Abstract
Yeast and humans share thousands of genes despite a billion years of evolutionary divergence. While many human genes can functionally replace their yeast counterparts, nearly half of the tested shared genes cannot. For example, most yeast proteasome subunits are “humanizable,” except subunits comprising the β-ring core, including β2c (HsPSMB7, a constitutive proteasome subunit). We developed a high-throughput pipeline to humanize yeast proteasomes by generating a large library of Hsβ2c mutants and screening them for complementation of a yeast β2 (ScPup1) knockout. Variants capable of replacing ScPup1 included (1) those impacting local protein–protein interactions (PPIs), with most affecting interactions between the β2c C-terminal tail and the adjacent β3 subunit, and (2) those affecting β2c proteolytic activity. Exchanging the full-length tail of human β2c with that of ScPup1 enabled complementation. Moreover, wild-type human β2c could replace yeast β2 if human β3 was also provided. Unexpectedly, yeast proteasomes bearing a catalytically inactive HsPSMB7-T44A variant that blocked precursor autoprocessing were viable, suggesting an intact propeptide stabilizes late assembly intermediates. In contrast, similar modifications in human β2i (HsPSMB10), an immunoproteasome subunit and the co-ortholog of yeast β2, do not enable complementation in yeast, suggesting distinct interactions are involved in human immunoproteasome core assembly. Broadly, our data reveal roles for specific PPIs governing functional replaceability across vast evolutionary distances.
Keywords: humanized yeast, suppressor screen, proteasome core assembly, orthology, functional replaceability or replacement–complement, trypsin-like activity
Introduction
Despite the divergence of humans and yeast from a common ancestor over a billion years ago, Saccharomyces cerevisiae (yeast) still shares nearly a third of its proteome with humans (Remm et al. 2001; Sonnhammer and Östlund 2015). Systematic studies have discovered many conserved human genes that complement a lethal growth defect conferred by the loss of the corresponding shared yeast gene, indicating functional conservation (Hamza et al. 2015, 2020; Kachroo et al. 2015, 2017; Sun et al. 2016; Yang et al. 2017; Garge et al. 2020; Laurent et al. 2020). Therefore, the replacement-competent human genes can assimilate within the yeast genetic and protein–protein interaction (PPI) networks. However, regardless of their identification as clear orthologs, more than 50% of the tested human genes could not replace the function of the yeast orthologs (Kachroo et al. 2015; Laurent et al. 2020). There could be several reasons for this lack of replaceability, such as the divergence of crucial amino acid residues or domains required for maintaining function in the yeast orthologs by coevolution with the rest of the cellular machinery, altered expression from constitutive promoters (Kachroo et al. 2017), or simply a change in function. The third mechanism is unlikely for most proteins in deeply conserved complexes and pathways such as DNA replication, transcription and translation, and proteasome-mediated protein degradation (Dolinski and Botstein 2007; Kachroo et al. 2022). For example, nearly all subunits of the yeast proteasome complex are individually replaceable by their human counterparts except for 2 “sub-modules,” a 5-subunit contiguous subset of the hetero-heptameric β-ring of the proteasome core (Supplementary Fig. 1a) and a pair of interacting proteins (Rpn3 and Rpn12) in the lid subcomplex (Kachroo et al. 2015).
The proteasome is a highly conserved molecular machine found in all domains of life. It is primarily responsible for selective protein degradation (Maupin-Furlow 2011). The eukaryotic 26S proteasome complex comprises 2 subcomplexes—the 19S regulatory particle (RP) and the 20S core particle (CP) (Supplementary Fig. 1a) (Tomko and Hochstrasser 2013). The 20S CP comprises 4 stacked heptameric rings. Among the 4 rings, the 2 outer rings bear 7 different α subunits, and the 2 inner rings each have 7 distinct β subunits. Three proteolytically active protease subunits are present in each β-ring, specifically β1, β2, and β5, which are responsible for catalyzing substrate cleavage (Groll et al. 1999) (Supplementary Fig. 1a). These subunits each harbor a catalytic threonine at their N-terminus that functions as the attacking nucleophile in peptide bond hydrolysis, but they are initially synthesized as inactive precursors. Activation occurs by autocatalytic N-terminal propeptide cleavage, which is highly regulated and is initiated only after successful CP assembly, thus only generating the active-site threonine residues after they are inside the CP central chamber (Chen and Hochstrasser 1996; Tanaka 2009). Notably, none of the human subunits requiring propeptide cleavage (β1, β2, β5, β6, and β7) can substitute for their yeast orthologs (Supplementary Fig. 1a).
Assembly of the proteasome core occurs through a combination of the self-assembly of subunits guided by the N-terminal and C-terminal extensions of specific subunits and external assembly chaperones (Tomko and Hochstrasser 2013). Subunits are added sequentially during the assembly process, creating a series of detectable intermediates (Ramos and Dohmen 2008). The incorporation of β subunits usually follows α-ring assembly, starting with the β2 subunit, at least in mammalian cells (Hirano et al. 2008). A unique C-terminal extension of β2 is crucial for viability and wraps around β3 and interacts with β4, followed by the incorporation of β5, β6, β1 and, lastly, β7 (Budenholzer et al. 2017).
Furthermore, humans possess duplicated gene copies encoding some β-CP subunits (2 copies each for β1 and β2 and β3 paralogs for β5 subunits). In the case of the constitutive proteasome, present in most human tissues, the CP utilizes the catalytic subunits β1c (HsPSMB6), β2c (HsPSMB7) and β5c (HsPSMB5). In contrast, human lymphoid tissues make an alternate “immunoproteasome” CP, substituting constitutive proteasome subunits with their paralogs β1i (HsPSMB9), β2i (HsPSMB10), and β5i (HsPSMB8) or β5t (HsPSMB11), respectively (Kloetzel 2001). The human and yeast β subunit orthologs have diverged relative to 1 other (such as Hsβ2c-Hsβ2i, 56% identity; Hsβ2c-Scβ2, 52%; and Hsβ2i-Scβ2, 44%). Yeast and human proteasome core subunits generally share only ∼50% identical residues, even at successfully humanized protein interfaces (Kachroo et al. 2015), making it challenging to pinpoint critical differences facilitating complementation.
Given the remarkably high conservation of proteasome complex genes, structure, and assembly mechanisms between humans and yeast, it is puzzling that most yeast β subunits are not functionally replaceable by their human counterparts (Supplementary Fig. 1a). We hypothesized that the replacement-incompetent human subunits might still be sufficiently functionally similar to their yeast counterparts to create human gene variants that enable replaceability. Indeed, a previous screen identified a single mutation (S214G) in the human β2c subunit conferring functional replaceability (Kachroo et al. 2015) (Supplementary Fig. 1b). However, the basis of the functional replacement is unclear, mainly since this serine residue is present in yeast (Ser200) and other orthologs, except in the co-ortholog HsPSMB10 (β2i), which harbors a glycine (Gly210) instead (Supplementary Fig. 1c). The S214G mutation might restore local PPIs by promoting the assembly of the human protein into the yeast CP because of the proximity of Ser214 (Hsβ2c) to the neighboring yeast β6 subunit or by altering epistatic interactions that change the protein conformation to fit in yeast CP (Supplementary Fig. 1d).
To systematically address the role of suppressor mutations in human β2c to functionally replace the yeast subunit and identify incompatible interfaces, we devised a novel screen to obtain replacement-competent human gene variants in a high-throughput manner, providing insights into the likely mechanisms of replaceability of human β2 in yeast. We identified multiple replacement-competent variants. Structural modeling of the human mutants indicated at least 2 modes of suppression: (1) mutations close to the interacting surfaces of neighboring proteins in the complex suggested multiple PPIs critical to β-core assembly. Specifically, the mutations in the C-terminal tail extension of human β2c suggested the importance of PPIs with the β3 subunit for optimal assembly. (2) Mutations that affected the catalytic activity of the human β2c also enabled its assembly into the yeast proteasome core, presumably through retention of the entire or partially trimmed 43-residue propeptide.
To further characterize the role of the C-terminal extension mutations in functional replaceability, we showed that swapping the β2 C-terminal tail from yeast Pup1 to human PSMB7 enables functional replacement of the yeast counterpart. Biochemical analysis of the human PSMB7-T44A variant revealed a catalytically inactive subunit with no trypsin-like activity. By contrast, variants such as HsPSMB7-S214G and HsPSMB7-tail swap conferred trypsin-like activities comparable to the orthologous yeast protein within the proteasome. Furthermore, we show that similar modifications in the human immunoproteasome subunit and co-ortholog of the yeast Pup1, Hsβ2i (HsPSMB10), did not enable functional complementation in yeast, suggesting distinct requirements for immunoproteasome core assembly. Finally, the presence of the Hsβ3 subunit enabled functional complementation by wild-type human β2c, generating a doubly humanized Hsβ2β3 yeast strain. Our data demonstrate that the local protein–protein interfaces in the human and yeast β subunits have evolved in a species-specific manner and are critical for CP assembly. Restoring these orthogonal interactions enables the humanization of yeast proteasomes while unveiling novel mechanistic insights into human proteasome complex assembly.
Materials and methods
Strains and primers
All primers and strains used in this study are listed in Supplementary Tables 2 and 3.
Constructing a PSMB7 mutant gene library in a yeast expression vector
The PSMB7 mutant gene library was previously generated (Kachroo et al. 2015) by error-prone PCR (GeneMorph II Random Mutagenesis Kit from Agilent) to introduce mutations and add attL1 and attL2 sites at the 5′ and 3′ ends of the gene (Reece-Hoyes and Walhout 2018). The library was cloned using the LR cloning strategy into the expression vector pAG416GPD-ccdB (URA3, CEN6) where the PSMB7 alleles are under the control of the constitutive GPD (TDH3) promoter (Gateway LR Clonase II enzyme mix kit from Invitrogen). The conditions for the error-prone PCR were selected to introduce 1–4 mutations per Kbp.
Transformation and selection of replacement-competent human gene suppressors (HsPSMB7) in yeast
Competent cells for the heterozygous knockout (HetKO) yeast (PUP1/pup1Δ::kanMX) with a Magic Marker (MM) selection were made using the Frozen-EZ Yeast Transformation II Kit (Zymo Research). For maximum representation of mutant human gene clones transformed in the strain, the transformation was performed in larger scale (4×) than conventional methods, and the transformation mix was plated on Q-trays (Corning, 245 mm Square BioAssay Dish) containing synthetic medium [SD-Ura with G418 (200 µg/ml)]. The trays were incubated at 30°C for 2–3 days. Next, >1,000 single yeast colonies were picked using the QPix 460 colony picker. The single colonies were spotted on presporulation GNA medium (5% glucose, 3% Difco nutrient broth, and 1% Difco yeast extract) with G418 selection (200 µg/ml) in a 96-spot format and incubated at 30°C for 1–2 days.
