Abstract
Ultraviolet-B irradiation is a common environmental stressor that has detrimental effects on human skin. Natural sunscreens are well-known for their ability to benefit inflamed sunburn and dry skin. This study examined the effect of formulated Ipomoea carnea herbal cream on UVB-induced skin damage. We screened the bioactive compounds of I. carnea crude extract, showing significant antioxidant activity. Additionally, we evaluated the cytotoxicity, revealing that I. carnea extract has less toxicity to vero cells (IC50 98.45 μg/mL) than to A375 cells (IC50 48.95 μg/mL). Based on this, we formulated the I. carnea herbal cream (FIHC) at 50, 100 and 200 mg concentrations and evaluated its organoleptic characteristics. Then, the rats were exposed to UVB radiation (32,800 J/m2) four times/week (on alternate days) before the cream was applied topically to the dorsal skin surface. Under UVB stress without treatment, rats showed deep dermal damage. In contrast, rats treated with the FIHC exhibited significantly reduced sunburn. Moreover, the histopathological and biochemical assays were confirmed by the topical application of FIHC, which had potentially reduced the skin elasticity and maintained the imbalanced enzyme and non-enzymatic antioxidant activity. Our findings amply demonstrate that the FIHC significantly accelerated the recovery of UVB-induced lesions through antioxidant and down-regulation of skin photodamage.
Keywords: UVB irradiation, Ipomoea carnea, Herbal cream, Anti-inflammation, Sagging and histology
Graphical abstract
1. Introduction
The sun is the primary source of life and energy. It generates a steady energy flow from electromagnetic radiation with wavelengths between 290 and 4000 nm that reaches the earth's surface. Approximately 40% of sunlight is visible, 50% is infrared, and 10% is ultraviolet [[1], [2], [3], [4]]. The UV region is categorized into three subcategories: UVC (200–280 nm), UVB (280–320 nm), and UVA (320–400 nm). Extended exposure to solar ultraviolet (UV) radiation can damage the skin. The main cause of skin damage is UVB radiation, with UVA radiation contributing to a lesser extent [5,6]. UVB radiation causes skin damage followed by sunburn, skin pigmentation, premature aging, and photo-carcinogenesis [7,8]. According to researchers, the sun has detrimental consequences, such as acute impacts (e.g., sunburn) and chronic dangers, such as wrinkling, melanoma, cancer and immunological suppression [9,10]. Reactive oxygen species (ROS) are principally responsible for skin damage caused by UVB radiation because they interact with proteins and lipids to change them [11]. When the production of reactive oxygen species (ROS) generated by UV radiation surpasses the skin's ability to eliminate them, damage occurs. Extensive research has demonstrated that the accumulation of ROS can trigger the production of inflammatory cytokines, metalloproteinases (MMPs), and the degradation of collagen, ultimately leading to skin photoaging [[12], [13], [14]]. To efficiently reduce the extent of ultraviolet (UV) radiation exposure and minimize the risk of sunburn on the skin, it is advisable to utilize topical sunscreen as a recommended approach [15].
Sunscreens shield the skin from the sun's harmful rays, which may cause erythema to briefly emerge, as well as actinic photoaging and skin cancer to develop over time [16,17]. Sunscreens may lessen the risk of sun-induced skin cancer by decreasing the intensity of UV radiation that reaches the skin [6]. An ideal sunscreen should provide adequate protection throughout the whole UV spectrum and be non-irritating, non-toxic, and allergen-free [18]. Due to their antioxidant properties and UV-R absorption, plant-derived extracts have lately been explored as possible sunscreen components [18,19]. Much of the research focuses on developing herbal plant products (cream, lotion, gel and paste), as natural substances are safer and more biocompatible than manufactured materials [20]. Herbal cream incorporate one or two natural ingredients for specific cosmetic benefits. These compositions are applied to the skin to reduce the ROS imbalance and protect it from the harmful effects of UV light [21,22]. The sun protection factor refers to the degree to which the sun's harmful effects are mitigated by sunscreens. Several clinical trials have demonstrated that sunscreens may reduce the incidence of skin cancer, primarily squamous cell carcinoma and melanoma [[23], [24], [25]].
The phytocompounds in antioxidant plant extracts work together to create a synergistic effect that is less harmful [26]. While referring to the context of plant drugs, topical application of Panax ginseng [27], Aloe saponaria [28], Acalypha indica [29], Viola tricolor [30], Annona muricata [31], Centella asiatica [32] and Hyptis mociniana [33] these plant parts used to formulate herbal treatments must possess anti-inflammatory, antioxidant, antimicrobial and also have potent ameliorative effect against to UVB induced skin damages [34]. Phyto-extract loaded creams are semi-solids frequently applied to the skin and consist of two immiscible phases: oily and aqueous. Due to the emulsified structure of the skin, cream-formulated medications interact effectively with the skin and penetrate biological membranes more readily [35].
Many of the popular medicinal plants have been explored to find out the therapeutic compounds which can provide remedy for UVB induced skin damage, but there has been limited progress in exploring weed plants for similar ameliorative purposes [36,37]. The weed plant of Ipomoea carnea is a member of the Convolvulaceae family commonly known as ‘Morning glory’. It can be found in various parts of India, including Tamil Nadu, Kerala, Chandigarh, Madhya Pradesh, West Bengal, Rajasthan, and Maharashtra [38]. I. carnea has rapid propagation, wide ecological range, and exceptional competitiveness [39]. The plant exhibits allelopathic effects, its boiled roots are used as a laxative and to stimulate menstruation. Traditional healers utilize various parts of the plant to treat skin diseases, while the milky juice is specifically employed for the treatment of Leucoderma and related skin conditions. It contains a variety of phytochemicals, including glycosides, reducing sugars, alkaloids, flavonoids, esters, fatty acids, alcohols, and tannins. The leaves specifically contain alkaloids, hexadecanoic acid, saponins, stearic acid, 1,2-diethyl phthalate, phenolic compounds, n-octadecanol, octacosane, hexatriacontane, tetracontane, 3-diethylamino-1-propanol, xanthoproteins, and flavonoids [40,41]. Some of these compounds are known to possess properties such as anti-diabetic, hypolipidemic, anti-inflammatory, antibacterial, hepatoprotective, and anti-cancer effects. Additionally, a significant chitinase/lysozyme activity has been observed during screening [[42], [43], [44], [45]]. To the best of our knowledge, there is a lack of scientific reports available in support of its traditional treatment. Although no notable herbal cream based studies have been conducted on I. carnea. Based on this background, we developed a herbal cream using I. carnea to assess its efficacy against UV-B irradiation induced skin damage in Rattus norvegicus under laboratory conditions.
2. Materials and methods
2.1. Plant collection and extraction
Ipomoea carnea leaves were collected in April around Sivakasi (latitude: 9.463898° N &77.760829° E), Tamil Nadu, India and confirmed by taxonomists at the Centre for Research and Postgraduate Studies in Botany, Ayya Nadar Janaki Ammal College, Sivakasi, Tamil Nadu, India. The voucher specimen was registered under the number TPH-1552. The leaves were shade-dried at 35–40 °C, ground into a fine powder, and 100 g of the fine powder were subjected to Soxhlet extraction (Borosil, Madurai, India) using 600 mL of 95% methanol (AR grade) for 12 h at 60 °C. The extracted solvent was then dried under vacuum, yielding the final extract used for further analysis.
2.1.1. Gas chromatography-mass spectrometry analysis
The dried plant residues were dissolved in methanol and subjected to Gas chromatography-mass spectrometry (GC-MS) (GC-QP2010, Shimadzu, Tokyo, Japan) was performed using Thermal Desorption. The GC-MS had an Rtx-5 capillary column (30 m × 0.25 mm x 0.25 μm film thickness). Helium was used as the carrier gas at a constant flow rate of 1.21 mL/min. The oven temperature was programmed to increase from an initial 60 °C–200 °C at a rate of 5 °C per minute and then to 280 °C. The electron multiplier was set to auto-tune, and the scan was performed over a mass range of 40–650 m/z Da. To identify the fragmentation, the retention time and mass spectra of each separated peak were compared to NIST 20 and the WILEY spectral library searching program, as well as the literature.
2.2. Antioxidant activity
To determine the free radical scavenging ability of the I. carnea crude extract, several non-biological and biological analyses were performed. These included the 2,2-diphenyl-1-picrylhydrazyl radical assay (DPPH) [46], the metal chelating activity assay [47], and assays for biological radicals such as superoxide anion (O2·-) [48], hydroxyl radical (OH-) [49], and reducing power [50]. (Details of these assays are mentioned in the supplementary file).
2.3. Cytotoxicity analysis I. carnea extract
The cytotoxic properties of the I. carnea crude extract were assessed using 3–4,5-(dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay in A375 and Vero cells. Briefly, A375 and Vero cells were seeded into 96-well microtiter plates and cultured at 37 °C with 5% CO2 for 24 h to reach confluency. The cells were further treated with various concentrations of the I. carnea crude extract (1.95, 3.9, 7.81, 15.62, 31.25, 62.5, 125, 250, 500, and 1000 μg/mL). After 48 h, MTT assay was performed according to the standard procedure [51]. Untreated cells served as control. Dimethyl sulfoxide (DMSO) served as a negative control. The absorbance was measured at 570 nm using a microplate reader (Bio-Rad, Hercules, CA, USA). The percentage of viability was calculated using the following formula (Eq. (1)).
| (1) |
where ODsample = absorbance of the treated sample, ODblank = absorbance of DMSO, and ODconrol = absorbance of non-treated sample. The IC50 values were calculated by using GraphPad Prism software by plotting the percentage of inhibition against the logarithm of the extract concentration.
