Abstract
Supramolecular chemistry introduces us to the macrocyclic host cyclodextrin, which has a hydrophobic cavity. The hydrophobic cavity has a higher affinity for hydrophobic guest molecules and forms host-guest complexation with non-covalent interaction. Three significant cyclodextrin kinds are α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin. The most often utilized is β-cyclodextrin (β-CD). An effective weapon against bacteria that are resistant to antibiotics is cyclodextrin. Several different kinds of cyclodextrin nanocarriers (β-CD, HP-β-CD, Meth-β-CD, cationic CD, sugar-grafted CD) are utilized to enhance the solubility, stability, dissolution, absorption, bioavailability, and permeability of the antibiotics. Cyclodextrin also improves the effectiveness of antibiotics, antimicrobial peptides, metallic nanoparticles, and photodynamic therapy (PDT). Again, cyclodextrin nanocarriers offer slow-release properties for sustained-release formulations where steady-state plasma antibiotic concentration is needed for an extended time. A novel strategy to combat bacterial resistance is a stimulus (pH, ROS)-responsive antibiotics released from cyclodextrin carrier. Once again, cyclodextrin traps autoinducer (AI), a crucial part of bacterial quorum sensing, and reduces virulence factors, including biofilm formation. Cyclodextrin helps to minimize MIC in particular bacterial strains, keep antibiotic concentrations above MIC in the infection site and minimize the possibility of antibiotic and biofilm resistance. Sessile bacteria trapped in biofilms are more resistant to antibiotic therapy than bacteria in a planktonic form. Cyclodextrin also involves delivering antibiotics to biofilm and resistant bacteria to combat bacterial resistance.
Keywords: Biofilm, Virulence factors, Quorum sensing (QS), Cyclodextrin, Antibiotics
1. Introduction
Numerous infectious disorders have been treated, and hundreds of antibacterial drugs have been made therapeutically available since Alexander Fleming's discovery of penicillin in 1928. However, due to their widespread use, antimicrobial resistance—one of current medicine's most critical issues—arises, reducing conventional therapy's therapeutic effect [1]. The “Golden Age” of antibiotic discovery is said to have occurred between 1940 and 1962, when more than 100 antibiotics, mostly derived from naturally occurring bacteria, were discovered [2]. But the rise of microbial resistance to already-existing medicines has prompted the ongoing discovery of new antibiotics. First-generation penicillin could be used to treat Staphylococcus aureus (S. aureus), but within a year, penicillin-resistant S. aureus started to emerge. New methicillin-based drugs were subsequently created to treat S. aureus resistant to penicillin, although Methicillin-resistant Staphylococcus aureus (MRSA) first appeared in 1986 [3]. The newly created vancomycin was crucial in the management of MRSA. However, vancomycin usage led to the development of vancomycin-resistant S. aureus (VRSA) [3]. Because of this, using recently created antibiotics may hasten the appearance of new antibiotic-resistant bacteria. It also implies that the likelihood of developing antibiotic-resistant bacteria increases with increasing antibiotic overuse [4]. The biggest threat to a patient's condition is hospital-acquired bacterial infections, often brought on by nosocomial pathogens such as Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, and others [5]. Recently developed community-acquired MRSA (CA-MRSA) variants quickly establish themselves as the community's predominant pathogens [6]. Switching to more expensive, broad-spectrum antibiotics has become necessary due to the persistence of resistant strains and the continuously high rates of antibiotic usage in hospitals, communities, and agriculture [7]. Antibiotic resistance among bacteria that risk human health has been caused by this misuse and abuse of antibiotics [8], which has resulted in treatment problems and elevated healthcare costs [7]. Once they start feeling better, many patients tend to stop their treatments, which can worsen antibiotic resistance. Additionally, many antibiotics have short half-lives and require frequent administration, promoting compliance to the patient. The lack of compliance frequently causes treatments to fail or raises the expense of healthcare resources by requiring additional drugs and hospital admission [9]. Increased livestock production results from rising demand for animal protein, which increases the use of antibiotics in the agricultural and livestock sectors and, eventually, breeds antibiotic resistance [10]. Antibiotic resistance is complex. It is known that horizontal transmission of genes on plasmids or transposons, as well as spontaneous gene mutation, are the two ways bacteria develop resistance to both single and multiple antibiotics [11]. The resistant genes to the drug include blaZ, mecA, parC, gyrA, gyrB, sulA, drfB, ermA, ermb, ermc, vat, and vatB [12]. The expression of the resistance gene results in the capacity to fight antibiotics by a variety of processes, including (a) altering the drug's drug targets, (b) degrading the antibiotic enzymatically, (c) reducing cell membrane permeability, and (d) efflux pumps [13]. Planktonic bacterial infections present acute threats and are getting harder and harder to cure as acquired antibiotic resistance rates rise. This problem is made more difficult when bacteria develop biofilms linked to recurrent and chronic bacterial infections [14]. Bacterial biofilms spur chronic infections because they are more resistant to phagocytosis and other defensive mechanisms, including phagocytosis and disinfectant chemicals [15]. Pathogenic biofilms are produced by populations of pathogens that attach to surfaces and are immersed in the extracellular matrix, including Pseudomonas aeruginosa, Staphylococcus aureus, Vibrio cholerae, and Nontuberculous mycobacteria [[16], [17], [18]]. The development of bacterial biofilms is one of the core aspects causing bacterial resistance [19]. The resistance of bacteria in biofilms to antibiotics can be up to 1000 times higher than planktonic bacteria [20]. Subinhibitory concentrations, which are antibiotic exposure levels below the minimum inhibitory concentration (MIC), might cause bacteria to be more capable of forming biofilms, which can reduce their sensitivity to antibiotics [21]. For example, it has recently been demonstrated that clinical Enterococcus faecalis isolates can develop biofilms more quickly when exposed to subinhibitory antibiotic doses [22]. The conventional antibiotics used for antibacterial therapy have certain drawbacks in contemporary medicine, such as limited bioavailability, little penetration into the infection nidus, and the emergence of drug-resistant bacteria (superbugs) [23]. Antibiotic resistance is currently being combated using various methods, including cationic polymers, nanoparticles, combinatorial therapy, antimicrobial photodynamic therapy (PDT), interference in bacterial quorum sensing, antimicrobial peptides, and chemical modification of antibiotics. By serving as either intrinsic therapies or nanocarriers for antimicrobial drugs, recent advancements in nanomaterial-based systems offer new ways to fight multi-drug-resistant planktonic and biofilm infections [24]. When used as antibacterial agents or as carriers for antibacterial drug loading, nanoparticles can increase the bioavailability and efficacy of antibiotics [25]. Compared to conventional therapy, the effectiveness of medication delivery using nano-systems increases while potential toxicity decreases. Because of their great attraction to bacteria, high specific surface area ratio, possibility for surface functionalization, and capacity to load drug molecules, nanoparticles play an important role in efficient antibacterial action [26]. Nanoparticles can shield medicines from enzyme attacks, prolong medication release, and boost half-life and bioavailability [27]. A successful strategy to raise the therapeutic index is the active targeting of nanoparticles at bacteria [28]. Reversible non-covalent interactions, a key component of supramolecular chemistry, such as electrostatic interactions, p-p stacking interactions, hydrophobic effects, van der Waals interactions, and hydrogen-bonding interactions, can successfully bring together like molecules through molecular recognition to create ordered supramolecular nanoarchitecture's with adjustable sizes, morphology, and functions, such as micelles, nanoparticles (NPs), vesicles, and hydrogels [[29], [30], [31]]. Cyclodextrin (CD) is a supramolecular macrocyclic host, also known as α-, β- or γ-cyclodextrins, are cyclic oligosaccharides made up of various numbers of glucose molecules [32] and include six, seven, or eight glucose monomers connected by α-1,4-glucose linkages [33]. Due to their capacity to modify the physical, chemical, and biological characteristics of guest molecules through the creation of inclusion (host-guest) complexes (Fig. 1), cyclodextrins are prospective candidates for drug carriers [34]. Based on hydrophobic interactions, the hollow portion of the center cavity may hold a large number of hydrophobic guest molecules [35]. Antibiotics [36], antimicrobial peptides [37], photosensitizing agents [38], metallic nanoparticles [39], various autoinducers [40], essential oils [41], and other hydrophobic guests can all be included in cyclodextrins. While permeability and dissolution are both rate-limiting factors for BCS Class IV drugs, the dissolution phase is the rate-limiting element for the absorption of BCS Class II drugs [42]. Developing inclusion complexes with cyclodextrins can considerably enhance the water solubility of poorly soluble drugs [43]. In photodynamic treatment (PDT), a chromophore known as a photosensitizer (PS) is utilized to create highly reactive oxygen species (ROS), such as hydroxyl radicals (Type I photo-process) and cytotoxic singlet oxygen, by “sensitizing” the surrounding triplet oxygen upon light absorption (Type II photo-process) [[44], [45], [46], [47]]. The rise in antibiotic resistance has shifted the scientific community's attention to using PDT to treat infections [48]. Due to excellent benefits such as the ease of application, the non-invasive nature of light, and, most importantly, the lack of potential ROS resistance in microbes, antimicrobial PDT (aPDT) has emerged as a major replacement for conventional antibiotic therapy [49]. Photodynamic therapy includes the systemic delivery of a photosensitizer, typically a porphyrin-based compound, which has been integrated into lipophilic drug carriers, such as liposomes, oil emulsions, or cyclodextrin inclusion complexes to reduce precipitation in the blood system or accumulation in a polar milieu and enhance PDT effectiveness [50,51]. Targeting bacteria's virulence factors, biofilm development, and protease and siderophore synthesis appear to be a more promising alternative strategy than focusing on them with bactericidal or bacteriostatic drugs. Such virulence factors as regulating virulence expression and bacterial adherence are necessary for infection [[52], [53], [54]]. Quorum sensing is a technique of cell-to-cell communication used by bacteria. Gram-negative bacteria often employ an acylated homoserine lactone (AHL) known as an autoinducer during quorum sensing. By trapping AHLs, cyclodextrin reduces quorum sensing [55]. Cyclodextrin nanocarrier also increases the QSI's (Quorum sensing inhibitor) efficacy by improving the solubility and bioavailability [56]. In addition to enhancing medication permeability across the membrane barrier when employed as complexing agents, CDs may make antibiotics or other antibacterial agents more soluble. It increases the guest molecule's bioavailability and alters the antibacterial activity and chemical stability [57]. For several bacterial strains, CD/antibiotic complexes displayed lower MIC values than antibiotics alone because CD or CD derivatives can increase the stability and permeability of antibiotics (Table 1) [58]. Frequent dosing of many antibiotics with short half-lives in conventional formulations is required to maintain antibacterial action. Otherwise, a concentration below the minimum inhibitory concentration (MIC) usually happens during anti-infective therapy, which results in antibiotic resistance. By sustaining a consistent plasma drug concentration over MIC over a prolonged length of time, extended-release dosage forms improve the therapeutic effectiveness of antibiotics while decreasing antibiotic resistance. Improved patient compliance is an unquestionable benefit of extended-release formulations [59]. Due to its unique glucose ring topologies and many hydroxyl groups with a hydrophobic interior chamber and a hydrophilic exterior [62], cyclodextrins have been widely employed in creating medical devices to provide prolonged antibiotic-release properties [60,61]. Antimicrobial medications can be captured by CD and released gradually at the necessary times [63]. We briefly address developments in the creation of cyclodextrin-based antibacterial agents in this review. The antibacterial activities of these supramolecular materials can be related to either the modified structure of macrocycles or the encapsulated antibiotics or other antibacterial agents in the cavities of these macrocycles, which further aid in activity enhancement for antimicrobials, decrease antibiotic resistance, disrupt biofilms, and minimize the chance of infection due to the host-guest complexation properties and easy and versatile functionality of cyclodextrin.
Fig. 1.
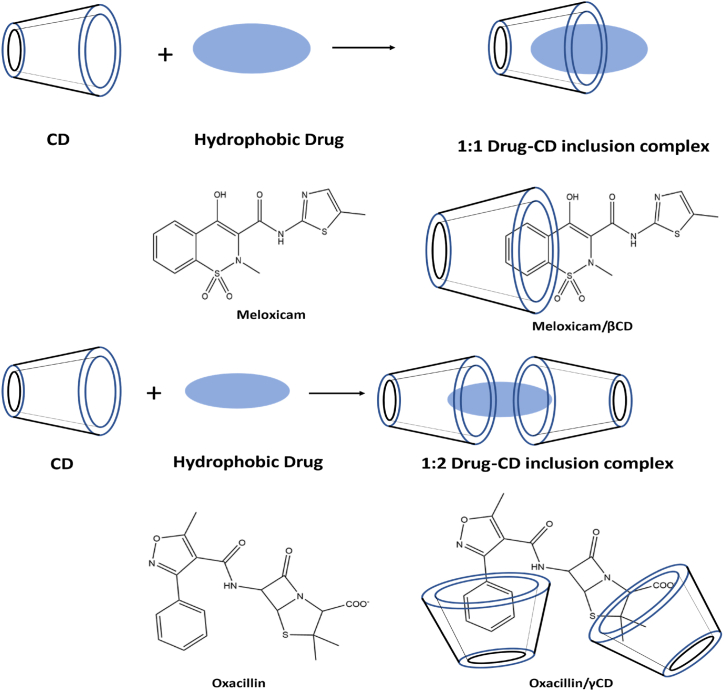
Cyclodextrin inclusion complex. When a drug or even other “guest” molecule is completely or partially incorporated into the cavity of the host, a complex is created.
Table 1.
Combination of various antibacterial agents with different CD carrier.
| Antibacterial agents | Class | Cyclodextrin carrier | Improvement | Antibacterial activity | Ref. |
|---|---|---|---|---|---|
| Tebipenem pivoxil (TP) | carbapenem analog, which belongs to the β-lactam antibiotics | β-CD | Enhancement of solubility and permeability | MIC values were dramatically lowered concerning the growth inhibition of Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, and Proteus mirabilis. | [212] |
| Ampicillin, Amoxicillin, Dicloxacillin | β-lactam antibiotics | γ-CD and octakis[6-(2- carboxyethyl)thio-6-deoxy]- γ-CD | Enhance the penicillin's chemical stability and lessen its allergenicity and propensity to cause bacterial resistance. | _ | [213] |
| Sodium Dicloxacillin |
soluble salt of semi-synthetic penicillin |
γ-CD or HP-β-CD | Improve solubility and stability. | _ | [214] |
| Meropenem | β-lactam antibiotic | 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) | Improve chemical stability | The MER-β-CD complex had higher bactericidal activity than the free form against P. aeruginosa, Rhodococcus equi, and Listeria ivanovii. But the MIC values for the majority of bacterial strains were the same. | [215] |
| Oxacillin | β-lactam antibiotic | octakis[6-(2-aminoethylthio)-6-deoxy]-γ-CD (γCys) | Improve stability against highly active oxa-1 β-lactamase | Compared to uncomplexed oxacillin, complexing oxacillin with γ-CD moderately reduces the viability of S. epidermidis biofilms (by around 50%). | [216] |
| Methicillin | β-lactam antibiotic | per-6-(4-methoxylbenzyl)-amino-6-deoxy-β-cyclodextrin (pMBA - βCD) | Improve solubility | When compared to methicillin alone, the MIC values of pMBA- βCD/methicillin were reduced 30-65-fold for methicillin-resistant Staphylococcus aureus. | [217] |
| Cefuroxime axetil (CA) | Oral cephalosporin prodrug | hydroxypropyl-β-cyclodextrin (HPβCD) | Improve solubility and dissolution profile | Clinical isolates of Klebsiella pneumonia and Pseudomonas aeruginosa exposed to the CA-HPβCD inclusion complex exhibited up to a four-fold increase in antibacterial activity. | [218] |
| Cefadroxil | cephalosporin class of antibiotic | β-CD HP-β-CD Meth- β-CD |
Enhance the permeability of antibiotics and resistance against β lactamase | Methicillin-sensitive S. aureus strain was 16 and 4 times more active than cefadroxil-HP βCD (1:2) and cefadroxil- βCD (1:2), respectively. E. coli strains were four times and two times more active to Cefadroxil-Meth-βCD (1:2), and P. aeruginosa strains were two as more active to cefadroxil-Meth-βCD (1:2) complex | [219] |
| Cefdinir | semi-synthetic third-generation broad-spectrum oral cephalosporin | β CD HP-β-CD |
Improve solubility, dissolution and bioavailability | increase the release rate of CEF to enhance its antibacterial efficacy against Gram-positive species S. aureus and Gram-negative species E. coli in vitro. | [220] |
| Rifampin/Rifampicin | ansamycin or macrocyclic antibiotics | Octakis(6-(2-aminoethylthio)-6-deoxy)-γ-cyclodextrin (γ Cys) | improve antibiotic delivery and efficacy |
Indicates a significant reduction in S. epidermidis biofilm viability compared to rifampicin alone. | [216] |
| hydroxy propyl- ß-cyclodextrin (HPßCD) and randomly methylated ß-cyclodextrin (RAMEB) | improve solubility | similar or higher bacteriostatic activity against A. Baumannii | [221] | ||
| Ciprofloxacin | Fluoroquinolone broad-spectrum antibiotic | mono-6-deoxy-6-aminoethylamino-β-cyclodextrin (Et-β-CD | enhance the solubility and bioavailability | significantly affect the growth curve and improve the antibacterial activity of CIP against MRSA | [222] |
| Natamycin | Polyene macrolide antibiotic | β-CD HP-β-CD |
enhance the solubility | The MIC of natamycin:β-CD complex and natamycin: HP β-CD complex is significantly reduced in Candida albicans and Saccharomyces cerevisiae strains. | [223] |
| Roxithromycin | semi-synthetic macrolide and an ether-oxime derivative of erythromycin | βCD HP-β-CD |
Improve solubility | βCD-ROX/PLGA NPs and HPβCD-ROX/PLGA NPs have shown significant antimicrobial effects against the selected multidrug-resistant Gram-positive MRSA bacteria. The HPβCD-ROX/PLGA NPs show significantly higher zones of inhibition |
[224] |
| Vancomycin Hamamelitannin |
glycopeptide antibiotics Quorum sensing inhibitor(QSI) |
HP-β-CD | Sustained delivery of antibiotics | HPβCD-functionalized cellulose gauzes containing the antibiotic VAN and the QSI HAM capable of affecting S. aureus and P. aeruginosa biofilm | [225] |
| Ciprofloxacin | Fluoroquinolone broad-spectrum antibiotic | β-CD | Sustained delivery of antibiotics | β-CD grafted fibers loading CipHCl possess excellent antibacterial activity against E. coli and S. aureus. | [226] |
| Alamethicin | Antimicrobial peptide (AMP) | γ-CD | Better solubility, temperature, and pH stability | The γ cyclodextrin/alamethicin 5:1 mol ratio was determined to have more excellent antibacterial action in L. monocytogenes strains than AMP alone | [227] |
| ZnO Cefepime |
Nanoparticles (NPs) Fourth-generation cephalosporin |
β-CD | Enhance the solubility and bioavailability of nanoparticles | ZnO-βCD-Cfp improves the anti-biofilm activity in E. coli strains | [228] |
| Cinnamon | essential oil | β-CD | Improvement of stability | β-CD:CLO inhibits the growth of Alternaria alternata more effectively than free CLO | [229] |
| Carvacrol, eugenol, linalool, 2-pentanoylfuran | essential oil | α-CD β-CD HP-β-CD |
Enhancement of solubility | The most significant improvement in the MICs of essential oil compounds against E. coli, B. subtilis, S. aureus, and S. cerevisiae was shown when hydroxypropyl-β-CD was used as the solubilizer. However, there was no improvement in the MIC with eugenol for S. aureus and S. cerevisiae using hydroxypropyl-β-CD as the solubilizer. α-CD and β-CD were effective solubilizers concerning eugenol and linalool for B. subtilis and S. aureus; and carvacrol and 2-pentanoylfuran for E. coli and S. cerevisiae, respectively. |
[230] |
2. Antibiotic resistance mechanisms
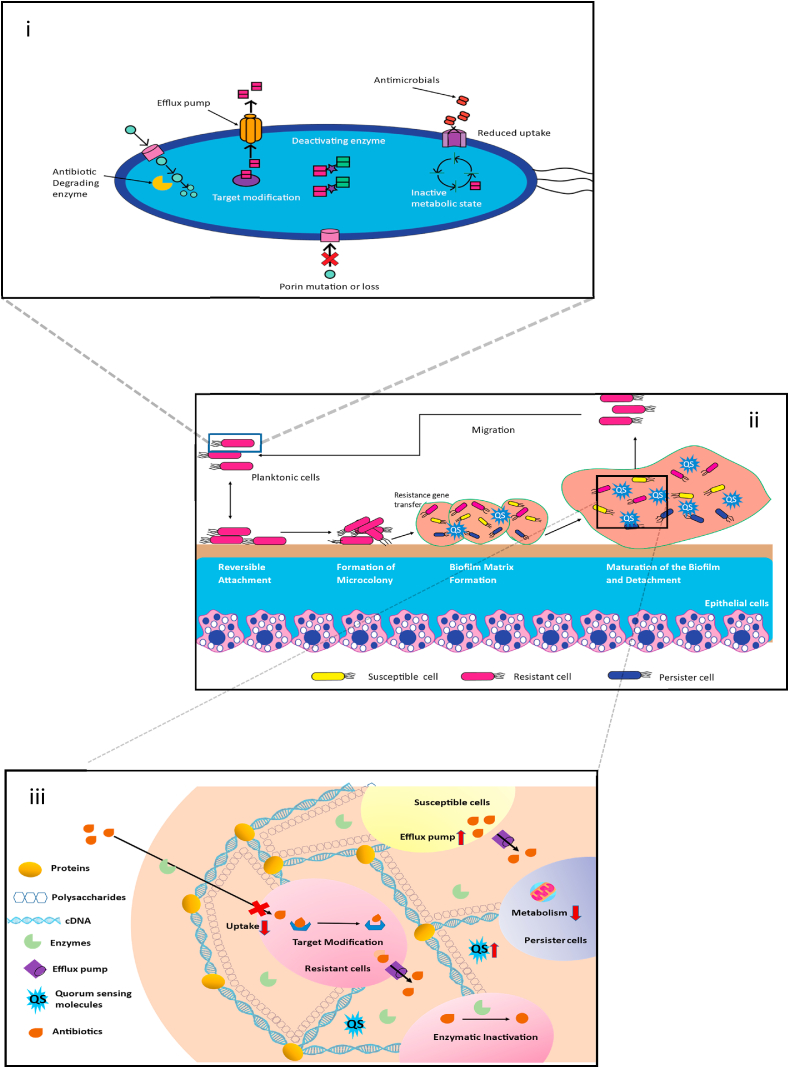
Bacteria adopt a range of tactics to combat antibiotics (Fig. 2).
