Abstract
Antibodies, as one of the most important components of host adaptive immune system, play an important role in defense of infectious disease, immune surveillance, and autoimmune disease. Due to the development of recombinant antibody technology, antibody therapeutics become the largest and rapidly expanding drug to provide major health benefits to patients, especially for the treatment of cancer patients. Many antibody-based therapeutic strategies have been developed including monoclonal antibodies, antibody-drug conjugates, bispecific and trispecific antibodies and pro-antibodies with promising results from both clinical and pre-clinical trials. However, the response rate and side-effect still vary between patients with undefined mechanisms. Here, we summarized the current and future perspectives of antibody-based cancer immunotherapeutic strategies for designing next-generation drugs.
Keywords: antibody, cancer, engineering, immunotherapy
Introduction
Since 1990s, over 100 antibody-based therapeutics have been approved for the treatment of serious human disease including infectious disease, cancer, and immune-related disorders [1], [2], [3]. However, the application of antibody treatment has also been extensively applied to other human disease including hematology, neurology, and metabolic disease. Recent approvals or emergency authorizations of anti-SARS-CoV-2 monoclonal antibodies (mAbs) (casirivimab plus imdevimab, bamlanivimab, bamlanivimab plus etesevimab) highlighted the importance of neutralization antibodies in treatment of acute respiratory virus infection [4, 5]. The research on monoclonal antibodies for cancer treatment started in 1970s, when the methods of producing antibodies using hybridomas were established [6, 7]. MAbs against melanoma showed antitumor efficacy in nude mice first followed by clinical trials of mAb treatment on lymphoma patients [8, 9]. However, the high immunogenicity of murine derived mAbs limited the further application of these therapeutic strategies. With the rapid development of yeast display, recombinant antibody and humanized antibody technologies, more and more antibody-based therapy has been studied for the treatment of cancer [10]. Since 1995, increasing numbers (1–8 per year) of antibody treatment has been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Antibodies as therapeutic agent for cancer treatment has several advantages. First, antibodies are relatively stable with extended in vivo half-life mediated by neonatal Fc receptor (FcRn) [11]. With such properties, antibodies can be given intravenously and penetrate to the targeted tissue with durable concentration. Second, antibodies can not only block the growth factor signal transduction in tumor cells, but also activate innate immune response to kill tumor cells through antibody-dependent cellular cytotoxicity (ADCC) [12]. Third, compared with traditional therapy, the specificity of antibody treatment is much higher. Recognition of antigens through variable region of antibody can elicit strong tumor specific immune response while minimizing toxicity and adverse events. Based on these properties, many advanced antibody-based therapeutic strategies have been developed including antibody-drug conjugate (ADC) which conjugate potent cytotoxic agents to specific sites on antibodies, bispecific antibody (BsAb) which can simultaneously bind two targets and antibody fusion protein which can target the tumor microenvironment to deliver cytokines.
Even though promising results have been observed in pre-clinical and clinal trials of antibody-based immunotherapies. There are still several major hurdles to overcome to benefit more patients suffering from different kind of cancers. For example, the “on-target off-tumor” distractions and the undefined mechanism of drug resistance [13, 14]. In addition, the innovation in antibody engineering, target screening and therapeutic regimen still have plenty of room for further improvement [15].
Here, we review the design of mAbs, ADCs, BsAb and antibody fusion proteins for cancer immunotherapy. We also discussed the mechanism of action, side-effect, clinical uses, combination treatment strategies and future perspectives in antibody-based therapeutics.
Therapeutic monoclonal antibodies
Therapeutic monoclonal antibodies targeting tumor associated antigens
Tumor associated antigens (TAAs) is a category of tumor antigens which overexpressed or accumulated in cancer cells compared with normal cells [16, 17]. Candidate targets are protein or glycoproteins which highly expressed on the surface of tumor cells. The growth factor receptors are one of the most common TAAs identified in a variety of cancer patients including epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) [18], [19], [20], [21]. Antibodies targeting EGFR have generated antitumor responses in colorectal cancer patients and four EGFR monoclonal antibody drugs (cetuximab, panitumumab, nimotuzumab, and necitumumab) are already approved by the FDA [22], [23], [24]. Similarly, trastuzumab (Herceptin) was first approved in 1998 for the treatment of HER2 positive breast cancer patients followed by other antibodies like pertuzumab and the most recent approval of margetuximab in 2020 [25, 26]. The mechanisms involved in anti-EGFR/HER2 mediated antitumor effect includes blocking the tyrosine kinases signaling induced malignant cell proliferation and differentiation, inhibiting hetero-dimerization and internalization of receptors. Host immune system also play an important role in anti-EGFR/Her2 mediated antitumor effect. ADCC, complement-dependent cytotoxicity (CDC) and antibody-dependent cellular phagocytosis (ADCP) are the most frequently reported mechanisms of innate immune system mediated tumor killing [27], [28], [29]. In a preclinical model, the antitumor effects of rituximab were completely abolished in complement cascade component C1q knockout mice [30]. In addition, Park et al. found that the antitumor effect of anti-HER2/neu antibody depends on both innate and adaptive immunity [31]. The antitumor effect of anti-neu was completely abolished in Rag knockout mice and dramatically reduced in wild-type (WT) mice after CD8 T cell depletion, indicating that host cytotoxic T cells also contribute to these monoclonal antibodies mediated tumor regression. Consistently, anti-CD8 depletion also abolished cetuximab mediated antitumor effect [32]. All these independent studies demonstrated the efficiency of mAbs in regulating host immune system to kill malignant cells.
Besides mAbs targeting growth factor receptors, mAbs targeting other TAAs has also been extensively studied. One of the most ideal targets is CD20, which expressed on B cell lymphoma. Rituximab was the first FDA approved anti-CD20 mAb, followed by ofatumumab and obinutuzumab. Rituximab alone or the combination with chemotherapy is still one of the first-line therapies of CD20 positive B-cell non-Hodgkin lymphomas [33], [34], [35]. Compared with targeting growth factor receptors which usually expressed on solid tumor, mAbs targeting CD20 has unique advantages. For example, the expression of CD20 is more specific than other TAAs, only on the surface of B cells in the late pre-B cell through mature memory B cell stages [36, 37]. Early pro-B cells and plasma cells are negative for CD20. Even though using anti-CD20 to treat lymphoma may deplete B cells as well, but antibody producing cells are preserved, thus minimizing the side effect [38, 39]. In addition, B cells can be constantly reconstituted by the bone marrow, which also prevent the severe toxicity. The mechanism of anti-CD20 mediated tumor clearance has been extensively studied by Ravetch et al. They demonstrated that Fc γ receptor (FcγR) expressed by CD11c+ antigen-presenting cells is required to generate anti-tumor T cell responses upon ADCC-mediated tumor clearance. The engagement of FcγR on both macrophage and dendritic cell (DC) can generate synergistic antitumor effect [40]. Recently, more and more specific targets for solid tumors have been identified for antibody therapeutics. For example, claudin18.2 (CLDN18.2), one of the tight junction proteins, is specifically expressed on differentiated epithelial cells of the gastric mucosa. However, abnormal overexpression has also been found in many cancer types, especially in digestive system malignancies [41]. Zolbetuximab (IMAB362), an anti-CLDN monoclonal antibody drug has been evaluated in a phase 3 clinical trial in combination with chemotherapy [42]. Besides proteins, other biomacromolecules can also be ideal target of mAbs. Mucin 1 (MUC1) is a glycoprotein that has been demonstrated to be involved in the metastasis and invasion of multiple tumor types [43]. Anti-MUC1 antibodies have also been extensively studied in multiple preclinical and clinical trials [44].
Even though the therapeutic benefits have been observed in TAA-targeting mAb treatment. Patients still responded to these treatments partially with relapse. Mechanisms involved in mAb treatment resistance includes: (1) mutation of the antibody target; (2) induction of alternative growth signaling pathways, studies have shown that c-MET promotes resistance to EGFR-targeting therapies; (3) adaptive immune editing; (4) the negative regulation of host immune response. Combination treatment with chemotherapy, targeted therapy (tyrosine kinase inhibitors) and immune checkpoint blockades (ICBs) might be required to overcome these resistance [45, 46].
Therapeutic monoclonal antibodies targeting immune checkpoints
Immune checkpoints are regulators of the immune systems. These pathways play an important role in T cell development and the maintenance of self-tolerance [47]. However, cancer cells also take advantages of these unique pathways to shut down host antitumor immune responses [48]. Therapeutic strategies that can interrupt these signal pathways in the tumor microenvironment may restore the antitumor immune response by “take the brakes off” effect [49, 50]. Since 1990s, many immune checkpoints have been identified including programmed cell death protein 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), T-cell immunoglobulin mucin receptor 3 (TIM-3), lymphocyte-activation gene 3 (LAG-3). And the most successful applications of immune checkpoint blockade for cancer treatment are antibodies targeting PD-L1 and CTLA-4 [51].
