Abstract
Introduction
Pelvic exenteration (PE) surgery represents the only potentially curative treatment option for patients with locally advanced or recurrent rectal cancer (LARRC). Given the potential morbidity, whether or not PE should be recommended for an individual patient presents a major decisional conflict. This study aims to identify the outcomes of PE for which there is consensus among patients, carers and clinicians regarding their importance in guiding treatment decision-making, and to develop a risk prediction tool which predicts these outcomes.
Methods and analysis
This study will be conducted at a specialist PE centre, and employ a mixed-methods study design, divided into three distinct phases. In phase 1, outcomes of PE will be identified through a comprehensive systematic review of the literature (phase 1a), followed by exploration of the experiences of individuals who have undergone PE for LARRC and their carers (phase 1b, target sample size 10–20 patients and 5–10 carers). In phase 2, a survey of patients, their carers and clinicians will be conducted using Delphi methodology to explore consensus around the outcomes of highest priority and the level of influence each outcome should have on treatment decision-making. In phase 3 a, risk prediction tool will be developed using data from a single PE referral centre (estimated sample size 500 patients) to predict priority outcomes using multivariate modelling, and externally validated using data from an international PE collaboration.
Ethics and dissemination
Ethical approval has been granted for phases 1 and 2 (X22-0422 and 2022/ETH02659) and for maintenance of the database used in phase 3 (X13-0283 and HREC/13/RPAH/504). Informed consent will be obtained from participants in phases 1b and 2; a waiver of consent for secondary use of data in phase 3 will be sought. Study results will be submitted for publication in international and/or national peer reviewed journals.
PROSPERO registration number
CRD42022351909.
Keywords: Colorectal surgery, Gastrointestinal tumours, Patient Reported Outcome Measures
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Define priority outcomes of pelvic exenteration according to individuals who have undergone surgery, their carers and clinicians.
Develop a risk prediction tool using prospective individual patient data from a high-volume centre.
External validation using international, multicentre data.
Potential limited generalisability beyond high-volume, specialist centres.
Introduction
Pelvic exenteration (PE) surgery represents the standard of care for selected patients presenting with locally advanced or recurrent rectal cancer, and the only potentially curative treatment option.1 This ultraradical surgical procedure involves en bloc resection of all anatomical structures contiguously involved by tumour, and typically requires excision of multiple pelvic viscera as well as pelvic bone and major neurovascular structures, followed by complex reconstruction. Refinement of surgical techniques in recent decades has made increasingly radical ‘higher and wider’ resections in all compartments of the pelvis safe and oncologically feasible.2 3 However, such radical surgery may be associated with major morbidity (32%–38%),4–6 functional impairment,7 at least a temporary reduction in quality of life,8 as well as substantial cost to the healthcare system.9 Therefore, whether or not radical surgery should be recommended for an individual patient with locally advanced or recurrent rectal cancer presents a major decisional conflict for the team of treating clinicians, where the consequences of surgery must be weighed against the potential for cure. The paradigm has shifted such that the decision to be made is no longer what can be technically resected, but rather what should be.10
Currently, the decision-making process around whether curative intent PE surgery is recommended for an individual patient tends to be based on individual clinician and centres’ experiences, rather than a reproducible, evidence-based process. This may lead to substantial variation in treatment decision-making within and between PE centres, as has been recently demonstrated by international comparative data,11 as well as variation in which patients with a new diagnosis of locally advanced or recurrent rectal cancer are referred to a PE centre for consideration of potentially curative surgery. Recommendations for or against surgery, and decisions around whether to refer a patient to a PE centre, are often made with survival as the primary outcome of interest, and which other outcomes of surgery (such as anticipated quality of life, functional outcomes and morbidity) are considered important by patients, carers and clinicians and how they should be incorporated into the decision-making process is not well understood.
This study aims to develop a risk prediction tool to predict the outcomes of PE that are considered most important by patients with locally advanced and recurrent rectal cancer, their carers and clinicians. The tool may be used at the time of diagnosis by referring clinicians, as well as those managing patients at a specialist PE centre, to access an evidence-based prediction of the anticipated outcomes of surgery for an individual patient. Development and implementation of this tool will assist clinicians to navigate the decisional conflict of whether to refer or recommend PE when managing a patient with a new diagnosis of advanced rectal cancer in a reproducible, evidence-based manner.
