Abstract
Immune cells are indispensable defenders of the human body, clearing exogenous pathogens and toxicities or endogenous malignant and aging cells. Immune cell dysfunction can cause an inability to recognize, react, and remove these hazards, resulting in cancers, inflammatory diseases, autoimmune diseases, and infections. Immune cells regulation has shown great promise in treating disease, and immune agonists are usually used to treat cancers and infections caused by immune suppression. In contrast, immunosuppressants are used to treat inflammatory and autoimmune diseases. However, the key to maintaining health is to restore balance to the immune system, as excessive activation or inhibition of immune cells is a common complication of immunotherapy. Nanoparticles are efficient drug delivery systems widely used to deliver small molecule inhibitors, nucleic acid, and proteins. Using nanoparticles for the targeted delivery of drugs to immune cells provides opportunities to regulate immune cell function. In this review, we summarize the current progress of nanoparticle-based strategies for regulating immune function and discuss the prospects of future nanoparticle design to improve immunotherapy.
Keywords: biomaterial, drug delivery, immune cell, immunotherapy, nanoparticle
Introduction
The immune system consists of immune cells, related molecules, and tissues that protect the body from external influences and maintain homeostasis. It maintains a dynamic balance between promoting immune or inflammatory processes and regulating or inhibiting functions, playing a crucial role in tissue homeostasis and disease progression [1]. Immunomodulatory biomaterials capable of manipulating innate and adaptive immunity hold great promise for many preventive and therapeutic purposes [2]. Nanotechnology enables engineered nanomaterials to target specific organs or tissues and deliver immunomodulators while avoiding adverse immunosuppressive or immunostimulatory effects. Various nanomaterials are in clinical trials or have been approved by the US Food and Drug Administration (FDA) [3, 4].
Nanoparticles can modulate the immune system for different therapeutic purposes (inflammation, autoimmune disorders, tumors). For example, when immune cell function is suppressed by stress, chronic infection or uncontrolled due to autoimmune disease, allergies, transplants, and hypersensitivity reactions, nanoparticles can be used as immunomodulators to modulate immunosuppression or activation. Immunosuppressive nanoparticles can block the adverse immune response (autoimmune or allergic disease). In contrast, immunoreactive nanoparticles can transport cytokines and molecules that enhance the immune response (useful for treatment in immunosuppressed patients) [5]. Nanoparticles for tumor immunotherapy and adjuvant nanoparticles for improved vaccines have also been developed [6]. For example, the innate immune response can kill tumor cells through macrophages, natural killer cells, neutrophils, and dendritic cells (DCs). Tumor-associated antigens stimulate the maturation and subsequent migration of DCs to the lymph nodes. Once in the lymph nodes, it stimulates T cell activation (the adaptive immune system), then the activated T cells migrate and recognize tumor cell antigens. Finally, tumor cells are killed by cytotoxic T cells [7].
To regulate immune cell function, nanocarriers can be applied via several approaches. (1) Nanoparticle as adjuvants can be used to alter the response of immune cell subsets. The adjuvant activity of nanoparticles depends on their physicochemical properties, and their ability to be internalized by different cells can modulate the degree of immune cell activation. (2) Nanoparticles can deliver drugs into immune cells. The delivery of immunomodulatory molecules via nanoparticles to stimulate the immune response may limit systemic toxicity. The characteristics of the nanocarriers (i.e., size, surface charge, or shape) can be optimized to facilitate binding to immune cells, and specific immune cell populations preferentially take up different modifications (e.g., mannose modification). Cell-specific activation and selective identification can only be achieved using actively targeted ligands. (3) Nanoparticles can be used to deliver immunomodulators to the surface of immune cells. They can block or activate signaling pathways mediated by relevant ligands and enhance the interaction between effector and target cells to regulate immune cell function.
Here we discuss the role of immune cells in the initiation and progression of various diseases, summarize the leading strategy used to regulate immune cells, and review drug delivery systems designed to target immune cells. Finally, we provide a prospectus of the future of immune cell-targeted drug delivery systems.
Relationship between immune cells and diseases
Abnormal immune responses, including excess immune suppression or activation, will inevitably lead to disease development. Recent studies have shown that the presence of both inflammatory processes (immune stimulation) and immune tolerance (no response to autoantigens or harmless foreign antigens) are influenced by the way antigens are presented [8]. When the mechanisms that regulate the balance between pathogen recognition and self-injury avoidance are compromised, inflammation can get out of control, resulting in sustained immune activation and tissue lesions.
Role of innate immunity in diseases
The innate immune system, consisting of physical mucosal barriers, proteins, and myeloid cells (including granulocytes, mast cells, monocytes, macrophages, DCs, and innate lymphoid cells), is the first line of defense against infectious agents and other environmental challenges. These cells initiate adaptive immune responses such as phagocytosis of macrophages and granulocytes and cytotoxicity of NK cells while producing effector responses. Through the opsonization of antibodies, innate immune cells also participate in the effect-response after antibody induction through antibody-dependent cytophagocytosis or antibody-dependent cytotoxicity and secrete specific cytokines at the reaction site to produce local inflammation [9–11]. For example, autoimmune diseases are chronic inflammatory diseases caused by immune dysregulation. DCs are directly involved in systemic autoimmune responses and disease as antigen-presenting cells and major producers of type 1 interferon [12]. Different subpopulations of DCs regulate humoral and cellular adaptive immunity [13]. Immunosuppressive factors in tumors also inhibit innate immune cells from maintaining an effective antitumor immune response. Infiltration of myeloid suppressor cells (MDSCs) or tumor-associated macrophages (TAMs) into a tumor may create a tolerant environment that inhibits antitumor immune activity, thereby protecting the continued growth of tumor cells. Excess circulating MDSCs and tumor-infiltrating MDSCs or TAMs have been associated with poor prognosis in various cancers [14, 15].
Role of adaptive immunity in diseases
The adaptive immune response follows the innate immune response via the activation of T and B lymphocytes, antigen-specific cells that target pathogens and immune system initiators. The two immune responses are interrelated and highly dependent on immune system activation [16]. T cells and B cells are the main and central force in mediating the adaptive immune response, but in some diseases, such as chronic inflammation and tumors, T cells become dysfunctional due to multiple inhibitory signals in the inflammatory microenvironment [17]. Prolonged exposure of T cells to homologous antigens leads to T cell receptor (TCR) signaling, resulting in sustained elevated expression of inhibitory receptors on these cells. In addition, T cells enter a state of dysfunction characterized by graded loss of effector function and proliferation and different transcriptional and metabolic changes [18]. Upregulation of immune checkpoints has been described as a marker of T cell depletion, a secondary characteristic of which is the progressive loss of effector function, including secretion of interleukin 2 (IL-2) and tumor necrosis factor α (TNF-α) [19, 20]. B cells also play a key role in disease progression. For example, tumor-infiltrating B cells mediate humoral and cellular immunity in tumor tissue, but their role in antitumor immunity is still under debate [21]. Some studies have shown that B cells induce and maintain beneficial antitumor activity. In contrast, others have found that B cells may perform tumor-suppressing functions due to their different immunosuppressive subtypes [22].
Strategies and challenges for regulating immune cell function
For chronic inflammatory diseases, including tumors, the key is to reverse immune cell inhibition and incapacitating depletion, reactivate existing immune responses, or generate new antigen-specific immune responses. For example, immune checkpoint blockade therapy has yielded unprecedented efficacy in a variety of tumor diseases by blocking immune checkpoints, but immunotherapy (especially monotherapy) remains ineffective in most patients, a finding that reflects the heterogeneity of immunobiology and the complex mechanisms of immune escape in chronic inflammation [23, 24]. Understanding the interactions between immune cells and the disease-associated microenvironment is crucial for analyzing the mechanisms of action and an effective strategy for improving the efficacy of current immunotherapies. We need a multi-pronged approach to induce an immune response, enhance the depth and persistence of the immune response, and achieve complete remission. Methods include (1) eliminating immunosuppression by weakening immunosuppressive mediators such as suppressive cells or blocking them via inhibitory receptors; (2) inducing immunogenic death of tumor cells (in combination with chemotherapy, radiotherapy, targeted therapy, and cell therapy); (3) enhancing antigen-presenting cell function (immune adjuvant); and (4) promoting the activation, enhancement, and survival of memory T cells and macrophage function.
Although immunotherapy improves outcomes for many patients by intervening in the immune system to treat various diseases, overstimulation can lead to autoimmune toxicity, also known as immune-related adverse events (irAEs). As a result, there remains a critical unmet need for alternative ways to drive the immune system response safely and effectively to targeting various diseases. The main challenge is to hit these targets safely at the right time and place [25–27].
To enable immune cell regulation, nanoparticles have emerged as a suitable vehicle to overcome the limitations of conventional pharmaceutical preparations and their associated pharmacokinetics. Various studies have reported that nanoparticles can regulate immune cell function [28, 29]. The properties of the nanoparticle (e.g., composition, size, shape, surface chemical modifications, peptides, or protein modifications) determine the interaction between the nanoparticle and immune cells. Due to the presence of different cells and molecules in other organs, such as epithelial cells (e.g., DCs, macrophages) or blood (e.g., granulocytes, mononuclear/macrophages, lymphocytes), the results of immune stimulation vary. A better understanding of immune responses and their interactions with nanoparticles is critical to developing nanoparticle-based immunoregulatory therapies.
Nanoparticle-based strategies for T cell regulation
T cells are one of the most important immune system lymphocytes and play an essential role in various diseases, including inflammation, cancers, and autoimmune diseases [30]. T cells originate in the bone marrow and mature in the thymus [31]. After migration to the thymus gland, the lymphoid precursors differentiate into helper CD4+ T cells, cytotoxic CD8+ T cells, regulatory CD4+ T cells, or become memory T cells. Cytotoxic CD8+ T cells can kill infected cells and cancer cells [32]. CD4+ T cells perform different functions depending on their effector subtypes, including activation of B lymphocytes, cytotoxic T cells, and the innate immune system [33]. Here we review nanotechnology applied to T cells, including nanoparticle-mediated T cell activation, T cell-targeting strategies for nanoparticle delivery, CAR-T cell engineering, and nano-bispecific-engagers of T cells.
Nanoparticles for T cell activation
Activation and expansion are important for T cell function. T cell activation requires three signals: (1) recognition of the peptide/major histocompatibility complex (MHC) by TCR on the T cell surface; (2) co-stimulation signals induced by interactions between co-signaling molecules expressed on antigen-presenting cells (APCs) and their paired T cell-expressed receptors; and (3) cytokines such as IL-2, IL-12, and Type I IFN. Effective T cell activation remains a challenge in tumor therapy [34]. Nanotechnology control of T cell activation offers solutions.
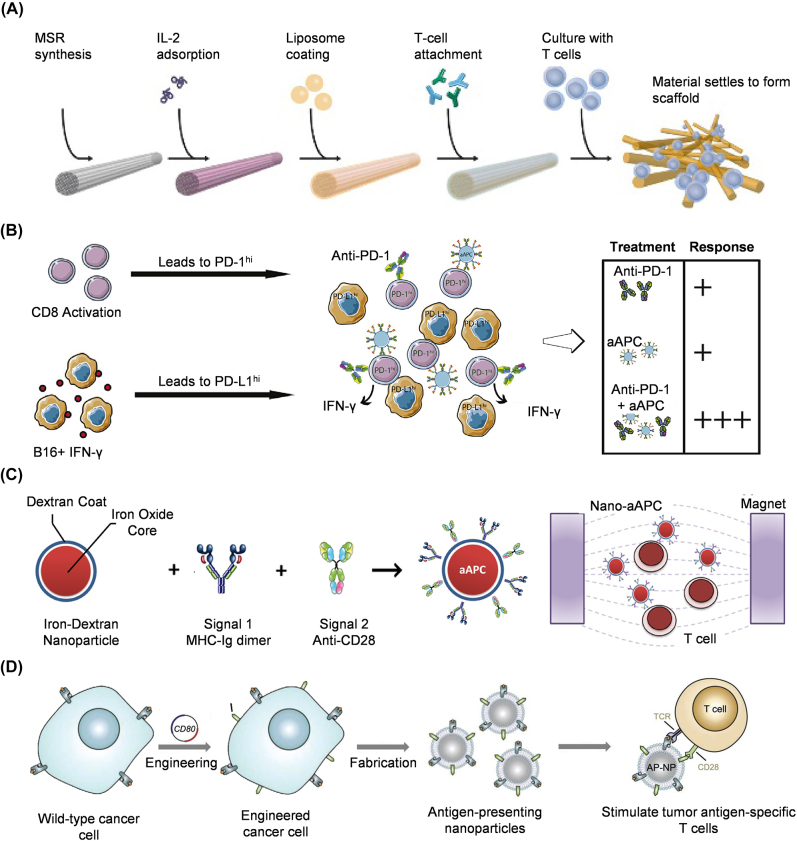
Artificial APC (aAPC) micro- or nanoparticle-based systems mimic natural APCs, mainly DCs, to activate adaptive immunity. To generate the three required activation signals, Cheung et al. developed fluid lipid bilayer-supported mesoporous silica micro-rods (MSRs) (Figure 1A). The APC-MSRs contained anti-CD3 antibody for T cell attachment, pMHC for signal 1, αCD28 antibody for signal 2, and cytokine IL-2 for signal 3. They induced 2- to 10-fold greater expansion of T cells than commercial expansion beads (Dynabeads) [35]. Many biomaterials-based nanoscale aAPCs have been developed. Poly (lactic-co-glycolic acid) (PLGA)-based nanoparticles were functionalized with an MHC Class I-Ig fusion protein to mimic signal one and an agonistic monoclonal anti-CD28 antibody (Figure 1B) [36]. Kosmides et al. showed that combining this polymeric aAPC and anti-PD-1 monoclonal antibody induced the greatest CD8+ T cell activation in vitro and delayed melanoma tumor growth and extended survival in vivo. Lipid-based and inorganic materials can also mimic the features of APCs to activate T cells [37, 38]. Compared to micro-aAPCs, nano-aAPCs have a small contact area with T cells, leading to less efficient activation. To enhance the area of contact between the T cell and nano-aAPC, nanoparticle clustering and shape alternation have been applied to nano-aAPC design. Perica et al. showed that magnetic-field-induced nanoparticle clustering enhanced T cell activation [39]. The magnetic field-mediated aggregation of nano-aAPCs increased TCR cluster size and T cell expansion in vitro and enhanced adaptive T cell immunotherapy (Figure 1C). The cellular fate of nano-aAPCs can also be altered by changing their shape. Ellipsoid particles reduce non-targeted uptake and increase specific uptake, thereby enhancing anticancer efficacy [40, 41]. Meyer et al. used ellipsoidal PLAG nano-aAPCs functionalized with an MHC Class I Ig dimer (signal 1) and anti-CD28 (signal 2) to reduce phagocytosis compared to spherical nano-aAPCs [42]. The use of large nano-aAPCs is an effective way to increase the contact area with T cells [43]. Biomimetics such as cell membrane-based aAPCs provide natural protein structures that may facilitate conjugation to solid nanoparticles. Jiang et al. reported a tumor cell-derived membrane-coated aAPC to directly stimulate T cells without APCs [44]. Wild-type B16 melanoma cells presenting antigen/MHC-I complex were engineered to co-express cytosolic OVA and costimulatory protein CD80. The membranes of these engineered tumor cells were then used to coat polymeric nanoparticles to generate aAPCs, which efficiently activated T cells ex vivo and significantly delayed tumor growth after intravenous injection (Figure 1D). aAPCs can also be used to induce regulatory T cells. Immune-tolerant aAPCs were developed by coating nanoparticles with autoimmune disease-related peptides/MHC complex [45, 46]. These aAPCs do not carry signal 2 for T cell activation and thus induce antigen-specific regulatory CD4+ and CD8+ T cells and antigen-specific immune tolerance for treating autoimmune disease.
