Abstract
Accurate detection of metastatic prostate cancer in the setting of preoperative staging as well as posttreatment recurrence is crucial to provide patients with appropriate and timely treatment of their disease. This has traditionally been accomplished with a combination of computed tomography, magnetic resonance imaging, and bone scan. Recently, more novel imaging techniques have been developed to help improve the detection of advanced and metastatic prostate cancer. This review discusses the efficacy of the traditional imaging modalities as well as the novel imaging techniques in detecting metastatic prostate cancer. Articles discussed were gathered through a formal PubMed search.
Keywords: Imaging, metastatic, prostate cancer
INTRODUCTION
Prostate cancer is the most common non-cutaneous cancer in men.[1] Although less aggressive than other cancers, prostate cancer continues to be a leading cause of death in men.[2] Detecting metastatic disease in prostate cancer, especially oligometastatic disease, is critical to disrupt the natural history of prostate cancer. In men who develop biochemical recurrence following radical prostatectomy, nearly half develop metastatic disease by 7 years.[3] If left untreated, over half of men die of prostate cancer within 5 years.[3] The ability to promptly and accurately identify patients with metastatic disease after evidence of biochemical recurrence (prostate-specific antigen [PSA] >0.2 ng/dL) is crucial to delay a fatal disease course.[4] In addition, accurate preoperative staging may help avoid localized treatment in patients with metastatic disease, evading morbidity of localized treatment, and expediting systemic therapy. As the treatment of prostate cancer evolves, imaging will play a vital role in determining optimal treatment.
Computed tomography (CT), magnetic resonance imaging (MRI), and Tc99 mMethylene Diphosphonate (MDP) bone scintigraphy have been the mainstay for preoperative staging, biochemical recurrence, and disease progression monitoring. Current National Comprehensive Cancer Network guidelines recommend obtaining imaging as a part of the initial staging workup for prostate cancer and in the setting of PSA persistence or recurrence following radical prostatectomy.[5] Initial imaging guidelines are based on risk stratification, where unfavorable intermediate or high-risk patients receive a bone scan with a CT abdomen/pelvis. Those with PSA persistence or recurrence may be assessed with a range of modalities including chest X-ray or CT, technetium-99 m-MDP bone scan, 18F sodium fluoride positron emission tomography (PET), abdominal/pelvic CT or MRI, and 11C choline or 18F fluciclovine PET/CT or PET/MRI.[5,6] Each of the traditional modalities has its shortfalls, and reported sensitivities and specificities vary considerably. New imaging modalities have been developed to address the shortcomings of current imaging regimens. Choline PET, fluciclovine PET, and prostate-specific membrane antigen (PSMA) PET have all demonstrated promising results in improving the detection of metastatic disease. This paper reviews the current data on both the traditional imaging modalities as well as newer imaging techniques for identifying and localizing metastatic prostate cancer [Table 1]. All articles discussed were gathered through a formal PubMed search utilizing the keywords prostate cancer, metastatic, and imaging.
Table 1.
Efficacy of various modalities in predicting status of metastatic prostate cancer
| Modality | Author | Year | Number of patients | Sensitivity (%) | Specificity (%) | Comparison |
|---|---|---|---|---|---|---|
| CT | Engeler et al.[8] | 1992 | 160 | 5 | 100 | PLND |
| Van Poppel et al.[7] | 1994 | 285 | 77 | 95 | PLND+FNA | |
| Hövels et al.[10] | 2008 | * | 43 | 39 | LN metastasis | |
| Briganti et al.[9] | 2012 | 1541 | 13 | 96 | PLND | |
| MRI | Biondetti et al.[13] | 1987 | 29 | 83 | 97 | PLND |
| Rifkin et al.[12] | 1990 | 185 | 4 | 95 | PLND | |
| Jager et al.[14] | 1996 | 63 | 59 | 97 | PLND+FNA | |
| Hövels et al.[10] | 2008 | * | 39 | 82 | PLND+FNA | |
| Tc99m MDP bone scintigraphy | Pyka et al.[21] | 2016 | 126 | 86.7-89.3 | 60.8-96.1 | Staging, recurrence, surveillance |
| (111) Indium-capromab pendetide SPECT-CT (ProstaScint®) | Rieter et al.[23] | 2011 | 43 | 60 | 97 | PLND |
| Rieter et al.[23] | 2011 | 188 | 17 | 80 | Seminal vesicle dissection | |
