Abstract
Objectives
To compare the annual and period prevalence of modifiable cardiovascular risk factors (MCVRFs) between populations with and without osteoarthritis (OA) in the UK over 25 years.
Methods
215 190 patients aged 35 years and over from the UK Clinical Practice Research Datalink GOLD database who were newly diagnosed OA between 1992 and 2017, as well as 1:1 age-matched, sex-matched, practice-matched and index year-matched non-OA individuals, were incorporated. MCVRFs including smoking, hypertension, type 2 diabetes, obesity and dyslipidaemia were defined by Read codes and clinical measurements. The annual and period prevalence and prevalence rate ratios (PRRs) of individual and clustering (≥1, ≥2 and ≥3) MCVRFs were estimated by Poisson regression with multiple imputations for missing values.
Results
The annual prevalence of MCVRFs increased in the population with OA between 1992 and 2017 and was consistently higher in the population with OA compared with the population without OA between 2004 and 2017. Trends towards increased or stable annual PRRs for individuals and clustering of MCVRFs were observed. A 26-year period prevalence of single and clustering MCVRFs was significantly higher in individuals with OA compared with non-OA individuals. Period PRRs were higher in Southern England, women and increased with age for most MCVRFs except for obesity, which has the higher PRR in the youngest age group.
Conclusions
A consistently higher long-term prevalence of MCVRFs was observed in individuals with OA compared to those without OA. The higher prevalence of obesity in the youngest age group with OA highlights the need for public health strategies. Further research to understand MCVRF management in OA populations is necessary.
Keywords: osteoarthritis, cardiovascular diseases, epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies have suggested that shown osteoarthritis (OA) might share risk factors with cardiovascular diseases (CVDs).
However, there is a limited research on the long-term prevalence of modifiable cardiovascular risk factors (MCVRFs) in OA populations in the UK.
WHAT THIS STUDY ADDS
This study found that individuals with OA have a consistently higher long-term prevalence of MCVRFs compared with those without OA.
The prevalence of obesity in the youngest age group with OA was also found to be higher, highlighting the need for public health strategies.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study emphasises the necessity for public health strategies aimed at addressing the higher prevalence of obesity in young individuals with OA and suggests that the findings could inform the development of targeted interventions and public health policies to manage MCVRFs and reduce the burden of CVD in this population.
Introduction
Osteoarthritis (OA) is the most common joint condition and is one of the leading reasons for disability in adults.1 In the UK, 8.7 million adults aged 45 and over have consulted primary care practices for OA between 2004 and 2010.2 OA is associated with a higher risk of cardiovascular disease (CVD).3 4 Recent studies have proposed that shared risk factors (eg, ageing and obesity) for OA and CVD explain some of the increased risk.5 Modifiable cardiovascular risk factors (MCVRFs), such as smoking, obesity, type 2 diabetes mellitus (T2DM), dyslipidaemia and hypertension, are the key targets of prevention programmes.6 7 The hypothesis regarding the differential clustering of cardiovascular risk factors between individuals with OA and those without OA is based on factors such as higher prevalence of pain and functional limitations leading to reduced physical activity,8 increased sedentary behaviour,9 comorbidities such as obesity and metabolic disorders often associated with OA10 and potential influence of OA medications on cardiovascular risk factor clustering.11 It is therefore important in understanding the pattern and the number of these risk factors with OA for formulating tailored preventive strategies and management plans. Individual risk factors, including obesity, hypertension, dyslipidaemia and diabetes,12–14 have been reported to be more common, while smoking has been reported to be less common in people with OA than those without OA.15 16 Based on a recent systematic review,17 only a limited number of studies have examined the long-term (over 20 years) temporal trend of the prevalence of MCVRFs among both populations with and without OA and investigated the long-term prevalence differences between the two populations, which can only be assessed using long-term longitudinal datasets. Such studies are crucial for enhancing our understanding of MCVRFs profiles and providing valuable insights for optimising CVD prevention strategies in populations with OA.
Electronic health records (EHRs) from primary care serve as a valuable longitudinal dataset that enables the monitoring of long-term trends of the prevalence of MCVRFs which are consistently recorded and managed in primary care settings. These estimates can be considered representative of the general population, as a large proportion of people (over 98%) are registered with a primary care GP in the UK. This provides the opportunity to assess MCVRFs in the general practice population and patient groups using EHRs from the primary care.18–20
Our aim is to analyse the temporal trend from 1992 to 2017 (the data availability period for our current study) in the prevalence and prevalence rate ratios (PRRs) of single and clustering MCVRFs in adults with newly diagnosed OA and without. We will consider overall trends as well as account for common population-level confounders (age and sex) and geographical region due to regional coding variations and geographical deprivation at primary care settings.21
Methods
Study population
In this study, matched retrospective cohorts were extracted from the Clinical Practice Research Datalink (CPRD) GOLD. The CPRD GOLD database contains anonymous EHR data of 11.3 million patients registered at 674 UK general practices, starting from 1987 to the present day.21 The database covers patients from the first until their last visit to a general practice contributing data to the CPRD. Eligible participants for this study were newly diagnosed OA individuals aged 35 and above between 1 January 1992 and 31 December 2017 with complete and up-to-standard (UTS) data and registered in the CPRD for at least 3 years prior to the incident OA diagnosis. The UTS date, calculated for each participating practice, is a practice-based quality metric that determines the latest date at which practices meet minimum quality criteria based on the continuity of recording and the number of recorded deaths.21 An incident OA individual was defined as those with the first OA (defined by Read Codes; accessible at https://www.keele.ac.uk/mrr/) consultation in each calendar year and without a recorded OA diagnosis within 3 years prior to the consultation. The selection of eligible participants is presented in online supplemental figure 1.