Figure 1:
Nano-aAPCs for T cell activation. (A) APC-ms prepared from MSRs can realize polyclonal T cell expansion and antigen-specific T cell expansion by binding different antibodies (image source: Li et al. [35] with permission). (B) In vitro tumor microenvironment model system for MHC IgG dimer and anti-CD28 antibody-conjugated PLGA nanoparticles [36]. (C) Nano-aAPC synthesis by coupling MHC-Ig dimers and costimulatory anti-CD28 to iron-dextran nanoparticles [39]. (D) Engineered cancer cell-membrane-coated nanoparticles for direct antigen presentation [44].
Nanoparticles for T cell-targeting delivery
After injection, nanoparticles are easily captured by the mononuclear phagocyte system or tissue-resident phagocytes. Some studies have shown that, without targeting strategies, T cells may take up fewer nanoparticles than other immune cells like DCs [47], [48], [49]. Therefore, nanoparticles with specific properties can be used for T cell delivery.
T cell delivery can be accomplished passively, mediated by the physical properties of the nanoparticles. Li et al. used cationic lipid-assisted polymeric nanoparticles encapsulating small interfering RNA (siRNA) targeting cytotoxic T lymphocyte-associated molecule-4 (CTLA-4) for T cell immunotherapy [50]. This nanoparticle delivery system could deliver siRNA into CD4+ and CD8+ T cells in vivo, enhancing T cell activation and proliferation, enhancing the antitumor immune response, and significantly inhibiting B16 melanoma. Huq et al. reported antioxidant carbon nanoparticles for T cell delivery [51]. Nontoxic poly (ethylene glycol)-functionalized hydrophilic carbon clusters (PEG-HCCs) are taken up by T cells over other splenic immune cells after systemic application in vivo. However, T cells in lymph nodes did not exhibit this preference. Hewitt et al. demonstrated that non-functionalized ultrasmall silica nanoparticles (USSNs, diameter 3.6–5.1 nm) strongly activated T cells but showed no T cell-specific uptake [52]. USSNs induced dose-dependent CD4+ and CD8+ T cell activation and INF-γ secretion but failed to induce secretion of IL-2, IL-10, IL-4, or T cell proliferation. Thiramanas et al. investigated the use of silica nanoparticles (SiNPs) of different particle sizes and physicochemical properties as T cell delivery vectors [53] by varying size, core hydrophobicity, surface charge, and surface function to modulate CD8+ T cell uptake and toxicity. Cellular uptake and toxicity are primarily size- and dose-dependent. SiNPs smaller than 100 nm had a significant toxic effect. Another study of nanoparticle size and T cell delivery [54] used a cationic deblock copolymer modified with triphenylphosphonium cations and small cationic nanohydrogel particles and tested them for siRNA delivery to T cells. Their nanoparticles achieved efficient complexation of siRNA and significant internalization into T cells without reducing cell viability in vitro.
T cell-targeting antibodies can functionalize nanoparticles to enhance T cell uptake. Anti-CD3 antibody-conjugated gelatin nanoparticles were developed for T cell-specific targeting [55] by binding biotinylated anti-CD3 antibodies to nanoparticles via NeutrAvidin-biotin-complex formation. Uptake efficiency by T cell leukemia cells via endocytosis was about 84% in vitro. Kheirolomoon et al. also demonstrated that anti-CD3 antibody-conjugated lipid nanoparticles (αCD3-LNPs) could be used for T cell delivery in vitro and in vivo [56]. αCD3-LNPs efficiently delivered mRNA to Jurkat T cells in vitro and, after systemic injection, αCD3-LNPs encapsulating mCherry mRNA accumulated in the spleen. The efficiency of mCherry expression was 2%–4% of all splenic T cells and 2%–7% of all circulating T cells. Ramishetti et al. described a CD4-targeting strategy to deliver siRNAs specifically to CD4+ T cells [57]. Anti-CD4 antibody-functionalized LNPs loaded with CD45 siRNA were taken up by CD4+ T cells in the spleen, inguinal lymph nodes, blood, and bone marrow, resulting in significant knockdown of CD45 expression. Tombacz et al. also reported anti-CD4 antibody-conjugated LNPs to enable specific targeting of mRNA interventions in CD4+ cells [58]. After systemic injection in mice, CD4-targeted mRNA-LNPs accumulated in the spleen and provided a 30-fold higher reporter mRNA signal in splenic T cells than non-targeted mRNA-LNPs. Intravenous administration of CD4-targeted LNPs loaded with mRNA encoding Cre recombinase led to highly specific loxP-mediated genetic recombination in CD4+ T cells (approximately 60% in the spleen and 40% in lymph nodes). CD7 and CD8 have also been used for T cell targeting due to their high expression levels on T cells [59, 60].
Nanoparticles for CAR-T cell engineering
CAR-T cell therapy has achieved a high response rate in treating hematological malignancies and is an important cellular immunotherapy for treating tumors. Currently, most CAR-T cells are engineered by transduction of T cells collected from patients and then infused back into the patients. The high cost and complexity of CAR-T cell manufacture make it difficult to achieve worldwide application. To date, FDA-approved CAR-T cell products are all transfected with CAR by lentivirus, which cannot be used directly in vivo. Nawaz et al. showed that an adeno-associated viral vector (AAV) carrying the CAR gene generates human CD4-targeting CAR-T cells and reduces human T cell leukemia in a humanized mouse model [61]. However, the AAV vector can be taken up by non-T cells in vivo, resulting in non-targeted toxicity. Therefore, there is a need for in vivo CAR-T-cell production methods that can target T cells more efficiently.
Nanoparticles are widely used for nucleic acid delivery and can be used for cell targeting by surface-modified antibodies. Smith et al. developed in-situ engineered CAR-T cells using polymeric nanoparticles [62]. Anti-CD3e F(ab’)2-modified nanoparticles encapsulating plasmids encoding murine anti-CD19 CAR and hyperactive transposase selectively deliver CAR genes into T cells in vivo, resulting in long-term disease remission (Figure 2A). The same strategy could be used for CAR mRNA delivery ex vivo and in vivo (Figure 2B) [63, 64]. Compared to plasmid DNA, therapeutic mRNA is pharmacologically and immunologically suited for clinical application. Lipid nanoparticle (LNP)-based mRNA delivery systems have emerged as a potential method for T cell engineering. LNP-mRNA technology has been successfully used in developing COVID-19 vaccines and has demonstrated potential for treating other diseases [65], [66], [67]. Billingsley et al. designed ionizable LNP for mRNA delivery to human T cells ex vivo [68]. A library of 24 ionizable lipids was formulated into LNPs and screened for reporter gene mRNA delivery into human Jurkat cells. The delivery efficiency of the best-performing LNP formulation is greater than the efficiency of lipofectamine and equivalent to electroporation but with low cytotoxicity. CAR-T cells were reprogrammed in vivo by CD5-targeting LNP-carrying CAR mRNA [69]. In a mouse model of heart failure, fibroblast activation protein (FAP)-targeting CAR mRNA was encapsulated in anti-CD5 antibody-functionalized LNP and then delivered into T cells to generate CAR-T cells against FAP (Figure 2C). This study also indicated that in vivo generation of CAR-T cells can be a therapeutic platform for cancer and other diseases.
Figure 2:
Nanoparticles for CAR-T cell engineering. (A) Poly (β-amino ester) (PBAE) nanoparticles functionalized with anti-CD3e F(ab’)2 to deliver plasmid DNA for CAR-T cell generation in vivo [62]. (B) Anti-CD8 antibody-modified PBAE nanoparticles to deliver mRNA for specific TCR-T or CAR-T cell generation in vivo [64]. (C) CD5-targeted LNPs delivering mRNA to FAP for CAR-T cell generation in vivo [69].
Nano-bispecific-engager for enhancing T cell function
T cell-mediated killing requires recognizing antigens on the surface of target cells by T cell surface receptors [70]. However, expression of MHC-I/antigen complex and other tumor cell markers are reduced, resulting in weak interaction between T cells and tumor cells [71]. Bispecific engagers to enhance recognition between T cells and tumor cells provide a feasible strategy to solve the problem. Nanoparticles are suitable for bispecific engager design due to their flexible, controllable surface modification and long-circulating time [72, 73].
Cheng et al. developed synthetic multivalent antibodies retargeted exosomes (SMART-Exos) by displaying two different types of antibodies on the exosomal surface [74]. To enhance immunotherapy in breast tumors, Expi293F cells were transfected to express epidermal growth factor receptor (EGFR, cancer cell targeting) and anti-CD3 antibody (T cell targeting), derived single-chain variable fragment (scFv) fusion constructs on the cell surface, then exosomes from these cells were collected (Figure 3A). The SMART-Exos induced cross-linking of T cells and EGFR-expressing breast cancer cells, resulting in potent antitumor immunity both in vitro and in vivo. Kosmides et al. described “immunoswitch” iron-dextran nanoparticles coated with two distinct antibodies to block the inhibitory checkpoint PD-L1 pathway and stimulate T cells through 4-1BB costimulatory signaling [75]. The antibodies to block PD-L1 and stimulate 4-1BB were also used to dual-target tumor cells and T cells (Figure 3B). The “immunoswitch” nanoparticles demonstrated tumor growth inhibition and prolonged survival in multiple tumor models, including melanoma and colon tumors. Jiang et al. established a versatile antibody immobilization nanoparticle called immunomodulating nano-adaptors (imNAs) to enhance T cell-, natural killer cell-, and macrophage-mediated antitumor immune responses [76]. In this strategy, two types of antibodies were immobilized onto single nanoparticles functionalized with anti-Fc antibodies, one for binding T cells (or other immune cells) and the other for tumor cell targeting (Figure 3C). Jiang et al. selected the immune checkpoint inhibitors anti-PD1 antibody and anti-PD-L1 antibody to construct imNAs to enhance T cell-mediated antitumor immunity.
Figure 3:
Nano-bispecific-engager for enhancing T cell function. (A) Design and generation of anti-CD3/anti-EGFR synthetic multivalent antibodies retargeted exosomes (SMART-Exos) [74]. (B) Immunoswitch particles link PD-L1 checkpoint blockade with 4-1BB costimulation [75]. (C) Nano-adaptors promote T cell-mediated cancer therapy [76].
Nanoparticle-based strategies for macrophage regulation
As an essential innate immune subgroup for maintaining homeostasis and defending against exogenous pathogens, macrophages were the first phagocytes discovered. Macrophages play a crucial regulatory role in development, disease (including cancer, inflammation, and autoimmune diseases), and tissue regeneration. Macrophages can circulate in the blood or exist in a stable state in tissues (resident macrophages).
Macrophages express specific proteins dynamically and reversibly, depending on their environment and degree of activation, resulting in different phenotypes. Activated macrophages are occasionally divided into two simple phenotypes, M1 and M2, representing polarized pro-inflammatory or anti-inflammatory phenotypes. In some diseases, however, macrophages can express markers for the cells’ M1 and M2 subtypes to varying degrees, reflecting that they are in an intermediate activation state. Normally, M1 macrophages have pro-inflammatory effects, characterized by the secretion of pro-inflammatory cytokines (such as IFN-1β, IL-12, and TNF-α), reactive nitrogen intermediates, and reactive oxygen species (ROS), which can clear pathogens and stimulate angiogenesis. In addition, excessive production of pro-inflammatory cytokines by M1 macrophages may cause damage to normal tissue.
In contrast, M2 macrophages have anti-inflammatory effects, characterized by increased secretion of anti-inflammatory cytokines (including IL-4, IL-10, and IL-13), which promote tissue remodeling and healing, extracellular matrix (ECM) deposition, etc. Excessive M2 behavior promotes tissue fibrosis. The balance of pro-inflammatory and anti-inflammatory responses is often key to the outcome of inflammatory diseases. For example, TAMs, M2 phenotype can promote tumor cell proliferation and metastasis. They promote angiogenesis via vascular endothelial growth factor (VEGF) signaling and inhibit immune function through PD-L1 and other immune checkpoints. Therefore, the development of tumor immunotherapies targeting TAMs has received much attention. The options are to remove it from the tumor and block its tumor-promoting function or restore its immune activation and tumor cytotoxicity functions.
Strategies for regulating macrophage function include (1) regulating the number of macrophages at the disease site and (2) local or systemic delivery of bioactive molecules to polarize macrophages. The macrophage-targeting accuracy of these immunomodulatory strategies can be optimized by exploiting the properties of the nano-delivery system.
Nanoparticles for regulating the number of macrophages
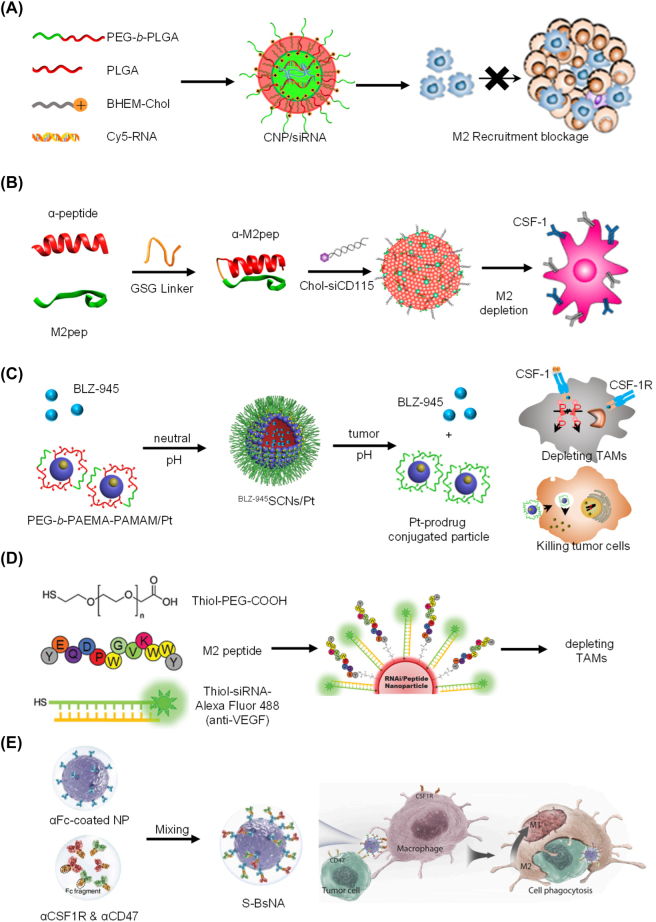
By targeting chemokines and receptors (such as colony-stimulating factor-1 (CSF-1)/CSF-1 receptor (CSF1R), C-C motif chemokine ligand 2 (CCL2)/C-C chemokine receptor 2(CCR2), VEGF/VEGFR), the aggregation of macrophages at the disease site can be controlled. For example, CCL2 is a potent chemokine and an essential mediator in the recruitment of myeloid cells. Majumdar et al. used nanoparticle-facilitated silencing of CCR2 to reduce the number of inflammatory (Ly6Chi) mononuclear cells and improved the healing of myocardial infarction in apolipoprotein E-deficient (apoE−/−) mice [77]. Macrophage cell membranes are generally negatively charged. Positively charged (cationic) particles are more likely to trigger inflammatory responses than negatively charged (anion) and neutral particles. Shen et al. used cationic nanoparticles to deliver CCR2 siRNA [78]. This delivery strategy effectively inhibits the recruitment of monocytes in the peripheral blood, bone marrow, and spleen and reduces TAM invasion in the tumor, thus reversing the immunosuppressive tumor microenvironment (Figure 4A).