2.4. Formulation of I. carnea herbal cream
The herbal cream was formulated by using the I. carnea extract and water/oil emulsion according to the adapted method [52] (Table 2 details are given in the supplementary file).
2.4.1. Evaluation of I. carnea cream
The formulated I. carnea herbal cream were evaluated through visual examination and measurements of various physicochemical characteristics, including color, physical appearance, odor, pH, homogeneity [53] spreadability [54], stability [55], viscosity [53] and in vitro permeation [56].
2.5. UVB irradiation and topical application
A total thirty number of Swiss female Wistar rats (Rattus norvegicus; weighing 170 ± 10 gm) were used in the study were performed at Dept. of Pharmacology, K.M. College of Pharmacy, Madurai. The animals were housed in polypropylene cages under controlled environmental conditions of a temperature maintained at 22 ± 1 °C, a regular light/dark cycle, and given free access to food and water. All experimental protocols were authorized by the Ethical Committee (No. 661/PO/Re/S/02/CPCSEA) in accordance with internationally accepted guidelines for the use and care of laboratory animals outlined by the NIH. The experimental study was approved by the institutional animal ethics committee (Process number-IAEC/SUNDAR.M/PhD/MKU/F9884/KMCP/70/2019).
The UVB chamber box setup was performed as described previously [57]. The UVB radiation source consisted of two UVB lamps (Philips 20 W Sunlamp, Holland). It was placed 30 cm above the animals and continuously produced a light spectrum with a peak emission of 315 nm in the UVB chamber. A spectroradiometer (IL-700, International Lights, USA) equipped with a broadband light sensor (SEE 400 type) was used to measure the energy delivered by the regulator [58]. To prevent the animals from moving during the exposure to UVB, they were injected intraperitoneally with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) for anesthesia prior to the procedure.
At the initiation of the study, the animals were shaved (5 cm2) in the dorsal skin surface, and divided into 6 experimental groups (n = 5 each group), as well as a control group of naive, untreated rats (n = 5 rats).
G1 (n - 5) control group (without UVB & creams).
G2 (n - 5) exposed UVB radiation only.
G3 (n - 5) exposed UVB with treated plain cream.
G4 (n - 5) exposed UVB with treated FIHC 50 mg.
G5 (n - 5) exposed UVB with treated FIHC 100 mg.
G6 (n - 5) exposed UVB with treated FIHC 200 mg.
For our studies, we slightly modified the methodology described earlier to adapt the short-term UVB irradiated dose [59,60]. Each rat received a total energy of 32,800 J/m2, administered as follows: 600 J/m2 during the 1st week, 1800 J/m2 during the 2nd week, 2200 J/m2 during the 3rd week, and 3600 J/m2 during the 4th week. The exposure lasted 5 min per dose, four times/week on alternate days. After the exposure of 30 min for each individual, 500 mg of formulated I. carnea herbal cream was topically applied to the dorsal skin of each rat. At the end of each week, the rat dorsal skin (5 cm2) was marked, and red spot occurrence and size were recorded by laying a transparent sheet over the skin and taking photographs using a Nikon-D90 DSLR camera with a macro 105 lens (DX-format, Nikon crop, Japan). At that time point, the dimensions of all the rashes and red spots on each rat were recorded and calculated (Eq. (2)).
| (2) |
(A = Area; r = radius).
2.5.1. Skin elasticity or pinch test
The flexibility of the rats dorsal skin was examined before and after treatment with a formulated cream using the skin recovery ability test, often known as the pinch test [2,61]. In brief, the fingers were used to raise the midline of the rat's dorsal skin until its feet were barely touching the desk. The pinch was then released, and the time taken for the skin to recover (in seconds) was immediately measured and calculated.
2.5.2. Histopathological analysis
At the end of the experiment period, animal dorsal skin was detached freshly and fixed in 10% neutral buffered formalin, followed by being embedded in paraffin and sectioned at 2 μm using a microtome (Weswox Optik-1090A). These sections were then affixed to slides and stained using Haematoxylin-eosin (H&E). The degree of skin structure alteration and elastosis were assessed microscopically (Olympus microscope, CH20iBIMP with micro view ×86 software).
2.5.3. Biochemical assays
After completing four-week UV exposure and formulation application as per the protocol, the skins were excised from the animals were homogenized in 50 mM phosphate buffer (pH 7.0). The homogenized samples were then centrifuged at 15,000 rpm for 15 min at 4 °C, and the resulting supernatant was utilized for biochemical analysis. The levels of enzymatic and non-enzymatic antioxidant status were analysed using the following standard protocols: estimation of protein content [62], superoxide dismutase activity (SOD) [63], catalase activity (CAT) [64], and lipid peroxidation (MDA) [65] (details are mentioned in the supplementary file).
2.6. Statistical analysis
The data were presented as mean ± SD. To identify the inter-group differences, multiple group comparisons were conducted using one-way analysis of variance (ANOVA) followed by Duncan's comparison test. A value of p < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS (version 21.0; IBM Corp. Armonk, NY, USA).
3. Results and discussion
3.1. Phytochemical analysis of Ipomoea carnea by GC-MS
The GCMS data showed that methanolic extract of I. carnea leaves contained a total of 54 phytocompounds (Fig. 1) (Table 3 provide in supplementary file). The compound 1,2,4-Butanetriol (62.95%), were found be predominating among the 54 compounds followed by n-Hexadecanoic acid (4.27%), Phytol (2.62%), Neophytadiene (2.28%), gamma.-Sitosterol (2.25%), Stigmasterol (2.14%), Squalene (1.44%), Caryophyllene oxide (1.09%), Ergost-5-en-3-ol (3. beta.,24r)- (0.91%), Phytyl palmitate respectively (0.65% with), Lupeol (0.65%), Isospathulenol (0.29%) and gamma-Tocopherol (0.24%) belonging to different classes like ester, steroid, fatty acid, terpene, aliphatic alcohol and flavonoids. Previous reports have shown that the compound 1,2,4-Butanetriol has potent wound healing activity [66,67]. The compound n-hexadecanoic acid has major potential anti-inflammatory properties [68] and antioxidant properties [69]. Phytol, the third major bioactive compound identified in the I. carnea extract, has been reported to possess remarkable antioxidant potential and the ability to inhibit free radical formation [70]. The compound squalene is an effective inhibitor of chemical carcinogens [71,72]. Additionally, vitamin E is a potent antioxidant that protects cells against free radicals and has anti-inflammatory properties [73,74]. The compound caryophyllene oxide exhibits significant analgesic and anti-inflammatory activities. Likewise, several phytocompounds such as phytol, neophytadiene, ɤ-sitosterol, and gamma-thionodecalactone phytol acetate were detected in Ipomoea horsfalliae, which have been reported to have anticancer potential [75].
Fig. 1.
Bioactive compounds of I. carnea were analysed by GC-MS. The peaks on the graph represent the percentage of identified phytoconstituents of I. carnea extracts.
3.2. Antioxidant activity
The antioxidant effect of I. carnea was assessed by various assays, including the DPPH assay, metal chelation assay, hydroxyl radical scavenging activity, and superoxide anion radical scavenging assay, as shown in Fig. 2. The antioxidant potential of the methanolic extract of I. carnea crude was compared to that of the standard ascorbic acid. The DPPH activity of I. carnea extract has significantly increased with the increase in its concentration and exhibited higher antioxidant activity of 83.02% at 1000 μg/mL. However, the standard Ascorbic acid demonstrated 94.26% inhibition in the same concentration (Fig. 2a). The I. carnea extract also showed significant hydroxyl radical scavenging activity of 72.2% and ascorbic acid to 85% at 1000 μg/mL (Fig. 2b), while the ferrous ion-chelating capacity was found to be increase with increasing the concentration at maximum concentration 1000 μg/mL the chelation was 75.6%, and 84.3% with Ipomoea extract and ascorbic acid standard. (Fig. 2c). Moreover, the maximum inhibition of superoxide radical scavenging activity was found to be 82.4% by I. carnea as compared to the activity of ascorbic acid 90.3% at a concentration of 1000 μg/mL (Fig. 2d). The reducing power of I. carnea was determined by the increase in absorbance of the reaction mixture, which indicated an increase in its reducing power (Fig. 2e). The results demonstrate that the antioxidant potential of I. carnea extract is significantly greater as compared to standard ascorbic acid. Similar to our study, previous reports of strong antioxidant capacity were noted from other plant species, including Aristolochia indica, Piper nigrum, Ocimum basilicum Aspalathus linearis, Tabernaemontana divaricata and Camellia oleifera [26,[76], [77], [78], [79], [80]]. Antioxidants have the ability to boost the endogenous antioxidant capacity of the skin and help neutralise reactive oxygen species (ROS) that are caused by external causes such as UV radiation from the sun. Almost every living thing on earth has some defense against the harmful effects of ultraviolet radiation [81].
Fig. 2.
Free radical scavenging effect of I. carnea (IC) extract. (a) DPPH radical scavenging test (b) Metal chelating test (c) Hydroxyl radical scavenging test (d) Superoxide anion radical scavenging test (e) Reducing power test. Each value was presented as the mean ± SD (n = 3).