Fig. 2.
(i) To lessen susceptibility to antibiotics, bacteria have evolved various drug resistance mechanisms, including antibiotic degradation by beta-lactamase, antibiotic modification by aminoglycoside-modifying enzymes, drug target modification, loss or mutation of porins, and increased expression of efflux pumps. (ii) Different stages of biofilm formation: (a) reversible attachment of bacteria to a surface with the help of pili, fimbriae, and glycocalyx, (b) irreversible attachment and microcolony formation, (c) formation of EPS matrix and elevated level of QS molecules help in further biofilm formation, (d) after the maturation of biofilm, the release of mature cells from it, (e) repeat the cycle. (iii) Mechanisms of biofilm that are resistant to antibiotics and the primary matrix components — polysaccharides, proteins, and DNA.
2.1. Reduced uptake and active antibacterial drug efflux
Pumps for drug efflux either prevent drug entrance or expel the antibacterial agent from the microbiological cell before it. Sub-toxic drug levels are present in the microbial cells due to transmembrane multi-drug efflux pump overexpression and decreased absorption [64]. Enhanced outpour occurs in P. aeruginosa when a regulatory protein that typically suppresses the gene that encodes efflux proteins is mutated. When porins in P. aeruginosa are expressed insufficiently or not at all, the permeability of the cell wall to carbapenems is decreased, which may impact antibiotic uptake or efflux [65]. TetR (Tet Repressor proteins), a repressor protein, prevents the regular expression of TetA, a gene that codes for a tetracycline efflux pump, in Gram-positive and Gram-negative bacteria. Contrarily, tetracycline attaches to and inactivates TetR, which causes TetA production to accelerate drug efflux [66].
2.2. Covalent modification of an antibacterial agent reduces its effectiveness
The primary mechanism of antibiotic resistance is believed to be hydrolysis or the transfer of a chemical group, both of which directly affect antibiotics. The degradation of penicillin antibiotics by the beta-lactamase enzyme is a well-known example. Bacteria also introduce chemical groups (acyl, phosphate, nucleotides, ribitol, etc.) into the antibiotic's active sites, preventing them from binding to the target due to steric hindrance [67,68]. Plasmids or transposons and rarely bacterial chromosomes have resistance genes for aminoglycoside-modifying enzymes, which change the aminoglycoside's OH or NH2 groups. It reduces the aminoglycoside's affinity for the 30S ribosomal subunit and its antimicrobial action. Gene transfer has led to the evolution of β-lactamases, which have broad-spectrum activity against most β-lactam antibiotics, including cephalosporins [69,70]. Chloramphenicol acetyltransferases alter chloramphenicol such that it can no longer bind to the 50S ribosomal subunit and prevent the production of proteins. Additionally, covalent modification confers resistance to macrolides, tetracyclines, quinolones, streptogramins, tobramycin, gentamycin, chloramphenicol, kanamycin, and other antibiotics [66,71].
2.3. Synthesis of a competitive antibiotic inhibitor
By making para-aminobenzoic acid, an inhibitor of the sulfonamide that competes with it for binding to the active site of bacterial dihydropteroate synthetase, S. aureus and Neisseria meningitidis gain sulfonamide resistance [66,72]. Sulfonamide-based therapies have been relegated to second or third-line choices due to mutations in this enzyme discovered in several clinical isolates [73].
2.4. Biofilm formation to avoid interaction with antibiotics
Pathogenic bacteria's essential virulence factors include biofilms; some biofilm infections are notoriously difficult to treat [74]. Most bacteria and fungi, including Pseudomonas aeruginosa [75], Staphylococcus epidermidis [76], Candida albicans [77], Acinetobacter baumannii [78], Helicobacter pylori [79], Staphylococcus aureus [80], Listeria monocytogenes [81], Vibrio cholerae [82], and Salmonella enterica [83], may produce biofilms. Medical devices include orthodontal prosthetics, contact lenses, endotracheal tubes, central venous catheters, intrauterine devices, needleless connectors, peritoneal dialysis catheters, prosthetic joints, urinary catheters, prosthetic joints, and breast implants, are broadly utilized and have become crucial for treatments in clinical work. Additional complications can occasionally arise from medical devices, the most common of which is an infection brought on by bacteria that break free from biofilms on the device; an example is catheter-associated biofilms [84]. Microorganisms may also cling to biotic surfaces and form biofilms in various host tissues, such as epidermal cells [85] and teeth [86], as well as in tissues like the mucus on mucosal membranes [87] or within chronic wounds [88]. Chronic infections and ongoing inflammation are linked to higher cancer risk [89]. Humans can become infected by bacteria that are in planktonic, biofilm, or intracellular forms. Bacteria that are free to move around are called planktonic bacteria. In their respective environments, they exist as floating microorganisms. The adhesive or sessile form of growth is the antithesis of planktonic bacteria's growth mode [90]. Sessile microbial colonies known as biofilms are immersed in a matrix of extracellular polymeric substances (EPS), such as proteins, exopolysaccharides, and nucleic acids they produce (Fig. 2) [91]. When bacteria form biofilms, these bacteria are highly resistant to antimicrobial drugs that kill cells of the same species in the planktonic state [90]. Although the exact causes of biofilm's resistance to antimicrobial therapy are still unknown, they have been linked to factors such as decreased drug diffusion through the EPS matrix, a slow growth rate, spatial heterogeneity, increased expression of enzymes that break down antibiotics, and the presence of drug-tolerant phenotypes like persister cells and small-colony variants (Fig. 2) [[92], [93], [94], [95], [96], [97]]. Antibiotics can't attack bacteria that are protected by biofilm and host cells. Antibiotic resistance spreads, and bacteria become more aggressive due to the favorable environment for gene transfer provided by biofilms [98]. The majority of antibacterial agents only have modest effects on intracellular microorganisms. Drug resistance develops due to antibiotic concentrations below the minimum inhibitory concentration (MIC) in the intracellular compartment [99]. It has been noted that a biofilm contains two microbial subpopulations with distinct morphologies, one of which is metabolically active and the other inactive. Sessile bacteria are buried in the EPS matrix within the biofilm with a sluggish rate of cell division, which makes them resistant to antibiotics. Planktonic bacteria are on the surface and in contact with the external environment, making them sensitive to antibiotics [100,101]. Bigger discovered a tiny number of persister cells in bacterial cultures in 1994 [102]. These cells were phenotypic variations of common bacteria that were inactive, meaning their development was sluggish or even stopped. Persister cells have a high antibiotic resistance because most available antibiotics only target metabolically active bacteria. Chronic infections and recurrence are caused when the persister cells return from the dormant condition to the state of dynamic growth when the stimulation intensity of antibiotics falls below a specific threshold value [[103], [104], [105], [106]]. Persist cells bring challenging bacterial resistance with them. More concerningly, it is exceedingly tricky for conventional medicines to effectively eliminate biofilms when persister cells are embedded in them [107].
2.5. Formation of biofilms
There are several stages to the biofilm generation process (Fig. 2).
2.5.1. Reversible attachment
A single planktonic cell can migrate and reversibly adhere to a surface under favorable conditions, initiating the process of biofilm formation [108]. Reversible attachment includes weak electrostatic, van der Waals, or hydrophobic interactions [109,110]. These surfaces are changed once microorganisms adhere to them, creating a surface charge that facilitates the attraction and adhesion of bacteria with a negative control [111]. The organisms may cling to the surface more intensely because of pili, fimbriae, and glycocalyx. Cells can loosely cluster during the adhesion and sessile development stages, but they are reversible, and they can also detach and go back to a planktonic state [112]. While the initial adhesion is reversible, it becomes irreversible if attraction is preferred over repulsion [113]. Antibiotics are still effective against bacteria at this stage.
2.5.2. Quasi-irreversible adhesion for microcolony formation
As porins and a systematic microcolony are formed at this stage, the planktonic cells are visibly more layered and create an irreversible attachment [114]. The polysaccharides needed to develop the EPS layer are transported more easily by porins [115]. Biofilm's virulence and dormancy are greatly influenced by colonization, one of their distinguishing features. Numerous microbes gather and release EPS, a sealant to fix the microorganisms after the cells firmly attach to the proper surface [114]. Environmental antimicrobials only eliminate bacteria that are not part of a biofilm. Suppose the bacteria inside the biofilm have antimicrobial resistance. In that case, the EPS-matrix permits the transport of nutrients and metabolic waste products through water channels but severely hinders the transport of antimicrobials [116].
2.5.3. Matrix formation in biofilms
The EPS of the adherent creates a matrix in which the bacteria cells develop their community and attain their maximal cell density. Extracellular DNA (eDNA), quorum sensing molecules (autoinducers), persister cells, lipids, proteins, and polysaccharides are only a few of the components that make up the EPS that encases the cells in a biofilm (Fig. 2) [[117], [118], [119], [120]]. The polysaccharides in the matrix provide the cells with biofilm strength, including adhesion, protection, and structural rigidity [121]. In addition to serving as a source of nutrients and a genetic material reservoir for horizontal gene transfer, EPS also protects against harmful elements, including drying, exposure to biocides, oxidation, certain metallic cations, antibiotics, UV radiation, and immunological reactions. The successful development of biofilm communities, as well as the proliferation and survival of the cells in their local surroundings, are represented by the generation of the matrix [122,123]. The microorganisms sense the cell population density and start to produce the polymeric matrix components of the biofilm [125] when the concentration of QS (Quorum sensing) signaling molecules in the surrounding medium reaches a threshold level, inducing and transcribing specific genes and horizontal gene transfer [124].
2.5.4. Biofilm maturation and detachment
The cells develop and differentiate in response to nutrient- and environment-rich environments, resulting in mature biofilms with a spatial architecture. This highly developed biofilm resembles a social community governed by quorum-sensing molecules released by the biofilm's microbial occupants [114]. As the biofilm matures, the cells inside release matrix components that promote cell-cell adhesion and disruptive agents such as nucleases, proteases, and phenol-soluble modules [126]. Additionally, these disrupting elements could encourage biofilm dissociation. During the detachment process, biofilms can lose individual cells and flake off fragments into the blood system and surrounding tissues, linked to several acute and chronic infections [127].
3. Cyclodextrin inclusion complexes
The unique complexation between hydroquinone and several volatile substances within the inclusion complexes were first detected by Mylius in 1886 [129]. Additionally, he observed that there was no chemical interaction between the molecules that were trapped within one another. Before Schlenk coined the word “inclusion compounds,” Other names included “occlusion compounds, adducts, and clathrates.” The complex of one guest molecule and the host molecule is an inclusion compound. The host molecule traps the hydrophobic drug component, and the existence of non-covalent contacts between the drug and the host molecule indicates this complexation. The guest molecule's size must equal the host molecule for the guest molecule to be trapped within the host molecule's cavity and form a stable complex with the appropriate physiochemical characteristics [[128], [129], [130]]. It is one of the critical requirements for this approach.
3.1. Cyclodextrin
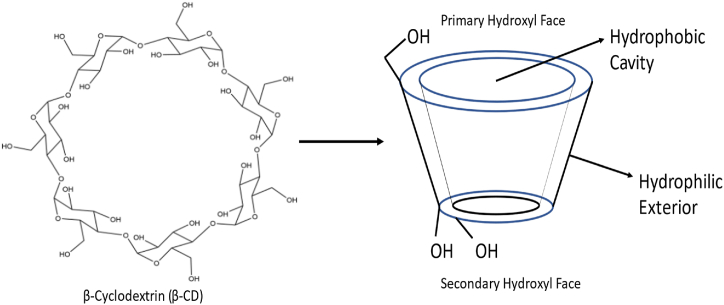
By using bacterial digestion, Antoine Villiers of France extracted potato starch in 1891. He called this experimental substance dextrin “cellulose” [131]. Franz Schardinger found the crystalline molecules α-dextrin and β-dextrin in 1903, and Villiers' “cellulose” were identified as β-dextrin. Today, these compounds are also known as α-cyclodextrin (α-CD) and β-cyclodextrin (β-CD) [132,133]. Freudenberg et al. discovered a different substance in 1935 that they called γ-cyclodextrin (CD) [134]. CDs can create supramolecular structures called inclusion complexes (ICs) when combined with other molecules, primarily small molecules. CDs are truncated cone-shaped molecules with an external hydrophilic shell and a hydrophobic core (Fig. 3). The driving forces of IC (inclusion complex) formation is influenced by interactions, including hydrophobic, van der Waals, and hydrogen bonding, which can change guest molecules' physical, chemical, and biological properties [135,136]. CDs are regarded as effective drug-carrying and drug-delivering devices due to their capacity to create ICs. CDs are also effective solubilizers, physical stabilizers, and protectors of guest molecules against gastrointestinal tract degradation, increasing medication bioavailability [137]. Piroxicam/β-CD (Brexin® tablets) was the first pharmaceutical formulation to be released in Europe in 1977, and itraconazole/2-hydroxypropyl-βCD oral solution (Sporanox®) was the first to get US approval [138].
Fig. 3.
CDs are truncated cone-shaped molecules with an external hydrophilic shell and a hydrophobic core.
3.1.1. α-Cyclodextrin (α-CD)
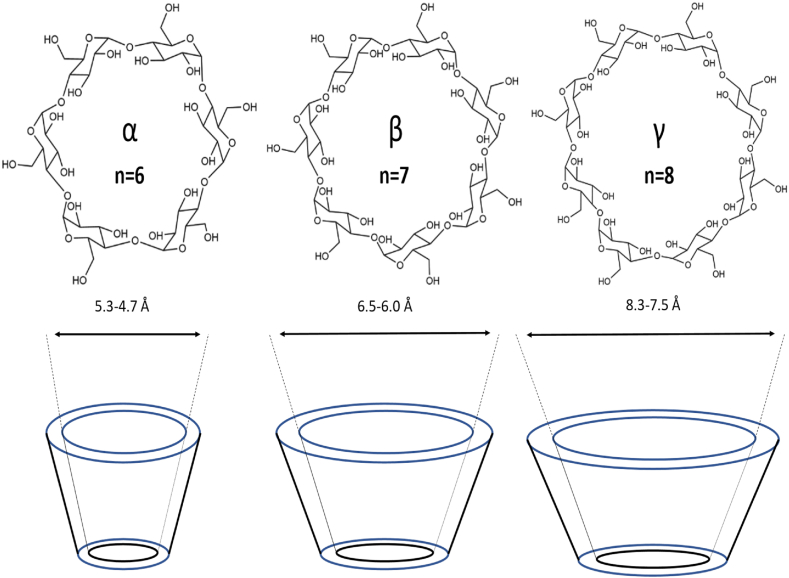
CDs are primarily categorized according to how many glucose units are in their structure; for example, molecules with six glucose units are called α-CDs (Fig. 4) [139]. There is weak hydrogen bonding on the outer edge between the 2-OH and 3-OH groups; this interaction is weak in α-CD and highest in γ-CD. While 2-OH serves as the acceptor and 3-OH as the donor in α-CD, the bonding in β- and γ-CD alternates between 3-OH (acceptor) and 2-OH (donor). CDs are amphipathic structures with 6-OH groups on the narrower rim and 3-OH and 2-OH groups on the broader rim. The hydrophilic groups surround the molecular cavity of cyclodextrins in contrast with the hydrophobic interior coated by ether-like anomeric oxygen atoms. Due to its inadequate size, drugs cannot be contained in the cavity of α-CD [[140], [141], [142]].
Fig. 4.
Different Cyclodextrin Structure (α-CD, β-CD, γ-CD).
3.1.2. β-Cyclodextrin (β-CD)
β-CD has frequently been used in the beginning phases of pharmaceutical applications because it is easily accessible and has a cavity size suitable for various pharmaceuticals [142]. Comparatively to other CDs, the cavity size of the β-CD is more suited to encapsulate a variety of compounds [143]. It is possible to employ β-CD to improve medication solubility, bioavailability, safety, and stability and as a carrier in drug formulation by forming inclusion complexes with drug molecules that are chemically bonded [144]. Hydrophilic, hydrophobic, and ionizable derivatives are the three classes into which β-CD derivatives are divided. Every group carries out a distinct task. Examples of hydrophilic CDs that can enhance the water solubility of weakly water-soluble molecules include 2,6-dimethyl-β-CD, 2,3,6, trimethyl-β-CD, 2-hydroxypropyl-β-CD (HP-β-CD), and maltosyl-β-CD. Similarly, CDs belonging to the hydrophobic group, such as 2,6-diethyl-β-CD, can slow down the dissolution rate of poorly water-soluble drugs [145]. Sulfobutyl-ether-β-cyclodextrin (SBECD), also known by the brand name Captisol®, carboxymethyl-β-cyclodextrin (CMCD), and hydroxypropyl-β-cyclodextrin (HPβCD) derivatives have all been used in drug formulation, especially for Class 2 and 4 drugs (low solubility and low permeability) [52], because they have a higher water solubility and better biocompatibility.
3.1.3. γ-Cyclodextrin (γ-CD)
Large internal cavity sizes and the ability to enclose larger molecules give the γ-CD a distinct advantage over the α- and β-CDs [146]. Compared to other natural cyclodextrins, γ-CD has the maximum water solubility due to its flexible and noncoplanar structure. This feature makes it a suitable host for the solubility improvement of less water-soluble drugs, further explaining its applicability in other industries [147]. Due to its difficulty passing across biological membranes, quick digestion in the gastrointestinal system, and unchanged excretion through the urine following parental delivery, γ-CD has a limited bioavailability [[148], [149], [150], [151], [152]]. The γ-CD is a superior choice to improve drug properties due to its high water solubility, greater cavity size, and most favorable toxicological profile [146].