| Choline PET | Beheshti et al.[33] | 2013 | 250 | 74 | - | Biochemical recurrence |
| Evangelista et al.[29] | 2013 | * | 49.2 | 95 | Pooled results | |
| Shen et al.[32] | 2014 | * | 91 | 99 | Per-patient | |
| Shen et al.[32] | 2014 | * | 84 | 93 | Per-lesion | |
| Fanti et al.[31] | 2016 | * | 89 | 89 | Pooled results | |
| Fluciclovine PET | Schuster et al.[25] | 2014 | 93 | 90.2 | 40 | Prostate/prostatic bed recurrence |
| Schuster et al.[25] | 2014 | 93 | 55 | 96.7 | Extra-prostatic recurrence | |
| Ren et al.[41] | 2015 | * | 87 | 66 | Pooled results | |
| Chen et al.[39] | 2019 | 106 | 100 | 98 | Pathology | |
| PSMA PET | Perera et al.[50] | 2016 | * | 86 | 86 | Per-patient, histologic confirmation |
| Perera et al.[50] | 2016 | * | 80 | 97 | Per-lesion, histologic confirmation | |
| MR lymphography | Bjurlin et al.[60] | 2018 | * | 82-93 | 93-98 | |
| Meta-analysis* |
*meta-analysis. PLND: Pelvic lymph node dissection, FNA: Fine needle aspiration, CT: Computed tomography, MRI: Magnetic resonance imaging, MDP: Methylene diphosphonate, PET: Positron emission tomography, PSMA: Prostate-specific membrane antigen, MR: Magnetic resonance, SPECT: Single-photon emission computed tomography
COMPUTED TOMOGRAPHY
CT of the abdomen and pelvis is widely used for both initial staging of prostate cancer and for restaging, in the setting of PSA recurrence or persistence following treatment. Current guidelines recommend supplementing CT imaging with technetium-99 m-MDP bone scan to increase detection of bone metastasis.[6] The diagnostic accuracy of CT for detecting lymph node (LN) metastasis, however, has been variable. Van Poppel demonstrated a sensitivity of 77% and a specificity of 96% comparing 285 preoperative staging CT scans with pelvic LN dissection (PLND), and fine-needle aspiration (FNA).[7] However, Engeler found a sensitivity of only 5% with a specificity of 100% in a series of 160 patients when comparing CT with PLND.[8] In a series of 1541 patients who underwent CT followed by retropubic prostatectomy with extended LND, Briganti reported a sensitivity of only 13% and a specificity of 96% for detection of positive LNs.[9] The sensitivity improved with increasing risk groups, with a sensitivity of 17.9% in high-risk patients.[9] A meta-analysis of 17 studies showed a pooled sensitivity of 42% (range: 5%–94%) and specificity of 39% (range: 59%–99%) in identifying LN metastasis.[10] With such a wide variability in the reported performance of CT, there remains significant room for improvement in both the initial staging of prostate cancer and for the detection of recurrence.
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) of pelvis has also played a vital role in both the initial staging of prostate cancer as well as the restaging in patients with PSA recurrence or persistence.[5,6] The majority of MRIs in prostate cancer evaluation are obtained to identify intraprostatic lesions at the time of diagnosis. A meta-analysis reviewing MRI and locally recurrent prostate cancer showed a sensitivity of 90% with a specificity of 81%.[11] However, MRI’s ability to accurately characterize pelvic LN metastasis is somewhat limited. In a study of 185 patients with preoperative staging MRI, Rifkin et al. reported only a 4% sensitivity of detecting positive LNs (1/23), but a specificity of 95% (155/163).[12] When looking at periprostatic extension, MRI had a sensitivity of 77%.[12] Comparing the area of invasion on MRI with that of the pathology specimen, the two matched 76% of the time.[12] The interpretation of the scans was consistent, with no significant variability.[12] Biondetti et al. showed a much higher sensitivity of 83% and specificity of 97% when comparing preoperative MRI to PLND pathology specimens; however, only 29 patients were enrolled in the study.[13] In a study of 63 patients using PLND combined with FNA as the gold standard, Jager et al. demonstrated a sensitivity of 59% and specificity of 97%.[14] A meta-analysis of ten studies comparing MRI to PLND ± FNA showed a pooled sensitivity of 39% (range: 6%–83%) and specificity of 82% (range: 65%–99%).[10] Multiparametric MRI (mpMRI) commonly involves utilizing diffusion-weighted imaging (DWI) and dynamic contrast-enhanced MRI and has been used for the detection and surveillance of prostate cancer.[15] Similar to CT scan, the performance of MRI is quite variable for the identification of prostate cancer metastasis.