PD-1 expression is driven by T-cell receptor (TCR) signaling and mainly expressed on activated T cells [52, 53]. One of the PD-1 ligands, PD-L1, was expressed by myeloid cells including macrophages and dendritic cells. Some cancer cells also intrinsically or extrinsically express PD-L1, and the latter usually induced by interferons (IFNs) [54]. Upon the binding of PD-L1 with PD-1, phosphatase like phosphatase and tensin homolog (PTEN) will inhibit the phosphoinositide 3-kinase (PI3K)-AKT signaling to restrain T cell activation and proliferation [55, 56]. This pathway has been demonstrated as a major mechanism of T cell exhaustion in multiple preclinical models of cancer and chronic infection disease. Many monoclonal antibodies targeting PD-L1/PD-1 has been studied in the clinical trials with six FDA approved drugs, including atezolizumab, avelumab and durvalumab for PD-L1 and pembrolizumab, nivolumab and cemiplimab for PD-1 [57, 58]. Even though these immunotherapies have observed promising antitumor effect in some patients [59, 60]. The overall response rate of anti-PD-1/L1 monotherapy is still below 25%. Several mechanisms have been reported to affect the efficacy of anti-PD-1/L1 treatment [61], [62], [63], [64]. First, the T cell infiltration level. Since the aim of blocking PD-L1/PD-1 interaction is to rescue T cells from exhaustion, the level of T cell infiltration, especially CD8 T cell, are critical for the therapeutic effect of anti-PD-1/L1 [65]. Second, lack of tumor antigens. The genetic mutations are key drivers in the oncogenic process, which may lead to tumor immunogenicity and provide an opportunity for cancer immunotherapy. The tumor immunogenicity is closely related to the ability of T cells to recognize tumor cells and thus affect the efficacy of PD-L1/PD-1 blockade. Studies have showed that mutation burden and the number of neoantigens is closely related to the outcome of PD-L1/PD-1 blockade [66, 67], which has been confirmed in tumors with DNA mismatch repair (dMMR) deficiency and microsatellite instability (MSI) high [68]. In addition, studies have also showed that dMMR deficiency tumor cells can induce more cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) activation on dendritic cells to enhance the antitumor effect of PD-L1/PD-1 blockade [69]. Third, the level of stimulatory cytokines. Some preclinical studies have shown that the level interleukin-2 (IL-2) can determine the efficacy of PD-L1/PD-1 blockade [70]. Fourth, host gut microbiome can also influence the efficacy of ICB treatment [71, 72]. Finally, the induction of other immune checkpoints expression. The terminal exhausted phenotype of T cells can be maintained by multiple inhibitory pathways. High expression of CTLA-4, TIM-3 and LAG-3 on T cells will contribute to acquired resistance to PD-L1/PD-1 blockade [73, 74]. Besides tumor tissue, PD-L1 can also restrain T cell function in the tumor draining lymph nodes (TDLNs) [75]. Thus, PD-L1/PD-1 blockade could work on both priming and effector stage of the cancer-immune cycle. Moreover, studies also found that dendritic cells but not tumor cells expressed PD-L1 contribute to the PD-L1 blockade induced antitumor immunity [76, 77]. Mechanistically, interruption of PD-1/PD-L1 interaction will reconstitute B7-CD28 dependent costimulatory signaling for proper T cell function [78, 79].
CTLA-4 is another well-identified immune checkpoint [80]. Different from PD-L1/PD-1, CTLA-4 expression is upregulated on regulatory T cells (Tregs) within 24–48 h after activation. Mechanistically, CTLA-4 can first bind to B7 molecules with higher affinity than CD28 and then remove these ligands by transendocytosis, which results in impaired costimulation of T cells via CD28 [81], [82], [83]. Anti-CTLA-4 mAbs have been extensively studied in both preclinical and clinical models. Currently, ipilimumab is the first and only anti-CTLA-4 mAb approved by FDA for the treatment of melanoma, renal cell carcinoma and colorectal cancer, which has a human IgG1 isotype [84]. Two possible mechanisms of anti-CTLA-4 mediated T cell activation includes enhancing the B7-CD28 signaling on effector T cells and the Treg depletion [85]. However, the Treg depletion mechanism is still controversial [86]. In mouse models, anti-CTLA-4 mediated Treg depletion has been observed, but similar observations have not been reported in clinical applications [86, 87]. The difference in the isotypes of anti-CTLA-4 mAbs can contribute to distinct efficacy of ADCC or CDC mediated Treg depletion. Further studies are required to define the detailed mechanism. Finally, studies have shown that combination treatment of anti-PD-L1 and anti-CTLA-4 can generate synergistic antitumor effect by coordinately enhancing the B7-CD28 signaling [88, 89]. Even though anti-PD-L1 and anti-CTLA-4 have observed promising antitumor effect. The efficacy of monoclonal antibodies against other immune checkpoints remains to be validated in clinical trials, including anti-TIM-3, anti-immunoreceptor with Ig and ITIM domains (TIGIT) and anti-LAG-3.
In addition to mAbs against co-inhibitory molecules, agonist mAbs that can activate co-stimulatory signaling has also been evaluated for cancer treatment. CD28, inducible T-cell costimulator (ICOS), CD137 (4-1BB) and CD40 are representative targets that have been broadly investigated [90], [91], [92]. In 2006, anti-CD28 monoclonal antibody TGN1412 was reported to induced strong cytokine storm in health volunteers [93], and was withdrawn from development. In 1997, Melero et al. have demonstrated that monoclonal antibodies against 4-1BB can eradicate established tumors in preclinical models [94]. However, clinical development of anti-CD137 is facing the same toxicity issue with anti-CD28. Clinical trial on 4-1BB agonist antibody, urelumab, had to be terminated because of treatment-related severe adverse effects in the form of liver inflammation [95]. Thus, severe side effect remains the major hurdle for systemic treatment of agonist antibody against costimulatory molecules.
Recent years, investigators started to develop antibodies to target “innate immune checkpoints”. The most representative molecule is CD47. CD47 is ubiquitously expressed in human cells and binds to signal regulatory protein α (SIRPα) expressed on macrophages to deliver a “don’t eat me” signaling to avoid macrophage mediated phagocytosis [96]. Many cancer cells can escape from this innate immune surveillance by overexpressing CD47 [97, 98]. Anti-CD47 blocking antibody can induce more macrophage-dependent phagocytosis and T-cell dependent antitumor immune responses [99]. However, Liu et al. also reported that the antitumor effect of anti-CD47 depends on dendritic cells and cGAS-STING dependent DNA sensing [100]. Thus, CD47 is a unique immune checkpoint which can bridge innate and adaptive immune system for effective tumor control. Even though CD47 is a promising target for antibody therapeutics, it still has the toxicity issues. Since CD47 is ubiquitously expressed in human, systemic delivery of anti-CD47 may induce severe adverse effect, as represented by red blood cell (RBC) depletion [101]. Alternative strategies have been investigated to improve the efficacy while reduce the toxicity. For example, Weiskopf et al. have engineered SIRPα variants with much higher affinity than wild-type SIRPα to enhance antibody mediated ADCC effect for cancer immunotherapy [102]. Bispecific antibodies targeting TAA and CD47 have also been engineered and studied [103, 104]. Clinical development of antibody therapeutics targeting CD47 have been extensively performed, but no drugs have been approved by the FDA yet. The safety and efficacy of magrolimab, a first-in-class anti-CD47 antibody, have not been established since FDA puts clinical hold on trials concerning the safety. Similar phagocytosis checkpoint has also been identified including Stanniocalcin 1, but the efficacy of antibody therapeutics against these target remains to be determined in clinical trials [105]. In summary, antibodies against innate and adaptive immune checkpoint have showed impressive potency (Table 1). Delivery strategies that can overcome systemic toxicity are required to improve the efficacy and broaden the application.
Table 1:
US FDA-approved monoclonal antibody for cancer treatment.
| Target | mAb name | 1st approval year | Format | Application |
|---|---|---|---|---|
| CD20 | Rituximab | 1997 | Chimeric IgG1 | Non-Hodgkin lymphoma |
| Ofatumumab | 2009 | Human IgG1 | Chronic lymphocytic leukemia | |
| Obinutuzumab | 2013 | Humanized IgG1 | Chronic lymphocytic leukemia | |
| HER2 | Trastuzumab | 1998 | Humanized IgG1 | Breast cancer |
| Pertuzumab | 2012 | Humanized IgG1 | Breast cancer | |
| EGFR | Cetuximab | 2004 | Chimeric IgG1 | Colorectal cancer |
| Necitumumab | 2015 | Human IgG1 | NSCLC | |
| Panitumumab | 2006 | Human IgG2 | Colorectal cancer | |
| PD-1 | Cemiplimab | 2018 | Human mAb | Cutaneous squamous cell carcinoma |
| Nivolumab | 2014 | Human IgG4 | Melanoma, NSCLC | |
| Pembrolizumab | 2014 | Human IgG4 | Melanoma | |
| CTLA-4 | Ipilimumab | 2011 | Human IgG1 | Metastatic melanoma |
| PD-L1 | Atezolizumab | 2016 | Humanized IgG1 | Bladder cancer |
| Avelumab | 2017 | Human IgG1 | Merkel cell carcinoma | |
| Durvalumab | 2017 | Human IgG1 | Bladder cancer | |
| SLAMF7 | Elotuzumab | 2014 | Humanized IgG1 | Multiple myeloma |
| CD38 | Daratumumab | 2015 | Human IgG1 | Multiple myeloma |
| Isatuximab | 2021 | Chimeric IgG1 | Multiple myeloma | |
| CD52 | Alemtuzumab | 2001 | Humanized IgG1 | Chronic myeloid leukemia |
| VEGF-A | Bevacizumab | 2004 | Humanized IgG1 | Colorectal cancer |
| VEGFR2 | Ramucirumab | 2014 | Human IgG1 | Gastric cancer |
| CD19 | Tafasitamab | 2020 | Humanized IgG1 | Diffuse large B-cell lymphoma |
| LAG-3 | Relatlimab | 2022 | Human IgG4 | Melanoma |
EGFR, epidermal growth factor receptor; HER2, epidermal growth factor receptor 2; PD-L1, programmed cell death 1 ligand 1; FDA, Food and Drug Administration.