rmdopen-2023-003298supp001.pdf (3.6MB, pdf)
Each patient with OA was matched with one non-OA individual based on age (35–44, 45–54, 55–64, 65–74, 85 years and over), sex and registered practice, using the risk set sampling method.22 The date of the incident OA diagnosis was defined as the index date, and non-OA individuals were assigned the same index date without any OA consultation in the 3 years prior to it. Patients with OA or non-OA individuals who were transferred out or died before the index date were excluded.
Definition of MCVRFs
Current smoking, hypertension and T2DM, recorded prior to the index consultation of each study participant, were identified using Read codes (available at https://www.keele.ac.uk/mrr/). Individuals without records of smoking status, hypertension diagnosis or T2DM diagnosis were considered as never smoked, non-hypertensive and non-diabetes, respectively. Obesity was identified using a body mass index (BMI)≥30 kg/m2.23 Dyslipidaemia was identified using a high total cholesterol level (≥5 mmol/L), high triglyceride level (≥1.7 mmol/L) or low HDL cholesterol level (<1.0 mmol/L for men and <1.2 mmol/L for women). The nearest BMI or lipid assessment to the index date was used when more than one measurement was recorded within 3 years before the index consultation.
The use of clinical measurements, such as body weight, height and lipid profiles, instead of relying solely on Read codes, improves the accuracy and sensitivity in defining obesity and dyslipidaemia prevalence, providing a more reliable representation of these risk factors in the underlying population. Individuals who had clustering of MCVRFs were defined as those with a record of at least one (≥1), two (≥2) or three (≥3) MCVRFs within 3-year prior to the index consultation.
Statistical analyses
Both period and annual prevalence were estimated to provide insights into the overall proportion of individuals with the specific CVRF over the study period and to assess the temporal trend of the proportion of individuals with the specific CVRF in each calendar year. The annual and period prevalence of single and clustering of MCVRFs were estimated with 95% CI by Poisson regression for the populations with and without OA between 1992 and 2017. To estimate the relative difference in the prevalence between the OA and non-OA populations during the specific periods (1 year for annual prevalence and 26 year for period prevalence) from 1992 to 2017, we calculated the annual and period PRRs using Poisson regressions. The PRRs represent the ratio of the prevalence in the OA population to the prevalence in the non-OA population. We considered overall trends as well as accounted for common population-level confounders (age and sex) and geographical regions due to regional coding variations and geographical deprivation at primary care settings.21 Stratified analyses for period prevalence and PRRs by age groups (35–44, 45–54, 55–64, 65–74, 75–84, 85 and over), sex and UK geographical regions (North East, North West, Yorkshire and The Humber, East Midlands, West Midlands, East of England, South West, South Central, London, South East Coast, Northern Ireland, Scotland, Wales) were conducted. The missing data in BMI and lipid assessment was imputed by multiple imputations using chained equations.24 Based on the worst scenario of 11% of patients with 1 more missing data, 11 imputed datasets were created for multiple imputations with the chained equation, and estimations were made by Robin’s rule.24 Stata MP V.17.0 (Stata Corporation, College Station, Texas, USA) was used for data management and statistical analyses.
Results
Between 1992 and 2017, 215 190 newly diagnosed patients with OA aged 35 and over were included in this study alongside 1:1 matched non-OA individuals (online supplemental figure 1). The demographic characteristics of OA and matched non-OA cohorts were presented in table 1. The mean age of OA population was 62.62±11.53 years and that of non-OA population was 62.41±11.87 years. Matched OA and non-OA populations had the same percentage of women (64.79%), in each geographical region, and each index year (online supplemental table S1).
Table 1.
