Abstract
Background
Immunoglobulin A nephropathy (IgAN) is a progressive inflammatory kidney disease requiring long-term treatment to reduce the risk of progression to kidney failure. Here, we present two systematic literature reviews (SLRs) to identify and summarize literature reporting the humanistic and economic burden of IgAN.
Methods
Electronic literature databases (Ovid Embase, PubMed, and Cochrane) were searched for relevant literature on 29 November 2021, supplemented with gray literature searches. Studies reporting any health-related quality of life (HRQoL) or health state utility outcomes in IgAN patients were included in the humanistic impact SLR, and studies reporting the costs and healthcare resource utilization associated with or economic models of IgAN disease management were included in the economic burden SLR. Narrative synthesis was used to discuss the heterogeneous studies included in the SLRs. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Cochrane guidelines were followed, and all included studies were assessed for risk of bias using the Center for Evidence-Based Management tool for Critical Appraisal of a Survey or the Drummond Checklist.
Results
A total of 876 and 1122 references were identified from electronic and gray literature searches for humanistic and economic burden, respectively. Three studies reporting humanistic impact and five studies reporting economic burden met criteria for inclusion in these SLRs. The included humanistic studies reported patient preferences in the USA and China, HRQoL for patients with IgAN in Poland, and impact of exercise on HRQoL for patients with IgAN in China. The five economic studies reported costs of IgAN treatment in Canada, Italy, and China, along with two economic models from Japan.
Discussion
Current literature suggests IgAN is associated with substantial humanistic and economic burdens. However, these SLRs demonstrate the paucity of research conducted to specifically describe the humanistic or economic burden of IgAN and highlight the need for further research.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41669-023-00415-0.
Key Points for Decision Makers
| Our systematic literature reviews highlight the paucity of peer-reviewed literature describing the impact of IgA nephropathy on health-related quality of life or the economic burden |
| From the literature that is available, IgA nephropathy is associated with substantial humanistic and economic burdens |
| There is a clear unmet need for further research into the humanistic and economic burdens associated with IgA nephropathy and for novel treatments that mitigate these burdens |
Introduction
Immunoglobulin A nephropathy (IgAN) is an immune complex-mediated inflammatory disease characterized by the mesangial deposition of galactose-deficient IgA, often with IgG [1, 2]. Structural damage resulting from immune complex deposition compromises the glomerular filtration barrier and leads to proteinuria, tubular atrophy and interstitial fibrosis, hematuria, progressive loss of glomerular filtration rate (GFR), and ultimately, kidney failure (KF) [2, 3]. Physical limitations and fatigue associated with KF have a profound impact on patients [4, 5].
The goal of the current therapy in IgAN, in accordance with the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, is to preserve kidney function through management of blood pressure and proteinuria, which is pivotal in slowing progression to KF [6]. Current guidelines for IgAN recommend initial therapy with either an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) and corticosteroid therapy for some patients who remain at high risk of progressive kidney disease despite maximal supportive care [6]. The currently recommended treatments are nontargeted, off-label treatments aimed at controlling symptoms and slowing progression to KF [7]. Following progression to KF, chronic kidney replacement therapy (KRT; dialysis or kidney transplant) are the remaining treatment options, both of which have substantial costs [8] and impacts on quality of life (QoL) [9, 10]. In the USA, diagnosis of IgAN typically occurs in the mid-thirties [11], meaning that patients may require KRT at a relatively young age if disease progression cannot be slowed or prevented with current treatments.
There is a need for development of IgAN-specific treatments that can effectively control proteinuria and slow, or prevent, progression to KF. While burden of illness of IgAN was discussed recently in an SLR [12],the authors included studies with mixed glomerular disease population, which does not give a specific understanding of the burden of IgAN. Here, we conducted two systematic literature reviews (SLRs) to identify and summarize current literature that specifically describes IgAN-associated (1) health-related quality of life (HRQoL) or (2) costs/healthcare resource use (HRU). Through these reviews we sought to provide greater understanding of the IgAN disease burden, and to highlight areas where further research may be required during the development of novel therapies.
Materials and Methods
Data Review Methods and Data Sources
Two SLRs were conducted to identify literature reporting (1) the humanistic impact and (2) the economic burden associated with IgAN. SLR protocols were developed and SLRs were performed in accordance with the PRISMA guidelines [13] and followed guidance from the Cochrane Handbook for Systematic Review of Interventions and Centre for Reviews and Dissemination’s Guidance for Undertaking Reviews in Health Care [14, 15]. The protocol for this SLR was not registered.
Key literature databases [Ovid Embase, PubMed, Cochrane Central register of controlled trials (CENTRAL), and the Cochrane database of systematic reviews] were searched on 29 November 2021. Bibliographies of relevant SLRs, meta-analyses and included studies were screened. Supplementary searches of the National Health Service Economic Evaluation Database (NHS EED), Cost-Effectiveness Analysis (CEA) register, School of Health and Related Research (ScHARR) Health Utilities Database, and Database of Abstracts of Reviews of Effects (DARE) and conferences [International Society for Pharmacoeconomics and Outcomes Research (ISPOR), American Society of Nephrology (ASN), European Renal Association–European Dialysis and Transplant Association (ERA–EDTA), International Society of Nephrology (ISN), UK Kidney Week, and National Kidney Foundation meetings 2019–2021] were used to supplement the electronic database searches. All screening and data extraction was undertaken by two independent reviewers, with final decisions on study inclusion being confirmed by a third reviewer if required.
Search Strategy, Study Selection, and Data Extraction
Full electronic search strings for Ovid Embase, PubMed, and Cochrane (CENTRAL and Database of Systematic Reviews) are presented in Supplementary Tables 1–3. Briefly, electronic searches used search strings combining disease specific terms (“Immunoglobulin A Nephropathy,” “IgA Nephropathy,” “IgAN,”, etc.) with key outcome descriptions of humanistic (“Quality of life,” “HRQoL,” “short form 36,” “QALY,” etc.) or economic (“HRU,” “Costs,” “economic burden,” etc.) studies.
