
A major problem in effectively treating tumor metastasis is identifying tissues with micrometastases. Matrix proteins that participate in this process become activated and undergo conformational changes that expose biologically active cryptic-specific sites [150]. Monoclonal antibodies are recruited to activate vitronectin preferentially localized to malignant tumors [150]. Antibodies directed to the extra domain (ED-B) of fibronectin localized selectively to new blood vessel development sites and malignant tumors. At the same time, little has been associated with non-proliferative tissues [151]. Moreover, the cryptic epitope in laminin-1 recognized by the STQ-peptide was selectively exposed in malignant melanoma in vivo [152]. Taken together, these studies suggest that cryptic ECM elements may represent highly selective targets for the development of novel imaging reagents for the detection of early metastasis [153].
The cross-talk between cancer metabolic pathways and the tumor ECM offers another set of potential imaging targets for cancer. For example, the dynamic contrast enhancement (DCE) MRI has been applied widely in screening high-risk populations and diagnosis and therapy evaluation of breast cancer [154]. A recent low-dose multitasking DCE MRI imaging has enabled extravascular extracellular space (EES) volume fraction as a marker for fibrosis in liver cancer [155]. Additionally, with the explosion of publications on deep learning applications for cancer diagnosis, prognosis, and treatment response [156], [157], [158], [159], we expect to see real-world medical utility of these ECM markers in the near future.
ECM in cancer treatment
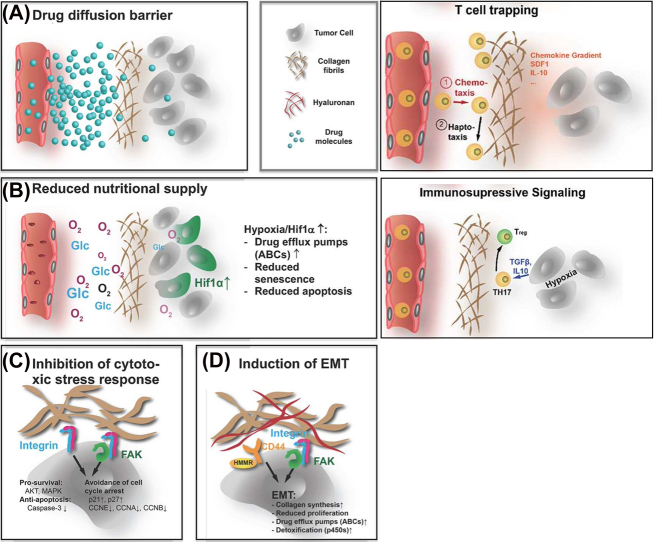
Drawing the parallel between tumor development and wound healing again, the tumor ECM is more abundant, denser, and stiffer than that in normal tissue, akin to but worse than pathological scars, which can negatively impact response to cancer therapy biophysically and biochemically. A recent review highlighted the current understanding of the physical, cellular, and molecular mechanisms by which the pathological tumor ECM affects the efficiency of radio-, chemo-, and immunotherapy [41] (Figure 2). The accumulation of dense and rigid ECM histologically often encapsulates tumors (e.g., the TACS in breast cancer [29]), acts as a barrier to reducing molecular diffusion, and shields the cells from chemotherapeutic agents. At the same time, reduced diffusion of oxygen, nutrients, and metabolites in and out of the tumor also increases hypoxia and metabolic stress, leading to activation of angiogenesis, antiapoptotic, and drug resistance pathways. Furthermore, via integrin-mediated FAK-signaling, increased tissue stiffness and different components of the ECM can contribute to chemoresistance by inhibiting cytotoxic stress response and triggering EMT of tumor cells. Adding to this complexity, ECM dynamics intersect other intracellular signaling networks through modulating activation thresholds, which have in turn become promising targets in increasing chemotherapy efficacy and improving patient outcome [160]. These mechanisms individually and in combination provide many potential targets for therapeutic tools.
Figure 2:
Four ways the ECM affects the efficacy of systemic treatment and immunotherapy.
(A) The abundant, rigid, and dense ECM is a diffusion barrier for drug molecules, thus shielding the tumor from the tumor therapy agents and T cells. (B) The ECM also reduces the diffusion of nutrients and oxygen, resulting in a hypoxic and stressful TME. Tumor cells increase the expression of drug efflux pumps, impair apoptosis and senescence, and activate and activate immunosuppressive signaling, rendering drugs that reach the cells less effective. (C) The cell-ECM contact mediated by integrin and FAK signaling inhibits the cytotoxic stress response that avoids cell cycle arrest. (D) Similarly, integrin, FAK, and hyaluronan-induced CD44/HMMR signals can lead to EMT. The mesenchymal state is less proliferative and more chemoresistant. The EMT further increases collagen production and crosslinking (Adapted from Henke et al. [41] with permission).
The physical barrier for drug diffusion: Distribution of drugs in the tumor occurs mainly by diffusion. Therefore, an abundance and highly cross-linked ECM can significantly reduce drug transport, especially for large molecules. In addition, the ECM barrier can result in only a small volume of the surrounding tissue being supplied by the individual vessels. Solid tumors are already characterized by low microvessel density [161, 162]. The abundant and dense ECM further aggravates the supply situation, leading to hypoxia and metabolic stress, which are directly linked to resistance vs. various forms of cytotoxic therapy and radiotherapy [163], [164], [165], [166], [167].