Demographical characteristics of the study participants
| Osteoarthritis | Non-osteoarthritis | |
| Patients, n | 215 190 | 215 190 |
| Age | ||
| Mean±SD years | 62.62±11.53 | 62.41±11.87 |
| 35–44, n (%) | 11 360 (5.28) | 11 360 (5.28) |
| 45–54, n (%) | 43 852 (20.38) | 43 852 (20.38) |
| 55–64, n (%) | 69 988 (32.52) | 69 988 (32.52) |
| 65–74, n (%) | 53 631 (24.92) | 53 631 (24.92) |
| 75–84, n (%) | 31 030 (14.42) | 31 030 (14.42) |
| 85+, n (%) | 5329 (2.48) | 5329 (2.48) |
| Sex, n (%) | ||
| Women | 139 426 (64.79) | 139 426 (64.79) |
| Men | 75 764 (35.21) | 75 764 (35.21) |
| Region, n (%) | ||
| North East | 4593 (2.13) | 4593 (2.13) |
| North West | 27 200 (12.64) | 27 205 (12.64) |
| Yorkshire and The Humber | 9277 (4.31) | 9275 (4.31) |
| East Midlands | 8960 (4.16) | 8957 (4.16) |
| West Midlands | 22 256 (10.34) | 22 250 (10.34) |
| East of England | 18 103 (8.41) | 18 101 (8.41) |
| South West | 18 251 (8.48) | 18 249 (8.48) |
| South Central | 20 656 (9.60) | 20 655 (9.60) |
| London | 15 763 (7.33) | 15 767 (7.33) |
| South East Coast | 19 290 (8.96) | 19 294 (8.97) |
| Northern Ireland | 6430 (2.99) | 6434 (2.99) |
| Scotland | 20 521 (9.54) | 20 519 (9.54) |
| Wales | 23 890 (11.10) | 23 891 (11.10) |
Annual prevalence and PRR of MCVRFs
The annual prevalence of certain risk factors exhibited an upward trend throughout the study period in both the OA and non-OA populations. In the OA population, the prevalence of current smoking increased from 18.34% in 1992 to 23.75% in 2017, while in the non-OA population, it rose from 12.60% to 21.72%. Hypertension prevalence showed an increase from 28.18% to 38.35% in the OA population and from 21.55% to 27.18% in the non-OA population. T2DM prevalence rose from 1.99% to 11.72% in the OA population and from 3.54% to 8.17% in the non-OA population. Dyslipidaemia prevalence increased from 50.83% to 68.24% in the OA population, whereas it remained relatively stable around 51.93% in the non-OA population. Obesity prevalence witnessed an increase from 27.73% to 39.12% in the OA population and from 20.11% to 29.92% in the non-OA population. The prevalence of having ≥1 MCVRFs rose from 75.91% to 89.00% in the OA population and remained constant at 75.91% in the non-OA population. Similarly, having ≥2 MCVRFs increased from 38.34% to 57.84% in the OA population and from 27.96% to 43.23% in the non-OA population. The prevalence of having ≥3 MCVRFs experienced an increase from 11.27% to 26.42% in the OA population and from 10.06% to 19.13% in the non-OA population (figure 1).
Figure 1.
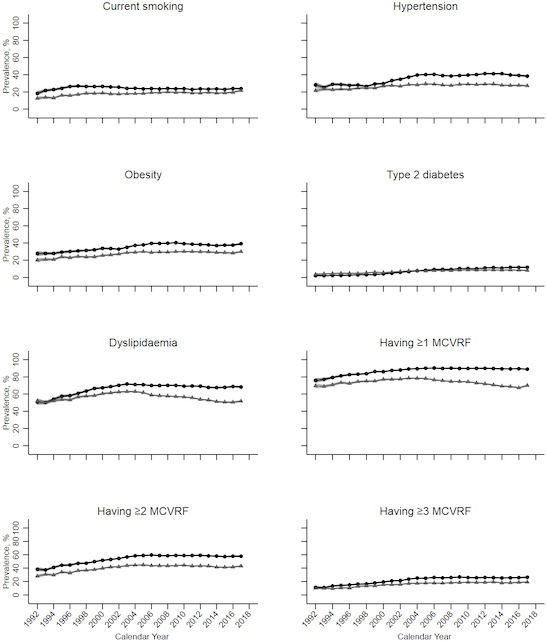
Annual prevalence of modifiable cardiovascular risk factors in osteoarthritis and non-osteoarthritis populations between 1992 and 2017 in the UK. The black circle lines indicate estimations for the osteoarthritis population; the grey triangle lines indicate estimations for non-osteoarthritis populations. MCVRFs, modifiable cardiovascular risk factors.
A consistent increasing trend in PRRs was observed between 1992 and 2017 for certain risk factors. Hypertension showed an increase from 1992 (PRR: 1.31) to 2017 (PRR: 1.41). T2DM exhibited an increase from 1992 (PRR: 0.56) to 2017 (PRR: 1.43). Dyslipidaemia showed an increase from 1992 (PRR: 0.98) to 2017 (PRR: 1.32). The PRRs for having ≥1 MCVRFs increased from 1992 (PRR: 1.09) to 2017 (PRR: 1.27). Having ≥2 MCVRFs increased from 1992 (PRR: 1.37) to 2017 (PRR: 1.34). Having ≥3 MCVRFs increased from 1992 (PRR: 1.12) to 2017 (PRR: 1.38). The PRR for T2DM remained relatively stable throughout the period, with values of 1.38 in 1992 and 1.31 in 2017. However, the PRR for current smoking decreased from 1992 (PRR: 1.46) to 2017 (PRR: 1.09) (figure 2).
Figure 2.