TC99 MMETHYLENE DIPHOSPHONATE BONE SCINTIGRAPHY
Tc99 mMDP Bone scintigraphy detects lesions based on mineral bone turnover and can detect a change in mineral bone turnover of 10% compared to a 50% change needed for detection by plain X-ray.[16] Tc99 mMDP is absorbed by the bone surface and is thought to represent skeletal bone blood flow and osteoblastic activity.[17] Bone scans can detect metastatic disease up to 18 months before plain X-ray and have an increased sensitivity.[18] After LNs, bone represents the second most common site of prostate cancer metastasis.[19] Skeletal metastases are found in roughly 80% of patients that had prostate cancer as the leading cause of death, showing the need to effectively detect these bone lesions in patients with potential metastatic disease.[20] A study of 126 prostate cancer patients undergoing bone scan for preoperative staging, biochemical recurrence, or surveillance of known metastatic disease, reported a sensitivity of 86.7%–89.3% and a specificity of 60.8%–96.1%.[21] Without a gold standard to compare the bone scan results to, it is hard to determine the real sensitivity and specificity of Tc99 mMDP bone scintigraphy. In addition, benign conditions, such as trauma, osteomyelitis, and osteoporosis, increase blood flow and osteoblastic activity, and show increased Tc99 mMDP uptake, complicating diagnosis in prostate cancer patients.[17] Still, bone scans are widely used to identify and follow bone metastases due to prostate cancer and form an essential part of management in the majority of patients with metastatic disease.
(111) INDIUM-CAPROMAB PENDETIDE SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY (PROSTASCINT®)
(111) Indium-capromab pendetide is a conjugate of a monoclonal antibody directed to PSMA, linked to a gamma-emitting isotope using a linker chelator.[22] The ProstaScint® scan capitalizes on the variation in PSMA expression between benign and malignant prostate tissue.[23] When compared to pathology following prostatectomy with LN dissection, ProstaScint® demonstrated a sensitivity of 60% and a specificity of 97% in the 43 LNs available for review.[23] Seminal vesicle involvement was more difficult to detect, with a sensitivity of only 17% and specificity of 80% in the 188 individual seminal vesicles reviewed.[23] It has also been used to help localize recurrence following prostatectomy, with 108/181 patients being found to have disease in the prostatic fossa versus LNs on ProstaScint® scan.[22] However, only half of these positive antibody detections were able to be confirmed with biopsy.[22] In order to increase sensitivity, ProstaScint® has been combined with diffusion-weighted MRI, which increased sensitivity from 40.0% to 88.9% while maintaining a specificity > 96%.[24] While some studies suggested Fluciclovine PET to be superior to ProstaScint® in detecting metastasis as discussed in previous sections, the combination of ProstaScint® with DWI-MRI was not studied.[25] ProstaScint® is also currently being used for pretreatment prognostic staging and localization of biologic target volumes for individualized image-guided radiotherapy dose escalation.[26] This modality has also been used to optimize patient selection before salvage cryotherapy, showing increased success versus clinical risk alone and sparing patients’ years of androgen deprivation therapy.[27] While the sensitivity of ProstaScint® scan for the detection of metastatic disease preoperatively is low, it’s performance for detecting recurrence disease after prostatectomy is promising.
CHOLINE POSITRON EMISSION TOMOGRAPHY
Choline is a precursor of the phospholipids incorporated into cell membranes during proliferation. In proliferating prostate cancer tissue, an intensification in choline kinase activity brings increased choline into cells.[28] Choline has been labeled with both 11Carbon and 18Flourine for its use in PET. Choline PET has been extensively studied in both primary and metastatic prostate cancer. A meta-analysis done by Evangelista et al. included ten studies that evaluated 18Flouromethylcholine PET/CT in the detection of LN disease during initial staging in prostate cancer patients. This study found a pooled sensitivity and specificity of 49.2% and 95%, respectively.[11,29] In 2016, Fanti et al. completed a meta-analysis including 18 studies that utilized choline PET/CT. They discovered a pooled sensitivity and specificity of 89%.[30,31] In a separate meta-analysis of 27 studies evaluating choline PET and other imaging modalities in the diagnosis of bone metastases, the pooled per-patient sensitivity was 91%, and specificity was 99%.[32] The pooled per-lesion sensitivity and specificity were 84% and 93%.[32] In a study by Beheshti et al. of 250 patients with biochemical recurrence, 74% of the patients had a positive choline PET.[33] The scan sensitivity was 77.5%, 80.7%, 85.2%, and 92.8% for PSA levels of 0.5, 1, 2, and 4 ng/mL, respectively.[33] Overall, the reported sensitivities and specificities for both 11C and 18F choline PET vary widely in the literature.[34] Although typically thought to be less sensitive and specific than newer modalities such as 68Ga-PSMA PET, recent studies have attempted to directly analyze the difference of utilizing 68Ga-PSMA versus 11C-choline in PET/CT.[35] In a study done in 2017, researchers noted a significant difference in detection rates between 68Ga-PSMA and 11C-choline PET when looking at 123 prostate cancer patients, especially at low PSA levels (PSA <1 ng/mL). 11C-choline showed more promise only in a few patients with higher PSA levels (PSA >1 ng/mL), a finding in line with literature that discusses a superiority over 68Ga-PSMA at such levels.[35] Given these findings, choline PET appears to be best suited to assist in distinguishing between locoregional versus distant prostate cancer, helping guide therapeutic decisions with that distinction.[30] In its recent prostate cancer guidelines, the European Association of Urology included choline PET in the imaging options for the evaluation of recurrent prostate cancer, especially when the PSA is >1 ng/mL and the doubling time is <6 months.[36]