Molecular mechanisms: The increased collagen production in the TME is strongly correlated with chemo-resistance and reduced survival in triple-negative breast cancer patients [168]. The complex process of collagen synthesis and maturation offers many potential targets for treatment. Inhibition of LOX reduces tissue stiffness on overall collagen deposition, as the maturation process in the form of LOX-induced cross-links further stabilizes collagens and protects them from degradation. Treatment with 2-aminopropionitrile reduced collagen deposition and drug accumulation in murine allograft models [41]. Hyaluronic acid (HA) production is increased in many cancers [168]. Stromal fibroblasts are often identified as the primary source of hyaluronic acid in the tumor. Acting as a ligand for CD44 and playing a role in EMT, HA levels are correlated with poor prognosis [169, 170].
Cellular mechanisms: As carcinoma- or tumor-associated fibroblasts (TAFs) are the primary producer of the ECM in tumors, it is necessary to have a closer look at the particularities of these cells [171], [172], [173]. CAFs are found in all solid tumors [174, 175]. They differ substantially from the quiescent, metabolically inactive fibroblasts found in normal connective tissue, as they are migratory, growth and immune response promoting, and synthetically active [41]. TAFs further contribute to more malignant tumor phenotype by driving epithelial-to-mesenchymal transition (EMT), resulting in invasion and metastasis.
The increased understanding of these physical, molecular, and cellular mechanisms has led to increased interest in targeting the ECM for improved therapeutic efficacy. For example, the degradation of the ECM to remove the physical barrier has been shown to improve drug uptake and response in preclinical trials [176]. However, as the ECM in tumors is constantly remodeled, removing the existent ECM might not be necessary. The perpetual turnover is signified by the high-level synthesis of ECM macromolecules and degradation of the ECM by tumor-secreted hydrolytic enzymes like MMPs and cathepsins. Therefore, it is possible to shift the ECM remodeling toward degradation by blocking or reducing ECM synthesis. This can be accomplished by either reducing cues that lead to increased expression of ECM molecules, like TGFβ signaling or hypoxia-response pathways, or by inhibiting the various modifying enzymes necessary for proper production, secretion, and maturation of ECM molecules [41]. This approach can be further specified by targeting collagen synthesis and maturation and hyaluronan synthesis. For example, inhibition of NFκb reversed the typical CAF phenotype, strongly reduced collagen deposition in the tumor matrix, and improved response to cisplatin in ovarian cancer xenografts [177].
On the other hand, the ECM and ECM-like materials have great potential as cell and drug carriers. Modulated and engineered ECM is an effective approach to improve drug delivery and normalize the tumor ECM by reducing matrix stiffness or reversing the cell–ECM alignment. Modulating collagen alignment using anti-lysyl oxidase-like 2 (LOXL2) antibodies interfered with the adhesion and invasion properties of human breast cancer cells [178]. Although targeting specific stages of collagen assembly in vivo presents a great challenge, additional studies capitalizing on these ideas can potentially lead to a more comprehensive understanding of this promising type of approach [144]. ECM-like materials, e.g., thermally responsive elastin-like polypeptides (ELPs), were synthesized by recombinant DNA techniques and conjugated to doxorubicin to enable release of the drug in the acidic environment of lysosomes, which was delivered to solid tumors as a cancer therapy [179]. Electrospun scaffolds from synthetic polymers, natural proteins, or hybrid materials are often used to deliver drug [180].
While the research efforts targeting ECM to improve the outcome of cancer therapies are still mainly in the realms of primary and early translational research, a number of proto-drugs are in various phases of clinical trial [41]. For example, LOXL2 antibody in combination with gemcitabine and FOLFIRI in PDAC and CRC patients, respectively, has completed phase II trials [181, 182]. Three major challenges in ECM-targeting therapies have been identified [41]. First, targeting ECM synthesis almost always affects other processes in the TME. Likewise, targeting other components of the TME, e.g., angiogenesis or immune cells, also affects the ECM. This mutual interdependence of various processes in the tumor often complicates the interpretation of experiments. Second, as the ECM is a complex mixture of macromolecules, the challenge is to select from this multitude of possible points of attack the right ones that promise the strongest effects and are least likely to cause problems with the functions of the ECM in physiological processes. Finally, the most likely application for ECM-targeted approaches is not a stand-alone therapy but as synergistic improvements in combination with other forms of treatment.
Summary
The concept of tumors as non-healing wounds helps to organize our understandings of the functional roles of ECM in cancer biology. It has long been recognized that tumors share properties with healing wounds. In the early 1970s, Haddow proposed that tumor formation might represent an “overhealing” [183]. Surgeons have reported the tendency of tumors to recur in healing resection margins and that the wound healing environment provides an opportunistic matrix for tumor growth [184, 185]. We compare the ECM of normal tissue to that of normal and pathological scars and propose that tumor ECM is the worst form of abnormal tissue scar that promotes cancer progression.
A dynamic interlocking mesh of water, minerals, proteoglycans, and fibrous proteins such as collagen, elastin, and laminin, secreted by resident cells, the ECM is unique in every tissue to serve the tissue-specific functional needs [10]. In stressful microenvironments, tumor cells co-opt the wound healing response to induce survival and growth stroma. As a result, the tumor ECM resembles the worst form of abnormal scar tissue that differs drastically from normal organs in composition, architecture, and mechanical properties, due to disrupted balance between ECM synthesis and secretion and altered expression of matrix-remodeling enzymes. The tumor ECM provides physical, mechanical, biochemical, and cellular cues to promote tumor growth and malignancy, regulate the course of the disease, and influence the response toward therapy.