Figure 4:
Nanoparticle-based strategies for macrophage regulation. (A) CNP/siCCR2-mediated tumor microenvironment modification and cancer therapy [78]. (B) Design of the M2NP for M2-like TAM-specific molecular-targeted immunotherapy [79]. (C) Tumor pH triggered release of BLZ-945 and Pt-prodrug conjugated small particles for spatial delivery to TAMs and tumor cells [80]. (D) Nanoparticle-based strategy to deliver RNAi for VEGF silencing in both TAMs and lung cancer cells [81], (E) S-BsNA improves the antitumor response of macrophage [82].
Furthermore, since the small molecule drug does not target TAMs, modifying macrophage-associated ligands on the nanoparticle’s surface can improve the specificity of targeting macrophages. Conde et al. designed potent and selective biohybrid RNA interference–M2 peptide nanoparticles to induce specific and long-lasting gene therapy in inflammatory TAMs. Qian et al. developed double-targeted nanoparticles modified with α-peptide (SR-B1 targeting peptide) and M2pep (M2 macrophage binding peptide), when loaded with anti-colony-stimulating factor 1 receptor (anti-CSF-1R) siRNA, it can specifically block the survival signals of M2-like TAMs and clear them from melanoma (Figure 4B) [79]. This strategy can inhibit tumor growth and prolong survival. Such systems reduce TAM infiltration at disease sites by inhibiting macrophage recruitment. Drugs can also be specifically delivered to TAMs via environmentally responsive nanoparticle release. Shen et al. designed acid-responsive clusters of nanoparticles (SCNs) loaded with BLZ-945 (a small molecule inhibitor of CSF-1) and platinum (IV) prodrugs (Figure 4C) [80], and Wang et al. designed light-sensitive BLZ@S-NP/Pt to achieve accurate delivery of BLZ-945 to TAMs and eliminate them [83]. Conde et al. present highly potent and selective biohybrid RNA interference (RNAi)-peptide nanoparticles (NPs) to induce specific and long-lasting gene therapy for regulating inflammatory TAMs (Figure 4D), which can reduce the recruitment of inflammatory TAMs in lung tumor tissue, reduces tumor size (about 95%), and increases animal survival (about 75%) in mice [81].
Nanoparticles for reprogramming macrophage phenotype
Regulating macrophage phenotypes may be more important than regulating the overall number of macrophages at the disease site. Small molecule inhibitors, cytokines, CpG oligonucleotides, and Toll-Like-Receptor (TLR) agonists can be used to reprogram TAMs. Polylysine, polyethylenimine (PEI), cationic gelatin, and glucan can activate M1 macrophages through the toll-like receptor pathway and specifically induce IL-12 secretion. Strong stimulation of the Th1 response results in the reversal of the M1 phenotype from M2 TAMs. Li et al. synthesized porous hollow iron oxide nanoparticles (PHNPs) loaded with P13 Kγ small molecule inhibitors (3-MA) and targeted TAMs by adding a mannitol modification. PHNPs combined with 3-MA was used for macrophage-activating inflammatory factor NF-κB p65 [84]. The synergistic conversion of TAMs into pro-inflammatory M1 macrophages activates the immune response and inhibits tumor growth in vivo. Huang et al. designed a pH-sensitive galactose-modified polymer nanoparticle to co-deliver CpG oligonucleotides and anti-IL-10 antibodies to repolarize M2 TAMs into M1 macrophages. CpG oligonucleotides enhance secretion of the tumor suppressor IL-12, and anti-IL-10 antibodies inhibit the IL-10 signaling axis, reversing the polarization of the anti-inflammatory TAMs. Notably, Awojoodu et al. found that the release of S1P3 receptor agonist FTY720 in PLGA scaffold in mice at the site of ischemic muscle injury preferred the local mobilization of monocyte subpopulations that were polarized into M2-like macrophages (as measured by CD206 expression), leading to increased arteriogenesis [85]. ROS are critical regulators of macrophage polarization and activation. Iron oxide- or photosensitizer-bearing nanoparticles can produce ROS and repolarize TAMs into M1 macrophages. Zanganeh et al. demonstrated that ROS from the Fenton reaction mediated by FDA-approved glucan-coated iron oxide nanoparticles (Ferumoxytol) inhibited breast cancer progression and lung and liver metastasis by inducing the repolarization of TAMs into M1 macrophages.
Nanoparticles can also be functionalized to serve as contrast agents in imaging modalities, enabling a dual-functional or diagnostic approach. For example, Harel-Adar et al. designed liposomes loaded with iron oxide and decorated with phosphatidylserine (PS). This apoptotic cell surface ligand triggers macrophage uptake and subsequent phenotypic transformation to an M2-like phenotype, enabling manipulation of macrophage phenotypes and imaging and tracking via magnetic resonance imaging [86].
The interaction between CD47 and SIRP-α inhibits phagocytosis of red blood cells and other normal cells. However, CD47 also protects cancer cells from phagocytosis, and excess expression of CD47 has been associated with poor prognosis in various solid tumors. Blocking the CD47/SIRP-α pathway is expected to restore the antitumor efficacy of TAMs and promote phagocytosis of tumor cells, which may also trigger macrophage repolarization. Chen et al. developed core–shell albumin nanoparticles loaded with anti-CD47 and anti-PD1 (aPD1@αCD47 complex) [87]. The nanoparticles first released αCD47 from the particle surface to block the tumor’s “don’t eat me” signal and then released aPD1 from the nuclear layer to increase lymphocyte infiltration of the tumor. The group also developed in-situ fibrin gels containing calcium carbonate nanoparticles loaded with anti-CD47 antibodies that repolarize TAMs into M1 macrophages and promote phagocytosis. Kulkarni et al. developed a supramolecular assembly consisting of amphiphilic molecules that inhibit colony-stimulating factor 1 receptor (CSF-1R) and contain SiRP-α-blocking antibodies [88]. This assembly enhances M2-M1 repolarization in the tumor microenvironment and significantly improves antitumor and anti-metastatic efficacy. Chen et al. describe dual-functional super bispecific nano-antibodies (S-BsNA) (Figure 4E) constructed by immobilizing two types of traditional monoclonal antibodies (αCSF1R and αCD47) onto a universal antibody-immobilization platform. The S-BsNA simultaneously reprogram the phenotype of TAMs and establish a close physical connection between effector cells and tumor cells, which can efficiently augment innate antitumor immunity both in vitro and in vivo [82].
Engineering macrophage for drug delivery
Macrophage-based drug carriers are also versatile. They circulate in the blood like red blood cells and neutrophils and specifically target tumor tissues by binding to the vascular cell adhesion molecule-1 (VCAM-1) via α4 and β1 integrins of macrophages. As a result, researchers are trying to use macrophages, macrophage-derived exosomes, or nanoparticles coated with macrophage cell membranes as drug-delivery vehicles to target and kill tumor cells. Guo et al. found that DOX-loaded M1-type macrophages inhibit tumor invasion and can transfer drugs to ovarian cancer cells via tunneling nanotubes. The liposome dox has no such effect [89]. Choi et al. developed peritoneal macrophages loaded with DOX-liposome (macrophages extracted from the inflamed intestinal abdominal cavity), which had a stronger inhibitory effect on tumor metastasis than DOX-liposome [90]. Macrophages can be further modified to enhance the targeting of these vectors or endow them with multiple functions. Cao et al. developed a transferable legume protein-reacting macrophage LD-MDS. In the tumor microenvironment, LD-MDS treated with legume protease can be transformed into exosome-like nanovesicles loaded with solanine (DM4), which is then internalized by metastatic 4T1 cancer cells and inhibits metastasis [91]. Macrophage-derived exosomes have surface membrane properties like those of macrophages. Therefore, M1-macrophage-derived exosomes (M1-exos) can be used to deliver various anticancer drugs for tumor therapy. Nie et al. used click chemistry to modify M1-exos with anti-CD47 and anti-SiRP-α antibodies via pH-sensitive linkers [92]. They demonstrated that M1-exo blocks CD47 and SiRP-α and transforms M2-type macrophages into M1-type macrophages, thereby enhancing tumor cell removal by phagocytosis.
Modulating TAMs alone is commonly not sufficient to inhibit disease progression completely and needs to combined with other treatment options such as immunotherapy or chemotherapy. In addition, nanoparticle design is vital in the use of engineered macrophages (CAR macrophages, etc.) for cancer immunotherapy. The size, shape, targeting, and drug-delivery behavior of nanocarriers must be carefully considered.
Nanoparticle-based strategies for DC regulation
DCs are the most efficient, potent, professional APCs. They are derived from the hematopoietic stem cells of the bone marrow, and play a vital role in modulating lymphocyte and inflammation [93]. DCs located in lymphoid organs are ideal for inducing T cell priming and amplification, since these DCs are close to T cells [94]. Besides activation, DCs also induce tolerance to self-antigens to avoid autoimmune reactions. Thus, DCs provide a connection between innate and adaptive immunity [95]. The potential of DCs as immunotherapy targets has been addressed in clinical studies, particularly as a target for vaccination [96]. The delivery of these drugs to DCs remains a challenge. Nanoparticles can be designed to encapsulate drugs and interfere with DC function, even as vaccine adjuvants, making this a promising strategy for pathogenic infection, cancer, and immune disease therapy. For instance, our group created a DC-targeting cancer vaccine [97], as well as two types of nanomedicine-delivered nucleic acids and peptides to ameliorate autoimmune diseases and induce transplant tolerance [98, 99].
Whether to activate the immune system or induce tolerance, DC-targeting nanoparticles are mainly used as vaccines to modulate DC function to achieve disease prevention and treatment. Here we describe the application of nanoparticles to induce immune activation and immune tolerance, focusing on research progress with nanotechnology to prepare DC-targeting vaccines, and summarize various models, including their mechanism of action, therapeutic strategies and results, and future prospects.
DC-targeting peptide/protein nanoparticles
DC-targeting vaccines can induce both cellular and humoral immune responses. In recent years, different strategies have been explored for efficient vaccine development [100]. Nanoparticle-based peptide or protein-delivery provides a flexible and effective platform for vaccine development by coating the particles with antigens via various methods. The primary methods for antigen presentation on nanoparticles are chemical conjugation, genetic fusion, and tag coupling. Shimp et al. reported that recombinant Pfs25H conjugated to a Pseudomonas aeruginosa exoprotein A (EPA) was able to overcome the poor immunogenicity of Pfs25H [101]. Pfs25-EPA provided a 75–110-fold increase in specific antibody generation compared to Psf25 alone and was used in a phase 1 human trial. The antigen was lysine-treated, and then conjugated to the cysteine of EPA [102]. Another common example is virus-like-particles (VLPs), which are virus capsid protein platforms with which stable nanoparticles are built [103]. We discuss applications of VLP vaccines later. Tag coupling typically involves binding of two proteins that are engineering with a tag or partner protein, such as the popular biotin-avidin, Halo Tag, and SpyTag/SpyCatcher [104]. Biotin-Avidin is one of the strongest non-covalent interactions known and enhances antibody production [105]. Decorating the surface of DCs with nanotechnology is also a promising strategy for vaccine development. Irvine et al. designed a series of amphiphile ligands (amph-ligands) carrying peptides that bind albumin after injection, are trafficked to lymph nodes, and inserted into the DC membrane to prime CAR-T cells (Figure 5A) [106].
Figure 5:
Nanoparticles for targeting DCs. (A) DC-targeting peptide-amph-ligand vaccine to boost CAR-T cells [106]. (B) The scheme of CLANmRNA vaccines induce anti-tumor immune response [97]. (C) The scheme of bioreducible nanogels for vaccination [107].
Proteins can also self-assemble into nanoparticles or be conjugated to other molecules. Irvine et al. developed two vaccines with heavily glycosylated human immunodeficiency virus (HIV) antigens that are shuttled to DCs in germinal centers via complement, mannose-binding lectin (MBL), and immunogen glycan, all of which were more effective than monomeric antigens. Follicular DC targeting was lost in MBL-deficient mice or after immunogen deglycosylation [108, 109]. In a subsequent study, they developed pharmacokinetically tuned, high-potency antitumor vaccines by fusing the peptide epitopes to albumin [110]. The new formulations of these vaccines exhibited more efficient antigen uptake, reduced proteolytic degradation, less antigen presentation in uninflamed distal lymphoid organs, and increased vaccine immunogenicity. Dye-binding endogenous albumin can be used to identify sentinel lymph nodes in cancer patients [111]. Given that albumin-bound compounds can avoid elimination by phagocytes and travel to lymph nodes by “albumin hitchhiking”, Irvine et al. developed safe and potent molecular vaccines by synthesizing amphiphiles (amph-vaccines) composed of the antigens or adjuvants and lipophilic albumin-binding tails [112]. Immunization of mice with these optimized CpG-DNA/peptide amph-vaccines resulted in marked vaccine accumulation in lymph nodes accompanied by high levels of T cell priming and enhanced antitumor efficacy.
DC targeting with polymeric nanoparticles
Polymeric nanoparticles have been exploited in vaccine development due to their biocompatibility, biodegradability, and reduced cytotoxicity. Polymer-based nanoparticles can protect nucleic acids from extracellular ribonucleases and mediate efficient antigen uptake and expression by DCs. Antigens can be encoded as RNA, so nanoparticle delivery of RNA targeting DCs can be used as a vaccine system [113]. Fan et al. used cationic lipid-assisted PEG-b-PLGA nanoparticles encapsulating mRNA to create a cancer vaccine [97]. They demonstrated that nanoparticles loaded with OVA mRNA enter and stimulate the maturation of DCs, promoting activation of OVA-specific T cells (Figure 5B). In an effort to reprogram DCs to induce strong antitumor immunity, they created a co-delivery immunotherapeutic strategy by encapsulating siRNA targeting phagocytosis checkpoint signal regulatory protein α (SIRPα) and stimulator of interferon genes (STING) (NPsiSIRPα/cGAMP) to induce DCs to actively capture tumor antigens and produce type I interferons (IFNs). Intravenous injection of NPsiSIRPα/cGAMP activated DCs and T cells to eradicate tumors [114].