FLUCICLOVINE POSITRON EMISSION TOMOGRAPHY
Fluciclovine is an alicyclic nonnatural amino acid that is taken up in higher levels by prostate cancer cells.[37] Fluciclovine has been labeled with 18Fluorine for use in PET scanning and has been studied extensively, mainly with regard to recurrent prostate cancer.[38] A recent clinical trial compared fluciclovine to ProstaScint® and included 93 patients with recurrent prostate cancer who underwent both imaging studies within 90 days of each other.[25] Of the 93 patients, 82.8% had a positive fluciclovine PET, while only 60.2% of the ProstaScint® scans were positive.[25] For patients with recurrence in the prostate or prostatic bed, the sensitivity and specificity were 90.2% and 40.0%.[25] The fluciclovine PET had a sensitivity and specificity of 55.0% and 96.7% for extraprostatic recurrences.[25] The PET scan outperformed the ProstaScint® scan in all measures and correctly upstaged 25.7% of patients.[25] In a comparative study from 2019, 106 patients were evaluated with both fluciclovine and Tc99 mMDP, with fluciclovine showing stronger detection overall and a greater number of disease sites than that detected from Tc99 mMDP. These findings were confirmed through pathology at 4-month follow-up visits. Overall sensitivity and specificity in fluciclovine were 100% and 98%, respectively, while only 79% and 86% in Tc99 mMDP.[39] In a study of fifty patients with recurrent prostate cancer, imaging with both 18F-fluciclovine and 11C-choline was undertaken within 7 days of each other.[27] On a per-patient, per-lesion, LN, bone metastasis, and local recurrence basis, the fluciclovine PET was statistically significantly superior at identifying prostate cancer metastasis.[40] It was also better at identifying recurrence at all PSA values.[40] A meta-analysis conducted by Ren et al., in 2016, attempted to analyze the performance of fluciclovine in the diagnosis of recurrent prostate carcinoma. They reviewed six studies on biochemical recurrence and found a pooled sensitivity of 87% with a specificity of 55%.[41] In 2018, Glaser performed a systematic review to look at evidence supporting the utility of fluciclovine PET for prostate cancer.[42] He found studies demonstrating a superior sensitivity and specificity compared to CT, for patients with histologically confirmed biochemical recurrent prostate cancer. Studies showed the detection of extraprostatic true-positive lesions in 29% of patients using Fluciclovine PET, whereas only 7% of true-positive lesions were discovered in patients using CT.[30,43] He also found studies demonstrating superiority of fluciclovine over mpMRI, with detection rates of 94.7% and 31.6%-36.8%, respectively, in a cohort of 24 men with clinical biochemical recurrent prostate cancer.[42] An 18F-fluciclovine compound was recently approved for commercial use by the FDA and is currently being marketed under the trade name Axumin®. Figure 1 demonstrates an example of recurrent cancer detected by fluciclovine scan in a patient with negative conventional imaging. Knowledge of normal physiologic distribution and typical variations, as well as common patterns of prostate cancer spread, are needed to ensure appropriate interpretation of Fluciclovine PET findings.[30]
Figure 1.
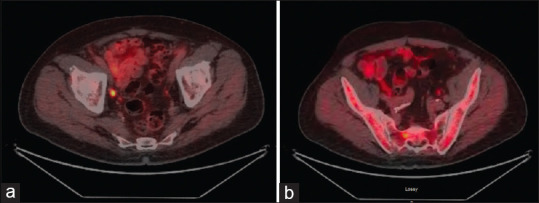
(a) Pt. is 66-year-old male with a history of Gleason 4+3 prostate cancer s/p = status post radical prostatectomy in 2013 followed by salvage radiation therapy. Patient presented with rising prostate-specific antigen and underwent Fluciclovine positron emission tomography scan for restaging. Imaging demonstrates abnormal Fluciclovine uptake involving R external lymph node, consistent with metastasis. Patient underwent salvage LN dissection and pathology of metastasis was confirmed. (b) Patient is 66-year-old male with a history of Gleason 4+3 prostate cancer s/p radical prostatectomy in 2013 followed by salvage radiation therapy. Patient presented with rising prostate-specific antigen and underwent Fluciclovine positron emission tomography scan for restaging. Subtle increased uptake in R aspect of sacrum, representing possible early bone metastases
PROSTATE-SPECIFIC MEMBRANE ANTIGEN POSITRON EMISSION TOMOGRAPHY
PSMA is a transmembrane protein expressed on the apical surface of the epithelium of prostatic ducts.[44] Expression of PSMA is increased in prostate cancer compared to benign hypertrophied tissue and has been shown to increase with increasing prostate cancer grade but does not correlate to clinical stage.[45,46] This variability in expression is the basis for the PSMA PET, as only 5%–10% of primary prostate cancer lesions have been shown to be PSMA negative.[47] PSMA PET/CT plays a critical role in the assessment of patients with prostate cancer shown through biochemical recurrence.[48] PSMA can be labeled with 68Gallium for its use in PET and has shown promise for diagnosing both primary and metastatic prostate cancer.[46,49] A meta-analysis from 2016, including 16 studies and over 1,300 patients, reported a 76% positive lesion rate in patients undergoing scans for biochemical recurrence (recurrence definition ranged from >0.2 to 0.5 ng/mL).