Antibody-drug conjugates for cancer therapy
Besides monoclonal antibodies, ADCs are the most common format for antibody-based cancer immunotherapeutic drugs [106]. To date, ten ADCs have been approved by the FDA. In addition, more than 80 ADCs are being developed in clinical as monotherapy or combination therapy for the treatment of different cancer types [107]. ADCs are complex molecules which conjugate a potent cytotoxic agent to specific sites on antibody via a chemical linker. The cytotoxic agent also named as “payload”, which includes anti-mitotic tubulin disruptors, DNA alkylating agents, RNA polymerase II and toll-like receptor agonists [108, 109]. Mechanistically, ADCs depend on the specificity of antibodies to target tumor cells first, followed by ADC internalization, and subsequent payload release to achieve the cytotoxicity. Even though most FDA approved ADCs are for the treatment of hematological malignancies, more ADCs are being developed to treat solid tumor (Table 2). For example, trastuzumab emtansine (T-DM1) and sacituzumab govitecan are well-defined ADCs for breast cancer, which can significantly prolong progression-free and overall survival in metastatic triple-negative breast cancer patients [110]. The major barrier for ADCs is still the ‘on-target off-tumor’ side effect, since ADCs can induce stronger cytotoxicity compared with mAbs. Future engineering directions for ADCs includes target selection, linker chemistry, novel payload development, conjugation method optimization and enhancing internalization. Recently, proteolysis-targeting chimera (PROTAC) has been applied as a payload for ADC and termed as degrader-antibody conjugates (DAC) [111]. Several preclinical studies have demonstrated the potential efficacy of this next-generation drug [112]. More data from clinical trials are required for better characterization of these novel ADCs.
Table 2:
US FDA-approved antibody-drug conjugates for cancer treatment.
| Name | Target | Application | FDA approval year |
|---|---|---|---|
| Loncastuximab tesirine-lpyl | CD19 | Large B-cell lymphoma | 2021 |
| Belantamab mafodotin-blmf | BCMA | Relapsed or refractory multiple myeloma | 2020 |
| Sacituzumab govitecan | Trop-2 | Metastatic triple-negative breast cancer | 2020 |
| Trastuzumab deruxtecan | HER2 | Unresectable or metastatic Her2-positive breast cancer | 2019 |
| Enfortumab vedotin | Nectin-4 | Advanced or metastatic urothelial cancer | 2019 |
| Polatuzumab vedotin | CD79b | Diffuse large B-cell lymphoma | 2019 |
| Moxetumomab pasudotox | CD22 | Relapsed or refractory hairy cell leukemia | 2018 |
| Inotuzumab ozogamicin | CD22 | Relapsed or refractory B-cell precursor ALL | 2017 |
| Trastuzumab emtansine | HER2 | HER2-positive metastatic breast cancer | 2013 |
| Brentuximab vedotin | CD30 | Relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma | 2011 |
| Gemtuzumab ozogamicin | CD33 | Acute myeloid leukemia | 2000/2017 |
BCMA, B-cell maturation antigen; HER2, epidermal growth factor receptor 2; FDA, Food and Drug Administration.
Bispecific antibodies for cancer immunotherapy
CD3-based bispecific antibodies for cancer immunotherapy
Since 1988, Jeffrey Bluestone group first reported that in vivo administration of anti-CD3 mAb can prevent malignant progressor tumor growth [113]. Antibody therapeutics targeting CD3 have been studied and developed for more than 30 years. To date, anti-CD3 based bispecific antibody is still the major topic among all the antibody therapeutics [114, 115]. In physiological conditions, the TCR/CD3 complex is cross-linked upon recognition of MHC-peptide complex, thus leading to specific activation of T cells [116]. However, anti-CD3ε mAb can directly cross-link CD3 complex to non-specifically activate T cells [117]. Due to this unique function, anti-CD3 has been broadly investigated and applied to prevent allograft rejection, to treat diabetes and for cancer immunotherapy [118]. Since systemic delivery of anti-CD3 can non-specifically activate all the T cells, the treatment related side-effect remains the major concern. In order to target tumor cells to activate T cells, bispecific T cell engager (BiTE) concept was introduced as early as in 1980s [119]. To use antibody to ‘cross-link’ the CD3 signaling, one additional binding site is required besides CD3. According to this unique feature, BiTE was designed as two single-chain variable fragment (ScFv) linked with a flexible linker which can simultaneously bind to CD3 and TAA. This structure has several advantages over traditional mAbs. First, it can bridge T cells with TAA expressing cell for effective killing. Second, it can target TAA expressing cells to cross-link TCR signaling, thus may reduce the systemic toxicity. To date, different formats of BiTE have been developed such as Fab2, diabody and dual-affinity re-targeting (DART) [120]. But only the CD19xCD3 BiTE, blinatumomab, has been approved by the FDA to treat adults and children with B-cell precursor acute lymphoblastic leukemia (ALL) who are in remission but still have minimal residual disease (MRD) [121]. Similar with mAbs, the major concern of BiTE treatment is still the ‘on-target off-tumor’ side effect. Since TAAs also expressed in some normal tissue, BiTE induced T cell activation may cause cytokine storm and tissue damage [122]. In addition, the short half-life of BiTE also limits its application in solid tumors. Even for blinatumomab treatment, patients need daily intravenous infusion of BiTE for 4 weeks as a treatment cycle. The penetration of BiTE against solid tumors is still problematic. To extend the in vivo half-life of BiTE, Fc region was engineered with BiTE and termed as bispecific antibody (BsAb). Several alternative formats of BsAb have also been investigated including ScFv-knobs-into-holes (KIH), dual variable domain (DVD)-IgG and CrossMab [123]. Among them, catumaxomab is a rat-mouse hybrid monoclonal antibody which simultaneously binds to CD3 and EpCAM. It was first approved by EMA to treat malignant ascites in 2009 but withdrawn 8 years later due to commercial reasons [124]. In early 2022, the US FDA approved tebentafusp-tebn, a bispecific gp100 peptide-HLA-directed CD3 T cell engager, for HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma [125]. This is a unique and next-generation design of bispecific antibody. Unlike traditional bispecific antibodies, tebentafusp-tebn binds to the gp100 peptide-HLA complex instead of TAAs. Thus, it can increase the specificity of tumor-targeting while reducing the side effect. This therapeutic strategy could benefit selective patients with well-identified mutant peptide and specific HLA alleles. Similar constructs are designed to target the common oncogenes such as p53 and KRAS with TCR mimic antibodies [126]. Based on the successful application of blinatumomab in leukemia, more and more bispecific antibodies have been developed to treat hemopoietic malignance. Most recently, the EMA has approved CD20xCD3 bispecific antibody (mosunetuzumab) for patients with relapsed or refractory disease of follicular lymphoma and BCMAxCD3 bispecific antibody (teclistamab) for patients with relapsed and refractory multiple myeloma [127, 128]. Compared with hemopoietic malignance, solid tumors usually form an inhibitory tumor microenvironment that limits the efficacy of bispecific antibodies [129]. T cell infiltration level, antibody penetration and the expression of immune checkpoint molecules are major barriers for BsAb treatment against solid tumors. Resistance to BiTE treatment in T cell–cold solid tumors can be overcome by combination treatment [130]. Many targets on solid tumors have been extensively evaluated as candidates for BsAb, including EGFR, carcinoembryonic antigen (CEA), prostate-specific membrane antigen (PSMA) and HER2 (Table 3). But the efficacy and safety have not been established yet [131, 132].
Table 3:
Summary of clinical bispecific antibodies for cancer treatment.
| Target 1 | Target 2 | Name | Application | Status |
|---|---|---|---|---|
| CD3 | CD19 | Blinatumomab | ALL | FDA approved |
| A-319 | ALL | Phase I (NCT04056975) | ||
| AMG562 | Lymphoma | Phase I (NCT03571828) | ||
| CD20 | Mosunetuzumab | NHL | Phase I (NCT05169658) | |
| Odronextamab | NHL | Phase II (NCT03888105) | ||
| Plamotamab | Diffuse large-cell B-cell lymphoma | Phase II (NCT05328102) | ||
| CD38 | ISB 1342 | Multiple myeloma | Phase I/II (NCT03309111) | |
| BCMA | AMG 420 | Multiple myeloma | Phase I (NCT03836053), completed | |
| AMG 701 | Multiple myeloma | Phase I (NCT03287908) | ||
| CD123 | APVO436 | AML | Phase I (NCT03647800) | |
| XmAb14045 | ALL | Phase I (NCT02730312) | ||
| CEA | RO6958688 | Solid tumor | Phase I (NCT02650713) | |
| PSMA | Acapatamab | Prostate cancer | Phase I (NCT03792841) | |
| BAY2010112 | Prostate cancer | Phase I (NCT01723475), completed | ||
| HER2 | M802 | Solid tumor | Phase I (NCT04501770) | |
| Runimotamab | Solid tumor | Phase I (NCT03448042) | ||
| MUC16 | REGN4018 | Ovarian cancer | Phase I/II (NCT 03564340) | |
| GPC3 | ERY974 | Solid tumor | Phase I (NCT02748837), completed | |
| EGFR vIII | AMG 596 | Glioblastoma or malignant Glioma | Phase I (NCT03296696), completed | |
| CLDN18.2 | AMG 910 | Gastric and Gastroesophageal junction adenocarcinoma | Phase I (NCT04260191), completed | |
| CD47 | PD-1 | HX009 | Relapsed/Refractory lymphoma | Phase I (NCT05189093) |
| PD-L1 | IBI322 | Advanced malignant tumor | Phase I (NCT04338659) | |
| CD19 | TG-1801 | B-cell lymphoma | Phase I (NCT03804996) | |
| PD-1 | ICOS | XmAb®23,104 | Advanced solid tumor | Phase I (NCT03752398) |
| CTLA-4 | MEDI5752 | Advanced RCC | Phase I (NCT04522323) | |
| PD-L1 | LY3434172 | Advanced cancer | Phase I (NCT03936959), completed | |
| TIM3 | RO7121661 | Advanced and/or metastatic solid tumor | Phase I (NCT03708328) | |
| PD-L1 | LAG-3 | FS118 | Advanced malignancies | Phase I (NCT03440437) |
| CTLA-4 | KN046 | Thymic carcinoma | Phase II (NCT04469725) | |
| EGFR | c-MET | Amivantamab | NSCLC | Phase I (NCT04077463) |
| HER2 | HER3 | MCLA-128 | Breast cancer | Phase II (NCT03321981) |
BCMA, B-cell maturation antigen; EGFR, epidermal growth factor receptor; HER2, epidermal growth factor receptor 2; PD-L1, programmed cell death 1 ligand 1; NSCLC, non-small cell lung cancer.