The scope of the reviews was defined using the patient, intervention, comparator, outcome, and study design (PICOS) framework (Table 1). Briefly, the populations of interest for both the humanistic and economic SLRs included patients of any age with IgAN. Studies with mixed populations were included only if relevant outcomes were reported specifically for patients with IgAN. Outcomes included HRQoL or health state utility measures (humanistic outcomes) or direct/indirect costs, cost–utility, cost–benefit, cost effectiveness, cost minimalization, budget impact analyses, or healthcare resource utilization (HRU) (economic outcomes). Studies published from 1980 onward were considered for inclusion in the humanistic SLR, while the economic SLR considered studies published from 2011 onward to focus on only more recent costs.
Table 1.
PICOS elements and databases used for reference searches
| Element | Humanistic studies | Economic studies |
|---|---|---|
| Patients |
Individuals of any age with immunoglobulin A nephropathy (IgAN) Studies reporting a mixed population were excluded unless data were reported separately for patients with IgAN |
|
| Interventions | Any or no intervention | |
| Comparison | Any or no comparators | |
| Outcomes |
Any HRQoL measure Any health state utility Any utility mapping algorithms |
Direct costs Indirect costs Cost–benefit outcomes Cost–utility outcomes Cost-effectiveness outcomes Cost-minimization outcomes Budget impact analyses Healthcare resource use |
| Study designs |
Systematic reviews conducted in the most recent 5 years (for record checking only) Prospective clinical trials (phases I–IV) Observational studies Cost–utility analyses Utility elicitation studies Utility mapping studies Exclude: Case reports Nonsystematic reviews |
Systematic reviews conducted in the most recent 5 years (for record checking only) Economic evaluations (cost–benefit analyses, cost-effectiveness analyses, cost–utility analyses, cost-minimization analyses) Prospective clinical trials (Phases I to IV) Observational studies Budget impact analyses Costing and resource use studies |
| Publication timeframe | Studies published from 1980 onward | Studies published from 2011 onward |
| Geographic limitations | None | |
| Language | English language abstract and full text. | |
| Databases to search |
PubMed Ovid Embase Cochrane Database of Systematic Reviews (Cochrane Library) Cochrane CENTRAL database (Cochrane Library) ClinicalTrials.gov International Clinical Trials Registry Platform (ICTRP) NHS Economic Evaluations Database (NHS EED) CEA register ScHARR Health Utilities Database Database of Abstracts of Reviews of Effects (DARE) |
|
| Other search approaches |
Searches of the following conferences for the period 2019–2021: American Society of Nephrology (ASN) The European Renal Association–European Dialysis and Transplant Association (ERA–EDTA) International Society of Nephrology (ISN) The Professional Society of Health Economics and Outcomes Research (ISPOR) UK Kidney Week National Kidney Foundation meetings Checking the reference lists of relevant systematic reviews published in the last 5 years Checking the reference lists of included studies |
CEA cost-effectiveness analysis, eGFR estimated glomerular filtration rate, HRQoL health-related quality of life, IgAN Immunoglobulin A nephropathy
Assessment of Quality and Risk of Bias
Risk of bias was assessed at the study level. Quality assessment of studies assessing the humanistic impact of IgAN were conducted using relevant questions from the Center for Evidence-Based Management tool for Critical Appraisal for a Survey (https://www.cebma.org/wp-content/uploads/Critical-Appraisal-Questions-for-a-Survey.pdf). The Drummond checklist was used for the quality assessments of economic evaluations [16]. Cost and HRU studies were quality assessed using the elements of the Drummond checklist that relate to the collection of those data.
Analysis
As the study designs and outcomes reported in the included studies were anticipated to be heterogeneous, a narrative synthesis was conducted to explore the findings. This approach has been recommended for the synthesis of findings from multiple, heterogeneous studies, when statistical meta-analysis or other forms of synthesis are not feasible. Narrative synthesis uses a textual approach, i.e., relies primarily on the use of words and text to summarize and explain the findings from different studies [17].
Results
Humanistic Impact of IgAN
Search Results
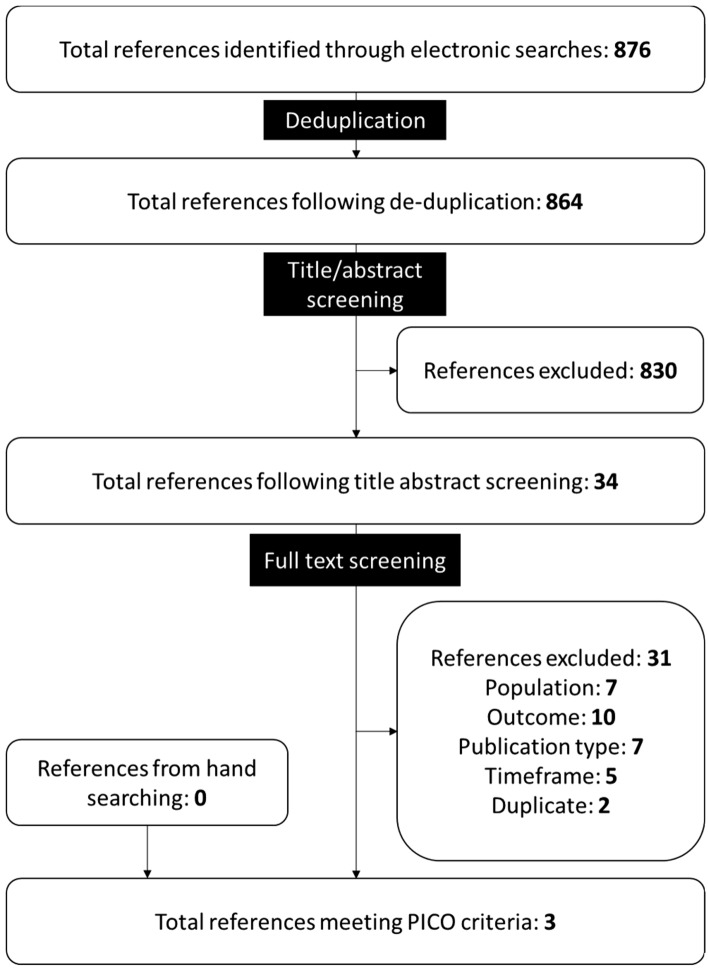
Electronic searches for studies reporting humanistic outcomes returned 876 references, three of which [18–20] met the PICOS criteria for inclusion into this systematic literature review. No additional references meeting the PICOS criteria were identified through hand searching (Fig 1). Studies excluded at full text review are listed in Supplementary Table 4.