Methods and analysis
Aims and study design overview
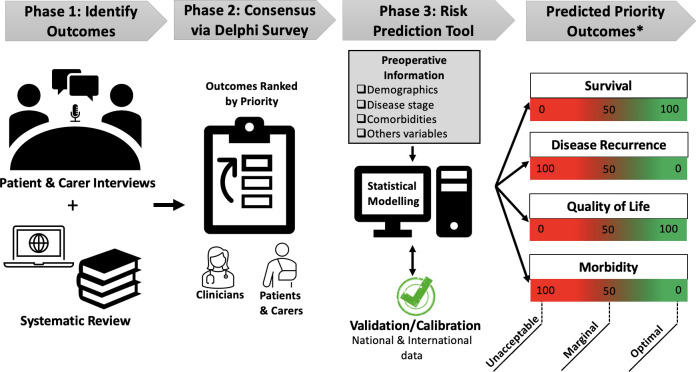
The general aim of this study is to develop and validate a risk prediction tool for patients with locally advanced and recurrent rectal cancer who undergo PE (figure 1). Specifically, this study aims to:
Figure 1.
Schematical representation for the development of the pelvic exenteration risk prediction tool. *Hypothesised priority outcomes are listed. Actual outcomes will be identified during phases 1 & 2.
Define ‘priority outcomes’ following PE for locally advanced and recurrent rectal cancer, based on patients, carers and clinicians consensus regarding their importance in guiding treatment decision-making.
Develop a risk prediction model which predicts the identified priority outcomes of PE for an individual patient based on information available preoperatively.
This study will employ a mixed-methods study design which will follow recommendations from the established methodology for development of core outcome sets (COS), such as that used for the core outcome research measures in anal cancer study,12 13 and be modified for the purposes of addressing the study objectives. COS development methodology has been outlined by the COMET Initiative and uses comprehensive consensus methods, involving patients and clinicians, to develop agreement around a minimum set of outcomes to be reported in all studies and trials for a specific clinical area.14 While the primary purpose of a COS is to define the minimum outcomes to be used in clinical trials, the purpose of this study is to identify the priority outcomes to be used for clinical decision-making by incorporation into a risk prediction tool, and therefore, the established COS methodology will be modified to account for this different objective.
There will be three distinct phases of this study. In phase 1, outcomes of PE will be identified through a comprehensive systematic review of the literature (phase 1a), followed by in depth exploration of experiences of individuals who have undergone PE for locally advanced or recurrent rectal cancer and their carers using qualitative research framework (phase 1b). In phase 2, a survey of individuals who have undergone PE, carers and clinicians will be conducted using the Delphi methodology to explore consensus around the outcomes of highest priority and the level of influence each outcome should have on treatment decision-making. In phase 3, a risk prediction tool will be developed to predict the identified priority outcomes using comprehensive multivariate modelling.
Multidisciplinary advisory committee
This study will be governed by a multidisciplinary advisory committee (MAC), which will comprise of cancer specialists (surgeons, medical and radiation oncologists, senior surgical and cancer nurses, allied health professionals), a health economist, statistician, epidemiologist, health policymaker, guideline and quality measurement developer, information technology professional, patients and carers. The MAC will guide development of the tool and in later phases (beyond those outlined in this protocol) advise on development of a surgical decision-making tool, which produces a recommendation for or against PE surgery based on the risk prediction model developed in this study. The MAC will also develop communication strategies and guide translation to clinical practice with implementation and long-term sustainability plans.
Patient and public involvement
Two consumers have been consulted during the development of the study concept and protocol. Individuals who have undergone PE and their nominated carer/family member will inform the development of PE priority outcomes through in-depth interviews (phase 1). The interviews will explore their experience of PE, the decision-making process and identify priorities and factors that informed the decision. Both are included as participant groups in the Delphi process (phase 2). One or more consumers will be members of the advisory group involved in reviewing the outcomes identified in phase 1 prior to those outcomes being distributed in the subsequent Delphi study.
Phase 1: identifying outcomes of PE
Phase 1a
This phase aims to identify all outcomes of PE for locally advanced or recurrent rectal cancer reported in the published literature. The review will be conducted according to the Cochrane Collaboration guidelines15 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.16 A comprehensive search strategy has been created in conjunction with an experienced medical librarian. The protocol has been published a priori on the PROSPERO registry.17
MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing & Allied Health Literature (CINAHL) and Scopus were searched from 1990 to 25 April 2023 for combinations of the following medical subject headings and keywords: pelvic exenteration or extended radical resection or multivisceral resection, and rectal neoplasms. The search was limited to studies published from 1990 onward, English language and adult patient. The complete search strategies are available in online supplemental file 1.
bmjopen-2023-075304supp001.pdf (32.4KB, pdf)
Retrospective and prospective cohort studies, cross-sectional studies, qualitative studies and randomised trials reporting outcomes of PE or multivisceral resection as the primary treatment for locally advanced or recurrent adenocarcinoma of the rectum will be included. Studies will be excluded if >10% of the population had non-rectal cancers or underwent less extensive resection and outcomes were not reported separately. Narrative reviews, case reports, case series including <5 patients, conference abstracts and letters will be excluded.