[50] In a subgroup of these studies that reported histologic confirmation of imaging findings, the per-patient sensitivity and specificity were both 86%.[50] The per-lesion sensitivity and specificity were reported as 80% and 97%, respectively.[50] PET positivity was demonstrated to increase with increasing PSA level.[50] With a PSA of <0.2 ng/mL, there was a 42% pooled positivity, which increased to 58%, 76%, and 95% with PSA levels of 0.2–0.99, 1.0–1.99, and >2.0 ng/mL, respectively.[50] In 2015, two retrospective studies looked at populations of 319 and 248 patients, finding positive detection rates of 88% and 90%, respectively.[51,52] Another retrospective study completed in 2017, looked at 1007 patients, finding a position detection rate of 79.5.[53] Several studies have compared the performance of 18F-choline PET to 68Ga-PSMA PET, with PSMA PET outperforming choline PET.[54] In one study, PSMA PET detected sites of recurrence in 44% of patients with a negative choline PET.[54] Novel labeling agents, including N-[N-[(S)-1,3-dicarboxypropyl] carbamoyl]-4-18F-fluorobenzyl-L-cysteine (18F-DCFBC) and 2-(3-{1-carboxy-5-[(6-[(18) F] fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL), have been developed to improve the performance of PSMA PET.[55,56] 18F-DCFBC was shown to have an improved sensitivity (90%) compared to CT scan (64%), Tc99 mMDP bone scan (40%), and CT + bone scan combined (71%) in patients with known metastatic disease in both hormone sensitive and castrate resistant settings.[55] The study was, however, limited as there was no histopathologic confirmation, only confirmation based on lesion response to treatment.[55] In a direct comparison study involving 14 patients with recurrent prostate cancer, staging shown by 18F-DCFPyL was equivalent to that with 68Ga-PSMA PET.[57] A study in 2017 showed 18F-DCFPyL to be superior to conventional imaging methods, detecting 131 sites of cancer in patients with known metastatic disease, compared to only 45 sites detected on x-ray, bone scan, and CT.[56] Although many studies show these novel agents to be superior, a prospective study from 2018 compared results in 23 patients with metastatic cancer in bone lesions, using PSMA-targeted 18F-DCFBC PET and 18F-NaF PET and showed a superior detection rate for 18F-NaF PET– 98.4% compared to 45.9%.[58] 68Gallium-labeled PSMA has not been approved by the FDA for use in PET scans but continues to become a clinically accepted technique for prostate cancer imaging worldwide.[47]
OTHER UPCOMING MODALITIES
The need for better imaging modalities has led to development of alternative techniques for detection. One such emerging modality involves radiolabeled bombesin analogs. Bombesin is a tetradecapeptide that antagonizes the gastrin-releasing peptide receptor, which is expressed in prostate cancer cells.[59] Radiolabeled bombesin analogs have been shown to be inferior to current imaging modalities, with studies showing 0%–40% detection rates compared to 100% in radiolabeled choline.[59] Other modalities include MR lymphography, a molecular imaging technique that distinguishes normal functioning tissues from metastatic tissue through the utilization of contrast agents. Contrast is taken up by macrophages and metastatic tissue has significantly fewer macrophages, allowing for a variation in image signaling.[60] Studies have shown MR lymphography to have varying sensitivity and specificity, with two studies showing a range of 82%–92% and 93%–98%, respectively.[60] Another imaging modality utilizes the humanized monoclonal antibody, J591, which targets the extracellular domain of PSMA.[61] This antibody has been studied with radiolabeling through Lutetium and Indium, as well as Zirconium. In 2014, Pandit-Taskar et al. reported the first data on the use of 89Zr-huJ591 in patients with metastatic prostate cancer. Results from their analysis showed superiority over conventional imaging methods, but further testing is needed to determine other analytical measures.[61]
CONCLUSIONS
Detecting low volume metastatic prostate cancer can be challenging. Traditional modalities of CT, MRI, and bone scan have been the mainstay for both preoperative imaging and detection of recurrent disease. Each modality has shortcomings, and as shown above, variable sensitivities and specificities. Perhaps these techniques would benefit from improvements in magnet field strength or protocol standardization.[62] However, new techniques have been developed which have shown promising results for detecting metastatic disease both in preoperative staging and in biochemical recurrence following treatment. Utility of these agents can help ensure patients receive appropriate therapy and avoid costly interventions.[63] As the availability of these modalities increases and more data are reported, their clinical usefulness will be better demonstrated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Street W. American Cancer Society. Cancer Facts and Figures 2020. Atlanta: American Cancer Society; 2020. [Google Scholar]
- 2.Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer:A review. JAMA. 2017;317:2532–42. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Tourinho-Barbosa R, Srougi V, Nunes-Silva I, Baghdadi M, Rembeyo G, Eiffel SS, et al. Biochemical recurrence after radical prostatectomy:What does it mean? Int Braz J Urol. 2018;44:14–21. doi: 10.1590/S1677-5538.IBJU.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Prostate Cancer (version 1.2018. 2018. [Last accessed on 2020 Sep 20]. Available from:https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf .
- 6.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer:AUA/ASTRO/SUO guideline. Part I:Risk stratification, shared decision making, and care options. J Urol. 2018;199:683–90. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 7.Van Poppel H, Ameye F, Oyen R, Van de Voorde W, Baert L. Accuracy of combined computerized tomography and fine needle aspiration cytology in lymph node staging of localized prostatic carcinoma. J Urol. 1994;151:1310–4. doi: 10.1016/s0022-5347(17)35238-2. [DOI] [PubMed] [Google Scholar]
- 8.Engeler CE, Wasserman NF, Zhang G. Preoperative assessment of prostatic carcinoma by computerized tomography. Weaknesses and new perspectives. Urology. 1992;40:346–50. doi: 10.1016/0090-4295(92)90386-b. [DOI] [PubMed] [Google Scholar]
- 9.Briganti A, Abdollah F, Nini A, Suardi N, Gallina A, Capitanio U, et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. Eur Urol. 2012;61:1132–8. doi: 10.1016/j.eururo.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer:A meta-analysis. Clin Radiol. 2008;63:387–95. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Smith CP, Laucis A, Harmon S, Mena E, Lindenberg L, Choyke PL, et al. Novel imaging in detection of metastatic prostate cancer. Curr Oncol Rep. 2019;21:31. doi: 10.1007/s11912-019-0780-8. [DOI] [PubMed] [Google Scholar]
- 12.Rifkin MD, Zerhouni EA, Gatsonis CA, Quint LE, Paushter DM, Epstein JI, et al. Comparison of magnetic resonance imaging and ultrasonography in staging early prostate cancer. Results of a multi-institutional cooperative trial. N Engl J Med. 1990;323:621–6. doi: 10.1056/NEJM199009063231001. [DOI] [PubMed] [Google Scholar]
- 13.Biondetti PR, Lee JK, Ling D, Catalona WJ. Clinical stage B prostate carcinoma:Staging with MR imaging. Radiology. 1987;162:325–9. doi: 10.1148/radiology.162.2.3797644. [DOI] [PubMed] [Google Scholar]
- 14.Jager GJ, Barentsz JO, Oosterhof GO, Witjes JA, Ruijs SJ. Pelvic adenopathy in prostatic and urinary bladder carcinoma:MR imaging with a three-dimensional TI-weighted magnetization-prepared-rapid gradient-echo sequence. AJR Am J Roentgenol. 1996;167:1503–7. doi: 10.2214/ajr.167.6.8956585. [DOI] [PubMed] [Google Scholar]
- 15.Patel P, Wang S, Siddiqui MM. The use of multiparametric magnetic resonance imaging (mpMRI) in the detection, evaluation, and surveillance of clinically significant prostate cancer (csPCa) Curr Urol Rep. 2019;20:60. doi: 10.1007/s11934-019-0926-0. [DOI] [PubMed] [Google Scholar]
- 16.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer:99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–97. [PubMed] [Google Scholar]
- 17.Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. J Nucl Med. 2005;46:1356–67. [PubMed] [Google Scholar]
- 18.Pagani JJ, Libshitz HI. Imaging bone metastases. Radiol Clin North Am. 1982;20:545–60. [PubMed] [Google Scholar]
- 19.Beheshti M, Langsteger W, Fogelman I. Prostate cancer:Role of SPECT and PET in imaging bone metastases. Semin Nucl Med. 2009;39:396–407. doi: 10.1053/j.semnuclmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Langsteger W, Rezaee A, Pirich C, Beheshti M. 18F-NaF-PET/CT and 99mTc-MDP bone scintigraphy in the detection of bone metastases in prostate cancer. Semin Nucl Med. 2016;46:491–501. doi: 10.1053/j.semnuclmed.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2114–21. doi: 10.1007/s00259-016-3435-0. [DOI] [PubMed] [Google Scholar]
- 22.Kahn D, Williams RD, Manyak MJ, Haseman MK, Seldin DW, Libertino JA, et al. 111Indium-capromab pendetide in the evaluation of patients with residual or recurrent prostate cancer after radical prostatectomy. The ProstaScint Study Group. J Urol. 1998;159:2041–6. doi: 10.1016/S0022-5347(01)63239-7. [DOI] [PubMed] [Google Scholar]
- 23.Rieter WJ, Keane TE, Ahlman MA, Ellis CT, Spicer KM, Gordon LL. Diagnostic performance of In-111 capromab pendetide SPECT/CT in localized and metastatic prostate cancer. Clin Nucl Med. 2011;36:872–8. doi: 10.1097/RLU.0b013e318219ae29. [DOI] [PubMed] [Google Scholar]
- 24.Hardie AD, Rieter WJ, Bradshaw ML, Gordon LL, Young MA, Keane TE. Improved performance of SPECT-CT In-111 capromab pendetide by correlation with diffusion-weighted magnetic resonance imaging for identifying metastatic pelvic lymphadenopathy in prostate cancer. World J Urol. 2013;31:1327–32. doi: 10.1007/s00345-013-1079-2. [DOI] [PubMed] [Google Scholar]