3.2. Improvement of the physicochemical properties of drugs
3.2.1. Improved solubility and dissolution rate
The water solubility of the drug is a crucial factor in drug absorption during oral drug delivery, and it also has the potential to have excellent bioavailability. Increasing the solubility and dissolution rate can improve the bioavailability of drugs in BCS Classes II and IV. The cyclodextrin complexes can dissolve drugs by creating non-covalent complexes in the solution [153]. Inclusion complexes of the antifungal medicine Voriconazole were built using hydroxypropyl-β-cyclodextrin (HPCD) and 2-O-methyl-β-cyclodextrin by spray drying, and the findings showed that they would have a more excellent solubility and dissolution rate than the pure drug [154]. The practically insoluble prostaglandin latanoprost was made into cyclodextrin complexes for ocular administration. Among all the cyclodextrin complexes, the latanoprost propylamino-β-CD formulation demonstrated the highest solubility and least irritative effects compared to the commercial latanoprost formulation used as a reference [155]. The solubility and effectiveness of three vitamins—cholecalciferol, ascorbic acid, and α-tocopherol—were increased by formulating with various cyclodextrins [156]. Nateglinide inclusion complexes with sulfobutyl ether β-CD were created, and they displayed higher aqueous solubility than the free drug [157].
3.2.2. Improvement of bioavailability
A drug's oral bioavailability is primarily influenced by the gastrointestinal tract's permeability and solubility. The formulation's oral bioavailability is improved by higher solubility in gastrointestinal fluids and excellent penetration across the gastrointestinal membrane. Cyclodextrin complexes can improve the API's solubility and bioavailability. Glimepiride cyclodextrin complexes were created to show the improvement of oral bioavailability and therapeutic effectiveness [158]. In vivo, tests on six healthy individuals revealed that the inclusion complexes of sulfamethoxazole with β- CD had a greater bioavailability than pure [159]. In-vivo tests showed that the orally disintegrating tablets of eslicarbazepine acetate with cyclodextrin inclusion complex had a two times greater bioavailability than commercial formulations [160].
3.2.3. Increased resistance to hydrolysis and heat degradation
When developing pharmaceutical formulations, stability is the primary factor that researchers should consider. Different stability studies must be established to control the formulation's shelf life [161]. Cyclodextrin complexes shield the host compound, minimize the impact of heat, light, or oxygen on the formulation, and may even increase the stability of the drug entrapped in the complexes [162]. Due to the addition of cyclodextrin complexes, composite films made of polylactic acid-βCD inclusion complex (PLA–IC–CFs) exhibit improved thermal stability compared to pure PLA composite films [163].
3.2.4. Chemical stability
Chemical stability must be considered while developing new products to prevent deterioration in the presence of various excipients. The effects of cyclodextrin complexes on the chemical stability of pharmaceuticals were the subject of a highly instructive review study published in 2017 by Popielec and Loftsson [164]. In a different investigation, inclusion complexes of famotidine were created using carboxymethyl-β-cyclodextrin, which significantly enhanced the drug's chemical stability in an acidic environment [165]. Making inclusion complexes with cyclodextrin led to an increase in the chemical stability of Voriconazole [154].
3.3. Cyclodextrin complexes formation methods
Generating inclusion complexes is crucial since it affects the product's functionality and morphometric properties [166].
3.3.1. Kneading technique
The guest material was dissolved in the solvent using kneading; cyclodextrin slurry was added to the solution in a mortar and pestle. The complexes were created in solid form and then vacuum-dried. Because the guest molecule was trapped inside the cyclodextrin, this approach produced cyclodextrin complexes with altered physicochemical properties, but it is effective for bulk manufacturing [167,168]. This technique created inclusion complexes of ibuprofen with cyclodextrins, and the solubility and bioavailability were significantly increased. The encapsulation of ibuprofen [169], Omega-3 fatty acids in thymol essential oil [170], and European anchovy (Engraulis encrasicolus L.) oil [171] have been complexed with cyclodextrins by kneading technique.
3.3.2. Supercritical carbon dioxide method
To carry out the supercritical carbon dioxide procedure, the required quantity of cyclodextrins and the guest compounds are put into a thermostatically regulated autoclave and pressed with carbon dioxide at a certain pressure and temperature. After then, the pressure drops rapidly, causing the carbon dioxide to evaporate, and the inclusion complex is separated. According to Banchero (2021), carbon dioxide is the most often utilized solvent because of its low critical point and low toxicity, among other characteristics. Supercritical fluids are often used in various industrial processes and scientific fields. Supercritical carbon dioxide, according to Banchero (2021), can be a substitute for conventional complexation methods like kneading or co-precipitation because they can have drawbacks in terms of process time, the presence of a leftover organic solvent, or encapsulation effectiveness. However, compared to other techniques employing water, the supercritical carbon dioxide process has the benefit of being scalable for commercial use, according to Wadhwa et al. (2017). Banchero (2021) further noted that the ability to create a precise separation between the processed products and the supercritical solvent makes supercritical fluid technology unique [166,172].
3.3.3. Co-precipitation technique
Hydrophobic drugs can form cyclodextrin complexes with one another through co-precipitation. Host molecules were dissolved in an aqueous phase, whereas hydrophobic drugs or guest molecules were dissolved in an organic phase. The organic solvent was dissolved in the aqueous medium solution with the right agitation. Complexes were washed with organic solvent once the solution had been cooled [[173], [174], [175]]. Drugs, including oxaprozin [176] and transanethole, a main component in anise and fennel essential oils [177], were encapsulated using the co-precipitation approach. Using two distinct techniques, such as co-precipitation and freeze-drying processes, the effectiveness of inclusion complexes of fenbufen and ibuprofen with β-cyclodextrin was compared [178].
3.3.4. Technique of solvent evaporation
This procedure involved separating the guest and host molecules into the soluble solvents and combining these solutions to produce molecular dispersion. The solid powdered inclusion complex was then collected after the solvent evaporated under a vacuum at 45 °C and the dry mass passed through a sieve. This method is straightforward, affordable, and applied in industrial and laboratory settings. It is regarded as a highly effective alternative to the spray drying process [179]. Another research found that cyclodextrin complexes of norfloxacin prepared using solvent evaporation had a more excellent solubility and dissolution rate than those prepared using physical trituration and kneading methods [180].
3.3.5. Lyophilization or freeze-drying
This method, which may have also been employed in large-scale manufacturing, is the most suited method for forming cyclodextrin complexes of heat-sensitive and water-soluble drugs [181]. In this procedure, the medication and polymer were dissolved in a suitable solvent while properly stirring, and the solution was then freeze-dried. Under vacuum, the solvent was evaporated, and cyclodextrin inclusion complexes of high quality were produced [182]. Many essential oils, including cinnamon, clove [183], estragole [184], and black pepper oil [185], were encapsulated by using cyclodextrin.
4. Different cyclodextrin carriers for currently available antibiotics
Antibiotic resistance develops when antibiotics are present at the infected site at sub-inhibitory concentrations because of poor solubility, bioavailability, penetration, or instability. By incorporating all or a portion of the drug molecule into their hydrophobic cavity and functioning as drug carriers, CDs improve lipophilic drugs' water solubility, stability, and bioavailability [186]. By directly interacting with mucosal membranes, solubilizing membrane lipids through inclusion complexation, and affecting membrane integrity, cyclodextrin also aids in enhancing the permeability and absorption of drugs at high dosages. Because of their cyclic three-dimensional structure, CDs are strong hosts that may construct drug-delivery systems with hydrophobic drugs of the correct sizes by forming inclusion complexes in their hydrophobic cavities [187,188]. The introduction of substituents at the narrow side of CDs can lead to three different effects: (i) cavity enlargement to accommodate larger guests; (ii) positively charged derivatives (terminal amino groups) that may be capable of traversing cell membranes to deliver their cargo [189]; and (iii) negatively charged derivatives (terminal carboxyl groups) that serve as hosts for appropriately sized cationic guests, such as sugammadex [190]. The effectiveness, solubility, and bioavailability of conventional antibiotics or antibacterial agents can be significantly increased by inclusion complexes generated by CDs (Table 1).
4.1. Cationic cyclodextrin drug carrier system
Recent reports on positively charged CDs have revealed new, intriguing, and pertinent properties that highlight their great potential as (i) bacterial membrane disruptors [192], (ii) inhibitors of bacterial toxicity due to their capacity to block trans-membrane pores found in the cell walls of deadly bacteria like Bacillus anthracis [191], (iii) potentiators of MET (methicillin) against MRSA in vitro [193], (iv) mimics of antimicrobial peptides [194], and (v) as DNA delivery agents [195]. The following chain of reactions occurs when cationic compounds are introduced to microorganisms: (i) adsorption and penetration of the agent into the cell wall; (ii) reaction with the cytoplasmic membrane (protein or lipid), which results in membrane disorganization; (iii) leakage of cytoplasmic low-molecular-weight content; (iv) protein and nucleic acid degradation; and (v) wall lysis brought on by autolytic enzymes [196,197]. In addition to other damaging impacts on the bacterial cell, the cytoplasmic membrane in bacteria would lose its structural organization and integrity, resulting in K+ leakage [198].
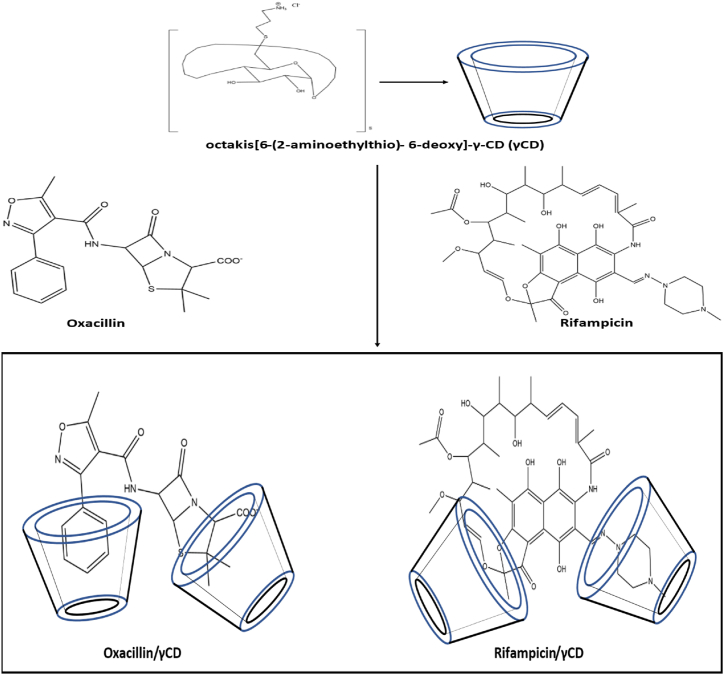
The key role of the positive charge in nanocarriers for biofilm penetration has been confirmed by the observation that in Staphylococcus epidermidis biofilms, positively charged cyclodextrin polymers are more effectively retained and pierced than neutral or negatively charged cyclodextrin polymers of the same molecular weight (∼50 kD) [199]. The supramolecular inclusion complexes of several positively charged γ-cyclodextrin (γ-CD) derivatives with common penicillins highlighted the sufficiently potent complexation of octakis[6-(2-aminoethylthio)- 6-deoxy]-γ-CD (γCD) with oxacillin (Fig. 5). When exposed to a particular, extremely active oxa-1 β lactamase in vitro, oxacillin was shielded from enzymatic breakdown via inclusion complexation. Oxacillin/γCD moderately decreases the S. epidermidis biofilm viability compared to non-complexed oxacillin. Octakis(6-(2-aminoethylthio)-6-deoxy)-γ-cyclodextrin (γ-CD) complexed with rifampicin to form γCD/rifampicin. γCD/rifampicin treatment shows a significant decrease in S. epidermidis biofilm viability compared to rifampicin alone [200].
Fig. 5.
Octakis[6-(2-aminoethylthio)- 6-deoxy]-γ-CD (γCD) inclusion complexation with Oxacillin and Rifampicin [200].
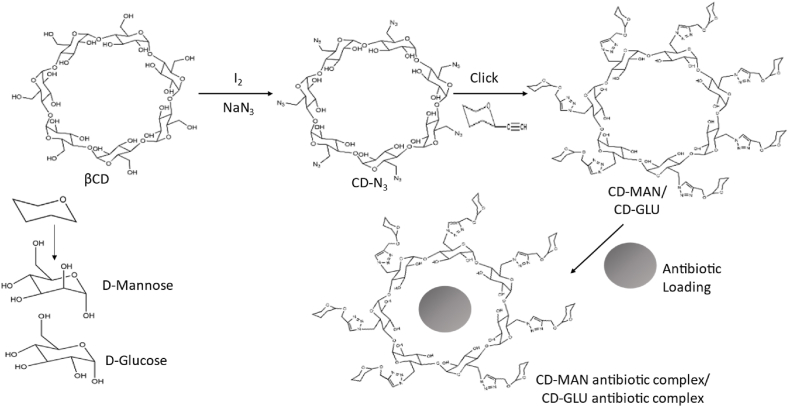
4.2. Sugar-grafted cyclodextrin drug carrier system
Azide-alkyne click reaction is used to create the antibiotic carriers CD-MAN and CD-GLU from ß-cyclodextrin grafted with d-mannose and d-glucose, respectively (Fig. 6). Additionally, d-mannose is a carbon source for many bacteria with active uptake systems and transporters (porins) on the cell membrane, just like d-glucose does. Bacteria will therefore be attracted to the sugar on the CD carriers via their sugar-sensing capabilities. CD-MAN and CD-GLU were created and served as nanocarriers for hydrophobic antibiotics (erythromycin, rifampicin, and ciprofloxacin). The minimum inhibitory concentration of the loaded antibiotics into sugar-grafted nanocarriers against Gram-negative Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii strains, as well as several Gram-positive Staphylococcus aureus strains, including the methicillin-resistant strains (MRSA), were lowered by a factor ranging from 3 to >100 when compared to free antibiotics. Additionally, the CD-MAN-antibiotic combination can extend the stability of the loaded antibiotic and its capacity to suppress bacterial growth [201]. This prevents S. aureus and E. coli from developing antibiotic resistance.
Fig. 6.
The preparation processes for the CD-MAN/CD-GLU and CD-MAN-antibiotic/CD-GLU-antibiotic complex are illustrated schematically [201].
4.3. Stimuli-responsive cyclodextrin drug carrier system
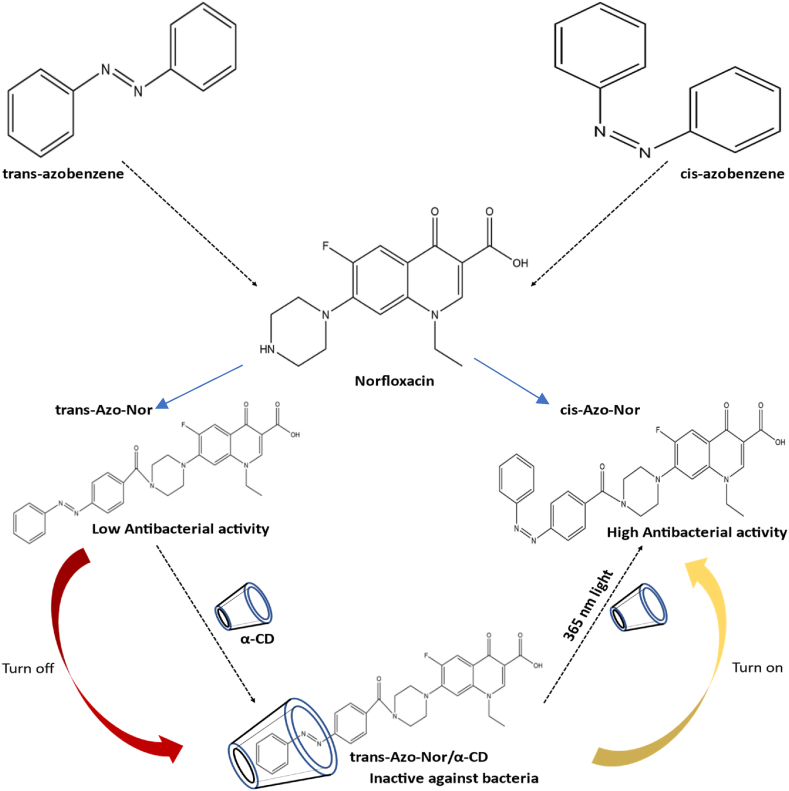
An optically controlled antibacterial platform was created using the light-responsive azobenzene/CD inclusion complex (Fig. 7). Azo-Nor, an antibiotic that responds to light, was made possible by conjugating norfloxacin with azobenzene. When exposed to 365 nm light, trans-Azo-Nor showed no biological activity, whereas the cis conformation showed strong antibacterial activity. The antibacterial property was turned off when trans-Azo-Nor was trapped by α-CD. The antibacterial activity was activated when the material was exposed to 365 nm light. This “on-off” technique offers a simple method to control antibacterial activity without adding a competitive guest to combat antibiotic-induced bacterial resistance [202]. A cyclodextrin inclusion complex is an excellent example of an enzyme-triggered release mechanism because, in the presence of the small intestine-found enzymes α-amylase and α-glucosidase, the cyclodextrins degrade into glucose [[203], [204], [205]].
Fig. 7.
Photo-responsive activation and inactivation approach to regulate antibacterial activity: the antibacterial effect was disabled as soon as trans - Azo-Nor was trapped by α-CD. The antibacterial activity was activated when the trans - Azo-Nor/α-CD complex was exposed to 365 nm light.
Additionally, current research demonstrates that cyclodextrins are quickly broken down in the blood and eliminated by urination. Compared to Fungizone (a commercially available amphotericin B formulation), the encapsulation of amphotericin B in hydrophobized cyclodextrin, specifically cholesterol-modified cyclodextrin nanoparticles, produced high plasma concentrations of amphotericin B with no hemolysis, which is frequently caused by amphotericin B [206]. It raises the possibility of adopting this delivery system to administer amphotericin B intravenously.
Nanoparticles (NPs) have been employed to encapsulate MXF (Moxifloxacin) to minimize the dosing frequency, the 4-(hydroxymethyl) phenylboronic acid pinacol ester (HPAP)-modified cyclodextrin (Oxi-αCD) was utilized as a carrier to encapsulate MXF to generate core-shell NPs (MXF/Oxi-αCD NPs) by a nanoprecipitation/self-assembly process. The surface of MXF/Oxi-αCD NPs was coated with DSPE-PEG-FA to form MXF/FA-Oxi-αCD. MXF/FA-Oxi-αCD NPs can quickly release MXF in the presence of reactive oxygen species (ROS) such as hydroxyl radicals (∙OH), hydrogen peroxide (H2O2), superoxide (O2−), and singlet oxygen (1O2). MXF-loaded Oxi-αCD NPs were found to have higher antibacterial activity than MXF against P. aeruginosa in an in vitro antibacterial assay. An important point to notice is that MXF/FA-Oxi-αCD NP's active targeting, mucus penetration, and controlled drug release abilities boosted the drug concentration in pathological tissues and improved the antibacterial activity in vivo [207].
4.4. Extended-release cyclodextrin drug carrier system
One of the studies being conducted to reduce the formation of antibiotic resistance is extended-release delivery systems. The extended release also goes by’ sustained release,’ ‘prolonged release,’ and ‘slow release.’ A significant amount of antibiotic exposure is required to ensure the eradication of the microorganism because antiinfection therapies sometimes include a lengthy course of medication. Frequent delivery can be reduced by maintaining a steady-state plasma drug concentration for an extended period [208]. The prolonged antibiotic-release properties of β-cyclodextrins (βCD) are well-known. Antimicrobial medication can be captured by CD and released slowly for the required times. The CD-grafted PP (polypropylene) mesh materials include the antibiotic levofloxacin HCL (LVFX) to prevent mesh infection. Levofloxacin HCL-captured CD-grafted PP meshes exhibit good antibacterial properties for ten days. The modified PP mesh devices showed effective antibacterial traits and long-lasting drug-release kinetics. Levofloxacin was discovered to release in bursts during the first several hours after being loaded onto CD-grafted PP meshes but afterward showed sustained drug release [209]. Curdlan-CD nanoparticles have the potential to be used as nanocarriers for the targeted delivery of drugs to macrophages, enhancing TB treatment and preventing drug resistance from forming due to the use of large dosages of anti-tuberculosis medications. The synthesis of curdlan-based nanoparticles containing the anti-tuberculosis drugs RIF (rifampicin) and LVX (Levofloxacin) through conjugation with cyclodextrin for macrophage targeting was simple. By using the solvent evaporation approach and the freeze-drying method, β cyclodextrin (βCD) inclusion complexes containing RIF and LVX were created, yielding inclusion complexes (βCD-RIF) and inclusion complexes (βCD-LVX).
