Abstract
Background
Brexucabtagene autoleucel (brexu-cel) is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved in the USA for adults with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) and in the European Union for patients ≥26 years with R/R B-ALL. After 2 years of follow-up in ZUMA-3, the overall complete remission (CR) rate (CR+CR with incomplete hematological recovery (CRi)) was 73%, and the median overall survival (OS) was 25.4 months in 78 Phase 1 and 2 patients with R/R B-ALL who received the pivotal dose of brexu-cel. Outcomes by prior therapies and subsequent allogeneic stem cell transplantation (alloSCT) are reported.
Methods
Eligible adults had R/R B-ALL and received one infusion of brexu-cel (1×10⁶ CAR T cells/kg) following conditioning chemotherapy. The primary endpoint was the CR/CRi rate per central review. Post hoc subgroup analyses were exploratory with descriptive statistics provided.
Results
Phase 1 and 2 patients (N=78) were included with median follow-up of 29.7 months (range, 20.7–58.3). High CR/CRi rates were observed across all prior therapy subgroups examined: 1 prior line of therapy (87%, n=15) and ≥2 prior lines (70%, n=63); prior blinatumomab (63%, n=38) and no prior blinatumomab (83%, n=40); prior inotuzumab (59%, n=17) and no prior inotuzumab (77%, n=61); and prior alloSCT (76%, n=29) and no prior alloSCT (71%, n=49). The frequency of Grade ≥3 cytokine release syndrome, neurological events, and treatment-related Grade 5 adverse events were largely similar among prior therapy subgroups.
Median duration of remission (DOR) in responders with (n=14) and without (n=43) subsequent alloSCT was 44.2 (95% CI, 8.1 to not estimable (NE)) and 18.6 months (95% CI, 9.4 to NE); median OS was 47.0 months (95% CI, 10.2 to NE) and not reached (95% CI, 23.2 to NE), respectively. Median DOR and OS were not reached in responders without prior or subsequent alloSCT (n=22).
Conclusions
In ZUMA-3, adults with R/R B-ALL benefited from brexu-cel, regardless of prior therapies and subsequent alloSCT status, though survival appeared better in patients without certain prior therapies and in earlier lines of therapy. Additional studies are needed to determine the impact prior therapies and subsequent alloSCT have on outcomes of patients who receive brexu-cel.
Keywords: Hematologic Neoplasms; Immunity, Cellular; Immunotherapy; Cytotoxicity, Immunologic; Cell Engineering
WHAT IS ALREADY KNOWN ON THIS TOPIC
It is unclear what the optimal sequencing of available salvage therapies is for patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) and whether allogeneic stem cell transplantation (alloSCT) following chimeric antigen receptor T-cell therapy provides patients with long-term benefits.
WHAT THIS STUDY ADDS
In ZUMA-3, adults with R/R B-ALL benefited from brexucabtagene autoleucel (brexu-cel), regardless of prior therapies and subsequent alloSCT status, though survival appeared better in patients without certain prior therapies and in earlier lines of therapy.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Together, these results suggest that meaningful clinical responses can be obtained with brexu-cel therapy in adult patients with R/R B-ALL following multiple prior therapies, with manageable safety, and that subsequent alloSCT may not be necessary to achieve long-term survival following brexu-cel therapy.
Introduction
Adult patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) have a poor prognosis with worsening outcomes for each subsequent salvage therapy.1 Although blinatumomab and inotuzumab have improved outcomes for adults with R/R B-ALL, the median overall survival (OS) following these therapies is <8.0 months, highlighting an important unmet need.2 3 A common treatment strategy to improve outcomes following salvage therapy is to consolidate with allogeneic stem cell transplantation (alloSCT), currently the only potentially curative therapy for B-ALL; however, this strategy relies on achieving remission with salvage therapy and for some patients, can be associated with certain toxicities such as graft-versus-host disease.4
Brexucabtagene autoleucel (brexu-cel, formerly known as KTE-X19) is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy approved in the USA to treat adults with R/R B-ALL and in the European Union to treat patients ≥26 years with R/R B-ALL based on the positive results of the pivotal ZUMA-3 study (NCT02614066).5 6 After 2 years of follow-up in ZUMA-3, brexu-cel demonstrated a high overall complete remission (CR) rate (CR+CR with incomplete hematological recovery (CRi)) of 73%, with a median duration of remission (DOR) of 18.6 months and a median OS of 25.4 months in 78 adults (age ≥18 years) with R/R B-ALL who received the pivotal dose of brexu-cel.7
With multiple salvage therapies now available for R/R B-ALL, questions remain regarding optimal sequencing of these therapies. It is unclear how prior exposure to CD19-targeting and CD22-targeting therapies such as blinatumomab and inotuzumab may impact outcomes with subsequent anti-CD19 CAR T-cell therapy.8 9 Additionally, it is not known whether consolidation with alloSCT following remission with autologous anti-CD19 CAR T-cell therapy in adults with R/R B-ALL is necessary to produce durable remissions, given the potential for long-term responses with CAR T-cell therapies alone in certain lymphomas.10–13 Therefore, to better understand how prior therapies and subsequent alloSCT may impact outcomes of patients with R/R B-ALL treated with brexu-cel, we performed exploratory post hoc assessments of efficacy and safety outcomes in various prior treatment subgroups and efficacy durability outcomes in subsequent alloSCT subgroups. Here, we report outcomes by prior exposure to blinatumomab, inotuzumab, or alloSCT and by subsequent alloSCT status after more than 2 years of follow-up in a pooled analysis of Phase 1 and 2 ZUMA-3 adult patients with R/R B-ALL who received the pivotal dose of brexu-cel.
Methods
Study design and patients
Detailed methodology for the single-arm, multicenter, Phase 1/2 ZUMA-3 study (NCT02614066) was previously reported (online supplemental methods).14 15 Briefly, patients were aged ≥18 years with R/R B-ALL (>5% blasts in the bone marrow (BM)). R/R disease was defined as primary refractory, first relapse after remission of ≤12 months, R/R after ≥2 previous lines of systemic therapy, or R/R after alloSCT. Patients with prior alloSCT could enroll if the alloSCT occurred at least 100 days before enrollment and the patient discontinued use of immunosuppressive medications ≥4 weeks before enrollment. Patients could have received previous blinatumomab given CD19 tumor expression from BM or circulating blasts was documented after completing the most recent prior line of therapy; if CD19 expression was quantified, then ≥90% CD19-positive blasts were required for inclusion. ZUMA-3 was conducted in accordance with the Declaration of Helsinki principles.15
jitc-2023-007118supp001.pdf (3MB, pdf)
Procedures
Patients underwent leukapheresis followed by conditioning chemotherapy (intravenous fludarabine 25 mg/m2 on Days −4, −3, and −2; and intravenous cyclophosphamide 900 mg/m2 on Day −2) and a single infusion of brexu-cel at a target dose of 1×106 CAR T cells/kg on Day 0. Bridging chemotherapy was allowed per physician’s discretion as previously reported and outlined in the protocol (online supplemental file 1).15 Following bridging therapy, BM blast levels were reevaluated by Day −4 pre-infusion. AlloSCT was allowed as subsequent consolidative therapy following brexu-cel treatment at physician’s discretion but was not protocol-defined.
Outcomes
The primary endpoint was the overall CR/CRi rate per independent central assessment. Key secondary endpoints included DOR and relapse-free survival (RFS) per independent central assessment, with patients undergoing new anticancer therapies (including alloSCT) censored; OS; and safety. Exploratory endpoints included CAR T-cell levels in the blood.
Statistical analyses
Efficacy, safety, and exploratory endpoints are reported in Phase 2 treated patients and pooled Phase 1 and 2 patients treated with the pivotal dose of brexu-cel (1×10⁶ CAR T cells/kg) in ZUMA-3. Post hoc subgroup efficacy and safety assessments were performed by prior number of therapy lines (1 prior line and ≥2 prior lines), prior blinatumomab therapy (yes or no), prior inotuzumab therapy (yes or no), and prior alloSCT (yes or no; all prior therapies were received prior to enrollment on study and not used as bridging therapy). Post hoc subgroup efficacy durability assessments were performed by subsequent alloSCT (yes or no).
Time-to-event endpoints were analyzed using the Kaplan-Meier method. Subgroup analyses were unplanned and conducted retrospectively. As such, no specific hypotheses were tested, and only descriptive statistics are reported.
Role of the funding source
The study sponsor, in collaboration with the authors, participated in designing the study; collecting, analyzing, and interpreting the data; and writing the report.
Results
Patients
At data cut-off, the median follow-up time was 26.8 months (range, 20.7–32.6) for Phase 2 treated patients (N=55). Baseline characteristics for Phase 2 patients were previously reported.15 A total of 78 patients in ZUMA-3 (23 Phase 1 patients and 55 Phase 2 patients) received the pivotal dose of brexu-cel (22 Phase 1 patients received other doses of brexu-cel and were not included in this analysis14), with a median follow-up duration of 29.7 months (range, 20.7–58.3) at data cut-off (July 23, 2021). The median age was 42.5 years (range, 18–84), with 12 patients aged 65 years or older (15%). Most patients had an Eastern Cooperative Oncology Group performance status score of 1 (72%) and had greater than 25% BM blasts at baseline (72%). The median number of prior therapies received was 2 (range, 1–8), with 63 patients (81%) receiving ≥2 prior therapies. Additional baseline characteristics are included in table 1.
