Dear Editor,
While the primary target of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the respiratory epithelium, it has been linked to a diverse range of neurological symptoms.1,2 Previous studies have found that this virus can cause complications in the central nervous system (CNS), such as ischemic stroke, necrotizing encephalopathy, encephalomyelitis, and CNS demyelinating disease.2 Cases of unclassified CNS demyelinating lesions have also been observed.3 Here we present a case of bilateral corticospinal tract (CST) lesions in a patient following COVID-19 infection, which is an unusual feature of possible CNS demyelinating disease.
A previously healthy 25-year-old male presented to our outpatient clinic with progressive gait disturbance. He initially experienced numbness at the bilateral finger and toe tips, followed by instability while running that progressed to inability to stand up straight and difficulty in typing. He had weakness and paresthesia in all four limbs, but no headache, blurred vision, dysarthria, cognitive disturbance, bladder or bowel dysfunction, or any relevant family history. He also denied any history of trauma, drug abuse or addiction, malnutrition, or neurotoxin or radiation exposure. He was diagnosed with COVID-19 using the SARS-CoV-2 polymerase chain reaction (PCR) 6 weeks before symptom onset, which lasted for 10 days and comprised fever, chills, malaise, sore throat, and cough.
A neurological examination revealed increased deep tendon reflex responses in the upper and lower limbs with bilateral spasticity. Muscular strength on the Medical Research Council Scale was grade 4+ in the upper limbs and grade 4 in the lower limbs. Positive Babinski’s signs were observed on both sides. He had sensory impairment to all modalities in the distal limbs. Initial blood tests were normal, including the complete blood cell count erythrocyte sedimentation rate, blood glucose, electrolytes, and C-reactive protein levels. SARS-CoV-2 was not detected by a real-time PCR, and a chest X-ray produced normal findings.
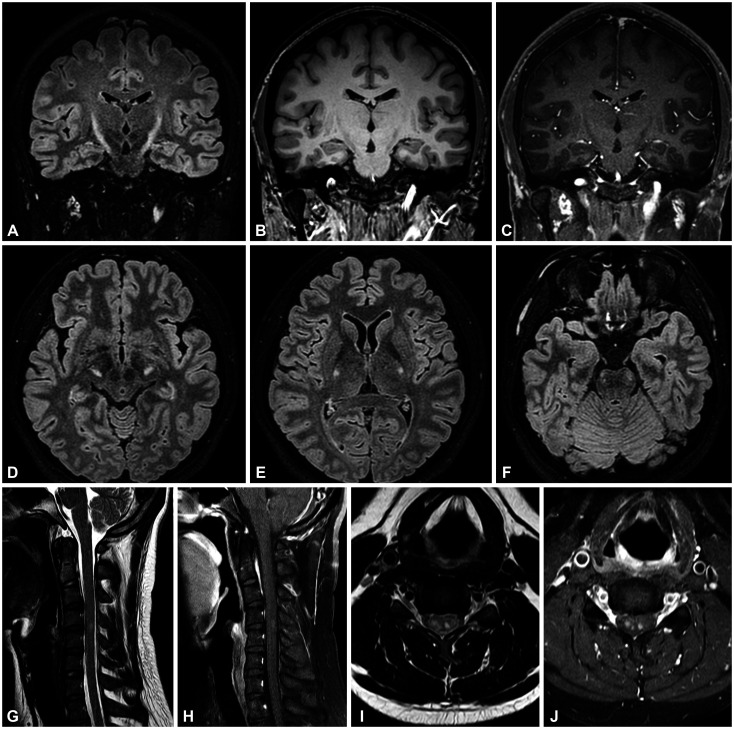
A fluid-attenuated inversion-recovery image in brain magnetic resonance imaging (MRI) revealed bilateral lesions along the CST that extended from the internal capsule to the cerebral peduncles and pons. Spine MRI revealed contrast-enhanced lesions that predominantly involved the bilateral lateral columns (Fig. 1). A nerve conduction study and needle electromyography produced no abnormal findings, and visual evoked potentials were also normal. A cerebrospinal fluid (CSF) study revealed normal protein levels without pleocytosis. Oligoclonal bands were not detected, and the IgG index was normal. The laboratory tests for other vasculitis, vitamin B12 or folate deficiency, paraneoplastic antibodies, human immunodeficiency virus, hepatitis B and C, and toxocara canis all produced negative results. The GALC gene mutation for Krabbe disease was not detected. Tests for aquaporin-4 protein and myelin oligodendrocyte glycoprotein autoantibodies in the serum were both negative.
Fig. 1. Brain and spine MRI of the patient. A-J: Brain MRI revealed bilateral symmetric hyperintense lesions extending from suspicious lesions in the corona radiata and continuing down to the pons. These lesions exhibited a characteristic ‘wine-glass’ appearance in the coronal plane (A). They appeared hypointense in T1-weighted images (B) and were not enhanced by gadolinium (C). Axial images of lesions involving bilateral the internal capsule (D), crus cerebri (E), and basis pontis (F). Spine MRI revealed hyperintense lesions at C4-5 level in T2-weighted images (G, I) with contrast enhancement (H, J). MRI, magnetic resonance imaging.