In contrast to burst releases, the dual-drug loaded nanoparticles showed a simultaneous sustained release of both medicines over an extended period. The medications on the CD remained active after being conjugated with curdlan nanoparticles and showed similar MIC values to free drugs [210]. Ciprofloxacin hydrochloride (CipHCl) produces an antibacterial paper when added to the cavity of grafted CD on cellulose fibers. These CipHCl-loaded grafted fibers showed slow, sustained, and affinity-based antibiotic release, which significantly extended their antibacterial efficacy against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) [211].
5. Photodynamic therapy
With appropriate wavelengths of visible light, photodynamic therapy (PDT) kills target pathogens by producing reactive oxygen species (ROS), such as singlet oxygen and free radicals produced by photosensitization in the presence of oxygen. PDT has been effective in treating cancer diseases and bacterial infections since the turn of the 20th century [231]. PDT is a promising method for treating viral, fungal, and parasite infections and gram-positive and gram-negative bacterial infections that have developed multi-drug resistance [232,233]. PDT has been investigated as an antibacterial treatment for body infections and a complement in surgeries [234] because of its strong antibacterial efficacy and lack of drug resistance. In this therapy method, a photosensitizer is administered. Photosensitizers mostly come from porphyrins, chlorines, phthalocyanines, Rose Bengal, phenothiazines, and acridines. The tetrapyrrole nucleus serves as the basis for the structures of porphyrins, chlorines, and phthalocyanines, but other molecules have various molecular forms [235]. The recommended course of treatment includes the (systemic) administration of a light-sensitive but safe drug (photosensitizer, PS), followed by the uptake of this compound and more or less selective accumulation/retention in the target cells or tissue, and the subsequent irradiation of the photosensitizer with the light of the recommended wavelength. Reactive oxygen species (ROS) are created upon quanta absorption and oxidize intracellular molecules, leading to cell death [[236], [237], [238], [239]].
PDT offers several benefits compared to other traditional therapies like radiation and antibiotic therapy. First, photodynamic therapy makes building treatment resistance more difficult for the pathogen. The ROS produced during photodynamic therapy can kill the pathogen through membrane and DNA damage. In the former, the ROS encourages non-specific activities like cellular content leakage or enzyme disruption, which hasten the pathogen's demise. Another benefit of PDT is its fewer side effects because it targets the area exposed to radiation. The effectiveness of PDT against bacteria can also be increased by combining it with other antibacterial therapies such as photothermal therapy, radiation, and conventional antibiotic treatments [234]. However, some PDT has specific difficulties. For instance, ROS typically have brief lifetimes (40 ns or less) and limited diffusion ranges (approximately 10 nm) [240,241]. Sometimes the photosensitizer's ROS contain excessive non-specific reactivity, which reduces the effectiveness of their antibacterial action. Second, the light used to activate the photosensitizer frequently isn't appropriate for penetrating deeply into biological tissues. Particularly, light with a wavelength over 700 nm penetrates deeper into tissues, while most widely used photosensitizers absorb best at lower wavelengths. Infected tissues often have limited oxygen concentrations, so oxygen consumption can result in incredibly low concentrations and a reduction in ROS formation [234].
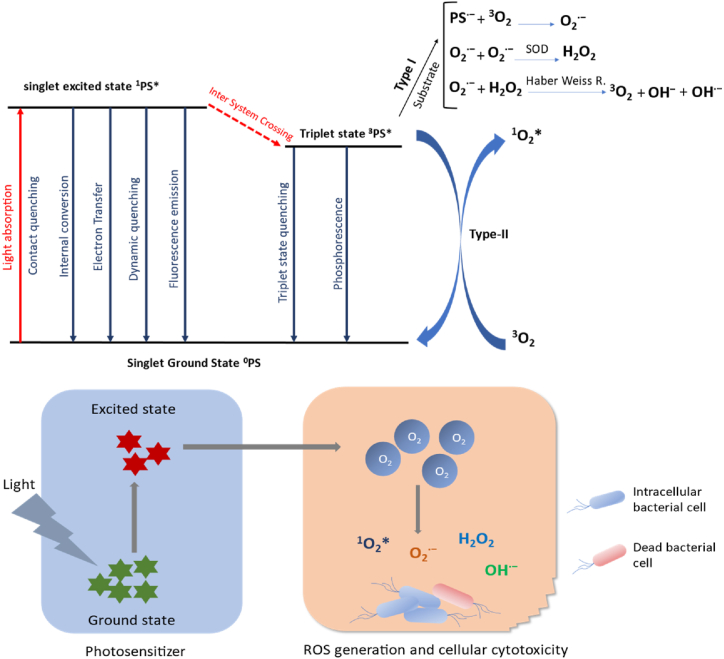
5.1. Mechanism of antimicrobial photodynamic therapy
To excite an electron from its ground state of 0PS to its singlet excited state of 1PS*, a photosensitizer must first absorb a particular wavelength of light. The ground state of the photosensitizer can be formed through several processes, including contact quenching, internal conversion, electron transfer, dynamic quenching, and fluorescence emission, from the excited singlet state of the photosensitizer. These mechanisms decrease the effectiveness of ROS generation. The photosensitizer's excited singlet state can undergo an inter-system crossing (ISC) process to create the equivalent triplet state 3 PS*. Finally, ROS generation occurs by reactions of the triplet state of the photosensitizer [234]. Since this triplet state facilitates the production of ROS via Type-I and Type-II photo-processes, the effectiveness of the ISC process and the longevity of 3PS* are two essential components of a successful PS (Fig. 8) [[242], [243], [244]].
Fig. 8.
The formation of reactive oxygen species (ROS) by a photosensitizer (PS) is depicted in a simplified Jablonski diagram after light absorption, intersystem crossover, and Type-I and –II processes. Finally, ROS cause dose-dependent cellular damage.
5.1.1. Type I mechanism
In the Type-I photo-process, 3PS* and a substrate (often a biomolecule) exchange electrons to create a radical anion PS∙−, which reacts with ground-state molecule oxygen (3O2) to generate a superoxide radical (O2∙−). In biological systems (lipid or proteins), superoxide radicals are not highly reactive, although they can react to form hydrogen peroxide (H2O2). Higher hydrogen peroxide concentrations can induce the highly reactive hydroxyl radical OH∙ ─ to develop when it reacts with superoxide anions. (Haber Weiss reaction in Fig. 10) [[242], [243], [244], [245], [246]].
Fig. 10.
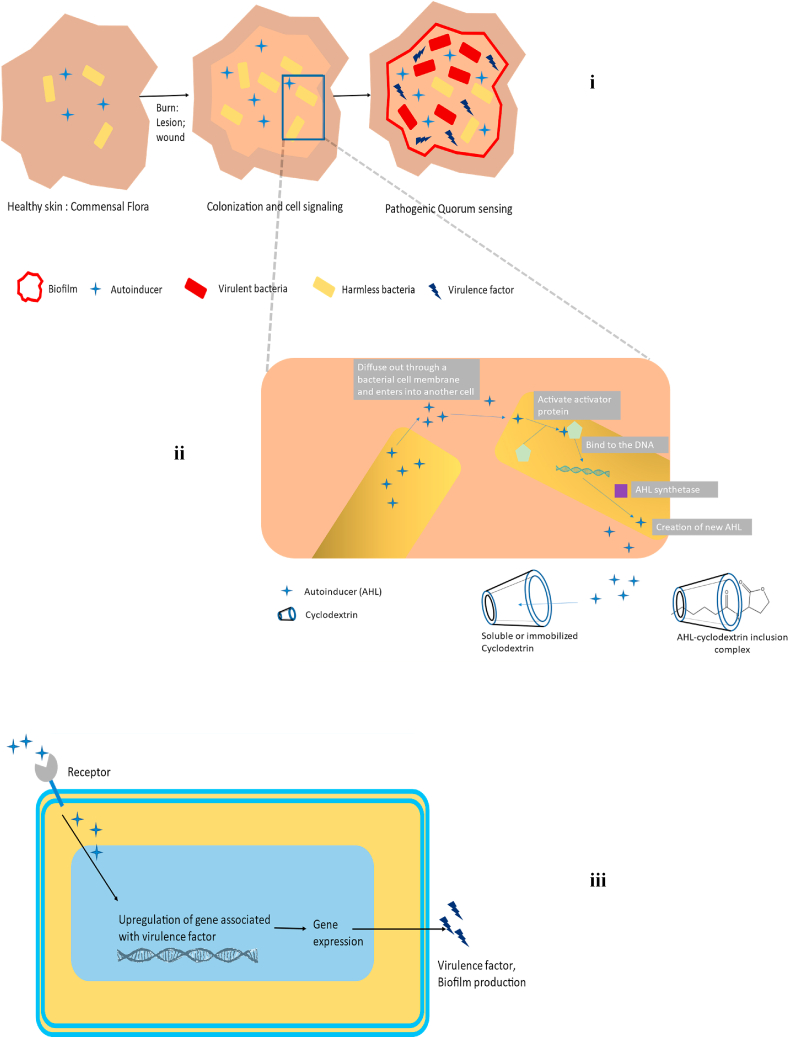
(i) Figure illustrates Quorum sensing in a tissue injury. Typically, the skin has a pathogenic-free natural and commensal flora (Left). Bacteria colonize the injured tissue when a wound or lesion forms (middle right). Bacteria create communication molecules when they expand (autoinducers). Bacteria may coordinate their behavior to emit virulence factors and create biofilms if the molecules are not broken down, which may reduce the effectiveness of antibiotic treatment (right). (ii) AHL is a substance produced by gram-negative bacteria that disperses from one bacterial cell to another, stimulating the activator protein in the other cell, which then connects to DNA and releases the enzyme known as AHL synthetase, catalyzing the production of even more AHL, leading to quorum sensing within the bacterial colony and assisting in colonizing them and forming biofilm. AHLs representing transported QS signals might be incorporated into the cyclodextrin's hydrophobic cavity. The sequential QS process is being blocked by a drop in the local AHL concentration. (iii) Mechanism of autoinducer for virulence factor synthesis: When autoinducers bind to their receptors, a signaling cascade is set off, upregulating the gene expression that produces virulence factors and biofilm.
5.1.2. Type II mechanism
Triplet-triplet energy transfer between 3PS* and 3O2 creates singlet molecular oxygen (1O2*), a fleeting and extremely cytotoxic molecule, in the Type-II mechanism (energetically, the transformation from 3O2 to 1O2* needs 94.3 kJ mol─1) [247]. The singlet oxygen can directly interact with nearby cellular molecules and generate more oxygen radicals [248]. The type-II photochemical reaction is the predominant process for most photosensitizers used in PDT [249].
Deleting the plasmid supercoiled fraction, both single- and double-stranded DNA break-in, which has been seen in both Gram-positive and Gram-negative species following PDT, occurs when bacteria are exposed to different photosensitizers and light [250,251].
More damage can be done by certain photosensitizers that can more quickly intercalate into double-stranded DNA [252]. DNA damage, however, could not be the main factor in bacterial cell death, considering that several DNA repair mechanisms may be able to reverse the damage [253]. The cytoplasmic membrane is, therefore, another important ROS damage site during PDT, allowing for the loss of cellular contents or the inactivation of membrane transport systems and enzymes. A multilamellar structure developing near the septum of dividing cells, changes in cytoplasmic membrane proteins, disruptions in cell-wall synthesis, and potassium ion loss from the cells have all been observed [[254], [255], [256]]. ROS, which has high reactivity and powerful oxidation abilities, quickly oxidizes the bacteria's lipids [257]. PDT was utilized as an antibacterial technique to prevent the growth of biofilms produced by various microorganisms by successfully destroying the biofilms [258]. Most PDT antibiofilm research has focused on dental plaque-related illnesses and chronic wound infections [259]. PDT has also been employed in several trials to treat chronic rhinosinusitis and ventilator-associated pneumonia biofilm-related infections [260,261].
5.2. Strengthening of the antimicrobial photosensitizing activity with cyclodextrin nanocarrier
An effective photosensitizer must have a large quantum yield to produce the long-lived triplet state, which can then generate cytotoxic substances such as reactive oxygen species (ROS) and, in particular, singlet oxygen (1O2*) or radical intermediates [262]. In an aqueous solution, organic photosensitizer has a significant tendency to agglomerate, effectively contacting singlet states.
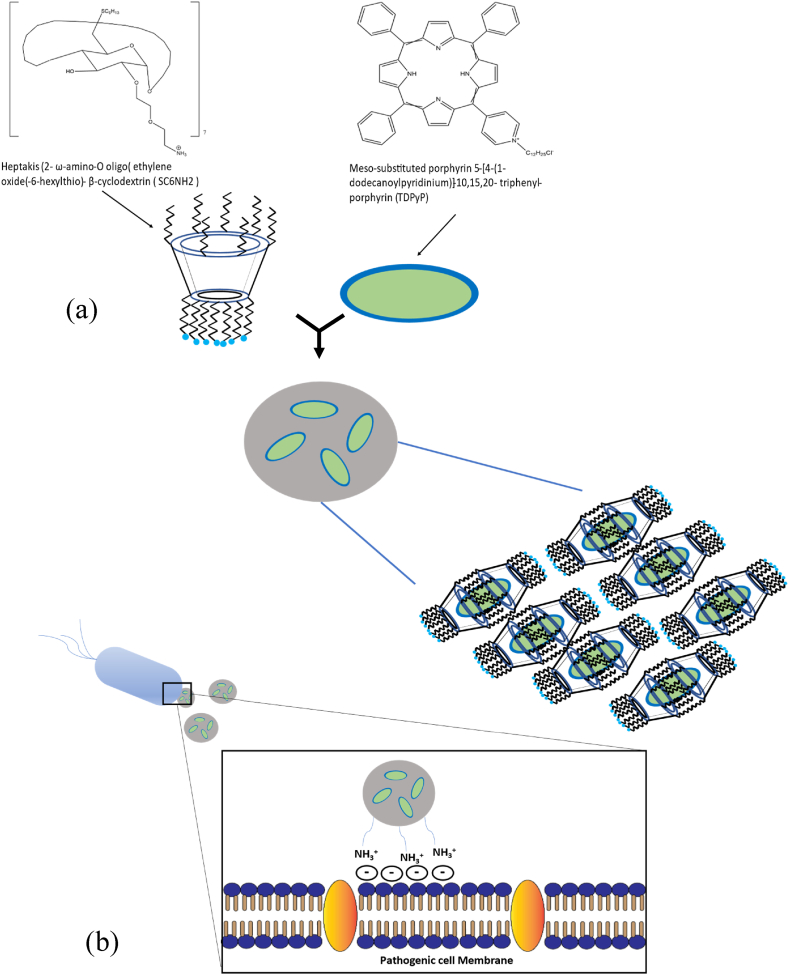
It is usual practice to include sterically bulky groups to lessen the aggregation of the photosensitizers. Additionally, cyclodextrin, a macrocyclic host that is water soluble, has been used for this [263,264]. Phenothiazines, porphyrins, phthalocyanines, and fullerenes are the photosensitizing agents with the highest antimicrobial efficacy [[265], [266], [267], [268], [269], [270]]. With the diversity of negative charges on the surface of bacterial cells, these dyes can swiftly form an electrostatic interaction [271]. Surprisingly, a strong photo-bactericidal effect can also be seen when the photosensitizer is given through a positively charged carrier, as was recently shown for the meso-substituted porphyrin 5-[4-(1-dodecanoylpyridinium)]-10,15,20- triphenyl-porphyrin (TDPyP) inserted into heptakis (2-ω-amino-O oligo (ethylene oxide(-6-hexylthio)- β-cyclodextrin (SC6NH2) (Fig. 9 (a) & (b)). Through host-guest interactions, TDPyP can be incorporated into the CD's cavity on the surfaces of nanoparticles. Due to less quenching of its singlet excited state, TDPyP as a guest in the CD cavity was shown to have a greater quantum yield (ΦΔ = 0.90) for ROS production. PDT utilizing this supramolecular structure significantly suppresses S. aureus and E. coli due to enhanced ROS production [234,262]. Similar methods were used to develop an adamantyl-modified Si (IV) phthalocyanine system serving as the guest photosensitizer and β-CD vesicles serving as the host photosensitizer carrier. By being incorporated into β-CD vesicles, the PDT acquires more biocompatibility and experiences less photosensitizer aggregation, improving its antibacterial efficacy [264].
Fig. 9.
(a) Meso-substituted porphyrin 5-[4-(1-dodecanoylpyridinium)]-10,15,20- triphenyl-porphyrin (TDPyP) inserted into positively charged carrier, heptakis (2- ω-amino-O oligo(ethylene oxide(-6-hexylthio)- β-cyclodextrin (SC6NH2) and Porphyrin entanglement in CD nanoassemblies is shown schematically (TDPyP/SC6NH2 system). (b) An illustration of how negatively charged pathogenic cell membranes interact with poly-cationic substances.
6. Interference in bacterial quorum sensing
A study on the bioluminescence phenomena observed in Vibrio fischeri, a marine bacterium linked to Hawaiian squid, initiated in the early 1970s is where sociomicrobiology was defined initially [272]. When the bacteria were cultured in shaking flasks, it was observed that the expression of the luminescence gene (lux) was very low during early exponential growth but that it quickly increased throughout the late exponential and early stationary phases. Cell-free fluid extracts from stationary phase cultures can be added to exponential phase cultures to activate the luminescence gene. These findings suggested that Vibrio fischeri possesses an environmental sensing system to track the density of its population, and a signaling compound known as “autoinducer,” subsequently identified as 3-oxohexanoyl-Homoserine lactone, triggers lux expression in high cell density cultures [273]. Cell-cell communication is crucial for the pathogenicity and growth of biofilms. Small diffusible molecules known as autoinducers (AIs) play a major role in this communication, sometimes called quorum sensing [274]. Quorum sensing (QS) is a biochemical process that bacteria use to communicate and coordinate their collective activity in response to cell density and the environment [275] (Fig. 10).
6.1. In gram-negative bacteria
Most Gram-negative species examined, including P. aueroginsa and V. fischeri, use N-acyl homoserine lactones (AHLs) as signaling molecules in their quorum-sensing systems. The inner and outer membranes allow the AHLs to disperse (Fig. 10) easily. With the cell population, the AHL concentration rises. AHLs diffuse back to the cell and bind to transcriptional protein receptors to trigger the transcription when their concentration reaches a high enough level [276]. Autoinducer 2 (AI-2) is a quorum-sensing signaling molecule in several Gram-negative organisms, such as V. cholera [277].
6.2. In gram-positive bacteria
Numerous Gram-positive bacteria use quorum sensing to communicate. Different types of signaling molecules are found in both Gram-negative and Gram-positive bacteria. AHL production by Gram-positive bacteria has never been previously documented. Gram-positive bacteria like S. aureus and other Streptococcus species use small post-translationally processed peptide signal molecules. They are known as autoinducing peptides (AIPs) [278].
Targeting the autoinducer molecules has shown to be a successful tactic for preventing the growth of biofilms and their pathogenicity [274]. More and more evidence suggests that bacteria control their virulence genes through the quorum-sensing mechanism (Fig. 10). Quorum sensing is a strategic tool that helps bacteria carry out their infection processes and endure in the host. Until a specific threshold population density is reached, the physiological advantage permits the bacterial cells to multiply without overt pathogenic behavior (Fig. 10). Therefore, a coordinated immune response by the host only occurs when the bacterial population is substantial, increasing the possibility that any defenses will be effectively overcome and improving the chances of the bacteria surviving [279].
Additionally, it has been demonstrated that quorum sensing affects the growth of biofilms. Because extracellular matrix-embedded biofilm cells are less sensitive to antibacterial agents than free-floating cells [[280], [281], [282]], biofilm is strongly linked to pathogenicity. As a result, biofilm infections are frequently recurring and challenging to treat. The primary infection in CF sufferers’ lungs is Pseudomonas aeruginosa. There, the bacteria are known to reside in a biofilm and generate a large number of quorum-sensing molecules [283].
Quorum quenching, or QQ, is the process of disrupting quorum signaling. Rather than killing bacteria, QQ has the potential to disarm their virulence, reducing the selection pressure put on pathogens and postponing the emergence of drug resistance to QQs [276]. As a result, this method is a promising one for anti-virulence treatment [284]. Quorum quenching is the term for interferences with QS (QQ). With the discovery of a QQ enzyme able to destroy AHL signals from Erwinia carotovora, QQ was initially recognized as a naturally occurring phenomenon in 2000 [285]. The QS signal was interfered with due to the enzymatic hydrolysis of AHL. There are several ways to stop bacterial communication: (i) using small molecules called quorum sensing inhibitors (QSIs) to prevent the production or perception of AIs [286], (ii) scavenging AIs with quorum quenching macromolecules such as cyclodextrins [287], or (iii) extracellularly hydrolyzing the AIs with QQ enzymes [288].