Since the treatment related severe adverse effects is one of the major barriers for BsAb. Several studies have investigated the potential mechanism. Li et al. showed that anti-HER2/CD3–induced cytokine release is not required for T cell cytotoxic activity. Anti-TNFα can prevent monocytes derived IL-1 and IL-6 to induce toxicity while keep the antitumor effect from T cells [133]. Cytokine release syndrome (CRS) was only observed at 24 h post the first dose of treatment in CD20xCD3 treatment [134, 135]. These reports indicate that the treatment-related side effect can be overcome by combination treatment. Besides toxicity, strategies to improve the efficacy have also been investigated. Belmontes et al. have shown that resistant to CLDN18.2 specific BiTE treatment can be overcome by the combination with either anti-PD-L1, anti-CTLA-4 or anti-4-1BB [130]. Bispecific antibody targeting the immune checkpoint PD-L1 and CD3 generate much better antitumor effect than traditional TAA-targeting BiTE by enhancing B7-CD28 mediated costimulatory signaling from dendritic cells [136]. These studies indicate that target the immune checkpoint may generate better T-cell activation than targeting TAAs on the cancer cells by enhancing the crosstalk between immune cells. In addition, several studies have also reported that TAA-targeting BiTE treatment can induce activation-induced cell death (AICD) on T cells, thus prevent the establishment of memory T cell response [137]. Concurrent delivery of costimulatory signaling by anti-CD28 can overcome this limitation by potentiating T cell activation [138, 139].
TAA and immune checkpoint targeting bispecific antibodies for cancer immunotherapy
Apart from CD3-based bispecific antibodies, other dual functional bispecific antibodies have also been developed. The first category is dual receptor blockade antibody. cMet/EGFR and HER2/HER3 bispecific antibodies are representative drugs under clinical investigation [140, 141]. These antibodies are designed to simultaneously block multiple growth factor signaling to limit the adaptive resistance of mAb treatment. In 2021, amivantamab-vmjw, the cMet/EGFR bispecific antibody was approved by FDA for patients with metastatic non-small cell lung cancer (NSCLC). The second category is dual immune checkpoint blockade antibody. Several drugs are being evaluated in phase I to III clinical trials including PD-1xCTLA-4, PD-L1xLAG3, PD-L1xCD47. The purpose of these drugs is to target tumor tissue first and then simultaneously block multiple immune checkpoints [104, 142, 143]. In addition, bispecific antibodies are also designed to deliver agonist or antagonist in the tumor tissue. Anti-4-1BB is a powerful but toxic drug. Like CD3 and some TNF receptor superfamily members, trimerization is required for 4-1BB signaling. According to this unique feature, 4-1BBxHer2 and 4-1BBxPD-L1 have been designed [144], [145], [146]. Finally, cytokine receptors are also ideal targets for bispecific antibodies. M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β have showed effective antitumor effect in multiple mouse tumor models [147]. Despite failing in NSCLC patients, bintrafusp alfa (M7824) will continue to be evaluated in several additional clinical trials for different cancer types.
Antibody-cytokine fusion proteins for cancer immunotherapy
Cytokines are soluble proteins that mediate cell-to-cell communication, especially between immune cells. Studies showed that several cytokines can limit cancer cell growth by both direct anti-proliferative or pro-apoptotic activity, and indirectly activating host adaptive immune responses [148]. IFNα, TNFα and IL-2 have been extensively studied and applied for cancer treatment [59, 149, 150]. Since antibodies can provide unique tumor-targeting property by binding either TAAs or the immune checkpoints. People also engineered cytokines to these antibodies to increase the efficacy and reduce the toxicity [151], [152], [153], [154]. Yang et al. reported that type-I interferon fused to anti-EGFR (cetuximab) can overcome therapeutic resistance to cetuximab by increasing DC function and CD8 T cell immune responses [155]. Similar result was also observed in CD20xIFNα treatment study [156, 157]. IL-2 is a potent cytokine for T cell activation and proliferation [158]. System delivery of recombinant IL-2 have been approved by FDA for patients with metastatic renal cell carcinoma and metastatic melanoma in 1990s. However, high dose IL-2 treatment is toxic and may also expand Tregs which express high levels of IL-2 receptor alpha [59, 159]. Several antibody-based IL-2 delivery strategies have been designed to overcome these limitations [160, 161]. In addition, Sun et al. designed an EGFR targeting super mutant IL-2 and demonstrated that tumor-targeting delivery of CD8-T cell-biased IL-2 generate better antitumor effect than WT IL-2 [162]. In another study, Qiao et al. demonstrated that EGFR-targeting delivery of interlukin-10 prevents dendritic cell mediated CD8+ tumor-infiltrating lymphocyte apoptosis through regulating IFN-γ production [163, 164]. In summary, antibodies can be a powerful platform to deliver cytokines in the tumor microenvironment. More clinical validations are required for establishing the efficacy and safety profile for cancer patients.
Strategies to overcome immune-related adverse events in antibody therapeutics
The fundamental rationale of antibody-based drugs is targeting tumor tissue to deliver therapeutic agents and activate immune responses. To date, very few cancer-specific antigens have been identified except some shared/public neoantigens and oncogenic viral antigens [165]. Most antibodies are still targeting the tumor-associated antigens which are also expressed in normal tissues. Systemic delivered antibody therapeutics may also penetrate to these normal tissue, induce inflammation and tissue damage [166]. To overcome this “on-target off-tumor” side effect, several strategies have been developed. The most common design is antibody prodrugs [167]. The principle of these prodrugs is using a protease substrate peptide to link a ‘mask’ to the antibody. Thus, the antibodies will be masked in the peripheral circulation to avoid off-tumor binding. When the antibodies penetrate to the tumor tissue, tumor associated protease will ‘cut’ the substrate linker, remove the mask, and release the drug [168, 169]. This two-step targeting approach could dramatically improve the tumor-targeting efficacy and reduce the toxicity. Representative constructs using this strategy is the dual variable domain Ig (DVD-Ig). Pai et al. demonstrated that anti-CTLA4 DVD-Ig markedly reduced multiorgan immune toxicity by preserving tissue-resident Tregs. In this study, the anti-CTLA4 antibody was shielded by an outer tumor-targeting anti-prostate stem cell antigen (PSCA) domain. Activated membrane type-serine protease 1 (MT-SP1), which was highly expressed in the tumor microenvironment can cleave this shield to expose the inner CTLA4-binding site [170]. Other advanced antibody prodrugs in development are the Probody™ Therapeutics [171], [172], [173]. This platform usually needs two steps screening. First, the identification of appropriate mask by screening the peptide libraries. Second, the identification of a suitable protease substrate [174, 175]. Many antibody therapeutics are developed and under clinical trials, including CX-072 (pacmilimab) for anti-PD-L1 [176], CX-904 for EGFRxCD3 and BMS-986249 for anti-CTLA4. The efficacy of peptide masking and protease digestion need to be future investigated for broader application. The immunogenicity of the peptide mask also needs to be identified for the safety.