Fig. 1.
PRISMA diagram summarizing screening of references reporting humanistic impact of immunoglobulin A nephropathy
Summary of Included Studies
Each of the three studies included in this SLR focused on different populations, used different study designs, and assessed different aspects of the humanistic impact of IgAN (Table 2). Marsh et al. [18] reported patient preference based on an interview swing weighting approach. The population included adults (aged ≥ 18 years) from the USA or China who self-reported as having biopsy-verified primary IgAN. Participants had been receiving ACEi/ARB treatment for at least 6 months (Table 2). Mizerska-Wasiak et al. [19] assessed patients aged ≥ 8 years old from the Polish IgAN Registry (2000–2015) whose disease developed during childhood. HRQoL was assessed using the Kidscreen-52 questionnaire, anger expression scale and personal competence (KompOs) scale (Table 2). Zhao et al. [20] assessed adult patients (≥ 18 years) diagnosed with primary IgAN on renal biopsy using the Beck Depression Inventory-II (BDI-II), Eysneck Personality Questionnaire (EPQ), Quality of Life Index (QLI), Life Science Index (LSI), and Short Form-36 (SF-36) (Table 2).
Table 2.
Study design and baseline characteristics of studies reporting humanistic impact of immunoglobulin A nephropathy
| Author (year) | Study design | Country | Year range for data collection | Measures reported | Inclusion criteria | Study population and patient characteristics |
|---|---|---|---|---|---|---|
| Marsh et al. (2021) [18] | Patient preference elicitation study | USA and China | NR | An interview-based swing weighting approach to assess patient preference elicitation | To be eligible, participants had to be ≥ 18 years of age at the time of consent, self-report having biopsy-verified primary IgAN, currently receiving ACEi/ARBs for at least 6 months, and be fluent in English (in the USA) or Mandarin (in China) |
Population USA, n = 25; China, n = 15 Mean age: 41.6 years Race/ethnicity (US population): 68% Caucasian; 16% Latino; 12% Black/African American; 4% Asian Sex: 32.5% male; 67.5% female |
| Mizerska-Wasiak et al. (2021) [19] | Multicenter cross-sectional study | Poland | 2000–2015 |
Kidscreen-52 questionnaire The Anger Expression Scale The Personal Competence (KompOs) Scale |
Patient age ≥ 8 years, no disease exacerbation during the last 3 months, and written informed consent for study participation |
Population: n = 51 Mean age: 15.5 years Mean duration of IgAN: 5 years Patients with hematuria/proteinuria: 76.5% |
| Zhao et al. (2020) [20] | Prospective observational | China | 2013–2018 |
The Beck Depression Inventory-II (BDI-II) Eysenck Personality Questionnaire (EPQ) Quality of Life Index (QLI) Life Satisfaction Index (LSI) Short Form-36 (SF-36) |
(1) Adults (age > 18 years); (2) patients who were diagnosed with IgAN on renal biopsy; and (3) Beck Depression Inventory-II (BDI-II) score > 14 |
Population: Physical activity (PA), n = 108; no intervention (NI) n = 108 Mean age: PA: 56.1 years; NI: 58.1 years Sex: PA: 62% female; NI: 58.3% female Receiving dialysis: PA: 35.2%; NI: 38.9% |
NR not reported, ACEi/ARBs angiotensin-converting enzyme inhibitors/ angiotensin receptor blockers, GN glomerulonephritis, BC British Columbia, HRU healthcare resource use, ESKD end-stage kidney disease
Patient Preference Elicitation, the Ability to Perform Usual Activities and the Level of Emotional Impact
An interview-based swing weighting approach was used to elicit preferences from 40 adult patients in the USA (N = 25) and China (N = 15) [18]. Patient preference described in Marsh et al. [18] was determined to be relevant to humanistic impact of IgAN as treatment decision-making was found to impact quality of life or perception of quality of life resulting from treatment decisions. Participants placed the greatest value on avoiding dialysis and assigned greater preference weight to reducing the likelihood of dialysis than improvements in all other attributes (p < 0.05). Similar weight was given to short-term quality-of-life improvements and avoiding infections. Treatment burden, defined as the number of vaccinations required before commencing treatment and at 5 year intervals after treatment, received the least weight [18]. Participants from China placed more importance on the risk of other adverse effects than participants from the USA (p < 0.05), and while the study did not describe a reason for this difference, the authors described the result as being consistent with previous studies in kidney diseases [18].
Physical and Psychological Well-Being in Children and Adolescents
Mizersak-Wasiak et al. ([19] discussed HRQoL of children and adolescents with IgAN (N = 51) in relation to the disease course, social status, and psychological factors. This included expressing anger and perceived personal competence, assessed using Kidscreen-52, KompOs, and the Skala Ekspresji Gniewu (SEG) anger expression scale [19].