Abstracts will be screened by two reviewers independently and one reviewer will complete the full text screening and data extraction, with all extracted outcomes then reviewed by a multidisciplinary team. Data (all verbatim outcomes and their definitions) will be extracted and entered into an electronic database using a custom-designed data entry form. Outcomes will be merged with similar outcomes using different wording to create ‘standardised outcomes’ and allocated to a domain, each of which will be reviewed at a multidisciplinary advisory meeting, attended by specialist clinicians and nurses, a surgical outcomes researcher, and two patient advocates.
Phase 1b
In this phase, an exploratory qualitative interpretive design will be used to investigate the perspectives of individuals who have undergone PE and their carers on the important outcomes of surgery for them individually. Individual interviews with people who have undergone PE and their carers will be conducted using opened semistructured interview format to maintain a participant-led dialogue. Two topic frameworks have been developed—for individuals who have undergone PE and for carers (online supplemental file 2). The interviews will aim to identify all outcome following PE which the participant considers important, by exploring the general experience of the individual who underwent PE, which information they did or did not access about PE, alternative treatments at the time of diagnosis, how the patient decided whether or not to undergo PE, how having undergone PE has impacted their life, and which factors they would view as most important if counselling someone about undergoing PE.
bmjopen-2023-075304supp002.pdf (33.2KB, pdf)
Participants, recruitment and setting
Individuals who have undergone PE for locally advanced or recurrent rectal cancer at Royal Prince Alfred Hospital, Sydney, Australia, will be identified from a prospectively maintained electronic PE database and invited to participate. The inclusion criteria are outlined below. The final sample size will be determined by iterative analysis for data saturation, with an estimated sample size required for saturation of 10–20 patients and 5–10 carers. A purposive sampling matrix was developed (table 1) to guide recruitment and in order to ensure the participants represent a broad group of individuals with diverse views. Characteristics used for selection will include age at time of surgery, gender, place of residence and tumour type. Carers for individuals who have undergone PE will also be invited to participate. According to participant preference, interviews will take place face to face or via telephone.
Table 1.
Purposive sampling criteria to guide recruitment in phase 1b
| Characteristics | Target no of participants |
| Age at surgery | |
| <50 years | 5–8 |
| ≥ 50 years | 5–8 |
| Sex | |
| Male | 5–8 |
| Female | 5–8 |
| Place of residence | |
| Local (metropolitan Sydney) | 6–10 |
| Rural/regional | 4–5 |
| Tumour | |
| Locally advanced primary rectal cancer | 5–8 |
| Locally recurrent rectal cancer | 5–8 |
| Total sample | 10–20 |
Inclusion criteria
Adults ≧18 years of age.
Patients who have undergone PE for locally advanced or locally recurrent rectal cancer.
Patients who are more than 6 months post PE surgery.
Patients who are fit to participate in an interview (according to their treating clinician).
Patients who are able to participate in an interview in English.
Patients who have the capacity to provide informed consent.
The nominated carer for a participating individual who has undergone PE. This may be a spouse, child or other close relative. Paid carers or those from a support agency will not be included.
Data collection and analysis
Baseline demographic characteristics of participating individuals will be extracted from the existing PE database. Interviews will be audiorecorded, transcribed verbatim and imported into NVivo qualitative analysis software (NVivo V.11, QSR International, Burlington, Massachusetts, USA). Template analysis will be used to analyse the interview content, where outcomes of PE identified in the interview transcripts will be coded using NVivo and themes will be identified. Coded data will be used to generate a list of outcomes of PE which are prioritised by patients and carers.
Participant consent and withdrawal
All participants will complete a consent form after they have read the approved participant information sheet and had time to consider participation, and consent will be confirmed verbally at the start of the interview. Participants will be able to take a break, end the interview or withdraw from the study at any time, without any impact on their relationship with their treating clinician(s) or hospital.