Summary and future prospect
To date, more than 100 antibody-based therapeutic drugs have been approved by the FDA and most of them are for cancer treatment [3]. Due to the advanced technology in recombinant and humanized antibody engineering, more and more candidates are being screened preclinically and clinically. However, detailed mechanism studies on efficacy and toxicity are required for the translation (Figure 1 and Table 4). Several drugs have observed good efficacy and limited toxicity in mouse models, but immune-related adverse events (irAE) were observed in human despite the efficacy [177, 178]. This means that there are still some undiscovered differences in the immune system between murine and human. Even though the mouse tumor models have been extensively optimized using human peripheral blood mononuclear cell (PBMC) and CD34+ stem cells. The reconstituted human immune system on mouse still have some differences compared with patients [179]. Cetuximab, rituximab, ipilimumab and nivolumab are successful mAbs that are being applied in patients broadly, lessons should be learnt from these drugs on the engineering and evaluation strategies. Some future investigation on antibody therapeutics will help more patients benefit from this therapy. First, more cancer antigens should be screened to identify more specific targets. This will be important for immune checkpoint blockade, antibody-cytokine fusion protein and agonist antibodies development. Second, optimization on the antibody sequence. The Fc region play controversial roles on the function of therapeutic antibodies. On one hand, it can extend the in vivo half-life of antibodies. On the other hand, it may also bind to different Fc receptors and mediate ADCC or CDC effect. For tumor cell targeting mAbs, Fc-FcR binding can promote innate immune activation [180]. However, for ICB treatment, Fc may also lead to T cell depletion. In the bispecific antibody design, mutations on Fc have been made to avoid Fc mediated non-specific cross-link of CD3 in the peripheral tissues [181, 182]. Further screening on Fc is essential to improve the efficacy. Third, development of computation protein design to antibody discovery. The generation of antibody repertoires deep-sequencing data and matched functional data can be used to train machine-learning-based models for affinity maturation and humanization [183, 184]. This will also be an important technology to discover ideal mask for prodrugs. Fourth, the application of novel technologies. To improve the tumor targeting ability and antitumor efficacy of BsAbs, several trispecific antibodies have been developed, including the CD3/CD28/CD38 trispecific antibody that can increase the costimulatory signaling and the “2 + 1” platform that can increase tumor targeting [134, 185, 186]. Other tumor-targeting strategies should also be considered for antibody delivery. For example, as tumor tissue usually have a lower pH than other tissues, pH sensitive nanoparticle can be a good platform for antibody encapsulation and targeted release [187, 188]. Tumor tropic cell and bacteria can also be engineered for antibody delivery [189]. Finally, the combination treatment. Antibody therapeutics may generate synergistic antitumor effect with traditional therapy. For example, neoadjuvant ICB may induce better and systemic antitumor effect with either radiation or surgery [190, 191]. The combination with ICB can also improve the efficacy of BiTE [192]. Blocking the inflammatory cytokines may reduce the irAE from ICB while enhancing the antitumor effect simultaneously [193]. In multiple ongoing clinical trials, antibodies are used as adjuvant therapy to chemotherapy and observed clinical responses. Thus, the combination of either different antibody therapeutics or antibody therapeutics with traditional therapies will be potential strategies to try. In conclusion, we have summarized the highlights of innovation with antibody therapeutics, the progress of antibodies engineering in recent years and discussed the limitation of antibody therapeutics and the potential strategies to overcome the hurdles. With more than 100 FDA approved antibody-based drugs, immunotherapy will benefit more cancer patients in the near future.
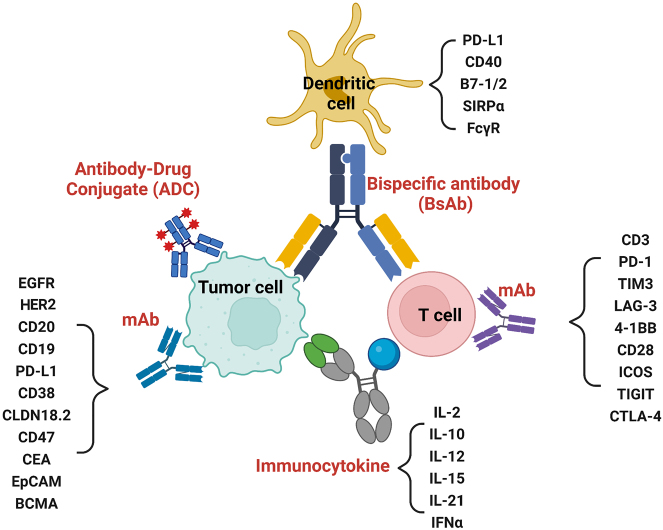
Figure 1:
Schematic overview showing current antibody-based therapeutics for the cancer treatment. There are four major categories of antibody therapeutics. First, monoclonal antibody (mAb), which includes anti-tumor associated antigen (TAA) and T-cell costimulatory/coinhibitory molecules. Second, antibody-drug conjugate. Third, bispecific antibodies. Fourth, immunocytokines. IL-10, interleukin-10; IL-12, interleukin-12; IL-15, interleukin-15; IL-21, interleukin-21; EpCAM, epithelial cell adhesion molecule; BCMA, B-cell maturation antigen; EGFR, epidermal growth factor receptor; HER2, epidermal growth factor receptor 2; PD-L1, programmed cell death 1 ligand 1.
Table 4:
Advantages and disadvantages of different therapeutic antibodies.
| Name | Advantage | Disadvantage |
|---|---|---|
| Monoclonal antibody |
|
|
| Antibody-drug conjugate |
|
“On-target off-tumor” side-effect |
| Bispecific antibody |
|
“On-target off-tumor” side-effect |
| Antibody fusion proteins |
|
Undefined mechanism of action |
Acknowledgments
We thank Chuanhui Han for helpful suggestions.
Footnotes
Research funding: None declared.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Not applicable.
Ethical approval: Not applicable.
References
- 1.Carter PJ, Rajpal A. Designing antibodies as therapeutics. Cell. 2022;185:2789–805. doi: 10.1016/j.cell.2022.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Kaplon H, Chenoweth A, Crescioli S, Reichert JM. Antibodies to watch in 2022. mAbs. 2022;14:2014296. doi: 10.1080/19420862.2021.2014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullard A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov. 2021;20:491–5. doi: 10.1038/d41573-021-00079-7. [DOI] [PubMed] [Google Scholar]
- 4.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody combination and outcomes in outpatients with covid-19. N Engl J Med. 2021;385:e81. doi: 10.1056/nejmoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, et al. Bamlanivimab plus etesevimab in mild or moderate covid-19. N Engl J Med. 2021;385:1382–92. doi: 10.1056/nejmoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwaber J, Cohen EP. Human x mouse somatic cell hybrid clone secreting immunoglobulins of both parental types. Nature. 1973;244:444–7. doi: 10.1038/244444a0. [DOI] [PubMed] [Google Scholar]
- 7.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 8.Koprowski H, Steplewski Z, Herlyn D, Herlyn M. Study of antibodies against human melanoma produced by somatic cell hybrids. Proc Natl Acad Sci U S A. 1978;75:3405–9. doi: 10.1073/pnas.75.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadler LM, Stashenko P, Hardy R, Kaplan WD, Button LN, Kufe DW, et al. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980;40:3147–54. [PubMed] [Google Scholar]
- 10.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 11.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 12.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Samaranayake H, Wirth T, Schenkwein D, Raty JK, Yla-Herttuala S. Challenges in monoclonal antibody-based therapies. Ann Med. 2009;41:322–31. doi: 10.1080/07853890802698842. [DOI] [PubMed] [Google Scholar]
- 14.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–33. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27:1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haen SP, Loffler MW, Rammensee HG, Brossart P. Towards new horizons: characterization, classification and implications of the tumour antigenic repertoire. Nat Rev Clin Oncol. 2020;17:595–610. doi: 10.1038/s41571-020-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–4. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wykosky J, Fenton T, Furnari F, Cavenee WK. Therapeutic targeting of epidermal growth factor receptor in human cancer: successes and limitations. Chin J Cancer. 2011;30:5–12. doi: 10.5732/cjc.010.10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos EDS, Nogueira KAB, Fernandes LCC, Martins JRP, Reis AVF, Neto JBV, et al. EGFR targeting for cancer therapy: pharmacology and immunoconjugates with drugs and nanoparticles. Int J Pharm. 2021;592:120082. doi: 10.1016/j.ijpharm.2020.120082. [DOI] [PubMed] [Google Scholar]
- 20.Congdon KL, Gedeon PC, Suryadevara CM, Caruso HG, Cooper LJ, Heimberger AB, et al. Epidermal growth factor receptor and variant III targeted immunotherapy. Neuro Oncol. 2014;16:viii20–5. doi: 10.1093/neuonc/nou236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Contr Release. 2010;146:264–75. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi: 10.1056/nejmoa071834. [DOI] [PubMed] [Google Scholar]
- 23.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34. doi: 10.1056/nejmoa1305275. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan MS, Eswaraiah A, Crombet T, Piedra P, Saurez G, Iyer H, et al. Nimotuzumab, a promising therapeutic monoclonal for treatment of tumors of epithelial origin. mAbs. 2009;1:41–8. doi: 10.4161/mabs.1.1.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/nejmra043186. [DOI] [PubMed] [Google Scholar]
- 26.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34. doi: 10.1056/nejmoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1–9. doi: 10.1111/j.1365-2249.2009.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubert P, Amigorena S. Antibody-dependent cell cytotoxicity in monoclonal antibody-mediated tumor immunotherapy. OncoImmunology. 2012;1:103–5. doi: 10.4161/onci.1.1.17963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiLillo DJ, Ravetch JV. Fc-receptor interactions regulate both cytotoxic and immunomodulatory therapeutic antibody effector functions. Cancer Immunol Res. 2015;3:704–13. doi: 10.1158/2326-6066.cir-15-0120. [DOI] [PubMed] [Google Scholar]