3.3. Cytotoxicity assay
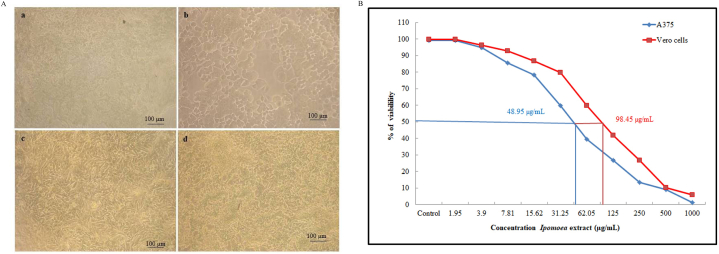
The MTT assay was employed to evaluate the cytotoxicity of the I. carnea extract. In this study, A375 cells and Vero cells were utilized in this investigation. I. carnea exhibited anti-proliferative activity against A375 with an IC50 value of 48.95 μg/mL. Plant extracts containing high levels of phenolics, flavonoids, and terpenes, which are major classes of secondary metabolites, have been found to increase cell death. The possible mechanism of phenolic compounds could be to enhanced the reactive oxygen species, which are amplified in cancer cells promote their proliferation and differentiation [82]. To validate the morphological features of apoptosis, the extract-treated cells were examined under a phase contrast light microscope (Fig. 3A & B). The treated cells appeared to undergo apoptosis, as evidenced by prominent features such as detachment from the culture plate, cell contraction, aggregation of nuclear chromatin, and loss of contact with neighboring cells [83,84]. In the case of the Vero cells, I. carnea extracts showed an IC50 value of 98.45 μg/mL. They were found to be non-toxic even at high concentrations. The non-toxicity was observed not only in cell morphology but also in proliferation rate (Fig. 3A and B). The non-toxic property plays a vital role in the successful formulation of cream and pharmaceutical products [85]. Moreover, the I. carnea extract exhibited anti-proliferative activity against A375 and was less toxic to Vero cell lines. Based on this property, the extract of I. carnea was used to formulate a herbal cream against UVB sunburns.
Fig. 3.
The morphological changes in A375 and Vero cells after 48-h treatment of I. carnea extract observed under phase contrast microscope (a- A375 cells control, b -A375 cells treated I. carnea extract, c –Vero cells control and d- Vero cells treated I. carnea extract).
Fig. 3B MTT assay to determine the IC50 value of the I. carnea and analyze their effect on A375 and Vero cell viability.
3.4. Physiochemical evaluations of herbal cream
Based on the pilot screening the I. carnea extract were used to formulate herbal creams in three different concentrations: FIHC-50, FIHC-100, and FIHC-200. Plain cream was also prepared without plant extract. The formulated creams were evaluated for their color, physical appearance, odor, homogeneity, spreadability, pH, stability, in-vitro permeation and viscosity analyses before topical application to experimental animals. From the physiochemical evaluation, the formulated creams were pale green, semisolid with a uniformly smooth texture and showed no phase separation (Table 1). The pH values of the formulated creams were slightly acidic, ranging from 6.1 to 6.3 throughout the observation period, whereas the plain cream had a pH of 6.2. The spreadability range of the formulated creams was observed at 12.66, 12.33, and 9.63 s/cm/g, while the spreadability of the plain creams was 18.3 s/cm/g. After 90 days, the spreadability rate was slightly changed to 13.1, 12.66, and 11.62 s/cm/g for the different concentrations of the formulated creams, while the plain cream remained at 18.6 s/cm/g (Table 1). The spreadability time values of the I. carnea creams were in the range of 9–12 s. The formulated creams of I. carnea had high stability, withstanding up to 90 days without losing their shelf life and consistency. The cream excipient had potent cling with the plant extract and the physical and chemical nature of the formulations [86].
Table 1.
Evaluation of stability and organoleptic parameter studies of I. carnea herbal cream.
|
Test |
Days | Plain cream | FIHC 50 mg concentration | FIHC 100 mg concentration | FIHC 200 mg concentration | Accelerated stability condition |
|---|---|---|---|---|---|---|
| Color | 0 | White | Pale green | Pale green | Dark green | 40 °C ± 2 °C/75% ± 5% RH |
| 90 | NCC | NCC | NCC | NCC | ||
| Physical appearance | 0 | Semi solid | Semi solid | Semi solid | Semi solid | 40 °C ± 2 °C/75% ± 5% RH |
| 90 | NCC | NCC | NCC | NCC | ||
| Odor | 0 | No odor | Herbal fragrance | Herbal fragrance | Herbal fragrance | 40 °C ± 2 °C/75% ± 5% RH |
| 90 | NCC | NCC | NCC | NCC | ||
| Homogeneity | 0 | Smooth | Smooth | Smooth | Smooth | 40 °C ± 2 °C/75% ± 5% RH |
| 90 | NCC | NCC | NCC | NCC | ||
| Spreadability (g/cm/sec) | 0 | 18.3 ± 0.5 | 12.66 ± 0.5 | 12.33 ± 0.5 | 9.63 ± 0.5 | 40 °C ± 2 °C/75% ± 5% RH |
| 90 | 18.6 ± 0.5 | 13.1 ± 0.5 | 12.66 ± 0.5 | 11.62 ± 0.5 | ||
| pH | 0 | 6.2 ± 0.1 | 6.1 ± 0.1 | 6.2 ± 0.1 | 6.3 ± 0.1 | 40 °C ± 2 °C/75% ± 5% RH |
| 90 | Within range | Within range | Within range | Within range |
NCC- Non characteristic changes, RH- Relative humidity.
3.4.1. In vitro permeation study
In vitro permeation is a laboratory technique used to assess the ability of substances to penetrate through biological barriers such as skin, membranes, or biological tissues [87]. This technique is commonly used in the pharmaceutical industry to evaluate the effectiveness of drug formulations and delivery systems [88]. Fig. 4 displays the permeation analysis results, which evaluated the duration of I. carnea herbal cream release across the dialysis membrane. The percentage of time taken for the control sample to release was used to determine the release time of the formulated creams through the membrane. Compared to the control group, FIHC-50 showed a gradual increase to 6.4% and 52.3% after the first and eighth hours, FIHC-100 had 7.7% and 59.6%, and FIHC-200 had 9% and 70.3% after the first and eighth hour of cream suspension. The sustained permeation of 200 mg of formulated I. carnea cream was 70.3%. (Fig. 4). The in vitro scavenging activity results of the formulations released through the artificial membrane towards DPPH substantiate the adequate release of polyphenolic compounds. According to the previous report the DPPH was found to be effective as a marker for detecting the release of the extract and for assessing the release in terms of antioxidant activity in the receptor solution [56]. Therefore, this experiment concluded that the I. carnea cream formulations could penetrate the skin well when applied.
Fig. 4.
A comparison of in vitro permeation profile of formulated 50 mg, 100 mg and 200 mg I. carnea cream. Each value represents the mean ± SD (n = 3).
3.4.2. Viscosity measurement
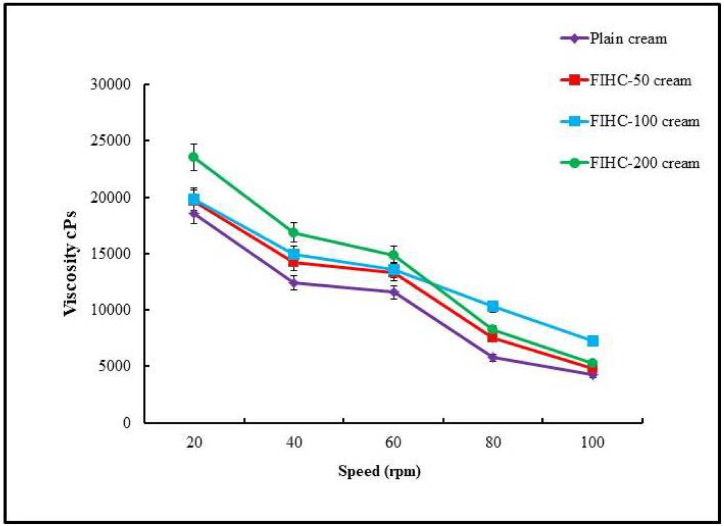
Viscosity is an important physical property of any topical cream formulation [89,90]. As the rpm increased, all the tested creams exhibited gradual changes in viscosity. FIHC-50 mg and FIHC-100 mg displayed a viscosity range from 19,650 to 4781.667 and 19,855 to 7283.333 cPs, respectively. These data showed that FIHC -50 mg and FIHC-100 mg creams had equal viscosity. The viscosity of FIHC-200 was found to be greater, ranging from 23,545 to 5293.33 cPs. In contrast, the plain cream had the least viscosity range from 18,538 to 4241 cPs (Fig. 5). Viscosity can be affected by temperature changes and other factors. Thus, the formulated cream is easier to apply smoothly onto the skin while still penetrating deep into body tissues for healing purposes [91].
Fig. 5.
The graph showing the viscosity profile of formulated I. carnea 50 mg, 100 mg, and 200 mg creams.