Phase 2: defining priority outcomes by consensus
Outcomes identified from the literature and interviews in phase 1 will be reviewed according to the method described by Fish et al.12 ‘Standardised outcome terms’ will be developed, where outcomes which have the same meaning but are described with different wording are combined. Similar standardised outcomes will then be grouped by domain. Standardised outcome terms and domains for each outcome will be ratified at an MAC subcommittee meeting, attended by cancer specialists, an academic with experience in surgical outcomes, senior PE nursing staff and consumer advocates. Outcomes will be excluded if considered to be of minimal clinical relevance by the MAC subcommittee. The resulting list of standardised outcomes will be used to populate the first of a three round iterative survey process using Delphi methodology.18
Participants, recruitment and setting
Participants will be recruited from three key participant groups:
Clinicians with experience in PE and the management of locally advanced and recurrent rectal cancer (including medical, nursing and allied health staff). Clinicians will be identified via the International PelvEx Collaborative (an international collaborative group made up of specialist surgeons/physicians with experience managing advanced pelvic cancer) and Australia and New Zealand Pelvic Exenteration Multi-Disciplinary teams.
Patients who have undergone PE for locally advanced primary or recurrent rectal cancer. Patients will be identified from an existing institutional PE database as for phase 1.
Carers for patients who have undergone PE for locally advanced primary or recurrent rectal cancer. Patients who participate will be asked to forward the invitation email to their carers.
Identified potential participants will be initially contacted via email to advise of the upcoming Delphi survey and provide a study information sheet. The first round of the survey will be emailed 5 days after the initial contact, followed by reminders at 10 and 20 days. Following the final reminder, non-responders will be excluded from the study. Late replies will be considered, if within the study time frame. The second and the third rounds of the survey will be emailed to all responders of the survey first round. The same reminder protocol will be used. The survey rounds will be approximately 30 days apart. Snowball sampling will be used where all participants will be invited to forward the first round invitation email to anyone who is eligible to participate.
Data collection and analysis
Survey first round: Participants will indicate whether they are a patient, carer or clinician, which will allow them to access a survey specifically designed for each of these participant groups. The first round of the survey will be divided into two main sections:
-
Section 1 will include demographic information specific to each participant group:
Patients: age, gender, tumour type (primary or recurrent rectal), months since surgery.
Carers: age, gender, relationship to patient with locally advanced or recurrent rectal cancer.
Clinicians: age group, gender, specialty, qualifications, whether a dedicated PE fellowship was undertaken, country of residency, number of years of experience treating locally advanced and recurrent rectal cancer, whether they practice within a dedicated pelvic oncology multidisciplinary team, the number of operations performed annually (in the case of surgeons).
Section 2 will present participants with the outcomes identified in the systematic review and interviews, grouped by domains. Participants will be asked to use a 9-point Likert scale to rate the importance of each outcomes as limited importance1–3; important but not critical4–6 and critically important.7–9 14 An open question will be included at the end of the survey to allow participants to list any additional outcomes that they do not feel have been identified or considered in the questionnaire. Each outcome will be described in medical (for clinicians) and lay terms (for patients and carers).
Survey second round: In round 2, a list of all outcomes with a mean score or 4–6 (important but not critical) and 7–9 (critically important) during round 1 will be collated with any additional unique outcomes suggested by participants and redistributed (those scoring 1–3 will be discarded). Participants will be provided with feedback from round 1 in the form of their previous score for each domain and a mean score from their participant group. Participants will be asked to reflect on the information presented before scoring each outcome again on the 9-point Likert scale.
Consensus around outcomes will be assessed prior to round 3, where consensus status for each outcome will be categorised according to Williamson et al19 as:
Consensus in: 70% or more respondents within a participant group rate the outcome as critically important7–9 and 15% or fewer rate the outcome as limited importance.1–3
Consensus out: 70% or more of respondents within a participant group rate the outcome as limited importance and 15% or fewer rate the outcome as critically important.7–9
No consensus: Neither of the above criteria are met.
Survey third round: In this final round, the refined list of ‘consensus in’ outcomes will be included. Participants will be asked to divide 100 points among the ‘consensus in’ outcomes according to the relative level of influence each outcome should have on treatment decision-making. The outcomes will be listed in rank order based on the mean number of points attributed to each. This list will form the provisional list of priority outcomes.
Descriptive statistics will be used to characterise the participants according to participant group. Means and SD will be used to rank the outcomes. The data from all rounds will be displayed in descriptive format, with mean responses, in order of overall ranking of importance.
Participant consent
All invited participants have no obligation to complete the study surveys and can withdraw from the study at any time. Completion of the study survey will be an indication of implied consent.