- 30.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–7. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–70. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Zhang X, Mortenson ED, Radkevich-Brown O, Wang Y, Fu YX. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21:91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maury S, Chevret S, Thomas X, Heim D, Leguay T, Huguet F, et al. Rituximab in B-lineage adult acute lymphoblastic leukemia. N Engl J Med. 2016;375:1044–53. doi: 10.1056/nejmoa1605085. [DOI] [PubMed] [Google Scholar]
- 34.Salles G, Mounier N, de Guibert S, Morschhauser F, Doyen C, Rossi JF, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 2008;112:4824–31. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. doi: 10.1200/jco.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 36.Klasener K, Jellusova J, Andrieux G, Salzer U, Bohler C, Steiner SN, et al. CD20 as a gatekeeper of the resting state of human B cells. Proc Natl Acad Sci U S A. 2021;118:e2021342118. doi: 10.1073/pnas.2021342118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forsthuber TG, Cimbora DM, Ratchford JN, Katz E, Stuve O. B cell-based therapies in CNS autoimmunity: differentiating CD19 and CD20 as therapeutic targets. Ther Adv Neurol Disord. 2018;11:1756286418761697. doi: 10.1177/1756286418761697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boross P, Leusen JH. Mechanisms of action of CD20 antibodies. Am J Cancer Res. 2012;2:676–90. [PMC free article] [PubMed] [Google Scholar]
- 39.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–68. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 40.DiLillo DJ, Ravetch JV. Differential fc-receptor engagement drives an anti-tumor vaccinal effect. Cell. 2015;161:1035–45. doi: 10.1016/j.cell.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, et al. Claudin18.2-Specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst. 2019;111:409–18. doi: 10.1093/jnci/djy134. [DOI] [PubMed] [Google Scholar]
- 42.Sahin U, Tureci O, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32:609–19. doi: 10.1016/j.annonc.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Singh R, Bandyopadhyay D. MUC1: a target molecule for cancer therapy. Cancer Biol Ther. 2007;6:481–6. doi: 10.4161/cbt.6.4.4201. [DOI] [PubMed] [Google Scholar]
- 44.Bose M, Mukherjee P. Potential of anti-MUC1 antibodies as a targeted therapy for gastrointestinal cancers. Vaccines. 2020;8:659. doi: 10.3390/vaccines8040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/nejm200103153441101. [DOI] [PubMed] [Google Scholar]
- 46.Zhao B, Wang L, Qiu H, Zhang M, Sun L, Peng P, et al. Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget. 2017;8:3980–4000. doi: 10.18632/oncotarget.14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660–9. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, et al. Defining ‘T cell exhaustion. Nat Rev Immunol. 2019;19:665–74. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;20:840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gravbrot N, Gilbert-Gard K, Mehta P, Ghotmi Y, Banerjee M, Mazis C, et al. Therapeutic monoclonal antibodies targeting immune checkpoints for the treatment of solid tumors. Antibodies. 2019;8:51. doi: 10.3390/antib8040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 52.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–25. doi: 10.1111/j.1600-065x.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–67. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 54.Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14:10. doi: 10.1186/s13045-020-01027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hudson K, Cross N, Jordan-Mahy N, Leyland R. The extrinsic and intrinsic roles of PD-L1 and its receptor PD-1: implications for immunotherapy treatment. Front Immunol. 2020;11:568931. doi: 10.3389/fimmu.2020.568931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vidotto T, Melo CM, Castelli E, Koti M, Dos Reis RB, Squire JA. Emerging role of PTEN loss in evasion of the immune response to tumours. Br J Cancer. 2020;122:1732–43. doi: 10.1038/s41416-020-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301. doi: 10.1056/nejmoa1716948. [DOI] [PubMed] [Google Scholar]
- 58.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376:2415–26. doi: 10.1056/nejmoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/jco.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 60.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90. doi: 10.1056/nejmoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022;29:3044–60. doi: 10.3390/curroncol29050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasan Ali O, Berner F, Bomze D, Fassler M, Diem S, Cozzio A, et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur J Cancer. 2019;107:8–14. doi: 10.1016/j.ejca.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 63.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–8. doi: 10.1200/jco.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 64.Sun JY, Zhang D, Wu S, Xu M, Zhou X, Lu XJ, et al. Resistance to PD-1/PD-L1 blockade cancer immunotherapy: mechanisms, predictive factors, and future perspectives. Biomark Res. 2020;8:35. doi: 10.1186/s40364-020-00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang H, Wang Y, Chlewicki LK, Zhang Y, Guo J, Liang W, et al. Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell. 2016;29:285–96. doi: 10.1016/j.ccell.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strickler JH, Hanks BA, Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin Cancer Res. 2021;27:1236–41. doi: 10.1158/1078-0432.ccr-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36:773–9. doi: 10.1200/jco.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 69.Lu C, Guan J, Lu S, Jin Q, Rousseau B, Lu T, et al. DNA sensing in mismatch repair-deficient tumor cells is essential for anti-tumor immunity. Cancer Cell. 2021;39:96–108 e6. doi: 10.1016/j.ccell.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren Z, Zhang A, Sun Z, Liang Y, Ye J, Qiao J, et al. Selective delivery of low-affinity IL-2 to PD-1+ T cells rejuvenates antitumor immunity with reduced toxicity. J Clin Invest. 2022;132:e153604. doi: 10.1172/JCI153604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 72.Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci U S A. 2018;115:157–61. doi: 10.1073/pnas.1712901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian T, Li Z. Targeting tim-3 in cancer with resistance to PD-1/PD-L1 blockade. Front Oncol. 2021;11:731175. doi: 10.3389/fonc.2021.731175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khair DO, Bax HJ, Mele S, Crescioli S, Pellizzari G, Khiabany A, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453. doi: 10.3389/fimmu.2019.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T, et al. The PD-1/PD-L1-checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell. 2020;38:685–700 e8. doi: 10.1016/j.ccell.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med. 2020;12:eaav7431. doi: 10.1126/scitranslmed.abd0088. [DOI] [PubMed] [Google Scholar]
- 77.Oh SA, Wu DC, Cheung J, Navarro A, Xiong H, Cubas R, et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat Can. 2020;1:681–91. doi: 10.1038/s43018-020-0075-x. [DOI] [PubMed] [Google Scholar]
- 78.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–7. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–33. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131:58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410:604–8. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 82.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–50. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 83.Yokosuka T, Kobayashi W, Takamatsu M, Sakata-Sogawa K, Zeng H, Hashimoto-Tane A, et al. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010;33:326–39. doi: 10.1016/j.immuni.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/nejmoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–86. doi: 10.1158/2159-8290.cd-18-0367. [DOI] [PubMed] [Google Scholar]
- 86.Sharma A, Subudhi SK, Blando J, Scutti J, Vence L, Wargo J, et al. Anti-CTLA-4 immunotherapy does not deplete FOXP3(+) regulatory T cells (Tregs) in human cancers. Clin Cancer Res. 2019;25:1233–8. doi: 10.1158/1078-0432.ccr-18-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei SC, Anang NAS, Sharma R, Andrews MC, Reuben A, Levine JH, et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U S A. 2019;116:22699–709. doi: 10.1073/pnas.1821218116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y, Lee CK, Lin CH, Gassen RB, Xu X, Huang Z, et al. PD-L1:CD80 Cis-Heterodimer triggers the Co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity. 2019;51:1059–73.e9. doi: 10.1016/j.immuni.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sainson RCA, Thotakura AK, Kosmac M, Borhis G, Parveen N, Kimber R, et al. An antibody targeting ICOS increases intratumoral cytotoxic to regulatory T-cell ratio and induces tumor regression. Cancer Immunol Res. 2020;8:1568–82. doi: 10.1158/2326-6066.cir-20-0034. [DOI] [PubMed] [Google Scholar]
- 91.Chester C, Ambulkar S, Kohrt HE. 4-1BB agonism: adding the accelerator to cancer immunotherapy. Cancer Immunol Immunother. 2016;65:1243–8. doi: 10.1007/s00262-016-1829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartkowiak T, Curran MA. 4-1BB agonists: multi-potent potentiators of tumor immunity. Front Oncol. 2015;5:117. doi: 10.3389/fonc.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/nejmoa063842. [DOI] [PubMed] [Google Scholar]
- 94.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 95.Segal NH, Logan TF, Hodi FS, McDermott D, Melero I, Hamid O, et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin Cancer Res. 2017;23:1929–36. doi: 10.1158/1078-0432.ccr-16-1272. [DOI] [PubMed] [Google Scholar]