3.5. UVB induces skin damage and topical treatment
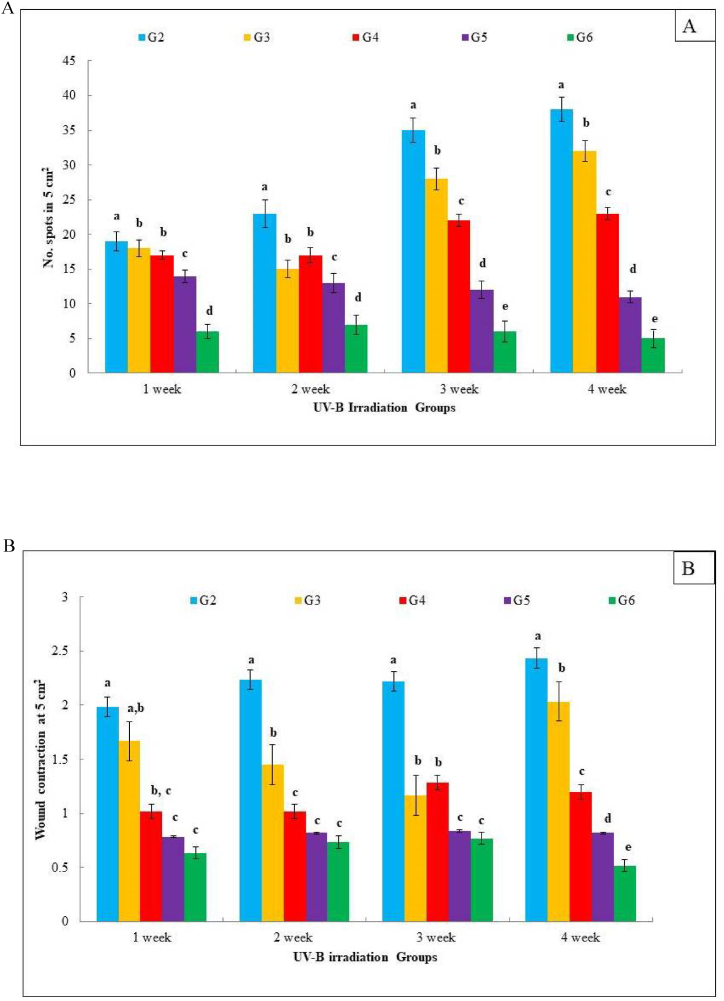
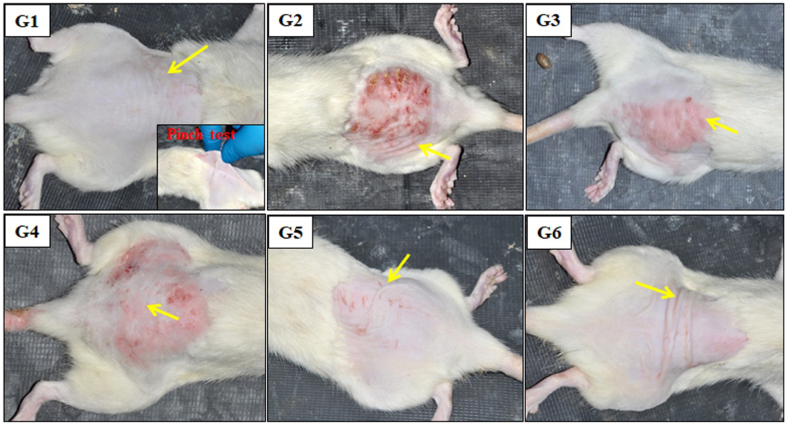
The impacts of UVB on the skin tissue of R. norvegicus female Wistar rats were studied. Initially, reddening of the skin was noticed on the exposed skin area. Later, lesions developed with severe sunburn on the surface of skin layers. The severity of skin damage and mitigation of this damage by the topical application of formulated I. carnea herbal creams are shown in Fig. 6 for G4, G5, and G6. At the same time, no such effect was observed in G2 and G3 rat skin. We observed significant changes in rats exposed to UVB but not provided any treatment (G2) compared to the unexposed UVB control group (G1). Due to UVB exposure, there is an increase in the number of red spots, the area of wrinkles, and skin color. Some epidemiological changes were also observed in the UVB-exposed animals (Fig. 7A & B). The structural changes of the epidermal layer lead to non-melanoma spots on the skin, appearing as coarse red spots by rapid generation of ROS level [92,93]. The overproduction of ROS results in the depletion of the tissue's inherent antioxidants, which curtails the cells' ability to protect themselves [[94], [95], [96], [97]]. The Duncan's test revealed that topical treatment of FIHC 200 cream was found to be a beneficial restoration of reddening (G6). It reduced the red spots and expedited wound contraction compared to the UVB irradiation alone (G2) in Fig. 7A & B. Conversely, the application of plain cream (without plant extract) over the irradiated skin surface failed to produce any positive effects in terms of restoration of damage (G3). The topical application of formulated creams to the irradiated surface offered protection only at high concentrations. While the treatment of 50 & 100 mg concentrations of FIHC cream had failed to produce significant changes in the reversal of UVB damages as noticed in the earlier weeks (G4 & 5). High concentration of 200 mg FIHC cream was effective enough to cause maximum reduction of UVB-induced damage on the dorsal skin surface (G6). In the healing process, numerous enzymatic and non-enzymatic pathways are engaged in reducing the elevated ROS levels in the skin [98]. Antioxidants like ascorbic acid (vitamin C), carotenoids, α-tocopherol (vitamin E) and plant phenols play a role in averting premature skin aging and cellular harm [25,28,33,99]. The primary reason for using sunscreen is to protect ourselves from harmful UV rays, which helps prevent and minimize premature aging, tanning, sunburn blotchiness on the face, promotes skin health, and reduces the risk of skin cancer [100]. An earlier study has indicated that the application of UV sunscreen can reduce the formation of free radicals by approximately 55% [101]. However, the presence of antioxidants in sunscreen has a greater ability to decrease free radicals compared to using sunscreen alone. Our research revealed that I. carnea crude possesses abundant antioxidant properties. Based on these findings, we prepared I. carnea herbal cream that may provide greater protection against shorter wavelengths.
Fig. 6.
Photographic images demonstrate the physical appearance of UVB irradiated dorsal skin with treatment group, G1-refers to normal control skin, unexposed UVB and without any treatment; G2-refers to skin exposed UVB without any treatment; G3-refers to skin that has been exposed to UVB radiation but treated with plain cream. G4-refers to skin that has been exposed to UVB radiation but treated with FIHC-50 mg cream. G5-refers to skin that has been exposed to UVB radiation but treated with FIHC-100 mg cream. G6-refers to skin that has been exposed to UVB radiation but treated with FIHC-200 mg cream. At the end of every week, the rat's dorsal skin was photographed, spots were noted and size was measured using an overlaying clear plastic sheet.
Fig. 7.
Histogram demonstrates the physical appearance of extent of skin damage in experimental rats with different treatments groups (A) The effect of I. carnea cream can reduce the visual score of red spots on the skin surface, (B) The effect of I. carnea cream treatment reflect the macroscopic changes of healing progression between phase (All groups except G-2). The data are expressed as mean ± SD, and significant differences among the groups were identified using one way ANOVA with Duncan's multiple range test at P < 0.05, indicated by different superscripts in the values.
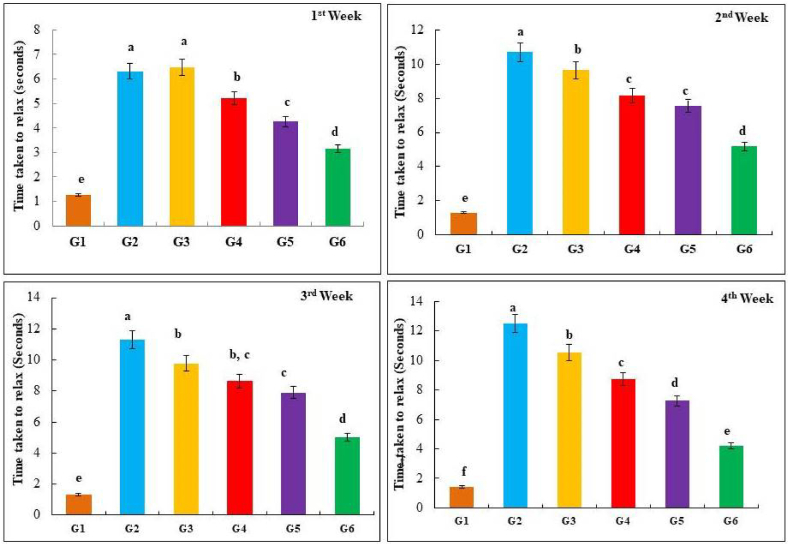
3.5.1. Skin elasticity/pinch test
To quantity the dorsal skin elasticity in rats, a pinch test was carried out every week for four weeks immediately after UVB treatment. The photographs of the dorsal skin after being stretched are illustrated in Fig. 8. The time taken for the skin to return to normalcy after pinching was measured in animals exposed to UVB and formulated cream for four weeks and results are shown in Fig. 9. The time taken by the untreated animals (G2) skin was much shorter compared to the treated animals (G3-6). The exposure to UVB radiation caused a reduction in skin elasticity initially and eventually led to the development of wrinkles [102]. One possible explanation could be that the elastases secreted by surrounding cells degrade the elastic fibers. Which are directly induced in fibroblasts by UVB radiation, resulting in the curling and/or reproduction of elastic fibers. Alternatively, it could be due to preventing the regeneration of elastic fibers after the breakdown of existing elastic fibers by the presence of collagen fibers, leading to a comparable convoluted pattern of newly formed elastic fibers [103]. The time for rat skin to regain its initial shape after UVB-induced deformation was significantly longer, up to 5-fold, compared to plain cream treated skin (G3). There was no significant difference between the UVB-induced without treatment group and the plain cream treatment group, indicating that plain cream had no ameliorative effect on the skin surface. However, the application of FIHC 200 mg cream had a tendency to enhance skin elasticity, and the recovery time was significantly shorter than the UVB-induced without treatment group. Interestingly, the effects of 50 and 100 mg FIHC cream treatment were also compared. In addition, the treatment of FIHC-50 and 100 mg cream showed no lesions, but a few shallow wrinkles were observed, and the recovery time was significantly longer than in the group of rats that were not exposed to UVB (G1). As shown in Fig. 9 at the end of this experiment, the expected results were observed in rats treated with FIHC 200 mg cream, which could promote skin elasticity. The presence of the inhibitor caused a decrease in skin elasticity and inhibited wrinkle appearance [104]..