To select for viable haploid yeast knockout strains, each colony from presporulation GNA medium was inoculated in 700 µL of liquid sporulation medium [0.1% potassium acetate (Sigma P1190) and 0.005% zinc acetate (Sigma Z0625) in 96-well deep well plates]. The mutant clones were incubated at room temperature (22–24°C) for 3–5 days while vigorously shaking at 230 rpm or by using a rotator. After confirming sporulation by bright-field microscopy, the spore mixes were plated on synthetic MM medium [-His -Arg -Leu -Ura +Can (60 µg/mL) with (yeast gene absent) or without G418 (yeast gene present) (200 µg/ml)] in a 96-well format and incubated at 30°C for 3–5 days. To further the growth of haploid yeast harboring replacement-competent HsPSMB7 mutants that grew similarly on MM-G418 and MM+G418, their corresponding spore mixes were diluted (1:20 dilution), plated on MM-G418 and MM+G418 petri plates, and incubated at 30°C for 3–5 days to obtain single colonies.
Human gene plasmid-dependency assays
To test the human gene dependency of viable haploid knockout yeast (pup1Δ::kanMX), the haploid spores that grew on MM+G418 medium were replica plated on synthetic medium containing 5-FOA (1 g/1L) from Thermo Fisher and uracil (50 mg/L) from Sigma Aldrich and incubated at 30°C for 1–2 days. Cells that did not grow on the 5-FOA medium were judged to be dependent on the plasmid-borne human gene variants for viability.
Plasmid preparation from yeast and Escherichia coli
Plasmids harboring the mutant human PSMB7 genes that passed the 5-FOA human gene dependency test were extracted from the original diploid HetKO strains. The cells were inoculated in YPD+G418 medium and incubated overnight at 30°C. The plasmids were extracted from yeast the following day using the QIAprep Spin Miniprep Kit. The plasmid yield from yeast was low. Therefore, the plasmids were transformed into E. coli and extracted from the resulting colonies using the QIAprep Spin Miniprep Kit.
Construction of HsPSMB7 and HsPSMB10 single-site mutants via site-directed mutagenesis
Single mutants were created using the Q5 Site-Directed Mutagenesis Kit from New England Biolabs. The primers for this experiment were designed using the software Geneious. We first created the wild-type human PSMB7 and PSMB10 entry clone in pDONR221 using the BP Gateway strategy followed by Sanger sequencing. Using the Q5 Site-Directed Mutagenesis Kit, primers were used to introduce specific single-nucleotide changes in the wild-type human genes cloned in the pDONR221 entry clone. The forward primer introduced a mutation, and in combination with a compatible reverse primer, the entire plasmid with a human gene was amplified. The linear plasmids were then treated with 3 enzymes from the kit: kinase, ligase, and DpnI to obtain circular human single mutant clones. Human point mutant clones were verified by restriction enzyme digestion (EcoRV and HindIII from New England Biolabs) followed by Sanger sequencing. The confirmed clones were moved into the yeast expression vector pAG416GPD-ccdB (URA3, CEN6) using the Gateway LR Clonase II enzyme mix kit.
Quantitative yeast growth assays and doubling time calculations
Yeast cells were inoculated in liquid SD-URA+G418 and grown overnight at 30°C. The yeast culture was inoculated in SD-URA+G418 at the initial OD (600 nm) of 0.01. The growth assay was performed with Biotek Synergy H1 plate reader for 48–72 h while continuously shaking at 282 rpm and measuring the OD600 at 20-min intervals. The OD600 measurements were then plotted to obtain growth curves for comparison using GraphPad Prism software version 9, San Diego, California, USA. The doubling times were calculated using inbuilt nonlinear regression and log of exponential growth curve analysis, and significance was calculated using unpaired t-parametric t-test in GraphPad Prism software.
Construction of human–yeast tail swap HsPSMB7 clones in a yeast expression vector
Pymol-based structural evaluation, multiple-sequence alignments, and human gene suppressor mutations were used to identify the C-terminal tails of the human and yeast β2 proteins. A common forward primer and 3 different reverse primers, each harboring a part of the Pup1 C-terminal tail region (3′ region) and a part of the PSMB7 and PSMB10 gene (5′ region), were designed and used to create different human–yeast hybrid genes by PCR (AccuPrime Pfx DNA polymerase from Invitrogen). The primers also add the attB1 and attB2 sites at the 5′ and 3′ ends of the PCR for cloning in pDONR221 entry clones using the BP cloning strategy. The unique reverse primers used for the construction of the tail-swap human PSMB7 or PSMB10 mutants are listed in Supplementary Table 1 (Supplementary file 1). The clones were confirmed by restriction digestion using EcoRV and HindIII (in the case of PSMB7) followed by Sanger sequencing. The verified hybrid human gene–yeast tail swap variants were cloned into the yeast expression vector pAG416GPD-ccdB (URA3, CEN6) by LR cloning (Gateway LR Clonase II enzyme mix kit from Invitrogen).
CRISPR-Cas9-based strategy to introduce a 3xFLAG tag at the endogenous RPN11 gene
To design synthetic guide (sg) RNAs targeting the yeast proteasome RPN11 gene, we used a built-in gRNA design tool in Geneious software (Kearse et al. 2012). We selected 2 guides with high ON-target and low OFF-target scores (Supplementary Fig. 5). The sgRNAs were synthesized as complementary oligos (IDT). After annealing the oligos, the 5′ and 3′ overhangs match the type IIS enzyme sites in a yeast expression vector pCAS9-GFPdo with sgRNA expression system (CEN6, G418) (Lee et al. 2015; Akhmetov et al. 2018). See Supplementary Table 1 (Supplementary file 1) for guide sequences and primers. Each sgRNA was cloned in the pCAS9-GFPdo expression vector using the Golden Gate strategy. The plasmid allows the expression of an sgRNA, Cas9 nuclease, and an auxotrophic (URA3) or antibiotic selection (Geneticin, Sigma) marker.
The Golden Gate reaction for cloning sgRNAs was performed in a 10 µl volume, with ∼20 fmol of annealed primer, 1 µl each of the BSA1 FD (fast digestion, Thermo) enzyme, 1 µl of T7 DNA ligase (NEB), and 1 µl of ATP (NEB), 1 µl of FD buffer (Thermo) and water to make up the volume. The Golden Gate reaction was performed in a PCR machine according to a previously published protocol (Akhmetov et al. 2018). The reaction mix was transformed into competent E. coli cells and plated on LB agar with kanamycin (50 µg/ml). Since the sgRNA primers exchange the GFP expression cassette, the correct clones were selected by screening for nonfluorescent colonies and verified by Sanger sequencing. The repair template for RPN11-3xFLAG was synthesized as a gBlock (IDT) with Golden Gate enzyme sites and cloned in pYTK001 and sequence-verified. The repair template was designed to harbor silent DNA sequence changes that allow efficient cloning in a vector using a Golden Gate reaction strategy (eliminates an internal enzyme site) while also carrying mutations in the sgRNA binding sites such that the engineered strains become resistant to DSBs (double-strand break) by CRISPR/Cas9 (Supplementary Fig. 6a).
Clones were initially screened using colony PCR and Phire plant direct master mix (Thermo). The forward primer for PCR screening was designed such that it binds outside of the ORF and homology used for HDR insertion of the repair template, and the reverse primer was designed to bind within the 3xFLAG tag. Following plasmid loss, clones were further verified by Sanger sequencing the entire RPN11 locus using primers outside of the homology used for HDR.
CRISPR-Cas9-based genome editing to introduce wild-type human genes or their variants at the corresponding native yeast loci
The sgRNAs to target yeast PUP1 and PUP3 loci were generated using Geneious and cloned in pCAS9-GFPdo as described above. See Supplementary Table 1 (Supplementary file 1) for guide sequences and primers. The human gene repair templates for wild-type HsPSMB7 (Hsβ2 subunit) and HsPSMB3 (Hsβ3) were synthesized as a gBlock with unique type IIS enzyme sites capable of generating distinct 4-base overhangs (IDT). To add native yeast locus homologous sequences, we amplified the 5′UTR (∼500 bp) and 3′UTR (∼150 bp) sequences of yeast PUP1 and PUP3 loci. The primers used to amplify the UTRs also harbor type IIS enzyme sites to clone (in YTK001) the UTRs with the corresponding human gene repair template gBlocks using Golden Gate reactions as described above. The clones were sequence-verified, BsaI-digested and directly used as repair templates for HDR. For HsPSMB7 variants, primers were designed with 80 bp homology to the 5′ and 3′ UTRs of the yeast loci. The high-fidelity Accuprime enzyme was used to amplify variants from pAG416GPD expression constructs. The PCR product was gel extracted and used as a repair template for HDR in yeast.
Western blotting to test expression of RPN11-3xFLAG tag in yeast
Yeast strains were grown to mid-exponential phase in YPD medium to ∼0.6 OD. The mixture was centrifuged for 3 min at 500 rpm followed by washing with 20 µl of 100 µM Tris–HCl (pH 8.0) containing a protease inhibitor cocktail (Millipore Sigma). The whole cell lysate was prepared by adding 200 µl of microbeads to the pellet and vortexed for 45 s followed by incubating on ice for 30 s. The procedure was repeated 5–8 times. The mixture was centrifuged at max speed (∼10,000 rpm, Eppendorf mini spin centrifuge) for 5 min. ∼20 µl of Laemmli sample buffer was added to the 100 µl of centrifuged supernatant and incubated at 65°C for 20 min. Twenty microliters of each sample were loaded on precast SDS-PAGE gels (Thermo) and transferred to activated 0.2 µm PVDF membrane (Millipore Sigma) for western blotting. RPN11-3xFLAG-tagged subunits were detected by monoclonal anti-FLAG antibodies (Monoclonal ANTI-FLAG M2 antibody #F3165, Millipore Sigma). The lysate from a wild-type strain with untagged RPN11 was used as a negative control. For secondary detection, an antimouse antibody was used (IRDye 800CW Goat anti-Mouse IgG Secondary Antibody, LI-COR) and imaged by Odyssey (LI-COR Odyssey 9120).
Yeast transformation
Yeast transformations were performed using the Frozen EZ Yeast Transformation II Kit from Zymo Research transformation according to the manufacturer's protocol.
Nondenaturing PAGE and SDS-PAGE of whole cell extracts
Yeast whole cell extracts were prepared as described previously (Li and Hochstrasser 2022). Mid-exponential phase yeast cells were washed twice with ice-cold sterile water and frozen in liquid nitrogen. The frozen cells were ground with mortar and pestle, and the resulting cell powder was thawed on ice and resuspended in an equal volume of extraction buffer (50 mM Tris–HCl, pH 7.5, 5 mM MgCl2, 10% glycerol, and 5 mM ATP). Extracts were centrifuged for 10 min at 21,000×g to remove cell debris. Protein concentrations were determined using a Pierce BCA protein assay kit (Thermo Scientific, catalog #23225, lot #SJ256254) according to the manufacturer's protocol. Equal amounts of total protein per sample (50 µg, except 10 µg for Pgk1) were loaded onto 4% native PAGE gels and resolved for 3 h at 100 V at 4°C for proteasome profile analysis. Alternatively, samples were resolved by 10% SDS-PAGE for analysis of HsPSMB7 expression and processing.