The patient received pulsed intravenous methylprednisolone therapy for 3 days due to the clinical diagnosis of possible COVID-19-associated CNS demyelinating lesions. Although brain MRI revealed no significant changes at the 6-month follow-up, the symptoms in the patient had improved significantly, with normal motor function and only mild numbness in both finger and toe tips remaining.
This was a case of CNS demyelinating disease with characteristic MRI findings that predominantly affected the bilateral CST. High signal intensities in the bilateral CST can be found in other conditions such as motor neuron disease, Krabbe disease, celiac disease, Lyme neuroborreliosis, and HTLV-1 infection. However, in the present case there were no clues for these differential diagnoses despite the potential doubts raised by the 6-week interval between COVID-19 infection and CNS demyelinating disease onset; CNS inflammation can still be present even 70 days after the initial onset of COVID-19 infection.4 We therefore diagnosed the patient with possible COVID-19-associated CNS demyelinating disease that predominantly affected the bilateral CST. Various mechanisms of COVID-19-associated CNS complications have been proposed, including a virus-induced hypercoagulable/pro-inflammatory state, direct viral invasion, and para-/postinfectious immune-mediated responses.1,5 Considering the 6-week interval between COVID-19 infection and neurological symptom onset, and the absence of febrile symptoms with SARS-CoV-2 negativity on PCR and a normal CSF profile, it was conceivable that postinfectious immune-mediated responses had been the primary mechanism related to the neurological manifestations in our patient.
Kremer et al.2 found that approximately 30% of patients with COVID-19 with neurological manifestations presented CST involvement. However, predominantly bilateral CST lesions are an uncommon CNS manifestation of COVID-19 infection. There have been a few cases of such lesions, including one that occurred during the acute phase of COVID-19 infection with fever, and lung lesions detected using chest computed tomography.3 Four cases have also been observed following COVID-19 vaccination.6,7 However, our case differed from these previous ones since there was no sign of inflammation in the CSF analysis and it did not occur after vaccination. Brain pathological studies conducted on COVID-19 autopsy cases have revealed the presence of perivascular inflammation and hemorrhagic destructive lesions in the internal capsule region.8,9 The underlying reasons for the selective involvement of this region in COVID-19-associated CNS demyelinating diseases remain largely unknown. Further studies are required to understand these bilateral CST lesions in COVID-19-associated CNS demyelinating diseases.
We present a case of CNS demyelinating disease that predominantly affected the bilateral CST following COVID-19 infection. Further studies are needed to elucidate the relationship between bilateral CST involvement in CNS demyelinating disease and COVID-19 infection.
Footnotes
Ethics Statement: This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2015-04-086), and written informed consent was obtained from the patient.
- Conceptualization: Hyunjin Ju, Byoung Joon Kim.
- Supervision: Jin Myoung Seok, Byoung Joon Kim.
- Visualization: Hyunjin Ju, Young Hun Kim.
- Writing—original draft: Hyunjin Ju, Young Hun Kim.
- Writing—review & editing: Jin Myoung Seok, Byoung Joon Kim.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Uversky VN, Elrashdy F, Aljadawi A, Ali SM, Khan RH, Redwan EM. Severe acute respiratory syndrome coronavirus 2 infection reaches the human nervous system: how? J Neurosci Res. 2021;99:750–777. doi: 10.1002/jnr.24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kremer S, Lersy F, Anheim M, Merdji H, Schenck M, Oesterlé H, et al. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology. 2020;95:e1868–e1882. doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 3.Zoghi A, Ramezani M, Roozbeh M, Darazam IA, Sahraian MA. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Scler Relat Disord. 2020;44:102324. doi: 10.1016/j.msard.2020.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakur KT, Miller EH, Glendinning MD, Al-Dalahmah O, Banu MA, Boehme AK, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. 2021;144:2696–2708. doi: 10.1093/brain/awab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodro M, Compta Y, Sánchez-Valle R. Presentations and mechanisms of CNS disorders related to COVID-19. Neurol Neuroimmunol Neuroinflamm. 2020;8:e923. doi: 10.1212/NXI.0000000000000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh R, Dutta S, Ghosh M, Benito-León J. ‘Wine glass’ sign following COVID-19 vaccination in a previously healthy adult. Neurologia. 2022;37:820–823. doi: 10.1016/j.nrleng.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahajan A, Nayak MK, Gaikwad SB, Sharma K, Padma Srivastava MV, Anand P, et al. Post-vaccination/post-COVID immune-mediated demyelination of the brain and spinal cord: a novel neuroimaging finding. Neurol India. 2023;71:86–91. doi: 10.4103/0028-3886.370449. [DOI] [PubMed] [Google Scholar]
- 8.Kantonen J, Mahzabin S, Mäyränpää MI, Tynninen O, Paetau A, Andersson N, et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020;30:1012–1016. doi: 10.1111/bpa.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eschbacher KL, Larsen RA, Moyer AM, Majumdar R, Reichard RR. Neuropathological findings in COVID-19: an autopsy cohort. J Neuropathol Exp Neurol. 2022;82:21–28. doi: 10.1093/jnen/nlac101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.