The more abundant, dense, and rigid ECM associated with the TME interacts with tumor cells and stromal cells, not only can induce tumorigenesis, regulate tumor metabolism, facilitates angiogenesis, induces EMT for invasion, forms pre-metastatic and metastatic niches to facilitate metastasis but can be a barrier for therapeutic agents. As our in this field grow, interfering with the synthesis and accumulation of ECM or related processes can potentially improve the outcome of concomitant therapeutic approaches, whether these are conventional cytotoxic treatments, radiotherapy, or targeted therapy, including immunotherapy. Furthermore, the ECM and ECM-like materials have great potential as cell and drug carriers, and modulating ECM and engineered ECM can improve drug delivery for enhanced therapeutic efficacy. Of course, the ECM not only affects the efforts to treat cancer but also is directly involved in tumor establishment and disease progression. Effects of ECM-targeted approach on the efficacy of antitumor therapy often cannot be distinguished from direct effects on tumor behavior or progression. Taken together, it is crucial that future studies further elucidate the role of ECM on all stages of cancer development to design therapies that most effectively treat or manage cancer.
Footnotes
Research funding: This work was partially supported by the National Institute of Health grants from NCI (R01CA201340) and NEI (1R01EY028450).
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Not applicable.
Ethical approval: Not applicable.
References
- 1.Kaushik S, Pickup MW, Weaver VM. From transformation to metastasis: deconstructing the extracellular matrix in breast cancer. Cancer Metastasis Rev. 2016;35:655–67. doi: 10.1007/s10555-016-9650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39:1987–94. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Northcott JM, Dean IS, Mouw JK, Weaver VM. Feeling stress: the mechanics of cancer progression and aggression. Front Cell Dev Biol. 2018;6:17. doi: 10.3389/fcell.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ketteler J, Klein D. Caveolin-1, cancer and therapy resistance. Int J Cancer. 2018;143:2092–104. doi: 10.1002/ijc.31369. [DOI] [PubMed] [Google Scholar]
- 5.Padhye A, Ungewiss C, Fradette JJ, Rodriguez BL, Albritton JL, Miller JS, et al. A novel ex vivo tumor system identifies Src-mediated invasion and metastasis in mesenchymal tumor cells in non-small cell lung cancer. Sci Rep. 2019;9:4819. doi: 10.1038/s41598-019-41301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlou M, Tzelepi V, Efstathiou E. Therapeutic targeting of the prostate cancer microenvironment. Nat Rev Urol. 2010;7:494–509. doi: 10.1038/nrurol.2010.134. [DOI] [PubMed] [Google Scholar]
- 7.Baskaran L, Maliakal G, Al’Aref SJ, Singh G, Xu Z, Michalak K, et al. Identification and quantification of cardiovascular structures from CCTA: an end-to-end, rapid, pixel-wise, deep-learning method. JACC Cardiovasc Imag. 2020;13:1163–71. doi: 10.1016/j.jcmg.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 10.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kai F, Laklai H, Weaver VM. Force matters: biomechanical regulation of cell invasion and migration in disease. Trends Cell Biol. 2016;26:486–97. doi: 10.1016/j.tcb.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–51. doi: 10.1530/joe-10-0377. [DOI] [PubMed] [Google Scholar]
- 15.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harbor Perspect Biol. 2011;3:1–24. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4:119–36. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo DD, Zimmermann AS, Nauta A, Longaker MT, Lorenz HP. Scarless fetal skin wound healing update. Birth Defects Res Part C Embryo Today – Rev. 2012;96:237–47. doi: 10.1002/bdrc.21018. [DOI] [PubMed] [Google Scholar]
- 18.Baisch A, und Keloide RFHN. Teil I: grundlagen und Prävention [Hyperplastic scars and keloids. Part I: basics and prevention] HNO. 2006;54:893–904. doi: 10.1007/s00106-006-1462-z. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich HP, Desmoulière A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105. [PMC free article] [PubMed] [Google Scholar]
- 20.Harold F, Dvorak M. Tumors: wounds that do not heal. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 21.Dvorak HF. Tumors: wounds that do not heal—redux. Cancer Immunol Res. 2015;3:1–11. doi: 10.1158/2326-6066.cir-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mammoto T, Jiang A, Jiang E, Panigrahy D, Kieran MW, Mammoto A. Role of collagen matrix in tumor angiogenesis and glioblastoma multiforme progression. Am J Pathol. 2013;183:1293–305. doi: 10.1016/j.ajpath.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer. 2013;108:1–8. doi: 10.1038/bjc.2012.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey T, Bond J, Tighe S, Hunter T, Lintault L, Patel O, et al. Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast Cancer Res Treat. 2009;114:47–62. doi: 10.1007/s10549-008-9982-8. [DOI] [PubMed] [Google Scholar]
- 26.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics. 2012;11:M111 014647. doi: 10.1074/mcp.M111.014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–63. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez JI, Kang I, You W-K, McDonald DM, Weaver VM. In situ force mapping of mammary gland transformation. Integr Biol. 2011;3:910–21. doi: 10.1039/c1ib00043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178:1221–32. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermanns T, Müller UB, Könen‐Waisman S, Howard JC, Steinfeldt T. The toxoplasma gondii rhoptry protein ROP18 is an Irga6-specific kinase and regulated by the dense granule protein GRA7. Cell Microbiol. 2016;18:244–59. doi: 10.1111/cmi.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee B, Konen J, Wilkinson S, Marcus AI, Jiang Y. Local alignment vectors reveal cancer cell-induced ECM fiber remodeling dynamics. Sci Rep. 2017;7:39498. doi: 10.1038/srep39498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomark Prev. 1998;7:1133–44. [PubMed] [Google Scholar]