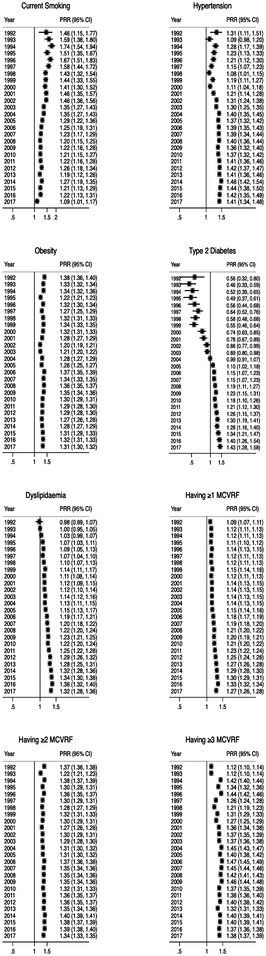
Annual prevalence rates ratio for single and clustering of modifiable risk factors between osteoarthritis and non-osteoarthritis populations in the UK between 1992 and 2017. MCVRF, modifiable cardiovascular risk factor; PRR, prevalence rate ratio.
Period prevalence and PRR of MCVRFs
Over the 26-year study period (1992–2017), the prevalence rates were estimated as follows in the OA population: 24.07% for current smoking, 37.45% for hypertension, 8.44% for T2DM, 37.00% for obesity, 68.33% for dyslipidaemia, 88.59% for having ≥1 MCVFR, 56.32% for having ≥2 MCVRFs and 23.85% for having ≥3 MCVRFs. In the non-OA population, the prevalence rates were estimated as follows: 18.66% for current smoking, 27.67% for hypertension, 7.61% for T2DM, 28.39% for obesity, 57.13% for dyslipidaemia, 74.32% for having ≥1 MCVRFs, 42.12% for having ≥2 MCVRFs and 17.12% for having ≥3 MCVRFs (table 2).
Table 2.
Period prevalence of single and clustering of modifiable cardiovascular risk factors in OA and non-OA samples in the UK between 1992 and 2017
| Risk factor | OA | Non-OA |
| Prevalence (95% CI), % | Prevalence (95% CI), % | |
| Current smoking | 24.07 (23.89 to 24.25) | 18.66 (18.50 to 18.83) |
| Hypertension | 37.45 (37.25 to 37.66) | 27.67 (27.49 to 27.86) |
| Type 2 diabetes mellitus | 8.44 (8.32 to 8.56) | 7.61 (7.50 to 7.72) |
| Obesity | 37.00 (36.80 to 37.21) | 28.39 (28.20 to 28.58) |
| Dyslipidaemia | 68.33 (68.13 to 68.52) | 57.13 (56.92 to 57.34) |
| Having ≥1 risk factors | 88.59 (88.46 to 88.73) | 74.32 (74.13 to 74.50) |
| Having ≥2 risk factors | 56.32 (56.11 to 56.53) | 42.12 (41.91 to 42.33) |
| Having ≥3 risk factors | 23.85 (23.67 to 24.03) | 17.12 (16.96 to 17.28) |
OA, osteoarthritis.
The PRR over the 26-year period was estimated as follows: 1.29 for current smoking, 1.35 for hypertension, 1.11 for T2DM, 1.30 for obesity, 1.20 for dyslipidaemia, 1.19 for having ≥1 MCVRF, 1.34 for having ≥2 MCVRFs and 1.39 for having ≥3 MCVRFs (figure 3).
Figure 3.
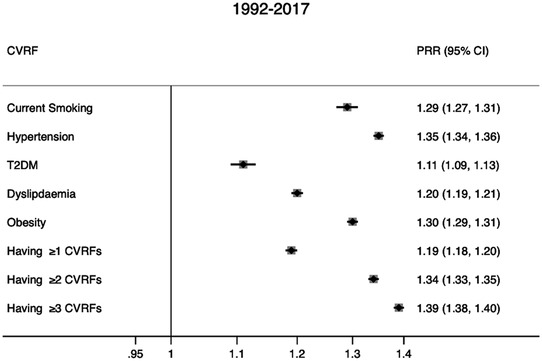
Period prevalence rates ratio for single and clustering of modifiable risk factors between osteoarthritis and non-osteoarthritis populations in the UK between 1992 and 2017. CVRF, cardiovascular risk factor; PRR, prevalence rate ratio.
The period prevalence of single and clustering of MCVRFs was higher in Northern English regions and Scotland for both the OA and non-OA populations (online supplemental table S2). Within each geographical region, the OA population had a higher period prevalence of single and clustering of MCVRFs compared with the non-OA population (table 3). Higher PRRs were more likely to be found in Southern regions, mainly due to the lower prevalence of single and clustering of MCVRFs in the non-OA populations in those regions figure 4(table 3).
Figure 4.