Native PAGE-separated proteins or SDS PAGE-separated proteins were then transferred to PVDF membranes (EMD Millipore, catalog #IPVH00010, lot #R1EB02212) and subjected to western blotting analysis as described previously (Li et al. 2016) with the following primary antibodies: rabbit anti-Pre6 (Jager et al. 2001) at 1:5000 dilution, rabbit anti-Rpn5 (a generous gift from Daniel Finley lab at Harvard University) at 1:5000 dilution, rabbit anti-Rpt5 (Enzo Life Sciences, catalog #PW8245, lot #Z01946) at 1:10,000 dilution, rabbit anti-HsPSMB7 (Novus Biologicals, catalog #NBP2-19954, lot #40275) at 1:1000 dilution, or an anti-Pgk1 monoclonal antibody (Abcam, catalog #ab113687, lot #GR3373682-5) at 1:10,000 dilution. Primary antibody binding was followed by antimouse-IgG (GE Healthcare, catalog #NXA931V, lot #17193521) or antirabbit-IgG (GE Healthcare, catalog #NA934V, lot #17212129) secondary antibody conjugated to horseradish peroxidase at the same dilution used with the primary antibodies. The membranes were incubated in ECL detection reagent (Mruk and Cheng 2011), and the ECL signals were detected using film (Thomas Scientific, catalog #1141J52).
Affinity purification of proteasomes and proteasome activity assays
Proteasomes were affinity-purified from yeast cells expressing Rpn11-3xFLAG as described previously (Li and Hochstrasser 2022). Briefly, about 7 ml of cell powder from the same samples as used above for nondenaturing PAGE analysis were thawed on ice, resuspended in 10 ml buffer A (50 mM Tris pH 7.5, 150 mM NaCl, 10% glycerol, 5 mM MgCl2, and 5 mM ATP, Roche complete EDTA-free protease inhibitor: catalog #11873580001, lot #53418500), and incubated for 15 min on ice. Cell debris was pelleted at 30,000×g for 20 min at 4°C. Total protein concentrations of the supernatants were determined using the BCA assay. Supernatant equivalent to ∼100 mg total protein was incubated with 200 µl (packed) resin of anti-FLAG M2 affinity gel (Sigma, catalog #A2220, lot #SLCH0130) for 2 h on a rotator at 4°C. The proteasome-bound resin was washed twice with 12 ml buffer A for 10 min and then incubated with 3 resin volumes of 200 µg·ml−1 3xFLAG peptide (Sigma, catalog #F4799, lot #SLCJ4916) for 45 min to elute proteasome complexes. Proteasomes were concentrated with 100 K MWCO centrifugal filters (Merk Millipore, catalog #UFC510024, lot #R1MB60377) and quantified with a BSA standard using a G:Box Chemi HR16 imager (Syngene).
Proteasome activity analyses were performed as previously described (Li et al. 2015), with minor modifications. 10 µg purified proteasomes were loaded onto 4% native PAGE gels and resolved for 3 h at 100 V at 4°C. The gels were incubated with developing buffer (50 mM Tris–HCl, pH 7.5, 5 mM MgCl2, 10% glycerol, and 1 mM ATP) containing 50 µM substrates Boc-LRR-AMC (Enzo Life Sciences, catalog #BML-BW8515-0005, lot #07232103) for trypsin-like activity or Suc-LLVY-AMC (Sigma, catalog #S6510, lot #BCBK9233V) for chymotrypsin-like activity analysis with a gentle shaking at 30 rpm for 30 min at 30°C. Gels were transferred to a UV trans-illuminator and exposed to 365 nm light in a G:Box Chemi HR16 imager (Syngene).
Structural analysis
Homology modeling of the human PSMB7 (Hsβ2; from PDB-1IRU) in the yeast 20S proteasome core (PDB-1RYP) or in 13S proteasome core (PDB-7LSX) was performed using Pymol (The Pymol molecular graphics system, version 2.5.2, Schrodinger, LLC). Some structures that show yeast proteasome core and humanized Hsβ2 and Hsβ3 were designed using ChimeraX (Pettersen et al. 2021).
HsPSMB10-GFP expression and visualization in yeast
The HsPSMB10 gene lacking stop codon was cloned into a gateway entry clone (pDONR221) following by cloning into the destination vector PAG416-GPD-ccdB-EGFP-Ura to create yeast expression vector using gateway LR cloning. The clones were verified by Sanger sequencing. The yeast strains were grown in selection conditions to maintain the plasmid (Ura+) and imaged (in log phase) using a DMi6000B microscope (Leica). The images were analyzed using ImageJ.
Results
A novel high-throughput pipeline to screen for yeast-complementing human gene variants
While successful, our previous screening strategy to identify replacement-competent human gene variants of the yeast gene was tedious and identified only 2 suppressors (Kachroo et al. 2015). The technique required manual isolation of colonies followed by tetrad dissection, and identification of suppressors required manual screening of hundreds of yeast colonies. Therefore, we developed a novel pipeline to screen for suppressor plasmids in an automated high-throughput manner (Fig. 1a. We specifically focused on screening for suppressor mutations in the noncomplementing human β2c (HsPSMB7) protein, a constitutively expressed proteasome subunit. The method allowed large-scale and error-free screening in a significantly shorter time. Assays were performed in a 96-well format and haploid-specific mutant isolation using synthetic genetic array (SGA) or MM selection to eliminate the need for tetrad dissection (Pan et al. 2004; Kuzmin et al. 2016). A plasmid-dependency assay using 5-FOA selection confirmed that complementation was associated with the variant human gene on the URA3-based plasmid. Using this strategy, we successfully obtained multiple replacement-competent suppressor mutations in the HsPSMB7 gene. Throughout the manuscript, we consistently use the terms “functional replacement” or “replaceability” or “replacement-competent” to refer to the capacity of the human gene or its variant to compensate for the function of its yeast ortholog.
Fig. 1.
High-throughput automated pipeline to screen for replacement-competent human gene suppressors in yeast. a) Workflow showing the generation and screening of a human gene mutant library made by error-prone PCR (0–4 mutations per kbp). The variant pool was cloned into the expression vector (CEN6, URA3) and transformed into the yeast heterozygous diploid knockout PUP1/pup1Δ::kanMX strain. b) Transformation of the mutant library was scaled (4×) and the mixture plated on a QTray. Individual colonies were picked by a QPix 460 colony-picking robot (up to 1,000 colonies) and spotted on a presporulation GNA-rich media followed by sporulation in a 96-well format. c) Each sporulation mix was spotted on MM medium with G418, indicating “the yeast gene ABSENT and human gene PRESENT condition,” for screening replacement-competent human gene variants. Alternatively, the sporulation mix plated without G418 “the yeast gene PRESENT or ABSENT and human gene PRESENT condition,” allows the growth of wild-type haploid yeast cells, serving as a sporulation efficiency control. For simplicity, we will refer to these conditions as “with (+)G418” to denote the “yeast gene ABSENT” condition and “without (−)G418” to denote the “yeast gene PRESENT condition.” The likely suppressors appear as spots growing in MM with G418, similar to the MM without G418 condition. d) Colonies growing on MM with G418 (yeast gene absent and human gene present condition) are further analyzed for plasmid-dependency assay using 5-fluoroorotic acid (5-FOA) selection against the URA3 plasmid. Representative examples show yeast strains with human gene suppressor that does not grow on a 5-FOA medium, indicating their plasmid dependence (suppressor 1, top panel) or growth on a 5-FOA medium, indicating plasmid independence (suppressor 2, bottom panel). Finally, each plasmid harboring a human gene suppressor was purified and retested to confirm functional replaceability and plasmid dependency, followed by Sanger sequencing to identify mutations in the human gene.
The screening pipeline was developed based on several considerations. Wild-type human PSMB7 (β2c) cannot functionally replace the orthologous yeast β2 gene, ScPUP1, as shown by the lethal phenotype observed when selecting for loss of the yeast gene (Supplementary Fig. 1b) (Kachroo et al. 2015). By contrast, if a human gene (or its variant) successfully replaces the function of the host gene, the strain will be able to grow, serving as a simple readout for functional replacement. The error-prone PCR strategy generated a HsPSMB7 mutant gene library, with an average of 1–4 mutations per gene, in a URA3-marked yeast expression vector (Fig. 1a). To determine if any mutant PSMB7 alleles can complement the lethality caused by deleting the orthologous yeast gene, we transformed the mutant library into a yeast diploid HetKO PUP1/pup1Δ::kanMX strain (Pan et al. 2004). The transformation protocol was scaled to obtain several thousand well-separated yeast colonies, each carrying a plasmid with a different PSMB7 mutant gene (Fig. 1b).
Several thousand colonies were picked in an automated manner using a QPix 460 robot and spotted in a 96-well format on the presporulation GNA medium with G418 selection for the kanMX-marked pup1Δ. After sporulation, MM medium (-Leu -Arg -His -Ura +CAN) allowed the selection of viable pup1Δ::kanMX haploid yeast spores (in the presence of G418) harboring plasmids with different human PSMB7 alleles (Fig. 1c, bottom panel). As an internal control for sporulation efficiency, we also tested the growth of wild-type PUP1 haploid spores on MM medium (in the absence of G418) (Fig. 1c, top panel). The screen identified 19 colonies that grew on MM+G418 (see representative images in Fig. 1d) that potentially carried complementing human PSMB7 variants. The haploid suppressor strains were then tested to determine if suppression was due to the presence of the plasmid-borne mutant human gene. The yeast cells were tested for plasmid dependency based on loss of growth on 5-FOA, which selects against the URA3 gene. Seven of the 19 suppressors did not survive on the 5-FOA medium, suggesting that the human gene variants in these strains were essential for their viability (Fig. 1d).
Identification and characterization of complementing human PSMB7 variants in yeast
To identify the relevant PSMB7 mutations and quantify pup1Δ complementation by the mutant human gene variants, we isolated the plasmid from each strain that had passed the test of plasmid dependency. The extracted human gene expression vectors were again tested for functional replaceability in a pup1Δ strain. Sanger sequencing of the 7 complementing human PSMB7 gene variants with each plasmid containing 1–4 mutations resulted in amino acid changes in the human protein. The screen also identified a previously characterized suppressor, HsPSMB7-S214G, further validating the functioning of the pipeline (Supplementary Fig. 2a and Table 1). Two independently isolated variants included an active-site Thr44Ala mutation (Table 1).