PLGA nanoparticles have also been used to deliver hepatitis-B virus antigen [115], tetanus toxoid [116], hydrophobic antigen [117], and other antigens using PLA, PCL, and other copolymers as vaccine carriers. Kanchan et al. reported that immunization with PLA particles loaded with antigen and alum generated a memory antibody response [118]. With a single immunization, a PLA nanoparticle-based vaccine entrapping tetanus or diphtheria toxin induced high levels of memory antibody compared to soluble antigen. Continuous and slow-release of antigen from nanoparticles are important features of a single immunization-induced memory antibody response. PCL-based nanoparticles have been tested for vaccine delivery of Schistosoma mansoni antigen [119]. Natural polymers are also used as adjuvants in vaccine. Li et al. developed a bioreducible alginate-PEI nanogel for antigen delivery and found that it exhibited great antigen-loading capacity without causing cytotoxicity (Figure 5C) [107].
DC targeting with inorganic nanoparticles
Inorganic nanoparticles such carbon, gold, and silica have been used for vaccine studies. Wang et al. reported a novel carbon nanoparticle adjuvant for oral vaccines [120]. Their optimized 470-nm carbon nanoparticle contained large mesopores and macropores for antigen loading. After oral immunization with the BSA-loaded carbon nanoparticle, the IgG titer reached levels commensurate to conventional immunization using Freund’s complete adjuvant through parenteral administration. Xu et al. developed gold nanorods engineered with cetyltrimethylammonium bromide (CTAB), poly (diallydimethylammonium chloride) (PDDAC), and polyethyleneimine (PEI) carrying plasmid as a DNA vaccine for HIV-1 [121]. Their plasmid-loaded gold nanorods activated APCs, resulting in significant T cell proliferation. Interestingly, different surface modifications induced variable immune activation. Tao et al. also used gold nanoparticles with M2 membrane protein to induce a strong antibody response to influenza A [122]. Silica-based nanoparticles are convenient for surface functionalization and vaccine delivery. An et al. developed a toll-like receptor 9 (TLR-9) agonist and antigen loaded, amine modified silica nanoparticle as tumor vaccine [123]. These silica nanoparticles accumulated in draining lymph nodes, and delivered antigen to APCs. More importantly, surface-modified silica nanoparticles reduced the systemic toxicity of TLR-9 and provided an effective vaccine platform.
DC targeting by lipid nanoparticles
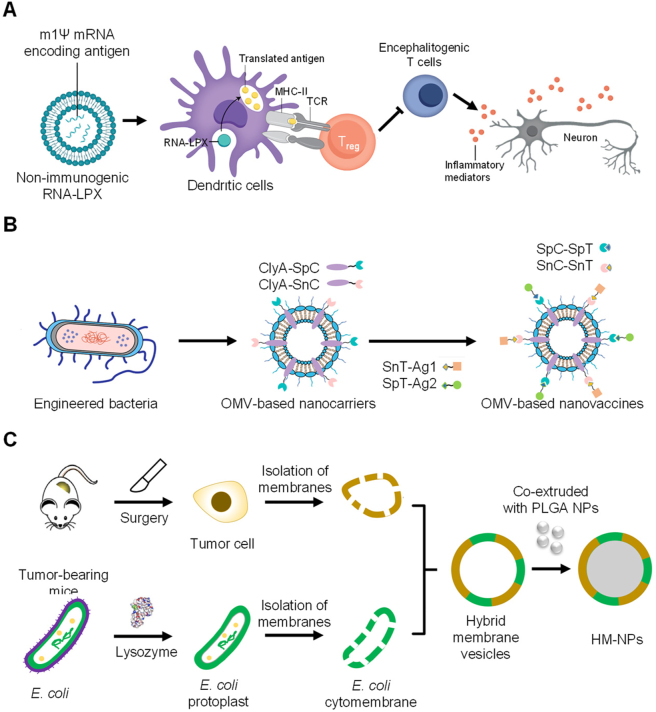
In recent years, lipid nanoparticles (LNPs) have seen exciting development as part of the COVID-19 mRNA vaccine initiative. LNPs protect and transport mRNA, can be used to transport hydrophobic or hydrophilic molecules, and are suitable for antigen delivery. Moon et al. reported using antigen-loaded multilamellar lipid nanoparticles as vaccines to induce T and B cell responses [124]. The interbilayer-crosslinked multilamellar lipid vesicles entrapped protein antigens in the vesicle, and adjuvant in the vesicle wall. Ichihashi et al. reported phosphatidylserine (PS)-liposomes as vaccines [125]. Antigens conjugated to PS-liposomes were effectively taken up by APCs and induced an antigen-specific CD8+ T cell response and a T helper cell-mediated immune response. Sahin et al. developed RNA-lipoplexes (RNA-LPX) to deliver vaccine antigens to DCs [126]. They developed a strategy for precisely and effectively targeting DCs in vivo by preparing lipid carries with an optimized net charge, without modifying particles with molecular ligands. The RNA-LPX led to the release of interferon-α (IFNα) by plasmacytoid DCs (pDCs) and the activation of inflammatory immune mechanisms in the early systemic phase of viral infection. Intravenous injection of RNA-LPX induced strong effector and memory T cell responses and triggered potent IFNα-dependent rejection of progressive tumors. They provided a universal vaccine strategy for systemic targeting of DCs and induction of adaptive as well as type-I-IFN-mediated innate immune responses for cancer immunotherapy. Türeci et al. developed individualized mutanome vaccines by implementing an RNA-based poly-neo-epitope against a spectrum of cancer mutations to solve the inefficient spontaneous immune recognition of mutations. This concept was applied in patients with melanoma and the results showed that individual mutations can be exploited as personalized immunotherapy in this patient population [127]. DCs also play a vital role in immune tolerance, preventing autoimmune disease [128]. Sahin et al. designed a safe and efficient method for noninflammatory delivery of mRNA-encoded antigens to control autoreactive T cells without disturbing normal immune reactions to treat autoimmune diseases. Systemic delivery of nanoparticle-formulated one methylpseudouridine-modified messenger RNA (m1Ψ mRNA) coding for disease-related autoantigens promotes antigen presentation on DCs in the spleen without costimulatory signals. The vaccines ameliorated disease in several multiple sclerosis mouse models with a reduction of effector T cells and the development of regulatory T cells (Figure 6A) [129, 130]. In addition to protein and mRNA, LNPs can also be used to deliver DNA vaccines. Mucker et al. reported lipid nanoparticles formulated using Andes virus or Zika virus DNA vaccines to promote immune responses in non-rodent laboratory animals [131].
Figure 6:
Nanotechnology for regulating DCs. (A) The scheme of anti-inflammatory mRNA vaccine to induce tolerance [130]. (B) Genetically engineered outer-membrane vesicles (OMVs), and (C) Hybrid membrane vesicles containing bacterial cytoplasmic membrane [132].
VLP nanoparticles for targeting DCs
VLPs are virus-mimic but are not infectious. VLPs are composed of several proteins, including viral capsids, cores or envelopes [133]. VLPs are effective vaccines because of their size and structural similarity to natural viruses [134]. VLPs also provide a platform to display additional fused proteins. Lusso et al. developed a VLP with a membrane-anchored HIV-1 envelope (Env) and SIV Gag proteins to prevent HIV infection. Immunization with these mRNA-carrying Env-Gag VLPs induced production of neutralized antibodies and triggered robust CD4+ T cell responses. The VLP mRNA platform thus provides a promising strategy for HIV-1 vaccine development [135]. Stephen et al. designed Tandem-HBc VLPs and expressed them in bacterial, yeast, and plant systems [136]. Chemical techniques can be used for VLP surface modifications. Lysine residues are a common target for conjugation, because free lysine forms stable amide bonds with N-hydroxysuccinimide esters [137]. Stefanetti et al. developed a glycoconjugate vaccine through click chemistry [138]. Different types of VLPs are classified by their structure. Enveloped VLPs consist of a cell membrane from the host cell and present viral protein on the surface [139]. Yao et al. produced enveloped VLPs containing SIV Gag protein and HIV Env protein [140]. The enveloped VLPs have been used in vaccines against retroviruses, influenza A, and hepatitis C virus [141]. Compared to lipid nanoparticles or other nanoparticle-based vaccines, enveloped VLPs have a larger size (>100 nm), which will limit their migration to lymph nodes [142]. Non-enveloped VLPs have a simple composition in comparison to enveloped VLPs, as they do not contain host cell membrane and are preferable for vaccines [143]. They are smaller than enveloped VLPs, and easily migrate to lymph nodes [137].
DC targeting by other nanoparticles
There are several versatile novel vaccine designs. Moon et al. developed personalized vaccines for immunotherapy to stimulate strong T cell responses. They co-delivered high-density lipoprotein-mimicking nanodiscs with antigen peptides and adjuvants to lymphoid organs to promote antigen presentation on DCs. This multi-epitope vaccination triggered broad-spectrum T cell responses and inhibited tumor growth when combined with immune checkpoint blocking antibodies [144]. Ding et al. developed a tubular DNA nanodevice vaccine composed of two molecular adjuvants with antigen peptide in the inner cavity to trigger long-term T cell activation and cancer cytotoxicity [145]. Liu et al. described a genetically engineered cell membrane nanovesicle that integrates antigen self-presentation and immunosuppression reversal (ASPIRE) for cancer immunotherapy. ASPIRE promotes antigen delivery to lymphoid organs and eliminates tumors by activating both native T cells and exhausted T cells [146]. Inspired by natural immune defenses against bacterial invasion, Nie et al. developed cancer vaccines based on bacterial membrane materials, including genetically engineered outer-membrane vesicles (OMVs) and hybrid membrane vesicles containing bacterial cytoplasmic membrane to induce strong tumor regression (Figure 6B–C) [132]. Li et al. developed potent vesicular cancer nanovaccines (BTs) by fusing bacterial OMVs and tumor cell membranes. The bacteria-derived pathogenic adjuvants in BTs can target DCs and confer an excellent tumor prophylactic effect [147].
DC-targeting nanoparticles can also be used for tracking and imaging. Pham et al. developed superparamagnetic iron oxide nanoparticle (SPIOs) for DC tracking without compromising cell function and investigated the migration of SPIO-labeled DCs in a syngeneic mouse model using magnetic resonance imaging. With polylysine, dextran-coated SPIOs can be taken up by DCs within 1 h of incubation, and injection of the SPIO-labeled DCs in the footpad showed migration from the injection site to the corresponding popliteal lymph node. Thus, SPIO-enhanced MR imaging can be used to track DC migration in vivo [148].
Nanoparticle-based strategies for granulocyte regulation
Granulocytes comprise the largest proportion of white blood cells. They survive a few hours after entering the bloodstream, then leave and die [149]. Granulocytes include eosinophils, neutrophils, and basophils, which are differentiated by granule staining. Neutrophils are most abundant in the peripheral circulation and are the main defense effector cells of antibody and complement-mediated immune responses [150]. They can be rapidly recruited to sites of tissue injury to perform antibacterial and inflammatory functions through phagocytosis, degranulation, release of neutrophil extracellular traps (NETs) and antigen presentation [151, 152].
Neutrophils participate in the pathogenesis of widespread human diseases and abnormal physiological functions of neutrophils, such as underactivity or overactivation, are likely to develop into disease [153]. Therefore, targeting or intervening with neutrophils and their related pathways in the treatment of inflammatory diseases and cancer is a new therapeutic approach. Nanoparticle-based strategies for neutrophil regulation include in vivo hijacking of neutrophils by multifunctional nanoparticles across the vascular barrier for targeted therapy. The goal is to control disease progression by regulating neutrophil infiltration.
Nanoparticles for hijacking neutrophils to across the vascular barrier
Neutrophils release cytotoxic substrates involving ROS and hydrogen peroxide (H2O2) to mediate the elimination of tumor cells, but also secrete factors that inhibit proliferation. Therefore, if neutrophils can be precisely targeted and manipulated in vivo, they could be used for tumor treatment and theragnostic [154, 155]. Li et al. constructed a nano-pathogen (NPN) mimic by coating nanoparticles with vesicles secreted by bacteria along with homogeneous pathological functions, thus hitchhiking neutrophil circulation [156]. In a mouse model, tumors were completely eradicated by a combined application of PTT and NPNs. In another study, Li et al. achieved active and precise regulation of the surface protein corona by changing the characteristics of the carrier, enabling hijacking of endogenous neutrophils and delivery of nanomedicine to the lung by taking advantage of the natural delivery of neutrophils for the precise treatment of pneumonia [157].
Tang et al. modified 5-HT on nanoparticles loaded with photosensitizer and Zileuton (a leukotriene inhibitor) for targeting MPO and neutrophils in a similar way [154]. Noninvasive positron emission tomography studies confirmed the MPO-targeting properties of 5-HT-modified NPs, then photodynamic therapy was used to launch an inflammatory response, which mediated the accumulation and retention of targeted neutrophils in breast cancer models.
Nanoparticles for restraining neutrophil infiltration and migration
The process of neutrophils entering inflammatory tissues through the vascular endothelium is an important innate immune defense mechanism against infections as well as acute and chronic inflammatory diseases involving sepsis, trauma, ischemia, and reperfusion. Therefore, targeting neutrophil infiltration may be an effective means to improve the treatment of inflammation-related diseases [158]. For instance, Liu and Cao et al. screened specific cationic lipid-assisted nanoparticles (CLANs) for optimized neutrophil targeting in high-fat diet (HFD)-induced type 2 diabetes (T2D) mice with chronic inflammation [159]. A CRISPR-Cas9 plasmid with guide RNA targeting neutrophil elastase (NE) was packaged into the optimized CLAN (CLANpCas9/gNE). In epididymal white adipose tissue (eWAT) and liver, CLANpCas9/gNE was expeditiously internalized by neutrophils, at which point the NE gene was knocked down, significantly decreasing neutrophil infiltration and insulin resistance, and disturbing inflammation (Figure 7A).
Figure 7:
Nanoparticles for restraining the infiltration and migration of neutrophils. (A) Neutrophil NE gene was knocked down by the optimized CLANpCas9/gNE for the treatment of HFD-induced T2D, significantly decreasing neutrophil infiltration and insulin resistance [159]. (B) Platelet-mimetic nanoparticles were recognized by neutrophils through platelet membrane coating, and piceatannol delivered by nanoparticles could detach adherent neutrophils, thereby restraining the migration of neutrophils and reducing infiltration [160].