Table 1.
Baseline disease and patient characteristics in pooled Phase 1 and 2 patients treated at the pivotal dose (N=78) by prior therapies
| Phase 1 and 2 treated patients (N=78)* | Prior number of therapies | Prior blinatumomab | Prior inotuzumab | Prior alloSCT | ||||||
| 1 (n=15) |
≥2 (n=63) |
Yes (n=38) |
No (n=40) |
Yes (n=17) |
No (n=61) |
Yes (n=29) |
No (n=49) |
|||
| Age, median (range), years | 42.5 (18–84) | 39 (21–65) | 44 (18–84) | 39 (18–84) | 44.5 (19-77) | 46 (18–84) | 39 (19–73) | 39 (19–69) | 45 (18–84) | |
| ≥65 years, n (%) | 12 (15) | 1 (7) | 11 (17) | 5 (13) | 7 (18) | 3 (18) | 9 (15) | 3 (10) | 9 (18) | |
| Male, n (%) | 42 (54) | 9 (60) | 33 (52) | 21 (55) | 21 (53) | 12 (71) | 30 (49) | 15 (52) | 27 (55) | |
| ECOG PS of 1, n (%) | 56 (72) | 9 (60) | 47 (75) | 28 (74) | 28 (70) | 13 (76) | 43 (70) | 21 (72) | 35 (71) | |
| Philadelphia chromosome-positive, n (%) | 17 (22) | 2 (13) | 15 (24) | 5 (13) | 12 (30) | 4 (24) | 13 (21) | 10 (34) | 7 (14) | |
| Extramedullary disease at screening, n (%) | 9 (12) | 0 | 9 (14) | 7 (18) | 2 (5) | 2 (12) | 7 (11) | 3 (10) | 6 (12) | |
| CNS-1 disease at baseline, n (%) | 78 (100) | 15 (100) | 63 (100) | 38 (100) | 40 (100) | 17 (100) | 61 (100) | 29 (100) | 49 (100) | |
| Bone marrow blasts at baseline, median (range) % | 63 (0–98) | 58 (0–95) | 65 (2–98) | 70 (2–98) | 54 (0–96) | 76 (2–98) | 52 (0–97) | 61 (0–96) | 65 (0–98) | |
| >25%, n (%) | 56 (72) | 10 (67) | 46 (73) | 28 (74) | 28 (70) | 14 (82) | 42 (69) | 20 (69) | 36 (73) | |
| CD19 lymphoblast baseline category per central laboratory, % | ||||||||||
| ≥95 | 58 (74) | 11 (73) | 47 (75) | 29 (76) | 29 (73) | 13 (76) | 45 (74) | 21 (72) | 37 (76) | |
| <95 | 15 (19) | 3 (20) | 12 (19) | 7 (18) | 8 (20) | 4 (24) | 11 (18) | 6 (21) | 9 (18) | |
| Missing | 5 (6) | 1 (7) | 4 (6) | 2 (5) | 3 (8) | 0 | 5 (8) | 2 (7) | 3 (6) | |
| Number of prior therapies, median (range) | 2 (1–8) | 1 (1–1) | 3 (2–8) | 3 (1–8) | 2 (1–5) | 3 (2–7) | 2 (1–8) | 3 (1–8) | 2 (1–7) | |
| ≥3 prior lines of therapy, n (%) | 37 (47) | 0 | 37 (59) | 22 (58) | 15 (38) | 14 (82) | 23 (38) | 19 (66) | 18 (37) | |
| Prior blinatumomab, n (%) | 38 (49) | 1 (7) | 37 (59) | 38 (100) | 0 | 10 (59) | 28 (46) | 14 (48) | 24 (49) | |
| Prior inotuzumab, n (%) | 17 (22) | 0 | 17 (27) | 10 (26) | 7 (18) | 17 (100) | 0 | 5 (17) | 12 (24) | |
| Prior alloSCT, n (%) | 29 (37) | 2 (13) | 27 (43) | 14 (37) | 15 (38) | 5 (29) | 24 (39) | 29 (100) | 0 | |
| Prior radiotherapy, n (%) | 19 (24) | 3 (20) | 16 (25) | 11 (29) | 8 (20) | 3 (18) | 16 (26) | 14 (48) | 5 (10) | |
| Primary refractory, n (%) | 24 (31) | 6 (40) | 18 (29) | 11 (29) | 13 (33) | 4 (24) | 20 (33) | 7 (24) | 17 (35) | |
| Relapsed or refractory to second or greater line of therapy, n (%) | 60 (77)† | 0 | 60 (95) | 35 (92) | 25 (63) | 17 (100) | 43 (70) | 25 (86) | 35 (71) | |
| First relapse with remission ≤12.0 months, n (%) | 22 (28) | 8 (53) | 14 (22) | 7 (18) | 15 (38) | 0 | 22 (36) | 7 (24) | 15 (31) | |
| Relapsed or refractory post-alloSCT, n (%) | 30 (38)‡ | 3 (20) | 27 (43) | 14 (37) | 16 (40) | 5 (29) | 25 (41) | 29 (100) | 1 (2)‡ | |
| Response to the last prior therapy, n (%) | ||||||||||
| CR | 25 (32) | 8 (53) | 17 (27) | 11 (29) | 14 (35) | 3 (18) | 22 (36) | 12 (41) | 13 (27) | |
| CRi | 2 (3) | 1 (7) | 1 (2) | 0 | 2 (5) | 0 | 2 (3) | 1 (3) | 1 (2) | |
| PR | 2 (3) | 0 | 2 (3) | 1 (3) | 1 (3) | 0 | 2 (3) | 1 (3) | 1 (2) | |
| NR | 24 (31) | 3 (20) | 21 (33) | 10 (26) | 14 (35) | 5 (29) | 19 (31) | 4 (14) | 20 (41) | |
| PD | 19 (24) | 3 (20) | 16 (25) | 13 (34) | 6 (15) | 5 (29) | 14 (23) | 6 (21) | 13 (27) | |
| Not evaluated | 6 (8) | 0 | 6 (10) | 3 (8) | 3 (8) | 4 (24) | 2 (3) | 5 (17) | 1 (2) | |
*Only includes patients who received the pivotal dose of brexu-cel.
†Two patients with relapsed or refractory disease to second or greater lines of therapy were erroneously not marked in the eCRF as such.
‡One patient had prior autologous transplantation but was erroneously marked in the eCRF as relapsed/refractory disease after alloSCT.
alloSCT, allogeneic stem cell transplantation; CNS, central nervous system; CR, complete remission; CRi, complete remission with incomplete hematological recovery; ECOG PS, Eastern Cooperative Oncology Group performance status; eCRF, electronic case report form; NR, no response; PD, progressive disease; PR, partial response.
Outcomes by number of prior therapy lines
Of the 78 pooled Phase 1 and 2 patients, 15 (19%) patients received 1 prior line of therapy and 63 (81%) patients received ≥2 prior lines of therapy at baseline, with a median age of 39 (range, 21–65) and 44 years (range, 18–84), respectively. Most baseline patient and disease characteristics were largely similar between 1 and ≥2 prior therapy subgroups, with some differences in the types of prior therapies received and response to last prior therapy (table 1).