6.3. Enhance the efficacy of quorum-sensing inhibitors (QSIs) with cyclodextrin nanocarrier
Recent research indicates that flavonoids are potent quorum-sensing inhibitors with several important benefits in natural anti-virulence applications [289]. As a model flavonoid payload, quercetin has a variety of pharmacological effects, such as acting as an antioxidant [290], an anticancer agent [291], an antiviral agent [292], and a blood pressure-lowering agent in hypertension patients [293,294]. When quercetin is delivered using chitosan/SBECD-based nanoparticles, its solubility is increased, its bioavailability is controlled, and its anti-QS and antibacterial actions are enhanced [295]. The apparent water solubility of Triclosan (TCS) increases due to its complexation with modified β-CDs, and its antibacterial and anti-QS activities are significantly improved [296]. In the Chromobacterium violaceum model system, TCS complexed with HP-β-CD and CM-β-CD display anti-QS action and antibiofilm activity by interfering with cell-to-cell communication processes. To prevent biofilm growth on medical devices and encourage the release of antimicrobial drugs, TCS-CD complexes can be employed to create antimicrobial hydrogels or hydrophilic coatings [297].
6.4. AI scavenging through signal trapping or quorum quenching with cyclodextrin
Vibrio fischeri cells could not communicate with one another when exposed to silicon dioxide nanoparticles coated with β-cyclodextrin. The population density of V. fischeri regulates its bioluminescence, which may be seen via the quorum-sensing signaling molecule acyl-homoserine lactone. Aryl-homoserine lactone bonds to the β-cyclodextrin group on silicon dioxide nanoparticles, inhibiting its activity. The outcome was a decrease in V. fischeri's luminous output. Additionally, it was discovered that the luminescence genes luxA and luxR were downregulated [298]. The combination between a transcription factor and an AHL molecule becomes stable in cells. It starts the expression of a certain gene when the concentration of AHLs (N- Acylhomoserine Lactones), produced by each bacterial cell, rises and exceeds a threshold. In many human infections, the QS system frequently controls the production of virulence factors, the development of biofilms, etc [299]. Several techniques for managing the QS system have been documented, such as competitive QS suppression using synthetic antagonists and enzymatic quenching with acyl-homoserine lactonase [300]. AHL trapping on acceptor molecules is a straightforward and efficient way to maintain low levels of AHLs (Fig. 10). One of the opportunistic pathogens that regulate the formation of the red pigment 2-methyl-3-pentyl-6-methoxy prodigiosin through the AHL-mediated QS system is Serratia marcescens [301]. Prodigiosin synthesis decreased, and the inclusion of complex formation of AHL and cyclodextrins (CD) suggested that the QS signal AHL was probably trapped in the hydrophobic cavity of the CD [302]. High levels of biofilm-inhibitory action against S. aureus Mu50 biofilms generated in the absence or presence of P. aeruginosa were seen in studies using hydroxypropyl-β-cyclodextrin (HPβCD)-functionalized gauzes loaded with QSI hamamelitannin (HAM) and vancomycin (VAN). And again, HPβCD-functionalized gauzes scavenge AHLs, which affects QS signaling and, in turn, reduces P. aeruginosa's ability to build biofilms [303].
7. Future perspective
The article comprised an overview and discussion of 150 studies on the delivery of antibacterial agents using the CD-antibiotic inclusion complex and how CD can increase the efficacy of antibiotics, photodynamic therapy, and QSIs. The impact of CD complexation on antibacterial activity was discussed in two-thirds (100) of the articles. Although many articles focus on the potential improvement of the efficiency, solubility, and permeability of antibacterial agents to the resistant bacteria, there are comparatively few studies on increasing the permeability and intracellular delivery of antibacterial agents (antibiotics, photosensitizers), as it is primarily required for treating intracellular infection like Mycobacterium tuberculosis, Salmonella typhimurium, etc. Furthermore, there is little likelihood that the cavity will contain macrolides, aminoglycosides, or glycopeptides, a group of substances with big molecules. The assertions expressed in several articles conflict; for example, while some authors claim that inclusion happens, others disagree. Therefore, more investigation is required to ascertain the possible geometry of the combination and the effectiveness of CD's intracellular delivery of antibacterial agent.
8. Conclusion
The macrocyclic host cyclodextrin, introduced to us through supramolecular chemistry, boosts the solubility, bioavailability, stability, and efficacy of antibacterial agents such as antibiotics, essential oils, and QSIs. Again, It also expands the effectiveness of photodynamic therapy. CD inclusion complexes have various applications, such as drug delivery, antimicrobial coatings on materials (e.g., biomedical devices and implants), and antimicrobial dressings that assist in preventing wound infections. Moreover, Cyclodextrin-antibiotic complexes display significant antibacterial action against MRSA and other resistant bacterial strains. It reduces the antibiotic's minimum inhibitory concentration (MIC) for a specific pathogenic strain. Cyclodextrin also exhibits anti-biofilm action by trapping the autoinducer (AHL). Although more research is needed to determine the mechanism of action of these antimicrobial CD inclusion complexes and derivatives, activity is frequently connected to their interaction with bacterial cell membranes.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made available on request.
Funding statement
No funding was received for this work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gao P., Nie X., Zou M., Shi Y., Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011 Sep;64(9):625–634. doi: 10.1038/ja.2011.58. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C., Cars O. Antibiotic resistance—problems, progress, and prospects. N. Engl. J. Med. 2014 Nov 6;371(19):1761–1763. doi: 10.1056/NEJMp1408040. [DOI] [PubMed] [Google Scholar]
- 3.Walsh C. Where will new antibiotics come from? Nat. Rev. Microbiol. 2003 Oct;1(1):65–70. doi: 10.1038/nrmicro727. [DOI] [PubMed] [Google Scholar]
- 4.Neu H.C. The crisis in antibiotic resistance. Science. 1992 Aug 21;257(5073):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 5.Al-Anazi K.A., Al-Jasser A.M. Infections caused by Acinetobacter baumannii in recipients of hematopoietic stem cell transplantation. Front. Oncol. 2014 Jul 14;4:186. doi: 10.3389/fonc.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller L.G., Diep B.A. Colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008 Mar 1;46(5):752–760. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- 7.Woodford N., Wareham D.W., Guerra B., Teale C. Carbapenemase-producing Enterobacteriaceae and non-Enterobacteriaceae from animals and the environment: an emerging public health risk of our own making? J. Antimicrob. Chemother. 2014 Feb 1;69(2):287–291. doi: 10.1093/jac/dkt392. [DOI] [PubMed] [Google Scholar]
- 8.Yeh Y.C., Huang T.H., Yang S.C., Chen C.C., Fang J.Y. Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front. Chem. 2020 Apr 24;8:286. doi: 10.3389/fchem.2020.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao P., Nie X., Zou M., Shi Y., Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011 Sep;64(9):625–634. doi: 10.1038/ja.2011.58. [DOI] [PubMed] [Google Scholar]
- 10.Jukes T.H. Antibiotics in animal feeds and animal production. Bioscience. 1972 Sep 1;22(9):526–534. [Google Scholar]
- 11.Giedraitienė A., Vitkauskienė A., Naginienė R., Pavilonis A. Antibiotic resistance mechanisms of clinically important bacteria. Medicina. 2011 Mar;47(3):19. [PubMed] [Google Scholar]
- 12.Alipiah N.M., Shamsudin M.N., Yusoff F.M., Arshad A. Membrane biosynthesis gene disruption in methicillin-resistant Staphylococcus aureus (MRSA) as potential mechanism for reducing antibiotic resistance. Indian J. Microbiol. 2015 Mar;55(1):41–49. [Google Scholar]
- 13.Wright G.D. Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005 Jul 29;57(10):1451–1470. doi: 10.1016/j.addr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Lebeaux D., Chauhan A., Rendueles O., Beloin C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens. 2013 May 13;2(2):288–356. doi: 10.3390/pathogens2020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010 Apr 1;35(4):322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Stacy A., McNally L., Darch S.E., Brown S.P., Whiteley M. The biogeography of polymicrobial infection. Nat. Rev. Microbiol. 2016 Feb;14(2):93–105. doi: 10.1038/nrmicro.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDougald D., Rice S.A., Barraud N., Steinberg P.D., Kjelleberg S. Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012 Jan;10(1):39–50. doi: 10.1038/nrmicro2695. [DOI] [PubMed] [Google Scholar]
- 18.Høiby N., Ciofu O., Johansen H.K., Song Z.J., Moser C., Jensen P.Ø., Molin S., Givskov M., Tolker‐Nielsen T., Bjarnsholt T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011 Apr;3(2):55–65. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999 May 21;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 20.Stewart P.S., Costerton J.W. Antibiotic resistance of bacteria in biofilms. Lancet. 2001 Jul 14;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 21.Stoitsova S.R., Paunova-Krasteva T.S., Borisova D.B. Modulation of biofilm growth by sub-inhibitory amounts of antibacterial substances. Microb. Biofilms-Imp. Appl. 2016;1:441–459. [Google Scholar]
- 22.Bernardi S., Anderson A., Macchiarelli G., Hellwig E., Cieplik F., Vach K., Al-Ahmad A. Subinhibitory antibiotic concentrations enhance biofilm formation of clinical Enterococcus faecalis isolates. Antibiotics. 2021 Jul 19;10(7):874. doi: 10.3390/antibiotics10070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizzolato-Cezar L.R., Okuda-Shinagawa N.M., Machini M.T. Combinatory therapy antimicrobial peptide-antibiotic to minimize the ongoing rise of resistance. Front. Microbiol. 2019 Aug 9;10:1703. doi: 10.3389/fmicb.2019.01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L.S., Gupta A., Rotello V.M. Nanomaterials for the treatment of bacterial biofilms. ACS Infect. Dis. 2016 Jan 8;2(1):3–4. doi: 10.1021/acsinfecdis.5b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baptista P.V., McCusker M.P., Carvalho A., Ferreira D.A., Mohan N.M., Martins M., Fernandes A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018 Jul 2;9:1441. doi: 10.3389/fmicb.2018.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zazo H., Colino C.I., Lanao J.M. Current applications of nanoparticles in infectious diseases. J. Contr. Release. 2016 Feb 28;224:86–102. doi: 10.1016/j.jconrel.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Walvekar P., Gannimani R., Govender T. Combination drug therapy via nanocarriers against infectious diseases. Eur. J. Pharmaceut. Sci. 2019 Jan 15;127:121–141. doi: 10.1016/j.ejps.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Yeh Y.C., Huang T.H., Yang S.C., Chen C.C., Fang J.Y. Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances. Front. Chem. 2020 Apr 24;8:286. doi: 10.3389/fchem.2020.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X.Y., Gao L., Mosel S., Ehlers M., Zellermann E., Jiang H., Knauer S.K., Wang L., Schmuck C. From supramolecular vesicles to micelles: controllable construction of tumor‐targeting nanocarriers based on host–guest interaction between a pillar [5] arene‐based prodrug and a RGD‐sulfonate guest. Small. 2018 Dec;14(52) doi: 10.1002/smll.201803952. [DOI] [PubMed] [Google Scholar]
- 30.Song N., Yang Y.W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem. Soc. Rev. 2015;44(11):3474–3504. doi: 10.1039/c5cs00243e. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z., Yao P. Versatile injectable supramolecular hydrogels containing drug loaded micelles for delivery of various drugs. Polym. Chem. 2014;5(3):1072–1081. [Google Scholar]
- 32.Cho E., Jeong D., Paik H.D., Jung S. Solubility enhancement of flavonols in the inclusion complex with thioether-bridged dimeric β-cyclodextrins. Bull. Kor. Chem. Soc. 2014;35(8):2487–2493. [Google Scholar]
- 33.Paczkowska M., Szymanowska-Powałowska D., Mizera M., Siąkowska D., Błaszczak W., Piotrowska-Kempisty H., Cielecka-Piontek J. Cyclodextrins as multifunctional excipients: influence of inclusion into β-cyclodextrin on physicochemical and biological properties of tebipenem pivoxil. PLoS One. 2019 Jan 25;14(1) doi: 10.1371/journal.pone.0210694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uekama K, Hirayama F, Irie T. Cyclodextrin Drug Carrier Systems. [DOI] [PubMed]
- 35.Szejtli J. Past, present and futute of cyclodextrin research. Pure Appl. Chem. 2004 Jan 1;76(10):1825–1845. [Google Scholar]
- 36.Athanassiou G., Michaleas S., Lada‐Chitiroglou E., Tsitsa T., Antoniadou‐Vyza E. Antimicrobial activity of β‐lactam antibiotics against clinical pathogens after molecular inclusion in several cyclodextrins. A novel approach to bacterial resistance. J. Pharm. Pharmacol. 2003 Mar;55(3):291–300. doi: 10.1211/002235702649. [DOI] [PubMed] [Google Scholar]
- 37.Brancaccio D., Pizzo E., Cafaro V., Notomista E., De Lise F., Bosso A., Gaglione R., Merlino F., Novellino E., Ungaro F., Grieco P. Antimicrobial peptide Temporin-L complexed with anionic cyclodextrins results in a potent and safe agent against sessile bacteria. Int. J. Pharm. 2020 Jun 30;584 doi: 10.1016/j.ijpharm.2020.119437. [DOI] [PubMed] [Google Scholar]
- 38.Fu X.J., Fang Y., Yao M. Antimicrobial photodynamic therapy for methicillin-resistant Staphylococcus aureus infection. BioMed Res. Int. 2013 doi: 10.1155/2013/159157. Oct;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martínez-Carmona M., Gun’Ko Y., Vallet-Regí M. ZnO nanostructures for drug delivery and theranostic applications. Nanomaterials. 2018 Apr 23;8(4):268. doi: 10.3390/nano8040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W., Sato T., Ito S., Kato N., Morohoshi T., Ikeda T. BM-P18 Inhibition of quorum sensing in gram-negative bacteria using AHL analogues and modified cyclodextrins (Section III Biomedical Engineering) J. Biosci. Bioeng. 2009 Nov;108(1):S38. [Google Scholar]
- 41.Hill L.E., Gomes C., Taylor T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT--Food Sci. Technol. 2013 Apr 1;51(1):86–93. [Google Scholar]
- 42.Loh Z.H., Samanta A.K., Heng P.W. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015 Jul 1;10(4):255–274. [Google Scholar]
- 43.Hanaei J, Dastmalchi S, Adibkia K, Mirzazadeh A, Barzegar JM, Jouyban AG. Solubility Prediction of Sulfonamides at Various Temperatures Using a Single Determination.