In addition, Cruz et al. constructed an innovative nano-platform with α1-antitrypsin-derived peptide to bind NE on activated neutrophils [161]. This modification specifically anchors activated neutrophils and platelet-neutrophil aggregates to nanoparticles. Neutrophils play an important role in chemically-mediated inflammatory responses via myeloperoxidase (MPO) and inflammation triggered by traumatic brain injury (TBI). Yao et al. obtained nanodrugs targeting MPO and neutrophils (T-Hes) by modifying 5-hydroxytryptamine (5-HT) on nanoparticles carrying hesperidin. They confirmed that T-Hes targeted by neutrophils rapidly accumulate in the brain tissue, reducing the production of inflammatory factors, microglia, and astrocytes. T-Hes significantly enhanced drug retention and release, improving TBI treatment [162].
Neutrophils prolifically infiltrate into ischemic areas of the brain and release ROS after acute ischemic stroke. Therefore, reducing neutrophil infiltration after acute ischemic stroke may be an effective treatment. Tang et al. constructed platelet-mimicking nanoparticles co-loaded with the spleen tyrosine kinase inhibitor piceatannol and the T2 contrast agent superparamagnetic iron oxide (SPIO) [160]. The particles were recognized by neutrophils due to their platelet membrane coating, and the piceatannol caused adherent neutrophils to detach, thereby reducing infiltration and infarct size (Figure 7B).
Nano-engineered neutrophils or neutrophil derivatives
Live cells or derivatives, such as exosomes, have become attractive as pharmaceutical carriers because of their biocompatibility, low clearance rate, natural ability to penetrate physiological barriers, and specific biological characteristics of the source cells [163]. For example, Wang et al. used neutrophil-exosomes (NEs-Exos) to carry doxorubicin (DOX) to glioma by taking advantage of their blood-brain barrier permeability, the inflammatory chemotaxis properties of neutrophils, and glioma-killing efficacy [164]. To endow the exosomes with more functionality, Zhang et al. modified SPIO nanoparticles (SPIONs) with neutrophil exosomes (SPION-Ex) to achieve better tumor targeting efficacy than conventional NEs-Exos [165].
Although endogenous living cells or exosomes inherit the native functions and features of the original cells, they typically do not meet the diverse needs of disease treatment and large-scale drug preparation due to their low levels of production and singular function. It may be possible to develop new multifunctional cellular drugs through nanoengineering strategies, which may provide new ideas for treating multiple diseases. Zhang et al. proposed the notion of cytopharmaceuticals, and physically modified neutrophils to carry out non-destructive loading of nanodrugs and to prepare neutrophil-based drugs that did not affect the physiological function and vitality of the neutrophils. This method has made breakthroughs in the therapy of orthotopic brain glioma in mice, postoperative chemotherapy, hyperthermia combined with chemotherapy for liver cancer, and radiotherapy combined with chemotherapy for gastric cancer. Compared with traditional targeted delivery systems, cyto-pharmaceuticals provide advantages in long circulation, targeting, and biocompatibility in vivo [68]. Xue et al. constructed a novel autologous cell-mediated drug-delivery system utilizing neutrophils to pre-ingest paclitaxel (PTX)-loaded cationic liposomes (PTX-CL) with a positive surface charge in vitro (PTX-CL/NEs). PTX-CL/NEs were intravenously injected into mice with surgically resected gliomas. Inflammatory factors produced by the surgical site induced neutrophils to cross the BBB into the brain and infiltrate into the surrounding tumor cells. The PTX-CL/NEs were over-activated, releasing NETs while releasing the liposome PTX-CL. Then, the liposome PTX-CL effectively delivered the PTX to the tumor interior, thereby killing brain tumor cells and limiting tumor recurrence [163].
Zhang et al. also developed a neutrophil membrane-coated polymer nanoparticle to treat rheumatoid arthritis [166, 167]. These nanoparticles neutralize pro-inflammatory cytokines, alleviating synovial inflammation and providing robust cartilage protection against joint damage. There has been a lot of work based on neutrophil-derived membranes in cancer. For instance, Li et al. designed a pseudoneutrophil cytokine sponge (pCSs) by coating the outer surface of degradable polymer nanoparticles with activated neutrophil cell membranes [168]. Receptors on the membrane surface adsorb and capture immunosuppressive cytokines, significantly inhibiting the expansion, recruitment, and activation of myelosuppressive cells, thus alleviating the immunosuppressive tumor microenvironment and enhancing the tumor-killing effect of T cells.
Nanoparticles for regulating other granulocytes
When stimulated by allergens, mast cells, a type of granulocyte, activate ROS production, degranulation to release allergic mediators, and pathological responses, leading to the development of allergic diseases. Based on the narrow therapeutic time window of regulating mast cells, Lin et al. developed a novel mast cell nano-stabilizer based on ceria nanoparticles to achieve long-term prevention of allergic diseases [169]. The nano-stabilizer continuously regulates degranulation-related phosphate signal cascades in allergen-stimulated mast cells to inhibit release of inflammatory mediators, thereby inhibiting the occurrence of an allergic response.
Eosinophils mediate the development of asthma by secreting a large number of inflammatory cytokines or programmed necrosis, which aggravates the condition. Sun et al. developed a ROS-responsive nanoparticle carrying Fedratinib (JAK2 inhibitor) with a modified Fc fragment of immunoglobulin G on the surface [170]. By targeting the FcRn of lung airway epithelial cells, the cargo was delivered across the airway barrier through Fc/FcRn-mediated receptor transport to achieve lysosomal escape. Fedratinib was released after being cleaved by ROS in the inflammatory microenvironment, relieving eosinophilic asthma.
Nanoparticle-based strategies for B cell regulation
As a type of lymphocyte, B cells also develop in the bone marrow, which are involved in humoral immunity. After encountering the foreign substance, B cells can differentiate into plasma cells, with the ability of producing antigen-specific antibodies to fight infection and regulate inflammation, or directly secreting cytokines to support or modulate effector immune functions. At the same time, B cells generate memory B cells to protect against pathogen re-exposure. B cells can also serve as APCs leading to optimal antigen-specific T cell expansion [171]. Regulating B cell function in vivo may have a profound impact on disease research, prevention, and treatment [172].
Nanoparticles for disrupting B cell function
B cells also have important functions in the pathogenesis of autoimmune diseases. B cells promote autoimmunity by producing autoantibodies, acting as APCs, producing cytokines, and other mechanisms. Therefore, B cells are a promising therapeutic target for autoimmune diseases [173].
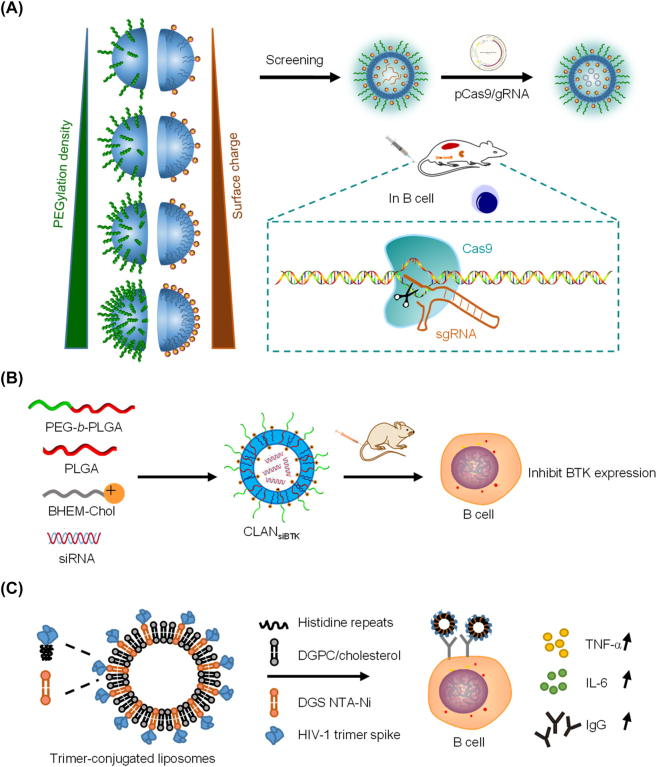
Our group developed PEG-b-PLA or PEG-b-PLGA-based CLANs to deliver nucleic acids (Figure 8A). To optimize CLAN delivery efficiency to B cells, we created a library of CLANs and compared them with different PEG-b-PLGA and cationic lipid formulations. By flow cytometry, we compared the uptake of CLAN by B cells in bone marrow, spleen, and lymph nodes, and screened for the best CLAN-40.30%NPB2.0 (40.30% PEG/PLGA and 2.0 mg BHEM-Chol). The obtained CLAN can effectively deliver CRISPR-Cas9 plasmids to B cells in vivo, and was used to successfully treat rheumatoid arthritis in mice by knocking out BAFFR in B cells [174]. We also delivered BTK siRNA via CLAN to treat rheumatoid arthritis in a CIA model and found that systemic administration of CLANsiBTK significantly reduced the expression of inflammatory cytokines and relieved symptoms of arthritis (Figure 8B) [175].
Figure 8:
Nanoparticle for regulating B cell function. (A) CLANs deliver CRISPR-Cas9 plasmid to B cells [174]. (B) CLANs deliver BTK siRNA to B cells [175]. (C) Env-trimer-conjugated liposomes activate B cell. The composition of the nanoparticles is shown in the figure [176].
Nanoparticles for activating or enhancing B cell function
Vaccines are an important means to fight infectious diseases. During antigen exposure resulting from vaccination, B cells rapidly proliferate and undergo somatic hypermutation to select better and more antigenically responsive B cell clones to produce high-affinity immunoglobulin-α (IgA) and immunoglobulin-γ (IgG) antibodies. Activated B cells leave the germinal centers as plasma cells and memory B cells, protecting against reinfection for months to years. Therefore, efficient eliciting of B cell responses is of great importance for the development of vaccines against viruses [177].
Temchura et al. developed biodegradable calcium phosphate nanoparticles displaying protein antigens on their surface and explored the efficacy of B cell activation after exposure to these nanoparticles [178]. First, they studied calcium phosphate nanoparticles modified with model antigen Hen Egg Lysozyme (CaP-HEL). When CaP-HEL was co-incubated with mouse SW-HEL B cells, nearly 49% of the B cells bound to CaP-HEL and surface expression of CD69 and CD86 increased approximately 100-fold compared to free HEL. In order to increase the immunogenicity of CaP-HEL nanoparticles, they also modified TLR ligands on the surface of CaP-HEL [179]. After injecting the nanoparticles into mice, they found that IgG levels were significantly elevated.
For highly variable influenza viruses, Kanekiyo et al. developed a mosaic array by co-localizing heterotypic influenza hemagglutinin antigens on a single nanoparticle [180]. They linked different hemagglutinin RBD sequences to engineered ferritin sequences, and then transfected the RBD-ferritin plasmids into 293F cells. Four days after transfection, they collected the supernatant to obtain self-assembled RBD nanoparticles (RBD-np). This ferritin-based modular self-assembling nanoparticle system has been applied in many studies for influenza vaccines [181, 182]. RBD-np carries different strains of influenza virus hemagglutinin antigens on its surface, effectively reducing the activation of strain-specific B cells and allowing selective engagement of B cells resistant to antigenic variation.
For HIV, which still has no effective drug, Wyatt et al. designed LNPs displaying well-ordered HIV Env spike trimers on their surface to serve as an HIV vaccine immunogen (Figure 8C) [176]. The liposomes were composed of DGPC, cholesterol, and DGS-NTA(Ni), and incubation with trimeric proteins yielded Env-trimer-conjugated liposomes. After incubation with Env-trimer-conjugated liposomes, CD69 expression increased, and more TNF-α and IL-6 secretion was observed, indicating that Env-trimer-conjugated liposomes activated the B cells. When Env-trimer-conjugated liposomes was injected into C57BL/6 mice for 14 days, germinal center B cells were also activated. In the HIV pseudovirus neutralization test, rabbits were immunized with soluble or Env-trimer-conjugated liposomes. Serum measurements showed that, compared with soluble trimers, the neutralization titer caused by Env-trimer-conjugated liposomes tended to increase.
Nanoparticle-based strategies for innate lymphocyte regulation
Natural killer cells, also known as NK cells or large granular lymphocytes, are a type of innate immune cell and perform a cytotoxic role in cellular immunity. NK cells develop and mature in bone marrow and secondary lymphoid tissues, including spleen, lymph nodes, and tonsils. NK cells are analogous to cytotoxic T cells and kill aberrant cells, but they do so without receptors for antigen recognition. In the absence of antigen specificity, NK cells have the ability to selectively recognize and kill stressed cells and cancer cells, allowing for a fast immune reaction [183]. NK cells can also produce a variety of cytokines and chemokines [184]. NK cells can be classified as CD56bright or CD56dim. CD56bright NK cells are the largest proportion of NK cells and mainly release cytokines, like T helper cells [185]. CD56dim NK cells are characterized by their killing ability [186]. Here we review direct NK cell targeting nanotechnology applications, including nanoparticle-mediated NK cell activation and a nano-engager for NK cells.
Nanoparticle-mediated NK cell activation
NK cells play important roles in cancer therapy, but poor infiltration and inhibition remain barriers to clinical translation that may be overcome by using nanoparticles. To direct NK cells to infiltrate tumors, Jang et al. used Cy5.5-conjugated Fe3O4/SiO2 nanoparticles to control human NK cells by an external magnetic field [187]. NK-92MI cells were incubated with Fe3O4/SiO2 nanoparticles to allow uptake, then the nanoparticle-loaded NK cells were injected into a mouse model of B cell lymphoma. Under an external magnetic field, this strategy increased NK-92MI cell infiltration of the tumor by more than 17-fold (Figure 9A). Similarly, polydopamine-coated magnetic nanoparticles directed NK cells to the tumor site to strengthen NK cell-mediated cancer therapy [188]. To activate NK cells and enhance NK cell-mediated cancer therapy, Wu et al. developed cell membrane-encapsulated magnetic nanoparticles [189]. Fe3O4@SiO2 nanoparticles were coated with cancer cell membrane and used to stimulate NK cells with cancer-specific antigens, resulting in release of cytotoxic cytokines such as perforin and granzyme B to enhance antitumor efficacy in hepatoma tumor cells. Kim et al. reported a facile and efficient method to activate NK cells through cationic magnetic nanoparticles [190]. Primary NK and NK-92MI cells were treated with cationic magnetic nanoparticles, and upregulated CCR4 and CXCR4 chemokine receptor expression (Figure 9B). In a model of triple-negative breast cancer, nanoparticle-activated NK cells inhibited tumor growth.
Figure 9:
Nanoparticle-mediated NK cell activation. (A) The Fe3O4/SiO2 nanoparticles modified with PET-silane and fluorescent dyes directed NK cells under an external magnetic field [187]. (B) Cationic nanoparticle-mediated NK cell activation via regulation of CCR4 and CXCR4 [190].
Other nanoparticles can also be used for NK cells activation. Loftus et al. developed a strategy to generate soluble nanoclusters of NK cell-activating antibodies using antibody-conjugated nanoscale graphene oxide as template [191]. These nanoclusters mimicked a number of natural cell surface proteins, successfully stimulated NK cells, and induced cellular activation via the CD16 receptor. Nakamura et al. used a multifunctional envelope-type nanodevice (MEND) containing YSK12-C4 (YSK12-MEND) to transfect human immune cell lines with siRNA [192]. In this work, Jurkat, THP-1, KG-1, and NK92 were transfected and showed 96, 96, 91, and 75% mRNA knockdown efficiencies, respectively. These results indicated that YSK12-MEND was suitable for delivering siRNA into immune cells, including NK cells. However, NK-92 cell transfection yielded significant cytotoxicity. Nakamura et al. decreased the total amount of lipids in YSK12-C4, which was the cause of cytotoxicity in NK-92 [193]. This improved YSK12-MEND and demonstrated more potential ways to regulate NK cells.