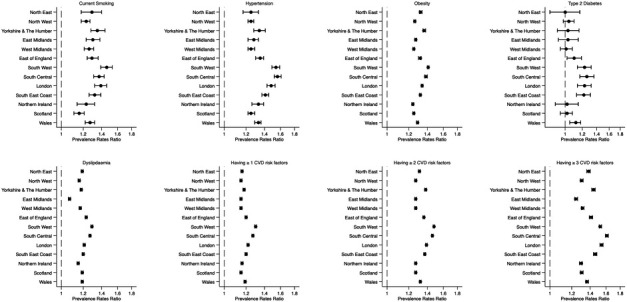
Period prevalence rates ratio of single and clustering of modifiable cardiovascular risk factors in osteoarthritis and non-osteoarthritis populations in the UK between 1992 and 2017
The sex–age-stratified period prevalence of single and clustering of MCVRFs revealed that higher prevalence was found in females of the elder age group (≥65 years) (online supplemental figure S2). The higher PRR of single and clustering of MCVRFs was found in women and the PRR increased with age for most single and clustering of MCVRFs, as the highest PPR was found in the eldest age group (age≥85 years). For obesity, the PRR decreased with age and the highest PRR was found in the youngest age group (age 35–44 years) (online supplemental figure S3).
Discussion
Our study, based on a large-matched cohort from the UK primary care database, shows that the annual prevalence of single and clustering MCVRFs significantly increased from 1992 to 2017 in both the population with OA and the matched non-OA population. This trend is seen in both men and women over the age of 35 and in all UK regions. The higher prevalence of MCVRFs in the OA population compared with the non-OA population was consistent over 26 years, ranging from an 11% difference for hypertension to a 39% difference for having 3 or more MCVRFs. The difference was higher in women and increased with age for most MCVRFs, but the largest difference for obesity was found in the youngest age group. The relative difference in prevalence varied among UK regions, with the highest difference seen in Southern England regions. This potentially reflects a lower prevalence of MCVRFs in the non-OA population (part of the general population) comparing with those in the Northern England regions and Scotland.
The prevalence of smoking in the OA population has been previously reported; however, few studies have compared the prevalence between OA and non-OA populations. In a study conducted by Leyland et al, the period prevalence of current smoking was found to be 12.5% among individuals with knee OA, with a mean age of 68 years, in Catalonia, Spain between 2006 and 2011.25 However, the estimate from our current study, which used a longer prevalence period of 26 years compared with their 6-year period, indicates a significantly higher prevalence of smoking at 24.07%. This difference can be attributed to the extended time frame examined in our study. The higher prevalence of smoking in the OA cohort suggests a possible lower socioeconomic status (SES), as smoking has long associated with lower SES.26 The persistent higher disparity in smoking prevalence between OA and non-OA populations, particularly among women across age groups, highlights the need to address lifestyle factors among women with OA. Although the causal relationship between smoking and OA has not been definitively established,27 it is important to consider implementing stop-smoking services in accordance with guidelines28 and promoting smoking cessation through public health campaigns29 for populations with OA. This is crucial due to the well-known strong association between smoking and adverse cardiovascular outcomes and mortality.30
The higher period prevalence of certain MCVRFs in individuals consulting for OA observed in this study is consistent with previous findings. Furthermore, the current study revealed consistent patterns in subgroups defined by sex, age and geographical regions. Previous studies, using primary care EHRs, have consistently demonstrated a higher prevalence of obesity, hypertension, diabetes and dyslipidaemia among individuals with OA compared with those without OA.31–33 These risk factors are considered to be shared between OA and CVD. Notably, obesity, a primary risk factor for knee and hip OA, along with the presence of hypertension, alterations in lipid profile, blood glucose levels and the use of OA medications such as non-steroidal anti-inflammatory drugs, collectively contribute to the inflammatory milieu underlying both OA and CVD.5 13 34 Particularly concerning is the significant disparity in obesity prevalence between individuals with OA and those without OA in the youngest age group (35–44 years) for both men and women. This finding raises concerns regarding future health and economic burdens, as well as the potential impact on healthy life expectancy and increased CVD risk.35 These observations align with the overall increased prevalence of obesity among young individuals due to the higher prevalence of sedentary lifestyles and unhealthy dietary habits in recent decades.36 The higher obesity rate observed in young individuals with newly diagnosed OA may be attributed to the shared biological link between obesity and OA.37–39 Thus, it is crucial to implement more robust public health strategies, such as promoting healthy eating habits40 and reducing the accessibility of fast food, specifically targeting the young population with early-onset OA.41
There do not appear to be previous reports of the temporal trends of MCVRFs in individuals with OA using primary care EHRs. In general populations, the prevalence of MCVRFs such as obesity, diabetes and hypertension is increasing in the UK.42 The current study showed an increase in the prevalence of MCVRFs in both individuals with and without OA over the study period. This study is the first to report the difference in the prevalence of MCVRFs between individuals with and without OA and revealed an increasing gap in hypertension, T2DM, dyslipidaemia and the clustering of MCVRFs. The temporary increase in the annual prevalence of T2DM in individuals consulting for OA in 2004 found in this study is likely due to the Quality and Outcomes Framework, an incentive scheme introduced in the same year to improve the identification of clinical conditions in the primary care.43 The continued increase in the prevalence ratio of T2DM between individuals with and without OA throughout the study period raises concerns that there may be a further increase in the burden of T2DM and a resulting increase in the risk of CVD in the UK population with OA.