Table 1.
Summary of the human replacement-competent PSMB7 mutations.
| Primary suppressor (HSPSMB7) | Amino acid change(s) | Growth profile | Single-site mutant | Growth profile |
|---|---|---|---|---|
| Mutant 1 | Lys → Arg (K249R) and Arg → Trp (R32W) | +++ | K249R | ++± |
| R32W | No growth | |||
| Mutant 2 | Ser → Thr (S161T), Thr → Ile (T260I*) and Glu → Lys (E263K*) | +++± | S161T | + |
| T260I* | No growth | |||
| E263K* | No growth | |||
| Mutant 3 | Thr → Arg (T233R), Asp → Val (D96V) | +++± | T233R | +++ |
| D96V | No growth | |||
| Mutant 4 | Thr → Ala (T44A), Leu → His (L116H) | ++++ | T44A | ++++ |
| L116H | No growth | |||
| Mutant 5 | Thr → Ala (T44A), Glu → Lys (E26K), Met → Val (M67V) and Met → Val (N244D) | ++++ | T44A | ++++ |
| E26K | No growth | |||
| M67V | No Growth | |||
| N244D | No Growth | |||
| Mutant 6 | Ala → Val (A70V) | ++++ | N.A. | N.A. |
| Mutant 7 | Ser → Gly (S214G) | +++++ | N.A. | N.A. |
| Mutant 8CSSUPSTART#CSSUPEND | Ser → Gly (S214G) Gly → Cys (G211C) | +++++ | S214G | +++++ |
| G211C | No Growth | |||
| Mutant 9CSSUPSTART#CSSUPEND | Asp → Asn (D71N)* Glu → Stop (E263-Stop)* | +++ | D71N* | No Growth |
| E263-Stop* | No Growth |
The table shows the growth rescue of yeast pup1Δ by replacement-competent primary variants and the corresponding point mutants of human PSMB7. The quantification of the growth profile was performed using the days needed to grow on a selection medium as a readout. The wild-type yeast gene on a plasmid shows optimal growth (+++++). In contrast, human gene variants show variable growth pattern with Hs-PSMB7-S214G (Mutant 7) showing a comparable growth profile to the wild-type yeast and HsPSMB7-K249R/R32W (Mutant 1) showing a significant growth defect. The single-site variants and their comparative growth profile relative to the primary suppressors are indicated. As a comparison, we also show 2 previously obtained variants indicated as “#” (Kachroo et al. 2015). The mutations marked with an asterisk (*) do not independently enable functional replaceability; however, they contribute to improved complementation, which is observed as a growth phenotype. N.A. indicates “not applicable” as these primary variants were obtained as single-site substitutions.
All 7 original suppressors were confirmed for functional rescue using quantitative growth assays (Fig. 2 and Supplementary Fig. 2). We first performed growth assays on solid agar medium. As a control, we used the plasmid-borne cognate yeast gene (pGPD-ScPUP1) as a benchmark for optimal functional replacement (Fig. 2a). The pup1 knockout strain with pGPD-ScPUP1 formed colonies after 2–3 days of incubation at 30°C. Expression of the wild-type HsPSMB7 failed to complement the PUP1 deletion (Fig. 2b). By contrast, the isolated primary variants caused growth rescue (Fig. 2b, representative assay for the T44A/L116H variant). We tested all 7 of the primary HsPSMB7 variants by extending the incubation times up to 6 days, observing a variable level of growth (Figure S2A). Quantitative growth assays show that pup1Δ cells harboring pGPD-HsPSMB7 variants grow significantly slower than the pGPD-ScPUP1 strain, indicating partial complementation of Pup1 function (Fig. 2c).
Fig. 2.
Confirmation and quantitative growth analysis of replacement-competent human gene suppressors in yeast. a) Post-sporulation selection to grow haploids on MM medium with G418 (yeast gene ABSENT) or without G418 (yeast gene PRESENT) enables selection for functional replaceability. The expression of yeast PUP1 under the control of the constitutive GPD promoter functionally complements the growth defect of the pup1Δ::kanMX strain, whereas the empty vector does not allow growth. b) The expression of the wild-type human PSMB7 under the control of the GPD promoter does not rescue the lethality of the PUP1 deletion in MM+G418 (yeast gene ABSENT). A representative example shows the expression of human PSMB7 suppressor T44A-L116H that rescues the growth defect rescue of the pup1Δ::kanMX strain after 3–4 days of incubation at 30°C. c) Quantitative growth assays plotted as doubling times confirm the growth deficiency of primary human gene suppressors relative to the wild-type yeast Pup1 expressing strain. d) Quantitative growth assays plotted as doubling times show the comparative growth rates of single-site mutations in HsPSMB7 relative to their primary suppressors. The HsPSMB7-T44A variant alone shows comparable growth to its primary suppressors. By contrast, humanized yeast with HsPSMB7 single-site mutants T233R, S161T, and K249R show delayed growth compared to the corresponding primary suppressors, suggesting an accessory role of these other mutations in functional replaceability. The mean doubling times are plotted with standard deviation (N = 3). P-values are indicated as asterisks; ns, not significant; *, <0.05; **, <0.01; ***, <0.001; ****, <0.0001.
To determine which mutations from the original HsPSMB7 suppressor alleles individually or in combination contributed to the ability to replace yeast PUP1 function, we performed site-directed mutagenesis to generate single-site amino acid substitutions in wild-type HsPSMB7. Each HsPSMB7 point mutant was tested again using the previously established pipeline for functional replaceability (Fig. 2d and Supplementary Fig. 2b and c). Eight of 14 single mutants failed to complement PUP1 loss-of-function (Table 1). The remaining 6 single-site substitutions were identified as suppressors (K249R, S161T, T44A, T233R, A70V, and S214G) of the yeast pup1 deletion (Fig. 2c and d and Table 1). Quantitative growth assays in liquid cultures revealed that the HsPSMB7-T44A variant grew similarly to its corresponding primary suppressors (Fig. 2d). Thus, in the case of T44A-harboring primary suppressors, the additional mutations (L116H, E26K, M67V, and N224D) were incidental products of the random mutagenesis and were not required for yeast Pup1/β2 replacement. By contrast, 3 single-point mutant HsPSMB7 variants (S161T, T233R, and K249R) that complemented pup1Δ showed delayed growth on solid agar and in liquid medium relative to the respective primary suppressors from which they derive (Fig. 2d and Supplementary Fig. 2b). These data suggest accessory roles for the mutations in the original suppressors (R32W*, D71N*, D96V*, T260I*, E263K*, and E263-Stop*; the enhancing ancillary function is denoted by an asterisk) (Fig. 2d, Supplementary Fig. 2b and c, and Table 1).
It is important to note that our previous plate-based assays failed to identify the HsPSMB7-T44A mutant as a replacement-competent variant (Kachroo et al. 2015). This anomaly resulted from its slower growth rate on synthetic medium agar plates. However, given that we obtained 2 independent suppressors with the T44A mutation by incubating the HsPSMB7-T44A variant for longer times (additional ∼12 h), we conclude that this mutation allows human β2 to (partially) complement the loss of its yeast ortholog (Fig. 2b and Supplementary Fig. 2a and b).
Structural modeling links mutations at subunit interfaces to the functionality of HsPSMB7 in yeast
The replacement-competent and accessory mutations in HsPSMB7 were scattered across different regions of the protein with a cluster in the C-terminal tail (Fig. 3a). To explore how these mutated residues might facilitate complementation, we modeled the HsPSMB7 structure within the yeast proteasome core structure. The homology modeling allowed the classification of the mutations based on their proximity to the neighboring yeast proteasome subunits or epistatic interactions within the protein (Fig. 3b and Supplementary Fig. 3). A subset of the mutations was classified as likely to promote critical PPIs with neighboring yeast proteasome subunits (such as β1, β3, and β6) (Supplementary Fig. 3a, b, and d). For example, HsPSMB7-S214G is close to the β6 subunit in the apposed dyad-related β-ring (numbering for the precursor form) (Supplementary Fig. 1d). Additionally, Ser214, through its interactions with β2residues Thr233 and Asn236, may also affect the positioning of the C-terminal tail (Supplementary Figs. 1d and 3c). Similarly, the weakly complementing S161T mutation in HsPSMB7 is close to the β1 subunit interface (Supplementary Fig. 3a). By contrast, Thr233 mostly makes polar contacts with a stretch of highly conserved residues (Ser212, Gly213, and Ser214) within human β2, likely affecting the flexibility/conformation of the C-terminal tail (Supplementary Fig. 3c).
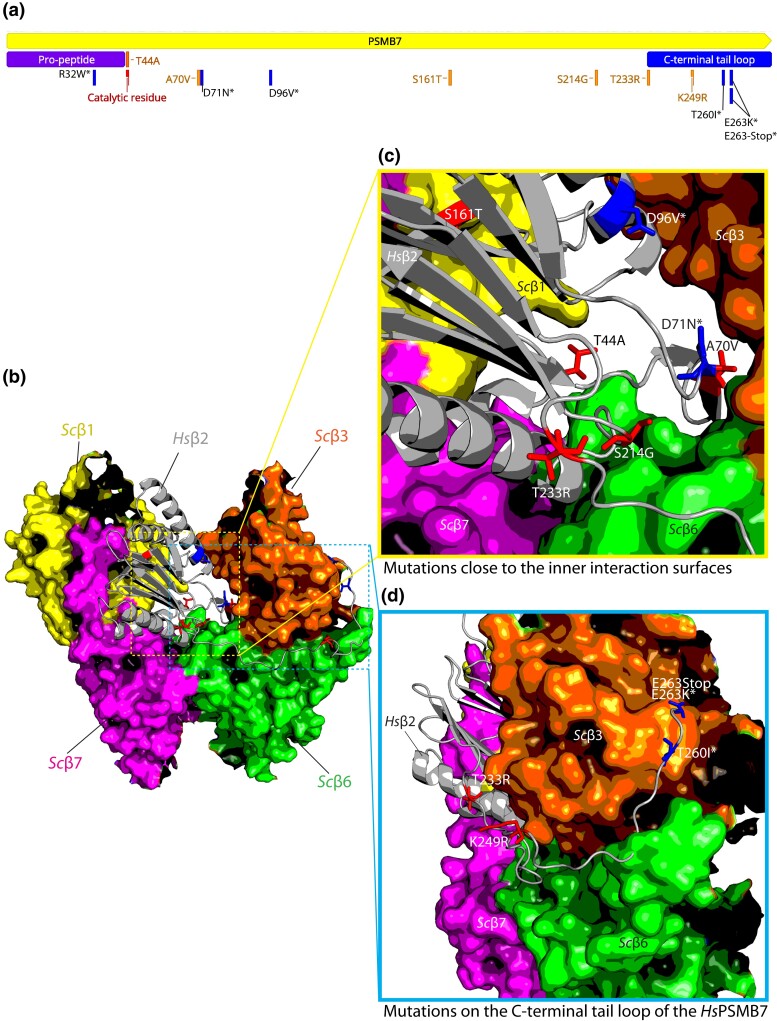
Fig. 3.