Extracellular vesicles contain different proteins with natural structures. Oyer et al. developed a cancer cell membrane-derived particle for cytotoxic NK cell expansion [194]. K562-mb21-4-1BBL cells, which express membrane-bound IL-21 and 4-1BB ligand, were prepared to generate PM21 particles, which showed highly efficient activation to produce 66-fold more human NK cells ex vivo, and 360-fold more in vivo.
Nano-engager for regulating NK cells
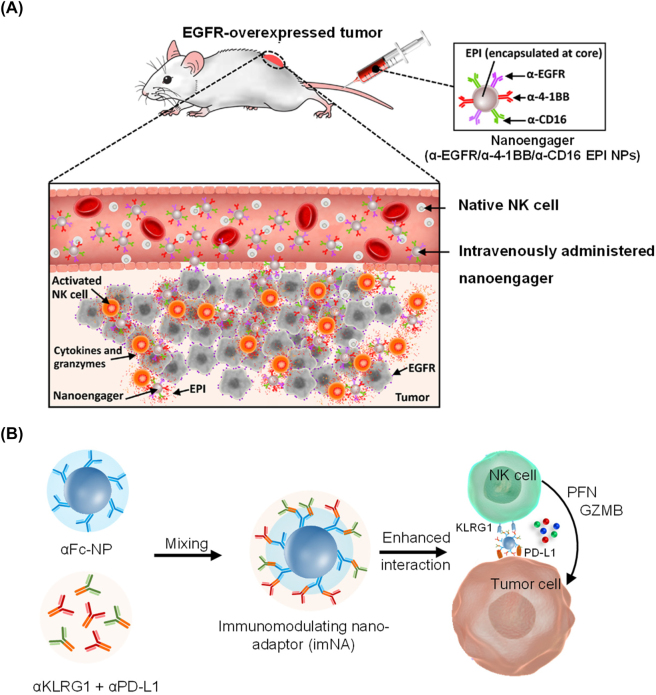
Like cytotoxic T cells, NK cell-mediated cell killing depends on recognition of aberrant cells by NK cells. Bispecific or trispecific antibody have been used as engagers between NK cells and tumor cells [195]. Nanotechnology provides a more flexible approach to direct NK cell interactions with target cells and enhance NK cell-mediated cytotoxicity. Au et al. reported a nanoparticle-based trispecific NK cell engager (nano-TriNKE) platform to promote NK cell-mediated cancer therapy [196]. In this work, PEG-PLGA nanoparticles were functionalized with three types of antibodies including antibody targeting activating receptors, CD16 and 4-1BB, on NK cells and EGFR on tumor cells. The nano-TriNKE enhanced the interaction between NK cells and tumor cells by targeting their specific surface proteins and promoted NK cell activation through CD16 and 4-1BB receptors (Figure 10A). The results revealed that nano-TriNKE activated NK cells more effectively than free antibodies, and improved tumor therapy in multiple tumor models. Jiang et al. developed a versatile imNA model that could be applied as a nano-engager for NK cells [76]. To construct NK cell-targeting imNAs, antibodies targeting KLRG1 (killer-cell lectin-like receptor G1) and PD-L1 were immobilized onto anti-Fc antibody-functionalized nanoparticles (Figure 10B). Anti-KLRG1 antibody served to block cadherin/KLRG1 signaling to enhance NK cell functions, and anti-PD-L1 antibody was used to bind tumor cells. This strategy significantly reduced metastatic melanoma formation in lung compared to free antibodies.
Figure 10:
Nano-engager for NK cells. (A) EGFR-targeted nanoparticle-based trispecific NK cell engagers (nano-TriNKEs) (αEGFR/αCD16/α4-1BB NPs) against EGFR-overexpressed cancer after systemic administration [196]. (B) Nano-adaptor in NK cell-mediated cancer therapy [76].
Conclusions and future perspectives
In summary, nanotechnology can improve the safety and efficacy of immunomodulatory compounds by altering their temporal and spatial release. Concentration and targeting of the dose to the appropriate site or cell type can improve therapeutic efficacy. Extended, controlled, localized drug release enables continuous reprogramming of the TME or post-surgery environment [197]. Although nanoparticle-based regulation strategies are promising, especially for disease treatment, the current perception of their role in disease is not comprehensive, including the fate and state of nanoparticles in vivo, the potential influence in immune cell subtypes with different functions, and the transformation between subtypes. The activation and abundance of immune cells change dynamically through disease development and inflammation. Accurate immune intervention often depends on updating technology to achieve real-time dynamic exploration of cellular function or behavior. Targeting of immune cells in vivo requires appropriate and rational design of nanomedicines and increased targeting specificity to mitigate nonspecific targeting, and the selection of drugs and carriers that do not affect cellular function should also be considered. Researchers can control the targeting functions of nanoparticles to different tissues or immune cells by adjusting the characteristics of nanoparticles, such as the types and proportions of lipids, which can be manipulated to target different tissues [198]. In addition, specific nucleic acid barcodes can be encapsulated into nanoparticles for high-throughput screening by deep sequencing of the barcodes, thereby finding the optimal nanoparticle for predicting the therapeutic potency of anticancer medicines in a personalized manner [199]. According to the location and characteristics of the diseases, it is promising to achieve the purpose of treating different diseases by more precisely targeting nanoparticles.
For in vitro engineered immune cells, the yield, activity, and safety of functional proteins require further attention. Manufacturing of clinical grade materials remains a key consideration that cannot be ignored. The formulation should be designed with a view to conversion to GMP standards. Particles, devices, and cells require reproducible and scalable chemistry, manufacturing, and control, so simplicity is extremely valuable [28]. The convergence of cancer immunotherapy, nanotechnology, and bioengineering is coming of age and could affect patient lives soon. To accelerate the clinical translation of nanocarriers that regulate immune cells, it is essential to engage researchers across the fields of materials science, engineering, pharmacology, and immunology. In order to rapidly screen nanomedicine with clinical application value, organoid technology platform can be used to achieve fast, accurate and high-throughput screening [200].
Footnotes
Research funding: This work was supported by the National Key R&D Program of China (2022YFB3808100), the National Natural Science Foundation of China (82072048, 52130301, and 32271442), Guangdong Basic and Applied Basic Research Foundation (2022B1515020025), the Science and Technology Program of Guangzhou, China (202103030004), and the Fundamental Research Funds for the Central Universities (2022ZYGXZR102).
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Conflicts of interests: Authors state no conflict of interest.
Ethical approval: Not applicable.
Contributor Information
Cong-Fei Xu, Email: xucf@scut.edu.cn.
Jun Wang, Email: mcjwang@scut.edu.cn.
References
- 1.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–89. doi: 10.1016/s0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 2.Feng X, Xu W, Li Z, Song W, Ding J, Chen X. Immunomodulatory nanosystems. Adv Sci. 2019;6:1900101. doi: 10.1002/advs.201900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14:85. doi: 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci. 2020;8:4653–64. doi: 10.1039/d0bm00558d. [DOI] [PubMed] [Google Scholar]
- 5.Ngobili TA, Daniele MA. Nanoparticles and direct immunosuppression. Exp Biol Med. 2016;241:1064–73. doi: 10.1177/1535370216650053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C, Merlin D. Nanoparticle-mediated drug delivery systems for the treatment of IBD: current perspectives. Int J Nanomed. 2019;14:8875–89. doi: 10.2147/ijn.s210315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irvine DJ, Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20:321–34. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–88. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akar-Ghibril N. Defects of the innate immune system and related immune deficiencies. Clin Rev Allergy Immunol. 2022;63:36–54. doi: 10.1007/s12016-021-08885-y. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Zhang Y, Yang L, Li H. The innate immune signaling in cancer and cardiometabolic diseases: friends or foes? Cancer Lett. 2017;387:46–60. doi: 10.1016/j.canlet.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Place DE, Kanneganti T-D. The innate immune system and cell death in autoinflammatory and autoimmune disease. Curr Opin Immunol. 2020;67:95–105. doi: 10.1016/j.coi.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–40. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 14.Haist M, Stege H, Grabbe S, Bros M. The functional crosstalk between myeloid-derived suppressor cells and regulatory T cells within the immunosuppressive tumor microenvironment. Cancers. 2021;13:210. doi: 10.3390/cancers13020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–10. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenberg GF, Hepworth MR. Functional interactions between innate lymphoid cells and adaptive immunity. Nat Rev Immunol. 2019;19:599–613. doi: 10.1038/s41577-019-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer Cell. 2018;33:547–62. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdon DJ, Mulazzani M, Jenkins MR. Cellular and molecular mechanisms of CD8(+) T cell differentiation, dysfunction and exhaustion. Int J Mol Sci. 2020;21:7357. doi: 10.3390/ijms21197357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linette GP, Carreno BM. Tumor-infiltrating lymphocytes in the checkpoint inhibitor era. Curr Hematol Malig Rep. 2019;14:286–91. doi: 10.1007/s11899-019-00523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Donnell JS, Teng MW, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 21.Engelhard V, Conejo-Garcia JR, Ahmed R, Nelson BH, Willard-Gallo K, Bruno TC, et al. B cells and cancer. Cancer Cell. 2021;39:1293–6. doi: 10.1016/j.ccell.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Wang SS, Liu W, Ly D, Xu H, Qu L, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol. 2019;16:6–18. doi: 10.1038/s41423-018-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Chan HL, Chen P. Immune checkpoint inhibitors: basics and challenges. Curr Med Chem. 2019;26:3009–25. doi: 10.2174/0929867324666170804143706. [DOI] [PubMed] [Google Scholar]
- 24.Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 2021;14:45. doi: 10.1186/s13045-021-01056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17:504–15. doi: 10.1038/s41571-020-0352-8. [DOI] [PubMed] [Google Scholar]
- 27.Kang JH, Bluestone JA, Young A. Predicting and preventing immune checkpoint inhibitor toxicity: targeting cytokines. Trends Immunol. 2021;42:293–311. doi: 10.1016/j.it.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg MS. Improving cancer immunotherapy through nanotechnology. Nat Rev Cancer. 2019;19:587–602. doi: 10.1038/s41568-019-0186-9. [DOI] [PubMed] [Google Scholar]
- 29.Bockamp E, Rosigkeit S, Siegl D, Schuppan D. Nano-enhanced cancer immunotherapy: immunology encounters nanotechnology. Cells. 2020;9:2102. doi: 10.3390/cells9092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–22. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 31.Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48:202–13. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philip M, Schietinger A. CD8(+) T cell differentiation and dysfunction in cancer. Nat Rev Immunol. 2022;22:209–23. doi: 10.1038/s41577-021-00574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4(+)T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdalla AM, Xiao L, Ullah MW, Yu M, Ouyang C, Yang G. Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics. 2018;8:533–48. doi: 10.7150/thno.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung AS, Zhang DKY, Koshy ST, Mooney DJ. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat Biotechnol. 2018;36:160–9. doi: 10.1038/nbt.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosmides AK, Meyer RA, Hickey JW, Aje K, Cheung KN, Green JJ, et al. Biomimetic biodegradable artificial antigen presenting cells synergize with PD-1 blockade to treat melanoma. Biomaterials. 2017;118:16–26. doi: 10.1016/j.biomaterials.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding Q, Chen J, Wei X, Sun W, Mai J, Yang Y, et al. RAFTsomes containing epitope-MHC-II complexes mediated CD4+ T cell activation and antigen-specific immune responses. Pharm Res (N Y) 2013;30:60–9. doi: 10.1007/s11095-012-0849-7. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33:64–72. doi: 10.1038/nbt.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano. 2014;8:2252–60. doi: 10.1021/nn405520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Florez L, Herrmann C, Cramer JM, Hauser CP, Koynov K, Landfester K, et al. How shape influences uptake: interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small. 2012;8:2222–30. doi: 10.1002/smll.201102002. [DOI] [PubMed] [Google Scholar]
- 41.Barua S, Yoo JW, Kolhar P, Wakankar A, Gokarn YR, Mitragotri S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc Natl Acad Sci U S A. 2013;110:3270–5. doi: 10.1073/pnas.1216893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, et al. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11:1519–25. doi: 10.1002/smll.201402369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hickey JW, Vicente FP, Howard GP, Mao HQ, Schneck JP. Biologically inspired design of nanoparticle artificial antigen-presenting cells for immunomodulation. Nano Lett. 2017;17:7045–54. doi: 10.1021/acs.nanolett.7b03734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Y, Krishnan N, Zhou J, Chekuri S, Wei X, Kroll AV, et al. Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv Mater. 2020;32:e2001808. doi: 10.1002/adma.202001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–40. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 46.Singha S, Shao K, Yang Y, Clemente-Casares X, Sole P, Clemente A, et al. Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat Nanotechnol. 2017;12:701–10. doi: 10.1038/nnano.2017.56. [DOI] [PubMed] [Google Scholar]
- 47.Glass JJ, Yuen D, Rae J, Johnston AP, Parton RG, Kent SJ, et al. Human immune cell targeting of protein nanoparticles--caveospheres. Nanoscale. 2016;8:8255–65. doi: 10.1039/c6nr00506c. [DOI] [PubMed] [Google Scholar]
- 48.Glass JJ, Li Y, De Rose R, Johnston AP, Czuba EI, Khor SY, et al. Thiol-reactive star polymers display enhanced association with distinct human blood components. ACS Appl Mater Interfaces. 2017;9:12182–94. doi: 10.1021/acsami.6b15942. [DOI] [PubMed] [Google Scholar]
- 49.Sivaram AJ, Wardiana A, Alcantara S, Sonderegger SE, Fletcher NL, Houston ZH, et al. Controlling the biological fate of micellar nanoparticles: balancing stealth and targeting. ACS Nano. 2020;14:13739–53. doi: 10.1021/acsnano.0c06033. [DOI] [PubMed] [Google Scholar]
- 50.Li SY, Liu Y, Xu CF, Shen S, Sun R, Du XJ, et al. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J Contr Release. 2016;231:17–28. doi: 10.1016/j.jconrel.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 51.Huq R, Samuel EL, Sikkema WK, Nilewski LG, Lee T, Tanner MR, et al. Preferential uptake of antioxidant carbon nanoparticles by T lymphocytes for immunomodulation. Sci Rep. 2016;6:33808. doi: 10.1038/srep33808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vis B, Hewitt RE, Faria N, Bastos C, Chappell H, Pele L, et al. Non-functionalized ultrasmall silica nanoparticles directly and size-selectively activate T cells. ACS Nano. 2018;12:10843–54. doi: 10.1021/acsnano.8b03363. [DOI] [PubMed] [Google Scholar]