Fig. 8.
Amelioration efficiency of formulated I. carnea creams on the UVB induced damages in skin elasticity assessed through pinch test. The yellow arrow denotes the skin sagging.
Fig. 9.
The time-response curve was examined to evaluate the anti-aging effects of I. carnea herbal cream on UVB radiation-induced skin damage. The topical application FIHC treatment G4-6 significantly decreased compared to G2 and G3.
Statistical analysis.
All the data are represented as mean ± SD. Values with different superscripts are significantly different among the groups by ANOVA with Duncan's multiple range test at P < 0.05.
a values statistically highly significant difference (P < 0.05) when compared with group-1 values.
b values statistically highly significant difference (P < 0.05) when compared with group −2 values.
c values statistically significant difference (P < 0.05) when compared with group −3 values
d values statistically highly significant difference (P < 0.05) when compared with group-4 values.
e values statistically highly significant difference (P < 0.05) when compared with group-5 values.
f values statistically highly significant difference (P < 0.05) when compared with group-6 values
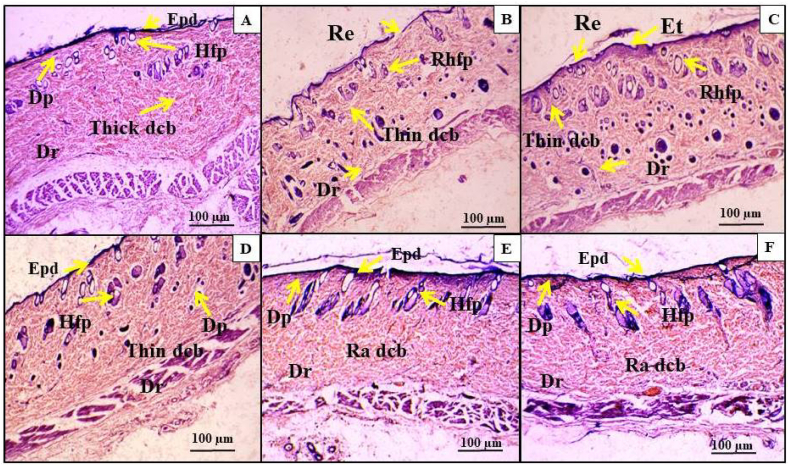
3.5.2. Histopathological analysis
Histological studies provide strong evidence that the treatment of I. carnea herbal cream had an ameliorative effect on UVB-induced skin alterations. Under microscopic assessment, the non-irradiated UVB group (G1) exhibited a relatively complete structure. Fig. 10A showed that the skin had a stratified epidermis consisting of the stratum corneum, stratum granulosum, stratum spinosum, and stratum basal layer, which were distinguishable. A thick connective dermis containing a papillary layer, a reticular layer with collagen fibers, and hair follicles was also evident. After UVB exposure, the tissue network was lost in the dermal papillary and reticular layers. The UVB induction of the metabolic activity of fibroblasts was noticed. The dermal vessels became enlarged and leaky, accumulating excessive basement membrane-like material. Inflammatory cells congregated around the vessels, proliferation of mast cells, and signs of degranulation were observed in connection with the UVB treatment of the dorsal side of R. norvegicus skin. Thus, the severity of damage caused by UVB to skin tissue is evident from our studies Fig. 10B. Upon application of plain cream to the UVB-exposed area resulted in a reduction in connective tissues and the loss of hair follicles (Fig. 10C). On the contrary, the application of herbal creams prepared from I. carnea was effective enough to restore the tissue structure in the irradiated area, especially at 200 mg concentrations, whereas 50 and 100 mg concentrations of the formulated creams were not as effective (Fig. 10D–F). Thus, histological observations confirm that the formulated creams from I. carnea extract were found to ameliorate the UVB-induced damage as far as the skin structure is concerned. The robust antioxidant properties and the presence of diverse phytochemicals in the cream may account for the potential mechanism underlying the healing process of epidermal repair through the scavenging of free radicals [105].
Figure 10.
Histological observation of topical treatment of I. carnea creams suppressed the UVB-induced skin epidermal changes. The H&E staining of all photographs of rat dorsal skin was magnified at (10X) view and scale bar: 100 μm. A-G1, B-G2, C-G3, D-G4, E-G5 and F-G6. The yellow arrow indicates damage to the epidermal layer of skin. (Dr- Dermal reticular layer, Dp- Dermal pupillary layer, Epd-Epidermis, Et-Epithelial tissue, Hfp- Hair follicle present, Ra dcb- Ruptured dermal collagen bundle Re- Ruptured epidermis, Rhfp- Ruptured hair follicle present, Thick dcb- Thickening of dermal collagen bundle and Thin dcb- Thinning of dermal collagen bundle).
3.5.3. Biochemical analysis
Typical changes in soluble protein, SOD, CAT activity, and MDA content of R. norvegicus skin that was exposed to UVB and I. carnea herbal cream treatments are shown in Fig. 11. Upon short-term UVB exposure, a downregulation of protein content, SOD, CAT activity was noticed in G2 in Fig. 11A, B, and C. Exposure to solar-simulated UVR caused a temporary decline in SOD activity in human skin, which was succeeded by an elevation in the level of conjugated diene double bonds, indicative of lipid peroxidation [106]. The overproduced free radicals from administering UVB irradiation to the rat skin spiked the lipid peroxidation, evidenced by the increased MDA level in Fig. 11D. It was also compared to unexposed UVB rats in G1. However, applying plain cream (without any plant extract) to the UVB-irradiated skin was found to enhance the level of protein content, SOD, and CAT compared to the UVB control. Proteins are known to be major oxidative modification targets. Protein amino acid alterations caused by oxygen radicals and other activated oxygen species frequently result in structural or enzymatic protein functioning changes [107]. Moreover, promoting the metastatic process by melanoma cells might involve an increase in oxidative damage to the surrounding tissue as one of its mechanisms [108,109].
Fig. 11.
Changes in soluble protein (A), SOD (B), CAT activity (C) and (D) MDA level of skin tissue exposed to various treatments.
Statistical analysis.
The data are expressed as mean ± SD, and significant differences among the groups were identified using one way ANOVA with Duncan's multiple range test at P < 0.05, indicated by different superscripts in the values.
a values statistically highly significant difference (P < 0.05) when compared with group-1 values.
b values statistically highly significant difference (P < 0.05) when compared with group −2 values.
c values statistically significant difference (P < 0.05) when compared with group −3 values
d values statistically highly significant difference (P < 0.05) when compared with group-4 values.
e values statistically highly significant difference (P < 0.05) when compared with group-5 values
f values statistically highly significant difference (P < 0.05) when compared with group-6 values.
4. Conclusion
Our findings suggest that topical application of formulated I. carnea herbal cream exhibited a stable and potent ameliorative effect on a UVB radiation-induced skin burn, due to the presence of bioactive compounds with higher antioxidant properties. The efficiency of creams can be improved by increasing the concentration of I. carnea extract. Clinically and histopathologically, the formulated I. carnea has a potent defense mechanism, and its topical application can diminish the UVB-induced damage on the skin surface. Similarly, the effectiveness of formulated I. carnea herbal cream in maintaining the imbalanced enzymatic and non-enzymatic activity in post-treatment of UVB-irradiated skin is supported by the results of biochemical assays. Thus, the cream showed the potential to mitigate the damages caused by UVB irradiation. Based on the current research findings, it is suggested that the I. carnea herbal cream shows promise as a viable option for medicinal applications.
Author contribution statement
Madasamy Sundar: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper. Krishnasamy Lingakumar: Conceived and designed the experiments.
Data availability statement
Data included in article/supp. Material/referenced in article:
Funding
No funding was availed from any funding sources.