Modeling human PSMB7 variants in the yeast proteasome core suggests a role for specific protein–protein interactions in functional replacement of yeast Pup1. a) The schematic shows the suppressors in a linear map of HsPSMB7. The C-terminal tail domain is indicated in blue. The mutations with an asterisk (*) do not enable functional replaceability independently but are needed for better replaceability/growth. b) Human PSMB7 (Hsβ2, gray ribbon; PDB 1IRU) modeled into the yeast proteasome core structure (labelled and indicated as coloredsubunits in yellow, Scβ1; orange, Scβ3; pink, Scβ7; and green, Scβ6; PDB 1RYP). c) Amino acid changes contributing to functional replaceability are highlighted (single amino acid substitutions alone in red and the residues playing an accessory role in blue). An active-site substitution T44A in HsPSMB7 confers functional replaceability in yeast; however, it does not show proximity to any interacting subunit surface, whereas the remaining mutant residues are close to several subunit interfaces. d) Replacement-competent HsPSMB7 variants, including T233R and K249R and the substitutions (T260I*, E263K*, and E263-Stop*), reside on the C-terminal tail of the human protein that wraps around the neighboring yeast β3 protein.
The mutations clustered in the C-terminal tail of HsPSMB7 likely affect interactions with the neighboring yeast β3 subunit (Fig. 3d). The β2 C-terminal tail wraps around the outside of the β3 subunit in the same ring and also makes contact with the β6 subunit in the apposed C2 symmetry-related β-ring. Mutations in the HsPSMB7 C-terminal tail (K249R, T260I*, E263K*, and E263-Stop*) may impact its interactions with the Scβ3 outer surface (Supplementary Figs. 3d and 4), whereas nontail mutations such as A70V could affect Hsβ2c binding to Scβ3 or Scβ6 (Supplementary Fig. 3b). The unexpected active-site mutation (T44A) is not close to any interface and is predicted to lead to a catalytically dead Hsβ2c subunit with an incompletely processed propeptide due to a block in autocleavage (see below).
C-terminal tail swaps from yeast β2 to human β2c show functional replaceability in yeast
The Hsβ2c subunit initiates the assembly of the β-ring in the proteasome core, at least in mammalian cells, by recruiting the β3 subunit (Murata et al. 2009; Tanaka 2009). As noted above, the unique C-terminal tail of β2c threads around the β3 subunit while also interacting with neighboring β4, β6, and α subunits (Ramos et al. 2004). Deletion of the yeast β2 C-terminal tail is lethal and, in heterozygous diploids, leads to a pronounced accumulation of assembly intermediates containing the unprocessed β2 precursor (Ramos et al. 2004). Therefore, the C-terminal tail of yeast β2 is essential for CP assembly.
Sequence alignment of β2 subunits across diverse species shows that the C-terminal tail has diverged more extensively relative to the rest of the protein (Fig. 4a). Therefore, the incompatibility between the human and yeast β2 may be a consequence of the divergent interactions of the C-terminal tail, leading to the assembly failure of humanized yeast proteasomes. Indeed, structural modeling of the human β2 subunit within the yeast proteasome core revealed 5 of 14 HsPSMB7 mutations [T233R, K249R, T260I*, E233K*, and the previously identified E263-STOP* (Kachroo et al. 2015)] that promoted functional replaceability occurred in the C-terminal tail (Fig. 4b).
Fig. 4.
Multiple-sequence alignment of β2 proteins across diverse species highlighting the divergence of C-terminal tail regions. a) The alignment of β2 protein sequences belonging to diverse organisms shows the divergence of the C-terminal tail of β2. C-terminal tails are critical for subunit interaction and sequential proteasome assembly. The identity score is represented as bars with high identity in dark green, medium in light green, and low in red. b) A magnified view of the aligned C-terminal tail regions highlights the divergent tails. The swaps replacing tail sequences of human β2 with corresponding yeast sequences are shown in cyan. Tail Swap220–261 (TS220–261) transplants the entire β2 tail from yeast to human PSMB7 (the numbers correspond to the amino acids of the yeast β2), whereas TS220–235, TS220–236, TS236–261, and TS247–261 carry C-terminal tail transplants of smaller regions.
Therefore, we asked if swapping human PSMB7 tail segments with the corresponding yeast Pup1 tail elements would allow functional replaceability. We engineered 5 human–yeast hybrid genes with different lengths of C-terminal tail swaps (Fig. 5a). We chose the lengths of the swapped C-terminal tails based on structural modeling and the aligned positions of suppressor mutations in the human C-terminal segment (T233R, K249R, T260I*, and E263K*) (Figs. 3 and 4). The clones with different lengths of the swapped sequences were designated as Tail Swap220–261 (TS220–261, which included the entire yeast Pup1 tail sequence—residues 220–261—and would cover all of the C-terminal tail suppressor mutations in HsPSMB7), TS236–261 (spanning K249R and downstream mutations), TS247–261 (spanning only the accessory mutations T260I* and E263K* and E263-Stop*), TS220–236 (spanning a region with T233R and K249R mutations), and TS220–235 (a stretch of 16 Pup1 residues that would span only the T233R mutation) (Figs. 4b and 5a and b). The functional replaceability assays were performed as described above. The assays revealed that TS220–261, TS220–236, and TS220–235 could functionally replace the yeast Pup1 (Fig. 5c), whereas the TS236–261 and TS247–261 chimeras failed to complement. The successful rescues were further confirmed by plasmid-dependency assays using 5-FOA. Quantitative growth assays indicated that cells carrying the human PSMB7-TS220–261 chimera grew similarly to the positive control (wild-type yeast β2), whereas TS220–236 and TS220–235-expressing strains showed progressively slower growth (Fig. 5d). Thus, our data demonstrate that the divergence of the C-terminal tails of human and yeast β2 subunits is likely to impact the assembly or activity of the proteasome core.
Fig. 5.
C-terminal tail swap from yeast to wild-type human β2c enables the functional replacement of yeast β2. a) The sequences of C-terminal tails of the wild-type human β2c (gray), wild-type yeast β2 (cyan), chimeric human Tail Swap220–261 (TS220–261, with the entire yeast C-terminal tail of 42 amino acids), TS247–261 (with last 15 amino acids of yeast β2), and TS236–261 (with last 26 residues of the yeast β2), TS220–235 (harboring 16 amino acids of the beginning of the yeast β2), and TS220–236 (harboring 17 amino acids of the beginning of the yeast β2) are shown. Human β2c amino acids, when mutated, contributing to functional replacement are shown in red, and amino acids that play an accessory role are shown in blue. b) Structure of human β2 (gray) (PDB 1IRU) modeled in the yeast β-core with neighboring yeast β3 (orange), yeast β1 (yellow), yeast β7 (magenta), and yeast β6 (green) subunits (PDB 1RYP). The structure shows the C-terminal tail of human β2 wrapping around the yeast β3 subunit. The C-terminal tail segments of yeast β2 that replaced the corresponding human β2 segments are shown in cyan. c) Growth assays were performed on MM medium without G418 (yeast gene PRESENT) and MM medium with G418 (yeast gene ABSENT) at 30°C. The data show that plasmid-based expression of yeast PUP1 successfully complements the deletion of the native yeast gene copy, whereas the wild-type human β2c (PSMB7) does not. The C-terminal tail swap from yeast to human β2c (TS220–261, TS220–236, and TS220–235) showed growth rescue after 3 days of incubation at 30°C. However, shorter tail swaps (TS236–261 and TS247–261) did not complement the deletion of PUP1. d) The doubling times of the human β2-TS220–261 and the positive control yeast β2 are comparable. In comparison, shorter tail swaps (TS220–236 and TS220–235) show a significantly slower growth rate. The mean doubling times are plotted with standard deviation (N = 3). P-values are indicated as asterisks; ns, not significant; **, <0.01; ***, <0.001.
Previous studies have shown that the C-terminal tail of yeast β2 plays a role in facilitating the organized assembly of the β-ring (Ramos et al. 2004). Furthermore, modeling of human β2c in the cryo-EM structure of the yeast 13S proteasome assembly intermediate reveals potential steric clashes between the C-terminal tail of Hsβ2c and the yeast β3 subunit (Schnell et al. 2021) (Supplementary Fig. 4). The exchange of the C-terminal tail from yeast to human β2 likely restores optimal interactions between the tail and the yeast β3 subunit.
HsPSMB7-like (Hsβ2c) modifications in HsPSMB10 (Hsβ2i) fail to functionally replace the yeast ortholog
We also asked if similar modifications in a duplicated copy of HsPSMB7, i.e. the HsPSMB10 (Hsβ2i) paralog, an immunoproteasome subunit and a co-ortholog of yeast Pup1, could enable functional replacement. We tested 2 mutations in Hsβ2i, an entire yeast β2 Tail Swap (Hs-PSMB10-TS220–261) and an active-site mutation (HsPSMB10-T40A). Wild-type Hsβ2i could not replace yeast PUP1 function (Supplementary Fig. 5a —top panel) despite robust expression (Supplementary Fig. 5b) (Laurent et al. 2020). Interestingly, wild-type HsPSMB10 harbors Gly210 instead of Ser214 in HsPSMB7; an S214G mutation in HsPSMB7 enables functional rescue in yeast (Supplementary Fig. 1c). Thus, suppressor mutations function in a context-specific manner. Furthermore, neither HsPSMB10-TS220–261 nor HsPSMB10-T40A enabled functional replaceability in yeast (Supplementary Fig. 5a —middle and bottom panels).
Biochemical characterization of the yeast-complementing human β2 variants reveals a catalytically active proteasome core
Yeast and human β2 proteins harbor the catalytic site for trypsin-like protease activity in the 20S proteasome (Arendt and Hochstrasser 1997; Harshbarger et al. 2015; Rut and Drag 2016). While several mutations allow human PSMB7 to complement the lethal growth defect of a yeast PUP1 gene deletion, the rescue may occur at the level of proteasome assembly rather than restoration of β2 proteolytic activity, which is not essential for viability. This is supported by previous findings demonstrating that the active-site mutant yeast pup1-T30A subunit can assemble into the proteasome core (Arendt and Hochstrasser 1997). To biochemically characterize human β2 in hybrid yeast-human proteasomes, we introduced a sequence encoding a 3xFLAG tag at the C-terminal coding sequence of yeast RPN11 at its native genomic locus using CRISPR-Cas9 and homology-directed recombination (HDR) (Supplementary Fig. 6a and b). Rpn11, a component of the RP of the 26S proteasome, is not functionally compromised by the 3xFLAG tag (Sakata et al. 2011). The RPN11-3xFLAG strains were confirmed to stably express the tagged protein and showed no growth defects (Supplementary Fig. 6c, d, and e). The humanized β2c strains were engineered to harbor the RPN11-3xFLAG tag to allow affinity purification of the full 26S proteasome (RP–CP).