- 53.Thiramanas R, Jiang S, Simon J, Landfester K, Mailander V. Silica Nanocapsules with different sizes and physicochemical properties as suitable nanocarriers for uptake in T-cells. Int J Nanomed. 2020;15:6069–84. doi: 10.2147/ijn.s246322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tabujew I, Willig M, Leber N, Freidel C, Negwer I, Koynov K, et al. Overcoming the barrier of CD8(+)T cells: two types of nano-sized carriers for siRNA transport. Acta Biomater. 2019;100:338–51. doi: 10.1016/j.actbio.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Dinauer N, Balthasar S, Weber C, Kreuter J, Langer K, von Briesen H. Selective targeting of antibody-conjugated nanoparticles to leukemic cells and primary T-lymphocytes. Biomaterials. 2005;26:5898–906. doi: 10.1016/j.biomaterials.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 56.Kheirolomoom A, Kare AJ, Ingham ES, Paulmurugan R, Robinson ER, Baikoghli M, et al. In situ T-cell transfection by anti-CD3-conjugated lipid nanoparticles leads to T-cell activation, migration, and phenotypic shift. Biomaterials. 2022;281:121339. doi: 10.1016/j.biomaterials.2021.121339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramishetti S, Kedmi R, Goldsmith M, Leonard F, Sprague AG, Godin B, et al. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano. 2015;9:6706–16. doi: 10.1021/acsnano.5b02796. [DOI] [PubMed] [Google Scholar]
- 58.Tombacz I, Laczko D, Shahnawaz H, Muramatsu H, Natesan A, Yadegari A, et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA-LNPs. Mol Ther. 2021;29:3293–304. doi: 10.1016/j.ymthe.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J, Yun KS, Choi CS, Shin SH, Ban HS, Rhim T, et al. T cell-specific siRNA delivery using antibody-conjugated chitosan nanoparticles. Bioconjugate Chem. 2012;23:1174–80. doi: 10.1021/bc2006219. [DOI] [PubMed] [Google Scholar]
- 60.Yang YS, Moynihan KD, Bekdemir A, Dichwalkar TM, Noh MM, Watson N, et al. Targeting small molecule drugs to T cells with antibody-directed cell-penetrating gold nanoparticles. Biomater Sci. 2018;7:113–24. doi: 10.1039/c8bm01208c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nawaz W, Huang B, Xu S, Li Y, Zhu L, Yiqiao H, et al. AAV-mediated in vivo CAR gene therapy for targeting human T-cell leukemia. Blood Cancer J. 2021;11:119. doi: 10.1038/s41408-021-00508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–20. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moffett HF, Coon ME, Radtke S, Stephan SB, McKnight L, Lambert A, et al. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat Commun. 2017;8:389. doi: 10.1038/s41467-017-00505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parayath NN, Stephan SB, Koehne AL, Nelson PS, Stephan MT. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat Commun. 2020;11:6080. doi: 10.1038/s41467-020-19486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–61. doi: 10.1158/0008-5472.can-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland ML, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385:493–502. doi: 10.1056/nejmoa2107454. [DOI] [PubMed] [Google Scholar]
- 67.Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–53. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 68.Billingsley MM, Singh N, Ravikumar P, Zhang R, June CH, Mitchell MJ. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020;20:1578–89. doi: 10.1021/acs.nanolett.9b04246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rurik JG, Tombacz I, Yadegari A, Mendez Fernandez PO, Shewale SV, Li L, et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375:91–6. doi: 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm Res (N Y) 2008;25:55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu Y, Fourniols T, Labrak Y, Preat V, Beloqui A, des Rieux A. Surface modification of lipid-based nanoparticles. ACS Nano. 2022;16:7168–96. doi: 10.1021/acsnano.2c02347. [DOI] [PubMed] [Google Scholar]
- 74.Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ, Zhang Y. Reprogramming exosomes as nanoscale controllers of cellular immunity. J Am Chem Soc. 2018;140:16413–7. doi: 10.1021/jacs.8b10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kosmides AK, Sidhom JW, Fraser A, Bessell CA, Schneck JP. Dual targeting nanoparticle stimulates the immune system to inhibit tumor growth. ACS Nano. 2017;11:5417–29. doi: 10.1021/acsnano.6b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang CT, Chen KG, Liu A, Huang H, Fan YN, Zhao DK, et al. Immunomodulating nano-adaptors potentiate antibody-based cancer immunotherapy. Nat Commun. 2021;12:1359. doi: 10.1038/s41467-021-21497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038–46. doi: 10.1161/circulationaha.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen S, Zhang Y, Chen KG, Luo YL, Wang J. Cationic polymeric nanoparticle delivering CCR2 siRNA to inflammatory monocytes for tumor microenvironment modification and cancer therapy. Mol Pharm. 2018;15:3642–53. doi: 10.1021/acs.molpharmaceut.7b00997. [DOI] [PubMed] [Google Scholar]
- 79.Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L, et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11:9536–49. doi: 10.1021/acsnano.7b05465. [DOI] [PubMed] [Google Scholar]
- 80.Shen S, Li HJ, Chen KG, Wang YC, Yang XZ, Lian ZX, et al. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett. 2017;17:3822–9. doi: 10.1021/acs.nanolett.7b01193. [DOI] [PubMed] [Google Scholar]
- 81.Conde J, Bao C, Tan Y, Cui D, Edelman ER, Azevedo HS, et al. Dual targeted immunotherapy via in vivo delivery of biohybrid RNAi-peptide nanoparticles to tumour-associated macrophages and cancer cells. Adv Funct Mater. 2015;25:4183–94. doi: 10.1002/adfm.201501283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen KG, Liu A, Jiang CT, Zhao DK, Ye QN, Liao YQ, et al. Dual-functional super bispecific nano-antibodies derived from monoclonal antibodies potentiate the antitumor effect of innate immune cells. Nano Today. 2021;39:101209. doi: 10.1016/j.nantod.2021.101209. [DOI] [Google Scholar]
- 83.Wang J, Shen S, Li J, Cao Z, Yang X. Precise depletion of tumor seed and growing soil with shrinkable nanocarrier for potentiated cancer chemoimmunotherapy. ACS Nano. 2021;15:4636–46. doi: 10.1021/acsnano.0c08996. [DOI] [PubMed] [Google Scholar]
- 84.Li K, Lu L, Xue C, Liu J, He Y, Zhou J, et al. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale. 2020;12:130–44. doi: 10.1039/c9nr06505a. [DOI] [PubMed] [Google Scholar]
- 85.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, et al. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A. 2013;110:13785–90. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harel-Adar T, Mordechai TB, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci USA. 2011;108:1827–32. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat Nanotechnol. 2019;14:89–97. doi: 10.1038/s41565-018-0319-4. [DOI] [PubMed] [Google Scholar]
- 88.Kulkarni A, Chandrasekar V, Natarajan SK, Ramesh A, Pandey P, Nirgud J, et al. A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer. Nat Biomed Eng. 2018;2:589–99. doi: 10.1038/s41551-018-0254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo L, Zhang Y, Yang Z, Peng H, Wei R, Wang C, et al. Tunneling nanotubular expressways for ultrafast and accurate M1 macrophage delivery of anticancer drugs to metastatic ovarian carcinoma. ACS Nano. 2019;13:1078–96. doi: 10.1021/acsnano.8b08872. [DOI] [PubMed] [Google Scholar]
- 90.Choi J, Kim H-Y, Ju EJ, Jung J, Park J, Chung H-K, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33:4195–203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 91.Cao H, Wang H, He X, Tan T, Hu H, Wang Z, et al. Bioengineered macrophages can responsively transform into nanovesicles to target lung metastasis. Nano Lett. 2018;18:4762–70. doi: 10.1021/acs.nanolett.8b01236. [DOI] [PubMed] [Google Scholar]
- 92.Nie W, Wu G, Zhang J, Huang LL, Ding J, Jiang A, et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew Chem Int Ed Engl. 2020;59:2018–22. doi: 10.1002/ange.201912524. [DOI] [PubMed] [Google Scholar]
- 93.Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zinkernagel RM, Ehl S, Aichele P, Oehen S, Kundig T, Hengartner H. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol Rev. 1997;156:199–209. doi: 10.1111/j.1600-065x.1997.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 95.Steinman RM. The control of immunity and tolerance by dendritic cells. Pathol Biol. 2003;51:59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 96.Zanna MY, Yasmin AR, Omar AR, Arshad SS, Mariatulqabtiah AR, Nur-Fazila SH, et al. Review of dendritic cells, their role in clinical immunology, and distribution in various animal species. Int J Mol Sci. 2021;22:8044. doi: 10.3390/ijms22158044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fan YN, Li M, Luo YL, Chen Q, Wang L, Zhang HB, et al. Cationic lipid–assisted nanoparticles for delivery of mRNA cancer vaccine. Biomater Sci. 2018;6:3009–18. doi: 10.1039/c8bm00908b. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, Shen S, Zhao G, Xu C-F, Zhang H-B, Luo Y-L, et al. In situ repurposing of dendritic cells with CRISPR/Cas9-based nanomedicine to induce transplant tolerance. Biomaterials. 2019;217:119302. doi: 10.1016/j.biomaterials.2019.119302. [DOI] [PubMed] [Google Scholar]
- 99.Luo YL, Liang LF, Gan YJ, Liu J, Zhang Y, Fan YN, et al. An all-in-one nanomedicine consisting of CRISPR-Cas9 and an autoantigen peptide for restoring specific immune tolerance. ACS Appl Mater Interfaces. 2020;12:48259–71. doi: 10.1021/acsami.0c10885. [DOI] [PubMed] [Google Scholar]
- 100.Saleh A, Qamar S, Tekin A, Singh R, Kashyap R. Vaccine development throughout history. Cureus. 2021;13:e16635. doi: 10.7759/cureus.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimp RL, Rowe C, Reiter K, Chen B, Vu N, Aebig J, et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine. 2013;31:2954–62. doi: 10.1016/j.vaccine.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qian F, Wu Y, Muratova O, Zhou H, Dobrescu G, Duggan P, et al. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine. 2007;25:3923–33. doi: 10.1016/j.vaccine.2007.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lopez-Sagaseta J, Malito E, Rappuoli R, Bottomley MJ. Self-assembling protein nanoparticles in the design of vaccines. Comput Struct Biotechnol J. 2016;14:58–68. doi: 10.1016/j.csbj.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brune KD, Howarth M. New Routes and opportunities for modular construction of particulate vaccines: stick, click, and glue. Front Immunol. 2018;9:1432. doi: 10.3389/fimmu.2018.01432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J Clin Invest. 2001;108:415–23. doi: 10.1172/jci11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma L, Dichwalkar T, Chang JY, Cossette B, Garafola D, Zhang AQ, et al. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365:162–8. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li P, Luo Z, Liu P, Gao N, Zhang Y, Pan H, et al. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J Contr Release. 2013;168:271–9. doi: 10.1016/j.jconrel.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 108.Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Chang JY, et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science. 2019;363:649–54. doi: 10.1126/science.aat9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilson JT. A sweeter approach to vaccine design. Science. 2019;363:584–5. doi: 10.1126/science.aav9000. [DOI] [PubMed] [Google Scholar]
- 110.Mehta NK, Pradhan RV, Soleimany AP, Moynihan KD, Rothschilds AM, Momin N, et al. Pharmacokinetic tuning of protein-antigen fusions enhances the immunogenicity of T-cell vaccines. Nat Biomed Eng. 2020;4:636–48. doi: 10.1038/s41551-020-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsopelas C, Sutton R. Why certain dyes are useful for localizing the sentinel lymph node. J Nucl Med. 2002;43:1377–82. [PubMed] [Google Scholar]
- 112.Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507:519–22. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–72. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu Z-D, Chen Y-F, Shen S, Xu C-F, Wang J. Co-delivery of phagocytosis checkpoint silencer and stimulator of interferon genes agonist for synergetic cancer immunotherapy. ACS Appl Mater Interfaces. 2021;13:29424–38. doi: 10.1021/acsami.1c08329. [DOI] [PubMed] [Google Scholar]
- 115.Thomas C, Rawat A, Hope-Weeks L, Ahsan F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm. 2011;8:405–15. doi: 10.1021/mp100255c. [DOI] [PubMed] [Google Scholar]
- 116.Diwan M, Tafaghodi M, Samuel J. Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. J Contr Release. 2002;85:247–62. doi: 10.1016/s0168-3659(02)00275-4. [DOI] [PubMed] [Google Scholar]
- 117.Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, et al. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kanchan V, Katare YK, Panda AK. Memory antibody response from antigen loaded polymer particles and the effect of antigen release kinetics. Biomaterials. 2009;30:4763–76. doi: 10.1016/j.biomaterials.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 119.Baras B, Benoit MA, Dupre L, Poulain-Godefroy O, Schacht AM, Capron A, et al. Single-dose mucosal immunization with biodegradable microparticles containing a Schistosoma mansoni antigen. Infect Immun. 1999;67:2643–8. doi: 10.1128/iai.67.5.2643-2648.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang T, Zou M, Jiang H, Ji Z, Gao P, Cheng G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur J Pharmaceut Sci. 2011;44:653–9. doi: 10.1016/j.ejps.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 121.Xu L, Liu Y, Chen Z, Li W, Liu Y, Wang L, et al. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12:2003–12. doi: 10.1021/nl300027p. [DOI] [PubMed] [Google Scholar]
- 122.Tao W, Gill HS. M2e-immobilized gold nanoparticles as influenza A vaccine: role of soluble M2e and longevity of protection. Vaccine. 2015;33:2307–15. doi: 10.1016/j.vaccine.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.An M, Li M, Xi J, Liu H. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl Mater Interfaces. 2017;9:23466–75. doi: 10.1021/acsami.7b06024. [DOI] [PubMed] [Google Scholar]
- 124.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–51. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ichihashi T, Satoh T, Sugimoto C, Kajino K. Emulsified phosphatidylserine, simple and effective peptide carrier for induction of potent epitope-specific T cell responses. PLoS One. 2013;8:e60068. doi: 10.1371/journal.pone.0060068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 127.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Loewer M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–6. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 128.Hilkens CMU, Isaacs JD, Thomson AW. Development of dendritic cell-based immunotherapy for autoimmunity. Int Rev Immunol. 2010;29:156–83. doi: 10.3109/08830180903281193. [DOI] [PubMed] [Google Scholar]
- 129.Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–53. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 130.Wardell CM, Levings MK. mRNA vaccines take on immune tolerance. Nat Biotechnol. 2021;39:419–20. doi: 10.1038/s41587-021-00880-0. [DOI] [PubMed] [Google Scholar]