- 44.Daniell M.D., Hill J.S. A history of photodynamic therapy. Aust. N. Z. J. Surg. 1991 May;61(5):340–348. doi: 10.1111/j.1445-2197.1991.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 45.DeRosa M.C., Crutchley R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002 Nov 1;233:351–371. [Google Scholar]
- 46.Castano A.P., Demidova T.N., Hamblin M.R. Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004 Dec 1;1(4):279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klausen M., Ucuncu M., Bradley M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules. 2020 Nov 10;25(22):5239. doi: 10.3390/molecules25225239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamblin M.R., Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004;3(5):436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tavares A., Carvalho C.M., Faustino M.A., Neves M.G., Tomé J.P., Tomé A.C., Cavaleiro J.A., Cunha Â., Gomes N.C., Alves E., Almeida A. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs. 2010 Jan 20;8(1):91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cassidy C.M., Tunney M.M., McCarron P.A., Donnelly R.F. Drug delivery strategies for photodynamic antimicrobial chemotherapy: from benchtop to clinical practice. J. Photochem. Photobiol., B. 2009 May 4;95(2):71–80. doi: 10.1016/j.jphotobiol.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Bombelli C., Bordi F., Ferro S., Giansanti L., Jori G., Mancini G., Mazzuca C., Monti D., Ricchelli F., Sennato S., Venanzi M. New cationic liposomes as vehicles of m-tetrahydroxyphenylchlorin in photodynamic therapy of infectious diseases. Mol. Pharm. 2008 Aug 4;5(4):672–679. doi: 10.1021/mp800037d. [DOI] [PubMed] [Google Scholar]
- 52.Thanh Nguyen H., Goycoolea F.M. Chitosan/Cyclodextrin/TPP nanoparticles loaded with quercetin as novel bacterial quorum sensing inhibitors. Molecules. 2017 Nov 15;22(11):1975. doi: 10.3390/molecules22111975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clatworthy A.E., Pierson E., Hung D.T. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007 Sep;3(9):541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 54.Heilmann S., Krishna S., Kerr B. Why do bacteria regulate public goods by quorum sensing?—how the shapes of cost and benefit functions determine the form of optimal regulation. Front. Microbiol. 2015 Jul 28;6:767. doi: 10.3389/fmicb.2015.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirakawa H., Tomita H. Interference of bacterial cell-to-cell communication: a new concept of antimicrobial chemotherapy breaks antibiotic resistance. Front. Microbiol. 2013 May 13;4:114. doi: 10.3389/fmicb.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thanh Nguyen H., Goycoolea F.M. Chitosan/Cyclodextrin/TPP nanoparticles loaded with quercetin as novel bacterial quorum sensing inhibitors. Molecules. 2017 Nov 15;22(11):1975. doi: 10.3390/molecules22111975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agrawal G.P., Bhargava S. Preparation & characterization of solid inclusion complex of cefpodoxime proxetil with β-cyclodextrin. Curr. Drug Deliv. 2008 Jan 1;5(1):1–6. doi: 10.2174/156720108783330998. [DOI] [PubMed] [Google Scholar]
- 58.Jeong D., Joo S.W., Shinde V.V., Cho E., Jung S. Carbohydrate-based host-guest complexation of hydrophobic antibiotics for the enhancement of antibacterial activity. Molecules. 2017 Aug 8;22(8):1311. doi: 10.3390/molecules22081311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vermet G., Degoutin S., Chai F., Maton M., Flores C., Neut C., Danjou P.E., Martel B., Blanchemain N. Cyclodextrin modified PLLA parietal reinforcement implant with prolonged antibacterial activity. Acta Biomater. 2017 Apr 15;53:222–232. doi: 10.1016/j.actbio.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 60.Cheirsilp B., Rakmai J. Inclusion complex formation of cyclodextrin with its guest and their applications. Biol. Eng. Med. 2016 Dec 12;2(1):1–6. [Google Scholar]
- 61.Yee E.M., Hook J.M., Bhadbhade M.M., Vittorio O., Kuchel R.P., Brandl M.B., Tilley R.D., Black D.S., Kumar N. Preparation, characterization and in vitro biological evaluation of (1: 2) phenoxodiol-β-cyclodextrin complex. Carbohydr. Polym. 2017 Jun 1;165:444–454. doi: 10.1016/j.carbpol.2017.02.081. [DOI] [PubMed] [Google Scholar]
- 62.Choi J.M., Park K., Lee B., Jeong D., Dindulkar S.D., Choi Y., Cho E., Park S., Yu J.H., Jung S. Solubility and bioavailability enhancement of ciprofloxacin by induced oval-shaped mono-6-deoxy-6-aminoethylamino-β-cyclodextrin. Carbohydr. Polym. 2017 May 1;163:118–128. doi: 10.1016/j.carbpol.2017.01.073. [DOI] [PubMed] [Google Scholar]
- 63.Szente L., Szemán J., Sohajda T. Analytical characterization of cyclodextrins: history, official methods and recommended new techniques. J. Pharmaceut. Biomed. Anal. 2016 Oct 25;130:347–365. doi: 10.1016/j.jpba.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Alcalde-Rico M., Hernando-Amado S., Blanco P., Martínez J.L. Multidrug efflux pumps at the crossroad between antibiotic resistance and bacterial virulence. Front. Microbiol. 2016 Sep 21;7:1483. doi: 10.3389/fmicb.2016.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaikh S., Fatima J., Shakil S., Rizvi S.M., Kamal M.A. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J. Biol. Sci. 2015 Jan 1;22(1):90–101. doi: 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pelgrift R.Y., Friedman A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013 Nov 30;65(13–14):1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 67.Blair J., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015 Jan;13(1):42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y., Jiang X. Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale. 2013;5(18):8340–8350. doi: 10.1039/c3nr01990j. [DOI] [PubMed] [Google Scholar]
- 69.Beyth N., Houri-Haddad Y., Domb A., Khan W., Hazan R. Alternative antimicrobial approach: nano-antimicrobial materials. Evid. base Compl. Alternative Med. 2015;2015 doi: 10.1155/2015/246012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaikh S., Fatima J., Shakil S., Rizvi S.M., Kamal M.A. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J. Biol. Sci. 2015 Jan 1;22(1):90–101. doi: 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cox G., Stogios P.J., Savchenko A., Wright G.D. Structural and molecular basis for resistance to aminoglycoside antibiotics by the adenylyltransferase ANT (2 ″)-Ia. mBio. 2015 Jan 6;6(1) doi: 10.1128/mBio.02180-14. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yun M.K., Wu Y., Li Z., Zhao Y., Waddell M.B., Ferreira A.M., Lee R.E., Bashford D., White S.W. Catalysis and sulfa drug resistance in dihydropteroate synthase. Science. 2012 Mar 2;335(6072):1110–1114. doi: 10.1126/science.1214641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hemeg H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017;12:8211. doi: 10.2147/IJN.S132163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Lounes-Hadj Sahraoui A, Fontaine J, Sanchez H, Hatfield RD, Ntambi JM. Novel entries in a fungal biofilm matrix encyclopedia. mBio 5: e01333-01314. [DOI] [PMC free article] [PubMed]
- 75.Olivares E., Badel-Berchoux S., Provot C., Prévost G., Bernardi T., Jehl F. Clinical impact of antibiotics for the treatment of Pseudomonas aeruginosa biofilm infections. Front. Microbiol. 2020 Jan 9;10:2894. doi: 10.3389/fmicb.2019.02894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sabaté Brescó M., Harris L.G., Thompson K., Stanic B., Morgenstern M., O'Mahony L., Richards R.G., Moriarty T.F. Pathogenic mechanisms and host interactions in Staphylococcus epidermidis device-related infection. Front. Microbiol. 2017 Aug 2;8:1401. doi: 10.3389/fmicb.2017.01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cavalcanti I.M., Nobbs A.H., Ricomini-Filho A.P., Jenkinson H.F., Del Bel Cury A.A. Interkingdom cooperation between Candida albicans, Streptococcus oralis and Actinomyces oris modulates early biofilm development on denture material. FEMS Path. Dis./ 2016 Apr 1;74(3):ftw002. doi: 10.1093/femspd/ftw002. [DOI] [PubMed] [Google Scholar]
- 78.Eze E.C., Chenia H.Y., El Zowalaty M.E. Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect. Drug Resist. 2018;11:2277. doi: 10.2147/IDR.S169894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yonezawa H., Osaki T., Kamiya S. Biofilm formation by Helicobacter pylori and its involvement for antibiotic resistance. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moormeier D.E., Bayles K.W. Staphylococcus aureus biofilm: a complex developmental organism. Mol. Microbiol. 2017 May;104(3):365–376. doi: 10.1111/mmi.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barbosa J., Borges S., Camilo R., Magalhães R., Ferreira V., Santos I., Silva J., Almeida G., Teixeira P. Biofilm formation among clinical and food isolates of Listeria monocytogenes. Int. J. Microb. 2013;2013 doi: 10.1155/2013/524975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bridges A.A., Bassler B.L. The intragenus and interspecies quorum-sensing autoinducers exert distinct control over Vibrio cholerae biofilm formation and dispersal. PLoS Biol. 2019 Nov 11;17(11) doi: 10.1371/journal.pbio.3000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fabrega A., Soto S.M., Ballesté-Delpierre C., Fernández-Orth D., Jiménez de Anta M.T., Vila J. Impact of quinolone-resistance acquisition on biofilm production and fitness in Salmonella enterica. J. Antimicrob. Chemother. 2014 Jul 1;69(7):1815–1824. doi: 10.1093/jac/dku078. [DOI] [PubMed] [Google Scholar]
- 84.Donlan R.M. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 2001 Oct 15;33(8):1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 85.Donlan R.M. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 2001 Oct 15;33(8):1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 86.Black C., Allan I., Ford S.K., Wilson M., McNab R. Biofilm-specific surface properties and protein expression in oral Streptococcus sanguis. Arch. Oral Biol. 2004 Apr 1;49(4):295–304. doi: 10.1016/j.archoralbio.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Cellini L., Grande R., Di Campli E., Traini T., Di Giulio M., Nicola Lannutti S., Lattanzio R. Dynamic colonization of Helicobacter pylori in human gastric mucosa. Scand. J. Gastroenterol. 2008 Jan 1;43(2):178–185. doi: 10.1080/00365520701675965. [DOI] [PubMed] [Google Scholar]
- 88.Akiyama H., Kanzaki H., Tada J., Arata J. Staphylococcus aureus infection on cut wounds in the mouse skin: experimental staphylococcal botryomycosis. J. Dermatol. Sci. 1996 Mar 1;11(3):234–238. doi: 10.1016/0923-1811(95)00448-3. [DOI] [PubMed] [Google Scholar]
- 89.Groeger S., Meyle J. Oral mucosal epithelial cells. Front. Immunol. 2019 Feb 14;10:208. doi: 10.3389/fimmu.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramos M.A., Da Silva P.B., Spósito L., De Toledo L.G., Bonifácio B.V., Rodero C.F., Dos Santos K.C., Chorilli M., Bauab T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: a review. Int. J. Nanomed. 2018;13:1179. doi: 10.2147/IJN.S146195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rabin N., Zheng Y., Opoku-Temeng C., Du Y., Bonsu E., Sintim H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015 Mar;7(4):493–512. doi: 10.4155/fmc.15.6. [DOI] [PubMed] [Google Scholar]
- 92.Singh R., Ray P., Das A., Sharma M. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J. Med. Microbiol. 2009 Aug 1;58(8):1067–1073. doi: 10.1099/jmm.0.009720-0. [DOI] [PubMed] [Google Scholar]
- 93.Qu Y., Daley A.J., Istivan T.S., Rouch D.A., Deighton M.A. Densely adherent growth mode, rather than extracellular polymer substance matrix build-up ability, contributes to high resistance of Staphylococcus epidermidis biofilms to antibiotics. J. Antimicrob. Chemother. 2010 Jul 1;65(7):1405–1411. doi: 10.1093/jac/dkq119. [DOI] [PubMed] [Google Scholar]
- 94.Singh R., Ray P., Das A., Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010 Sep 1;65(9):1955–1958. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 95.Singh R., Ray P., Das A., Sharma M., Mrt Letter Spatial distribution of vancomycin‐induced damage in Staphylococcus epidermidis biofilm: an electron microscopic study. Microsc. Res. Tech. 2010 Jul;73(7):662–664. doi: 10.1002/jemt.20871. [DOI] [PubMed] [Google Scholar]
- 96.Singh R., Ray P., Das A., Sharma M. Enhanced production of exopolysaccharide matrix and biofilm by a menadione-auxotrophic Staphylococcus aureus small-colony variant. J. Med. Microbiol. 2010 May 1;59(5):521–527. doi: 10.1099/jmm.0.017046-0. [DOI] [PubMed] [Google Scholar]
- 97.Singh R., Ray P. Vancomycin modulated autolysis in Staphylococcus aureus: does it vary with the susceptibility and planktonic or biofilm phenotype? FEMS Immunol. Med. Microbiol. 2012 Jun 1;65(1):1–4. doi: 10.1111/j.1574-695X.2012.00951.x. [DOI] [PubMed] [Google Scholar]
- 98.Lebeaux D., Ghigo J.M., Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014 Sep;78(3):510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Armstead A.L., Li B. Nanomedicine as an emerging approach against intracellular pathogens. Int. J. Nanomed. 2011;6:3281. doi: 10.2147/IJN.S27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith A.W. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Deliv. Rev. 2005 Jul 29;57(10):1539–1550. doi: 10.1016/j.addr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 101.Soto S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence. 2013 Apr 1;4(3):223–229. doi: 10.4161/viru.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dhar N., McKinney J.D. Microbial phenotypic heterogeneity and antibiotic tolerance. Curr. Opin. Microbiol. 2007 Feb 1;10(1):30–38. doi: 10.1016/j.mib.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Smith P.A., Romesberg F.E. Combating bacteria and drug resistance by inhibiting mechanisms of persistence and adaptation. Nat. Chem. Biol. 2007 Sep;3(9):549–556. doi: 10.1038/nchembio.2007.27. [DOI] [PubMed] [Google Scholar]
- 104.Mulcahy L.R., Burns J.L., Lory S., Lewis K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 2010 Dec 1;192(23):6191–6199. doi: 10.1128/JB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keren I., Kaldalu N., Spoering A., Wang Y., Lewis K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004 Jan 1;230(1):13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 106.Jung S.H., Ryu C.M., Kim J.S. Bacterial persistence: fundamentals and clinical importance. J. Microbiol. 2019 Oct;57(10):829–835. doi: 10.1007/s12275-019-9218-0. [DOI] [PubMed] [Google Scholar]
- 107.Yan J., Bassler B.L. Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe. 2019 Jul 10;26(1):15–21. doi: 10.1016/j.chom.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berne C., Ducret A., Hardy G.G., Brun Y.V. Adhesins involved in attachment to abiotic surfaces by Gram‐negative bacteria. Microb. Biofilms. 2015 Oct 7:163–199. doi: 10.1128/microbiolspec.MB-0018-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee C.K., De Anda J., Baker A.E., Bennett R.R., Luo Y., Lee E.Y., Keefe J.A., Helali J.S., Ma J., Zhao K., Golestanian R. Multigenerational memory and adaptive adhesion in early bacterial biofilm communities. Proc. Natl. Acad. Sci. USA. 2018 Apr 24;115(17):4471–4476. doi: 10.1073/pnas.1720071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Armbruster C.R., Parsek M.R. New insight into the early stages of biofilm formation. Proc. Natl. Acad. Sci. USA. 2018 Apr 24;115(17):4317–4319. doi: 10.1073/pnas.1804084115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Evans L.V. CRC press; 2000 Dec 21. Biofilms: Recent Advances in Their Study and Control. [Google Scholar]
- 112.Kumar A., Alam A., Rani M., Ehtesham N.Z., Hasnain S.E. Biofilms: survival and defense strategy for pathogens. Int. J. Med. Microb. 2017 Dec 1;307(8):481–489. doi: 10.1016/j.ijmm.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 113.Michael JR W., Dunne J. Bacterial adhesion: seen any good biofilms lately. Clin. Microbiol. Rev. 2002;15(2) doi: 10.1128/CMR.15.2.155-166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nadar S., Khan T., Patching S.G., Omri A. Development of antibiofilm therapeutics strategies to overcome antimicrobial drug resistance. Microorganisms. 2022 Jan 27;10(2):303. doi: 10.3390/microorganisms10020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thoendel M., Kavanaugh J.S., Flack C.E., Horswill A.R. Peptide signaling in the staphylococci. Chem. Rev. 2011 Jan 12;111(1):117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y., Shi L., Su L., van der Mei H.C., Jutte P.C., Ren Y., Busscher H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019;48(2):428–446. doi: 10.1039/c7cs00807d. [DOI] [PubMed] [Google Scholar]
- 117.Hooshdar P., Kermanshahi R.K., Ghadam P., Khosravi-Darani K. A review on production of exopolysaccharide and biofilm in probiotics like lactobacilli and methods of analysis. Biointerf. Res. Appl. Chem. 2020;10:6058–6075. [Google Scholar]
- 118.Flemming H.C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010 Sep;8(9):623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 119.Decho A.W., Gutierrez T. Microbial extracellular polymeric substances (EPSs) in ocean systems. Front. Microbiol. 2017 May 26;8:922. doi: 10.3389/fmicb.2017.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nishanth S., Bharti A., Gupta H., Gupta K., Gulia U., Prasanna R. Cyanobacterial extracellular polymeric substances (EPS): biosynthesis and their potential applications. InMicrob. Nat. Macromol. 2021 Jan 1:349–369. (Academic Press) [Google Scholar]
- 121.Karygianni L., Ren Z., Koo H., Thurnheer T. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol. 2020 Aug 1;28(8):668–681. doi: 10.1016/j.tim.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 122.O'Toole G., Kaplan H.B., Kolter R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000;54:49. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 123.Castro-Rosas J., Escartin E.F. Increased tolerance of Vibrio cholerae O1 to temperature, pH, or drying associated with colonization of shrimp carapaces. Int. J. Food Microbiol. 2005 Jul 15;102(2):195–201. doi: 10.1016/j.ijfoodmicro.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 124.Zhu L., Chen T., Xu L., Zhou Z., Feng W., Liu Y., Chen H. Effect and mechanism of quorum sensing on horizontal transfer of multidrug plasmid RP4 in BAC biofilm. Sci. Total Environ. 2020 Jan 1;698 doi: 10.1016/j.scitotenv.2019.134236. [DOI] [PubMed] [Google Scholar]
- 125.Landini P., Antoniani D., Burgess J.G., Nijland R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl. Microbiol. Biotechnol. 2010 Apr;86(3):813–823. doi: 10.1007/s00253-010-2468-8. [DOI] [PubMed] [Google Scholar]
- 126.Graf A.C., Leonard A., Schäuble M., Rieckmann L.M., Hoyer J., Maass S., Lalk M., Becher D., Pané-Farré J., Riedel K. Virulence factors produced by Staphylococcus aureus biofilms have a moonlighting function contributing to biofilm integrity*[S] Mol. Cell. Proteomics. 2019 Jun 1;18(6):1036–1053. doi: 10.1074/mcp.RA118.001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davies D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003 Feb;2(2):114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 128.Kurkov S.V., Loftsson T. Cyclodextrins. Int. J. Pharm. 2013 Aug 30;453(1):167–180. doi: 10.1016/j.ijpharm.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 129.Budhwar V. Cyclodextrin complexes: an approach to improve the physicochemical properties of drugs and applications of cyclodextrin complexes. Asian J. Pharm. 2018 Aug 18;(2):12. [Google Scholar]
- 130.Uekama K, Hirayama F, Irie T. Cyclodextrin Drug Carrier Systems. [DOI] [PubMed]
- 131.Villiers A. Sur la fermentation de la fécule par l’action du ferment butyrique. Compt. Rend. Acad. Sci. 1891;112:536–538. [Google Scholar]
- 132.Schardinger F. Bildung kristallisierter polysaccharide (dextrine) aus stärkekleister durch microben. Zentralbl. Bakteriol. Parasitenk. Abt. II. 1911;29:188–197. [Google Scholar]
- 133.Schardinger F. Über thermophile Bakterien aus verschiedenen Speisen und Milch. Zeitschrift für Untersuchung der Nahrungs-und Genussmittel, sowie der Gebrauchsgegenstände. 1903 Oct;6(19):865–880. [Google Scholar]
- 134.Freudenberg K., Jacobi R. Über schardingers dextrine aus stärke. Justus Liebigs Ann. Chem. 1935;518(1):102–108. [Google Scholar]
- 135.Acuña-Rougier C., Olea-Azar C. Thermodynamic and geometric study of diasteroisomeric complexes formed by racemic flavanones and three cyclodextrins through NMR. J. Inclusion Phenom. Macrocycl. Chem. 2013 Feb;75(1):119–136. [Google Scholar]