The Kidscreen-52 questionnaire is validated for use in chronic illness [21] and is designed to be answered by children and adolescents aged 8–18 years. It measures ten dimensions of HRQoL (including physical well-being, mental health as evaluated in three dimensions, and social functioning as evaluated in five dimensions). Outcomes from the study population were compared with Polish population reference values [21]. Participants with IgAN (N = 51) rated their psychological well-being as significantly worse than external reference values (62.8 ± 20.2 versus 72.2 ± 17.3; p < 0.05). Physical well-being, self-perception, and social support and peers were rated significantly worse by boys in the study group compared with reference values (65.78 ± 21.44 versus 72.71 ± 18.50, 71.09 ± 20.90 versus 78.85 ± 17.78, 63.54 ± 21.56 versus 73.03 ± 19.37, respectively; p < 0.05). Social acceptance was rated higher in children with IgAN than in healthy peers, where the difference was statistically significant among girls (91.67 ± 16.67 versus 86.34 ± 18.83; p < 0.05). No significant differences were found when the results of the Kidscreen-52 questionnaire were compared between residents of urban and rural areas [19].
KompOs was used to measure general perception of personal competence [19]. KompOs consists of two subscales, subscale A measures the perceived emotional strength needed to initiate actions whilst subscale B measures the persistence in continuing actions. The study population was compared with a reference population of healthy Polish children (11–17 years old) [19]. Children with IgAN included in the study (N = 46) were characterized by significantly higher perceived strength than the reference population (18.98 ± 4.04 versus 17.09; p < 0.05) [19].
Using the SEG scale to evaluate the severity of anger and expression, children with IgAN (N = 46) were characterized by a significantly lower intensity of expressed anger (24.11 ± 7.49 versus 27.65 ± 6.81; p < 0.001) and a significantly higher intensity of suppressed anger (32.19 ± 6.64 versus 28.06 ± 7.07 p < 0.001) compared with a healthy reference population [19].
Effect of Physical Activity on Depression Symptoms
A prospective, observational study performed in China aimed to assess the improvement in levels of depression among patients with IgAN who engaged in regular physical activity and to examine the relationship between physical activity, depression, and quality of life [20]. BDI-II, EPQ, QLI, LSI, and SF-36 were administered to all participants prior to and after completing a physical exercise program for 6 months [20].
At enrollment, scores across all measures were similar between the two groups (p > 0.15 in all measures). Patients in the physical activity (PA; N = 108) group had significantly lower levels of severe depression compared with the no intervention (NI; N = 108) group after following the physical activity program (PA: 33.4 versus NI: 46.1; p < 0.001) measured by BDI-II scores [20]. Additionally, there was a significant difference in median LSI score (PA: 56.1 versus NI: 41.1; p = 0.001), median SF-36 PCS (PA: 47.5 versus NI: 44.6; p = 0.007) and median QLI (PA: 9.6 vs. NI: 8.9; p = 0.002) between the groups [20]. Multiple logistic regression analysis showed that regular physical activity, extended hemodialysis intervals, and increased SF-36 scores (Physical Component, Mental Component, and Life Satisfaction Index), were independently associated with improved QLI [20].
Quality Assessment and Risk of Bias
Quality assessments were carried out on all publications using relevant questions from the Center for Evidence-Based Management (CEBM) tool for studies reporting humanistic outcomes. All three studies addressed a clearly focused research question, used an appropriate method for answering the research question, and clearly described the method of selection of the subjects [18–20]. Validated tools were used, and statistical significance was assessed in all the studies. All three studies had small study populations, this is likely due to difficulties involved with studying a rare disease. Overall, the studies reporting the humanistic impact of IgAN are of high quality with low risk of bias, despite low population size.
Economic Burden of IgAN
Search Results
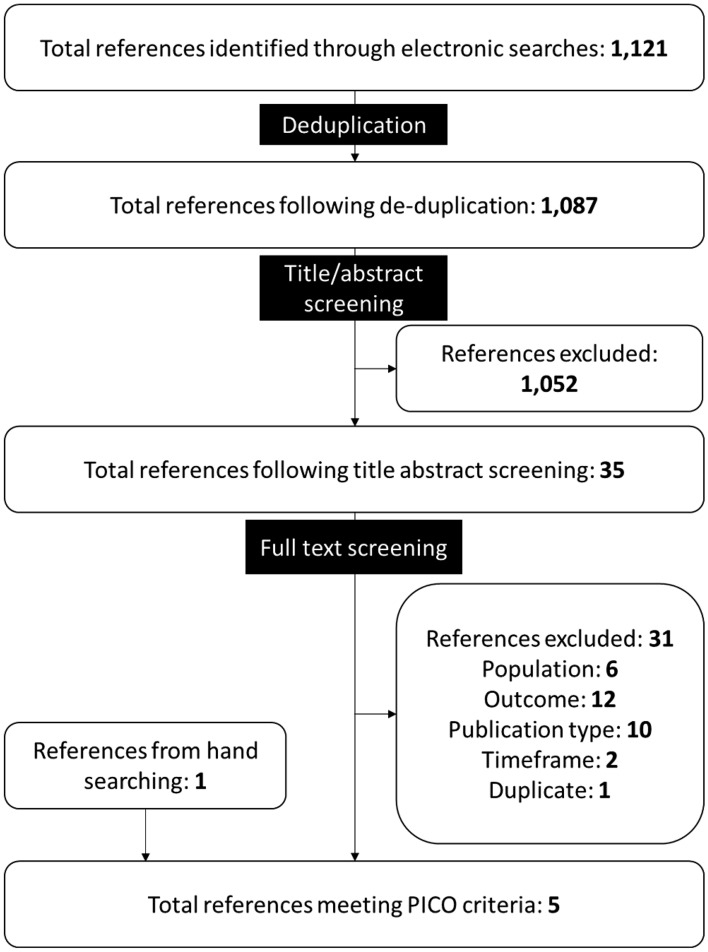
Electronic searches for studies reporting economic outcomes returned 1121 references, four of which met the PICOS criteria for inclusion in this SLR [22–25]. One additional reference meeting the PICOS criteria was found during hand searches [26] (Fig 2). Four of the five identified references assessing the economic burden were peer-reviewed journal articles [22, 24–26] and one was conference abstract [23]. Studies excluded at full text review are listed in Supplementary Table 5.