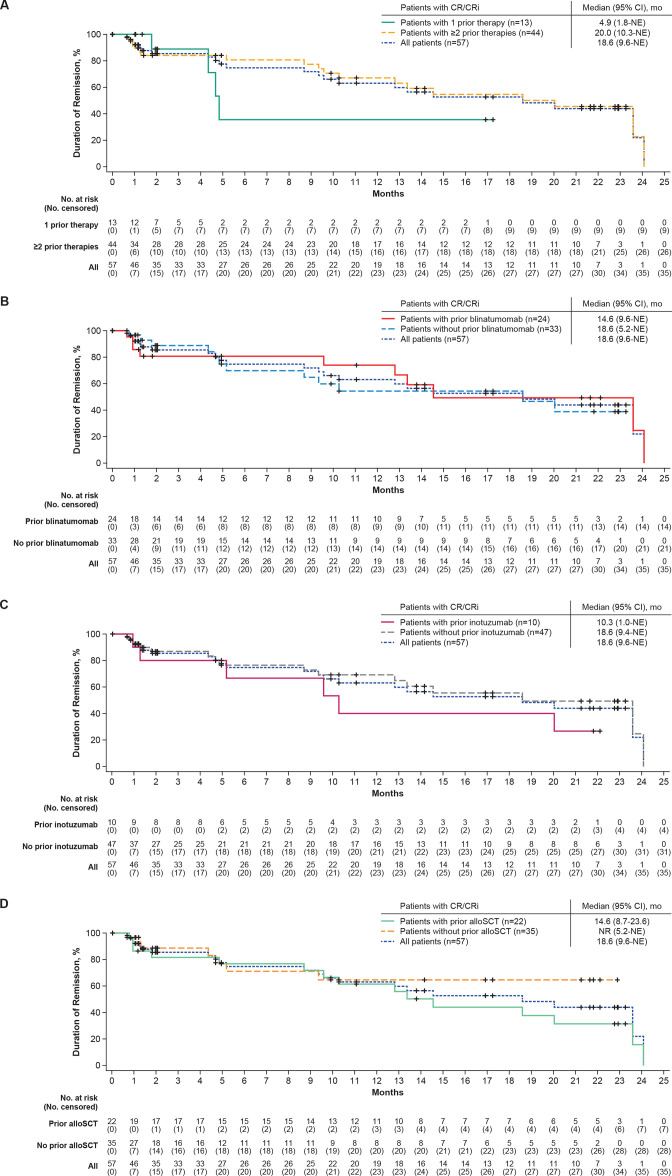
Overall CR/CRi rates, per independent central review, were 87% (95% CI, 60% to 98%; 80% CR rate) in patients who received 1 prior therapy and 70% (95% CI, 57% to 81%; 56% CR rate) in those who received ≥2 prior therapies (table 2). In patients who achieved a response, the median DOR censored at subsequent therapy, including alloSCT, was 4.9 months (95% CI, 1.8 to not estimable (NE); n=13) for those with 1 prior therapy and was 20.0 months (95% CI, 10.3 to NE) for those with ≥2 prior therapies (n=44; table 2; figure 1A). Median DOR not censored at subsequent alloSCT was 5.7 months (95% CI, 4.4 to NE) and 20.0 months (95% CI, 12.8 to 24.1) for responders with 1 or ≥2 prior therapies, respectively (online supplemental figure S1A). At data cut-off, 1 of the 13 responders (CR or CRi) with 1 prior therapy (8%) was in ongoing remission without subsequent therapy, 3 (23%) proceeded to subsequent alloSCT, 4 (31%) proceeded to other anticancer therapies (online supplemental table S1), 1 (8%) was lost to follow-up, and 4 (31%) relapsed. Of the 44 responders who had received ≥2 prior lines of therapy, 11 (25%) were in ongoing remission without subsequent therapy, 11 (25%) proceeded to subsequent alloSCT, 4 (9%) proceeded to other anticancer therapies (online supplemental table S1), 15 (34%) relapsed, and 3 (7%) died (1 due to COVID-19, 1 due to progressive disease, and 1 due to an adverse event (AE)).
Table 2.
Efficacy and durability outcomes by prior therapies for pooled Phase 1 and 2 patients treated at the pivotal dose (N=78) and Phase 2 treated patients (N=55)
| N | CR/CRi rate, n (%) | CR, n (%) | CRi, n (%) | BFBM, n (%) | No response, n (%) | Median DOR, mo. (95% CI) | Median RFS, mo. (95% CI) | Median OS, mo. (95% CI) | |
| Phase 1 and 2 patients treated at pivotal dose | 78 | 57 (73) | 47 (60) | 10 (13) | 6 (8) | 12 (15) | 18.6 (9.6 to NE) | 11.7 (6.1 to 20.5) | 25.4 (16.2 to NE) |
| Prior number of therapies | |||||||||
| 1 | 15 | 13 (87) | 12 (80) | 1 (7) | 1 (7) | 1 (7) | 4.9 (1.8 to NE) | 6.1 (2.8 to NE) | NR (7.6 to NE) |
| ≥2 | 63 | 44 (70) | 35 (56) | 9 (14) | 5 (8) | 11 (17) | 20.0 (10.3 to NE) | 11.7 (2.7 to 20.5) | 25.4 (15.9 to NE) |
| Prior blinatumomab | |||||||||
| Yes | 38 | 24 (63) | 18 (47) | 6 (16) | 4 (11) | 8 (21) | 14.6 (9.6 to NE) | 7.3 (0 to 15.5) | 15.9 (8.3 to 25.4) |
| No | 40 | 33 (83) | 29 (73) | 4 (10) | 2 (5) | 4 (10) | 18.6 (5.2 to NE) | 11.7 (6.1 to NE) | 47.0 (18.6 to NE) |
| Prior inotuzumab | |||||||||
| Yes | 17 | 10 (59) | 7 (41) | 3 (18) | 2 (12) | 3 (18) | 10.3 (1.0 to NE) | 2.2 (0 to 12.3) | 8.8 (2.2 to NE) |
| No | 61 | 47 (77) | 40 (66) | 7 (11) | 4 (7) | 9 (15) | 18.6 (9.4 to NE) | 14.2 (6.1 to 25.4) | 47.0 (18.6 to NE) |
| Prior alloSCT | |||||||||
| Yes | 29 | 22 (76) | 17 (59) | 5 (17) | 2 (7) | 4 (14) | 14.6 (8.7 to 23.6) | 12.3 (2.7 to 20.5) | 25.4 (14.2 to NE) |
| No | 49 | 35 (71) | 30 (61) | 5 (10) | 4 (8) | 8 (16) | NR (5.2 to NE) | 10.3 (2.7 to NE) | 47.0 (10.9 to NE) |
| Phase 2 treated patients | 55 | 39 (71) | 31 (56) | 8 (15) | 4 (7) | 9 (16) | 14.6 (9.4 to NE) | 11.6 (2.7 to 20.5) | 25.4 (16.2 to NE) |
| Prior number of therapies | |||||||||
| 1 | 10 | 9 (90) | 8 (80) | 1 (10) | 1 (10) | 0 | 4.7 (1.8 to NE) | 5.6 (0 to NE) | NR (2.1 to NE) |
| ≥2 | 45 | 30 (67) | 23 (51) | 7 (16) | 3 (7) | 9 (20) | 14.6 (9.4 to NE) | 11.0 (1.8 to 15.5) | 25.4 (14.2 to NE) |
| Prior blinatumomab | |||||||||
| Yes | 25 | 15 (60) | 10 (40) | 5 (20) | 2 (8) | 6 (24) | 19.1 (1.3 to NE) | 11.6 (0 to 25.4) | 14.2 (3.2 to 26.0) |
| No | 30 | 24 (80) | 21 (70) | 3 (10) | 2 (7) | 3 (10) | 10.3 (5.2 to NE) | 11.7 (2.8 to 22.1) | NR (18.6 to NE) |
| Prior inotuzumab | |||||||||
| Yes | 12 | 8 (67) | 5 (42) | 3 (25) | 0 | 2 (17) | 9.6 (1.0 to 20.0) | 4.2 (0 to 12.3) | 15.9 (0.5 to NE) |
| No | 43 | 31 (72) | 26 (60) | 5 (12) | 4 (9) | 7 (16) | 18.6 (9.4 to NE) | 14.2 (2.7 to 25.4) | 26.0 (18.6 to NE) |
| Prior alloSCT | |||||||||
| Yes | 23 | 16 (70) | 13 (57) | 3 (13) | 2 (9) | 4 (17) | 14.6 (8.7 to 23.6) | 11.7 (0 to 20.5) | 25.4 (14.2 to NE) |
| No | 32 | 23 (72) | 18 (56) | 5 (16) | 2 (6) | 5 (16) | NR (4.7 to NE) | 6.1 (2.2 to NE) | NR (9.0 to NE) |
alloSCT, allogeneic stem cell transplantation; BFBM, blast-free hypoplastic or aplastic bone marrow; CR, complete remission; CRi, complete remission with incomplete hematological recovery; DOR, duration of remission; mo, month; NE, not estimable; NR, not reached; OS, overall survival; RFS, relapse-free survival.
Figure 1.
Duration of remission (censored at subsequent alloSCT) in Phase 1 and 2 treated patients by (A) prior number of therapy lines, (B) prior blinatumomab exposure, (C) prior inotuzumab exposure, and (D) prior alloSCT exposure. Kaplan-Meier estimates of the duration of remission by central assessment, with censoring of patients at subsequent alloSCT. alloSCT, allogeneic stem cell transplantation; CR, complete remission; CRi, complete remission with incomplete hematological recovery; mo, month; NE, not estimable; No, number; NR, not reached.