We tested proteasome activity and assembly in the yeast strains expressing the wild-type yeast Pup1, catalytically inactive pup1-T30A, or human PSMB7-T44A, PSMB7-S214G, or PSMB7-TS220–261 variants of the Hsβ2c protein (Fig. 6). As expected, trypsin-like activity was abolished in both affinity-purified proteasomes with active-site β2 mutations, PSMB7-T44A, and pup1-T30A. By contrast, trypsin-like activity was observed in the humanized PSMB7-S214G and PSMB7-TS220–261 proteasomes, similar to the wild-type yeast Pup1-containing particles (Fig. 6a).
Fig. 6.
Complementing human β2c (PSMB7) variants in yeast reveal defects in proteasome assembly or PSMB7 autoprocessing. a) Yeast pup1Δ strains bearing plasmids expressing wild-type ScPUP1, Scpup1-T30A, HsPSMB7-T44A, HsPSMB7-S214G, or HsPSMB7-TS220–261 genes and harboring chromosomally tagged RPN11-3xFLAG were used to affinity purify 26S proteasomes via the 3xFLAG tag. The purified proteasomes were fractioned by native gel electrophoresis, and trypsin-like and chymotrypsin-like (from the β5 subunit) activities were tested using overlays with the fluorogenic Boc-LRR-AMC and Suc-LLVY-AMC substrates, respectively (top 2 panels). RP2–CP and RP–CP are doubly and singly capped 26S proteasomes, respectively. The purified proteasomes were also separated by SDS-PAGE and analyzed by anti-PSMB7 immunoblotting. Anti-PSMB7 antibody might weakly cross-react with ScPup1; the band in lane 2 might therefore represent an unprocessed or partially processed Pup1, but this has not been verified. The anti-FLAG blot shows similar loading of samples. b) Whole-cell lysates obtained from the same yeast strains as in panel (a) were directly fractionated by SDS-PAGE and analyzed by anti-PSMB7 immunoblotting. An anti-Pgk1 immunoblot was used as a loading control. c) Native-PAGE immunoblot analyses of the cell lysates from panel (b) using antibodies to the indicated CP (Pre6/α4), lid (Rpn5), and base (Rpt5) subunits.
Analysis of the purified humanized proteasome particles by SDS-PAGE followed by anti-PSMB7 immunoblotting revealed that the unprocessed PSMB7-T44A precursor assembled into hybrid 26S proteasomes and the particles had no detectable trypsin-like activity; by contrast, the PSMB7 propeptide had been cleaved in the PSMB7-S214G and PSMB7- TS220–261 proteasomes (Fig. 6a, PSMB7 blot). Chymotrypsin-like activity, which derives from the yeast β5 subunit, was seen in all the purified proteasomes (Fig. 6a). Interestingly, evaluation of PSMB7 processing activity in whole cell lysates without prior purification showed most of the human β2 protein still in its precursor form in cells expressing PSMB7-S214G and PSMB7-TS220–261 (Fig. 6b). This observation might be due either to a reduced rate of autoprocessing in the hybrid proteasomes or to the majority of human β2 protein being unincorporated or present in immature assembly intermediates.
To test this last idea, we evaluated the assembly state of proteasomes in yeast whole cell lysates by nondenaturing-PAGE immunoblot analysis (Fig. 6c). The human PSMB7 variants were able to assemble into mature yeast proteasomes, but this was accompanied by 20S CP assembly defects and likely concomitant RP base assembly defects. Specifically, an anti-Pre6 (yeast ɑ4) blot revealed little or no free CP. Instead, it showed the accumulation of sub-CP species in cells with the humanized PSMB7 variants (T44A, S214G, and possibly TS220–261), suggesting limiting amounts of mature CP relative to RP. Anti-Rpt5 blotting revealed an excess of the Rpt4-Rpt5 assembly intermediate (lower band, Fig. 6c) and other particles smaller than full 26S proteasomes. An excess of free RP lid complexes accumulated due to the deficit in fully assembled base intermediates (Fig. 6c, middle panel). Similar proteasome assembly defects, including of the RP, were previously observed in CP assembly mutants (Kusmierczyk et al. 2008).
Assembly defects were less prominent in the HsPSMB7-TS220–261 humanized proteasomes. Because the C-terminal tail threads along the interface between the 2 β-rings, the processing defect observed in this mutant might reflect slower autoprocessing since this only occurs after 2 half-proteasomes have associated properly (Chen and Hochstrasser 1996). These results indicate that human PSMB7 variants (S214G and TS220–261) can replace yeast Pup1 catalytic activity and assemble into yeast 26S proteasomes. Furthermore, the data confirm that Thr44 of the human PSMB7/β2c is essential for autoprocessing and proteasomal trypsin-like activity.
Wild-type human β2c can incorporate into yeast proteasomes if human β3 is also present
Ideally, humanizing yeast to functionally characterize human genes would utilize the wild-type human alleles in yeast. However, the suppressor screen and the C-terminal tail swap data suggest that human β2c requires mutations to interact with neighboring yeast subunits, particularly Scβ3, to assemble properly into the yeast CP. Thus, a strategy that restores these human-specific subunit interactions might enable the integration of the wild-type HsPSMB7 into yeast proteasomes. To test this hypothesis, we asked if the wild-type human β2c can functionally replace its yeast counterpart in a strain expressing the human β3 subunit.
We developed a CRISPR-Cas9 methodology to target the yeast ScPUP1 (β2) and ScPUP3 (β3) genes. Transformation of plasmids expressing Cas9-sgRNAScPUP1 or Cas9-sgRNAScPUP3 was lethal in yeast (Supplementary Fig. 7a). Co-transformation of human gene repair templates harboring homology at the 5′ and 3′ termini of the corresponding yeast loci would be predicted to yield viable cells if the human genes could functionally replace the yeast orthologs. We first tested whether wild-type HsPSMB7 could replace ScPUP1 at the native locus but failed to obtain any viable colonies, as expected (Fig. 7a). However, using HsPSMB7 variants, such as those with a T44A or S214G mutation, repair templates allowed functional replacement of the yeast PUP1 gene (Fig. 7b). Previously, we had demonstrated that the yeast β3 (PUP3) gene is functionally replaceable by its human ortholog (HsPSMB3) when expressed on a plasmid (Kachroo et al. 2015). Using the CRISPR-Cas9-based strategy of HDR (Akhmetov et al. 2018), we successfully replaced the genomic yeast β3 gene with human β3 (HsPSMB3) (Fig. 7c). Thus, the yeast β2 subunit can recruit human β3 to the yeast proteasome core, but human β2c is unable to perform this function with yeast β3.
Fig. 7.
Provision of both wild-type human β2c and neighboring human β3 enables functional replacement of their yeast orthologs. a) Co-transformation of the pCas9-sgRNAScPUP1 and human wild-type PSMB7 gene repair template as a PCR fragment fails to obtain viable humanized strains. b) However, the co-transformation of pCas9-sgRNAScPUP1 and human PSMB7-T44A or PSMB7-S214G gene variants as a repair template yields viable yeast with genomically integrated human gene variants. c) Transformation of the pCas9-sgRNAScPUP3 and human wild-type PSMB3 (β3) gene repair template as a PCR fragment yields viable humanized β3 strains. d) Using humanized β3 strains as a background, the co-transformation of pCas9-sgRNAScPUP1 and human wild-type PSMB7 gene as a repair template yield viable yeast with genomically integrated wild-type human β2-β3 in yeast. A single yeast β-proteasome core ring of 7 subunits is shown (PDB-1RYP) using ChimeraX software. The replacement-competent human subunits are indicated in yellow, and the nonreplaceable subunits are shown in blue. Representative petri plates show colonies growing on selection media after 3–5 days of incubation at 30°C.
Starting with the humanized β3 yeast strain, CRISPR-Cas9-based genome editing now enabled the replacement of yeast β2 with the wild-type human β2c (HsPSMB7) gene. The doubly humanized Hsβ2c-Hsβ3 yeast strain was viable, as verified by locus-specific PCR and Sanger sequencing (Fig. 7d). Thus, by providing its neighboring human subunit, i.e. human β3, the normally noncomplementing human β2c could now function in the yeast proteasome. However, providing human β3 in yeast did not allow complementation of yeast β2 by Hsβ2i (not shown). Quantitative growth assays revealed modest fitness defects of the engineered strains. The humanized Hsβ2c-S214G strain showed a cold-sensitive growth defect at 23°C, whereas the humanized Hsβ2c-T44A strain grew slower at 23°C and 37°C compared to the wild-type strain. In addition, while the humanized Hsβ3 strain grew comparably to wild-type yeast, the Hsβ2c-Hsβ3 strain manifested a cold-sensitive phenotype at 23°C and grew slower at 30°C in liquid culture (Supplementary Fig. 7b).
Discussion
Using an automated high-throughput pipeline and a large pool of mutant human PSMB7 genes, we identified human β2c variants that enable functional replacement of the yeast β2 (Pup1) ortholog (7 of 19 suppressors). The variants reveal protein residues and domains critical for assembling human β2c into the yeast proteasome core. While this study focused on the human gene mutations that enable human gene function in yeast, our assay also discovered viable yeast cells (12 of 19 suppressors) independent of the mutation in a human gene. Although these suppressors may likely be due to segregation defects (cells harboring both the knockout and the wild-type allele of the yeast gene), some may have mutations in yeast genes that allow a wild-type human gene to function in yeast. Indeed, recent efforts to humanize yeast histones have identified several mutations in yeast genes, enabling wild-type human histones to function in yeast and providing mechanistic insights into the diverged roles of highly conserved proteins (Truong and Boeke 2017; Haase et al. 2023). In the future, our pipeline may allow the discovery of similar yeast gene modifications to allow nonreplaceable human β subunit functioning in yeast, providing tools to explore new proteasome biology (Kachroo et al. 2022).
Structural analysis and homology modeling show that all amino acid substitutions in human PSMB7, except T44A, that allow replacement of yeast Pup1 are at or near interfaces with neighboring subunits, suggesting the contribution of multiple PPIs to the assembly of the CP. In particular, several variants appear to promote the interaction with the yeast β3 subunit. Modeling of the human β2c in a fully assembled CP indicates that the mutations affect residues within an internal loop (A70V and D71N*) or the C-terminal tail (K249R, E263K*, and E236-Stop*) of β2c that may stabilize intramolecular contacts and impact interactions with the yeast β3 or β6 subunits (Supplementary Fig. 3).