Fig. 2.
PRISMA diagram summarizing screening of references reporting economic burden of immunoglobulin A nephropathy
Summary of Included Studies
Barbour et al. [22] reported population-level costs in Canadian dollars (CAD) for adult patients (≥ 18 years) with IgAN treated with immunosuppressive medications in British Columbia, Canada, between 2000 and 2012 (Table 3). Carlassara et al. [23] reported the total costs in Euros for hemodialysis (including equipment, transportation, and healthcare workers) for a single patient with IgAN in Italy in 2013 (Table 3). As this study included a single patient, it has not been included in further narrative synthesis. Hiragi et al. [24] compared two approaches modeling the cost effectiveness of immunosuppressive therapy for IgAN. The cost effectiveness models included inputs from Japan, Europe, and Canada [24] (Table 3). Cost analysis of biomarker screening for IgAN in Japanese Yen was reported by Ishida et al. [25] and used Japanese 40-year-old patients with first-time hematuria and suspected IgAN to analyze renal disease-related expenses (Table 3). Li et al. [26] reported healthcare resource utilization (HRU) for hospitalized patients with IgAN (n = 11,569) in China. The retrospective cohort study assessed costs in Chinese Yuan and HRU using the Chinese national inpatient database between 2010 and 2015 (Table 3).
Table 3.
Study design and baseline characteristics of studies reporting economic burden of immunoglobulin A nephropathy
| Author (year) | Study design | Country | Year range for data collection | Measures reported | Inclusion criteria | Study population and patient characteristics |
|---|---|---|---|---|---|---|
| Barbour et al. (2018) [22] | Population-level, observational, retrospective, cohort study using administrative health data | BC, Canada | 2000–2013 |
Population-level costs in 2016 Canadian dollars, of immunosuppressive medications used to treat IgAN The primary outcome was the mean cost of immunosuppressive medications per treated patient each year after kidney biopsy |
All adult patients in BC (18 years of age or older) with a diagnosis of GN on a native kidney biopsy between 1 January 2000 and 31 December 2012. |
Population: n = 756 Mean age: 44.6 years Sex: 61.6% male Mean time from diagnosis: 3.7 years Stage of disease: 23.7% progressed to ESKD |
| Carlassara et al., (2021) [23] | Assessment of the economic costs of non-assisted home hemodialysis using the case of a single IgAN patient in rural Italy | Italy | 2013–NR |
The total amount of the economic resources reported in euros for hemodialysis (HD) comprising: HD equipment, healthcare workers, and ambulance transportation. Reported in Euros (cost year not reported) |
NR |
Population: n = 1 Age: 50 years Sex: 100% male |
| Hiragi et al. (2018) [24] |
Cost-effectiveness models Base-case analysis used previously reported IgAN epidemiology data; survival analysis used the European VALIGA study IgAN cohort Mortality data obtained complete life tables from Ontario, Canada (2009–2011). Mortality data for dialysis patients obtained from Japanese Society of Dialysis Therapy (2013) |
Models had Japanese perspective IgAN inputs from European studies |
NR |
Cost-effectiveness model selection for CKD Primary outcomes: QALYs, costs, and ICER of no intervention versus intervention in CKD patients in USD Secondary outcomes: renal survival rate of virtual CKD cohort using VALIGA-derived parameters as well as base-case [31] parameters for both models following immunosuppressant therapy Cost year, source of costs, and model perspective not reported |
Model represented CKD progression using IgAN inputs |
Population: n = 30,000 Initial age: 37 years |
| Ishida et al. (2022) [25] |
Cost analysis using an analytical decision model. Decision tree compared two clinical scenarios: the intervention strategy as screening with novel biomarkers (group N) and the control strategy as conventional screening (group C). |
Japan | NR |
Cost analysis of biomarker screening for IgA nephropathy in Japanese Yen. Cost year not reported. Base-case and sensitivity analysis |
NA | NA |
| Li et al. (2018) [26] | Retrospective cohort study using the national inpatient database | China | January 2010–December 2015 | Costs and HRU in 2015 Chinese Yuan | Patients with the presence of nephrotic syndrome or glomerulonephritis were defined as having GN. |
Population: n = 11,569 Mean age: 37.7 years Sex: 51.1% male Disease severity: 2% acute kidney injury; 6.4% nephrotic syndrome |
NR not reported, ACEi/ARBs angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, GN glomerulonephritis, BC British Columbia, HRU healthcare resource use, ESKD end-stage kidney disease
Population-Level Costs of Immunosuppressive Medications
Barbour et al. [22] reported population-level costs in CAD for treatment of patients with IgAN using immunosuppressive medications. The primary outcome was mean immunosuppressive medication cost per treated patient each year, which increased from CA$158 in 2000 to CA$221 in 2013, although the change was not statistically significant (p = 0.08). Only direct costs associated with immunosuppressive therapy for IgAN and other glomerular nephropathies were reported. Prednisone was the most common immunosuppressive treatment for patients with IgAN (between 89.3% and 100% over study years), and the yearly per-patient medication costs for prednisone did not change significantly (p = 0.06) [22].
Cost-Effectiveness Model Selection for CKD Using an IgAN Patient Cohort
Hiragi et al. [24] compared two approaches modeling the cost effectiveness of immunosuppressive therapy. Both models used clinical inputs from an IgAN patient cohort. The first model used disease grade-based microsimulation (MSM-dg) with health states based on CKD stage, requirement for dialysis, or death. The second model used kidney function-based microsimulation (MSM-kf) where patients could be alive or dead and kidney function in the “alive” state was based on estimated glomerular filtration rate (eGFR) [24]. The MSM-kf model resulted in a greater number of life years (MSM-kf: 78.8 years versus MSM-dg: 76.35 years), more utilities (MSM-kf: 21.12 years versus MSM-dg: 20.34 years), and lower costs (MSM-kf: US$122,990 versus MSM-dg: US$199,980) than MDM-dg. This resulted in higher threshold prices to achieve cost effectiveness at a US$50,000 per QALY threshold using MDM-kf [24].