- 96.Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the CD47/SIRPalpha Axis. Front Immunol. 2020;11:18. doi: 10.3389/fimmu.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–99. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110:11103–8. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–15. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pietsch EC, Dong J, Cardoso R, Zhang X, Chin D, Hawkins R, et al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. 2017;7:e536. doi: 10.1038/bcj.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiskopf K, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science. 2013;341:88–91. doi: 10.1126/science.1238856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piccione EC, Juarez S, Liu J, Tseng S, Ryan CE, Narayanan C, et al. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. mAbs. 2015;7:946–56. doi: 10.1080/19420862.2015.1062192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X, Liu L, Ren Z, Yang K, Xu H, Luan Y, et al. Dual targeting of innate and adaptive checkpoints on tumor cells limits immune evasion. Cell Rep. 2018;24:2101–11. doi: 10.1016/j.celrep.2018.07.062. [DOI] [PubMed] [Google Scholar]
- 105.Lin H, Kryczek I, Li S, Green MD, Ali A, Hamasha R, et al. Stanniocalcin 1 is a phagocytosis checkpoint driving tumor immune resistance. Cancer Cell. 2021;39:480–93 e6. doi: 10.1016/j.ccell.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody-drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18:3–19. doi: 10.1158/1541-7786.mcr-19-0582. [DOI] [PubMed] [Google Scholar]
- 107.Dean AQ, Luo S, Twomey JD, Zhang B. Targeting cancer with antibody-drug conjugates: promises and challenges. mAbs. 2021;13:1951427. doi: 10.1080/19420862.2021.1951427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35:e00225. doi: 10.1042/BSR20150089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su D, Zhang D. Linker design impacts antibody-drug conjugate pharmacokinetics and efficacy via modulating the stability and payload release efficiency. Front Pharmacol. 2021;12:687926. doi: 10.3389/fphar.2021.687926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–41. doi: 10.1056/nejmoa2028485. [DOI] [PubMed] [Google Scholar]
- 111.Ocana A, Pandiella A. Proteolysis targeting chimeras (PROTACs) in cancer therapy. J Exp Clin Cancer Res. 2020;39:189. doi: 10.1186/s13046-020-01672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maneiro MA, Forte N, Shchepinova MM, Kounde CS, Chudasama V, Baker JR, et al. Antibody-PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem Biol. 2020;15:1306–12. doi: 10.1021/acschembio.0c00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ellenhorn JD, Hirsch R, Schreiber H, Bluestone JA. In vivo administration of anti-CD3 prevents malignant progressor tumor growth. Science. 1988;242:569–71. doi: 10.1126/science.2902689. [DOI] [PubMed] [Google Scholar]
- 114.Ma J, Mo Y, Tang M, Shen J, Qi Y, Zhao W, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi: 10.3389/fimmu.2021.626616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93:290–6. doi: 10.1038/icb.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shah K, Al-Haidari A, Sun J, JU K. T cell receptor (TCR) signaling in health and disease. Signal Transduct Targeted Ther. 2021;6:412. doi: 10.1038/s41392-021-00823-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsoukas CD, Landgraf B, Bentin J, Valentine M, Lotz M, Vaughan JH, et al. Activation of resting T lymphocytes by anti-CD3 (T3) antibodies in the absence of monocytes. J Immunol. 1985;135:1719–23. doi: 10.4049/jimmunol.135.3.1719. [DOI] [PubMed] [Google Scholar]
- 118.Gaglia J, Kissler S. Anti-CD3 antibody for the prevention of type 1 diabetes: a story of perseverance. Biochemistry. 2019;58:4107–11. doi: 10.1021/acs.biochem.9b00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goebeler ME, Bargou RC. T cell-engaging therapies - BiTEs and beyond. Nat Rev Clin Oncol. 2020;17:418–34. doi: 10.1038/s41571-020-0347-5. [DOI] [PubMed] [Google Scholar]
- 120.Tian Z, Liu M, Zhang Y, Wang X. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J Hematol Oncol. 2021;14:75. doi: 10.1186/s13045-021-01084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Przepiorka D, Ko CW, Deisseroth A, Yancey CL, Candau-Chacon R, Chiu HJ, et al. FDA approval: blinatumomab. Clin Cancer Res. 2015;21:4035–9. doi: 10.1158/1078-0432.ccr-15-0612. [DOI] [PubMed] [Google Scholar]
- 122.Leclercq G, Servera LA, Danilin S, Challier J, Steinhoff N, Bossen C, et al. Dissecting the mechanism of cytokine release induced by T-cell engagers highlights the contribution of neutrophils. OncoImmunology. 2022;11:2039432. doi: 10.1080/2162402x.2022.2039432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vafa O, Trinklein ND. Perspective: designing T-cell engagers with better therapeutic windows. Front Oncol. 2020;10:446. doi: 10.3389/fonc.2020.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–21. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196–206. doi: 10.1056/nejmoa2103485. [DOI] [PubMed] [Google Scholar]
- 126.Duan Z, Ho M. Targeting the cancer neoantigens p53 and KRAS with TCR mimic antibodies. Antib Ther. 2021;4:208–11. doi: 10.1093/abt/tbab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Budde LE, Assouline S, Sehn LH, Schuster SJ, Yoon SS, Yoon DH, et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J Clin Oncol. 2022;40:481–91. doi: 10.1200/jco.21.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moreau P, Garfall AL, van de Donk N, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387:495–505. doi: 10.1056/nejmoa2203478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Middelburg J, Kemper K, Engelberts P, Labrijn AF, Schuurman J, van Hall T. Overcoming challenges for CD3-bispecific antibody therapy in solid tumors. Cancers. 2021;13:287. doi: 10.3390/cancers13020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Belmontes B, Sawant DV, Zhong W, Tan H, Kaul A, Aeffner F, et al. Immunotherapy combinations overcome resistance to bispecific T cell engager treatment in T cell-cold solid tumors. Sci Transl Med. 2021;13:eabd1524. doi: 10.1126/scitranslmed.abd1524. [DOI] [PubMed] [Google Scholar]
- 131.Hummel HD, Kufer P, Grullich C, Seggewiss-Bernhardt R, Deschler-Baier B, Chatterjee M, et al. Pasotuxizumab, a BiTE((R)) immune therapy for castration-resistant prostate cancer: phase I, dose-escalation study findings. Immunotherapy. 2021;13:125–41. doi: 10.2217/imt-2020-0256. [DOI] [PubMed] [Google Scholar]
- 132.Haense N, Atmaca A, Pauligk C, Steinmetz K, Marme F, Haag GM, et al. A phase I trial of the trifunctional anti Her2 x anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Cancer. 2016;16:420. doi: 10.1186/s12885-016-2449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li J, Piskol R, Ybarra R, Chen YJ, Li J, Slaga D, et al. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci Transl Med. 2019;11:eaax8861. doi: 10.1126/scitranslmed.aax8861. [DOI] [PubMed] [Google Scholar]
- 134.Bacac M, Colombetti S, Herter S, Sam J, Perro M, Chen S, et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24:4785–97. doi: 10.1158/1078-0432.ccr-18-0455. [DOI] [PubMed] [Google Scholar]
- 135.Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, et al. Anti-CD20/CD3 T cell-dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med. 2015;7:287ra70. doi: 10.1126/scitranslmed.aaa4802. [DOI] [PubMed] [Google Scholar]
- 136.Liu L, Chen J, Bae J, Li H, Sun Z, Moore C, et al. Rejuvenation of tumour-specific T cells through bispecific antibodies targeting PD-L1 on dendritic cells. Nat Biomed Eng. 2021;5:1261–73. doi: 10.1038/s41551-021-00800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Benonisson H, Altintas I, Sluijter M, Verploegen S, Labrijn AF, Schuurhuis DH, et al. CD3-Bispecific antibody therapy turns solid tumors into inflammatory sites but does not install protective memory. Mol Cancer Therapeut. 2019;18:312–22. doi: 10.1158/1535-7163.mct-18-0679. [DOI] [PubMed] [Google Scholar]
- 138.Skokos D, Waite JC, Haber L, Crawford A, Hermann A, Ullman E, et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci Transl Med. 2020;12:eaaw7888. doi: 10.1126/scitranslmed.aaw7888. [DOI] [PubMed] [Google Scholar]
- 139.Wu L, Seung E, Xu L, Rao E, Lord DM, Wei RR, et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat Can (Que) 2020;1:86–98. doi: 10.1038/s43018-019-0004-z. [DOI] [PubMed] [Google Scholar]
- 140.Moores SL, Chiu ML, Bushey BS, Chevalier K, Luistro L, Dorn K, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76:3942–53. doi: 10.1158/0008-5472.can-15-2833. [DOI] [PubMed] [Google Scholar]
- 141.Geuijen CAW, De Nardis C, Maussang D, Rovers E, Gallenne T, Hendriks LJA, et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2-guided ligand blockade. Cancer Cell. 2018;33:922–36.e10. doi: 10.1016/j.ccell.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 142.Kraman M, Faroudi M, Allen NL, Kmiecik K, Gliddon D, Seal C, et al. FS118, a bispecific antibody targeting LAG-3 and PD-L1, enhances T-cell activation resulting in potent antitumor activity. Clin Cancer Res. 2020;26:3333–44. doi: 10.1158/1078-0432.ccr-19-3548. [DOI] [PubMed] [Google Scholar]
- 143.Dovedi SJ, Elder MJ, Yang C, Sitnikova SI, Irving L, Hansen A, et al. Design and efficacy of a monovalent bispecific PD-1/CTLA4 antibody that enhances CTLA4 blockade on PD-1(+) activated T cells. Cancer Discov. 2021;11:1100–17. doi: 10.1158/2159-8290.cd-20-1445. [DOI] [PubMed] [Google Scholar]
- 144.Peper-Gabriel JK, Pavlidou M, Pattarini L, Morales-Kastresana A, Jaquin TJ, Gallou C, et al. The PD-L1/4-1BB bispecific antibody-anticalin fusion protein PRS-344/S095012 elicits strong T-cell stimulation in a tumor-localized manner. Clin Cancer Res. 2022;28:3387–99. doi: 10.1158/1078-0432.ccr-21-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hinner MJ, Aiba RSB, Jaquin TJ, Berger S, Durr MC, Schlosser C, et al. Tumor-localized costimulatory T-cell engagement by the 4-1BB/HER2 bispecific antibody-anticalin fusion PRS-343. Clin Cancer Res. 2019;25:5878–89. doi: 10.1158/1078-0432.ccr-18-3654. [DOI] [PubMed] [Google Scholar]
- 146.Muller D, Frey K, Kontermann RE. A novel antibody-4-1BBL fusion protein for targeted costimulation in cancer immunotherapy. J Immunother. 2008;31:714–22. doi: 10.1097/cji.0b013e31818353e9. [DOI] [PubMed] [Google Scholar]
- 147.Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-beta. Sci Transl Med. 2018;10:eaan5488. doi: 10.1126/scitranslmed.aan5488. [DOI] [PubMed] [Google Scholar]
- 148.Lode HN, Reisfeld RA. Targeted cytokines for cancer immunotherapy. Immunol Res. 2000;21:279–88. doi: 10.1385/ir:21:2-3:279. [DOI] [PubMed] [Google Scholar]