Availability of data and materials
This datasets analysed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19161.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Kligman A.M. Early destructive effect of sunlight on human skin. JAMA. 1969;210:2377–2380. https://doi:10.1001/jama.1969.03160390039008 [PubMed] [Google Scholar]
- 2.Tsukahara K., Moriwaki S., Hotta M., Fujimura T., Sugiyama-Nakagiri Y., Sugawara S., Kitahara T., Takema Y. The effect of sunscreen on skin elastase activity induced by ultraviolet-A irradiation. Biol. Pharm. Bull. 2005;28:2302–2307. doi: 10.1248/bpb.28.2302. [DOI] [PubMed] [Google Scholar]
- 3.Dehelean C.A., Soica C., Pinzaru I., Coricovac D., Danciu C., Pavel I., Borcan F., Spandidos D.A., Tsatsakis A.M., Baderca F. Sex differences and pathology status correlated to the toxicity of some common carcinogens in experimental skin carcinoma. Food Chem. Toxicol. 2016;95:149–158. doi: 10.1016/j.fct.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Meinhardt M., Krebs R., Anders A., Heinrich U., Tronnier H. Wavelength-dependent penetration depths of ultraviolet radiation in human skin. J. Biomed. Opt. 2008;13 doi: 10.1117/1.2957970. [DOI] [PubMed] [Google Scholar]
- 5.Imam S., Azhar I., Mahmood Z.A. 2015. In-vitro Evaluation of Sun Protection Factor of a Cream Formulation Prepared from Extracts of Musa Accuminata (L.), Psidium Gujava (L.) and Pyrus Communis (L.), IN-VITRO; p. 8. [Google Scholar]
- 6.Chanchal D., Swarnlata S. Herbal photoprotective formulations and their evaluation. Open Nat. Prod. J. 2009;2 doi: 10.2174/1874848100902010071. [DOI] [Google Scholar]
- 7.Wolf R. T zn B. and T zn Y. Dermatol. Ther. 2001;14:208–214. [Google Scholar]
- 8.Lakhdar H., Zouhair K., Khadir K., Essari A., Richard A., Seité S., Rougier A. Evaluation of the effectiveness of a broad‐spectrum sunscreen in the prevention of chloasma in pregnant women. J. Eur. Acad. Dermatol. Venereol. 2007;21:738–742. doi: 10.1111/j.1468-3083.2007.02185.x. [DOI] [PubMed] [Google Scholar]
- 9.Bishop T., Hewson D.W., Yip P.K., Fahey M.S., Dawbarn D., Young A.R., McMahon S.B. Characterisation of ultraviolet-B-induced inflammation as a model of hyperalgesia in the rat. Pain. 2007;131:70–82. doi: 10.1016/j.pain.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Peres P.S., Terra V.A., Guarnier F.A., Cecchini R., Cecchini A.L. Photoaging and chronological aging profile: understanding oxidation of the skin. J. Photochem. Photobiol. B Biol. 2011;103:93–97. doi: 10.1016/j.jphotobiol.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs J., Huflejt M.E., Rothfuss L.M., Wilson D.S., Carcamo G., Packer L. Impairment of enzymic and nonenzymic antioxidants in skin by UVB irradiation. J. Invest. Dermatol. 1989;93:769–773. doi: 10.1111/1523-1747.ep12284412. [DOI] [PubMed] [Google Scholar]
- 12.Bang E., Kim D.H., Chung H.Y. Protease-activated receptor 2 induces ROS-mediated inflammation through Akt-mediated NF-κB and FoxO6 modulation during skin photoaging. Redox Biol. 2021;44 doi: 10.1016/j.redox.2021.102022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu P., Xin Y., Zhang Z., Zou X., Xue K., Zhang H., Zhang W., Liu K. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res. Ther. 2020;11:1–14. doi: 10.1186/s13287-020-01777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subedi L., Lee T.H., Wahedi H.M., Baek S.-H., Kim S.Y. 2017. Resveratrol-enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades, Oxidative Medicine and Cellular Longevity. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lionetti N., Rigano L. The new sunscreens among formulation strategy, stability issues, changing norms, safety and efficacy evaluations. Cosmetics. 2017;4:15. doi: 10.3390/cosmetics4020015. [DOI] [Google Scholar]
- 16.Saucedo G.M.G., Vallejo R.S., Giménez J.C.M. Effects of solar radiation and an update on photoprotection. Anales de Pediatría (English Edition) 2020;92 doi: 10.1016/j.anpede.2020.04.003. 377-e1. [DOI] [PubMed] [Google Scholar]
- 17.Reis-Mansur M.C.P.P., da Luz B.G., dos Santos E.P. Consumer behavior, skin phototype, sunscreens, and tools for photoprotection: a review. Cosmetics. 2023;10:39. doi: 10.3390/cosmetics10020039. [DOI] [Google Scholar]
- 18.Suva M.A. Evaluation of sun protection factor of Zingiber officinale Roscoe extract by ultraviolet spectroscopy method. J. Pharmaceut. Sci. Technol. 2014;3:95–97. [Google Scholar]
- 19.Ngo H.T.T., Hwang E., Seo S.-A., Park B., Sun Z., Zhang M., Shin Y.-K., Yi T.-H. Topical application of neem leaves prevents wrinkles formation in UVB-exposed hairless mice. J. Photochem. Photobiol. B Biol. 2017;169:161–170. doi: 10.1016/j.jphotobiol.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Carriço C., Ribeiro H.M., Marto J. Converting cork by-products to ecofriendly cork bioactive ingredients: novel pharmaceutical and cosmetics applications. Ind. Crop. Prod. 2018;125:72–84. doi: 10.1016/j.indcrop.2018.08.092. [DOI] [Google Scholar]
- 21.Nayak S., Chaudhari A., Vaidhun B. Synthesis, characterization and ameliorative properties of food, formulation and cosmetic additives: case study of Zinc oxide nanoparticles. J. Excipients Food Chem. 2020;11:79–92. [Google Scholar]
- 22.Melo C.P.B., Saito P., Vale D.L., Rodrigues C.C.A., Pinto I.C., Martinez R.M., Bezerra J.R., Baracat M.M., Verri W.A., Fonseca-Bazzo Y.M. Protection against UVB deleterious skin effects in a mouse model: effect of a topical emulsion containing Cordia verbenacea extract. Photochem. Photobiol. Sci. 2021;20:1033–1051. doi: 10.1007/s43630-021-00079-x. [DOI] [PubMed] [Google Scholar]
- 23.Green A.C., Williams G.M., Logan V., Strutton G.M. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J. Clin. Oncol. 2011;29:257–263. doi: 10.1200/jco.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- 24.Vilela F.M.P., Oliveira F.M., Vicentini F.T.M.C., Casagrande R., Verri W.A., Jr., Cunha T.M., V Fonseca M.J. Commercial sunscreen formulations: UVB irradiation stability and effect on UVB irradiation-induced skin oxidative stress and inflammation. J. Photochem. Photobiol. B Biol. 2016;163:413–420. doi: 10.1016/j.jphotobiol.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Becker G., Brusco I., Casoti R., Marchiori M.C.L., Cruz L., Trevisan G., Oliveira S.M. Copaiba oleoresin has topical antinociceptive activity in a UVB radiation-induced skin-burn model in mice. J. Ethnopharmacol. 2020;250 doi: 10.1016/j.jep.2019.112476. [DOI] [PubMed] [Google Scholar]
- 26.Anand T., Sundararajan M., Anbukkarasi M., Thomas P.A., Geraldine P. A methanolic extract of Ocimum basilicum exhibits antioxidant effects and prevents selenite-induced cataract formation in cultured lenses of wistar rats. Phcog. J. 2019;11 doi: 10.5530/pj.2019.11.79. [DOI] [Google Scholar]
- 27.Lee H.J., Kim J.S., Song M.S., Seo H.S., Moon C., Kim J.C., Jo S.K., Jang J.S., Kim S.H. Photoprotective effect of red ginseng against ultraviolet radiation‐induced chronic skin damage in the hairless mouse. Phytother Res.: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2009;23:399–403. doi: 10.1002/ptr.2640. [DOI] [PubMed] [Google Scholar]
- 28.Silva M.A., Trevisan G., Hoffmeister C., Rossato M.F., Boligon A.A., Walker C.I.B., Klafke J.Z., Oliveira S.M., Silva C.R., Athayde M.L. Anti-inflammatory and antioxidant effects of Aloe saponaria Haw in a model of UVB-induced paw sunburn in rats. J. Photochem. Photobiol. B Biol. 2014;133:47–54. doi: 10.1016/j.jphotobiol.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Martanti A., Weta W., Wiraguna A. The akar kucing (Acalypha indica l.) Leaves extract cream 4% prevented the formed melanin cell of Guinea pigs (Cavia porcellus) skin that exposed to ultraviolet B. IJAAM (Indonesian Journal of Anti-Aging Medicine) 2021;5:27–29. [Google Scholar]
- 30.Piana M., Silva M.A., Trevisan G., de Brum T.F., Silva C.R., Boligon A.A., Oliveira S.M., Zadra M., Hoffmeister C., Rossato M.F. Antiinflammatory effects of Viola tricolor gel in a model of sunburn in rats and the gel stability study. J. Ethnopharmacol. 2013;150:458–465. doi: 10.1016/j.jep.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Byun E.-B., Song H.-Y., Kim W.S. Polysaccharides from Annona muricata leaves protect normal human epidermal keratinocytes and mice skin from radiation-induced injuries. Radiat. Phys. Chem. 2020;170 doi: 10.1016/j.radphyschem.2019.108672. [DOI] [Google Scholar]
- 32.Rahmawati Y.D., Prasetyawan S. Effects of oral and topical application of Centella asiatica extracts on the UVB-induced photoaging of hairless rats. The Journal of Pure and Applied Chemistry Research. 2019;8:7–14. doi: 10.21776/ub.jpacr.2019.008.01.430. [DOI] [Google Scholar]