Cost Analysis of Biomarker Screening for IgAN
Ishida et al. [25] reported a simulated cost analysis of two clinical strategies for diagnosis of IgAN over a 40 year period: novel biomarkers (galactose-deficient IgA1, IgA1 antibodies, and immune complexes) and conventional screening based on results of a renal biopsy alone. Medical expenses were estimated using an analytical decision model that started with patients at 40 years old with first-time hematuria and suspected IgAN. The cost year was not reported; however, base-case expected medical expenses over the 40-year time horizon were lower with novel biomarker screening than conventional [¥31.2 million (US$291,000 per patient) versus ¥33.4 million (US$312,000) per patient] [25]. Additional scenarios were assessed: (1) where the rate of tonsillectomy and steroid pulse (TSP) therapy was 80% and participants were in CKD stage 1 or 2, (2) where the proportion of good prognosis was 99% when participants with CKD stage 1 or received TSP therapy, or (3) combines scenarios 1 and 2. In all three scenarios, the expected medical expenses were lower when screening with biomarkers than conventional screening (scenario 1: ¥29.5 million versus ¥32.0 million; scenario 2: ¥26.4 million versus ¥29.2 million; scenario 3: ¥22.6 million versus ¥25.9 million per patient) [25]. The number of patients expected to be diagnosed with IgAN was greater with novel than conventional screening (48.44% versus 42.77%), while the proportion of patients requiring dialysis was described as being lower (novel: 19.06% versus conventional: 19.91%) and expected duration of dialysis was shorter (novel: 4.86 years versus conventional: 5.07 years) with novel biomarker screening [25].
Costs and HRU among Hospitalized Patients in China
Li et al. [26] reported costs (2015, Chinese yuan) and HRU outcomes in a large cohort (n = 11,569) of hospitalized patients with IgAN, using the Chinese national database—Hospital Quality Monitoring System (HQMS). Patients with IgAN were identified by ICD-10 codes [N02.801; N00.801, N02.002, N02.101, N02.201, N02.501, N02.701 (Beijing Version 4.0 only); N02.802, N04.303 (National Standard Version 1.0 only)]. Patients with IgAN secondary to a known systemic disease were excluded [26]. The median costs of hospitalized patients with IgAN were 8,000 (6000–12,000) yuan and median length of stay was 10 days [26]. The majority of admissions were routine (86.5%), with some admissions from emergency (8%) and other (5.5%) routes. A minority were admitted to the intensive care unit (ICU) (0.4%) [26].
Quality Assessment and Risk of Bias
Of the five studies included in the SLR, four were available as peer-reviewed manuscripts for full quality assessment [22, 24–26]. The quality of the four peer-reviewed studies was generally high, with sources of costs and/or resource data clearly stated, clearly focused research questions addressed, and appropriate methods used to answer the research questions. Year and currency for costs were reported in Barbour et al. [22] and Li et al. [26]. Details of statistical tests and confidence intervals for stochastic data were provided for two studies [22, 26] and two studies reported sensitivity analysis methods [24, 25]. Lastly, the conference abstract, Carlassara et al. [23], reported limited detail of study methodology and included only a single patient with IgAN, hence the risk of bias is high.
Discussion
All three studies included in the humanistic impact SLR highlight the considerable humanistic impact of IgAN. The studies used a range of methods across different populations and different locations. In the patient preference elicitation study performed in the USA and China, participants placed the greatest value on avoiding dialysis, with similar weight given to short-term quality-of-life improvements and avoiding infections [18]. The Polish study of adolescents and children reported that psychological well-being in all children with IgAN was rated worse in comparison to healthy peers [19]. The prospective observational study performed in China demonstrated improvement in depression symptoms and other HRQoL indicators among adult patients with IgAN who engage in regular physical activity [20]. The three studies included describing the humanistic impact of IgAN described a small sample of patients reflecting the unique challenges in patient accrual of studying rare diseases. Additionally, each of the three studies included here reported different measures of the humanistic impact of IgAN, making comparisons between studies impossible, and highlighting the need for further study.
Our findings are consistent with those reported in a recent SLR that included searches conducted in 2021 [12], and we further highlight a lack of HRQoL data specifically for IgAN. Kwon et al. included studies with mixed populations of patients, including those with IgAN, whereas our SLR was designed to identify IgAN-specific studies. Inclusion of mixed population studies may disguise specific humanistic and economic burdens of IgAN. Our searches identified one IgAN-specific paper [20], which was also included by Kwon and two more recent studies [18, 19] published since the search date of Kwon et al. [12]. While very few studies have been identified that report the specific humanistic impact of IgAN, Cannetta et al. [27] assessed the relationship between HRQoL and demographic and clinical characteristics in a mixed cohort from the CureGN study. As Cannetta et al. [27] included a mixed population of glomerular disease, it was excluded from this SLR; however, it provides a valuable analysis of quality-of-life impact of glomerular diseases, including IgAN. The population included 478 children and 1115 adults with primary glomerular diseases [minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), IgA vasculitis, and IgAN]. Patients with IgAN constituted 30% of the cohort assessed by Cannetta et al. [27]. Primary diagnosis in the CureGN cohort was not associated with differences in HRQoL, assessed using PROMIS, while factors such as edema, eGFR, weight, and sex were associated with some HRQoL domains. Assessment of longitudinal HRQoL data in the CureGN cohort (469 children and 1146 adults; 26% with an IgAN diagnosis) further demonstrated the limited association between diagnosis and HRQoL [28]. Diagnosis was independently associated with sleep impairment and mental health, but not overall HRQoL in adults and there was no association between diagnosis and any HRQoL domain in children. Sleep and mental health scores were best in adult patients diagnosed with IgA vasculitis, followed by MN, IgAN, FSGS, and MCD. The lack of association between diagnosis and HRQoL suggests that individual clinical and patient characteristics are more important determinants of HRQoL than the underlying disease. Cannetta et al. [27] and Kwon et al. [12] illustrate a key issue in studying rare diseases and provide a potential solution by including a mixed population of patients with glomerular diseases to increase the study population size. However, this may result in reduced specificity in the study results.