Postmenopausal women face an increased risk of CVD due to hormonal factors that decrease the cardioprotective effects of oestrogen, as well as an increased likelihood of obesity, dyslipidaemia and hypertension.44 These factors are shared pathways with OA.37–39 Notably, the larger disparity in the prevalence of having three or more MCVRFs between women with OA and those without across age groups in women, compared with men, highlights the gender-specific nature of this issue. It emphasises the need to address the clustering of MCVRF in women with OA. Therefore, it is essential to consider targeted public health promotion initiatives aimed at improving lifestyle factors specifically for women with OA.
Geographic inequalities in CVD risk, characterised by a pronounced North–South gradient, have been highlighted in previous studies in the UK. This gradient reflects disparities in the quality of local services (such as coding behaviour at primary care settings) and the prevalence of CVD risk factors.45 Certain CVD risk factors such as hypertension and obesity demonstrate higher prevalence in areas of socioeconomic deprivation. Additionally, a North–South divide is observed in the UK, with higher rates of these risk factors in the North of England (North East, North West and Yorkshire and Humber) and Scotland compared with the South of England (South West, South East and London).46 This North–South divide was also observed in populations with OA, exhibiting a higher prevalence of MCVRFs in the Northern regions. Socioeconomic deprivation might increase not only the risk factors but also the incidence of CVD in populations with OA through its impact on education, income, health services access and resource availability.47 However, the regional data used in this study may miss important variations in deprivation at a smaller area level. For example, the North West of England includes both affluent and less affluent rural areas, with diverse populations.46 Further studies using data at a smaller area level are needed to better understand whether cardiovascular risk factors are influenced by socioeconomic deprivation in populations with OA.
The study findings had potential limitations. First, this study did not include some MCVRFs such as physical inactivity, drinking and an unhealthy diet. However, this study covered five common modifiable risk factors with advantages in the completeness of recording and being managed in the primary care setting. This provides the basis for the assessment of healthcare needs for MCVRF treatment. Second, there was a lack of validation of each MCVRF in people with and without OA specifically. No resource was available to check for misclassification and whether it is differential or not. Third, a common issue related to selection bias in EHR-based studies was also highly likely in the current study. The non-OA individuals were those who consulted primary care for non-OA reasons and might be less healthy than the general population. This might lead to an underestimated difference in the prevalence of MCVRFs between people with OA and non-OA. Fourth, the PRR reported here should not be used to indicate the causality between OA and MCVRFs as it can only tell the prevalence difference between people with and without OA and there was no temporal sequence of OA and MCVRF in the current study. Although matching was used in the current study, there remained unmeasured confounders (eg, genetics, lifestyles, environmental factors) that could explain the difference in the prevalence of MCVRFs between people with OA and non-OA. Fifth, previous studies have revealed the high specificity48 and low sensitivity49 of OA diagnosis in primary care records, which may result in some misclassification, primarily affecting the non-OA population. Finally, comparisons of prevalence estimates and PRRs between regions and years might be treated with caution due to the differences in various potential confounders (eg, age and sex distribution, socioeconomic deprivation, completeness of recording) that could influence the occurrence of MCVRFs between regions and calendar years.
Conclusion
In conclusion, the study found that people with newly diagnosed OA had a consistently higher annual prevalence of individual and clustering of MCVRFs compared with those who did not consult for OA, especially between 2004 and 2017. This difference was seen in various age groups, gender and regions. The increasing gap in the prevalence of MCVRFs between the two groups underscores the importance of primary care providers assessing and treating CVD risk factors in line with current guidelines for patients with OA. Further research is needed to understand the influence of socioeconomic factors on CVD risk in OA populations and to determine the clinical effectiveness, cost-effectiveness and acceptability of potential preventive care strategies.
Acknowledgments
This study is based in part on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusions contained in this study are those of the author/s alone. We acknowledge that parts of the results presented in this paper were extracted from the PhD thesis of our cofirst author, XH.
Footnotes
DY and XH contributed equally.
Contributors: DY, XH, RW and MAM conceived and designed the study. DY and XH acquired the data. DY and XH performed the analysis. All authors interpreted the results. DY, XH, RW and MAM drafted the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content. DY and RW supervised the study. DY is responsible for the overall content as guarantor and accepts full responsibility for the work.
Funding: DY and RW hold Honorary Academic Consultant Contracts from the Office for Health Improvement and Disparities. XH was supported by Keele University ACORN PhD studentship.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. We used anonymised data on individual patients on which the analysis, results and conclusions reported in the paper are based. The CPRD data is not distributable under licence. However, the relevant data can be obtained directly from the agency (https://www.cprd.com/).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. The study was approved by the Independent Scientific Advisory Committee for CPRD research (protocol reference: 18_031R2). No further ethical permissions were required for the analyses of these anonymised patient-level data. Participants gave informed consent to participate in the study before taking part.
References
- 1.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 2.Arthritis Research UK . Osteoarthritis in general practice. Available: https://www.versusarthritis.org/media/2115/osteoarthritis-in-general-practice.pdf [Accessed 18 Jun 2023].