- 25.Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18) F] FACBC positron emission tomography-computerized tomography and (111) In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma:results of a prospective clinical trial. J Urol. 2014;191:1446–53. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellis RJ, Kaminsky DA, Zhou EH, Fu P, Chen WD, Brelin A, et al. Ten-year outcomes:The clinical utility of single photon emission computed tomography/computed tomography capromab pendetide (Prostascint) in a cohort diagnosed with localized prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:29–34. doi: 10.1016/j.ijrobp.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 27.El-Zawahry AM. Capromab pendetide scanning has a potential role in optimizing patient selection for salvage cryosurgical ablation of the prostate. Urology. 2010;76:1162–7. doi: 10.1016/j.urology.2010.01.082. [DOI] [PubMed] [Google Scholar]
- 28.Ramírez de Molina A, Rodríguez-González A, Gutiérrez R, Martínez-Piñeiro L, Sánchez J, Bonilla F, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun. 2002;296:580–3. doi: 10.1016/s0006-291x(02)00920-8. [DOI] [PubMed] [Google Scholar]
- 29.Evangelista L, Guttilla A, Zattoni F, Muzzio PC, Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer:A systematic literature review and meta-analysis. Eur Urol. 2013;63:1040–8. doi: 10.1016/j.eururo.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 30.Parent EE, Schuster DM. Update on 18F-fluciclovine PET for prostate cancer imaging. J Nucl Med. 2018;59:733–9. doi: 10.2967/jnumed.117.204032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fanti S, Minozzi S, Castellucci P, Balduzzi S, Herrmann K, Krause BJ, et al. PET/CT with (11) C-choline for evaluation of prostate cancer patients with biochemical recurrence:Meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging. 2016;43:55–69. doi: 10.1007/s00259-015-3202-7. [DOI] [PubMed] [Google Scholar]
- 32.Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer:A meta-analysis. Skeletal Radiol. 2014;43:1503–13. doi: 10.1007/s00256-014-1903-9. [DOI] [PubMed] [Google Scholar]
- 33.Beheshti M, Haim S, Zakavi R, Steinmair M, Waldenberger P, Kunit T, et al. Impact of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence:Influence of androgen deprivation therapy and correlation with PSA kinetics. J Nucl Med. 2013;54:833–40. doi: 10.2967/jnumed.112.110148. [DOI] [PubMed] [Google Scholar]
- 34.Mapelli P. 11C- or 18F-Choline PET/CT for imaging evaluation of biochemical recurrence of prostate cancer. J Nucl Med. 2016;57(Suppl 3):43–8. doi: 10.2967/jnumed.115.169755. [DOI] [PubMed] [Google Scholar]
- 35.Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. doi: 10.1007/s00259-016-3490-6. [DOI] [PubMed] [Google Scholar]
- 36.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II:Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Oka S, Okudaira H, Yoshida Y, Schuster DM, Goodman MM, Shirakami Y. Transport mechanisms of trans-1-amino-3-fluoro[1-(14) C] cyclobutanecarboxylic acid in prostate cancer cells. Nucl Med Biol. 2012;39:109–19. doi: 10.1016/j.nucmedbio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Savir-Baruch B, Zanoni L, Schuster DM. Imaging of prostate cancer using fluciclovine. PET Clin. 2017;12:145–57. doi: 10.1016/j.cpet.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Chen B, Wei P, Macapinlac HA, Lu Y. Comparison of 18F-Fluciclovine PET/CT and 99mTc-MDP bone scan in detection of bone metastasis in prostate cancer. Nucl Med Commun. 2019;40:940–6. doi: 10.1097/MNM.0000000000001051. [DOI] [PubMed] [Google Scholar]
- 40.Nanni C, Schiavina R, Brunocilla E, Boschi S, Borghesi M, Zanoni L, et al. 18F-Fluciclovine PET/CT for the detection of prostate cancer relapse:A comparison to 11C-Choline PET/CT. Clin Nucl Med. 2015;40:e386–91. doi: 10.1097/RLU.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 41.Ren J, Yuan L, Wen G, Yang J. The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma:A meta-analysis. [Last accessed on 2020 Jun 11];Acta Radiol. 2015 57:487–93. doi: 10.1177/0284185115581541. Available from:https://journals.sagepub.com/doi/10.1177/0284185115581541 . [DOI] [PubMed] [Google Scholar]