- 34.Kauppila S, Stenbäck F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. 1998;186:262–8. doi: 10.1002/(sici)1096-9896(1998110)186:3<262::aid-path191>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Ajeti V, Nadiarnykh O, Ponik SM, Keely PJ, Eliceiri KW, Campagnola PJ. Structural changes in mixed Col I/Col V collagen gels probed by SHG microscopy: implications for probing stromal alterations in human breast cancer. Biomed Opt Express. 2011;2:2307–16. doi: 10.1364/boe.2.002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miskolczi Z, Smith MP, Rowling EJ, Ferguson J, Barriuso J, Wellbrock C. Collagen abundance controls melanoma phenotypes through lineage-specific microenvironment sensing. Oncogene. 2018;37:3166–82. doi: 10.1038/s41388-018-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol. 2014;23:457–63. doi: 10.1111/exd.12435. [DOI] [PubMed] [Google Scholar]
- 38.Chitty JL, Setargew YFI, Cox TR. Targeting the lysyl oxidases in tumour desmoplasia. Biochem Soc Trans. 2019;47:1661–78. doi: 10.1042/bst20190098. [DOI] [PubMed] [Google Scholar]
- 39.Kuczek DE, Larsen AMH, Thorseth ML, Carretta M, Kalvisa A, Siersbæk MS, et al. Collagen density regulates the activity of tumor-infiltrating T cells. J Immunother Cancer. 2019;7:1–15. doi: 10.1186/s40425-019-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Garcia A, Palazon A, Noguera-Ortega E, Powell DJ, Guedan S. CAR-T cells hit the tumor microenvironment: strategies to overcome tumor escape. Front Immunol. 2020;11:1109. doi: 10.3389/fimmu.2020.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henke E, Nandigama R, Ergun S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci. 2019;6:160. doi: 10.3389/fmolb.2019.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafaeva M, Erler JT. Framing cancer progression: influence of the organ- and tumour-specific matrisome. FEBS J. 2020;287:1454–77. doi: 10.1111/febs.15223. [DOI] [PubMed] [Google Scholar]
- 43.Hynes RO, Naba A. Overview of the matrisome-an inventory of extracellular matrix constituents and functions. Cold Spring Harbor Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bateman JF, Boot-Handford RP, Lamande SR. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nat Rev Genet. 2009;10:173–83. doi: 10.1038/nrg2520. [DOI] [PubMed] [Google Scholar]
- 46.Winkler J, Roessler S, Sticht C, DiGuilio AL, Drucker E, Holzer K, et al. Cellular apoptosis susceptibility (CAS) is linked to integrin β1 and required for tumor cell migration and invasion in hepatocellular carcinoma (HCC) Oncotarget. 2016;7:22883. doi: 10.18632/oncotarget.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. doi: 10.1038/s41467-020-18794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Socovich AM, Naba A. The cancer matrisome: from comprehensive characterization to biomarker discovery. Semin Cell Dev Biol. 2019;89:157–66. doi: 10.1016/j.semcdb.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 50.Izzi V, Davis MN, Naba A. Pan-cancer analysis of the genomic alterations and mutations of the matrisome. Cancers. 2020;12:2046. doi: 10.3390/cancers12082046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo H, Tu G, Liu Z, Liu M. Cancer-associated fibroblasts: a multifaceted driver of breast cancer progression. Cancer Lett. 2015;361:155–63. doi: 10.1016/j.canlet.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 52.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 53.Raz Y, Erez N. An inflammatory vicious cycle: fibroblasts and immune cell recruitment in cancer. Exp Cell Res. 2013;319:1596–603. doi: 10.1016/j.yexcr.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, et al. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxidants Redox Signal. 2012;16:1264–84. doi: 10.1089/ars.2011.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482–97. [PMC free article] [PubMed] [Google Scholar]
- 56.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–8. doi: 10.1158/0008-5472.can-07-3127. [DOI] [PubMed] [Google Scholar]
- 57.Polanska UM, Acar A, Orimo A. Experimental generation of carcinoma-associated fibroblasts (CAFs) from human mammary fibroblasts. J Vis Exp. 2011;56:e3201. doi: 10.3791/3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA. 2010;107:20009–14. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–91. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–96. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–17. doi: 10.1158/2159-8290.cd-12-0429. [DOI] [PubMed] [Google Scholar]
- 62.Su S, Chen J, Yao H, Liu J, Yu S, Lao L, et al. CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–56 e16. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–79 e10. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Mongiat M, Buraschi S, Andreuzzi E, Neill T, Iozzo RV. Extracellular matrix: the gatekeeper of tumor angiogenesis. Biochem Soc Trans. 2019;47:1543–55. doi: 10.1042/bst20190653. [DOI] [PubMed] [Google Scholar]