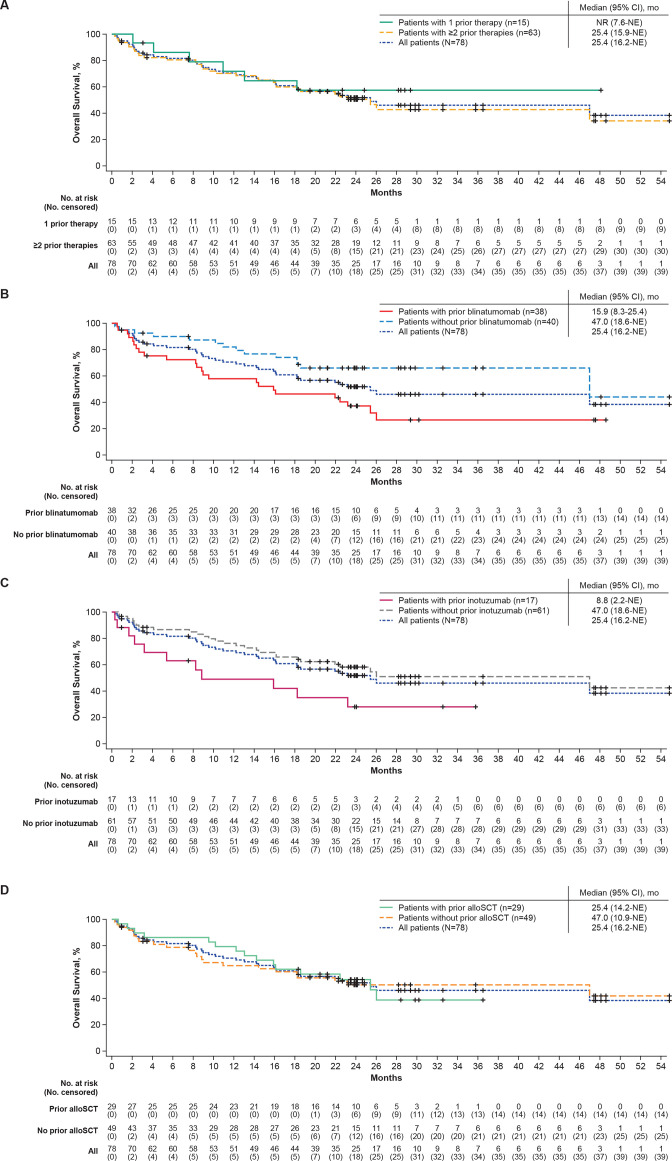
Median RFS censored at subsequent therapy, including alloSCT, was 6.1 months (95% CI, 2.8 to NE) for patients with 1 prior therapy and 11.7 months (95% CI, 2.7 to 20.5) for patients with ≥2 prior therapies (table 2 and online supplemental figure S2A). Median RFS not censored at subsequent alloSCT was 7.6 months (95% CI, 2.8 to NE) for patients with 1 prior therapy and 12.3 months (95% CI, 4.0 to 20.5) for patients with ≥2 prior therapies (online supplemental figure S1B). The median OS was not reached (NR; 95% CI, 7.6 to NE) in patients with 1 prior therapy and was 25.4 months (95% CI, 15.9 to NE) in patients with ≥2 prior therapies (table 2; figure 2A).
Figure 2.
Overall survival in Phase 1 and 2 treated patients by (A) prior number of therapy lines, (B) prior blinatumomab exposure, (C) prior inotuzumab exposure, and (D) prior alloSCT exposure. Kaplan-Meier estimates of the overall survival. alloSCT, allogeneic stem cell transplantation; mo, month; NE, not estimable; No, number; NR, not reached.
Of the patients with 1 prior therapy, 1 (7%) experienced a Grade 5 AE that was deemed not related to brexu-cel treatment (progressive disease), 2 (13%) experienced Grade ≥3 cytokine release syndrome (CRS), and 6 (40%) experienced Grade ≥3 neurological events (table 3). Of the patients with ≥2 prior therapies, 13 (21%) experienced a Grade 5 AE, 2 of which were related to brexu-cel treatment (brain herniation and septic shock as previously reported) and 11 of which were deemed not related to brexu-cel treatment (progressive disease (4), sepsis (2), graft-versus-host disease (1), herpes simplex viremia (1), pneumonia (1), pneumonia fungal (1), respiratory failure (1)15; 18 patients (29%) experienced Grade ≥3 CRS, and 19 (30%) experienced Grade ≥3 neurological events (table 3).
Table 3.
Summary of cytokine release syndrome and neurological events in pooled Phase 1 and 2 patients treated at pivotal dose (N=78)
| Prior number of therapies | Prior blinatumomab | Prior inotuzumab | Prior alloSCT | |||||
| 1 (n=15) |
≥2 (n=63) |
Yes (n=38) |
No (n=40) |
Yes (n=17) |
No (n=61) |
Yes (n=29) |
No (n=49) |
|
| Cytokine release syndrome, n (%)* | 14 (93) | 58 (92) | 34 (89) | 38 (95) | 16 (94) | 56 (92) | 26 (90) | 46 (94) |
| Grade 1 | 5 (33) | 10 (16) | 4 (11) | 11 (28) | 3 (18) | 12 (20) | 6 (21) | 9 (18) |
| Grade 2 | 7 (47) | 30 (48) | 18 (47) | 19 (48) | 7 (41) | 30 (49) | 15 (52) | 22 (45) |
| Grade ≥3 | 2 (13) | 18 (29) | 12 (32) | 8 (20) | 6 (35) | 14 (23) | 5 (17) | 15 (31) |
| Neurological events, n (%)† | 11 (73) | 42 (67) | 24 (63) | 29 (73) | 13 (76) | 40 (66) | 18 (62) | 35 (71) |
| Grade 1 | 3 (20) | 6 (10) | 4 (11) | 5 (13) | 4 (24) | 5 (8) | 2 (7) | 7 (14) |
| Grade 2 | 2 (13) | 17 (27) | 11 (29) | 8 (20) | 3 (18) | 16 (26) | 8 (28) | 11 (22) |
| Grade ≥3 | 6 (40) | 19 (30) | 9 (24) | 16 (40) | 6 (35) | 19 (31) | 8 (28) | 17 (35) |
Of the 55 Phase 2 treated patients, 10 (18%) had 1 prior line of therapy and 45 (82%) had ≥2 prior lines (online supplemental table S2). Efficacy outcomes in Phase 2 treated patients with 1 or ≥2 prior therapies were consistent with those reported in the larger pooled Phase 1 and 2 analysis (table 2). Kaplan-Meier estimates of DOR, RFS (both censored at subsequent therapy including alloSCT), and OS in Phase 2 treated patients by number of prior therapy lines are provided in online supplemental figures S3A, S4A, and S5A, respectively. Incidence of Grade ≤3 CRS and neurological events are reported in online supplemental table S3.
Outcomes by prior blinatumomab
Of the 78 pooled Phase 1 and 2 treated patients, 38 (49%) received prior blinatumomab therapy with a median time from blinatumomab initiation to brexu-cel infusion of 5.6 months (range 2.3–45.7). Seventeen of these patients (45%) received blinatumomab as their last prior therapy before receiving brexu-cel, with a median time from blinatumomab to brexu-cel of 3.4 months (range 2.3–45.7; best overall responses for patients with blinatumomab as last prior therapy are summarized in online supplemental table S4. Forty (51%) patients did not receive prior blinatumomab. For patients with and without prior blinatumomab therapy, respectively, the median age was 39 years (range, 18–84) and 44.5 years (range, 19–77); the median number of prior therapies was 3 (range, 1–8) and 2 (range, 1–5; table 1). Most baseline patient and disease characteristics were largely similar among patients with and without prior blinatumomab therapy, with notable differences in the median BM blast levels at baseline (70% vs 54%, respectively), the proportion of patients with R/R disease after second or greater lines of therapy (92% vs 63%, respectively), the proportion of patients with three or more prior lines of therapy (58% vs 38%, respectively), and the proportion of patients experiencing their first relapse within 12.0 months of remission (18% vs 38%, respectively; table 1).
Overall CR/CRi rates by independent central review were 63% (95% CI, 46% to 78%; CR rate, 47%) in patients with prior blinatumomab and 83% (95% CI, 67% to 93%; CR rate, 73%) in patients without prior blinatumomab therapy (table 2). Medians for DOR and RFS (both censored at subsequent therapy, including alloSCT) and OS in patients with prior blinatumomab therapy were 14.6 (95% CI, 9.6 to NE), 7.3 (95% CI, 0 to 15.5), and 15.9 (95% CI, 8.3 to 25.4) months, respectively, and were 18.6 (95% CI, 5.2 to NE), 11.7 (95% CI, 6.1 to NE), and 47.0 (95% CI, 18.6 to NE) months for patients without prior blinatumomab therapy (table 2, figure 1B, online supplemental figure S2B, and figure 2B). At data cut-off, 5 of the 24 patients with CR/CRi who had received prior blinatumomab therapy (21%) were in ongoing remission without subsequent therapy, 5 (21%) proceeded to subsequent alloSCT, 4 (17%) proceeded to other anticancer therapies, 7 (29%) relapsed, and 3 (13%) died. Of the 33 patients with CR/CRi who did not receive prior blinatumomab therapy, 7 (21%) were in ongoing remission without subsequent therapy, 9 (27%) proceeded to subsequent alloSCT, 4 (12%) proceeded to other anticancer therapies, 12 (36%) relapsed, and 1 (3%) was lost to follow-up.
Grade 5 AEs were reported in 9 patients (24%) with prior blinatumomab therapy and 5 patients (13%) without prior blinatumomab therapy; of these, 1 AE per subgroup was deemed related to brexu-cel therapy, as previously reported.15 Grade ≥3 CRS and neurological events occurred in 12 (32%) and 9 patients (24%) with prior blinatumomab therapy, respectively, and in 8 (20%) and 16 (40%) patients without prior blinatumomab therapy, respectively (table 3).