- 3.Hall AJ, Stubbs B, Mamas MA, et al. Association between osteoarthritis and cardiovascular disease: systematic review and meta-analysis. Eur J Prev Cardiolog 2016;23:938–46. 10.1177/2047487315610663 [DOI] [PubMed] [Google Scholar]
- 4.Veronese N, Stubbs B, Solmi M, et al. Osteoarthristis increases the risk of cardiovascular disease: data from the osteoarthritis initiative. J Nutr Health Aging 2018;22:371–6. 10.1007/s12603-017-0941-0 [DOI] [PubMed] [Google Scholar]
- 5.Fernandes GS, Valdes AM. Cardiovascular disease and osteoarthritis: common pathways and patient outcomes. Eur J Clin Invest 2015;45:405–14. 10.1111/eci.12413 [DOI] [PubMed] [Google Scholar]
- 6.Robson J, Dostal I, Sheikh A, et al. The NHS health check in England: an evaluation of the first 4 years. BMJ Open 2016;6:e008840. 10.1136/bmjopen-2015-008840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public Health England [Internet] . Health matters: preventing cardiovascular disease. Available: https://www.gov.uk/government/publications/health-matters-preventing-cardiovascular-disease/health-matters-preventing-cardiovascular-disease1 [Accessed 19 Jun 2023].
- 8.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013;21:1145–53. 10.1016/j.joca.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sliepen M, Mauricio E, Lipperts M, et al. Objective assessment of physical activity and sedentary behaviour in knee osteoarthritis patients - beyond daily steps and total sedentary time. BMC Musculoskelet Disord 2018;19:64. 10.1186/s12891-018-1980-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain S, Sarmanova A, Coupland C, et al. Comorbidities in osteoarthritis: a systematic review and meta-analysis of observational studies. Arthritis Care Res (Hoboken) 2020;72:991–1000. 10.1002/acr.24008 [DOI] [PubMed] [Google Scholar]
- 11.Booker SQ, Content VG. Chronic pain, cardiovascular health and related medication use in ageing African Americans with osteoarthritis. J Clin Nurs 2020;29:2675–90. 10.1111/jocn.15292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baudart P, Louati K, Marcelli C, et al. Association between osteoarthritis and dyslipidaemia: a systematic literature review and meta-analysis. RMD Open 2017;3:e000442. 10.1136/rmdopen-2017-000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Clanche S, Bonnefont-Rousselot D, Sari-Ali E, et al. Inter-relations between osteoarthritis and metabolic syndrome: a common link? Biochimie 2016;121:238–52. 10.1016/j.biochi.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 14.Louati K, Vidal C, Berenbaum F, et al. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 2015;1:e000077. 10.1136/rmdopen-2015-000077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon HM, Yang IH, Park KK, et al. Cigarette smoking and knee osteoarthritis in the elderly: data from the Korean national health and nutritional examination survey. Exp Gerontol 2020;133:110873. 10.1016/j.exger.2020.110873 [DOI] [PubMed] [Google Scholar]
- 16.Singh G, Miller JD, Lee FH, et al. Prevalence of cardiovascular disease risk factors among US adults with self-reported osteoarthritis: data from the third national health and nutrition examination survey. Am J Manag Care 2002;8:S383–91. [PubMed] [Google Scholar]
- 17.Huang X. Exploring cardiovascular risk and outcomes in primary care consulters for osteoarthritis using longitudinal electronic primary care data. PhD thesis.03/2023. Keele University, Available: https://eprints.keele.ac.uk/id/eprint/12045 [Google Scholar]
- 18.Booth HP, Prevost AT, Gulliford MC. Validity of smoking prevalence estimates from primary care electronic health records compared with national population survey data for England, 2007 to 2011. Pharmacoepidemiol Drug Saf 2013;22:1357–61. 10.1002/pds.3537 [DOI] [PubMed] [Google Scholar]
- 19.Herrett E, Shah AD, Boggon R, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ 2013;346:f2350. 10.1136/bmj.f2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tate AR, Dungey S, Glew S, et al. Quality of recording of diabetes in the UK: how does the GP’s method of coding clinical data affect incidence estimates? Cross-sectional study using the CPRD database. BMJ Open 2017;7:e012905. 10.1136/bmjopen-2016-012905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borgan O, Goldstein L, Langholz B. Methods for the analysis of sampled cohort data in the Cox proportional hazards model. Ann Statist 1995;23:1749–78. 10.1214/aos/1176324322 [DOI] [Google Scholar]
- 23.World Health Organization . Obesity and overweight. Available: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [Accessed 19 Jun 2023].