- 65.Hayward MK, Muncie JM, Weaver VM. Tissue mechanics in stem cell fate, development, and cancer. Dev Cell. 2021;56:1833–47. doi: 10.1016/j.devcel.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papalazarou V, Zhang T, Paul NR, Juin A, Cantini M, Maddocks ODK, et al. The creatine-phosphagen system is mechanoresponsive in pancreatic adenocarcinoma and fuels invasion and metastasis. Nat Metabol. 2020;2:62–+. doi: 10.1038/s42255-019-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bertero T, Oldham WM, Grasset EM, Bourget I, Boulter E, Pisano S, et al. Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metabol. 2019;29:124–40 e10. doi: 10.1016/j.cmet.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nallanthighal S, Rada M, Heiserman JP, Cha J, Sage J, Zhou B, et al. Inhibition of collagen XI alpha 1-induced fatty acid oxidation triggers apoptotic cell death in cisplatin-resistant ovarian cancer. Cell Death Dis. 2020;11:258. doi: 10.1038/s41419-020-2442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metabol. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–9. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 71.Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–21. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 72.Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39:453–63. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- 73.Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, et al. Overexpression of alpha(v)beta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis. 2002;23:237–44. doi: 10.1093/carcin/23.2.237. [DOI] [PubMed] [Google Scholar]
- 74.Folkman J. Proceedings: tumor angiogenesis factor. Cancer Res. 1974;34:2109–13. [PubMed] [Google Scholar]
- 75.Mongiat M, Andreuzzi E, Tarticchio G, Paulitti A. Extracellular matrix, a hard player in angiogenesis. Int J Mol Sci. 2016;17:1822. doi: 10.3390/ijms17111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whelan MC, Senger DR. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem. 2003;278:327–34. doi: 10.1074/jbc.m207554200. [DOI] [PubMed] [Google Scholar]
- 77.Kamei M, Brian Saunders W, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–6. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 78.De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29:639–49. doi: 10.1161/atvbaha.109.185165. [DOI] [PubMed] [Google Scholar]
- 79.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Yuen SM, et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–80. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemostasis. 2007;5(1 Suppl):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- 81.Hielscher A, Ellis K, Qiu C, Porterfield J, Gerecht S. Fibronectin deposition participates in extracellular matrix assembly and vascular morphogenesis. PLoS One. 2016;11:e0147600. doi: 10.1371/journal.pone.0147600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12:165–75. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou X, Rowe RG, Hiraoka N, George JP, Wirtz D, Mosher DF, et al. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes Dev. 2008;22:1231–43. doi: 10.1101/gad.1643308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–8. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bleuel K, Popp S, Fusenig NE, Stanbridge EJ, Boukamp P. Tumor suppression in human skin carcinoma cells by chromosome 15 transfer or thrombospondin-1 overexpression through halted tumor vascularization. Proc Natl Acad Sci USA. 1999;96:2065–70. doi: 10.1073/pnas.96.5.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, et al. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci USA. 1999;96:14888–93. doi: 10.1073/pnas.96.26.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–85. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quintero-Fabian S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V, Lara-Riegos J, et al. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–107. doi: 10.1161/01.res.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 90.Ives C, Eskin SG, McIntire LV. Mechanical effects on endothelial cell morphology: in vitro assessment. Vitro Cell Dev Biol. 1986;22:500–7. doi: 10.1007/bf02621134. [DOI] [PubMed] [Google Scholar]
- 91.Carosi JA, Eskin SG, McIntire LV. Cyclical strain effects on production of vasoactive materials in cultured endothelial cells. J Cell Physiol. 1992;151:29–36. doi: 10.1002/jcp.1041510106. [DOI] [PubMed] [Google Scholar]
- 92.Patrick C, McIntire LV. Shear stress and cyclic strain modulation of gene expression in vascular endothelial cells. Blood Purif. 1995;13:112–24. doi: 10.1159/000170194. [DOI] [PubMed] [Google Scholar]
- 93.Vernon RB, Sage EH. A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res. 1999;57:118–33. doi: 10.1006/mvre.1998.2122. [DOI] [PubMed] [Google Scholar]
- 94.Wang WY, Jarman EH, Lin D, Baker BM. Dynamic endothelial stalk cell-matrix interactions regulate angiogenic sprout diameter. Front Bioeng Biotechnol. 2021;9:620128. doi: 10.3389/fbioe.2021.620128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Deakin AS. Model for initial vascular patterns in melanoma transplants. Growth. 1976;40:191–201. [PubMed] [Google Scholar]
- 96.Balding D, McElwain DL. A mathematical model of tumour-induced capillary growth. J Theor Biol. 1985;114:53–73. doi: 10.1016/s0022-5193(85)80255-1. [DOI] [PubMed] [Google Scholar]
- 97.Sant M, Allemani C, Berrino F, Coleman MP, Aareleid T, Chaplain G, et al. Breast carcinoma survival in Europe and the United States: a population-based study. Cancer. 2004;100:715–22. doi: 10.1002/cncr.20038. [DOI] [PubMed] [Google Scholar]
- 98.McDougall SR, Anderson AR, Chaplain MA. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. J Theor Biol. 2006;241:564–89. doi: 10.1016/j.jtbi.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 99.Pore N, Gupta AK, Cerniglia GJ, Jiang Z, Bernhard EJ, Evans SM, et al. Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66:9252–9. doi: 10.1158/0008-5472.can-06-1239. [DOI] [PubMed] [Google Scholar]
- 100.Jones SW, Hill RJ, Krasney PA, O’Conner B, Peirce N, Greenhaff PL. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004;18:1025–7. doi: 10.1096/fj.03-1228fje. [DOI] [PubMed] [Google Scholar]