Of the 55 Phase 2 treated patients, 25 patients (45%) had prior blinatumomab and 30 (55%) did not. Efficacy outcomes in Phase 2 treated patients with or without prior blinatumomab therapy were largely similar to those reported in the pooled Phase 1 and 2 prior blinatumomab subgroups (table 2). Kaplan-Meier estimates of DOR, RFS (both censored at subsequent therapy including alloSCT), and OS in Phase 2 treated patients who received prior blinatumomab are provided in online supplemental figure S3B, S4B and S5B, respectively. Incidence of Grade ≤3 CRS and neurological events in Phase 2 patients are reported in online supplemental table S3.
Outcomes by prior inotuzumab
Of the 78 pooled Phase 1 and 2 treated patients, 17 patients (22%) had prior inotuzumab therapy, with a median time from inotuzumab to brexu-cel of 4.7 months (range, 2.0–13.9); 61 patients (78%) did not receive prior inotuzumab therapy. Nine patients received inotuzumab as their last prior therapy before brexu-cel infusion, with a median time from inotuzumab to brexu-cel of 2.6 months (range, 2.0–6.4). Patients who did and did not receive prior inotuzumab therapy had a median age of 46 and 39 years, a median baseline BM blast level of 76% and 52%, and a median number of prior therapies of three and two, respectively. Notable differences in baseline characteristics for patients with and without prior inotuzumab include the proportion of patients who were R/R to second or greater line of therapy (100% and 70%, respectively) and the proportion of patients who had their first relapse within 12.0 months of remission (0% vs 36%, respectively; table 1).
Patients with prior inotuzumab therapy had an overall CR/CRi rate of 59% (95% CI, 33% to 82%; CR rate, 41%), whereas patients who did not receive prior inotuzumab therapy had an overall CR/CRi rate of 77% (95% CI, 65% to 87%; CR rate, 66%). Medians for DOR (censored at subsequent anticancer therapy including alloSCT) were 10.3 months (95% CI, 1.0 to NE; n=10) and 18.6 months (95% CI, 9.4 to NE; n=47), respectively (table 2; figure 1C). At data cut-off, 2 of the 10 patients with prior inotuzumab therapy with CR/CRi (20%) were in ongoing remission, 1 (10%) proceeded to subsequent alloSCT, 1 (10%) proceeded to other anticancer therapy, and 6 (60%) relapsed. Of the 47 patients without prior inotuzumab therapy with CR/CRi, 10 (21%) were in ongoing remission, 13 (28%) proceeded to subsequent alloSCT, 7 (15%) proceeded to other anticancer therapies, 13 (28%) relapsed, 3 (6%) died, and 1 (2%) was lost to follow-up. Medians for RFS (censored at subsequent anticancer therapy including alloSCT) and OS were 2.2 (95% CI, 0 to 12.3) and 8.8 (95% CI, 2.2 to NE) months, respectively, in patients with prior inotuzumab therapy and 14.2 (95% CI, 6.1 to 25.4) and 47.0 (95% CI, 18.6 to NE) months, respectively, in patients who did not receive prior inotuzumab therapy (table 2, online supplemental figure S2C, figure 2C).
Grade 5 AEs occurred in 4 patients (24%) with prior inotuzumab therapy and in 10 patients (16%) without prior inotuzumab therapy; of these, 1 Grade 5 AE in each subgroup was deemed brexu-cel-related. Grade ≥3 CRS was experienced by 6 patients (35%) with prior inotuzumab therapy and 14 patients (23%) without prior inotuzumab therapy. Six (35%) of the patients with prior inotuzumab therapy and 19 (31%) of the patients without prior inotuzumab therapy experienced Grade ≥3 neurological events (table 3).
Of the 55 Phase 2 treated patients, 12 (22%) had prior inotuzumab therapy and 43 (78%) did not. Prior inotuzumab response rates and median DOR and RFS (censored at subsequent anticancer therapy including alloSCT) in Phase 2 were consistent with the pooled Phase 1 and 2 analysis; though for Phase 2 patients, the difference in median OS between patients with and without prior inotuzumab (15.9 months vs 26.0 months, respectively) was less pronounced compared with the reported difference between pooled Phase 1 and 2 prior inotuzumab subgroups (8.8 months vs 47.0 months, respectively; table 2, online supplemental figures S3C,S4C and S5C). Incidence of Grade ≤3 CRS and neurological events in Phase 2 patients are reported in online supplemental table S3.
Outcomes by prior alloSCT
Of the 78 pooled Phase 1 and 2 treated patients, 29 (37%) had prior alloSCT and 49 (63%) did not. Six of the 29 patients with prior alloSCT received alloSCT as their last prior therapy with a median time from alloSCT to brexu-cel of 11.4 months (range, 7.0–45.3). Baseline patient and disease characteristics were largely similar between prior alloSCT subgroups (yes or no), with slight differences in median age (39 vs 45, respectively), median number of prior therapies (three vs two, respectively), the proportion of patients with Philadelphia chromosome-positive disease (34% vs 14%, respectively), and the proportion of patients with prior radiotherapy (48% vs 10%, respectively; table 1).
Patients who received prior alloSCT had an overall CR/CRi rate of 76% (95% CI, 56% to 90%; CR rate, 59%) whereas patients who did not receive prior alloSCT had an overall CR/CRi rate of 71% (95% CI, 57% to 83%, CR rate, 61%; table 2). Median DOR (censored at subsequent therapy including alloSCT) was 14.6 months (95% CI, 8.7 to 23.6) for patients with prior alloSCT and NR (95% CI 5.2 to NE) for patients without prior alloSCT (table 2 and figure 1D). At data cut-off, 4 (18%) of the 22 patients with prior alloSCT with CR/CRi were in ongoing remission without subsequent therapy, 1 (5%) proceeded to subsequent alloSCT, 2 (9%) proceeded to other anticancer therapy, 12 (55%) relapsed, and 3 (14%) died. Of the 35 patients without prior alloSCT with CR/CRi, 8 (23%) were in ongoing remission without subsequent therapy, 13 (37%) proceeded to subsequent alloSCT, 6 (17%) proceeded to other anticancer therapies, 7 (20%) relapsed, none died, and 1 (3%) was lost to follow-up. Medians for RFS (censored at subsequent therapy including alloSCT) were similar between prior alloSCT subgroups (yes or no) at 12.3 months (95% CI, 2.7 to 20.5) and 10.3 months (95% CI, 2.7 to NE), respectively (table 2; Online supplemental figure S2D), whereas median OS was 25.4 months (95% CI, 14.2 to NE) in patients with prior alloSCT compared with 47.0 months (95% CI, 10.9 to NE) in patients without prior alloSCT (table 2; figure 2D).
Incidence of Grade ≥3 CRS in patients with prior alloSCT (n=5) was 17% and 31% in patients without prior alloSCT (n=15) (table 3). Grade ≥3 neurological events occurred in 8 patients (28%) with prior alloSCT and 17 patients (35%) without prior alloSCT (table 3). Grade 5 AEs were experienced by 5 patients (17%) who received prior alloSCT and 9 patients (18%) who did not receive prior alloSCT; of these, 1 Grade 5 AE in each subgroup was deemed brexu-cel-related.
Of the 55 Phase 2 treated patients, 23 (42%) had prior alloSCT, and 32 (58%) did not. Efficacy outcomes for prior alloSCT subgroups were similar between Phase 2 and pooled Phase 1 and 2 patients, with median DOR not reached in patients who did not receive prior alloSCT in both patient populations (table 2, online supplemental figures S3D,S4D and S5D). Incidence of Grade ≤3 CRS and neurological events is reported in online supplemental table S3.
Outcomes by subsequent alloSCT
Of the 78 pooled Phase 1 and 2 treated patients, 15 (19%) proceeded to subsequent alloSCT with a median time to alloSCT of 95.0 days (range, 60-390) for those with CR (n=11) and 134.0 days (range, 65–175) for those with CRi (n=3); and 63 patients (81%) did not proceed to subsequent alloSCT. Baseline characteristics were largely similar between responders who did and did not receive subsequent alloSCT; though responders who did receive subsequent alloSCT had a lower median number of prior therapies (two vs three), had a lower median level of BM blasts at baseline (37% vs 60%), and were less likely to have received a prior alloSCT (7% vs 49%) compared with responders who did not receive subsequent alloSCT (online supplemental table S5).