- 149.Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Solal-Celigny P, Lepage E, Brousse N, Reyes F, Haioun C, Leporrier M, et al. Recombinant interferon alfa-2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. Groupe d’Etude des Lymphomes de l’Adulte. N Engl J Med. 1993;329:1608–14. doi: 10.1056/nejm199311253292203. [DOI] [PubMed] [Google Scholar]
- 151.Murer P, Neri D. Antibody-cytokine fusion proteins: a novel class of biopharmaceuticals for the therapy of cancer and of chronic inflammation. Nat Biotechnol. 2019;52:42–53. doi: 10.1016/j.nbt.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Balza E, Mortara L, Sassi F, Monteghirfo S, Carnemolla B, Castellani P, et al. Targeted delivery of tumor necrosis factor-alpha to tumor vessels induces a therapeutic T cell-mediated immune response that protects the host against syngeneic tumors of different histologic origin. Clin Cancer Res. 2006;12:2575–82. doi: 10.1158/1078-0432.ccr-05-2448. [DOI] [PubMed] [Google Scholar]
- 153.Gillies SD, Lan Y, Williams S, Carr F, Forman S, Raubitschek A, et al. An anti-CD20-IL-2 immunocytokine is highly efficacious in a SCID mouse model of established human B lymphoma. Blood. 2005;105:3972–8. doi: 10.1182/blood-2004-09-3533. [DOI] [PubMed] [Google Scholar]
- 154.Pasche N, Wulhfard S, Pretto F, Carugati E, Neri D. The antibody-based delivery of interleukin-12 to the tumor neovasculature eradicates murine models of cancer in combination with paclitaxel. Clin Cancer Res. 2012;18:4092–103. doi: 10.1158/1078-0432.ccr-12-0282. [DOI] [PubMed] [Google Scholar]
- 155.Yang X, Zhang X, Fu ML, Weichselbaum RR, Gajewski TF, Guo Y, et al. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell. 2014;25:37–48. doi: 10.1016/j.ccr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liao J, Luan Y, Ren Z, Liu X, Xue D, Xu H, et al. Converting lymphoma cells into potent antigen-presenting cells for interferon-induced tumor regression. Cancer Immunol Res. 2017;5:560–70. doi: 10.1158/2326-6066.cir-16-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xuan C, Steward KK, Timmerman JM, Morrison SL. Targeted delivery of interferon-alpha via fusion to anti-CD20 results in potent antitumor activity against B-cell lymphoma. Blood. 2010;115:2864–71. doi: 10.1182/blood-2009-10-250555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Becker JC, Pancook JD, Gillies SD, Furukawa K, Reisfeld RA. T cell-mediated eradication of murine metastatic melanoma induced by targeted interleukin 2 therapy. J Exp Med. 1996;183:2361–6. doi: 10.1084/jem.183.5.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gutbrodt KL, Casi G, Neri D. Antibody-based delivery of IL2 and cytotoxics eradicates tumors in immunocompetent mice. Mol Cancer Therapeut. 2014;13:1772–6. doi: 10.1158/1535-7163.MCT-14-0105. [DOI] [PubMed] [Google Scholar]
- 161.Eigentler TK, Weide B, de Braud F, Spitaleri G, Romanini A, Pflugfelder A, et al. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17:7732–42. doi: 10.1158/1078-0432.ccr-11-1203. [DOI] [PubMed] [Google Scholar]
- 162.Sun Z, Ren Z, Yang K, Liu Z, Cao S, Deng S, et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8(+) T-cell response and effective tumor control. Nat Commun. 2019;10:3874. doi: 10.1038/s41467-019-11782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qiao J, Liu Z, Dong C, Luan Y, Zhang A, Moore C, et al. Targeting tumors with IL-10 prevents dendritic cell-mediated CD8(+) T cell apoptosis. Cancer Cell. 2019;35:901–15 e4. doi: 10.1016/j.ccell.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 164.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–96. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 165.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 166.Conroy M, Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun. 2022;13:392. doi: 10.1038/s41467-022-27960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mahato R, Tai W, Cheng K. Prodrugs for improving tumor targetability and efficiency. Adv Drug Deliv Rev. 2011;63:659–70. doi: 10.1016/j.addr.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vasiljeva O, Hostetter DR, Moore SJ, Winter MB. The multifaceted roles of tumor-associated proteases and harnessing their activity for prodrug activation. Biol Chem. 2019;400:965–77. doi: 10.1515/hsz-2018-0451. [DOI] [PubMed] [Google Scholar]
- 169.Drag M, Salvesen GS. Emerging principles in protease-based drug discovery. Nat Rev Drug Discov. 2010;9:690–701. doi: 10.1038/nrd3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pai CS, Simons DM, Lu X, Evans M, Wei J, Wang YH, et al. Tumor-conditional anti-CTLA4 uncouples antitumor efficacy from immunotherapy-related toxicity. J Clin Invest. 2019;129:349–63. doi: 10.1172/JCI123391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Donaldson JM, Kari C, Fragoso RC, Rodeck U, Williams JC. Design and development of masked therapeutic antibodies to limit off-target effects: application to anti-EGFR antibodies. Cancer Biol Ther. 2009;8:2147–52. doi: 10.4161/cbt.8.22.9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kavanaugh WM. Antibody prodrugs for cancer. Expet Opin Biol Ther. 2020;20:163–71. doi: 10.1080/14712598.2020.1699053. [DOI] [PubMed] [Google Scholar]
- 173.Desnoyers LR, Vasiljeva O, Richardson JH, Yang A, Menendez EE, Liang TW, et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci Transl Med. 2013;5:207ra144. doi: 10.1126/scitranslmed.3006682. [DOI] [PubMed] [Google Scholar]
- 174.Chen IJ, Chuang CH, Hsieh YC, Lu YC, Lin WW, Huang CC, et al. Selective antibody activation through protease-activated pro-antibodies that mask binding sites with inhibitory domains. Sci Rep. 2017;7:11587. doi: 10.1038/s41598-017-11886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang Y, Guo Q, Chen X, Zhang J, Guo H, Qian W, et al. Preclinical studies of a Pro-antibody-drug conjugate designed to selectively target EGFR-overexpressing tumors with improved therapeutic efficacy. mAbs. 2016;8:405–13. doi: 10.1080/19420862.2015.1127491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Naing A, Thistlethwaite F, De Vries EGE, Eskens F, Uboha N, Ott PA, et al. CX-072 (pacmilimab), a Probody ((R)) PD-L1 inhibitor, in advanced or recurrent solid tumors (PROCLAIM-CX-072): an open-label dose-finding and first-in-human study. J Immunother Cancer. 2021;9:e002447. doi: 10.1136/jitc-2021-002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952–6. doi: 10.1001/jamaoncol.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37:2730–7. doi: 10.1200/jco.19.00318. [DOI] [PubMed] [Google Scholar]
- 179.Yin L, Wang XJ, Chen DX, Liu XN, Wang XJ. Humanized mouse model: a review on preclinical applications for cancer immunotherapy. Am J Cancer Res. 2020;10:4568–84. [PMC free article] [PubMed] [Google Scholar]
- 180.Gogesch P, Dudek S, van Zandbergen G, Waibler Z, Anzaghe M. The role of Fc receptors on the effectiveness of therapeutic monoclonal antibodies. Int J Mol Sci. 2021;22:8947. doi: 10.3390/ijms22168947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schlothauer T, Herter S, Koller CF, Grau-Richards S, Steinhart V, Spick C, et al. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng Des Sel. 2016;29:457–66. doi: 10.1093/protein/gzw040. [DOI] [PubMed] [Google Scholar]
- 182.Carpenter PA, Pavlovic S, Tso JY, Press OW, Gooley T, Yu XZ, et al. Non-Fc receptor-binding humanized anti-CD3 antibodies induce apoptosis of activated human T cells. J Immunol. 2000;165:6205–13. doi: 10.4049/jimmunol.165.11.6205. [DOI] [PubMed] [Google Scholar]
- 183.Saka K, Kakuzaki T, Metsugi S, Kashiwagi D, Yoshida K, Wada M, et al. Antibody design using LSTM based deep generative model from phage display library for affinity maturation. Sci Rep. 2021;11:5852. doi: 10.1038/s41598-021-85274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Akbar R, Bashour H, Rawat P, Robert PA, Smorodina E, Cotet TS, et al. Progress and challenges for the machine learning-based design of fit-for-purpose monoclonal antibodies. mAbs. 2022;14:2008790. doi: 10.1080/19420862.2021.2008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bacac M, Klein C, Umana P, CEA TCB. A novel head-to-tail 2:1 T cell bispecific antibody for treatment of CEA-positive solid tumors. OncoImmunology. 2016;5:e1203498. doi: 10.1080/2162402x.2016.1203498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Seung E, Xing Z, Wu L, Rao E, Cortez-Retamozo V, Ospina B, et al. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. Nature. 2022;603:328–34. doi: 10.1038/s41586-022-04439-0. [DOI] [PubMed] [Google Scholar]
- 187.He X, Li J, An S, Jiang C. pH-sensitive drug-delivery systems for tumor targeting. Ther Deliv. 2013;4:1499–510. doi: 10.4155/tde.13.120. [DOI] [PubMed] [Google Scholar]
- 188.Navya PN, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, Daima HK. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019;6:23. doi: 10.1186/s40580-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu X, Li T, Shen S, Wang J, Abdou P, Gu Z, et al. Advances in engineering cells for cancer immunotherapy. Theranostics. 2019;9:7889–905. doi: 10.7150/thno.38583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nguyen AT, Shiao SL, McArthur HL. Advances in combining radiation and immunotherapy in breast cancer. Clin Breast Cancer. 2021;21:143–52. doi: 10.1016/j.clbc.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vos JL, Elbers JBW, Krijgsman O, Traets JJH, Qiao X, van der Leun AM, et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat Commun. 2021;12:7348. doi: 10.1038/s41467-021-26472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sam J, Colombetti S, Fauti T, Roller A, Biehl M, Fahrni L, et al. Combination of T-cell bispecific antibodies with PD-L1 checkpoint inhibition elicits superior anti-tumor activity. Front Oncol. 2020;10:575737. doi: 10.3389/fonc.2020.575737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hailemichael Y, Johnson DH, Abdel-Wahab N, Foo WC, Bentebibel SE, Daher M, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. 2022;40:509–23.e6. doi: 10.1016/j.ccell.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]