- 101.Bauer AL, Jackson TL, Jiang Y. A cell-based model exhibiting branching and anastomosis during tumor-induced angiogenesis. Biophys J. 2007;92:3105–21. doi: 10.1529/biophysj.106.101501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shirinifard A, Gens JS, Zaitlen BL, Popławski NJ, Swat M, Glazier JA. 3D multi-cell simulation of tumor growth and angiogenesis. PLoS One. 2009;4:e7190. doi: 10.1371/journal.pone.0007190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zheng X, Wise SM, Cristini V. Nonlinear simulation of tumor necrosis, neo-vascularization and tissue invasion via an adaptive finite-element/level-set method. Bull Math Biol. 2005;67:211–59. doi: 10.1016/j.bulm.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Olsen L, Sherratt JA, Maini PK, Arnold F. A mathematical model for the capillary endothelial cell-extracellular matrix interactions in wound-healing angiogenesis. IMA J Math Appl Med Biol. 1997;14:261–81. doi: 10.1093/imammb/14.4.261. [DOI] [PubMed] [Google Scholar]
- 105.Bentley K, Gerhardt H, Bates PA. Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J Theor Biol. 2008;250:25–36. doi: 10.1016/j.jtbi.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 106.Merks RMH, Brodsky SV, Goligorksy MS, Newman SA, Glazier JA. Cell elongation is key to in silico replication of in vitro vasculogenesis and subsequent remodeling. Dev Biol. 2006;289:44–54. doi: 10.1016/j.ydbio.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun S, Wheeler M, Obeyesekere M, Patrickjr C. A deterministic model of growth factor-induced angiogenesis. Bull Math Biol. 2005;67:313–37. doi: 10.1016/j.bulm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 108.Bauer AL, Jackson TL, Jiang Y. Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput Biol. 2009;5:e1000445. doi: 10.1371/journal.pcbi.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Oers RF, Rens EG, LaValley DJ, Reinhart-King CA, Merks RMH. Mechanical cell-matrix feedback explains pairwise and collective endothelial cell behavior in vitro. PLoS Comput Biol. 2014;10:e1003774. doi: 10.1371/journal.pcbi.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ruehle MA, Eastburn EA, LaBelle SA, Krishnan L, Weiss JA, Boerckel JD. Mechanical regulation of microvascular angiogenesis. bioRxiv. 2020 doi: 10.1101/2020.01.14.906354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carr JM, Dvorak AM, Dvorak HF. Circulating membrane vesicles in leukemic blood. Cancer Res. 1985;45:5944–51. [PubMed] [Google Scholar]
- 112.Donnelly SK, Cabrera R, Mao SPH, Christin JR, Wu B, Guo W, et al. Rac3 regulates breast cancer invasion and metastasis by controlling adhesion and matrix degradation. J Cell Biol. 2017;216:4331–49. doi: 10.1083/jcb.201704048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cox TR. The matrix in cancer. Nat Rev Cancer. 2021;21:217–38. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 114.Welch DR, Hurst DR. Defining the hallmarks of metastasis. Cancer Res. 2019;79:3011–27. doi: 10.1158/0008-5472.can-19-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–22. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–27. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 117.Livshits G, Kobielak A, Fuchs E. Governing epidermal homeostasis by coupling cell-cell adhesion to integrin and growth factor signaling, proliferation, and apoptosis. Proc Natl Acad Sci USA. 2012;109:4886–91. doi: 10.1073/pnas.1202120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pathak A. Scattering of cell clusters in confinement. Biophys J. 2016;111:1496–506. doi: 10.1016/j.bpj.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Venning FA, Wullkopf L, Erler JT. Targeting ECM disrupts cancer progression. Front Oncol. 2015;5:224. doi: 10.3389/fonc.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Helvert S, Storm C, Friedl P. Mechanoreciprocity in cell migration. Nat Cell Biol. 2018;20:8–20. doi: 10.1038/s41556-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brentnall TA. Arousal of cancer-associated stromal fibroblasts: palladin-activated fibroblasts promote tumor invasion. Cell Adhes Migrat. 2012;6:488–94. doi: 10.4161/cam.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Niedbala MJ, Sartorelli AC. Plasminogen activator mediated degradation of subendothelial extracellular matrix by human squamous carcinoma cell lines. Cancer Commun. 1990;2:189–99. [PubMed] [Google Scholar]
- 123.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–97. doi: 10.1200/jco.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Przybylo JA, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition: tumor progression at Snail’s pace. Int J Biochem Cell Biol. 2007;39:1082–8. doi: 10.1016/j.biocel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 126.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. JCB (J Cell Biol) 1997;139:1861–72. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–7. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jolly MK, Ware KE, Gilja S, Somarelli JA, Levine H. EMT and MET: necessary or permissive for metastasis? Mol Oncol. 2017;11:755–69. doi: 10.1002/1878-0261.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Konen J, Summerbell E, Dwivedi B, Galior K, Hou Y, Rusnak L, et al. Image-guided genomics of phenotypically heterogeneous populations reveals vascular signalling during symbiotic collective cancer invasion. Nat Commun. 2017;8:15078. doi: 10.1038/ncomms15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li CF, Chen J-Y, Ho Y-H, Hsu W-H, Wu L-C, Lan H-Y, et al. Snail-induced claudin-11 prompts collective migration for tumour progression. Nat Cell Biol. 2019;21:251–62. doi: 10.1038/s41556-018-0268-z. [DOI] [PubMed] [Google Scholar]