- 24.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons, 2004. [Google Scholar]
- 25.Leyland KM, Judge A, Javaid MK, et al. Obesity and the relative risk of knee replacement surgery in patients with knee osteoarthritis: a prospective cohort study. Arthritis Rheumatol 2016;68:817–25. 10.1002/art.39486 [DOI] [PubMed] [Google Scholar]
- 26.Hiscock R, Dobbie F, Bauld L. Smoking cessation and socioeconomic status: an update of existing evidence from a national evaluation of English stop smoking services. Biomed Res Int 2015;2015:274056. 10.1155/2015/274056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong L, Wang L, Meng F, et al. Association between smoking and risk of knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis and Cartilage 2017;25:809–16. 10.1016/j.joca.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence [Internet] . Tobacco: preventing uptake, promoting quitting and treating dependence. Available: https://www.nice.org.uk/guidance/ng209 [Accessed 19 Jun 2023]. [PubMed]
- 29.Department of Health . Towards a Smokefree generation: a tobacco control plan for England. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/630217/Towards_a_Smoke_free_Generation_-_A_Tobacco_Control_Plan_for_England_2017-2022__2_.pdf [Accessed 19 Jun 2023].
- 30.Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020;395:795–808. 10.1016/S0140-6736(19)32008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prieto-Alhambra D, Judge A, Javaid MK, et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014;73:1659–64. 10.1136/annrheumdis-2013-203355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman MM, Kopec JA, Anis AH, et al. Risk of cardiovascular disease in patients with osteoarthritis: a prospective longitudinal study. Arthritis Care Res (Hoboken) 2013;65:1951–8. 10.1002/acr.22092 [DOI] [PubMed] [Google Scholar]
- 33.Nielen MMJ, van Sijl AM, Peters MJL, et al. Cardiovascular disease prevalence in patients with inflammatory arthritis, diabetes mellitus and osteoarthritis: a cross-sectional study in primary care. BMC Musculoskelet Disord 2012;13:150. 10.1186/1471-2474-13-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol 2010;22:533–7. 10.1097/BOR.0b013e32833b4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecker J, Freijer K, Hiligsmann M, et al. Burden of disease study of overweight and obesity; the societal impact in terms of cost-of-illness and health-related quality of life. BMC Public Health 2022;22:46. 10.1186/s12889-021-12449-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai H, Alsalhe TA, Chalghaf N, et al. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990-2017: an analysis of the Global Burden of Disease Study. PLoS Med 2020;17:e1003198. 10.1371/journal.pmed.1003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kluzek S, Newton JL, Arden NK. Is osteoarthritis a metabolic disorder? Br Med Bull 2015;115:111–21. 10.1093/bmb/ldv028 [DOI] [PubMed] [Google Scholar]
- 38.Dickson BM, Roelofs AJ, Rochford JJ, et al. The burden of metabolic syndrome on osteoarthritic joints. Arthritis Res Ther 2019;21:289. 10.1186/s13075-019-2081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford) 2015;54:588–600. 10.1093/rheumatology/keu464 [DOI] [PubMed] [Google Scholar]
- 40.The King’s Fund . Tackling obesity: the role of the NHS in a whole-system approach. Available: https://www.kingsfund.org.uk/publications/tackling-obesity-nhs [Accessed 19 Jun 2023].
- 41.Department of Health and Social Care . Tackling obesity: empowering adults and children to live healthier lives. Available: https://www.gov.uk/government/publications/tackling-obesity-government-strategy/tackling-obesity-empowering-adults-and-children-to-live-healthier-lives [Accessed 19 Jun 2023].
- 42.Zghebi SS, Steinke DT, Carr MJ, et al. Examining trends in type 2 diabetes incidence, prevalence and mortality in the UK between 2004 and 2014. Diabetes Obes Metab 2017;19:1537–45. 10.1111/dom.12964 [DOI] [PubMed] [Google Scholar]
- 43.Roland M, Guthrie B. Quality and outcomes framework: what have we learnt? BMJ 2016;354:i4060. 10.1136/bmj.i4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maas AHEM, Appelman YEA. Gender differences in coronary heart disease. Neth Heart J 2010;18:598–602. 10.1007/s12471-010-0841-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.TheKing’sFund . Cardiovascular disease in England. 2022. Available: https://www.kingsfund.org.uk/sites/default/files/2022-11/CVD_Report_Web.pdf
- 46.UK Parliament . Health inequalities: income deprivation and North/South divides. Available: https://commonslibrary.parliament.uk/health-inequalities-income-deprivation-and-north-south-divides/ [Accessed 19 Jun 2023].
- 47.Pujades-Rodriguez M, Timmis A, Stogiannis D, et al. Socioeconomic deprivation and the incidence of 12 cardiovascular diseases in 1.9 million women and men: implications for risk prediction and prevention. PLoS One 2014;9:e104671. 10.1371/journal.pone.0104671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferguson RJ, Prieto-Alhambra D, Walker C, et al. Validation of hip osteoarthritis diagnosis recording in the UK clinical practice research Datalink. Pharmacoepidemiol Drug Saf 2019;28:187–93. 10.1002/pds.4673 [DOI] [PubMed] [Google Scholar]
- 49.Yu D, Jordan KP, Peat G. Underrecording of osteoarthritis in United Kingdom primary care electronic health record data. Clin Epidemiol 2018;10:1195–201. 10.2147/CLEP.S160059 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003298supp001.pdf (3.6MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. We used anonymised data on individual patients on which the analysis, results and conclusions reported in the paper are based. The CPRD data is not distributable under licence. However, the relevant data can be obtained directly from the agency (https://www.cprd.com/).