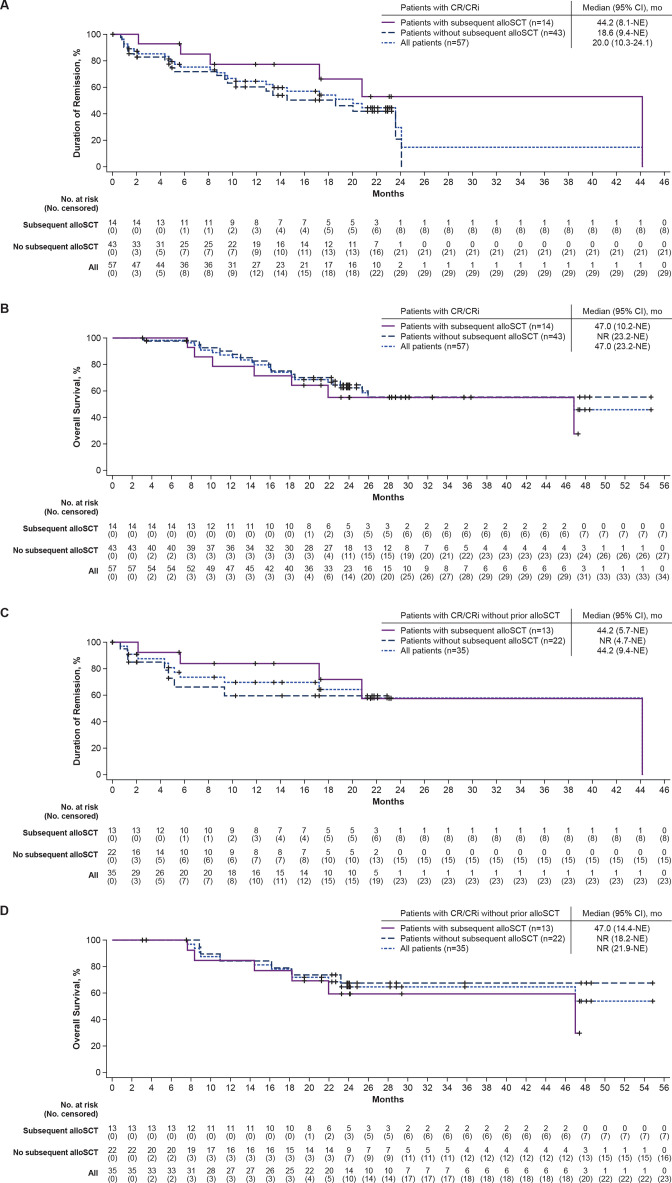
The median DOR (not censored at subsequent alloSCT) was 44.2 months (95% CI, 8.1 to NE) among responders who received subsequent alloSCT (n=14) and 18.6 months (95% CI, 9.4 to NE) among responders who did not receive subsequent alloSCT (n=43; figure 3A). At data cut-off, 7 (50%) of the 14 responders with subsequent alloSCT were in ongoing remission, 1 (7%) patient had proceeded to other anticancer therapies, 1 (7%) patient had relapsed, and 5 (36%) patients had died. Twelve (28%) of the 43 responders without subsequent alloSCT were in ongoing remission, 8 (19%) had proceeded to other anticancer therapies, 19 (44%) had relapsed, 3 (7%) had died, and 1 (2%) was lost to follow-up. The median OS in responders with subsequent alloSCT (n=14) was 47.0 months (95% CI, 10.2 to NE) and was NR (95% CI, 23.2 to NE) in responders without subsequent alloSCT (n=43; figure 3B).
Figure 3.
Duration of remission (not censored at subsequent alloSCT) and overall survival in pooled Phase 1 and 2 patients with response by subsequent alloSCT (A, B) and in pooled Phase 1 and 2 patients with response who did not receive prior alloSCT by subsequent alloSCT (C, D). Kaplan-Meier estimates of the duration of remission by central assessment, without censoring of patients at subsequent alloSCT, and overall survival in pooled Phase 1 and 2 patients with response by subsequent alloSCT and in pooled Phase 1 and 2 patients with response who did not receive prior alloSCT by subsequent alloSCT. alloSCT, allogeneic stem cell transplantation; CR, complete remission; CRi, complete remission with incomplete hematological recovery; mo, month; NE, not estimable; No, number; NR, not reached.figure 3B
Given that prior alloSCT may have confounding effects on these analyses, DOR and OS were also assessed in responders who did not receive prior alloSCT. For responders who did not receive prior alloSCT but did receive subsequent alloSCT (n=13), the median DOR (not censored at subsequent alloSCT) was 44.2 months (95% CI, 5.7 to NE), and for responders who did not receive prior or subsequent alloSCT (n=22), the median DOR was NR (95% CI, 4.7 to NE; figure 3C). At data cut-off, 7 (54%) of the 13 responders with no prior alloSCT who received subsequent alloSCT were in ongoing remission; 1 (8%) proceeded to other anticancer therapy, 1 (8%) relapsed, and 4 (31%) died. Of the 22 responders who did not receive prior or subsequent alloSCT, 8 (36%) were in ongoing remission, 7 (32%) relapsed, 6 (27%) started new anticancer therapy, none died, and 1 (5%) was lost to follow-up. For responders without prior alloSCT but with subsequent alloSCT (n=13), the median OS was 47.0 months (95% CI, 14.4 to NE), and for responders without prior or subsequent alloSCT (n=22), the median OS was NR (95% CI, 18.2 to NE; figure 3D).
Of the 55 Phase 2 treated patients, 11 (20%) proceeded to subsequent alloSCT, and 44 (80%) did not. Medians for DOR and OS for Phase 2 patients, per subsequent alloSCT subgroups, were largely similar to those values for corresponding alloSCT subgroups in pooled Phase 1 and 2 patients, with several medians not yet reached at data cut-off. Kaplan-Meier estimates of DOR (not censored at subsequent alloSCT) in responders with and without subsequent alloSCT and in those who did not receive prior alloSCT are depicted in online supplemental figure S6A and S6C. Kaplan-Meier estimates of OS in patients with and without subsequent alloSCT and in those who did not receive prior alloSCT are depicted in online supplemental figure S6B and S6D.
CAR T-cell levels by prior blinatumomab and inotuzumab
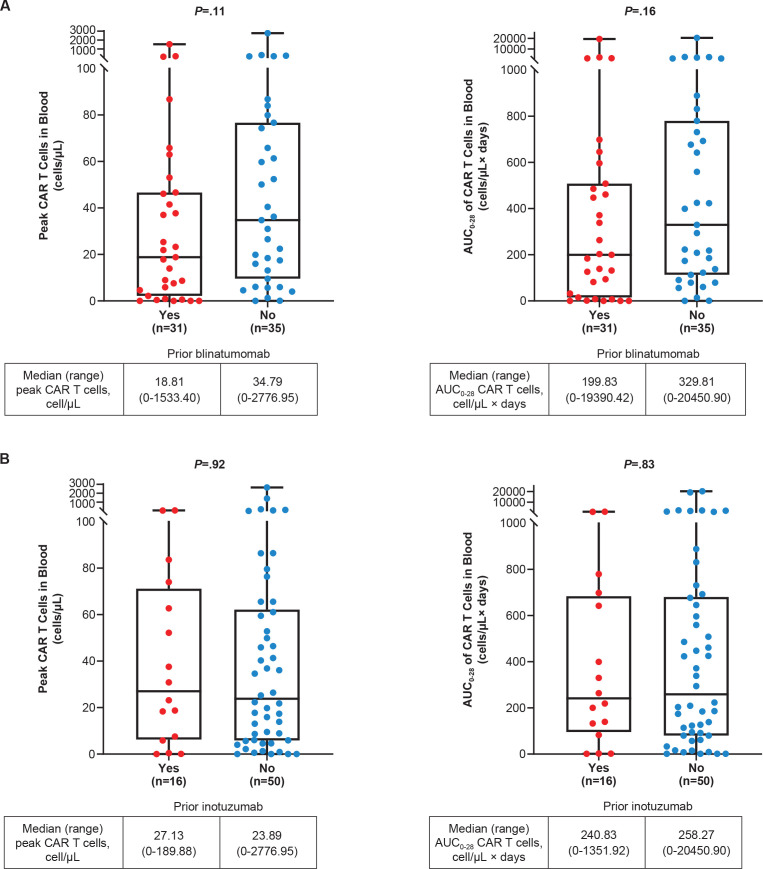
As previously reported, median peak CAR T-cell levels were significantly higher in Phase 2 responders compared with non-responders and were modestly higher in patients with ongoing responses compared with patients who had relapsed before data cut-off (16.4 months of median follow-up).15 In evaluable pooled Phase 1 and 2 treated patients, the median peak CAR T-cell levels in blood by prior blinatumomab (yes or no) were 18.8 cells/µL (range, 0–1533.4; n=31) and 34.8 cells/µL (range, 0–2777; n=35), respectively; and the median area under the curve from time of dose to 28 days (AUC0−28) CAR T-cell levels in blood were 199.8 cell/µL×days (range, 0–19390.4; n=31) and 329.8 cell/µL×days (0–20450.9; n=35), respectively (figure 4A). These differences may have been confounded by differences in baseline BM blast levels, which were previously shown to be inversely related to CAR T-cell expansion.15 Peak and AUC0–28 CAR T-cell levels in blood in pooled Phase 1 and 2 treated patients by BM blast levels at baseline in patients with and without prior blinatumomab are listed in online supplemental table S6. Peak and AUC0−28 CAR T-cell levels by blinatumomab as last prior therapy in evaluable pooled Phase 1 and 2 patients are shown in online supplemental figure S7.
Figure 4.