- 131.Mercedes SVA, Bocci F, Levine H, Onuchic JN, Jolly MK, Wong PK. Decoding leader cells in collective cancer invasion. Nat Rev Cancer. 2021;21:592–604. doi: 10.1038/s41568-021-00376-8. [DOI] [PubMed] [Google Scholar]
- 132.Ilina O, Gritsenko PG, Syga S, Lippoldt J, La Porta CAM, Chepizhko O, et al. Cell-cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nat Cell Biol. 2020;22:1103–15. doi: 10.1038/s41556-020-0552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, et al. Neutrophil extracellular traps sequester circulating tumor cells via beta1-integrin mediated interactions. Int J Cancer. 2017;140:2321–30. doi: 10.1002/ijc.30635. [DOI] [PubMed] [Google Scholar]
- 135.Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–32. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 136.Hoye AM, Erler JT. Structural ECM components in the premetastatic and metastatic niche. Am J Physiol Cell Physiol. 2016;310:C955–67. doi: 10.1152/ajpcell.00326.2015. [DOI] [PubMed] [Google Scholar]
- 137.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–26. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deyell M, Garris CS, Laughney AM. Cancer metastasis as a non-healing wound. Br J Cancer. 2021;124:1491–502. doi: 10.1038/s41416-021-01309-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen D, Chen D, Cao D, Hu J, Yao Y. A signature based on survival-related genes identifies high-risk glioblastomas harboring immunosuppressive and aggressive ECM characteristics. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2018;43:368–82. doi: 10.11817/j.issn.1672-7347.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 141.Bergamaschi A, Tagliabue E, Sørlie T, Naume B, Triulzi T, Orlandi R, et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J Pathol. 2008;214:357–67. doi: 10.1002/path.2278. [DOI] [PubMed] [Google Scholar]
- 142.Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol. 2015;7:1120–34. doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takai K, Le A, Weaver VM, Werb Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. 2016;7:82889–901. doi: 10.18632/oncotarget.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Malik R, Lelkes PI, Cukierman E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015;33:230–6. doi: 10.1016/j.tibtech.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–30. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 146.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–15. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J. 2008;95:5374–84. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bredfeldt JS, Liu Y, Conklin MW, Keely PJ, Mackie TR, Eliceiri KW. Automated quantification of aligned collagen for human breast carcinoma prognosis. J Pathol Inf. 2014;5:28. doi: 10.4103/2153-3539.139707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Konen J, Wilkinson S, Lee B, Fu H, Zhou W, Jiang Y, et al. LKB1 kinase-dependent and -independent defects disrupt polarity and adhesion signaling to drive collagen remodeling during invasion. Mol Biol Cell. 2016;27:1069–84. doi: 10.1091/mbc.e15-08-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bloemendal HJ, de Boer HC, Koop EA, van Dongen AJ, Goldschmeding R, Landman WJM, et al. Activated vitronectin as a target for anticancer therapy with human antibodies. Cancer Immunol Immunother. 2004;53:799–808. doi: 10.1007/s00262-004-0506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Menrad A, Menssen HD. ED-B fibronectin as a target for antibody-based cancer treatments. Expert Opin Ther Targets. 2005;9:491–500. doi: 10.1517/14728222.9.3.491. [DOI] [PubMed] [Google Scholar]
- 152.Akalu A, Roth JM, Caunt M, Policarpio D, Liebes L, Brooks PC. Inhibition of angiogenesis and tumor metastasis by targeting a matrix immobilized cryptic extracellular matrix epitope in laminin. Cancer Res. 2007;67:4353–63. doi: 10.1158/0008-5472.can-06-0482. [DOI] [PubMed] [Google Scholar]
- 153.Cretu A, Brooks PC. Impact of the non-cellular tumor microenvironment on metastasis: potential therapeutic and imaging opportunities. J Cell Physiol. 2007;213:391–402. doi: 10.1002/jcp.21222. [DOI] [PubMed] [Google Scholar]
- 154.Menezes GL, Knuttel FM, Stehouwer BL, Pijnappel RM, van den Bosch MA. Magnetic resonance imaging in breast cancer: a literature review and future perspectives. World J Clin Oncol. 2014;5:61–70. doi: 10.5306/wjco.v5.i2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang N, Xie Y, Fan Z, Ma S, Saouaf R, Guo Y, et al. Five-dimensional quantitative low-dose multitasking dynamic contrast-enhanced MRI: preliminary study on breast cancer. Magn Reson Med. 2021;85:3096–111. doi: 10.1002/mrm.28633. [DOI] [PubMed] [Google Scholar]
- 156.Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500–10. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vamathevan J, Clark D, Czodrowski P, Dunham I, Ferran E, Lee G, et al. Applications of machine learning in drug discovery and development. Nat Rev Drug Discov. 2019;18:463–77. doi: 10.1038/s41573-019-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A. Artificial intelligence in digital pathology – new tools for diagnosis and precision oncology. Nat Rev Clin Oncol. 2019;16:703–15. doi: 10.1038/s41571-019-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kleppe A, Skrede O-J, De Raedt S, Liestøl K, Kerr DJ, Danielsen HE. Designing deep learning studies in cancer diagnostics. Nat Rev Cancer. 2021;21:199–211. doi: 10.1038/s41568-020-00327-9. [DOI] [PubMed] [Google Scholar]
- 160.Vennin C, Murphy KJ, Morton JP, Cox TR, Pajic M, Timpson P. Reshaping the tumor stroma for treatment of pancreatic cancer. Gastroenterology. 2018;154:820–38. doi: 10.1053/j.gastro.2017.11.280. [DOI] [PubMed] [Google Scholar]