Five references were identified that report economic burden of IgAN, reporting different aspects of the costs and HRU associated with IgAN in different populations and locations. Barbour et al. [22] reported the first population-level estimates of the immunosuppressive treatment costs for patients with IgAN, demonstrating costs per patient associated with treatment of IgAN from 2000 to 2013 in BC, Canada (p = 0.08). The wider generalizability of the findings is limited because of different drug costs and funding models implemented in other countries and regions. Carlassara et al. [23] demonstrated cost savings associated with home-based hemodialysis, driven by reduced transportation costs. However, a very specific case is presented in the study based on a single patient living in a rural mountainous region of Italy, which may not be applicable to wider populations, in a conference abstract that lacks methodological detail [23]. Hiragi et al. [24] compared two model structures for CKD using an IgAN cohort for clinical inputs. Cost inputs were not IgAN specific; however, the authors reported modeling based on kidney function resulted in longer life years and more QALYs, and with lower costs, providing a more realistic representation of CKD [24]. However, the authors noted the simplification of their model by ignoring major parameters that affect CKD progression, such as race, ethnicity, and major cardiovascular risk factors, in addition to assuming a linear decline in eGFR, as limitations to their method [24]. Ishida et al. [25] demonstrated that biomarker screening for IgAN leads to a reduction in renal disease-related expenses compared with conventional screening. The results of this study may have limited applicability outside of Japan because of differences in screening practices. Both Hiragi et al. [24] and Ishida et al. [25] used models requiring certain assumptions to be made regarding factors affecting disease progression in the former and the potential for over simplification of complex medical problems in the decision tree analysis of the latter. Finally, Li et al. [26] reported median costs for hospitalized patients with IgAN in China in 2015, which were lower than costs associated with other forms of primary glomerular disease. The findings are specific to the Chinese healthcare system and may not be applicable to other healthcare systems.
Since this SLR was completed, two additional conference abstracts presenting economic burden of IgAN were presented [29, 30]. Hwang et al. [29] and Ramjee et al. [30] provide economic analyses of Nefecon to treat IgAN in the USA.
Limitations
The literature searches used here were designed to identify studies that reported humanistic and economic outcomes, using a combination of index terms and free-text (title/abstract/keyword) terms. Studies identified by this search would therefore be expected to have a major focus on humanistic and economic outcomes, but the inclusion of free-text terms adds to the sensitivity of the search strategy. It was possible this approach could omit studies that primarily focus on other outcomes but report some aspect of relevant data within the manuscript. However, this is mitigated with supplementary hand searching, such as checking the reference lists of included studies and relevant systematic reviews, meaning that this review is unlikely to have missed important studies. Additionally, searches were limited to studies published in the English language, which excludes studies published in any other language, thus biasing the search results in favor of studies focused on English-speaking countries.
Our SLRs identified very few studies for inclusion, which is reflective of the key finding that there is very little published data on the humanistic and economic burden or evaluations of interventions for IgAN, and the studies that were identified had small study populations. This limits the ability to draw broad conclusions from the available studies as each study reported different outcomes and were conducted in different countries, with limited applicability outside of the individual setting of each study.
Conclusions
These SLRs identified three references reporting the humanistic impact and five references reporting the economic burden of IgAN, highlighting the extreme paucity of available information regarding the burden of IgAN. This SLR did not identify any utilities for patients with IgAN nor any algorithms designed to map utility values from HRQoL data. Despite this, the available research suggests IgAN poses a significant humanistic and economic burden. These SLRs highlight the need for further research to fully characterize the humanistic and economic burden of IgAN and the potential benefits that could be provided by novel therapeutic approaches.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
SLR screening, data extraction, and risk-of bias assessment was supported by Katherine McAllister and Jessica Adams, employees of Genesis Research (Newcastle upon Tyne, UK), which received compensation from Travere Therapeutics, Inc. for conducting this study. This study was funded by Travere Therapeutics, Inc.
Declarations
Funding
This study was funded by Travere Therapeutics, Inc.
Conflict of interest
KDJ is a founder and co-president of the American Society of Onco-Nephrology; reports consultancy agreements with Secretome, George Clinicals, PMV pharmaceuticals and Calliditas; reports honoraria from the American Society of Nephrology, the International Society of Nephrology, and UpToDate.com; reports serving on the editorial boards of American Journal of Kidney Diseases, CJASN, Clinical Kidney Journal, Journal of Onconephrology, Kidney International, and Nephrology Dialysis Transplantation; reports serving as Editor-in-Chief of ASN Kidney News and section editor for onconephrology for Nephrology Dialysis Transplantation. MEB is a consultant for Travere Therapeutics, Inc. and reports an additional consultancy with Amgen, Inc.; MB is a consultant for Travere Therapeutics, Inc.; JAB and DMWC are employees of Genesis Research, which received compensation from Travere Therapeutics, Inc. for conducting this study. AJ declared that they have no conflict of interest.
Availability of data and material
All data supporting the findings of these systematic literature reviews are available within the article. All data were obtained from the references included and cited in these reviews.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
Study design and concept: Mark Bensink, Martin Bunke, David Cork. Evidence synthesis including search strategy development, abstract and full text screening, and data validation: Jonathon Briggs, David Cork. All authors contributed to interpretation of results, writing and/or review of manuscript drafts, and approved the final manuscript.