Phase 3: predicting priority outcomes
This phase aims to develop a risk prediction model, which can be used at the time of diagnosis to predict each priority outcome (identified in phase 2) for individual patients using information available at the time of treatment decision. An MAC subcommittee will review and ratify the provisional list of priority outcomes prior to this phase of the study.
Participants, recruitment and setting
Patients who underwent PE for locally advanced primary or recurrent rectal cancer at Royal Prince Alfred Hospital, Sydney, Australia between 1994 and 2023 will be identified from the authors' institutional PE database. This database is prospectively maintained and includes extensive preoperative, intraoperative, postoperative, long-term survival and quality of life data. The estimated number of eligible patients is 500.
Preoperative variables
Preoperative variables to be used to calculate patient-specific risk scores for each of the priority outcomes will be selected from the PE database based on demonstrable predictive value and face validity according to expert opinion. Due to the design of this study, preoperative variables cannot be selected a priori as the priority outcomes to be predicted will not be identified until the end of phase 2. If required, multiple imputation will be used for missing values.
Data collection and analysis
For eligible patients, priority outcome data (eg, survival, quality of life, complication rate) and all potential preoperative risk factors for those outcomes (eg, demographics, comorbidities and tumour factors) will be extracted from the PE database. Using this individual patient data, risk prediction models for each of the priority outcomes of PE will be developed. An experienced biostatistician will be involved in conducting and interpreting these analyses. Two main approaches will be used, including traditional multivariate regression techniques and machine learning approaches. The accuracy of the models will be compared by the computed sensitivity, specificity, negative predictive value, positive predictive value, accuracy and area under the curve. A separate statistical analysis plan will be developed for internal validation and external validation (using Australian wide and international individual patient data via the International PelvEx Collaborative Group).
Ethics and dissemination
Phase 1 involves a systematic review of the literature, semistructured interviews with patients and their carers, and a Delphi survey study of clinicians, patients and carers. Ethics approval for phase 1 has been granted by the Sydney Local Health District HREC (X22-0422 and 2022/ETH02659). Ethics approval for the Pelvic Exenteration Quality Improvement database, which will be used for the statistical modelling in phase 2, is current (X13-0283 and HREC/13/RPAH/504). Other than the patients/carer interviews and Delphi survey, where the risk to the participant is that of inconvenience or distress, this project is observational and does not involve any therapeutic intervention. Therefore, there are no other potentially ethically adverse consequences. Informed consent will be obtained from participants in phases 1b and 2; a waiver of consent for secondary use of data in phase 3 will be sought prior to commencement of phase 3.
The results of this study will be submitted for publication in scientific journals and for presentation at scientific meetings.
Beyond phase 3, future investigation will focus on development of a surgical decision-making tool which produces a patient-specific recommendation for or against PE, in a reproducible fashion. This recommendation will be based on the predicted priority outcomes for an individual patient according to the risk prediction model developed in the current study. This will involve using consensus methods among experts to define the threshold values for the predicted priority outcomes at which a recommendation for or against surgery is made.
Study status and planned timeline
Phase 1a: January 2023–September 2023 (manuscript under peer review).
Phase 1b: May 2023–November 2023 (data collection underway).
Phase 2: December 2023–April 2024 (not commenced).
Phase 3: May 2024–December 2024 (not commenced).
Supplementary Material
Footnotes
Twitter: @SOuRCe_RPA
Collaborators: The EviSurg Research Group: Dr Nabila Ansari, Dr Wendy Brown, Professor Phyllis Butow, A/Professor Sharon Carey, Dr Alix Dumitrescu, Professor Lisa Horvath, A/Professor Kate Mahon, Dr Kate McBride, Professor Glen Salkeld, A/Professor Charbel Sandroussi, Professor Des Winter, Dr David Yeo, Professor Jane Young.
Contributors: All authors made significant contributions to the design and development of this study and achieving ethical approval. KB was a major contributor in writing this paper. MS, DS, K-SN, PS, CK and KW contributed to the drafting and editing of this paper and approved the final manuscript.