- 161.Offersen BV, Borre M, Overgaard J. Immunohistochemical determination of tumor angiogenesis measured by the maximal microvessel density in human prostate cancer. APMIS. 1998;106:463–9. doi: 10.1111/j.1699-0463.1998.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 162.da Silva BB, Lopes-Costa PV, dos Santos AR, de Sousa-Júnior EC, Alencar AP, Pires CG, et al. Comparison of three vascular endothelial markers in the evaluation of microvessel density in breast cancer. Eur J Gynaecol Oncol. 2009;30:285–8. [PubMed] [Google Scholar]
- 163.Moeller BJ, Dewhirst MW. Raising the bar – how HIF-1 helps determine tumor radiosensitivity. Cell Cycle. 2004;3:1107–10. doi: 10.4161/cc.3.9.1099. [DOI] [PubMed] [Google Scholar]
- 164.Doublier S, Belisario DC, Polimeni M, Annaratone L, Riganti C, Allia E, et al. HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: a potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast. BMC Cancer. 2012;12:4. doi: 10.1186/1471-2407-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–22. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57:i90–8. doi: 10.1093/jrr/rrw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Graham K, Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int J Nanomed. 2018;13:6049–58. doi: 10.2147/ijn.s140462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xiong G, Stewart RL, Chen J, Gao T, Scott TL, Samayoa LM, et al. Collagen prolyl 4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC chemoresistance. Nat Commun. 2018;9:1–16. doi: 10.1038/s41467-018-06893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Heldin P, Basu K, Kozlova I, Porsch H. HAS2 and CD44 in breast tumorigenesis. Adv Cancer Res. 2014;123:211–29. doi: 10.1016/b978-0-12-800092-2.00008-3. [DOI] [PubMed] [Google Scholar]
- 170.Zhang L, Wang Y, Xia T, Yu Q, Zhang Q, Yang Y, et al. Suppression for lung metastasis by depletion of collagen I and lysyl oxidase via losartan assisted with paclitaxel-loaded pH-sensitive liposomes in breast cancer. Drug Deliv. 2016;23:2970–9. doi: 10.3109/10717544.2015.1132798. [DOI] [PubMed] [Google Scholar]
- 171.Bagordakis E, Sawazaki-Calone I, Macedo CCS, Carnielli CM, de Oliveira CE, Rodrigues PC, et al. Secretome profiling of oral squamous cell carcinoma-associated fibroblasts reveals organization and disassembly of extracellular matrix and collagen metabolic process signatures. Tumor Biol. 2016;37:9045–57. doi: 10.1007/s13277-015-4629-y. [DOI] [PubMed] [Google Scholar]
- 172.Pankova D, Chen Y, Terajima M, Schliekelman MJ, Baird BN, Fahrenholtz M, et al. Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol Cancer Res. 2016;14:287–95. doi: 10.1158/1541-7786.mcr-15-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pasanen I, Lehtonen S, Sormunen R, Skarp S, Lehtilahti E, Pietilä M, et al. Breast cancer carcinoma-associated fibroblasts differ from breast fibroblasts in immunological and extracellular matrix regulating pathways. Exp Cell Res. 2016;344:53–66. doi: 10.1016/j.yexcr.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 174.Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171:1611–24 e24. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao Q, Eichten A, Parveen A, Adler C, Huang Y, Wang W, et al. Single-cell transcriptome analyses reveal endothelial cell heterogeneity in tumors and changes following antiangiogenic treatment. Cancer Res. 2018;78:2370–82. doi: 10.1158/0008-5472.can-17-2728. [DOI] [PubMed] [Google Scholar]
- 176.Eikenes L, Tari M, Tufto I, Bruland ØS, de Lange Davies C. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx (TM)) in human osteosarcoma xenografts. Br J Cancer. 2005;93:81–8. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu S, Yang Z, Jin P, Yang X, Li X, Wei X, et al. Metformin suppresses tumor progression by inactivating stromal fibroblasts in ovarian cancer. Mol Cancer Therapeut. 2018;17:1291–302. doi: 10.1158/1535-7163.mct-17-0927. [DOI] [PubMed] [Google Scholar]
- 178.Grossman M, Ben-Chetrit N, Zhuravlev A, Afik R, Bassat E, Solomonov I, et al. Tumor cell invasion can be blocked by modulators of collagen fibril alignment that control assembly of the extracellular matrix. Cancer Res. 2016;76:4249–58. doi: 10.1158/0008-5472.can-15-2813. [DOI] [PubMed] [Google Scholar]
- 179.Dreher MR, Raucher D, Balu N, Michael Colvin O, Ludeman SM, Chilkoti A. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Contr Release. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 180.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 181.Benson AB, Wainberg ZA, Hecht JR, Vyushkov D, Dong H, Bendell J, et al. A phase II randomized, double-blind, placebo-controlled study of simtuzumab or placebo in combination with gemcitabine for the first-line treatment of pancreatic adenocarcinoma. Oncologist. 2017;22:241. doi: 10.1634/theoncologist.2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hecht JR, Benson AB, Vyushkov D, Yang Y, Bendell J, Verma U. A phase II, randomized, double-blind, placebo-controlled study of simtuzumab in combination with FOLFIRI for the second-line treatment of metastatic KRAS mutant colorectal adenocarcinoma. Oncologist. 2017;22:243-e23. doi: 10.1634/theoncologist.2016-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Haddow A. Addendum to “molecular repair, wound healing, and carcinogenesis: tumor production a possible overhealing?”. Adv Cancer Res. 1974;20:343–66. doi: 10.1016/s0065-230x(08)60113-x. [DOI] [PubMed] [Google Scholar]
- 184.Carmeliet P, Dor Y, Herbert J-M, Fukumura D, Brusselmans K, Dewerchin M, et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 185.Cheng SC, Ying MTC, Kwong DLW, Wu VWC. Sonographic appearance of parotid glands in patients treated with intensity-modulated radiotherapy or conventional radiotherapy for nasopharyngeal carcinoma. Ultrasound Med Biol. 2011;37:220–30. doi: 10.1016/j.ultrasmedbio.2010.11.002. [DOI] [PubMed] [Google Scholar]