References
- 1.Suzuki H, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol. 2011;22(10):1795–1803. doi: 10.1681/ASN.2011050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368(25):2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 3.Yeo SC, Cheung CK, Barratt J. New insights into the pathogenesis of IgA nephropathy. Pediatr Nephrol. 2018;33(5):763–777. doi: 10.1007/s00467-017-3699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artom M, et al. Fatigue in advanced kidney disease. Kidney Int. 2014;86(3):497–505. doi: 10.1038/ki.2014.86. [DOI] [PubMed] [Google Scholar]
- 5.Mayo Clinic. IgA Nephropathy. 2021 [cited Retrieved 24th September 2021; https://www.mayoclinic.org/diseases-conditions/iganephropathy/symptomscauses/syc-20352268. Accessed 24 Sep 2022.
- 6.Rovin BH, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Xu G. An update on targeted treatment of IgA nephropathy: an autoimmune perspective. Front Pharmacol. 2021;12:1–14. [DOI] [PMC free article] [PubMed]
- 8.Golestaneh L, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. 2017;23(10 Suppl):S163–s172. [PubMed] [Google Scholar]
- 9.Baek HS, et al. Impact of end-stage renal disease in children on their parents. Nephrology (Carlton) 2018;23(8):764–770. doi: 10.1111/nep.13083. [DOI] [PubMed] [Google Scholar]
- 10.Gayle F, et al. Quality of life in end stage renal disease: a multicentre comparative study. West Indian Med J. 2009;58(3):235–242. [PubMed] [Google Scholar]
- 11.Hastings MC, et al. Life expectancy for patients from the southeastern United States with IgA nephropathy. Kidney Int Rep. 2018;3(1):99–104. doi: 10.1016/j.ekir.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon CS, et al. A systematic literature review of the epidemiology, health-related quality of life impact, and economic burden of immunoglobulin A nephropathy. J Health Econ Outcomes Res. 2021;8(2):36–45. doi: 10.36469/jheor.2021.26129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centre for Reviews and Dissemination. Systematic Reviews: CRD's guidance for undertaking reviews in health care, 3rd edition. University of York; 2009.
- 15.Higgins J, Thomas J. Cochrane handbook for systematic reviews of interventions. Chapter 8: Assessing risk of bias in a randomized trial. 2021 [cited 2022 15/02/2022]; Available from: https://training.cochrane.org/handbook/current/chapter-08. Accessed 15 Feb 2022.
- 16.Drummond MF, et al. Methods for the economic evaluation of health care programmes. Oxford University Press; 2015. p. 461. [Google Scholar]
- 17.Popay J, et al. Guidance on the conduct of narrative synthesis in systematic reviews: A product from the ESRC Methods Programme, 1st ed. Lancaster University 2006. 10.13140/2.1.1018.4643.
- 18.Marsh K, et al. Assessing patient preferences in rare diseases: direct preference elicitation in the rare chronic kidney disease, immunoglobulin A nephropathy. The Patient Patient-Cent Outcomes Res. 2021;14(6):837–847. doi: 10.1007/s40271-021-00521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizerska-Wasiak M, et al. Health-related quality of life in children with immunoglobulin A nephropathy—results of a multicentre national study. Arch Med Sci. 2021;17(1):84–91. doi: 10.5114/aoms.2020.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, et al. Effect of physical activity on depression symptoms in patients with IgA nephropathy. J Int Med Res. 2020;48(1):1–9. [DOI] [PMC free article] [PubMed]
- 21.Mazur J, et al. Polska wersja kwestionariuszy do badania jakości życia związanej ze zdrowiem dzieci i młodzieży (KIDSCREEN) Instytut Matki i Dziecka, Warszawa; 2008.
- 22.Barbour S, et al. The population-level costs of immunosuppression medications for the treatment of glomerulonephritis are increasing over time due to changing patterns of practice. Nephrol Dial Transplant. 2018;33(4):626–634. doi: 10.1093/ndt/gfx185. [DOI] [PubMed] [Google Scholar]
- 23.Carlassara L, et al. MO898 New organisational model of home hemodialysis: the experience of the province of Belluno. In: Nephrology dialysis transplantation. 2021;36(Suppl. 1), gfab100.0023.
- 24.Hiragi S, et al. The effect of model selection on cost-effectiveness research: a comparison of kidney function-based microsimulation and disease grade-based microsimulation in chronic kidney disease modeling. BMC Med Inform Decis Mak. 2018;18(1):94. doi: 10.1186/s12911-018-0678-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishida M, et al. Cost analysis of screening for IgA nephropathy using novel biomarkers. Value Health Reg Issues. 2022;29:8–15. doi: 10.1016/j.vhri.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Li J, et al. Primary glomerular nephropathy among hospitalized patients in a national database in China. Nephrol Dial Transplant. 2018;33(12):2173–2181. doi: 10.1093/ndt/gfy022. [DOI] [PubMed] [Google Scholar]
- 27.Canetta PA, et al. Health-related quality of life in glomerular disease. Kidney Int. 2019;95(5):1209–1224. doi: 10.1016/j.kint.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy SL, et al. Longitudinal changes in health-related quality of life in primary glomerular disease: results from the CureGN Study. Kidney Int Rep. 2020;5(10):1679–1689. doi: 10.1016/j.ekir.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang S, et al. EE336 Budget Impact Analysis of Nefecon for the Treatment of Primary IgA Nephropathy in the United States. Value in Health. 2022;25(7):S400.
- 30.Ramjee L, et al. EE440 economic evaluation of nefecon in primary IgA nephropathy in The United States. Value in Health 2022;25(7):S421.
- 31.Tesar V, et al. Corticosteroids in IgA nephropathy: a retrospective analysis from the VALIGA study. J Am Soc Nephrol. 2015;26(9):2248–2258. doi: 10.1681/ASN.2014070697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.