- 33.Espinosa-González A.M., Estrella-Parra E.A., Nolasco-Ontiveros E., García-Bores A.M., García-Hernández R., López-Urrutia E., Campos-Contreras J.E., González-Valle M. del R., Benítez-Flores J. del C., Céspedes-Acuña C.L. Hyptis mociniana: phytochemical fingerprint and photochemoprotective effect against UV-B radiation-induced erythema and skin carcinogenesis. Food Chem. Toxicol. 2021;151 doi: 10.1016/j.fct.2021.112095. [DOI] [PubMed] [Google Scholar]
- 34.Abu Hajleh M.N., Al‐Samydai A., Al‐Dujaili E.A.S. Nano, micro particulate and cosmetic delivery systems of polylactic acid: a mini review. J. Cosmet. Dermatol. 2020;19:2805–2811. doi: 10.1111/jocd.13696. [DOI] [PubMed] [Google Scholar]
- 35.Jadoon S., Karim S., Bin Asad M.H.H., Akram M.R., Kalsoom Khan A., Malik A., Chen C., Murtaza G. 2015. Anti-aging Potential of Phytoextract Loaded-Pharmaceutical Creams for Human Skin Cell Longetivity, Oxidative Medicine and Cellular Longevity. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takshak S., Agrawal S.B. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol. B Biol. 2019;193:51–88. doi: 10.1016/j.jphotobiol.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Korać R.R., Khambholja K.M. Potential of herbs in skin protection from ultraviolet radiation. Phcog. Rev. 2011;5:164. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frey R. Ipomoea carnea ssp. fistulosa (Martius ex Choisy) Austin: taxonomy, biology and ecology reviewed and inquired. Trop. Ecol. 1995;36:21–48. [Google Scholar]
- 39.Ahmed Z.U., Hassan M.A., Begum Z.N.T., Khondker M., Kabir S.M.H., Ahmad M., Ahmed A.T.A., Rahman A.K.A., Haque E.U. vol. 7. 2008. p. 546. (Encyclopedia of Flora and Fauna of Bangladesh: Angiosperms: Dicotyledons: Balsaminaceae-Euphorbiaceae). Dhaka (Bangladesh): Asiatic Society of Bangladesh. [Google Scholar]
- 40.Chowdhury A.K.A., Ali M.S., Khan M.O.F. Antimicrobial activity of Ipomoea fistulosa extractives. Fitoterapia. 1997;68:379–380. [Google Scholar]
- 41.Adsul V., Khatiwora E., Kulkarni M., Tambe A., Pawar P., Deshpande N. GC-MS study of fatty acids, esters, alcohols from the leaves of Ipomoea carnea. Int. J. Pharm. Tech. Res. 2009;1:1224–1226. [Google Scholar]
- 42.Khalid M.S., Singh R.K., Kumar S.J., Suresh D.K., Rao S.K., V Reddy N.I. Antidiabetic activity of aqueous extract of Ipomoea carnea leaves in streptozotocin induced diabetic rats. International Journal of Pharmacology and Biological Sciences. 2011;5:45. [Google Scholar]
- 43.Suresh K., Singh R.K. Anti-hepatotoxic and antioxidant influence of Ipomoea carnea against anti-tubercular drugs induced acute hepatopathy in experimental rodents. J Coast Life Med. 2013;1:293–299. doi: 10.12980/JCLM.1.2013B1359. [DOI] [Google Scholar]
- 44.Dubey A., Yadav P., Verma P., Kumar R. Investigation of proapoptotic potential of ipomoea carnea leaf extract on breast cancer cell line. J. Drug Deliv. Therapeut. 2022;12:51–55. doi: 10.22270/jddt.v12i1.5172. [DOI] [Google Scholar]
- 45.Patel A.K., Singh V.K., Yadav R.P., Moir A.J.G., V Jagannadham M. Purification and characterization of a new chitinase from latex of Ipomoea carnea. Process Biochem. 2010;45:675–681. doi: 10.1016/j.procbio.2009.12.016. [DOI] [Google Scholar]
- 46.Brand-Williams W., Cuvelier M.-E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT--Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 47.Mathew S., Abraham T.E. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem. Toxicol. 2006;44:198–206. doi: 10.1016/j.fct.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 48.Rajeshwar Y., Kumar G.P.S., Gupta M., Mazumder U.K. Studies on in vitro antioxidant activities of methanol extract of Mucuna pruriens (Fabaceae) seeds. Eur Bull Drug Res. 2005;13:31–39. [Google Scholar]
- 49.Halliwell B., Gutteridge J.M.C., Aruoma O.I. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 50.Oyaizu M. Studies on products of browning reaction. The Japanese Journal of Nutrition and Dietetics. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 51.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 52.Carter S.J. twelfth ed. CBS Publishing and Distributors; New Delhi, India: 2008. Cooper and Gunns Dispensing for Pharmaceutical Students. [Google Scholar]
- 53.Sundar M., Suresh S., Lingakumar K. Preparation and optimization of medicated cold cream using Caralluma adscendens var. attenuata for the treatment of Candida skin infection. BioTechnologia. Journal of Biotechnology Computational Biology and Bionanotechnology. 2022;103 doi: 10.5114/bta.2022.118668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kp M.H., Saraswathi R., Mohanta G.P., Nayar C. Formulation and evaluation of herbal gel of Pothos scandens Linn. Asian Pac. J. Tropical Med. 2010;3:988–992. doi: 10.1016/S1995-7645(11)60015-1. [DOI] [Google Scholar]
- 55.Bajaj S., Singla D., Sakhuja N. Stability testing of pharmaceutical products. J. Appl. Pharmaceut. Sci. 2012;2:129–138. doi: 10.7324/JAPS.2012.2322. [DOI] [Google Scholar]
- 56.Mahdi E.S., Noor A.M., Sakeena M.H., Abdullah G.Z., Abdulkarim M.F., Sattar M.A. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int. J. Nanomed. 2011;6:2499. doi: 10.2147/ijn.s22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sundar M., Suresh S., Lingakumar K. Influence of Caralluma adscendens Var. attenuata cold cream on UV-B damaged skin epidermal cells: a novel approach. 3 Biotech. 2021;11:1–16. doi: 10.1007/s13205-021-02694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lingakumar K., Kulandaivelu G. Changes induced by ultraviolet-B radiation in vegetative growth, foliar characteristics and photosynthetic activities in Vigna unguiculata. Funct. Plant Biol. 1993;20:299–308. doi: 10.1071/PP9930299. [DOI] [Google Scholar]
- 59.Kim S.-Y., Kim S.-J., Lee J.-Y., Kim W.-G., Park W.-S., Sim Y.-C., Lee S.-J. Protective effects of dietary soy isoflavones against UV-induced skin-aging in hairless mouse model. J. Am. Coll. Nutr. 2004;23:157–162. doi: 10.1080/07315724.2004.10719356. [DOI] [PubMed] [Google Scholar]
- 60.Lee C.-W., Ko H.-H., Lin C.-C., Chai C.-Y., Chen W.-T., Yen F.-L. Artocarpin attenuates ultraviolet B-induced skin damage in hairless mice by antioxidant and anti-inflammatory effect. Food Chem. Toxicol. 2013;60:123–129. doi: 10.1016/j.fct.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 61.Agrawal R., Kaur I.P. Inhibitory effect of encapsulated curcumin on ultraviolet-induced photoaging in mice. Rejuvenation Res. 2010;13:397–410. doi: 10.1089/rej.2009.0906. [DOI] [PubMed] [Google Scholar]
- 62.Lowry O., Rosebrough N., Farr A.L., Randall R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 63.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 64.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 65.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 66.Sushmitha H.S., Roy C.L., Gogoi D., Velagala R.D., Nagarathna A., Balasubramanian S., Rajadurai M. Phytochemical and pharmacological studies on hylocereus undatus seeds: an in vitro approach. World J. Pharmaceut. Res. 2018;7:986–1006. [Google Scholar]
- 67.Ambika A.P., Nair S.N. Wound healing activity of Plants from the Convolvulaceae family. Adv. Wound Care. 2019;8:28–37. doi: 10.1089/wound.2017.0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aparna V., V Dileep K., Mandal P.K., Karthe P., Sadasivan C., Haridas M. Anti‐inflammatory property of n‐hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012;80:434–439. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- 69.Zahid M., Arif M., Rahman M.A., Singh K., Mujahid M. Solvent extraction and gas chromatography–mass spectrometry analysis of Annona squamosa L. seeds for determination of bioactives, fatty acid/fatty oil composition, and antioxidant activity. J. Diet. Suppl. 2018;15:613–623. doi: 10.1080/19390211.2017.1366388. [DOI] [PubMed] [Google Scholar]
- 70.de MP Santos C.C., Salvadori M.S., Mota V.G., Costa L.M., de Almeida A.A.C., de Oliveira G.A.L., Costa J.P., de Sousa D.P., de Freitas R.M., de Almeida R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neuroscience Journal. 2013:2013. doi: 10.1155/2013/949452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith T.J. Squalene: potential chemopreventive agent. Expet Opin. Invest. Drugs. 2000;9:1841–1848. doi: 10.1517/13543784.9.8.1841. [DOI] [PubMed] [Google Scholar]
- 72.Abd Rahim E.N.A., Ismail A., Omar M.N., Rahmat U.N., Ahmad W.A.N.W. GC-MS analysis of phytochemical compounds in Syzygium polyanthum leaves extracted using ultrasound-assisted method. Phcog. J. 2018;10 doi: 10.5530/pj.2018.1.20. [DOI] [Google Scholar]