The C-terminal tail of yeast β2 is known to help guide the ordered assembly of the β-ring (Ramos et al. 2004). Modeling human β2c in the yeast 13S proteasome assembly, intermediate structure shows potential steric clashes between the Hsβ2c C-terminal tail and yeast β3 (Supplementary Fig. 4). Furthermore, the C-terminal domains have diverged across species, suggesting the evolution of unique species-specific contacts. Replacing the sections of the C-terminal tail of human β2c with the tail of yeast β2 enables functional replaceability in yeast. Swapping a 16-residue C-terminal tail section from yeast to human β2 was sufficient to confer functional replaceability (Fig. 5). Indeed, similar approaches that generate human–yeast chimeras enabled the functional replacement of the corresponding β5 and β6 subunits in yeast, demonstrating their general applicability (Huber et al. 2016). Notably, by providing a human-like PPI interface using a humanized β3 strain, wild-type Hsβ2/PSMB7 could replace its yeast ortholog (Fig. 7). These data reveal that restoring local PPIs via mutations or providing a humanized neighbor enables an otherwise replacement-incompetent human gene to complement the orthologous yeast gene function. However, similar approaches to functionally replace the immunoproteasome Hsβ2i subunit in yeast failed, suggesting either that distinct PPIs govern the assembly of the immunoproteasome core or that the modifications operate in a context-specific manner; alternatively, Hsβ2i may simply have diverged too far relative to Scβ2 to allow its incorporation into the yeast CP. Similar comparative functional replaceability assays could be performed in other model organisms such as flies, worms, and particularly in Xenopus, a nonmammalian vertebrate encoding duplicated copies of immunoproteasomes (β5i and β1i) (Namikawa et al. 1995; Nonaka et al. 1997), to elucidate evolutionary diverged assembly process and catalytic functions of the proteasome.
We assessed the functional replacement of yeast β2 by human PSMB7 variants via growth assays and biochemical characterization. While the human β2c variants rescue the lethal growth defect of the knockout of the yeast ortholog, the humanized proteasome shows assembly defects based on the accumulation of distinct assembly intermediates and altered β2c precursor processing. These defects are more pronounced in the yeast cells expressing the HsPSMB7-T44A variant compared to the S214G and TS220–261alleles. The trypsin-like catalytic activity of the HsPSMB7-S214G and HsPSMB7-TS220–261 variants is also comparable to that of wild-type yeast Pup1-bearing proteasomes, whereas the HsPSMB7-T44A variant is catalytically inactive and accumulates in precursor form within proteasomes (Fig. 6).
Surprisingly, the catalytically dead PSMB7-T44A permits functional replaceability in yeast, whereas the unmutated PSMB7 does not. This observation was unexpected. The structure of the 13S assembly intermediate shows an uncleaved propeptide of yeast β2 interacting with yeast β3 and apparently aiding in assembly (Supplementary Fig. 4) (Schnell et al. 2021). We show that the human PSMB7-T44A retains its propeptide, which might assist in assembling human β2c into the yeast CP. Since wild-type β2 will also have the propeptide until autocleavage at the very end of CP assembly after 2 half-mers have come together (Chen and Hochstrasser 1996), the retained propeptide in the PSMB7-T44A mutant might stabilize β2c-β3 within the preholoproteasome at late stages of maturation. The R32W* mutation in the β2c propeptide may similarly stabilize subunit interactions of assembly intermediates in the humanized yeast CP before propeptide cleavage. The human β2c propeptide is known to play a vital role in the cooperative assembly of the human β-ring, unlike the shorter propeptide of the yeast ortholog (De et al. 2003; Hirano et al. 2008; Tanaka 2009; Budenholzer et al. 2017). Our data suggest that the human β2c propeptide, despite its sequence divergence, has retained the ability to interact with the yeast β3 subunit (Fig. 4). More broadly, the data show the importance of the human proβ2c structure that precedes its catalytic role. The significance of the scaffolding roles of proteins rather than their catalytic functions may be relevant to other conserved orthologous systems, such as protein kinases (Kung and Jura 2016) or metabolic enzymes in the human eye (Piatigorsky 1998). Hence, our findings caution against making assumptions solely based on catalytic site mutations when assessing the significance of protein function in orthologous systems, particularly in light of the structural roles that proteins may also play.
The biochemical analysis reveals that several replacement-competent human β2c variants are proteolytically active. Further characterization of the incompatibilities associated with nonreplaceable human β subunits should identify a path to full humanization of the yeast proteasome core while distinguishing divergent ortholog functions (Abdullah et al. 2023). Yeast with a humanized catalytically active proteasome core provide a synthetic platform to characterize proteasome functionality in vivo. Furthermore, the strategy should enable the generation of distinct types of human proteasome cores (i.e. constitutive and immunoproteasomes) (Huber et al. 2012; Nathan et al. 2013), allowing the characterization of their functions in a simplified cellular context. Proteasomes play a vital role in maintaining protein homeostasis and are implicated in human diseases ranging from cancer to age-related neurodegenerative disorders (Almond and Cohen 2002; Vilchez et al. 2014; Manasanch and Orlowski 2017). Although an attractive drug target, only a handful of FDA-approved drugs that inhibit the proteasome activity are available (Chen et al. 2011; Park et al. 2018), and none are known to increase human CP catalytic activity (Njomen and Tepe 2019) or target proteasome assembly. Yeast with a humanized functionally active proteasome core provide a unique platform for discovering novel therapeutics that inhibit or enhance proteasome activity (Xin et al. 2019) or alter proteasome assembly. The humanized proteasome in yeast will enable rapid assays deciphering human variant effects and gene–drug interactions that might alter proteasome function in cells. Such tools should be helpful in stratifying patients for different therapies—a step towards “personalized medicine.”
Supplementary Material
Acknowledgments
The authors thank Smita Amarnath and Nicholas Gold at the Concordia genome foundry for setting up and automating the human gene suppressor screen using the QPix robot. Authors thank Concordia CMC (Chris Law) for help with imaging yeast cells.
Contributor Information
Sarmin Sultana, Centre for Applied Synthetic Biology, Department of Biology, Concordia University, 7141 Sherbrooke St. W, Montreal, QC H3G 1M8, Canada.
Mudabir Abdullah, Centre for Applied Synthetic Biology, Department of Biology, Concordia University, 7141 Sherbrooke St. W, Montreal, QC H3G 1M8, Canada.
Jianhui Li, Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520, USA.
Mark Hochstrasser, Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, CT 06520, USA.
Aashiq H Kachroo, Centre for Applied Synthetic Biology, Department of Biology, Concordia University, 7141 Sherbrooke St. W, Montreal, QC H3G 1M8, Canada.
Data availability
The humanized yeast strains, CRISPR plasmids, and human gene yeast expression vectors will be made available by lead contact, Aashiq Kachroo (aashiq.kachroo@concordia.ca), upon request. The authors affirm that the conclusions of the article are fully contained within the article, figures, and tables. This paper does not report original code.
Supplemental material available at GENETICS online.
Funding
This research was funded by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC, Discovery grant #RGPIN-2018-05089), Canada Research Chairs (CRC) Tier 2 (NSERC/CRSNG-950-231904), Canada Foundation for Innovation and Québec Ministère de l’Économie, de la Science et de l’Innovation (#37415), and Fonds de recherche du Québec – Nature et technologies (FRQNT) support for New Academics to A.H.K., National Institutes of Health grant GM136325 to M.H., and fellowship support from School of Graduate Studies (SGS), Faculty of Arts and Sciences, Concordia University to S.S. and M.A.
Literature Cited
- Abdullah M, Greco BM, Laurent JM, Garge RK, Boutz DR, Vandeloo M, Marcotte EM, Kachroo AH. Rapid, scalable, combinatorial genome engineering by marker-less enrichment and recombination of genetically engineered loci in yeast. Cell Rep Methods. 2023;3(5):100464. doi: 10.1016/j.crmeth.2023.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmetov A, Laurent JM, Gollihar J, Gardner EC, Garge RK, Ellington A, Kachroo A, Marcotte E. Single-step precision genome editing in yeast using CRISPR-cas9. Bio Protoc. 2018;8(6):e2765. doi: 10.21769/BioProtoc.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia 2002;16(4):433–443. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- Arendt CS, Hochstrasser M. Identification of the yeast 20S proteasome catalytic centers and subunit interactions required for active-site formation. Proc Natl Acad Sci U S A. 1997;94(14):7156–7161. doi: 10.1073/pnas.94.14.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budenholzer L, Cheng CL, Li Y, Hochstrasser M. Proteasome structure and assembly. J Mol Biol. 2017;429(22):3500–3524. doi: 10.1016/j.jmb.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11(3):239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hochstrasser M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell 1996;86(6):961–972. doi: 10.1016/s0092-8674(00)80171-3. [DOI] [PubMed] [Google Scholar]
- De M, Jayarapu K, Elenich L, Monaco JJ, Colbert RA, et al. Beta 2 subunit propeptides influence cooperative proteasome assembly. J Biol Chem. 2003;278(8):6153–6159. doi: 10.1074/jbc.M209292200. [DOI] [PubMed] [Google Scholar]
- Dolinski K, Botstein D. Orthology and functional conservation in eukaryotes. Annu Rev Genet. 2007;41(1):465–507. doi: 10.1146/annurev.genet.40.110405.090439. [DOI] [PubMed] [Google Scholar]