Funding: KB is the recipient of the Mitchell J Notaras Fellowship in Colorectal Surgery from the University of Sydney. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: EviSurg Research Group, Nabila Ansari, Wendy Brown, Phyllis Butow, Sharon Carey, Alix Dumitrescu, Lisa Horvath, Kate Mahon, Kate McBride, Glen Salkeld, Charbel Sandroussi, Des Winter, David Yeo, and Jane Young
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Brown KGM, Solomon MJ, Koh CE. Pelvic exenteration surgery: the evolution of radical surgical techniques for advanced and recurrent pelvic malignancy. Dis Colon Rectum 2017;60:745–54. 10.1097/DCR.0000000000000839 [DOI] [PubMed] [Google Scholar]
- 2.Solomon MJ. Redefining the boundaries of advanced pelvic oncology surgery. Br J Surg 2021;108:453–5. 10.1093/bjs/znab047 [DOI] [PubMed] [Google Scholar]
- 3.Harji DP, Griffiths B, McArthur DR, et al. Surgery for recurrent rectal cancer: higher and wider Colorectal Dis 2013;15:139–45. 10.1111/j.1463-1318.2012.03076.x [DOI] [PubMed] [Google Scholar]
- 4.PelvEx Collaborative . Factors affecting outcomes following pelvic exenteration for locally recurrent rectal cancer. Br J Surg 2018;105:650–7. 10.1002/bjs.10734 [DOI] [PubMed] [Google Scholar]
- 5.Collaborative P. Surgical and survival outcomes following pelvic exenteration for locally advanced primary rectal cancer: results from an international collaboration. Ann Surg 2019;269:315–21. 10.1097/SLA.0000000000002528 [DOI] [PubMed] [Google Scholar]
- 6.Venchiarutti RL, Solomon MJ, Koh CE, et al. Pushing the boundaries of pelvic exenteration by maintaining survival at the cost of morbidity. Br J Surg 2019;106:1393–403. 10.1002/bjs.11203 [DOI] [PubMed] [Google Scholar]
- 7.Makker PGS, Koh CE, Solomon MJ, et al. Functional outcomes following pelvic exenteration: results from a prospective cohort study. Colorectal Dis 2021;23:2647–58. 10.1111/codi.15834 [DOI] [PubMed] [Google Scholar]
- 8.Steffens D, Solomon MJ, Young JM, et al. Cohort study of long-term survival and quality of life following pelvic exenteration. BJS Open 2018;2:328–35. 10.1002/bjs5.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh CE, Badgery-Parker T, Salkeld G, et al. Cost-effectiveness of pelvic exenteration for locally advanced malignancy. Br J Surg 2016;103:1548–56. 10.1002/bjs.10259 [DOI] [PubMed] [Google Scholar]
- 10.Solomon MJ, Brown KGM. Extended radical resection: the standard of care for patients with advanced pelvic malignancy. Ann Surg Oncol 2020;27:323–4. 10.1245/s10434-019-07817-7 [DOI] [PubMed] [Google Scholar]
- 11.Denost Q, Solomon M, Tuech J-J, et al. International variation in managing locally advanced or recurrent rectal cancer: prospective benchmark analysis. Br J Surg 2020;107:1846–54. 10.1002/bjs.11854 [DOI] [PubMed] [Google Scholar]
- 12.Fish R, Sanders C, Williamson PR, et al. Core outcome research measures in anal cancer (CORMAC): protocol for systematic review, qualitative interviews and Delphi survey to develop a core outcome set in anal cancer. BMJ Open 2017;7:e018726. 10.1136/bmjopen-2017-018726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fish R, Sanders C, Adams R, et al. A core outcome set for clinical trials of chemoradiotherapy interventions for anal cancer (CORMAC): a patient and health-care professional consensus. Lancet Gastroenterol Hepatol 2018;3:865–73. 10.1016/S2468-1253(18)30264-4 [DOI] [PubMed] [Google Scholar]
- 14.Williamson PR, Altman DG, Bagley H, et al. The COMET handbook: version 1.0. Trials 2017;18:280. 10.1186/s13063-017-1978-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochrane Handbook for systematic reviews of interventions. 2 Ed. Chichester (UK) John Wiley & Sons; 2019. [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Brown KGM, Pisaniello J, Ng KS, et al. Systematic review of outcomes measured and reported in studies of pelvic exenteration for the treatment of locally advanced and recurrent rectal cancer. PROSPERO CRD42022351909. 2022. Available: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022351909
- 18.Jünger S, Payne SA, Brine J, et al. Guidance on conducting and reporting Delphi studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med 2017;31:684–706. 10.1177/0269216317690685 [DOI] [PubMed] [Google Scholar]
- 19.Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. 10.1186/1745-6215-13-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-075304supp001.pdf (32.4KB, pdf)
bmjopen-2023-075304supp002.pdf (33.2KB, pdf)