- 73.Traber M.G., Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manosso L.M., Camargo A., Dafre A.L., Rodrigues A.L.S. Vitamin E for the management of major depressive disorder: possible role of the anti-inflammatory and antioxidant systems. Nutr. Neurosci. 2020:1–15. doi: 10.1080/1028415X.2020.1853417. [DOI] [PubMed] [Google Scholar]
- 75.Ashraf V.K.M., Kalaichelvan V.K., V Venkatachalam V., Ragunathan R. In vitro anticancer potential of aerial parts of Ipomoea horsfalliae hook in different human cancer cell lines. Ind. Crop. Prod. 2020;155 doi: 10.1016/j.indcrop.2020.112746. [DOI] [Google Scholar]
- 76.Karan S.K., Mishra S.K., Pal D., Mondal A. Isolation of-sitosterol and evaluation of antidiabetic activity of Aristolochia indica in alloxan-induced diabetic mice with a reference to in-vitro antioxidant activity. J. Med. Plants Res. 2012;6:1219–1223. doi: 10.5897/JMPR11.973. [DOI] [Google Scholar]
- 77.Jeena K., Liju V.B., Umadevi N.P., Kuttan R. Antioxidant, anti-inflammatory and antinociceptive properties of black pepper essential oil (Piper nigrum Linn) Journal of Essential Oil Bearing Plants. 2014;17:1–12. doi: 10.1080/0972060X.2013.831562. [DOI] [Google Scholar]
- 78.Awoniyi D.O., Aboua Y.G., Marnewick J., Brooks N. The effects of rooibos (Aspalathus linearis), green tea (Camellia sinensis) and commercial rooibos and green tea supplements on epididymal sperm in oxidative stress‐induced rats. Phytother Res. 2012;26:1231–1239. doi: 10.1002/ptr.3717. [DOI] [PubMed] [Google Scholar]
- 79.Anbukkarasi M., Thomas P.A., Sundararajan M., Geraldine P. Gas chromatography-mass spectrometry analysis and in vitro antioxidant activity of the ethanolic extract of the leaves of Tabernaemontana divaricata. Phcog. J. 2016;8 doi: 10.5530/pj.2016.5.7. [DOI] [Google Scholar]
- 80.Tsai C.-E., Lin L.-H. DPPH scavenging capacity of extracts from Camellia seed dregs using polyol compounds as solvents. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saewan N., Jimtaisong A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015;14:47–63. doi: 10.1111/jocd.12123. [DOI] [PubMed] [Google Scholar]
- 82.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 83.Saraste A., Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000;45:528–537. doi: 10.1016/S0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 84.Rajagopal G., Nivetha A., Sundar M., Panneerselvam T., Murugesan S., Parasuraman P., Kumar S., Ilango S., Kunjiappan S. 2021. Mixed Phytochemicals Mediated Synthesis of Copper Nanoparticles for Anticancer and Larvicidal Applications. Heliyon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shah U., Shah R., Acharya S., Acharya N. Novel anticancer agents from plant sources. Chin. J. Nat. Med. 2013;11:16–23. doi: 10.1016/S1875-5364(13)60002-3. [DOI] [Google Scholar]
- 86.Dhyani A., Chander V., Singh N. Formulation and evaluation of multipurpose herbal cream. J. Drug Deliv. Therapeut. 2019;9:341–343. doi: 10.22270/jddt.v9i2.2540. [DOI] [Google Scholar]
- 87.Hung C.-F., Fang C.-L., Liao M.-H., Fang J.-Y. The effect of oil components on the physicochemical properties and drug delivery of emulsions: tocol emulsion versus lipid emulsion. Int. J. Pharm. 2007;335:193–202. doi: 10.1016/j.ijpharm.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 88.Souto E.B., Fangueiro J.F., Fernandes A.R., Cano A., Sanchez-Lopez E., Garcia M.L., Severino P., Paganelli M.O., V Chaud M., Silva A.M. 2022. Physicochemical and Biopharmaceutical Aspects Influencing Skin Permeation and Role of SLN and NLC for Skin Drug Delivery. Heliyon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leblanc G.E., Secco R.A., Kostic M. The Measurement, Instrumentation, and Sensors. CRC Press; Boca Raton, FL, USA: 1999. Viscosity measurement. [Google Scholar]
- 90.Walters K., Jones W.M. Instrumentation Reference Book. Elsevier; 2003. Measurement of viscosity; pp. 45–52. [Google Scholar]
- 91.Cheng Y.S., Lam K.W., Ng K.M., Ko R.K.M., Wibowo C. An integrative approach to product development—a skin-care cream. Comput. Chem. Eng. 2009;33:1097–1113. doi: 10.1016/j.compchemeng.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hakozaki T., Date A., Yoshii T., Toyokuni S., Yasui H., Sakurai H. Visualization and characterization of UVB-induced reactive oxygen species in a human skin equivalent model. Arch. Dermatol. Res. 2008;300:51–56. doi: 10.1007/s00403-007-0804-3. [DOI] [PubMed] [Google Scholar]
- 93.Kawada S., Ohtani M., Ishii N. Increased oxygen tension attenuates acute ultraviolet-B-induced skin angiogenesis and wrinkle formation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R694. doi: 10.1152/ajpregu.00199.2010. –R701. [DOI] [PubMed] [Google Scholar]
- 94.Xu W., Chi L., Xu R., Ke Y., Luo C., Cai J., Qiu M., Gozal D., Liu R. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord. 2005;43:204–213. doi: 10.1038/sj.sc.3101674. [DOI] [PubMed] [Google Scholar]
- 95.Svobodova A., Walterova D., Vostalova J. Ultraviolet light induced alteration to the skin. Biomedical Papers-Palacky University in Olomouc. 2006;150:25. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 96.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C.J., Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 97.Fonseca V.A. Effects of β-blockers on glucose and lipid metabolism. Curr. Med. Res. Opin. 2010;26:615–629. doi: 10.1185/03007990903533681. [DOI] [PubMed] [Google Scholar]
- 98.Hruza L.L., Pentland A.P. Mechanisms of UV-induced inflammation. J. Invest. Dermatol. 1993;100 doi: 10.1038/jid.1993.21. S35–S41. [DOI] [PubMed] [Google Scholar]
- 99.Kim Y.H., Yang H.E., Park B.K., Heo M.Y., Jo B.K., Kim H.P. The extract of the flowers of Prunus persica, a new cosmetic ingredient, protects against solar ultraviolet-induced skin damage in vivo. J. Cosmet. Sci. 2002;53:27–34. [PubMed] [Google Scholar]
- 100.Geoffrey K., Mwangi A.N., Maru S.M. Sunscreen products: rationale for use, formulation development and regulatory considerations. Saudi Pharmaceut. J. 2019;27:1009–1018. doi: 10.1016/j.jsps.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haywood R., Wardman P., Sanders R., Linge C. Sunscreens inadequately protect against ultraviolet-A-induced free radicals in skin: implications for skin aging and melanoma? J. Invest. Dermatol. 2003;121:862–868. doi: 10.1046/j.1523-1747.2003.12498.x. [DOI] [PubMed] [Google Scholar]
- 102.Imokawa G. Journal of Investigative Dermatology Symposium Proceedings. Elsevier; 2009. Mechanism of UVB-induced wrinkling of the skin: paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase; pp. 36–43. [DOI] [PubMed] [Google Scholar]
- 103.Imayama S., Nakamura K., Takeuchi M., Hori Y., Takema Y., Sakaino Y., Imokawa G. Ultraviolet-B irradiation deforms the configuration of elastic fibers during the induction of actinic elastosis in rats. J. Dermatol. Sci. 1994;7:32–38. doi: 10.1016/0923-1811(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 104.Tsukahara K., Takema Y., Moriwaki S., Tsuji N., Suzuki Y., Fujimura T., Imokawa G. Selective inhibition of skin fibroblast elastase elicits a concentration-dependent prevention of ultraviolet B-induced wrinkle formation. J. Invest. Dermatol. 2001;117:671–677. doi: 10.1046/j.0022-202x.2001.01450.x. [DOI] [PubMed] [Google Scholar]
- 105.Majumder P., Paridhavi M. A novel poly‐herbal formulation hastens diabetic wound healing with potent antioxidant potential: a comprehensive pharmacological investigation. Phcog. J. 2019;11 doi: 10.5530/pj.2019.11.48. [DOI] [Google Scholar]
- 106.Punnonen K., Autio P., Kiistala U., Ahotupa M. In‐vivo effects of solar‐simulated ultraviolet irradiation on antioxidant enzymes and lipid peroxidation in human epidermis. Br. J. Dermatol. 1991;125:18–20. doi: 10.1111/j.1365-2133.1991.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 107.Stadtman E.R. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 108.Bittinger F., Gonzalez-Garcia J.L., Klein C.L., Brochhausen C., Offner F., Kirkpatrick C.J. Production of superoxide by human malignant melanoma cells. Melanoma Res. 1998;8:381–387. doi: 10.1097/00008390-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 109.Yin S., Wang Y., Liu N., Yang M., Hu Y., Li X., Fu Y., Luo M., Sun J., Yang X. Potential skin protective effects after UVB irradiation afforded by an antioxidant peptide from Odorrana andersonii. Biomed. Pharmacother. 2019;120 doi: 10.1016/j.biopha.2019.109535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. Material/referenced in article:
This datasets analysed during the current study are available from the corresponding author on reasonable request.