- Garge RK, Laurent JM, Kachroo AH, Marcotte EM. Systematic humanization of the yeast cytoskeleton discerns functionally replaceable from divergent human genes. Genetics 2020;215(4):1153–1169. doi: 10.1534/genetics.120.303378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groll M, Heinemeyer W, Jäger S, Ullrich T, Bochtler M, Wolf DH, Huber R. The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proc Natl Acad Sci USA. 1999;96(20):10976–10983. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase MAB, Ólafsson G, Flores RL, Boakye-Ansah E, Zelter A, Dickinson MS, Lazar-Stefanita L, Truong DM, Asbury CL, Davis TN, et al. DASH/Dam1 complex mutants stabilize ploidy in histone-humanized yeast by weakening kinetochore-microtubule attachments. EMBO J. 2023;42(8):e112600. doi: 10.15252/embj.2022112600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza A, Driessen MRM, Tammpere E, O’Neil NJ, Hieter P. Cross-species complementation of nonessential yeast genes establishes platforms for testing inhibitors of human proteins. Genetics 2020;214(3):735–747. doi: 10.1534/genetics.119.302971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza A, Tammpere E, Kofoed M, Keong C, Chiang J, Giaever Guri, Nislow C, Hieter P. Complementation of yeast genes with human genes as an experimental platform for functional testing of human genetic variants. Genetics 2015;201(3):1263–1274. doi: 10.1534/genetics.115.181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshbarger W, Miller C, Diedrich C, Sacchettini J. Crystal structure of the human 20S proteasome in complex with carfilzomib. Structure 2015;23(2):418–424. doi: 10.1016/j.str.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Kaneko T, Okamoto K, Bai M, Yashiroda H, Furuyama K, Kato K, Tanaka K, Murata S. Dissecting beta-ring assembly pathway of the mammalian 20S proteasome. EMBO J. 2008;27(16):2204–2213. doi: 10.1038/emboj.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber EM, Basler M, Schwab R, Heinemeyer W, Kirk CJ, Groettrup M, Groll M. Immuno- and constitutive proteasome crystal structures reveal differences in substrate and inhibitor specificity. Cell 2012;148(4):727–738. doi: 10.1016/j.cell.2011.12.030. [DOI] [PubMed] [Google Scholar]
- Huber EM, Heinemeyer W, de Bruin G, Overkleeft HS, Groll M. A humanized yeast proteasome identifies unique binding modes of inhibitors for the immunosubunit β5i. EMBO J. 2016;35(23):2602–2613. doi: 10.15252/embj.201695222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Strayle J, Heinemeyer W, Wolf DH. Cic1, an adaptor protein specifically linking the 26S proteasome to its substrate, the SCF component Cdc4. EMBO J. 2001;20(16):4423–4431. doi: 10.1093/emboj/20.16.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo AH, Laurent JM, Akhmetov A, Szilagyi-Jones M, McWhite CD, Zhao A, Marcotte EM. Systematic bacterialization of yeast genes identifies a near-universally swappable pathway. Elife 2017;6(e25093):1–26. doi: 10.7554/eLife.25093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo AH, Laurent JM, Yellman CM, Meyer AG, Wilke CO, Marcotte EM. Evolution. Systematic humanization of yeast genes reveals conserved functions and genetic modularity. Science 2015;348(6237):921–925. doi: 10.1126/science.aaa0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo AH, Vandeloo M, Greco BM, Abdullah M. Humanized yeast to model human biology, disease and evolution. Dis Model Mech. 2022;15(6):dmm049309. doi: 10.1242/dmm.049309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel PM. Antigen processing by the proteasome. Nat Rev Mol Cell Biol. 2001;2(3):179–187. doi: 10.1038/35056572. [DOI] [PubMed] [Google Scholar]
- Kung JE, Jura N. Structural basis for the non-catalytic functions of protein kinases. Structure 2016;24(1):7–24. doi: 10.1016/j.str.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmierczyk AR, Kunjappu MJ, Funakoshi M, Hochstrasser M. A multimeric assembly factor controls the formation of alternative 20S proteasomes. Nat Struct Mol Biol. 2008;15(3):237–244. doi: 10.1038/nsmb.1389. [DOI] [PubMed] [Google Scholar]
- Kuzmin E, Costanzo M, Andrews B, Boone C. Synthetic genetic array analysis. Cold Spring Harb Protoc. 2016;2016(4):pdb.prot088807. doi: 10.1101/pdb.prot088807. [DOI] [PubMed] [Google Scholar]
- Laurent JM, Garge RK, Teufel AI, Wilke CO, Kachroo AH, Marcotte EM. Humanization of yeast genes with multiple human orthologs reveals functional divergence between paralogs. PLoS Biol. 2020;18(5):e3000627. doi: 10.1371/journal.pbio.3000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ME, DeLoache WC, Cervantes B, Dueber JE. A highly characterized yeast toolkit for modular, multipart assembly. ACS Synth Biol. 2015;4(9):975–986. doi: 10.1021/sb500366v. [DOI] [PubMed] [Google Scholar]
- Li J, Fuchs S, Zhang J, Wellford S, Schuldiner M, Wang X. An unrecognized function for COPII components in recruiting the viral replication protein BMV 1a to the perinuclear ER. J Cell Sci. 2016;129(19):3597–3608. doi: 10.1242/jcs.190082. [DOI] [PubMed] [Google Scholar]
- Li J, Hochstrasser M. Selective microautophagy of proteasomes is initiated by ESCRT-0 and is promoted by proteasome ubiquitylation. J Cell Sci. 2022;135(4):jcs259393. doi: 10.1242/jcs.259393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tomko RJ, Hochstrasser M. Proteasomes: isolation and activity assays. Curr Protoc Cell Biol. 2015;67(1):3.43.1–3.43.20. doi: 10.1002/0471143030.cb0343s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14(7):417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin-Furlow J. Proteasomes and protein conjugation across domains of life. Nat Rev Microbiol. 2011;10(2):100–111. doi: 10.1038/nrmicro2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting: an inexpensive alternative to commercially available kits. Spermatogenesis 2011;1(2):121–122. doi: 10.4161/spmg.1.2.16606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata S, Yashiroda H, Tanaka K. Molecular mechanisms of proteasome assembly. Nat Rev Mol Cell Biol. 2009;10(2):104–115. doi: 10.1038/nrm2630. [DOI] [PubMed] [Google Scholar]
- Namikawa C, Salter-Cid L, Flajnik MF, Kato Y, Nonaka M, Sasaki M. Isolation of Xenopus LMP-7 homologues. Striking allelic diversity and linkage to MHC. J Immunol. 1995;155(4):1964–1971. doi: 10.4049/jimmunol.155.4.1964. [DOI] [PubMed] [Google Scholar]
- Nathan JA, Spinnenhirn V, Schmidtke G, Basler M, Groettrup M, Goldberg AL. Immuno- and constitutive proteasomes do not differ in their abilities to degrade ubiquitinated proteins. Cell 2013;152(5):1184–1194. doi: 10.1016/j.cell.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njomen E, Tepe JJ. Proteasome activation as a new therapeutic approach to target proteotoxic disorders. J Med Chem. 2019;62(14):6469–6481. doi: 10.1021/acs.jmedchem.9b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka M, Namikawa-Yamada C, Sasaki M, Salter-Cid L, Flajnik MF. Evolution of proteasome subunits delta and LMP2: complementary DNA cloning and linkage analysis with MHC in lower vertebrates. J Immunol. 1997;159(2):734–740. doi: 10.4049/jimmunol.159.2.734. [DOI] [PubMed] [Google Scholar]
- Pan X, Yuan DS, Xiang D, Wang X, Sookhai-Mahadeo S, Bader JS, Hieter P, Spencer F, Boeke JD. A robust toolkit for functional profiling of the yeast genome. Mol Cell. 2004;16(3):487–496. doi: 10.1016/j.molcel.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Park JE, Miller Z, Jun Y, Lee W, Kim KB. Next-generation proteasome inhibitors for cancer therapy. Transl Res. 2018;198:1–16. doi: 10.1016/j.trsl.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, Ferrin TE. UCSF Chimerax: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30(1):70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J. Multifunctional lens crystallins and corneal enzymes. More than meets the eye. Ann N Y Acad Sci. 1998;842(1 SALIVARY GLAN):7–15. doi: 10.1111/j.1749-6632.1998.tb09626.x. [DOI] [PubMed] [Google Scholar]
- Ramos PC, Dohmen RJ. PACemakers of proteasome core particle assembly. Structure 2008;16(9):1296–1304. doi: 10.1016/j.str.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Ramos PC, Marques AJ, London MK, Dohmen RJ. Role of C-terminal extensions of subunits beta2 and beta7 in assembly and activity of eukaryotic proteasomes. J Biol Chem. 2004;279(14):14323–14330. doi: 10.1074/jbc.M308757200. [DOI] [PubMed] [Google Scholar]
- Reece-Hoyes JS, Walhout AJM. Gateway recombinational cloning. Cold Spring Harb Protoc. 2018;2018(1):pdb.top094912. doi: 10.1101/pdb.top094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remm M, Storm CEV, Sonnhammer ELL. Automatic clustering of orthologs and in-paralogs from pairwise species comparisons. J Mol Biol. 2001;314(5):1041–1052. doi: 10.1006/jmbi.2000.5197. [DOI] [PubMed] [Google Scholar]
- Rut W, Drag M. Human 20S proteasome activity towards fluorogenic peptides of various chain lengths. Biol Chem. 2016;397(9):921–926. doi: 10.1515/hsz-2016-0176. [DOI] [PubMed] [Google Scholar]
- Sakata E, Stengel F, Fukunaga K, Zhou M, Saeki Y, Förster F, Baumeister W, Tanaka K, Robinson CV. The catalytic activity of Ubp6 enhances maturation of the proteasomal regulatory particle. Mol Cell. 2011;42(5):637–649. doi: 10.1016/j.molcel.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Schnell HM, Walsh RM, Rawson S, Kaur M, Bhanu MK, Tian G, Prado MA, Guerra-Moreno A, Paulo JA, Gygi SP, et al. Structures of chaperone-associated assembly intermediates reveal coordinated mechanisms of proteasome biogenesis. Nat Struct Mol Biol. 2021;28(5):418–425. doi: 10.1038/s41594-021-00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnhammer ELL, Östlund G. Inparanoid 8: orthology analysis between 273 proteomes, mostly eukaryotic. Nucleic Acids Res. 2015;43(D1):D234–D239. doi: 10.1093/nar/gku1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Yang F, Tan G, Costanzo M, Oughtred R, Hirschman J, Theesfeld CL, Bansal P, Sahni N, Yi S, et al. An extended set of yeast-based functional assays accurately identifies human disease mutations. Genome Res. 2016;26(5):670–680. doi: 10.1101/gr.192526.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(1):12–36. doi: 10.2183/pjab.85.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RJ, Hochstrasser M. Molecular architecture and assembly of the eukaryotic proteasome. Annu Rev Biochem. 2013;82(1):415–445. doi: 10.1146/annurev-biochem-060410-150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong DM, Boeke JD. Resetting the yeast epigenome with human nucleosomes. Cell 2017;171(7):1508–1519.e13. doi: 10.1016/j.cell.2017.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D, Saez I, Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat Commun. 2014;5(1):5659. doi: 10.1038/ncomms6659. [DOI] [PubMed] [Google Scholar]
- Xin B-T, Huber EM, de Bruin G, Heinemeyer W, Maurits E, et al. Structure-Based design of inhibitors selective for human proteasome β2c or β2i subunits. J Med Chem. 2019;62(3):1626–1642. doi: 10.1021/acs.jmedchem.8b01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Sun S, Tan G, Costanzo M, Hill DE, Vidal M, Andrews BJ, Boone C, Roth FP. Identifying pathogenicity of human variants via paralog-based yeast complementation. PLoS Genet. 2017;13(5):e1006779. doi: 10.1371/journal.pgen.1006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The humanized yeast strains, CRISPR plasmids, and human gene yeast expression vectors will be made available by lead contact, Aashiq Kachroo (aashiq.kachroo@concordia.ca), upon request. The authors affirm that the conclusions of the article are fully contained within the article, figures, and tables. This paper does not report original code.
Supplemental material available at GENETICS online.