- 131.Mucker EM, Karmali PP, Vega J, Kwilas SA, Wu H, Joselyn M, et al. Lipid nanoparticle formulation increases efficiency of DNA-vectored vaccines/immunoprophylaxis in animals including transchromosomic bovines. Sci Rep. 2020;10:8764. doi: 10.1038/s41598-020-65059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao X, Zhao R, Nie G. Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat Protoc. 2022;17:2240–74. doi: 10.1038/s41596-022-00713-7. [DOI] [PubMed] [Google Scholar]
- 133.Banskota S, Raguram A, Suh S, Du SW, Davis JR, Choi EH, et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell. 2022;185:250–65. doi: 10.1016/j.cell.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tariq H, Batool S, Asif S, Ali M, Abbasi BH. Virus-like particles: revolutionary platforms for developing vaccines against emerging infectious diseases. Front Microbiol. 2022;12:790121. doi: 10.3389/fmicb.2021.790121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang P, Narayanan E, Liu Q, Tsybovsky Y, Boswell K, Ding S, et al. A multiclade env-gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat Med. 2021;27:2234–45. doi: 10.1038/s41591-021-01574-5. [DOI] [PubMed] [Google Scholar]
- 136.Stephen SL, Beales L, Peyret H, Roe A, Stonehouse NJ, Rowlands DJ. Recombinant expression of tandem-HBc virus-like particles (VLPs) Methods Mol Biol. 2018;1776:97–123. doi: 10.1007/978-1-4939-7808-3_7. [DOI] [PubMed] [Google Scholar]
- 137.Mohsen MO, Zha L, Cabral-Miranda G, Bachmann MF. Major findings and recent advances in virus like particle (VLP)-based vaccines. Semin Immunol. 2017;34:123–32. doi: 10.1016/j.smim.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 138.Stefanetti G, Saul A, MacLennan CA, Micoli F. Click chemistry applied to the synthesis of salmonella typhimurium O-antigen glycoconjugate vaccine on solid phase with sugar recycling. Bioconjugate Chem. 2015;26:2507–13. doi: 10.1021/acs.bioconjchem.5b00521. [DOI] [PubMed] [Google Scholar]
- 139.Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ, et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnol. 2021;19:59. doi: 10.1186/s12951-021-00806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yao QZ, Kuhlmann FM, Eller R, Compans RW, Chen CY. Production and characterization of simian-human immunodeficiency virus-like particles. AIDS Res Hum Retrovir. 2000;16:227–36. doi: 10.1089/088922200309322. [DOI] [PubMed] [Google Scholar]
- 141.Wetzel D, Barbian A, Jenzelewski V, Schembecker G, Merz J, Piontek M. Bioprocess optimization for purification of chimeric VLP displaying BVDV E2 antigens produced in yeast Hansenula polymorpha. J Biotechnol. 2019;306:203–12. doi: 10.1016/j.jbiotec.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 142.Keikha R, Daliri K, Jebali A. The use of nanobiotechnology in immunology and vaccination. Vaccines. 2021;9:74. doi: 10.3390/vaccines9020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Naskalska A, Pyrc K. Virus like particles as immunogens and universal nanocarriers. Pol J Microbiol. 2015;64:3–13. doi: 10.33073/pjm-2015-001. [DOI] [PubMed] [Google Scholar]
- 144.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–96. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu S, Jiang Q, Zhao X, Zhao R, Wang Y, Wang Y, et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat Mater. 2021;20:431–3. doi: 10.1038/s41563-020-0793-6. [DOI] [PubMed] [Google Scholar]
- 146.Liu C, Liu X, Xiang X, Pang X, Chen S, Zhang Y, et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy. Nat Nanotechnol. 2022;17:531–40. doi: 10.1038/s41565-022-01098-0. [DOI] [PubMed] [Google Scholar]
- 147.Li M, Zhou H, Jiang W, Yang C, Miao H, Wang YC. Nanovaccines integrating endogenous antigens and pathogenic adjuvants elicit potent antitumor immunity. Nano Today. 2020;35:101007. doi: 10.1016/j.nantod.2020.101007. [DOI] [Google Scholar]
- 148.Kobukai S, Baheza R, Cobb JG, Virostko J, Xie J, Gillman A, et al. Magnetic nanoparticles for imaging dendritic cells. Magn Reson Med. 2010;63:1383–90. doi: 10.1002/mrm.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19:253–75. doi: 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- 150.Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The neutrophil. Immunity. 2021;54:1377–91. doi: 10.1016/j.immuni.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 151.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134–47. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 152.Wigerblad G, Kaplan MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol. 2022:1–15. doi: 10.1038/s41577-022-00787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20:485–503. doi: 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 154.Tang L, Wang Z, Mu Q, Yu Z, Jacobson O, Li L, et al. Targeting neutrophils for enhanced cancer theranostics. Adv Mater. 2020;32:e2002739. doi: 10.1002/adma.202002739. [DOI] [PubMed] [Google Scholar]
- 155.Hedrick CC, Malanchi I. Neutrophils in cancer: heterogeneous and multifaceted. Nat Rev Immunol. 2022;22:173–87. doi: 10.1038/s41577-021-00571-6. [DOI] [PubMed] [Google Scholar]
- 156.Li M, Li S, Zhou H, Tang X, Wu Y, Jiang W, et al. Chemotaxis-driven delivery of nano-pathogenoids for complete eradication of tumors post-phototherapy. Nat Commun. 2020;11:1126. doi: 10.1038/s41467-020-14963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li S, Li M, Huo S, Wang Q, Chen J, Ding S, et al. Voluntary-opsonization-enabled precision nanomedicines for inflammation treatment. Adv Mater. 2021;33:e2006160. doi: 10.1002/adma.202006160. [DOI] [PubMed] [Google Scholar]
- 158.Wu J, Ma T, Zhu M, Huang T, Zhang B, Gao J, et al. Nanotechnology reinforced neutrophil-based therapeutic strategies for inflammatory diseases therapy. Nano Today. 2022;46:101577. doi: 10.1016/j.nantod.2022.101577. [DOI] [Google Scholar]
- 159.Liu Y, Cao ZT, Xu CF, Lu ZD, Luo YL, Wang J. Optimization of lipid-assisted nanoparticle for disturbing neutrophils-related inflammation. Biomaterials. 2018;172:92–104. doi: 10.1016/j.biomaterials.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 160.Tang C, Wang C, Zhang Y, Xue L, Li Y, Ju C, et al. Recognition, intervention, and monitoring of neutrophils in acute ischemic stroke. Nano Lett. 2019;19:4470–7. doi: 10.1021/acs.nanolett.9b01282. [DOI] [PubMed] [Google Scholar]
- 161.Cruz MA, Bohinc D, Andraska EA, Alvikas J, Raghunathan S, Masters NA, et al. Nanomedicine platform for targeting activated neutrophils and neutrophil-platelet complexes using an alpha(1)-antitrypsin-derived peptide motif. Nat Nanotechnol. 2022;17:1004–14. doi: 10.1038/s41565-022-01161-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yao K, Mu Q, Zhang Y, Cheng Q, Cheng X, Liu X, et al. Hesperetin nanoparticle targeting neutrophils for enhanced TBI therapy. Adv Funct Mater. 2022;32:2205787. doi: 10.1002/adfm.202205787. [DOI] [Google Scholar]
- 163.Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 164.Wang J, Tang W, Yang M, Yin Y, Li H, Hu F, et al. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials. 2021;273:120784. doi: 10.1016/j.biomaterials.2021.120784. [DOI] [PubMed] [Google Scholar]
- 165.Zhang J, Ji C, Zhang H, Shi H, Mao F, Qian H, et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv. 2022;8:eabj8207. doi: 10.1126/sciadv.abj8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13:1182–90. doi: 10.1038/s41565-018-0254-4. [DOI] [PubMed] [Google Scholar]
- 167.Fang RH, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat Rev Clin Oncol. 2022;20:33–48. doi: 10.1038/s41571-022-00699-x. [DOI] [PubMed] [Google Scholar]
- 168.Li S, Wang Q, Shen Y, Hassan M, Shen J, Jiang W, et al. Pseudoneutrophil cytokine sponges disrupt myeloid expansion and tumor trafficking to improve cancer immunotherapy. Nano Lett. 2020;20:242–51. doi: 10.1021/acs.nanolett.9b03753. [DOI] [PubMed] [Google Scholar]
- 169.Zhong Y, Ye M, Huang L, Hu L, Li F, Ni Q, et al. A fibrin site-specific nanoprobe for imaging fibrin-rich thrombi and preventing thrombus formation in venous vessels. Adv Mater. 2022;34:e2109955. doi: 10.1002/adma.202109955. [DOI] [PubMed] [Google Scholar]
- 170.Sun W, Song S, Li G, Zhou L, Xie J, Guo Y, et al. FcRn-targeting and ROS-responsive Fedratinib-incorporated nanoparticles alleviate asthma by inducing eosinophil apoptosis. Allergy. 2022 doi: 10.1111/all.15575. [DOI] [PubMed] [Google Scholar]
- 171.Claes N, Fraussen J, Stinissen P, Hupperts R, Somers V. B cells are multifunctional players in multiple sclerosis pathogenesis: insights from therapeutic interventions. Front Immunol. 2015;6:642. doi: 10.3389/fimmu.2015.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Castiglioni P, Gerloni M, Zanetti M. Genetically programmed B lymphocytes are highly efficient in inducing anti-virus protective immunity mediated by central memory CD8 T cells. Vaccine. 2004;23:699–708. doi: 10.1016/j.vaccine.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 173.Rubin SJS, Bloom MS, Robinson WH. B cell checkpoints in autoimmune rheumatic diseases. Nat Rev Rheumatol. 2019;15:303–15. doi: 10.1038/s41584-019-0211-0. [DOI] [PubMed] [Google Scholar]
- 174.Li M, Fan YN, Chen ZY, Luo YL, Wang YC, Lian ZX, et al. Optimized nanoparticle-mediated delivery of CRISPR-Cas9 system for B cell intervention. Nano Res. 2018;11:6270–82. doi: 10.1007/s12274-018-2150-5. [DOI] [Google Scholar]
- 175.Zhao G, Liu A, Zhang Y, Zuo ZQ, Cao ZT, Zhang HB, et al. Nanoparticle-delivered siRNA targeting Bruton’s tyrosine kinase for rheumatoid arthritis therapy. Biomater Sci. 2019;7:4698–707. doi: 10.1039/c9bm01025d. [DOI] [PubMed] [Google Scholar]
- 176.Ingale J, Stano A, Guenaga J, Sharma SK, Nemazee D, Zwick MB, et al. High-density array of well-ordered HIV-1 spikes on synthetic liposomal nanoparticles efficiently activate B cells. Cell Rep. 2016;15:1986–99. doi: 10.1016/j.celrep.2016.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Singh A. Eliciting B cell immunity against infectious diseases using nanovaccines. Nat Nanotechnol. 2021;16:16–24. doi: 10.1038/s41565-020-00790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Temchura VV, Kozlova D, Sokolova V, Ueberla K, Epple M. Targeting and activation of antigen-specific B-cells by calcium phosphate nanoparticles loaded with protein antigen. Biomaterials. 2014;35:6098–105. doi: 10.1016/j.biomaterials.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 179.Zilker C, Kozlova D, Sokolova V, Yan H, Epple M, Ueberla K, et al. Nanoparticle-based B-cell targeting vaccines: tailoring of humoral immune responses by functionalization with different TLR-ligands. Nanomedicine. 2017;13:173–82. doi: 10.1016/j.nano.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 180.Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, et al. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol. 2019;20:362–72. doi: 10.1038/s41590-018-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kanekiyo M, Wei C-J, Yassine HM, McTamney PM, Boyington JC, Whittle JR, et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. 2013;499:102–6. doi: 10.1038/nature12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kelly HG, Tan H-X, Juno JA, Esterbauer R, Ju Y, Jiang W, et al. Self-assembling influenza nanoparticle vaccines drive extended germinal center activity and memory B cell maturation. JCI Insight. 2020;5:e136653. doi: 10.1172/jci.insight.136653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252–9. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 184.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 185.Pfefferle A, Jacobs B, Haroun-Izquierdo A, Kveberg L, Sohlberg E, Malmberg KJ. Deciphering natural killer cell homeostasis. Front Immunol. 2020;11:812. doi: 10.3389/fimmu.2020.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19:120. doi: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jang ES, Shin JH, Ren G, Park MJ, Cheng K, Chen X, et al. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials. 2012;33:5584–92. doi: 10.1016/j.biomaterials.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu L, Zhang F, Wei Z, Li X, Zhao H, Lv H, et al. Magnetic delivery of Fe(3)O(4)@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater Sci. 2018;6:2714–25. doi: 10.1039/c8bm00588e. [DOI] [PubMed] [Google Scholar]
- 189.Wu D, Shou X, Zhang Y, Li Z, Wu G, Wu D, et al. Cell membrane-encapsulated magnetic nanoparticles for enhancing natural killer cell-mediated cancer immunotherapy. Nanomedicine. 2021;32:102333. doi: 10.1016/j.nano.2020.102333. [DOI] [PubMed] [Google Scholar]
- 190.Kim KS, Han JH, Choi SH, Jung HY, Park JD, An HJ, et al. Cationic nanoparticle-mediated activation of natural killer cells for effective cancer immunotherapy. ACS Appl Mater Interfaces. 2020;12:56731–40. doi: 10.1021/acsami.0c16357. [DOI] [PubMed] [Google Scholar]
- 191.Loftus C, Saeed M, Davis DM, Dunlop IE. Activation of human natural killer cells by graphene oxide-templated antibody nanoclusters. Nano Lett. 2018;18:3282–9. doi: 10.1021/acs.nanolett.8b01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nakamura T, Kuroi M, Fujiwara Y, Warashina S, Sato Y, Harashima H. Small-sized, stable lipid nanoparticle for the efficient delivery of siRNA to human immune cell lines. Sci Rep. 2016;6:37849. doi: 10.1038/srep37849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nakamura T, Yamada K, Fujiwara Y, Sato Y, Harashima H. Reducing the cytotoxicity of lipid nanoparticles associated with a fusogenic cationic lipid in a natural killer cell line by introducing a polycation-based siRNA core. Mol Pharm. 2018;15:2142–50. doi: 10.1021/acs.molpharmaceut.7b01166. [DOI] [PubMed] [Google Scholar]
- 194.Oyer JL, Pandey V, Igarashi RY, Somanchi SS, Zakari A, Solh M, et al. Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: clinical implications for cancer treatment. Cytotherapy. 2016;18:653–63. doi: 10.1016/j.jcyt.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 195.Gauthier L, Morel A, Anceriz N, Rossi B, Blanchard-Alvarez A, Grondin G, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019;177:1701–13. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 196.Au KM, Park SI, Wang AZ. Trispecific natural killer cell nanoengagers for targeted chemoimmunotherapy. Science advance. 2020;6:eaba8564. doi: 10.1126/sciadv.aba8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Goldberg MS. Immunoengineering: how nanotechnology can enhance cancer immunotherapy. Cell. 2015;161:201–4. doi: 10.1016/j.cell.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 198.Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15:313–20. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yaari Z, da Silva D, Zinger A, Goldman E, Kajal A, Tshuva R, et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat Commun. 2016;7:13325. doi: 10.1038/ncomms13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hu YW, Sui XZ, Song F, Li YQ, Li KY, Chen ZY, et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat Commun. 2021;12:2581. doi: 10.1038/s41467-021-22676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]