- 42.Glaser ZA, Rais-Bahrami S. Fluciclovine positron emission tomography in the setting of biochemical recurrence following local therapy of prostate cancer. Transl Androl Urol. 2018;7:824–30. doi: 10.21037/tau.2018.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odewole OA, Tade FI, Nieh PT, Savir-Baruch B, Jani AB, Master VA, et al. Recurrent prostate cancer detection with anti-3-[(18) F] FACBC PET/CT:Comparison with CT. Eur J Nucl Med Mol Imaging. 2016;43:1773–83. doi: 10.1007/s00259-016-3383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955–64. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 45.Wright GL, Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28. doi: 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 46.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma:A study of 184 cases. Cancer. 1998;82:2256–61. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 47.Schwarzenboeck SM, Rauscher I, Bluemel C, Fendler WP, Rowe SP, Pomper MG, et al. PSMA ligands for PET imaging of prostate cancer. J Nucl Med Med. 2017;58:1545–52. doi: 10.2967/jnumed.117.191031. [DOI] [PubMed] [Google Scholar]
- 48.Keidar Z, Gill R, Goshen E, Israel O, Davidson T, Morgulis M, et al. 68Ga-PSMA PET/CT in prostate cancer patients –Patterns of disease, benign findings and pitfalls. [Last accessed on 2020 Jun 01];Cancer Imaging. 2018 18:39. doi: 10.1186/s40644-018-0175-3. Available from:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6211573/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer:biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 50.Perera M. Sensitivity, specificity, and predictors of positive (68) Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer:A Systematic review and meta-analysis. Eur Urol. 2016;70:926–37. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68) Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 53.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer:Evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bluemel C, Krebs M, Polat B, Linke F, Eiber M, Samnick S, et al. 68Ga-PSMA-PET/CT in patients with biochemical prostate cancer recurrence and negative 18F-Choline-PET/CT. Clin Nucl Med. 2016;41:515–21. doi: 10.1097/RLU.0000000000001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rowe SP, Macura KJ, Ciarallo A, Mena E, Blackford A, Nadal R, et al. Comparison of prostate-specific membrane antigen-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone-naïve and castration-resistant metastatic prostate cancer. J Nucl Med. 2016;57:46–53. doi: 10.2967/jnumed.115.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowe SP, Macura KJ, Mena E, Blackford AL, Nadal R, Antonarakis ES, et al. PSMA-based [(18) F] DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol. 2016;18:411–9. doi: 10.1007/s11307-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomäcker K, et al. Comparison of [18F] DCFPyL and [68Ga] Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17:575–84. doi: 10.1007/s11307-015-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harmon SA, Bergvall E, Mena E, Shih JH, Adler S, McKinney Y, et al. A Prospective comparison of 18F-sodium fluoride PET/CT and PSMA-targeted 18F-DCFBC PET/CT in metastatic prostate cancer. J Nucl Med. 2018;59:1665–71. doi: 10.2967/jnumed.117.207373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mertan FV, Lindenberg L, Choyke PL, Turkbey B. PET imaging of recurrent and metastatic prostate cancer with novel tracers. Future Oncol. 2016;12:2463–77. doi: 10.2217/fon-2016-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bjurlin MA, Turkbey B, Rosenkrantz AB, Gaur S, Choyke PL, Taneja SS. Imaging the high-risk prostate cancer patient:Current and future approaches to staging. Urology. 2018;116:3–12. doi: 10.1016/j.urology.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Pandit-Taskar N, O'Donoghue JA, Beylergil V, Lyashchenko S, Ruan S, Solomon SB, et al. 89Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:2093–105. doi: 10.1007/s00259-014-2830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdellaoui A, Iyengar S, Freeman S. Imaging in prostate cancer. Future Oncol. 2011;7:679–91. doi: 10.2217/fon.11.43. [DOI] [PubMed] [Google Scholar]
- 63.Ware RE, Williams S, Hicks RJ. Molecular imaging of recurrent and metastatic prostate cancer. Semin Nucl Med. 2019;49:280–93. doi: 10.1053/j.semnuclmed.2019.02.005. [DOI] [PubMed] [Google Scholar]