Peak and AUC0−28 CAR T-cell levels in pooled Phase 1 and 2 treated patients by (A) prior blinatumomab a and (B) prior inotuzumab. b Peak and AUC0−28 CAR T-cell levels assessed in the blood post brexu-cel infusion in pooled Phase 1 and 2 patients by prior blinatumomab (A) and prior inotuzumab (B). Subgroup n is the number of patients with documented CAR T cells in blood. Peak is defined as the maximum number of CAR T cells in blood measured after infusion. AUC0−28 is defined as the AUC in a plot of number of CAR T cells in blood against scheduled visit from Day 0 to Day 28. P value is calculated by Wilcoxon rank-sum test, a rank-based non-parametric test for two groups. a A similar proportion of patients with and without prior blinatumomab had >75% BM blasts at baseline (39% vs 34%, respectively). b For patients with and without prior inotuzumab, the proportion of patients with >75% BM blasts at baseline was 50% and 29%, respectively. AUC0−28, area under the curve from time of dose to 28 days; BM, bone marrow; CAR, chimeric antigen receptor.
The median peak and AUC0−28 CAR T-cell levels in blood were similar between evaluable pooled Phase 1 and 2 patients who received prior inotuzumab (n=16; peak, 27.1 cells/µL (range, 0–190); AUC0−28, 240.8 cell/µL×days (range, 0–1351.9)) and patients who did not receive prior inotuzumab (n=50; peak 23.9 cells/µL (range, 0–2777.0); AUC0−28, 258.3 cell/µL×days (range, 0–20450.9); figure 4B). Peak and AUC0−28 CAR T-cell levels in blood among pooled Phase 1 and 2 treated patients by BM blast levels at baseline in patients with and without prior inotuzumab are listed in online supplemental table S7.
Discussion
With >2 years of follow-up in an expanded Phase 1 and 2 analysis of brexu-cel at the pivotal dose in ZUMA-3, adults with R/R B-ALL continued to benefit from brexu-cel regardless of prior therapies, with a median OS of nearly 4 years in some subgroups. Some differences in efficacy outcomes were observed between post hoc univariate assessments of non-prespecified subgroups; however, it is important to note that differences in baseline patient and disease characteristics and small, unmatched sample sizes in some subgroups may have confounded these results. Therefore, these results should be interpreted with caution.
The analysis of patients by number of lines of prior therapy (1 or ≥2), in particular, may have been impacted by the large sample size disparity between subgroups. Patients with 1 line of prior therapy (n=15) had more favorable overall CR/CRi rates and median OS compared with those who had ≥2 prior therapies (n=63); however, they appeared to have shorter median DOR and RFS compared with those who had ≥2 prior therapies. Within the smaller subgroup, specifically, censorship based on the protocol-defined Kaplan-Meier calculations may have attributed to these observed differences.
Patients without prior blinatumomab or inotuzumab appeared to fare better across all efficacy outcomes reported compared with patients who received these prior therapies. One interpretation of these data is that using brexu-cel in earlier lines of therapy may provide greater benefit to patients; however, these comparisons do not account for patients who achieved long-term remission with these prior therapies. An analysis that accounts for successes of earlier lines of therapy would be required to determine the optimal sequencing of these agents. It is also possible that differences in baseline characteristics such as more aggressive disease, higher BM blast levels, and higher median number of prior therapies in patients with prior blinatumomab or inotuzumab may have impacted these results. In addition, the observed median OS in ZUMA-3 for these patient subgroups (15.9 and 8.8 months, respectively) are longer than the median OS historically observed with blinatumomab or inotuzumab therapy in adults with R/R B-ALL (<8.0 months), though differences in trial designs, eligibility criteria, and patient populations limit the interpretability of cross-trial comparisons.2 3
Given that documented OS rates for patients with R/R B-ALL decline with each subsequent salvage therapy, it is expected that patients with multiple prior therapies would have poorer outcomes in ZUMA-3; however, efficacy outcomes in the prior therapy subgroups examined remained only marginally lower than the overall ZUMA-3 population, suggesting benefit with brexu-cel in patients who would typically have a poor prognosis with salvage therapies.1 Even so, additional questions remain regarding the impact that prior blinatumomab and inotuzumab may have on the effectiveness of CD19-targeting CAR T cells.1 Despite somewhat lower response rates in patients with prior blinatumomab and inotuzumab, median peak and AUC0−28 CAR T-cell levels in blood were not significantly different from patients who did have these prior therapies (though trended lower in patients with prior blinatumomab), suggesting that the prior use of CD19-targeting and CD22-targeting therapies did not significantly impact anti-CD19 CAR T-cell expansion in brexu-cel-treated patients. Other possibilities reported in the literature include potential modulation or loss of the CD19 antigen from exposure to prior blinatumomab therapy, which may result in a less-targetable antigen and, therefore, reduced efficacy of subsequent CD19-targeting therapies8; however, baseline lymphoblast CD19 expression was similar regardless of prior blinatumomab exposure in ZUMA-3, as baseline CD19 positivity (>90% blast positivity in quantitative assessments) was required for inclusion in ZUMA-3 for patients with prior blinatumomab therapy. Factors contributing to the slightly lower response rate observed in blinatumomab or inotuzumab-exposed patients in ZUMA-3 remain to be determined.
Patients with prior alloSCT had a similar CR/CRi rate but numerically shorter median OS compared with patients without prior alloSCT; however, patients who had prior alloSCT experienced the same median OS as the overall population (25.4 months), thus benefiting greatly from brexu-cel therapy. Patients with subsequent alloSCT experienced favorable long-term response durability, with a median DOR of 44.2 months. Although median DOR in responders without subsequent alloSCT was 18.6 months, long-term benefit of brexu-cel was observed in these patients as well, with a median OS not yet reached after more than 2 years of follow-up. Of note, the median time to alloSCT after brexu-cel infusion (≥4 months) may have had a confounding impact on the DOR analysis; however, a landmark analysis of DOR was not assessed due to the limited number of patients with subsequent alloSCT (14 responders). In addition, the median DOR and OS in responders without prior or subsequent alloSCT were not yet reached, suggesting that brexu-cel alone can induce durable responses in patients with R/R B-ALL. Moreover, these findings were corroborated in the analysis of Phase 2 treated patients. Nevertheless, alloSCT is still considered standard of care and has an important role in treating patients with R/R B-ALL; its role as subsequent consolidative treatment after CAR T-cell therapy remains to be determined and represents an important area for further study.
The frequency of CRS, neurological events, and treatment-related Grade 5 AEs was largely similar between prior therapy subgroups, suggesting that prior therapies, including blinatumomab, inotuzumab, and alloSCT may have minimal impact on the incidence of CAR T-cell therapy-related AEs and long-term safety following treatment with brexu-cel in patients with R/R B-ALL.
Limitations of this analysis include the post hoc nature of the subgroup comparisons, which were limited to univariate analyses due to small patient numbers in some subgroups. Therefore, assessments were descriptive and potentially confounding factors such as baseline BM blast count and prior lines of therapy cannot be excluded.
Together, these results suggest that meaningful clinical responses can be obtained with brexu-cel therapy in adult patients with R/R B-ALL following multiple prior therapies, with manageable safety, though survival appeared most favorable in patients without prior blinatumomab and inotuzumab therapy and in earlier lines of therapy. Subsequent alloSCT was not necessary to achieve long-term survival with brexu-cel, though patients with subsequent alloSCT appeared to have longer response duration than patients without subsequent alloSCT. Additional studies are needed to better understand the impact prior and subsequent therapies may have on outcomes of CAR T-cell therapy in R/R B-ALL.
Acknowledgments
We thank the patients who participated in this trial and their families, caregivers, and friends; the trial investigators, coordinators, and healthcare staff at each site; Jinghui Dong, PhD, for supporting the development of the manuscript. Medical writing support was provided by Ashly Pavlovsky, PhD, from Nexus Global Group Science and was funded by Kite, a Gilead Company.
Footnotes
Twitter: @bshah, @RyanCassaday
BDS and AG contributed equally.
Contributors: The study was conceived and designed in collaboration between Kite (study sponsor) and the authors. BS, RC, JHP, RH, OOO, ACL, NB, TL, MRBi, MST, DT, KMO, MLA, YL, MRBa, GJS, MS, MA, MCM, WW, DJD, PJS, DJ, and AG enrolled and treated patients, and gathered data. DM and LZ completed the statistical analyses. All authors participated in drafting the article (with medical writing support funded by the Sponsor), provided feedback throughout the development process, and approved the final submitted version. BDS and AG are responsible for the overall content as guarantors.
Funding: This study was funded by Kite, a Gilead Company.