- 136.Folch-Cano C., Yazdani-Pedram M., Olea-Azar C. Inclusion and functionalization of polymers with cyclodextrins: current applications and future prospects. Molecules. 2014 Sep 9;19(9):14066–14079. doi: 10.3390/molecules190914066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Otero-Espinar F.J., Torres-Labandeira J.J., Alvarez-Lorenzo C., Blanco-Méndez J. Cyclodextrins in drug delivery systems. J. Drug Deliv. Sci. Technol. 2010 Jan 1;20(4):289–301. [Google Scholar]
- 138.Loftsson T., Duchêne D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007 Feb 1;329(1–2) doi: 10.1016/j.ijpharm.2006.10.044. 1-1. [DOI] [PubMed] [Google Scholar]
- 139.Rasheed A. Cyclodextrins as drug carrier molecule: a review. Sci. Pharm. 2008 Dec;76(4):567–598. [Google Scholar]
- 140.Zhou J., Ritter H. Cyclodextrin functionalized polymers as drug delivery systems. Polym. Chem. 2010;1(10):1552–1559. [Google Scholar]
- 141.Zafar N., Fessi H., Elaissari A. Cyclodextrin containing biodegradable particles: from preparation to drug delivery applications. Int. J. Pharm. 2014 Jan 30;461(1–2):351–366. doi: 10.1016/j.ijpharm.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 142.Varan G., Varan C., Erdoğar N., Hıncal A.A., Bilensoy E. Amphiphilic cyclodextrin nanoparticles. Int. J. Pharm. 2017 Oct 15;531(2):457–469. doi: 10.1016/j.ijpharm.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 143.Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998 Jul 30;98(5):1743–1754. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 144.Connors K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997 Aug 5;97(5):1325–1358. doi: 10.1021/cr960371r. [DOI] [PubMed] [Google Scholar]
- 145.Matsuda H., Arima H. Cyclodextrins in transdermal and rectal delivery. Adv. Drug Deliv. Rev. 1999 Mar 1;36(1):81–99. doi: 10.1016/s0169-409x(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 146.Del Valle E.M. Cyclodextrins and their uses: a review. Process Biochem. 2004 May 31;39(9):1033–1046. [Google Scholar]
- 147.Szejtli J. Cyclodextrins and their inclusion complexes. Akademiai Kiado. 1982;25 [Google Scholar]
- 148.Marshall J.J., Miwa I. Kinetic difference between hydrolyses of γ-cyclodextrin by human salivary and pancreatic α-amylases. Biochim. Biophys. Acta Enzymol. 1981 Sep 15;661(1):142–147. doi: 10.1016/0005-2744(81)90093-0. [DOI] [PubMed] [Google Scholar]
- 149.Kondo H., Nakatani H., Hiromi K. In vitro action of human and porcine α-amylases on cyclomalto-oligosaccharides. Carbohydr. Res. 1990 Sep 5;204:207–213. doi: 10.1016/0008-6215(90)84036-t. [DOI] [PubMed] [Google Scholar]
- 150.De Bie A.T., Van Ommen B., Bär A. Disposition of [14C] γ-Cyclodextrin in germ-free and conventional rats. Regul. Toxicol. Pharmacol. 1998 Apr 1;27(2):150–158. doi: 10.1006/rtph.1998.1219. [DOI] [PubMed] [Google Scholar]
- 151.Lai C.S., Chow J., Wolf B.W., inventors, Abbott Laboratories, assignee Methods of using gamma cyclodextrin to control blood glucose and insulin secretion. United States patent US. 2008 Sep 9;7:423. 027. [Google Scholar]
- 152.Li Z., Wang M., Wang F., Gu Z., Du G., Wu J., Chen J. γ-Cyclodextrin: a review on enzymatic production and applications. Appl. Microbiol. Biotechnol. 2007 Nov;77(2):245–255. doi: 10.1007/s00253-007-1166-7. [DOI] [PubMed] [Google Scholar]
- 153.Brewster M.E., Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007 Jul 30;59(7):645–666. doi: 10.1016/j.addr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 154.Miletic T., Kyriakos K., Graovac A., Ibric S. Spray-dried voriconazole–cyclodextrin complexes: solubility, dissolution rate and chemical stability. Carbohydr. Polym. 2013 Oct 15;98(1):122–131. doi: 10.1016/j.carbpol.2013.05.084. [DOI] [PubMed] [Google Scholar]
- 155.Rodriguez-Aller M., Guinchard S., Guillarme D., Pupier M., Jeannerat D., Rivara-Minten E., Veuthey J.L., Gurny R. New prostaglandin analog formulation for glaucoma treatment containing cyclodextrins for improved stability, solubility and ocular tolerance. Eur. J. Pharm. Biopharm. 2015 Sep 1;95:203–214. doi: 10.1016/j.ejpb.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 156.Braithwaite M.C., Kumar P., Choonara Y.E., du Toit L.C., Tomar L.K., Tyagi C., Pillay V. A novel multi-tiered experimental approach unfolding the mechanisms behind cyclodextrin-vitamin inclusion complexes for enhanced vitamin solubility and stability. Int. J. Pharm. 2017 Oct 30;532(1):90–104. doi: 10.1016/j.ijpharm.2017.08.109. [DOI] [PubMed] [Google Scholar]
- 157.Xu J., Zhang Y., Li X., Zheng Y. Inclusion complex of nateglinide with sulfobutyl ether β-cyclodextrin: preparation, characterization and water solubility. J. Mol. Struct. 2017 Aug 5;1141:328–334. [Google Scholar]
- 158.Ammar H.O., Salama H.A., Ghorab M., Mahmoud A.A. Formulation and biological evaluation of glimepiride–cyclodextrin–polymer systems. Int. J. Pharm. 2006 Feb 17;309(1–2):129–138. doi: 10.1016/j.ijpharm.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 159.Özdemir N., Erkin J. Enhancement of dissolution rate and bioavailability of sulfamethoxazole by complexation with β-cyclodextrin. Drug Dev. Ind. Pharm. 2012 Mar 1;38(3):331–340. doi: 10.3109/03639045.2011.604327. [DOI] [PubMed] [Google Scholar]
- 160.Desai S., Poddar A., Sawant K. Formulation of cyclodextrin inclusion complex-based orally disintegrating tablet of eslicarbazepine acetate for improved oral bioavailability. Mater. Sci. Eng. C. 2016 Jan 1;58:826–834. doi: 10.1016/j.msec.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 161.Schultheiss N., Newman A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009 Jun 3;9(6):2950–2967. doi: 10.1021/cg900129f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dhall M., Madan A.K. Comparison of cyclodextrins and urea as hosts for inclusion of drugs. J. Inclusion Phenom. Macrocycl. Chem. 2017 Dec;89(3):207–227. [Google Scholar]
- 163.Byun Y., Rodriguez K., Han J.H., Kim Y.T. Improved thermal stability of polylactic acid (PLA) composite film via PLA–β-cyclodextrin-inclusion complex systems. Int. J. Biol. Macromol. 2015 Nov 1;81:591–598. doi: 10.1016/j.ijbiomac.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 164.Popielec A., Loftsson T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017 Oct 15;531(2):532–542. doi: 10.1016/j.ijpharm.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 165.Mady F.M., Abou-Taleb A.E., Khaled K.A., Yamasaki K., Iohara D., Taguchi K., Anraku M., Hirayama F., Uekama K., Otagiri M. Evaluation of carboxymethyl-β-cyclodextrin with acid function: improvement of chemical stability, oral bioavailability and bitter taste of famotidine. Int. J. Pharm. 2010 Sep 15;397(1–2):1–8. doi: 10.1016/j.ijpharm.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 166.Yuan C., Du L., Jin Z., Xu X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013 Jan 2;91(1):385–389. doi: 10.1016/j.carbpol.2012.08.059. [DOI] [PubMed] [Google Scholar]
- 167.Budhwar V. Cyclodextrin complexes: an approach to improve the physicochemical properties of drugs and applications of cyclodextrin complexes. Asian J. Pharm. 2018 Aug 18;(2):12. [Google Scholar]
- 168.Budhwar V. Cyclodextrin complexes: an approach to improve the physicochemical properties of drugs and applications of cyclodextrin complexes. Asian J. Pharm. 2018 Aug 18;(2):12. [Google Scholar]
- 169.Pereva S., Sarafska T., Bogdanova S., Spassov Т. Efficiency of “cyclodextrin-ibuprofen” inclusion complex formation. J. Drug Deliv. Sci. Technol. 2016 Oct 1;35:34–39. [Google Scholar]
- 170.Tao F., Hill L.E., Peng Y., Gomes C.L. Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT--Food Sci. Technol. 2014 Nov 1;59(1):247–255. [Google Scholar]
- 171.Ünlüsayin M., Hădărugă N.G., Rusu G., Gruia A.T., Păunescu V., Hădărugă D.I. Nano-encapsulation competitiveness of omega-3 fatty acids and correlations of thermal analysis and Karl Fischer water titration for European anchovy (Engraulis encrasicolus L.) oil/β-cyclodextrin complexes. LWT--Food Sci. Technol. 2016 May 1;68:135–144. [Google Scholar]
- 172.Banchero M. Supercritical carbon dioxide as a green alternative to achieve drug complexation with cyclodextrins. Pharmaceuticals. 2021 Jun;14(6):562. doi: 10.3390/ph14060562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saenger W. Cyclodextrin inclusion compounds in research and industry. Angew Chem. Int. Ed. Engl. 1980 May;19(5):344–362. [Google Scholar]
- 174.Uekama K., Hirayama F. In: Cyclodextrin and Their Industrial Uses. Santé Paris., editor. France; 1987. Methods of investigating and preparing inclusion compounds; pp. 133–172. [Google Scholar]
- 175.Duchěne D., Wouessidjewe D. Pharmaceutical uses of cyclodextrins and derivatives. Drug Dev. Ind. Pharm. 1990 Jan 1;16(17):2487–2499. [Google Scholar]
- 176.Mennini N., Maestrelli F., Cirri M., Mura P. Analysis of physicochemical properties of ternary systems of oxaprozin with randomly methylated-ß-cyclodextrin and l-arginine aimed to improve the drug solubility. J. Pharmaceut. Biomed. Anal. 2016 Sep 10;129:350–358. doi: 10.1016/j.jpba.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 177.Cao H., Jiang Y., Zhang H., Nie K., Lei M., Deng L., Wang F., Tan T. Enhancement of methanol resistance of Yarrowia lipolytica lipase 2 using β-cyclodextrin as an additive: insights from experiments and molecular dynamics simulation. Enzym. Microb. Technol. 2017 Jan 1;96:157–162. doi: 10.1016/j.enzmictec.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 178.Bratu I., Hernanz A., Gavira J.M., Bora G.H. FT-IR spectroscopy of inclusion complexes of beta-cyclodextrin with fenbufen and ibuprofen. Rom. J. Phys. 2005;50(9/10):1063. [Google Scholar]
- 179.Kumar S.K., Sushma M., Raju P.Y. Dissolution enhancement of poorly soluble drugs by using complexation technique-a review. J. Pharmaceut. Sci. Res. 2013 May 1;5(5):120. [Google Scholar]
- 180.Loh G.O., Tan Y.T., Peh K.K. Enhancement of norfloxacin solubility via inclusion complexation with β-cyclodextrin and its derivative hydroxypropyl-β-cyclodextrin. Asian J. Pharm. Sci. 2016 Aug 1;11(4):536–546. [Google Scholar]
- 181.Jones S.P., Grant D.W., Hadgraft J., Parr G.D. Cyclodextrins in the pharmaceutical sciences. I: preparation, structure and properties of cyclodextrins and cyclodextrin inclusion compounds. Acta Pharm. Technol. 1984;30(3):213–223. [Google Scholar]
- 182.Junco S., Casimiro T., Ribeiro N., Nunes Da Ponte M., Cabral Marques H. A comparative study of naproxen–beta cyclodextrin complexes prepared by conventional methods and using supercritical carbon dioxide. J. Inclusion Phenom. Macrocycl. Chem. 2002 Dec;44(1):117–121. [Google Scholar]
- 183.Hill L.E., Gomes C., Taylor T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT--Food Sci. Technol. 2013 Apr 1;51(1):86–93. [Google Scholar]
- 184.Kfoury M., Auezova L., Ruellan S., Greige-Gerges H., Fourmentin S. Complexation of estragole as pure compound and as main component of basil and tarragon essential oils with cyclodextrins. Carbohydr. Polym. 2015 Mar 15;118:156–164. doi: 10.1016/j.carbpol.2014.10.073. [DOI] [PubMed] [Google Scholar]
- 185.Rakmai J., Cheirsilp B., Mejuto J.C., Torrado-Agrasar A., Simal-Gándara J. Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocolloids. 2017 Apr 1;65:157–164. [Google Scholar]
- 186.Maffeo D., Leondiadis L., Mavridis I.M., Yannakopoulou K. Positive effect of natural and negatively charged cyclodextrins on the stabilization of penicillins towards β-lactamase degradation due to inclusion and external guest–host association. An NMR and MS study. Org. Biomol. Chem. 2006;4(7):1297–1304. doi: 10.1039/b517275f. [DOI] [PubMed] [Google Scholar]
- 187.Schneider H.J., Agrawal P., Yatsimirsky A.K. Supramolecular complexations of natural products. Chem. Soc. Rev. 2013;42(16):6777–6800. doi: 10.1039/c3cs60069f. [DOI] [PubMed] [Google Scholar]
- 188.Chen Y., Liu Y. Cyclodextrin-based bioactive supramolecular assemblies. Chem. Soc. Rev. 2010;39(2):495–505. doi: 10.1039/b816354p. [DOI] [PubMed] [Google Scholar]
- 189.Mourtzis N., Paravatou M., Mavridis I.M., Roberts M.L., Yannakopoulou K. Synthesis, characterization, and remarkable biological properties of cyclodextrins bearing guanidinoalkylamino and aminoalkylamino groups on their primary side. Chem.--Eur. J. 2008 May 9;14(14):4188–4200. doi: 10.1002/chem.200701650. [DOI] [PubMed] [Google Scholar]
- 190.Bom A., Bradley M., Cameron K., Clark J.K., Van Egmond J., Feilden H., MacLean E.J., Muir A.W., Palin R., Rees D.C., Zhang M.Q. A novel concept of reversing neuromuscular block: chemical encapsulation of rocuronium bromide by a cyclodextrin‐based synthetic host. Angew. Chem. Int. Ed. 2002 Jan 18;41(2):265–270. doi: 10.1002/1521-3773(20020118)41:2<265::aid-anie265>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 191.Karginov V.A., Nestorovich E.M., Moayeri M., Leppla S.H., Bezrukov S.M. Blocking anthrax lethal toxin at the protective antigen channel by using structure-inspired drug design. Proc. Natl. Acad. Sci. USA. 2005 Oct 18;102(42):15075–15080. doi: 10.1073/pnas.0507488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamamura H., Sugiyama Y., Murata K., Yokoi T., Kurata R., Miyagawa A., Sakamoto K., Komagoe K., Inoue T., Katsu T. Synthesis of antimicrobial cyclodextrins bearing polyarylamino and polyalkylamino groups via click chemistry for bacterial membrane disruption. Chem. Commun. 2014;50(41):5444–5446. doi: 10.1039/c3cc49543d. [DOI] [PubMed] [Google Scholar]
- 193.Deng J.Z. Methicillin/per-6-(4-methoxylbenzyl)-amino-6-deoxy-β-cyclodextrin 1: 1 complex and its potentiation in vitro against methicillin-resistant Staphylococcus aureus. J. Antibiot. 2013 Sep;66(9):517–521. doi: 10.1038/ja.2013.51. [DOI] [PubMed] [Google Scholar]
- 194.Yamamura H., Suzuki K., Uchibori K., Miyagawa A., Kawai M., Ohmizo C., Katsu T. Mimicking an antimicrobial peptide polymyxin B by use of cyclodextrin. Chem. Commun. 2012;48(6):892–894. doi: 10.1039/c1cc16369h. [DOI] [PubMed] [Google Scholar]
- 195.Mourtzis N., Eliadou K., Aggelidou C., Sophianopoulou V., Mavridis I.M., Yannakopoulou K. Per (6-guanidino-6-deoxy) cyclodextrins: synthesis, characterisation and binding behaviour toward selected small molecules and DNA. Org. Biomol. Chem. 2007;5(1):125–131. doi: 10.1039/b614899a. [DOI] [PubMed] [Google Scholar]
- 196.Salton M.R. Lytic agents, cell permeability, and monolayer penetrability. J. Gen. Physiol. 1968 Jul 1;52(1):227–252. [PMC free article] [PubMed] [Google Scholar]
- 197.Denyer S.P. Mechanisms of action of antibacterial biocides. Int. Biodeterior. Biodegrad. 1995 Oct 1;36(3–4):227–245. [Google Scholar]
- 198.Campanhã M.T., Mamizuka E.M., Carmona-Ribeiro A.M. Interactions between cationic liposomes and bacteria: the physical-chemistry of the bactericidal action. J. Lipid Res. 1999 Aug 1;40(8):1495–1500. [PubMed] [Google Scholar]
- 199.Thomsen H., Benkovics G., É Fenyvesi, Farewell A., Malanga M., Ericson M.B. Delivery of cyclodextrin polymers to bacterial biofilms—an exploratory study using rhodamine labelled cyclodextrins and multiphoton microscopy. Int. J. Pharm. 2017 Oct 15;531(2):650–657. doi: 10.1016/j.ijpharm.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 200.Thomsen H., Agnes M., Uwangue O., Persson L., Mattsson M., Graf F.E., Kasimati E.M., Yannakopoulou K., Ericson M.B., Farewell A. Increased antibiotic efficacy and noninvasive monitoring of Staphylococcus epidermidis biofilms using per-cysteamine-substituted γ-cyclodextrin–A delivery effect validated by fluorescence microscopy. Int. J. Pharm. 2020 Sep 25;587 doi: 10.1016/j.ijpharm.2020.119646. [DOI] [PubMed] [Google Scholar]
- 201.Li M., Neoh K.G., Xu L., Yuan L., Leong D.T., Kang E.T., Chua K.L., Hsu L.Y. Sugar-grafted cyclodextrin nanocarrier as a “trojan horse” for potentiating antibiotic activity. Pharmaceut. Res. 2016 May;33(5):1161–1174. doi: 10.1007/s11095-016-1861-0. [DOI] [PubMed] [Google Scholar]
- 202.Fu X., Bai H., Qi R., Zhao H., Peng K., Lv F., Liu L., Wang S. Optically-controlled supramolecular self-assembly of an antibiotic for antibacterial regulation. Chem. Commun. 2019;55(96):14466–14469. doi: 10.1039/c9cc07999h. [DOI] [PubMed] [Google Scholar]
- 203.Kaneo Y., Taguchi K., Tanaka T., Yamamoto S. Nanoparticles of hydrophobized cluster dextrin as biodegradable drug carriers: solubilization and encapsulation of amphotericin B. J. Drug Deliv. Sci. Technol. 2014 Jan 1;24(4):344–351. [Google Scholar]
- 204.Lumholdt L.R., Holm R., Jørgensen E.B., Larsen K.L. In vitro investigations of α-amylase mediated hydrolysis of cyclodextrins in the presence of ibuprofen, flurbiprofen, or benzo [a] pyrene. Carbohydr. Res. 2012 Nov 15;362:56–61. doi: 10.1016/j.carres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 205.Kondo H., Nakatani H., Hiromi K. In vitro action of human and porcine α-amylases on cyclomalto-oligosaccharides. Carbohydr. Res. 1990 Sep 5;204:207–213. doi: 10.1016/0008-6215(90)84036-t. [DOI] [PubMed] [Google Scholar]
- 206.Kaneo Y., Taguchi K., Tanaka T., Yamamoto S. Nanoparticles of hydrophobized cluster dextrin as biodegradable drug carriers: solubilization and encapsulation of amphotericin B. J. Drug Deliv. Sci. Technol. 2014 Jan 1;24(4):344–351. [Google Scholar]
- 207.Wang Y., Yuan Q., Feng W., Pu W., Ding J., Zhang H., Li X., Yang B., Dai Q., Cheng L., Wang J. Targeted delivery of antibiotics to the infected pulmonary tissues using ROS-responsive nanoparticles. J. Nanobiotechnol. 2019 Dec;17(1):1–6. doi: 10.1186/s12951-019-0537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gao P., Nie X., Zou M., Shi Y., Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011 Sep;64(9):625–634. doi: 10.1038/ja.2011.58. [DOI] [PubMed] [Google Scholar]
- 209.Sanbhal N., Saitaer X., Li Y., Mao Y., Zou T., Sun G., Wang L. Controlled levofloxacin release and antibacterial properties of β-cyclodextrins-grafted polypropylene mesh devices for hernia repair. Polymers. 2018 May 3;10(5):493. doi: 10.3390/polym10050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Basha R.Y., Ts S.K., Doble M. Dual delivery of tuberculosis drugs via cyclodextrin conjugated curdlan nanoparticles to infected macrophages. Carbohydr. Polym. 2019 Aug 15;218:53–62. doi: 10.1016/j.carbpol.2019.04.056. [DOI] [PubMed] [Google Scholar]
- 211.Dong C., Ye Y., Qian L., Zhao G., He B., Xiao H. Antibacterial modification of cellulose fibers by grafting β-cyclodextrin and inclusion with ciprofloxacin. Cellulose. 2014 Jun;21(3):1921–1932. [Google Scholar]
- 212.Paczkowska M., Szymanowska-Powałowska D., Mizera M., Siąkowska D., Błaszczak W., Piotrowska-Kempisty H., Cielecka-Piontek J. Cyclodextrins as multifunctional excipients: influence of inclusion into β-cyclodextrin on physicochemical and biological properties of tebipenem pivoxil. PLoS One. 2019 Jan 25;14(1) doi: 10.1371/journal.pone.0210694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Maffeo D., Leondiadis L., Mavridis I.M., Yannakopoulou K. Positive effect of natural and negatively charged cyclodextrins on the stabilization of penicillins towards β-lactamase degradation due to inclusion and external guest–host association. An NMR and MS study. Org. Biomol. Chem. 2006;4(7):1297–1304. doi: 10.1039/b517275f. [DOI] [PubMed] [Google Scholar]
- 214.Echezarreta-Lopez M.M., Otero-Mazoy I., Ramírez H.L., Villalonga R., Torres-Labandeira J.J. Solubilization and stabilization of sodium dicloxacillin by cyclodextrin inclusion. Curr. Drug Discov. Technol. 2008 Jun 1;5(2):140–145. doi: 10.2174/157016308784746328. [DOI] [PubMed] [Google Scholar]
- 215.Paczkowska M., Mizera M., Szymanowska-Powałowska D., Lewandowska K., Błaszczak W., Gościańska J., Pietrzak R., Cielecka-Piontek J. β-Cyclodextrin complexation as an effective drug delivery system for meropenem. Eur. J. Pharm. Biopharm. 2016 Feb 1;99:24–34. doi: 10.1016/j.ejpb.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 216.Thomsen H., Agnes M., Uwangue O., Persson L., Mattsson M., Graf F.E., Kasimati E.M., Yannakopoulou K., Ericson M.B., Farewell A. Increased antibiotic efficacy and noninvasive monitoring of Staphylococcus epidermidis biofilms using per-cysteamine-substituted γ-cyclodextrin–A delivery effect validated by fluorescence microscopy. Int. J. Pharm. 2020 Sep 25;587 doi: 10.1016/j.ijpharm.2020.119646. [DOI] [PubMed] [Google Scholar]
- 217.Deng J.Z. Methicillin/per-6-(4-methoxylbenzyl)-amino-6-deoxy-β-cyclodextrin 1: 1 complex and its potentiation in vitro against methicillin-resistant Staphylococcus aureus. J. Antibiot. 2013 Sep;66(9):517–521. doi: 10.1038/ja.2013.51. [DOI] [PubMed] [Google Scholar]
- 218.Mizera M., Szymanowska D., Stasiłowicz A., Siąkowska D., Lewandowska K., Miklaszewski A., Plech T., Tykarska E., Cielecka-Piontek J. Computer-aided design of cefuroxime axetil/cyclodextrin system with enhanced solubility and antimicrobial activity. Biomolecules. 2019 Dec 23;10(1):24. doi: 10.3390/biom10010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Athanassiou G., Michaleas S., Lada‐Chitiroglou E., Tsitsa T., Antoniadou‐Vyza E. Antimicrobial activity of β‐lactam antibiotics against clinical pathogens after molecular inclusion in several cyclodextrins. A novel approach to bacterial resistance. J. Pharm. Pharmacol. 2003 Mar;55(3):291–300. doi: 10.1211/002235702649. [DOI] [PubMed] [Google Scholar]
- 220.Aleem O., Kuchekar B., Pore Y., Late S. Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. J. Pharmaceut. Biomed. Anal. 2008 Jul 15;47(3):535–540. doi: 10.1016/j.jpba.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 221.Tewes F., Brillault J., Couet W., Olivier J.C. Formulation of rifampicin–cyclodextrin complexes for lung nebulization. J. Contr. Release. 2008 Jul 14;129(2):93–99. doi: 10.1016/j.jconrel.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 222.Choi J.M., Park K., Lee B., Jeong D., Dindulkar S.D., Choi Y., Cho E., Park S., Yu J.H., Jung S. Solubility and bioavailability enhancement of ciprofloxacin by induced oval-shaped mono-6-deoxy-6-aminoethylamino-β-cyclodextrin. Carbohydr. Polym. 2017 May 1;163:118–128. doi: 10.1016/j.carbpol.2017.01.073. [DOI] [PubMed] [Google Scholar]
- 223.Koontz J.L., Marcy J.E. Formation of natamycin: cyclodextrin inclusion complexes and their characterization. J. Agric. Food Chem. 2003 Nov 19;51(24):7106–7110. doi: 10.1021/jf030332y. [DOI] [PubMed] [Google Scholar]
- 224.Masood F., Yasin T., Bukhari H., Mujahid M. Characterization and application of roxithromycin loaded cyclodextrin based nanoparticles for treatment of multidrug resistant bacteria. Mater. Sci. Eng. C. 2016 Apr 1;61:1–7. doi: 10.1016/j.msec.2015.11.076. [DOI] [PubMed] [Google Scholar]
- 225.Brackman G., Garcia‐Fernandez M.J., Lenoir J., De Meyer L., Remon J.P., De Beer T., Concheiro A., Alvarez‐Lorenzo C., Coenye T. Dressings loaded with cyclodextrin–hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromol. Biosci. 2016 Jun;16(6):859–869. doi: 10.1002/mabi.201500437. [DOI] [PubMed] [Google Scholar]
- 226.Dong C., Ye Y., Qian L., Zhao G., He B., Xiao H. Antibacterial modification of cellulose fibers by grafting β-cyclodextrin and inclusion with ciprofloxacin. Cellulose. 2014 Jun;21(3):1921–1932. [Google Scholar]
- 227.Zhang M.K., Lyu Y., Zhu X., Wang J.P., Jin Z.Y., Narsimhan G. Enhanced solubility and antimicrobial activity of alamethicin in aqueous solution by complexation with γ-cyclodextrin. J. Funct.Foods. 2018 Jan 1;40:700–706. [Google Scholar]
- 228.Oprea A.E., Pandel L.M., Dumitrescu A.M., Andronescu E., Grumezescu V., Chifiriuc M.C., Mogoantă L., Bălşeanu T.A., Mogoşanu G.D., Socol G., Grumezescu A.M. Bioactive zno coatings deposited by maple—an appropriate strategy to produce efficient anti-biofilm surfaces. Molecules. 2016 Feb 16;21(2):220. doi: 10.3390/molecules21020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ayala-Zavala J.F., Soto-Valdez H., González-León A., Alvarez-Parrilla E., Martin-Belloso O., González-Aguilar G.A. Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in β-cyclodextrin. J. Inclusion Phenom. Macrocycl. Chem. 2008 Apr;60(3):359–368. [Google Scholar]
- 230.Liang H., Yuan Q., Vriesekoop F., Lv F. Effects of cyclodextrins on the antimicrobial activity of plant-derived essential oil compounds. Food Chem. 2012 Dec 1;135(3):1020–1027. doi: 10.1016/j.foodchem.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 231.Lovell J.F., Liu T.W., Chen J., Zheng G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010 May 12;110(5):2839–2857. doi: 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- 232.Hamblin M.R., Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004;3(5):436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jori G., Fabris C., Soncin M., Ferro S., Coppellotti O., Dei D., Fantetti L., Chiti G., Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Laser Surg. Med.: Off. J. Am. Soci. Las. Med. Surg. 2006 Jun;38(5):468–481. doi: 10.1002/lsm.20361. [DOI] [PubMed] [Google Scholar]
- 234.Li X., Bai H., Yang Y., Yoon J., Wang S., Zhang X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv. Mater. 2019 Feb;31(5) doi: 10.1002/adma.201805092. [DOI] [PubMed] [Google Scholar]
- 235.Maisch T. A new strategy to destroy antibiotic resistant microorganisms: antimicrobial photodynamic treatment. Mini Rev. Med. Chem. 2009 Jul 1;9(8):974–983. doi: 10.2174/138955709788681582. [DOI] [PubMed] [Google Scholar]
- 236.Dolmans D.E., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003 May;3(5):380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 237.Dougherty T.J., Gomer C.J., Henderson B.W., Jori G., Kessel D., Korbelik M., Moan J J., Peng Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Henderson B.W., Dougherty T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992 Jan;55(1):145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 239.McCaughan J.S. Photodynamic therapy. Drugs Aging. 1999 Jul;15(1):49–68. doi: 10.2165/00002512-199915010-00005. [DOI] [PubMed] [Google Scholar]
- 240.Hopper C. Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol. 2000 Dec 1;1(4):212–219. doi: 10.1016/s1470-2045(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 241.Jiang L., Gan C.R., Gao J., Loh X.J. A perspective on the trends and challenges facing porphyrin‐based anti‐microbial materials. Small. 2016 Jul;12(27):3609–3644. doi: 10.1002/smll.201600327. [DOI] [PubMed] [Google Scholar]
- 242.DeRosa M.C., Crutchley R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002 Nov 1;233:351–371. [Google Scholar]
- 243.Klausen M., Ucuncu M., Bradley M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules. 2020 Nov 10;25(22):5239. doi: 10.3390/molecules25225239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Plaetzer K., Krammer B., Berlanda J., Berr F., Kiesslich T. Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Laser Med. Sci. 2009 Mar;24(2):259–268. doi: 10.1007/s10103-008-0539-1. [DOI] [PubMed] [Google Scholar]
- 245.Bergamini C.M., Gambetti S., Dondi A., Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharmaceut. Des. 2004 May 1;10(14):1611–1626. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- 246.Sharman W.M., Allen C.M., van Lier J.E. [35] Role of activated oxygen species in photodynamic therapy. Methods Enzymol. 2000 Jan 1;319:376–400. doi: 10.1016/s0076-6879(00)19037-8. [DOI] [PubMed] [Google Scholar]
- 247.Klausen M., Ucuncu M., Bradley M. Design of photosensitizing agents for targeted antimicrobial photodynamic therapy. Molecules. 2020 Nov 10;25(22):5239. doi: 10.3390/molecules25225239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Redmond R.W., Gamlin J.N. A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 1999 Oct 1;70(4):391–475. [PubMed] [Google Scholar]
- 249.Ito T. Cellular and subcellular mechanisms of photodynamic action: the 1 O2 hypothesis as a driving force in recent research. Photochem. Photobiol. 1978 Oct;28(4‐5):493–506. doi: 10.1111/j.1751-1097.1978.tb06957.x. [DOI] [PubMed] [Google Scholar]
- 250.Fiel R.J., Datta-Gupta N., Mark E.H., Howard J.C. Induction of DNA damage by porphyrin photosensitizers. Cancer Res. 1981 Sep;41(9_Part_1):3543–3545. [PubMed] [Google Scholar]
- 251.Menezes S., Capella M.A., Caldas L.R. Photodynamic action of methylene blue: repair and mutation in Escherichia coli. J. Photochem. Photobiol. B Biol. 1990 May 1;5(3–4):505–517. doi: 10.1016/1011-1344(90)85062-2. [DOI] [PubMed] [Google Scholar]
- 252.Hass B.S., Webb R.B. Photodynamic effects of dyes on bacteria III. Mutagenesis by acridine orange and 500-nm monochromatic light in strains of Escherichia coli that differ in repair capability. Mutat. Res. Fund Mol. Mech. Mutagen. 1979 Mar 1;60(1) doi: 10.1016/0027-5107(79)90204-5. 1-1. [DOI] [PubMed] [Google Scholar]
- 253.Imray F.P., MacPhee D.G. The role of DNA polymerase I and the rec system in survival of bacteria and bacteriophages damaged by the photodynamic action of acridine orange. Mol. Gen. Genet. MGG. 1973 Dec;123(4):289–298. doi: 10.1007/BF00433647. [DOI] [PubMed] [Google Scholar]
- 254.Valduga G., Breda B., Giacometti G.M., Jori G., Reddi E. Photosensitization of wild and mutant strains ofEscherichia colibymeso-tetra (N-methyl-4-pyridyl) porphine. Biochem. Biophys. Res. Commun. 1999 Mar 5;256(1):84–88. doi: 10.1006/bbrc.1999.0190. [DOI] [PubMed] [Google Scholar]
- 255.Bertolini G., Rossi F., Valduga G., Jori G., Van Lier J. Photosensitizing activity of water-and lipid-soluble phthalocyanines on Escherichia coli. FEMS Microbiol. Lett. 1990 Sep 1;71(1–2):149–155. doi: 10.1111/j.1574-6968.1990.tb03814.x. [DOI] [PubMed] [Google Scholar]
- 256.Nitzan Y., Gutterman M., Malik Z., Ehrenberg B. Inactivation of gram‐negative bacteria by photosensitized porphyrins. Photochem. Photobiol. 1992 Jan;55(1):89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 257.Qi M., Chi M., Sun X., Xie X., Weir M.D., Oates T.W., et al. Novel nanomaterial-based antibacterial photodynamic therapies to combat oral bacterial biofilms and infectious diseases. Int. J. Nanomed. 2019;14:6937–6956. doi: 10.2147/IJN.S212807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wood S., Nattress B., Kirkham J., Shore R., Brookes S., Griffiths J., Robinson C. An in vitro study of the use of photodynamic therapy for the treatment of natural oral plaque biofilms formed in vivo. J. Photochem. Photobiol. B Biol. 1999 May 1;50(1):1–7. doi: 10.1016/S1011-1344(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 259.Zanin I.C., Goncalves R.B., Junior A.B., Hope C.K., Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J. Antimicrob. Chemother. 2005 Aug 1;56(2):324–330. doi: 10.1093/jac/dki232. [DOI] [PubMed] [Google Scholar]
- 260.Krespi Y.P., Kizhner V. Phototherapy for chronic rhinosinusitis. Laser Surg. Med. 2011 Mar;43(3):187–191. doi: 10.1002/lsm.21042. [DOI] [PubMed] [Google Scholar]
- 261.Geralde M.C., Leite I.S., Inada N.M., Salina A.C., Medeiros A.I., Kuebler W.M., Kurachi C., Bagnato V.S. Pneumonia treatment by photodynamic therapy with extracorporeal illumination‐an experimental model. Phys. Rep. 2017 Mar;5(5) doi: 10.14814/phy2.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ferro S., Jori G., Sortino S., Stancanelli R., Nikolov P., Tognon G., Ricchelli F., Mazzaglia A. Inclusion of 5-[4-(1-dodecanoylpyridinium)]-10, 15, 20-triphenylporphine in supramolecular aggregates of cationic amphiphilic cyclodextrins: physicochemical characterization of the complexes and strengthening of the antimicrobial photosensitizing activity. Biomacromolecules. 2009 Sep 14;10(9):2592–2600. doi: 10.1021/bm900533r. [DOI] [PubMed] [Google Scholar]
- 263.Ferro S., Jori G., Sortino S., Stancanelli R., Nikolov P., Tognon G., Ricchelli F., Mazzaglia A. Inclusion of 5-[4-(1-dodecanoylpyridinium)]-10, 15, 20-triphenylporphine in supramolecular aggregates of cationic amphiphilic cyclodextrins: physicochemical characterization of the complexes and strengthening of the antimicrobial photosensitizing activity. Biomacromolecules. 2009 Sep 14;10(9):2592–2600. doi: 10.1021/bm900533r. [DOI] [PubMed] [Google Scholar]
- 264.Galstyan A., Kauscher U., Block D., Ravoo B.J., Strassert C.A. Silicon (IV) phthalocyanine-decorated cyclodextrin vesicles as a self-assembled phototherapeutic agent against MRSA. ACS Appl. Mater. Interfaces. 2016 May 25;8(20):12631–12637. doi: 10.1021/acsami.6b02132. [DOI] [PubMed] [Google Scholar]
- 265.Jori G., Brown S.B. Photosensitized inactivation of microorganisms. Photochem. Photobiol. Sci. 2004 May;3(5):403–405. doi: 10.1039/b311904c. [DOI] [PubMed] [Google Scholar]
- 266.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J. Antimicrob. Chemother. 1998 Jul 1;42(1):13–28. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 267.Soukos N.S., Ximenez-Fyvie L.A., Hamblin M.R., Socransky S.S., Hasan T. Targeted antimicrobial photochemotherapy. Antimicrob. Agents Chemother. 1998 Oct 1;42(10):2595–2601. doi: 10.1128/aac.42.10.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jori G. Photodynamic therapy of microbial infections: state of the art and perspectives. J. Environ. Pathol. Toxicol. Oncol. 2006;25(1–2) doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.320. [DOI] [PubMed] [Google Scholar]
- 269.Jori G., Coppellotti O. Inactivation of pathogenic microorganisms by photodynamic techniques: mechanistic aspects and perspective applications. Anti-Infect. Agents Med. Chem. 2007 Apr 1;6(2):119–131. [Google Scholar]
- 270.Sharma S K., Dai T., B Kharkwal G., Huang Y.Y., Huang L., J Bil De Arce V., P Tegos G., R Hamblin M. Drug discovery of antimicrobial photosensitizers using animal models. Curr. Pharmaceut. Des. 2011 May 1;17(13):1303–1319. doi: 10.2174/138161211795703735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wilson M., Burns T., Pratten J., Pearson G.J. Bacteria in supragingival plaque samples can be killed by low‐power laser light in the presence of a photosensitizer. J. Appl. Bacteriol. 1995 May;78(5):569–574. doi: 10.1111/j.1365-2672.1995.tb03101.x. [DOI] [PubMed] [Google Scholar]
- 272.Nealson K.H., Platt T., Hastings J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Eberhard A., Burlingame A.L., Eberhard C., Kenyon G.L., Nealson K.H., Oppenheimer N.J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 1;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 274.Banerjee D., Shivapriya P.M., Gautam P.K., Misra K., Sahoo A.K., Samanta S.K. A review on basic biology of bacterial biofilm infections and their treatments by nanotechnology-based approaches. Proc. Natl. Acad. Sci. India B Biol. Sci. 2020 Jun;90(2):243–259. [Google Scholar]
- 275.Heilmann S., Krishna S., Kerr B. Why do bacteria regulate public goods by quorum sensing?—how the shapes of cost and benefit functions determine the form of optimal regulation. Front. Microbiol. 2015 Jul 28;6:767. doi: 10.3389/fmicb.2015.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Annous B.A., Fratamico P.M., Smith J.L. Scientific status summary. J. Food Sci. 2009 Jan 1;74(1):R24–R37. doi: 10.1111/j.1750-3841.2008.01022.x. [DOI] [PubMed] [Google Scholar]
- 277.Zhu J., Miller M.B., Vance R.E., Dziejman M., Bassler B.L., Mekalanos J.J. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA. 2002 Mar 5;99(5):3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Thoendel M., Kavanaugh J.S., Flack C.E., Horswill A.R. Peptide signaling in the staphylococci. Chem. Rev. 2011 Jan 12;111(1):117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hirakawa H., Tomita H. Interference of bacterial cell-to-cell communication: a new concept of antimicrobial chemotherapy breaks antibiotic resistance. Front. Microbiol. 2013 May 13;4:114. doi: 10.3389/fmicb.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nickel J.C., Ruseska I., Wright J.B., Costerton J.W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 1985 Apr;27(4):619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mah T.F., O'Toole G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001 Jan 1;9(1):34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 282.Drenkard E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microb. Infect. 2003 Nov 1;5(13):1213–1219. doi: 10.1016/j.micinf.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 283.Singh P.K., Schaefer A.L., Parsek M.R., Moninger T.O., Welsh M.J., Greenberg E.P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000 Oct;407(6805):762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 284.Henke J.M., Bassler B.L. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 2004 Oct 15;186(20):6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Dong Y.H., Xu J.L., Li X.Z., Zhang L.H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA. 2000 Mar 28;97(7):3526–3531. doi: 10.1073/pnas.060023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tang K., Zhang X.H. Quorum quenching agents: resources for antivirulence therapy. Mar. Drugs. 2014 May 30;12(6):3245–3282. doi: 10.3390/md12063245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kato N., Morohoshi T., Nozawa T., Matsumoto H., Ikeda T. Control of gram-negative bacterial quorum sensing with cyclodextrin immobilized cellulose ether gel. J. Inclusion Phenom. Macrocycl. Chem. 2006 Oct;56(1):55–59. [Google Scholar]
- 288.Fetzner S. Quorum quenching enzymes. J. Biotechnol. 2015 May 10;201:2–14. doi: 10.1016/j.jbiotec.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 289.Vandeputte O.M., Kiendrebeogo M., Rasamiravaka T., Stevigny C., Duez P., Rajaonson S., Diallo B., Mol A., Baucher M., El Jaziri M. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology. 2011 Jul 1;157(7):2120–2132. doi: 10.1099/mic.0.049338-0. [DOI] [PubMed] [Google Scholar]
- 290.Gopu V., Meena C.K., Shetty P.H. Quercetin influences quorum sensing in food borne bacteria: in-vitro and in-silico evidence. PLoS One. 2015 Aug 6;10(8) doi: 10.1371/journal.pone.0134684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Noori-Daloii M.R., Momeny M., Yousefi M., Shirazi F.G., Yaseri M., Motamed N., Kazemialiakbar N., Hashemi S. Multifaceted preventive effects of single agent quercetin on a human prostate adenocarcinoma cell line (PC-3): implications for nutritional transcriptomics and multi-target therapy. Med. Oncol. 2011 Dec;28(4):1395–1404. doi: 10.1007/s12032-010-9603-3. [DOI] [PubMed] [Google Scholar]
- 292.Souza M.P., Vaz A.F., Correia M.T., Cerqueira M.A., Vicente A.A., Carneiro-da-Cunha M.G. Quercetin-loaded lecithin/chitosan nanoparticles for functional food applications. Food Bioprocess Technol. 2014 Apr;7(4):1149–1159. [Google Scholar]
- 293.Edwards R.L., Lyon T., Litwin S.E., Rabovsky A., Symons J.D., Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007 Nov 1;137(11):2405–2411. doi: 10.1093/jn/137.11.2405. [DOI] [PubMed] [Google Scholar]
- 294.Galindo P., Rodriguez-Gomez I., Gonzalez-Manzano S., Duenas M., Jimenez R., Menendez C., Vargas F., Tamargo J., Santos-Buelga C., Perez-Vizcaino F., Duarte J. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS One. 2012 Mar 12;7(3) doi: 10.1371/journal.pone.0032673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Thanh Nguyen H., Goycoolea F.M. Chitosan/Cyclodextrin/TPP nanoparticles loaded with quercetin as novel bacterial quorum sensing inhibitors. Molecules. 2017 Nov 15;22(11):1975. doi: 10.3390/molecules22111975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Du Preez J., Yang W.E. Improving the aqueous solubility of triclosan by solubilization, complexation, and in situ salt formation. J. Cosmet. Sci. 2003 Nov;54:537–550. [PubMed] [Google Scholar]
- 297.Fidaleo M., Zuorro A., Lavecchia R. Enhanced antibacterial and anti-quorum sensing activities of triclosan by complexation with modified β-cyclodextrins. World J. Microbiol. Biotechnol. 2013 Sep;29(9):1731–1736. doi: 10.1007/s11274-013-1335-z. [DOI] [PubMed] [Google Scholar]
- 298.Makabenta J.M., Nabawy A., Li C.H., Schmidt-Malan S., Patel R., Rotello V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021 Jan;19(1):23–36. doi: 10.1038/s41579-020-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Smith R.S., Iglewski B.H.P. Aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 2003 Feb 1;6(1):56–60. doi: 10.1016/s1369-5274(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 300.Dong Y.H., Wang L.H., Xu J.L., Zhang H.B., Zhang X.F., Zhang L.H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001 Jun;411(6839):813–817. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 301.Dauenhauer S.A., Hull R.A., Williams R.P. Cloning and expression in Escherichia coli of Serratia marcescens genes encoding prodigiosin biosynthesis. J. Bacteriol. 1984 Jun;158(3):1128–1132. doi: 10.1128/jb.158.3.1128-1132.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Kato N., Morohoshi T., Nozawa T., Matsumoto H., Ikeda T. Control of gram-negative bacterial quorum sensing with cyclodextrin immobilized cellulose ether gel. J. Inclusion Phenom. Macrocycl. Chem. 2006 Oct;56(1):55–59. [Google Scholar]
- 303.Brackman G., Garcia‐Fernandez M.J., Lenoir J., De Meyer L., Remon J.P., De Beer T., Concheiro A., Alvarez‐Lorenzo C., Coenye T. Dressings loaded with cyclodextrin–hamamelitannin complexes increase Staphylococcus aureus susceptibility toward antibiotics both in single as well as in mixed biofilm communities. Macromol. Biosci. 2016 Jun;16(6):859–869. doi: 10.1002/mabi.201500437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.