Competing interests: BS reports honoraria from Acrotech Biopharma, BeiGene, Gilead Sciences, Janssen, Pharmacyclics, and Spectrum; consulting/advisory role for Adaptive Biotechnologies; Amgen; Celgene/BMS; Kite, a Gilead Company; Novartis; Pfizer; and Precision BioSciences; research funding from Gilead Sciences, Incyte, Jazz Pharmaceuticals, and Kite; and travel support from Celgene, Janssen, Kite, Novartis, Pfizer, Seattle Genetics, and Stemline Therapeutics. RC reports spouse employment with Seagen; stock or other ownership in Seagen; honoraria from Amgen, Autolus (board member), PeproMene Bio (board member), Pfizer, and Kite; consultancy/advisory role for Amgen and Jazz; and research funding from Pfizer, Merck, Amgen, Servier, Kite, and Vanda. JHP reports consulting/advisory role for AstraZeneca, Kite, and Novartis and research funding from Amgen, Genentech, and Juno. RH reports honoraria from ADC therapeutics, Bristol Myers Squibb, Celgene, Gilead Sciences, Janssen, Kite, MSD, Novartis, and Roche; and consulting/advisory role for Kite/Gilead. OOO reports consulting/advisory role for Pfizer, Kite, Gilead, AbbVie, Janssen, TG Therapeutics, ADC, Novartis, Epizyme, Curio Science, Nektar, and Syncopation; research funding from Kite, Pfizer, Daiichi Sankyo, and Allogene; and honoraria from Pfizer and Gilead. ACL reports consulting/advisory role for AbbVie, Bristol Myers Squibb, and Pfizer; research funding from Amgen, Amphivena, Astellas, Autolus, Jazz, Kadmon, Kite, and Pharmacyclics. NB reports honoraria from and consulting/advisory role for Gilead Sciences. TL reports consultancy/advisory role for Amgen and Servier. MRBi reports honoraria from Incyte, Bristol Myers Squibb, Sanofi, and ADC Therapeutics; consulting/advisory role for Novartis, Kite/Gilead, Bristol Myers Squibb, Sana Bio, and CRISPR Therapeutics; and speakers’ bureau participation for Incyte, Bristol Myers Squibb, Sanofi, and ADC Therapeutics. MST reports consulting/advisory role for Amgen, Celgene, Kite, Regeneron, and Roche and research funding from Amgen, Kite, MacroGenics, Regeneron, and Roche. DT reports consulting/advisory role for Bristol Myers Squibb, EUSA, Partner, and Takeda; and research funding from Bristol Myers Squibb. KMO reports consulting/advisory role for Beam Therapeutics. MLA reports consulting/advisory role for Kite and Syndax Pharmaceuticals, Inc. and research funding from Kite (institutional PI). YL reports consulting/advisory role for Kite/Gilead, Celgene/BMS, Juno/BMS, bluebird bio, Janssen, Legend Biotech, Gamida Cell, Novartis, Iovance, Takeda, Fosun Kite, and Pfizer and research funding from Kite/Gilead, Celgene/BMS, bluebird bio, Janssen, Legend Biotech, Merck, Takeda, and Boston Scientific. MRBa reports research funding from AbbVie, Ascentage, Kite, Kura, and Takeda. GJS reports honoraria and research funding from and speakers’ bureau participation for Kite. MS reports consulting/advisory role for Amgen, Celgene/BMS, Gilead Sciences, Janssen, and Novartis; speakers’ bureau participation for Celgene/BMS, Gilead Sciences, Janssen, and Novartis; research funding from Amgen, Gilead Sciences, Miltenyi Biotec, MorphoSys, Roche, and Seattle Genetics; and travel support from Takeda. MA reports stock or other ownership in CytoDyn; consulting/advisory role for Celgene; and speakers’ bureau participation for Celgene, Bristol Myers Squibb, AbbVie, and Kite. MCM reports consulting/advisory role for Gilead Sciences and Janssen-Cilag and speakers’ bureau participation for Janssen-Cilag and Medscape. WW reports consulting/advisory role for Genzyme and Sanofi and research funding from AbbVie, Acerta, Cyclacel, Genentech, Gilead Sciences, GSK, Janssen, Juno, Karyopharm, Loxo Oncology, miRagen, Novartis, Oncternal, Pharmacyclics, Sunesis, and Xencor. DJD reports consulting or advisory role for Agios, Amgen, Autolus, Blueprint Medicines, Forty Seven, Gilead Sciences, Incyte, Jazz, Novartis, Pfizer, Shire, and Takeda and research funding from AbbVie, Glycomimetics, Novartis, and Blueprint Medicines. PJS reports honoraria from and consulting or advisory role for MorphoSys and CRISPR Therapeutics and research funding from Amgen, Gamida Cell, Pfizer, Karyopharm, Gilead Sciences, Incyte, Seagen, and Cellectar. DJ reports research funding from Jazz Pharmaceuticals and Pfizer. DM has nothing to report. SA reports employment with and stock or other ownership in Kite. LZ reports employment with Kite and ownership of AbbVie RSU or stock in the past 2 years. reports employment with Kite; stock or other ownership in Gilead Sciences; and travel support from Kite and Gilead Sciences. RDK reports employment, stock, or other ownership in Kite. AG reports consulting/advisory role for Amgen, Atara, Bristol Myers Squibb, CRISPR Therapeutics, Kite, and Wugen Inc.; research funding from Amgen, Genentech, and Kite; and honoraria from Kite.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by University of South Florida (IRB#:Ame31_Pro00022671) and ADVARRA (IRB # 00000971). Participants gave informed consent to participate in the study before taking part.
References
- 1.Gökbuget N, Dombret H, Ribera J-M, et al. International reference analysis of outcomes in adults with B-precursor pH-negative Relapsed/refractory acute Lymphoblastic leukemia. Haematologica 2016;101:1524–33. 10.3324/haematol.2016.144311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute Lymphoblastic leukemia. N Engl J Med 2017;376:836–47. 10.1056/NEJMoa1609783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus standard of care in Relapsed or refractory acute Lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019;125:2474–87. 10.1002/cncr.32116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldoss I, Yang D, Malki MMA, et al. Allogeneic hematopoietic cell transplantation for Relapsed and refractory Philadelphia negative B cell ALL in the era of novel salvage therapies. Transplant Cell Ther 2021;27:255. 10.1016/j.jtct.2020.12.020 [DOI] [PubMed] [Google Scholar]
- 5.Kite Pharma, Inc . TECARTUS® (brexucabtagene autoleucel) [prescribing information]. Santa Monica, CA, 2021. [Google Scholar]
- 6.Kite Pharma EU B.V . TECARTUS® (autologous anti-CD19-transduced CD3+ cells) [Summary of Product Characteristics]. Hoofddorp, The Netherlands, 2021. [Google Scholar]
- 7.Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with Relapsed or refractory adult B-cell acute Lymphoblastic leukemia in ZUMA-3 and its Contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol 2022;15:170. 10.1186/s13045-022-01379-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers RM, Taraseviciute A, Steinberg SM, et al. Blinatumomab Nonresponse and high-disease burden are associated with inferior outcomes after Cd19-CAR for B-ALL. J Clin Oncol:Jco2101405 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillai V, Muralidharan K, Meng W, et al. CAR T-cell therapy is effective for Cd19-dim B-Lymphoblastic leukemia but is impacted by prior Blinatumomab therapy. Blood Adv 2019;3:3539–49. 10.1182/bloodadvances.2019000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westin JR, Kersten MJ, Salles G, et al. Efficacy and safety of Cd19-directed CAR-T cell therapies in patients with Relapsed/refractory aggressive B-cell Lymphomas: observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am J Hematol 2021;96:1295–312. 10.1002/ajh.26301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldsmith SR, Ghobadi A, DiPersio JF. Hematopoeitic cell transplantation and CAR T-cell therapy: complements or competitors Front Oncol 2020;10:608916. 10.3389/fonc.2020.608916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldsmith SR, Ghobadi A, Dipersio JF, et al. Chimeric antigen receptor T cell therapy versus hematopoietic stem cell transplantation: an evolving perspective. Transplantation and Cellular Therapy 2022;28:727–36. 10.1016/j.jtct.2022.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah BD, Ghobadi A, Oluwole OO, et al. Two-year follow-up of KTE-X19 in patients with Relapsed or refractory adult B-cell acute Lymphoblastic leukemia in ZUMA-3 and its Contextualization with SCHOLAR-3, an external historical control study. J Hematol Oncol 2022;15. 10.1186/s13045-022-01379-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah BD, Stock W, Wierda WG, et al. Phase 1 results of ZUMA-3: KTE-C19, an anti-Cd19 Chimeric antigen receptor (CAR) T cell therapy, in adult patients with Relapsed/refractory acute Lymphoblastic leukemia (R/R ALL). Blood 2017;130:888. 10.1182/blood.V130.Suppl_1.888.888 [DOI] [Google Scholar]
- 15.Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for Relapsed or refractory adult B-cell acute Lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, Multicentre ZUMA-3 study. Lancet 2021;398:491–502. 10.1016/S0140-6736(21)01222-8 [DOI] [PubMed] [Google Scholar]
- 16.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–95. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topp MS, Göckbuget N, Stein AS. Correction to lancet Oncol 2015; 16: 60, 61. safety and activity of Blinatumomab for adult patients with Relapsed or refractory B-precursor acute Lymphoblastic leukaemia: a multi-centre, single-arm, phase 2 study. Lancet Oncol 2015;16. 10.1016/S1470-2045(15)70154-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007118supp001.pdf (3MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health, and access can be requested by contacting medinfo@kitepharma.com.